Abstract
Background and aims:
The natural history of lean nonalcoholic fatty liver disease (NAFLD) is not well understood. Consequently, patient counseling and disease management are limited. We aimed to compare the natural history of lean, overweight and obese NAFLD in a US population with long term follow-up.
Methods:
All adults diagnosed with NAFLD in Olmsted County, MN between 1996–2016 were identified and all subsequent medical events were ascertained using a medical record linkage system. Subjects were divided based on body mass index (BMI) at NAFLD diagnosis into 3 groups: normal, overweight, and obese. The probability to develop cirrhosis, decompensation, malignancies, cardiovascular events or death among the 3 groups was estimated using the Aalen-Johansen method, treating death as a competing risk. The impact of BMI categories on these outcomes was explored using Cox proportional hazards regression analysis.
Results:
A total of 4,834 NAFLD individuals were identified: 414 normal BMI, 1,189 overweight and 3,231 obese. Normal BMI-NAFLD individuals were characterized by a higher proportion of women (66 vs 47%) and lower prevalence of metabolic comorbidities than the other 2 groups. In reference to obese, those with normal BMI NAFLD had a nonsignificant trend towards lower risk of cirrhosis (HR=0.33, 95%CI 0.1–1.05). There were no significant differences in the risk of decompensation (HR=0.79, 95%CI 0.11–5.79), cardiovascular events (HR=1.05, 95%CI 0.73–1.51) or malignancy (HR=0.87, 95%CI 0.51–1.48). Compared to obese, normal BMI NAFLD had higher risk of all-cause mortality (HR=1.96, 95%CI 1.52–2.51).
Conclusions:
NAFLD with normal BMI is associated with a healthier metabolic profile and possibly a lower risk of liver disease progression but similar risk of cardiovascular disease and malignancy than obese NAFLD.
Keywords: NAFLD, mortality, lean, BMI, non-obese
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and is commonly associated with excess weight and metabolic abnormalities, such as diabetes mellitus, hypertension and dyslipidemia (1). NAFLD is known to increase the overall mortality and risk of morbidity and mortality from cardiovascular diseases (CVD), malignancies, and liver disease (2,3). Hepatic steatosis is present in up to 90% of morbidly obese patients and 25–30% of the general population (4–6). However, a significant proportion of patients diagnosed with NAFLD have a normal body mass index (BMI) (7–10). The metabolic risk profile of this group, conventionally described as “lean NAFLD”, remains controversial with some studies showing lower prevalence of metabolic risk factors (11–14), and others demonstrating similar metabolic profile to the non-lean NAFLD patients (7,8,15,16).
Data reporting long-term outcomes are even more scarce and controversial. An abstract by Cruz et al demonstrated increased risk of mortality in biopsy-proven lean NAFLD compared to the non-lean NAFLD patients (11). Another study from Europe showed no significant difference in mortality risk in lean NAFLD, despite higher risk of progression of liver disease when compared to the overweight and obese NAFLD patients (12). A recent meta-analysis pooling data from several studies showed that the long-term outcomes for lean NAFLD appear worse than obese (17).
Hence, the natural history of NAFLD with normal BMI in the United States remains obscure and this gap limits patient management and counseling in clinical practice. In this work, we use a well-characterized NAFLD cohort from a US community to examine the risk of liver disease progression, non-liver related outcomes and mortality among 3 BMI groups: normal, overweight and obese, over 20 years of follow-up.
METHODS
Study population
We assembled a cohort of all adults diagnosed with NAFLD in Olmsted County, Minnesota between January 1996 and December 2016. The study participants were identified using the medical record linkage system of the Rochester Epidemiology Project (REP). REP is an integrated electronic infrastructure corroborating the health data of all residents in Olmsted County, Minnesota, which includes all the medical providers in the county. Medical information from these providers for each individual resident of Olmsted County is linked to a single identification number allowing linkage across health care systems and longitudinal follow up of patients’ entire health care history throughout their lifetime.
Residents diagnosed with NAFLD were identified using a 3-step algorithm: a code-based algorithm previously described (18), followed by natural language processing of abdominal imaging reports confirming presence of steatosis or cirrhosis, then internal validation by in-depth medical record review of a large subset of the cohort, as detailed in Figure 1 and Supplemental Methods.
Figure 1:
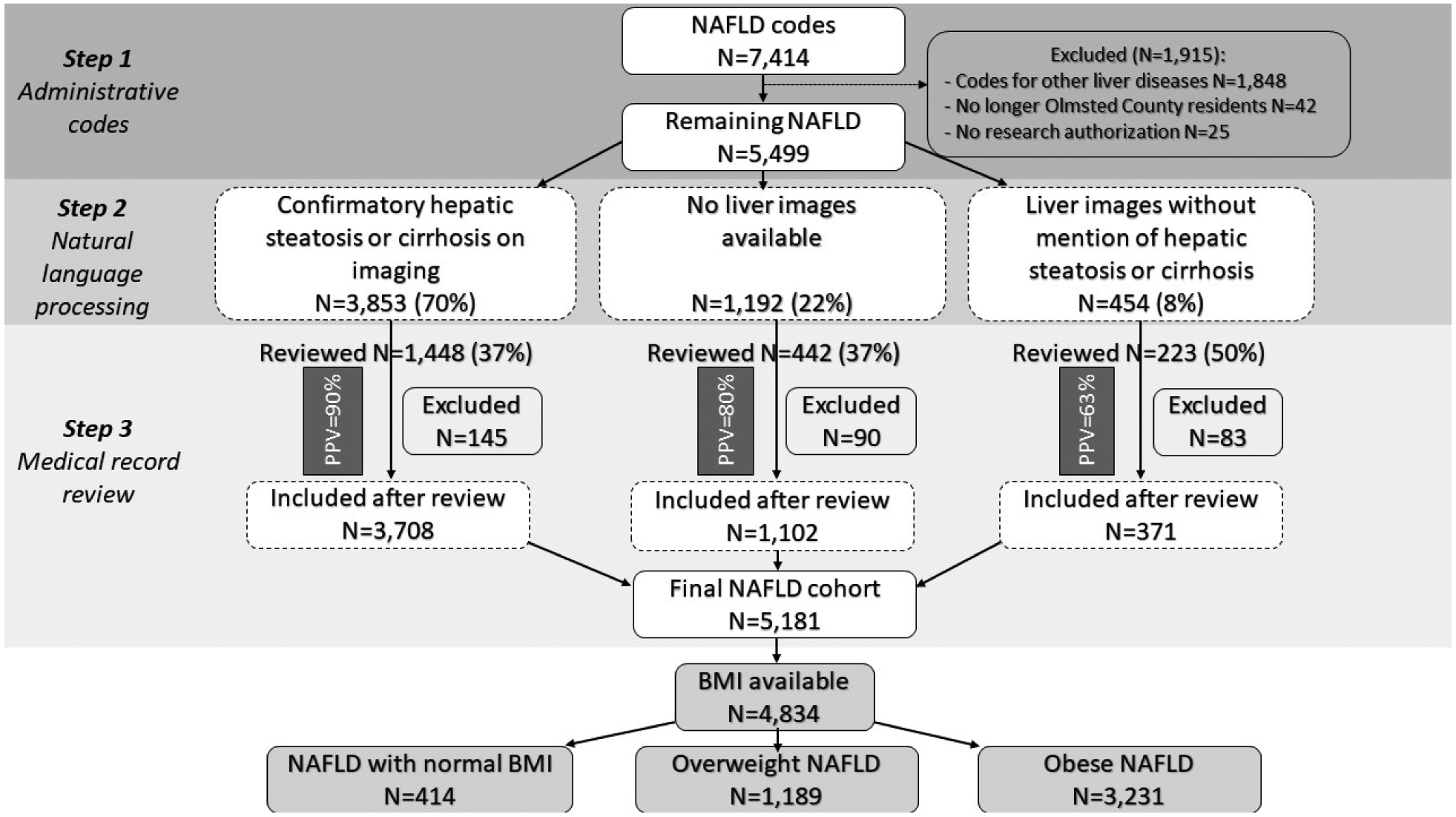
Study flowchart PPV: positive predictive value.
Ultimately, individual chart review was performed in approximately 40% of the total NAFLD cohort. This included all individuals with BMI <18.5 kg/m2 and 70% of those with BMI 18.5–24.9 kg/m2, who were specifically searched for any underlying causes that would explain recent/rapid weight loss (e.g., bariatric surgery) or increased risk of mortality (e.g., malignancies). Subjects were followed from study entry date (first NAFLD diagnosis date) until death, last date of follow up or study end-date (May 31, 2019).
Study outcomes and definitions
During individual chart review for validation of NAFLD diagnosis, NAFLD was defined as: 1. presence of steatosis on biopsy/imaging in the absence of competing etiologies OR 2. cirrhosis with coexistent metabolic comorbidities (obesity or diabetes mellitus) in the absence of competing etiologies. The primary outcomes were development of cirrhosis and decompensation, liver transplantation and death. Cirrhosis was identified using at least one code for cirrhosis or a decompensation event (i.e., ascites, encephalopathy, bleeding/non-bleeding esophageal varices and jaundice (Supplementary Table 2) or natural language processing of radiology reports containing keywords for cirrhosis (e.g., ‘cirrho’, ‘cirh’, ‘nodular’, and ‘nod’) or presence of FIB 4≥2.67 at any point during the follow up. All medical records meeting at least one of these criteria were individually reviewed. Cirrhosis was confirmed if at least one of the following criteria were met: histologic evidence of stage 4 fibrosis; imaging evidence of cirrhotic liver morphology and/or portal hypertension (splenomegaly, portosystemic shunting); increased liver stiffness concordant with cirrhosis (>5kPa on MRE).
Decompensating events were defined as ascites (evidenced on imaging), esophageal variceal bleeding (proven by esophagogastroduodenoscopy), hepatic encephalopathy based on physician notes and jaundice (based on physician notes and total bilirubin >1.5 mg/dl). Hepatocellular carcinoma and liver transplantation were also identified from patient notes, imaging and biopsy reports.
Secondary outcomes were: development of malignancies (hepatic, colon, stomach, esophageal, pancreatic, breast, prostate, uterine, ovarian, and lung) and cardiovascular events (angina, myocardial infarction (MI), stroke, atrial fibrillation (Afib), and cardiac arrest). These outcomes were validated in this cohort in previous studies (18, 19). These events, as well as comorbidities including diabetes mellitus (DM), hypertension (HTN), hyperlipidemia (HLD) documented at any time during the study timeframe were identified using the HICDA and ICD codes (see Supplemental Table 3).
Statistical analysis
The NAFLD cohort was divided into three subgroups based on the BMI value closest to the initial NAFLD diagnosis date, using the conventional World Health Organization (WHO) criteria and the revised cutoffs for Asians and Pacific Islanders (A/PI): normal BMI NAFLD (BMI <25 kg/m2 and <23 kg/m2 for A/PI), overweight NAFLD (BMI 25–29.9 kg/m2 and 23–24.9 kg/m2 for A/PI) and obese NAFLD (BMI ≥30 kg/m2 and ≥25 kg/m2 for A/PI). Those with missing BMI data were excluded from the analysis.
As BMI can vary during long follow-up periods, participants’ BMI category could change between normal, overweight and obese. Significant variations would require handling BMI as a time-dependent variable to allow transitions of participants between different BMI categories during follow-up. We explored this scenario by plotting all serial BMI values of each participant recorded over the health care visits since the date of NAFLD diagnosis (Supplementary Figure 1). The BMI variations over time were not sufficiently large to cause transitions between groups in this cohort. Therefore, we used the BMI closest to the NAFLD diagnosis date to establish the 3 groups for this analysis.
The probability to develop cirrhosis, decompensation, malignancies, cardiovascular events or death among the 3 groups was estimated using the Aalen-Johansen method, which allows for death as a competing risk. The impact of BMI categories on these outcomes was explored using Cox proportional hazards regression analysis, adjusted for conventional risk factors such as age, sex and time-dependent DM, HTN and dyslipidemia when applicable. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Inc.; Cary, NC) and R version 3.6.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). The study was approved by the Institutional Review Board.
RESULTS
Study flowchart and baseline characteristics
Of the 7,414 adults with at least one code for NAFLD, 1,915 were excluded due to presence of other non-NAFLD codes documented before the NAFLD diagnosis, lack of research authorization or Olmsted County residency on the date of diagnosis. The remaining 5,499 participants underwent the subsequent steps of natural language processing and individual chart review for validation of NAFLD codes (details in Figure 1). Among the 5,178 adults with confirmed NAFLD, BMI values were available in 4,834 subjects and they comprised the final study cohort for the analysis. Of these, 414 had normal BMI (8.6%), 1,189 were overweight (24.6%) and 3,231 were obese (66.8%). Subjects were followed for a median of 6.4 (range 0–20) years.
Noteworthy characteristics of the normal BMI NAFLD group were the female predominance (66%), a higher proportion of Asian and African American race (13%) and a lower prevalence of metabolic comorbidities than the other 2 groups. There was no difference in the proportion of those with cirrhosis at the time of initial diagnosis/study entry between the 3 BMI groups (total n=59). Baseline characteristics are summarized in Table 1.
TABLE 1:
Patients’ characteristics at the study entry/NAFLD diagnosis
| Variables | Normal (n = 414) |
Overweight (n = 1,189) | Obese (n = 3,231) | p value |
|---|---|---|---|---|
| Mean Age, years (SD) | 51.5 (18.0) | 54.1 (15.7) | 51.0 (14.1) | <0.001 |
| Gender, Female, n (%) | 273 (65.9%) | 561 (47.2%) | 1780 (55.1%) | <0.001 |
| Race | ||||
| White, n (%) | 336 (81.2%) | 1036 (87.1%) | 2790 (86.4%) | <0.001 |
| Asian American, n (%) | 30 (7.2%) | 27 (2.3%) | 144 (4.5%) | <0.001 |
| African American, n (%) | 25 (6.0%) | 50 (4.2%) | 93 (2.9%) | <0.001 |
| Others, n (%) | 23 (5.6%) | 76 (6.4%) | 204 (6.2%) | <0.001 |
| Mean BMI (SD) | 22.5 (2.0) | 27.7 (1.5) | 37.0 (6.2) | <0.001 |
| Diabetes, n (%) | 96 (23.2%) | 306 (25.7%) | 1270 (39.3%) | <0.001 |
| HTN, n (%) | 121 (29.2%) | 444 (37.3%) | 1437 (44.5%) | <0.001 |
| HLD, n (%) | 204 (49.3%) | 772 (64.9%) | 2235 (69.2%) | <0.001 |
| Cirrhosis, n (%) | 3 (0.7%) | 13 (1.1%) | 43 (1.3%) | 0.514 |
Risk of cirrhosis and decompensation
Of the 4,775 individuals with non-cirrhotic NAFLD at study entry, 107 developed cirrhosis during follow-up: 3 normal BMI, 23 overweight and 81 obese NAFLD (Table 2), at incidence rate of 0.11, 0.25 and 0.31 per 100 person-years, respectively. The probability of cirrhosis development since NAFLD diagnosis among the 3 groups is illustrated in Figure 2A. In reference to obese, those with normal BMI NAFLD were less likely to develop cirrhosis: hazard ratio (HR)=0.33 (95% confidence interval (CI) 0.10–1.05, p=0.06), although the relative risk difference did not reach statistical significance. There was no risk difference between obese and overweight NAFLD: HR=0.81 (95%CI 0.51–1.29, p=0.37). Due to the low number of incident cirrhosis outcomes in the non-obese groups, multivariable analyses were not feasible.
TABLE 2:
Primary and secondary outcomes at the end of the follow-up period.
| Outcomes | Normal (N= 414) | Overweight (N= 1,189) | Obese (N= 3,231) |
|---|---|---|---|
| NAFLD with cirrhosis patients | 6 | 36 | 124 |
| Compensated patients | 1 | 15 | 43 |
| Decompensated patients | 5 | 21 | 81 |
| Ascites events | 4 | 18 | 69 |
| Bleeding Varices events | 1 | 5 | 12 |
| Hepatic Encephalopathy events | 2 | 4 | 45 |
| Jaundice events | 3 | 9 | 42 |
| Any CV event * | 120 | 373 | 988 |
| Any Cancer event | 68 | 206 | 446 |
| Colon Cancer | 12 | 37 | 58 |
| Liver Cancer | 3 | 6 | 16 |
| Pancreatic Cancer | 7 | 8 | 27 |
| Stomach Cancer | 2 | 5 | 10 |
| Esophageal Cancer | 1 | 4 | 4 |
| Breast Cancer | 30 | 54 | 160 |
| Prostate Cancer | 10 | 65 | 84 |
| Uterine Cancer | 5 | 13 | 72 |
| Ovarian Cancer | 4 | 8 | 23 |
| Lung Cancer | 12 | 28 | 51 |
| Death | 74 | 148 | 347 |
Cardiovascular (CV) events: myocardial infarction, stroke, angina, atrial fibrillation, or cardiac arrest.
Figure 2:
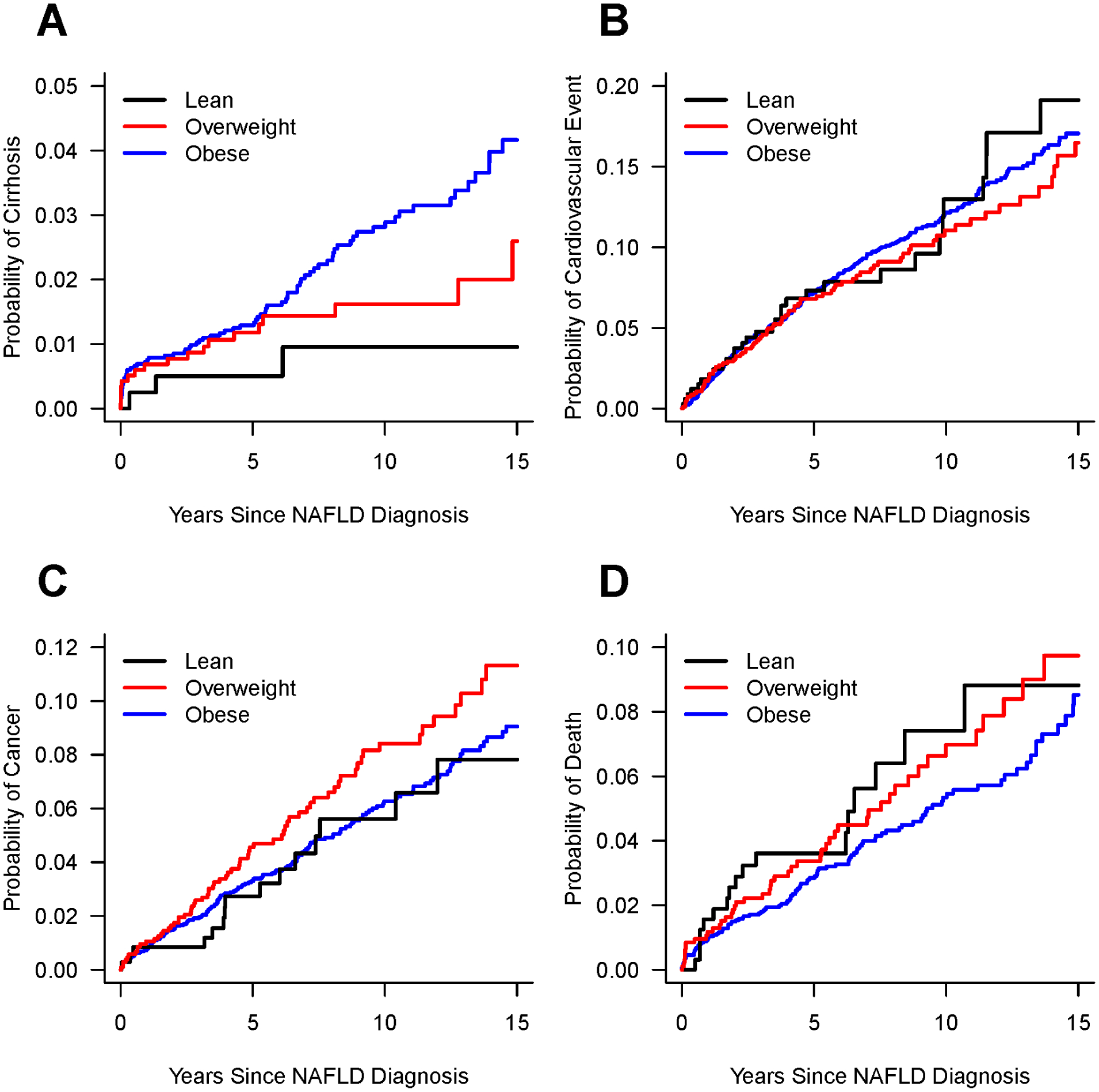
Probabilities of developing A: Cirrhosis; B: Cardiovascular event (myocardial infarction, stroke, angina, atrial fibrillation, or cardiac arrest); C: Incident cancers, all with death as a competing risk; and D: Kaplan Meier survival curves, since NAFLD diagnosis for lean, overweight and obese NAFLD.
The number of patients with decompensated cirrhosis and type of decompensation events are shown in Table 2. Decompensation analysis was performed only in those who did not have decompensation at study entry. The incidence rates of decompensation were 13.98, 8.22 and 16.0 per 100 person-years in the normal BMI, overweight and obese NAFLD with cirrhosis patients, respectively. There was no significant difference in the risk of decompensation in those with normal BMI and overweight NAFLD in reference to obese: HR=0.79 (95%CI 0.11–5.79, p=0.82) and HR=0.52 (95%CI 0.22–1.22, p=0.13), respectively.
Risk of cardiovascular events and malignancies
A total of 1481 CV events occurred during the study: 120 in normal BMI, 373 in overweight and 988 in obese NAFLD participants (Table 2), at incidence rate of 1.51, 1.37 and 1.40 per 100 person-years, respectively. The probabilities of CV events among the 3 groups are illustrated in Figure 2B. In reference to obese, after adjusting for age, sex, diabetes, hypertension, and dyslipidemia, there was no significant difference in CV risk for the normal BMI (aHR=1.23, 95%CI 0.85–1.78, p=0.28) or overweight (aHR=0.92, 95%CI 0.72–1.17, p=0.49) NAFLD individuals.
There were 720 incident diagnoses of malignancy: 68 among normal BMI, 206 in overweight and 446 in obese NAFLD individuals, at incidence rate of 0.61, 0.91 and 0.70 per 100 person-years, respectively. The probabilities of incident cancers among the 3 groups are illustrated in Figure 2C. There was no significant difference in malignancy risk among normal BMI compared to obese NAFLD (aHR=0.79, 95%CI 0.46–1.36, p=0.40). There was a trend towards increased malignancy risk in overweight NAFLD, which diminished after multivariable adjustment (aHR=1.10, 95%CI 0.82–1.46, p=0.53) (Table 3).
TABLE 3:
Showing the Hazard Ratio for development of cirrhosis, decompensation events, any cardiovascular event, malignancy, and death, with reference to obese NAFLD, by univariate or multivariable analysis (adjusted for sex, diabetes, HTN, and dyslipidemia).
| Variable | Normal | Overweight | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Cirrhosis | ||||||
| Univariate | 0.33 | 0.1–1.05 | 0.06 | 0.81 | 0.51–1.29 | 0.37 |
| Multivariable | - | - | - | - | - | - |
| Decompensation | ||||||
| Univariate | 0.79 | 0.11–5.79 | 0.82 | 0.52 | 0.22–1.22 | 0.13 |
| Multivariable | - | - | - | - | - | - |
| Any CV event | ||||||
| Univariate | 1.05 | 0.73–1.51 | 0.78 | 0.97 | 0.77–1.23 | 0.81 |
| Multivariable | 1.23 | 0.85–1.78 | 0.28 | 0.92 | 0.72–1.17 | 0.49 |
| Malignancy | ||||||
| Univariate | 0.87 | 0.51–1.48 | 0.61 | 1.29 | 0.98–1.71 | 0.07 |
| Multivariable | 0.79 | 0.46–1.36 | 0.40 | 1.10 | 0.82–1.46 | 0.53 |
| Death | ||||||
| Univariate | 1.96 | 1.52–2.51 | <0.001 | 1.23 | 1.02–1.49 | 0.03 |
| Multivariable | 1.63 | 1.25–2.13 | <0.001 | 0.99 | 0.81–1.2 | 0.90 |
Risk of death
A total of 569 individuals died: 74 normal BMI, 148 overweight and 347 obese, at incidence rate of 2.58, 1.61 and 1.31 per 100 person-years, respectively. The Kaplan Meier survival curves are shown in Figure 2D. In reference to obese, normal BMI NAFLD had a higher risk of death: HR=1.96 (95%CI 1.52–2.51, p<0.001), which persisted after adjusting for other covariates (Table 3). Overweight NAFLD was associated with higher mortality risk than obese in univariate analysis (HR=1.23, 95%CI 1.02–1.49, p=0.03) but not in multivariable analysis. The most common causes of death were malignancy and CVD in all groups (Table 4). Liver-related deaths represented 1.4%, 2% and 10% of all deaths in normal BMI, overweight and obese NAFLD, respectively.
TABLE 4:
Causes of death of all deceased patients in each BMI group.
| Causes of death | Normal | Overweight | Obese |
|---|---|---|---|
| Total deaths | 74 | 148 | 347 |
| Malignancy | 19 (25.7%) | 45 (30.4%) | 103 (29.7%) |
| Cardiovascular | 16 (21.6%) | 29 (19.6%) | 94 (27.1%) |
| Infection | 10 (13.5%) | 13 (8.8%) | 30 (8.6%) |
| Liver Disease | 1 (1.4%) | 3 (2.0%) | 36 (10.4%) |
| Neurodegenerative | 8 (10.8%) | 13 (8.8%) | 8 (2.3%) |
| Others * | 9 (12.2%) | 17 (11.5%) | 31 (8.9%) |
| Accident / Suicide | 8 (10.8) | 15 (10.1%) | 24 (6.9%) |
| Undetermined | 3 (4.1%) | 13 (8.8%) | 21 (6.1%) |
Others causes of death included renal failure and respiratory failure.
DISCUSSION
In this longitudinal study with up to 20 years of follow-up, NAFLD with normal BMI was associated with a trend towards lower progression to cirrhosis and decompensation than obese NAFLD. In this cohort, normal BMI NAFLD subjects had a higher risk of all-cause mortality despite a less severe metabolic risk profile. The main causes of death in all groups were malignancies and cardiovascular disease, while liver-related causes explained 1.4% of deaths in normal BMI versus 10% in obese NAFLD.
In this population, 8.6% of the adults diagnosed with NAFLD had normal BMI by conventional BMI criteria. This proportion is lower than the 12–20% prevalence estimated in previous studies (6–12,20). This may be explained by differences in selection criteria (most studies included biopsy-proven NAFLD, which can introduce spectrum bias due to higher likelihood to biopsy those with a less conventional phenotype, such as normal BMI) and race/ethnicity between populations. Our cohort was less enriched with Asians and African Americans, in whom normal BMI NAFLD is more likely.
The distinct clinical profile with a more favorable metabolic phenotype and the milder liver disease was a finding consistent with most of the other studies, which adds to the validity of this work. This supports the hypothesis that normal BMI NAFLD represents a distinct phenotype and requires perhaps a different management than conventional obese NAFLD, where weight loss and control of diabetes, hypertension and hyperlipidemia is the mainstay of treatment. Recent data demonstrating differences in metabolic, genetic and gut microbiota between lean and non-lean NAFLD may explain the pathophysiological distinction which translates to the different outcomes we noted in our study. Lean patients have increased bile acid levels and fibroblast growth factor (FGF19), as well as changes in microbiota which mediate resistance to diet-induced obesity through metabolic adaptation and improvement of glucose and lipid homeostasis (20).
Despite milder liver disease progression, lean NAFLD was associated with worse survival. This finding is concordant to another long-term study of 126 lean NAFLD patients published in abstract form (11) and conflicting with another long- term study of 123 European lean NAFLD cohort which had similar mortality to the non-lean counterparts (12). Due to differences between cohorts, settings (population-based versus referral center), NAFLD definition and possible selection bias in studies including only subjects with clinical liver biopsies, direct comparison cannot be used to explain the different results.
Nevertheless, NAFLD individuals with normal BMI had a 63% higher risk of death even after controlling for main confounders. Malignancy and CVD are the two most common causes of death in NAFLD (2,3), and this was constant across all 3 groups. However, liver-related mortality affects a very small proportion of normal BMINAFLD (1.4% of deaths vs 10% in obese), in whom infections account for the third most common cause (14%) of deaths. The reasons for the survival differential remain to be elucidated, specifically to determine if it is related to the NAFLD pathophysiology or the obesity paradox, in which obese individuals have better survival than the lean. This has been described in multiple disease settings such as cardiovascular diseases (21,22), ICU admissions (23,24), cancer (25) and hospitalized patients with cirrhosis (26).
This is the largest contemporary study comparing the natural history of NAFLD with normal BMI in the United States, comprising 414 among 4,834 individuals with NAFLD. The NAFLD etiology was confirmed using a rigorous algorithm with high PPV followed by internal validation by medical record review of almost 40% of the entire cohort. Cirrhosis status and liver-related outcomes were individually reviewed in 100% of cases. The use of a population-based cohort as opposed to a referral-based one minimizes selection bias, whereas the use of composite criteria to define NAFLD (clinical, imaging and histological) minimized disease spectrum bias and was reflective of real-world data. The database encompasses all the health outcomes of this population over 20 years of follow-up. Limitations include a small number of liver-related complications in the individuals with normal BMI, which is probably reflective of the natural history of the disease and would require much larger populations to allow exploration of outcomes while controlling for multiple covariates. As with most observational cohort studies, the risk of bias due to differential scenarios of NAFLD detection in those with normal BMI versus those with traditional risk factors (overweight or obese) can potentially impact the validity of the results. Lean individuals could have had health issues leading to incidental NAFLD diagnosis (selection bias, which could potentially inflate the mortality risk) but also more intense follow-up (medical surveillance bias, which would facilitate detection of cirrhosis, cardiovascular disease and malignancy detection during the follow-up). While we cannot account for all factors impacting selection bias and mortality, we were reassured by the lack of higher detection of events that impact mortality, such as cirrhosis, malignancy or cardiovascular rates in lean versus obese (which can be impacted by exposure to medical follow-up). Mitigation of these biases would require prospective enrollment of large populations undergoing systematic screening for NAFLD with long-term follow-up and thorough ascertainment of all their subsequent medical events, which would be much needed but less feasible. We did not include smoking, genetic, microbiome or bile acid-related biomarkers to explore mechanistic differences in the pathophysiology of lean NAFLD. Additionally, BMI was used to categorize patients, which may not be best the representative of body fat content and is especially inaccurate in patients with cirrhosis and ascites. Other measures of body composition and visceral fat were not uniformly available in this cohort, given that the data were collected during clinical care. Moreover, the Caucasian preponderance of the Olmsted County population limits the generalizability of this study to other races.
In conclusion, the natural history of NAFLD with normal, overweight and obese BMI is not identical. ‘Lean’ NAFLD appears to be a distinct pathophysiological entity, with a healthier metabolic profile, a trend towards milder liver disease progression and lower risk of liver-related outcomes, but worse survival. As metabolic comorbidities are less prevalent, the management of NAFLD with normal BMI is likely to be different than the conventional life-style changes of weight loss, control of diabetes, HTN and dyslipidemia. Further studies exploring the potential explanations for these findings would be crucial to guide the management of NAFLD with normal BMI and ultimately impact the patient outcomes.
Supplementary Material
Supplementary Figure 1: Smoothed fit of raw serial BMI values obtained for each subject since NAFLD diagnosis (smoothing is a statistical method used to detect trends of increase/decrease in the presence of fluctuating data).
What You Need to Know.
Background:
Data on the natural history of nonalcoholic fatty liver disease (NAFLD) with normal body mass index (BMI) and its long-term outcomes are scarce and controversial. Consequently, patient counseling and disease management are limited.
Findings:
NAFLD with normal BMI was associated with a healthier metabolic profile, a trend towards milder liver disease progression, but similar risk of cardiovascular disease and malignancy, and worse survival than obese NAFLD.
Implications for patient care:
The management of NAFLD with normal BMI is likely to be different than the conventional management of obese NAFLD. Further studies exploring the potential explanations for these findings would be crucial to guide the management of NAFLD patients with normal BMI.
Grant support:
Alina M. Allen: National Institute of Diabetes and Digestive and Kidney Diseases (K23DK115594); American College of Gastroenterology Junior Faculty Development Grant.
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The funding sources did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- Afib
Atrial fibrillation
- aHR
Adjusted hazard ratio
- aka
Also known as
- A/PI
Asian and Pacific Islanders
- BMI
Body mass index
- CI
Confidence interval
- CV
Cardiovascular
- CVD
Cardiovascular disease
- DM
Diabetes millets
- FGF19
Fibroblast growth factor-19
- HICDA
Hospital International Classification of Diseases Adapted
- HLD
Hyperlipidemia
- HR
Hazard ratio
- HTN
Hypertension
- ICD-9/10-CM
International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification
- ICU
Intensive care unit
- MI
Myocardial infarction
- NAFLD
Nonalcoholic fatty liver disease
- REP
Rochester epidemiology project
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All authors disclose no conflicts.
REFERENCES
- 1.Maximos M, Bril F, Portillo Sanchez P, et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatol. Baltim. Md 2015;61:153–160. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 3.Wong VW-S, Wong GL-H, Yip GW-K, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–1727. [DOI] [PubMed] [Google Scholar]
- 4.Fazel Y, Koenig AB, Sayiner M, et al. Epidemiology and natural history of nonalcoholic fatty liver disease. Metabolism. 2016;65:1017–1025. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc 2011;9:524–530.e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 7.Feng R-N, Du S-S, Wang C, et al. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J. Gastroenterol 2014;20:17932–17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Kim HJ, Lee KE, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch. Intern. Med 2004;164:2169–2175. [DOI] [PubMed] [Google Scholar]
- 9.Nishioji K, Sumida Y, Kamaguchi M, et al. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011–2012. J. Gastroenterol 2015;50:95–108. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore). 2012;91:319–327. [DOI] [PubMed] [Google Scholar]
- 11.Cruz ACD, Bugianesi E, George J, et al. 379 characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:S–909. [Google Scholar]
- 12.Hagström H, Nasr P, Ekstedt M, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol. Commun 2018;2:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fracanzani AL, Petta S, Lombardi R, et al. Liver and Cardiovascular Damage in Patients With Lean Nonalcoholic Fatty Liver Disease, and Association With Visceral Obesity. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc 2017;15:1604–1611.e1. [DOI] [PubMed] [Google Scholar]
- 14.Leung JC-F, Loong TC-W, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64. [DOI] [PubMed] [Google Scholar]
- 15.Alam S, Gupta UD, Alam M, et al. Clinical, anthropometric, biochemical, and histological characteristics of nonobese nonalcoholic fatty liver disease patients of Bangladesh. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol 2014;33:452–457. [DOI] [PubMed] [Google Scholar]
- 16.Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for nonalcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment. Pharmacol. Ther 2017;46:85–95. [DOI] [PubMed] [Google Scholar]
- 17.Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol 2020; [DOI] [PubMed] [Google Scholar]
- 18.Allen AM, Therneau TM, Larson JJ, et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatol. Baltim. Md 2018;67:1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen AM, Hicks SB, Mara KC, et al. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - A longitudinal cohort study. J. Hepatol 2019;71:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatol. Baltim. Md 2020;71:1213–1227. [DOI] [PubMed] [Google Scholar]
- 21.Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur. J. Epidemiol 2014;29:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet Lond. Engl 2006;368:666–678. [DOI] [PubMed] [Google Scholar]
- 23.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit. Care Med 2008;36:151–158. [DOI] [PubMed] [Google Scholar]
- 24.Hogue CWJ, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med 2009;35:1152–1170. [DOI] [PubMed] [Google Scholar]
- 25.Lennon H, Sperrin M, Badrick E, et al. The Obesity Paradox in Cancer: a Review. Curr. Oncol. Rep 2016;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagozian R, Bhardwaj G, Wakefield DB, et al. Obesity paradox in advanced liver disease: obesity is associated with lower mortality in hospitalized patients with cirrhosis. Liver Int. Off. J. Int. Assoc. Study Liver 2016;36:1450–1456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Smoothed fit of raw serial BMI values obtained for each subject since NAFLD diagnosis (smoothing is a statistical method used to detect trends of increase/decrease in the presence of fluctuating data).


