Abstract
Objective.
To describe technical aspects and surgical outcomes of endoscopic resection and mucosal reconstitution with epidermal grafting (ie, the Maddern procedure) in the treatment of idiopathic subglottic stenosis.
Study Design.
Medical record abstraction.
Setting.
Johns Hopkins Hospital.
Methods.
Retrospective series of 9 adults with idiopathic subglottic stenosis who underwent the Maddern procedure by a single surgeon over a 5-year period. Prespecified outcomes included (1) perioperative outcomes (Clavien-Dindo grade 4/5 complications, need for staged tracheostomy, hospital length of stay), (2) postoperative outcomes (peak expiratory flow rate [PEFR], need for subsequent airway surgery, tracheostomy at follow-up), and (3) patient-reported quality-of-life outcomes (Clinical COPD Questionnaire, Voice Handicap Index–10, Eating Assessment Tool–10, and 12-Item Short Form Version 2). Wilcoxon matched-pairs signed rank test and Kaplan-Meier analysis were performed.
Results.
There were no Clavien-Dindo grade 4/5 complications; 2 patients required unplanned staged tracheostomy; and the median length of stay was 3 days. Following endoscopic resection and stent removal, a median of 2 laser resurfacing procedures were required. Two patients developed recurrent stenosis requiring cricotracheal resection (CTR). There were significant improvements in PEFR, Clinical COPD Questionnaire, and Voice Handicap Index–10, without significant difference in Eating Assessment Tool–10. The 12-Item Short Form Version 2 approximated the population norm. Kaplan-Meier analysis demonstrated significant improvement in time to surgery after the final laser resurfacing.
Conclusion.
The Maddern procedure has a low complication rate and offers durable physiologic improvement in PEFR, limiting need for additional procedures. Risks included need for CTR salvage, temporary tracheostomy, phlegm accumulation, and laryngospasm. It is a surgical option for patients with short dilation intervals who prefer to avoid the risks of CTR.
Keywords: laryngotracheal stenosis, idiopathic subglottic stenosis, skin graft, endoscopic laryngotracheoplasty, minimally invasive surgery
Idiopathic subglottic stenosis (iSGS) is a rare but progressive fibroinflammatory narrowing of the subglottic airway. Patients present with dyspnea and stridor due to airway narrowing and are almost exclusively healthy Caucasian females.1-3 The pathogenesis of iSGS is poorly understood, although recent studies support immunologic mechanisms.4-6
Current treatment for iSGS is surgical and traditionally involves endoscopic intervention, open airway procedures, or tracheostomy, with considerable heterogeneity in management patterns.2,7,8 Limited retrospective case series also suggest benefit from repeated intralesional steroid injections.9-11 Endoscopic management typically involves endoscopic dilation (ED) or endoscopic resection and medical therapy (ERMT).7 Although effective in the short term, endoscopic techniques likely do not address the underlying pathophysiology of iSGS and often require repetition.
Open airway surgery involves cricotracheal resection (CTR) with end-to-end anastomosis or laryngotracheoplasty with cartilaginous augmentation. In an international cohort, 28.0% of patients treated with ED required recurrent intervention as compared with 12.4% with EMRT and only 1.2% with CTR.7 Multiple series demonstrate excellent long-term responses in carefully selected patients with iSGS.7,12,13 However, even CTR possesses a 10%-15% rate of long-term recurrence (>5 years). Additionally, improved disease control with CTR must be balanced with greater surgical morbidity and inferior voice.2,7,12
Translational science has recently implicated aberrant fibroblast function and adaptive immunologic cell subsets in iSGS.4-6,14 Integrating these insights with the clinical observation that iSGS is a disease of subglottic mucosa rather than a cartilaginous framework, surgeons have developed 2 novel procedures aimed at resecting the diseased mucosa while leaving the unaffected framework intact.1,15 In both, the mucosa is reconstituted with a split-thickness skin graft (STSG) to optimize wound healing and prevent restenosis following excision of mucosal scar. The retrograde endoscopically assisted cricoid hypertrophic epithelial resection (REACHER) procedure, developed by Dr Robert Lorenz, is conducted through open neck and tracheal incisions. The endoscopic Maddern procedure, developed by Dr Guri Sandhu and named after the first patient, endoscopically extirpates diseased mucosa and reconstitutes the airway lining with a STSG.15,16 In this article, we describe a pilot study of a single surgeon’s experience performing the Maddern series of procedures in 9 patients with iSGS.
Materials and Methods
Study Design
This is a retrospective pilot case series. Informed written consent was obtained from all participants in accordance with the Institutional Review Board (NA_00078310) of the Johns Hopkins University School of Medicine.
Eligibility Criteria
All patients with iSGS who underwent the Maddern procedure at Johns Hopkins between May 2016 and May 2020 were included. Diagnosis of iSGS was established with previously defined criteria.1,2
Surgical Technique
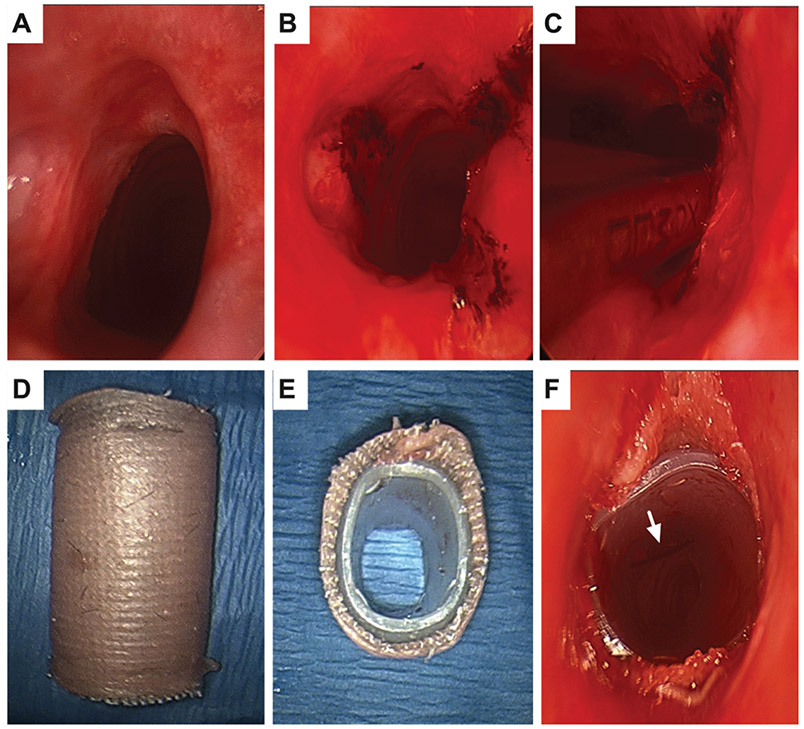
All Maddern procedures were performed by the senior author (A.T.H.) under general anesthesia. Suspension laryngoscopy with the universal modular glottiscope is performed to expose the airway (Figure 1A), and supraglottic jet ventilation maintains oxygenation. Under bronchoscopic guidance, radial excisions are performed with carbon dioxide laser (Lumenis; Figure 1B) to establish the depth by cutting down to the inner perichondrium. The microdebrider is used to excise stenosis down to the perichondrium of the cricoid and first tracheal ring, creating an airway of equal caliber to the healthy trachea below (Figure 1C). Balloon dilation may be performed, and the stent is sized to the airway. An STSG is harvested from the thigh (in all patients except No. 5, in whom a nasal mucosal graft was harvested). An endoluminal stent is fashioned from a silastic T-tube limb cut to the size of the stenotic segment and then wrapped with Adaptic (Medtronic) and STSG with dermis facing outward. The STSG is sutured to both edges of the stent, ensuring overhang at both ends to avoid direct contact of the stent with the trachea (Figure 1, D and E), which may cause granulation. The stent is placed endoscopically into the subglottis. Position is verified via bronchoscopy, and the stent is secured with a 2 nylon suture on a 75 mm needle (490T Suture, Ethicon, Johnson & Johnson, New Brunswick, NJ) placed transcervically (Figure 1F). The airway is managed with mask ventilation or laryngeal mask airway during emergence.
Figure 1.
Procedural images: (A) Stenosis. (B) Laser cuts to establish depth to perichondrium. (C) Microdebrider excision. (D, E) Modified T-tube wrapped with adaptic and skin graft. (F) After endoscopic placement across stenosis, secured with transcervical stitch (arrow).
Postoperatively, patients are admitted to the intensive care unit for 1 night with an anticipated 2- to 3-day hospital stay for pain control and to ensure adequate breathing and deglutition. Humidified air via a face tent is administered during the inpatient stay. Saline nebulizer (3 times per day) and guaifenesin (1200 mg, 2 times per day) are utilized for 2 weeks to limit secretion buildup within the stent. Augmentin (875 mg, 2 times per day) is given for the first week, with a transition to ciprofloxacin (500 mg, 2 times per day) the second week to prevent pseudomonas infection of the graft. Epithelial areas that were not debrided tend not to embed STSG; therefore, removal of excess graft is performed at the time of stent removal in the operating room after 12 to 16 days. Subsequent laser resurfacing, with pulsed KTP (potassium titanyl phosphate) or carbon dioxide, is conducted under anesthesia 4 to 6 weeks after stent removal to ablate the most superficial keratin-generating level of STSG epithelium from 2 opposing quadrants to avoid creation of contiguous raw surfaces leading to recurrent scar. Such resurfacing prevents accumulation of keratin within the subglottis and may be repeated if keratin production continues.
Data Extraction
Electronic medical records of patients meeting inclusion criteria were reviewed. Patient demographics, comorbidities, and body mass index were extracted. Stenosis characteristics, operative details, and treatment history before and after the Maddern procedure were also recorded.
Outcomes
Perioperative outcomes included Clavien-Dindo grade 4/5 complications (life-threatening complications or death),17 need for staged tracheostomy, and hospital length of stay. Postoperative outcomes were peak expiratory flow rate (PEFR),18,19 need for subsequent airway surgery, and tracheostomy at last follow-up. Validated patient-reported outcomes included Clinical COPD Questionnaire (CCQ),20 Voice-Related Quality of Life,21 Eating Assessment Tool–10,22 Voice Handicap Index–10 (VHI-10),23 and 12-Item Short-Form Version 2.24
Follow-up was defined as the time between the Maddern procedure and the last patient contact. Preoperative surgery-free interval (SFI) was defined as the time between the most recent 2 endoscopic procedures prior to the Maddern procedure, to reflect progressively shortening interdilation intervals that prompted alternate intervention. This was not significantly different from the average interdilation interval calculated over all previous procedures for each patient (4.5 vs 8.6 months, P = .0703). Postoperative SFI was defined as the time between the last laser resurfacing procedure and subsequent airway intervention or last patient contact. The preoperative patient-reported outcomes and spirometry were collected from the earliest patient encounter, and the postoperative findings were obtained at last follow-up (before subsequent intervention) or by survey at the time of the study.
Statistical Analysis
A nonparametric Wilcoxon matched-pairs signed rank test was used to assess differences in continuous variables before and after the Maddern procedure. Kaplan-Meyer analysis was performed to compare time to next surgery before and after completion of the Maddern series. P < .05 was considered statistically significant. Data analysis was performed with Prism 8 (GraphPad Inc).
Results
Patient Demographics and Treatment History
From May 2016 to May 2020, 9 patients with iSGS underwent the Maddern procedure. Eight (88.9%) were female; the median age was 51 years (95% CI, 39-65); and the median Charlson Comorbidity Index was 1 (95% CI, 0-3; Table 1). Before the Maddern procedure, patients had undergone a median 8 airway procedures (95% CI, 4-12). Six (66.7%) were treated exclusively with ED prior to the Maddern procedure, and 3 had prior complex airway interventions that failed.
Table 1.
Patient Demographics and Treatment History.
| No.a | Age, y | Sex | BMI | DM | GERD | CCI | Treatment History |
|---|---|---|---|---|---|---|---|
| 1 | 46 | M | 29.8 | No | No | 0 | Dilation, excision, steroid (2); office steroid injection (2) |
| 2b | 46 | F | 27.8 | No | No | 0 | Tracheostomy and stent; dilation, excision, steroid; operative steroid injection (2) |
| 3 | 51 | F | 31.8 | No | Yes | 1 | Dilation, excision, mitomycin C (7); dilation, mitomycin C |
| 4 | 58 | F | 32.1 | No | Yes | 1 | Dilation; open T-tube placement; dilation, excision, steroid (8) |
| 5b | 39 | F | 19.1 | No | No | 0 | Dilation, excision (3); dilation, excision, steroid (3); operative steroid injection |
| 6 | 61 | F | 31.1 | Yes | No | 3 | Dilation, excision, steroid (12) |
| 7 | 65 | F | 32.8 | No | Yes | 3 | Dilation, excision, steroid (5); dilation, excision, steroid, mitomycin C (2) |
| 8 | 39 | F | 19.2 | No | No | 0 | Dilation, excision, steroid (9) |
| 9 | 72 | F | 24.3 | No | Yes | 3 | Dilation, mitomycin C (6); office steroid injection; laryngoplasty with auricular cartilage; dilation, excision, steroid (4) |
| Overallc | 51 (39-65) | 89% F | 29.8 (19.2-32.1) | 11% Yes | 44% Yes | 1 (0-3) | 8 Procedure (4-12) |
Abbreviations: BMI, body mass index; CCI: Charlson Comorbidity Index; DM, diabetes mellitus; F, female; GERD, gastroesophageal reflux; M, male.
All patients were Caucasian.
Patient required additional open airway surgery after the Maddern procedure.
Values are presented as median (95% CI) or percentage.
Operative Details
See Table 2 and Appendix 1 (available online).
Table 2.
Operative Details.a
| No. | Distance from glottis, cm |
Length, cm |
Stenosis, % |
Stent size, mm |
LOS, d | Graft take, % |
Laser resurfacing proceduresb |
Other procedures |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.5 | 2 | 60 | 14 | 2 | 100 | 3 (2.6, 5.8, 8.0) | |
| 2c | 1 | 1.8 | 50 | 12 | 3 | 25 (anterior) | 1 (1.4) | CTR and tracheostomy |
| 3d | 1 | 1.5 | 40 | 12 | 9 | 100 | 4 (1.6, 2.8, 6.1, 14.2) | |
| 4 | 2 | 1.5 | 90 | 13 | 3 | 100 | 2 (1.9, 5.1) | |
| 5c | 1 | 1 | 95 | 12 | 5 | 100 (nasal mucosa) | Revision Maddern with STSG (60% take), CTR, and tracheostomy | |
| 6 | 0 | 2.5 | 60 | 12 | 2 | 100 | 1 (1.6) | |
| 7d | 0.5 | 2 | 75 | 12 | 7 | 100 | 2 (1.9, 5.1) | |
| 8 | 1 | 1.5 | 70-75 | 12 | 5 | 100 | 1 (2.1) | |
| 9 | 1 | 1.75 | 60-70 | 12 | 3 | 100 | 2 (1.6, 4.3) | |
| Overalle | 1 (0.5-1.5) | 1.75 (1.5-2) | 65 (50-90) | 12 (12-13) | 3 (2-7) | 100 (100-100) | 2 (1-4) | 2 (1-5) |
Abbreviations: CTR, cricotracheal resection; LOS, length of stay; STSG, split-thickness skin graft.
Blank cells indicate not applicable.
Values are presented as No. (months after index procedure).
Patient required additional open airway surgery after the Maddern procedure.
Patient had intraoperative tracheostomy.
Values are presented as median (95% CI).
Outcomes
There were no Clavien-Dindo grade 4/5 complications. Specifically, there were no 30-day mortalities, need for alternative routes of feeding, new vocal fold hypomobility, or unplanned returns to the operating room during initial hospitalization. There was one 30-day readmission (Appendix 2, available online). Frequent phlegm production was the most common complaint after the Maddern procedure. Specifically, in response to question 6 on the CCQ asking “On average, during the past week, how often did you produce phlegm?” the median score was 3 out of 6 (range, 3-5), corresponding to “several times.”
None of the patients had a tracheostomy at the time of the first Maddern procedure, and 2 required tracheostomies during the initial surgery (Appendix 2, available online). No patients had a tracheostomy at last follow-up. Postoperative length of stay ranged from 2 to 9 days (median 3) and was longest in patients who underwent tracheostomy.
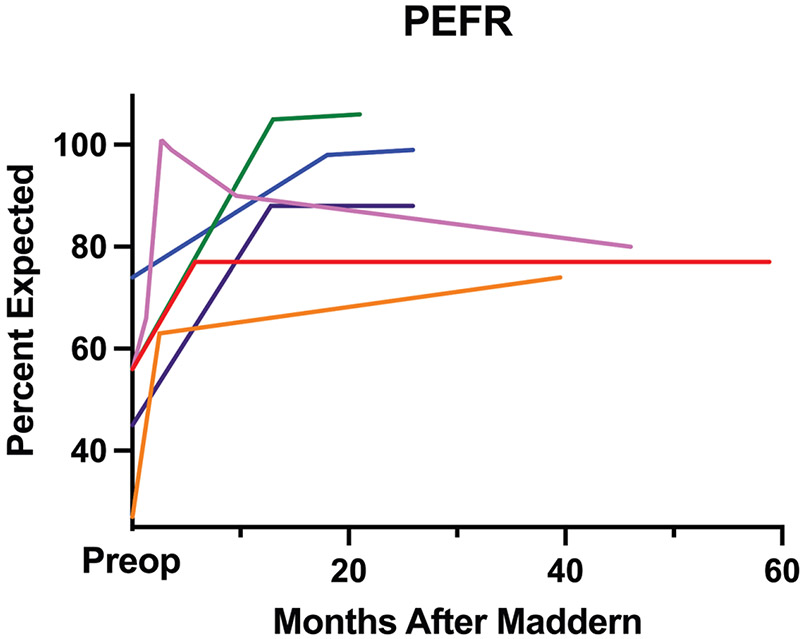
Patients were followed for a median 25.9 months (95% CI, 19.6-46) after the Maddern procedure (Table 3). Two patients (Nos. 2 and 6) experienced recurrent stenosis, ultimately requiring CTRs. None of the remaining 7 patients required recurrent dilation after the Maddern procedure. Pre- and postoperative spirometry was available for 6 of these patients, and PEFR improved significantly from a median 3.35 L/s (95% CI, 1.75-5.58) to 5.75 L/s (95% CI, 4.85-7.58; P = .0312). This corresponds to a median preoperative PEFR of 56% expected (95% CI, 27%-74%) and 84% expected (95% CI, 74%-106%; P = .0312) after the Maddern procedure. Improvements in PEFR were sustained over a median 32.7 months (Figure 2).
Table 3.
Pre- and Post-Maddern Objective Outcomes.a
| SFI, mo |
PEFR, L/s (% predicted) |
||||
|---|---|---|---|---|---|
| No. | Pre-Maddern | Post-Maddern | Pre-Maddern | Post-Maddern | Follow-up, mo |
| 1 | 4.5 | 50.8 | 5.58 (56) | 7.58 (77) | 58.8 |
| 2b | 1.9 | 7.7 | 2.22 (32) | 4.72 (71) | 19.6 |
| 3 | 6.1 | 31.8 | 3.56 (56) | 5.00 (80) | 46.0 |
| 4 | 8.6 | 34.3 | 1.75 (27) | 4.85 (74) | 39.5 |
| 5b | 1.4 | 0.77 | 2.24 (31) | 15.6 | |
| 6 | 5.1 | 24.6 | 2.56 (43) | 26.1 | |
| 7 | 3.5 | 20.5 | 4.38 (74) | 5.67 (99) | 25.9 |
| 8 | 11.2 | 19.0 | 3.00 (45) | 5.83 (88) | 21.1 |
| 9 | 3.7 | 16.8 | 3.14 (56) | 5.83 (106) | 21.1 |
| Overallc | 4.5 (1.9-8.6) | 20.5 (7.7-34.3) | 3.35 (1.75-5.58) | 5.75 (4.85-7.58) | 25.9 (19.6-46) |
| 56 (27-74) | 84 (74-106) | ||||
| P value | .0078d | .0312d | |||
Abbreviations: PEFR, peak expiratory flow rate; SFI, surgery-free interval.
Blank cells indicate not applicable.
Patient required additional open airway surgery after the Maddern procedure.
Values are presented as median (95% CI).
P < .05.
Figure 2.
Sustained peak expiratory flow rate (PEFR) improvement after the Maddern procedure in 6 patients with pre- and postoperative spirometry who did not require cricotracheal resection.
A median 2 additional procedures (95% CI, 1-5) were required after stent removal. The 7 patients who did not require subsequent CTR underwent 1 to 4 laser resurfacing procedures (median, 2) with no airway interventions required after each patient’s final laser procedure during the study period.
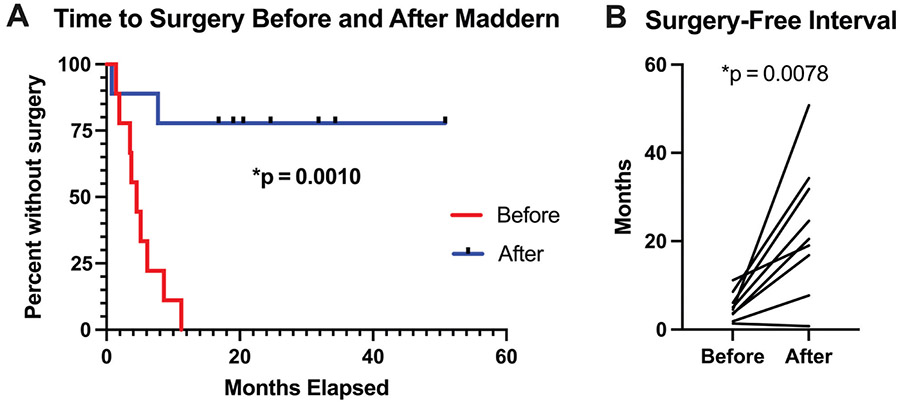
Kaplan-Meier analysis demonstrated significant improvement in time to surgery after the Maddern procedure as compared with presurgery (P = .0010; Figure 3A). The SFI increased significantly from a median 4.5 months (95% CI, 1.9-7.7) preoperatively to 20.5 months (95% CI, 7.7-34.2; P = .0078) after the Maddern procedure (Figure 3B, Table 3).
Figure 3.
Need for additional surgery was significantly reduced after the Maddern procedure and associated laser procedures for all 9 patients by (A) Kaplan-Meier analysis and (B) comparison of surgery-free intervals.
Patient-reported outcomes are presented in Table 4 and Figure 4.
Table 4.
Pre- and Post-Maddern Patient-Reported Outcomes.a
| CCQ |
V-RQOL |
EAT-10 |
Post-Maddern |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Pre-Maddern | Post-Maddern | Pre-Maddern | Post-Maddern | Pre-Maddern | Post-Maddern | VHI-10 | SF-12v2 Physical | SF-12v2 Mental |
| 1 | 5.2 | 0.9 | 16 | 10 | 0 | 1 | 3 | 50 | 60 |
| 2b | 1.2 | 5.1 | 12 | 50 | 0 | 0 | |||
| 3 | 4.1 | 1.3 | 22 | 12 | 0 | 2 | 8 | 51 | 57 |
| 4 | 5.0 | 2.2 | 34 | 28 | 2 | 9 | 35 | 53 | 56 |
| 5b | 2.6 | 17 | 0 | ||||||
| 6 | 3.5 | 0.9 | 10 | 11 | 0 | 1 | 2 | 47 | 62 |
| 7 | 1.8 | 0.5 | 12 | 10 | 0 | 0 | 0 | 51 | 64 |
| 8 | 4.0 | 0.8 | 13 | 12 | 2 | 1 | 5 | 58 | 57 |
| 9 | 4.6 | 1.4 | 32 | 11 | 14 | 3 | 0 | 46 | 43 |
| Overallc | 4.1 (1.8-5.2) | 0.9 (0.5-2.2) | 16 (10-34) | 11 (10-28) | 0 (0-14) | 1 (0-9) | 3 (0-35) | 51 (46-58) | 57 (43-64) |
| P value | .0156d | .0469d | .7188 | ||||||
Abbreviations: CCQ, Clinical COPD Questionnaire; EAT-10, Eating Assessment Tool–10; SF-12v2, 12-Item Short-Form Version 2; VHI-10, Voice Handicap Index–10; V-RQOL, Voice-Related Quality of Life.
Blank cells indicate not applicable.
Patient required additional open airway surgery after the Maddern procedure.
Values are presented as median (95% CI).
P < .05.
Figure 4.
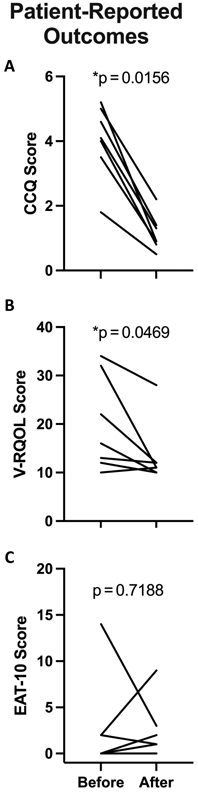
Patient-reported outcomes before and after the Maddern procedure for the 7 patients who did not ultimately require cricotracheal resection. CCQ, Clinical COPD Questionnaire; EAT-10, Eating Assessment Tool–10; V-RQOL, Voice-Related Quality of Life.
Discussion
This study documents the first adult proof-of-concept series of 9 patients with iSGS to undergo the Maddern series of procedures at a single institution. In carefully selected patients, the procedure had a low perioperative complication rate (Table 5). After the Maddern procedure (including stent removal and planned laser resurfacing), 7 patients (77.8%) required no additional surgery. These 7 also experienced significant and lasting improvement in physiologic ventilation (measured via PEFR) and subjective assessments of breathing, voice, and global quality of life (Tables 3 and 4, Figures 2 and 4). Two patients (22.2%) developed recurrent stenosis that was successfully managed with CTR.
Table 5.
Complications.a
| No.b | Complication | Consequence | Phlegm scorec |
|---|---|---|---|
| 1 | Mucous plug | None (coughed out in shower) | 3 |
| 2d | Recurrent stenosis | CTR | |
| 3 | 5 | ||
| 4 | 5 | ||
| 5d | Recurrent stenosis | Revision Maddern, also complicated by restenosis; ultimately required CTR | |
| 6 | 3 | ||
| 7 | 3 | ||
| 8 | Laryngospasm after discharge while stent in place | Readmission for observation (5 d) | 4 |
| 9 | 3 |
Abbreviation: CTR, cricotracheal resection.
Blank cells indicate not applicable.
No patient had a tracheostomy at last follow-up.
Postoperative Clinical COPD Questionnaire (0-6).
Patient required additional open airway surgery after the Maddern procedure.
While the Maddern procedure was not included in the recent international prospective trial of iSGS, its outcomes may be compared with the surgical treatment groups in that study.7 In comparison with CTR, the recurrent surgery rate following the Maddern series of procedures was higher (22.2% vs 1.2%, Maddern vs CTR); breathing outcomes were slightly worse based on the CCQ (0.9 vs 0.75); and voice outcomes were improved according to the VHI-10 (3 vs 13). Relative to ERMT, the recurrent surgery rate after the Maddern procedure was higher (22.2% vs 12.4%, Maddern vs ERMT), while breathing outcomes based on the CCQ were improved (0.9 vs 1.25) and VHI-10 voice outcomes were similar (3 vs 3).7 Finally, when compared with ED, recurrent surgery (22.2% vs 28.0%, Maddern vs ED), CCQ breathing (0.9 vs 1.8), and VHI-10 voice (3 vs 10) were improved with the Maddern procedure.7 There was no difference in swallowing outcomes, which are largely unaffected by management strategy. Similarly, the overall physical health of patients after Maddern procedures, as indicated by a median physical health score of 51 (12-Item Short-Form Version 2), is similar to the population norm of 50 and the median following ED (49), while slightly worse than ERMT (53) and CTR (54).7
There are key considerations when assessing Maddern candidates. Generally, iSGS is treated with serial endoscopic excision and dilation, with some surgeons preferring ERMT and CTR. CTR is utilized in refractory cases with short operative intervals and/or patients willing to take on higher surgical risks and expected voice changes in exchange for definitive therapy. The Maddern series of procedures provides a less invasive endoscopic option for patients with refractory disease, as demonstrated by the median preoperative SFI of 4.5 months in this series, which is far lower than the average 12.6 months in a recent multi-institutional cohort.2 In our opinion, it is not well suited for more routine cases of iSGS with mild disease requiring infrequent endoscopic management given the multiple procedures (3-6) required. For refractory cases such as those in this series, the Maddern procedure offers an alternative to CTR, with lower perioperative risk and less impact on voice to achieve durable benefit. If the Maddern procedure is unsuccessful, these patients can be effectively salvaged with CTR. Interestingly, previous open surgery is not a contraindication to the Maddern procedure, as shown by 3 patients in this series who had prior open laryngotracheoplasty and ultimately derived durable benefit from subsequent Maddern procedures. Finally, the etiology of stenosis should be considered. The frequent loss of cartilaginous integrity encountered in postintubation stenosis render this etiology less ideally suited to endoscopic procedures that address only mucosal disease.3 While it could be a surgical option for refractory SGS associated with granulomatosis with polyangiitis, such patients usually have longer dilation intervals with appropriate systemic therapy and/or suffer from concurrent glottic stenosis, which is inadequately addressed with the Maddern procedure.25
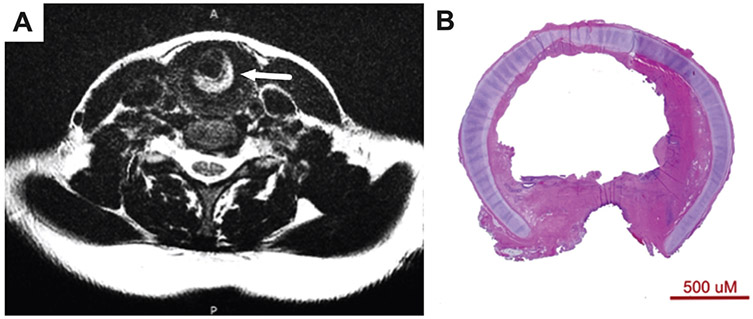
Additionally, in our limited series, characteristics of the airway scar appear to affect the operative outcome. Both patients in this series whose Maddern procedure failed had dense scar on CTR (Figure 5); however, they were not the patients with a history of the most invasive or numerous surgical procedures. Dense scar, especially posterior, is difficult to fully excise with the microdebrider due to instrument positioning through the laryngoscope. While dense scar may be dilated to fit the stent, the lack of sufficient excision may result in recurrent stenosis. Furthermore, extensive posterior tracheal involvement (Figure 5B) is a relative contraindication due to (1) hesitancy to aggressively debride without the safety of the posterior cricoid plate and (2) the cylindrical shape of the stent, which is restrictive to the width of the subglottis rather than the trachea. Our current strategy is to perform an endoscopic excision and dilation in advance of a Maddern procedure to assess for dense scar, whether the dilated subglottic airway fits a size 12 stent, and for proximity of scar to the vocal folds. Patients with dense scar and desire for definitive treatment are now offered CTR rather than the Maddern procedure. Patients with small subglottic airways who are expected to tolerate stent size <12 and stent placement above the conus elasticus are counseled on the probability of temporary tracheostomy, which is definitively placed for stent size ≤10.
Figure 5.
(A) T2 axial magnetic resonance image demonstrates dense posterior airway scar (arrow) prior to the Maddern procedure in patient 2. (B) Histology slide from cricotracheal resection shows dense scar in patient 5.
Intraoperative tracheostomy was required in 2 patients due to laryngeal edema, who both underwent decannulation at the time of stent removal. All patients were tracheostomy free at last follow-up (Table 4). This temporary tracheostomy rate of 22.2% is higher than the 9.3% rate in a cohort of 86 patients with CTR.7 This difference may be due to increased risk of laryngeal edema with endoscopic stent placement and a lower threshold to secure the airway surgically in a novel procedure involving stent placement. Observations common to the 2 patients who required tracheostomy were higher body mass index (>30) and greater neck soft tissue, suggesting that these characteristics may increase risk for tracheostomy.
The low rate of operative complications represents another important consideration with the Maddern procedure. One patient reported mucus plugging, which resolved with humification and expectoration, while another experienced episodic laryngospasm, in part due to the high location of the stent abutting her inferior vocal folds. There were no reports of vocal fold paralyses or unplanned returns to the operating room, which were encountered in 9.3% and 8.1% of patients with CTR, respectively.7 Furthermore, the 10%-20% risk of anastomotic complications after CTR was avoided in this alternative approach.7,12,26 It is worth noting that the 2 patients who developed recurrent stenosis after the Maddern procedure were successfully salvaged with CTR.
While 7 patients have not required additional iSGS surgery, a common postoperative concern was mucus accumulation. In addition to subjective complaints, there was elevation in response to the CCQ item “phlegm production several times over the last week.” This increase in mucus after the Maddern procedure is likely due to the lack of mucociliary transport afforded by the STSG, which lacks the cilia found on the pseudostratified columnar cells of the physiologic subglottic and tracheal epithelium. While laser resurfacing procedures help destroy the keratinizing stratified squamous epithelium in the STSG, the result is likely a heterogenous mixture of the 2 types. In response to these concerns, patient 5 was offered reconstruction with a cilia-bearing nasal mucosal graft. However, this graft developed obstructive edema and produced copious secretions, necessitating excision followed by an unsuccessful revision Maddern procedure and ultimately a successful CTR. On the basis of this experience, we would not recommend nasal mucosa for airway reconstruction. Buccal mucosa has been used by others with reported success in airway reconstruction and may represent an alternative to reduce phlegm burden.27 Potential alternative scaffolds that have been used in tympanic membrane and nasal mucosal or septal reconstruction include temporalis fascia, temporoparietal fascia, vascularized tensor fascia lata, and allogeneic materials.28-30 Although none of these linings would restore native mucociliary clearance, they may lead to less keratin buildup than an STSG. Identification of the ideal autologous graft or tissue-engineered construct for resurfacing the airway represents an opportunity for further research.
Limitations of this study include its retrospective nature, leading to incomplete availability of some data. The sample size is also small; however, given the rarity of iSGS and the dramatic impact of this procedure, it was enough to detect significant changes in disease course. In addition, the median follow-up of 25.4 months may be inadequate to detect late failures, such as those observed with CTR after 5 to 10 years.12,26 Based on the short SFI experienced by this patient population prior to the Maddern procedure, this follow-up is still adequate to demonstrate significant improvement in time to surgery. Monitoring of these patients is ongoing, and future studies will evaluate the long-term success of this procedure.
Conclusion
In a series of 9 patients with iSGS treated at a single institution, endoscopic resection and mucosal reconstitution with epidermal grafting (ie, the Maddern procedure) appeared to have a low burden of perioperative complications. Of 9 patients, 7 (77%) derived durable long-term benefit when measured via clinical, physiologic, and patient-reported end points. Risks included need for salvage with CTR, temporary tracheostomy, phlegm accumulation, and laryngospasm. The Maddern procedure represents an endoscopic alternative to CTR for patients with iSGS, with reduced perioperative risk and improved voice outcomes.
Supplementary Material
Funding source:
Research reported in his publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under awards 1R01DC018567 and R21DC017225. This study was also financially supported by the Triological Society and American College of Surgeons (Alexander T. Hillel).
Footnotes
Competing interests: Alexander T. Hillel has a sponsored research agreement with Medtronic.
Supplemental Material
Additional supporting information is available in the online version of the article.
Sponsorships: None.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Nouraei SAR, Sandhu GS. Outcome of a multimodality approach to the management of idiopathic subglottic stenosis. Laryngoscope. 2013;123(10):2474–2484. doi: 10.1002/lary.23949 [DOI] [PubMed] [Google Scholar]
- 2.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope. 2016;126(6):1390–1396. doi: 10.1002/lary.25708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2015;125(5):1137–1143. doi: 10.1002/lary.24956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelbard A, Katsantonis N-G, Mizuta M, et al. Idiopathic subglottic stenosis is associated with activation of the inflammatory IL-17A/IL-23 axis. Laryngoscope. 2016;126(11):E356–E361. doi: 10.1002/lary.26098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison RJ, Katsantonis N-G, Motz KM, et al. Pathologic fibroblasts in idiopathic subglottic stenosis amplify local inflammatory signals. Otolaryngol Neck Surg. 2019;160(1):107–115. doi: 10.1177/0194599818803584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motz KM, Yin LX, Samad I, et al. Quantification of inflammatory markers in laryngotracheal stenosis. Otolaryngol Head Neck Surg. 2017;157(3):466–472. doi: 10.1177/0194599817706930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelbard A, Anderson C, Berry LD, et al. Comparative treatment outcomes for patients with idiopathic subglottic stenosis. JAMA Otolaryngol Neck Surg. 2020;146(1):20. doi: 10.1001/jamaoto.2019.3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado F, Loiselle A, Depew ZS, et al. Idiopathic subglottic stenosis: an evolving therapeutic algorithm. Laryngoscope. 2014;124(2):498–503. doi: 10.1002/lary.24287 [DOI] [PubMed] [Google Scholar]
- 9.Hoffman MR, Francis DO, Mai JP, Dailey SH. Office-based steroid injections for idiopathic subglottic stenosis: patient-reported outcomes, effect on stenosis, and side effects. Ann Otol Rhinol Laryngol. 2020;129(4):361–368. doi: 10.1177/0003489419889066 [DOI] [PubMed] [Google Scholar]
- 10.Bertelsen C, Shoffel-Havakuk H, O’Dell K, Johns MM, Reder LS. Serial in-office intralesional steroid injections in airway stenosis. JAMA Otolaryngol Neck Surg. 2018;144(3):203. doi: 10.1001/jamaoto.2017.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song SA, Franco RA. Serial intralesional steroid injection for subglottic stenosis. Laryngoscope. 2020;130(3):698–701. doi: 10.1002/lary.28015 [DOI] [PubMed] [Google Scholar]
- 12.Carpenter PS, Pierce JL, Smith ME. Outcomes after cricotracheal resection for idiopathic subglottic stenosis. Laryngoscope. 2018;128(10):2268–2272. doi: 10.1002/lary.27263 [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Wright CD, Wain JC, Ott HC, Mathisen DJ. Idiopathic subglottic stenosis: factors affecting outcome after single-stage repair. Ann Thorac Surg. 2015;100(5):1804–1811. doi: 10.1016/j.athoracsur.2015.05.079 [DOI] [PubMed] [Google Scholar]
- 14.Hillel AT, Ding D, Samad I, Murphy MK, Motz K. T-helper 2 lymphocyte immunophenotype is associated with iatrogenic laryngotracheal stenosis. Laryngoscope. 2019;129(1):177–186. doi: 10.1002/lary.27321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniero JJ, Ekbom DC, Gelbard A, Akst LM, Hillel AT. Inaugural symposium on advanced surgical techniques in adult airway reconstruction: proceedings of the North American Airway Collaborative (NoAAC). JAMA Otolaryngol Head Neck Surg. 2017;143(6):609–613. doi: 10.1001/jamaoto.2016.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowe SN, Wentland CJ, Sandhu GS, Hartnick CJ. Management of complex pediatric laryngotracheal stenosis with skin graft reconstruction. Int J Pediatr Otorhinolaryngol. 2018;108:46–48. doi: 10.1016/j.ijporl.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 17.Clavien PA, Barkun J, De Oliveira ML, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 18.Carpenter DJ, Ferrante S, Bakos SR, Clary MS, Gelbard AH, Daniero JJ. Utility of routine spirometry measures for surveillance of idiopathic subglottic stenosis. JAMA Otolaryngol Head Neck Surg. 2019;145(1):21–26. doi: 10.1001/jamaoto.2018.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 20.Nouraei S, Randhawa P, Koury E, et al. Validation of the clinical COPD questionnaire as a psychophysical outcome measure in adult laryngotracheal stenosis. Clin Otolaryngol. 2009;34(4):343–348. doi: 10.1111/J.1749-4486.2009.01969.X [DOI] [PubMed] [Google Scholar]
- 21.Hogikyan N, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL). J Voice. 1999;13(4):557–569. doi: 10.1016/S0892-1997(99)80010-1 [DOI] [PubMed] [Google Scholar]
- 22.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–924. doi: 10.1177/000348940811701210 [DOI] [PubMed] [Google Scholar]
- 23.Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the Voice Handicap Index–10. Laryngoscope. 2004;114(9):1549–1556. doi: 10.1097/00005537-200409000-00009 [DOI] [PubMed] [Google Scholar]
- 24.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Del Pero MM, Jayne D, Chaudhry A, Sivasothy P, Jani P. Long-term outcome of airway stenosis in granulomatosis with polyangiitis (Wegener granulomatosis): an observational study. JAMA Otolaryngol Head Neck Surg. 2014;140(11):1038–1044. doi: 10.1001/jamaoto.2014.2430 [DOI] [PubMed] [Google Scholar]
- 26.Menapace DC, Modest MC, Ekbom DC, Moore EJ, Edell ES, Kasperbauer JL. Idiopathic subglottic stenosis: long-term outcomes of open surgical techniques. Otolaryngol Head Neck Surg. 2017;156(5):906–911. doi: 10.1177/0194599817691955 [DOI] [PubMed] [Google Scholar]
- 27.Liu IY, Mendelsohn AH, Ching H, Long J, Chhetri DK, Berke GS. Staged laryngotracheoplasty in adult laryngotracheal stenosis: predictors of long-term decannulation. JAMA Otolaryngol Head Neck Surg. 2015;141(3):211–218. doi: 10.1001/jamaoto.2014.3283 [DOI] [PubMed] [Google Scholar]
- 28.Sheehy JL, Glasscock ME. Tympanic membrane grafting with temporalis fascia. Arch Otolaryngol. 1967;86(4):391–402. doi: 10.1001/archotol.1967.00760050393008 [DOI] [PubMed] [Google Scholar]
- 29.Tellioğlu AT, Tekdemir I, Erdemli EA, Tüccar E, Ulusoy G. Temporoparietal fascia: an anatomic and histologic reinvestigation with new potential clinical applications. Plast Reconstr Surg. 2000;105(1):40–45. doi: 10.1097/00006534-200001000-00007 [DOI] [PubMed] [Google Scholar]
- 30.Karaaltin MV, Orhan KS, Demirel T. Fascia lata graft for nasal dorsal contouring in rhinoplasty. J Plast Reconstr Aesthetic Surg. 2009;62(10):1255–1260. doi: 10.1016/j.bjps.2008.03.053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.