Abstract
Diabetic cardiomyopathy is associated with an increased risk for heart failure and death in patients with diabetes. We investigated here whether and how GIP attenuated cardiac hypertrophy and fibrosis in diabetic mice with obesity. Diabetic db/db mice at 7 weeks old were infused with vehicle or GIP (50 nmol/kg/day) for 6 weeks, and hearts were collected for histological and RT-PCR analyzes. Cardiomyocytes isolated from neonatal mice were incubated with or without 300 nM [D-Ala2]-GIP, 30 mM glucose, or 100 μg/mL advanced glycation end products (AGEs) for RT-PCR and lucigenin assays. Compared with non-diabetic mice, diabetic mice exhibited larger left ventricle wall thickness and cardiomyocyte sizes and more fibrotic areas in association with up-regulation of myosin heavy chain β (β-Mhc) and transforming growth factor-beta2 (Tgf-β2) mRNA levels, all of which were inhibited by GIP infusion. High glucose increased NADPH oxidase-driven superoxide generation and up-regulated β-Mhc, Tgf-β2, and receptor for AGEs mRNA levels in cardiomyocytes, and augmented the AGE-induced β-Mhc gene expression. [D-Ala2]-GIP attenuated all of the deleterious effects of high glucose and/or AGEs on cardiomyocytes. Our present findings suggest that GIP could inhibit cardiac hypertrophy and fibrosis in diabetic mice via suppression of TGF-β2.
Keywords: AGEs, diabetic cardiomyopathy, GIP, oxidative stress, RAGE
Introduction
The prevalence of diabetes and associated increased morbidity and mortality has been increasing worldwide in the past few decades. 1 Epidemiological studies have revealed that diabetes (DM) patients are at increased risk for heart failure, 2 and the prognosis of DM patients with heart failure is poor with a survival rate approximately half that compared with non-DM individuals even after adjusting for conventional risk factors. 3
Heart failure can occur in DM patients without major causative disease, such as ischemic coronary artery disease, valvular disease, or hypertension. 4 Although the underlying mechanism remains unclear, this type of heart failure could be associated with diabetic cardiomyopathy, which is characterized by cardiac hypertrophy and fibrosis. 4 However, effective pharmacological therapies to improve clinical outcomes or attenuate the progression of diabetic cardiomyopathy have not been established. 5
GIP is a gut peptide hormone, secreted from intestinal K cells in response to nutrient stimuli. 6 In addition to its well-known insulinotropic action on pancreatic β cells, 6 GIP exerts pleiotropic effects through interactions with its receptor, which is widely expressed in extra-pancreatic organs, including heart and vessels.6,7 We have previously reported that the GIP receptor is expressed in mouse cardiomyocytes and that GIP inhibits transforming growth factor (Tgf)-β1 gene expression in angiotensin II-exposed cardiomyocytes. 8 Furthermore, we have found that GIP treatment attenuates cardiac hypertrophy and fibrosis in association with decreased expression levels of TGF-β protein in hypertensive mice receiving angiotensin II. 8 Since TGF-β plays a role in pathogenesis of diabetic cardiomyopathy, 9 our previous observations led us to speculate that GIP may be a novel therapeutic target for diabetic cardiomyopathy. In this study, we evaluated whether and how GIP treatment inhibited cardiac hypertrophy and fibrosis in db/db mice, a mouse model of DM with obesity.
Materials and methods
Agents
Chemical agents were obtained as follows: human GIP (1–42) from AnaSpec (Fremont, CA, USA), a dipeptidyl peptidase-4 (DPP-4) inhibitor from Merck Milliproe (Burlington, MA, USA), human [D-Ala2]-GIP from Phoenix Pharmaceuticals (Burlingame, CA, USA), NADPH from Sigma-Aldrich Japan (Shinagawa, Tokyo, Japan), Lucigenin from Tokyo Chemical Industry (Chuo, Tokyo, Japan).
Animal experiments
The design of animal experiments was approved by the Animal Care Committee of Showa University School of Medicine (Approval numbers: 02073). The experiments were conducted under strict adherence to the Guide for the Care and Use of Laboratory Animals as previously described.8,10 Male db/db mice (BKS.Cg-+Leprdb/+Leprdb/Jcl) at 5 weeks of age were purchased from CLEA Japan (Meguro, Tokyo, Japan) and maintained on a standard rodent chow diet. Seven-week-old db/db mice were divided into two groups: a diet-restricted (5 g/day) and free-feeding group. In the subset of mice, we confirmed that diet-restricted db/db mice exhibited similar body weights and plasma glucose levels to non-DM wild-type mice prior to this animal experiments (Supplemental Table 1). Therefore, in this study we used diet-restricted db/db mice as non-DM controls to match the genetic background to that of DM db/db mice, as deficiency of the leptin receptor may affect cardiac hypertrophy. 11 Free-fed db/db mice were further assigned to treatment with vehicle (DM-Vehicle) or GIP (50 nmol/kg/day, DM-GIP). The dose of GIP was determined based on our previous studies.8,10 Vehicle and GIP were continuously infused to mice via osmotic pumps implanted under dorsal skin.8,10 After 6-weeks intervention, the hearts were carefully isolated from surrounding connective tissues, and weighed to calculate heart index as follows: heart weight (mg)/left tibial length (mm). The apexes of isolated hearts were excised and snap-frozen for reverse transcription polymerase chain reaction (RT-PCR) and western blot analyzes, and the remaining heart tissue was immersed in 4% paraformaldehyde for histological analysis.
Measurement of plasma assays and blood pressure
Blood samples were collected after 6 h of fasting, and immediately mixed with a DPP-4 inhibitor to avoid GIP degradation.8,10 Blood levels of glycated hemoglobin (HbA1c) were measured by immunoassay (Roche Diagnostics, Minato, Tokyo). Plasma levels of glucose, and lipids were determined by enzyme electrode (Sanwa Kagaku, Nagoya, Aichi, Japan) and colorimetric (Fuji Film WAKO, Osaka, Osaka, Japan) assays, respectively. Plasma levels of insulin and total GIP were assessed by enzyme-linked immunosorbent assays (Ultrasensitive Insulin ELISA, Product ID: M1105, Morinaga Institute of Biological Science, Yokohama, Kanagawa, Japan, and Mouse GIP EIA kit, Product ID: EIAM-GIP, Ray Biotech, Peachtree Corners, GA, USA, respectively). Systolic and diastolic blood pressure and pulse rates were measured a few days prior to the end of experiments using a non-invasive tail-cuff method as previously described.8,10
Assessment of cardiac hypertrophy and fibrosis
Cardiac hypertrophy and fibrosis were evaluated as previously described. 8 In brief, cryosections of the hearts at the papillary muscle level were stained with Hematoxylin & Eosin (H&E) for the assessment of left ventricular wall thickness and cardiomyocyte sizes and Masson’s trichrome for left ventricular interstitial fibrosis area. Left ventricular wall thickness was determined in the sepal wall. The images were analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
Real-time RT-PCR
Total RNA extracted from tissues and cells were used to synthesize cDNA as previously described.8,10 Quantitative real-time RT-PCR was performed using the TaqMan gene expression assay and sequence detection system (StepOne Plus; Life Technologies Japan, Minato, Tokyo, Japan).8,10 The pre-designed TaqMan probe sets were used as follows: connective tissue growth factor (Ctgf), Mm1192932_g1; myosin heavy chain beta (β-Mhc), Mm00600555_m1; transforming growth factor-beta1 (Tgf-β1), Mm01178820_m1; Tgf-β2, Mm00436955_m1; receptor for advanced glycation end products (Rage), Mm00545815_m1. The 18S ribosomal RNA probe (18s rna, Mm03928990_g1) was used as an internal control.
Western blot analysis
Protein expression levels were determined by western blot analysis as previously described. 10 In brief, 10 μg of proteins extracted from the hearts was electrophoresed in polyacrylamide gels and transferred to polyvinylidene fluoride membranes. The membranes were incubated with blocking reagent for 1 h, primary antibodies overnight, and then secondary antibody for 1 h. The following antibodies were used for western blot analyzes: phosphorylated p38Thr180/Tyr182 (p-p38) (Cell Signaling Technology Japan, Yokohama, Kanagawa, Japan; Product ID: 9211, RRID: AB_331641; rabbit monoclonal antibody; dilution: 1:5000), β-actin (Santa Cruz Biotechnology, Dallas, TX, USA; Product ID: sc-47778; RRID: AB_2714189; dilution: 1:1000), and donkey polyclonal antibody raised against anti-rabbit IgG conjugated with horseradish peroxidase (GE Healthcare Japan; Product ID: NA9341; RRID: AB_772206; dilution: 1:40,000).
Cell culture experiments
Cardiomyocytes isolated from neonatal ICR mice were obtained from Cosmo Bio (Koto, Tokyo, Japan). Cell culture plates were coated with fibronectin (Cosmo bio) prior to the experiments. 8 Cells were seeded onto 24-well plates at the cell density of 8 × 104 per well, and cultured until 80%–90% confluence in DMEM (Gibco, Waltham, MA, USA) containing 10% fetal bovine serum (FBS) and 5.5 mM glucose. After serum starvation for 6 h, cells were pre-treated with or without degradation-resistant [D-Ala2]-GIP (300 nM) for 1 h, and further cultured under normal (5.5 mM) or high (30 mM) glucose conditions for 24 h.8,10 In other experiments, cells were cultured in DMEM containing either 5.5 or 30 mM glucose for 24 h, and cells were serum-starved for 6 h. After incubation with or without [D-Ala2]-GIP (300 nM) for 1 h, the cells were stimulated with or without AGEs (100 μg/mL) for 24 h. 12 The concentration of [D-Ala2]-GIP was determined based on our previous in vitro experiments.8,10 AGEs were prepared from bovine serum albumin as previously described. 13
Measurement of NADPH oxidase-driven superoxide generation
NADPH oxidase-driven superoxide generation was measured by previously described methods with some modification. 12 In brief, cells were lysed with buffer composed of 20 mM HEPES (pH 7.0), 100 mM KCl, 1 mM EDTA, and 1% protease inhibitor mixtures (Nakarai Tesque, Kyoto, Kyoto, Japan). To measure NADPH oxidase activity, the cell lysates were incubated with 50 mM phosphate buffer (pH 7.0) containing 1 mM EGTA, 150 mM sucrose, 250 μM lucigenin, and 100 μM NADPH for 10 min. Luminescence intensity was measured using SpectraMax i3X (Molecular Device, San Jose, CA, USA).
Statistics
Data are expressed as mean ± standard deviation (SD). Statistic comparison was performed using JMP software (version 13; SAS Institute Inc., NC, USA). Comparisons of two and three groups were tested by unpaired t-test and One-way ANOVA followed by Tukey’s test, respectively. Correlation was tested by Pearson correlation coefficient. The significance level was defined as p < 0.05.
Results
GIP suppressed cardiac hypertrophy and interstitial fibrosis in diabetic db/db mice
DM db/db mice (free-feeding group) were treated with vehicle or GIP for 6 weeks. Metabolic parameters are shown in Table 1. Final body weights, heart index, systolic and diastolic blood pressure, blood HbA1c, and plasma glucose and total cholesterol levels were significantly higher in DM mice than non-DM mice (food restriction group). There were no significant differences in biochemical and anthropometric parameters between vehicle and GIP groups of DM mice, except for plasma GIP levels.
Table 1.
Morphological and biochemical measures of non-diabetic and diabetic mice treated with vehicle or GIP.
| Non-DM | DM-vehicle | DM-GIP | |
|---|---|---|---|
| Number | 6 | 6 | 6 |
| Food intake (g/day) | 4.0 ± 0.0 | 7.1 ± 1.2 ** | 7.4 ± 1.7 ** |
| Final body weight (g) | 25.7 ± 0.4 | 41.3 ± 1.9 ** | 41.7 ± 2.2 ** |
| Heart index | 6.1 ± 0.3 | 7.2 ± 0.7 ** | 7.4 ± 0.4 ** |
| Pulse rate (/min) | 528 ± 27 | 557 ± 26 | 539 ± 58 |
| Systolic blood pressure BP (mmHg) | 106 ± 27 | 140 ± 17 * | 130 ± 17 * |
| Diastolic blood pressure (mmHg) | 71 ± 27 | 108 ± 16 * | 105 ± 18 * |
| HbA1c (%) | <4.0 | 11.7 ± 0.3 ** | 11.5±0.5 ** |
| Plasma glucose (mg/dL) | 113 ± 21 | 590 ± 81 ** | 560 ± 80 ** |
| Plasma insulin (ng/mL) | 0.48 ± 0.27 | 0.37 ± 0.21 | 0.24 ± 0.10 |
| Plasma total cholesterol (mg/dL) | 68 ± 6 | 92 ± 10 ** | 86 ± 9 ** |
| Plasma triglycerides (mg/dL) | 115 ± 7 | 151 ± 43 | 119 ± 28 |
| Plasma total GIP (pg/mL) | 2.7 ± 2.2 | 1.5 ± 1.5 | 6.8 ± 4.6 † |
Mean ± SD. * and **, p < 0.05 and p < 0.01 versus Non-DM, respectively.
p < 0.05 versus DM-vehicle. HbA1c levels in non-DM mice were below the detectable limit of assay used, and their values were rounded up to 4.0 for statistical comparisons.
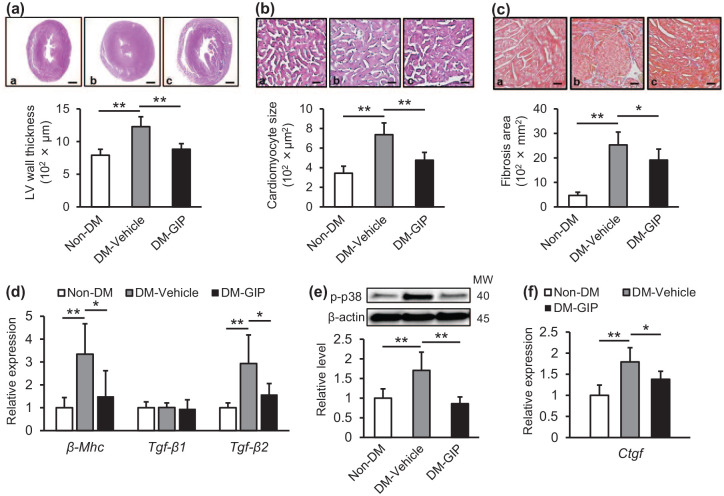
As shown in Figure 1(a)–(d), compared with non-DM mice, DM mice exhibited larger left ventricular wall thickness and cardiomyocyte sizes, and a greater fibrotic area, all of which were accompanied by increased gene expression levels of β-Mhc, a marker of cardiomyocyte hypertrophy, and Tgf-β2, a master regulator of fibrotic responses.9,13 After 6-week intervention, GIP treatment significantly attenuated all of these changes (Figure 1(a)–(d)). There was a positive correlation between cardiac β-Mhc and Tgf-β2 gene expression levels (r = 0.768, p < 0.01). When we evaluated downstream molecules of the TGF-β2 signaling pathway in the hearts, 9 we found that compared with non-DM mice, cardiac protein levels of p-p38 and gene expression levels of Ctgf were significantly increased in DM mice, both of which were prevented by GIP treatment (Figure 1(e) and (f)).
Figure 1.
Effects of GIP treatment on the hearts of diabetic db/db mice: Diabetic db/db mice were treated with vehicle (DM-Vehicle) or GIP (DM-GIP) for 6 weeks. Left ventricular wall thickness (a) and cardiomyocyte sizes (b). Upper panels show representative microscopic images of the left ventricle stained with H&E. (c) Interstitial fibrosis. Upper panels show representative microscopic images of the left ventricle stained with Masson’s trichrome. a–c: a, Non-DM; b, DM-Vehicle; c, DM-GIP. Scale bars: a, 1 mm; b, 0.05 mm; c, 0.1 mm. (d) Cardiac gene expression levels of β-Mhc, Tgf-β1, and Tgf-β2. (e) Cardiac protein levels of p-p38. (f) Cardiac gene expression levels of Ctgf. d–f: Gene expression and protein levels of target molecules were normalized by those of internal control 18 s rna and β-actin, respectively.
Data were shown as relative levels to the control mice. n = 6 per group. *p < 0.05, **p < 0.01.
GIP inhibited NADPH oxidase-driven superoxide generation and reduced β-Mhc and Tgf-β2 mRNA levels in high glucose-exposed cardiomyocytes
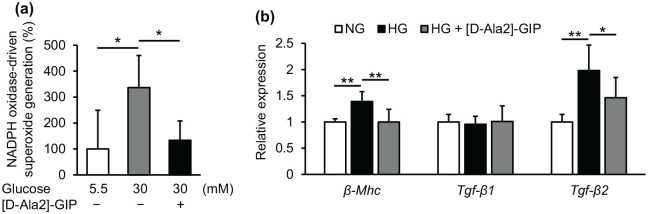
As shown in Figure 2(a) and (b), high glucose significantly increased NADPH oxidase-driven superoxide generation and up-regulated β-Mhc and Tgf-β2 mRNA levels in mouse cultured cardiomyocytes. Pre-treatment with [D-Ala2]-GIP suppressed all of these effects of high glucose on cardiomyocytes (Figure 2(a) and (b)).
Figure 2.
Effects of GIP on NADPH oxidase-driven superoxide generation and gene expression of β-Mhc, Tgf-β1, and Tgf-β2 in mouse cultured cardiomyocytes: Mouse cultured cardiomyocytes were pre-incubated with or without [D-Ala2]-GIP at 300 nM for 1 h, and then cultured under normal glucose (5.5 mM, NG) or high glucose (30 mM HG) for 24 h. (a) NADPH oxidase-driven superoxide generation. (b) Gene expression levels of β-Mhc, Tgf-β1, and Tgf-β2. a: The data were shown as % to the control. b: Gene expression levels of target molecules was normalized by those of internal control 18 s rna, and the data were shown as relative levels to the controls.
n = 4–9 per group. *p < 0.05, **p < .01.
GIP reduced Rage mRNA levels and blocked the effects of AGEs on β-Mhc gene expression in high glucose-exposed cardiomyocytes
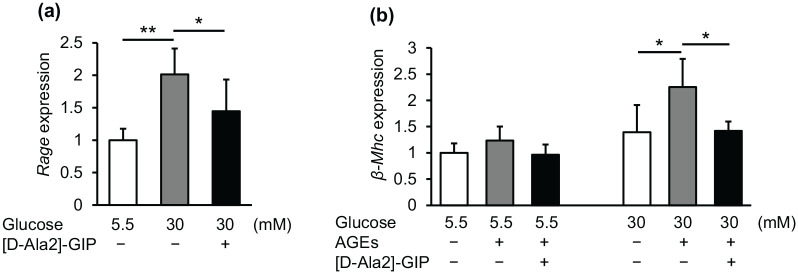
High glucose significantly up-regulated cardiomyocyte Rage mRNA levels and resultantly augmented the AGE-induced β-Mhc gene expression in high glucose-exposed cardiomyocytes, both of which were inhibited by [D-Ala2]-GIP treatment (Figure 3(a) and (b)).
Figure 3.
Effects of GIP on high-glucose-induced Rage gene expression and AGE-induced β-Mhc gene expression in mouse cultured cardiomyocytes: a: mouse cultured cardiomyocytes were pre-incubated with or without [D-Ala2]-GIP at 300 nM for 1 h, and then cultured under normal glucose (5.5 mM, NG) or high glucose (30 mM HG) for 24 h. (a) Gene expression levels of Rage. b: after cultured under normal or high glucose conditions for 24 h, mouse cardiomyocytes were incubated with or without [D-Ala2]-GIP at 300 nM for 1 h, and then stimulated with AGEs (100 μg/mL) for 24 h. (b) Gene expression levels of β-Mhc. Gene expression levels of target molecules was normalized by those of internal control 18 s rna, and the data were shown as relative levels to the controls.
n = 4–9 per group. *p < 0.05, **p < 0.01.
Discussion
An accumulating body of evidence has suggested that DM is associated with an increased risk for heart failure and death,2,3 and that diabetic cardiomyopathy, which is histologically characterized as cardiac hypertrophy and fibrosis, is considered one of the main causes of heart failure in patients with DM. 4 However, so far, no pharmacological therapy has been established to attenuate the progression of DM cardiomyopathy.4,5 In this study, obese and insulin resistant DM mice were found to exhibit left ventricular wall thickening, cardiomyocyte hypertrophy, and interstitial fibrosis compared with non-DM mice. We demonstrated for the first time that a continuous infusion of GIP for 6 weeks significantly inhibited all of these changes without affecting anthropometric and biochemical parameters, including plasma levels of insulin. Exposure to high glucose has been shown to impair the insulinotropic effect of GIP in rodents and humans. 6 However, we have previously reported that vascular effects of GIP are preserved under hyperglycemic conditions in both cell culture and animal models. 7 Therefore, the present findings suggest that GIP may inhibit cardiac hypertrophy and fibrosis in obese and diabetic mice partly through a pleiotropic effect. A previous study has shown that db/db mice at 12 weeks of age developed both systolic and diastolic dysfunction of hearts. 14 Since we could not evaluate the effect of GIP on cardiac function by echocardiography due to technical issues, it remains unclear whether GIP-induced histological improvement in diabetic hears can result in the prevention of systolic and/or diastolic dysfunction.
TGF-β is a multifunctional cytokine, which is secreted from many types of cells and organs in response to various stimuli, including hyperglycemia. 9 In cell culture studies, TGF-β has been shown to induce hypertrophic responses in cardiomyocytes via the TAK-1 pathway and promote extracellular matrix production in cardiac fibroblasts via the Smad2/3 pathway.15,16 In addition, TGF-β secreted from cardiomyocytes has been reported to induce hypertrophic responses by an autocrine mechanism. 17 Furthermore, cardiac hypertrophy is induced in transgenic mice overexpressing TGF-β, which is accompanied with interstitial fibrosis, 18 whereas cardiomyocyte-specific knockout of the TGF-β receptor prevents the development of pressure-overload-induced cardiac hypertrophy and fibrosis. 19 These findings indicate that TGF-β could play a crucial role in the pathogenesis of cardiac hypertrophy and fibrosis. In the present study, we found that mRNA levels of Tgf-β2, an isoform of TGF-β, were significantly up-regulated in the hearts of diabetic mice, and which were strongly associated with those of β-Mhc, a marker of cardiomyocyte hypertrophy. 13 GIP infusion for 6 weeks inhibited up-regulation of mRNA levels of Tgf-β2 and β-Mhc in the hearts of DM mice, which were associated with histological improvement of cardiac hypertrophy and interstitial fibrosis. In addition, GIP infusion prevented cardiac protein levels of p-p38 and mRNA levels of Ctgf in DM mice, which are downstream molecules of the TAK-1 and Smad2/3 pathways evoked by TGF-β2, respectively. Moreover, in mouse cultured cardiomyocytes, high glucose increased mRNA levels of Tgf-β2 and β-Mhc, both of which were inhibited by pre-treatment with a degradation-resistant [D-Ala2]-GIP. Since we have found in the previous and present studies that mouse cardiomyocytes express the GIP receptor 8 and that metabolic parameters in DM db/db mice are not affected by GIP treatment, GIP may directly act on cardiomyocytes and suppress high-glucose-induced gene expression levels of cardiac Tgf-β2, which could partly account for the protective role of GIP against cardiac hypertrophy and interstitial fibrosis in our mouse model.
Oxidative stress plays an important role in the pathogenesis of diabetic complications, including diabetic cardiomyopathy via modulation of redox-sensitive signaling pathways. 20 Exposure to high levels of glucose induces oxidative stress generation via activation of NADPH oxidase in cardiomyocytes, which could induce cellular hypertrophy. 21 Indeed, treatment with an antioxidant N-acetyl-L-cysteine is reported to prevent the progression of cardiac hypertrophy in diabetic mice. 22 In addition, oxidative stress has been shown to increase Tgf-β mRNA expression levels in rat cultured mesangial cells and fibroblasts, 23 whereas overexpression of an antioxidant-enzyme superoxide dismutase-1 decreases TGF-β protein expression in the kidneys of diabetic mice. 24 In this study, we found that high glucose significantly increased NADPH oxidase-driven superoxide generation in mouse cultured cardiomyocytes, changes which were prevented by [D-Ala2]-GIP treatment with concomitant reductions in Tgf-β2 mRNA levels. Among the isoforms of NADPH oxidase, cardiomyocytes have been shown to express NADPH oxidase 2 (NOX2), which is activated by high glucose via protein kinase C β (PKCβ)-dependent mechanisms. 25 A previous study has reported that stimulation of cyclic AMP (cAMP)-protein kinase A pathway prevents PKC-induced NADPH oxidase-driven oxidative stress generation in human neutrophils by inducing the phosphorylation of the NOX2-cytosolic C-terminal region, which results in the inhibition of its assembly with other components of NADPH oxidase. 26 Since we have previously shown that GIP increases cAMP levels in cardiomyocytes, 8 GIP may reduce the NADPH oxidase-driven superoxide generation in high glucose-exposed cardiomyocytes via cAMP-dependent inhibition of NOX2 activity, which could lead to the suppression of Tgf-β2 gene expression. This is an area worthy of further study.
Besides high glucose, AGEs, senescent macroprotein derivatives formed at an accelerated rate under DM conditions also stimulate the generation of oxidative stress in various types of cells through interaction with RAGE via the activation of NADPH oxidase. 27 In addition, RAGE has been shown to be involved in the pathogenesis of cardiac hypertrophy and fibrosis. 28 Indeed, RAGE protein levels are up-regulated in the hearts of mice that have undergone transverse aortic constriction, while knockout of the RAGE gene prevents pressure-overload-induced cardiac hypertrophy and fibrosis in mice. 28 We have previously shown that a cAMP analog 8-Br-cAMP or cAMP-elevating agents, such as GIP and glucagon like peptide-1 down-regulate RAGE mRNA levels in human cultured endothelial and mesangial cells, which results in the suppression of AGE-induced oxidative stress generation in these cell types.29,30 In the present study, we found that high glucose significantly up-regulated Rage gene expression in cardiomyocytes, and subsequently augmented the AGE-induced increase in gene expression of β-Mhc, a marker of cardiac hypertrophy, both of which were attenuated by [D-Ala2]-GIP treatment. Since NADPH oxidase-driven oxidative stress has been involved in up-regulation of RAGE expression in various kinds of cells,27,29,30 the present findings suggest that GIP may suppress the deleterious effects of AGEs on cardiomyocytes by suppressing Rage gene expression in high glucose-exposed cardiomyocytes via cAMP-induced inhibition of NADPH oxidase activity.
Conclusions
Our present findings suggest that GIP may inhibit cardiac hypertrophy and fibrosis in DM mice through the suppression of Tgf-β2 expression via blockade of the harmful effects of high glucose and/or AGEs on cardiomyocytes. GIP may be a potential therapeutic target for diabetic cardiomyopathy.
Supplemental Material
Supplemental material, sj-pdf-1-dvr-10.1177_1479164121999034 for Glucose-dependent insulinotropic polypeptide inhibits cardiac hypertrophy and fibrosis in diabetic mice via suppression of TGF-β2 by Munenori Hiromura, Yusaku Mori, Michishige Terasaki, Hideki Kushima, Tomomi Saito, Naoya Osaka, Hironori Yashima, Makoto Ohara, Tomoyasu Fukui, Takanori Matsui and Sho-ichi Yamagishi in Diabetes & Vascular Disease Research
Footnotes
Authors’ contributions: MH performed experiments, analyzed data, interpreted results of experiments, drafted, and revised manuscript. YM conceived and designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, drafted manuscript, edited, and revised manuscript. MT, HK, TS, NO, HY, MO, TF, TM, SY interpreted results of experiments, and edited and revised manuscript. All authors have approved the final version of the manuscript. YM is the guarantors of this work and are responsible for its integrity.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: YM have received financial supports from MSD Life Science Foundation.
ORCID iDs: Yusaku Mori  https://orcid.org/0000-0002-1734-0605
https://orcid.org/0000-0002-1734-0605
Naoya Osaka  https://orcid.org/0000-0002-9824-8164
https://orcid.org/0000-0002-9824-8164
Tomoyasu Fukui  https://orcid.org/0000-0002-8535-008X
https://orcid.org/0000-0002-8535-008X
Takanori Matsui  https://orcid.org/0000-0001-9506-7571
https://orcid.org/0000-0001-9506-7571
Supplemental material: Supplemental material for this article is available online.
References
- 1. Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol 2019; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004; 27: 699–703. [DOI] [PubMed] [Google Scholar]
- 3. Varela-Roman A, Grigorian Shamagian L, Barge Caballero E, et al. Influence of diabetes on the survival of patients hospitalized with heart failure: a 12-year study. Eur J Heart Fail 2005; 7: 859–864. [DOI] [PubMed] [Google Scholar]
- 4. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 2018; 122: 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Udell JA, Cavender MA, Bhatt DL, et al. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2015; 3: 356–366. [DOI] [PubMed] [Google Scholar]
- 6. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 7. Mori Y, Matsui T, Hirano T, et al. GIP as a potential therapeutic target for atherosclerotic cardiovascular disease-a systematic review. Int J Mol Sci 2020; 21: 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hiromura M, Mori Y, Kohashi K, et al. Suppressive effects of glucose-dependent insulinotropic polypeptide on cardiac hypertrophy and fibrosis in Angiotensin II-Infused mouse models. Circ J 2016; 80: 1988–1997. [DOI] [PubMed] [Google Scholar]
- 9. Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 2011; 51: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mori Y, Kushima H, Koshibu M, et al. Glucose-dependent insulinotropic polypeptide suppresses peripheral arterial remodeling in male mice. Endocrinology 2018; 159: 2717–2732. [DOI] [PubMed] [Google Scholar]
- 11. Karmazyn M, Rajapurohitam V. Leptin as a cardiac pro-hypertrophic factor and its potential role in the development of heart failure. Curr Pharm Des 2014; 20: 646–651. [DOI] [PubMed] [Google Scholar]
- 12. Yamagishi S, Nakamura K, Matsui T, et al. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J Biol Chem 2006; 281: 20213–20220. [DOI] [PubMed] [Google Scholar]
- 13. Schiaffino S, Samuel JL, Sassoon D, et al. Nonsynchronous accumulation of alpha-skeletal actin and beta-myosin heavy chain mRNAs during early stages of pressure-overload-induced cardiac hypertrophy demonstrated by in situ hybridization. Circ Res 1989; 64: 937–948. [DOI] [PubMed] [Google Scholar]
- 14. Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol 2002; 283: H976–H982. [DOI] [PubMed] [Google Scholar]
- 15. Watkins SJ, Borthwick GM, Oakenfull R, et al. Angiotensin II- induced cardiomyocyte hypertrophy in vitro is TAK1-dependent and Smad2/3-independent. Hypertens Res 2012; 35: 393–398. [DOI] [PubMed] [Google Scholar]
- 16. Khalil H, Kanisicak O, Prasad V, et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017; 127: 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlüter KD, Zhou XJ, Piper HM. Induction of hypertrophic responsiveness to isoproterenol by TGF-beta in adult rat cardiomyocytes. Am J Physiol 1995; 269: C1311–1316. [DOI] [PubMed] [Google Scholar]
- 18. Rosenkranz S, Flesch M, Amann K, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 2002; 283: H1253–H1262. [DOI] [PubMed] [Google Scholar]
- 19. Koitabashi N, Danner T, Zaiman AL, et al. Pivotal role of cardiomyocyte TGF-β signalin in the murine pathological response to sustained pressure overload. J Clin Invest 2011; 121: 2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cave AC, Brewer AC, Narayanapanicker A, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 2006; 8: 691–728. [DOI] [PubMed] [Google Scholar]
- 21. He X, Kan H, Cai L, et al. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol 2009; 46: 47–58. [DOI] [PubMed] [Google Scholar]
- 22. Fiordaliso F, Bianchi R, Staszewsky L, et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol 2004; 37: 959–968. [DOI] [PubMed] [Google Scholar]
- 23. Nath KA, Grande J, Croatt A, et al. Redox regulation of renal DNA synthesis, transforming growth factor-beta1 and collagen gene expression. Kidney Int 1998; 53: 367–381. [DOI] [PubMed] [Google Scholar]
- 24. DeRubertis FR, Craven PA, Melhem MF, et al. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide- nitric oxide interaction. Diabetes 2004; 53: 762–768. [DOI] [PubMed] [Google Scholar]
- 25. Prakoso D, De Blasio MJ, Qin C, et al. Phosphoinositide 3-kinase (p110α) gene delivery limits diabetes-induced cardiac NADPH oxidase and cardiomyopathy in a mouse model with established diastolic dysfunction. Clin Sci (Lond) 2017; 131: 1345–1360. [DOI] [PubMed] [Google Scholar]
- 26. Raad H, Mouawia H, Hassan H, et al. The protein kinase A negatively regulates reactive oxygen species production by phosphorylating gp91phox/NOX2 in human neutrophils. Free Radic Biol Med 2020; 160: 19–27. [DOI] [PubMed] [Google Scholar]
- 27. Yamagishi SI. Role of Advanced Glycation Endproduct (AGE)-Receptor for Advanced Glycation Endproduct (RAGE) axis in cardiovascular disease and its therapeutic intervention. Circ J 2019; 83: 1822–1828. [DOI] [PubMed] [Google Scholar]
- 28. Gao W, Zhou Z, Liang B, et al. Inhibiting receptor of advanced glycation end products attenuates pressure overload-induced cardiac dysfunction by preventing excessive autophagy. Front Physiol 2018; 9: 1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishibashi Y, Nishino Y, Matsui T, et al. Glucagon-like peptide-1 suppresses advanced glycation end product-induced monocyte chemoattractant protein-1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 2011; 60: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 30. Ojima A, Matsui T, Maeda S, et al. Glucose-dependent insulinotropic polypeptide (GIP) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm Metab Res 2012; 44: 501–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dvr-10.1177_1479164121999034 for Glucose-dependent insulinotropic polypeptide inhibits cardiac hypertrophy and fibrosis in diabetic mice via suppression of TGF-β2 by Munenori Hiromura, Yusaku Mori, Michishige Terasaki, Hideki Kushima, Tomomi Saito, Naoya Osaka, Hironori Yashima, Makoto Ohara, Tomoyasu Fukui, Takanori Matsui and Sho-ichi Yamagishi in Diabetes & Vascular Disease Research