Abstract
With its strong effect on vector-borne diseases, and insecticidal effect on mosquito vectors of malaria, inhibition of sporogonic and blood-stage development of Plasmodium falciparum, as well as in vitro and in vivo impairment of the P. berghei development inside hepatocytes, ivermectin (IVM) continues to represent an antimalarial therapeutic worthy of investigation. The in vitro activity of the first-generation IVM hybrids synthesized by appending the IVM macrolide with heterocyclic and organometallic antimalarial pharmacophores, against the blood-stage and liver-stage infections by Plasmodium parasites prompted us to design second-generation molecular hybrids of IVM. Here, a structural modification of IVM to produce novel molecular hybrids by using sub-structures of 4- and 8-aminoquinolines, the time-tested antiplasmodial agents used for treating the blood and hepatic stage of Plasmodium infections, respectively, is presented. Successful isolation of regioisomers and epimers has been demonstrated, and the evaluation of their in vitro antiplasmodial activity against both the blood stages of P. falciparum and the hepatic stages of P. berghei have been undertaken. These compounds displayed structure-dependent antiplasmodial activity, in the nM range, which was more potent than that of IVM, its aglycon or primaquine, highlighting the superiority of this hybridization strategy in designing new antiplasmodial agents.
Subject terms: Structure-based drug design, Synthetic chemistry methodology
Introduction
Malaria is a protozoan infection caused by the parasites of the genus Plasmodium1, 2. While five species of Plasmodium, P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi are responsible for human malaria, severe and complicated malaria is mostly caused by P. falciparum and P. vivax1–4. Further, P. falciparum is accountable for most of the malaria-related deaths worldwide, estimated at about 409,000 deaths arising from approximately 229 million cases of malaria in 2019, as reported by the World Health Organization (WHO)5. Children under five years and pregnant women are among the most vulnerable group affected by malaria1, 5–7. An obligatory step of infection by malaria parasites starts with the clinically silent liver-stage infection, wherein Plasmodium sporozoites, delivered by female Anopheles mosquitoes invade hepatocytes and replicate inside a parasitophorous vacuole, inside the hepatic host cell. The latter provides protection as well as resources to the infectious parasite to multiply into thousands of daughter merozoites, which are eventually released to the bloodstream where they infect erythrocytes, leading to the onset of the symptomatic, cyclic blood stage of infection1–4. P. vivax infection tends to relapse even after successful treatment due to the persistence of uninucleate parasites known as hypnozoites, long-lived, dormant, hepatic parasite forms that can reactivate months or even years after initial infection1, 8–10. Malaria control efforts are additionally complicated by the coexistence of P. vivax with P. falciparum infection in endemic regions11.
The key strategies used to manage malaria include preventive measures, such as insecticidal treated bed nets12, mosquito repellent indoor sprays13, and chemoprevention1, as well as therapeutic interventions, through the use of antimalarial medicines14. The most advanced malaria vaccine candidate, RTS, S/AS01(RTS,S), affords only partial protection, with efficacy as little as ∼34%, when tested on children living in P. falciparum-endemic areas, suggesting the need for the administration of multiple booster doses15, 16. Very recently, in October 2021, The WHO has recommended the use of RTS,S among children in sub-Saharan Africa and in regions with moderate to high P. falciparum malaria transmission17. However, this vaccine shows only moderate protection against severe disease caused by P. falciparum, while efforts to develop vaccines against P. vivax remain limited, despite the enormous burden caused by this parasite species16, 18. Traditional antimalarials based on chloroquine (1, CQ, Fig. 1) and 4-aminoquinolines14 are restricted by the development of drug resistance19–21, mainly due to over-use and single major mode of action i.e. inhibition of heme polymerization19–22. This leaves artemisinin-based combination therapies (ACTs)14, 23, representing fixed combinations of artemisinin or its analogs with a slow-acting antimalarial drug, as the most effective therapeutics for efficacious treatment of malaria23, 24. However, ACTs exclusively target the blood stage of infection, and only limited success has thus far been achieved in the development of drugs targeting the liver and transmission stages of the complex life cycle of malaria parasites14, 23, 24. It is thought that drug-resistant mutations arise in the sexual stages of the parasite’s life cycle where they are diploid and enter the asexual stages19–21. The 8-aminoquinolines, primaquine25 (2, PQ, Fig. 1) and tafenoquine 326, are the only drugs approved to eliminate P. vivax hypnozoites. However, these drugs face limitations of long dosage regimens, non-compliance with patients suffering from glucose-6-phosphate dehydrogenase deficiency (causes intravascular haemolysis, often requiring prescreening), and the fact that they are not recommended during pregnancy and lactation, restricting their widespread use27. Only two approved drugs, atovaquone (4, ATQ, Fig. 1) and pyronaridine (5, Fig. 1), have demonstrated multistage antiplasmodial activity14, 28, 29. Thus, finding new chemotherapeutic interventions that target the malaria parasite and/or the host, through the rational design of new drugs30 and/or repurposing of the time-tested drugs31, constitute one of the attractive strategies at the disposal of medicinal chemists.
Figure 1.
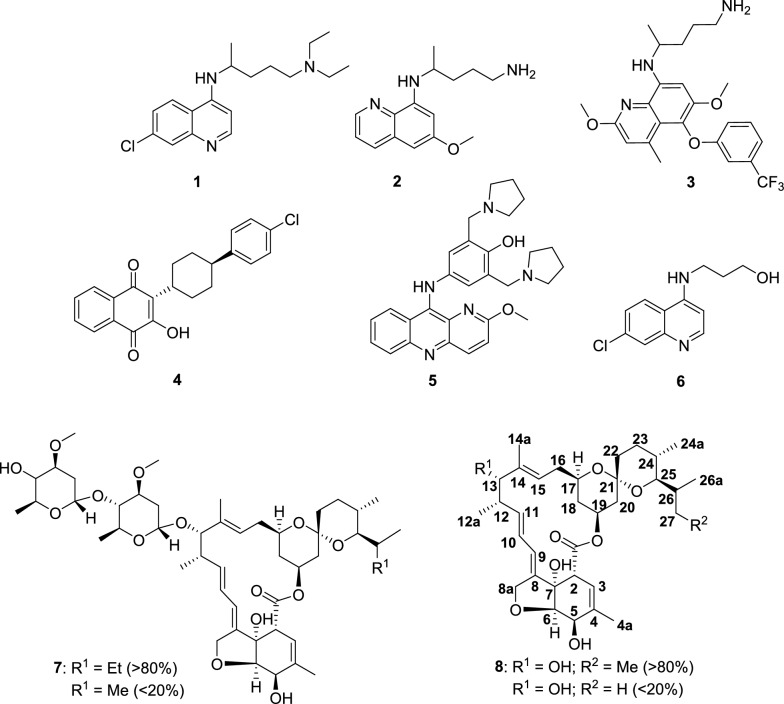
Structures of chloroquine 1, primaquine 2, tafenoquine 3, atovaquone 4, pyronaridine 5, chloroquine analogue 6, IVM 7, IVM aglycon 8 and numbering scheme.
Ivermectin (7, IVM, Fig. 1) (R1 = Et (B1a, > 80%); R1 = Me (B1b, < 20%), a semi-synthetic derivative of avermectin B1, is a powerful endectocide used in mass drug administration (MDA) against onchocerciasis32, lymphatic filariasis33, and several other parasitic diseases in humans34–36. The impact of IVM on malaria control is predominantly due to its insecticidal effect on the mosquito vectors carrying Plasmodium parasites37, 38, as well as to its inhibitory activity against mammalian and sporogonic39 parasite stages. IVM showed in vitro inhibitory effects against blood stages of P. falciparum infection (IC50 1.56–2.85 μM) of both the chloroquine-resistant (CQR) and chloroquine-sensitive (CQS) strains of the parasite40, and suppressed parasitemia by 40% relative to placebo-treated controls in P. berghei-infected mice. In an independent study41, in vitro inhibitory effects of IVM against asexual and sexual stages of the P. falciparum (IC50 100 nM and 500 nM, respectively) have been reported. However, in vivo studies revealed contradictory results, as no effect on parasitemia or gametocytemia was observed41. Further, IVM effectively reduces P. berghei infection of infected human hepatoma cells in vitro, with antiplasmodial activity comparable to the standard drug, 2 (IC50 = 2.1 and 2.4 μM, respectively). Additionally, IVM reduced liver infection in P. berghei-infected mice by 80% 44–46 h post IVM treatment42.
Further, IVM reduced in vitro development of P. cynomolgy schizonts (IC50 = 10.42 μM) and hypnozoites (IC50 = 29.24 μM) in rhesus macaque hepatocytes43. Finally, a recently-developed bioluminescence-based assay44 enabled demonstrating that IVM and other avermectins inhibit the sporogonic development of P. berghei in an in vitro setting45. Employing the rationale of hybrid drugs46, 47 by covalently linking of IVM sub unit with an antimalarial pharmacophore, “first-generation” molecular hybrids displayed structure-dependent “dual-channel” antiplasmodial activity in vitro against both the erythrocytic stages of P. falciparum and the hepatic stages of P. berghei infection48. However, these lacked a substantial insecticidal effect against A. stephensi mosquitoes in laboratory conditions, although, in a manner similar to IVM48, 49, it showed allosteric binding (in silico docking) to glutamate-gated chloride channels (GluCl) of the Cys-loop family, a primary target of IVM in A. gambiae50.
Inspired by these results48, we designed a set of “second-generation” IVM molecular hybrids using co-partners with established antiplasmodial activity and good pharmacokinetic properties. Herein, the first synthesis and improved antiplasmodial activity of the molecular hybrids of 7 in combination with CQ analogue 6 and 2, linked through the primary hydroxyl51 and amine function, respectively to the C13-OH position of the IVM aglycon 8 is reported. We have also, for the first time isolated regioisomers and epimers of these hybrids (abbreviated as IVM-CQ and IVM-PQ) and tested their antiplasmodial activity. The “second-generation” hybrids are distinctly more active than their “first-generation” counterparts48, and demonstrate significant improvement of the “dual-channel” antiplasmodial activity. These results also provide a deeper understanding of the structure dependent antiplasmodial effects of molecular hybrids of 7 with other pharmacophores.
Results and discussion
Chemistry
Synthesis and isolation
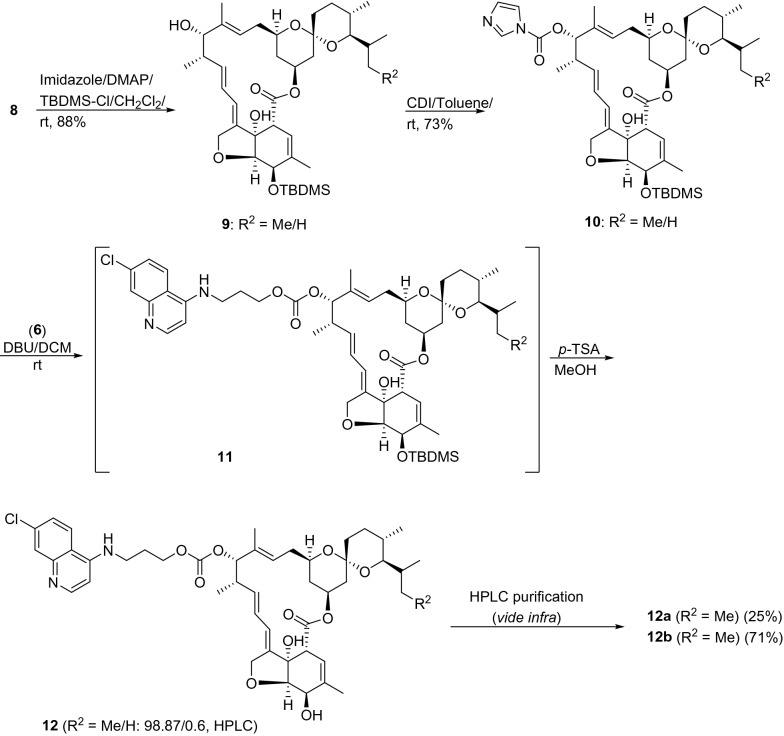
Routes for the synthesis of hybrids 12 and 15 from the common precursor 8 (Fig. 2) in combination with 6 and 2 are shown in Figs. 2 and 4. Compound 8 was prepared from 7 by treatment with 5% H2SO4 in methanol, as previously described48. Compound 8 was converted into a common precursor, IVM aglycon-1H-imidazole-1-carboxylate 10, by sequentially treating 8 with (1) tert-butyl dimethylsilyl chloride (TBDMS-Cl) in the presence of imidazole as an activator of TBDMS-Cl and 4-dimethylamino pyridine (DMAP) as a nucleophilic base48, 52, and (2) an excess of carbonyldiimidazole (CDI, 2.0 equiv) in dry benzene/dry toluene (Fig. 2)53. Intermediate 10 was reacted with 6 in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), a non-nucleophilic base, to obtain 11. Decomposition was noticed during the purification of 11 by column chromatography. Thus, 11 was rapidly passed through the column and the residue deprotected using p-toluene sulphonic acid (p-TSA) in methanol.
Figure 2.
Synthesis of IVM-CQ conjugates 12 from 8.
Figure 4.
Synthesis of IVM-PQ conjugates 15 from intermediate 10 derived from 8.
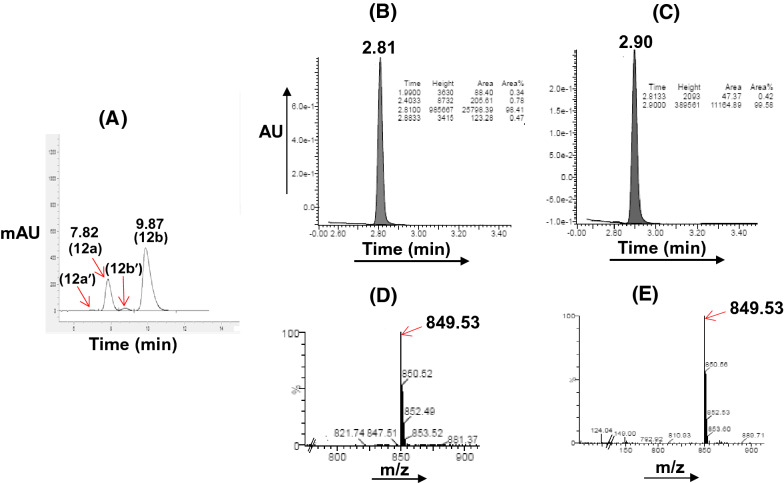
The residue was purified by column chromatography to obtain IVM-CQ hybrid 12, comprising of a mixture of the two inherent (R2 = Me and H) components in the ratio 98.87:0.6, as revealed from the high-performance liquid chromatography (HPLC) analysis (see Supplementary information, Fig. S16). Additionally, the HPLC chromatograms also indicated the presence of two isomeric peaks corresponding to each of the two inherent components. Thus, the isomeric mixture of 12, as isolated above was subjected to preparative HPLC using reverse phase (X Select CSH C18) chromatography, and the two isomers (12a and 12b/ 25.0% and 71.0%, Fig. 3) were isolated. The ultra-performance liquid chromatography (UPLC, see Supplementary information, Figs S17, S18) analysis of the two isomers revealed an analytical purity of 98.41% and 99.58% for 12a and 12b, respectively. The minor components of the isomeric mixture of 12 (R2 = H: 12a′ and 12b′, Fig. 3) however were eliminated during preparative HPLC purification. Interestingly, both 12a and 12b were stable and depicted parent ion peaks at identical mass (m/z 849.53) suggesting 12a and 12b to be structural isomers, whose structures could very convincingly be assigned using high-field NMR analysis (vide infra).
Figure 3.
HPLC (A) chromatogram of a mixture of isomers of IVM-CQ 12. UPLC chromatograms of isomers (B: 12a; C: 12b) after separation. UPLC-MS chromatograms: (D) 12a and (E) 12b.
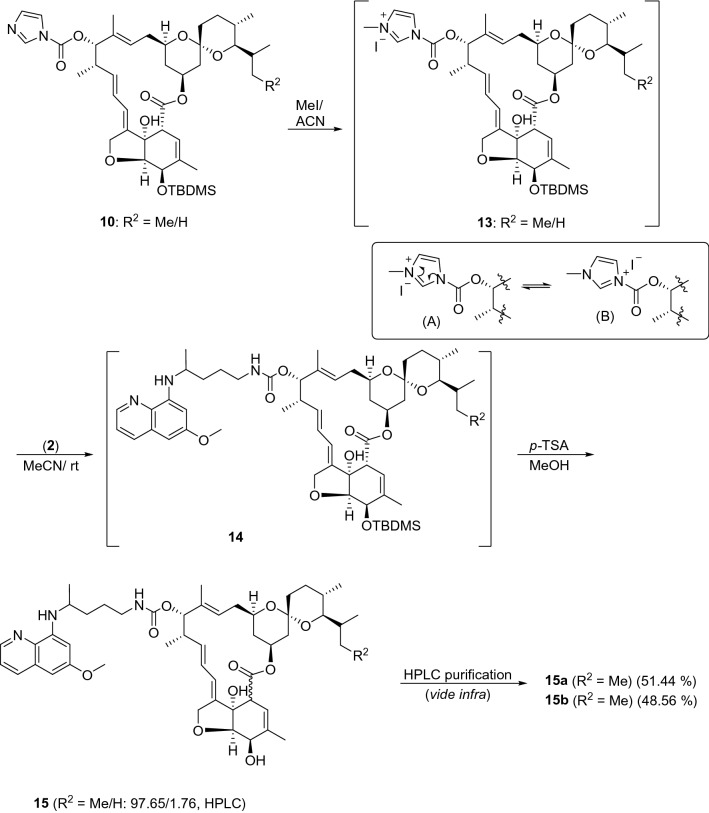
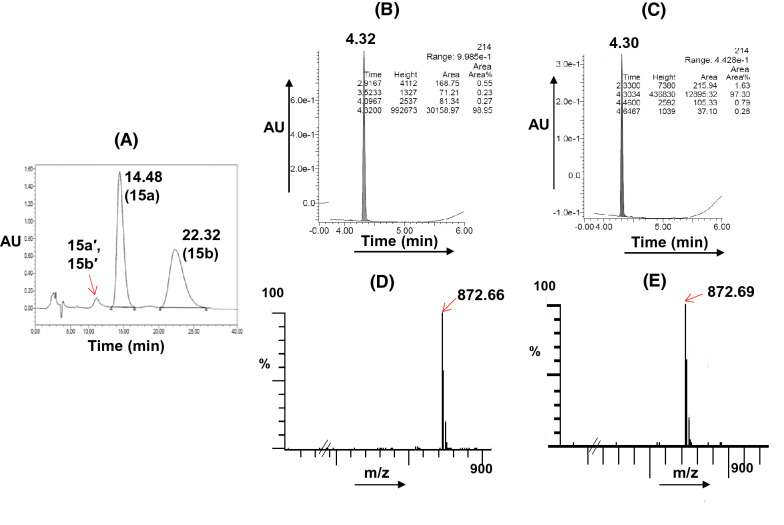
For the synthesis of IVM-PQ conjugates 15, our attempts at the use of the chemistry described in Fig. 2, by replacing 6 with 2 were not successful. Thus, treating 10 with 2 using different reaction conditions yielded the desired 14 in very poor yield. We envisaged that quaternization of the imidazole in 10 to create 13 might incorporate a better leaving group due to structural tautomerism (A ↔ B, Fig. 4) and increased yield of the desired product54. Thus, the reaction of intermediate 10 with iodomethane in anhydrous acetonitrile furnished the iodide salt, which was hygroscopic and washed with anhydrous ether. The reaction of 13 with 2 (vide experimental) straightway furnished 14 in good yield. Intermediate 14 was then deprotected using p-TSA to isolate the product, which was purified by column chromatography. HPLC analysis of the purified product using Chiralpak-IC column indicated the presence of the isomeric components (identified as 15a and 15b) of the major (R2 = Me) component in the ratio 51.44:48.56 (Fig. 5 and see Supplementary information, Fig. S19). The minor inherent component of 15 (R2 = H) was eliminated during column chromatographic purification, as it was not detected in the HPLC chromatogram. Preparative HPLC using Chiralpak-IC column was used to resolve the mixture of 15a and 15b, and the components (15a: 99.89% and 15b: 99.74%, see Supplementary information, Figs S20, S21) were isolated in analytically pure form. However, LCMS analysis of 15a and 15b indicated baseline achiral impurities. Therefore, 15a and 15b were subjected to preparative HPLC using reverse phase (X Select CSH C18) chromatography. The UPLC (see Supplementary information, Figs S22, S23) chromatograms of the isolated 15a and 15b indicated 98.95% and 97.30% analytical purity, respectively.
Figure 5.
HPLC (A) chromatogram of original mixture of isomers of IVM-PQ 15. UPLC chromatograms of individual isomers (B: 15a; C: 15b). UPLC-MS chromatograms: (D): 15a and (E): 15b.
Characterization of isomers of 12 and 15
All compounds were unambiguously characterized by spectroscopic techniques and the spectral data is presented in the Supplementary information (Figs S1–S15, S24–S27). 1H NMR spectral assignments of all intermediates and compounds were performed based on their coupling connectivity as seen in the 2D 1H–1H homonuclear correlated spectroscopy (COSY) spectrum and confirmed by High Resolution Mass Spectrometry (HRMS) and/or microanalytical data. Nuclear Overhauser effect spectroscopy (NOESY) was additionally used to identify relative orientations of H in 15a and 15b.
The antiplasmodial activity of hybrids of IVM is strongly structure-dependent. Therefore, the unnerving yet obligatory challenge was to assign the structures of the two isomers of both 12 (12a and 12b) and 15 (15a and 15b). In this context, high-field NMR data presented itself as a dependable tool, as contrasting differences in the chemical shift (δ ppm), as well as multiplicity of the comparable protons was observed, leading to the unambiguous assignment of structures.
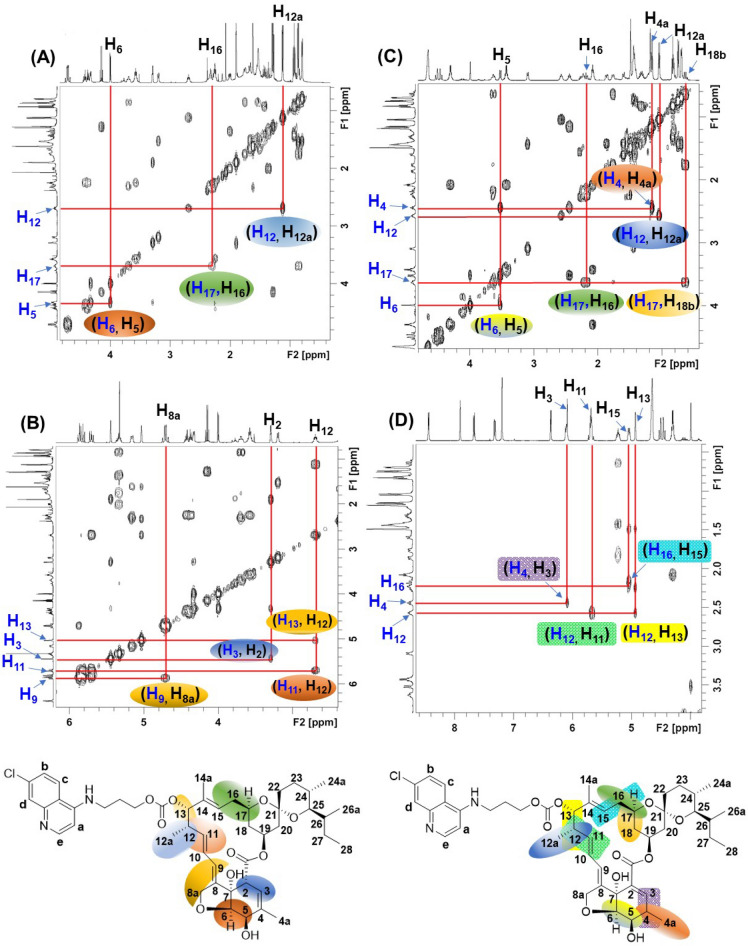
The 1H-1H COSY spectrum was quite useful in finding correlations leading to further simplification of the complex NMR data. In the 1H NMR spectrum of 12a, the signal corresponding to C-2 H (Fig. 6) of the macrolide at δ 3.27 (br, 1H) was absent in the 1H NMR spectrum of 12b. Instead, a 1H quintet at δ 2.54 (1H, J = 7.5 Hz) was observed in the NMR spectrum of 12b. Based on the coupling relationship revealed by the 1H-1H COSY spectrum of 12b (Fig. 6C), the quintet signal was assigned to the C-4 H of the macrolide. Quite convincingly, the corresponding change in the chemical shift and multiplicity of protons corresponding to C-4a of the macrolide was also observed. In 1H NMR of 12a, the C-4a protons appeared as a 3H singlet at δ 1.87, while a 3H doublet at δ 1.23 (d, J = 7.0 Hz) was observed for the C-4a protons of 12b. This change in the NMR spectrum hinted at the shifting of the double bond from Δ3 to Δ4 positions in the oxahydrindene ring of the macrolide. Thus, as expected, an upfield shift of the C5-H from δ 4.33 (d, J = 6.0 Hz) in 12a to δ 3.61 (dd, J = 2.0, 7.5, 1H) was observed in the 1H NMR spectrum of 12b. Other significant changes in the NMR spectra included: a downfield shift (Δδ = 0.73 ppm) of the C3-H, and the olefin C-9 H (Δδ = 0.41 ppm) of 12a and 12b. Based on similar proton couplings and correct mass spectral data (vide experimental), the structures of the isomers 12a and 12b were ascertained. The formation of the regioisomer 12b could be traced back to the synthetic step where DBU was used as a base. The C-2 H in 12a being acidic (due to the ester function) would yield a thermodynamically stable carbanion (compared to kinetically formed oxygen anions). Thus, re-protonation would yield both 12a and 12b.
Figure 6.
1H-1H COSY spectrum: (A, B): 12a and (C, D): 12b.
Quite surprisingly, no significant differences were observed between the NMR spectra of isomers 15a and 15b (see Supplementary information, Figs S4, S5). However, the assignment of the complex signals could be readily achieved from the COSY spectra of 15a and 15b. For example, the cross-peaks of H5 with H6, H12 with H12a and H13, H2 and H3 helped identify the coupling partners (see Supplementary information, Figs S24, S25). Given the close similarity in the NMR spectra of both 15a and 15b, the formation of regioisomers in analogy with 12a and 12b was ruled out.
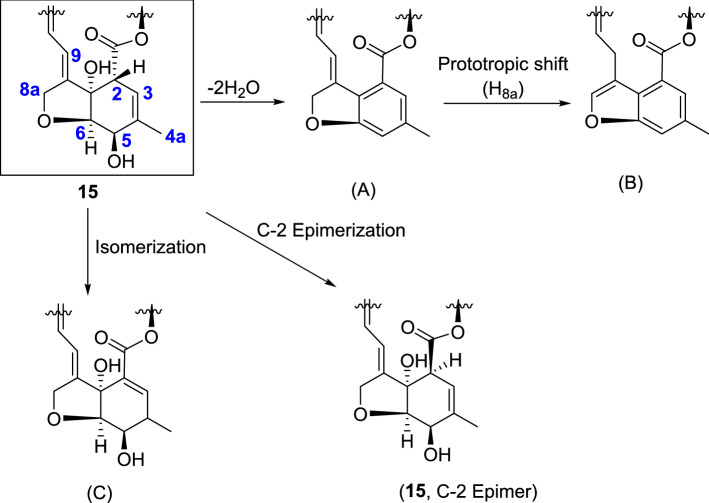
The oxahydrindene part of the macrolide ring of the IVM hybrids having two hydroxyl groups adjacent to sp3 hybridized carbons is prone to transformation into the benzenoid structure (A, Fig. 7) through double β-elimination of water, which, upon prototropic shift of a 8a-H, would result in an aromatic benzofuran ring (B). Interestingly, the spiroketal moiety of the macrolide remained intact.
Figure 7.
Possible chemical transformations of the oxahydrindene unit of 15.
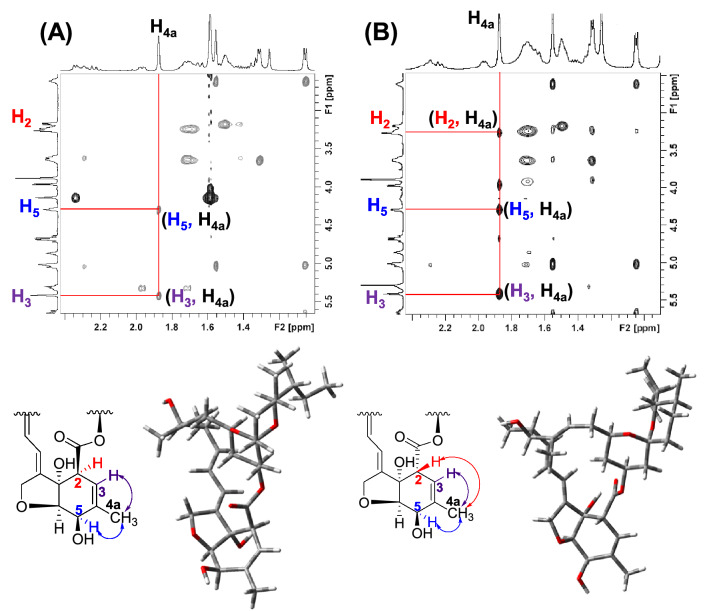
The presence of signals corresponding to C2-H, C3-H, C5-H, and C6-H, in the NMR spectra of both the isomers of 15 (15a and 15b) led us to rule out the formation of A-C (Fig. 7). It was further corroborated by HRMS where a peak (m/z 871) corresponding to the molecular formula C50H69N3O10 of the 15a and 15b was observed. However, in the NOESY spectra of 15a and 15b, the absence of through space coupling between C4a-H with C2-H (Fig. 8 and see Supplementary information, Figs. S26, S27) in the former strongly suggested the epimerization55, 56 at the C-2 of the macrolide. The nOe’s between C4a-H and the other relevant protons, C3-H, C5-H, could be identified in both isomers.
Figure 8.
NOESY spectra (in part) of (A): 15a; (B): 15b showing oxahydrindene unit and the corresponding DFT optimized structures depicting aglycon units.
Antiplasmodial activity
In vitro activity of “second-generation” IVM hybrids against Plasmodium hepatic infection
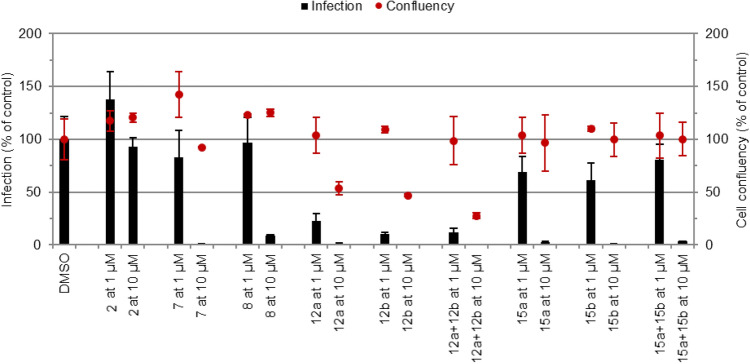
IVM Hybrids 12a,b and 15a,b were initially screened at 10 and 1 µM for their in vitro activity against the hepatic stage of P. berghei infection (Fig. 9). Pristine 7, 8, and 2 were employed as controls in these experiments. All compounds of interest dramatically impacted infection at 10 µM. However, in the case of the CQ hybrids 12a,b this was accompanied by a reduction in cell confluence, indicative of toxicity towards the host cells at this concentration. However, hybrids 12a,b were also the most active compounds at 1 µM. Given their potentially interesting activity, which was comparable to, or even higher than that of 7, all compounds were selected for IC50 determination.
Figure 9.
Assessment of in vitro activity of compounds against the hepatic stage of P. berghei infection. Total parasite load (infection scale, bars) and cell viability (cell confluency scale, dots) are shown. Results were normalized to the negative control, the drug vehicle dimethyl sulphoxide (DMSO), and are represented as mean ± SD of technical triplicates, n = 1.
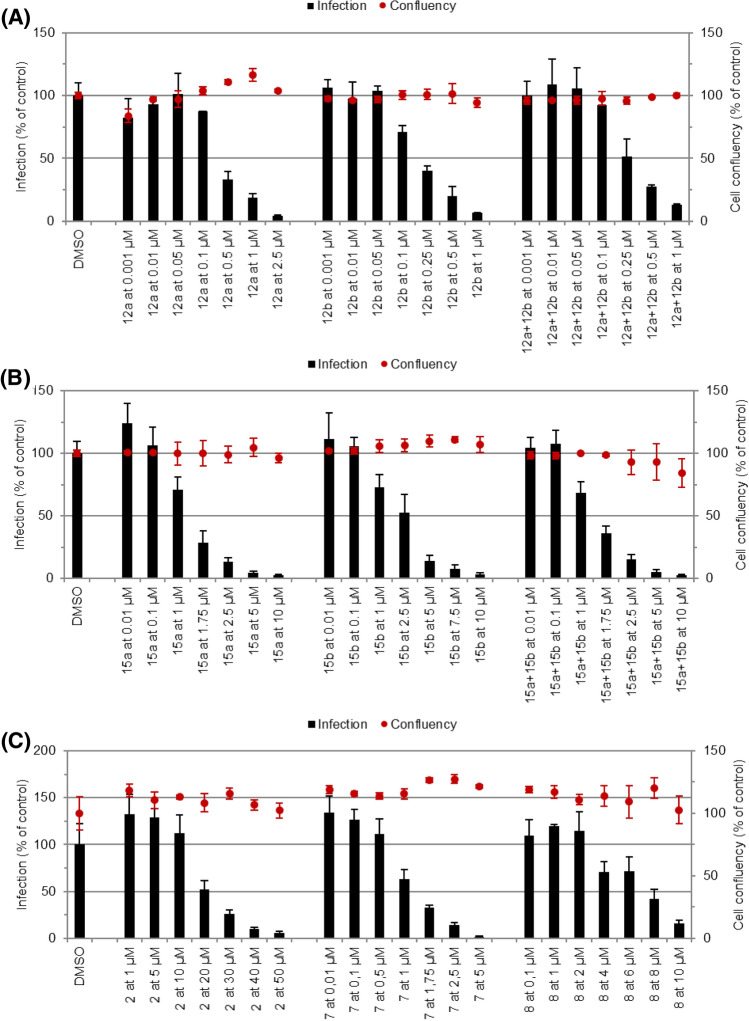
Dose-dependent responses of each compound against P. berghei hepatic infection were obtained (Fig. 10), which enabled the determination of their IC50 (Table 1). In agreement with the data from the initial screen, CQ hybrids 12a,b displayed the highest activity, with IC50 values ranging from 0.186 to 0.317 µM, whereas PQ hybrids 15a,b were less active, with IC50 values ranging from 1.291 to 2.057 µM. Comparing the activity of the most potent member of the first-generation IVM hybrids48 with compound 12b, the most active member of the current second-generation series, shows that the latter is nearly threefold more active than the former. The fact that the IVM hybrids are significantly more potent antiplasmodial agents than IVM warrants the chemical modification of IVM to produce antiplasmodial agents with enhanced potency. Further, the complete loss of the antiplasmodial activity of the 8 strongly suggests some role of the substitution at the C-13 position of the macrolide structure.
Figure 10.
Dose-dependent response of compounds against the hepatic stage of P. berghei infection. The dose-dependent response of (A) IVM-CQ hybrids, 12a,b (B) IVM-PQ hybrids, 15a,b, and (C) 7, 8 and standard drug 2 were evaluated against P. berghei-infected Huh7 cells. Total parasite load (infection scale, bars) and cell viability (cell confluence scale, dots) are shown, n ≥ 2. Calculated IC50 values are shown in Table 1.
Table 1.
IC50 values of selected compounds against the hepatic stage of P. berghei and the erythrocytic stage of P. falciparum NF54 infections.
| Compound | IC50 (µM)a P. berghei | IC50 (nM)a PfNF54 |
|---|---|---|
| 12a | 0.274 ± 0.103 | 48.2 ± 3.1 |
| 12b | 0.186 ± 0.025 | 64.8 ± 27.5 |
| 12a + 12bb | 0.317 ± 0.035 | 74.3 ± 33.0 |
| 15a | 1.291 ± 0.042 | ND |
| 15b | 2.057 ± 0.159 | ND |
| 15a + 15bb | 1.360 ± 0.006 | ND |
| 7 | 1.321 ± 0.011 | 359.6 ± 65.7 |
| 8 | 6.375 ± 0.909 | ND |
| 2 | 8.428 ± 3.38957 | ND |
| 1 | ND | 23.7 ± 10.148 |
aResults are represented as mean ± SD, n ≥ 2. ND: not determined.
bOriginal isolated mixture, before HPLC.
In vitro activity against P. falciparum erythrocytic infection
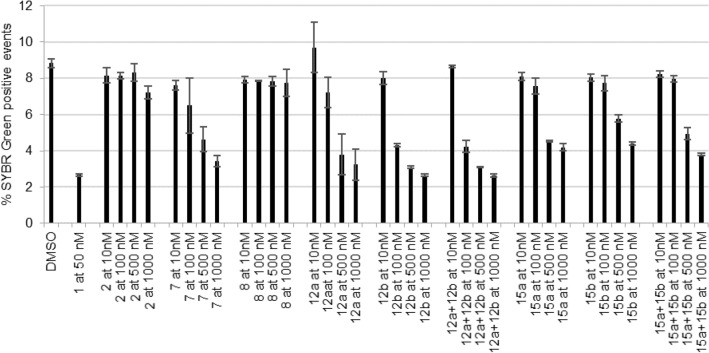
To assess their activity against the blood stage of P. falciparum (PfNF54) infection, compounds were initially screened at 1000, 500, 100, and 10 nM (Fig. 11). Compounds 7, 8, 2, and 1 were employed as controls. Compound 8 and 2 were not active against the parasite as shown by comparison with the DMSO control. Similar to what was observed for the hepatic stage, CQ hybrids 12a,b showed the highest activity against P. falciparum blood stages. For that reason, 12a, 12b, mixture 12a + 12b, and 7 were selected for IC50 determination.
Figure 11.
Assessment of in vitro activity of compounds against the blood stage of P. falciparum infection. Percentage of SYBR Green positive events (parasitemia) against each compound at 1000 nM, 500 nM, 100 nM, and 10 nM is shown. Chloroquine (CQ) at 50 nM was used as the positive control. Results were compared with the negative control (DMSO) and are represented as the mean ± SD of technical triplicates, n = 1.
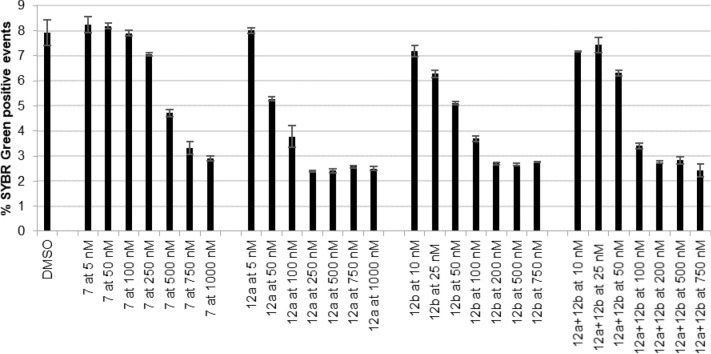
Dose-dependent values of the % of SYBR Green-positive events were obtained at selected concentrations (Fig. 12) for IC50 calculation (Table 1). CQ hybrids, 12a, 12b displayed IC50 values between 48.2 and 74.3 nM, an activity lower than that previously determined for the CQ control (23.7 nM ± 10.1)48. Compound 7 was the least active compound tested, with an estimated IC50 of 359.6 nM.
Figure 12.
Inhibition of P. falciparum in the presence of different concentrations of 12a + 12b mixture, 12a, 12b, and 7. A ring stage synchronized culture of P. falciparum (PfNF54) was incubated for 48 h with increasing concentrations of the compounds, DMSO (vehicle control). The percentage of SYBR Green positive events per concentration is represented. DMSO is represented as a negative control. Each time point represents the mean value of triplicate measurements (± one SD), n = 3.
It is interesting to note that the IVM-PQ hybrids 15a and 15b display lower activity than their CQ counterparts 12a and 12b against both stages of Plasmodium infection. This is unsurprising in what concerns the parasite’s blood stages, since 1 is a known blood stage antiplasmodial, whereas 2 does not display significant activity against this stage of the parasite’s life cycle.
The fact that 12a and 12b are also more active than 15a and 15b against hepatic infection is somewhat puzzling. However, it should be noted that the in vitro hepatic stage antiplasmodial activity of 7 (~ 2 μM) is higher than that of 2 (~ 10 μM) and, as such, the IVM moiety of the PQ hybrids 15a and 15b is the main contributor towards hepatic stage activity. The data (Table 1) suggests that the hepatic stage activity is potentiated by CQ analogue more than PQ in the hybrids of IVM. Evidently, when hybridized with IVM, the enhanced synergization of the former leads to the enhanced hepatic stage antiplasmodial activity, whereas such enhancement is absent in the PQ hybrids 15a and 15b, where the activity primarily results from the IVM moiety.
Conclusions
The second-generation IVM hybrids synthesized through molecular hybridization of 7 with the CQ analogue 6 and antiplasmodial drug 2 display higher potency against the hepatic and blood stages of Plasmodium infection than their first-generation counterparts. IVM-CQ hybrids 12a,b were the most active compounds against P. berghei hepatic infection in vitro. Interestingly, IVM hybrids [IC50 = 0.274 µM (12a) and 0.186 µM (12b)] displayed higher activity against P. berghei infection than 7 (IC50 = 1.321 µM) and the standard liver-stage drug, 2 (IC50 = 8.428 µM). Surprisingly, the IVM-PQ hybrids were less active [IC50 = 1.291 µM (15a) and 2.057 µM (15b)] against P. berghei hepatic infection than the IVM-CQ hybrids.
The blood-stage antiplasmodial activity of the compounds against the P. falciparum NF54 strain was significantly higher than that observed against the hepatic infection. Compound 12a was the most active (IC50 = 48.2 nM) displaying over sevenfold higher potency than the pristine IVM, and more active than the comparable member of the series of the first-generation IVM hybrids, emphasizing the advantage of the hybridization approach described here. IVM-PQ hybrids were not active enough to determine IC50 values.
Compound 8 displayed the lowest activity of all the compounds synthesized and paralleled the trend observed in the series of the first-generation IVM hybrids. Additionally, we evaluated the activity of the mixture of isomers in order to determine whether it is necessary to isolate the isomers. Reassuringly, our results showed that the in vitro antiplasmodial activities against both the hepatic and blood-stage infections by Plasmodium were generally lower than those of the individual isomers.
Overall, our data highlights the enhancement of the antiplasmodial activity of these structurally modified compounds through appending hybrid partners at C-13 of the macrolide unit. Work towards designing and synthesizing additional structurally modified IVM hybrids is currently in progress.
Experimental
General
All liquid reagents were dried/purified following recommended drying agents and/or distilled over 4 Å molecular sieves. CH3CN was dried by refluxing over P2O5. DCM was dried over fused calcium chloride. TBDMS-Cl, DMAP, and CDI were bought from Spectrochem. Imidazole was bought from Sigma Aldrich. K2CO3 was dried overnight in the furnace. 1H NMR and 13C NMR spectra were recorded on Bruker Biospin Avance III HD at 500 MHz and JEOL-FT NMR-AL at 400 MHz with TMS as an internal standard using CDCl3 as deuterated solvent. Data are reported as follows: chemical shift in δ (ppm), integration, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), coupling constant J (Hz). High-Resolution Mass spectra were recorded on a Bruker LC–MS MICROTOF II spectrometer. IR spectra were recorded on Agilent Technologies Cary 630 FTIR spectrometer. HPLC was performed in Reverse Phase mode using Agilent 1260 Infinity series HPLC (Agilent Technologies, USA) equipped with Quaternary Pump VL (G1311C) and degasser, 1260 ALS auto sampler (G1329B), and 1260 DAD VL detector (G1315D). The column used was the ZORBAX Eclipse C18 column (4 × 100 mm) (Agilent Technologies, USA). The mobile phase was comprised of a mixture of acetonitrile-methanol–water (49.2:32.8:18 v/v/v) and the flow rate was set to 1.0 mL/min. Agilent 1260 Infinity auto purification system with 1260 DAD VL-UV detector was used for preparative HPLC analysis. For reverse-phase prep purification of 12, the mobile phase was comprised of a mixture of 5 mM ammonium acetate in water-acetonitrile (10:90 v/v) and the flow rate was set to 18.0 mL/min. The column used was X SELECT CSH C18 (10 × 250 mm, 10 μm). For chiral purification of 15, the mobile phase comprised of a mixture of ethanol-0.1% TEA in n-hexane (30:70 v/v). The column used was Chiralpak-IC (21 × 250 mm, 5 µm) and the flow rate was set to 1.0 mL/min. For reverse-phase prep purification, the mobile phase was comprised of a mixture of 5 mM ammonium acetate in water-acetonitrile (10:90 v/v) and the flow rate was set to 18.0 mL/min. The column used was X SELECT CSH C18 (10 × 250 mm, 10 μm). DFT optimization of geometry was done using Gaussian09 software (STO 3G basis set). GaussView 5.0.9 molecular software was used for visualizing the optimized structures. All tested compounds are > 95% pure by HPLC/UPLC analysis. Optical rotations were determined with an AUTOPOL IV polarimeter at 25 °C using sodium D light in methanol (HPLC grade). Concentration “c” is depicted in g/mL.
Synthesis of 10
To the suspension of CDI (0.23 g, 1.4 mmol) in dry toluene (10 ml), a solution of 9 (0.5 g, 0.7 mmol) in dry toluene (5 ml) was added dropwise. The resulting mixture was stirred at room temperature for 12 h. The reaction mixture was poured into water and extracted with chloroform (2 × 20 ml). The organic extract was washed with water, dried over anhydrous sodium sulfate, filtered, and evaporated to obtain a brown oil. The crude product was purified by column chromatography over silica gel (60–120 mesh) using hexane/ethyl acetate (75:25, v/v) as eluent to afford 10 as a white solid in 73% yield. IR. 2929, 1766, 1461, 1394, 1282, 1177 cm−1. 1H NMR (500 MHz, CDCl3, 25 °C): δ 8.22 (s, 1H, imidazole), 7.49 (br, 1H, imidazole), 7.14 (br, 1H, imidazole), 5.81–5.87 (m, 2H, H9, H10), 5.61–5.69 (1H, m, H11), 5.27–5.33 (m, 3H, H19, H3, H13), 5.07 (t, 1H, J = 7.5 Hz, H15), 4.72 (ABq, 2H, J = 15.0 Hz, 2 × H8a), 4.44 (br, 1H, H5), 4.19 (s, 1H, C7-OH), 3.84 (d, 1H, J = 5.5 Hz, H6), 3.57–3.63 (m, 1H, H17), 3.38 (br, 1H, H2), 3.14 (d, 1H, J = 8.0 Hz, H25), 2.74–2.81 (m, 1H, H12), 2.28 (t, 2H, J = 8.5 Hz, 2 × H16), 2.02 (dd, 1H, J = 4.5, 8.0 Hz, H18a), 1.79 (s, 3H, 3 × H4a), 1.31–1.71 (m, 13H, 2 × H22, 2 × H23, H24, H26, 2 × H27, 2 × H20, 3 × H14a), 1.15 (d, 3H, J = 7.0 Hz, 3 × H12a), 0.93 (s, 9H, (CH3)3C), 0.80–0.86 (m, 7H, 3 × H28, 3 × H26a, H18b), 0.76 (d, 3H, J = 5.0 Hz, 3 × H24a), 0.14 (s, 6H, (CH3)2Si). 13C NMR (100 MHz, CDCl3, 25 °C): δ 173.9, 148.1, 142.3, 137.6, 137.2, 134.5, 133.7, 131.1, 126.4, 118.7, 118.7, 117.3, 116.9, 97.6, 83.3, 80.3, 80.1, 77.3, 69.5, 68.7, 67.9, 66.9, 45.7, 41.3, 39.2, 36.8, 35.8, 35.5, 34.2, 31.2, 28.1, 27.4, 25.9, 20.1, 18.9, 18.5, 17.5, 14.8, 12.5, 11.9, -4.5, -4.8. HRMS: m/z [M + Na]+ for C44H66N2O9Si, calculated 817.4430; observed 817.3509.
Synthesis of 12a and 12b
DBU (0.086 g, 0.57 mmol) was added to the suspension of 6 (0.02 g, 0.57 mmol) in 5 ml dry DCM and the resulting clear solution was stirred at room temperature for 15 min before dropwise addition of a solution (5 ml) of 10 (0.300 g, 0.38 mmol) in DCM. The reaction mixture was stirred at room temperature for 12 h. Upon completion, the reaction mixture was poured into water and extracted with water. The organic layer was dried over anhydrous sodium sulfate and the solvent was evaporated to brown oil (11, 0.310 g). For deprotection, 10 ml solution of p-TSA in methanol (0.02 g mL-1) was added to the solution of 11 (0.310 g) in methanol (10 ml) dropwise. The reaction mixture was stirred for 30 min at room temperature. Upon completion, DCM (30 ml) was added to the reaction mixture and washed with aqueous sodium bicarbonate, water, dried over anhydrous sodium sulfate, and the solvent was evaporated to give the brown crude product which was purified by column chromatography over silica gel (60–120 mesh) using chloroform/methanol (95:5, v/v) as the eluent afford 12 as a white solid in 39.9% yield. Characteristic data of isolates of 12 obtained after prep purification is given below.
12a White solid. IR. 3302, 3255, 2914, 1729, 1587, 1468, 1177 cm-1. 1H NMR (500 MHz, CDCl3, 25 °C): δ 8.51 (d, 1H, J = 5.0 Hz, ArH), 7.97 (br, 1H, ArH), 7.73 (d, 1H, J = 9.0 Hz, ArH), 7.38 (dd, 1H, J = 2.0, 7.0 Hz, ArH), 6.43 (d, 1H, J = 5.5 Hz, ArH), 5.67–5.87 (m, 3H, H9, H10, H11), 5.65 (br, 1H, NH), 5.42 (s, 1H, H3), 5.28–5.35 (m, 1H, H19), 5.16 (d, 1H, J = 3.5, 7.0 Hz, H15), 5.02 (s, 1H, H13), 4.72 (ABq, 2H, J = 2.0, 8.0, 12.5 Hz, 2 × H8a), 4.53 (br, 1H, C7-OH), 4.33–4.41 (m, 2H, CH2O-), 4.30 (d, 1H, J = 6.0 Hz, H5), 3.98 (d, J = 6.0 Hz, H6), 3.64–3.69 (m, 1H, H17), 3.45–3.52 (m, 2H, CH2NH), 3.27 (br, 1H, H2), 3.17 (d, J = 8.0, H25), 2.64–2.71 (m, 1H, H12), 2.24–2.33 (m, 2H, 2 × H16), 2.11–2.17 (m, 2H, CH2), 2.0 (dd, 1H, J = 4.5, 8.0 Hz, H18a), 1.87 (s, 3H, 3 × H4a), 1.72–1.75 (m, 2H, H20a, C5-OH), 1.66 (d, 1H, J = 12.5 Hz, H20b), 1.60 (s, 3H, 3 × H14a), 1.25–1.52 (m, 8H, 2 × H22, 2 × H23, H24, H26, 2 × H27), 1.12 (d, 3H, J = 6.5 Hz, 3 × H12a), 0.91 (t, 3H, J = 7.5 Hz, 3 × H28), 0.84 (d, 3H, J = 7.0 Hz, 3 × H26a), 0.80–0.83 (m, 1H, H18b), 0.77 (d, 3H, J = 5.5 Hz, 3 × H24a). 13C NMR (125 MHz, CDCl3, 25 °C): δ 173.6, 155.1, 153.4, 141.0, 140.9, 140.7, 137.9, 135.9, 134.2, 125.7, 125.7, 121.1, 120.0, 118.1, 118.0, 117.0, 98.8, 97.5, 83.1, 80.3, 79.1, 77.1, 68.5, 68.4, 67.7, 67.0, 66.5, 45.7, 41.2, 39.7, 39.2, 36.8, 35.8, 35.5, 34.2, 31.2, 28.0, 27.9, 27.4, 19.9, 18.7, 17.4, 14.6, 12.5, 11.9. HRMS: m/z [M + H]+ for C47H61ClN2O10, calculated 849.4087; observed 849.4038. UPLC purity: 98.41%. tR (HPLC) = 7.82 min.
12b White solid. IR. 3302, 3235, 2914, 1729, 1468, 1177 cm-1. 1H NMR (500 MHz, CDCl3, 25 °C): δ 8.51 (d, 1H, J = 5.5 Hz, ArH), 7.97 (br, 1H, ArH), 7.73 (d, 1H, J = 9.0 Hz, ArH), 7.40 (dd, 1H, J = 1.5, 7.5 Hz, ArH), 6.44 (d, 1H, J = 5.5 Hz, ArH), 6.15–6.19 (m, 2H, H9, H3), 5.71–5.80 (m, 2H, H10, H11), 5.65 (br, 1H, NH, D2O Exchangeable), 5.26–5.32 (m, 1H, H19), 5.12 (d, 1H, J = 10.5 Hz, H15), 5.00 (s, 1H, H13), 4.85 (br, 1H, C7-OH, D2O Exchangeable), 4.60 (ABq, 2H, J = 2.0, 12.0, 18.5 Hz, 2 × H8a), 4.33–4.41 (m, 2H, CH2O), 4.06 (br, H6), 3.67–3.74 (m, 1H, H17), 3.61 (dd, 1H, J = 2.0, 7.5 Hz, H5), 3.46–3.54 (m, 2H, CH2NH), 3.17 (d, J = 8.5 Hz, H25), 2.61–2.67 (m, 1H, H12), 2.54 (quintet, 1H, J = 7.5 Hz, H4), 2.20–2.36 (m, 2H, 2 × H16), 2.18 (quintet, 2H, J = 6.5 Hz, CH2), 1.95 (dd, 1H, J = 4.5, 7.5 Hz, H18a), 1.82–1.85 (m, 2H, H20a, C5-OH, D2O Exchangeable), 1.68 (d, 1H, J = 11.5 Hz, H20b), 1.56 (s, 3H, 3 × H14a), 1.33–1.52 (m, 8H, 2 × H22, 2 × H23, H24, H26, 2 × H27), 1.23 (d, 3H, J = 7.0 Hz, 3 × H4a) 1.12 (d, 3H, J = 6.5 Hz, 3 × H12a), 0.91 (t, 3H, J = 7.5 Hz, 3 × H28), 0.82 (d, 3H, J = 7.0 Hz, 3 × H26a), 0.77 (d, 3H, J = 5.5 Hz, 3 × H24a), 0.67–0.74 (m, 1H, H18b). 13C NMR (125 MHz, CDCl3, 25 °C): δ 168.6, 155.1, 140.3, 138.8, 136.0, 133.9, 129.8, 126.4, 125.7, 122.5, 121.1, 117.9, 117.0, 98.8, 97.4, 83.3, 82.9, 78.5, 72.3, 68.7, 68.2, 66.9, 66.4, 60.4, 53.4, 40.3, 39.7, 39.1, 36.8, 35.8, 35.5, 34.5, 33.4, 31.2, 29.7, 28.0, 27.9, 27.4, 21.0, 18.7, 17.4, 16.9, 14.6, 14.1, 12.5, 11.8. HRMS: m/z [M + H]+ for C47H61ClN2O10, calculated 849.4087; observed 849.3968. UPLC purity: 99.58%. tR (HPLC) = 9.87 min.
Synthesis of 13
Compound 10 (0.200 g, 0.25 mmol) was dissolved in 2 ml of dry ACN, and MeI (0.08 ml, 1.25 mmol) was added. The resulting colorless solution was stirred at 40 °C for 2 h. Upon completion, the excess of ACN was evaporated under reduced pressure to yield yellow solid 13, which was used as such without any further purification in the subsequent reactions as it displayed significant degradation in contact of air.
Synthesis of 15a and 15b
IVM-based intermediate 13 (0.100 g, 0.12 mmol) was dissolved in dry ACN (5 ml). To this, 5 ml solution of neutralized PQ (2, 0.048 g, 0.18 mmol) in dry ACN was added. The reaction mixture was stirred for 4–6 h in dark. Upon the completion, the excess of ACN was evaporated under vacuum, followed by the addition of DCM (30 ml). The resulting solution was washed with water, dried over anhydrous sodium sulfate, evaporated to obtain a dark brown oil (14, 0.130 g). For deprotection, 10 ml solution of p-TSA in methanol (0.02 g mL-1) was added to the solution of a 14 (0.130 g) in methanol (10 ml). The reaction mixture was stirred for 30 min at room temperature. Upon completion, DCM (30 ml) was added to the reaction mixture and washed with aqueous sodium bicarbonate, water, dried over anhydrous sodium sulfate and the solvent was evaporated to give the brown crude product which was purified by column chromatography over silica gel (60–120 mesh) using hexane/ethyl acetate (60:40, v/v) as the eluent afford to 15 as a white solid in 48% yield. Characteristic data of isolates of 15 obtained after prep purification is given below.
15a White solid. IR. 3302, 3235, 2914, 1729, 1617, 1520, 1468, 1177 cm-1. 1H NMR (500 MHz, CDCl3, 25 °C): δ 8.53 (dd, 1H, J = 3.0 Hz, ArH), 7.93 (d, 1H, J = 8.0 Hz, ArH), 7.30–7.32 (m, 1H, ArH), 6.34 (d, 1H, J = 2.5 Hz, ArH), 6.29 (d, 1H, J = 2.0 Hz, ArH), 6.00 (s, 1H, Ar–NH, D2O Exchangeable), 5.61–5.84 (m, 3H, H9, H10, H11), 5.41 (s, 1H, H3), 5.29–5.35 (m, 1H, H19), 5.01–5.04 (m, 2H, H15, H13), 4.87 (br, 1H, NH, D2O Exchangeable), 4.71 (ABq, 2H, J = 9.5, 14.5 Hz, 2 × H8a), 4.30 (d, 1H, J = 6.0 Hz, H5), 4.22 (br, 1H, C7-OH, D2O Exchangeable), 3.97 (d, J = 6.0 Hz, H6), 3.88 (s, 3H, OCH3), 3.59–3.64 (m, 2H, H17, CHCH3), 3.23–3.26 (m, 3H, H2, CH2), 3.18 (d, J = 8.5 Hz, H25), 2.59–2.62 (m, 1H, H12), 2.20–2.36 (m, 3H, 2 × H16, C5-OH, D2O Exchangeable), 1.98 (dd, 1H, J = 4.0, 7.5 Hz, H18a), 1.87 (s, 3H, 3 × H4a), 1.33–1.72 (m, 20H, 2 × H22, 2 × H23, H24, H26, 2 × H27, 2 × H20, 3 × H14a, CHCH3, CH2CH2), 1.06 (m, 3H, J = 7.0 Hz, 3 × H12a), 0.91 (t, 3H, J = 7.5 Hz, 3 × H28), 0.84 (d, 3H, J = 7.0 Hz, 3 × H26a), 0.80–0.82 (m, 1H, H18b), 0.77 (d, 3H, J = 5.5 Hz, 3 × H24a). 13C NMR (125 MHz, CDCl3, 25 °C): δ 173.7, 159.4, 155.9, 144.9, 144.3, 140.2, 137.9, 137.5, 135.4, 134.8, 129.9, 124.9, 121.9, 120.3, 118.1, 117.4, 97.5, 96.8, 91.7, 80.3, 79.1, 79.0, 76.9, 68.6, 68.5, 67.7, 67.2, 55.2, 47.8, 45.7, 41.3, 41.1, 39.2, 36.8, 35.8, 35.5, 34.2, 33.9, 31.2, 28.0, 27.3, 26.7, 20.6, 19.9, 18.7, 17.4, 14.6, 12.5, 12.0. HRMS: m/z [M + H]+ for C50H69N3O10, calculated 872.5055; observed 872.5011. UPLC purity: 98.95%. tR (HPLC) = 14.48 min. [α]D25 + 26.667° (c 0.0015, CH3OH).
15b White solid. IR. 3302, 3235, 2914, 1729, 1595, 1468, 1177 cm-1. 1H NMR (500 MHz, CDCl3, 25 °C): δ 8.53 (d, 1H, J = 3.5 Hz, ArH), 7.93 (d, 1H, J = 8.0 Hz, ArH), 7.29–7.32 (m, 1H, ArH), 6.34 (s, 1H, ArH), 6.28 (s, 1H, J = 2.0 Hz, ArH), 6.00 (s, 1H, Ar–NH, D2O Exchangeable), 5.63–5.84 (m, 3H, H9, H10, H11), 5.41 (s, 1H, H3), 5.29–5.33 (m, 1H, H19), 5.01–5.04 (m, 2H, H15, H13), 4.86 (br, 1H, NH, D2O Exchangeable), 4.71 (ABq, 2H, J = 9.0, 14.5 Hz, 2 × H8a), 4.30 (d, 1H, J = 5.0 Hz, H5), 3.89 (s, 3H, OCH3), 3.97 (d, J = 6.0 Hz, H6), 3.62–3.63 (m, 2H, H17, CHCH3), 3.21–3.26 (m, 3H, H2, CH2), 3.18 (d, J = 9.0 Hz, H25), 2.59–2.61 (m, 1H, H12), 2.19–2.36 (m, 3H, 2 × H16, C5-OH D2O Exchangeable), 1.97 (m, 1H, H18a), 1.87 (s, 3H, 3 × H4a), 1.33–1.72 (m, 20 H, 2 × H22, 2 × H23, H24, H26, 2 × H27, 2 × H20, 3 × H14a, CHCH3, CH2CH2), 1.05 (d, 3H, J = 6.5 Hz, 3 × H12a), 0.89 (t, 3H, J = 7.0 Hz, 3 × H28), 0.84 (d, 3H, J = 6.0 Hz, 3 × H26a), 0.81–0.82 (m, 1H, H18b), 0.77 (d, 3H, J = 4.5 Hz, 3 × H24a). 13C NMR (125 MHz, CDCl3, 25 °C): δ 173.7, 159.4, 155.9, 144.9, 144.4, 140.2, 137.9, 135.4, 134.8, 129.9, 124.9, 121.9, 120.3, 118.1, 117.4, 97.5, 96.7, 91.7, 80.3, 79.1, 79.0, 75.0, 68.6, 68.5, 67.7, 67.2, 55.2, 47.8, 45.7, 41.2, 41.1, 39.2, 36.8, 35.8, 35.4, 34.2, 33.9, 31.2, 28.0, 27.2, 26.8, 20.6, 19.9, 18.7, 17.4, 14.6, 12.5, 12.0. HRMS: m/z [M + H]+ for C50H69N3O10, calculated 872.5055; observed 872.4967. UPLC purity: 97.30%. tR (HPLC) = 22.32 min. [α]D25 + 29.412° (c 0.0017, CH3OH).
Biology
Parasites
Luciferase-expressing P. berghei sporozoites58 were obtained by dissection of salivary glands of female Anopheles stephensi mosquitoes, reared at Instituto de Medicina Molecular.
Cell culture
Huh7 cells, a human hepatic cell line was provided by Cenix Bioscience GmbH, and were cultured in complete culture medium, i.e. RPMI 1640 supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) glutamine, 1% (v/v) penicillin/streptomycin, 1% non-essential amino acids, and 10 mM HEPES at 37 °C, 5% CO2. Cells were plated in 96-well plates at 1 × 104 cells/well 24 h prior to infection.
In vitro liver-stage activity assessment
The activity of compounds against P. berghei-infected Huh7 cells was assessed in vitro by bioluminescence, as previously described58. Briefly, Huh7 cells were seeded as indicated above on the day prior to infection. Compound stock solutions were prepared in DMSO. Test concentrations were obtained by serial dilution of compound stock solutions in infection medium, i.e. complete culture medium supplemented with 50 µg/mL of gentamicin and 0.8 µg/mL of fungizone. After 1 h of incubation with selected compound dilutions, 1 × 104 luciferase-expressing P. berghei sporozoites were added per well. Plates were centrifuged and incubated for 46 h at 37 °C, 5% CO2. At this timepoint, the impact of the compounds on cell viability was assessed by the AlamarBlue (Invitrogen) assay, according to the manufacturer’s recommendations. Next, compounds’ impact on parasite load was assessed by bioluminescence, employing the Firefly Luciferase Assay Kit 2.0 (Biotium).
In vitro blood-stage activity assessment
Ring-stage synchronized cultures of P. falciparum NF54 at 2.5% hematocrit and at approximately 1% parasitemia were incubated with drugs or DMSO (vehicle control) in 96 well-plates, for 48 h, at 37 °C in a 5% CO2 and 5% O2 atmosphere. Stock solutions of all compounds were prepared in DMSO. Working solutions were prepared from the stock solutions in complete malaria culture medium (CMCM), which consists of RPMI 1640 supplemented with 25 mM HEPES, 2.4 mM L-glutamine, 50 μg/mL gentamicin, 0.5% w/v Albumax, 11 mM glucose, 1.47 mM hypoxanthine and 37.3 mM NaHCO3. For each measurement 5 µl of the culture (approximately 800 000 cells) was stained with the DNA-specific dye SYBR green I at 1 ×. After 20 min of incubation, in the dark, the stained sample was analyzed by flow cytometry using Cyflow Cube 6 (Sysmex, Germany). Approximately 100,000 events were analyzed in each flow cytometry measurement. All samples were analyzed in triplicate and three different experiments were performed.
Statistical analyses
Nonlinear regression analysis using GraphPad Prism 8 (GraphPad software, La Jolla California, USA) was employed to fit the normalized results of the dose–response curves for IC50 determinations of in vitro hepatic and blood stage activities.
Supplementary Information
Acknowledgements
KS thanks SERB, DST for the grant (EMR/2017/000520) and Guru Nanak Dev University, Amritsar for funding under the RUSA-II scheme as well as facilities. MP acknowledges Fundação para a Ciência e Tecnologia, Portugal, for Grant PTDC-SAU-INF-29550/2017. LS is thankful to University Grants Commission (UGC), New Delhi for funding under Rajiv Gandhi National Fellowship. We are grateful to Dr. Prasant K. Deb and his team at Jubilant Biosys for helping us with preparative HPLC and UPLC-MS analyses.
Abbreviations
- CQ
Chloroquine
- PQ
Primaquine
- ACT
Artemisinin-based combination therapy
- WHO
World Health Organization
- COSY
Correlation spectroscopy
- NOESY
Nuclear overhauser effect spectroscopy
- TBDMS
tert-Butyldimethylsilyl
- CMCM
Complete malaria culture medium
- CDI
1,1'-Carbonyldiimidazole
- DMSO
Dimethyl sulfoxide
Author contributions
K.S. initiated the project and designed the compounds. L.S. did synthesis, purification and characterization, under the supervision of K.S. M.P. Supervised the biological assays. D.F. Performed hepatic stage activity assays. D.F. performed asexual blood-stage activity assays. The manuscript was written through the contributions of all authors. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-04532-w.
References
- 1.Phillips MA, et al. Malaria. Nat. Rev. Dis. Primers. 2017;3:17050. doi: 10.1038/nrdp.2017.50. [DOI] [PubMed] [Google Scholar]
- 2.Neafsey DE, Taylor AR, Maclnnis BL. Advances and opportunities in malaria population genomics. Nat. Rev. Genet. 2021;22:502–517. doi: 10.1038/s41576-021-00349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White NJ, et al. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 4.Loy DE, et al. Out of Africa: Origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. Int. J. Parasitol. 2017;47:87–97. doi: 10.1016/j.ijpara.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Malaria Report 2020; World Health Organization (2020).
- 6.Roberts D, Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar. J. 2016;15:246. doi: 10.1186/s12936-016-1290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yimam Y, Nateghpour M, Mohebali M, Afshar MJ. A systematic review and meta-analysis of asymptomatic malaria infection in pregnant women in Sub-Saharan Africa: A challenge for malaria elimination efforts. PLoS One. 2021;16:e0248245. doi: 10.1371/journal.pone.0248245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrick CJ. Hypnozoites in Plasmodium: do parasites parallel plants? Trends Parasitol. 2021;37:273–282. doi: 10.1016/j.pt.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli LR, et al. The immunology of Plasmodium vivax malaria. Immunol Rev. 2020;293:163–189. doi: 10.1111/imr.12816. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad SS, Rahi M, Sharma A. Relapses of Plasmodium vivax malaria threaten disease elimination: time to deploy tafenoquine in India? BMJ Glob. Health. 2021;6:e004558. doi: 10.1136/bmjgh-2020-004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo E, et al. Transmission dynamics of co-endemic Plasmodium vivax and P. falciparum in Ethiopia and prevalence of antimalarial resistant genotypes. PLoS Negl. Trop. Dis. 2017;11:e000580. doi: 10.1371/journal.pntd.0005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamre KES, Ayodo G, Hodges JS, John CC. A mass insecticide-treated bed net distribution campaign reduced malaria risk on an individual but not population level in a highland epidemic-prone area of Kenya. Am. J. Trop. Med. Hyg. 2020;103:2183–2188. doi: 10.4269/ajtmh.19-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangena JA, et al. Indoor residual spraying for malaria control in sub-Saharan Africa 1997 to 2017: An adjusted retrospective analysis. Malar. J. 2020;19:150. doi: 10.1186/s12936-020-03216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tse EG, Korsik M, Todd MH. The past, present and future of anti-malarial medicines. Malar. J. 2019;18:93. doi: 10.1186/s12936-019-2724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Rts SCTP. Efficacy and safety of the RTS, S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med.11, e1001685 (2014). [DOI] [PMC free article] [PubMed]
- 16.Duffy PE, Gorres JP. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. 2020;5:48. doi: 10.1038/s41541-020-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO recommends groundbreaking malaria vaccine for children at risk (2021). https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk.
- 18.Thriemer K, Ley B, von Seidlein L. Towards the elimination of Plasmodium vivax malaria: Implementing the radical cure. PLoS Medicine. 2021;18:e1003494. doi: 10.1371/journal.pmed.1003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross LS, Fidock DA. Elucidating mechanisms of drug-resistant Plasmodium falciparum. Cell Host Microbe. 2019;26:35–47. doi: 10.1016/j.chom.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White NJ. Antimalarial drug resistance. J. Clin. Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibeshi MA, Kifle ZD, Atnafie SA. Antimalarial drug resistance and novel targets for antimalarial drug discovery. Infect. Drug Resist. 2020;13:4047–4060. doi: 10.2147/IDR.S279433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coronado LM, Nadovich CT, Spadafora C. Malarial hemozoin: From target to tool. Biochim. Biophys. Acta. 2014;1840:2032–2041. doi: 10.1016/j.bbagen.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eastman RT, Fidock DA. Artemisinin-based combination therapies: A vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyu H-N, et al. Study towards improving artemisinin-based combination therapies. Nat. Prod. Rep. 2021;38:1243–1250. doi: 10.1039/d0np00079e. [DOI] [PubMed] [Google Scholar]
- 25.Popovici J, Tebben K, Witkowski B, Serre D. Primaquine for Plasmodium vivax radical cure: What we do not know and why it matters. Int. J. Parasitol. Drugs Drug Resist. 2021;15:36–42. doi: 10.1016/j.ijpddr.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nekkab N, et al. Estimated impact of tafenoquine for Plasmodium vivax control and elimination in Brazil: A modelling study. PLoS Med. 2021;18:e1003535. doi: 10.1371/journal.pmed.1003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baird JK. 8-aminoquinoline therapy for latent malaria. Clin. Microbiol. Rev. 2019;32:e00011–19. doi: 10.1128/CMR.00011-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raphemot R, Posfai D, Derbyshire ER. Current therapies and future possibilities for drug development against liver-stage malaria. J. Clin. Invest. 2016;126:2013–2020. doi: 10.1172/JCI82981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailly C. Pyronaridine: An update of its pharmacological activities and mechanisms of action. Biopolymers. 2021;112:e23398. doi: 10.1002/bip.23398. [DOI] [PubMed] [Google Scholar]
- 30.Mishra M, Mishra VK, Kashaw V, Iyer AK, Kashaw SK. Comprehensive review on various strategies for antimalarial drug discovery. Eur. J. Med. Chem. 2017;125:1300–1320. doi: 10.1016/j.ejmech.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Pazhayam NM, Chibber-Goel J, Sharma A. New leads for drug repurposing against malaria. Drug. Discov. Today. 2019;24:263–271. doi: 10.1016/j.drudis.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Cupp EW, Sauerbrey M, Richards F. Elimination of human onchocerciasis: History of progress and current feasibility using ivermectin (mectizan®) monotherapy. Acta. Trop. 2011;120(suppl. 1):S100–S108. doi: 10.1016/j.actatropica.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: Health impact after 8 years. PLoS Negl. Trop. Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naquira C, et al. Ivermectin for human strongyloidiasis and other intestinal helminths. Am. J. Trop. Med. Hyg. 1989;40:304–309. doi: 10.4269/ajtmh.1989.40.304. [DOI] [PubMed] [Google Scholar]
- 35.Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: Effective in crusted scabies induced by corticosteroids but ineffective in nail scabies. J. Dermatol. 2003;30:411–416. doi: 10.1111/j.1346-8138.2003.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 36.Curie MJ, Reynolds GJ, Glasgow NI, Bowden FJ. A pilot study of the use of oral ivermectin to treat head lice in primary school students in Australia. Pedriatr. Dermatol. 2010;27:595–599. doi: 10.1111/j.1525-1470.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 37.Billingsley P, et al. A roadmap for the development of ivermectin as a complementary malaria vector control tool. Am. J. Trop. Med. Hyg. 2020;102(suppl. 2):3–24. doi: 10.4269/ajtmh.19-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh K, Singh L. Ivermectin: A promising therapeutic for fighting malaria. Current status and perspective. J. Med. Chem. 2021;64:9711–9731. doi: 10.1021/acs.jmedchem.1c00498. [DOI] [PubMed] [Google Scholar]
- 39.Kobylinski KC, et al. Ivermectin susceptibility and sporontocidal effect in Greater Mekong subregion Anopheles. Malar. J. 2017;16:280. doi: 10.1186/s12936-017-1923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panchal M, et al. Plasmodium falciparum signal recognition particle components and anti-parasitic effect of ivermectin in nucleo-cytoplasmic shuttling of SRP. Cell Death Dis. 2015;5:e994. doi: 10.1038/cddis.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Carvalho LP, et al. Ivermectin impairs the development of sexual and asexual stages of Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 2019;63:e00085–e119. doi: 10.1128/AAC.00085-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendes AM, et al. Inhibition of Plasmodium liver infection by ivermectin. Antimicrob. Agents Chemother. 2017;61:2e02005–2e0200516. doi: 10.1128/AAC.02005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanachayangkul P, et al. Safety, pharmacokinetics, and activity of high-dose ivermectin and chloroquine against the liver-stage of Plasmodium cynomolgi infection in rhesus macaques. Antimicrob. Agents Chemother. 2020;64:e00741–e820. doi: 10.1128/AAC.00741-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azevedo R, et al. A bioluminescence method for in vitro screening of Plasmodium transmission-blocking compounds. Antimicrob. Agents Chemother. 2017;61:202699–202716. doi: 10.1128/AAC.02699-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azevedo R, Mendes AM, Prudêncio M. Inhibition of Plasmodium sporogonic stages by ivermectin and other avermectins. Parasit. Vectors. 2019;12:594. doi: 10.1186/s13071-019-3805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meunier B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008;41:69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal D, Gupta RD, Awasthi SK. Are antimalarial hybrid molecules a close reality or a distant dream? Antimicrob. Agents Chemother. 2017;61:e00249–e317. doi: 10.1128/AAC.00249-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh L, et al. Molecular design and synthesis of ivermectin hybrids targeting hepatic and erythrocytic stages of Plasmodium parasites. J. Med. Chem. 2020;63:1750–1762. doi: 10.1021/acs.jmedchem.0c00033. [DOI] [PubMed] [Google Scholar]
- 49.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyers JI, et al. Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J. Exp. Biol. 2015;218:1478–1486. doi: 10.1242/jeb.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh K, Kaur H, de Kock C, Chibale K, Balzarini J. Quinoline-pyrimidine hybrids: Synthesis, antiplasmodial activity, SAR and mode of action studies. J. Med. Chem. 2014;57:435–448. doi: 10.1021/jm4014778. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J-H, Xu X-J, Ji M-H, Cheng J-L, Zhu G-N. Design, synthesis, and biological activities of milbemycin analogues. J. Agric. Food Chem. 2011;59:4836–4850. doi: 10.1021/jf2001926. [DOI] [PubMed] [Google Scholar]
- 53.Blizzard T, Margiatto G, Linn B, Mrozik H, Fisher MH. Avermectin analogs with a spacer between the aglycone and the disaccharide. Bioorg. Med. Chem. Lett. 1991;11:369–372. [Google Scholar]
- 54.Batey RA, Yoshina-Ishii C, Taylor SD, Santhakumar V. A new protocol for the formation of carbamates and thiocarbamates using carbamoyl imidazolium salts. Tetrahedron Lett. 1999;40:2669–2672. [Google Scholar]
- 55.All attempts to grow crystals for recording single crystal X-ray analysis were futile. However, density functional theorem (DFT) calculations were performed on both the epimers and the best optimized structures are shown in Fig. 8. The assignment of absolute stereochemistry at C-2 as R (15b) and S (15a) is based on the DFT optimized 3D structures of the epimers of the 8. Formation of regioisomers and epimers has also previously been observed in avermectins.
- 56.Fraser-Reid B, Wolleb H, Faghih R, Barchi J., Jr Avermectin chemistry: Problems of conjugation, deconjugation and epimerization. J. Am. Chem. Soc. 1987;109:933–935. [Google Scholar]
- 57.Kaur H, et al. Primaquine-pyrimidine hybrids: Synthesis and dual stage antiplasmodial activity. Eur. J. Med. Chem. 2015;101:266–273. doi: 10.1016/j.ejmech.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 58.Ploemen IH, et al. Visualisation and quantitative analysis of the rodent malaria liver-stage by real time imaging. PLoS One. 2009;4:e7881. doi: 10.1371/journal.pone.0007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.