Abstract
Objective
To investigate the effect of platelet‐rich plasma on tendon‐bone healing after anterior cruciate ligament reconstruction.
Methods
This retrospective study included 85 patients (range, 18–50 years; mean age, 33.95 ± 10.53 years; male/female, 49/36) who underwent anterior cruciate ligament reconstruction using autologous hamstring tendons between August 2017 and June 2019 at our institute. The participants in the study group (n = 42) were injected with platelet‐rich plasma at both ends of the tendon graft, while those in the control group (n = 43) received an injection of normal saline. Magnetic resonance imaging signal/noise quotient values of the femoral and tibial ends, knee Lysholm scores, and International Knee Documentation Committee scores were compared at 3, 6, and 12 months postoperatively.
Results
The signal/noise quotient values of the femoral and tibial ends in both groups were higher at 6 months than at 3 and 12 months postoperatively. The signal/noise quotient values of the tibial end were significantly lower in the platelet‐rich plasma group than in the normal saline group at all follow‐up time points (P < 0.05). The signal/noise quotient values of the tibial and femoral ends in both groups were significantly different at 3, 6, and 12 months postoperatively (P < 0.05). Additionally, the signal/noise quotient values of the tibia were significantly lower than those of the femur in both groups (P < 0.05). The Lysholm and International Knee Documentation Committee scores were significantly better in the platelet‐rich plasma group than in the normal saline group only at 3 months postoperatively. No complications, such as knee joint infection or vascular and nerve injuries, occurred in any of the 85 patients. The knee flexion of all patients were more than 90°, and the straight degree was 0°. No joint stiffness was observed in all patients.
Conclusion
Platelet‐rich plasma can promote tendon‐bone healing in grafts and can improve early postoperative knee joint function.
Keywords: Anterior cruciate ligament reconstruction, Magnetic resonance imaging, Platelet‐rich plasma, Signal/noise quotient, Tendon‐bone healing
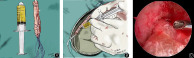
Preparation of PRP fluid and woven autologous hamstring tendon. (A) Image of the PRP fluid. (B) In the study group, 2.5 mL of PRP was injected into the tibial and femoral ends of the graft. (C) The graft was positioned properly after anterior cruciate ligament reconstruction.
Introduction
Successful anterior cruciate ligament (ACL) reconstruction with an autologous or allogeneic tendon graft requires sufficient healing of the tendon graft in the bone tunnel 1 , 2 . However, adequate tendon‐bone healing is not always easily achieved and poses great challenges to patients and orthopaedic physicians. In the past few decades, a number of regenerative strategies have been developed to improve the process of tendon‐bone healing, including the delivery of growth factors 3 , 4 and stem cells 5 , 6 , as well as the use of various biomaterials 7 , 8 , 9 , 10 .
The natural tendon‐bone junction consists of four regions: the bone, calcified fibrocartilage, non‐calcified fibrocartilage, and the tendon. This unique transition interface between the tissue and minerals can effectively transfer stress from the tendon to the bone or vice versa. Therefore, it is believed that the inward growth of a new bone is essential to ensure a firm graft‐to‐bone fixation. Interestingly, many bone inducible growth factors have been tested to achieve this goal, such as transforming growth factor‐β 11 , bone morphogenetic protein 12 , and granulocyte colony‐stimulating factor 13 .
However, because of the high cost and short shelf life of biological materials for tendon‐bone healing, the clinical availability of these methods remains limited. In contrast, many bone composite materials have been used to promote osseointegration of tendon grafts, including tricalcium phosphate 14 , hydroxyapatite 15 , and magnesium‐based bone adhesives 16 . However, these substances have no osteoinductive potential; hence, their ability to achieve physiological bridging between the tendon grafts and the bone is limited.
Platelet‐rich plasma (PRP) contains high levels of growth factors, including transforming growth factor‐β, bone morphogenetic protein‐2, insulin‐like growth factor, and platelet‐derived growth factor 17 . When PRP is implanted in vivo, a microenvironment favorable to cell growth and proliferation is created. Previous animal experiments have reported that PRP can promote tendon‐bone healing 18 , 19 ; however, clinical studies on this topic are limited 20 .
Weiler et al. 21 found that the signal/noise quotient (SNQ) value was negatively correlated with the maximum failure load, tensile strength, and stiffness of grafts. They also showed that the signal changes on magnetic resonance imaging (MRI) could adequately indicate the process of vascularization and ligament formation of the grafts. Since then, there have been more studies using MRI to evaluate graft maturity and tendon‐bone healing. The SNQ in MRI, which is proportional to the signal intensity of the graft, is a good index that can be used to evaluate tendon‐bone healing 22 . It has been reported that a lower SNQ value indicates a lower water content and better healing of the graft 23 .
Therefore, the purpose of this study was (i) to evaluate the effect of PRP on tendon‐bone healing in the procedure of ACL reconstruction; (ii) to investigate whether SNQ value can be used to evaluate the degree of maturation of grafts.
Methods
Inclusion and Exclusion Criteria
The inclusion criteria used in this study were as follows: (i) preoperative diagnosis of ACL rupture based on imaging and physical examination findings (anterior drawer test); (ii) age, 18–50 years; and (iii) initial unilateral ACL reconstruction. The exclusion criteria were as follows: (i) severe osteoarthritis of the affected knee joint (Kellgren–Lawrence grade III and above); (ii) severe meniscus injuries of the affected knee joint that needed suturing with the aid of an arthroscope; (iii) presence of serious cartilage damage confirmed using arthroscopy (Outerbridge grade III and above); and (iv) multiple ligament injuries or knee joint infection.
Study Design and Patients
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committee of our institution (No. 2019172). All study participants provided informed consent. A surgeon at the hospital began using PRP to enhance ACL reconstruction in April 2019. Prior to this, ACL reconstruction was performed without PRP enhancement. From April to June 2019, a total of 42 patients underwent PRP‐enhanced ACL reconstruction, constituting the PRP group. Forty‐three patients were selected as the normal saline group from the ACL reconstruction database of the same surgeon. These patients underwent ACL reconstruction from December 2017 to February 2018, without undergoing PRP enhancement. When selecting the normal saline group, the investigators were blinded to the results to avoid selection bias. The factors considered were age, sex, and whether initial ACL reconstruction was performed. From December 2017 to June 2019, there were no other changes in the ACL reconstruction technique of the surgeon.
PRP Preparation
The RegenACR®‐C Kit (Regen lab SA, Vaud, Switzerland) was used to prepare the PRP according to the manufacturer's instructions. Approximately 8 mL of venous blood was obtained from the patient and placed in a centrifuge for 10 min, at a rotating speed of 3220 rpm. After standing for 2 min, 4.5–5 mL of PRP was obtained. The required PRP was collected using a syringe (Fig. 1A).
Fig. 1.
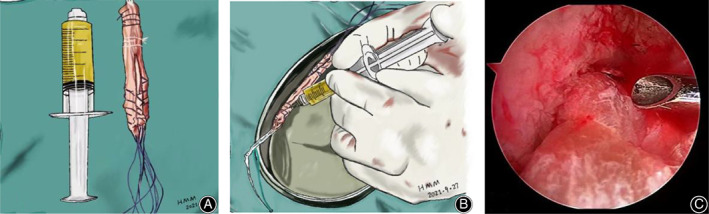
Preparation of PRP fluid and woven autologous hamstring tendon. (A) Image of the PRP fluid. (B) In the PRP group, 2.5 mL of PRP was injected into the tibial and femoral ends of the graft. (C) The graft was positioned properly after anterior cruciate ligament reconstruction. PRP, platelet‐rich plasma.
Anterior Drawer Test
The anterior drawer test was used to examine ACL injury. The patient lay flat on the bed with knees flexed at 90°, hip flexed at 45°, and both feet on the bed in a neutral position and remained relaxed. The examiner sat on the bed and held the patient's feet to fix them. Afterwards, the examiner held the tibial end of the knee joint with both hands and pulled the leg forward. In cases where the tibia moved forward ≥5 mm compared to the healthy side, the test was considered to be positive.
Surgical Methods
Anesthesia and Position
Under general or epidural anesthesia, the patients were placed in the supine position; the healthy knee adopted the lithotomy position and the affected knee naturally drooped.
Approach and Exposure
After ACL rupture was confirmed by anteromedial and anterolateral arthroscopic approaches, a 3‐cm longitudinal skin incision was made approximately 2 cm medial to the tibial tubercle. Parts of the semitendinosus and gracilis tendons were cut and woven to prepare the tendon graft, and its diameter was measured.
Application of PRP or Saline
In the PRP group, 2.5 mL of PRP was injected into the tibial and the femoral ends of the graft with a fine‐needle syringe (Fig. 1B), then the tendon was soaked in the rest of the PRP for about 10 min. After pulling the tendon into the bone tunnel, the joint fluid is fully absorbed by aspirator and the unabsorbed PRP was injected into both ends of the joint to ensure that the PRP adheres to the transplanted tendon to the greatest extent. In the normal saline group, the same volume of normal saline was injected into both ends of the tendon graft.
Graft Positioning
According to the diameter of the graft, tibial and femoral tunnels were drilled into the tibial and femoral anatomical footprints of the ACL. The femoral and tibial sides were fixed using a titanium plate and an absorbable interface screw, respectively. We confirmed that the graft was positioned properly after the ACL reconstruction (Fig. 1C).
Postoperative Rehabilitation
The PRP group and normal saline groups had the same postoperative rehabilitation training plan. On the day of the surgery, after recovering from anesthesia, straight leg raises and ankle pump training were performed in bed. Knee flexion activity began on postoperative day 2, and knee flexion reached the normal levels at 10–12 weeks postoperatively. Knee braces were fixed for 2 months after the surgery. According to the patient's tolerance, weight‐bearing activities were performed stepwise. Jogging and swimming were commenced 4–6 months postoperatively, and competitive sports resumed 9–12 months postoperatively.
Outcome Measures
Signal/Noise Quotient (SNQ) Value
We performed 3.0 T MRI (scanning series: repetition time/echo time, 3000/41 ms; field of view: 15 cm × 15 cm; matrix: 240 × 320; slice thickness: 3.0 mm; Magnetom, Verio, Siemens, Germany) at 3, 6, and 12 months postoperatively. All images were imported into the RadiAnt DICOM viewer 5.0 (Medixant, Poznan, Poland), and the data were analyzed in the oblique‐sagittal fat‐suppressed middle‐level imaging. The signal intensity was measured in the regions of the femoral and tibial ends of the graft, the quadriceps tendon, and the background (approximately 2 cm in front of the patellar tendon). The region of interest, which was also the area of the selected sites, was 0.2 cm2 (Fig. 2). Then, the signal intensity of each site was quantified and used in the SNQ value formula as follows: SNQ value = (signal intensity of ACL graft − signal intensity of quadriceps femoris tendon) / background signal intensity 8 , 13 . Two physicians participated in the MRI measurements of the grafts. Each physician independently measured the value of each region twice, with an interval of 2 weeks, to eliminate the memory effect. Then, the average value of the measurements performed by the two physicians was used to calculate the SNQ. The intra‐ and inter‐observer reliabilities were calculated using the results of the measurements.
Fig. 2.
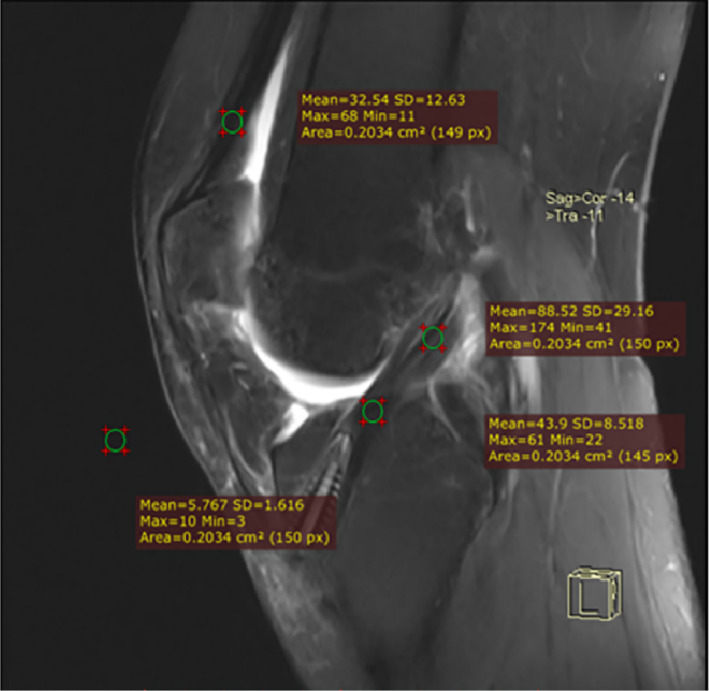
Measurement of SNQ values at the femoral and tibial ends of the graft. The SNQ value was calculated as follows: SNQ value of each graft site = (anterior cruciate ligament graft signal intensity – quadriceps tendon signal intensity)/background signal intensity. SNQ, signal/noise quotient.
Lysholm Scores
Lysholm scores were used to assess the outcomes after ACL repair. Lysholm scores range from 0 to 100 based on eight domains: pain, limping, stair climbing, locking, supporting, swelling, instability, and squatting.
International Knee Documentation Committee (IKDC) Evaluation
The IKDC Subjective Assessment Form, the standardized international documentation system for knee surgery, consists of 18 questions that emphasize the effects of symptoms, activities of daily living, and physical activity on the knee. The form also assessed the total knee function on a 0–100 conversion scale. A score of 100 indicates no symptoms and no restriction in activities of daily living or physical activity.
Statistical Analyses
The chi‐squared test was used to analyze categorical parameters, sex, and the affected knee. An independent sample t‐test was used to compare the age, last follow‐up time point, IKDC score, Lysholm score, and SNQ value of the two groups. The SNQ value, IKDC score, and Lysholm score within each group were analyzed using repeated‐measures analysis of variance, and pairwise comparisons are expressed as means ± standard deviations. The intraclass correlation coefficient (ICC) was used to study the inter‐ and intra‐observer reliabilities. The reliability was high, medium, and low when the ICC was >0.75, 0.4–0.75, and <0.4, respectively. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA) software. The level of significance was set at P < 0.05.
Results
General Results
All patients were followed up for a minimum of 12 months. There were no significant differences in age, sex, the affected knee (left or right), and body mass index between the two groups (Table 1).
TABLE 1.
Patients' characteristics
| Platelet‐rich plasma group (n = 42) | Normal saline group (n = 43) | P‐value | |
|---|---|---|---|
| Age (years) | 32.01 ± 11.23 | 35.90 ± 10.31 | 0.52 |
| Sex (male/female) | 23/19 | 26/17 | 0.41 |
| Affected knee (left/right) | 20/22 | 27/16 | 0.32 |
| Body mass index (kg/m2) | 20.12 ± 2.34 | 21.23 ± 1.98 | 0.20 |
SNQ Values
The intra‐ and inter‐observer ICCs were 0.893 and 0.882, respectively, indicating that the MRI measurement method had high reliability and could be used to assess graft healing. The SNQ values of the femur and tibia at 6 months after surgery were higher than those at 3 and 12 months in both groups (P < 0.05) (Tables 2 and 3). There were no significant differences in the SNQ values of the femoral end between the PRP group and the normal saline groups at 3, 6, and 12 months postoperatively (Table 2). However, a significant difference was observed in the SNQ values of the tibia between the PRP group and normal saline groups at 3, 6, and 12 months postoperatively (P < 0.05) (Table 3). Upon comparison of the SNQ values of the tibia and the femur in the PRP group, a significant difference was observed at 3, 6, and 12 months postoperatively, with the SNQ value of the tibial end of the graft being significantly lower than that of the femoral end (P < 0.05) (Table 4). The SNQ values of the tibia and the femur in the normal saline group were also significantly different at 3, 6, and 12 months postoperatively (P < 0.05) (Table 5). The associations between the MRI findings of the graft signal intensity and the time after ACL reconstruction in the PRP group and normal saline groups are presented in Fig. 3.
TABLE 2.
Signal/noise quotient values of the femoral ends of the grafts
| Postoperative time | Platelet‐rich plasma group | Normal saline group | P‐value * |
|---|---|---|---|
| 3 months | 11.08 ± 5.71 | 12.51 ± 5.30 | 0.60 |
| 6 months | 16.31 ± 7.52 | 18.32 ± 5.42 | 0.37 |
| 12 months | 14.35 ± 8.86 | 15.82 ± 3.71 | 0.51 |
| P‐value * | 0.00 | 0.00 |
P < 0.05 indicates statistical significance.
Table 3.
Signal/noise quotient values of the tibial ends of the grafts
| Postoperative time | Platelet‐rich plasma group | Normal saline group | P‐value * |
|---|---|---|---|
| 3 months | 7.33 ± 4.28 | 9.81 ± 2.84 | 0.04 |
| 6 months | 9.13 ± 5.79 | 12.26 ± 4.26 | 0.04 |
| 12 months | 8.11 ± 4.31 | 10.41 ± 7.50 | 0.02 |
| P‐value † | 0.03 | 0.01 |
P < 0.05 indicates a statistically significant difference in signal/noise quotient values between the platelet‐rich plasma group and normal saline groups.
P < 0.05 indicates a statistically significant difference in signal/noise quotient values at 6 months after surgery compared with the corresponding at 3 and 12 months after surgery.
TABLE 4.
Signal/noise quotient values of the tibial and femoral ends in the platelet‐rich plasma group
| Postoperative time | Tibial end | Femoral end | P‐value * |
|---|---|---|---|
| 3 months | 7.33 ± 4.28 | 11.08 ± 5.71 | 0.00 |
| 6 months | 9.13 ± 5.79 | 16.31 ± 7.52 | 0.00 |
| 12 months | 8.11 ± 4.31 | 14.35 ± 8.86 | 0.00 |
| P‐value † | 0.03 | 0.00 |
P < 0.05 indicates a statistically significant difference in signal/noise quotient values between the tibial and femoral ends.
P < 0.05 indicates a statistically significant difference in signal/noise quotient values at 6 months after surgery compared with the corresponding at 3 and 12 months after surgery.
TABLE 5.
Signal/noise quotient values of the tibial and femoral ends in the normal saline group
| Postoperative time | Tibial end | Femoral end | P‐value * |
|---|---|---|---|
| 3 months | 9.81 ± 2.84 | 12.51 ± 5.30 | 0.00 |
| 6 months | 12.26 ± 4.26 | 18.32 ± 5.42 | 0.00 |
| 12 months | 10.41 ± 7.50 | 15.82 ± 3.71 | 0.00 |
| P‐value † | 0.01 | 0.00 |
P < 0.05 indicates a statistically significant difference in signal/noise quotient values between the tibial and femoral ends.
P < 0.05 indicates a statistically significant difference in signal/noise quotient values at 6 months after surgery compared with the corresponding at 3 and 12 months after surgery.
Fig. 3.
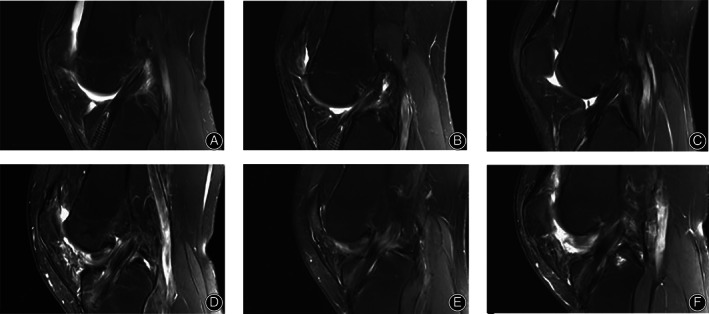
Changes in graft signal intensity with time after anterior cruciate ligament reconstruction. (A–C) present the magnetic resonance images of the right knee joint of a 37‐year‐old male patient in the platelet‐rich plasma group at 3, 6, and 12 months, respectively; (D–F) present the magnetic resonance images of the right knee joint of a 30‐year‐old male patient in the normal saline group at 3, 6, and 12 months, respectively.
Lysholm Scores
There was no significant difference in the Lysholm scores of the PRP group and normal saline groups before surgery; however, there was a significant difference at 3 months postoperatively (P < 0.05), the score of the PRP group was 15.61% higher than that of the normal saline group. Furthermore, no significant differences were observed at 6 and 12 months postoperatively (Table 6).
TABLE 6.
Lysholm scores
| Platelet‐rich plasma group | Normal saline group | P‐value | Percentage of increase | |
|---|---|---|---|---|
| Preoperative | 39.59 ± 4.19 | 38.21 ± 2.13 | 0.37 | ‐ |
| 3 months postoperative | 65.08 ± 4.93* | 56.29 ± 4.65 | 0.01 | 15.61% |
| 6 months postoperative | 68.93 ± 8.26 | 66.24 ± 8.17 | 0.52 | ‐ |
| 12 months postoperative | 82.29 ± 2.18 | 81.62 ± 3.61 | 0.09 | ‐ |
P < 0.05 indicates a statistically significant difference in scores between the platelet‐rich plasma group and normal saline groups.
IKDC Scores
There was no significant difference in the IKDC scores of the PRP group and the normal saline groups before surgery, whereas a significant difference was observed at 3 months postoperatively (P < 0.05), the score of the PRP group was 19.53% higher than that of the normal saline group. No significant differences were observed at 6 and 12 months postoperatively (Table 7).
TABLE 7.
International Knee Documentation Committee scores
| Platelet‐rich plasma group | Normal saline group | P‐value | Percentage of increase | |
|---|---|---|---|---|
| Preoperative | 50.02 ± 3.25 | 49.68 ± 1.24 | 0.09 | ‐ |
| 3 months postoperative | 61.31 ± 3.38 * | 51.29 ± 4.36 | 0.03 | 19.53% |
| 6 months postoperative | 63.62 ± 7.81 | 62.83 ± 6.28 | 0.57 | ‐ |
| 12 months postoperative | 84.78 ± 2.12 | 82.45 ± 2.09 | 0.50 | ‐ |
P < 0.05 indicates a statistically significant difference in scores between the platelet‐rich plasma group and normal saline groups.
Complications
No complications, such as knee joint infection or vascular and nerve injuries, occurred in any of the 85 patients. The knee flexions of all patients were more than 90°, and the straight degree was 0°. No joint stiffness was observed in all patients.
Discussion
PRP Could Promote Tendon‐Bone Healing in ACL Reconstruction
The main finding of this study was that the use of PRP in ACL reconstruction could promote tendon‐bone healing, which was consistent with our hypothesis. Despite the increasing use of hamstring grafts worldwide, many studies have reported that hamstring grafts have a relatively higher incidence of surgical complications concerning graft failure and revision rates compared to bone‐patellar tendon grafts 24 , 25 . One possible explanation for this is the fundamental difference between the two types of migration. Unlike a bone‐patellar tendon graft, which has a bone plug that can be quickly incorporated into the bone tunnel, a hamstring tendon graft has challenges pertaining to tendon‐bone integration 26 . According to Walters et al. 27 , irrespective of whether they were randomized to receive PRP in their patellar defect or not, the patients continued to have similar levels of kneeling pain and patellar defect sizes after autograft bone‐patellar tendon ACL reconstruction. However, Alves et al. 28 demonstrated that patients who received PRP presented texture changes when compared to the normal saline group, and PRP interferes with the ACL morphological parameters. Despite some positive findings in graft maturation and clinical outcomes, further studies are needed to determine whether PRP contributes to tendon‐bone healing in ACL reconstruction cases. In this study, PRP was injected into the tendons at both ends of the graft so that the PRP could fully penetrate the tendon fibers. Then, the graft was fixed to ensure a high concentration of PRP in the tunnel and a good biological environment for the healing of the graft. The concentration of PRP is the key point in this study. Theoretically, different PRP concentrations have different effects on the tendon‐bone healing. Therefore, it is very important to suck up the intra‐articular fluid before injecting the remaining PRP into the joint to ensure the intra‐articular PRP concentration. Whether there is an optimal PRP concentration to promote tendon‐bone graft healing is worthy of further study, we are conducting additional research on the effects of different PRP concentration on tendon bone healing. The SNQ values of the tibial end of the PRP group were lower than those of the normal saline group after the same duration, indicating that PRP promoted tendon‐bone healing of grafts. This finding was consistent with those of Radice et al. 29 , which stated that ACL reconstruction with the use of PRP can shorten the healing time of grafts.
SNQ Value is a Good Index to Evaluate the Degree of Maturation of Grafts
In this study, there was no significant difference in the SNQ values of the femoral end between the PRP group and normal saline groups. However, the SNQ values of the femoral end of the grafts were higher than those of the tibial end in both groups. This finding indicated that the maturation of the femoral end of the graft was slightly slower than that of the tibial end, and tendon‐bone healing was not improved by the injection of PRP in the femoral end. A previous study showed that a greater bending angle between the femoral end of the graft and the intra‐articular cavity of the graft could affect the graft tendon‐bone healing 30 . In addition, we believe that greater stress at the local turning point could affect the distribution of PRP in the tendon grafts and alter the effect of PRP on the grafts. In the future, we aim to analyze the effects of stress distribution in grafts on the efficacy of PRP.
Seijas et al. 31 previously divided the MRI signal intensity of the grafts into low, mild high, moderate high, severe high, and diffuse high signals, to evaluate the degree of graft healing; however, they found a large error in the MRI grading of the grafts. In this study, the signal intensity of the regions of interest on the grafts was calculated as SNQ values using the MRI workstation, and the degree of tendon‐bone healing of the graft after using PRP in ACL reconstruction was quantitatively measured to minimize the errors caused by previous MRI grading and indirect measurement. Some researchers have divided the maturity of grafts into three stages: the early, remodeling, and mature stages 32 , 33 . A previous study reported that the grafts were in the remodeling stage 3–6 months postoperatively, with the grafts undergoing cell regeneration and reconstruction of blood vessels. The water content was also observed to be at its highest level at this stage of maturation 22 . The results of this study showed that the SNQ values of the grafts at 6 months were higher than those at 3 and 12 months postoperatively. In fact, the histopathological analysis is the gold standard for the healing of tendon‐bone; however, its clinical implementation is inadequate. The maturity of the graft in the joint is positively associated with tendon‐bone graft healing. Therefore, the degree of tendon‐bone graft healing can also be defined by the maturity of the graft. This further confirms that the SNQ value is a good index to evaluate the degree of healing of tendon grafts and bones after ACL reconstruction.
The Healing of Bone‐Tendon has Great Clinical Significance for ACL Reconstruction
The IKDC and Lysholm scores in the PRP group were superior to those in the normal saline group at 3 months after surgery, indicating that using PRP in ACL reconstruction could improve knee joint healing in the early postoperative period. Studies have shown that graft tendon‐bone healing might affect the recovery of patients, and earlier commencement of rehabilitation exercises could result in better graft tendon‐bone healing 34 , 35 , 36 . During the follow‐up period, we found that tendon‐bone healing in the PRP group was better than that in the normal saline group at any time, indicating that PRP could accelerate graft remodeling, which was conducive to more active rehabilitation training in patients.
This study had some limitations. First, the number of cases was small. Thus, analysis of a larger sample is needed. Second, as PRP must be prepared from autologous blood, individual differences of the concentration of PRP may affect the degree of tendon‐bone healing. Third, the histopathological analysis is the most accurate for evaluating the healing of tendon‐bone; however, its clinical implementation is difficult. Therefore, we chose intra‐articular grafts for maturity analysis using the signal/noise quotient (SNQ) value to further estimate the degree of tendon‐bone graft healing and another technique could be adopted in the future to evaluate the maturity of the grafts.
In conclusion, PRP could promote tendon‐bone healing in grafts and improve early postoperative knee joint function as indicated by the MRI SNQ values and the IKDC and Lysholm scores that were used to evaluate tendon‐bone healing after ACL reconstruction with autologous grafts. The findings presented in this study provide an important reference value for orthopaedic clinicians regarding the treatment and evaluation of patients with ACL injury enhanced with PRP.
Author Contributions
Chen Rongjin and Zhu Haozhong contributed equally to this work. Chen Rongjin contributed to the design of the study, clinical assessment, analysis, interpretation of data, and revision of the manuscript. Zhu Haozhong and Gu Xinyi contributed to the design of the study, data interpretation, and revision of the manuscript for important intellectual content. Xiang Xianxiang contributed to the design of the study, data acquisition, and critical revision of the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Authorship Declaration
All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors. All authors are in agreement with the manuscript.
Grant Sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Disclosure: The authors declare that they have no conflicts of interest.
Data Availability Statement
The data and materials are available from the medical records department of the Affiliated Zhongshan Hospital of Dalian University. The datasets used and analyzed during the study are available from the corresponding author on reasonable request.
References
- 1. Wong CC, Yeh YY, Yang TL, Tsuang YH, Chen CH. Augmentation of tendon graft‐bone tunnel Interface healing by use of bioactive platelet‐rich fibrin scaffolds. Am J Sports Med, 2020, 48: 1379–1388. [DOI] [PubMed] [Google Scholar]
- 2. Lu H, Chen C, Xie S, Tang Y, Qu J. Tendon healing in bone tunnel after human anterior cruciate ligament reconstruction: a systematic review of histological results. J Knee Surg, 2019, 32: 454–462. [DOI] [PubMed] [Google Scholar]
- 3. Han B, Jones IA, Yang Z, Fang W, Vangsness CT Jr. Repair of rotator cuff tendon defects in aged rats using a growth factor injectable gel scaffold. Art Ther, 2020, 36: 629–637. [DOI] [PubMed] [Google Scholar]
- 4. Wei B, Wang C, Yan C, et al. Osteoprotegerin/bone morphogenetic protein 2 combining with collagen sponges on tendon‐bone healing in rabbits. J Bone Miner Metab, 2020, 38: 432–441. [DOI] [PubMed] [Google Scholar]
- 5. Zhou Y, Xie S, Tang Y, et al. Effect of book‐shaped acellular tendon scaffold with bone marrow mesenchymal stem cells sheets on bone‐tendon interface healing. J Orthop Translat, 2021, 26: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franklin A, Gi Min J, Oda H, et al. Homing of adipose‐derived stem cells to a tendon‐derived hydrogel: a potential mechanism for improved tendon‐bone interface and tendon healing. J Hand Surg Am, 2020, 45: 1180.e1181–1180.e1112. [DOI] [PubMed] [Google Scholar]
- 7. Su W, Wang Z, Jiang J, Liu X, Zhao J, Zhang Z. Promoting tendon to bone integration using graphene oxide‐doped electrospun poly(lactic‐co‐glycolic acid) nanofibrous membrane. Int J Nanomedicine, 2019, 14: 1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mutsuzaki H, Kinugasa T, Ikeda K, Sakane M. Morphological changes in the femoral and tibial bone tunnels after anatomic single‐bundle anterior cruciate ligament reconstruction using a calcium phosphate‐hybridized tendon graft in 2 years of follow‐up. Orthop Traumatol Surg Res, 2019, 105: 653–660. [DOI] [PubMed] [Google Scholar]
- 9. Kim DM, Shim IK, Shin MJ, et al. A combination treatment of raloxifene and vitamin D enhances bone‐to‐tendon healing of the rotator cuff in a rat model. Am J Sports Med, 2020, 48: 2161–2169. [DOI] [PubMed] [Google Scholar]
- 10. Zhang H, Yang L, Yang XG, et al. Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg, 2019, 11: 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoon JP, Lee CH, Jung JW, et al. Sustained delivery of transforming growth factor beta1 by use of absorbable alginate scaffold enhances rotator cuff healing in a rabbit model. Am J Sports Med, 2018, 46: 1441–1450. [DOI] [PubMed] [Google Scholar]
- 12. Chen P, Ouyang J, Xiao J, et al. Co‐injection of human adipose stromal cells and rhBMP‐2/fibrin gel enhances tendon graft osteointegration in a rabbit anterior cruciate ligament‐reconstruction model. Am J Transl Res, 2018, 10: 535–544. [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi Y, Kida Y, Kabuto Y, et al. Healing effect of subcutaneous administration of G‐CSF on acute rotator cuff injury in a rat model. Tissue Eng Part A, 2021, 27: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 14. Mao G, Qin Z, Li Z, Li X, Qiu Y, Bian W. A tricalcium phosphate/polyether ether ketone anchor bionic fixation device for anterior cruciate ligament reconstruction: safety and efficacy in a beagle model. J Biomed Mater Res B Appl Biomater, 2019, 107: 554–563. [DOI] [PubMed] [Google Scholar]
- 15. You X, Shen Y, Yu W, He Y. Enhancement of tendonbone healing following rotator cuff repair using hydroxyapatite with TGFbeta1. Mol Med Rep, 2018, 17: 4981–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Xu J, Wang X, et al. Magnesium‐pretreated periosteum for promoting bone‐tendon healing after anterior cruciate ligament reconstruction. Biomaterials, 2021, 268: 120576. [DOI] [PubMed] [Google Scholar]
- 17. Yang FA, Liao CD, Wu CW, Shih YC, Wu LC, Chen HC. Effects of applying platelet‐rich plasma during arthroscopic rotator cuff repair: a systematic review and meta‐analysis of randomised controlled trials. Sci Rep, 2020, 10: 17171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng C, Lu H, Tang Y, et al. Autologous freeze‐dried, platelet‐rich plasma carrying icariin enhances bone‐tendon healing in a rabbit model. Am J Sports Med, 2019, 47: 1964–1974. [DOI] [PubMed] [Google Scholar]
- 19. Zhang M, Zhen J, Zhang X, et al. Effect of autologous platelet‐rich plasma and gelatin sponge for tendon‐to‐bone healing after rabbit anterior cruciate ligament reconstruction. Art Ther, 2019, 35: 1486–1497. [DOI] [PubMed] [Google Scholar]
- 20. Davey MS, Hurley ET, Withers D, Moran R, Moran CJ. Anterior cruciate ligament reconstruction with platelet‐rich plasma: a systematic review of randomized control trials. Art Ther, 2020, 36: 1204–1210. [DOI] [PubMed] [Google Scholar]
- 21. Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast‐enhanced magnetic resonance imaging. A two‐year study in sheep. Am J Sports Med, 2001, 29: 751–761. [DOI] [PubMed] [Google Scholar]
- 22. Ahn JH, Lee SH, Choi SH, Lim TK. Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med, 2010, 38: 1768–1777. [DOI] [PubMed] [Google Scholar]
- 23. Ma Y, Murawski CD, Rahnemai‐Azar AA, Maldjian C, Lynch AD, Fu FH. Graft maturity of the reconstructed anterior cruciate ligament 6 months postoperatively: a magnetic resonance imaging evaluation of quadriceps tendon with bone block and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc, 2015, 23: 661–668. [DOI] [PubMed] [Google Scholar]
- 24. Laboute E, James‐Belin E, Puig PL, et al. Graft failure is more frequent after hamstring than patellar tendon autograft. Knee Surg Sports Traumatol Arthrosc, 2018, 26: 3537–3546. [DOI] [PubMed] [Google Scholar]
- 25. Smith AH, Capin JJ, Zarzycki R, et al. Athletes with bone‐patellar tendon‐bone autograft for anterior cruciate ligament reconstruction were slower to meet rehabilitation milestones and return‐to‐sport criteria than athletes with hamstring tendon autograft or soft tissue allograft: secondary analysis from the ACL‐SPORTS trial. J Orthop Sports Phys Ther, 2020, 50: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen CH, Chen WJ, Shih CH, et al. Enveloping the tendon graft with periosteum to enhance tendon‐bone healing in a bone tunnel: a biomechanical and histologic study in rabbits. Art Ther, 2003, 19: 290–296. [DOI] [PubMed] [Google Scholar]
- 27. Walters BL, Porter DA, Hobart SJ, et al. Effect of intraoperative platelet‐rich plasma treatment on postoperative donor site knee pain in patellar tendon autograft anterior cruciate ligament reconstruction: a double‐blind randomized controlled trial. Am J Sports Med, 2018, 46: 1827–1835. [DOI] [PubMed] [Google Scholar]
- 28. Alves AFF, de Arruda Miranda JR, de Souza SAS, et al. Texture analysis to differentiate anterior cruciate ligament in patients after surgery with platelet‐rich plasma. J Orthop Surg Res, 2021, 16: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radice F, Yanez R, Gutierrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet‐derived growth factors. Art Ther, 2010, 26: 50–57. [DOI] [PubMed] [Google Scholar]
- 30. Chen L, Wu Y, Lin G, et al. Graft bending angle affects allograft tendon maturity early after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc, 2018, 26: 3048–3054. [DOI] [PubMed] [Google Scholar]
- 31. Seijas R, Ares O, Catala J, Alvarez‐Diaz P, Cusco X, Cugat R. Magnetic resonance imaging evaluation of patellar tendon graft remodelling after anterior cruciate ligament reconstruction with or without platelet‐rich plasma. J Orthop Surg (Hong Kong), 2013, 21: 10–14. [DOI] [PubMed] [Google Scholar]
- 32. Claes S, Verdonk P, Forsyth R, Bellemans J. The "ligamentization" process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med, 2011, 39: 2476–2483. [DOI] [PubMed] [Google Scholar]
- 33. Pauzenberger L, Syre S, Schurz M. "Ligamentization" in hamstring tendon grafts after anterior cruciate ligament reconstruction: a systematic review of the literature and a glimpse into the future. Art Ther, 2013, 29: 1712–1721. [DOI] [PubMed] [Google Scholar]
- 34. Biercevicz AM, Akelman MR, Fadale PD, et al. MRI volume and signal intensity of ACL graft predict clinical, functional, and patient‐oriented outcome measures after ACL reconstruction. Am J Sports Med, 2015, 43: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Figueroa D, Melean P, Calvo R, et al. Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus‐gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Art Ther, 2010, 26: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 36. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long‐term follow‐up–correlation with functional and clinical evaluation. Radiology, 2008, 249: 581–590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available from the medical records department of the Affiliated Zhongshan Hospital of Dalian University. The datasets used and analyzed during the study are available from the corresponding author on reasonable request.


