Abstract
The objective of this study is to detect the number of different subsets of TFH and B cells in renal transplant recipients (RTR) with antibody-mediated acute rejection (AMR), acute rejection (AR), chronic rejection (CR), or transplant stable (TS). The present study was a prospective study. The numbers of ICOS +, PD-1+ and IL-21+ TFH, CD86+, CD38+, CD27+, and IgD- B cells in 21 patients with end-stage renal disease (ESRD) and post-transplant times were measured by flow cytometry. The level of serum IL-21 was detected by ELISA. The numbers of circulating CD4+CXCR5+, CD4+CXCR5+ICOS+, CD4+CXCR5+PD-1+, CD4+CXCR5+IL-21+ TFH, CD19+CD86+, and CD19 +CD86+CD38+ B cells as well as the level of serum IL-21 in the AMR, AR, and CR groups at post-transplantation were significantly higher than those at pre-transplantation. In contrast, the number of circulating CD19+CD27+IgD B cells was significantly increased in the TS groups in respect to the other groups. Moreover, the numbers of circulating CD4+CXCR5+IL-21+ TFH cells, CD19+CD86+CD38+ B cells as well as the level of serum IL-21 were positive related to the level of serum Cr while showing negative correlated with the values of eGFR in the AMR groups at post-transplantation for 4 and 12 weeks. Circulating TFH cells may be a biomarker in RTR with AMR, which can promote the differentiation of B cells into plasma cells by activating B cells, thereby promoting disease progression.
Keywords: Renal transplant, TFH cell, B cell, IL-21, CD38, inducible T-cell co-stimulator
Introduction
Renal transplant is the preferable choice for patients with end-stage renal disease (ESRD), as it improves survival and quality of life. 1 However, renal rejection episodes still are one of the major causes of the differential diagnosis of graft dysfunction. Previous studies have shown that the alloimmune response mediated by anti-HLA antibodies is the main cause of late allograft dysfunction. 2 Moreover, there has been a growing awareness that a variety of immune mechanisms play a critical role in acute and chronic allograft damage, in particular T and B cells.3–6 Therefore, understanding the influence of immune factors on allograft function is important for reducing graft rejection and improving graft survival rate.
Follicular helper T cells (TFH cells), a subset of CD4+T lymphocytes, which provide help to antigen-primed B cells, particularly within germinal centers (GCs), to produce high-affinity antibodies and develop immunological memory in response to foreign antigens. 7 TFH cells were defined as surface-expressing CXCR5, inducible T-cell co-stimulator (ICOS), programmed cell death protein 1 (PD-1), and regulatory cytokine interleukin (IL)-21.8,9 IL-21, despite its roles in other systems, is a cytokine secreted by TFH cell and is one of the most necessary stimulators of B cell proliferation, differentiation, and generating high-affinity antibodies.10,11 Previous experiments have confirmed that CD4+T cells are essential for the generation of DSA, and TFH is also regarded as one of the most promising targets to prevent DSA generation. 12 Although, Th1, Th2, and Th17 cells are instrumental in initiating allograft rejection after renal transplantation, little is known concerning the involvement of TFH cells in renal transplant rejection.13–17 Existing research has suggested that circulating TFH cells can play a role in predicting DSA generation. 18 However, little is known of the quantity, functionality, and subset composition of TFH cells in different types of rejection in renal transplant recipients (RTR). Consequently, a better understanding of what the number of circulating TFH cells is and the association with clinical measures between pre- and post-transplant will be instrumental for the development of effective strategies for clinical renal transplantation rejection.
Many studies have shed light on the emerging role of B cells subsets in the tolerance and rejection of renal transplant, although more attention has been focused on regulatory cells function.19–24 It is generally believed that B cell is recognized as a major determinant of long-term graft survival of renal transplant. Alternatively, Viklicky et al. 25 have found that the expression of peripheral blood B cell signatures are higher in the rejection-free patients and in patients with borderline changes compared to patients with acute rejection. The fractions of the B cell compartment were shown to play a key role in determining graft outcome. Plasma cells play an important role in the secretion of antibodies. TFH cells and their secreted IL-21 can stimulate the activation of B cells and differentiate into plasma cells. Circulating plasma cells can be identified as CD19+CD38+. CD86 is a marker of B cell activation. However, little is known about the expression of activated circulating B cell in different types of rejection in RTR and the relationship between circulating TFH cells and activated circulating B cells.
In summary, in this study, we examined the numbers of circulating TFH and B cells in RTR with AMR, AR, CR, or TS at post-transplant for 4 and 12 weeks, as well as the potential association with the renal function measures in the 21 patients. In this study, we attempt to show the role of circulating TFH and B cells in the tolerance and rejection of renal transplant.
Materials and methods
Subjects
The present study was a prospective study. Total 21 patients (13 males, eight females; 32–49 years old, median 36.3) with end-stage renal disease (ESRD) and 19 healthy controls (HC) were recruited in the study between 2013 and 2017 from the First Hospital of Jilin University (Changchun, China). Patients and controls are matched for age, gender, and ethnic (Table 1). According to the criteria, 26 patients with ESRD were diagnosed. All patients had compatible HLA gene matches. Exclusive criteria: 1) Participants had a history of surgical procedure, 2) Participants received renal transplants developed malignancy and infective complications. The study protocol was approved by the ethics review board of the Human Ethics Committee of Jilin University, China (No. 2013-250). All enrolled patients signed informed consent. All of the procedures were performed in accordance with the Declaration of Helsinki and relevant policies in China. No executed prisoners were used in any part of this study.
Table 1.
The demographic and clinical characteristics of subjects.
| Parameters | HC | ESRD |
|---|---|---|
| Number | 19 | 21 |
| Age (years) | 37.2 (30–47) | 36.3 (32–49) |
| F/M (n) | 8/11 | 8/13 |
| BUN (mmol/L) | 4.4 (3.5–7.0) | 17.1 (7.2–31.6)* |
| Cr (μmol/L) | 88 (65–112) | 846 (499–1790)* |
| eGFR (mL/min) | 115 (98–124) | 11 (6–13)* |
| WBC (×109/L) | 6.7 (3.7–9.3) | 10.4 (8.5–13.2)* |
| PBMCs (×109/L) | 2.6 (0.9–4.5) | 2.7 (1.1–4.3) |
Data shown are median (range) of each group of subjects. ESRD: end-stage renal disease; BUN: blood urea nitrogen (normal range: 3.2 –6.0 mmol/L); Cr: serum creatinine (normal ranges: men: 44–133 μmol/L; women: 70–108 μmol/L); eGFR: glomerular filtration rate (normal value: 125 mL/min); WBC: white blood cell (normal range: 4–10×109/L); *p < 0.05 vs. HC.
Study groups
In this study, we collected samples from all renal transplant patients before transplant, 4 weeks and 12 weeks after surgery. All individuals were divided into four groups in which those data were grouped according to the pathological results and analyzed uniformly the Banff Classification. 27 (a) Transplant Stable (TS) (n = 5): There are no clinical or laboratory features to suggest rejection in recipients (serum creatinine (scr): <150 μmol/L; eGFR: ≥50) in 12 weeks. (b) Acute antibody-mediated acute rejection (AMR) (n = 6): Recipients’ graft function deteriorated (scr: ≥150 μmol/L; eGFR: <50) within 1 week; (c) Acute rejection (AR) (n = 6): Recipients’ graft function deteriorated (scr: ≥150 μmol/L; eGFR: <50) within 2–12 weeks; (d) Chronic rejection (CR) (n = 4): Recipients’ graft function deteriorated (scr: ≥150 μmol/L; eGFR: <50) after 12 weeks. The AMR, AR, and CR group of patients were confirmed by biopsy. The clinical information of the four groups is shown in Table 2.
Table 2.
The demographic and clinical characteristics of subjects.
| BUN (mmol/L) | Cr (μmol/L) | eGFR (ml/min) | WBC (×109/L) | PBMCs (×109/l) | ||
|---|---|---|---|---|---|---|
| AMR (n = 6) | Pre- | 29.8* (19.1–38.9) | 1373* (1098–1790) | 36* (18–48) | 18.5* (11.2–20.8) | 0.3* (0.1–0.7) |
| Post-4 weeks | 21.2* (16.8–34.2) | 972* (642–1024) | 43* (29–61) | 12.1* (9.13–13.6) | 1.1* (0.2–1.4) | |
| Post-12 weeks | 20.3 (16.2–30.5) | 896 (689–1145) | 44 (31–60) | 9.4 (8.6–14.2) | 1.8 (1.0–3.4) | |
| AR (n = 6) | Pre- | 25.9* (18.2–34.5) | 1078* (794–1678) | 41* (29–49) | 13.3* (9.7–16.2) | 0.9* (0.3–1.2) |
| Post-4 weeks | 19.0* (14.3–31.7) | 885* (545–1345) | 51* (25–78) | 10.8* (7.0–12.6) | 1.2* (0.5–2.6) | |
| Post-12 weeks | 16.3 (11.4–28.4) | 823 (678–1456) | 47 (33–59) | 9.5 (8.8–12.3) | 1.5 (1.1–3.20) | |
| CR (n = 4) | Pre- | 18.0 (11.4–27.1) | 804 (597–1342) | 53 (29–67) | 9.7 (8.8–13.4) | 1.6 (1.0–2.7) |
| Post-4 weeks | 15.3* (9.5–23.5) | 598* (502–754) | 71* (48–85) | 11.3* (9.5–14.1) | 1.0* (0.7–1.2) | |
| Post-12 weeks | 13.0* (5.6–17.5) | 462* (326–678) | 73* (53–86) | 10.4* (9.4–11.6) | 1.1* (1.0–2.0) | |
| TS (n = 5) | Pre- | 15.4 (7.4–31.7) | 778 (513–1467) | 54 (28–67) | 9.9 (8.3–14.6) | 1.5 (0.9–3.0) |
| Post-4 weeks | 8.1* (3.5–16.4) | 88* (56–145) | 85* (59–103) | 7.7* (6.1–9.8) | 0.7* (0.4–1.1) | |
| Post-12 weeks | 6.4* (3.5–11.4) | 79 (47–135) | 85 (61–113) | 7.3* (5.6–9.3) | 1.6* (1.1–2.7) | |
Clinical measurements
The clinical data including age, sex, and laboratory tests were collected from hospital records. Blood samples were harvested by standard procedures from individual participants. The laboratory investigations included full blood counts, the levels of serum Cr, BUN, and estimated glomerular filtration rate (eGFR) were determined by nephelometry using a Siemens special protein analyzer (Siemens, GmbH, Germany).
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were separated from peripheral venous blood of participants. PBMCs at 5 ×106/tube were stained in duplicate with fluorescent-labeled antibodies CXCR5 (Biolegend, San Diego, USA), CD4, ICOS, PD-1, CD19, CD38, CD86, and CD27 using IgG1 as the isotype-matched controls (BD BioSciences, San Jose, USA). Flow cytometry analysis was performed using BD FACSAria II.
Stimulation of PBMC and FACS
The function of TFH cells secrete IL-21 were determined by flow cytometry. PBMCs (5 ×106/well) were stimulated as previously described. 28 The cells were stained in CXCR5 for 30 min. And then, the cells washed and fixed and permeabilized, followed by staining with IL-21 (BD PharMingen).
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to measure the concentrations of serum IL-21 (R&D system, USA). Individual sera from participants at 1:4 dilutions were tested by ELISA. The level of serum IL-21 is based on the standard curve established. The limitation of detection for IL-21 was 10 pg/mL.
Statistical analysis
Statistical differences between groups were analyzed by the Kruskal–Wallis test for unpaired comparisons and Chi-square test for unpaired and paired comparisons. Spearman rank correlation test was adopted for two variables. All survey data were analyzed by SPSS 16.0 software. If a two-side p value < 0.05, a statistically significant was defined.
Results
Pre-transplant characteristics of renal candidates
21 patients (13 males, eight females; 32–49 years old, median 36.3) with ESRD and 19 age and gender-matched HC were recruited. And demographics and clinical index of those subjects are shown in Table 1. Compared with the controls, those patients showed a higher level of serum Cr and BUN as well as the values of WBC and lower levels of eGFR.
Alterations in the number of circulating CD19+CD86+CD38+ B cells after transplantation
RTR were divided into four groups. Those clinical characters were shown in Table 2. Compared to those pre-transplant, patients with TS showed a significant reduction in levels of BUN and serum Cr and a significant increase in the valves of eGFR. In addition, the concentration of serum Cr and BUN in the AMR, AR, and CR groups were significantly higher than those in the TS or ESRD patients at post-transplant for 4 and 12 weeks.
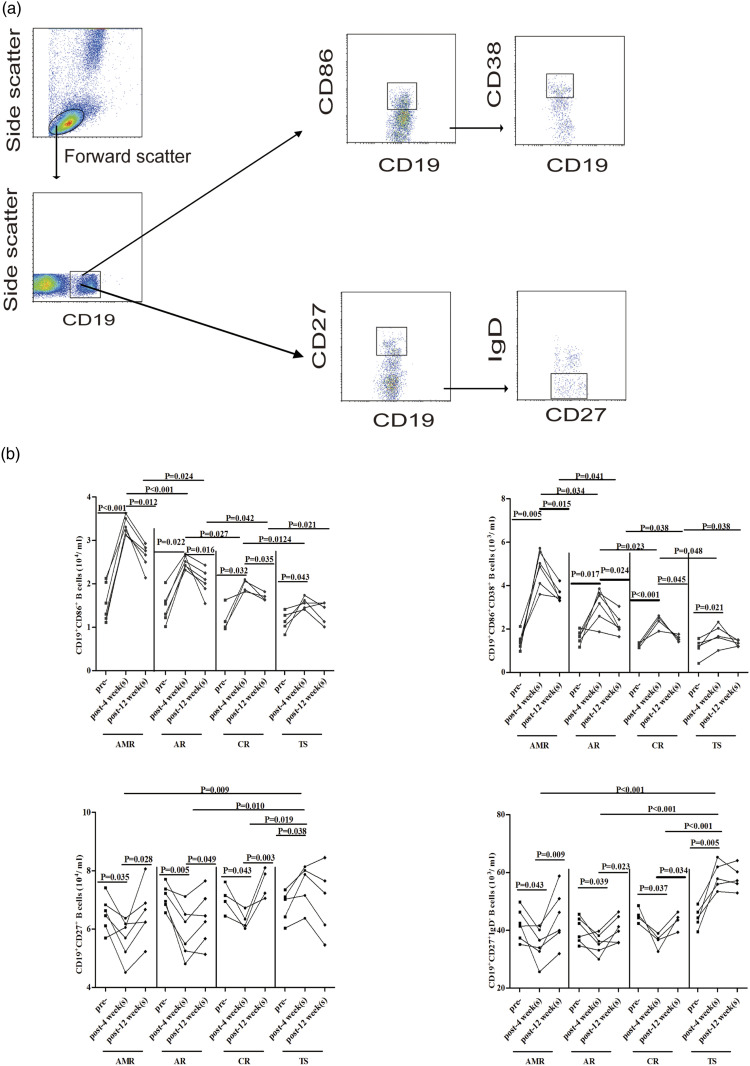
We first compared the circulating CD86+, CD38+, CD27+, and IgD+ B cells observed in the four groups RTR to evaluate the alterations between pre- and post-transplantation (Figure 1). We found that the number of circulating CD19+CD86+ and CD19+CD86+CD38+ B cells in the four groups was greater at post-transplantation than those at pre-transplantation as well as significantly higher in the AMR, AR, and CR groups than those in the TS groups. In contrast, the number of circulating CD19+CD27+ and CD19+CD27+IgD-memory B cells was significantly lower in the AMR, AR, and CR groups than those in the TS groups. Furthermore, there were no significant differences in the numbers of B cells subsets between the four groups. These data indicate a significantly higher number of circulating CD19+CD86+ and CD19+CD86+CD38+ B cells were detected in RTR patients.
Figure 1.
FACS analysis of the number of circulating CD19+ B cells profile; (a) flow cytometry analysis of B cells; (b) the numbers of CD19+CD86+ B cells, CD19+CD86+CD38+ B cells, CD19+CD27+ B cells, and CD19+CD27+IgD-memory B cells in the four groups patients with pre- and post-transplantation for 4 and 12 weeks, respectively.
Alterations in the number of IL-21+ TFH cells after transplantation
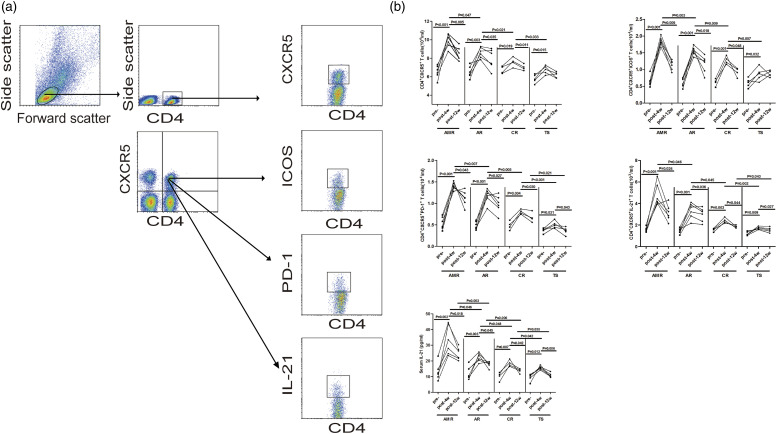
TFH cells are specialized in providing help to B cells and are necessary for maturation of B cells into plasma cells and memory B cells. Next, we compared the TFH cells observed in the four groups RTR to evaluate the alterations between pre- and post-transplantation (Figure 2). We found that the number of circulating CD4+CXCR5+, CD4+CXCR5+ICOS+, CD4+CXCR5+PD-1+, and CD4+CXCR5+IL-21+ TFH cells in the four group at post-transplant was greater than those at the pre-transplant as well as the levels of serum IL-21. On the other hand, the AMR, AR, and CR patients were all significantly higher in comparison to the TS patients. These results suggest that circulating TFH cells may be involved in the pathogenesis of RTR.
Figure 2.
FACS analyses of the number of circulating CD4+CXCR5+ TFH cells; (a) Flow cytometry analysis of TFH cells; (b) the numbers of CD4+CXCR5+ T cells, ICOS+, PD-1+, or IL-21+ TFH cells and the concentrations of serum IL-21 in the four groups patients with pre- and post-transplantation for 4 and 12 weeks, respectively.
The relationships between the number of circulating CD19+CD86+CD38+ B cells and the concentrations of eGFR or serum Cr in the AMR groups
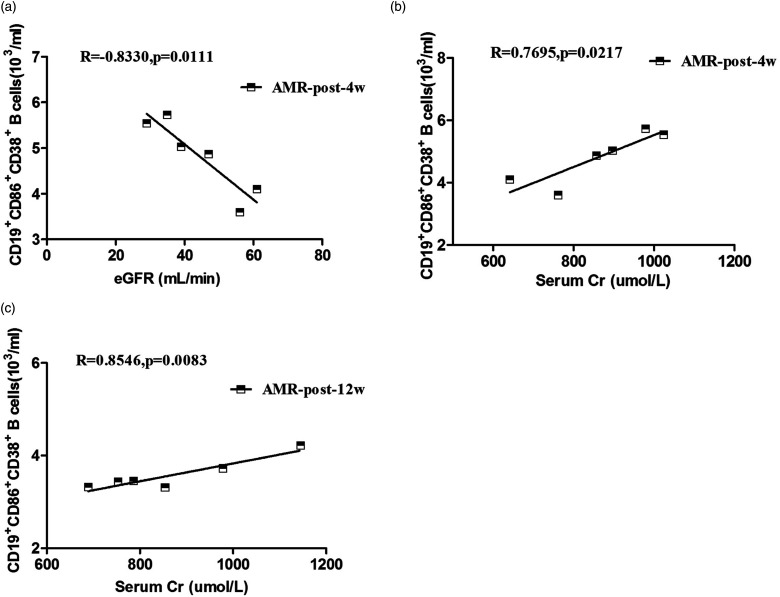
In order to determine which group did B cells play a more significant role, we further analyzed the relationships between B cells and clinical values in different groups. However, correlations between the number of circulating CD19+CD86+CD38+ B cells and the values of clinical measures tested only in the AMR group (Figure 3). There was not a statistically significant correction in the other three groups (data not shown). Renal damage often arises from acute antibody-mediated rejection, which has a bad effect on long-term graft survival. 29 Our data revealed that the number of circulating CD19+CD86+CD38+ B cells was positively correlated with the concentrations of serum Cr (R = 0.7695, p = 0.0217, Figure 3(b) for 4 weeks; R = 0.8546, p = 0.0083, Figure 3(c) for 12 weeks) and negatively corrected with eGFR (R = −0.8330, p = 0.0111, Figure 3(a) for 4 weeks) in the AMR groups. There was not a statistically significant association between the number of memory B cells and the values of clinical measures tested in this population (data not shown). Collectively, these data suggest that increased number of circulating CD19+CD86+CD38+ B cells may be responsible for producing rejection in RTR patients with AMR.
Figure 3.
The relationship between the number of circulating CD19+CD86+CD38+ B cells and the concentrations of eGFR or serum Cr in the AMR group; (a–c) correlation between the numbers of CD19+CD86+CD38+ B cells and the valves of eGFR and the levels of serum Cr for post-transplantation 4 weeks or 12 weeks, respectively.
The relationships between the number of circulating IL-21+ TFH cells or the level of serum IL-21 and the concentrations of eGFR or serum Cr in the AMR group
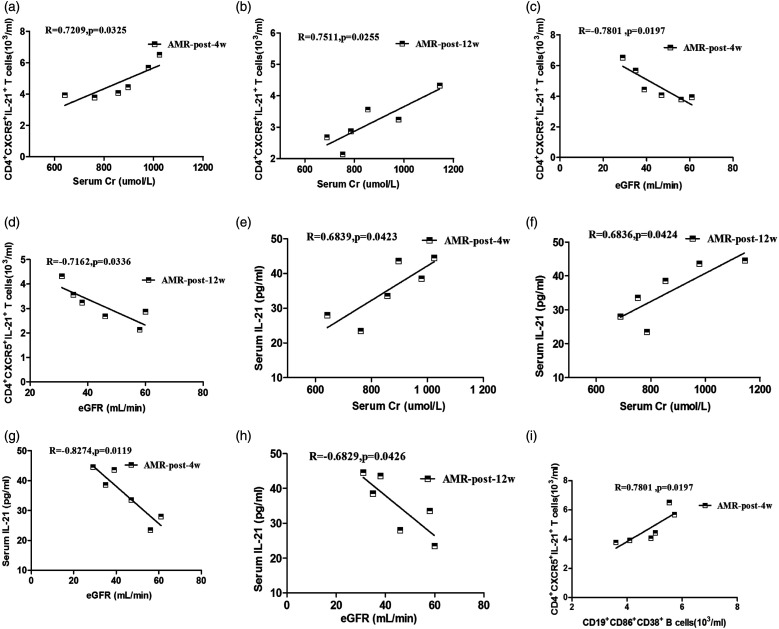
To explore the significance of TFH cells, we analyzed the relationships between the number of circulating TFH cells and the values of clinical parameters in the AMR group (Figure 4). The number of circulating IL-21+ TFH cells showed a positive related with the levels of serum Cr (R = 0.7209, p = 0.0325, Figure 4(a) for 4 weeks; R = 0.07,511, p = 0.0255, Figure 4(b) for 12 weeks) and a negative related with eGFR (R = −0.7801, p = 0.0197, Figure 4(c) for 4 weeks; R = −0.7162, p = 0.0336, Figure 4(d) for 12 weeks) in the AMR group. Furthermore, the levels of serum IL-21 positively related with the concentrations of serum Cr (R = 0.6839, p = 0.0423, Figure 4(e) for 4 weeks; R = 0.6836, p = 0.0424, Figure 4(f) for 12 weeks) and a negative related with eGFR (R = −0.8274, p = 0.0119, Figure 4(g) for 4 weeks; R = −0.6829, p = 0.0426, Figure 4(h) for 12 weeks). However, there was no significant association between the number of circulating ICOS+ TFH cells, PD-1+ TFH cells and the values of other laboratory test results (data not shown). Unfortunately, no significant statistical significance was observed in other groups (data not shown). In summary, these data suggest that the alterations of the number of circulating IL-21+ TFH cells and the level of serum IL-21 may be involved in production of rejection in AMR patients.
Figure 4.
The relationship between the number of circulating IL-21+ TFH cells or the levels of serum IL-21 and the concentrations of eGFR or serum Cr in the AMR group. (a–h) correlation between the number of IL-21+ TFH cells or the levels of serum IL-21 and the valves of eGFR and the levels of serum Cr for post-transplantation 4 weeks or 12 weeks, respectively. (i) Correlation between the number of IL-21+ TFH cells and CD19+CD86+CD38+ B cells for post-transplantation 4 weeks.
Conventional immunosuppression therapy not only reduces the number of circulating IL-21+ TFH cells and CD19+CD86+CD38+ B cells in the AMR, AR, and CR groups, but may stabilize the TS group
The conventional immunosuppression therapy has been recognized as a standard therapy for patients with renal transplantation. We found that the number of circulating CD19+CD86+CD38+ B cells and IL-21+ TFH cells were significantly decreased at post-transplantation for 12 weeks in comparison with 4 weeks and pre-transplantation in the AMR, AR, and CR groups, while was no significant differences between the post-transplantation for 4 and 12 weeks in the TS group (Figure 1). The number of circulating IL-21+ TFH cells showed a positive related with the number of circulating CD19+CD86+CD38+ B cells (R = 0.7801, p = 0.0197, Figure 4(i) for 4 weeks). Therefore, circulating TFH cells may promote the differentiation of B cells into plasma cells by activating B cells, and conventional immunosuppression therapy can improve the rejection after renal transplantation.
Discussion
There has recently been an increasing interest in graft rejection and the treatment after renal transplantation.30,31 Furthermore, it is now widely accepted that antibody-mediated injury is a major factor of renal-allograft loss.32–34 However, little is known about whether B cells or TFH cells are involved in the rejection after renal transplantation and how the numbers of those cells change in the ESRD and RTR.
Nickerson and Rush 35 have indicated that de novo donor-specific antibody (DSA) requires both B cell and CD4+T cell activation, which this coordinate interaction may lead to memory and effector T and B responses. 35 Furthermore, DSA poses a significant barrier to successful kidney transplantation because of the increased risk of both early and late graft loss.36–38 Most importantly, TFH cell has been regarded as one of the most promising targets to prevent DSA generation. 12 Indeed, in an effort to gain understanding of the rejection response, we attempted to assess the number of circulating TFH cells and B cells in RTR with the AMR, AR, CR, or TS.
The relative roles of B lymphocytes and plasma cells during allograft rejection remain obscure since B cells carry out multiple functions in immune responses. Moreover, the B cells are increasingly identified as a critical factor for graft outcome. 39 Consequently, we first examined the role of B cells in RTR with the AMR, AR, CR, and TS. We discovered that the number of circulating CD19+CD86+CD38+ B cells in the four groups at post-transplant was greater than those at pre-transplant as well as was higher in the AMR, AR, and CR group than those in the TS group, while the number of CD19+CD27+IgD-memory B cells was lower in the AMR, AR, and CR groups than those in the TS group. This indicates that B cells are in an activated state and consume a large number of memory cells and B cells can affect graft survival in a number of ways. It is able to differentiate into plasma cells or memory B cells.40,41 CD27 is expressed on somatically mutated B cells and includes the post-switch memory CD27+IgD-B cells. 42 The level of serum DSA can predict the occurrence of rejection reaction, and the Banff score can indicate the extent of damage to kidney rejection. However, eGFR is the most direct indicator of renal function 43 and can reflect the long-term survival of the graft. 44 Furthermore, the number of circulating CD19+CD86+CD38+ B cells was also positively correlated with the levels of serum Cr and negatively the levels of eGFR in AMR patients. We next found the numbers of circulating CD4+CXCR5+ T cells, ICOS+ TFH cells, PD-1+ TFH cells, and IL-21+ TFH cells in the four groups at post-transplant were greater than those at pre-transplant as well as the level of serum IL-21. Moreover, the AMR, AR, and CR groups were all significantly higher in comparison with the TS group. The number of circulating CD4+CXCR5+IL-21+ TFH cells was positively correlated with the level of serum Cr, while negatively correlated with the level of eGFR. In conclusion, we speculate that these factors may be associated with the progression of RTR.
TFH cells secrete a large amount of IL-21, which also involves in the differentiation and function of TFH and acts on B cells. We also found that the level of serum IL-21 was positively correlated with the level of serum Cr in the AMR group, while negatively correlated with the level of eGFR in the AMR group. Our data confirms the previous research that IL-21 is a key regulator of functional B cells differentiation and promotes rejection production. Therefore, IL-21 may be involved in RTR rejection, in particular AMR patients.
It seems mean that during the rejection process of RTR patients, TFH cells promote the differentiation of B cells and mediated humoral immune, leading to the impairment of renal function. Simultaneously, TFH cells secrete high level of IL-21. In conclusion, TFH cell may be a crucial factor contributing to the progression of rejection in RTR patients. Commonly used immunosuppressive drugs work mainly by suppressing the helper signals of T cells. 45 The main reason for the large amount of DSA generated after transplantation is also believed to be caused by insufficient immunosuppression to prevent the function of TFH. Although TFH plays an important role in post-transplant rejection, current immunosuppressive drugs do not adequately block it. 12 The molecular mechanism is also unclear. It also provides us with the direction of future research.
In this study, we examined the numbers of circulating TFH and B cells in RTR with AMR, AR, CR, or TS at post-transplant for 4 and 12 weeks, as well as the potential association with the renal function measures in the 21 patients. In summary, the numbers of circulating TFH cells and B cells in RTR with AMR, AR, and CR showed increased. However, the numbers of CD4+CXCR5+IL-21+ TFH and CD19+CD86+CD38+ B cells and level of serum IL-21 were positively correlated with the level of serum Cr while negatively related with eGFR in the RTR with AMR. There are certain limitations to our study, such as the sample size is insufficient, the lack of functional explorations and molecular mechanisms research. In addition, there is a lack of evidence about the direct causation between the B cell and cytokine profile and RTR rejections.
Conclusion
In summary, circulating TFH cells may be a biomarker in RTR with AMR, and promote the differentiation of B cells into plasma cells by activating B cells, thereby promoting disease progression. We think that it will bring new insights into the pathogenesis of RTR with ARM.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Natural Science Foundation of China (nos. 30972610, 81273240, 91742107, and 81570002), National Key Research and Development Program (nos. 2017YFC0910000 and 2017YFD0501300), Jilin Province Science and Technology Agency (nos. JJKH20211210KJ, JJKH20211164KJ, 20200403084SF, JLSWSRCZX2020-009, 20200901025SF, 20190101022JH, 2019J026, 20170622009JC, 2017C021, 2017J039, SXGJXX2017-8, JJKH20180197KJ, DBXM154-2018, and 2018SCZWSZX-015).
Ethical approval and consent to participate: Permission from the Human Ethics Committee of Jilin University 2013-250 was obtained for the study. In addition, all enrolled patients signed informed consent.
ORCID iD
Yanfang Jiang https://orcid.org/0000-0002-2478-1521
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. New England Journal of Medicine 341: 1725–1730. DOI: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Loupy A, Lefaucheur C, Vernerey D, et al. (2013) Complement-binding anti-HLA antibodies and kidney-allograft survival. New England Journal of Medicine 369: 1215–1226. DOI: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 3.Halloran PF, Pereira AB, Chang J, et al. (2013) Potential impact of microarray diagnosis of T cell-mediated rejection in kidney transplants: The INTERCOM study. American Journal of Transplantation 13: 2352–2363. DOI: 10.1111/ajt.12387. [DOI] [PubMed] [Google Scholar]
- 4.Clatworthy MR. (2013) B cell responses to allograft-more common than we thought? American Journal of Transplantation 13: 1629–1630. DOI: 10.1111/ajt.12309. [DOI] [PubMed] [Google Scholar]
- 5.Gorbacheva V, Fan R, Fairchild RL, et al. (2016) Memory CD4 T cells induce antibody-mediated rejection of renal allografts. Journal of the American Society of Nephrology 27: 3299–3307. DOI: 10.1681/ASN.2015080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeekens SP, van den Hoogen MWF, Kamburova EG, et al. (2013) The effects of in vivo B-cell depleting therapy on ex-vivo cytokine production. Transplant Immunology 28: 183–188. DOI: 10.1016/j.trim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Crotty S. (2011) Follicular helper CD4 T cells (TFH). Annual Review of Immunology 29: 621–663. DOI: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 8.Nutt SL, Tarlinton DM. (2011) Germinal center B and follicular helper T cells: Siblings, cousins or just good friends? Nature Immunology 12: 472–477. DOI: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt N, Ueno H. (2013) Blood Tfh cells come with colors. Immunity 39: 629–630. DOI: 10.1016/j.immuni.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crotty S. (2014) T follicular helper cell differentiation, function, and roles in disease. Immunity 41: 529–542. DOI: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard WJ, Spolski R. (2005) Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nature Reviews Immunology 5: 688–698. DOI: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 12.Chen C-C, Koenig A, Saison C, et al. (2018) CD4+ T cell help is mandatory for naive and memory donor-specific antibody responses: impact of therapeutic immunosuppression. Frontiers in Immunology 9: 275. DOI: 10.3389/fimmu.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang S, Herrera O, Lechler RI. (2004) New spectrum of allorecognition pathways: Implications for graft rejection and transplantation tolerance. Current Opinion in Immunology 16: 550–557. DOI: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Soltaninejad E, Nicknam MH, Nafar M, et al. (2015) Differential expression of microRNAs in renal transplant patients with acute T-cell mediated rejection. Transplant Immunology 33: 1–6. DOI: 10.1016/j.trim.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Chung BH, Kim KW, Kim B-M, et al. (2015) Increase of Th17 cell phenotype in kidney transplant recipients with chronic allograft dysfunction. PLoS One 10: e0145258. DOI: 10.1371/journal.pone.0145258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena A, Panigrahi A, Gupta S, et al. (2012) Frequency of T cell expressing Th1 and Th2 associated chemokine receptor in patients with renal allograft dysfunction. Transplantation Proceedings 44: 290–295. DOI: 10.1016/j.transproceed.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Luo F, Shi Q, et al. (2015) Increased circulating follicular helper T cells with decreased programmed death-1 in chronic renal allograft rejection. BMC Nephrology 16: 182. DOI: 10.1186/s12882-015-0172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Muraglia GM, 2nd, Wagener ME, Ford ML, et al. (2020) Circulating T follicular helper cells are a biomarker of humoral alloreactivity and predict donor‐specific antibody formation after transplantation. American Journal of Transplantation 20: 75–87. DOI: 10.1111/ajt.15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XC, Turka LA. (2010) An update on regulatory T cells in transplant tolerance and rejection. Nature Reviews Nephrology 6: 577–583. DOI: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 20.Krystufkova E, Sekerkova A, Striz I, et al. (2012) Regulatory T cells in kidney transplant recipients: the effect of induction immunosuppression therapy. Nephrology Dialysis Transplantation 27: 2576–2582. DOI: 10.1093/ndt/gfr693. [DOI] [PubMed] [Google Scholar]
- 21.Hu M, Wang YM, Wang Y, et al. (2016) Regulatory T cells in kidney disease and transplantation. Kidney International 90: 502–514. DOI: 10.1016/j.kint.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Burton H, Dorling A. (2017) Transitional B cell subsets-a convincing predictive biomarker for allograft loss? Kidney International 91: 18–20. DOI: 10.1016/j.kint.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Svachova V, Sekerkova A, Hruba P, et al. (2016) Dynamic changes of B-cell compartments in kidney transplantation: Lack of transitional B cells is associated with allograft rejection. Transplant International 29: 540–548. DOI: 10.1111/tri.12751. [DOI] [PubMed] [Google Scholar]
- 24.Cherukuri A, Salama AD, Carter CR, et al. (2017) Reduced human transitional B cell T1/T2 ratio is associated with subsequent deterioration in renal allograft function. Kidney International 91: 183–195. DOI: 10.1016/j.kint.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Viklicky O, Krystufkova E, Brabcova I, et al. (2013) B-cell-related biomarkers of tolerance are up-regulated in rejection-free kidney transplant recipients. Transplantation 95: 148–154. DOI: 10.1097/TP.0b013e3182789a24. [DOI] [PubMed] [Google Scholar]
- 26.Meijers RW, Litjens NH, de Wit EA, et al. (2012) Uremia causes premature ageing of the T cell compartment in end-stage renal disease patients. Immunity & Ageing 9: 19. DOI: 10.1186/1742-4933-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loupy A, Haas M, Solez K, et al. (2017) The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. American Journal of Transplantation 17: 28–41. DOI: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Ji H, Zhao P, et al. (2019) Follicular helper T cell and memory B cell immunity in CHC patients. Journal of Molecular Medicine 97: 397–407. DOI: 10.1007/s00109-018-01735-z. [DOI] [PubMed] [Google Scholar]
- 29.Davis S, Cooper JE. (2017) Acute antibody-mediated rejection in kidney transplant recipients. Transplantation Reviews 31: 47–54. DOI: 10.1016/j.trre.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Orandi BJ, Chow EHK, Hsu A, et al. (2015) Quantifying renal allograft loss following early antibody-mediated rejection. American Journal of Transplantation 15: 489–498. DOI: 10.1111/ajt.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicora F, Paz M, Mos F, et al. (2013) Use of bortezomib to treat anti-HLA antibodies in renal transplant patients: a single-center experience. Transplant Immunology 29: 7–10. DOI: 10.1016/j.trim.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Thomas KA, Valenzuela NM, Reed EF. (2015) The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection. Trends in Molecular Medicine 21: 319–329. DOI: 10.1016/j.molmed.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hörmann M, Dieplinger G, Rebellato LM, et al. (2016) Incidence and impact of anti-HLA-DP antibodies in renal transplantation. Clinical Transplantation 30: 1108–1114. DOI: 10.1111/ctr.12794. [DOI] [PubMed] [Google Scholar]
- 34.Dragun D, Catar R, Philippe A. (2013) Non-HLA antibodies in solid organ transplantation. Current Opinion in Organ Transplantation 18: 430–435. DOI: 10.1097/MOT.0b013e3283636e55. [DOI] [PubMed] [Google Scholar]
- 35.Nickerson PW, Rush DN. (2013) Rejection: an integrated response. American Journal of Transplantation 13: 2239–2240. DOI: 10.1111/ajt.12365. [DOI] [PubMed] [Google Scholar]
- 36.Lachmann N, Terasaki PI, Budde K, et al. (2009) Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 87: 1505–1513. DOI: 10.1097/TP.0b013e3181a44206. [DOI] [PubMed] [Google Scholar]
- 37.Cross AR, Lion J, Loiseau P, et al. (2016) Donor Specific Antibodies are not only directed against HLA-DR: Minding your Ps and Qs. Human Immunology 77: 1092–1100. DOI: 10.1016/j.humimm.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Kauke T, Oberhauser C, Lin V, et al. (2017) De novodonor-specific anti-HLA antibodies after kidney transplantation are associated with impaired graft outcome independently of their C1q-binding ability. Transplant International 30: 360–370. DOI: 10.1111/tri.12887. [DOI] [PubMed] [Google Scholar]
- 39.Nouël A, Ségalen I, Jamin C, et al. (2014) B cells display an abnormal distribution and an impaired suppressive function in patients with chronic antibody-mediated rejection. Kidney International 85: 590–599. DOI: 10.1038/ki.2013.457. [DOI] [PubMed] [Google Scholar]
- 40.Colvin RB, Hirohashi T, Farris AB, et al. (2010) Emerging role of B cells in chronic allograft dysfunction. Kidney International 78: S13–S17. DOI: 10.1038/ki.2010.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arpin C, Dechanet J, Van Kooten C, et al. (1995) Generation of memory B cells and plasma cells in vitro. Science 268: 720–722. DOI: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 42.Agematsu K. (2000) Author index for volume 15. Molecular and Cellular Neuroscience 15: 573–574. DOI: 10.14670/HH-15.573. [DOI] [Google Scholar]
- 43.Mahajan S, Mukhiya GK, Singh R, et al. (2005) Assessing suitability for renal donation: can equations predicting glomerular filtration rate substitute for a reference method in the Indian population? Nephron Clinical Practice 101: c128–c133. DOI: 10.1159/000086683. [DOI] [PubMed] [Google Scholar]
- 44.Fernández‐Fresnedo G, Palomar R, Escallada R, et al. (2001) Hypertension and long‐term renal allograft survival: Effect of early glomerular filtration rate. Nephrology Dialysis Transplantation 16(Suppl 1): 105–109. DOI: 10.1093/ndt/16.suppl_1.105. [DOI] [PubMed] [Google Scholar]
- 45.Heidt S, Roelen DL, Eijsink C, et al. (2010) Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clinical & Experimental Immunology 159: 199–207. DOI: 10.1111/j.1365-2249.2009.04051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]