Abstract
The obesity pandemic increasingly causes morbidity and mortality from type 2 diabetes, cardiovascular diseases and many other chronic diseases. Fat cell size (FCS) predicts numerous obesity-related complications such as lipid dysmetabolism, ectopic fat accumulation, insulin resistance, and cardiovascular disorders. Nevertheless, the scarcity of systematic literature reviews on this subject is compounded by the use of different methods by which FCS measurements are determined and reported. In this paper, we provide a systematic review of the current literature on the relationship between adipocyte hypertrophy and obesity-related glucose and lipid dysmetabolism, ectopic fat accumulation, and cardiovascular disorders. We also review the numerous mechanistic origins of adipocyte hypertrophy and its relationship with metabolic dysregulation, including changes in adipogenesis, cell senescence, collagen deposition, systemic inflammation, adipokine secretion, and energy balance. To quantify the effect of different FCS measurement methods, we performed statistical analyses across published data while controlling for body mass index, age, and sex.
Keywords: Adipocyte hypertrophy, diabetes, obesity, cardiometabolic disorders, systematic review, meta-analysis
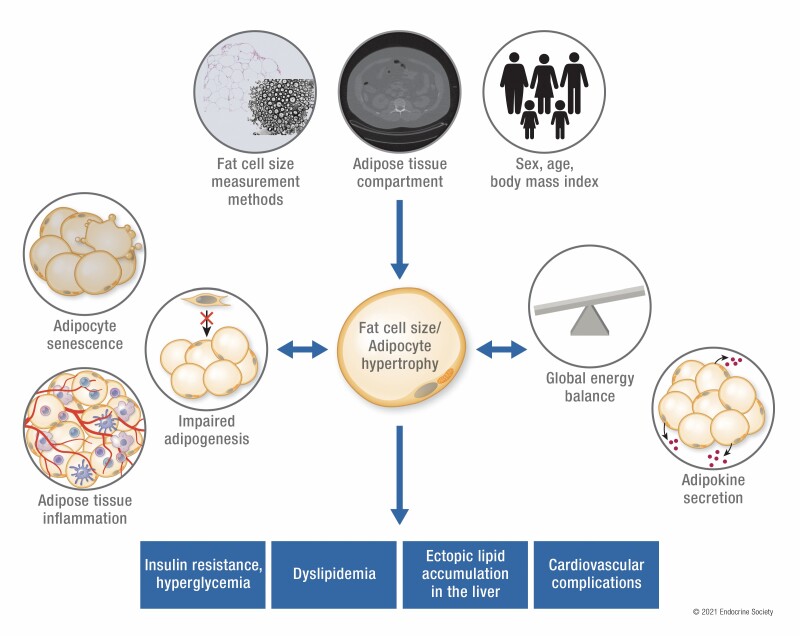
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
Meta-analyses showed that fat cell size vary according to measurement methods, adipose tissue depots, as well as age and body mass index.
Adipocyte hypertrophy is associated with dysregulations in glucose metabolism, lipid metabolism, ectopic fat accumulation, and cardiovascular endpoints independently of body mass index.
Adipocyte hypertrophy is related to impaired adipose tissue differentiation potential and rate of adipogenesis, adipose tissue inflammation, and altered adipokine secretion.
Metabolic interventions resulting in negative and positive energy balance have led respectively to reduced and increased fat cell size.
The obesity pandemic has gained increasing attention over the last 2 decades. However, the severity of obesity-related metabolic complications does not seem to depend solely on the quantity of excess adipose tissue. In this regard, this differential effect of obesity can be explained by differences in adipose tissue dysmetabolism. An important indicator of such dysmetabolism of adipose tissue is adipocyte hypertrophy. Fat cell size (FCS) predicts numerous obesity-related complications such as lipid dysmetabolism, ectopic fat accumulation, insulin resistance, and cardiovascular disorders.
Nevertheless, the scarcity of systematic literature reviews on this subject is compounded by diverse methods used to report FCS measurements. Therefore, we performed statistical analyses across published studies to quantify the effects of FCS measurement methods, while also introducing a novel technique to standardize the FCS measurements reported in different dimensions. Furthermore, we present herein an extensive review of the relationships between adipocyte hypertrophy and systemic metabolic dysregulation. Finally, we also review the mechanistic origins of adipocyte hypertrophy and its relationships with metabolic dysregulation.
Methods for the Systematic Review
Articles identified through primary database screening were assessed for eligibility if they were original, peer-reviewed research conducted on humans and published in English. All articles that were deemed eligible for meta-analysis had to contain measurements of adipocyte size as well as information pertaining to (1) the site(s) at which adipose tissue (AT) samples were collected; (2) the type of method employed for the measurement of cell size; and (3) the type of FCS data (eg, diameter, volume, and cross-sectional area).
The PubMed database was searched up to January 25, 2021, using both Medical Subject Heading terms and keywords. A complete description of the strategy used can be found elsewhere (1).
The study selection process and the assessment for eligibility of articles were done following the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines. The evaluation of potential articles, examination of the title, abstract, full text, and supplementary materials were done by the first author. The following data were extracted from the selected articles: general information (ie, title, year published, DOI, study design), characteristics of participants (ie, number of participants in each subgroup, if any, number of women and men, average age, body mass index (BMI), weight, fat mass, percent fat, waist and hip circumference), measures of AT adiposity (ie, adipocyte size, the site of AT biopsy, and the type of methodology employed).
For studies that only analyzed data from an existing cohort that had already been previously evaluated, we made sure that no other study that used the same dataset was included in our quantitative analysis. Furthermore, for studies that investigated clinical samples including pathological conditions, only data from the participants from the control group or those with prediabetes or type 2 diabetes (T2D) were extracted.
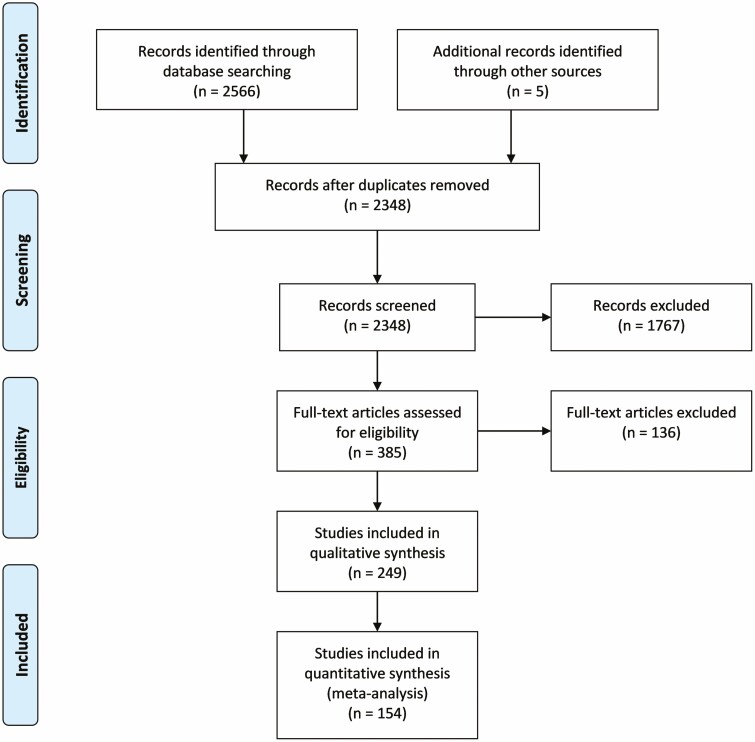
Two thousand five hundred and seventy-one articles were identified; after removal of duplicates, 2348 records were obtained. Examination of titles and abstracts allowed the inclusion of 385 articles for eligibility assessment. After full text screening of these articles, 249 articles were retained for qualitative synthesis, and, among these, 154 met the criteria for inclusion in quantitative analysis (Fig. 1). Data from 3681 men and 8434 women, which included 12 705 distinct biopsy samples from different sites, were used for statistical analyses.
Figure 1.
Prisma flowchart showing the number of articles at each step of the literature review and meta-analysis.
Collagenase digestion of adipose tissue followed by microscopic examination (CD) constitutes the most frequently used method and was employed in 89 of the 154 studies; 38 studies used the histological section (HS) technique, while the remaining 26 used the osmium fixation (OF) method (ie, osmium fixation of adipocytes followed by size measurement via a Coulter counter); 1 study used all 3 methods. For adipocyte size information presented in graphical form, an online utility, WebPlotDigitizer (2), was used. This tool is highly reliable for numerical data extraction from published figures (3).
The reporting of adipocyte size across various studies was not standardized; indeed, some expressed average size in terms of diameter (in μm), others reported it as volume (in pL) or mass (in μg of lipid per cell), and still others used cross-sectional area (in μm2). Therefore, for quantitative analysis, all data were converted to measures of diameter. However, an equation exists only to calculate average volume from average diameter (4), which was employed in a previous meta-analysis on adipocyte size by Murphy et al. (5); we have therefore expanded on this idea by developing formulae to solve the reverse problem (1). Similar formulae were also found to convert cell area to cell diameter (1). For adipocyte sizes expressed in terms of micrograms of lipid per cell, the density of triolein (0.915 g/mL) was used to change measurements of mass to volume. Details the statistical approaches used for nonlinear modeling of FCS and tests of group comparisons can be found elsewhere (1).
Adipose Tissue Expansion
Adipose tissues grow through a process known as adipogenesis, which can be defined as the ability of preadipocytes to multiply and to differentiate into mature adipocytes. During the development of obesity as a result of chronic positive energy balance, adipose tissue volume increases via 2 main processes: adipocyte hypertrophy (increase in FCS) and hyperplasia (increase in fat cell number). In contrast to adipocyte hyperplasia, hypertrophy of fat cells portends worse insulin sensitivity, glucose disposal, lipid metabolism, and cardiovascular outcomes independently of the effect of obesity alone.
One of the key factors that may pave the way to fat cell hypertrophy is impaired adipogenesis. By inducing differentiation of preadipocytes from abdominal subcutaneous adipose tissue (SCAT), Isakson et al. showed that differentiation potential of these cells was negatively associated with FCS and was reduced in individuals with obesity despite a higher number of CD133-positive preadipocytes (6). Park et al. later corroborated this finding by showing that SC CD34+/CD31– preadipocyte differentiation was negatively correlated with both SCAT and visceral FCS (7). The rate of production of new adipocytes was also significantly lower in individuals with adipocyte hypertrophy than in those with hyperplasia (8). In a sample of women who are healthy, the negative association between SCAT rate of adipogenesis and visceral FCS was found even after matching for BMI (9).
Furthermore, FCS and the rate of in vitro differentiation of SC adipocytes is influenced by several regulators of adipogenesis. FCS in children was positively correlated with expression of the meteorin-like protein, which decreases PPAR-γ expression and adipocyte differentiation (10). In omental ATs of individuals with severe obesity, FCS is strongly associated with AT content of Pref-1, which inhibits differentiation of preadipocytes (11). In addition, other negative regulators of adipogenesis, such as Wnt (6, 12, 13), Notch (14), and retinoid-related orphan receptor gamma (15), have also been implicated in adipocyte hypertrophy in numerous human studies. Induction of PPAR-γ constitutes an important mediator of adipocyte differentiation; as such, individuals with obesity have higher expression of mitogen-activated protein kinase 4, an enzyme which is stimulated by TNF-α and blunts the activation of PPAR-γ (6).
In summary, adipocyte hypertrophy is related with impaired AT differentiation potential and rate of adipogenesis. Causality of this association is uncertain. Moreover, there is conflicting data regarding the effects of induction of adipogenesis on FCS in humans.
Relation Between Adipocyte Hypertrophy and Adipose Tissue Pathophysiological Processes
Adipocyte Senescence
Not only has FCS been correlated with reduced adipogenesis, it was also associated with adipocyte death and senescence. In SCAT adipocytes, Gustafson et al. (16) showed that adipocyte hypertrophy was positively correlated with markers of cell senescence, notably plasminogen activator inhibitor-1 (PAI1), TP53, and transforming growth factor beta 1 (TGFB1). In epicardial adipocytes, similar relationships have been found with the expression of p53 (17).
By measuring the number of crown-like structures in AT samples as a surrogate for the frequency of cell death, Cinti et al. (18) found that adipocyte hypertrophy was associated with adipocyte necrosis. Telomere shortening is also known to be a marker of cell senescence. Accordingly, adipocyte telomere length was shorter in individuals with fat cell hypertrophy (19-21). Therefore, the current limited evidence suggests that adipocyte hypertrophy does accompany cell senescence, cell necrosis, and telomere shortening in AT.
Inflammation
A mounting body of evidence has also implicated adipocyte hypertrophy in the development of the inflammatory response. Indeed, there appears to be a stronger correlation between adipocyte size and inflammation than between obesity and inflammation, in terms of macrophage content in ATs (22) and of IL-6 and TNF-α levels (23). In a randomized controlled trial comparing a 26-week treatment with valsartan (an angiotensin receptor antagonist mainly used as an antihypertensive medication) vs placebo, no weight change was observed between the 2 groups; nevertheless, valsartan significantly reduced both adipocyte size and expression of inflammatory genes. Moreover, the change in FCS induced by valsartan was positively correlated with the change in inflammatory gene expression (24).
Higher FCS, determined with histological analysis, was also associated with an increased number of infiltrating macrophages in human AT (22, 25). Using osmium fixation, however, the number of CD68+ and CD163/MAC2+ macrophages was negatively correlated with the size of small and medium-sized fat cells (26). This result is, in fact, consistent with the capacity of osmium fixation to detect small adipocytes. Acosta et al. found that adipocyte hypertrophy was associated with increases in the ratio of M1/M2 macrophages (27). Other studies have also suggested a potential relationship between adipocyte hypertrophy and increased AT M1 macrophages.
Adipocyte hypertrophy is associated with increased gene expression of numerous proinflammatory factors. FCS was correlated with the expression of CEBPB (CCAAT Enhancer Binding Protein Beta) mRNA (28), CD68 (cluster of differentiation 68) (24, 28-31), TNF-α (tumor necrosis factor-α) (29), TNF (tumor necrosis factor) receptor (32), biglycan (33), MCP-1 (30, 34), MCP-2 (30), MIP-1α (macrophage inflammatory protein-1α) (30), MIF (Macrophage migration inhibitory factor) (35), and RANTES (Regulated on Activation, Normal T Cell Expressed and Secreted) (36). Using osmium fixation, adipocyte hypertrophy was characterized by an increase in the proportion of small adipocytes. Accordingly, 2 studies that employed osmium fixation to measure FCS found that the fraction of small cells correlated positively with the expression of inflammatory factors (37), including MIP-1α and MCP-1α (26). In addition, larger adipocytes expressed more serum amyloid A (SAA) gene than smaller adipocytes (38), and serum level of SAA was also correlated with SCAT FCS (39).
The alteration in inflammatory gene expression related to adipocyte hypertrophy is also reflected by changes in downstream secretion of inflammatory factors. Adipocyte enlargement is associated with increased IL-6 (13, 23, 29, 40-42), IL-8 (13, 41), CRP (25, 42-45), TNF-α (23, 27, 41, 42), MCP-1 (13, 41), and MIP-1β (41) secretion. Furthermore, increased FCS is associated with reduction in IL-10 production (41), which may allow M2 polarization of macrophages.
In summary, our review of the literature reveals strong relationships between adipocyte hypertrophy and AT inflammation which could not be merely explained by obesity or increased BMI.
Adipokine Secretion
Adipokines are hormones secreted by adipose tissues that mediate numerous systemic effects. Leptin and adiponectin are the 2 main adipokines that have been extensively investigated in relation to adipocyte hypertrophy.
Leptin is an anorectic hormone that regulates food intake and energy expenditure, but also reduces fat accumulation in the liver and muscles. Many studies have found that individuals with enlarged fat mass had higher plasma concentrations of leptin (29, 43, 46-52), higher AT leptin content (53), increased leptin secretion (41, 51), and higher AT LEP mRNA levels (54). Although leptin concentration is positively correlated with BMI (48, 55) and fat mass (46, 48) and decreases proportionally to weight loss (51, 56), leptin level is associated with adipocyte hypertrophy independently of AT mass and BMI. In a large cohort (46), FCS was correlated with leptin levels even with adjustment for AT mass. Along with AT mass, FCS accounted for more than 60% of the variation in leptin levels. AT gene expression of leptin decreased (57) with diet-induced weight reduction and leptin receptor expression increased after Roux-en-Y gastric bypass (58), suggesting an increased leptin sensitivity with caloric restriction.
Adiponectin is associated with increased insulin sensitivity, increased adipogenesis, and decreased inflammation. In various studies, higher FCS was associated with lower serum adiponectin concentrations (19, 28, 33, 42, 49, 52, 59), reduced expression of adiponectin (54, 60), and lower secretion (41) and release (61) of adiponectin in AT. With weight reduction, improvements in insulin sensitivity (62) and BMI (63) were associated with increased secretion of adiponectin. However, there is insufficient data to conclude whether the association between FCS and adiponectin production is independent of obesity.
The correlation between FCS and adiponectin levels was also weaker and more difficult to detect than that between FCS and serum leptin. This was especially the case when using SCAT and when the histological section or collagenase digestion method was employed. Indeed, a comparative study of the 3 sizing methods revealed that osmium fixation yielded the strongest signals for the correlations between FCS and both plasma adiponectin and leptin (52). At least 3 studies (13, 61, 64) failed to detect any statistically significant correlation between FCS and adiponectin; all of them used the collagenase digestion method and SCAT biopsies.
In conclusion, increased FCS is strongly associated with AT leptin expression, production, secretion, and plasma leptin level. These associations are independent of obesity and cannot be entirely accounted for by genetic and/or epigenetic factors. On the contrary, increased FCS is correlated with reduced adiponectin production and release by AT.
Variation in Fat Cell Size Due to Methodological Factors
The use of different techniques, ie, CD, OF, or HS, to ascertain mean adipocyte size has been shown to yield different results. Independent of the adipose tissue sample and of the BMI of the participant, CD and OF both appear to produce higher cell size estimates than HS; moreover, OF also provides marginally higher measurements than CD, although this difference diminishes with larger BMI. A study directly comparing these 3 methods published by Laforest et al. (52) corroborated the above observations.
Our meta-regression analysis demonstrates that compared to CD, HS yielded significantly lower values of abdominal subcutaneous (SC) adipocyte diameter (β = –20.2385, P < .0001), while OF resulted in higher estimates of cell size (β = 6.3998, P = .0166) (Table 1), after accounting for sex, age, and BMI. For visceral adipocytes, similar results were found, with the HS method revealing smaller average cell diameters than CD (β = –20.3486, P < .0001) (Table 2).
Table 1.
Mixed effects model multiple meta-regression of abdominal subcutaneous adipocyte diameter and cell measurement methods (HS and of compared with CD as the reference method)
| Dependent variable: abdominal subcutaneous adipocyte diameter (μm) | ||||
|---|---|---|---|---|
| Predictor | Coefficient (β) | Lower bound (95% CI) | Upper bound (95% CI) | P value |
| (Intercept) | 65.6814 | 55.6909 | 75.6719 | <.0001 |
| HS (compared to CD) | –20.2385 | –24.1850 | -16.2920 | <.0001 |
| OF (compared to CD) | 6.3998 | 1.1725 | 11.6271 | .0166 |
| BMI | 0.8813 | 0.6626 | 1.0999 | <.0001 |
| Age | 0.2659 | 0.1094 | 0.4224 | .0009 |
| % Women | –0.0039 | –4.5339 | 4.5261 | .9986 |
Abbreviations: BMI, body mass index; CD, collagenase digestion; CI, confidence interval; HS, histological section; OF, osmium fixation; % Women, percentage of women.
Table 2.
Mixed effects model multiple meta-regression of visceral adipocyte diameter and cell measurement methods (HS and OF compared with CD as the reference method)
| Dependent variable: visceral adipocyte diameter (μm) | ||||
|---|---|---|---|---|
| Predictor | Coefficient (β) | Lower bound (95% CI) | Upper bound (95% CI) | P-value |
| (Intercept) | 26.7406 | -0.4224 | 53.9035 | .0536 |
| HS (compared to CD) | –20.3486 | –26.2228 | –14.4744 | <.0001 |
| OF (compared to CD) | 5.7024 | –6.3914 | 17.7961 | .3511 |
| BMI | 1.1628 | 0.8698 | 1.4558 | <.0001 |
| Age | 0.7394 | 0.3419 | 1.1368 | .0004 |
| % Women | –7.2799 | –15.5429 | 0.9830 | .0834 |
Abbreviations: BMI, body mass index; CD, collagenase digestion; CI, confidence interval; HS, histological section; OF, osmium fixation; % Women, percentage of women.
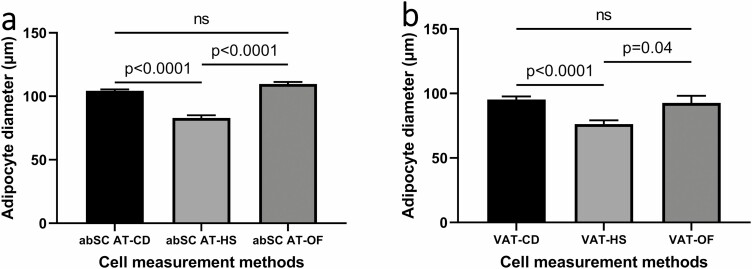
Analysis of variance also showed statistically significant differences between results obtained with the 3 methods not only in SCAT (P < .0001), but also in visceral adipose tissue (VAT) (P < .0001). Multiple comparison tests confirmed variations in median values (Fig. 2A and 2B), except for adipocyte measurements between CD and OF, where the differences were nonsignificant.
Figure 2.
Adipocyte diameter according to different size measurement methods. (A) Abdominal subcutaneous adipocyte diameter. (B) Visceral adipocyte diameter. abSC, abdominal subcutaneous; CD, collagenase digestion; HS, histological section; OF, osmium fixation; VAT, visceral adipose tissue.
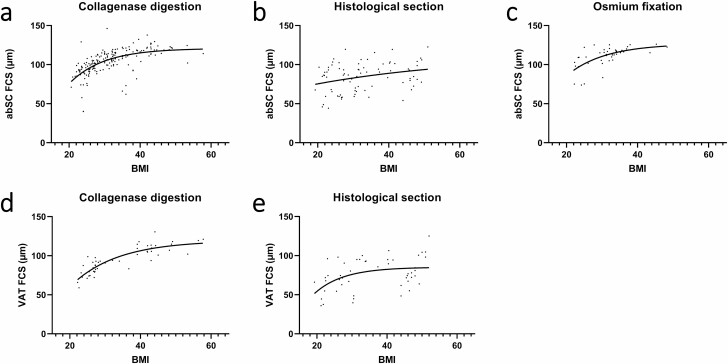
In addition, nonlinear regression analysis of abdominal subcutaneous adipocyte diameter with respect to BMI using an exponential plateau model, resulted in different maximum plateau diameter values (Dmax) for the 3 methods: 120.8 vs 112.5 vs 126.7 μm for the CD, HS, and OF methods, respectively (Fig. 3A-3C). In the case of visceral adipocytes, similar results were found, with the CD method yielding higher Dmax than HS (119.4 vs 85.21 μm) (Fig. 3D and 3E). However, due to the limited number of data points with OF for visceral adipocytes, regression analysis was not performed.
Figure 3.
Adipocyte diameter in relation to body mass index (BMI) according to different size measurement methods. (A) Abdominal subcutaneous adipocyte diameter (in µm) assessed using collagenase digestion. (B) Abdominal subcutaneous adipocyte diameter (in µm) assessed using histological section. (C) Abdominal subcutaneous adipocyte diameter (in µm) assessed using osmium fixation. (d) Visceral adipocyte diameter (in µm) assessed using collagenase digestion. (E) Visceral adipocyte diameter (in µm) assessed using histological section. abSC, abdominal subcutaneous; CD, collagenase digestion; FCS, fat cell size; HS, histological section; OF, osmium fixation; VAT, visceral adipose tissue.
Our meta-analysis corroborated the influence of measurement techniques on average cell diameter. The fact that these differences did not depend on BMI, sex, or age also suggests that they are dependent on the specific methodology. Indeed, many sources of bias in each method may contribute to these observed variations. In both CD and OF, there is the risk of disruption of larger adipocytes due to their increased fragility (65). With OF, there is an added advantage of being able to study cells with very small diameters (<50 μm) (37, 66, 67); nevertheless, because it is the only method in which adipocytes are not directly visualized, there is also the possibility of including small cell fragments. Furthermore, OF is known to cause swelling in a variety of tissues, a phenomenon which has been well documented (68, 69) and reviewed (70, 71). This swelling is thought to be caused by increase in tissue weight due to osmium tetroxide uptake and osmotic pressure gradient and may explain the consistently higher estimates of FCS by OF. The most significant source of bias that accounts for size underestimation with HS is the fact that most cells are likely not cut through their geometric center, hence yielding smaller cross-sectional areas.
In summary, we found that the 3 techniques for FCS measurement generate quantitatively different results, regardless of sex, age, and BMI. HS always produces lower estimates than CD. OF results in slightly higher size measurements than CD in SCAT. These variations are likely due to differences in cell fragility, physical, chemical, statistical, and/or geometric factors.
Regional variations in adipocyte size in relation to cell measurement method
The existing literature suggests that adipocytes are smaller in abdominal SCAT than in femoral (72, 73) and gluteal SCAT (74). However, using the CD method, Berman et al found no differences in cell size between abdominal and gluteal SCAT of women with obesity who were postmenopausal and Caucasian (75). Later, another study using the same method showed smaller abdominal than gluteal SC adipocytes in women with obesity who were African-American but not women with obesity who were non-Hispanic Caucasian (76). Using the HS method, Joffe et al. have provided further evidence supporting this racial difference in regional variations by observing increased cell size in gluteal relative to the abdominal region in women of African descent with and without obesity (77).
Cell size variations between the SC and visceral depots are also very well established. Overall, omental VAT contains adipocytes with smaller diameters than does abdominal SCAT, regardless of the technique used to determine cell size, be it with CD (78-82), HS (83, 84), or OF (85). Nonetheless, van Beek et al. studied abdominal SCAT and VAT in women with obesity and challenged this observation by finding no difference in cell size between these depots (86). Moreover, SC adipocytes are also larger than epicardial AT cells, as demonstrated using both the CD (87) and HS methods (88). Finally, studies using all 3 methods (CD (52), HS (52, 89), and OF (52)) have shown positive correlations between visceral and SC adipocyte diameter.
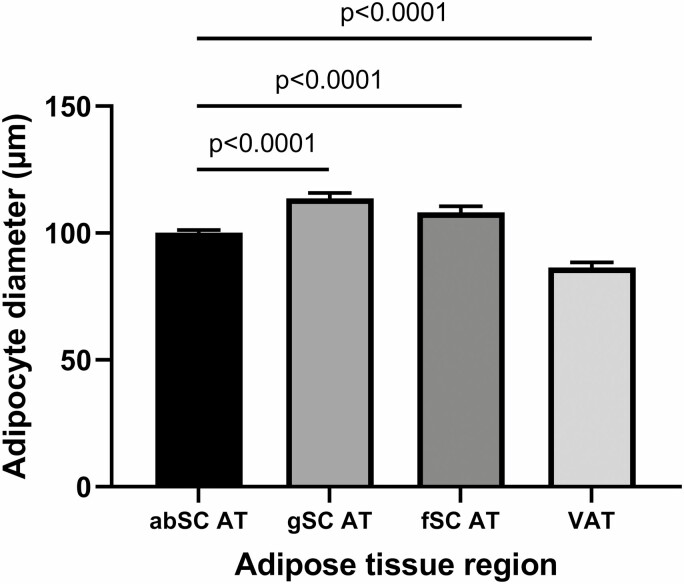
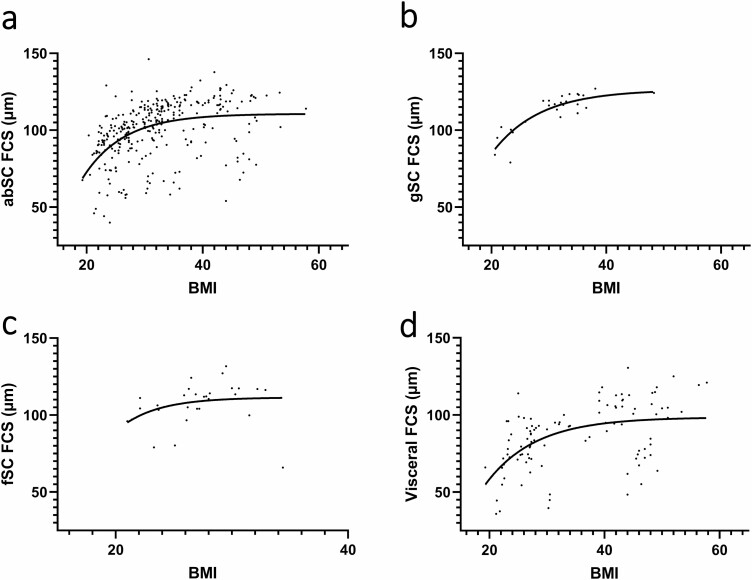
Because studies that reported cell size other than that of abdominal SC adipocytes usually also measured abdominal SC FSC, we assessed differences in FCS across various AT regions. Results showed that abdominal SCAT FCS was significantly smaller than gluteal SCAT (P < .0001) and femoral SCAT FCS (P < .0001), and larger than visceral AT FCS (P < .0001) (Fig. 4). Furthermore, gluteal SC and femoral SC FCS also plateaued at higher values of Dmax (126.3 μm and 111.4 μm, respectively) with increasing level of obesity than did abdominal SC FCS (110.7 μm) and visceral FCS (98.51 μm) (Fig. 5).
Figure 4.
Adipocyte diameter across different adipose tissue regions. abSC, abdominal subcutaneous; AT, adipose tissue; fSC, femoral subcutaneous; gSC, gluteal subcutaneous; VAT, visceral adipose tissue.
Figure 5.
Fat cell size as a function of BMI in different adipose tissue depots. (a) Abdominal subcutaneous fat cell size as a function of BMI. (B) Gluteal subcutaneous fat cell size as a function of BMI. (C)Femoral subcutaneous fat cell size as a function of BMI. (D) Visceral fat cell size as a function of BMI. abSC, abdominal subcutaneous; AT, adipose tissue; BMI, body mass index; FCS, fat cell size; fSC, femoral subcutaneous; gSC, gluteal subcutaneous; VAT, visceral adipose tissue.
Multiple meta-regression was then performed to assess the relationships between abdominal SC FCS and FCS from the other depots. Results (Table 3) showed that adipocyte size from the abdominal SC region, independent of sex, was a strong predictor of cell diameter from the gluteal, femoral, and visceral depots.
Table 3.
Mixed effects model multiple meta-regression of gluteal, femoral, and visceral adipocyte diameter as a function of subcutaneous adipocyte diameter and sex
| Predictor | Coefficient (β) | Lower bound (95% CI) | Upper bound (95% CI) | P value |
|---|---|---|---|---|
| Dependent variable: gluteal subcutaneous adipocyte diameter (μm) | ||||
| (Intercept) | 22.5476 | 14.9479 | 30.1474 | <.0001 |
| abSC FCS | 0.8129 | 0.7424 | 0.8833 | <.0001 |
| % Women | 1.7878 | –1.9485 | 5.5242 | .3363 |
| Dependent variable: femoral subcutaneous adipocyte diameter (μm) | ||||
| (Intercept) | 13.6637 | –1.7442 | 29.0715 | .0801 |
| abSC FCS | 0.9127 | 0.7639 | 1.0614 | <.0001 |
| % Women | 3.4501 | –0.7970 | 7.6973 | .1074 |
| Dependent variable: visceral subcutaneous adipocyte diameter (μm) | ||||
| (Intercept) | 3.8491 | –4.7361 | 12.4344 | .3747 |
| abSC FCS | 0.9325 | 0.8535 | 1.0114 | <.0001 |
| % Women | –11.2152 | –15.5459 | –6.8845 | <.0001 |
Abbreviations: abSC, abdominal subcutaneous; CI, confidence interval; FCS, fat cell size; % Women, percentage of women.
To summarize, it is clear from individual studies that femoral and gluteal AT contain larger adipocytes than does abdominal SCAT, whereas VAT contains smaller cells than abdominal SCAT. Furthermore, FCS in abdominal SCAT is positively correlated with FCS in all other ATs. These finding were confirmed by our numerical meta-analyses.
Sex differences in adipocyte size
In contrast to regional differences, sexual dimorphism in FCS was less clearly demonstrated. All 3 cell measurement techniques (CD (90), HS (54), and OF (91)) have shown larger abdominal SC FCS in men. Nevertheless, among studies using CD, Hellström et al. (55) observed no sex variation in abdominal SC FCS, while Votruba et al. (92) found larger adipocytes in femoral SC and gluteal SCAT of women and Couillard et al. (47) have shown increased abdominal SC and femoral SC FCS in women. Using a group of healthy, nondiabetic participants, McLaughlin et al. (93) confirmed this finding by observing higher diameter of large adipocytes in women than in men.
In our meta-regression analysis, there was no statistically significant difference between abdominal SC FCS of the 2 sexes (Table 1). Studies with a higher proportion of women, however, did tend to reveal reduced FCS in visceral AT (β = –7.2799, P = .0834) (Table 2). Additionally, when corrected for abdominal SC FCS, visceral FCS was significantly lower with increasing proportion of women (β = –11.2152, P < .0001). The above findings were also confirmed using partial correlation controlling for abdominal SC FCS (Table 4).
Table 4.
Partial correlation between FCS and sex
| Proportion of Women | Spearman’s Rho | P-value |
|---|---|---|
| Control Variable: abSC FCS | ||
| gSC FCS | 0.115 | .531 |
| fSC FCS | 0.300 | .096 |
| Visceral FCS | -0.456 | <.0001 |
Abbreviations: abSC, abdominal subcutaneous; FCS, fat cell size; fSC, femoral subcutaneous; gSC, gluteal subcutaneous.
In summary, data are conflicting regarding possible differences in FCS between men and women; our meta-analyses also failed to demonstrate statistically significant variations. However, we found lower visceral FCS in women, independently of abdominal SC FCS. This finding indicates that for any given degree of SC adipocyte hypertrophy, women are less prone to visceral fat cell hypertrophy.
Adipocyte size and age
We found few results regarding the relationship between adipocyte size and age, although it is known that, during childhood, fat cells are subject to a steady enlargement from ages 1-9 years (94) and that obesity leads to increased FCS throughout adolescence (95). Evidence of an association between age and adipocyte size in adults is scarce (16, 96). Therefore, we used multiple regression to illustrate increased FSC in the abdominal SC (β = 0.2659, P = .0009) and visceral (β = 0.7394, P = .0004) depots as a function of age, independently of BMI, sex, and cell measurement method (Tables 1 and 2).
Adipocyte size and obesity
The relationship between adipocyte hypertrophy and obesity has already been reviewed elsewhere (97) and a plethora of studies exists that demonstrated the strong positive correlation between FCS and increasing body weight (16, 19, 25, 30, 34, 44, 47, 49-51, 54, 62-64, 66, 67, 76, 77, 81, 83, 89, 93, 96, 98-129). Moreover, the relationship between adipocyte hypertrophy and increased BMI does not appear to be dependent on the presence of dysglycemia, because it was found both in participants with normoglycemia and those with T2D (30, 64). Further supporting evidence of this association is the well-established beneficial effect of weight loss on fat cell shrinkage in individuals with varying demographic profiles. In twin studies, the co-twins with obesity showed greater fat cell hypertrophy than their respective co-twins who were leaner (44), thus underscoring the critical role of acquired environmental and life-style factors in adipocyte enlargement.
Figure 3 shows BMI in relation to abdominal SC and visceral adipocyte size with the 3 different sizing methods (CD, HS, and OF). With multiple meta-regression, BMI is a strong contributor to changes in FCS, regardless of the location of adipose tissue or adjustment for cofounders.
Adipocyte size, insulin resistance, and hyperglycemia
There exists a relatively strong body of evidence supporting an important contribution of adipocyte hypertrophy to the exacerbation of insulin resistance that is independent of the degree of obesity, although a few studies have challenged this assertion.
Among 24 studies that used the CD technique, only 2 found no association between FCS and diabetes or insulin resistance (86, 107). Among 10 studies that have controlled for obesity, either by adjusting for fat area or by matching for BMI (8, 27, 44, 48, 100, 130-134), only 2 found no association between T2D and FCS after adjustment (130, 131). Indeed, when men who were nonobese and had T2D were matched with participants from the control group for BMI, there were significantly higher values of both insulin resistance and abdominal SC FCS in the diabetic group (27). Other studies have reached similar findings but did not control for obesity (102, 103, 135-140).
Since the expandability of adipocytes varies between individuals and is clearly associated with obesity and insulin resistance, it may be posited that genetically inherited factors may be the source of this relationship. In a study of twins who were young, Heinonen et al. (44) showed that, within each pair of twins, intertwin differences in abdominal SC FCS was correlated with differences in Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) independently of body fat variations. While this result indicates that genetic and epigenetic factors are not the sole contributors of adipocyte hypertrophy and insulin resistance, FCS is, in part, dependent on family history of T2D and genetic factors. Indeed, in a large cohort of women after menopause (105), the size of abdominal SC and femoral SC adipocytes was greater in participants with a family history of T2D than those without. Similar results have been found in women after menopause (141) and in men who were middle-aged (60). In addition, Henninger et al. (13) have shown, in a small group of first-degree relatives of patients with T2D who were nonobese and participants from the control group who were nondiabetic and nonobese, that abdominal SC cell size correlated with decreased insulin sensitivity only in first-degree relatives. Furthermore, differences also exist between individuals of African and European descent; in women after menopause with obesity (134), 2-hour postoral glucose tolerance test insulin and glucose levels were positively correlated with SC and visceral FCS in participants of African but not Caucasian descent.
Among 21 studies that used the HS method to investigate the relationship between FCS and insulin resistance, only 2 found no association. The absence of association in 1 of these 2 (77) may be explained by small sample size, while the other study sampled SCAT from the thoracic instead of the abdominal SC area (88). Furthermore, histomorphological analyses have also revealed that insulin resistance correlates more closely with visceral than SCAT FCS. Indeed, while many studies have shown relationships between increased insulin resistance or the presence of diabetes and increased adipocyte size from both depots (19, 83, 114, 142), a substantial number of investigations have demonstrated an association only with visceral FCS (89, 113, 117, 143-145) or a stronger correlation with visceral than with SCAT FCS (83, 142).
In line with the findings by Svensson et al. (133), Rojas-Rodriguez et al. observed higher SCAT adipocyte size in women who were pregnant with gestational diabetes compared to those with normal glucose tolerance (143). Consistent with studies that have employed CD, results using the HS technique also support the notion that the influence of adipocyte hypertrophy on insulin resistance and glucose tolerance is independent of obesity and fat mass (19, 23, 59, 83, 146, 147). Moreover, FCS measured using HS is not only associated with levels of fasting insulin (96, 110, 148), but also with fasting glucose (148), HOMA-IR (15, 149), glucose disposal rate (59, 146), and 2-hour postoral glucose tolerance test glucose (149).
All except 1 of 17 studies that used OF have concluded that AT hypertrophy contributes to impaired insulin sensitivity and T2D. As discussed previously, 1 of the key distinguishing features of the OF method is that it allows for the identification and quantification of very small adipocytes. The inclusion of this population of cells, which cannot be detected by the HS and CD techniques (52), results in a bimodal distribution of cell size. Indeed, Azuma et al. have shown that the average cell diameter did not vary between individuals with and without diabetes (125). This result was in agreement with an investigation by McLaughlin et al. (150), who also observed that average cell size did not differ between the insulin sensitive and the insulin resistant groups matched for BMI, but that the insulin-resistant group had a higher proportion of very small cells. Many studies have therefore also analyzed the mode of and the size distribution of the large and small adipocyte populations. These have concluded that insulin resistance and T2D are associated both with an increased large adipocyte size (50, 52, 62, 67, 151, 152) and a higher relative number of small adipocytes (127, 150, 153-155). Nonetheless, some studies that have used the OF method also found association between the presence of diabetes (42, 156, 157) or impaired insulin response (85, 157, 158) and increased average cell size.
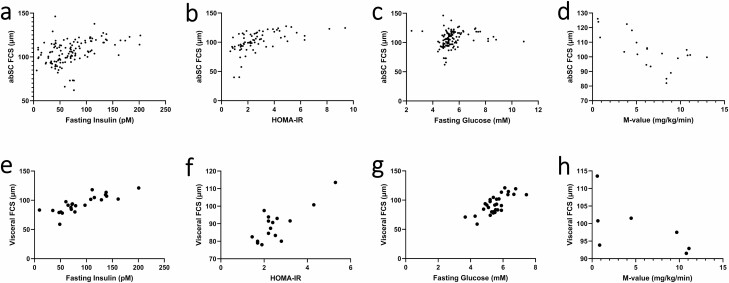
Meta-regression analysis corroborated the positive relationship between FCS and impaired insulin sensitivity and glucose intolerance (Fig. 6). Abdominal SCAT FCS was predicted by fasting insulin (β = 0.1089, P < .0001), HOMA-IR (β = 3.9202, P < .0001), and M-value (β = –1.8483, P < .0001) independently of the percentage of women, age, and FCS methodology. Abdominal SC FCS was, however, not predicted by fasting glucose (P > .1). Similar results have been reached between visceral FCS and fasting insulin (β = 0.2712, P < .0001), fasting glucose (β = 4.9258, P = .0006), and HOMA-IR (β = 5.0827, P = .001). When BMI was included in the model, no significant relationship could be found between abdominal SC FCS and any of the above indexes. For visceral FCS, however, fasting glucose (β = 2.5795, P = .03) and HOMA-IR (β = 3.4731, P = .0054) remained significant predictors, while fasting insulin (β = 0.1132, P = .0787) was of borderline statistical significance.
Figure 6.
Relationships between abdominal subcutaneous fat cell size and (A) fasting insulin; (B) HOMA-IR; (C) fasting glucose; (D) M-value. Relationship between visceral fat cell size and (E) fasting insulin; (F) HOMA-IR; (G) fasting glucose; and (H) M-value. abSC, abdominal subcutaneous; FCS, fat cell size; HOMA-IR, homeostatic model assessment of insulin resistance.
In summary, FCS increases with diabetes status, impaired glucose tolerance, and insulin resistance. These associations are stronger in VAT than SCAT but are not independent of obesity or BMI in all studies. Genetic, epigenetic, and family history of diabetes all seem to contribute to this relationship. We confirmed these correlations in meta-regression analyses and showed that visceral FCS is associated with hyperglycemia and insulin resistance independently of BMI.
Adipocyte size and lipid metabolism
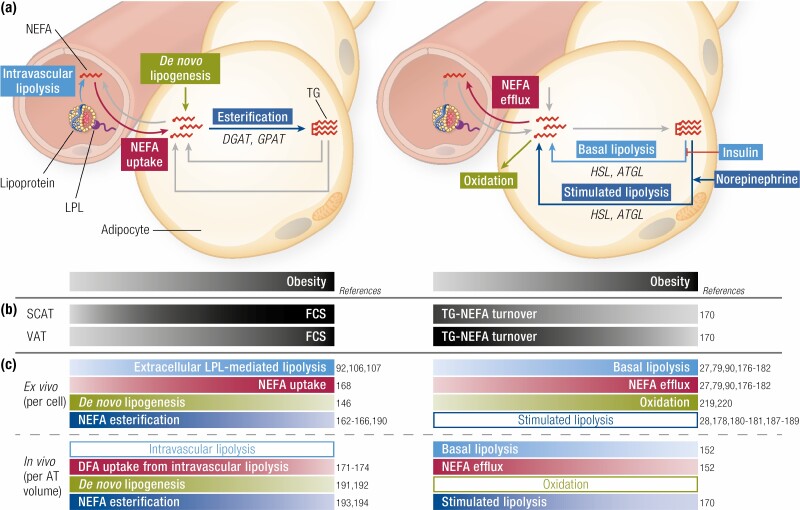
Adipocyte hypertrophy may exert its influence on systemic lipid metabolism through changes in adipose tissue lipid uptake, storage and lipolytic rates (Fig. 7). Under physiological conditions, triglyceride (TG) transported by blood lipoprotein particles bind to lipoprotein lipase (LPL) and are then hydrolyzed as nonesterified fatty acids (NEFAs), incorporated into adipocytes to be either oxidized or re-esterified and stored as TG, or released into the circulation (ie, NEFA spillover) (159). Change in LPL activity with fat cell hypertrophy has thus been the topic of numerous investigations. Indeed, individuals who are obese have higher AT LPL activity than those who are lean due to an increased amount of fat mass and adipocyte size (106). In women and men who are nonobese or overweight, there exists a significant, positive relationship between fasting LPL activity and SCAT FCS (92). Furthermore, there are also variations in LPL activity with respect to the location of AT. In women who are nonobese, abdominal SCAT has lower LPL activity than gluteal SCAT (160) but LPL mRNA expression is higher in abdominal SCAT than visceral AT (109). This result agrees with the notion that during nutritional excess, the surplus of TG is preferentially stored in subcutaneous rather than visceral depots. This has been shown directly in vivo after only a 7-day overfeeding in healthy subjects using molecular imaging of dietary fat uptake in all organs of the body (161). In an elegant study in women with obesity, Serra et al. showed that abdominal SCAT of those who had low proportions of visceral fat relative to total fat demonstrated a significant and positive correlation between adipocyte volume and LPL activity. The strength of this association was substantially attenuated in abdominal SCAT of those with higher proportions of visceral fat relative to total fat and was absent altogether in gluteal SCAT (107). Therefore, the increase in LPL activity in AT as a function of FCS was more manifest in abdominal SCAT and in individuals who were leaner and metabolically healthier.
Figure 7.
Adipocyte size and lipid metabolism. (A) Left panel: mechanisms leading to adipocyte triglyceride (TG) accumulation include nonesterified fatty acids (NEFAs) uptake from the circulatory pool or from lipoprotein lipase (LPL)-mediated hydrolysis of TG-rich lipoproteins, and de novo synthesis of fatty acids from carbohydrates (de novo lipogenesis). Right panel: mechanisms leading to adipocyte TG mobilization include basal and norepinephrine-stimulated intracellular TG lipolysis leading to NEFA efflux into the circulation or NEFA oxidation. (B) Change in fat cell size in relation to obesity is depicted for abdominal subcutaneous adipose tissue (SCAT) vs visceral adipocyte tissue (VAT) along with change in triglyceride-nonesterified fatty acid (TG-NEFA) turnover based on in vivo studies. (C) Change in the mechanisms leading to adipocyte TG accumulation (left panels) or mobilization (right panels) according to the degree of obesity and fat cell size (FCS) measured ex vivo (upper panels) or in vivo (lower panels). Changes are expressed by the color tone, light tones for low metabolic rates or smaller levels and dark tones for high metabolic rates or higher levels. Empty bands indicate conflicting results or insufficient data to indicate increase or decrease with obesity or fat cell size change. ACS, acetyl-CoA synthetase; ATGL, adipose triglyceride lipase; DGAT, diglyceride acyltransferase; DFA, dietary fatty acid; GPAT, glycerol-3-phosphate acyltransferase; HDL-c, high-density lipoprotein cholesterol; HSL, hormone sensitive lipase; LDL-c, low-density lipoprotein-cholesterol.
Furthermore, FCS is also associated with decreased intracellular enzymes involved in TG synthesis, namely acetyl-CoA synthetase (162), diglyceride acyltransferase (162-165), and glycerol-3-phosphate acyltransferase (166), as well as reduced expression of genes involved in de novo lipogenesis (146), suggesting a potential role of adipocyte hypertrophy in impaired TG storage. In a study of men and women with overweight who were middle-aged, however, Rajjo et al. found that NEFA storage rate was higher in large adipocytes in proportion to their larger volume (167). It has also been shown that obesity is associated with increased adipose tissue fatty acid uptake (115, 168) and that SCAT is responsible for the majority of total AT very-low-density lipoprotein-TG storage (169). The increase in LPL activity in the presence of less efficient NEFA esterification would theoretically increase NEFA spillover. However, this phenomenon may be attenuated through the concomitant reduction in the expression of factors involved in de novo lipogenesis seen with adipocyte hypertrophy. Furthermore, since the visceral adipose tissue maintains its metabolic flexibility prior to the establishment of severe obesity, the increase in subcutaneous adipose tissue NEFA spillover may also be curbed by increased NEFA uptake in visceral adipose tissue due to the higher flux of circulating NEFA (159).
By measuring visceral and SCAT TG age, Spalding et al. estimated TG storage capacity of ATs of a large cohort consisting of individuals who were lean, overweight, or morbidly obese (170). Among those with smaller average abdominal FCS, individuals who were metabolically unhealthy had slower lipid turnover rates than the metabolically healthy. Nonetheless, among individuals with more pronounced SCAT adipocyte hypertrophy, no difference of lipid turnover was observed between the metabolically healthy and unhealthy groups. Across all participants, participants who were metabolically healthy had smaller cells than those who were metabolically unhealthy. Furthermore, although individuals who were lean had significantly higher subcutaneous lipid turnover rates, there was no difference in the turnover rate among those who were overweight, obese, or morbidly obese. However, in visceral AT, lipid turnover rate decreased only in participants who had reached the morbidly obese status. Because slower lipid turnover reflects increased storage capacity relative to lipid removal capacity, this suggests that, early on during the development of obesity, SC adipocytes adapt by slowing down their lipid turnover to accommodate excess lipids; this adaptive response may become saturated during the late stages of obesity, during which visceral adipocytes play a more important role. In morbidly obese individuals, this metabolic flexibility is overwhelmed in both SC and visceral adipose tissue depots, leading to reduced uptake of dietary fatty acid and increased dietary fatty acid spillover that are reversible only upon caloric restriction (171). This is consistent with the finding of impaired dietary fatty acid uptake and storage capacity with impaired glucose tolerance (172-174). In addition, in a large cross-sectional study, Sato et al. showed that the cross-sectional area of SCAT measured by abdominal computed tomography (CT) increased as a function of VAT cross-sectional area. However, after VAT cross-sectional area reaches a critical point (at 100 cm2), increases in SCAT area became less pronounced despite continued expansion of VAT (175). Together, these results corroborate the assumption that excess lipids are stored primarily in subcutaneous tissues during the early stages of obesity, as we found with short-term overfeeding in individuals who were healthy (161); the visceral depots appear to retain their expansion capacity after SC adipocytes slow their expansion and have developed some degree of hypertrophy and increased adipogenesis.
Obesity is generally associated with higher rates of adipose tissue lipolysis, and numerous studies have documented increased ex vivo basal lipolytic rate with larger adipocytes (27, 79, 90, 91, 176-182). Accordingly, increased basal lipolysis has been associated with overexpression of hormone sensitive lipase (45, 176, 183, 184) and adipose triglyceride lipase (45), as well as decreased perilipin levels (81, 176) in larger adipocytes. The increased basal lipolytic activity in hypertrophic fat cells is also associated with a blunted ex vivo antilipolytic effect of insulin (90, 177, 185). In support of these observations, we have also recently shown that the increase in postprandial NEFA flux in individuals with impaired glucose tolerance was entirely due to increase in NEFA flux originating from AT intracellular lipolysis, not dietary fatty acid spillover (186). In the latter study, insulin resistance was however not associated with increased postprandial NEFA flux and we found inverse relationship between insulin resistance and glycerol flux, a marker of adipose tissue lipolysis.
Whether FCS affects catecholamine-stimulated lipolysis is more controversial, with some studies observing increased (28, 178, 180, 181, 187, 188) and others showing decreased (179, 189) lipolysis rates after stimulation with adrenergic agonists. Whether these observations made from ex vivo experiments can predict adipose tissue lipolysis in vivo, however, is unclear. For example, we found an inverse association between change in SCAT FCS and change in plasma glycerol appearance rate or change in NEFA spillover induced by bariatric surgery in individuals with morbid obesity without or with T2D (152). This demonstrates that those patients with the greatest reduction in SCAT FCS display the lowest reduction in in vivo adipose tissue lipolytic rate. In addition, the increase in NEFA flux associated with insulin resistance may also be a result of impaired re-esterification of NEFAs originating from intracellular lipolysis (159). Accordingly, it has been shown that NEFA re-esterification was reduced in participants who had diabetes (165, 190) and that TG storage was impaired in those with insulin resistance (191-193) or obesity (194).
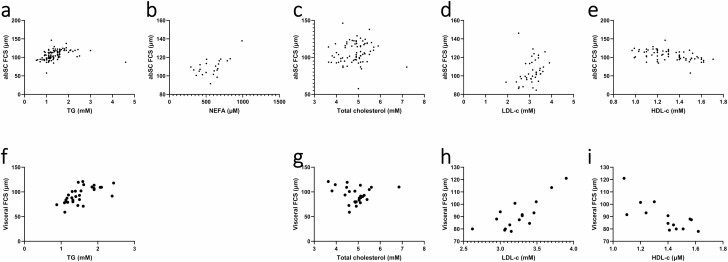
Increased abdominal adipocyte size, especially in visceral AT, has also been associated with decreased HDL-c (19, 132, 134) and increased circulating TG (19, 45, 89, 131, 132, 134, 139, 148, 151, 195), NEFAs (165), total cholesterol (19, 195), and LDL-c (44, 139, 195). Results from meta-regression analyses are in line with the association between adipocyte hypertrophy and poor circulating lipid profile (Fig. 8). For abdominal SCAT, FCS was predicted by TG (β = 5.9983, P = .0098), NEFAs (β = 0.0371, P = .0077), total cholesterol (β = 6.2174, P = .0044), and by low HDL-c (β = -24.3195, P = .0029), independently of age, sex, and cell measurement methodology. However, when BMI was also included in the model, only total cholesterol remained a significant predictor (β = 6.1120, P = .0025). For visceral AT, FSC was predicted by TG (β = 19.4356, P = .0004) and low HDL-c (β = –52.6697, P = .0026); both remained significant after controlling for BMI.
Figure 8.
Relationships between abdominal subcutaneous fat cell size and (A) plasma triglycerides; (B) nonesterified fatty acid; (C) total cholesterol; (D) low-density lipoprotein-cholesterol; (E) and high-density lipoprotein-cholesterol. Relationships between visceral fat cell size and (F) triglycerides; (G) total cholesterol; (H) low-density lipoprotein-cholesterol; and (I), high-density lipoprotein-cholesterol. abSC, abdominal subcutaneous; FCS, fat cell size; NEFA, free fatty acid; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; TG, triglyceride.
To summarize, as adipocytes expand, basal lipolysis measured by ex vivo methods also increases, possibly due to greater amounts of intracellular TG substrates. However, increased insulin levels and impaired catecholamine action lead to reduced lipolysis rate per adipose tissue TG mass in vivo (159). The rapid adipocyte expansion followed by a decrease and stabilization in turnover suggests an early recruitment and loss of adipocyte expandability in the subcutaneous adipose tissue. In visceral adipose tissue, lipid turnover rate decreases only in individuals who have reached the morbidly obese status. The relationships between FCS and TG, NEFA, total cholesterol, and reduced HDL-c were confirmed using meta-regression and are not solely dependent on BMI. Furthermore, adipocyte hypertrophy is associated with AT LPL activity and impairment of lipid storage, all of which may eventually contribute to ectopic lipid accumulation.
Adipocyte size and ectopic lipid accumulation
It is hypothesized that adipocyte hypertrophy resulting from chronic energy imbalance may lead to insulin resistance via accumulation of ectopic fat in the liver and skeletal muscles. However, existing literature has delineated a more complex interplay between FCS and increased lipid content in these ectopic sites. In a large cohort of individuals who were obese, abdominal SCAT FCS explained 21% of the variance of liver fat content measured by proton magnetic resonance spectroscopy, independently of age, sex, BMI, and the ratio of visceral vs SC abdominal fat mass (196). Similar positive correlations between intrahepatic lipid levels and abdominal SCAT FCS have been observed in groups of participants with overweight (126), obesity (127), severe obesity, and morbid obesity (64, 89, 124). Furthermore, Anand et al. found that individuals of South Asian descent had more body fat, visceral adiposity, and liver fat than people of Caucasian descent and that abdominal SCAT FCS was associated with the observed difference in liver fat between these 2 ethnic groups (197). By studying a group of participants who were monozygotic twins, Pietiläinen et al. have also shown that variations in intrahepatic lipids within each pair of twins were associated with variations in leptin levels, which were, in turn, predicted by SCAT FCS (29). Despite these findings, other investigators have reported no significant correlation between SCAT adipocyte size and liver fat in smaller cohorts of individuals who were lean (123), overweight (198), or obese (199). As shown in Table 5, these have generally included participants with lower BMIs; it can be speculated that the relationship between SCAT FCS and ectopic lipid accumulation in the liver is, in part, dependent on BMI and is observed mostly in those with more severe obesity. In particular, 1 of these studies (198) investigated the effect of a hypercaloric diet on liver fat in participants with a BMI below 32 kg/m2 and found that despite increases in both abdominal SCAT FCS and intrahepatic lipid with weight gain, no correlation was found between these variables. The investigators have further shown that regardless of fat mass, having larger adipocytes at baseline curbed hepatic fat accumulation induced by overfeeding. It may be argued, therefore, that in individuals with relatively low BMI, those who have larger SCAT adipocytes may display efficient adipose tissue fat storage that protects against liver fat accretion. A more recent twin study (44) showed that although twins with obesity and more pronounced adipocyte hypertrophy had increased liver fat, differences in SCAT FCS were not correlated with differences in hepatic lipid content.
Table 5.
Relationships between liver and muscle fat content and FCS, insulin resistance, and adipogenesis
| Findings | FCS-liver fat corr. | FCS-muscle fat corr. | AT | N | Age | Sex | BMI | T2D | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Histology assessment of ectopic lipid content | |||||||||
| • Visceral FCS correlated with: liver injury (ALT, AST) and NAS severity (histology) | ↑ | NA | Vis. | 93 | 43 | 72 W | 52 | Unk. | (43) |
| HS | |||||||||
| • Visceral and SC FCS correlated with degree of hepatic steatosis | ↑ | NA | Abd. | 35 | 41.8 | 25 W | 49.5 | 6 | (89) |
| • Visceral FCS: predictor of liver fibrosis | HS and CD | ||||||||
| • Visceral FCS correlated with Pref-1 expression, which correlated with degree of hepatic steatosis | ↑ (?) | NA | Abd. | 29 | 42.3 | 20 W | 48.6 | Unk. | (11) |
| HS and CD | |||||||||
| Proton magnetic resonance spectroscopy assessment of ectopic lipid content | |||||||||
| • Compared to individuals with IS, individuals with IR have ↑ VAT and intrahepatic lipid | ↑ | NA | Abd. | 31 | 30-60 | 15 W | 25-35 | 0 | (127) |
| • Δ peak diameter predicted Δ intrahepatic lipid with overfeeding | OF | Mean ≈ 56 | Mean ≈ 30 | ||||||
| • FCS correlated with serum leptin, which correlated with liver fat | ↑ (?) | NA | Abd. | 34 | 24-27 | 16 W | Mean ≈ 26.8 | 0 | (29) |
| • The co-twins who was heavier had ↑ SAT, ↑ FCS, and ↑ liver fat | CD | ||||||||
| • Liver fat correlated with serum insulin | |||||||||
| • Co-twins with hypoplastic obesity: ↑ liver fat | ↑ | NA | Abd. | 40 | 28.4 | 30 W | 28 | 0 | (44) |
| • Co-twins with hypertrophic obesity: ↓ fat cell number, ↑ liver fat | CD | ||||||||
| • Δ FCS: not correlated with Δ liver fat | |||||||||
| • FCS explained 21% of liver fat variation | ↑ | NA | Abd. | 119 | 40 | 83 W | 30 | 0 | (196) |
| CD | |||||||||
| • FCS: correlated with VAT and liver fat, but not muscle fat | ↑ | — | Abd. | 46 | 37.5 | Unk. | 25-30 | 0 | (126) |
| OF | Mixed. | Mean ≈ 27.7 | |||||||
| • Participants of South Asian descent have more liver fat than those of Caucasian descent. Adjustment for FCS and fat distribution decreased the difference in liver fat | ↑ (?) | NA | Abd. | 108 | 35.5 | Unk. | Mean ≈ 27.5 | 0 | (197) |
| HS | Mixed. | ||||||||
| • No correlation between FCS and liver fat | — | NA | Abd. | 27 | 61 | 9 W | 31.4 | 27 | (199) |
| • AT adiponectin correlated negatively with liver fat | HS | ||||||||
| • Liver fat: correlated with crown-like structures and macrophages in SCAT, correlated with PAI-1 | |||||||||
| • In high visceral fat group: ↑ hepatic fat | NA | NA | Abd. | 38 | 15.1 | 14 W | 36.8 | Unk. | (124) |
| OF | |||||||||
| • FCS is not correlated with hepatic, muscle, or visceral fat | — | — | Abd. | 75 | 26.8 | 37 W | 22.9 | 0 | (123) |
| OF | |||||||||
| • No association between FCS and liver or muscle fat | — | — | Abd. | 29 | 26.8 | 0 W | 25.5 | 0 | (198) |
| OF | |||||||||
| • No difference in liver fat between T2D and control | NA | NA | Abd. | 30 | 58 | 10 W | 30.8 | 15 | (165) |
| CD | |||||||||
| • FCS: positively correlated with liver fat, but not muscle fat | ↑ | — | Abd. | 53 | 25.8 | 21 W | 35.4 | 0 | (64) |
| CD | |||||||||
| • FCS: positively correlated with liver lipids | ↑ | — | Abd. | 53 | 42 | 0 W | Unk. | 0 | (225) |
| CD | |||||||||
| CT assessment of ectopic lipid content | |||||||||
| • In high liver fat group: ↑ CD68, chemokines monocyte chemoattractant protein-1, and PAI-1, ↓ PPAR-γ and adiponectin | NA | NA | SC | 20 | Mean ≈ 40.5 | 20 W | Mean ≈ 36 | 0 | (226) |
| CD | |||||||||
| • In individuals with T2D: ↑ liver fat, ↑ muscle fat | ↑ (?) | ↑ (?) | Abd. | 102 | 58 | 59 W | Mean ≈ 33.6 | 67 | (125) |
| OF | |||||||||
| • Women with higher proportions of visceral fat have higher fat accumulation in the muscle | NA | ↑ (?) | Abd., g. | 48 | Mean ≈ 60 | 48 W | Mean ≈ 31.5 | 0 | (107) |
| CD | |||||||||
| • Liver fat: associated with IL-6 and number of macrophages in SCAT | NA | NA | Abd. | 36 | 37 | 17 W | 26 | 0 | (227) |
| HS |
Abbreviations: ↑ (in first column), increase in; ↓ (in first column): decrease in; ↑ (in second and third columns, positive correlation; Δ, change in; abd., abdominal subcutaneous; AT, adipose tissue assessment; BMI, body-mass index; CD, collagenase digestion; corr., correlation; CT, computed-tomography; FCS, fat cell size; HS, histological section; IR, insulin resistant; IS, insulin sensitive; N, number of participants; NA, not applicable or not reported; NAS, nonalcoholic steatosis; OF, osmium fixation; Ref., reference number; SAT, subcutaneous adipose tissue; Sex, number of women; T2D, type 2 diabetes; Unk., unknown; VAT, visceral adipose tissue; W, women.
Visceral adiposity is a better predictor of metabolic endpoints. Furthermore, it has also been hypothesized that due to the venous drainage of omental and mesenteric AT to the liver, hypertrophy of the visceral AT may be better associated with liver fat content than would SCAT fat cell enlargement. This is in agreement with findings in individuals with morbid obesity, in which both visceral and SCAT FCS correlated with hepatic steatosis severity assessed by liver biopsy, while only visceral AT FCS was associated with hepatic fibrosis (89). These observations were later corroborated by Wree et al. (43) in a larger cohort of participants with more severe obesity.
In contrast to the liver, the relationship between lipid accumulation in muscles and SCAT FCS has not been well demonstrated. Four studies (64, 123, 126, 198) found no significant correlation; 1 of them (126) revealed a positive association between SCAT FCS and liver fat but not intramuscular lipid content. Nevertheless, CT densitometry showed increased muscle fat accumulation in women with higher percentage of visceral fat mass relative to total fat mass than those with less visceral adiposity (107). As discussed earlier, the former had larger SCAT FCS and lower rate of increase in AT LPL activity with respect to SCAT adipocyte size.
We found no study investigating the possible link between muscle fat content and visceral FCS. However, compared with SCAT FCS, visceral AT FCS is more strongly correlated with decreased adiponectin, an adipokine that is associated with increased insulin sensitivity, lower risk of T2D, and increased lipid oxidation in muscles and liver (203, 204). In line with these findings, plasma adiponectin is negatively correlated with fat levels in skeletal myocytes (64). Furthermore, although the NEFA fractional extraction rate was similar in the skeletal muscles of individuals with vs without obesity, it was correlated with serum TG and was increased in individuals with obesity and T2D (115).
In summary, abdominal SCAT adipocyte size is positively associated with liver fat in large cross-sectional studies. The mechanism explaining the association between SCAT adipocyte hypertrophy and liver fat content is currently unclear. In contrast, visceral AT FCS appears to be more closely associated with the severity of hepatic steatosis. Nevertheless, FCS in the SCAT does not seem to be associated with muscle lipid accumulation.
Adipocyte size and cardiovascular complications
In general, increased FCS accompanies poor cardiovascular endpoints. A positive association between blood pressure and adipocyte hypertrophy was observed in numerous studies (48, 103, 107, 117, 137, 205). This relationship seems to be stronger for visceral FCS, consistent with the fact that cardiometabolic dysfunctions are better correlated with visceral than SC fat mass. Furthermore, a recent study showed a strong, positive correlation between SCAT FCS and the Framingham risk score for cardiovascular disease; this increased risk may be caused by higher NEFA levels due to increased lipolysis and decreased lipogenesis (177). The accumulation of lipids in cardiomyocytes may then lead to altered cardiac function. Another causal mechanism of hypertension implicating adipocyte hypertrophy is stiffening of arteries. Using pulse wave velocity as a proxy for arterial stiffness, Arner et al. showed that pulse wave velocity was positively correlated with SCAT and visceral FCS (206).
Due to their proximity to the myocardial tissue and vessels, epicardial and perivascular ATs are of particular interest when studying the effects of AT dysregulation of lipid metabolism and adipokine secretion on cardiac dysfunction. In a given individual, the average size of epicardial adipocyte is smaller than that of visceral and SCAT adipocytes (34, 87, 88, 207). In addition, the epicardial AT fat cells were hypertrophied in patients with coronary artery disease (CAD) relative to those without CAD (87, 208, 209). Epicardial AT FCS was also higher in those with T2D compared to individuals who were nondiabetic (88). In line with these findings, patients with CAD had reduced expression of adiponectin in their epicardial AT (87, 88), which may limit adipocyte hyperplasia and promote inflammation. Moreover, blood pressure is also associated with increased Pref-1 expression in omental AT (11) and decreased adiponectin in epicardial AT (210), indicating reduced adipogenesis. The proinflammatory profile in epicardial AT of patients with CAD or epicardial fat cell hypertrophy was evidenced by higher expression of MCP-1 (88), CD68 (88), IL-6 (211), TLR-2 (208), and TLR-4 (208), more CD11c-positive macrophages (208), and lower expression of adiponectin (17). Nevertheless, in a cohort of 22 patients with or without CAD, Eiras et al. found reduced epicardial MCP-1 expressions in those with larger epicardial FCS, despite higher MCP-1 plasma concentrations (34). Furthermore, elevated PAI-1 level increases the risk for CAD (212-214) and is positively correlated with FCS of both the SC and visceral depots (16, 54, 215).
Hence, there is solid evidence that SCAT adipocyte hypertrophy accompanies a wide range of dysregulations in cardiometabolic health, with high blood pressure, increased Framingham risk score, and arterial stiffening. In line with these findings, epicardial FCS is also associated with CAD and diabetes status. These associations may be mediated through a proinflammatory profile of hypertrophied epicardial fat cells.
Effect of energy balance and therapeutic interventions on adipocyte hypertrophy
Energy balance and therapeutic interventions.
Long-term energy imbalance is among the most important determinants of excessive weight and, perhaps, adipocyte hypertrophy. Changes in energy intake have been shown to affect FCS in numerous studies. In women with obesity, dietary caloric restriction resulted in decreased SCAT FCS to the level of control participants (74). Dietary weight loss also reduced the size of both gluteal and abdominal SCAT adipocyte size in men with obesity (216). Other subsequent studies have reached similar conclusions with different diets and in different test populations (57, 99, 178, 217, 218). One study in adults who were healthy, but insulin-resistant and overweight showed that changes in FCS were significantly associated with changes in weight (weight loss of 4.3 kg) and in insulin resistance (151). In the latter study, changes in FCS, but not in BMI, predicted improvement in insulin sensitivity.
The effects of weight fluctuation on FCS and adipocyte number are specific to the type of adipose depots. Increase in weight (by 3.1 kg (99) or by 4.6 kg (219)) was associated with increase in the quantity of both upper-body and lower-body SCAT. However, whereas the increase in upper-body SCAT mass (by 1.9 kg) was associated with adipocyte hypertrophy, the increase in lower-body SCAT mass (by 1.6 kg) did not appear to be associated with cell hypertrophy, but with cell hyperplasia (219). With subsequent weight reduction (of 2.4 kg) in the same participants, FCS in both regions decreased, but adipocyte numbers did not change (99). With a 15% weight loss, FCS decrease was more important in the abdominal SCAT than in the gluteal AT (218). In addition, another study showed that dietary intervention in men with obesity (resulting in an average weight loss of 13.1 kg) reduced abdominal FCS, with modest decrease in gluteal FCS that was not statistically significant (216). Hence, it seems that upper-body FCS is more reactive to weight fluctuations, while lower-body AT responds to weight gain through adipogenesis, which confers more durable increase in lipid storage capacity. Other investigators have found that AT cell numbers in the femoral (74) and abdominal (115, 137) depots were not affected by weight loss interventions (with losses of 25 kg (115) and 33% (137)).
Significant decrease in abdominal SCAT FCS was observed after bariatric surgery with Roux-en-Y gastric bypass (58, 115, 137), duodenal switch (152), and sleeve gastrectomy (115). Initial FCS was also an important negative predictor of the beneficial effects of bariatric surgery on the diabetes status and metabolic dysfunctions (hyperinsulinemia, hyperglycemia, hypertension, dyslipidemia, and central obesity). Indeed, patients with larger FCS displayed decreased effectiveness of gastric bypass on remission of diabetes status and improvement of diabetes risk factors (141). The rates of basal and stimulated ex vivo AT lipolysis were reduced with gastric banding and lifestyle interventions (178), which may thus explain the decrease in lean tissue steatosis with weight reduction. However, in vivo NEFA appearance rate did not change despite very important weight loss and reduction in SCAT FCS after duodenal switch in patients without or with T2D (152).
The level of physical activity constitutes a determinant of overall energy expenditure that is associated with adipocyte size. Individuals with a sedentary lifestyle have larger adipocytes than individuals who were physically active (148); this difference, however, may be due to higher BMI in the former group. Nonetheless, while dietary restriction did reduce body weight (by 10.4 kg) in a study conducted by You et al., it did not change SCAT FCS, which was decreased only with the addition of exercise (220). A 3-month aerobic exercise program reduced gluteal and abdominal SC FCS in men (216). In individuals with overweight, FCS was correlated with intrahepatic lipid content; both were significantly reduced following dietary restriction with physical exercise (126). Finally, in 7 pairs of volunteers who were monozygotic twins, Ravussin et al observed a decrease in FCS with a negative energy balance imposed by exercise concomitantly with a slight increase in plasma ghrelin concentration (221).
In addition, thiazolidinediones are antidiabetic medications known to mediate their effects through activation of PPAR-γ and induction of adipogenesis. Accordingly, 2 studies have found significant increases in the proportion and absolute number of small cells in individuals treated with thiazolidinediones (222, 223). Other studies have concluded that the induction of PPAR-γ using pioglitazone (14) and troglitazone (224) was associated with increased FCS by measuring the average size of adipocytes.
In summary, metabolic interventions involving dietary restriction result in reduced FCS with weight reduction and suggest an effect of FCS correction on insulin resistance that is independent of change in BMI. On the contrary, caloric excess with overfeeding leads to increased FCS. Furthermore, dietary interventions appear to have different effects depending on the AT depot: abdominal SCAT reacted to weight change through change in FCS whereas lower-body SCAT responded to weight gain preferentially through change in the number of adipocytes. Other interventions aimed at restricting excess energy balance, including bariatric surgery and physical activity, have also consistently led to reduced FCS. Induction of adipogenesis via treatment with thiazolidinediones may also increase AT storage capacity by increasing the number of small cells and by promoting subsequent enlargement of these adipocytes.
AT oxidative metabolism.
Adipose tissue of individuals with obesity generally consumes less oxygen than those of individuals who are lean; this reduced oxygen consumption seems to be related to altered mitochondrial function in fat cells (225, 226). However, it is not entirely clear whether this discrepancy is merely due to diminished mitochondrial function in both small and large fat cells in obesity or if it is caused by a relative decrease in oxygen consumption per gram of lipid as adipocytes enlarge. Fischer et al. measured the mitochondrial oxidative phosphorylation capacity in adipocytes, which was negatively correlated with BMI. They also determined that respiratory capacity was not significantly different between small and large fat cells (227). Yet in another study, oxygen consumption rate was higher in AT of participants who were obese compared to those who were lean, when it was expressed per number of adipocytes. Nevertheless, it was lower in obesity when expressed as a function of quantity of adipose tissue (226). Furthermore, a more recent study found that the oxygen consumption rate and citrate synthase activity were reduced in AT of individuals who were obese vs whose who were lean. When expressed per number of cells, oxygen consumption rate and citrate synthase activity were higher in large adipocytes than in small adipocytes of the same individuals, although these differences did not reach statistical significance (225). However, while not explicitly presented in the article, the oxygen consumption rate and citrate synthase activity were lower in large cells when expressed per gram of lipids. It thus appears that, at the cellular level, adipocyte hypertrophy is associated with an increase in oxygen consumption, although such an increase is not commensurate with the growth in cell volume.
In conclusion, the few studies on AT oxidative metabolism and FCS indicate that the reduced oxidative metabolism in hypertrophied AT of individuals with obesity may be a result of increases of adipocyte volume that surpass the increase in oxygen consumption displayed individually by larger adipocytes. The relationship between impaired oxidative metabolism and adipocyte hypertrophy may be caused by acquired variations in expression of genes implicated in mitochondrial function.
Conclusion
To summarize, we first assessed variations in FCS due to measurement methods and demographic factors. We found that, regardless of sex, age, and BMI, HS produces lower FCS estimates than CD, while OF results in slightly higher size measurements than CD in SCAT. These variations are probably the result of differences in cell fragility, physical, chemical, statistical, and/or geometrical factors. Numerical analyses confirmed that FCS in SCAT is positively correlated with cell size in all other AT compartments and that femoral and gluteal AT contain the largest adipocytes, whereas visceral AT contains smaller cells compared to abdominal SCAT. Nevertheless, data are conflicting regarding possible differences in FCS between men and women in SCAT; our meta-analyses also failed to demonstrate statistically significant variations between sexes. Despite the lack of studies directly measuring the relationship between age and adipocyte size, we were able to show, using meta-regression, that FCS increases with age in both SCAT and visceral AT independently of measurement method, sex, and BMI. This finding is in line with the observation that increases in FCS is associated with adipocyte senescence.
Our review of the physiopathological processes associated with adipocyte hypertrophy revealed that adipocyte hypertrophy is related to impaired AT differentiation potential and rate of adipogenesis. Furthermore, hypertrophy of fat cells also accompanies cell senescence, with increased cell necrosis and reduced telomere length. We also found an extensive body of evidence for the strong relationships between adipocyte hypertrophy and AT inflammation. Moreover, we have also consistently found studies indicating that the associations between FCS and these physiopathological processes are not solely the result of obesity or increased BMI.
Next, we reviewed the relationships between adipocyte hypertrophy and systemic metabolic endpoints. FCS increases with diabetes status, impaired glucose tolerance, and insulin resistance. Our meta-regression analyses confirmed these correlations and showed that visceral AT (but not SCAT) FCS is associated with glucose intolerance and insulin resistance independently of BMI. In terms of lipid metabolism, adipocyte hypertrophy is associated with AT LPL activity, impaired lipid storage, and increased intracellular lipolysis, all of which may contribute to ectopic lipid accumulation. Consistent with this observation, we found that abdominal SCAT adipocyte size is positively associated with liver fat content in large cross-sectional studies. In contrast, visceral FCS appears to be more closely associated with the severity of hepatic steatosis, possibly due to the direct venous drainage from visceral AT. Finally, SCAT adipocyte hypertrophy is related to numerous indicators of cardiometabolic dysregulations, including high blood pressure, increased Framingham risk score, and arterial stiffening.
Metabolic interventions involving dietary restriction and overfeeding resulted respectively in reduced and increased FCS. Other interventions aimed at inducing negative energy imbalance, including bariatric surgery and physical activity, have also consistently led to reduced FCS. In line with these observations with change in energy expenditure, studies also suggest that the reduced oxidative metabolism in hypertrophied AT of individuals with obesity may be the result of the increase of adipocyte volume that is proportionally greater than the increase in oxygen metabolism at the individual cell level. This may lead to decreased overall AT oxidative metabolism per mass of AT.
Acknowledgments
A.C.C. holds the Canada Research Chair in Molecular Imaging of Diabetes. This work was supported by the Canadian Institutes of Health Research (Grant MOP 53094) and by the Canada Research Chair in Molecular Imaging of Diabetes (held by A.C.C.).
Financial Support: ACC holds the Canada Research Chair in Molecular Imaging of Diabetes and is funded by operating grants from the Canadian Institutes of Health Research (CIHR) (MOP-53094) and by the Canada Research Chair in Molecular Imaging of Diabetes (held by ACC).
Glossary
Abbreviations
- AT
adipose tissue
- BMI
body mass index
- CAD
coronary artery disease
- CD
collagenase digestion
- CT
computed tomography
- FCS
fat cell size
- HDL-c
high-density lipoprotein cholesterol
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- HS
histological section
- LDL-c
low-density lipoprotein cholesterol
- LPL
lipoprotein lipase
- NEFA
nonesterified fatty acid
- OF
osmium fixation
- SCAT
subcutaneous adipose tissue
- T2D
type 2 diabetes
- TG
triglyceride
- VAT
visceral adipose tissue
Additional Information
Disclosures: R.Z.Y., G.R., and N.G. have nothing to declare. A.C.C. received research funding by Eli Lilly (2019-2021) and NovoNordisk (2021—ongoing) and consultation fees by HLS Therapeutics, Janssen Inc., Novartis Pharmaceuticals Canada Inc., and Novo Nordisk Canada Inc. A.T. receives research funding from Johnson & Johnson Medical Companies, Medtronic and Bodynov. A.T. received consultation fees from Novo Nordisk and Bausch Health. None of these commercial relationships are relevant to the present review.
References
- 1. Ye RZ, Richard G, Gévry N, Tchernof A, Carpentier AC. Supplementary material to Adipocyte hypertrophy: measurement methods, pathophysiological origins, and relationships with metabolic dysregulations.2021. ProMED-mail website. https://figshare.com/s/4f70a4c8e2edc7e032c9
- 2. WebPlotDigitizer [computer program]. Version 4.1. Austin, TX,2018. https://automeris.io/WebPlotDigitizer/ [Google Scholar]
- 3. Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of webplotdigitizer in extracting graphed data. Behav Modif. 2017;41(2):323-339. [DOI] [PubMed] [Google Scholar]
- 4. Goldrick RB. Morphological changes in the adipocyte during fat deposition and mobilization. Am J Physiol. 1967;212(4):777-782. [DOI] [PubMed] [Google Scholar]
- 5. Murphy J, Moullec G, Santosa S. Factors associated with adipocyte size reduction after weight loss interventions for overweight and obesity: a systematic review and meta-regression. Metabolism. 2017;67:31-40. [DOI] [PubMed] [Google Scholar]
- 6. Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park HT, Lee ES, Cheon YP, et al. The relationship between fat depot-specific preadipocyte differentiation and metabolic syndrome in obese women. Clin Endocrinol (Oxf). 2012;76(1):59-66. [DOI] [PubMed] [Google Scholar]
- 8. Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lessard J, Laforest S, Pelletier M, Leboeuf M, Blackburn L, Tchernof A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte. 2014;3(3):197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Löffler D, Landgraf K, Rockstroh D, et al. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int J Obes (Lond). 2017;41(1):112-119. [DOI] [PubMed] [Google Scholar]
- 11. O’Connell J, Lynch L, Hogan A, Cawood TJ, O’Shea D. Preadipocyte factor-1 is associated with metabolic profile in severe obesity. J Clin Endocrinol Metab. 2011;96(4):E680-E684. [DOI] [PubMed] [Google Scholar]
- 12. Konings E, Timmers S, Boekschoten MV, et al. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes. 2014;38(3):470-473. [DOI] [PubMed] [Google Scholar]
- 13. Henninger AM, Eliasson B, Jenndahl LE, Hammarstedt A. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS One. 2014;9(8):e105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spencer M, Yang L, Adu A, et al. Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLoS One. 2014;9(7):e102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meissburger B, Ukropec J, Roeder E, et al. Adipogenesis and insulin sensitivity in obesity are regulated by retinoid-related orphan receptor gamma. EMBO Mol Med. 2011;3(11):637-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gustafson B, Nerstedt A, Smith U. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun. 2019;10(1):2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agra RM, Fernández-Trasancos Á, Sierra J, González-Juanatey JR, Eiras S. Differential association of S100A9, an inflammatory marker, and p53, a cell cycle marker, expression with epicardial adipocyte size in patients with cardiovascular disease. Inflammation. 2014;37(5):1504-1512. [DOI] [PubMed] [Google Scholar]
- 18. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347-2355. [DOI] [PubMed] [Google Scholar]
- 19. Monickaraj F, Gokulakrishnan K, Prabu P, et al. Convergence of adipocyte hypertrophy, telomere shortening and hypoadiponectinemia in obese subjects and in patients with type 2 diabetes. Clin Biochem. 2012;45(16-17):1432-1438. [DOI] [PubMed] [Google Scholar]
- 20. el Bouazzaoui F, Henneman P, Thijssen P, et al. Adipocyte telomere length associates negatively with adipocyte size, whereas adipose tissue telomere length associates negatively with the extent of fibrosis in severely obese women. Int J Obes (Lond). 2014;38(5):746-749. [DOI] [PubMed] [Google Scholar]
- 21. Lakowa N, Trieu N, Flehmig G, et al. Telomere length differences between subcutaneous and visceral adipose tissue in humans. Biochem Biophys Res Commun. 2015;457(3):426-432. [DOI] [PubMed] [Google Scholar]
- 22. Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277-2286. [DOI] [PubMed] [Google Scholar]
- 23. Maffeis C, Silvagni D, Bonadonna R, Grezzani A, Banzato C, Tatò L. Fat cell size, insulin sensitivity, and inflammation in obese children. J Pediatr. 2007;151(6):647-652. [DOI] [PubMed] [Google Scholar]
- 24. Goossens GH, Moors CC, van der Zijl NJ, et al. Valsartan improves adipose tissue function in humans with impaired glucose metabolism: a randomized placebo-controlled double-blind trial. PLoS One. 2012;7(6):e39930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang HM, Chen LL, Wang L, et al. Macrophage infiltrates with high levels of Toll-like receptor 4 expression in white adipose tissues of male Chinese. Nutr Metab Cardiovasc Dis. 2009;19(10):736-743. [DOI] [PubMed] [Google Scholar]
- 26. Pasarica M, Gowronska-Kozak B, Burk D, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94(12):5155-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Acosta JR, Douagi I, Andersson DP, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560-570. [DOI] [PubMed] [Google Scholar]
- 28. Michaud A, Boulet MM, Veilleux A, Noël S, Paris G, Tchernof A. Abdominal subcutaneous and omental adipocyte morphology and its relation to gene expression, lipolysis and adipocytokine levels in women. Metabolism. 2014;63(3):372-381. [DOI] [PubMed] [Google Scholar]
- 29. Pietiläinen KH, Kannisto K, Korsheninnikova E, et al. Acquired obesity increases CD68 and tumor necrosis factor-alpha and decreases adiponectin gene expression in adipose tissue: a study in monozygotic twins. J Clin Endocrinol Metab. 2006;91(7):2776-2781. [DOI] [PubMed] [Google Scholar]
- 30. Murdolo G, Hammarstedt A, Sandqvist M, et al. Monocyte chemoattractant protein-1 in subcutaneous abdominal adipose tissue: characterization of interstitial concentration and regulation of gene expression by insulin. J Clin Endocrinol Metab. 2007;92(7):2688-2695. [DOI] [PubMed] [Google Scholar]
- 31. Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86(6):1670-1679. [DOI] [PubMed] [Google Scholar]
- 32. Hube F, Birgel M, Lee YM, Hauner H. Expression pattern of tumour necrosis factor receptors in subcutaneous and omental human adipose tissue: role of obesity and non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1999;29(8):672-678. [DOI] [PubMed] [Google Scholar]
- 33. Kim J, Lee SK, Shin JM, et al. Enhanced biglycan gene expression in the adipose tissues of obese women and its association with obesity-related genes and metabolic parameters. Sci Rep. 2016;6:30609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eiras S, Teijeira-Fernandez E, Salgado-Somoza A, et al. Relationship between epicardial adipose tissue adipocyte size and MCP-1 expression. Cytokine. 2010;51(2):207-212. [DOI] [PubMed] [Google Scholar]
- 35. Koska J, Stefan N, Dubois S, et al. mRNA concentrations of MIF in subcutaneous abdominal adipose cells are associated with adipocyte size and insulin action. Int J Obes (Lond). 2009;33(8):842-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skurk T, Mack I, Kempf K, Kolb H, Hauner H, Herder C. Expression and secretion of RANTES (CCL5) in human adipocytes in response to immunological stimuli and hypoxia. Horm Metab Res. 2009;41(3):183-189. [DOI] [PubMed] [Google Scholar]
- 37. McLaughlin T, Deng A, Yee G, et al. Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia. 2010;53(2):369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jernås M, Palming J, Sjöholm K, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20(9):1540-1542. [DOI] [PubMed] [Google Scholar]
- 39. Sjöholm K, Lundgren M, Olsson M, Eriksson JW. Association of serum amyloid A levels with adipocyte size and serum levels of adipokines: differences between men and women. Cytokine. 2009;48(3):260-266. [DOI] [PubMed] [Google Scholar]
- 40. Morisset AS, Huot C, Légaré D, Tchernof A. Circulating IL-6 concentrations and abdominal adipocyte isoproterenol-stimulated lipolysis in women. Obesity (Silver Spring). 2008;16(7):1487-1492. [DOI] [PubMed] [Google Scholar]
- 41. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023-1033. [DOI] [PubMed] [Google Scholar]
- 42. Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30(3):210-214. [DOI] [PubMed] [Google Scholar]
- 43. Wree A, Schlattjan M, Bechmann LP, et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metabolism. 2014;63(12):1542-1552. [DOI] [PubMed] [Google Scholar]
- 44. Heinonen S, Saarinen L, Naukkarinen J, et al. Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int J Obes (Lond). 2014;38(11):1423-1431. [DOI] [PubMed] [Google Scholar]
- 45. De Naeyer H, Ouwens DM, Van Nieuwenhove Y, et al. Combined gene and protein expression of hormone-sensitive lipase and adipose triglyceride lipase, mitochondrial content, and adipocyte size in subcutaneous and visceral adipose tissue of morbidly obese men. Obes Facts. 2011;4(5):407-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wåhlen K, Sjölin E, Löfgren P. Role of fat cell size for plasma leptin in a large population based sample. Exp Clin Endocrinol Diabetes. 2011;119(5):291-294. [DOI] [PubMed] [Google Scholar]
- 47. Couillard C, Mauriege P, Imbeault P, et al. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int J Obes Relat Metab Disord. 2000;24(6):782-788. [DOI] [PubMed] [Google Scholar]
- 48. Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50(3):625-633. [DOI] [PubMed] [Google Scholar]
- 49. Vargas G, Chandalia M, Jiang Y, Davila H, Motamedi M, Abate N. Heterogeneity in subcutaneous adipose tissue morphology and metabolic complications in overweight and obese women. Metab Syndr Relat Disord. 2013;11(4):276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landgraf K, Rockstroh D, Wagner IV, et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes. 2015;64(4):1249-1261. [DOI] [PubMed] [Google Scholar]
- 51. Löfgren P, Andersson I, Adolfsson B, et al. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab. 2005;90(11):6207-6213. [DOI] [PubMed] [Google Scholar]
- 52. Laforest S, Michaud A, Paris G, et al. Comparative analysis of three human adipocyte size measurement methods and their relevance for cardiometabolic risk. Obesity (Silver Spring). 2017;25(1):122-131. [DOI] [PubMed] [Google Scholar]
- 53. Lee MJ, Wang Y, Ricci MR, Sullivan S, Russell CD, Fried SK. Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am J Physiol Endocrinol Metab. 2007;292(3): E858-E864. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y, Zitsman JL, Hou J, et al. Fat cell size and adipokine expression in relation to gender, depot, and metabolic risk factors in morbidly obese adolescents. Obesity (Silver Spring). 2014;22(3):691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hellström L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. 2000;247(4):457-462. [DOI] [PubMed] [Google Scholar]
- 56. Kohrt WM, Landt M, Birge SJ Jr. Serum leptin levels are reduced in response to exercise training, but not hormone replacement therapy, in older women. J Clin Endocrinol Metab. 1996;81(11):3980-3985. [DOI] [PubMed] [Google Scholar]
- 57. Adechian S, Balage M, Remond D, et al. Protein feeding pattern, casein feeding, or milk-soluble protein feeding did not change the evolution of body composition during a short-term weight loss program. Am J Physiol Endocrinol Metab. 2012;303(8): E973-E982. [DOI] [PubMed] [Google Scholar]
- 58. Tamez M, Ramos-Barragan V, Mendoza-Lorenzo P, et al. Adipocyte size and leptin receptor expression in human subcutaneous adipose tissue after Roux-en-Y Gastric bypass. Obes Surg. 2017;27(12):3330-3332. [DOI] [PubMed] [Google Scholar]
- 59. Chandalia M, Lin P, Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One. 2007;2(8):e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang X, Jansson PA, Nagaev I, et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun. 2004;317(4):1045-1051. [DOI] [PubMed] [Google Scholar]
- 61. Drolet R, Bélanger C, Fortier M, et al. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity (Silver Spring). 2009;17(3):424-430. [DOI] [PubMed] [Google Scholar]
- 62. Pasarica M, Tchoukalova YD, Heilbronn LK, et al. ; Look AHEAD Adipose Research Group . Differential effect of weight loss on adipocyte size subfractions in patients with type 2 diabetes. Obesity (Silver Spring). 2009;17(10):1976-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hoffstedt J, Andersson DP, Eriksson Hogling D, et al. Long-term protective changes in adipose tissue after gastric bypass. Diabetes Care. 2017;40(1):77-84. [DOI] [PubMed] [Google Scholar]
- 64. Koska J, Stefan N, Permana PA, et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87(2):295-302. [DOI] [PubMed] [Google Scholar]
- 65. Monteiro R, de Castro PM, Calhau C, Azevedo I. Adipocyte size and liability to cell death. Obes Surg. 2006;16(6):804-806. [DOI] [PubMed] [Google Scholar]
- 66. Salans LB, Cushman SW, Weismann RE. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest. 1973;52(4):929-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity (Silver Spring). 2012;20(5):932-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bahr GF, Bloom G, Friberg U. Volume changes of tissues in physiological fluids during fixation in osmium tetroxide or formaldehyde and during subsequent treatment. Exp Cell Res. 1957;12(2):342-355. [DOI] [PubMed] [Google Scholar]
- 69. Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968;9(1):110-119. [PubMed] [Google Scholar]
- 70. Hayat MA. Fixation for Electron Microscopy. 1st ed. Academic Press, Inc.; 1981:170-171. [Google Scholar]
- 71. Fried SK. Adipocyte size redux. Obesity (Silver Spring). 2017;25(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vogel MAA, Jocken JWE, Sell H, et al. Differences in upper and lower body adipose tissue oxygen tension contribute to the adipose tissue phenotype in humans. J Clin Endocrinol Metab. 2018;103(10):3688-3697. [DOI] [PubMed] [Google Scholar]
- 73. Santosa S, Jensen MD. Adipocyte fatty acid storage factors enhance subcutaneous fat storage in postmenopausal women. Diabetes. 2013;62(3):775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Björntorp P, Carlgren G, Isaksson B, Krotkiewski M, Larsson B, Sjöström L. Effect of an energy-reduced dietary regimen in relation to adipose tissue cellularity in obese women. Am J Clin Nutr. 1975;28(5):445-452. [DOI] [PubMed] [Google Scholar]
- 75. Berman DM, Nicklas BJ, Rogus EM, Dennis KE, Goldberg AP. Regional differences in adrenoceptor binding and fat cell lipolysis in obese, postmenopausal women. Metabolism. 1998;47(4):467-473. [DOI] [PubMed] [Google Scholar]
- 76. Johnson JA, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol Endocrinol Metab. 2001;280(1):E40-E49. [DOI] [PubMed] [Google Scholar]
- 77. Joffe BI, Goldberg RB, Feinstein J, Kark A, Seftel HC. Adipose cell size in obese Africans: evidence against the existence of insulin resistance in some patients. J Clin Pathol. 1979;32(5):471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van Harmelen V, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47(6):913-917. [DOI] [PubMed] [Google Scholar]
- 79. Reynisdottir S, Dauzats M, Thörne A, Langin D. Comparison of hormone-sensitive lipase activity in visceral and subcutaneous human adipose tissue. J Clin Endocrinol Metab. 1997;82(12):4162-4166. [DOI] [PubMed] [Google Scholar]
- 80. Despres JP, Fong BS, Julien P, Jimenez J, Angel A. Regional variation in HDL metabolism in human fat cells: effect of cell size. Am J Physiol. 1987;252(5 Pt 1):E654-E659. [DOI] [PubMed] [Google Scholar]
- 81. Ray H, Pinteur C, Frering V, Beylot M, Large V. Depot-specific differences in perilipin and hormone-sensitive lipase expression in lean and obese. Lipids Health Dis. 2009;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hube F, Lietz U, Igel M, et al. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm Metab Res. 1996;28(12):690-693. [DOI] [PubMed] [Google Scholar]
- 83. Meena VP, Seenu V, Sharma MC, et al. Relationship of adipocyte size with adiposity and metabolic risk factors in Asian Indians. PLoS One. 2014;9(9):e108421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Quinkler M, Bujalska IJ, Tomlinson JW, Smith DM, Stewart PM. Depot-specific prostaglandin synthesis in human adipose tissue: a novel possible mechanism of adipogenesis. Gene. 2006;380(2):137-143. [DOI] [PubMed] [Google Scholar]
- 85. Shimabukuro M, Sato H, Izaki H, et al. Depot- and gender-specific expression of NLRP3 inflammasome and toll-like receptors in adipose tissue of cancer patients. Biofactors. 2016;42(4):397-406. [DOI] [PubMed] [Google Scholar]
- 86. van Beek L, Lips MA, Visser A, et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism. 2014;63(4):492-501. [DOI] [PubMed] [Google Scholar]
- 87. Bambace C, Telesca M, Zoico E, et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc Pathol. 2011;20(5):e153-e156. [DOI] [PubMed] [Google Scholar]
- 88. Bambace C, Sepe A, Zoico E, et al. Inflammatory profile in subcutaneous and epicardial adipose tissue in men with and without diabetes. Heart Vessels. 2014;29(1):42-48. [DOI] [PubMed] [Google Scholar]
- 89. O’Connell J, Lynch L, Cawood TJ, et al. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One. 2010;5(4):e9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dahlman I, Ryden M, Arner P. Family history of diabetes is associated with enhanced adipose lipolysis: evidence for the implication of epigenetic factors. Diabetes Metab. 2018;44(2):155-159. [DOI] [PubMed] [Google Scholar]
- 91. Sparks LM, Pasarica M, Sereda O, et al. Effect of adipose tissue on the sexual dimorphism in metabolic flexibility. Metabolism. 2009;58(11):1564-1571. [DOI] [PubMed] [Google Scholar]
- 92. Votruba SB, Jensen MD. Sex differences in abdominal, gluteal, and thigh LPL activity. Am J Physiol Endocrinol Metab. 2007;292(6):E1823-E1828. [DOI] [PubMed] [Google Scholar]
- 93. McLaughlin T, Lamendola C, Coghlan N, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring). 2014;22(3):673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Soriguer Escofet FJ, Esteva de Antonio I, Tinahones FJ, Pareja A. Adipose tissue fatty acids and size and number of fat cells from birth to 9 years of age – a cross-sectional study in 96 boys. Metabolism. 1996;45(11):1395-1401. [DOI] [PubMed] [Google Scholar]
- 95. Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979;63(2):239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ktotkiewski M, Sjöström L, Björntorp P, Smith U. Regional adipose tissue cellularity in relation to metabolism in young and middle-aged women. Metabolism. 1975;24(6):703-710. [DOI] [PubMed] [Google Scholar]
- 97. Laforest S, Labrecque J, Michaud A, Cianflone K, Tchernof A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit Rev Clin Lab Sci. 2015;52(6):301-313. [DOI] [PubMed] [Google Scholar]
- 98. Vertemati M, Goffredi M, Moscheni C, Callegari S, Vizzotto L. Human visceral fat in different anthropometric patterns and in diabetes: a morphometric study. Anal Quant Cytol Histol. 2008;30(1):39-46. [PubMed] [Google Scholar]
- 99. Singh P, Somers VK, Romero-Corral A, et al. Effects of weight gain and weight loss on regional fat distribution. Am J Clin Nutr. 2012;96(2):229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Serra MC, Blumenthal JB, Addison OR, Miller AJ, Goldberg AP, Ryan AS. Effects of weight loss with and without exercise on regional body fat distribution in postmenopausal women. Ann Nutr Metab. 2017;70(4):312-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50(1):151-157. [DOI] [PubMed] [Google Scholar]
- 102. Ukropec J, Penesová A, Skopková M, et al. Adipokine protein expression pattern in growth hormone deficiency predisposes to the increased fat cell size and the whole body metabolic derangements. J Clin Endocrinol Metab. 2008;93(6):2255-2262. [DOI] [PubMed] [Google Scholar]
- 103. Eriksson-Hogling D, Andersson DP, Bäckdahl J, et al. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int J Obes (Lond). 2015;39(6):893-898. [DOI] [PubMed] [Google Scholar]
- 104. Mottagui-Tabar S, Rydén M, Löfgren P, et al. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia. 2003;46(6):789-797. [DOI] [PubMed] [Google Scholar]
- 105. Anthanont P, Ramos P, Jensen MD, Hames KC. Family history of type 2 diabetes, abdominal adipocyte size and markers of the metabolic syndrome. Int J Obes (Lond). 2017;41(11):1621-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nellemann B, Gormsen LC, Christiansen JS, Jensen MD, Nielsen S. Postabsorptive VLDL-TG fatty acid storage in adipose tissue in lean and obese women. Obesity (Silver Spring). 2010;18(7):1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Serra MC, Ryan AS, Sorkin JD, Favor KH, Goldberg AP. High adipose LPL activity and adipocyte hypertrophy reduce visceral fat and metabolic risk in obese, older women. Obesity (Silver Spring). 2015;23(3):602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87(1):56-63. [DOI] [PubMed] [Google Scholar]
- 109. Drolet R, Richard C, Sniderman AD, et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes (Lond). 2008;32(2):283-291. [DOI] [PubMed] [Google Scholar]
- 110. Björntorp P, Bengtsson C, Blohmé G, et al. Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism. 1971;20(10):927-935. [DOI] [PubMed] [Google Scholar]
- 111. Echiburú B, Pérez-Bravo F, Galgani JE, et al. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids. 2018;130:15-21. [DOI] [PubMed] [Google Scholar]
- 112. Krotkiewski M, Garellick G, Sjöström L, Persson G, Bjurö T, Sullivan L. Fat cell number, resting metabolic rate, mean heart rate, and insulin elevation while seeing and smelling food as predictors of slimming. Metabolism. 1980;29(11):1003-1012. [DOI] [PubMed] [Google Scholar]
- 113. Nway NC, Sitticharoon C, Chatree S, Maikaew P. Correlations between the expression of the insulin sensitizing hormones, adiponectin, visfatin, and omentin, and the appetite regulatory hormone, neuropeptide Y and its receptors in subcutaneous and visceral adipose tissues. Obes Res Clin Pract. 2016;10(3):256-263. [DOI] [PubMed] [Google Scholar]
- 114. Varady KA, Tussing L, Bhutani S, Braunschweig CL. Degree of weight loss required to improve adipokine concentrations and decrease fat cell size in severely obese women. Metabolism. 2009;58(8):1096-1101. [DOI] [PubMed] [Google Scholar]
- 115. Dadson P, Ferrannini E, Landini L, et al. Fatty acid uptake and blood flow in adipose tissue compartments of morbidly obese subjects with or without type 2 diabetes: effects of bariatric surgery. Am J Physiol Endocrinol Metab. 2017;313(2): E175-E182. [DOI] [PubMed] [Google Scholar]
- 116. Mårin P, Andersson B, Ottosson M, et al. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism. 1992;41(11):1242-1248. [DOI] [PubMed] [Google Scholar]
- 117. Ledoux S, Coupaye M, Essig M, et al. Traditional anthropometric parameters still predict metabolic disorders in women with severe obesity. Obesity (Silver Spring). 2010;18(5):1026-1032. [DOI] [PubMed] [Google Scholar]
- 118. Ezure T, Amano S. Increment of subcutaneous adipose tissue is associated with decrease of elastic fibres in the dermal layer. Exp Dermatol. 2015;24(12):924-929. [DOI] [PubMed] [Google Scholar]
- 119. Cancello R, Zulian A, Gentilini D, et al. Permanence of molecular features of obesity in subcutaneous adipose tissue of ex-obese subjects. Int J Obes (Lond). 2013;37(6):867-873. [DOI] [PubMed] [Google Scholar]
- 120. Wan D, Amirlak B, Giessler P, et al. The differing adipocyte morphologies of deep versus superficial midfacial fat compartments: a cadaveric study. Plast Reconstr Surg. 2014;133(5):615e-622e. [DOI] [PubMed] [Google Scholar]
- 121. Srdić B, Stokić E, Korać A, Ukropina M, Veličković K, Breberina M. Morphological characteristics of abdominal adipose tissue in normal-weight and obese women of different metabolic profiles. Exp Clin Endocrinol Diabetes. 2010;118(10):713-718. [DOI] [PubMed] [Google Scholar]
- 122. Bravo-Flores E, Mancilla-Herrera I, Espino YSS, et al. Macrophage populations in visceral adipose tissue from pregnant women: potential role of obesity in maternal inflammation. Int J Mol Sci. 2018;19(4):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Raja GK, Sarzynski MA, Katzmarzyk PT, et al. Commonality versus specificity among adiposity traits in normal-weight and moderately overweight adults. Int J Obes (Lond). 2014;38(5):719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kursawe R, Eszlinger M, Narayan D, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59(9):2288-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Azuma K, Heilbronn LK, Albu JB, Smith SR, Ravussin E, Kelley DE; Look AHEAD Adipose Research Group . Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007;293(1):E435-E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McLaughlin T, Craig C, Liu LF, et al. Adipose cell size and regional fat deposition as predictors of metabolic response to overfeeding in insulin-resistant and insulin-sensitive humans. Diabetes. 2016;65(5):1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Brook CG, Lloyd JK. Adipose cell size and glucose tolerance in obese children and effects of diet. Arch Dis Child. 1973;48(4):301-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Glastonbury CA, Pulit SL, Honecker J, et al. Machine Learning based histology phenotyping to investigate the epidemiologic and genetic basis of adipocyte morphology and cardiometabolic traits. PLoS Comput Biol. 2020;16(8):e1008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mundi MS, Karpyak MV, Koutsari C, Votruba SB, O’Brien PC, Jensen MD. Body fat distribution, adipocyte size, and metabolic characteristics of nondiabetic adults. J Clin Endocrinol Metab. 2010;95(1):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60(5):1504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rydén M, Andersson DP, Bergström IB, Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab. 2014;99(10):E1870-E1876. [DOI] [PubMed] [Google Scholar]
- 133. Svensson H, Wetterling L, Bosaeus M, et al. Body fat mass and the proportion of very large adipocytes in pregnant women are associated with gestational insulin resistance. Int J Obes (Lond). 2016;40(4):646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tittelbach TJ, Berman DM, Nicklas BJ, Ryan AS, Goldberg AP. Racial differences in adipocyte size and relationship to the metabolic syndrome in obese women. Obes Res. 2004;12(6):990-998. [DOI] [PubMed] [Google Scholar]
- 135. Hammarstedt A, Graham TE, Kahn BB. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol Metab Syndr. 2012;4(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Després JP, Nadeau A, Tremblay A, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38(3):304-309. [DOI] [PubMed] [Google Scholar]
- 137. Andersson DP, Eriksson Hogling D, Thorell A, et al. Changes in subcutaneous fat cell volume and insulin sensitivity after weight loss. Diabetes Care. 2014;37(7):1831-1836. [DOI] [PubMed] [Google Scholar]
- 138. Sandqvist M, Strindberg L, Schmelz M, Lönnroth P, Jansson PA. Impaired delivery of insulin to adipose tissue and skeletal muscle in obese women with postprandial hyperglycemia. J Clin Endocrinol Metab. 2011;96(8):E1320-E1324. [DOI] [PubMed] [Google Scholar]
- 139. Hoffstedt J, Arner E, Wahrenberg H, et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia. 2010;53(12):2496-2503. [DOI] [PubMed] [Google Scholar]
- 140. Mannerås-Holm L, Leonhardt H, Kullberg J, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96(2):E304-E311. [DOI] [PubMed] [Google Scholar]
- 141. Cotillard A, Poitou C, Torcivia A, et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99(8):E1466-E1470. [DOI] [PubMed] [Google Scholar]
- 142. Muir LA, Neeley CK, Meyer KA, et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity (Silver Spring). 2016;24(3):597-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rojas-Rodriguez R, Lifshitz LM, Bellve KD, et al. Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia. 2015;58(9):2106-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kranendonk ME, van Herwaarden JA, Stupkova T, et al. Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis. 2015;239(2):419-427. [DOI] [PubMed] [Google Scholar]
- 145. Muir LA, Baker NA, Washabaugh AR, et al. Adipocyte hypertrophy-hyperplasia balance contributes to weight loss after bariatric surgery. Adipocyte. 2017;6(2):134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Roberts R, Hodson L, Dennis AL, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52(5):882-890. [DOI] [PubMed] [Google Scholar]
- 147. Lönn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24(1):326-331. [DOI] [PubMed] [Google Scholar]
- 148. Björntorp P, Grimby G, Sanne H, Sjöström L, Tibblin G, Wilhelmsen L. Adipose tissue fat cell size in relation to metabolism in weight-stabile, physically active men. Horm Metab Res. 1972;4(3):182-186. [DOI] [PubMed] [Google Scholar]
- 149. Bredella MA, Karastergiou K, Bos SA, et al. GH administration decreases subcutaneous abdominal adipocyte size in men with abdominal obesity. Growth Horm IGF Res. 2017;35:17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. McLaughlin T, Sherman A, Tsao P, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50(8):1707-1715. [DOI] [PubMed] [Google Scholar]
- 151. McLaughlin T, Abbasi F, Lamendola C, Yee G, Carter S, Cushman SW. Dietary weight loss in insulin-resistant non-obese humans: Metabolic benefits and relationship to adipose cell size. Nutr Metab Cardiovasc Dis. 2019;29(1):62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Grenier-Larouche T, Carreau AM, Geloën A, et al. Fatty acid metabolic remodeling during type 2 diabetes remission after bariatric surgery. Diabetes. 2017;66(11):2743-2755. [DOI] [PubMed] [Google Scholar]
- 153. Weyer C, Wolford JK, Hanson RL, et al. Subcutaneous abdominal adipocyte size, a predictor of type 2 diabetes, is linked to chromosome 1q21–q23 and is associated with a common polymorphism in LMNA in Pima Indians. Mol Genet Metab. 2001;72(3):231-238. [DOI] [PubMed] [Google Scholar]
- 154. Balakrishnan P, Grundy SM, Islam A, Dunn F, Vega GL. Influence of upper and lower body adipose tissue on insulin sensitivity in South Asian men. J Investig Med. 2012;60(7):999-1004. [DOI] [PubMed] [Google Scholar]
- 155. Pasarica M, Xie H, Hymel D, et al. Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes Care. 2009;32(5):900-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Dubois SG, Heilbronn LK, Smith SR, Albu JB, Kelley DE, Ravussin E; Look AHEAD Adipose Research Group . Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity (Silver Spring). 2006;14(9):1543-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498-1506. [DOI] [PubMed] [Google Scholar]
- 158. Abbott WG, Foley JE. Comparison of body composition, adipocyte size, and glucose and insulin concentrations in Pima Indian and Caucasian children. Metabolism. 1987;36(6):576-579. [DOI] [PubMed] [Google Scholar]
- 159. Carpentier AC. 100(th) Anniversary of the discovery of insulin Perspective: Insulin and Adipose Tissue Fatty Acid Metabolism. Am J Physiol Endocrinol Metab. 2021;320(4):E653-E670. [DOI] [PubMed] [Google Scholar]
- 160. Ferrara CM, Lynch NA, Nicklas BJ, Ryan AS, Berman DM. Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinol Metab. 2002;87(9):4166-4170. [DOI] [PubMed] [Google Scholar]
- 161. Noll C, Montastier É, Amrani M, et al. Seven-day overfeeding enhances adipose tissue dietary fatty acid storage and decreases myocardial and skeletal muscle dietary fatty acid partitioning in healthy subjects. Am J Physiol Endocrinol Metab. 2020;318(2):E286-E296. [DOI] [PubMed] [Google Scholar]
- 162. Hames KC, Koutsari C, Santosa S, Bush NC, Jensen MD. Adipose tissue fatty acid storage factors: effects of depot, sex and fat cell size. Int J Obes (Lond). 2015;39(6):884-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mundi MS, Koutsari C, Jensen MD. Effects of increased free fatty acid availability on adipose tissue fatty acid storage in men. J Clin Endocrinol Metab. 2014;99(12):E2635-E2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity (Silver Spring). 2009;17(6):1129-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pereira MJ, Skrtic S, Katsogiannos P, et al. Impaired adipose tissue lipid storage, but not altered lipolysis, contributes to elevated levels of NEFA in type 2 diabetes. Degree of hyperglycemia and adiposity are important factors. Metabolism. 2016;65(12):1768-1780. [DOI] [PubMed] [Google Scholar]
- 166. Morgan-Bathke M, Chen L, Oberschneider E, Harteneck D, Jensen MD. Sex and depot differences in ex vivo adipose tissue fatty acid storage and glycerol-3-phosphate acyltransferase activity. Am J Physiol Endocrinol Metab. 2015;308(9): E830-E846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rajjo TI, Harteneck DA, Jensen MD. Direct free fatty acid storage in different sized adipocytes from the same depot. Obesity (Silver Spring). 2014;22(5):1275-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Petrescu O, Fan X, Gentileschi P, et al. Long-chain fatty acid uptake is upregulated in omental adipocytes from patients undergoing bariatric surgery for obesity. Int J Obes (Lond). 2005;29(2):196-203. [DOI] [PubMed] [Google Scholar]
- 169. Søndergaard E, Nellemann B, Sørensen LP, et al. Similar VLDL-TG storage in visceral and subcutaneous fat in obese and lean women. Diabetes. 2011;60(11):2787-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Spalding KL, Bernard S, Näslund E, et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat Commun. 2017;8:15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Carreau AM, Noll C, Blondin DP, et al. Bariatric surgery rapidly decreases cardiac dietary fatty acid partitioning and hepatic insulin resistance through increased intra-abdominal adipose tissue storage and reduced spillover in type 2 diabetes. Diabetes. 2020;69(4):567-577. [DOI] [PubMed] [Google Scholar]
- 172. Carpentier AC. Abnormal myocardial dietary fatty acid metabolism and diabetic cardiomyopathy. Can J Cardiol. 2018;34(5):605-614. [DOI] [PubMed] [Google Scholar]
- 173. Labbé SM, Grenier-Larouche T, Noll C, et al. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes. 2012;61(11):2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Noll C, Carpentier AC. Dietary fatty acid metabolism in prediabetes. Curr Opin Lipidol. 2017;28(1):1-10. [DOI] [PubMed] [Google Scholar]
- 175. Sato S, Demura S, Nakai M. Storage capacity of subcutaneous fat in Japanese adults. Eur J Clin Nutr. 2015;69(8):933-938. [DOI] [PubMed] [Google Scholar]
- 176. Laurencikiene J, Skurk T, Kulyté A, et al. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab. 2011;96(12):E2045-E2049. [DOI] [PubMed] [Google Scholar]
- 177. Rydén M, Arner P. Cardiovascular risk score is linked to subcutaneous adipocyte size and lipid metabolism. J Intern Med. 2017;282(3):220-228. [DOI] [PubMed] [Google Scholar]
- 178. Löfgren P, Hoffstedt J, Näslund E, Wirén M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia. 2005;48(11):2334-2342. [DOI] [PubMed] [Google Scholar]
- 179. Gao H, Mejhert N, Fretz JA, et al. Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab. 2014;19(6):981-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jacobsson B, Smith U. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res. 1972;13(5):651-656. [PubMed] [Google Scholar]
- 181. Arner P, Ostman J. Relationship between the tissue level of cyclic AMP and the fat cell size of human adipose tissue. J Lipid Res. 1978;19(5):613-618. [PubMed] [Google Scholar]
- 182. Arner P, Engfeldt P, Ostman J. Relationship between lipolysis, cyclic AMP, and fat-cell size in human adipose tissue during fasting and in diabetes mellitus. Metabolism. 1979;28(3):198-209. [DOI] [PubMed] [Google Scholar]
- 183. Yu J, Yu HC, Kim KA, et al. Differences in the amount of lipolysis induced by atrial natriuretic peptide in small and large adipocytes. J Pept Sci. 2008;14(8):972-977. [DOI] [PubMed] [Google Scholar]
- 184. Large V, Arner P, Reynisdottir S, et al. Hormone-sensitive lipase expression and activity in relation to lipolysis in human fat cells. J Lipid Res. 1998;39(8):1688-1695. [PubMed] [Google Scholar]
- 185. Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95(8):4052-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Montastier E, Ye RZ, Noll C, et al. Increased postprandial nonesterified fatty acid efflux from adipose tissue in prediabetes is offset by enhanced dietary fatty acid adipose trapping. Am J Physiol Endocrinol Metab. 2021;320(6):E1093-E1106. [DOI] [PubMed] [Google Scholar]
- 187. Bray GA, Glennon JA, Salans LB, Horton ES, Danforth E Jr, Sims EA. Spontaneous and experimental human obesity: effects of diet and adipose cell size on lipolysis and lipogenesis. Metabolism. 1977;26(7):739-747. [DOI] [PubMed] [Google Scholar]
- 188. Ek I, Arner P, Rydén M, et al. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes. 2002;51(2):484-492. [DOI] [PubMed] [Google Scholar]
- 189. Large V, Reynisdottir S, Langin D, et al. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res. 1999;40(11):2059-2066. [PubMed] [Google Scholar]
- 190. Jönsson C, Castor Batista AP, Kjølhede P, Strålfors P. Insulin and β-adrenergic receptors mediate lipolytic and anti-lipolytic signalling that is not altered by type 2 diabetes in human adipocytes. Biochem J. 2019;476(19):2883-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Allister CA, Liu LF, Lamendola CA, et al. In vivo 2H2O administration reveals impaired triglyceride storage in adipose tissue of insulin-resistant humans. J Lipid Res. 2015;56(2):435-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tuvdendorj D, Chandalia M, Batbayar T, et al. Altered subcutaneous abdominal adipose tissue lipid synthesis in obese, insulin-resistant humans. Am J Physiol Endocrinol Metab. 2013;305(8):E999-E1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Frayn KN, Humphreys SM, Coppack SW. Net carbon flux across subcutaneous adipose tissue after a standard meal in normal-weight and insulin-resistant obese subjects. Int J Obes Relat Metab Disord. 1996;20(9):795-800. [PubMed] [Google Scholar]
- 194. McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60(1):47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Imbeault P, Lemieux S, Prud’homme D, et al. Relationship of visceral adipose tissue to metabolic risk factors for coronary heart disease: is there a contribution of subcutaneous fat cell hypertrophy? Metabolism. 1999;48(3):355-362. [DOI] [PubMed] [Google Scholar]
- 196. Petäjä EM, Sevastianova K, Hakkarainen A, Orho-Melander M, Lundbom N, Yki-Järvinen H. Adipocyte size is associated with NAFLD independent of obesity, fat distribution, and PNPLA3 genotype. Obesity (Silver Spring). 2013;21(6):1174-1179. [DOI] [PubMed] [Google Scholar]
- 197. Anand SS, Tarnopolsky MA, Rashid S, et al. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One. 2011;6(7):e22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Johannsen DL, Tchoukalova Y, Tam CS, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “adipose tissue expandability” hypothesis. Diabetes Care. 2014;37(10):2789-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Jansen HJ, Vervoort GM, van der Graaf M, Stienstra R, Tack CJ. Liver fat content is linked to inflammatory changes in subcutaneous adipose tissue in type 2 diabetes patients. Clin Endocrinol (Oxf). 2013;79(5):661-666. [DOI] [PubMed] [Google Scholar]
- 200. Rawshani A, Eliasson B, Rawshani A, et al. Adipose tissue morphology, imaging and metabolomics predicting cardiometabolic risk and family history of type 2 diabetes in non-obese men. Sci Rep. 2020;10(1):9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56(8):1960-1968. [DOI] [PubMed] [Google Scholar]
- 202. Tam CS, Viardot A, Clément K, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59(9):2164-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179-188. [DOI] [PubMed] [Google Scholar]
- 204. Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6(1):13-21. [DOI] [PubMed] [Google Scholar]
- 205. Rizkalla SW, Prifti E, Cotillard A, et al. Differential effects of macronutrient content in 2 energy-restricted diets on cardiovascular risk factors and adipose tissue cell size in moderately obese individuals: a randomized controlled trial. Am J Clin Nutr. 2012;95(1):49-63. [DOI] [PubMed] [Google Scholar]
- 206. Arner P, Bäckdahl J, Hemmingsson P, et al. Regional variations in the relationship between arterial stiffness and adipocyte volume or number in obese subjects. Int J Obes (Lond). 2015;39(2):222-227. [DOI] [PubMed] [Google Scholar]
- 207. Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398):1-12. [DOI] [PubMed] [Google Scholar]
- 208. Vianello E, Dozio E, Arnaboldi F, et al. Epicardial adipocyte hypertrophy: Association with M1-polarization and toll-like receptor pathways in coronary artery disease patients. Nutr Metab Cardiovasc Dis. 2016;26(3):246-253. [DOI] [PubMed] [Google Scholar]
- 209. Silaghi A, Silaghi H, Scridon T, Pais R, Achard V. Glucocorticoid receptors in human epicardial adipose tissue: role of coronary status. J Endocrinol Invest. 2012;35(7):649-654. [DOI] [PubMed] [Google Scholar]
- 210. Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Fernandez A, Adrio B, Gonzalez-Juanatey JR. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens. 2008;22(12):856-863. [DOI] [PubMed] [Google Scholar]
- 211. Eiras S, Teijeira-Fernández E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine. 2008;43(2):174-180. [DOI] [PubMed] [Google Scholar]
- 212. Hamsten A, de Faire U, Walldius G, et al. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1987;2(8549):3-9. [DOI] [PubMed] [Google Scholar]
- 213. Song C, Burgess S, Eicher JD, O’Donnell CJ, Johnson AD. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J Am Heart Assoc. 2017;6(6):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Tofler GH, Massaro J, O’Donnell CJ, et al. Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thromb Res. 2016;140:30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Eriksson P, Van Harmelen V, Hoffstedt J, et al. Regional variation in plasminogen activator inhibitor-1 expression in adipose tissue from obese individuals. Thromb Haemost. 2000;83(4):545-548. [PubMed] [Google Scholar]
- 216. Schwartz RS. The independent effects of dietary weight loss and aerobic training on high density lipoproteins and apolipoprotein A-I concentrations in obese men. Metabolism. 1987;36(2):165-171. [DOI] [PubMed] [Google Scholar]
- 217. Verhoef SP, van Dijk P, Westerterp KR. Relative shrinkage of adipocytes by paraffin in proportion to plastic embedding in human adipose tissue before and after weight loss. Obes Res Clin Pract. 2013;7(1):e8-13. [DOI] [PubMed] [Google Scholar]
- 218. Presta E, Leibel RL, Hirsch J. Regional changes in adrenergic receptor status during hypocaloric intake do not predict changes in adipocyte size or body shape. Metabolism. 1990;39(3):307-315. [DOI] [PubMed] [Google Scholar]
- 219. Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107(42):18226-18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes (Lond). 2006;30(8):1211-1216. [DOI] [PubMed] [Google Scholar]
- 221. Ravussin E, Tschöp M, Morales S, Bouchard C, Heiman ML. Plasma ghrelin concentration and energy balance: overfeeding and negative energy balance studies in twins. J Clin Endocrinol Metab. 2001;86(9):4547-4551. [DOI] [PubMed] [Google Scholar]
- 222. McLaughlin TM, Liu T, Yee G, et al. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring). 2010;18(5):926-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Boden G, Cheung P, Mozzoli M, Fried SK. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism. 2003;52(6):753-759. [DOI] [PubMed] [Google Scholar]
- 224. Ciaraldi TP, Kong AP, Chu NV, et al. Regulation of glucose transport and insulin signaling by troglitazone or metformin in adipose tissue of type 2 diabetic subjects. Diabetes. 2002;51(1):30-36. [DOI] [PubMed] [Google Scholar]
- 225. Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab. 2014;99(2):E209-E216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Hallgren P, Sjöström L, Hedlund H, Lundell L, Olbe L. Influence of age, fat cell weight, and obesity on O2 consumption of human adipose tissue. Am J Physiol. 1989;256(4 Pt 1):E467-E474. [DOI] [PubMed] [Google Scholar]
- 227. Fischer B, Schöttl T, Schempp C, et al. Inverse relationship between body mass index and mitochondrial oxidative phosphorylation capacity in human subcutaneous adipocytes. Am J Physiol Endocrinol Metab. 2015;309(4):E380-E387. [DOI] [PubMed] [Google Scholar]