Abstract
Purpose:
To examine a) whether there are significant differences in gut microbial diversity and in the abundance of gut microbial taxa; and b) differences in predicted functional pathways of the gut microbiome between those participants with high co-occurring symptoms and those with low co-occurring symptoms, prior to neoadjuvant chemotherapy and radiation therapy (CRT) for rectal cancer.
Methods:
Rectal cancer patients (n = 41) provided stool samples for 16 S rRNA gene sequencing and symptom ratings for fatigue, sleep disturbance, and depressive symptoms prior to CRT. Descriptive statistics were computed for symptoms. Gut microbiome data were analyzed using QIIME2, LEfSe, and the R statistical package.
Results:
Participants with high co-occurring symptoms (n = 19) had significantly higher bacterial abundances of Ezakiella, Clostridium sensu stricto, Porphyromonas, Barnesiella, Coriobacteriales Incertae Sedis, Synergistiaceae, Echerichia-Shigella, and Turicibacter compared to those with low co-occurring symptoms before CRT (n = 22). Biosynthesis pathways for lipopolysaccharide, L-tryptophan, and colanic acid building blocks were enriched in participants with high co-occurring symptoms. Participants with low co-occurring symptoms showed enriched abundances of Enterococcus and Lachnospiraceae, as well as pathways for β-D-glucoronosides, hexuronide/hexuronate, and nicotinate degradation, methanogenesis, and L-lysine biosynthesis.
Conclusion:
A number of bacterial taxa and predicted functional pathways were differentially abundant in patients with high co-occurring symptoms compared to those with low co-occurring symptoms before CRT for rectal cancer. Detailed examination of bacterial taxa and pathways mediating co-occurring symptoms is warranted.
Keywords: gut microbiome, rectal cancer, co-occurrence of symptoms, pre-treatment, compositions, biosynthesis/functional pathways, mechanisms
Rectal cancer is one of the most common cancer diagnoses affecting both women and men; more than 43,000 individuals in the United States were diagnosed with rectal cancer in 2020 (Siegel et al., 2020). While preoperative concomitant chemotherapy and radiation therapy (CRT) has improved local control of rectal cancer and survival, it often produces acute adverse effects including gastrointestinal (e.g., diarrhea, nausea, vomiting), micturition problems, sexual dysfunction, pain, as well as behavioral symptoms such as fatigue, sleep disturbance and depressive symptoms with negative impacts on quality of life (Herman et al., 2013; O’Gorman et al., 2014). However, even before CRT treatment begins, patients with rectal cancer experience co-occurring symptoms associated with their initial recent cancer diagnosis, their tumors and/or psychological stress (Gunn et al., 2013; Lee et al., 2018; Tantoy et al., 2016). High pretreatment symptom burden can lead to delays in starting curative therapy, poorer tolerance of radiation or chemoradiation, or exacerbation of expected symptoms during therapy (Gunn et al., 2013). To the best of our knowledge, there is a paucity of literature on symptom co-occurrence of fatigue, sleep disturbance and depressive symptoms reported by rectal cancer patients before treatment initiation. These three symptoms can form a psychoneurological symptom cluster (George et al., 2020). High pretreatment co-occurrence of psychoneurological symptoms can be long-lasting and are also associated with worse quality of life (Tometich et al., 2018). To date, effective interventions targeting co-occurring symptoms are lacking in part because biological mechanisms that lead to co-occurrence of psychoneurological symptoms have not been identified. A better understanding of the biological underpinnings of psychoneurological symptom co-occurrence may lead to effective treatments and/or better symptom management.
The etiology of and mechanisms driving the co-occurrence of cancer-related symptoms remain poorly understood. Recently, the role of maladaptive alterations in gut microbiota composition (referred to as dysbiosis) resulting in unbalanced production of microbiota byproducts has been linked to gut inflammation, aberrant gut-brain communications (Skonieczna-Żydecka et al., 2018), and co-occurrence of symptoms (González-Mercado, Henderson, et al., 2020). Gut dysbiosis can be caused by environmental, psychological and physical stressors (Bajinka et al., 2020; Carter et al., 2019; Clark & Mach, 2016; Galley et al., 2014). Preclinical studies demonstrated that stress leads to dysbiosis and altered hypothalamic-pituitary-adrenal (HPA) axis activity in mice (Neufeld et al., 2011). In rectal cancer patients, we previously reported the association between fatigue or sleep-disturbance scores and gut microbial alpha diversity during CRT (Gonzalez-Mercado et al., 2019; Gonzalez-Mercado, Pérez-Santiago, et al., 2020), as well as significant correlations between the abundances of Bacteroides and Blautia and co-occurring symptoms at the end of CRT (González-Mercado, Henderson, et al., 2020). This pilot study aims to build on our recent work by examining differences in gut microbial diversity (alpha diversity and taxa abundances) between those with high co-occurring symptoms and those with low co-occurring symptoms prior to initiation of CRT. We also investigated the predicted functional pathways of the gut bacterial taxa that differed between those with high and those with low co-occurring symptoms prior to initiation of CRT. Our results provide preliminary evidence of associations of gut dysbiosis with symptom co-occurrence and on phenotypic characteristics of patients at-risk for higher cancer-related symptoms.
Methods
All work was approved by the Southeastern Academic Medical Center prior to data collection. Study participants were newly diagnosed adult rectal cancer patients scheduled to receive CRT, and recruited between July 2017 and April 2019. Informed consent was obtained from each participant. The inclusion criteria are as follows: male and female patients aged ≥18 years scheduled to receive CRT for locally advanced rectal cancer and able to provide written informed consent in Spanish or English. The exclusion criteria included: history of other cancers; history of digestive disorders such as: irritable bowel syndrome; diagnosed psychiatric and/or sleep disorders; comorbidities associated with sleep disturbance (e.g., sleep apnea); and use of insomnia medications, antibiotics, probiotics, prebiotics, steroids, or immune-suppressant agents within 1 month prior to sample-collection. Data from 41 participants who completed the study procedures for the pre-CRT visit were used.
Fatigue was assessed using the seven-item Patient-Reported Outcome Measures Information System-Fatigue (PROMIS-F; scores range 7–35) and sleep disturbance with the eight-item PROMIS-Sleep Disturbance (PROMIS-SD; scores range 8–40). Scores were totaled and divided by the number of items answered to compute the mean score. A conversion table available in the manual was then used to translate this score into a T-score for each participant. The T-score re-scales the raw score into a standardized score with a mean of the US general population equal to 50 and standard deviation of 10 (PROMIS, 2015). Depression was assessed by the clinician-rated 17-item Hamilton Rating Scale for Depression (HAM-D17; Hamilton, 1960), using the conventional Structured Interview Guide for the HAM-D (Williams, 1988) with scores ranging from 0 to 50 for the HAM-D17. For the three instruments, higher scores reflect more severe symptoms. The psychometric properties of the instruments are well documented (Bagby et al., 2004; Buysse et al., 2010; Cella et al., 2002, 2019; Yang et al., 2019). The Cronbach α values for the PROMIS-F, PROMIS-SD, and the HAM-D17 were 0.90, 0.92, and 0.86 at the pre-treatment visit. A cutoff score of ≥15, has been used previously in cancer patients to screen for depression, with higher scores indicating more symptoms of depression (Lydiatt et al., 2008). Study participants were dichotomized into two groups based on the T-scores for the PROMIS and the total score for the HAM-D17. Those with a T-score < 55 in both the PROMIS-F, PROMIS-SD, and < 14 the HAM-D17 were assigned to the “low co-occurring symptoms” (n = 22 or 54% of all participants) group and those with at least two of the three symptom measures exceeding the cut-off for greater symptom severity (i.e., T-score > 55 for either PROMIS measures, ≥15 for the HAM-D17) were assigned to the “high co-occurring symptoms” group (n = 19 or 46% of all participants).
16S rRNA Gene Sequencing
Briefly, total DNA was isolated from 250 mg stool aliquots collected prior to initiation of CRT using the Power Soil DNA Isolation kit (MoBio, Carlsbad, CA), per manufacturer’s protocol. The V3-V4 regions of the 16 S rRNA gene were amplified and sequenced using Illumina’s 16 S rRNA gene library preparation and MiSeq 2× 300 bp sequencing platform. Reads were trimmed at a Q = 25 threshold using Trim Galore! v0.44, denoized into amplicon sequence variants (ASVs) using the DADA2 plugin in QIIME2-2019.17, and the resulting ASVs were taxonomically assigned against the SILVA v132 database using a naïve Bayes classifier. The ASV table was rarefied to 5,929 sequences per sample and used for alpha and beta diversity calculations in QIIME2. MetaCyc (Caspi et al., 2020) pathway abundances were predicted from the rarefied table using QIIME2’s q2-picrust2 plugin (Langille et al., 2013).
Statistical Analysis
Participants were grouped into two subgroups for statistical analyses: those with low co-occurring symptoms and those with two or more (high) co-occurring symptoms reported before CRT. Descriptive statistics including frequency, percentages, medians, and interquartile ranges, were calculated for participant demographic and disease characteristics. We used a two-tailed Fisher’s Exact or Mann-Whitney test to assess statistical differences between group characteristics.
For 16 S rRNA sequencing analysis, the linear discriminant analysis (LDA) effect size (LEfSe) method (Segata et al., 2011) was used to identify bacterial genera significantly different between the co-occurring symptom groups. Metagenomic inference based on MetaCyc pathways was performed on the ASV count table collapsed at the genus level using QIIME2’s q2-picrust2 plugin (Langille et al., 2013). Differentially abundant predicted gene pathways between the co-occurring symptom groups were identified with LEfSe. All statistical tests were performed with a p-value significance threshold of 0.05.
Results
Study participants (n = 41) had a mean age of 60.2 ± 11.4 years and 61% were male. Before CRT, 19 participants (46%) experienced high co-occurring symptoms, while 22 participants (54%) experienced low co-occurring symptom. Participants reporting high co-occurring symptoms did not differ significantly from participants with low co-occurring symptoms by age, pre-treatment hemoglobin levels, or body mass index (BMI) (p > 0.05). Descriptive statistics for participant characteristics and symptom ratings are reported in Table 1.
Table 1.
Demographic and Clinical Characteristics of Study Participants (n = 41).
| Characteristics |
Low Co-Occurring symptom
Group (n = 22) |
High Co-Occurring Symptom
Group (n = 19) |
p-Value* |
|---|---|---|---|
| Sex (male), n (%) | 13 (59) | 12 (63) | |
| Stage, n (%) | |||
| II | 7 (32) | 3 (16) | |
| III | 15 (68) | 16 (84) | |
| Race, n (%) | |||
| Non-Hispanic White | 9 (41) | 10 (54) | |
| Hispanic White | 10 (45) | 7 (38) | |
| African American | 3 (14) | 1 (4) | |
| Asian | 0 | 1 (4) | |
| Marital status, n (%) | |||
| Married/partnered | 16 (72) | 10 (54) | |
| Single/divorced | 6 (28) | 9 (46) | |
| Occupation, n (%) | |||
| Working | 13 (59) | 11 (58) | |
| Retired | 6 (27) | 5 (26) | |
| Disability | 3 (14) | 4 (16) | |
| Pretreatment symptoms exceeding cut-point, n (%) | |||
| All low | 22 (100) | ||
| Fatigue + SD + Depression | 9 (46) | ||
| Fatigue + SD | 7 (38) | ||
| Fatigue + Depression | 3 (16) | ||
| Raw symptom scores | |||
| PROMIS-F; Median (IQR) | 12.0 (10, 16) | 23.0 (21,26) | 0.001 |
| PROMIS-SD; Median (IQR) | 17.5 (12, 22) | 32.0 (29, 36) | 0.001 |
| HAM-D17; Median (IQR) | 6.0 (1,12.5) | 15.5 (5, 19) | 0.06 |
| Age, Median (IQR) | 58.0 (52, 72) | 60.0 (50,60) | 0.80 |
| HgB, Median (IQR) | 13.3 (11.3, 14) | 11.8 (11.2, 13.7) | 0.84 |
| BMI, Median (IQR) | 25.5 (21.9, 27.7) | 26.7 (22.9, 31.8) | 0.36 |
Note. BMI = body mass index; CRT = neoadjuvant chemotherapy and radiation therapy; F = fatigue; HAM-D = Hamilton Rating Scale for Depression; Hgb = hemoglobin levels; IQR = interquartile range; PROMIS = Patient-Reported Outcome Measures Information System; SD = sleep disturbance. Possible raw score ranges for the PROMIS-F, PROMIS-SD, and HAM-D17 are 7–35, 7–35, and 0–50, respectively.
*p-value for two-tailed Fisher’s Exact or Mann-Whitney test.
Bacterial Diversity and Composition
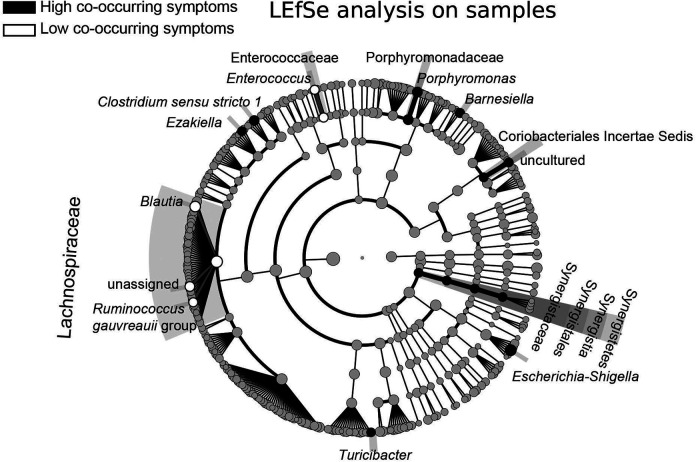
Three of four indices of bacterial alpha diversity (i.e., Shannon, Pielou’s Evenness, Faith’s PD) in stool samples collected from participants in the high co-occurring symptom group were lower than those in the low co-occurring symptom group, but the difference was not statistically significant (Table 2). LEfSe analysis (Segata et al., 2011) showed that participants in the high co-occurring symptom group had significantly higher bacterial abundances of Ezakiella, Clostridium sensu stricto, Porphyromonas and Barnesiella (family Porphyromonadaceae), Coriobacteriales Incertae Sedis, Synergistaceae/Synergistales/Synergistia/Synergistestes, Escherichia-Shigella, and Turicibacter in comparison to participants in the low co-occurring symptom group (Figure 1).
Table 2.
Comparison of Alpha Diversity Indexes Between Participants Who Had Developed High Co-Occurring Symptoms and Those Who Developed Low Co-Occurring Symptoms Before CRT for Rectal Cancer (n = 41).
| Diversity Index |
Low Co-Occurring Symptom
Group (n = 22) |
High Co-Occurring Symptom
Group (n = 19) |
p-Value* |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Shannon | 6.66 | 6.49 | 0.87 |
| Observed OTU | 157.5 | 158 | 0.60 |
| Pielou’s Evenness | 0.91 | 0.89 | 0.28 |
| Faith’s PD | 12.47 | 12.28 | 0.89 |
Note. CRT = chemotherapy and radiation therapy; HAM-D = Hamilton Rating Scale for Depression; OTU = operational taxonomic units, PD = phylogenetic diversity; PROMIS-F = Patient-Reported Outcome Measures Information System-fatigue; PROMIS-SD = Patient-Reported Outcome Measures Information System-sleep disturbance.
*p-value for two-tailed Kruskal-Wallis test.
Figure 1.
Cladogram showing differentially abundant taxa between participants with high co-occurring symptoms and participants with low co-occurring symptoms before chemotherapy and radiation therapy (CRT), based on linear discriminant analysis (LDA) for effect size. Bacterial taxa significantly enriched (p < 0.05) in participants with high-occurring symptoms were indicated with black nodes, while those enriched in participants with low co-occurring symptoms were indicated with white nodes.
Predicted Functional Pathways
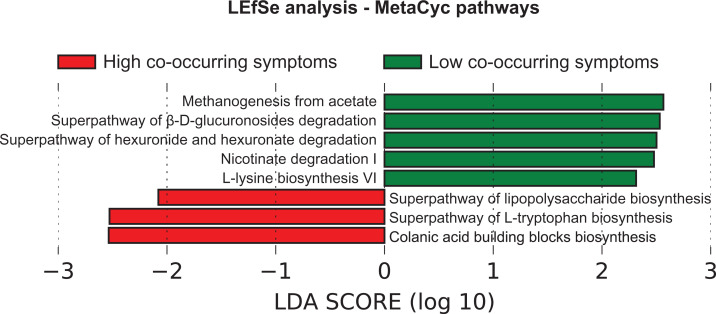
In addition to examination of bacterial abundances, the functional potential (i.e., the impact of the production of physiologically active synthesis products produced by gut bacterial taxa) estimated from the 16 S rRNA gene content in the participants’ gut was done. MetaCyc pathway abundances were predicted from bacterial general abundances (Langille et al., 2013). LEfSe analysis (Segata et al., 2011) on MetaCyc pathway abundances indicated that participants with high co-occurring symptoms were enriched for lipopolysaccharide (LPS), L-tryptophan, and colanic acid building blocks biosynthesis pathways (Kruskal-Wallis p < 0.05). The low co-occurring symptom group were enriched for β-D-glucoronosides, hexuronide/hexuronate, and nicotinate degradation pathways, as well as pathways for methanogenesis and L-lysine biosynthesis (Kruskal-Wallis p < 0.05; Figure 2).
Figure 2.
Predicted MetaCyc pathways that were differentially abundant between participants with high-occurring symptoms and participants with low co-occurring systems before chemotherapy and radiation therapy (CRT), based on linear discriminant analysis (LDA) for effect size.
Discussion
Why some rectal cancer patients report experiencing co-occurring symptoms prior to treatment while others do not is an unanswered question. Although the reasons for co-occurring symptoms before treatment are thought to be multifactorial (Gunn et al., 2013; Langford et al., 2016; Lin et al., 2020), prior work by our group showed an association between gut microbial composition and co-occurring symptoms during CRT (González-Mercado, Henderson, et al., 2020). We conducted the current prospective clinical study to investigate whether there are significant differences in gut microbial diversity, taxonomic composition, and predicted functional pathways between rectal cancer patients with high as compared to low co-occurring symptoms before CRT. While our findings must be treated as preliminary until they are independently replicated, our findings suggest that abundance of some gut microbial taxa and predicted pathways may play a role in symptom occurrence prior to treatment for rectal cancer. Although LEfSe analysis (Segata et al., 2011) predicted several candidate taxa and pathways that were enriched in participants with high or low co-occurring symptoms, we focus our discussion on taxa and pathways that were either intercorrelated or taxa/pathways previously shown to be relevant to gut microbiome functions.
Low Bacterial Diversity Indices Do Not Associate With Co-Occurring Symptoms
Recent evidence by our group (González-Mercado et al. 2019; González-Mercado, Pérez-Santiago, et al., 2020) and others (Wang et al., 2015) suggests that gut microbial dysbiosis may play an important role in the pathogenesis of common symptoms including depressive symptoms, sleep disturbance, and fatigue. In this study, we observed no significant differences in microbial alpha diversity between rectal cancer patients with high as compared to low co-occurring symptoms before CRT. Species richness and evenness, except for observed OTUs, were slightly lower in patients with co-occurring symptoms. This observation is also consistent with our preliminary data in which we observed no significant differences in alpha diversity in rectal cancer patients at the end of CRT with high as compared to those with low co-occurring symptom severity (González-Mercado, Henderson, et al. 2020). In contrast, we (González-Mercado, Pérez-Santiago, et al., 2020) and others (Kleiman et al., 2015; Wang et al., 2015) have also observed reduced fecal microbiome diversity in associated with fatigue, depressive symptoms, or sleep disturbance among oncology patients. Although gut microbiota perturbation is typically associated with a lower species diversity, this is not always the case. In some cases, an increase in the low abundance species (Triplett et al., 2020), resulting in altered composition or abundance while diversity is maintained.
Participants With High Co-Occurring Symptoms Have Greater Abundance of Gammaproteobacteria Genera and Predicted Lipopolysaccharide Functional Pathways
We observed increased abundances of Gram-negative anaerobic or facultative anaerobic bacteria, specifically Escherichia-Shigella (phylum Proteobacteria) and Synergistaceae in participants with high compared to low co-occurring symptoms. We previously observed higher abundances of Escherichia in patients reporting CRT-related fatigue in a small sample (n = 13) of rectal cancer participants (González-Mercado, Pérez-Santiago, et al., 2020). Escherichia–Shigella IgA coating index was positively correlated with anxiety and depression in individuals with chronic inflammatory disease (Liu, Yuan, et al., 2020). Induction of Proteobacteria is a potential hallmark of intestinal inflammation (Kaser et al., 2010), which may facilitate the translocation of bacteria and microbial-mediated metabolites (e.g., LPS) into systemic circulation, inducing aberrant activation of the immune system that can influence neural processes and contribute to the development of cancer-related symptoms (Jakobsson et al., 2010; Jordan et al., 2018; Rea et al., 2020). Future research focusing on host and bacterial markers indicative of inflammation (e.g., C-reactive protein, tumor necrosis factor-alpha [TNFα], interleukin (IL)-6, IL-1β)) and intestinal permeability (e.g. fatty acid binding protein, fecal calprotectin) will be instrumental in connecting gut microbial activity with gut permeability, brain function and co-occurring symptoms.
Consistent with the enriched presence of Gram-negative bacteria in the gut of participants with high co-occurring symptoms, biosynthetic pathways for LPS, a component of the Gram-negative bacterial outer membrane, and colanic acid, an extracellular polysaccharide specific to Enterobacteriaceae and Proteobacteria (Nikaido, 2003), were similarly enriched in this group of participants. Biosynthetic pathways for LPS have been widely characterized in E. coli (Bertani & Ruiz, 2018), and the development of inhibitors of LPS biogenesis are being considered to treat infections caused by Gram-negative bacteria (Bertani & Ruiz, 2018). Also, LPS, present solely in Gram-negative bacteria, has been proposed as a marker of microbial translocation (Giloteaux et al., 2016). Among oncology patients, elevated serum LPS levels was associated with the development of diarrhea and worsening of fatigue after pelvic RT (Wang et al., 2015). Enrichment of biosynthetic pathways for LPS production have also been associated with depressive symptoms among young adults (Liu, Rowan-Nash, et al., 2020). Of central relevance to the finding that enrichment in biosynthetic pathways that produce LPS is the fact that many studies have now demonstrated that LPS is capable of inducing sickness behavior and the constellation of symptoms experience in oncology patients, including disrupted sleep, fatigue, and depressive symptoms (Lasselin et al., 2020). Interestingly, previous studies using shotgun metagenomic sequencing of stool samples from colorectal cancer patients revealed the positive correlation between the abundances of certain bacteria (e.g., Porphyromonas found in our study) and LPS biosynthetic pathways (Dai et al., 2018). This suggests that cancer and its treatment can exacerbate the occurrence and severity of symptoms in part via microbial dysbiosis.
Participants With High Co-Occurring Symptoms Have Greater Abundance of Turicibacter Genera and Potential Enrichment of the Biosynthetic Pathway for L-Tryptophan
Abundance of the Turicibacter genus, which were recently linked to depression (Kelly et al., 2016), was also identified among participants with high as compared to low co-occurring symptoms. Some strains in the genus Turicibacter (e.g. Turicibacter sanguinis) have been associated with increased fecal serotonin levels (Hoffman & Margolis, 2020), a neurotransmitter involved in a wide range of functions in the central nervous system and periphery. Serotonin dysregulation has been proposed as a mechanism contributing to cancer-related symptoms including fatigue, sleep disturbance, and depression (O’Higgins et al., 2018). It is increasingly clear that gut neurocompetent metabolites, including serotonin, can affect gut-brain axis signaling centrally and impact symptoms (Skonieczna-Żydecka, et al., 2020). One hypothesis is that low levels of serotonin (5-HT) in the brain may occur as a result of elevated proinflammatory cytokines activating the tryptophan- and 5-HT degrading enzyme indoleamine2,3-dioxygenase. This may lead to depressed HPA axis activity and cortisol activity, possibly causing cancer related-sleep disturbances and mood disorders (O’Higgins, 2018).
Enrichment in the biosynthetic pathway for L-tryptophan was also observed in participants with high co-occurring symptoms. Tryptophan is a serotonergic precursor (Desbonnet et al., 2008). Because tryptophan biosynthesis activity is negatively regulated by tryptophan (Yanofsky, 1981), the enrichment of tryptophan biosynthesis pathways in participants with high co-occurring symptoms may reflect low tryptophan levels in the gut. This finding, if validated in an independent cohort and extended by measurement of peripheral serotonin, would have important implications for symptom management. Evidence of improvements in a wide variety of conditions related to serotonin imbalance (e.g. depression, insomnia, fibromyalgia) have been reported after treatment with the serotonin precursor, 5-hydroxytryptophan (5HTP) (Birdsall, 1998; Mora-Villalobos & Zeng, 2018). Additionally, some evidence from animal models suggest that the consumption of probiotics, such as Bifidobacteria infantis, can increase plasma levels of tryptophan and reduce levels of pro-inflammatory cytokines such as IL-6 and TNFα with evidence of an antidepressant effect (Desbonnet et al., 2008). Clinical trials of probiotics and/or pharmacology interventions targeting the biosynthetic pathway for L-tryptophan may offer innovative tools for prophylaxis of cancer-related symptoms.
Participants With Low Co-Occurring Symptoms Have Greater Abundance of Lachnospiraceae Family and Genus Enterococcus and Predicted Lysine Biosynthesis Functional Pathways
Consistent with our prior study, the present study found a significantly higher abundance of the Lachnospiraceae family and genera Blautia and Ruminococcus in the stool of participants from the low co-occurring symptom group (González-Mercado, Henderson, et al., 2020). This is a relevant factor as, according to Markowiak-Kopeć and Śliżewska (2020), Blautia and Ruminococcus are predominant genera involved in the production of short-chain fatty acids (SCFAs). SCFAs, such as acetate, propionate, and butyrate (90%–95% of the SCFAs present in the colon), are metabolites formed by gut microbiota from complex dietary carbohydrates that play a pivotal role in maintaining homeostasis in humans (Parada Venegas et al., 2019; Vinolo, Rodrigues, Hatanaka, et al., 2011). Besides their important role as fuel for intestinal epithelial cells, SCFAs regulate several leukocyte functions, including the suppression of pro-inflammatory cytokine production (Park et al., 2007; Vinolo, Rodrigues, Hatanaka, et al., 2011). Indeed, animal studies have shown that propionate and butyrate inhibit the expression of pro-inflammatory mediators (e.g., TNF-α) in rat neutrophils, an effect that seems to involve attenuation of NF-κB activation (Vinolo, Rodrigues, Hatanaka, et al., 2011; Vinolo, Rodrigues, Nachbar, & Curi, 2011) while butyrate administration has been associated to higher levels of the anti-inflammatory cytokine IL-10 (Vinolo, Rodrigues, Nachbar, & Curi, 2011; Zhang et al., 2016). Other investigations have shown that butyrate and valproic acid (inhibitors of histone deacetylase [HDAC] activity) appear to exert promising anti-neuroinflammatory and neuroprotective effects in the central nervous system (H. J. Kim et al., 2007; Singh et al., 2014). Also, there is evidence from in vitro models that SCFAs (e.g., hexanoic and octanoic acids) inhibit the development of pathogenic microorganisms such as Escherichia coli likely due to the damaging effects of SCFAs on Escherichia coli membranes (Rodríguez-Moyá, & Gonzalez, 2015; Royce et al., 2013). As such, it is plausible that higher abundance of SCFA-producing bacteria (Blautia and Ruminococcus) may be a critical element in the gut microbiome of rectal cancer patients as it may trigger the strong anti-inflammatory response associated with lower symptoms.
Recently, the effectiveness of therapeutics and nutritional interventions containing SCFAs or their derivatives in the treatment of behavioral symptoms and in inflammatory conditions has been a subject of extensive investigations and reviews (Stilling et al., 2016; Vinolo, Rodrigues, Nachbar, & Curi, 2011). One study of health volunteers (n = 30) suggest that consumption of γ-aminobutyric acid (GABA)-containing beverages was associated with a significant reduction of both psychological and physical fatigue and improvement in task-solving ability (Kanehira et al., 2011). Neuropharmacological agents containing butyrate (e.g., GABA) are being tested for its anti-fatigue (Chen et al., 2016) and sleep quality improving (S. Kim et al., 2019) effects. Beta-hydroxybutyrate has been examined for the treatment of depressive symptoms in animal models (Yamanashi et al., 2017). Administration of sodium propionate (also a SCFA) induces antidepressant-like effects in animal models (Li et al., 2018). Interestingly, the stool from participants with low co-occurring symptoms had a greater abundance of the genus Enterococcus, which includes strains (e.g., Enterococcus faecium) that have been associated with the production of an acidic environment (Hanchi et al., 2018), shown to have beneficial effects (e.g., protection of the intestinal villi morphology, improvements in immunity, and optimization of the intestinal flora) when used in conjunction with Clostridium butyricum as probiotics in weaned piglets (Wang et al., 2019). Enterococcus and Porphyromonadaceae abundances may also be affected by rectal cancer itself or by treatment side effects. For example, abundance of the genera Enterococcus, which includes strains (e.g., Enterococcus faecalis) and the family Porphyromonadaceae (abundant in high co-occurring symptoms participants) has been previously associated with colorectal cancer (Balamurugan et al., 2008).
Participants with low co-occurring symptoms had a greater abundance of taxa predicted to be enriched in lysine biosynthesis pathways. The fact that the lysine pathway is one of the main pathways for butyrate synthesis suggests that amino acids could also play an important role in butyrate synthesis (Vital et al., 2014). Metagenomic analysis revealed that a high percentage of publicly available genomes contained a butyrate-producing pathway (mean, 19.1%; range, 3.2% to 39.4%), with the lysine pathway (mean, 11.2%) being among the most prevalent ones (Vital et al., 2014). Further, the conversion of lysine to the beneficial SCFAs butyrate and acetate by Intestinimonas AF211 in a synthetic medium have been demonstrated (Bui et al., 2015). These findings also have potential implications for future interventional studies since L-lysine and L-arginine supplementation is effective in treating anxiety-related conditions in humans (Lakhan & Vieira, 2010). Of note, lysine and leucine supplementation significantly reduced fatigue in animal models after treadmill stress test and forced swimming test (H. Y. Kim et al., 2016).
Limitations
The main limitation of the study is its modest sample size, which limited the sophistication of the statistical modeling permitted (i.e., controlling for potential covariates such as age, sex, body mass index). Replication of these findings in a larger, independent sample may permit more comprehensive analyses of gut microbiome measures in relation to symptoms in this population. If replicated, similar studies of microbial dysbiosis in relation to other symptoms and cancers would be warranted. Another limitation is the insufficient resolution of bacterial species with 16 S rRNA gene sequence analysis. Future studies employing shotgun metagenomic sequencing may resolve gut bacterial taxa beyond the genus level. Additionally, pathway predictions based on 16 S rRNA data may not provide an accurate overview of the functional potential and expressed functions of the gut microbiome. These predictions should be validated through metagenomics, metatranscriptomics, and other experimental approaches. Our study and future follow-up studies could inform the development of biomarkers for the diagnosis and management of pre-treatment symptom burden.
Conclusion
This pilot study highlights, for the first time, significant differences in gut microbial communities between participants with high co-occurring symptoms and those with low co-occurring symptoms before CRT for rectal cancer. We observed enriched abundances of pro-inflammatory gram-negative bacterial taxa, including Escherichia-Shigella and Synergistaceae and pathways related to LPS biosynthesis in patients with co-occurring symptoms. Patients with low co-occurring symptoms showed increased abundances of SCFA-producing Lachnospiraceae and Enterococcus and pathways related to bacterial growth on carbon substrates. Large-scale, prospective studies that include species and pathway identification are needed to verify the clinical significance of our findings.
Acknowledgments
We thank all the staff at Tampa General Hospital Cancer Center, the Cancer Care team at AdventHealthTampa, and the St. Joseph’s Hospital Cancer Institute for the collaborative clinical recruitment support. This study would not have been possible without the participants. We also thank the Biobehavioral Laboratory at University of South Florida’s College of Nursing for Illumina sequencing support.
Footnotes
Author Contributions: Gonzalez-Mercado, V. contributed to conception and design, contributed to acquisition and interpretation, drafted manuscript, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Lim, J. contributed to design, contributed to analysis and interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Yu, G contributed to design, contributed to analysis and interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Penedo, F. contributed to interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Bernabe, R. contributed to interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Tirado-Gomez, M. contributed to interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Pedro, E. contributed to acquisition, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Aouizerat, B. contributed to conception and design, contributed to interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was made possible by the National Institute of Nursing Research (NINR) of the National Institutes of Health (NIH) under award number F32NR016618. Research reported in this publication was supported by the University of Puerto Rico NIH–funded awards 2U54MD007587 and CA096297/CA096300. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Velda J. González-Mercado  https://orcid.org/0000-0002-7236-0182
https://orcid.org/0000-0002-7236-0182
References
- Bagby R. M., Ryder A. G., Schuller D. R., Marshall M. B. (2004). The Hamilton Depression Rating Scale: Has the gold standard become a lead weight? American Journal of Psychiatry, 161(12), 2163–2177. 10.1176/appi.ajp.161.12.2163 [DOI] [PubMed] [Google Scholar]
- Bajinka O., Tan Y., Abdelhalim K. A., Özdemir G., Qiu X. (2020). Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express, 10(1), 130. 10.1186/s13568-020-01066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan R., Rajendiran E., George S., Samuel G. V., Ramakrishna B. S. (2008). Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. Journal of Gastroenterology and Hepatology, 23(8 Pt 1), 1298–1303. 10.1111/j.1440-1746 [DOI] [PubMed] [Google Scholar]
- Bertani B., Ruiz N. (2018). Function and biogenesis of lipopolysaccharides. EcoSal Plus, 8(1). 10.1128/ecosalplus.ESP-0001-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall T. C. (1998). 5-Hydroxytryptophan: A clinically-effective serotonin precursor. Alternative Medicine Review, 3(4), 271–280. [PubMed] [Google Scholar]
- Bui T. P., Ritari J., Boeren S., de Waard P., Plugge C. M., de Vos W. M. (2015). Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nature Communications, 6, 10062. 10.1038/ncomms10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D. J., Yu L., Moul D. E., Germain A., Stover A., Dodds N. E., Johnston K. L., Shablesky-Cade M. A., Pilkonis P. A. (2010). Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep, 33(6), 781–792. 10.1093/slee [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. J., Hunter G. R., Blackston J. W., Liu N., Lefkowitz E. J., Van Der Pol W. J., Morrow C. D., Paulsen J. A., Rogers L. Q. (2019). Gut microbiota diversity is associated with cardiorespiratory fitness in post-primary treatment breast cancer survivors. Experimental Physiology, 104(4), 529–539. 10.1113/ep087404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R., Billington R., Keseler I. M., Kothari A., Krummenacker M., Midford P. E., Ong W. K., Paley S., Subhraveti P., Karp P. D. (2020). The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Research, 48(D1), D445–D453. 10.1093/nar/gkz862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Choi S. W., Condon D. M., Schalet B., Hays R. D., Rothrock N. E., Yount S., Cook K. F., Gershon R. C., Amtmann D., DeWalt D. A., Pilkonis P. A., Stone A. A., Weinfurt K., Reeve B. B. (2019). PROMIS(®) Adult health profiles: Efficient short-form measures of seven health domains. Value Health, 22(5), 537–544. 10.1016/j.jval.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Eton D. T., Lai J. S., Peterman A. H., Merkel D. E. (2002). Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. Journal of Pain Symptom Manage, 24(6), 547–561. 10.1016/s0885-3924(02)00529 [DOI] [PubMed] [Google Scholar]
- Chen H., He X., Liu Y., Li J., He Q., Zhang C., Wei B., Zhang Y., Wang J. (2016). Extraction, purification and anti-fatigue activity of γ-aminobutyric acid from mulberry (Morus alba L.) leaves. Scientific Reports, 6, 18933. 10.1038/srep18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Mach N. (2016). Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. Journal of the International Society of Sports Nutrition, 13, 43. 10.1186/s12970-016-0155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Coker O. O., Nakatsu G., Wu W. K. K., Zhao L., Chen Z., Chan F. K. L., Kristiansen K., Sung J. J. Y., Wong S. H., Yu J. (2018). Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome, 6(1), 70. 10.1186/s4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L., Garrett L., Clarke G., Bienenstock J., Dinan T. G. (2008). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of Psychiatric Research, 43(2), 164–174. 10.1016/j.jpsychires.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Galley J. D., Yu Z., Kumar P., Dowd S. E., Lyte M., Bailey M. T. (2014). The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes, 5(6), 748–760. 10.4161/19490976.2014.972241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M. A., Lustberg M. B., Orchard T. S. (2020). Psychoneurological symptom cluster in breast cancer: The role of inflammation and diet. Breast Cancer Research and Treatment, 184(1), 1–9. 10.1007/s10549-020-05808-x [DOI] [PubMed] [Google Scholar]
- Giloteaux L., Goodrich J. K., Walters W. A., Levine S. M., Ley R. E., Hanson M. R. (2016). Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome, 4(1), 30. 10.1186/s40168-016-0171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mercado V. J., Henderson W. A., Sarkar A., Lim J., Saligan L. N., Berk L., Dishaw L., McMillan S., Groer M., Sepehri F., Melkus G. D. (2020). Changes in gut microbiome associated with co-occurring symptoms development during chemo-radiation for rectal cancer: A proof of concept study. Biological Research for Nursing, 1099800420942830. 10.1177/1099800420942830 [DOI] [PMC free article] [PubMed]
- González-Mercado V. J., Pérez-Santiago J., Lyon D., Dilán-Pantojas I., Henderson W., McMillan S., McMillan S., Groer M., Kane B., Marrero S., Pedro E., Saligan L. N. (2020). The role of gut microbiome perturbation in fatigue induced by repeated stress from chemoradiotherapy: A proof of concept study. Advances in Medicine, 2020, 6375876. 10.1155/2020/6375876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mercado V. J., Sarkar A., Penedo F. J., Pérez-Santiago J., McMillan S., Marrero S. J., Marrero-Falcón M. A., Munro C. L. (2019). Gut microbiota perturbation is associated with acute sleep disturbance among rectal cancer patients. Journal of Sleep Research, 29(3), e12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn G. B., Mendoza T. R., Fuller C. D., Gning I., Frank S. J., Beadle B. M., Hanna E. Y., Lu C., Cleeland C. S, Rosenthal D. I. (2013). High symptom burden prior to radiation therapy for head and neck cancer: A patient-reported outcomes study. Head and Neck, 35(10), 1490–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchi H., Mottawea W., Sebei K., Hammami R. (2018). The genus enterococcus: Between probiotic potential and safety concerns-an update. Frontiers in Microbiology, 9, 1791. 10.3389/fmicb.2018.01791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. M., Narang A. K., Griffith K. A., Zalupski M. M., Reese J. B., Gearhart S. L., Azad N. S., Chan J., Olsen L., Efron J. E., Lawrence T. S., Ben-Josef E. (2013). The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. International Journal of Radiation Oncology, Biology, Physics, 85(1), e15–19. 10.1016/j.ijrobp.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. M., Margolis K. G. (2020). Building community in the gut: A role for mucosal serotonin. Nature Reviews Gastroenterology & Hepatology, 17(1), 6–8. 10.1038/s415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson S., Ahlberg K., Taft C., Ekman T. (2010). Exploring a link between fatigue and intestinal injury during pelvic radiotherapy. Oncologist, 15(9), 1009–1015. 10.1634/theoncologist.2010-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K. R., Loman B. R., Bailey M. T., Pyter L. M. (2018). Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer, 124(20), 3990–3999. 10.1002/cncr.31584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehira T., Nakamura Y., Nakamura K., Horie K., Horie N., Furugori K., Sauchi Y., Yokogoshi H. (2011). Relieving occupational fatigue by consumption of a beverage containing γ-amino butyric acid. Journal of Nutritional Science and Vitaminology (Tokyo), 57(1), 9–15. 10.3177/jnsv.57.9 [DOI] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R. S. (2010). Inflammatory bowel disease. Annual Review of Immunology, 28, 573–621. 10.1146/annurev-immunol-030409-101225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. R., Borre Y., O’ Brien C., Patterson E., El Aidy S., Deane J., Kennedy P. J., Beers S., Scott K., Moloney G., Hoban A. E., Scott L., Fitzgerald P., Ross P., Stanton C., Clarke G., Cryan J. F., Dinan T. G. (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatry Research, 82, 109–118. 10.1016/j.jpsychires.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Kim H. J., Rowe M., Ren M., Hong J. S., Chen P. S., Chuang D. M. (2007). Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. The Journal of Pharmacology and Experimental Therapeutics, 321(3), 892–901. 10.1124/jpet.107.120188 [DOI] [PubMed] [Google Scholar]
- Kim H. Y., Han N. R., Kim N. R., Lee M., Kim J., Kim C. J., Jeong H. J., Kim H. M. (2016). Effect of fermented porcine placenta on physical fatigue in mice. Experimental Biology and Medicine (Maywood), 241(17), 1985–1996. 10.1177/1535370216659945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jo K., Hong K. B., Han S. H., Suh H. J. (2019). GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharmaceutical Biology, 57(1), 65–73. 10.1080/13880209.2018.1557698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman S. C., Watson H. J., Bulik-Sullivan E. C., Huh E. Y., Tarantino L. M., Bulik C. M., Carroll I. M. (2015). The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosomatic Medicine, 77(9), 969–981. 10.1097/psy.0000000000000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan S. E., Vieira K. F. (2010). Nutritional and herbal supplements for anxiety and anxiety-related disorders: Systematic review. Nutrition Journal, 9, 42. 10.1186/1475-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D. J., Paul S. M., Cooper B., Kober K. M., Mastick J., Melisko M., Levine J. D., Wright F., Hammer M. J., Cartwright F., Lee K. A., Aouizerat B. E., Miaskowski C. (2016). Comparison of subgroups of breast cancer patients on pain and co-occurring symptoms following chemotherapy. Support Care Cancer, 24(2), 605–614. 10.1007/s00520-015-2819-1 [DOI] [PubMed] [Google Scholar]
- Langille M. G., Zaneveld J., Caporaso J. G., McDonald D., Knights D., Reyes J. A., Clemente J. C., Burkepile D. E., Vega Thurber R. L., Knight R., Beiko R. G., Huttenhower C. (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology, 31(9), 814–821. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J., Schedlowski M., Karshikoff B., Engler H., Lekander M., Konsman J. P. (2020). Comparison of bacterial lipopolysaccharide-induced sickness behavior in rodents and humans: Relevance for symptoms of anxiety and depression. Neuroscience and Biobehavioral Reviews, 115, 15–24. 10.1016/j.neubiorev.2020.05.001 [DOI] [PubMed] [Google Scholar]
- Lee M. S., Tyson D. M., Gonzalez B. D., Small B. J., Lechner S. C., Antoni M. H., Vinard A., Krause M., Meade C., Jacobsen P. B. (2018). Anxiety and depression in Spanish-speaking Latina cancer patients prior to starting chemotherapy. Psychooncology, 27(1), 333–338. 10.1002/pon.4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hou L., Wang C., Jia X., Qin X., Wu C. (2018). Short term intrarectal administration of sodium propionate induces antidepressant-like effects in rats exposed to chronic unpredictable mild stress. Frontiers in Psychiatry, 9, 454. 10.3389/fpsyt [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Docherty S. L., Porter L. S., Bailey D. E. (2020). Common and Co-occurring symptoms experienced by patients with gastric cancer. Oncology Nursing Forum, 47(2), 187–202. 10.1188/20.onf.187-202 [DOI] [PubMed] [Google Scholar]
- Liu R. T., Rowan-Nash A. D., Sheehan A. E., Walsh R. F. L., Sanzari C. M., Korry B. J., Belenky P. (2020). Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behavior and Immunity, 88, 308–324. 10.1016/j.bbi.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yuan X., Li L., Lin L., Zuo X., Cong Y., Li Y. (2020). Increased ileal immunoglobulin a production and immunoglobulin a-coated bacteria in diarrhea-predominant irritable bowel syndrome. Clinical and Translational Gastroenterology, 11(3), e00146. 10.14309/ctg.0000000000000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiatt W. M., Denman D., McNeilly D. P., Puumula S. E., Burke W. J. (2008). A randomized, placebo-controlled trial of citalopram for the prevention of major depression during treatment for head and neck cancer. Archives Otolaryngology Head Neck Surgery, 134(5), 528–535. 10.1001/archotol.134.5.528 [DOI] [PubMed] [Google Scholar]
- Markowiak-Kopeć P., Śliżewska K. (2020). The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients, 12(4). 10.3390/nu12041107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Villalobos J. A., Zeng A. P. (2018). Synthetic pathways and processes for effective production of 5-hydroxytryptophan and serotonin from glucose in Escherichia coli. Journal of Biological Engineering, 12, 3. 10.1186/s13036-018-0094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K. M., Kang N., Bienenstock J., Foster J. A. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology & Motility, 23(3), 255–264, e119. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Nikaido H. (2003). Molecular basis of bacterial outer membrane permeability revisited. Microbiology and Molecular Biology Reviews, 67(4), 593–656. 10.1128/mmbr.67.4.593-656.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman C., Denieffe S., Gooney M. (2014). Literature review: Preoperative radiotherapy and rectal cancer—Impact on acute symptom presentation and quality of life. Journal of Clinical Nursing, 23(3–4), 333–351. 10.1111/jocn.12138 [DOI] [PubMed] [Google Scholar]
- O’Higgins C. M., Brady B., O’Connor B., Walsh D., Reilly R. B. (2018). The pathophysiology of cancer-related fatigue: Current controversies. Support Care Cancer, 26(10), 3353–3364. 10.1007/s00520-018-4318-7 [DOI] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M. K., Landskron G., González M. J., Quera R., Dijkstra G., Dijkstra G., Harmsen H. J. M., Faber K. N., Hermoso M. A. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology, 10, 277. 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Lee E. J., Lee J. C., Kim W. K., Kim H. S. (2007). Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-kappaB and ERK signaling pathways. International Immunopharmacology, 7(1), 70–77. 10.1016/j.intimp.2006.08.015 [DOI] [PubMed] [Google Scholar]
- PROMIS Scoring Manuals.(2015). PROMIS Fatigue Scoring Manual 2015. https://www.assessmentcenter.net/documents/PROMIS%20Fatigue%20Scoring%20Manual.pdf
- Rea K., Dinan T. G., Cryan J. F. (2020). Gut microbiota: A perspective for psychiatrists. Neuropsychobiology, 79(1), 50–62. 10.1159/000504495 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moyá M., Gonzalez R. (2015). Proteomic analysis of the response of Escherichia coli to short-chain fatty acids. Journal of Proteomics, 122, 86–99. 10.1016/j.jprot. [DOI] [PubMed] [Google Scholar]
- Royce L. A., Liu P., Stebbins M. J., Hanson B. C., Jarboe L. R. (2013). The damaging effects of short chain fatty acids on Escherichia coli membranes. Applied Microbiology and Biotechnology, 97(18), 8317–8327. 10.1007/s00253-013-5113-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., Huttenhower C. (2011). Metagenomic biomarker discovery and explanation. Genome Biology, 12(6), R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(1), 7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Singh V., Bhatia H. S., Kumar A., de Oliveira A. C., Fiebich B. L. (2014). Histone deacetylase inhibitors valproic acid and sodium butyrate enhance prostaglandins release in lipopolysaccharide-activated primary microglia. Neuroscience, 265, 147–157. 10.1016/j.neuroscience.2014.01.037 [DOI] [PubMed] [Google Scholar]
- Skonieczna-Żydecka K., Jakubczyk K., Maciejewska-Markiewicz D., Janda K., Kaźmierczak-Siedlecka K., Kaczmarczyk M., Łoniewski I., Marlicz A. W. (2020). Gut biofactory-neurocompetent metabolites within the gastrointestinal tract. A scoping review. Nutrients, 12(11), E3369. 10.3390/nu12113369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonieczna-Żydecka K., Marlicz W., Misera A., Koulaouzidis A., Łoniewski I. (2018). Microbiome—The missing link in the gut-brain axis: Focus on its role in gastrointestinal and mental health. Journal of Clinical Medicine, 7(12). 10.3390/jcm7120521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling R. M., van de Wouw M., Clarke G., Stanton C., Dinan T. G., Cryan J. F. (2016). The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochemistry International, 99, 110–132. 10.1016/j.neuint.2016.06.011 [DOI] [PubMed] [Google Scholar]
- Tantoy I. Y., Cataldo J. K., Aouizerat B. E., Dhruva A., Miaskowski C. (2016). A review of the literature on multiple co-occurring symptoms in patients with colorectal cancer who received chemotherapy alone or chemotherapy with targeted therapies. Cancer Nursing, 39(6), 437–445. 10.1097/ncc.0000000000000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tometich D. B., Small B. J., Carroll J. E., Zhai W., Luta G., Zhou X., Kobayashi L. C., Ahles T., Saykin A. J., Clapp J. D., Jim H., Jacobsen P. B., Hurria A., Graham D., McDonald B. C., Denduluri N., Extermann M., Isaacs C., Dilawari A., Root J. Thinking and Living with Cancer (TLC) Study. (2018). Pretreatment psychoneurological symptoms and their association with longitudinal cognitive function and quality of life in older breast cancer survivors. Journal of Pain and Symptom Management, 57(3), 596–606. 10.1016/j.jpainsymman.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett J. D., Shelly S., Livne G., Milone M., Kassardjian C. D., Liewluck T., Kelly C., Naddaf E., Laughlin R. S., Lamb C. J., Rubin D., Dimberg E. L., Dubey D., Mills J. R., Mandrekar J., Klein C. J. (2020). Temporal and region-specific effects of sleep fragmentation on gut microbiota and intestinal morphology in Sprague Dawley rats. Gut Microbes, 11(4), 706–720. 10.1080/19490976.2019.1701352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo M. A., Rodrigues H. G., Hatanaka E., Sato F. T., Sampaio S. C., Curi R. (2011). Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. The Journal of Nutritional Biochemistry, 22(9), 849–855. 10.1016/j.jnutbio.20 [DOI] [PubMed] [Google Scholar]
- Vinolo M. A., Rodrigues H. G., Nachbar R. T., Curi R. (2011). Regulation of inflammation by short chain fatty acids. Nutrients, 3(10), 858–876. 10.3390/nu3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital M., Howe A. C., Tiedje J. M. (2014). Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio, 5(2), e00889. 10.1128/mBio [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Ling Z., Yang Z., Kiela P. R., Wang T., Wang C., Cao L., Geng F., Shen M., Ran X., Su Y., Cheng T., Wang J. (2015). Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: A pilot study. PLoS One, 10(5), e0126312. 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Cao G., Zhang H., Li Q., Yang C. (2019). Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food & Function, 10(12), 7844–7854. https://doi.org/.1039/c9fo01650c [DOI] [PubMed] [Google Scholar]
- Williams J. B. (1988). A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry, 45(8), 742–747. 10.1001/archpsyc.1988.018003200580 [DOI] [PubMed] [Google Scholar]
- Yamanashi T., Iwata M., Kamiya N., Tsunetomi K., Kajitani N., Wada N., Iitsuka T., Yamauchi T., Miura A., Pu S., Shirayama Y., Watanabe K., Duman R. S., Kaneko K. (2017). Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Scientific Reports, 7(1), 7677. 10.1038/s41598-017-08055-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Keller S., Lin J. S. (2019). Psychometric properties of the PROMIS(®) Fatigue Short Form 7a among adults with myalgic encephalomyelitis/chronic fatigue syndrome. Quality of Life Research, 28(12), 3375–3384. 10.1007/s11136-019-02289-4 [DOI] [PubMed] [Google Scholar]
- Yanofsky C. (1981). Attenuation in the control of expression of bacterial operons. Nature, 289(5800), 751–758. 10.1038/289751a0 [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhou Q., Dorfman R. G., Huang X., Fan T., Zhang H., Zhang J., Yu C. (2016). Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterology, 16(1), 84. 10.1186/s12876-016-0500-x [DOI] [PMC free article] [PubMed] [Google Scholar]