Abstract
Obesity surgery remains the most effective treatment for obesity and its complications. Weight loss was initially attributed to decreased energy absorption from the gut but has since been linked to reduced appetitive behavior and potentially increased energy expenditure. Implicated mechanisms associating rearrangement of the gastrointestinal tract with these metabolic outcomes include central appetite control, release of gut peptides, change in microbiota, and bile acids. However, the exact combination and timing of signals remain largely unknown. In this review, we survey recent research investigating these mechanisms, and seek to provide insights on unanswered questions over how weight loss is achieved following bariatric surgery which may eventually lead to safer, nonsurgical weight-loss interventions or combinations of medications with surgery.
Keywords: Obesity surgery, weight loss, eating behavior, gut hormones, energy expenditure
Graphical Abstract
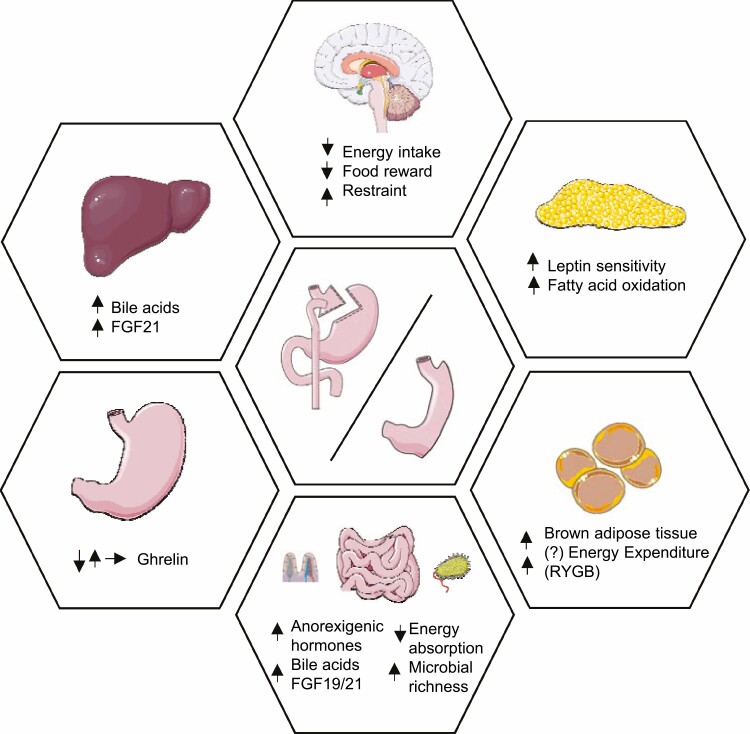
Graphical Abstract.
Representation of the main physiological mechanisms underlying weight loss following vertical sleeve gastrectomy (VSG) and Roux-n-Y gastric bypass (RYGB). GLP-1, glucagon like peptide-1; PYY, peptide YY. Figure was created using Servier Medical Art.
Essential Points.
Obesity surgery induces significant weight loss, yet the exact mechanisms remain unclear.
Changes in food selection take place after obesity surgery and this mechanism could compliment reduction in hunger and increase in satiety.
Enhanced energy expenditure may be a contributing mechanism to weight loss but reports are controversial.
Postprandial elevated secretion of anorectic gut peptides is considered to be a key mediator of the observed postoperative increase in satiety.
Obesity surgery induces an increase in gut microbiota richness, which may play a direct role in the control of adiposity by regulating lipid metabolism.
Obesity surgery over the past 6 decades has been successful not only in providing a means of achieving substantial weight loss but also in giving us many novel insights on the pathophysiology of obesity. Obesity surgery was first described in the 1960s, when it was observed that patients with subtotal gastrectomy for cancer lost a considerable amount of weight (1). Several modifications to the technique led to the first laparoscopic gastric bypass in 1994 (2), and the establishment of the 3 techniques most widely used in clinical practice today.
The 2 main approaches that are currently performed widely are Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG). RYGB involves the creation of a small gastric pouch (~30 mL) that is anastomosed to the proximal jejunum, which has been transected at 30 to 75 cm from the ligament of Treitz, to form the “alimentary limb.” The continuity of the intestine is restored via a jejuno-jejunal anastomosis between the alimentary limb and the excluded biliopancreatic limb approximately 75 to 150 cm distal to the gastrojejunostomy (3). As a result, food bypasses most of the stomach, the entire duodenum, and the proximal jejunum. VSG involves dividing the stomach along its vertical length to create a sleeve and removing ~75% of its volume (4). Although decreasing in popularity, the adjustable gastric banding (AGB) involves placing a silicone ring around the proximal stomach, bellow the gastroesophageal junction. The ring pressure is adjusted through fluid injected or withdrawn from a subcutaneous port (5).
Efficacy is not the same among procedures, as RYGB and VSG cause more weight than AGB (6). Patients benefit not only from weight loss, but more vitally from improvements in glycemic control (7), reduced cardiovascular morbidity and mortality (8), and reduced incidence of cancer (9). All 3 procedures cause no mechanical restriction with little or no macronutrient malabsorption. Instead, weight loss is due to changes in the physiology of body weight regulation.
In this review, we will explore the biological mechanisms underpinning weight loss. We will not discuss the mechanisms underlying glycemic/metabolic improvements as they fall outside the already wide scope of this review. The impact of obesity surgery on metabolism appears to be predominantly because of the substantial and sustained weight loss, but given the large number of mechanisms which are not weight loss related we expect that the beneficial metabolic outcome at individual level may be a composite of the weight loss together with nonweight loss–related mechanisms.
We will focus on mechanistic studies in humans and animal models focusing on RYGB, VSG, and AGB, as they are the most commonly performed operations. While animal data may not always apply to humans, they also raise new questions that can be answered in humans and answer questions that cannot be answered in humans.
Mechanisms Underlying Weight Loss After Obesity Surgery (RYGB, VSG, AGB)
Eating Behavior
Reduction in energy intake
The setpoint theory supports the notion that an individual’s body weight trajectory during life is predominantly influenced by their genetic make-up, which interacts with nonbiological factors (eg, social, psychological) to determine the final phenotype (10). Any weight loss below or above the setpoint is perceived as an alarm signal by the areas of the brain that regulate energy intake and expenditure, such as the hypothalamus and brainstem (11). These areas are located in the subcortical areas of the brain involved in automated function like respiration or body temperature. The hypothalamus and brainstem receive continuous and highly accurate humoral and neural signals from adipose tissue, stomach, intestine, and pancreas regarding body energy stores and acute energy intake respectively. Upon weight loss, these messengers change and (12) signal depletion of body energy stores, which is disadvantageous from an evolutionary perspective. The final common pathway of this mechanism is the defense of the individual’s body weight setpoint through an increase in hunger and reduction in satiety, which trigger the executive function areas located in the cortical areas of the brain to seek and consume food.
A good example of how this system is activated is intentional weight loss through caloric restrictive diets. People on severe caloric restriction frequently report a decrease in hunger and increase in satiety during the acute phase of negative energy balance. However, the vast majority find it difficult to maintain the weight they have lost when it plateaus during the stable energy balance phase. This is despite the cortical areas of the brain that control dietary restraint working intensely to maintain the body weight lost. The increase in hunger and decrease in satiety signaled by the hypothalamus/brainstem results in an increase in caloric intake which eventually leads to the regain of weight lost and in many cases the establishment of a new setpoint which is higher than the original baseline (12). Repeated cycles of this process increase body weight setpoint, making it progressively harder to achieve sustained weight loss (13). Consequently, any successful weight loss and maintenance therapy should be sophisticated enough, from a biological perspective, to counteract this elaborate body weight regulation system.
Obesity surgery has proven to be biologically very sophisticated and is thus an effective therapy. Similar to caloric restriction during the acute negative balance phase, patients after surgery report a decrease in hunger and increase in satiety (14). The key difference between dieting and obesity surgery is that after surgery the body weight setpoint is reduced by approximately 20% to 30% (15). Manipulation of the stomach and the small intestine result in favorable changes in humoral and neural signals from the gut to the brain that are conducive to the maintenance of this newly established body weight setpoint.
The comparison of patients’ reports and actual weight during the plateau phase of weight loss during dieting vs obesity surgery is intriguing. Even after surgery, patients report an “alarming” increase in hunger and decrease in satiety during the stable energy balance phase and indeed this translates in both higher energy intake during meals and an increase in meal frequency (16). Yet, body weight increases only marginally and never reaches the preoperative value in the majority of cases. While at this new setpoint, the intensity of the internal feelings of hunger and satiety might return to almost preoperative levels, altered signaling from the gut acts continuously to reduce total energy intake during a 24-hour period in order to robustly defend the new normal (12).
Patients losing weight through pharmacotherapy (eg, with glucagon-like peptide 1 [GLP-1] receptor agonists) report very similar changes in their appetite during the acute and chronic phase of their weight loss journey (17). The only difference is that the effect size of pharmacotherapy is lower than that of surgery, and that is because medications change only 1 or few of the signaling pathways in the appetite centers of the brain.
Weight loss after the biliopancreatic diversion further highlights that the mechanisms through which these operations work are physiological and not “cognitive” in nature. This procedure is the most effective operation for weight loss, but rarely performed these days due to the associated severe nutritional complications. The very long intestinal bypass in this procedure results in frank macronutrient malabsorption and weight loss. The brain appetite centers rapidly detect this and compensate by increasing hunger. Patients after the biliopancreatic diversion commonly consume more calories compared to before their operation. However, even this hyperphagia is not enough to compensate for the severe loss of calories through the gut which is therefore the dominant mechanism causing weight loss.
Neural correlates of reduction in energy intake.
The hypothalamus is a critical brain area that controls energy intake and expenditure via 2 sets of antagonistic neurons: agouti-related peptide (AgRP) neurons to promote hunger and pro-opiomelanocortin (POMC) neurons to promote satiety (18) (Fig. 1). Neuropeptide Y (NPY) is secreted by AgRP neurons and is an orexigenic factor. Hypothalamic gene expression of Agrp, Npy, and Pomc changes following RYGB surgery (19, 20), but the findings are not consistent and often lack a weight-matched calorie restricted model. Expression levels of hypothalamic Agrp in obese female rats are upregulated compared with lean controls, but go down to levels similar to lean animals following RYGB. Gene expression of Pomc does not change (21). A recent study investigated the expression of hypothalamic NPY and AgRP in obese mice, following RYGB and compared the results with a weight-matched model. During the first 2 postoperative weeks, when the peak weight loss was observed, hypothalamic Agrp and Npy gene expression did not increase compared with mice undergoing sham surgery, suggesting that compensatory hunger signals in the RYGB mice were not activated. In contrast, when the same amount of weight loss was achieved by caloric restriction in a different group of mice, increased expression of Agrp and Npy was observed. Of note, Pomc expression was not altered to a similar degree as Agrp, indicating that RYGB suppresses the adaptive hunger response triggered by weight loss (22-24). Similarly, VSG does not change Npy and Agrp gene expression in obese rats 4 weeks after surgery (25). A study that compared VSG and AGB-treated obese rats 6 weeks after surgery showed that the hypothalamic expression of Npy was significantly lower and the expression of Pomc was significantly higher in the VSG group (26). Given the similar postoperative time points, any discrepancies between these studies’ findings on Agrp, Npy, and Pomc may be due to rodent strain, differences, diet type and length of exposure, and variations in surgical technique.
Figure 1.
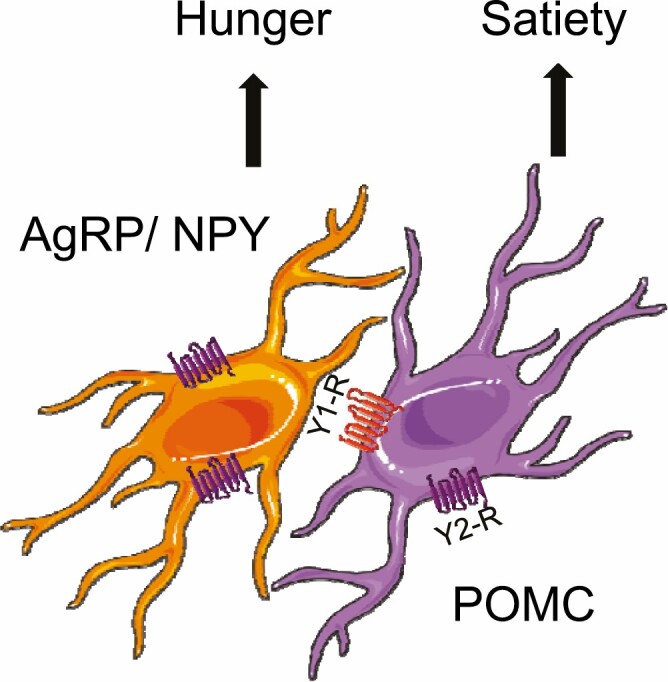
The “AgRP-POMC” model of gut–brain cross-talk. AgRP, agouti-related peptide; POMC, pro-opiomelanocortin; NPY, neuropeptide Y. Figure was created using Servier Medical Art.
The brainstem is the other key player in the obesity surgery–induced suppression of hunger. The strong orexigenic drive stemming from arcuate AgRP/NPY neurons may partly result from inhibition of an equally strong feeding anorexia circuit organized around the lateral parabrachial nucleus and brainstem (27, 28). Measurement of meal-induced neuronal activation by means of c-Fos in obese mice showed that brainstem anorexia circuit may have a potential role in adaptive neural and behavioral changes involved in the strong early suppression of energy intake after RYGB (29).
These findings from animal models support the observations from humans in that the direction of change in expression of neuropeptides in the hypothalamus and brainstem after RYGB and VSG is opposite to dieting and favor the maintenance of a lower body weight setpoint.
Neural signaling.
The mechanism of action of AGB is thought to be exclusively through vagal signaling. Injection of fluid through the subcutaneous port increases the extraluminal pressure on vagal afferents, sending an anorexigenic signal to the brainstem, even in the fasting state (30). This mechanism is further exaggerated through the increase in fundal intraluminal pressure exerted by the consumption food, leading to early satiety during a meal. It is common for healthcare professionals to inject progressively more fluid in the AGB in patients not losing enough weight. This eventually leads to mechanical restriction and vomiting. This is a preventable complication that should be avoided, and instead an early decision should be made to remove the AGB in patients who do not respond. More patients do not respond to the AGB compared to RYGB/VSG (31) because the AGB activates only 1 signaling system to the brain, as opposed to the plethora of anorexigenic signals after RYGB/VSG. A study in rats suggested that signals carried by vagal afferents from the mid and lower small intestine contribute to the early RYGB-induced body weight loss and reduction of food intake (32). Disruption of vagal afferents and/or efferents takes place during RYGB and VSG surgery; whether this affects appetite and postoperative weight loss remains unclear. Some studies suggested that vagal sparing surgical technique affects body weight loss in rodents, and therefore the vagal nerve should be preserved during the gastric bypass operation (33, 34). However, there are limited data on the role of vagus nerve dissection in RYGB and VSG with regards to body weight in humans (35).
Food selection
After RYGB and VSG surgery, but not AGB, some patients also change their food selection (36). This includes a shift in preference from energy-dense sweet and fatty food to less energy-dense options. The majority of research in this area has used indirect measures of behavior, such as questionnaires, food diaries, and verbal report at recall sessions. While these have suggested that the reduction of the consumption of energy dense food might be an additional weight loss mechanism after surgery, they have also demonstrated large variations in response and substantial heterogeneity in findings (37). This is particularly noticeable in the longer-term measurements of eating behavior, 5 to 10 years after surgery when any early changes in macronutrient selection tend to dissipate.
Only a small number of studies have used direct measurements of eating behavior, in other words observing the participant’s choices during an ad libitum meal or an eating behavior task. The best evidence so far suggests that patients who lost more weight were those who consumed a lower percentage of fat and low glycemic index foods, and higher percentage of protein as a proportion of their total daily caloric intake (38).
The reduction in the rewarding properties of food is 1 of the mechanisms that underpins the changes in food selection (Fig. 2). This mechanism has been investigated using functional neuroimaging. Functional magnetic resonance imaging and positron emission tomography studies provide information both with respect to the direction of change and the areas of the brain reward system that correlate with changes in observed or reported eating behavior. Notwithstanding discrepancies between studies, there is some agreement that there is a reduction in the activation of brain areas that respond to the involved cues with rewarding properties (eg, food pictures) after RYGB and VSG (39, 40). The effect size of this reduction is more pronounced after RYGB compared with VSG (41). Gut hormones are mediators that underlie this observation, as the blockage partly reverses the reduction in activation of these brain regions (42). This is in line with animal and human data demonstrating that gut hormones such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) do not just reduce hunger and increase fullness, but reduce the rewarding properties of food through their direct action on their receptors in brain reward areas (43). It should be noted that functional neuroimaging findings should be interpreted with some caution as they only measure neural correlates of eating behavior, not behavior itself. The available paradigms also do not allow enough granularity as to whether measured brain responses to food pictures reflect appetitive or consummatory behavior.
Figure 2.
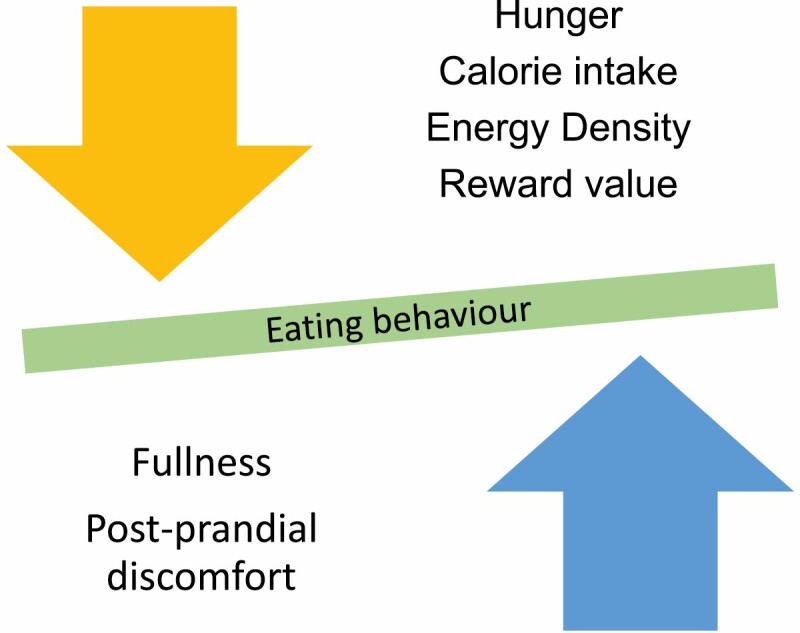
Changes in eating behavior following obesity surgery.
Altered taste function is another mechanism underlying the changes in food selection after RYGB and VSG. With regards to the sensory domain of taste, acuity for sweet taste is heightened only in the early postoperative period. (44). It is therefore unlikely to be responsible for long-term effects. Selective changes in the appetitive reward value of sweet/fatty taste have also been reported in humans 3 months after RYGB and VSG, namely during the acute phase of negative energy balance (45, 46), but these findings have not been replicated in animal models of RYGB during the stable energy balance phase (47). The valid measurement of the consummatory reward value of taste is challenging in humans as it relies entirely on the use of indirect measures such as a visual analog scale. Studies using a visual analog scale after RYGB surgery have shown discrepant results (44, 48). There is more consistency in the rodent literature, in which orofacial responses, a good marker of consummatory responses, increase for low concentrations of glucose, and decrease for high concentrations of glucose after RYGB (49, 50). The third domain of taste function is termed digestive preparation and salivation is a marker of this reflex response to tastants. Rates of salivation correlate with the rewarding aspects of the tastant and people with obesity demonstrate higher salivation rates to normal-weight controls (51). Attempts have been made to measure salivation rates after obesity surgery but with mixed results (52). Our group’s experience with measuring salivation rates in humans is that the available methodologies suffer from low reproducibility (unpublished data).
Neural signaling also contributes to changes in the rewarding value of fat and sugar after surgery. This was investigated in obese rats undergoing RYGB as they were found produce less of the fat satiety molecule oleoylethanolamide in the small intestine, and this effect was associated with vagus nerve–driven increases in dorsal striatal dopamine release (53). Inhibition of local oleoylethanolamide, vagal, and dorsal striatal dopamine-1 receptor signaling was inhibited, the beneficial effects of RYGB on fat intake and preferences was reversed.
Postingestive neural signaling, in the form of what is widely known as dumping syndrome, may contribute to the underlying reductions in high glycemic index or fatty food after RYGB, and less so after VSG. Patients report unpleasant sensations of nausea, sweating, and dizziness early after consumption of sugary or fatty foods, which in some people may result in conditioned taste avoidance (54). During this learning process, these unpleasant symptoms are presumably generated through osmotic shifts between the intestine and circulation, and altered neural signaling. These symptoms are usually associated with the ingestion of specific foods. This does not lead to the complete extinction of these foods from regular consumption, namely aversion, but rather their avoidance. Thus the foods remain pleasant to the subject but only when consumed in smaller quantities (54). It should be noted that dumping syndrome is not present in all patients after RYGB and it may indeed be the case that its impact dissipates over time. This might be due to intestinal adaptation that continues to take place for years after surgery. Dumping is less common after VSG and AGB (55), operations not involving duodenal bypass.
Overall, the available data suggest that changes in food selection take place in a proportion of people after RYGB and VSG, but not after AGB. In the former, this mechanism could compliment the reduction in hunger and increase in satiety to cause additional weight loss. Whether this mechanism persists over time or dissipates following intestinal adaptation remains uncertain. The process of learning to avoid foods that generate unpleasant postingestive effects has a greater impact than taste function in shaping food preferences after surgery. Some of the above unresolved questions could be answered using residential stays in facilities that allow human eating behavior to be as close to normal as possible. Such experiments could be conducted both early and late after surgery and complimented by studies in animal models of surgery.
Energy Expenditure
Enhanced energy expenditure after obesity surgery may be a contributing mechanism to weight loss. Resting energy expenditure has been measured in humans following RYGB, and most recent studies using indirect calorimetry show resting energy expenditure to either decrease within the first postoperative year (56-58), remain stable (59), or even slightly increase (60). These changes are reported to be highly dependent on organ tissue body composition as RYGB patients maintain a larger high metabolic rate organ mass than nonoperated controls (59). Moreover, the acute weight loss following obesity surgery was found to affect the accuracy of energy expenditure predictive equations (61).
A small number of studies used 24-hour indirect calorimetry, a method that is optimal for measuring substrate oxidation because each subject can freely move, consume meals, and engage in physical activity. One study reported that diet-induced energy expenditure in patients 20 months after RYGB was increased, which resulted in an increased contribution to total energy expenditure over 24 hours from an average 12.9 cal/min/kg to 14.7 cal/min/kg, when corrected for total tissue mass, including total adipose tissue, lean body mass, bone mineral density, and bone mineral content (62). Another study reported no changes in 24-hour or diet-induced energy expenditure 11 weeks after RYGB, although this was not corrected for total tissue mass (63). Nine years after RYGB, patients had greater diet-induced energy expenditure and total 24-hour energy expenditure at an average of 16.9 cal/min/kg than vertical banded gastroplasty patients, a procedure similar to AGB, at 14.9 cal/min/kg (64). At a shorter follow-up period, 24-hour energy expenditure was significantly decreased from baseline to 8 weeks after treatment in patients who underwent RYGB, VSG, AGB, and very low–calorie diet, following adjustment for decreases in fat-free mass and fat mass. However, this effect persisted up to 1 year only after RYGB and VSG (RYGB, −124 ± 42; VSG, −155 ± 118 kcal/day) (65). Additionally, patients who underwent biliopancreatic diversion (consisting of a horizontal gastrectomy with a distal Roux-en-Y reconstruction resulting in an alimentary limb of 250 cm and a common channel of 50-100 cm) demonstrated increased diet-induced (11.0% at baseline to 19.9% of caloric intake) and physical activity–related (8344.3 at baseline to 9701.4 kcal/24 hour) thermogenesis at 6 months postoperatively when compared with an unoperated control group (66). One mechanism which may contribute to increased energy expenditure during a meal in humans may be the enhanced glucose utilization by the hypertrophied small intestine (67). However, absolute energy expenditure is reduced after surgery in humans and the increase in energy expenditure expressed per total body mass may be at least in part explained by change in body composition (ie, increased lean to fat mass ratio).
The type of diet may also affect measurements of energy expenditure. A randomized clinical trial in patients following diet-induced weight loss showed that lowering dietary carbohydrate intake increases energy expenditure during weight loss maintenance (68). However, meta-analysis of 32 controlled feeding studies with isocaloric substitution of carbohydrate for fat found that both energy expenditure and fat loss are greater with lower dietary fat (69).
Contrary to observations in humans, the majority of studies in rodent models of RYGB report an increase in total energy expenditure when compared with ad libitum–fed shams and weight-matched shams. This has been measured at different postoperative time points using indirect calorimetry or validated mathematical formulae (70-73). VSG appears to induce no change in total energy expenditure (25, 71, 73). However, indirect calorimetry produces an absolute error as high as 38% when compared with standard direct calorimetry (74). A recent study (75) used a combination of sensitive direct and indirect calorimetry to overcome this limitation and demonstrated an increase in resting energy expenditure after RYGB, but not VSG.
Brown adipose tissue (BAT) is a major player in regulating energy metabolism by thermogenesis and triglyceride clearance (76, 77) and plays a role in energy expenditure changes after obesity surgery. A decrease in triglyceride content, coupled with increased proportion of BAT in the supraclavicular fat depot was found in women 6 months after RYGB and VSG (78). However, the role of BAT in energy expenditure following obesity surgery has mainly been studied in rodents. The expression of key BAT thermoregulatory genes such as uncoupling protein-1 (UCP-1) remain unchanged following RYGB but are reduced in caloric-restricted weight-matched animals (79), and that the bypassed duodenum has a key role in the observed postoperative metabolic profile (80). The volume and metabolic activity of BAT, as recorded by micro-positron emission tomography/computed tomography increased following RYGB, but not after AGB and VSG (81). A proposed mechanism for the metabolic activity of BAT is an observed increase in growth hormone/insulin-like growth factor-1, which regulates adipocyte differentiation (81). Unlike VSG, RYGB causes an increase in total resting metabolic rate, as well as a specific increase in splanchnic sympathetic nerve activity and “browning” of visceral mesenteric fat via endocannabinoid signaling within the small intestine (75). Although in vivo studies are vital to unravel the mechanisms of energy expenditure difference after obesity surgery, it is important to note the species difference between mice and rats, as well as strain differences in a single species. There are also differences between rodent and human BAT, in terms of depot locations, beige adipose tissue and BAT amount, and thermogenic capacity (82). Despite this, UCP1 content and function are similar between human and mouse BAT (83).
Overall, it remains unclear from the existing evidence to what extent, if at all, postoperative weight loss is driven by enhanced energy expenditure after RYGB and VSG vs dietary calorie restriction, as energy metabolism is closely associated with body weight changes. The discrepancy on energy expenditure values reported in the discussed studies could indeed be due to differences in diet, patient body composition and energy expenditure measurement. These uncertainties suggest to us that the physiological contribution of energy expenditure to weight loss after RYGB and VSG is small in comparison to the dominant contribution of reduced energy intake.
Mediators Underlying Changes in Energy Intake and Expenditure After Obesity Surgery
Gut Hormones
Gut hormones are secreted in response to nutrient ingestion and regulate energy balance and glucose homeostasis by signaling to the pancreas but also by direct and indirect action in the brainstem and the hypothalamic arcuate nuclei (84). Two anorexigenic gut hormones that have been widely investigated after obesity surgery are GLP-1 and PYY, which are secreted by the enteroendocrine L-cells across the gastrointestinal tract (14).
Both GLP-1 and PYY are elevated postprandially after RYGB and VSG, and the enhanced secretion has been hypothesized to be a key mediator of the observed postoperative increase in satiety (85). Fasting concentrations do not change significantly, suggesting that they are not the mechanisms underlying the reduction in hunger. The absence of mechanical restriction at the level of the gastrojejunal anastomosis after RYGB enables the rapid delivery of nutrients to the jejunum and ileum, where the highest number of enteroendocrine (primarily GLP-1 secreting) L-cells are located, triggering the enhanced secretion of anorexigenic gut hormones (86). These exert their action in the brainstem/hypothalamic system through stimulation of intestinal vagal afferents and by crossing the blood-brain barrier. Despite the absence of intestinal bypass, VSG is thought to engage the same mechanism through the rapid emptying of the high-pressure gastric remnant (87), thus creating a functional intestinal bypass. However, the postprandial increase in anorexigenic gut hormones after VSG is lower than that observed after RYGB (88). This might explain differences in the weight loss efficacy of the 2 interventions and substantial weight regain after VSG at long-term follow-up. Despite the persistent rapid delivery of nutrients to the distal small intestine, there is no compensatory decrease in the L-cell numbers after RYGB (86). In contrast, following intestinal adaptation L-cell numbers increase, further amplifying anorexigenic signaling. The density of enteroendocrine cells in the distal small intestine does not change as the intestinal volume also increases through hypertrophy (86).
Combined blockade of both GLP-1 and PYY via single infusion of antagonists increases energy intake, pointing at their appetite-suppressing role in humans after RYGB (89). These findings are in line with experiments in which the administration of the somatostatin analogue octreotide following RYGB and ABG in humans resulted in suppression of postprandial secretion of PYY and GLP-1 and reduction in energy intake only in the RYGB group (14).
Chronic infusion of the selective GLP-1 receptor antagonist exendin-(9-30) into the lateral cerebral ventricle significantly increased energy intake and body weight in both RYGB and sham-operated rats, while chronic infusion of a selective Y2 receptor antagonist had no effect in either group (90). However, obese GLP-1R–deficient mice (GLP-1–/–) lost the same amount of body weight and fat mass and maintained a similarly lower body weight than wild-type mice, following RYGB (90). This observation indicates low importance of GLP-1R in appetite regulation and this was further confirmed by blocking peripheral and central GLP-1R action in RYGB and sham obese mice using exendin-(9-30), which did not reverse the weight loss effect of RYGB or influence the weekly body weight gain in sham mice (91). Similarly, obese Y2 receptor–deficient mice (PYY–/–) also responded similarly to RYGB compared with wild-type mice for up to 20 weeks after surgery, with initial hypophagia and sustained body weight loss. Weight-matched Y2-R knockout mice showed the same improvements to RYGB as seen in wild-type mice, suggesting that PYY signaling through Y2 receptor alone is not responsible for the appetite-suppressing and body weight–lowering effects of RYGB (92). However, acute administration of exendin-(9-30) with a selective Y2-R antagonist increased high-fat food preference additively in RYGB-operated but not in sham-operated diet-induced obese rats (93). This is in agreement with human studies (89, 94) and indicates a differential effect of antagonists when administered alone vs in combination, as well as acutely vs infused chronically. This also contrasts an acquired effect associated with antagonist infusion compared with the genetic state associated with deficiency of the Y2 receptor or GLP-1 receptor in knockout models.
Recent studies have focused on 2 additional gut hormones, oxyntomodulin and glicentin; products of the preproglucagon gene also released from enteroendocrine in response to food transit. Oxyntomodulin is a dual agonist of glucagon and GLP-1 receptors that may act additively to GLP-1 to reduce food intake and appetite (95). Glicentin protein sequence contains the sequence of oxyntomodulin and although its biological role is not yet clear, it is hypothesized to be the most stable of the proglucagon peptides and therefore may serve as the best marker of the secretion of L-cell hormones, such as GLP-1 (88). Postprandial levels of oxyntomodulin and glicentin were significantly increased 3 months after VSG or RYGB, but not after AGB, in humans, and these elevated concentrations were positively associated with feeling of satiety and weight loss (96). These results were later replicated by Nielsen et al., who reported that elevated circulating levels of oxyntomodulin and glicentin predicted weight loss and were positively associated with a decreased preference for energy-dense foods (88).
Changes to plasma concentration of the orexigenic hormone ghrelin after RYGB remains controversial. Studies in humans have demonstrated that hormone levels are increased, decreased or stay the same (97). The results of studies measuring ghrelin after VSG are more consistently demonstrating a decrease in the postprandial concentrations of the hormone (97). Thus, the contribution of ghrelin reductions in weight loss might be more relevant after VSG than RYGB.
Bile Acids
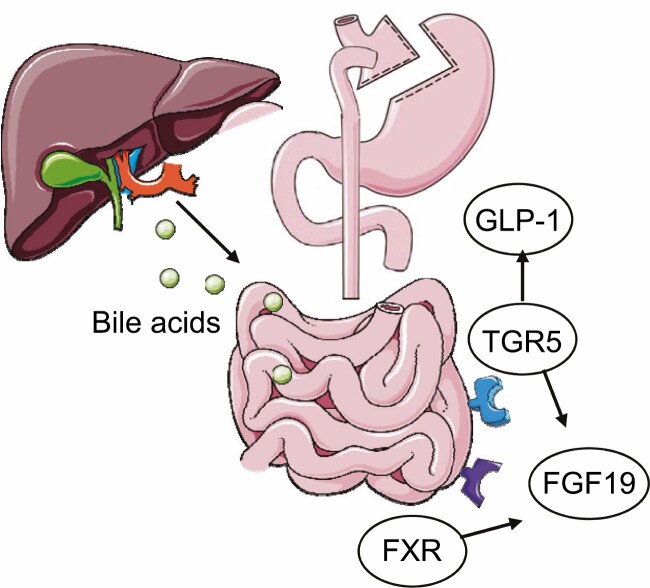
Bile acids have long been known to play an important role in dietary lipid absorption and cholesterol catabolism and have been shown to increase energy expenditure by promoting intracellular thyroid hormone activation (98). Bile acid function is mediated by 2 primary gut receptors, Takeda G-protein receptor 5 (TGR5) and farnesoid X receptor A (FXR). These receptors stimulate the postprandial release of fibroblast growth factors 19 and 21 (FGF19/21) (99). FGF19 is released from the small intestine postprandially and decreases bile acid secretion, while FGF21 is secreted by the liver during fasting and has a role in energy homeostasis maintenance, as well as controls glucose and lipid metabolism (Fig. 3). Circulating FGF19 levels have been shown to be lower in people with obesity than in healthy control subjects (100), while administration of human FGF19 in obese mice induced a significant dose-dependent decrease in body mass which was associated with a decrease in the concentrations of triglycerides, as well as increased fatty acid oxidation and brown tissue mass (101). Following the release of FGF19, the role of subsequent neuronal FGF receptor activation has also been linked to body weight regulation, as it signals an energy-replete state to hypothalamic AgRP/NPY neurons (102). In contrast, FGF21 is elevated in people with obesity (103), and obese mice are insensitive to exogenous FGF21 administration, suggesting that obesity is an FGF21-resistant state (104). However, FGF21 sensitivity is restored in humans following weight loss (105). Although not directly correlated with obesity, FGF21 variants are associated with increased sweet consumption, as plasma FGF21 levels increase acutely after oral sucrose ingestion. This indicates that FGF21 could influence eating behavior (106).
Figure 3.
Bile acid synthesis and receptor activation following obesity surgery. FXR, farnesoid X receptor; TGR5, G protein-coupled bile acid receptor 5; GLP-1, glucagon-like peptide 1; FGF19, fibroblast growth factor 19. Figure was created using Servier Medical Art.
Total bile acids and FGF19 increase after RYGB and VSG in humans and rodents (107). Specifically, glycine-conjugated serum bile acids increased acutely following RYGB in humans, while both conjugated and unconjugated bile acids increased after VSG, an effect not replicated in an unoperated calorie restriction control group (108, 109). The bile acid increase is sustained 5 years after surgery, with higher levels associated with greater weight loss and lower total cholesterol (110). Apart from their role in energy expenditure increase (98) and fatty acid oxidation (101), bile acids are thought to have an appetite inhibitory effect, as they stimulate the secretion of GLP-1 and PYY (111). However, serum bile acids, FGF19 and GLP-1 concentration all decreased in patients who achieved lifestyle-induced weight loss, further pointing to the fact that dieting and obesity surgery-induced changes in body weight are triggered by different mechanisms (112). Discrepancies exist regarding the postoperative timing of bile acid increase, as some studies report an acute effect (109) and others observe a gradual increase 1 year following surgery (113, 114). Concentrations of FGF21 after surgery remain more controversial between different studies, possibly because circulating concentration and sensitivity changes are shown to be secondary to weight loss which can differ widely (99, 115-117).
A growing body of evidence suggests that circulating bile acids act as signaling molecules that control both their own synthesis and multiple metabolic pathways by targeting the transcription factor FXR and the membrane protein TGR5. FXR appears to be key in postoperative weight loss, as it controls the transcription of genes involved in fatty acid and triglyceride synthesis and lipoprotein metabolism (118) and promotes adipose tissue browning (119). In vivo studies involving genetic disruption of FXR in mice that then underwent VSG demonstrated that the receptor is a molecular target for beneficial effects of surgery as it contributes to the maintenance of weight loss following VSG. Specifically, FXR knockout VSG mice consumed more energy than sham-operated controls, suggesting that FXR signaling is necessary for the repression of rebound hyperphagia following caloric restriction initially achieved by VSG (120). Studies in mice also investigated the role of TGR5 receptor in postoperative weight loss, as its activation can increase postprandial GLP-1 secretion in the lower intestine (121, 122). Similar to FXR studies, TGR5 knockout mice demonstrated reduced weight loss following VSG. Moreover, body composition analysis revealed no differences between wild-type TGR5 knockout sham and VSG mice at 14 weeks after surgery, indicating that TGR5 is required to maintain weight loss and fat mass reduction after VSG (123). A possible mechanism of this is a TGR5-driven mitochondrial separation and turnover of white adipose tissue to beige, as administration of TGR5-selective bile acid mimetics to thermoneutral housed mice led to the appearance of beige adipocyte markers and an increase in mitochondrial content (124). However, not all studies report a reduction of weight loss following VSG and RYGB in TGR5 knockout mice (125, 126). A possible explanation for this is the rate of weight regain following obesity surgery, and, as a result, the type and length of exposure to high-fat diet in preoperative mice. Most studies investigating the role of the TGR5 and FXR receptors were conducted in animal models, and their roles may be different in humans.
Overall, the role of bile acids on postoperative weight loss is not yet fully understood. As the extent to which energy expenditure drives weight reduction following obesity surgery remains unclear, the ability of bile acids to increase GLP-1 secretion (111) and the role of FGF19 on hypothalamic AgRP/NPY neurons (102) indicate an indirect anorectic effect as the main course of action after RYGB and VSG.
Gut Microbiota
Gut microbiota have a vital role in both energy harvesting and energy expenditure. They can metabolize indigestible complex carbohydrates by fermentation, leading to the production of short-chain fatty acids, as well as control the absorption of nutrients (127, 128). Gut microbiota also play a role in the thermogenic capacity of BAT and the turnover of beige adipocytes, as mice lacking gut microbiota have been reported to have impaired UCP1-dependent thermogenesis in the cold, and oral gavage of the bacterial metabolite butyrate was able to rescue the effect with BAT recruitment (129).
Obesity is often characterized by gut dysbiosis, as defined by substantial modifications in the gut microbiota composition and low microbial gene richness (130). Firmicutes and Bacteroidetes are the 2 dominant gut microphyla associated with obesity (131), and the Firmicutes/Bacteroidetes ratio correlates with increased body weight (132). Together these phyla account for 90% of the microbiome, with the remaining groups separated mainly into Actinobacteria, Proteobacteria, and Verrucomicrobia (133). Akkermansia muciniphila of class Verrucomicrobia has also been correlated with obesity in humans (134).
The mechanism via which obesity surgery achieves weight loss may include changes in gut microbiota. Transfer of gut microbiota from RYGB-treated mice to nonoperated, germ-free mice resulted in weight loss and decreased fat mass in the recipient animals when compared with recipients of microbiota induced by sham surgery (135). In rats transplanted with the RYGB microbiota, this decrease in adiposity and body weight was not associated with a change in food intake, further suggesting that the RYGB-associated gut microbiota either increase energy expenditure or have reduced ability to harvest energy from nutrients (135). Stool transplantation from patients after RYGB or vertical banded gastroplasty to germ-free mice promoted reduced fat deposition and weight gain when compared with a control group that was colonized with stools from patients with obesity (136). Mice colonized with obesity surgery microbiota also had a lower respiratory quotient, indicating decreased utilization of carbohydrates as fuel (136).
Although human studies (137, 138) have reported differences in gut microbiota postoperatively, the extent of these changes varies. This could be due to patient inclusion criteria, such as glycemia state and medication, but also diet and type of procedure. However, studies in humans consistently demonstrate an increase in gut microbiota diversity, spatial organization and stability, and specifically Proteobacteria after RYGB (Table 1). Gut microbiota diversity is a measure of how many different species exist and how evenly distributed they are in the gut community, and low diversity is a sign of dysbiosis (139). Some studies also reported a decrease in Firmicutes and Bacteroidetes phyla in humans and rats post RYGB (136, 140, 141). Increase in gut microbiota diversity, stability, and resilience is important, as a large number of associations between gut microbiota and adipose tissue gene regulation as early as 3 months after surgery (136, 140, 142) have been reported, further demonstrating that gut microbiota may play a direct role in the control of adiposity by regulating lipid metabolism. Moreover, gut microbiota lead to the production of short-chain fatty acids, which stimulate GLP-1 secretion via free fatty acid receptor-2, and therefore may also reduce energy intake (143).
Table 1.
Summary of selected publications reporting gut microbiota changes following bariatric surgery
| Author | Species | Procedure | Increased phyla | Comparison | Timepoint |
|---|---|---|---|---|---|
| Liou et al., 2014 (135) | Mouse | RYGB | Proteobacteria Verrucomicrobia | Obesity, pair fed | 0-12 weeks |
| Tremaroli et al., 2015 (136) | Human | RYGB, VBG | Proteobacteria | Obesity | 9 years |
| Graessler et al., 2013 (137) | Human | RYGB | Proteobacteria | Obesity | 0, 3 months |
| Palmisano et al., 2020 (138) | Human | RYGB | Proteobacteria Fusobacteria | Normal weight | 0, 3, 6 months |
| Zhang et al., 2009 (140) | Human | RYGB | Proteobacteria Verrucomicrobia | Obesity, normal weight | 8, 15 months |
| Li et al., 2011 (141) | Rat (lean) | RYGB | Proteobacteria | Normal weight | 2 weeks |
| Kong et al., 2013 (142) | Human | RYGB | Proteobacteria | Obesity | 0, 3, 6 months |
| Lee et al., 2019 (145) | Human | RYGB, LAGB | Proteobacteria Actinobacteria | Medically induced weight loss | 9 months |
| Furet et al., 2010 (150) | Human | RYGB | Bacteroides | Obesity, normal weight | 0, 3, 6 months |
A decrease in Proteobacteria was recorded in patients following VSG (144) and AGB (145). This differential effect between VSG and RYGB could be a result of duodenal exclusion in RYGB, as duodenal-jejunal bypass with minimal gastric resection in obese rats increased microbial richness and abundancy when compared with rats treated with GLP-1R agonists (146, 147). This has also been observed in humans following treatment with the endoscopic duodenal-jejunal bypass liner (148, 149). Comparison of AGB, pharmacologically induced weight loss and RYGB demonstrated that at similar weight loss, the greatest alteration in gut microbiota diversity occurred after RYGB (145, 150).
Despite the positive effect on weight loss through a combination of mechanisms discussed above, RYGB is unable to fully reverse the decreased gut microbial gene richness and compositional changes observed in patients with obesity (151). Interventions such as fecal transplantation from lean donors to patients with obesity revealed that weight-lowering beneficial effects are linked to changes in plasma metabolites and driven by baseline fecal microbiota composition (152). Moreover, gut microbiota diversity alteration accelerates postdieting weight regain (153), suggesting that microbiome-targeting approaches may help enhance weight loss after surgery or prevent weight regain.
Genetics and Obesity Surgery
Patient selection for surgery (“personalized medicine”) may provide an additional refinement for existing procedures and could lead to the identification of genes or pathways which might provide useful therapeutic targets. Candidate gene studies have explored roles for the melacocortin-4 receptor (154), revealing greater weight loss in patients whose obesity is in part driven by mutations in this gene. A more recent genome-wide association study (155) has reported 17 single nucleotide polymorphisms in weight loss 2 years post RYGB, implicating roles for the 5-hydroxytriptamine receptor 1A and other genes. Whether the strength and number of these associations is substantial enough to provide predictive power is unclear.
Conclusion
The anatomical manipulations during the most frequently used obesity surgery procedures cause weight loss through changes in the biology of the gut. Altered signaling from the gut to the brain, the organ responsible for the disease of obesity, facilitate reductions in energy intake, and in some people changes in food selection. Increased or unaltered energy expenditure in the context of weight loss may also contribute to the defense of a new body weight setpoint. The precise mechanisms underlying these profound changes are not completely understood. Unravelling of the elusive physiology of the gut after surgery will help optimize surgical procedures, develop nonsurgical therapies, address weight regain after surgery, but also understand the pathophysiology of the disease of obesity itself.
Glossary
Abbreviations
- AGB
adjustable gastric banding
- AgRP
agouti-related peptide
- BAT
brown adipose tissue
- FGF
fibroblast growth factor
- FXR
farnesoid X receptor A
- GLP-1
glucagon like peptide-1
- NPY
neuropeptide Y
- POMC
pro-opiomelanocortin
- PYY
peptide YY
- RYGB
Roux-en-Y gastric bypass
- TGR5
Takeda G-protein receptor 5
- UCP-1
uncoupling protein-1
- VSG
vertical sleeve gastrectomy
Acknowledgments
Financial Support: E.A. was supported by a grant from the Rosetrees Trust (M825) and from the British Society for Neuroendocrinology. A.D.M. has been supported from grants from the JP Moulton Charitable Foundation, National Institute of Health Research, Imperial College Healthcare Charity and Novo Nordisk. The Section of Investigative Medicine is funded by grants from the MRC, BBSRC, NIHR, an Integrative Mammalian Biology Capacity Building Award, an FP7-HEALTH-2009-241592 EuroCHIP grant and is supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care. G.A.R. was supported by a Wellcome Trust Investigator Award (212625/Z/18/Z), MRC program grants (MR/R022259/1, MR/J0003042/1, MR/L020149/1), an Experimental Challenge Grant (DIVA, MR/L02036X/1), a Diabetes UK Project grant (BDA16/0005485). This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking, under grant agreement no. 115881 (RHAPSODY). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA. C.lR. is funded by the Irish Research Council (IRCLA/2017/234) and The Health Research Board (USIRL-2016-2).
Additional Information
Disclosures: The authors have no competing interest to disclose.
References
- 1. Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47(6):1345-1351. [DOI] [PubMed] [Google Scholar]
- 2. Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4(4):353-357. [DOI] [PubMed] [Google Scholar]
- 3. Olbers T, Lönroth H, Fagevik-Olsén M, Lundell L. Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obes Surg. 2003;13(3):364-370. [DOI] [PubMed] [Google Scholar]
- 4. Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5(4):469-475. [DOI] [PubMed] [Google Scholar]
- 5. Burton PR, Brown WA. The mechanism of weight loss with laparoscopic adjustable gastric banding: induction of satiety not restriction. Int J Obes (Lond). 2011;35(Suppl 3):S26-S30. [DOI] [PubMed] [Google Scholar]
- 6. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737. [DOI] [PubMed] [Google Scholar]
- 7. Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339-50; discussion 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sjöström L, Narbro K, Sjöström CD, et al. ; Swedish Obese Subjects Study . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741-752. [DOI] [PubMed] [Google Scholar]
- 9. Anveden Å, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145(2):224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farias MM, Cuevas AM, Rodriguez F. Set-point theory and obesity. Metab Syndr Relat Disord. 2011;9(2):85-89. [DOI] [PubMed] [Google Scholar]
- 11. Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S37-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597-1604. [DOI] [PubMed] [Google Scholar]
- 13. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24(8):1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laurenius A, Larsson I, Melanson KJ, et al. Decreased energy density and changes in food selection following Roux-en-Y gastric bypass. Eur J Clin Nutr. 2013;67(2):168-173. [DOI] [PubMed] [Google Scholar]
- 16. Laurenius A, Larsson I, Bueter M, et al. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond). 2012;36(3):348-355. [DOI] [PubMed] [Google Scholar]
- 17. Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661-671. [DOI] [PubMed] [Google Scholar]
- 19. Cavin JB, Voitellier E, Cluzeaud F, et al. Malabsorption and intestinal adaptation after one anastomosis gastric bypass compared with Roux-en-Y gastric bypass in rats. Am J Physiol Gastrointest Liver Physiol. 2016;311(3):G492-G500. [DOI] [PubMed] [Google Scholar]
- 20. Barkholt P, Pedersen PJ, Hay-Schmidt A, Jelsing J, Hansen HH, Vrang N. Alterations in hypothalamic gene expression following Roux-en-Y gastric bypass. Mol Metab. 2016;5(4):296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herrick MK, Favela KM, Simerly RB, Abumrad NN, Bingham NC. Attenuation of diet-induced hypothalamic inflammation following bariatric surgery in female mice. Mol Med. 2018;24(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patkar PP, Hao Z, Mumphrey MB, Townsend RL, Berthoud HR, Shin AC. Unlike calorie restriction, Roux-en-Y gastric bypass surgery does not increase hypothalamic AgRP and NPY in mice on a high-fat diet. Int J Obes (Lond). 2019;43(11):2143-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nadreau E, Baraboi ED, Samson P, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes (Lond). 2006;30(3):419-429. [DOI] [PubMed] [Google Scholar]
- 24. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027-1031. [DOI] [PubMed] [Google Scholar]
- 25. Stefater MA, Pérez-Tilve D, Chambers AP, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426-36, 2436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawasaki T, Ohta M, Kawano Y, et al. Effects of sleeve gastrectomy and gastric banding on the hypothalamic feeding center in an obese rat model. Surg Today. 2015;45(12):1560-1566. [DOI] [PubMed] [Google Scholar]
- 27. Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77(5):810-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mumphrey MB, Hao Z, Townsend RL, et al. Eating in mice with gastric bypass surgery causes exaggerated activation of brainstem anorexia circuit. Int J Obes (Lond). 2016;40(6):921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stefanidis A, Forrest N, Brown WA, et al. An investigation of the neural mechanisms underlying the efficacy of the adjustable gastric band. Surg Obes Relat Dis. 2016;12(4):828-838. [DOI] [PubMed] [Google Scholar]
- 31. NBSR. National bariatric surgery register.https://www.bomss.org.uk/wp-content/uploads/2018/11/Extract_from_the_NBSR_2014_Report-2.pdf. Accessed July 1, 2021.
- 32. Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR. Vagal innervation of intestine contributes to weight loss after Roux-en-Y gastric bypass surgery in rats. Obes Surg. 2014;24(12):2145-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bueter M, Löwenstein C, Ashrafian H, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20(5):616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ballsmider LA, Vaughn AC, David M, Hajnal A, Di Lorenzo PM, Czaja K. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015;2015:601985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perathoner A, Weiss H, Santner W, et al. Vagal nerve dissection during pouch formation in laparoscopic Roux-Y-gastric bypass for technical simplification: does it matter? Obes Surg. 2009;19(4):412-417. [DOI] [PubMed] [Google Scholar]
- 36. Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes. 1981;5(5):457-464. [PubMed] [Google Scholar]
- 37. Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav. 2012;107(4):476-483. [DOI] [PubMed] [Google Scholar]
- 38. Nielsen MS, Christensen BJ, Ritz C, et al. Roux-En-Y gastric bypass and sleeve gastrectomy does not affect food preferences when assessed by an ad libitum buffet meal. Obes Surg. 2017;27(10):2599-2605. [DOI] [PubMed] [Google Scholar]
- 39. Scholtz S, Miras AD, Chhina N, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63(6):891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baboumian S, Pantazatos SP, Kothari S, McGinty J, Holst J, Geliebter A. Functional magnetic resonance imaging (fMRI) of neural responses to visual and auditory food stimuli pre and post Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). Neuroscience. 2019;409:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith KR, Papantoni A, Veldhuizen MG, et al. Taste-related reward is associated with weight loss following bariatric surgery. J Clin Invest. 2020;130(8):4370-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldstone AP, Miras AD, Scholtz S, et al. Link between increased satiety gut hormones and reduced food reward after gastric bypass surgery for obesity. randomized controlled trial research support, non-U.S. Gov’t. J Clin Endocrinol Metab. 2016;101(2):599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bueter M, Miras AD, Chichger H, et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104(5):709-721. [DOI] [PubMed] [Google Scholar]
- 45. Miras AD, Jackson RN, Jackson SN, et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr. 2012;96(3):467-473. [DOI] [PubMed] [Google Scholar]
- 46. Abdeen G, Miras A, Alqahtani A, Roux Cl. Vertical sleeve gastrectomy in adolescents reduces the appetitive reward value of a sweet and fatty reinforcer in a progressive ratio task, in press. Surg Obes Relat Dis. 2018;15(2):194-199. [DOI] [PubMed] [Google Scholar]
- 47. Mathes CM, Bohnenkamp RA, Blonde GD, et al. Gastric bypass in rats does not decrease appetitive behavior towards sweet or fatty fluids despite blunting preferential intake of sugar and fat. Physiol Behav. 2015;142:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring). 2014;22(5):E13-E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond). 2011;35(5):642-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berthoud HR, Zheng H, Shin AC. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Ann N Y Acad Sci. 2012;1264(1):36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bond DS, Raynor HA, Vithiananthan S, et al. Differences in salivary habituation to a taste stimulus in bariatric surgery candidates and normal-weight controls. Obes Surg. 2009;19(7):873-878. [DOI] [PubMed] [Google Scholar]
- 52. Farias TMCP, Vasconcelos BCDE, SoutoMaior JR, Lemos CAA, de Moraes SLD, Pellizzer EP. Influence of bariatric surgery on salivary flow: a systematic review and meta-analysis. Obes Surg. 2019;29(5):1675-1680. [DOI] [PubMed] [Google Scholar]
- 53. Hankir MK, Seyfried F, Hintschich CA, et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25(2):335-344. [DOI] [PubMed] [Google Scholar]
- 54. Mathes CM, Bohnenkamp RA, le Roux CW, Spector AC. Reduced sweet and fatty fluid intake after Roux-en-Y gastric bypass in rats is dependent on experience without change in stimulus motivational potency. Am J Physiol Regul Integr Comp Physiol. 2015;309(8):R864-R874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramadan M, Loureiro M, Laughlan K, et al. Risk of dumping syndrome after sleeve gastrectomy and Roux-en-Y gastric bypass: early results of a multicentre prospective study. Gastroenterol Res Pract. 2016;2016:2570237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lamarca F, Melendez-Araújo MS, Porto de Toledo I, Dutra ES, de Carvalho KMB. Relative Energy expenditure decreases during the first year after bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2019;29(8):2648-2659. [DOI] [PubMed] [Google Scholar]
- 57. Wolfe BM, Schoeller DA, McCrady-Spitzer SK, Thomas DM, Sorenson CE, Levine JA. Resting metabolic rate, total daily energy expenditure, and metabolic adaptation 6 months and 24 months after bariatric surgery. Obesity (Silver Spring). 2018;26(5):862-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chu L, Steinberg A, Mehta M, et al. Resting energy expenditure and metabolic adaptation in adolescents at 12 months after bariatric surgery. J Clin Endocrinol Metab. 2019;104(7):2648-2656. [DOI] [PubMed] [Google Scholar]
- 59. Heshka S, Lemos T, Astbury NM, et al. Resting energy expenditure and organ-tissue body composition 5 years after bariatric surgery. Obes Surg. 2020;30(2):587-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilms B, Ernst B, Thurnheer M, Schmid SM, Spengler CM, Schultes B. Resting energy expenditure after Roux-en Y gastric bypass surgery. Surg Obes Relat Dis. 2018;14(2):191-199. [DOI] [PubMed] [Google Scholar]
- 61. Ravelli MN, Schoeller DA, Crisp AH, et al. Accuracy of total energy expenditure predictive equations after a massive weight loss induced by bariatric surgery. Clin Nutr ESPEN. 2018;26:57-65. [DOI] [PubMed] [Google Scholar]
- 62. Werling M, Fändriks L, Olbers T, et al. Roux-en-Y gastric bypass surgery increases respiratory quotient and energy expenditure during food intake. PLoS One. 2015;10(6):e0129784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schmidt JB, Pedersen SD, Gregersen NT, et al. Effects of RYGB on energy expenditure, appetite and glycaemic control: a randomized controlled clinical trial. Int J Obes (Lond). 2016;40(2):281-290. [DOI] [PubMed] [Google Scholar]
- 64. Werling M, Olbers T, Fändriks L, et al. Increased postprandial energy expenditure may explain superior long term weight loss after Roux-en-Y gastric bypass compared to vertical banded gastroplasty. PLoS One. 2013;8(4):e60280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tam CS, Redman LM, Greenway F, LeBlanc KA, Haussmann MG, Ravussin E. Energy metabolic adaptation and cardiometabolic improvements one year after gastric bypass, sleeve gastrectomy, and gastric band. J Clin Endocrinol Metab. 2016;101(10):3755-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Iesari S, le Roux CW, De Gaetano A, Manco M, Nanni G, Mingrone G. Twenty-four hour energy expenditure and skeletal muscle gene expression changes after bariatric surgery. J Clin Endocrinol Metab. 2013;98(2):E321-E327. [DOI] [PubMed] [Google Scholar]
- 67. Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ebbeling CB, Feldman HA, Klein GL, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152(7):1718-1727.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bueter M, Löwenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138(5):1845-1853. [DOI] [PubMed] [Google Scholar]
- 71. Hao Z, Townsend RL, Mumphrey MB, Morrison CD, Münzberg H, Berthoud HR. RYGB produces more sustained body weight loss and improvement of glycemic control compared with VSG in the diet-induced obese mouse model. Obes Surg. 2017;27(9):2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zechner JF, Mirshahi UL, Satapati S, et al. Weight-independent effects of Roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144(3):580-590.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring). 2009;17(10):1839-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Walsberg GE, Hoffman TC. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. J Exp Biol. 2005;208(Pt 6):1035-1043. [DOI] [PubMed] [Google Scholar]
- 75. Ye Y, Abu El Haija M, Morgan DA, et al. Endocannabinoid receptor-1 and sympathetic nervous system mediate the beneficial metabolic effects of gastric bypass. Cell Rep. 2020;33(4):108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200-205. [DOI] [PubMed] [Google Scholar]
- 78. Dadson P, Hannukainen JC, Din MU, et al. Brown adipose tissue lipid metabolism in morbid obesity: effect of bariatric surgery-induced weight loss. Diabetes Obes Metab. 2018;20(5):1280-1288. [DOI] [PubMed] [Google Scholar]
- 79. Hankir MK, Bronisch F, Hintschich C, Krügel U, Seyfried F, Fenske WK. Differential effects of Roux-en-Y gastric bypass surgery on brown and beige adipose tissue thermogenesis. Metabolism. 2015;64(10):1240-1249. [DOI] [PubMed] [Google Scholar]
- 80. Baraboi ED, Li W, Labbé SM, et al. Metabolic changes induced by the biliopancreatic diversion in diet-induced obesity in male rats: the contributions of sleeve gastrectomy and duodenal switch. Endocrinology. 2015;156(4):1316-1329. [DOI] [PubMed] [Google Scholar]
- 81. Chen Y, Yang J, Nie X, Song Z, Gu Y. Effects of bariatric surgery on change of brown adipocyte tissue and energy metabolism in obese mice. Obes Surg. 2018;28(3):820-830. [DOI] [PubMed] [Google Scholar]
- 82. Vosselman MJ, van Marken Lichtenbelt WD, Schrauwen P. Energy dissipation in brown adipose tissue: from mice to men. Mol Cell Endocrinol. 2013;379(1-2):43-50. [DOI] [PubMed] [Google Scholar]
- 83. Porter C, Herndon DN, Chondronikola M, et al. Human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metab. 2016;24(2):246-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dimitriadis GK, Randeva MS, Miras AD. Potential hormone mechanisms of bariatric surgery. Curr Obes Rep. 2017;6(3):253-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10(10):575-584. [DOI] [PubMed] [Google Scholar]
- 86. Larraufie P, Roberts GP, McGavigan AK, et al. Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep. 2019;26(6):1399-1408.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):E424-E432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nielsen MS, Ritz C, Wewer Albrechtsen NJ, Holst JJ, le Roux CW, Sjodin A. Oxyntomodulin and glicentin may predict the effect of bariatric surgery on food preferences and weight loss. J Clin Endocrinol Metab. 2020;105(4):e1064-e1074. [DOI] [PubMed] [Google Scholar]
- 89. Svane MS, Jørgensen NB, Bojsen-Møller KN, et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2016;40(11):1699-1706. [DOI] [PubMed] [Google Scholar]
- 90. Ye J, Hao Z, Mumphrey MB, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306(5):R352-R362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Carmody JS, Muñoz R, Yin H, Kaplan LM. Peripheral, but not central, GLP-1 receptor signaling is required for improvement in glucose tolerance after Roux-en-Y gastric bypass in mice. Am J Physiol Endocrinol Metab. 2016;310(10):E855-E861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Boland B, Mumphrey MB, Hao Z, et al. The PYY/Y2R-deficient mouse responds normally to high-fat diet and gastric bypass surgery. Nutrients. 2019;11(3). doi: 10.3390/nu11030585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dischinger U, Corteville C, Otto C, Fassnacht M, Seyfried F, Hankir MK. GLP-1 and PYY3-36 reduce high-fat food preference additively after Roux-en-Y gastric bypass in diet-induced obese rats. Surg Obes Relat Dis. 2019;15(9):1483-1492. [DOI] [PubMed] [Google Scholar]
- 94. Goldstone AP, Miras AD, Scholtz S, et al. Link between increased satiety gut hormones and reduced food reward after gastric bypass surgery for obesity. J Clin Endocrinol Metab. 2016;101(2):599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Perakakis N, Mantzoros CS. The role of glicentin and oxyntomodulin in human metabolism: new evidence and new directions. J Clin Endocrinol Metab. 2020;105(8):e3003-e3005. [DOI] [PubMed] [Google Scholar]
- 96. Perakakis N, Kokkinos A, Peradze N, et al. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: evidence from two independent trials. Metabolism. 2019;101:153997. [DOI] [PubMed] [Google Scholar]
- 97. Papamargaritis D, le Roux CW. Do gut hormones contribute to weight loss and glycaemic outcomes after bariatric surgery? Nutrients. 2021;13(3). doi: 10.3390/nu13030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484-489. [DOI] [PubMed] [Google Scholar]
- 99. Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36(7):1859-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gallego-Escuredo JM, Gómez-Ambrosi J, Catalan V, et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes (Lond). 2015;39(1):121-129. [DOI] [PubMed] [Google Scholar]
- 101. Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594-2603. [DOI] [PubMed] [Google Scholar]
- 102. Liu S, Marcelin G, Blouet C, et al. A gut-brain axis regulating glucose metabolism mediated by bile acids and competitive fibroblast growth factor actions at the hypothalamus. Mol Metab. 2018;8:37-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53(5):934-940. [DOI] [PubMed] [Google Scholar]
- 104. Díaz-Delfín J, Hondares E, Iglesias R, Giralt M, Caelles C, Villarroya F. TNF-α represses β-Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology. 2012;153(9):4238-4245. [DOI] [PubMed] [Google Scholar]
- 105. Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. J Clin Endocrinol Metab. 2012;97(6):2143-2150. [DOI] [PubMed] [Google Scholar]
- 106. Søberg S, Sandholt CH, Jespersen NZ, et al. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 2017;25(5):1045-1053.e6. [DOI] [PubMed] [Google Scholar]
- 107. Albaugh VL, Banan B, Ajouz H, Abumrad NN, Flynn CR. Bile acids and bariatric surgery. Mol Aspects Med. 2017;56:75-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jahansouz C, Xu H, Hertzel AV, et al. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann Surg. 2016;264(6):1022-1028. [DOI] [PubMed] [Google Scholar]
- 109. Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab. 2015;100(9):E1225-E1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Risstad H, Kristinsson JA, Fagerland MW, et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg Obes Relat Dis. 2017;13(9):1544-1553. [DOI] [PubMed] [Google Scholar]
- 111. Kuhre RE, Wewer Albrechtsen NJ, Larsen O, et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab. 2018;11:84-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Biemann R, Penner M, Borucki K, et al. Serum bile acids and GLP-1 decrease following telemetric induced weight loss: results of a randomized controlled trial. Sci Rep. 2016;6:30173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring). 2013;21(12):E660-E668. [DOI] [PubMed] [Google Scholar]
- 114. Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab. 2015;100(3):E396-E406. [DOI] [PubMed] [Google Scholar]
- 115. Haluzíková D, Lacinová Z, Kaválková P, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (Silver Spring). 2013;21(7):1335-1342. [DOI] [PubMed] [Google Scholar]
- 116. Fjeldborg K, Pedersen SB, Møller HJ, Richelsen B. Reduction in serum fibroblast growth factor-21 after gastric bypass is related to changes in hepatic fat content. Surg Obes Relat Dis. 2017;13(9):1515-1523. [DOI] [PubMed] [Google Scholar]
- 117. Crujeiras AB, Gomez-Arbelaez D, Zulet MA, et al. Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: a marker of metabolic stress? Int J Obes (Lond). 2017;41(10):1570-1578. [DOI] [PubMed] [Google Scholar]
- 118. Yuan ZQ, Li KW. Role of farnesoid X receptor in cholestasis. J Dig Dis. 2016;17(8):501-509. [DOI] [PubMed] [Google Scholar]
- 119. Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46(4):302-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chaudhari SN, Harris DA, Aliakbarian H, et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat Chem Biol. 2021;17(1):20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ding L, Sousa KM, Jin L, et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. 2016;64(3):760-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Velazquez-Villegas LA, Perino A, Lemos V, et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun. 2018;9(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. McGavigan AK, Garibay D, Henseler ZM, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66(2):226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hao Z, Leigh Townsend R, Mumphrey MB, et al. Roux-en-Y gastric bypass surgery-induced weight loss and metabolic improvements are similar in TGR5-deficient and wildtype mice. Obes Surg. 2018;28(10):3227-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39(2):338-342. [DOI] [PubMed] [Google Scholar]
- 128. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li B, Li L, Li M, et al. Microbiota depletion impairs thermogenesis of brown adipose tissue and browning of white adipose tissue. Cell Rep. 2019;26(10):2720- 2737 e5. [DOI] [PubMed] [Google Scholar]
- 130. Le Chatelier E, Nielsen T, Qin J, et al. ; MetaHIT consortium . Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541-546. [DOI] [PubMed] [Google Scholar]
- 131. Qin J, Li R, Raes J, et al. ; MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787-8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522. [DOI] [PubMed] [Google Scholar]
- 138. Palmisano S, Campisciano G, Silvestri M, et al. Changes in gut microbiota composition after bariatric surgery: a new balance to decode. J Gastrointest Surg. 2020;24(8):1736-1746. [DOI] [PubMed] [Google Scholar]
- 139. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li JV, Reshat R, Wu Q, et al. Experimental bariatric surgery in rats generates a cytotoxic chemical environment in the gut contents. Front Microbiol. 2011;2:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kong LC, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16-24. [DOI] [PubMed] [Google Scholar]
- 143. Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Campisciano G, Palmisano S, Cason C, et al. Gut microbiota characterisation in obese patients before and after bariatric surgery. Benef Microbes. 2018;9(3):367-373. [DOI] [PubMed] [Google Scholar]
- 145. Lee CJ, Florea L, Sears CL, et al. Changes in gut microbiome after bariatric surgery versus medical weight loss in a pilot randomized trial. Obes Surg. 2019;29(10):3239-3245. [DOI] [PubMed] [Google Scholar]
- 146. Kashihara H, Shimada M, Yoshikawa K, et al. Duodenal-jejunal bypass changes the composition of the gut microbiota. Surg Today. 2017;47(1):137-140. [DOI] [PubMed] [Google Scholar]
- 147. Yu X, Wu Z, Song Z, et al. Single-anastomosis duodenal jejunal bypass improve glucose metabolism by regulating gut microbiota and short-chain fatty acids in Goto-Kakisaki rats. Front Microbiol. 2020;11:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. de Jonge C, Fuentes S, Zoetendal EG, et al. Metabolic improvement in obese patients after duodenal-jejunal exclusion is associated with intestinal microbiota composition changes. Int J Obes (Lond). 2019;43(12):2509-2517. [DOI] [PubMed] [Google Scholar]
- 149. Ruban A, Miras AD, Glaysher MA, et al. Duodenal-jejunal bypass liner for the management of type 2 diabetes mellitus and obesity: a multicenter randomized controlled trial. Ann Surg. 2021. doi: 10.1097/sla.0000000000004980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Aron-Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68(1):70-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611-619.e6. [DOI] [PubMed] [Google Scholar]
- 153. Thaiss CA, Itav S, Rothschild D, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540(7634):544-551. [DOI] [PubMed] [Google Scholar]
- 154. Elkhenini HF, New JP, Syed AA. Five-year outcome of bariatric surgery in a patient with melanocortin-4 receptor mutation. Clin Obes. 2014;4(2):121-124. [DOI] [PubMed] [Google Scholar]
- 155. Hatoum IJ, Greenawalt DM, Cotsapas C, Daly MJ, Reitman ML, Kaplan LM. Weight loss after gastric bypass is associated with a variant at 15q26.1. Am J Hum Genet. 2013;92(5):827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]