Abstract
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders affecting cortisol biosynthesis. Reduced activity of an enzyme required for cortisol production leads to chronic overstimulation of the adrenal cortex and accumulation of precursors proximal to the blocked enzymatic step. The most common form of CAH is caused by steroid 21-hydroxylase deficiency due to mutations in CYP21A2. Since the last publication summarizing CAH in Endocrine Reviews in 2000, there have been numerous new developments. These include more detailed understanding of steroidogenic pathways, refinements in neonatal screening, improved diagnostic measurements utilizing chromatography and mass spectrometry coupled with steroid profiling, and improved genotyping methods. Clinical trials of alternative medications and modes of delivery have been recently completed or are under way. Genetic and cell-based treatments are being explored. A large body of data concerning long-term outcomes in patients affected by CAH, including psychosexual well-being, has been enhanced by the establishment of disease registries. This review provides the reader with current insights in CAH with special attention to these new developments.
Keywords: Steroid biosynthesis, 21-hydroxylase deficiency, CYP21A2, glucocorticoid, mineralocorticoid, cortisol, aldosterone
Graphical Abstract
Graphical Abstract.
Essential points.
Congenital adrenal hyperplasia (CAH) is most often caused by deficiency of steroid 21 hydroxylase encoded by CYP21A2
Allelic variants are associated with a spectrum of phenotypes
CAH in its severe, classic form includes cortisol and aldosterone deficiencies, as well as androgen excess
Newer concepts in steroid biosynthesis, hormonal and genetic diagnostic tools, and novel therapeutics have expanded our understanding of CAH
Long-term sequelae of this disease have been reported in detail and strategies are being developed to improve quality of life for these patients
Congenital adrenal hyperplasia (CAH) is an inherited inability to synthesize cortisol. Approximately 90% to 99% of cases of CAH are caused by 21-hydroxylase deficiency (21OHD) caused by mutations in the CYP21A2 gene (1, 2); the terms CAH and 21OHD will be used interchangeably in this article. The literature has historically described classic and nonclassic (NC) forms of this disorder, although current thinking views CYP21A2 allelic variants and their phenotypic manifestations as a continuum. The classic form, occurring in 1 in 14 000 to 18 000 based on newborn screening (Table 1), is defined by severely reduced or absent enzyme activity with impaired cortisol production manifesting clinically in the neonatal period. In the most severe, salt-wasting (SW) form of classic CAH, there is little or no residual enzymatic activity, resulting in cortisol and aldosterone deficiency. Lack of negative feedback on the hypothalamic–pituitary–adrenal axis leads to excess adrenal androgen production as elevated precursor steroids are shifted to the nonaffected androgen pathways. If not promptly treated, infants with this form of CAH quickly develop potentially fatal “salt-wasting crises” with hyponatremia, hyperkalemia, acidosis, and shock. Those infants who produce slightly more aldosterone are less likely to suffer acute SW crisis, but such patients still have severe cortisol deficiency and markedly elevated adrenal androgen production. They are said to have “simple virilizing” (SV) CAH, associated with residual enzymatic activity of 1% to 5% of normal. All infants affected with classic CAH benefit from glucocorticoid plus adjunctive mineralocorticoid treatment at least within the first year of life, when there is relative renal tubular resistance to the salt-retaining effects of aldosterone in early infancy (28) and low sodium content of infant diets (29).
Table 1.
Incidence of CAH in different countries
| Country | Complete national data? | Sample size | 1/Incidence | PPV (term infants or overall) | Reference |
|---|---|---|---|---|---|
| Argentina (Buenos Aires) | No | 80 436 | 8937 | 50 | (3) |
| Australia* | Yes | 18 034 | N/A | (4) | |
| Australia (New South Wales) | No | 185 854 | 15 488 | 1.8 | (4) |
| Australia (Western Australia)* | No | 550 153 | 14 869 | N/A | (5) |
| Brazil | No | 748 350 | 14 967 | (6) | |
| Brazil (Goias state) | No | 82 603 | 10 325 | 28.6 | (7) |
| Brazil (Minas Gerais state) | No | 159 415 | 19 927 | 2.1 | (8) |
| Brazil (Rio Grande do Sul state) | No | 108 409 | 13 551 | 1.6 | (9) |
| China | No | 30 000 | 6084 | (10) | |
| China (Beijing) | No | 44 360 | 7393 | 3.0 | (11) |
| Croatia | Yes | 532 942 | 14 403 | (12) | |
| Cuba | Yes | 621 303 | 15 931 | 0.3 | (13) |
| Czech Republic | Yes | 888 891 | 12 520 | 1.6 | (14) |
| France | Yes | 6 012,798 | 15 699 | 2.3 | (15) |
| Germany (Bavaria) | No | 1 420,102 | 12 457 | 5 | (16) |
| India | No | 55 627 | 6334 | (17) | |
| Israel | Yes | 1 378,132 | 16 910 | 16.5 | (18) |
| Japan (Sapporo) | No | 498 147 | 20 756 | 8 | (19) |
| Japan (Tokyo) | No | 2 105,108 | 21 264 | 25.8 | (20) |
| Netherlands | Yes | 2 235,931 | 17 468 | 24.7 | (21) |
| New Zealand | Yes | 1 175,988 | 26 727 | (22) | |
| Sweden | Yes | 2 737,932 | 14 260 | 25.1 | (2) |
| Turkey | No | 241 083 | 15 067 | 1.9 | (23) |
| United Arab Emirates | Yes | 750 365 | 9030 | (24) | |
| United Kingdom* | Yes | 18 248 | N/A | (25) | |
| Uruguay | Yes | 190 053 | 15 800 | (26) |
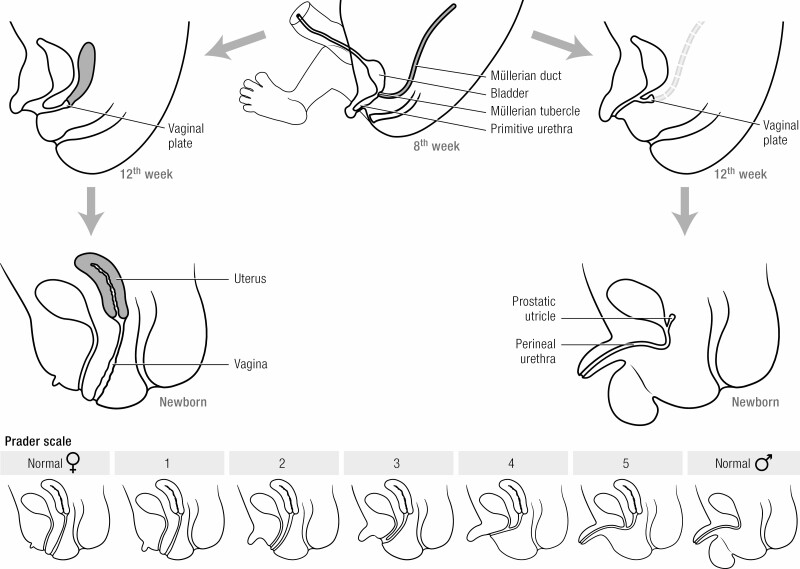
Whereas gonadal development is normal, severely increased prenatal adrenal androgen production leads to virilization of the female external genitalia (30), including variable degrees of clitoral enlargement and labial fusion. The genital appearance of affected 46,XX infants is occasionally indistinguishable from that of male genitals with penis and scrotum but empty of gonads. Müllerian duct development is normal, except for the formation of a urogenital sinus with conjoined urethra and vagina. Thus, reproductive potential exists in females despite atypical external genitalia. Males have normal external genitalia. Wolffian duct development is normal in males but absent in females, who continue to produce COUP-TFII (Chicken Ovalbumin Upstream Promoter-Transcription Factor-2), which induces Wolffian duct involution (31).
Adverse sequelae in CAH patients occur as a result of adrenal hormone imbalance and from chronic glucocorticoid therapy (32). Androgen excess can cause inappropriately rapid somatic growth, accelerated skeletal maturation, and reduced adult height. A systematic review and meta-analysis for >1000 classic CAH patients found shorter than average stature for mid-parental heights (–1.03 standard deviations, corresponding to ~7 cm) (33), but many of these children were diagnosed before the implementation of neonatal screening and did not receive the benefit of early initiation of treatment.
Elevated levels of adrenal androgens affect the hypothalamic–pituitary–gonadal axis. Central precocious puberty is a risk in patients experiencing prolonged periods of poor hormonal control. Young women with well-controlled CAH usually experience normal menarche (34), but poor control is associated with acne, female hirsutism, male pattern baldness, altered body habitus, irregular menses, and subnormal fertility (35). Males with poor hormonal control may develop small testes and benign testicular adrenal rest tumors (TARTs) (see section “Long-term sequelae,” “Gonadal function in males,” “Testicular adrenal rest tumors”) (36).
Individuals affected with milder allelic variants (ie, NC CAH) tend to present to medical attention after infancy, hence the former term “late-onset” CAH. The associated alleles encode enzymes with residual activity of 20% to 50%. Thus, these individuals typically have normal basal cortisol and aldosterone production but mildly elevated levels of adrenal androgens; however, suboptimal cortisol levels after adrenocorticotropic hormone (ACTH) stimulation are reported in up to 30% of patients (37). Children may present with symptoms due to elevated adrenal androgens such as premature adrenarche, acne, and accelerated skeletal maturation but many, especially males, are asymptomatic. Adolescent girls or adult women may present with hirsutism, oligomenorrhea, acne, and subnormal fertility (37). Because NC CAH is not the primary target of neonatal screening and is rarely detected by that strategy, the true prevalence of this milder disorder is unclear. The estimated prevalence is ~1 in 200 in the Caucasian population (38).
Since the last publication summarizing CAH in Endocrine Reviews in 2000 (1), there have been numerous new developments. These include more detailed understanding of steroidogenic pathways, refinements in neonatal screening, improved diagnostic measurements utilizing high-throughput liquid chromatography-tandem mass spectrometry (LC-MS/MS) coupled with steroid profiling, and improved genotyping methods. Clinical trials of alternative medications and modes of delivery have been recently completed or are under way, with the nearer prospect of genetic and cell-based treatments and a large body of data concerning long-term outcomes in patients affected by CAH, including psychosexual well-being, enhanced by the establishment of disease registries.
Much remains to be learned in several other domains spanning fetal life through adulthood. Both human and animal studies have illuminated risks of antenatal dexamethasone (Dex) treatment. Noninvasive prenatal diagnosis of CAH in families with known CYP21A2 pathogenic genotypes has been accomplished by analysis of circulating free fetal DNA in maternal blood in proof-of-concept studies, but is not yet widely available. Genital reconstructive surgery in affected females is no longer viewed as an emergency procedure, and indeed the practice of genital surgery in infancy has been questioned. Shared decision making among parents, patients, surgeons, endocrinologists, mental health providers, and support groups has been promoted as model for optimal care. Benefit-to-risk ratio for no surgery, or early or late genital surgery for females with CAH remains to be determined. Unfortunately, even in advanced societies, medical care for CAH is neglected, increasing the risk for cardiovascular or metabolic morbidities due to suboptimal corticosteroid therapy. Methods to improve transition of care from pediatric to adult healthcare, as well as patient and provider education, are important goals.
This multiauthored review is the result of a planned European CAH Symposium, which was postponed due to the Covid-19 pandemic. The large international group of authors contributed innovative approaches to understanding and managing this condition.
Basic Principles of Steroid Synthesis and Adrenal Enzymatic Defects
Physiology and Pathophysiology of Steroidogenesis
Steroidogenesis in the adrenal cortex takes place in 3 concentric zones: the outermost zona glomerulosa (mineralocorticoid biosynthesis), the zona fasciculata (glucocorticoid biosynthesis), and the innermost zona reticularis (sex steroid precursor biosynthesis). It entails conversion of cholesterol to active steroid hormones, and involves many enzymes, cofactors, and accessory proteins (Fig. 1). Most of these are expressed in the appropriate zones of the adrenal cortex, with others expressed in the gonads, placenta, and some “peripheral” tissues; these factors and the conditions caused by their mutations have been studied in detail (39). Mutations have been described in most of the genes encoding these proteins; those that disrupt cortisol synthesis with compensatory elevations in ACTH cause CAH, but in common parlance “CAH” refers to 21OHD. This section describes all enzymatic conversions required to synthesize cortisol.
Figure 1.
Adrenal steroidogenesis. Enzymes are boxed with dotted lines extending to arrows denoting each enzymatic conversion; 2 enzymes, CYP11B2 and CYP17, catalyze several successive enzymatic conversions. Accessory proteins required for activity of cytochrome P450 enzymes are shown next to each such enzyme: POR, P450 oxidoreductase, required by CYP enzymes in the endoplasmic reticulum; FDXR/FDX1, ferredoxin reductase and ferredoxin, required by mitochondrial CYP enzymes. Cytochrome B5 (CYP5A) is required for full 17,20-lyase activity of CYP17A1. There are 2 11β-hydroxysteroid dehydrogenase isozymes; HSD11B1, expressed mainly in the liver, catalyzes reduction (eg, cortisone to cortisol), whereas HSD11B2, expressed mainly in the kidney, catalyzes oxidation (eg, cortisol to cortisone). The steps affected by 21OHD, including steroids secreted in increased amounts in this disease, are denoted by red lines and red lettering. Steps taking place only in the adrenal glands are in unshaded boxes; steps taking place partly or predominantly outside the adrenal cortex are denoted by shaded boxes. Planar structures of cholesterol, aldosterone, cortisol, and testosterone are illustrated; the position of the 11-oxo (11-keto) group in 11-ketotestosterone is illustrated in green. Colored rectangles indicate the following: gray, early steps of steroidogenesis common to all zones of the cortex; orange, steps in the zona glomerulosa leading to aldosterone; blue, steps in the zona fasciculata leading to cortisol; magenta; steps in the zona reticularis and extra-adrenal tissues leading to androgens; purple, the “backdoor” or alternate pathway from 17-OH progesterone to dihydrotestosterone (for clarity, the alternative pathway from progesterone is not shown); green, conversions leading to 11-oxo androgens.
Cholesterol side-chain cleavage
Steroidogenesis is initiated by the conversion of cholesterol to pregnenolone, catalyzed by the cholesterol side-chain cleavage enzyme, CYP11A1 (P450scc). To initiate steroidogenesis, cholesterol from cytoplasmic storage depots must reach CYP11A1 on the inner mitochondrial membrane; this cholesterol influx requires the steroidogenic acute regulatory protein (StAR), acting on the outer mitochondrial membrane (OMM) (40). The action of StAR requires its phosphorylation and interaction with some other proteins, but the exact mechanism of StAR’s action remains under investigation (41, 42). Mutations in StAR cause another rare form of CAH, congenital lipoid adrenal hyperplasia, in which virtually no steroid hormones are made and 46,XY fetuses are phenotypically female due to impaired testicular steroidogenesis (43, 44). CYP11A1 defects were once considered incompatible with term pregnancy; however, more than 30 cases of such defects have been reported (40). These 2 conditions are clinically and hormonally indistinguishable, but lipoid CAH is typically associated with very large adrenals, whereas CYP11A1 deficiency is not; gene sequencing is needed for definitive diagnosis. Milder “nonclassical” forms of these conditions have been reported with intermediate phenotypes (45-48). CYP11A1 is one of 7 human mitochondrial cytochrome P450 (CYP) enzymes, all of which require electron donation via ferredoxin and ferredoxin reductase (49). Mutations in ferredoxin have not been reported, but several patients have been described with ferredoxin reductase mutations that disrupt synthesis of iron/sulfur centers, causing neuropathic deafness, optic atrophy, encephalopathy, and developmental delay (50-52); impaired steroidogenesis is to be expected but not yet reported.
3β-Hydroxysteroid dehydrogenase
Once pregnenolone is produced, it may be converted to progesterone by 3β-hydroxysteroid dehydrogenase (HSD3B, 3β-HSD). There are 2 human HSD3B genes: HSD3B1 encodes an isozyme found in the placenta, brain, liver, and elsewhere; HSD3B2 encodes an isoenzyme found in the adrenals and gonads. Both of these isozymes can convert the ∆ 5-steroids (pregnenolone, 17-hydroxypregnenolone [17OHPreg], dehydroepiandrosterone [DHEA], and androstenediol) to the corresponding ∆ 4-steroids (progesterone, 17OH-progesterone [17OHP], androstenedione, testosterone) (53), but the placental/hepatic HSD3B1 has a low Michaelis–Menten constant (Km), permitting it to act on low concentrations of steroids in the circulation (54), whereas the Km for the adrenal/gonadal HSD3B2 is 10-fold higher (55), so it acts only on locally produced, intraglandular steroids. Mutations in HSD3B2 cause a rare form of CAH, characterized by elevated ratios of ∆ 5/∆ 4 steroids, notably 17OH-Preg/17OH-progesterone (17OHP), that are >8 SD above normal (56, 57). The low Km of hepatic HSD3B1 permits it to convert some of the elevated 17OH-Preg to 17OHP, engendering false positives in newborn screening programs for 21OHD (58). HSD3B2 deficiency causes DSD in both sexes: genetic females are mildly virilized because some fetal adrenal DHEA is converted to testosterone by HSD3B1; genetic males synthesize some androgens by peripheral conversion of DHEA, but these are insufficient for complete male genital development (59).
17α-Hydroxylase/17,20-lyase
Pregnenolone can also be converted to 17OH-Preg by 17α-hydroxylase (CYP17A1, P450c17). CYP17A1 catalyzes both 17 α -hydroxylase and 17,20-lyase activities. The 17 α -hydroxylase activity converts pregnenolone to 17OHPreg and progesterone to 17OHP. The 17,20-lyase activity can convert 17OH-Preg to DHEA, but very little 17OHP is converted to androstenedione because the human enzyme catalyzes this reaction poorly (60, 61). The activities of CYP17A1 are expressed in a zone-specific fashion: the enzyme is absent in the adrenal zona glomerulosa, hence pregnenolone yields mineralocorticoids; only the 17 α -hydroxylase activity is found in the zona fasciculata, thus pregnenolone yields cortisol; both activities are present in the zona reticularis, hence pregnenolone yields 19-carbon (C19) precursors of sex steroids (Fig. 1). The principal factor regulating 17,20-lyase activity is electron transport from NADPH via cytochrome P450 oxidoreductase (POR) with the assistance of cytochrome b5 (b5). CYP17A1 mutations causing 17-hydroxylase deficiency (17OHD) are rare except in Brazil and China. Lack of CYP17A1 prevents sex steroid biosynthesis, yielding a female phenotype in 46,XY males and sexual infantilism in both sexes; overproduction of 11-deoxycorticosterone (DOC) in the zona fasciculata typically causes mineralocorticoid hypertension; cortisol is not produced, but corticosterone substitutes for glucocorticoid requirements (62). Rare cases of apparently isolated 17,20-lyase deficiency may be attributable to mutations in CYP17A1, b5 (CYB5 gene) or POR (63-65).
The enzymology of adrenal 21-hydroxylase (CYP21A2, P450c21, encoded by CYP21A2 within the HLA locus), is discussed in section “Basic principles of steroid synthesis and adrenal enzymatic defects,” “Enzymology of CYP21A2.”
P450 oxidoreductase
All microsomal cytochrome P450 (CYP) enzymes, including CYP17A1, CYP21A2, CYP19A1 (aromatase, P450aro), as well as the drug-metabolizing CYP enzymes of the liver, require the activity of POR, a flavoprotein that transfers electrons from NADPH to all microsomal CYP enzymes (49). Mutations in POR cause POR deficiency; patients have been described with highly variable clinical and hormonal findings depending on the underlying mutations (66-72). Most POR mutations impair CYP17A1, especially 17,20-lyase activity (including the G539R POR variant with a phenotype simulating isolated 17,20 lyase deficiency) (63, 68, 73), with CYP21A2 and CYP19A1 being affected variably, depending on the POR mutation. It is difficult to reach definitive conclusions about phenotype–genotype correlations with such rare disorders, although there is a suggestion that compound heterozygotes carrying R457H in trans with null mutations tend to have a more severe phenotype (72). Findings range from severely affected infants with 46,XX and 46,XY disorders/differences of sex development (DSD), cortisol deficiency, and the Antley–Bixler skeletal malformation syndrome to mildly affected women who appear to have polycystic ovary syndrome, or mildly affected men with gonadal insufficiency. The skeletal phenotype probably results from diminished activity of CYP26B1, a POR-dependent enzyme that degrades retinoic acid (74). POR mutations also result in clinically relevant disruption of hepatic CYP enzyme activity (75). Patients with POR deficiency typically have normal electrolytes and mineralocorticoid function, nearly normal cortisol levels that respond poorly to ACTH stimulation, increased levels of 17OHP that respond variably to ACTH, and low levels of sex steroids. Impaired CYP21A2 activity may generate levels of 17OHP detected by newborn screening for 21OHD (66, 76). Atypical genital development occurs in both sexes, with considerable variability. The 17,20-lyase activity of CYP17A1 is especially sensitive to disrupted electron transport (77), thus POR defects typically affect fetal testicular steroidogenesis. Virilization of 46,XX females has 2 causes. First, POR deficiency diverts steroids into the “backdoor pathway” of dihydrotestosterone biosynthesis (Fig. 1), contributing to the prenatal female virilization (69, 78-80). Second, as placental CYP19 (aromatase) requires POR, pregnant women carrying a fetus with the POR mutation R457H (but not POR A287P) may experience virilization during pregnancy (66-68), similarly to women carrying an aromatase-deficient fetus (81, 82). The POR polymorphism A503V, which mildly affects many P450 activities, is found commonly—from 19% among African Americans to 37% of Chinese Americans (83)—but does not affect the presentation of 21OHD (84).
11β-Hydroxylase and aldosterone synthase
Steroid 11-hydroxylase (CYP11B1, P450c11β) and aldosterone synthase (CYP11B2, P450c11AS, P450aldo) are closely related enzymes that catalyze the final steps in the synthesis of glucocorticoids and mineralocorticoids, respectively; they are encoded by duplicated genes (39, 85). Like CYP11A1, these are mitochondrial enzymes that require ferredoxin and ferredoxin reductase to receive electrons from NADPH. CYP11B1 is expressed abundantly in the zona fasciculata, where it converts 11-deoxycortisol to cortisol and DOC to corticosterone, and also in the zona reticularis, where it initiates the 11-oxo-pathway (see later) (86). CYP11B2 expression is less abundant and confined to the zona glomerulosa where it catalyzes the 11 β -hydroxylase, 18-hydroxylase, and 18-methyloxidase activities needed to convert DOC to aldosterone (87, 88). Mutations in CYP11B1 cause 11 β -hydroxylase deficiency (11OHD), with deficient cortisol, increased adrenal sex steroids, female virilization, and increased DOC, causing mineralocorticoid hypertension; 17OHP may be elevated in the newborn, leading to misdiagnosis of 21OHD (85, 89). Mutations in CYP11B2 selectively impair aldosterone synthesis, causing hyponatremia and hyperkalemia with normal cortisol production (39, 85). However, hyponatremia is typically less severe than in 21OHD because of continued DOC and cortisol secretion.
17β-Hydroxysteroid dehydrogenases
The synthesis of sex steroids requires the action of 1 of the 17 β -hydroxysteroid dehydrogenases (17 β -HSD, HSD17B). These enzymes differ in their structures, cofactor requirements, reactions catalyzed, and tissue-specific expression (39). Several are important in steroidogenesis. HSD17B1 is required for the synthesis of ovarian estradiol and placental estrogens (90-92). No genetic deficiency syndrome for HSD17B1 has been described. HSD17B2 inactivates estradiol to estrone and testosterone to androstenedione in the placenta, liver, small intestine, prostate, secretory endometrium, and ovary. Whereas HSD17B1 is found in placental syncytiotrophoblast cells, HSD17B2 is expressed in endothelial cells of placental intravillous vessels, consistent with a role in defending the fetal circulation from transplacental passage of maternal estrogens and androgens. No deficiency state for 17 β HSD2 has been reported. HSD17B3 is the testicular form of 17 β HSD that completes the synthesis of testosterone from androstenedione; its mutations cause a form of 46,XY DSD (93, 94). HSD17B5 (AKR1C3, an aldo-keto reductase enzyme), which is also a 3α-hydroxysteroid dehydrogenase, reduces androstenedione to testosterone (95) in the ovary and several nonsteroidogenic tissues. AKR1C3 is expressed at low levels in the zona reticularis, accounting for the small amount of adrenally produced testosterone (96). HSD17B6, also known as RoDH for its homology to retinol dehydrogenases (97), is expressed at low levels in the fetal testes, where it appears to catalyze oxidative 3 α HSD activities in the alternative or “backdoor” pathway to 5α -dihydrotestosterone (DHT) synthesis (79, 98)(see later).
Aromatase
Aromatase (CYP19A1) converts 19-carbon androgens to 18-carbon estrogens (99). Aromatase is abundantly expressed in the ovary and placenta and is slightly expressed in fat, but is only expressed in the adrenal in certain malignancies. Nevertheless, it is central to the pathophysiology of fetal development in CAH. The fetus with CAH fetus is only virilized by its own adrenal androgens; even when maternal testosterone concentrations reach 300 ng/dL in a mother who herself has CAH, the female fetus is not virilized because placental aromatase inactivates the androgens from the maternal circulation (100).
Enzymology of CYP21A2
CYP21A2 (P450c21), like CYP17A1, is a microsomal or type II cytochrome P450, which catalyzes 2 essential reactions in adrenal steroidogenesis (39). The major substrate of CYP21A2 is 17OHP, which is converted to 11-deoxycortisol in the zona fasciculata during the biosynthesis of cortisol. In the zona glomerulosa, CYP21A2 21-hydroxylates progesterone to 11-deoxycorticosterone within the aldosterone pathway. Other hepatic cytochrome P450 enzymes have some 21-hydroxylase activity with progesterone as a substrate (101), but this activity does not rescue glucocorticoid deficiency in patients with classic CAH.
As with other microsomal P450s, CYP21A2 utilizes 2 electrons donated by POR to reduce molecular oxygen, producing a hydroxylated substrate and water. The enzymology of CYP21A2 is unusual for a cytochrome P450 in that the primary site of oxygenation is a methyl group, which is a kinetically disfavored site of hydrogen atom abstraction in the reaction cycle. The C-H bond breaking step is partially rate-limiting, and deuterium substitution at C-21 of progesterone shifts hydroxylation partially to the 16α-hydrogen (102). The x-ray crystal structures of bovine (103) and human CYP21A2 (104) with 17OHP bound to the active site explain this activity profile. The steroid substrate is held perpendicular to the heme ring with the A-ring 3-keto oxygen hydrogen bonded to arginine-234 (R234) furthest from the reactive iron–oxygen complex, with C-21 dangling just close enough for the reaction to occur. On the side of the active site, valine-359 (V359) holds the steroid substrate with hydrophobic interactions in the geometry required for 21-hydroxylation and limits access of other reaction sites, principally the C-16 protons; mutagenesis of V359 to the smaller amino acids alanine and glycine progressively shifts progesterone hydroxylation to the 16α-hydrogen (105). The crystal structures also contain a second molecule of steroid outside the active site where the F-G loop that forms the roof of the active site abuts the α-helical domain (103). Whether this second molecule reflects an intermediate state in substrate binding or simply a hydrophobic interaction that favors crystal formation is not known.
The common mutations that cause 21OHD have been compared with wild-type CYP21A2 as recombinant native enzymes in transfected mammalian cells (106), vaccinia-infected mammalian cells (107, 108) and yeast (109) or as purified proteins modified for expression in Escherichia coli and reconstitution in vitro (110). The catalytic activities of the mutants are reduced generally in proportion to the severity of the deficiency observed in patients with CAH. The studies of purified, reconstituted enzyme assays enable more detailed kinetic studies, which demonstrate that most mutations variably impair substrate binding, catalytic efficiency, and thermal stability in some combination. Extrapolation of these systems to the human adrenal in affected patients should be considered a good approximation but with limitations.
When using purified, reconstituted assay systems, investigators must add phospholipid and purified POR, in addition to the steroid substrate and NADPH. The phospholipid used does not exactly replicate the endoplasmic reticulum of adrenal cortex cells but does bring together CYP21A2 and POR in a proteoliposome to enable electron transfer and catalysis. The phospholipid composition is known to influence the reconstituted activity of CYP17A1 and other steroidogenic P450 enzymes (111), although CYP21A2 has not been studied well in this regard.
New Pathways; New Steroids
The alternative or “backdoor” pathway to dihydrotestosterone
In addition to the classic pathway via DHEA, androstenedione, and testosterone, the most potent endogenous androgen, DHT, can also be synthesized via an alternative or “backdoor” pathway that bypasses the classical pathway intermediates (71, 79, 112-116). This alternative pathway is physiologically active during the major period of human sexual differentiation in the sixth to tenth week of human fetal development (79) and into the second trimester (117). To enter the alternative pathway to DHT, progesterone, or 17OHP are 5α-reduced by steroid 5α-reductase type 1 (SRD5A1) to yield 5α-dihydroprogesterone and 17α-hydroxydihydroprogesterone, respectively (for clarity, only the alternative pathway from 17OHP is shown in Fig. 1). These 3-ketosteroids are subsequently 3α-reduced to allopregnanolone and 17α-hydroxyallopregnanolone by isoforms of the AKR1C enzyme family. CYP17A1 converts allopregnanolone to 17α-hydroxyallopregnanolone and then to androsterone by its 17,20-lyase activity, serving as its preferred substrate. Androsterone, which is also an inactive metabolite of androstenedione and testosterone, can then be activated to DHT by sequential 17β-reduction and 3α-oxidase reactions (118) (Fig. 1).
Because excessive 17OHP accumulation is a key characteristic of 21OHD, it is highly likely that the alternative pathway to DHT is a major contributor to fetal female virilization in 21OHD. Alternative pathway steroid metabolites can be detected in patients of all ages with 21OHD, most prominently in the neonate (119). These studies indicate that the high concentrations of 17OHP in individuals with 21OHD drive DHT production by the alternative pathway. The alternative pathway intermediate 17α-hydroxydihydroprogesterone (also termed 5α-17-hydroxypregnanolone) can be detected directly by urinary steroid profiling and indicates the activity of the alternative pathway (119, 120).
The role of 11-oxo-androgens in CAH
After cleavage of the side chain by 17,20-lyase activity of CYP17A1 in the zona reticularis, the major 19-carbon product of the human adrenal cortex is DHEA and its sulfate ester DHEAS. Whereas the latter is not a precursor to testosterone, DHEA is efficiently converted to androstenedione and also within the adrenal to lesser amounts of testosterone (121). Both androstenedione and testosterone are good substrates for CYP11B1. Precursor steroids accumulate in the adrenals of patients with 21OHD, and CYP17A1 and CYP11B1 activities are high owing to chronic ACTH stimulation, hence the system can synthesize large quantities of 11OH-androstenedione, with concentrations exceeding that of androstenedione in both 21OHD patients and unaffected controls (122). 11-ketotestosterone (11KT) is primarily generated from circulating 11OH-androstenedione via the sequential action of 11β-HSD type 2 (which converts the 11β-hydroxyl to a keto group) and AKR1C3 (123). 11KT, which is in fact the major testicular androgen in teleost fishes (124), is nearly as potent as testosterone in transactivating the human androgen receptor (125). The intermediate 11-ketoandrostenedione—but not 11OH-androstenedione—is a much better substrate for AKR1C3 than androstenedione itself (126), which explains why 11KT is the second-most abundant circulating 11-oxo-androgen in both 21OHD patients and unaffected individuals. In addition, 11KT is a substrate for the 5α-reductases (123), yielding 11-ketoDHT (11KDHT), which appears to be a more potent androgen than 11KT (reviewed in (86)), but is not detected in relevant concentrations in circulation.
In women with 21OHD, 11KT rises roughly proportionately to testosterone (122), reflecting the adrenal rather than gonadal origin of these androgens. Furthermore, 11-oxo-androgens are poor substrates for aromatase; whereas 11-oxo-androgens can be converted to 11-oxygenated estrogens, the latter do not contribute substantially to the circulating estrogen pool (127). In contrast, 11KT is inversely proportional to testosterone in men (122) and in boys Tanner stage 3 to 5 (128) with 21OHD. This is because men with poor disease control produce more 11OH-androstenedione, which is preferentially metabolized to 11KT, which then suppresses the hypothalamic–pituitary–testicular axis, thereby decreasing testicular secretion of testosterone. In men with good disease control, 11KT synthesis is low, whereas testicular testosterone synthesis is normal. Hence, a low 11KT/testosterone ratio in a man with 21OHD indicates both good disease control and good testicular function.
It is difficult to evaluate long-term disease control in adults with classic CAH. Assessing adrenal size, which might be the ultimate assessment, requires cross-sectional imaging with associated cost and radiation exposure. The 11-oxo-androgens (and 21-deoxycortisol), correlate better with adrenal size than traditional biomarkers of short-term disease control, such as androstenedione and 17OHP (128). Elevated 11-oxo-androgens are also predictive of menstrual irregularity in women and of TARTs in men with CAH (128). In contrast to DHEAS, androstenedione, and testosterone, 11KT does not decline with age in women from 20 to 80 years old, and 11KT declines very gradually in men over the same age range (129). These data suggest that 11-oxo-androgens may be useful biomarkers of 21OHD control well into adulthood and in hypogonadal states. In patients with NC CAH, 11-oxo-androgens are elevated about 2-fold compared with women with clinical features of androgen excess, although 11-oxo-androgens alone cannot be used to establish the diagnosis of NC CAH (130). Finally, limited data suggest that 11-oxo-androgens are rather specific for 21OHD and are not elevated in other androgen excess forms of CAH such as 11β-hydroxylase deficiency and 3βHSD2 deficiency, because either CYP11B1 activity or intra-adrenal androstenedione production are low, respectively (86, 122).
In summary, androgens are generated in CAH patients via all 3 major pathways (131). First, classic pathway androgen synthesis is enhanced through increased conversion of accumulating 17OHP to androstenedione via the 17,20-lyase activity of CYP17A1, an ordinarily minor reaction compared with the preferred conversion of 17OH-Preg to DHEA (60). Second, the androstenedione so generated consequently drives increased substrate flow to the 11-oxoandrogen pathway, through conversion of androstenedione to 11β-hydroxyandrostenedione. Third, while the alternative pathway to DHT contributes to excess androgen generation in 21OHD, its relative contribution appears to be more limited than that of classic and 11-oxo-pathways, as indicated by in vivo urinary steroid metabolite profiling in CAH patients during glucocorticoid therapy (132).
Biological activities of steroidal intermediates
Aside from defects in StAR and CYP11A1, in which essentially no steroids are secreted, a hallmark of inherited enzymatic defects in adrenal steroidogenesis is the accumulation of “upstream” steroids, proximal to the affected enzymatic step, which provide useful diagnostic markers. In 21OHD, 17OHP, the steroid before 21-hydroxylase, accumulates and is traditionally used to diagnose 21OHD (1, 133, 134). Besides 17OHP, several other “upstream” steroids such as pregnenolone, 17OH-Preg, and progesterone, and may also accumulate but are not diagnostically specific. In the absence of 21-hydroxylase activity, a substantial portion of 17OHP is converted into 21-deoxycortisol by CYP11B1 (Fig. 1). 21-deoxycortisol is a potentially useful marker for the diagnosis of 21OHD (135).
Some steroids that accumulate in 21OHD, including 21-deoxycortisol, progesterone and 17OHP, may also bind to glucocorticoid or mineralocorticoid receptors and act variously as either agonists or antagonists. In vitro, 21-deoxycortisol, corticosterone, 17OHP, and progesterone bind the glucocorticoid receptor with 24% to 43% of the affinity of cortisol. However, the transactivation activities of progesterone and 17OHP were only 0.2 to 0.8% of that for cortisol, whereas the transactivation activity of 21-deoxycortisol was 8.5% and 17% in 2 different assays (136, 137). By contrast, 17OHP and progesterone inhibit aldosterone-mediated transactivation of the mineralocorticoid receptor in a dose-dependent fashion, explaining the strong antimineralocorticoid effect of 17OHP and progesterone in vitro (138, 139). Androstenedione and testosterone had no effect on mineralocorticoid receptor transactivation (139).
The clinical implications of these findings are not yet completely understood. Some adult classic CAH patients stop glucocorticoid medication without developing symptoms and signs of adrenal insufficiency (136, 140). Perhaps elevated levels of other steroids partially compensate for the low cortisol concentrations. Moreover, 21-hydroxylation of progesterone by hepatic cytochrome P450 enzymes other than CYP21A2 may permit some mineralocorticoid (11-deoxycorticosterone) synthesis (101). Clinical consequences of treatment lapses include androgen excess in women and TARTs in men, adrenal hyperplasia, and/or tumors, as well as the theoretical risk of adrenal crisis in all patients.
Genetics in CAH
21OHD is caused by inactivating mutations in the gene coding for adrenal 21-hydroxylase (CYP21A2, older nomenclature CYP21, CYP21B, P450c21B; GeneID 1589).
The CYP21 Genes and the Surrounding Genetic Region
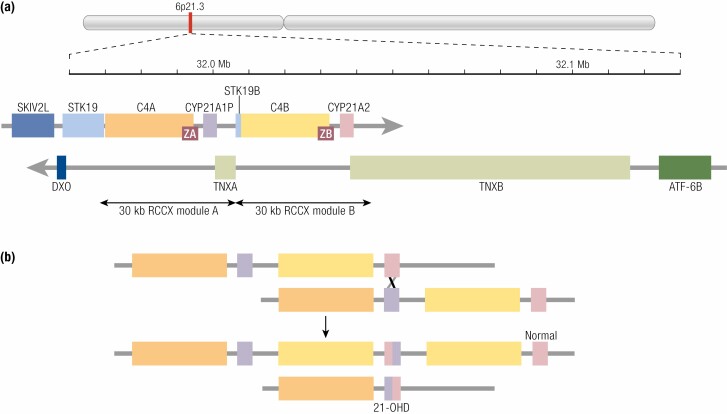
The CYP21A2 gene encodes the microsomal P450 enzyme, 21-hydroxylase (CYP21A2, P450c21), a protein of 495 amino acids. CYP21A2 is located in the Human Leukocyte Antigen (HLA) Class III region on the short arm of chromosome 6ß (6p21.3), approximately 30 kilobases apart from the nonfunctional CYP21A1P pseudogene (Fig. 2). CYP21A2 and CYP21A1P both consist of 10 exons and share high nucleotide homology of about 98% and 96% in exons and introns respectively (143, 144). CYP21A1P and CYP21A2 are arranged in tandem with the C4A and C4B genes encoding the fourth complement factor (145). There are additional sense and antisense transcripts of unknown significance near or overlapping the CYP21 genes (141, 142). The C4/CYP21 unit is flanked by the serine-threonine kinase-19 (STK19, RP1) gene on the telomeric side and by the tenascin-X gene (TNXB, which encodes an extracellular matrix protein on the opposite DNA strand) (146) on the centromeric side, and their pseudogenes, STK19B and TNXA, forming a 30 kb tandem repeat sometimes referred to as an RCCX module (RP-C4-CYP21-TNX) (147). The STK19, C4, and CYP21 genes are transcribed in the telomeric to centromeric direction, whereas TNXB is transcribed from the opposite strand. Most chromosomes have 2 copies of the module with a CYP21A1P pseudogene in the telomeric module and a CYP21A2 gene in the centromeric module. However, this locus shows high structural variability with monomodular, trimodular, or even quadrimodular haplotypes detected (148, 149). The TNXA and STK19B pseudogenes were truncated during the duplication of the ancestral RCCX module. The last exons of TNXA and TNXB overlap the 3′ untranslated regions of exon 10 of CYP21A1P and CYP21A2, respectively.
Figure 2.
Genetics of the CYP21 genes. (A) the genetic region on chromosome 6p21.3, using data from the Human Genome database (http://genome.ucsc.edu/). The location of this region is indicated on a schematic of the entire chromosome. A scale is marked every 10 kb, with positions in the genome assembly numbered every 0.1 Mb. Genes transcribed in the telomeric-to- centromeric direction (left to right) are on the strand denoted by a right-facing arrow: SKIV2L, Ski2 like RNA helicase; STK19, serine/threonine kinase 19; C4A, complement component C4A; CYP21A1P, cytochrome P450 family 21 subfamily A member 1 (21-hydroxylase) pseudogene; STK19B, serine/threonine kinase 19 pseudogene; C4B, complement component C4B, CYP21A2, cytochrome P450 family 21 subfamily A member 2 (21-hydroxylase). Genes transcribed from the opposite strand (right to left in the figure) are immediately below: ATF6B, activating transcription factor 6 beta; TNXB, tenascin XB; TNXA, tenascin XA pseudogene; DXO, Decapping and exoribonuclease protein. ZA and ZB are adrenal-specific noncoding transcripts overlapping the C4 genes in the sense direction (141, 142); additional transcripts exist but are not shown. The 30 kb duplication of part of STK19, all of C4, all of CYP21, and part of TNX (a so-called RCCX module) is indicated. (B) An illustration of unequal meiotic crossing-over generating a deletion representing a salt-wasting 21-hydroxylase deficiency allele. The other chromosome has 3 copies of the RCCX tandem and is not associated with disease. The scale is expanded from Fig. 1A. For clarity, only the C4 and CYP21 genes are illustrated.
CYP21A1P is transcribed but its mRNA cannot encode a functional protein owing to at least 10 deleterious mutations (143, 144) including 2 frameshifts (8 bp deletion in exon 3, 1 bp insertion in exon 7, a nonsense mutation (p.Gln318stop; Q318X) (150), and a mutation in intron 2 that activates a cryptic splice site and causes an extra 19 nucleotides to be included in the mRNA (151). Missense mutations in the pseudogene include p.Pro30Leu (P30L) (107), p.Ile172Asn (I172N) (152), a cluster of missense mutations in exon 6, p.Ile236Asn, Val237Glu, Met239Lys (I236N, V237E, M239K), p.Val281Leu (V281L) (153), and p.Arg356Trp (R356W). Additionally, 4 single nucleotide differences in the 5′ flanking region of CYP21A1P reduce its transcriptional activity to 20% of that of CYP21A2 (see section “Genetics in CAH,” “CYP21A2 gene expression,” “Transcriptional control of CYP21A1P and CYP21A2” ) (141). Note that there is a polymorphism of no functional significance in the hydrophobic leader sequence at the amino terminus of CYP21A2, consisting of a single amino acid insertion. Consequently, some publications and databases list mutations with positions incremented by 1; eg, P31L instead of P30L.
CYP21A2 Gene Expression
Pattern of CYP21A2 expression
By immunohistochemistry, CYP21A2 expression is first detected robustly at 50 to 52 days postconception within the nascent inner fetal zone. Within the outer definitive zone, CYP21A2 is more weakly detected and persists up to 14 weeks postconception. All other enzymes required for cortisol biosynthesis are present as well, and cortisol concentrations within the fetal adrenal are high during the first trimester (154). From 14 to 22 weeks, CYP21A2 is expressed only in the fetal and transitional zones, but not the definitive zone, and cortisol secretion is relatively low; definitive zone expression is detected starting at 23 weeks and continuing through the remainder of gestation, as cortisol secretion increases (155). Cortisol secretion in the first trimester suppresses DHEA production and thus minimizes fetal androgen secretion until placental aromatase expression increases in the second trimester, by which time differentiation of the external genitalia is complete and cannot be affected by DHEA levels. Cortisol is again required in the third trimester to support lung maturation, including surfactant production (156). Low expression of CYP21A2 during the second trimester partially explains the high incidence of false-positives in newborn screens for CAH in premature infants (see section “Diagnostics,” “Neonatal screening”) (157).
In normal adult adrenal glands, CYP21A2 immunoreactivity is detected in all 3 cortical layers, particularly the zonae glomerulosa and reticularis, with variegated expression in the zona fasciculata. The immunoreactivity is more intense in adrenals from patients with Cushing disease and at sites of regeneration in normal adrenal glands (158).
In addition to the adrenal cortex, CYP21A2 is detected in other tissues by RT-PCR. These include lymphocytes, which also express an additional 21-hydroxylase activity that is not mediated by CYP21A2 (159). CYP21A2 is expressed throughout the human heart at levels approximately 0.1% those in the adrenal cortex. Expression patterns of other steroidogenic enzymes suggest autocrine or paracrine roles for corticosterone and deoxycorticosterone, but not cortisol or aldosterone, in the normal adult human heart (160).
Regulation of CYP21A2 expression
Cortisol secretion is regulated mainly by ACTH, which acts via the Gα-protein coupled MC2R receptor to increase activity of adenylyl cyclase and thus increase intracellular levels of cyclic adenosine monophosphate (cAMP). This in turn increases activity of protein kinase A. The main effect of corticotropin-releasing hormone (CRH) secreted by the hypothalamus is to increase ACTH secretion by the pituitary gland, but additionally, it may act directly on adrenocortical cells to increase cortisol secretion, and expression of CYP21A2 and other steroidogenic enzymes (161). Infection, fever, and pyrogens stimulate the release of interleukin (IL)-1 and IL-6, promoting secretion of CRH, and stimulate IL-2 and tumor necrosis factor promoting release of ACTH, increasing cortisol secretion during inflammation (162); IL-6 can also directly stimulate adrenal synthesis and release of cortisol (163).
In contrast, aldosterone secretion is regulated mainly by angiotensin II, which activates the Gq-protein coupled angiotensin 2 receptor (AT2R), which acts primarily through the protein kinase C pathway but also through Ca2+ signaling (164). Additionally, high extracellular potassium levels trigger voltage sensitive calcium channels that also increase intracellular calcium levels. Calcium then increases activity of protein kinase C (165).
Regulation of CYP21A2 expression is consistent with these tropic stimuli. In the H295R human adrenocortical cell line (166, 167) and also in primary cultures of human adrenocortical cells (167, 168), mRNA, and/or protein expression of CYP21A2 are induced by increases in cAMP analogs and by angiotensin II or tetradecanoyl phorbol acetate, which stimulate protein kinase C. Insulin and IGF-I are additional trophic stimuli (168). Additional hormone and environmental factors may regulate CYP21A2 expression. These include orexins, which stimulate secretion of cortisol (169), and potential endocrine disruptors including brominated flame retardants (170, 171) and organic freshwater contaminants (172).
Transcriptional control of CYP21A1P and CYP21A2
The most important CYP21A2 transcript begins 10 to 11 nucleotides before the initial AUG codon (143). CYP21A1P is also transcribed specifically in the intact adrenal cortex at a level 10% to 20% that of CYP21A2 (141). However, the first 2 introns are inconsistently spliced out, and an uncertain proportion of transcripts include additional exons in the region between the end of CYP21A1P and the beginning of C4B. Some of these exons may overlap the truncated TNXA gene. Adrenal transcripts in the same direction as CYP21A2 have also been detected overlapping TNXB; these are also of uncertain functional significance (173).
Similarly, CYP21A1P transcripts cannot be detected in primary cultures of human adrenocortical cells, whereas CYP21A2 is appropriately expressed under the same conditions (168, 174). In cultured mouse Y-1 or human H295 adrenocortical cells, the 5′ flanking region of human CYP21A2 drives basal expression of reporter constructs at levels 2.5 to 8 times higher than the corresponding region of CYP21A1P (174-176). Sequences responsible for this difference have been localized to the first 176 nucleotides (174), although sequences upstream of this region are required for full expression. There are only 4 nucleotide differences (–126C>T, –113G>A, –110T>C, and –103A>G) between CYP21A1P and CYP21A2 in this region. The first 2 listed affect binding of the Sp1 transcription factor. In patients with 21OHD, gene conversions involving this region reduce but do not eliminate CYP21A2 expression. In isolation with no additional mutations present, they can be associated with NC CAH (177). When the gene conversion extends to the P30L missense mutation (which is usually a Group C, ie, NC allele; see section “Genetics in CAH,” “Genotype–phenotype correlation”), it becomes a SV (Group B) allele (178).
The most important transcription factor for adrenal-specific expression of CYP21A2 is steroidogenic factor-1 (SF-1, Ad4BP, NR5A1). This protein is also required for development of the adrenal gland and gonads (179, 180). It interacts with specific DNA elements both within the proximal promoter and in intron 35 of the linked C4B gene (181).
Additional relevant transcription factors include nerve growth factor induced-B (NGFI-B, Nur77, NR4A1) (167, 182), and Nur-related factor 1 (NURR1) (NR4A2); they may overlap in their functions (183). NGFI-B is phosphorylated under basal conditions and dephosphorylated in response to ACTH, which activates it. Thus it may help to mediate ACTH regulation of CYP21A2 expression. These transcription factors may also be important for mediating gene regulation by angiotensin II (165). A third closely related transcription factor, neuron-derived orphan receptor 1 (NOR1, NR4A3), is also expressed in the adrenal cortex and may function similarly (184).
Molecular Genetics of CAH
Over 90% of mutations causing 21OHD are the consequence of intergenic recombinations within the 30 kb tandem repeat (Fig. 2), promoted by the high recombination rate in the HLA region along with the nucleotide identity shared across the 30 kb repeat. These include both deletions generated by unequal meiotic crossing-over during gametogenesis, and gene conversions between CYP21A2 and the CYP21A1P, generated by either meiotic or mitotic events (185). Unequal crossovers, owing to misalignment of the 30 kb tandem duplication, can occur with break points anywhere along the duplicated region. Breakpoints originating in STK19 or C4A lead to a net deletion of 1 of the C4 genes and CYP21A1P but leave CYP21A2 unaffected. Such a configuration occurs on at least 5% of normal chromosomes (145). Breakpoints originating in CYP21A1P yield a deletion of C4B and a single chimeric CYP21 gene that has a 5′ end corresponding to CYP21A1P and a 3′ end corresponding to CYP21A2. This chimeric gene usually (but not always (186)) includes CYP21A1P mutations that prevent synthesis of a functional protein, so it represents a null allele and thus is usually referred to as a CYP21A2 deletion. Occasionally a breakpoint occurs in the TNX genes, leading to complete deletion of C4B and CYP21A2, and a TNXB/TNXA chimeric gene (147, 148, 187). Homozygosity for such a chimera leads to a contiguous gene syndrome consisting of CAH and Ehlers–Danlos syndrome (188), which is rarely clinically reported in patients with severe CAH. However, 7% to 14% of patients with CAH have heterozygous TNXB mutations (189, 190). This extended phenotype has been termed the CAH-X syndrome. CAH-X is associated with joint hypermobility, chronic arthralgia, joint subluxations, hernias, and cardiac defects (191, 192). Deletions account for approximately 20% of mutant alleles in 21OHD (187, 193-211). CYP21A2 gene duplications are relatively common in some populations (212, 213). Many of these alleles carrying a CYP21A2 gene duplication have a p.Gln318X (Q318X) mutation in the duplicated CYP21A2 gene next to the TNXB gene, and a wild-type CYP21A2 gene next to the TNXA pseudogene. Importantly, such alleles are nondisease causing, but can be easily misinterpreted (212).
Approximately 70% to 75% of disease-causing CYP21A2 mutations arise from the transfer of deleterious mutations from CYP21A1P, namely gene conversion (Fig. 3). In addition, over 200 pseudogene-independent mutations are listed in the Human Gene Mutation Database (HGMD, http://www.hgmd.cf.ac.uk) and the Pharmacogene Variation Consortium (https://www.pharmvar.org/gene/CYP21A2). Most of these rare mutations are sporadic. However, due to founder effects increased frequencies of some pseudogene-independent mutations are observed in some populations. Deletions, the splice site mutation in intron 2 (c.293-13A/C>G) and p.Ile172Asn (I172N) are the most common mutations in most populations (187, 193-211). The p.Val281Leu (V281L) mutation is by far the most common allele detected in patients with NC CAH and is highly prevalent in Ashkenazi Jews (153). Novel or rare mutations account for about 3% to 5% of detected mutations in large cohorts. The vast majority of these rare mutations have been identified in single families or small populations. Approximately 1% to 2% of CYP21A2 disease causing mutations arise de novo.
Figure 3.
(A) Structure of the CYP21 genes. Exons are numbered. Mutations affecting enzymatic function that are normally present in the CYP21A1P pseudogene are shown. They are positioned vertically to show the severity of CAH they cause when transferred to CYP21A2 in gene conversion events. These are grouped into 4 mutation groups (0, A-C) and are associated with particular forms of CAH, as indicated. (B) Associations between mutation groups and forms of CAH. These are displayed in tabular form on the left and as histograms on the right.
Genotype–Phenotype Correlation
In descending order of compromised 21-hydroxylase activity, 4 general groups of CYP21A2 mutations have been established to predict the phenotype (Fig. 3) (193, 194, 199, 201, 206, 211, 214). Deletions, large gene conversions, nonsense mutations, frameshifts, and missense mutations that totally abolish enzymatic activity are SW classic alleles, comprising mutation Group 0 (“null”). A single nucleotide mutation that alters splicing of intron 2 (c.293-13A/C>G, “intron 2 G” mutation) (151) is particularly common, comprising 20% to 25% of mutant alleles in most populations (Table 2). It has been seen in both SW and SV patients, suggesting that there is a small amount of normally spliced mRNA; it is generally considered its own separate Group A (in the first analysis of this sort (193), Groups 0 and A were referred to as Groups A1 and A2, respectively). A nonconservative amino acid substitution, p.Ile172Asn (I172N) (152) reduces enzymatic activity to <5% of normal and is associated with the SV form of the disorder (mutation Group B) (106, 108, 219). Finally, missense mutations such as p.Val281Leu (V281L) and p.Pro30Leu (P30L) (107) reduce enzyme activity to ~20% to 50% of normal (mutation Group C), and are associated with NC CAH. Although enzyme function in vitro appears to be similar (110), clinical observations suggest that patients carrying the P30L allele are somewhat more symptomatic, straddling the border between SV and NC forms of CAH (211, 220). As noted in section “Genetics in CAH,” “CYP21A2 gene expression,” “Transcriptional control of CYP21A1P and CYP21A2,” in many cases this may be a consequence of gene conversions extending into the 5′ flanking transcriptional regulatory region, thus impairing gene expression (178).
Table 2.
Allele frequencies in various regions
| North America | South America | Europe | China | Total | |
|---|---|---|---|---|---|
| References | (193, 206, 211, 215) | (198, 216) | (195, 201, 210, 217) | (214, 218) | |
| Allele | |||||
| Deletion/conversion | 21.1% | 11.1% | 28.8% | 21.9% | 21.5% |
| P30La | 2.4% | 1.0% | 1.2% | 1.1% | 1.8% |
| I2G | 23.1% | 20.6% | 26.7% | 33.8% | 25.3% |
| E3Δ8bp | 2.3% | 0.9% | 2.4% | 0.3% | 1.8% |
| I172N | 9.0% | 9.4% | 15.6% | 15.1% | 11.4% |
| E6 | 2.2% | 1.8% | 2.4% | 1.9% | 2.1% |
| V281La | 22.2% | 24.5% | 6.2% | 1.4% | 15.7% |
| Q318X | 3.6% | 6.5% | 3.5% | 5.3% | 4.2% |
| R356W | 3.8% | 4.8% | 4.3% | 6.6% | 4.5% |
| Other | 10% | 10% | 8.9% | 12.4% | 10.2% |
| Alleles analyzed | 3527 | 1094 | 1338 | 1142 | 7101 |
Gene conversion mutations occur with similar frequencies in most populations (Table 2).
a P30L and V281L are found mainly in patients with nonclassic CAH and therefore their allele frequencies depend on the proportions of nonclassic patients included in each study.
CAH due to 21OHD is an autosomal recessive condition. About 65% to 75% of 21OHD patients are compound heterozygotes; ie, they carry different mutations on each allele. In cohort studies, the clinical phenotype of CAH strongly correlates with the less severely impaired CYP21A2 allele (Fig. 3); 96% of individuals carrying 2 Group 0 alleles have SW CAH, whereas 97% of those with at least 1 Group C allele have NC CAH. The correlation is somewhat less strong for the groups with enzymatic impairment of intermediate severity (Groups A and B, and the P30L mutation). To some extent, this reflects the fact that the distinctions between SW and SV CAH, or SV and NC CAH, are a continuum and not absolute. For example, many SV CAH patients nevertheless require mineralocorticoid supplementation early in life and might be classified as SW, whereas the distinction between the SV and NC forms can be particularly challenging in males. Without exhaustive sequencing, it is difficult to rule out the existence of additional mutations in introns or flanking regions that might affect mRNA processing or gene expression; as noted in section “Genetics in CAH,” “CYP21A2 gene expression,” “Transcriptional control of CYP21A1P and CYP21A2,” 1 transcriptional control region is several kilobases away from CYP21A2, in the C4B gene (181). Data correlating genotype with intermediate phenotypes are limited and often are not presented in a way that permits meta-analysis. In both American (193, 221) and German (199) data, median (interquartile range) Prader virilization scores (Fig. 4) for females in Groups 0, A, B, and C are 4 (3-5), 4 (3-5), 3 (2-4), and 0 (0-2) respectively (199). A similar correlation of severity with genotype is seen when evaluating genital appearance in adult women (222). No factors modulating androgen effects have been demonstrated to influence the degree of virilization associated with each genotype group. Basal levels of 17OHP are also correlated with genotype (193, 198, 199, 223), with different studies reporting mean levels (in ng/dL) in Groups 0/A, B, and C of 23-41 000, 10-18 000, and 3-8000, respectively. However, there is substantial overlap in values between genotype groups. Adult height and mean hydrocortisone (HC) doses are also influenced by genotype (224). There are limited data directly correlating psychosexual functioning with genotype (222, 223), but gender dysphoria does tend to be most severe in women with SW CAH, which in turn is highly correlated with group 0 and A genotypes (225). Long-term health outcomes in adults do not correlate well with the genotype (210). However, girls and women with more severe CYP21A2 genotypes appear to have an increased risk for psychiatric conditions (226) and variations of the complement component C4 may influence the risk of psychopathology (227). In summary, genotype–phenotype correlations are strong but not absolute, and clinical management should be based on clinical and hormonal data.
Figure 4.
Genital development. Top, Differentiation of male and female reproductive systems are illustrated in schematic cross-section (not to scale). Bottom, the Prader scale of genital virilization.
By analyzing the CYP21A2 crystal structure, novel insights into the underlying molecular pathology have been gained (104, 228). Null and other severely deleterious mutations commonly disrupt heme and/or substrate binding domains, the anchoring of the protein to the membrane, or impair protein stability. Mutations categorized as group B partially impair membrane anchoring or affect conserved hydrophobic clusters within the protein. Milder mutations (group C) result in less severe alterations, often interfering with electron transfer from POR, salt bridge and hydrogen-bonding networks, and nonconserved hydrophobic clusters (104). However, other factors potentially influence enzymatic activity including mRNA expression, splicing and stability, and protein stability.
Diagnostics
Neonatal Screening
Benefits
Neonatal screening for classic CAH was introduced to prevent morbidity and mortality due to adrenal crisis. Currently, all 50 states in the United States, 35 other countries, and portions of 17 additional countries screen for CAH (229, 230). Results of these screens indicate that the incidence of classic CAH in most populations is approximately 1:14 000 to 1:18 000. Table 1 summarizes data since 2008; data reviewed 1997-2004 are similar (231-233). Although newborn screening for CAH is now performed in an increasing number of countries, protocols and reported outcomes vary widely (234).
Screening markedly reduces the time to diagnosis of infants with classic CAH (89, 235-237), consequently reducing morbidity and mortality. Diagnosis is more likely to be delayed in males owing to the lack of genital ambiguity. Thus, a relative paucity of males in a patient population may be taken as indirect evidence of unreported deaths from SW crises. Females do outnumber males in some (12, 238, 239) although not all (240) retrospective studies in which CAH was clinically diagnosed without neonatal screening. Moreover, there is a greater preponderance of severe genotypes in screened infants than in those ascertained prior to screening, again suggesting extra deaths of severely affected infants prior to screening (2, 241). Nevertheless, infant deaths from CAH are now rare (0-4%) in advanced economies even without screening (242, 243).
Infants ascertained through screening have less severe hyponatremia and shorter hospitalizations (236, 240, 244, 245). The delay before correct sex assignment of severely virilized females is also markedly reduced (231). Moreover, males with SV CAH, and (mildly) virilized females, may otherwise not be diagnosed until later in childhood, at which time height may already be compromised. Although not an aim of screening, children with NC CAH are occasionally diagnosed. In some cases, the consequent close monitoring and, if necessary, treatment may improve adult height.
Initial screening methodology
First-tier screens for CAH due to 21OHD employ immunoassays to measure 17OHP in dried blood spots on the same filter paper (“Guthrie”) cards used for other newborn screening tests (236, 246, 247). Radioimmunoassay was the first method developed (248), but automated time-resolved dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) has almost completely supplanted other immunoassays (249).
The main drawback to screening is that false positive rates are high, leading to substantial costs for evaluation and increasing parental concern. Several factors limit accuracy of these tests. First, premature, sick, or stressed infants tend to have higher levels of 17OHP than term infants; as studied by high-performance liquid chromatography, preterm infants have a functional deficiency of several adrenal steroidogenic enzymes with a nadir in function at 29 weeks of gestation (157). This “adrenal prematurity” can generate many false positives unless screening programs use higher screening cut-offs for preterm infants. For example, in 26 years of operation of the Swedish screening program, the positive predictive value for full-term infants was 25%, whereas it was only 1.4% for preterm infants, and it correlated very strongly with gestational age (250). There are no universally accepted standards for stratifying infants. Most laboratories use a series of birth weight-adjusted cut-offs (251, 252) but actual gestational age, or both, might be preferable, because gestational age correlates much better with 17OHP levels (27, 253).
Second, 17OHP levels are normally high after birth, decreasing rapidly during the first few postnatal days in healthy infants. In contrast, 17OHP levels increase with time in infants with 21OHD. Thus, diagnostic accuracy is poor in the first 2 days, and screening a second sample several days later improves both sensitivity and positive predictive value with the risk of delaying treatment (89, 247, 254). Moreover, a comparison of 1-screen vs 2-screen state programs found a higher incidence of 21OHD when a second screen was employed (255). It has been suggested that preterm infants should have additional samples rescreened at 2 and 4 weeks of age, most practical in a hospitalized population where potential SW can be monitored (252).
Multiple courses of antenatal corticosteroids might reduce 17OHP levels and thus potentially increase the likelihood of false negative screens. Studies have reported inconsistent effects of antenatal corticosteroid administration in practice (256, 257). Testing of later samples should minimize this problem, but the delay may increase the risk of SW crisis.
Female infants have lower mean circulating 17OHP levels than males, slightly reducing screening sensitivity (258). Because almost all females with SW CAH are virilized, most of them are diagnosed based on clinical symptoms, and therefore the reduced sensitivity is not usually problematic. However, even severely virilized girls can be missed as virilization is not always noticed at physical examination (2, 22, 259, 260). Finally, immunoassays may lack specificity; this is discussed in section “Diagnostics,” “Biochemical evaluation.”
Second-tier screening
Because 21OHD is a rare disease, the positive predictive value of neonatal screening is low, even though the specificity and sensitivity of the tests are very high (230). The positive predictive value might be improved with a second-tier screen.
Biochemical second screens.
Direct biochemical analysis of steroids in blood samples using LC-MS/MS can obviate the specificity problems of immunoassays (261-263) and both heel stick blood samples (264) and urine samples (265, 266) can also be analyzed by mass spectrometric methods. Measuring 21-deoxycortisol instead of 17OHP may improve diagnostic accuracy (135). Measuring steroid ratios further improves the screening specificity of LC-MS/MS. Such ratios have included (17OHP+androstenedione)/cortisol (261, 267, 268), 17OHP/11-deoxycortisol (269), and (17OHP+21-deoxycortisol)/cortisol (263). Some (270, 271) but not all (252) laboratories have reported markedly superior results with these approaches, with 1 recent report claiming a positive predictive value of 71% (268). A recent statistical approach using principal component analysis of 6 steroid levels (17OHP, both first and second tier, 11-deoxycortisol, androstenedione, 21-deoxycortisol, and cortisol) achieved a positive predictive value of 67% (272). Consistency of results might be improved by mandating participation in national proficiency testing programs (273). However, caution should be exercised in developing reference ranges for assays using dried blood spots that have been stored for prolonged periods at room temperature, because cortisol and 11-deoxycortisol are not stable past 4 weeks of such storage (274).
Molecular genetic second screens.
CYP21A2 mutations can be detected in DNA extracted from the same dried blood spots used for hormonal screening (see section “Diagnostics,” “Neonatal screening,” “Initial screening methodology”). Because >90% of mutant alleles carry 1 or more of a discrete number of mutations, we can assume with >99% confidence that samples that carry none of these mutations are unaffected. Several studies of genotyping of samples from screening programs have suggested that this is a potentially useful adjunct to hormonal measurements (275-280), but there has been no large-scale study of its efficacy as a second-tier screen in actual use.
Biochemical Evaluation
Determining levels of steroid hormones and their precursors is a mainstay of diagnosis and management of CAH. Currently, the determination of steroid hormones rests on analytical techniques either based on the principle of immunoassay or on chromatographic methods coupled to mass spectrometry (281).
The specificity of the antibody is crucial for the reliability of an immunoassay. Inefficient discrimination between the analyte and structurally closely related substances will lead to cross reactivity with consequent overestimation of the amount of analyte. The overestimation of 17OHP in serum or plasma of premature infants, neonates, or young infants by immunoassay techniques used in screening procedures or clinical routine with the consecutive risk of overdiagnosing 21OHD, presents a typical and important example of this phenomenon (282). Crossreactivity has been documented with 17-OH pregnenolone sulfate, a steroid originating in extremely high amounts from the fetal zone of the fetal adrenal (283), and 15β-hydroxylated compounds apparently generated by gut bacteria and resorbed through the entero-hepatic circulation (284). There may be additional substances in dried blood spots that interfere with immunoassays (matrix effect) (285). The DELFIA was reformulated in late 2009 to make it less sensitive to crossreacting compounds in premature infants (286). This modification improved the positive predictive value from 0.4% to 3.7% for the first screen (247). The specificity of immunoassays may be further improved with organic extraction to remove crossreacting substances, such as steroid sulfates. Additional preparative steps such as extraction, chromatographic prepurification, or dilution can help to circumvent matrix effects.
Currently, MS represents the most versatile and exact of all analytical techniques for steroids. Initial separation by LC or gas chromatography (GC) can consistently improve specificity, and it also permits multicomponent analysis, namely the simultaneous determination of multiple analytes in a single run. This development laid the foundation for the field of metabolomics, which presents the unbiased and systematic study of small molecules present in a biological sample. If mass spectrometry records all ions of a particular mass range, this is called an “untargeted” mode. If operated in “targeted” mode, mass spectrometry only records preselected ions (281).
Of all separation techniques, GC provides the best resolution of steroids. In combination with MS as the detection method, GC-MS presents a robust analytical tool, unsurpassed in determining simultaneously a multitude of steroids including precursors or metabolites of progestins, glucocorticoids, mineralocorticoids (all C21-steroids), androgens (C19-steroids), and estrogens (C18-steroids) (287, 288). GC-MS is particularly useful for urinary steroid metabolome analysis, but it can also be applied to the analysis of blood or tissues (289) or be used as a gold standard in quality assurance (290). As over two-thirds of steroid hormones and their metabolites are excreted into urine, the measurement of these urinary steroids provides an integrated picture of a patient’s steroid hormonal status (steroidal fingerprint) and has enormous diagnostic power. Adrenal enzyme defects show unique metabolic patterns (disease signatures, metabotypes) (291). Usually, a spot urine sample is sufficient for diagnosing an adrenal enzyme defect (266, 292, 293). Timed samples (eg, 24-hr urine) provide additional information on hormonal production rates via determination of steroid excretion rates (294, 295). This information aids the diagnosis of hormonal overproduction syndromes, such as Cushing syndrome or tumors, as well as in assessing compliance with hormonal therapy in CAH (296, 297). Moreover, this approach has been used to dissect the contribution of the 3 androgen biosynthesis pathways discussed in section “Basic principles of steroid synthesis and adrenal enzymatic defects,” “New pathways; new steroids” (132, 298, 299). Unbiased systems biology approaches allow for clustering and describing various metabotypes (300), reclassifying hitherto uncharacterized conditions (301) or improving metabolic monitoring of 21OHD (302, 303).
LC-MS is a more recent technique than GC-MS (301). MS/MS provides an extra level of filtering, thus improving the relatively poor separation properties of LC. Simple work up procedures and short run times permit much greater throughput than with GC-MS (281). Currently, determination of most clinically relevant steroid hormones in plasma or serum can be carried out by LC-MS/MS. It is the technique of choice for determining conjugated steroids (304). However, factors such as relatively low chromatographic resolution and lower ionization fraction, compared with electron impact in GC-MS, can impair the specificity of LC-MS/MS. Thus, GC-MS and LC-MS/MS should be considered complementary techniques.
Whatever analytical method is used, thorough method validation is a sine qua non. Important aspects of method validation comprise assessment of sensitivity, precision, reproducibility, accuracy, limits of quantification and detection, recovery, stability, carryover, and matrix effects. Recommendations have been published for the hormonal diagnosis of steroid related disorders (305).
Molecular Genetic Testing for CYP21A2 Gene Mutations
Southern blot analysis was originally the gold standard for the detection of CYP21A2 gene deletions but is no longer used clinically because it requires relatively large amounts of high-quality DNA, is labor intensive, and time consuming. Moreover, CYP21A1P duplications and certain other rearrangements at this locus may impede the detection of CYP21A2 gene deletions or duplications (306). The most widely used current approach for gene dosage determination is multiplex ligation–dependent probe amplification (MLPA). MLPA requires only small amounts of DNA for detection of gene deletions, rearrangements, and fusion genes (210, 307-309). However, complex rearrangements can lead to challenges interpreting the correct genotype. The design of CYP21A2-specific primers for PCR-based amplification is crucial to avoid amplification of the pseudogene and allele dropout by nonamplifying PCR fragments. This represents a challenge due to the high number of polymorphisms within CYP21A2 and the high sequence identity with its CYP21AP1 pseudogene. A variety of targeted molecular genetic strategies for detecting the common mutations have been published and are established in diagnostic laboratories. However, direct sequencing of the amplified PCR products combined with a method for the detection of gene deletions and chimeric genes are the only available strategies that allow for the detection of close to 100% of CYP21A2 mutated alleles. If possible, carrier testing should be performed in the parents to set phase (ie, confirm the parental origin of each mutation), which is required to determine compound heterozygosity, distinguish hemizygosity from homozygosity in the index case, and estimate the recurrence risk.
Prenatal Diagnosis
Prenatal diagnosis can be performed when both parents are carriers of CYP21A2 mutations; most often this situation arises when they have a previous child with 21OHD. The possible methods for prenatal diagnosis have increased over the past decades. However, methods involving invasive sampling should only be performed if the results will lead to changes in approach or treatment (310).
Analysis of fetal hormones in amniotic fluid was the initial method available for prenatal diagnosis (311-313). Fetal cells obtained this way were originally used for HLA typing to determine the inheritance of maternal and paternal haplotypes (CYP21A2 is located in the HLA complex) (314) but can also be used for genetic analysis, although cells must first be cultured, a time-consuming process.
Chorionic villus sampling to obtain fetal DNA can be performed as early as gestational week 10-11, compared with week 12-14 for amniocentesis (315). This method is available in many countries and centers today. Both amniocentesis and chorionic villus sampling are associated with a small but increased risk of fetal loss (316).
Noninvasive methods
Cell-free fetal DNA can be isolated from maternal plasma (317). Unlike fetal cells, it disappears from the maternal circulation shortly after delivery (317, 318) and therefore does not confound prenatal genetic investigations in subsequent pregnancies (319, 320). Prenatal sex typing (SRY detection) can be performed using PCR amplification of cell-free fetal DNA as early as week 6-9 (321) and may be useful in decisions regarding prenatal treatment with Dex to minimize treatment of male fetuses (see section “Management,” “Prenatal treatment”) (322). Next generation sequencing of cell-free fetal DNA can ascertain mutations, but it is challenging to detect CYP21A2 mutations in this manner because the vast majority of such mutations are already present in the CYP21A1P pseudogene. Instead, targeted massive parallel sequencing of cell-free fetal DNA in maternal plasma can identify SNPs flanking CYP21A2 that are specific for the mother, father, and proband (previous child), thus constructing haplotype blocks to determine the maternal and paternal alleles inherited by the fetus (323). The technique is promising but costly; it requires specific resources and personnel and is not yet available as part of routine clinical care.
Preimplantation genetic diagnosis
Preimplantation genetic diagnosis (PGD) is available in many countries for families at risk of having a child with a severe genetic condition including 21OHD. PGD requires an in vitro fertilization approach and enables implantation only of embryos without the specific genetic disorder. It may present ethical challenges beyond the scope of this review (324). The preferred approach to obtain DNA for PGD is a biopsy at day 5-6 from the trophectoderm of the blastocyst when it comprises about 120 cells. The inner cell mass that will develop into the fetus, from which 5 to 10 cells are required, can then be separated from the trophectoderm (310). If the first polar body of the oocyte is used in PGD, the procedure is performed before fertilization occurs, and the analysis offers the unique possibility of preconceptional diagnosis. The disadvantage for autosomal recessive disorders is that only the oocyte is assessed and the paternal allele is not included in the assessment. The rate of fetal anomalies is not increased with PGD, rather it is thought that if damage occurs during the procedure it is lethal to the embryo (325).
Management
Hormonal Treatment of Classic CAH
Treatment of classic CAH is intended to replace both glucocorticoid and if necessary, mineralocorticoid hormones to prevent adrenal and SW crisis and to reduce excessive corticotropin driving adrenal androgen secretion (133). Clinical goals are normal growth and development and pubertal maturation from birth to adolescence, and prevention of adrenal crisis, virilization, and other long-term complications discussed below (133, 326, 327). The levels of evidence for management guidelines are detailed in The Endocrine Society’s Clinical Practice Guideline published in 2018 (133) and are not repeated here. There are no large-scale prospective randomized trials for any therapies discussed here. As such the evidence is generally of low or moderate quality and rests to some extent on expert opinion, values, and preferences. Glucocorticoid replacement in CAH faces 3 particular challenges. First, it aims to replace cortisol, but current treatment strategies cannot completely mimic the circadian rhythm of cortisol with a typical early morning rise of cortisol leading to a peak concentration at 6 to 8 am and a nadir at midnight (328). Second, it aims to mimic the adaptation to stress (329). These distinctive features of physiological cortisol biosynthesis could only be closely mimicked by an infusion pump (330, 331) which, however, is neither practical nor cost-effective and thus not routinely available. Third, it aims to restore negative feedback on pituitary ACTH drive thereby controlling adrenal androgen excess (332). Normalizing ACTH levels in CAH patients requires supraphysiological glucocorticoid doses compared with the mere replacement doses of cortisol required in other forms of adrenal insufficiency. Treatment in classic CAH therefore constantly struggles to prevent overtreatment with multiple adverse side effects on growth and on metabolic, cardiovascular, and bone health, or undertreatment, which carries risks life-threatening adrenal crises and virilization or, in children, accelerated skeletal maturation with consequently reduced adult height. Both over- and undertreatment also affect reproductive function in both sexes.
Treatment in the neonatal period and early infancy
In growing children with classic CAH, the preferred glucocorticoid is the synthetic form of cortisol, HC, because its shorter half-life minimizes the adverse side effects typical of longer-acting, more potent glucocorticoids, especially growth suppression (133). As cortisone must be converted to cortisol for bioactivity (333, 334) and differences in conversion rates may influence drug effectiveness, cortisone acetate is not recommended (335, 336). HC should be given in 3 to 4 divided doses totaling 10 to 15 mg/m2 daily (Table 3), a supraphysiological dose under which many patients show satisfactory control of adrenal androgen production. Although there are data to suggest that 4 daily doses are preferable (337), this may not be practical for many patients or their families. Data remain inconclusive regarding morning vs evening dose weighting (338, 339). The total dosage should be individualized based on adequate monitoring (see below) and may need to be increased for short periods in certain circumstances; such increased needs are described below. Therefore, all children with CAH should be under the care of a pediatric endocrinologist (340).
Table 3.
Maintenance therapy in patients with CAH classic CAH
| Drug | Recommended total daily dose | Divided dosing frequency (times daily) |
|---|---|---|
| Children | ||
| Hydrocortisone | 10-15 mg/m2 | 3-4 |
| Fludrocortisone | 0.05-0.2 mg | 1-2 |
| Sodium chloride supplements | 1-2 g (17-24 mEq/day) in infancy | Several |
| Adults | ||
| Glucocorticoids | ||
| Hydrocortisone | 15-25 mg | 2-3 |
| Prednisone | 5-7.5 mg | 2 |
| Prednisolone | 4-6 mg | 2 |
| Methylprednisolone | 4-6 mg | 2 |
| Modified-release hydrocortisone (Plenadren®) | 15-25 mg | No published data in CAH patients, clinical experience shows that in addition to the morning dose a second GC dose is required in the evening |
| Modified- and delayed-release hydrocortisone (Chronocort®)a | 15-25 mg | 2 (2/3 of dose at 2300 and 1/3 of dose at 0700)a |
| Dexamethasoneb | 0.25-0.5 mg | 1 |
| Fludrocortisone | 0.05-0.2 mg | 1 |
a Not yet launched, currently only available within the extension phase of the phase III study.
b Avoid if possible or limit to a short time. Adapted from Speiser et al. (133).
In the neonatal period, some clinicians exceed the recommended glucocorticoid dose in order to reduce elevated androgen levels as quickly as possible. If this treatment strategy is adopted, more frequent monitoring is necessary to rapidly reduce the dose when target levels of monitored steroids are achieved, to avoid adverse effects of high doses of glucocorticoids (341). After a few months, maintenance daily totals of about 3 to 4 mg HC divided in 3 doses (ie, 1-2 mg 3 times daily) are usually sufficient. Infants have low sensitivity to androgens, and completely suppressed adrenal androgens should not be the main goal in the first year of life. Commercial HC tablets, which may be extemporaneously crushed and mixed into food or suspended, are preferred as there have been reports of variable dose accuracies in compounded preparations (342, 343). A suspension would be preferable for small children, but the commercial suspension was withdrawn 20 years ago owing to unreliable therapeutic effects, although in some countries reliable solutions are now available (344). An immediate-release granule formulation of HC (Infacort®/Alkindi®, Diurnal Ltd) available in 0.5, 1, 2, and 5 mg preparations has been approved for use in the EU and the United States (345, 346).
Mineralocorticoid replacement is achieved with fludrocortisone. Monitoring is discussed in section “Management,” “Hormonal treatment of classic CAH,” “Monitoring.” Subclinical or overt aldosterone deficiency is present in all forms of classic CAH (347, 348). Therefore, fludrocortisone is given to all newborns with classic CAH detected in neonatal screening programs even before hyponatremia develops. Due to relative mineralocorticoid resistance and the antimineralocorticoid effects of elevated 17OHP in this period, neonates and young infants require higher fludrocortisone doses than older children, typically 100 to 200 µg/day but occasionally more, divided in 1 or 2 oral doses. However, this treatment requires frequent monitoring of electrolytes, plasma renin, and blood pressure and tapering the fludrocortisone dose in order to avoid iatrogenic hypertension. Because of a lower glomerular filtration rate, immature renal tubules, and the low sodium concentration in breast milk and infant formula, infants often require additional supplemental sodium chloride to maintain sodium balance. The recommended dosage is approximately 1 to 2 g (4-8 mEq/kg/day) of NaCl given in divided doses ideally using a standard saline solution (349) or crushed, aliquoted sodium chloride tablets. In patients receiving high doses of fludrocortisone, NaCl supplementation may not be needed (350). Moreover, 0.1 mg of fludrocortisone has the glucocorticoid potency of ~1 mg of HC, so high fludrocortisone doses may permit (or require) a reduction of the HC dose in young children.
Treatment during childhood
Children younger than 18 months should be monitored at least every 3 months, while older children should be monitored every 4 to 6 months or more frequently after a change in dosing. The suggested target 17OHP range is 100 to 1200 ng/dL (3-36 nmol/L) when measured in the early morning before medication (335, 336), and/or age-appropriate androstenedione levels. Attempts to normalize 17OHP levels should be avoided because of the risk of HC overdosing causing iatrogenic Cushing syndrome. Because prepubertal children normally have low circulating sex steroid levels, adequate androgen suppression is important to achieve normal growth and puberty. Table 3 provides suggested dosing guidelines (133). HC dosing requirements may vary and depend on differences in HC absorption and half-life (351). Long-acting glucocorticoids should be avoided in growing children except for short intervals when necessary to restore hormonal control, or if HC is unavailable (Table 4). If used, care must be taken to avoid overdosing, which will suppress growth (352-354), and the dose should be decreased as quickly as possible once hormonal control is achieved.
Table 4.
Indications for different glucocorticoid (GC) preparations
| Steroid | Clinical indication | Pros | Cons |
|---|---|---|---|
| Hydrocortisone | Preferred option for GC replacement. | Best long-term outcome with regard to metabolic, cardiovascular, and bone health | Short half-life. Needs to be given 3 times daily. Adrenal androgen suppression overnight may escape. |
| Prednisolone (Prednisone) | Might be a preferred option for regulation of menstrual cycles or fertility induction, or if patient adherence is poor with thrice daily hydrocortisone | Longer half-life, twice daily regimen. Potentially better patient adherence compared with 3 times daily regimen | Potential higher rate of adverse effects on metabolic, cardiovascular, and bone health compared with hydrocortisone |
| Dexamethasone | Fertility induction TART treatment | Strong adrenal suppressive effect, longest half-life, once daily regimen often possible | Highest rate of adverse effects on metabolism, bone health. Traverses placenta barrier |
Aldosterone deficiency is described in up to 75% to 90% of all classic CAH patients, now viewed as a continuum of phenotypes rather than strict divisions between SV and SW disease. In classic CAH after infancy, fludrocortisone is usually given in doses ranging between 50 and 200 µg. Fludrocortisone has a biological half-life of approximately 18 to 36 hours. Therefore, low doses can be administered once a day, although doses above 0.1 mg may still be divided to be given twice daily. In hot and humid weather conditions, some endocrinologists suggest a seasonal increase in fludrocortisone, although increased salt intake may suffice. In contrast to glucocorticoid treatment fludrocortisone does not need to be increased during illness (section “Management,” “Hormonal treatment of classic CAH,” “Management of adrenal emergency in CAH”).
Treatment during puberty and adolescence
Puberty is often associated with difficult hormonal control, even if the replacement dose seems adequate and there is good adherence to the medication regimen. During puberty, the pharmacokinetics of HC may be altered by increased clearance due to decreased activity of 11β-HSD1. Therefore, higher glucocorticoid doses are necessary during puberty (355). However, as adult height of patients with CAH correlates negatively with the glucocorticoid dose administered in early puberty (354), HC doses exceeding 17 mg/m2 per day should be used with care. Treatment should be continued with the lowest effective dose to achieve treatment goals, prioritizing height attainment over arbitrary steroid measurements. At or near the completion of growth, long-acting glucocorticoids might be considered but are not preferred.
Management of CAH during adolescence and the transition from pediatric to adult healthcare is challenging (356). CAH patients may have poorer health, beginning in adolescence and persisting into adulthood, highlighting the importance of this period in patient care (357). Multidisciplinary transition clinics involving pediatric and adult endocrinologists, gynecologists, urologists, and psychologists may promote good medical adherence among adults with CAH (133). Uninterrupted glucocorticoid and mineralocorticoid administration at the transition from adolescence to adulthood is required to prevent increased morbidity and mortality, particularly from adrenal crises. Treatment regimens should be reassessed and adapted to the recommendations for adult CAH patients. Importantly, mineralocorticoid requirements, which change from birth to adolescence, should be reassessed in adolescence/young adulthood to avoid mineralocorticoid over- and under-replacement (358). Transition, however, is more than just prescribing steroid replacement for primary adrenal insufficiency and must address sex- and gender-specific issues (359, 360). In females, obesity and hyperandrogenism are common problems leading to menstrual irregularities and hirsutism (357). Gynecologic evaluation should be offered to all adolescents with CAH at transition, particularly in cases of blocked menstrual flow, planned penetrative vaginal intercourse, or desired pregnancy (133, 361). Boys should have a testicular ultrasound upon completion of puberty and regular examination for TARTs (358). All patients should be aware of the risk of reduced fertility with poor medical adherence (133). Psychosexual and genetic counseling of the adolescent patient are strongly recommended during transition (362).
Treatment of adults
Treatment of adults with classic CAH aims to replace the missing cortisol and aldosterone, and ameliorate adrenal androgen excess (332). Optimal hormone replacement theoretically should enable normal quality of life and life expectancy. However, this aim is not always achieved, and adults with classic CAH suffer from multiple disease-associated comorbidities (363-365), reduced health-related quality of life (358), and increased mortality (366).
The decision of which preparation to use for glucocorticoid treatment in adults with classic CAH is based on the clinical experience of the individual physician and on the needs of each patient (Table 4). In general, the lowest possible doses should be prescribed that minimize risk of adrenal crises and control androgen excess. HC is associated with better bone mineral density (BMD) and better metabolic and cardiovascular outcome than Dex in both sexes; prednisolone and prednisone have adverse effect profiles intermediate between HC and Dex (364, 367). Therefore, immediate-release HC remains the preferred option for glucocorticoid treatment in adulthood. Due to its short half-life of 4 to 6 hours, however, it needs to be taken 3 to 4 times per day and requires reliable adherence.
When patient adherence to a 3 times daily regimen is difficult, a twice daily regimen with prednisolone or prednisone (eg, 1-5 mg per dose, for a total daily dose of 20% to 25% of the previous HC dose) might be used instead (358, 368, 369). Prednisolone/prednisone also has been used for fertility induction when it might be more effective and can be continued throughout pregnancy (370). Dex also effectively helps to establish regular menstrual cycles, but it is long-acting and associated with more adverse metabolic side effects (371). In contrast to prednisolone, prednisone, or HC, Dex traverses the placenta and therefore should be avoided during pregnancy (the use of Dex for prenatal treatment of a possibly affected fetus is discussed in section “Management,” “Prenatal treatment”) (133, 372-375). Due to its strong adrenal-suppressive effect, Dex is preferred in the treatment of TARTs (376, 377). For this purpose, it needs to be given in supraphysiological doses, and should be given for short duration to avoid adverse metabolic effects such as weight gain, striae, edema, and glucose intolerance.
Sustained-release HC preparations have been developed as an alternative to longer-acting synthetic corticosteroids such as prednisone/prednisolone or Dex. A modified-release HC formulation Plenadren® (Shire Services BVBA, Belgium), is approved in Europe for treatment of adrenal insufficiency in adults. When given once daily to patients with primary adrenal insufficiency, it significantly improves metabolic variables such as body weight, body mass index (BMI), and HbA1c compared with conventional HC replacement of the same daily dose (378, 379). However, data on its use in CAH patients are lacking. Clinical experience shows that once daily HC therapy fails to control early morning rise of ACTH with subsequent excess of adrenal androgens in CAH, requiring an additional glucocorticoid dose in the evening. Excessive nighttime glucocorticoid administration has potential adverse metabolic consequences (369, 380). Another modified-release preparation (Chronocort®, Diurnal, UK) addressing this CAH-specific challenge is currently under regulatory review for the treatment of CAH. It exerts a delayed (4 hours following intake) and sustained action (381). If taken at 23.00 (11 pm), the delayed release mimics the overnight rise and following morning peak of cortisol (381, 382). A second dose is given in the morning (7 am) ensuring cortisol supply during the day. A phase III trial including 122 patients with classic CAH revealed superior hormonal control during the early morning and early afternoon compared with patients receiving standard glucocorticoid therapy (336, 383).
Monitoring
Regular follow-up should include measurement of height, weight, blood pressure, and physical examination. In children, special attention should be paid to accelerated or reduced height velocity, rapid weight gain, skin and mucosal hyperpigmentation, signs of virilization, pubic hair onset, development of apocrine odor, and signs of central precocious puberty such as breast development or testicular enlargement. Medical history concerning symptoms of salt craving, phases of unusual fatigue during the day, irregular menstrual cycles in girls, and skin hyperpigmentation point to the need for medication titration (Table 5).
Table 5.
Biochemical monitoring of glucocorticoid replacement in children and adults
| Sample | Variable | Goals and Commentsa |
|---|---|---|
| Serum | Androstenedione | Normal values for sex and age (often useful to assess together with testosterone in males) |
| Testosterone | Normal values for sex and age (assess in the context of gonadotropins and androstenedione) | |
| Sex hormone–binding globulin | For calculation of free and bioavailable testosterone | |
| DHEAS | Low to suppressed, not a good marker of disease control, but can be used to check for compliance/adherence | |
| 17OHP | Normal values indicate overtreatment, aim at ULN to 400-1200 ng/dL (12-36 nmol/L) | |
| ACTH | Not a useful parameter for disease control; normal values indicate overtreatment | |
| Androstenedione/Testosterone ratio | Healthy woman: <2 | |
| Women with CAH: >4 indicates testosterone mainly of adrenal origin | ||
| Healthy males: <0.2 | ||
| Men with CAH: >0.5 indicates testosterone mainly of adrenal origin | ||
| Men with CAH: >1.0 + LH, FSH suppressed indicates testosterone only of adrenal origin due to poor disease control | ||
| Progesterone (females) | Goal is <2 nmol/L (<0.6 ng/mL) in follicular phase for women trying to conceive | |
| 11-oxygenated C19 steroids (11-ketotestosterone, 11-hydroxytestosterone, 11-hydroxyandrostenedione, 11-ketoandrostenedione) | Translational method; not yet established in clinical care | |
| Saliva | Androstenedione | Normal values for sex and age |
| 17OHP | Up to ~3 times upper normal limit | |
| Urine | GC-MS urinary steroid metabolome analysis (C21-, C19-, C18-steroids) | Translational method; not yet established in clinical care |
a These goals are derived from clinical experience and based on expert opinion as there are no established optimal biomarkers nor target values for treatment monitoring.
Laboratory monitoring traditionally relies on consistently timed serum 17OHP, androstenedione, and plasma renin levels, whereas ACTH measurements are superfluous (Table 6). Plasma renin activity and direct renin levels are extremely variable and should be used along with standing blood pressure and electrolytes to titrate mineralocorticoid dosing (133, 384). Other hormonal monitoring approaches have been suggested but are not yet used routinely. Adrenal-specific metabolites such as 21-deoxycortisol (385) and 11-oxygenated androgens (86) may provide more direct evidence for adrenal androgen production in CAH. Steroids can be measured in blood, urine (266, 303), saliva (386) and dried filter paper blood samples (387, 388) and fluctuate with both the circadian rhythm and the timing of glucocorticoid intake (296, 389-391).
Table 6.
Monitoring glucocorticoid replacement by history and clinical/technical examination (generally every 4-6 months in adults, every 3-4 months in children >18 months old)
| Parameter | Goals and Comments |
|---|---|
| History | |
| Symptoms of adrenal insufficiency (fatigue, headache, nausea, abdominal pain, postural dizziness, frequent stress dosing) | No signs of adrenal insufficiency |
| Adrenal crisis prevention | Well-educated and equipped patient with knowledge of sick day rules, and possession of steroid emergency card and injection kit; medical alert identification worn at all time |
| Menstrual cycle | Regular menstrual cycles |
| Libido, erections (males) | Normal |
| Sexual health (females) | Pain-free intercourse |
| Physical examination | |
| Height (children) | Linear growth within target range |
| Pubertal development/Tanner stage (children and adolescents) | Normal pubertal development |
| Blood pressure | Within age- and sex-dependent reference range |
| BMI | Within age- and sex-dependent reference range |
| Cushingoid features, Striae distensae | No clinical signs of hypercortisolism |
| Gynecological assessment only if indicated | |
| Imaging | |
| Bone age yearly (children >2 years old/adolescents) | Bone age within 2 SD |
| Scrotal ultrasound every 2-5 years | No gonadal masses |
| Ovarian ultrasound only indicated in unexplained hyperandrogenism | |
| Bone mineral density every 3-5 years (adults treated with high GC doses) | Within age- and sex-dependent reference range |
| Others | |
| Semen analysis if indicated, ie, presence of TARTs (males) | Normal results (WHO guideline) |
| Genetic assessment and counselling | Confirmation diagnosis CAH; counselling for family planning |
Regular bone age X-rays in growing children beyond 2 years of age are helpful to detect unwanted bone age advancement as a result of cumulative exposure to excess adrenal androgens. The clinician should be alert to signs of central precocious puberty (eg, testicular enlargement in boys, breast development in girls) because elevated adrenal androgens may activate the hypothalamic–pituitary–gonadal axis (133). The decision to adjust HC and fludrocortisone doses should consider clinical symptomatology and should not solely rely on laboratory data. Monitoring for reproductive complications are discussed below including reduced fertility in females (see section “Long-term sequelae”), and TARTs in males (see section “Long-term sequelae,” “Gonadal function in males,” “Testicular adrenal rest tumors”).
Management of adrenal emergency in CAH
The overwhelming majority of patients with CAH survive into adulthood, but with shortened life expectancy. Adrenal crises were responsible for 42% of deaths in 588 patients with CAH in a Swedish population-based study; those with the SW form were especially at risk, as they had the lowest cortisol and aldosterone reserves (366). In a retrospective matched-cohort study in the UK, all-cause mortality rates were higher in patients with CAH, with a mean age at death of 54.8 years vs 72.8 years in controls (392). The incidence of adrenal crisis in adults with adrenal insufficiency is estimated to be 5 to 10 crises/100 patient years with a mortality rate of 0.5/100 patient years (393). Studies of children report similar findings. Two German studies estimated the incidence of adrenal crisis after the neonatal period to be 4.9 to 6.5 adrenal crises/100 patient years (394, 395). In an American series, 55/155 children with SW CAH were hospitalized a total of 105 times over a 14-year period (396). In an Australian population-based study of children and adolescents with CAH, both hospital admission and the risk of adrenal crisis decreased with age (397). A large multicenter international study of 518 children from low- and middle-income countries as well as high-income countries reported an adrenal crisis rate of 2.6/100 patient years (398).
Adrenal crisis is most often triggered by infectious illness (395, 399, 400). A population-based retrospective cohort study (drug prescriptions and clinical diagnoses) in the UK reported increased rates of infectious illnesses in patients with CAH (399). Gastrointestinal illnesses and upper respiratory tract illnesses are the most common precipitants of adrenal crises in both children and adults (395, 396, 399, 400). Socioeconomic factors influence risk; in the United States, patients with government insurance (reflecting low family income) were twice as likely to be hospitalized as patients with commercial insurance (396). Preschool children, adolescents, males, and those with SW CAH were more likely to experience sick days requiring stress dosing. Patients treated with higher glucocorticoid doses were less likely to suffer illness requiring stress dosing. The frequency of adrenal crises has decreased over time, perhaps due to greater awareness of this risk during sick days. None of the adrenal crises reported in a multicenter study were fatal (398).
Hypoglycemia can occur unexpectedly (394), may be associated with seizures, and can occasionally result in permanent neurologic sequelae, especially in children (400, 401). Patients with SW CAH have adrenomedullary dysfunction with epinephrine deficiency (402) and this contributes to the risk of hypoglycemia especially in young children.
Protocols for the prevention and treatment of adrenal crisis are based on expert opinion and clinical experience (133, 403-405). “Sick day rules” aim to prevent acute deterioration and a life-threatening adrenal crisis. However, the definition and reporting of sick days is more variable than that of adrenal crises, with evidence of systematic variation between centers (398). Adverse outcomes in children are related more to hypoglycemia than to electrolyte disturbances (394, 400, 406); thus, frequent intake of carbohydrates is important (400). Oral stress doses (2-3 times usual doses) of glucocorticoid cannot always prevent the progression to adrenal crisis and the occurrence of hypoglycemia (406, 407). Increased HC doses are suggested with infectious illnesses (Table 7). HC sodium succinate for intramuscular injection should be prescribed with instructions for home use if oral medication is not tolerated during episodes of major stress (eg, febrile illness with vomiting), especially for patients residing far from medical facilities. Once brought to emergency care, intravenous HC and isotonic fluids should be given. Continuous intravenous infusion of HC sodium succinate might have a theoretical advantage over intermittent bolus administration because of lower variability and avoidance of regular troughs in plasma cortisol levels (383, 408), but these 2 approaches have not been compared directly and clinical outcomes are likely similar. Stress dosing is indicated for pregnant women in active labor, similar to that used in major surgical stress (403). Stress dosing is not recommended for everyday mental and emotional stress, minor illness, or before routine exercise (133, 403, 404). Serum cortisol did not exceed 10 µg/dL (276 nmol/L) in healthy children undergoing minor surgical procedures; therefore, stress dosing for minor procedures (eg, brief medical or dental procedures performed under local anesthetic with or without light sedation) should be individualized (247, 409).
Table 7.
Suggested management and glucocorticoid stress dosing for patients with adrenal insufficiency due to congenital adrenal hyperplasia (393, 396, 397, 400)
| Clinical Scenario | Glucocorticoid Management | Additional considerations |
|---|---|---|
| At home | ||
| Major illness or high-grade fever (>39°C children) | Children: Three times the usual dose of hydrocortisone divided into 4 doses (given every 6 hours). | Drink regularly and increase fluida intake for concentrated (dark) urine |
| Adults: 20 mg of hydrocortisone orally 3 times daily in addition to usual glucocorticoid or triple usual glucocorticoid. | Eat regularly simple and complex carbohydrates. 15 g (children) or 30 g (adults) | |
| Adults with severe infections should divide dose every 6 hours | ||
| Gastroenteritis with diarrhea ± vomiting (with or without fever) | Children: 3 times the usual dose of hydrocortisone divided into 4 doses (given every 6 hours); | Consider early parenteral hydrocortisone |
| Adults: 10-20 mg of hydrocortisone 3 to 4 times daily in addition to usual glucocorticoid or double or triple usual glucocorticoid; dose depends on severity of diarrhea | If unable to tolerate fluids, call emergency services for evaluation following glucocorticoid injection | |
| Repeat oral dose if vomiting occurs within 1 hour of medication. If vomiting reoccurs, parenteral hydrocortisone 100 mg (children 50-100 mg/m2) | Return to usual dose within 1-2 days of recovery with return to usual diet | |
| Minor illness or low-grade fever (>38°C in children) | Children: 2 to 3 times the usual dose of hydrocortisone divided into 3-4 doses (given every 6-8 hours) | Drink regularly and increase fluida intake for concentrated (dark) urine |
| Adults: 10 mg of hydrocortisone orally 3 times daily in addition to usual glucocorticoid, or double usual glucocorticoid | Eat regularly simple and complex carbohydrates. 15 g (children) or 30 g (adults) | |
| Return to usual dose within 1 day of recovery | ||
| Exhausting physical exercise | Add 1 usual dose (children) or 10 mg hydrocortisone (adults) 30 to 60 minutes before exercise | For unusual activities beyond normal routines. Not for routine use |
| Can repeat dose(s) if extended time period of strenuous exercise (eg, marathon) | ||
| Procedures | ||
| Major surgery | Hydrocortisone intravenous bolus 50-100 mg (children 50-100 mg/m2) followed by continuous intravenous hydrocortisone infusion 100-200 mg (children 100 mg/m2) over 24 hours. Alternatively, divided doses every 6 hours, intravenous hydrocortisone 100-200 mg/day (children 100 mg/m2/day) | Taper over 2-3 days with return to usual dose |
| Short surgeries | Hydrocortisone intramuscularly or intravenous bolus 50-100 mg (children 50 mg/m2) just before general anesthesia. Alternatively, give triple the usual morning dose before oral intake is held | Rapid return to oral regimen |
| Labor and delivery | As for surgical procedures | |
| Bowel procedures requiring overnight laxative | Double or triple usual glucocorticoid dose prior to laxative and repeat every 6 hours if oral medication tolerable and allowed. Alternatively, hydrocortisone 50-100 mg (children 50 mg/m2) intramuscularly with laxative | |
| Hydrocortisone 50 mg (children 50 mg/m2) intramuscularly or intravenous prior to procedure | ||
| Dental surgery | Extra morning dose 1 hour prior to surgery | Can repeat dose depending on recovery |
| No additional doses for routine dental procedures | ||
| Minor procedures with no sedation | No adjustment needed | |
| Acute emergency | Rapid infusion of intravenous fluids: 1000 mL of 0.9% sodium chloride (children 20 mL/kg normal saline, repeat up to 60 mL/kg) during the first 60 minutes, further fluid administration guided by individual patient needs | Measurement of glucose and electrolytes |
| Hydrocortisone bolus 100 mg (children 50-100 mg/m2) followed by continuous intravenous infusion 200 mg over 24 hours or 50 mg every 6 hours (children 50-100 mg/m2/day divided every 6 hours). Reduce to 100 mg (children 50 mg/m2/day) over 24 hours the following day | Cardiac monitoring | |
| Consider antibiotics | ||
| For hypoglycemia: dextrose 0.5-1g/kg dextrose or 2-4 mL/kg of D25W (maximum single dose 25 g) infused slowly at rate of 2 to 3 mL/minute Alternatively, 5-10 mL/kg of D10W for children <12 yrs old | Rapid hydrocortisone tapering and switch to oral regimen depending on clinical status |
a Electrolyte- and sugar-containing fluids recommended. If hydrocortisone sodium succinate is unavailable, another parenteral glucocorticoid, such as dexamethasone, methylprednisolone, or prednisolone, may be used in equivalent doses. Fludrocortisone replacement is not required if hydrocortisone doses exceed 50 mg every 24 hours but is generally administered, in those normally on fludrocortisone, when oral hydrocortisone is started.
Approximately one-third of patients with NC CAH have mild but clinically silent cortisol impairment (410, 411) and the risk of adrenal crisis is unknown. Adrenal crisis has only been reported in NC CAH patients receiving glucocorticoid therapy in the setting of iatrogenic tertiary hypothalamic–pituitary–adrenal axis suppression (133, 400). Thus, stress dosing for the prevention of adrenal crisis is recommended for glucocorticoid-treated patients with NC CAH. The Endocrine Society Clinical Practice Guideline (133) suggests HC stress dosing in the case of severe illness, major surgery, major trauma, or childbirth for untreated individuals with a suboptimal ACTH test (in adults, cortisol below 14 to 18 µg/dL, <400-500 nmol/L).
In general, prevention of adrenal crisis in patients with known adrenal insufficiency is best accomplished through repeated structured patient education regarding “sick day rules” (412, 413). All patients should wear medical alert identification tags or have an emergency card (and/or emergency information on their mobile phones) indicating adrenal insufficiency. A medical card developed by the European Society of Endocrinology is downloadable and includes guidance for healthcare providers as well (https://adrenals.eu/emergency-card/). A UK version including a QR code rapidly linking emergency personnel to instructions on adrenal crisis treatment is available (https://www.endocrinology.org/media/3652/steroid-nhs-card.jpg).
Treatment of NC CAH
In NC CAH the estimated residual enzymatic activity of CYP21A2 is about 20% to50% based on in vitro or in silico studies, resulting in a generally mild but highly variable phenotype (210, 215, 414). In contrast to classic CAH, no general guidelines exist for the management and follow-up of these patients and the overall evidence of recommendations for NC CAH is low (133, 340, 415-417). Decisions about starting treatment should be individualized and based mainly on clinical symptoms; the Endocrine Society guidelines do not recommend routine treatment with glucocorticoid in asymptomatic individuals (133). The general treatment goals in children are to maintain normal growth and pubertal development and to minimize risk of therapies; children should be regularly monitored clinically for height, weight, signs of androgen excess, puberty, and bone age advancement (418).
When glucocorticoid treatment is required, HC is preferred, as with classic CAH. Patients receiving glucocorticoid therapy require stress dosing per guidelines (see section “Management,” “Hormonal treatment of classic CAH,” “Management of adrenal emergency in CAH”). Mineralocorticoid supplementation with fludrocortisone is not required.
Growth
In contrast to untreated children with SV CAH, children with NC CAH may not have increased growth velocity although bone age maturation can be accelerated, potentially leading to reduced adult height (419, 420). However, most studies describe nearly normal adult height in NC CAH patients (415, 421-423). Glucocorticoid treatment should be reserved for patients who suffer from androgen excess, although criteria for deciding when symptomatology warrants treatment are not well defined. Supraphysiological dosages of glucocorticoids similar to those used to treat classic CAH patients may be necessary to suppress adrenal androgen production (424). Treatment will suppress the hypothalamic–pituitary–adrenal axis requiring stress dosing in case of illness. In many cases glucocorticoid treatment may be discontinued after reaching adult height, if the individual is otherwise asymptomatic (133). Adverse effects such as excess weight gain may make continued glucocorticoid treatment less desirable.
Puberty
Children with NC CAH can present with signs of increased adrenal androgen production such as premature pubarche, acne, mild hirsutism, and menstrual disturbances that can progress over time (425), but in contrast to classic CAH, central precocious puberty is infrequently observed (37). Glucocorticoids can lower adrenal androgen concentrations ameliorating signs of hyperandrogenism, but prolonged glucocorticoid treatment may have long-term adverse effects. Alternative treatment options in adolescent and young adult females to induce menstrual cycles and improve acne and hirsutism include oral contraceptives containing progestins with low androgenic activity such as desogestrel (426). Antiandrogens can be considered as an add-on for patient-important hirsutism that persists despite oral contraceptives (see the next section).
NC CAH in adult women
Most patients diagnosed with NC CAH are females suffering from mild adrenal androgen excess without clinically relevant deficiencies of gluco- and mineralocorticoids (133, 427). Typical symptoms in affected women are hirsutism, oligo- and amenorrhea, acne, alopecia, and sub- or infertility (425). Sometimes the diagnosis is made within the course of evaluation for adrenal incidentalomas (428-430). The main treatment goal is to reduce adrenal androgens and symptoms of androgen excess. Clinical studies comparing different treatment approaches in adults with NC CAH are lacking; treatment should only be started in symptomatic patients desiring treatment (133). The risks, benefits, and effectiveness of various treatment options should be discussed. Fertility and childbearing in women with NC CAH are discussed in the section “Long-term sequelae,” “Reproductive function in women”).
Additional treatments for signs of androgen excess
Hirsutism is the most prevalent symptom in women with NC CAH, but also the most difficult to treat (431). Clinical experience suggests that a combination of oral contraceptives, topical eflornithine, and cosmetic treatment (shaving, chemical depilatories, plucking, tweezing, threading, waxing or epilation therapy, electrolysis, and intense pulsed light) might be the most effective treatment approach (432). For the treatment of acne and androgenic alopecia a dermatologist should be consulted.
Oral contraceptives act on the production, transport (increase of sex hormone binding globulin) and action of androgens. Antiandrogenic oral contraceptives containing cyproterone acetate, chlormadinone acetate, dienogest, or drospirenone effectively reduce androgenic symptoms. If hirsutism is the leading symptom, oral contraceptives are the preferred treatment (133). One randomized study in 30 women with NC CAH found cyproterone acetate to be more effective than HC for isolated hirsutism (433).
Spironolactone, flutamide, and finasteride can be used to treat hirsutism (431), acne, and androgenic alopecia (434) but are teratogenic and not approved for this use. Eflornithine hydrochloride cream is used as topical therapy for facial hirsutism (431). It prevents hair growth by inhibiting the anagen phase of hair production. Eflornithine irreversibly binds to ornithine decarboxylase and thus prevents the natural substrate, ornithine, from accessing the active site. It is most effective when combined with physical means of hair removal, such as topical lasers.
Treatment of adult men with NC CAH
As androgen production in the testis far outweighs adrenal androgen production, men generally do not experience symptoms of androgen excess requiring treatment, and therefore remain undiagnosed. In rare cases, severe acne, reduced fertility, or adrenal incidentaloma lead to the diagnosis of NC CAH in men (427). TARTs are rare in men with NC CAH (435-437). Therefore, routine scrotal ultrasound is not recommended in NC CAH males.
Prenatal treatment
Purpose
Since the mid-1980s, prenatal treatment with high doses of Dex has been proposed for to pregnant women with a fetus at risk for classic CAH using a treatment protocol of 20 µg/kg/day, maximum 1.5 mg/day, with the aim of preventing prenatal virilization of the external genitalia in affected girls (311, 315, 438). The treatment is effective in ameliorating virilization of the external genitalia if started by gestational week 6-7 (439); in most centers this is before a fetal diagnosis can be made (see section “Diagnostics,” “Prenatal diagnosis”). If prenatal diagnosis, most often by chorionic villus biopsy obtained in week 10-11, shows that the fetus is a girl with classic CAH, the treatment is continued until term, but otherwise stopped. The treatment is controversial due to safety concerns (440). Risk–benefit assessments must consider that, on average, 8 pregnancies at risk for CAH must be treated for every affected female who might benefit from the treatment (441-444). Endocrine societies and others have stated that the treatment is experimental and should only be performed in centers taking part in long-term research studies of these treated pregnancies (133, 326, 373, 375, 445).
Fetal safety
There have not been randomized studies of prenatal Dex treatment, and so all discussions of adverse effects are based on animal or retrospective data. Dex is a pluripotent gene regulator and its introduction at a critical stage of embryonic development may impact much more than the developing hypothalamic–pituitary–adrenal axis. Numerous studies (Table 8) have delineated adverse outcomes affecting brain, cardiovascular, renal, reproductive, thyroid, and metabolic functions in nonhuman mammalian species exposed to glucocorticoids in utero (reviewed in (373)).
Table 8.
Animal models of prenatal glucocorticoid exposure
| Animal | Medication/Dosinga | Outcome | Reference |
|---|---|---|---|
| Mouse | NK1R antagonists 30-300 mg/kg/dayb | 9% cleft palate in higher dosage | (446) |
| Rat | Dex 0.1 mg/kg/day during the whole pregnancy | Lower body weight and kidney size, postnatal hypertension, albuminuria, sodium retention, and decreased glomerular filtration | (447) |
| Spiny mouse | Mini osmotic pump with Dex 125 µg/kg | Decreased the number of nephrons and altered expression of genes involved in nephron development in the spiny mouse | (448) |
| Rat | Dex | Impaired thyroid development with fewer follicular cells and C cells | (449) |
| Rat | Carbenoxolonec 12.5 mg/day | Lower birth weight and increased blood pressure | (450) |
| Rat | Dex 100 µg/kg/day SC in late pregnancy | Glucose intolerance, 25% increase in hepatic expression of glucocorticoid receptor | (451) |
| Rat | Dex 100 µg/kg/day SC in late pregnancy | Lower birth weight, fatty acid esterification, and triglyceride synthesis | (452) |
| Rodents | Dex 50-120-200 µg/kg/day | Impaired glucose tolerance, hyperinsulinism increased blood pressure, reduced postnatal growth at 1 year of age despite normal birth weight | (453) |
| Rat | Carbenoxolone 12.5 mg/day | Reduced birth weight | (454) |
| 11 β HSD mutant mouse | None | Mice lacking Hsd11b2 had lower birthweights and increased anxiety compared with wild type littermates | (455) |
| Sheep | Betamethasone 0.5 mg/kg during 3 days | Retardation of fetal brain development | (456) |
| Sheep | Single or repeated betamethasone injections | Reduced brain weight | (457) |
| Sheep | Repeated betamethasone injections | Reduced neuronal myelinization | (458) |
| Rhesus macaque | Dex 0.5 or 5 or 10 mg/kg or repeated injections | Decreased numbers of pyramidal neurons in the hippocampal CA regions | (459) |
| Neural stem cells of newborn rat | Dex in vitro | Impairment of neuron and oligodendrocyte size and differentiation | (460) |
| Fetal guinea pig | Betamethasone 1 mg/kg for 4 days | Changes in GR DNA binding and DNA methylation in the fetal hippocampus | (461) |
| Guinea pig | Betamethasone 1 mg/kg for 4 days | Reduced locomotor activity; effect on programming HPA axis and hippocampal glucocorticoid feedback | (462) |
| Spiny mouse | 125 µg/kg Dex SC for 60 hours using mini pump | Reduction of adrenal steroidogenesis, decrease in plasma DHEA reduced adrenal expression of steroidogenic enzymes in adulthood | (463) |
| Guinea pig | Betamethasone 1 mg/kg for 4 days | Altered DNA methylation underlies both the long-term effects of glucocorticoids and of maternal stress on the fetus | (464) |
a Note that the doses given to animals exceed the typical doses given in pregnancies at risk for CAH.
b NK1R antagonists modulate the hypothalamic-pituitary-adrenal axis leading to increased corticosterone secretion.
c Carbenoxolone is a glycyrrhetinic acid derivative with a steroid-like structure. It inhibits placental Hsd11b2 activity, thereby increasing fetal exposure to maternal glucocorticoids (465).
With respect to human teratogenicity, systematic review and meta-analysis found an odds ratio of 1.41 (95% CI 1.14-1.74) for cleft lip and palate in case–control series of infants whose mothers were treated with glucocorticoids in the first trimester (466). Even when exposed later in gestation, multiple doses of antenatal steroids for preterm labor increased the number of infants with birth weight <10th percentile and the risk for cerebral palsy (467). Among pregnancies at risk for CAH, prenatally Dex-treated newborns have lower, but nominally normal, birth weights than untreated controls (468); the decrease averages ~400 g (469). Other adverse events including failure to thrive, stroke-like events and midline defects have been observed in both short-term and full-term treated cases at risk for CAH (470-472).
Prenatal Dex treatment has shown inconsistent long-term effects on cognition and behavior (Table 9). One study showed no cognitive differences but increased shyness and emotionality in treated children (473). A larger follow-up study from the same group of 126 non-CAH and 48 with CAH short-term exposed children and 8 girls with CAH treated until term did not show any effects on motor, cognitive, and social development or scholastic competence using parental questionnaires (474). In a later report including 2 different age groups (5-12 and 11-24 years) using neuropsychological testing, there were no significant findings in children without CAH (478). Swedish studies of healthy non-CAH children exposed to Dex only during the first trimester have shown negative effects on cognition, especially verbal working memory (475); a sexually dimorphic effect with a more pronounced negative effect on working memory and executive function was observed in the girls (481). A follow-up with a second neuropsychological testing in a subgroup of the cohort as adults showed less pronounced effects indicating a possibility for compensatory mechanisms over time (483).
Table 9.
Studies of human prenatal exposure to glucocorticoids
| Study group | Results | |||||
|---|---|---|---|---|---|---|
| Dex exposed | Controls | Age at study | Questionnaire findings | Psychological tests | Laboratory and MRI | Reference |
| First trimester exposure only a | ||||||
|
26 total
3 with CAH |
14 total
3 with CAH |
6 mo-5.5 yrs Mean 2.5 ± 1.3 yrs |
NS overall development Dex treated higher shyness, emotionality, lower sociability (EAS), internalizing (CBCL) (parental Q) |
(473) | ||
|
174 total
48 with CAH |
313 total
195 with CAH |
1-12 yrs 3 diff age groups |
No developmental differences NS CBCL school scale (parental Q) |
(474) | ||
|
22 total
10 F 7 M and 5 M with CAH |
35 total
all healthy |
7-17 yrs Median 11 yrs |
NS CBCL school scale (parental Q) Poorer scholastic competence (self-reported) |
NS IQ, but Poorer working memory (WISC-III) NS learning, memory (NEPSY) |
(475) | |
| Same study population | NS behavior (CBCL), or shyness (SPAI-CP). Higher scores sociability (EAS) (parental) more social anxiety (self-reported Q) | (476) | ||||
| Same study population | M reported more neutral behavior KI-GRB (self-reported Q) F, NS |
(477) | ||||
|
Study 1
67 total 51non-CAH (35 F, 16 M) 8 M CAH (8 F full term Dex) Study 2 7 total (1 CAH F Full term Dex) |
73 total
31 F 16 M 15 CAH F 11 CAH M 13 total 2 F 1 M 4 CAH F 6 CAH M |
Study 1
5-12 yrs Study 2 11-24 yrs |
Study 1
Few significant findings K-ABC Sequential Processing positive for M alone P = .095 Study 2 Non-CAH F performed significantly less well on Faces & Places M, NSerence |
(478) | ||
| 9 F | 8 non-CAH 9 CAH non-DEX All F |
Mean 12 yrs But CAH untreated 16 yrs |
NS psychopathology (CBCL) (parental Q) | Lower scores in non-CAH Dex treated F (WAIS-R-PL, WISC-R) |
(479) | |
|
34 total
16 F 18 M |
66 total
36 F 30 M |
7-17 yrs Mean age 10.5 yrs |
Poorer working memory (WISC-III) Sex difference with larger neg effects in F for executive functions and psychometric intelligence (WISC-III, WMS-III) |
(480) | ||
|
34
15 F 19 M |
67 total
36 F 31 M |
7-17 yrs Mean age 10.5 yrs |
CBCL, SPAI-R, and EAS (parental Q) SASC-R (self-reported Q)) |
NS. Generally well adjusted. | (481) | |
|
23 Adults
12 F 11 M |
Population controls
31 F 27 M |
16-24 yrs Mean age 20-21 |
No significant neuropsychological changes; no increase in anxiety, depression or autistic traits. | (482) | ||
|
29 total
12 F 17 M |
37 total
18 F 19 M |
Mean age 16.5-17 yrs |
Methylation in BDNF, FKBP5, and NR3C1 genes were associated with the performance on WAIS | Altered DNA methylation in peripheral CD4+ T-cells | (483) | |
|
16 total
9 F 7 M |
15 total
8 F 7 M |
Mean 24 yrs |
Lower insulin secretion by 17-22%. Lower glucagon |
(484) | ||
|
40 total
18 F 22 M |
75 total
35 F 40 M |
Mean age 16.3 ± 6.2 Age groups Young <16 Older ≥16 |
HOMA-β index, lower β-cell function in younger F Glucose level, higher in younger age group Higher cholesterol, and LDL in older age group |
(485) | ||
|
19 total
9 F 10 M |
43 total
26 F 17 M |
16-26 yrs | MRI Alterations in brain structure Enlarged amygdala, surface area and volume of left sup frontal gyrus, widespread white matter changes |
(481) | ||
| Girls with CAH, Dex-treated until term | ||||||
| 4 | 1 not able to perform neuropsychological testing Generally low IQ |
(475) | ||||
| Study 1 8 Study 2 1 |
Study 1 15 CAH girls Study 2 4 CAH F |
Study 1 5-12 yrs Study 2 11-24 yrs |
Performed more poorly on K-ABC Mental Processing Composite (P = 0.09) Performed (marginally) less well on Hand Movements (subtest Sequential Processing) and Spatial Memory (Simultaneous Processing). |
(478) | ||
| 9 | 8 non-CAH 9 CAH untreated |
Mean 12 yrs and CAH untreated 16 yrs |
NS psychopathology (CBCL) (parental Q) | Higher scores IQ in Dex treated F. Lower in non-CAH Dex treated F (WAIS-R-PL, WISC-R) |
(479) | |
| 4 | 25 F CAH | Diff in self-perceived deficits in executive function (B-DEFS-SF) |
Broad deficits in most measures of cognition in Dex treated F (WAIS-IV, WMS-III) |
(486) | ||
HOMA-β homeostasis model assessment of beta cell function; LDL, low-density lipoprotein; MRI magnetic resonance imaging.
Abbreviations: NS, not significantly different; Dex, dexamethasone; CAH, congenital adrenal hyperplasia; CBCL, Child Behavior Checklist; EAS Temperament Survey for Children; WISC-III, Wechsler Intelligence Scales for Children; NEPSY, Developmental Neuropsychological Assessment; SPPC, Self-Perception SPAI-C-P, Social Phobia and Anxiety Inventory for Children—Parent Report; K-ABC, Kaufman Assessment Battery for Children; WAIS, Wechsler Adult Intelligence Scale; WMS, Wechsler Memory Scale; KI-GRB, The Karolinska Inventory of Gender Role Behavior; B-DEFS-SF, Barkley Deficit in Executive Functioning Scale—Short Form.
a The earlier studies reported results from mixed cohorts, short-term treated boys and girls without CAH, and boys with CAH, while more recent studies have assessed individuals with and without CAH, males and females, separately.
In 2 cohorts of girls with CAH treated throughout gestation, neurocognitive outcomes were negatively affected for mental processing and spatial memory (478) and broad deficits were found in most measures of cognition (486). In contrast, a Polish study reported better cognitive results in general in 9 girls with CAH who were treated throughout pregnancy, but 8 unaffected girls who had been treated with Dex had worse results than controls (479).
Possible imprinting effects of prenatal exposure to Dex have only begun to be explored. Differences in DNA methylation in peripheral CD4+ T cells seemed to be related to sex (483). Of particular interest were methylation effects of the genes BDNF and FKBP5, relevant for the development of the central nervous system, and NR3C1 encoding the glucocorticoid receptor. There were also associations between DNA methylation and performance on cognitive tasks.
Moreover, first trimester Dex exposure of non-CAH fetuses is associated with differences in brain morphology (487). Magnetic resonance imaging (MRI) studies in adults showed enlargement of the amygdala, increased left superior frontal gyrus, and widespread white matter changes. The pathophysiology behind the observed neuropsychological effects of early Dex exposure are largely unknown. Infants prenatally exposed to betamethasone have altered responses of the HPA axis and a higher incidence of mental and behavioral disorders (488, 489).
Negative effects on glucose and lipid metabolism in childhood and in young adulthood have been reported in individuals without CAH but exposed to Dex during the first trimester. Lower insulin secretion, followed by lower glucagon secretion was reported in a French study (484). A lower HOMA-β was reported in the Swedish cohort, significant in girls but not in boys. Plasma glucose levels were higher in the younger treated group with no sex difference. In older adolescents and young adults, total cholesterol and low-density lipoprotein cholesterol were higher in the treated individuals (485). It is unknown if this implies an increased risk of developing metabolic syndrome later in life.
In order to minimize the exposure to Dex, efforts have been made to develop diagnostic techniques using cell-free fetal DNA in maternal blood samples, but these are not yet routinely available (see section “Diagnostics,” “Prenatal diagnosis”) (323). Dose adjustments, with lower doses during the later phases of pregnancy, have been discussed (156) but such studies have not been reported.
New Medical Strategies
The treatment goals for classic CAH include both hormonal replacement and reducing adrenal androgen production. Glucocorticoid therapy is used to achieve both goals, but normalizing adrenal androgen production requires supraphysiologic doses that are higher than required to replace the cortisol deficiency, contributing to comorbidities. Modified and delayed-release HC formulations were discussed in section “Management,” “Hormonal treatment of classic CAH,” “Treatment of adults” (490). Continuous subcutaneous delivery of HC is also suitable for mimicking physiologic cortisol secretion patterns and is useful in patients with rapid cortisol metabolism or impaired gut absorption (491), but this approach is less practical than oral drugs for widespread chronic use (Fig. 5).
Figure 5.
New therapeutic approaches target different aspects of the pathophysiology of CAH. Circadian cortisol replacement with a modified-release glucocorticoid or subcutaneous hydrocortisone infusion aim to control corticotropin-driven hyperandrogenism by replacing cortisol in a physiological manner. Other approaches to reduce androgen production without chronic supraphysiological glucocorticoid exposure include corticotropin-releasing hormone receptor-1 antagonists, adrenocorticotropic hormone (corticotropin, ACTH) antibodies, adrenocorticotropic hormone receptor (MC2R) antagonists, adrenolytic agents, adrenalectomy, and pharmacological inhibition of steroidogenic enzymes or steroid receptors in the adrenal or peripheral tissues. Since CAH owing to 21OHD is a monogenic disorder, gene therapy with cell-based and gene-editing technologies may be able to restore defective steroidogenesis. CRH denotes corticotropin-releasing hormone (sometimes referred to as corticotropin-releasing factor [CRF]). From New England Journal of Medicine, Merke DP, Auchus RJ, Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency, Volume 83, Page 1258. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission.
Alternatively, medications that lower androgen production and/or action can be added to physiologic glucocorticoid therapy, similar to doses used to treat primary adrenal insufficiency. The combination of testolactone (an aromatase inhibitor) and flutamide (an androgen receptor antagonist) with 8 mg/m2/day HC normalized growth and bone maturation in a 2-year randomized trial of 28 children (492). A long-term study of this combination to determine the efficacy of this regimen on improving adult height will soon be completed (NCT00001521). Abiraterone acetate is a potent CYP17A1 inhibitor used to treat prostate cancer (493). When added to HC 20 mg/day, 6 days of treatment with 100 to 250 mg/day of abiraterone acetate normalized androstenedione in 6 adult women (494) with parallel reductions in testosterone, androgen metabolites, and 11-oxo-androgens (495). Abiraterone acetate therapy can cause DOC accumulation and consequent hypertension and/or hypokalemia in patients with prostate cancer via CYP21A2-mediated 21-hydroxylation of intra-adrenal progesterone (495); however, this conversion cannot occur in patients with classic CAH (495). Abiraterone acetate is likely to be most useful in prepubertal children with classic CAH to suppress androgens and estrogens until the anticipated age of puberty, and a phase I trial testing this approach is underway (NCT02574910). Abiraterone acetate monotherapy might cause DOC accumulation in patients with NC CAH if not combined with glucocorticoid therapy or a mineralocorticoid receptor antagonist. Moreover, its use in pubertal girls would require concomitant estrogen treatment, for example with oral contraceptive pills. Third-generation antiandrogens such as enzalutamide, apalutamide, and darolutamide have not been tested in CAH patients but also might be useful treatments in women of reproductive age willing to use contraception.
Agents that reduce the ACTH-mediated drive for androgen production are possible approaches. The binding of CRH to its type 1 receptor (CRHR1) is a major input to corticotropes, raising intracellular cAMP and stimulating ACTH secretion. A single-dose, fixed-sequence study of 8 women given 300 or 600 mg of the CRHR1 antagonist NBI77860 at 22.00 (10 pm) showed significant reductions in ACTH and 17OHP over the ensuing 16 hours relative to a control period during which glucocorticoid treatment was withheld (496). The CRHR1 antagonists tildacerfont and crinecerfont were tested in 14-day continuous-dosing trials (NCT03257462 and NCT04045145, respectively), and tildacerfont therapy was extended in a 3-month trial (NCT03687242). Peer-reviewed results are unavailable as yet. Additional trials are required to further assess the long-term benefits of these treatments. Theoretically, an anti-ACTH antibody (497) or an antagonist of the melanocortin type 2 receptor (MC2R, the ACTH receptor) (498) might also reduce adrenal androgen synthesis in patients with classic CAH, but these approaches have only been tested in preclinical models (499). It should be kept in mind that most of these approaches do not eliminate the need to treat with, and monitor adequacy of, glucocorticoid replacement, albeit perhaps in lower doses.
Unilateral or bilateral adrenalectomy has been suggested as an approach to long-term management of classic CAH to limit adrenal androgens (500). A recent meta-analysis of 48 CAH cases, 34 (71%) described symptomatic improvement after bilateral adrenalectomy but with 5 cases (10%) reporting short-term and 13 cases (27%) long-term adverse outcomes, including an increased risk of adrenal crisis (501). The subsequent development of adrenal rest tumors due to elevated ACTH levels even in women has been reported (502, 503), which defeats the purpose and allows the recrudescence of androgen excess. Consequently, this approach has fallen out of favor (133). Adrenolytic therapy with mitotane has been reported in men with TARTs as an approach to restore fertility (504), but long-term outcomes have not been published. The adrenolytic drug nevanimibe was testing in a dose escalation study of 14-day treatment periods interrupted with 14-day placebo periods, up to 1000 mg twice daily (505). The median 17OHP was consistently lower in treatment periods and rose during placebo periods, consistent with a reversible effect on steroidogenesis, but only 20% met the primary endpoint (17OHP ≤2× upper limit of normal). A study using longer treatment periods in order to achieve greater and more sustained reductions in adrenal-derived androgens was initiated (NCT03669549) but terminated after an interim analysis (https://clinicaltrials.gov/ct2/show/NCT03669549, accessed December 22, 2020). Thus current data do not support the approach of “medical adrenalectomy.”
Growth hormone has been used to improve height in children with CAH (506-508). Growth hormone treatment for a mean duration of 5.6 years achieved nearly adult height in 34 children with CAH (12 NC-CAH patients). In some of the patients, GnRH analogue was also used to delay puberty (507). Controlled studies in larger groups of patients are lacking. Therefore, growth hormone, with or without GnRH analogue therapy, cannot be generally recommended as adjunctive therapy.
Novel Cell- and Gene-based Therapies
Potential cell-based therapies for CAH
Cellular reprogramming is the process whereby a fully differentiated, specialized cell type is forced to acquire a different phenotype that it would not reach under normal physiological conditions. Somatic cells can be induced to dedifferentiate to an embryonic stem cell (ESC)-like phenotype by forcing the expression of specific transcription factors; these cells, termed inducible pluripotent stem cells (iPSCs), are donor-specific and phenotypically highly similar to ESCs (509). An example of cell therapy used ESC- and iPSC-derived pancreatic beta cells for potential treatment for type 1 diabetes (510).
An alternative strategy for reprogramming somatic cells without an intermediate state is through lineage-conversion (also known as direct reprogramming or transdifferentiation), which entails the forced expression of lineage-determining transcription factors (511). Various human and mouse cell types have been used for lineage conversion to an adrenocortical phenotype (512). Adrenocortical-like cells have also been established from cells derived from human skin, blood, and urine cells in humans using a combination of steroidogenic factor-1 (SF-1, NR5A1) expression (through lentiviral delivery) and activation of protein kinase A and gonadotropin-releasing hormone (GnRH) pathways (513). These reprogrammed cells displayed ultrastructural features resembling steroid-secreting cells (such as larger mitochondria with a densely packed inner mitochondrial membrane), de novo expressed steroidogenic enzymes and secreted steroid hormones in response to physiologic (such as ACTH) and pharmacologic (such as nondegradable cAMP-dependent protein kinase A activators) stimuli. They are also viable when transplanted into the mouse kidney capsule or intra-adrenally. Importantly, the hypocortisolism observed in cells reprogrammed from epithelial cells recovered from the urine of patients with CAH (due to mutations in CYP21A2, STAR, HSD3B2, and CYP11A1) was rescued by expressing the wild-type version of the defective enzyme. These studies model CAH in a dish to test personalized interventions. In the future, one could attempt to apply gene-editing on cells reprogrammed from patients to achieve normal steroidogenesis. The same approach could be employed in vivo, through delivery of gene-editing tools to the adrenal with viral vectors. Other, as yet untested, methodologies include establishment of adrenocortical-like cells from iPSCs and adrenocortical organoids capable of self-renewal.
Potential gene-based therapies for CAH
Gene therapy using adeno-associated viruses (AAVs) is an alternative option, tested in an animal model of 21OHD. The active murine gene is named Cyp21a1, while the duplicated pseudogene is Cyp21a2-p. Mice bearing a deletion of approximately 80 kilobases of chromosome 17 (514), including the Cyp21 locus showed perinatal lethality, elevated ACTH, cortical hyperplasia with lack of proper zonation, accumulation of steroid precursors, and both glucocorticoid and mineralocorticoid deficiency (515). Intra-adrenal injection of AAVs carrying human CYP21A2 reverted the CAH-like phenotype for 40 days. A drawback of AAVs is their induction of an inflammatory response, but adrenals of mice treated with gene therapy did not show active inflammation, possibly due to high intra-adrenal levels of glucocorticoids. (515). Restoration of adrenocortical function in Cyp21a1-null mice was also achieved through AAV-mediated delivery of murine Cyp21a1 to the thigh muscles, suggesting that functional 21-hydroxylase enzymatic activity does not have to be confined in the adrenal (516). Intravenous injections of AAVrh10-CAG-human CYP21A2-HA vector endowed with adrenocortical tropism efficiently restored near-normal adrenal function; cells in the zona fasciculata, but not in the zona glomerulosa or capsule, were efficiently transduced 2 weeks after a single AAV injection, and this was concomitant with a reduction of progesterone and ACTH levels (517). However, the restoration of proper steroidogenesis was only transient. A likely explanation of such phenomenon lies within the biology of the gland; through the use of specific transgenic mouse model (lineage tracing) we now know that the adrenal cortex undergoes a self-renewal process, and key paracrine effectors supporting a dynamic centripetal streaming of adrenocortical cells have been identified (518). Adrenocortical self-renewal relies on the differentiation of at least 2 cell populations of progenitor cells, located in capsular (expressing the transcription factor Gli1) and subcapsular compartments (secreting the morphogen Sonic Hedgehog, Shh). These 2 cell populations are able to differentiate and become fully mature steroidogenic cells forming the distinct histological and functional layers of the zona glomerulosa and zona fasciculata. If Cyp21--AAVs are not able to transduce adrenocortical stem/progenitor cells, as suggested from the studies cited above, newly formed steroidogenic cells will therefore be Cyp21a1-deficient and mice revert to a CAH phenotype. In the future, it will be important to determine AAVs serotypes that are able to efficiently transduce stem/progenitor cells in order to offer a long-term curative solution.
In considering the applicability of animal models for gene therapy of 21OHD, it must be kept in mind that mice do not express Cyp17a1 in their adrenal glands and consequently cannot synthesize sex steroid precursors in the adrenals. Thus, mice cannot be used to model the efficacy of suppression of adrenal androgen secretion with gene therapy. Moreover, enzyme kinetics suggest that extra-adrenal expression of CYP21A2 using gene therapy is likely to produce adequate amounts of cortisol only with very high levels of precursor steroids, which means that this approach will be of limited utility in controlling adrenal androgen secretion in humans.
Psychological Risk Factors, Surveillance, and Intervention
Historically, psychological studies in CAH have emphasized the role of prenatal androgens on gender development (ie, gender identity, gender role, and sexual orientation) and other domains exhibiting sex-related variability (eg, cognitive abilities) (519-522). Most studies only concern females with classic CAH, as only they provide opportunities to test hormonal hypotheses of gender development (523). However, this emphasis may promote the belief that atypical gender behavior or nonheterosexual attractions are causes for clinical concern to the extent that they are linked to the pathophysiology of CAH. Historically it has been assumed that men with CAH require little attention directed to their mental health because prenatal androgen exposure is typical of males.
Advances in therapeutics contribute to a disease-specific (“categorical”) approach to care (524, 525). In CAH, this approach has facilitated a fuller understanding of its genetics and pathophysiology and refinement of medical and surgical interventions (133, 526). A disease-specific approach also emphasizes the psychosexual aspects of CAH in affected females (520). Yet, a substantial body of evidence suggests that successful developmental trajectories in people with chronic medical conditions are influenced as much by the psychosocial environment, supports, and organization of healthcare delivery as by the specific nature of the person’s medical condition (524, 525). A more generic (or “noncategorical”) approach emphasizes the effects of repeated hospitalizations on the person’s psychosocial adaptation, irrespective of whether the hospitalizations were for asthma or CAH. Relatively neglected topics in CAH are those routinely addressed in more prevalent conditions, including effects on parenting and family, factors influencing adherence to the medical regimen, frequent doctor visits, impact on the person’s body- and self-image, and transition from pediatric to adult healthcare (Table 10).
Table 10.
Selected generic and CAH-specific risk factors for mental health, psychosocial/psychosexual adaptation and well-being
| Illustrative studies | |
|---|---|
| A. Generic (noncategorical) | |
| Males and females | |
| Challenges to parenting with | (527) |
| accompanying caregiver psychological distress | (528, 529) |
| negative emotional spillover effects from parent to child | (530-532) |
| perceived child vulnerability and overprotectiveness | (533, 534) |
| Burdens of clinic visits and adherence to sometimes complex and changing treatment regimens; emergency room visits and hospitalizations | (535-537) |
| Threats to body-image and self-esteem | (538, 539) |
| Higher rates of missed school and peer victimization | (540, 541) |
| Academic challenges | (542) |
| Problems of psychosocial adaptation (ie, increased psychological symptomatology in youth and adults compared with healthy comparison groups | (540, 543-545) |
| Systemic weaknesses in the process of transitioning from pediatric to adult care | (546-548) |
| Career barriers for people with chronic illness | (549) |
| B. CAH-specific (categorical) | |
| Female-specific | |
| Early reactions to newborn with atypical genitalia (experiences in medical environment) | (547, 550) |
| Stigma (anticipated or experienced) stemming from atypical genitalia and its modulation by culture | (551-561) |
| Tension between person-first (ie, CAH as a medical condition) vs identity-first (intersex and LGBT advocacy); and related human rights perspectives | (562-564) |
| Secrecy | (560, 565-568) |
| Genital examinations and medical photography | (548, 569) |
| Gender of rearing in Prader V cases | (133, 570-572) |
| Genital surgery decision making and | (573) |
| consequences for sexual function | (526) |
| outcomes of postponing surgery | (574) |
| Gender identity | (575-577) |
| Effects on social support | (578, 579) |
| Model of care | (340) |
| Males and females | |
| Terminology | (580-582) |
| Early puberty/attenuated adult height; growth hormone therapy | (33, 507) |
| Neurocognitive sequelae | (486, 583, 584) |
| prenatal dexamethasone | (372, 480, 487) |
| hyponatremic episodes | (585) |
| Fertility problems (testicular adrenal rest tumors in males; low levels of fecundity in females) | (586-590) |
Generic (or noncategorical) factors
Parental reactions to learning that their child has a serious and chronic medical condition—expressed as shock, panic, worry, and sometimes feelings of guilt—are common generic stressors (591). The mental health of patients with CAH is another example; although most studies focus on females, increased psychiatric symptomatology in both sexes mirrors observations for a wide range of chronic medical conditions (545, 592-595).
CAH-specific (or categorical) factors
Patient reactions to repeated genital examinations potentially threaten mental health and well-being (596). Apart from the effects of prenatal androgens on female reproductive anatomy, the influence of early androgen exposure on brain and gender development garners significant attention. Prenatal exposure to testosterone increases the expression of behaviors and interests more typical of males than females. The largest differences between females with CAH and unaffected females are observed for childhood toy preferences and adolescent and adult hobbies and interests. The majority of girls and women with CAH experience a female binary gender identity (576), yet there is evidence that the strength of that identity may be reduced (575, 597). Although the sexual orientation of women with CAH is less likely to be exclusively heterosexual than is true for unaffected women, the majority are heterosexual (225, 598). Though prenatal androgen exposure may play a role in the development of these outcomes, its influence is much smaller than effects on gender-role behavior (521, 594, 597, 599).
Psychological assessment and interventions
In the general pediatric population, the base rate for having a psychiatric disorder at any time is about 20% (600), and is similar in European adults (601, 602), yet many with mental health problems are neither identified nor referred for specialized treatment (603, 604). Specialists treating patients with CAH should consider that many of their patients (and/or their caregivers) may be struggling with mental health problems which can impact the effectiveness of medical care provided. Consequently, regular screening of patient (and family) for risk and resilience factors are indicated along with evaluating the developmental, behavioral, emotional, social, and educational status of the patient as part of ongoing clinical care. Pediatric assessments should also encompass self-perceptions of domain-specific competencies, body image, and experiences of gender typicality and contentedness (605). Comparable surveillance in adulthood is recommended (358). Psychosocial screening should be both general (psychiatric symptoms, coping with illness) and specific (negative body image related to challenges of endocrine management, anticipated or experienced stigma, distress over nonheterosexual interests or behaviors, avoidance of potential romantic relationships as maladaptive coping strategy, sexual dysfunction potentially related to genital surgery, and fertility concerns). Adult healthcare providers need to be comfortable in assessing these topics and refer to knowledgeable specialists who understand the psychological issues in CAH. A recommendation to connect with peer support can also be extremely useful although careful consideration of where to direct patients is warranted (578). There is specific guidance for clinicians regarding the psychological aspects of CAH that warrant evaluation and possible intervention (522).
Because optimal care in CAH involves multiple subspecialties, it is recommended that clinical services be comprehensive and integrated (340, 606, 607), but inclusion of medical psychologists in interdisciplinary healthcare teams for CAH is inconsistent. There are no mental health interventions specifically designed for CAH. Psychoeducational counseling that includes detailed discussion of CAH with the patient and caregivers should be provided in an iterative and developmentally sensitive manner. For girls with genital virilization, such counseling necessarily involves education regarding the process of sex development and the influence of excess androgens on genital growth. A recent Cochrane review of psychological interventions for parents of youth with chronic illness provides clinicians with evidence-based strategies for managing parenting challenges and enhancing psychosocial adaptation in both the parent and the child (608). Interventions to promote treatment adherence in other chronic conditions should be transferable to CAH (609). Although preparation for and assessment of readiness for transition from pediatric to adult care (610, 611) does not guarantee physical health and well-being in adulthood, reports of major morbidities in adult patients with CAH (358) warrant continued efforts to improve outcomes.
Urogenital Surgery
Decisions concerning feminizing surgery
Most girls with classic CAH are born with virilized external genitalia. Virilization may consist of fusion of the outer labia, a single opening of a common urogenital sinus, a recessed vagina that enters into the common channel and clitoromegaly. The degree of virilization is variable, and is influenced by the severity of the enzymatic defect. To indicate the severity of virilization the Prader classification, or similar scales, may be used (Fig. 4). These anatomical variations affect the decisions regarding surgery: the timing, 1- or 2-stage surgery, the technique and the extensiveness of the procedure and risk for complications (612).
Feminizing surgery is often performed in early infancy/childhood in order to provide a female appearance of the genitalia in childhood, and to enable sexual intercourse in adult life. This complex surgery may lead to short- and long-term complications. Early surgery for girls with CAH has become controversial. Many surgeons prefer complete surgical repair at an early age because of good elasticity of the tissue, prevention of possible hydrometrocolpos and reduction of parents’ distress (613). However, concerns have been raised regarding body integrity and the inability of children to provide informed consent for early surgery. Unsatisfactory outcomes regarding genital sensation and sexual function, and greater acceptance of gender nonbinary status have led some to advocate that surgery be postponed until patients can express their gender and wishes (614). However, the effects of growing up with atypical genitalia on mental health or on sexual satisfaction are unknown and may vary in different cultures. The majority of patients and their parents in an American survey endorsed early reconstructive surgery (615). Families should be informed about surgical options including avoiding or delaying surgery. There should be a shared decision-making process including the family, endocrinologist, surgeon, and mental health professionals, and the surgery should be performed by an experienced surgeon (133, 579, 616, 617).
Surgical techniques, outcomes, and complications
Feminizing genitoplasty involves clitoroplasty, opening of the vaginal introitus, and labioplasty. When the patient has a high urethra–vaginal confluence, vaginoplasty may be postponed to later in life. Surgical techniques have been adjusted for best preservation of clitoral sensitivity and least vaginal stenosis (618). However, functional results of the current techniques can only be evaluated after many years.
In tandem with the Endocrine Society’s 2018 Guideline, a systematic review and meta-analysis found no data to support 1 approach over another (526). The data included 29 observational studies (1178 CAH women, mean age at the time of surgery 2.7 ± 4.7 years, mostly classic CAH). After an average follow-up of 10.3 years, the majority who underwent surgery had a female gender identity (88.7%) and were heterosexual (76.2%). Women who underwent surgery reported a lower than optimal Female Sexual Function Index Score of 25.13 out of a maximum possible score of 36, with 26 being the threshold accepted for risk for sexual dysfunction (619). Many patients reported impairment of clitoral sensitivity (620, 621), uncomfortable vaginal penetrative intercourse, and low frequency of intercourse (21). The majority of patients (79.4%) and treating healthcare professionals (71.8%) were satisfied with the surgical outcomes. The most common clinical finding was vaginal stenosis, whereas other surgical complications, such as fistulas, urinary incontinence, and urinary tract infections, were less common (622). Data on quality of life were sparse and inconclusive. To date there are no systematic prospective studies documenting outcomes in girls and women with CAH who did not undergo urogenital reconstruction; until recently most nonoperated girls have been those who were only mildly virilized. Reoperations are usually much less extensive surgical procedures than the initial genitoplasty. Most consist of widening the vaginal introitus; clitoroplasty has also been performed.
There are no studies comparing different techniques of feminizing surgery nor studies comparing early vs late surgery (526). The Endocrine Society’s Guideline (133) cites urogenital mobilization with or without neurovascular-sparing clitoroplasty as the techniques now preferred by many surgeons. No evidence-based guidelines for surgical management exist, and further long-term follow-up studies are needed.
Long-term Sequelae
Gonadal Function in Males
In men with CAH, gonadal and reproductive function are often impaired due to primary gonadal failure from TARTs and/or secondary gonadal failure due to suppressed hypothalamic-pituitary-gonadal axis as a consequence of high adrenal androgen concentrations (376, 435, 436, 623).
Testicular adrenal rest tumors
TARTs are benign testicular tumors typically found in males with classic CAH (376, 435). TARTs have histological similarities to adrenocortical cells and are believed to originate from aberrant adrenal like cells in the testes but the etiology is not yet fully understood (376). TARTs are usually bilateral (70-100% of the cases) and painless (128, 435, 436, 624-629), but discomfort can occur, especially in patients with extensive tumors (630). TARTs less than 2 cm diameter are difficult to detect by palpation (435). Both ultrasound and MRI can be used to detect/confirm TARTs with similar sensitivity down to a few millimeters, but ultrasound costs less (437, 628, 631). The reported prevalence of TARTs in CAH ranges from 14% to 86% (435, 632), with an average of 25% in adolescents, and 46% in men (376). TARTs are found occasionally in patients with NC CAH (435, 437). They occur not only in 21OHD but also in 11β-hydroxylase and 3β-hydroxysteroid dehydrogenase type 2 deficiencies (633, 634).
Elevated ACTH concentrations may play an important role in the development of TARTs. Suppression of ACTH secretion by increased doses of glucocorticoid can decrease the size of TARTs in some cases and may restore fertility (635-637). However, TARTs also occur in well-controlled patients and only a few studies have found a clear association between hormonal control, and TARTs (435, 638, 639). Moreover, there seems to be no correlation between TARTs and bilateral adrenalectomy, a condition that usually leads to high ACTH concentrations (501, 640).
It is important to discriminate Leydig cell tumors from TARTs, due to the malignant potential of Leydig cell tumors, but this cannot be done by either palpation or imaging (630, 641). TARTs are usually bilateral whereas Leydig cell tumors are mostly unilateral and often produce estrogens (376). TART size may decrease in some cases after intensified glucocorticoid dosing (586, 630, 635-637). Additionally, characteristic histologic structures called Reinke crystalloids can sometimes be found in Leydig cell tumors but never in TARTs (376, 630, 631). Furthermore, Leydig cell tumors are very rare in CAH while TARTs are very common.
The central location of TARTs in the testes may result in mechanical obstruction of the seminiferous tubules with azoospermia and irreversible peritubular fibrosis (642). Moreover, the paracrine effects of steroids produced by TARTs may destroy the surrounding Sertoli or germ cells (376). Testis sparing surgery has been described in TARTs but usually does not improve gonadal function, probably owing to irreversible damage to the testis (635). Regular testicular ultrasound is recommended (every 2-5 years if TARTs are small and stable) and if an increased TART burden is found, glucocorticoid therapy should be optimized and cryopreservation of sperm offered (435).
Secondary gonadal failure
Poor hormonal control in CAH results in increased risk of hypogonadotropic hypogonadism (435, 624), because high levels of adrenal androgen precursors will be aromatized to estrogens and suppress the hypothalamic–pituitary–gonadal axis. Steroids produced by TARTs can also suppress gonadotropin secretion (436). Even though most males with CAH and secondary gonadal failure will compensate for reduced testicular testosterone production with increased adrenal testosterone, low testosterone levels are found in some patients (436, 643). Overtreatment with glucocorticoids in men with CAH may also induce gonadal failure (587); optimizing glucocorticoid therapy will usually reverse this.
Paternity
There are few controlled studies of fertility in men with CAH. In a Finnish study of 29 young men with classic CAH a child rate of 0.07 children per adult male was reported, which was significantly lower than the 0.34 in the entire Finnish male population with a similar age distribution (644). In a similar Swedish study of 30 men with CAH the child rate was 0.9 compared with 1.8 in the entire age-matched Swedish population (435). Of 30 US men with CAH only 7% had fathered children (636), and of 22 German men with CAH, 23% had children (587). Of 65 British men with CAH, 25% had become fathers, 2 after fertility treatment, but only 37% had tried to become fathers (358). Finally, of 219 French men with classic CAH, 24% had children (11% after in vitro fertilization), and this fertility rate was lower than the national reference population (624). Men with CAH seem to be less sexually active than matched controls (366). However, of 221 Swedish men with CAH and 22 100 matched controls, only those born before neonatal screening had a reduced child rate (odds ratio 0.5, ie, half as likely to have fathered a child), suggesting that fertility may not be reduced for most men with CAH in the future. Men with NC CAH had a normal child rate and of those who, irrespective of genotype or phenotype, had succeeded in having children, the number of offspring was similar to controls (588). Men with CAH adopted children more often (odds ratio 2.9) (588).
Reproductive Function in Women
CAH affects gonadal function and fertility in women. In general, there is an association between the severity of the CAH phenotype and the level of gonadal dysfunction and fertility (623).
Pubertal development
Age of menarche is normal in well-controlled girls, with no difference between SW, SV, and NC CAH (34, 354, 370, 423, 645). However, when glucocorticoid therapy is withheld or inadequate, menarche is delayed (646). Irregular menstrual patterns in CAH are associated with other hyperandrogenic signs such as acne and hirsutism and signs of insulin resistance. This clinical picture closely resembles polycystic ovary syndrome (427). Sonographic findings of polycystic ovarian appearance have been reported in adult women with CAH (about 20-50%) and in a minority of adolescent patients (647-651). Breast development can be impaired in case of inadequate androgen control (646, 652). The European multicenter dsd-LIFE study showed that only 68% of adults with CAH had reached Tanner stage B5 compared with 90% in women without DSD (653).
Fertility
Compared with age-matched controls, women with CAH have fewer pregnancies and children. In a Finnish study, the mean child rate was 0.34 vs 0.91 in the general Finnish female population, and was lower in SW than in non-SW women (654). In a Swedish study, the number of pregnancies was 50% lower than in age-matched controls (370); 16 of 19 women who attempted pregnancy succeeded in becoming pregnant and there was a clear relationship between more severe genotypes and fewer children. Of 106 CAH patients in the UK, 25 considered motherhood and 23 had actively attempted conception, of whom 21 achieved 34 pregnancies (589). The pregnancy rate in this subgroup was similar to that in the normal UK population (95%), and similar in the SW (88.9%) and non-SW (92.9%) subgroups. However, women with SW were less likely to seek motherhood. More recently, the dsd-LIFE study reported that only 14.7% of 221 CAH women had 1 or more children without assisted reproduction techniques (ART), and 1.9% with ART (655). In a recent Swedish epidemiological study using the national CAH registry, 272 females with CAH (aged 14 years or above) were compared with 27 200 matched controls (656). Only 25.4% of women with CAH had given birth compared with 45.8% of controls. Furthermore, mothers with CAH were older and had fewer children.
All studies have emphasized that the major cause for low child rates is that women with CAH are less likely to seek motherhood. Women with the SW phenotype show the lowest interest in motherhood. This may be caused by the effects of prenatal androgen exposure on gender role behavior, including reduced interest in infants (590, 657), the lack of a partner, dissatisfaction with genital appearance, decreased sexual satisfaction, and urogenital and sexual dysfunction as a result of corrective surgery (363). When patients attempt pregnancy, the success rate seems to have increased in the last twenty years, as a result of various factors, including increased understanding of the effect of androgen and progesterone levels, and the level of mineralocorticoid substitution (35).
Optimizing fertility in women
A large review of case reports of women with classic CAH included 159 pregnancies since 1999. In 84 pregnancies the mode of conception was reported, and 62/84 pregnancies were spontaneous (363). When pregnancy is attempted and especially when spontaneous conception fails, the first approach is to optimize glucocorticoid therapy, aiming at normal androgen and follicular-phase progesterone levels (589, 658, 659). Second, optimizing mineralocorticoid treatment appears to improve fertility SW and SV patients (363, 589, 660), but the exact mechanism remains unknown.
If needed, and especially when the above approaches are unsuccessful, assisted reproduction techniques can be used for ovulation induction and conception (661, 662).
Most (53-68%) women with NC CAH conceive spontaneously without any treatment (647, 663). Of 190 women with NC CAH, 95 wanted pregnancy and 187 pregnancies occurred in 85 women. Of these pregnancies, 99 occurred before the diagnosis of NC CAH (96/99 spontaneous), and 88 (47%) after the diagnosis (11/88 spontaneous) (647). Therefore, in case of subfertility (or recurrent miscarriages) there is a clear indication for temporary glucocorticoid treatment in NC CAH (37, 133). Glucocorticoid treatment shortens the time to pregnancy from about 1 year to less than 6 months (664). If conception cannot be achieved with glucocorticoids, ovulation induction is usually successful. The course of pregnancy is usually uneventful; however, the miscarriage rate in women with NC CAH is substantially higher (25%) than in the general population (6%) in some (647, 663) but not all (664) studies. The miscarriage rate can be reduced to normal in women treated with low to moderate doses of HC (647), prednisolone, or prednisone (663) prior to and during pregnancy.
Pregnancy outcome
Pregnancy outcome is good in women with CAH (363). Placental aromatase activity protects the fetus from maternal androgens (100). Gestational diabetes has been described relatively frequently (370, 656). Adjustments in glucocorticoid (and fludrocortisone) dose are usually necessary, especially in the third trimester (133), similar to pregnancies in women with primary adrenal insufficiency (665, 666). In the offspring, the rate for small for gestational age seems to be increased in some (363), but not all (656) studies, and no other problems are seen at follow-up (667).
Cardiovascular and Metabolic Morbidity
Metabolic consequences
The prevalence of overweight and obesity are greater in adults with CAH in the UK (358) and Sweden (668) but similar to the general population in the US (215) and France (624, 669). Increased abdominal adiposity, with a higher proportion of proinflammatory visceral adipose tissue compared with subcutaneous adipose tissue, was present in adolescents and young adults with CAH compared with age-, sex-, and BMI-matched controls (670). Metabolic syndrome was observed in nearly 20% of adults in the NIH’s cross-sectional study cohort (215), associated with older age but not with androgens, glucocorticoid type, or dose. The Endocrine Society’s systematic review of relevant literature published through early 2016 included 20 observational studies (14 longitudinal, 6 cross-sectional) with a moderate to high risk of bias (671). The average dose of glucocorticoids (in HC equivalents) was 9 to 26.5 mg/m2/day. In the meta-analysis (416 patients, 14 months-63 years old), compared with controls, individuals with CAH had increased values for the homeostatic model assessment of insulin resistance (HOMA-IR; weighted mean difference [WMD] 0.49; 95% CI 0.02-0.96); however, no differences were noted in fasting blood glucose, insulin level, and glucose or insulin level after 2-hour glucose load, or serum lipids.
Blood pressure
Some studies report normal resting (215, 624, 672) and 24-hour blood pressure profiles (673) whereas others report a slight increase in either diurnal or both diurnal and nocturnal systolic blood pressure compared with matched controls even in childhood (674, 675). There are minimal data on the prevalence of hypertension in adults with CAH (676, 677), with inconsistent results in individual studies conducted in different locales (624, 668, 678). The systematic review and meta-analysis (671) found that individuals with CAH had modestly increased systolic blood pressure (WMD 4.44 mmHg; 95% CI 3.26-5.63 mmHg) and diastolic blood pressure (WMD 2.35 mmHg; 95% CI 0.49-4.20 mmHg). The authors were unable to draw conclusions regarding the effects of several important variables such as sex, glucocorticoid type and dose, fludrocortisone dose, and genotype, and bias in the individual reports was moderate to high.
Cardiovascular consequences
Cardiovascular morbidity and mortality are difficult to assess in CAH, as few of the studied patients are older than 50 years (679). Results for carotid intima media thickness (cIMT), a surrogate marker of cardiac dysfunction, vary in existing studies (674, 675, 680), without correlation between cIMT and cumulative glucocorticoid doses or androgen levels (680). A systematic review and meta-analysis showed slight but significantly greater carotid intima thickness (WMD 0.08 mm; 95% CI 0.01-0.15 mm) (671). In adolescent and adult CAH patients, normal left ventricular morphology has been reported (674, 681), but mild diastolic dysfunction and impaired exercise performance were shown. Recently, a French group reported the complex interactions between gonadotropins and steroid hormones on the duration of ventricular repolarization. QT interval duration was shorter in women with CAH than in control women (682). A Swedish study analyzed cardiovascular and metabolic morbidity in CAH patients, finding increases in both cardiovascular and metabolic disorders including higher frequencies of hypertension, dyslipidemia, and atrial fibrillation (668). Obesity was consistently increased in all subgroups while diabetes was increased in females, SV and NC phenotypes, and those above 40 years of age. However, the nonobese patients were similarly affected by hypertension and diabetes as the entire CAH cohort. This study also found an increased frequency of venous thromboembolic events, which should be studied further to determine if, as reported in both Cushing syndrome and glucocorticoid use, there is a higher risk of venous thromboembolism due to hypercoagulability that should prompt a lower threshold for thrombosis prophylaxis in this population.
Thus, CAH may be associated with higher cardiovascular risk (683, 684). Increased cardiovascular mortality has been reported in CAH in Sweden, second only to adrenal crisis as a cause of death (366). Data on cardiac events are sparse, and most of the literature has focused on surrogate outcomes, rather than episodes of acute myocardial infarction, heart failure, or death. Some subgroups of patients seem to be more affected by cardiovascular risk factors. Regular follow-up is needed, along with lifestyle interventions, to limit weight gain, prevent obesity, and screen for diabetes (especially gestational diabetes), and dyslipidemia. Close monitoring of glucocorticoid and mineralocorticoid doses is important. Further prospective studies on larger cohorts are necessary to clarify the mechanisms leading to metabolic and cardiovascular abnormalities, and to understand the respective roles of adrenal sex hormones, lifelong glucocorticoid and mineralocorticoid treatment (364), and the impact of genetic background, such as glucocorticoid receptor gene polymorphisms, and other loci contributing to adverse cardio-metabolic risk profiles (685).
Neurological aspects
Early hormonal alterations affect the development of mammalian neural circuits. Widespread expression of androgen and glucocorticoid receptors in the brain suggest that fetal and postnatal imbalances in androgen and glucocorticoid exposure characteristic of CAH might influence brain development and function, with the potential to impact mental health (686). Compared with controls, patients with classic CAH have higher prevalence of anxiety, depression, alcohol misuse, suicidality, and adjustment disorders (392, 545, 595) (also see section “Management,” “Psychological risk factors, surveillance, and intervention”). Males diagnosed beyond the neonatal period and women with the most severe null genotype are especially at risk for mental health issues (545, 592, 595).
Neuroimaging studies in patients with CAH have revealed alterations in brain structure and function. In a functional MRI study of 14 adolescents with classic CAH compared with age-matched controls, girls with CAH showed a similar pattern of amygdala activation to control boys, suggesting an androgen effect on amygdala function in girls with CAH (687). Glucocorticoid therapy has been implicated in the development of white matter hyperintensities which reflect reduction of white matter structural integrity (688). White matter hyperintensities are found in patients with CAH, but are an uncommon finding in healthy adults aged <45 years (689, 690). Glucocorticoid therapy in CAH has been reported to affect working memory and digit span scores; patients on higher glucocorticoid doses have worse performance (688). Memory impairment is similarly found among patients with Cushing disease and Cushing syndrome (691).
Structural differences in gray matter morphometry in the medial temporal lobe were found in a cross-sectional MRI study of 27 adolescents with CAH (692). Young people with classic CAH had smaller regional volumes in the prefrontal cortex, amygdala, and hippocampus and overall smaller brain volumes than age-matched controls. In a study of 37 young adults with CAH, alterations in gray matter structure, including the middle frontal gyrus and the parietal and superior occipital cortex were found in CAH patients compared with controls (693). These regions play a role in visuospatial working memory and patients performed worse in visuospatial working memory tasks.
All of the neuroimaging studies are hindered by small sample size (686). Decreased brain volume has been observed in patients compared with controls in multiple studies and this needs to be accounted for when evaluating individual brain regions. Moreover, sex matching is essential since human male/female differences have been found in total brain volume (694), gray matter brain volume in specific regions (695) and brain connectivity (696); sexual dimorphism of the brain has also been found during childhood (697) (reviewed in (686)). In CAH, there are multiple hormonal imbalances including in utero glucocorticoid deficiency and androgen excess, postnatal androgen excess and iatrogenic glucocorticoid excess, and epinephrine deficiency, all possibly occurring during different developmental periods and with varying potential impact on neural circuits.
Bone
Since patients with CAH are on lifelong glucocorticoid supplementation, reduced BMD and osteoporosis are potential long-term outcomes. Epidemiological and other studies have demonstrated that glucocorticoids cause secondary osteoporosis and increase fracture risk (698, 699). Both direct and indirect effects by glucocorticoids on bone result in initial increased resorption and later deceased bone formation leading to micro-architectural distortion and fracture risk (700). Moreover, glucocorticoids may cause secondary hyperparathyroidism by decreasing intestinal calcium absorption and increasing renal calcium excretion. Despite the known negative effects of glucocorticoids on BMD, studies with patients with CAH have reported inconsistent finding. A few studies have reported normal (701-706) or even high BMD (707), but most have shown low BMD at all or at least some sites (207, 215, 358, 643, 708-719). These differences may be due to both glucocorticoid and androgen exposure, since androgens stimulate osteoblast proliferation and differentiation in both genders (720). Adrenal androgens, including DHEAS, affect bone metabolism throughout life, especially during adrenarche, with effects mainly on cortical bone (721). Thus, late diagnosis and/or poor hormonal control may improve BMD due to high androgen concentrations (643, 715). Moreover, different glucocorticoid regimens may affect BMD differently; HC seems to affect BMD less than longer acting glucocorticoids, especially Dex (367). A recent meta-analysis comparing patients with CAH and matched controls found slightly decreased BMD in patients with CAH (722). Furthermore, adult women with CAH had more fractures than matched controls (715) whereas men with CAH did not (643). Patients with classic CAH had more nontraumatic fractures than those with NC CAH (721). However, osteoporosis-related fractures typically occur after 50 years of age and very few older patients have been included in studies of BMD and fractures. BMD screening is recommended by the Endocrine Society in adults with CAH and a prolonged period of higher than average glucocorticoid dosing, or in patients who have had a nontraumatic fracture (133). Others have also suggested screening any patient upon transfer to adult care and every 2 to 5 years thereafter (679).
Adrenal tumors
Approximately 20% to30% of adult patients with CAH have adrenal masses (723). Almost a quarter of these are benign adrenal myelolipomas, which generally occur in patients with a history of poor hormonal control, suggesting that persistent ACTH stimulation may play a role in pathogenesis (626, 723, 724). There is no evidence that adrenocortical carcinoma, a rare malignancy with poor prognosis, is more prevalent in CAH. Adrenocortical carcinomas can be distinguished from benign adrenal masses by their characteristic steroid profile as assessed with mass spectrometry-based methods (725).
CAH in Developing Countries—Challenges and Limitations
CAH management in developing countries is challenging. Newborn screening for CAH is not available in many developing countries (726, 727), delaying diagnosis and increasing mortality, particularly in boys who lack atypical genitalia (728, 729). Pediatric endocrinologists are scarce (558, 727, 730), and late referral to specialized centers may delay diagnosis and treatment. Hormonal assays for diagnosis and follow-up have limited availability and are expensive (727, 730). Needed medications may be available only on the black market (136, 554, 730). Delayed diagnosis (555, 731), emotional (728), and gender assignment problems (729, 732) negatively influence quality of life (558, 733).
There are also socioeconomic and cultural issues (730). Myths and misconceptions about ambiguous genitalia in certain communities may lead to discrimination against patients and families (554, 558). Gender reassignments in late-identified patients may be met with resistance or refusal because of social stigma and cultural pressure (727). Moreover, many developing countries also face poverty and insufficient basic medical knowledge (554, 730). These issues imply the need for better primary healthcare education. Educational materials in the local language may increase understanding of CAH among families and communities. Clinical guidelines for developing countries are needed, along with advocacy to encourage government policy to improve access to essential medications and implementation of newborn screening.
Future Directions
Basic Science
As discussed previously, there has been much recent progress in adrenal steroidogenesis as regards the alternative “backdoor” pathway to androgens and the importance of 11-oxo-androgens. Other unanswered questions in steroidogenesis remain (summarized in (42)). Areas requiring further study include more detailed understandings of how StAR imports cholesterol to the mitochondria inner membrane, and how the 17,20-lyase activity of CYP17A1 is regulated. Secretion of androgens and androgen precursors by the fetal adrenal gland is a key component of the pathophysiology of CAH, yet regulation of fetal adrenal growth and postnatal involution of the fetal zone are poorly understood, and teleologically it is unclear why primate adrenal glands normally secrete DHEA and androgens either prenatally or at adrenarche. Steroid synthesizing enzymes, including CYP21A2, are found in nonglandular tissues, but the functional significance of extraglandular steroidogenesis remains uncertain.
Clinical Management
Given the relative rarity of CAH, national and international registries are valuable in developing and testing best practices throughout the lifespan (734). Whereas it may be unrealistic to expect that every clinical site caring for CAH patients possesses a comprehensive, multidisciplinary team, networks of expert centers can ensure access to specialty care when necessary. Criteria defining a comprehensive expert level of care for CAH have been published (340, 735). Surveys show that patient satisfaction, provider training, research, and quality improvement activities vary among medical centers (736, 737); thus, there is a need for clinical benchmarks in management. Real world data including patient and family satisfaction, as well as peer observation of clinical care can help develop guidelines and decision support tools. By providing robust data on epidemiology, patients’ characteristics, and current standard of care, registries have the potential to shape healthcare policy and, by engaging with patients, increase stakeholder involvement and improve the patient-centered experience (738). One example of outcomes from the I-CAH Registry has been to define acute adverse events associated with adrenal insufficiency including sick day episodes, adrenal crises, and hospitalizations among CAH patients (399). The challenge for rare disease registries is to ensure that the data represent the widest range of patients, and that the data are findable, accessible, interoperable, and reusable (FAIR) within a rigorous framework of data governance, integrated with other data sources through multiomics technology (739). With anticipated therapeutic advances over the next decade, the use of registries for measuring therapeutic effectiveness, as well as maintaining clinician and patient engagement, will become imperative (399). Given the relative rarity of CAH, national and international registries are valuable in developing and testing best practices throughout the lifespan (734). As it may be unrealistic to expect that every clinical site caring for CAH patients possesses a comprehensive, multidisciplinary team, networks of expert centers can ensure access to specialty care when necessary. Criteria defining a comprehensive expert level of care for CAH have been published (340, 735). Surveys show that patient satisfaction, provider training, research, and quality improvement activities vary among medical centers (736, 737), thus there is a need for clinical benchmarks in disease management. (399, 738). Other areas that could benefit from large-scale collaborative data collection include prenatal and neonatal diagnosis and treatment. With recent data pointing to potential serious adverse outcomes, long-term follow-up studies should closely monitor both CAH patients and unaffected siblings subjected to prenatal Dex treatment. As discussed in section “Diagnostics,” “Neonatal screening,” the suboptimal positive predictive value for immunoassay in many newborn screening programs mandates further studies to determine the most cost effective strategies to improve screening sensitivity and specificity. Clinical trials for novel drug targets and potential gene therapy are in progress or planned that should provide additional treatment options. At the same time, more widespread availability of mass spectrometry-based assays for new steroid biomarkers, such as 11-oxo-androgens, may improve monitoring and titrating existing medication regimens.
Long-term management should emphasize the importance of a smooth transition from pediatric to adult medical care, with continued emphasis on risk assessment for adverse reproductive, psychosexual, cardiovascular, metabolic, and musculoskeletal outcomes. To this end, implementation of telemedicine services have lately been recognized as a valuable resource in managing patients living in remote areas or lacking access to specialty centers.
Much discourse and debate has centered on whether and when surgical intervention ought to be considered. A systematic review and meta-analysis found scant sound evidence to favor early surgery, delayed surgery, or no surgery (526). More work is needed to develop evidence-based guidelines for surgical treatment of CAH, including ideal timing of surgery, surgical technique, risk of incontinence, risk of additional surgery (such as repair of vaginal stenosis at puberty), risk of loss of sexual function, and extent of clitoral surgery. Given that the likelihood of performing randomized controlled trials in this area is minimal, long-term surveillance using commonly agreed and routinely collected clinical and patient reported outcome measures should be prioritized.
Not least among desired goals is for mental health professionals in collaboration with other specialists to develop and validate quality of life instruments specific to CAH. In summary, based on what has been learned from collective clinical and basic research, the outlook is optimistic for improved modes of CAH treatment and consequently better quality of life.
Acknowledgments
P.T. would like to thank Professor A. Bachelot for her role as investigator in different studies and Jerome Dulon for his technical assistance. We would like to thank Lukas Claahsen for preparing the graphical abstract.
Financial Support: H.C. received grant support from the International Funding CAH (IFCAH) and the Innovatiefonds zorgverzekeraars for CAH related research. P.W.S. received grant support from the National Institutes of Health (R01HD093450 subcontract). H.F. received grant support from Magnus Bergvall Foundation. L.G. received grant support from the BBSRC, Barts Charity, IFCAH and Rosetrees Trust. A.H. received grant support from the Deutsche Forschungsgemeinschaft (DFG, 314061271 CRC-TRR205). This research was supported in part by the Intramural Research Program of the NIH (D.P.M.). A.N. received grant support from Stockholm County Council and Karolinska Institutet. N.R. received grant support from the Deutsche Forschungsgemeinschaft (DFG Heisenberg Professorship 325768017 and 314061271 CRC-TRR205). D.E.S. received support from R01 HD093450 (the DSD Translational Research Network) and R01 HD086583 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. S.A.W. acknowledges support from DFG grant WU 148/7-1 and -2). P.C.W. is the Audry Newman Rapoport Distinguished Chair in Pediatric Endocrinology, and received grant support from the National Institutes of Health (U01-HD083493).
Glossary
Abbreviations
- 11KT
11-ketotestosterone
- 11OHD
11-hydroxylase deficiency
- 17OH-Preg
17-hydroxypregnenolone
- 17OHD
17-hydroxylase deficiency
- 17OHP
17-hydroxyprogesterone
- 21OHD
21-hydroxylase deficiency
- AAV
adeno-associated virus
- ACTH
adrenocorticotropic hormone, corticotropin
- BMD
bone mineral density
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- cAMP
cyclic adenosine monophosphate
- cIMT
carotid intima media thickness
- COUP-TFII
Chicken Ovalbumin Upstream Promotor-Transcription Factor-2
- CRF
corticotropin-releasing factor
- CRH
corticotropin-releasing hormone
- CYP21A2
21-hydroxylase
- CYP19A1
Aromatase
- Dex
dexamethasone
- DHEA
dihydroeoiandrostenedione
- DHEAS
dehydroepiandrosterone sulfate
- DELFIA
dissociation-enhanced lanthanide fluoroimmunoassay
- DHT
5-dihydrotestosterone
- DOC
11 deoxycorticosterone
- DSD
differences in sex development
- ESC
embryonic stem cell
- FSH
follicle-stimulating hormone
- GC
gas chromatography
- GnRH
gonadotropin-releasing hormone
- HC
hydrocortisone
- HOMA-β
homeostatic model assessment
- HSD3B
3β-hydroxysteroid dehydrogenase
- HSD17B
17β-hydroxysteroid dehydrogenase
- IL
interleukin
- iPSC
inducible pluripotent stem cell
- LC
liquid chromatography
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LH
luteinizing hormone
- MC2R
adrenocorticotropic hormone receptor
- MLPA
multiplex ligation-dependent probe amplification
- MR
mineralocorticoid receptor
- MRI
magnetic resonance imaging
- MS
mass spectrometry
- NC
nonclassic
- OMM
outer mitochondrial membrane
- PGD
preimplantation genetic diagnosis
- POR
P450 oxidoreductase
- TARTs
testicular adrenal rest tumors
- SF-1
steroidogenic factor-1
- SRD5A1
5α-reductase type 1
- StAR
steroidogenic acute regulatory protein
- SV
simple virilizing
- SW
salt wasting
- TNXB
tenascin-X
- WMD
weighted mean difference
Additional Information
Disclosures: S.F.A. has received unrestricted educational grants from Diurnal and Neurocrine Biosciences. P.W.S. has served as a consultant to Neurocrine Biosciences, Adrenas Therapeutics, OMASS Therapeutics, Certara and Gerson Lehman Group, and has received lecture fees from Sandoz/ Novartis. C.E.F. has previously consulted for Adrenas Therapeutics, Inc. W.A. consults for Diurnal Ltd and Spruce Biosciences. R.J.A. has previously consulted for Neurocrine Biosciences and Spruce Biosciences; consults for Adrenal Therapeutics, OMass Therapeutics, and Crinetics Pharmaceuticals; and receives contracted research support (2013-current) from Neurocrine Biosciences and Spruce Biosciences. H.C. has consulted for Neurocrine Bioscience and Spruce Bioscience. A.N. received lecture fees from Merck and has previously consulted for Diurnal. N.P.K. has consulted for Neurocrine Biosciences, Inc., Diurnal Ltd., Novo Nordisk, and Adrenas Therapeutics. N.P.K. also reports speaker fees from Sandoz, Merck, and Novo Nordisk. N.P.K. has received grant support from the Medical Research council, UK, International Fundraising CAH (IFCAH), and the Deutsche Forschungsgemeinschaft (DFG; KR3363/3–1). A.H. received grant support (IIT study) from Pfizer Pharma GmbH. WLM has previously consulted for Spruce Biosciences, Adrenas Therapeutics, EicOsis LLC, and BioMarin Pharmaceutical Inc. D.M. received unrelated research funds from Diurnal Limited through the National Institutes of Health Cooperative Research and Development Agreement. N.R. has consulted for Diurnal. HF has consulted for Neurocrine Biosciences, Inc., Diurnal Limited, Roche Diagnostics International Ltd and Adrenas Therapeutics. P.C.W. previously obtained investigator-initiated research support from Janssen Pharmaceuticals; he consults for Neurocrine Biosciences and Crinetics Pharmaceuticals, and receives contracted research support from Neurocrine Biosciences.
References
- 1. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21(3):245-291. [DOI] [PubMed] [Google Scholar]
- 2. Gidlöf S, Falhammar H, Thilén A, et al. One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2013;1(1):35-42. [DOI] [PubMed] [Google Scholar]
- 3. Gruñieiro-Papendieck L, Chiesa A, Mendez V, Prieto L. Neonatal screening for congenital adrenal hyperplasia: experience and results in Argentina. J Pediatr Endocrinol Metab. 2008;21(1):73-78. [DOI] [PubMed] [Google Scholar]
- 4. Gleeson HK, Wiley V, Wilcken B, et al. Two-year pilot study of newborn screening for congenital adrenal hyperplasia in New South Wales compared with nationwide case surveillance in Australia. J Paediatr Child Health. 2008;44(10):554-559. [DOI] [PubMed] [Google Scholar]
- 5. Shetty VB, Bower C, Jones TW, Lewis BD, Davis EA. Ethnic and gender differences in rates of congenital adrenal hyperplasia in Western Australia over a 21 year period. J Paediatr Child Health. 2012;48(11):1029-1032. [DOI] [PubMed] [Google Scholar]
- 6. Nascimento ML, Cristiano AN, Campos Td, et al. Ten-year evaluation of a Neonatal Screening Program for congenital adrenal hyperplasia. Arq Bras Endocrinol Metabol. 2014;58(7):765-771. [DOI] [PubMed] [Google Scholar]
- 7. Silveira EL, dos Santos EP, Bachega TA, van der Linden Nader I, Gross JL, Elnecave RH. The actual incidence of congenital adrenal hyperplasia in Brazil may not be as high as inferred–an estimate based on a public neonatal screening program in the state of Goiás. J Pediatr Endocrinol Metab. 2008;21(5):455-460. [DOI] [PubMed] [Google Scholar]
- 8. Pezzuti IL, Barra CB, Mantovani RM, Januário JN, Silva IN. A three-year follow-up of congenital adrenal hyperplasia newborn screening. J Pediatr (Rio J). 2014;90(3):300-307. [DOI] [PubMed] [Google Scholar]
- 9. Kopacek C, de Castro SM, Prado MJ, da Silva CM, Beltrão LA, Spritzer PM. Neonatal screening for congenital adrenal hyperplasia in Southern Brazil: a population based study with 108 409 infants. BMC Pediatr. 2017;17(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhong K, Wang W, He F, Wang Z. The status of neonatal screening in China, 2013. J Med Screen. 2016;23(2):59-61. [DOI] [PubMed] [Google Scholar]
- 11. Gong LF, Gao X, Yang N, Zhao JQ, Yang HH, Kong YY. A pilot study on newborn screening for congenital adrenal hyperplasia in Beijing. J Pediatr Endocrinol Metab. 2019;32(3):253-258. [DOI] [PubMed] [Google Scholar]
- 12. Dumic K, Krnic N, Skrabic V, et al. Classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency in Croatia between 1995 and 2006. Horm Res. 2009;72(5):310-314. [DOI] [PubMed] [Google Scholar]
- 13. González EC, Carvajal F, Frómeta A, et al. Newborn screening for congenital adrenal hyperplasia in Cuba: six years of experience. Clin Chim Acta. 2013;421(6):73-78. [DOI] [PubMed] [Google Scholar]
- 14. David J, Chrastina P, Pešková K, et al. Epidemiology of rare diseases detected by newborn screening in the Czech Republic. Cent Eur J Public Health. 2019;27(2):153-159. [DOI] [PubMed] [Google Scholar]
- 15. Coulm B, Coste J, Tardy V, et al. ; DHCSF Study Group . Efficiency of neonatal screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency in children born in mainland France between 1996 and 2003. Arch Pediatr Adolesc Med. 2012;166(2):113-120. [DOI] [PubMed] [Google Scholar]
- 16. Odenwald B, Dörr HG, Bonfig W, et al. Classic congenital adrenal hyperplasia due to 21-hydroxylase-deficiency: 13 years of neonatal screening and follow-up in Bavaria. Klin Padiatr. 2015;227(5):278-283. [DOI] [PubMed] [Google Scholar]
- 17. Kaur G, Thakur K, Kataria S, et al. Current and future perspective of newborn screening: an Indian scenario. J Pediatr Endocrinol Metab. 2016;29(1):5-13. [DOI] [PubMed] [Google Scholar]
- 18. Pode-Shakked N, Blau A, Pode-Shakked B, et al. Combined gestational age- and birth weight-adjusted cutoffs for newborn screening of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2019;104(8):3172-3180. [DOI] [PubMed] [Google Scholar]
- 19. Morikawa S, Nakamura A, Fujikura K, et al. Results from 28 years of newborn screening for congenital adrenal hyperplasia in Sapporo. Clin Pediatr Endocrinol. 2014;23(2):35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsuji A, Konishi K, Hasegawa S, et al. Newborn screening for congenital adrenal hyperplasia in Tokyo, Japan from 1989 to 2013: a retrospective population-based study. BMC Pediatr. 2015;15(12):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kreukels BPC, Cohen-Kettenis PT, Roehle R, et al. Sexuality in adults with differences/disorders of sex development (DSD): findings from the dsd-LIFE Study. J Sex Marital Ther. 2019;45(8):688-705. [DOI] [PubMed] [Google Scholar]
- 22. Heather NL, Seneviratne SN, Webster D, et al. Newborn screening for congenital adrenal hyperplasia in New Zealand, 1994-2013. J Clin Endocrinol Metab. 2015;100(3):1002-1008. [DOI] [PubMed] [Google Scholar]
- 23. Güran T, Tezel B, Çakır M, et al. Neonatal screening for congenital adrenal hyperplasia in Turkey: outcomes of extended pilot study in 241 083 infants. J Clin Res Pediatr Endocrinol. 2020;12(3):287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al Hosani H, Salah M, Osman HM, et al. Expanding the comprehensive national neonatal screening programme in the United Arab Emirates from 1995 to 2011. East Mediterr Health J. 2014;20(1):17-23. [PubMed] [Google Scholar]
- 25. Al-Harbi T, Al-Shaikh A. Apparent mineralocorticoid excess syndrome: report of one family with three affected children. J Pediatr Endocrinol Metab. 2012;25(11-12):1083-1088. [DOI] [PubMed] [Google Scholar]
- 26. Larrandaburu M, Matte U, Noble A, et al. Ethics, genetics and public policies in Uruguay: newborn and infant screening as a paradigm. J Community Genet. 2015;6(3):241-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Kamp HJ, Oudshoorn CG, Elvers BH, et al. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J Clin Endocrinol Metab. 2005;90(7):3904-3907. [DOI] [PubMed] [Google Scholar]
- 28. Martinerie L, Pussard E, Foix-L’Hélias L, et al. Physiological partial aldosterone resistance in human newborns. Pediatr Res. 2009;66(3):323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gattineni J, Baum M. Developmental changes in renal tubular transport-an overview. Pediatr Nephrol. 2015;30(12):2085-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Straaten S, Springer A, Zecic A, et al. The external genitalia score (EGS): a European multicenter validation study. J Clin Endocrinol Metab. 2020;105(3):e222-e230. [DOI] [PubMed] [Google Scholar]
- 31. Zhao F, Franco HL, Rodriguez KF, et al. Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science. 2017;357(6352):717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383(13):1248-1261. [DOI] [PubMed] [Google Scholar]
- 33. Muthusamy K, Elamin MB, Smushkin G, et al. Clinical review: adult height in patients with congenital adrenal hyperplasia: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2010;95(9):4161-4172. [DOI] [PubMed] [Google Scholar]
- 34. Völkl TM, Öhl L, Rauh M, Schöfl C, Dörr HG. Adrenarche and puberty in children with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2011;76(6):400-410. [DOI] [PubMed] [Google Scholar]
- 35. Gomes LG, Bachega TASS, Mendonca BB. Classic congenital adrenal hyperplasia and its impact on reproduction. Fertil Steril. 2019;111(1):7-12. [DOI] [PubMed] [Google Scholar]
- 36. Mendes-Dos-Santos CT, Martins DL, Guerra-Júnior G, et al. Prevalence of testicular adrenal rest tumor and factors associated with its development in congenital adrenal hyperplasia. Horm Res Paediatr. 2018;90(3):161-168. [DOI] [PubMed] [Google Scholar]
- 37. Falhammar H, Nordenström A. Nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency: clinical presentation, diagnosis, treatment, and outcome. Endocrine. 2015;50(1):32-50. [DOI] [PubMed] [Google Scholar]
- 38. Hannah-Shmouni F, Morissette R, Sinaii N, et al. Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians. Genet Med. 2017;19(11):1276-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2017;165(Pt A):18-37. [DOI] [PubMed] [Google Scholar]
- 41. Selvaraj V, Stocco DM, Clark BJ. Current knowledge on the acute regulation of steroidogenesis. Biol Reprod. 2018;99(1):13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller WL. Steroidogenesis: unanswered questions. Trends Endocrinol Metab. 2017;28(11):771-793. [DOI] [PubMed] [Google Scholar]
- 43. Bose HS, Sugawara T, Strauss JF 3rd, Miller WL; International Congenital Lipoid Adrenal Hyperplasia Consortium . The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335(25):1870-1878. [DOI] [PubMed] [Google Scholar]
- 44. Lin D, Sugawara T, Strauss JF 3rd, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267(5205):1828-1831. [DOI] [PubMed] [Google Scholar]
- 45. Baker BY, Lin L, Kim CJ, et al. Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab. 2006;91(12):4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab. 2009;94(3):936-939. [DOI] [PubMed] [Google Scholar]
- 47. Sahakitrungruang T, Tee MK, Blackett PR, Miller WL. Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2011;96(3):792-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishii T, Tajima T, Kashimada K, et al. Clinical features of 57 patients with lipoid congenital adrenal hyperplasia: criteria for nonclassic form revisited. J Clin Endocrinol Metab. 2020;105(11):e3929-e3937. [DOI] [PubMed] [Google Scholar]
- 49. Miller WL. Minireview: regulation of steroidogenesis by electron transfer. Endocrinology. 2005;146(6):2544-2550. [DOI] [PubMed] [Google Scholar]
- 50. Paul A, Drecourt A, Petit F, et al. FDXR mutations cause sensorial neuropathies and expand the spectrum of mitochondrial Fe-S-synthesis diseases. Am J Hum Genet. 2017;101(4):630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peng Y, Shinde DN, Valencia CA, et al. Biallelic mutations in the ferredoxin reductase gene cause novel mitochondriopathy with optic atrophy. Hum Mol Genet. 2017;26(24):4937-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Slone J, Peng Y, Chamberlin A, et al. Biallelic mutations in FDXR cause neurodegeneration associated with inflammation. J Hum Genet. 2018;63(12):1211-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005;26(4):525-582. [DOI] [PubMed] [Google Scholar]
- 54. Thomas JL, Mason JI, Brandt S, Spencer BR Jr, Norris W. Structure/function relationships responsible for the kinetic differences between human type 1 and type 2 3beta-hydroxysteroid dehydrogenase and for the catalysis of the type 1 activity. J Biol Chem. 2002;277(45):42795-42801. [DOI] [PubMed] [Google Scholar]
- 55. Lee TC, Miller WL, Auchus RJ. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab. 1999;84(6):2104-2110. [DOI] [PubMed] [Google Scholar]
- 56. Moisan AM, Ricketts ML, Tardy V, et al. New insight into the molecular basis of 3beta-hydroxysteroid dehydrogenase deficiency: identification of eight mutations in the HSD3B2 gene eleven patients from seven new families and comparison of the functional properties of twenty-five mutant enzymes. J Clin Endocrinol Metab. 1999;84(12):4410-4425. [DOI] [PubMed] [Google Scholar]
- 57. Mermejo LM, Elias LL, Marui S, Moreira AC, Mendonca BB, de Castro M. Refining hormonal diagnosis of type II 3beta-hydroxysteroid dehydrogenase deficiency in patients with premature pubarche and hirsutism based on HSD3B2 genotyping. J Clin Endocrinol Metab. 2005;90(3):1287-1293. [DOI] [PubMed] [Google Scholar]
- 58. Jeandron DD, Sahakitrungruang T. A novel homozygous Q334X mutation in the HSD3B2 gene causing classic 3β-hydroxysteroid dehydrogenase deficiency: an unexpected diagnosis after a positive newborn screen for 21-hydroxylase deficiency. Horm Res Paediatr. 2012;77(5):334-338. [DOI] [PubMed] [Google Scholar]
- 59. Burckhardt MA, Udhane SS, Marti N, et al. Human 3β-hydroxysteroid dehydrogenase deficiency seems to affect fertility but may not harbor a tumor risk: lesson from an experiment of nature. Eur J Endocrinol. 2015;173(5):K1-K12. [DOI] [PubMed] [Google Scholar]
- 60. Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273(6):3158-3165. [DOI] [PubMed] [Google Scholar]
- 61. Flück CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J Clin Endocrinol Metab. 2003;88(8):3762-3766. [DOI] [PubMed] [Google Scholar]
- 62. Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am. 2001;30(1):101-19, vii. [DOI] [PubMed] [Google Scholar]
- 63. Miller WL. The syndrome of 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97(1):59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Idkowiak J, Randell T, Dhir V, et al. A missense mutation in the human cytochrome b5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97(3):E465-E475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marsh CA, Auchus RJ. Fertility in patients with genetic deficiencies of cytochrome P450c17 (CYP17A1): combined 17-hydroxylase/17,20-lyase deficiency and isolated 17,20-lyase deficiency. Fertil Steril. 2014;101(2):317-322. [DOI] [PubMed] [Google Scholar]
- 66. Flück CE, Tajima T, Pandey AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36(3):228-230. [DOI] [PubMed] [Google Scholar]
- 67. Fukami M, Horikawa R, Nagai T, et al. Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab. 2005;90(1):414-426. [DOI] [PubMed] [Google Scholar]
- 68. Huang N, Pandey AV, Agrawal V, et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76(5):729-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Krone N, Reisch N, Idkowiak J, et al. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2012;97(2):E257-E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dean B, Chrisp GL, Quartararo M, et al. P450 oxidoreductase deficiency: a systematic review and meta-analysis of genotypes, phenotypes, and their relationships. J Clin Endocrinol Metab. 2020;105(3):e42-e52. [DOI] [PubMed] [Google Scholar]
- 71. Arlt W, Walker EA, Draper N, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363(9427):2128-2135. [DOI] [PubMed] [Google Scholar]
- 72. Fukami M, Nishimura G, Homma K, et al. Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab. 2009;94(5):1723-1731. [DOI] [PubMed] [Google Scholar]
- 73. Hershkovitz E, Parvari R, Wudy SA, et al. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab. 2008;93(9):3584-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Laue K, Pogoda HM, Daniel PB, et al. Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic acid. Am J Hum Genet. 2011;89(5):595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol. 2010;163(6):919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sahakitrungruang T, Huang N, Tee MK, et al. Clinical, genetic, and enzymatic characterization of P450 oxidoreductase deficiency in four patients. J Clin Endocrinol Metab. 2009;94(12):4992-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Geller DH, Auchus RJ, Mendonça BB, Miller WL. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet. 1997;17(2):201-205. [DOI] [PubMed] [Google Scholar]
- 78. Homma K, Hasegawa T, Nagai T, et al. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab. 2006;91(7):2643-2649. [DOI] [PubMed] [Google Scholar]
- 79. Reisch N, Taylor AE, Nogueira EF, et al. Alternative pathway androgen biosynthesis and human fetal female virilization. Proc Natl Acad Sci U S A. 2019;116(44):22294-22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Flück CE, Parween S, Rojas Velazquez MN, Pandey AV. Inhibition of placental CYP19A1 activity remains as a valid hypothesis for 46,XX virilization in P450 oxidoreductase deficiency. Proc Natl Acad Sci U S A. 2020;117(26):14632-14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84(12):4677-4694. [DOI] [PubMed] [Google Scholar]
- 82. Reisch N, Auchus RJ, Shackleton CHL, Hanley NA, Arlt W. Reply to Flück et al. : Alternative androgen pathway biosynthesis drives fetal female virilization in P450 oxidoreductase deficiency. Proc Natl Acad Sci U S A. 2020;117(26):14634-14635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang N, Agrawal V, Giacomini KM, Miller WL. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci U S A. 2008;105(5):1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gomes LG, Huang N, Agrawal V, Mendonça BB, Bachega TA, Miller WL. The common P450 oxidoreductase variant A503V is not a modifier gene for 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2008;93(7):2913-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. White PC, Curnow KM, Pascoe L. Disorders of steroid 11 beta-hydroxylase isozymes. Endocr Rev. 1994;15(4):421-438. [DOI] [PubMed] [Google Scholar]
- 86. Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol. 2020;16(5):284-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Curnow KM, Tusie-Luna MT, Pascoe L, et al. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol. 1991;5(10):1513-1522. [DOI] [PubMed] [Google Scholar]
- 88. Mulatero P, Curnow KM, Aupetit-Faisant B, et al. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab. 1998;83(11):3996-4001. [DOI] [PubMed] [Google Scholar]
- 89. Therrell BL Jr, Berenbaum SA, Manter-Kapanke V, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101(4 Pt 1):583-590. [DOI] [PubMed] [Google Scholar]
- 90. Moeller G, Adamski J. Integrated view on 17 β -hydroxysteroid dehydrogenases. Mol Cell Biol. 2009;301(1-2):7-19. [DOI] [PubMed] [Google Scholar]
- 91. Penning TM. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr Rev. 1997;18(3):281-305. [DOI] [PubMed] [Google Scholar]
- 92. Tremblay Y, Ringler GE, Morel Y, et al. Regulation of the gene for estrogenic 17-ketosteroid reductase lying on chromosome 17cen----q25. J Biol Chem. 1989;264(34):20458-20462. [PubMed] [Google Scholar]
- 93. Moghrabi N, Andersson S. 17beta-hydroxysteroid dehydrogenases: physiological roles in health and disease. Trends Endocrinol Metab. 1998;9(7):265-270. [DOI] [PubMed] [Google Scholar]
- 94. Mendonca BB, Inacio M, Arnhold IJ, et al. Male pseudohermaphroditism due to 17 beta-hydroxysteroid dehydrogenase 3 deficiency. Diagnosis, psychological evaluation, and management. Medicine (Baltimore). 2000;79(5):299-309. [DOI] [PubMed] [Google Scholar]
- 95. Penning TM, Burczynski ME, Jez JM, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(Pt 1):67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nakamura Y, Hornsby PJ, Casson P, et al. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94(6):2192-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Biswas MG, Russell DW. Expression cloning and characterization of oxidative 17beta- and 3alpha-hydroxysteroid dehydrogenases from rat and human prostate. J Biol Chem. 1997;272(25):15959-15966. [DOI] [PubMed] [Google Scholar]
- 98. Flück CE, Meyer-Böni M, Pandey AV, et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011;89(2):201-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342-355. [DOI] [PubMed] [Google Scholar]
- 100. Lo JC, Grumbach MM. Pregnancy outcomes in women with congenital virilizing adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30(1):207-229. [DOI] [PubMed] [Google Scholar]
- 101. Gomes LG, Huang N, Agrawal V, Mendonça BB, Bachega TA, Miller WL. Extraadrenal 21-hydroxylation by CYP2C19 and CYP3A4: effect on 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2009;94(1):89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yoshimoto FK, Zhou Y, Peng HM, et al. Minor activities and transition state properties of the human steroid hydroxylases cytochromes P450c17 and P450c21, from reactions observed with deuterium-labeled substrates. Biochemistry. 2012;51(36):7064-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhao B, Lei L, Kagawa N, et al. Three-dimensional structure of steroid 21-hydroxylase (cytochrome P450 21A2) with two substrates reveals locations of disease-associated variants. J Biol Chem. 2012;287(13):10613-10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pallan PS, Lei L, Wang C, Waterman MR, Guengerich FP, Egli M. Research resource: correlating human cytochrome P450 21A2 crystal structure and phenotypes of mutations in congenital adrenal hyperplasia. Mol Endocrinol. 2015;29(9):1375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mizrachi D, Wang Z, Sharma KK, et al. Why human cytochrome P450c21 is a progesterone 21-hydroxylase. Biochemistry. 2011;50(19):3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hu MC, Chung BC. Expression of human 21-hydroxylase (P450c21) in bacterial and mammalian cells: a system to characterize normal and mutant enzymes. Mol Endocrinol. 1990;4(6):893-898. [DOI] [PubMed] [Google Scholar]
- 107. Tusie-Luna MT, Speiser PW, Dumic M, New MI, White PC. A mutation (Pro-30 to Leu) in CYP21 represents a potential nonclassic steroid 21-hydroxylase deficiency allele. Mol Endocrinol. 1991;5(5):685-692. [DOI] [PubMed] [Google Scholar]
- 108. Tusie-Luna MT, Traktman P, White PC. Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem. 1990;265(34):20916-20922. [PubMed] [Google Scholar]
- 109. Wu DA, Chung BC. Mutations of P450c21 (steroid 21-hydroxylase) at Cys428, Val281, and Ser268 result in complete, partial, or no loss of enzymatic activity, respectively. J Clin Invest. 1991;88(2):519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang C, Pallan PS, Zhang W, et al. Functional analysis of human cytochrome P450 21A2 variants involved in congenital adrenal hyperplasia. J Biol Chem. 2017;292(26):10767-10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Peng HM, Barlow C, Auchus RJ. Catalytic modulation of human cytochromes P450 17A1 and P450 11B2 by phospholipid. J Steroid Biochem Mol Biol. 2018;181(7):63-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15(9):432-438. [DOI] [PubMed] [Google Scholar]
- 113. Wilson JD, Auchus RJ, Leihy MW, et al. 5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate. Endocrinology. 2003;144(2):575-580. [DOI] [PubMed] [Google Scholar]
- 114. Shaw G, Fenelon J, Sichlau M, Auchus RJ, Wilson JD, Renfree MB. Role of the alternate pathway of dihydrotestosterone formation in virilization of the Wolffian ducts of the tammar wallaby, Macropus eugenii. Endocrinology. 2006;147(5):2368-2373. [DOI] [PubMed] [Google Scholar]
- 115. Wilson JD, Shaw G, Renfree MB, Auchus RJ, Leihy MW, Eckery DC. Ontogeny and pathway of formation of 5alpha-androstane-3alpha,17beta-diol in the testes of the immature brushtail possum Trichosurus vulpecula. Reprod Fertil Dev. 2005;17(6):603-609. [DOI] [PubMed] [Google Scholar]
- 116. Wilson JD, Renfree MB, Auchus RJ, Pask AJ, Shaw G. Formation of 5alpha-reduced androgens in the testes and urogenital tract of the grey short-tailed opossum, Monodelphis domestica. Reprod Fertil Dev. 2009;21(5):649-654. [DOI] [PubMed] [Google Scholar]
- 117. O’Shaughnessy PJ, Antignac JP, Le Bizec B, et al. Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. 2019;17(2):e3000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3alpha-hydroxysteroid dehydrogenase in human prostate that converts 5alpha-androstane-3alpha,17beta-diol to 5alpha-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol Endocrinol. 2006;20(2):444-458. [DOI] [PubMed] [Google Scholar]
- 119. Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97(3):E367-E375. [DOI] [PubMed] [Google Scholar]
- 120. Schiffer L, Barnard L, Baranowski ES, et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J Steroid Biochem Mol Biol. 2019;194:105439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Turcu AF, Nanba AT, Chomic R, et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Swart AC, Storbeck KH. 11β-Hydroxyandrostenedione: downstream metabolism by 11βHSD, 17βHSD and SRD5A produces novel substrates in familiar pathways. Mol Cell Endocrinol. 2015;408(6):114-123. [DOI] [PubMed] [Google Scholar]
- 124. Borg B. Androgens in teleost fishes. Comp Biochem Physiol. 1994;109C(3):219-245. [Google Scholar]
- 125. Rege J, Turcu AF, Kasa-Vubu JZ, et al. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Barnard M, Quanson JL, Mostaghel E, Pretorius E, Snoep JL, Storbeck KH. 11-Oxygenated androgen precursors are the preferred substrates for aldo-keto reductase 1C3 (AKR1C3): implications for castration resistant prostate cancer. J Steroid Biochem Mol Biol. 2018;183(10):192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Barnard L, Schiffer L, Louw du-Toit R, Tamblyn JA, Chen S, Africander D, et al. 11-Oxygenated estrogens are a novel class of human estrogens but do not contribute to the circulating estrogen pool. Endocrinology. 2021;162(3):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Turcu AF, Mallappa A, Elman MS, et al. 11-Oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2017;102(8):2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Davio A, Woolcock H, Nanba AT, Rege J, O’Day P, Ren J, et al. Sex differences in 11-oxygenated androgen patterns across adulthood. J Clin Endocrinol Metab. 2020;105(8):e2921-e2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Turcu AF, El-Maouche D, Zhao L, et al. Androgen excess and diagnostic steroid biomarkers for nonclassic 21-hydroxylase deficiency without cosyntropin stimulation. Eur J Endocrinol. 2020;183(1):63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Storbeck KH, Schiffer L, Baranowski ES, et al. Steroid metabolome analysis in disorders of adrenal steroid biosynthesis and metabolism. Endocr Rev. 2019;40(6):1605-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jones CM, Mallappa A, Reisch N, et al. Modified-release and conventional glucocorticoids and diurnal androgen excretion in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2017;102(6):1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Falhammar H, Wedell A, Nordenström A. Biochemical and genetic diagnosis of 21-hydroxylase deficiency. Endocrine. 2015;50(2):306-314. [DOI] [PubMed] [Google Scholar]
- 135. Miller WL. Congenital adrenal hyperplasia: time to replace 17OHP with 21-deoxycortisol. Horm Res Paediatr. 2019;91(6):416-420. [DOI] [PubMed] [Google Scholar]
- 136. Engels M, Pijnenburg-Kleizen KJ, Utari A, et al. Glucocorticoid activity of adrenal steroid precursors in untreated patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2019;104(11):5065-5072. [DOI] [PubMed] [Google Scholar]
- 137. Pijnenburg-Kleizen KJ, Engels M, Mooij CF, et al. Adrenal steroid metabolites accumulating in congenital adrenal hyperplasia lead to transactivation of the glucocorticoid receptor. Endocrinology. 2015;156(10):3504-3510. [DOI] [PubMed] [Google Scholar]
- 138. Quinkler M, Meyer B, Bumke-Vogt C, et al. Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur J Endocrinol. 2002;146(6):789-799. [DOI] [PubMed] [Google Scholar]
- 139. Mooij CF, Parajes S, Pijnenburg-Kleizen KJ, Arlt W, Krone N, Claahsen-van der Grinten HL. Influence of 17-hydroxyprogesterone, progesterone and sex steroids on mineralocorticoid receptor transactivation in congenital adrenal hyperplasia. Horm Res Paediatr. 2015;83(6):414-421. [DOI] [PubMed] [Google Scholar]
- 140. Pijnenburg-Kleizen KJ, Noordam C, Otten BJ, Claahsen-van der Grinten HL. A delayed diagnosis of salt-wasting congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2016;85(3):497-499. [DOI] [PubMed] [Google Scholar]
- 141. Bristow J, Gitelman SE, Tee MK, Staels B, Miller WL. Abundant adrenal-specific transcription of the human P450c21A “pseudogene”. J Biol Chem. 1993;268(17):12919-12924. [PubMed] [Google Scholar]
- 142. Tee MK, Babalola GO, Aza-Blanc P, Speek M, Gitelman SE, Miller WL. A promoter within intron 35 of the human C4A gene initiates abundant adrenal-specific transcription of a 1 kb RNA: location of a cryptic CYP21 promoter element? Hum Mol Genet. 1995;4(11):2109-2116. [DOI] [PubMed] [Google Scholar]
- 143. White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986;83(14):5111-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986;83(9):2841-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. White PC, Grossberger D, Onufer BJ, et al. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci U S A. 1985;82(4):1089-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bristow J, Tee MK, Gitelman SE, Mellon SH, Miller WL. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol. 1993;122(1):265-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Blanchong CA, Zhou B, Rupert KL, et al. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in Caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J Exp Med. 2000;191(12):2183-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yang Z, Mendoza AR, Welch TR, Zipf WB, Yu CY. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. J Biol Chem. 1999;274(17):12147-12156. [DOI] [PubMed] [Google Scholar]
- 149. Chen W, Xu Z, Nishitani M, Van Ryzin C, McDonnell NB, Merke DP. Complement component 4 copy number variation and CYP21A2 genotype associations in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Genet. 2012;131(12):1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Globerman H, Amor M, Parker KL, New MI, White PC. Nonsense mutation causing steroid 21-hydroxylase deficiency. J Clin Invest. 1988;82(1):139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Higashi Y, Tanae A, Inoue H, Hiromasa T, Fujii-Kuriyama Y. Aberrant splicing and missense mutations cause steroid 21-hydroxylase [P-450(C21)] deficiency in humans: possible gene conversion products. Proc Natl Acad Sci U S A. 1988;85(20):7486-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Amor M, Parker KL, Globerman H, New MI, White PC. Mutation in the CYP21B gene (Ile-172----Asn) causes steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1988;85(5):1600-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Speiser PW, New MI, White PC. Molecular genetic analysis of nonclassic steroid 21-hydroxylase deficiency associated with HLA-B14,DR1. N Engl J Med. 1988;319(1):19-23. [DOI] [PubMed] [Google Scholar]
- 154. Goto M, Piper Hanley K, Marcos J, et al. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest. 2006;116(4):953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol. 2001;174(1-2):111-120. [DOI] [PubMed] [Google Scholar]
- 156. White PC. Ontogeny of adrenal steroid biosynthesis: why girls will be girls. J Clin Invest. 2006;116(4):872-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nomura S. Immature adrenal steroidogenesis in preterm infants. Early Hum Dev. 1997;49(3):225-233. [DOI] [PubMed] [Google Scholar]
- 158. Sasano H, White PC, New MI, Sasano N. Immunohistochemical localization of cytochrome P-450C21 in human adrenal cortex and its relation to endocrine function. Hum Pathol. 1988;19(2):181-185. [DOI] [PubMed] [Google Scholar]
- 159. Zhou Z, Agarwal VR, Dixit N, White P, Speiser PW. Steroid 21-hydroxylase expression and activity in human lymphocytes. Mol Cell Endocrinol. 1997;127(1):11-18. [DOI] [PubMed] [Google Scholar]
- 160. Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab. 2000;85(7):2519-2525. [DOI] [PubMed] [Google Scholar]
- 161. Sirianni R, Rehman KS, Carr BR, Parker CR Jr, Rainey WE. Corticotropin-releasing hormone directly stimulates cortisol and the cortisol biosynthetic pathway in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90(1):279-285. [DOI] [PubMed] [Google Scholar]
- 162. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351-1362. [DOI] [PubMed] [Google Scholar]
- 163. Weber MM, Michl P, Auernhammer CJ, Engelhardt D. Interleukin-3 and interleukin-6 stimulate cortisol secretion from adult human adrenocortical cells. Endocrinology. 1997;138(5):2207-2210. [DOI] [PubMed] [Google Scholar]
- 164. Bird IM, Mason JI, Rainey WE. Protein kinase A, protein kinase C, and Ca(2+)-regulated expression of 21-hydroxylase cytochrome P450 in H295R human adrenocortical cells. J Clin Endocrinol Metab. 1998;83(5):1592-1597. [DOI] [PubMed] [Google Scholar]
- 165. Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2004;18(2):279-290. [DOI] [PubMed] [Google Scholar]
- 166. Bird IM, Mason JI, Rainey WE. Battle of the kinases: integration of adrenal responses to cAMP, DG and Ca2+ at the level of steroidogenic cytochromes P450 and 3betaHSD expression in H295R cells. Endocr Res. 1998;24(3-4):345-354. [DOI] [PubMed] [Google Scholar]
- 167. Kelly SN, McKenna TJ, Young LS. Modulation of steroidogenic enzymes by orphan nuclear transcriptional regulation may control diverse production of cortisol and androgens in the human adrenal. J Endocrinol. 2004;181(2):355-365. [DOI] [PubMed] [Google Scholar]
- 168. Endoh A, Yang L, Hornsby PJ. CYP21 pseudogene transcripts are much less abundant than those from the active gene in normal human adrenocortical cells under various conditions in culture. Mol Cell Endocrinol. 1998;137(1):13-19. [DOI] [PubMed] [Google Scholar]
- 169. Wenzel J, Grabinski N, Knopp CA, et al. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1601-R1609. [DOI] [PubMed] [Google Scholar]
- 170. Ding L, Murphy MB, He Y, et al. Effects of brominated flame retardants and brominated dioxins on steroidogenesis in H295R human adrenocortical carcinoma cell line. Environ Toxicol Chem. 2007;26(4):764-772. [DOI] [PubMed] [Google Scholar]
- 171. Song R, He Y, Murphy MB, et al. Effects of fifteen PBDE metabolites, DE71, DE79 and TBBPA on steroidogenesis in the H295R cell line. Chemosphere. 2008;71(10):1888-1894. [DOI] [PubMed] [Google Scholar]
- 172. Bláha L, Hilscherová K, Mazurová E, et al. Alteration of steroidogenesis in H295R cells by organic sediment contaminants and relationships to other endocrine disrupting effects. Environ Int. 2006;32(6):749-757. [DOI] [PubMed] [Google Scholar]
- 173. Speek M, Barry F, Miller WL. Alternate promoters and alternate splicing of human tenascin-X, a gene with 5’ and 3’ ends buried in other genes. Hum Mol Genet. 1996;5(11):1749-1758. [DOI] [PubMed] [Google Scholar]
- 174. Chang SF, Chung BC. Difference in transcriptional activity of two homologous CYP21A genes. Mol Endocrinol. 1995;9(10):1330-1336. [DOI] [PubMed] [Google Scholar]
- 175. Kyllo JH, Collins MM, Donohoue PA. Constitutive human steroid 21-hydroxylase promoter gene and pseudogene activity in steroidogenic and nonsteroidogenic cells with the luciferase gene as a reporter. Endocr Res. 1995;21(4):777-791. [DOI] [PubMed] [Google Scholar]
- 176. Chin KK, Chang SF. The -104G nucleotide of the human CYP21 gene is important for CYP21 transcription activity and protein interaction. Nucleic Acids Res. 1998;26(8):1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Araújo RS, Mendonca BB, Barbosa AS, et al. Microconversion between CYP21A2 and CYP21A1P promoter regions causes the nonclassical form of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(10):4028-4034. [DOI] [PubMed] [Google Scholar]
- 178. Araujo RS, Billerbeck AE, Madureira G, Mendonca BB, Bachega TA. Substitutions in the CYP21A2 promoter explain the simple-virilizing form of 21-hydroxylase deficiency in patients harbouring a P30L mutation. Clin Endocrinol (Oxf). 2005;62(2):132-136. [DOI] [PubMed] [Google Scholar]
- 179. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18(3):361-377. [DOI] [PubMed] [Google Scholar]
- 180. Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24(7):1322-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Wijesuriya SD, Zhang G, Dardis A, Miller WL. Transcriptional regulatory elements of the human gene for cytochrome P450c21 (steroid 21-hydroxylase) lie within intron 35 of the linked C4B gene. J Biol Chem. 1999;274(53):38097-38106. [DOI] [PubMed] [Google Scholar]
- 182. Wilson TE, Mouw AR, Weaver CA, Milbrandt J, Parker KL. The orphan nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21-hydroxylase. Mol Cell Biol. 1993;13(2):861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Crawford PA, Sadovsky Y, Woodson K, Lee SL, Milbrandt J. Adrenocortical function and regulation of the steroid 21-hydroxylase gene in NGFI-B-deficient mice. Mol Cell Biol. 1995;15(8):4331-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Fernandez PM, Brunel F, Jimenez MA, Saez JM, Cereghini S, Zakin MM. Nuclear receptors Nor1 and NGFI-B/Nur77 play similar, albeit distinct, roles in the hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141(7):2392-2400. [DOI] [PubMed] [Google Scholar]
- 185. Tusié-Luna MT, White PC. Gene conversions and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc Natl Acad Sci U S A. 1995;92(23):10796-10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. L’Allemand D, Tardy V, Grüters A, Schnabel D, Krude H, Morel Y. How a patient homozygous for a 30-kb deletion of the C4-CYP 21 genomic region can have a nonclassic form of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2000;85(12):4562-4567. [DOI] [PubMed] [Google Scholar]
- 187. Lee HH, Lee YJ, Wang YM, et al. Low frequency of the CYP21A2 deletion in ethnic Chinese (Taiwanese) patients with 21-hydroxylase deficiency. Mol Genet Metab. 2008;93(4):450-457. [DOI] [PubMed] [Google Scholar]
- 188. Burch GH, Gong Y, Liu W, et al. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet. 1997;17(1):104-108. [DOI] [PubMed] [Google Scholar]
- 189. Merke DP, Chen W, Morissette R, et al. Tenascin-X haploinsufficiency associated with Ehlers-Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98(2):E379-E387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Gao Y, Lu L, Yu B, et al. The prevalence of the chimeric TNXA/TNXB gene and clinical symptoms of Ehlers-Danlos Syndrome with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2020;105(7):2288-2299. [DOI] [PubMed] [Google Scholar]
- 191. Morissette R, Chen W, Perritt AF, et al. Broadening the spectrum of Ehlers Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(8):E1143-E1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Miller WL, Merke DP. Tenascin-X, congenital adrenal hyperplasia, and the CAH-X syndrome. Horm Res Paediatr. 2018;89(5):352-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Speiser PW, Dupont J, Zhu D, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992;90(2):584-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wedell A, Thilén A, Ritzén EM, Stengler B, Luthman H. Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab. 1994;78(5):1145-1152. [DOI] [PubMed] [Google Scholar]
- 195. Barbat B, Bogyo A, Raux-Demay MC, et al. Screening of CYP21 gene mutations in 129 French patients affected by steroid 21-hydroxylase deficiency. Hum Mutat. 1995;5(2):126-130. [DOI] [PubMed] [Google Scholar]
- 196. Carrera P, Bordone L, Azzani T, et al. Point mutations in Italian patients with classic, non-classic, and cryptic forms of steroid 21-hydroxylase deficiency. Hum Genet. 1996;98(6):662-665. [DOI] [PubMed] [Google Scholar]
- 197. Jääskeläinen J, Levo A, Voutilainen R, Partanen J. Population-wide evaluation of disease manifestation in relation to molecular genotype in steroid 21-hydroxylase (CYP21) deficiency: good correlation in a well defined population. J Clin Endocrinol Metab. 1997;82(10):3293-3297. [DOI] [PubMed] [Google Scholar]
- 198. Bachega TA, Billerbeck AE, Madureira G, et al. Molecular genotyping in Brazilian patients with the classical and nonclassical forms of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1998;83(12):4416-4419. [DOI] [PubMed] [Google Scholar]
- 199. Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000;85(3):1059-1065. [DOI] [PubMed] [Google Scholar]
- 200. Baumgartner-Parzer SM, Schulze E, Waldhäusl W, et al. Mutational spectrum of the steroid 21-hydroxylase gene in Austria: identification of a novel missense mutation. J Clin Endocrinol Metab. 2001;86(10):4771-4775. [DOI] [PubMed] [Google Scholar]
- 201. Stikkelbroeck NM, Hoefsloot LH, de Wijs IJ, Otten BJ, Hermus AR, Sistermans EA. CYP21 gene mutation analysis in 198 patients with 21-hydroxylase deficiency in The Netherlands: six novel mutations and a specific cluster of four mutations. J Clin Endocrinol Metab. 2003;88(8):3852-3859. [DOI] [PubMed] [Google Scholar]
- 202. Loidi L, Quinteiro C, Parajes S, et al. High variability in CYP21A2 mutated alleles in Spanish 21-hydroxylase deficiency patients, six novel mutations and a founder effect. Clin Endocrinol (Oxf). 2006;64(3):330-336. [DOI] [PubMed] [Google Scholar]
- 203. Friães A, Rêgo AT, Aragüés JM, et al. CYP21A2 mutations in Portuguese patients with congenital adrenal hyperplasia: identification of two novel mutations and characterization of four different partial gene conversions. Mol Genet Metab. 2006;88(1):58-65. [DOI] [PubMed] [Google Scholar]
- 204. Wilson RC, Nimkarn S, Dumic M, et al. Ethnic-specific distribution of mutations in 716 patients with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Mol Genet Metab. 2007;90(4):414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Baş F, Kayserili H, Darendeliler F, et al. CYP21A2 gene mutations in congenital adrenal hyperplasia: genotype-phenotype correlation in Turkish children. J Clin Res Pediatr Endocrinol. 2009;1(3):116-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Finkielstain GP, Chen W, Mehta SP, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96(1):E161-E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Nermoen I, Brønstad I, Fougner KJ, et al. Genetic, anthropometric and metabolic features of adult Norwegian patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(4):507-516. [DOI] [PubMed] [Google Scholar]
- 208. Rabbani B, Mahdieh N, Ashtiani MT, et al. Mutation analysis of the CYP21A2 gene in the Iranian population. Genet Test Mol Biomarkers. 2012;16(2):82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ben Charfeddine I, Riepe FG, Clauser E, et al. Steroid 21-hydroxylase gene mutational spectrum in 50 Tunisian patients: characterization of three novel polymorphisms. Gene. 2012;507(1):20-26. [DOI] [PubMed] [Google Scholar]
- 210. Krone N, Rose IT, Willis DS, et al. ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE) . Genotype-phenotype correlation in 153 adult patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency: analysis of the United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE) cohort. J Clin Endocrinol Metab. 2013;98(2):E346-E354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. New MI, Abraham M, Gonzalez B, et al. Genotype-phenotype correlation in 1507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 2013;110(7):2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Parajes S, Quinteiro C, Domínguez F, Loidi L. High frequency of copy number variations and sequence variants at CYP21A2 locus: implication for the genetic diagnosis of 21-hydroxylase deficiency. PLoS One. 2008;3(5):e2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kleinle S, Lang R, Fischer GF, et al. Duplications of the functional CYP21A2 gene are primarily restricted to Q318X alleles: evidence for a founder effect. J Clin Endocrinol Metab. 2009;94(10):3954-3958. [DOI] [PubMed] [Google Scholar]
- 214. Xu C, Jia W, Cheng X, et al. Genotype-phenotype correlation study and mutational and hormonal analysis in a Chinese cohort with 21-hydroxylase deficiency. Mol Genet Genomic Med. 2019;7(6):e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Marino R, Ramirez P, Galeano J, et al. Steroid 21-hydroxylase gene mutational spectrum in 454 Argentinean patients: genotype-phenotype correlation in a large cohort of patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2011;75(4):427-435. [DOI] [PubMed] [Google Scholar]
- 217. Wedell A. Molecular genetics of congenital adrenal hyperplasia (21-hydroxylase deficiency): implications for diagnosis, prognosis and treatment. Acta Paediatr. 1998;87(2):159-164. [DOI] [PubMed] [Google Scholar]
- 218. Hou L, Liang L, Lin S, et al. Analysis of phenotypes and genotypes in 84 patients with 21-Hydroxylase deficiency in southern China. Steroids. 2019;151(11):108474. [DOI] [PubMed] [Google Scholar]
- 219. Chiou SH, Hu MC, Chung BC. A missense mutation at Ile 172----Asn or Arg356----Trp causes steroid 21-hydroxylase deficiency. J Biol Chem. 1990;265(6):3549-3552. [PubMed] [Google Scholar]
- 220. Kocova M, Anastasovska V, Falhammar H. Clinical outcomes and characteristics of P30L mutations in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocrine. 2020;69(2):262-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Gurgov S, Bernabé KJ, Stites J, et al. Linking the degree of virilization in females with congenital adrenal hyperplasia to genotype. Ann N Y Acad Sci. 2017;1402(1):56-63. [DOI] [PubMed] [Google Scholar]
- 222. Nordenström A. Adult women with 21-hydroxylase deficient congenital adrenal hyperplasia, surgical and psychological aspects. Curr Opin Pediatr. 2011;23(4):436-442. [DOI] [PubMed] [Google Scholar]
- 223. Hall CM, Jones JA, Meyer-Bahlburg HF, et al. Behavioral and physical masculinization are related to genotype in girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89(1):419-424. [DOI] [PubMed] [Google Scholar]
- 224. Balsamo A, Cicognani A, Baldazzi L, et al. CYP21 genotype, adult height, and pubertal development in 55 patients treated for 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2003;88(12):5680-5688. [DOI] [PubMed] [Google Scholar]
- 225. Meyer-Bahlburg HF, Dolezal C, Baker SW, New MI. Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Arch Sex Behav. 2008;37(1):85-99. [DOI] [PubMed] [Google Scholar]
- 226. Strandqvist A, Falhammar H, Lichtenstein P, et al. Suboptimal psychosocial outcomes in patients with congenital adrenal hyperplasia: epidemiological studies in a nonbiased national cohort in Sweden. J Clin Endocrinol Metab. 2014;99(4):1425-1432. [DOI] [PubMed] [Google Scholar]
- 227. Lao Q, Jardin MD, Jayakrishnan R, Ernst M, Merke DP. Complement component 4 variations may influence psychopathology risk in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Genet. 2018;137(11-12):955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Haider S, Islam B, D’Atri V, et al. Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc Natl Acad Sci U S A. 2013;110(7):2605-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Therrell BL, Padilla CD, Loeber JG, et al. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015;39(3):171-187. [DOI] [PubMed] [Google Scholar]
- 230. White PC. Neonatal screening for congenital adrenal hyperplasia. Nat Rev Endocrinol. 2009;5(9):490-498. [DOI] [PubMed] [Google Scholar]
- 231. Pang S, Shook MK. Current status of neonatal screening for congenital adrenal hyperplasia. Curr Opin Pediatr. 1997;9(4):419-423. [DOI] [PubMed] [Google Scholar]
- 232. Therrell BL. Newborn screening for congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30(1):15-30. [DOI] [PubMed] [Google Scholar]
- 233. van der Kamp HJ, Wit JM. Neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol. 2004;151(Suppl 3):U71-U75. [DOI] [PubMed] [Google Scholar]
- 234. Speiser PW, Chawla R, Chen M, et al. Newborn screening protocols and positive predictive value for congenital adrenal hyperplasia vary across the United States. Int J Neonatal Screen. 2020;6(2):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Balsamo A, Cacciari E, Piazzi S, et al. Congenital adrenal hyperplasia: neonatal mass screening compared with clinical diagnosis only in the Emilia-Romagna region of Italy, 1980-1995. Pediatrics. 1996;98(3 Pt 1):362-367. [PubMed] [Google Scholar]
- 236. Brosnan PG, Brosnan CA, Kemp SF, et al. Effect of newborn screening for congenital adrenal hyperplasia. Arch Pediatr Adolesc Med. 1999;153(12):1272-1278. [DOI] [PubMed] [Google Scholar]
- 237. Thil’en A, Nordenström A, Hagenfeldt L, von Döbeln U, Guthenberg C, Larsson A. Benefits of neonatal screening for congenital adrenal hyperplasia (21-hydroxylase deficiency) in Sweden. Pediatrics. 1998;101(4):E11. [DOI] [PubMed] [Google Scholar]
- 238. Nordenström A, Ahmed S, Jones J, et al. Female preponderance in congenital adrenal hyperplasia due to CYP21 deficiency in England: implications for neonatal screening. Horm Res. 2005;63(1):22-28. [DOI] [PubMed] [Google Scholar]
- 239. Liu SY, Lee CT, Tung YC, Chien YH, Hwu WL, Tsai WY. Clinical characteristics of Taiwanese children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency detected by neonatal screening. J Formos Med Assoc. 2018;117(2):126-131. [DOI] [PubMed] [Google Scholar]
- 240. Hird BE, Tetlow L, Tobi S, Patel L, Clayton PE. No evidence of an increase in early infant mortality from congenital adrenal hyperplasia in the absence of screening. Arch Dis Child. 2014;99(2):158-164. [DOI] [PubMed] [Google Scholar]
- 241. Riedl S, Röhl FW, Bonfig W, et al. ; AQUAPE CAH Study Group . Genotype/phenotype correlations in 538 congenital adrenal hyperplasia patients from Germany and Austria: discordances in milder genotypes and in screened versus prescreening patients. Endocr Connect. 2019;8(2):86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Grosse SD, Van Vliet G. How many deaths can be prevented by newborn screening for congenital adrenal hyperplasia? Horm Res. 2007;67(6):284-291. [DOI] [PubMed] [Google Scholar]
- 243. Dörr HG, Wollmann HA, Hauffa BP, Woelfle J; German Society of Pediatric Endocrinology and Diabetology . Mortality in children with classic congenital adrenal hyperplasia and 21-hydroxylase deficiency (CAH) in Germany. BMC Endocr Disord. 2018;18(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Van der Kamp HJ, Noordam K, Elvers B, Van Baarle M, Otten BJ, Verkerk PH. Newborn screening for congenital adrenal hyperplasia in the Netherlands. Pediatrics. 2001;108(6):1320-1324. [DOI] [PubMed] [Google Scholar]
- 245. Fox DA, Ronsley R, Khowaja AR, et al. Clinical impact and cost efficacy of newborn screening for congenital adrenal hyperplasia. J Pediatr. 2020;220(5):101-108.e2. [DOI] [PubMed] [Google Scholar]
- 246. Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(5 Pt 2):S287-S295. [DOI] [PubMed] [Google Scholar]
- 247. Chan CL, McFann K, Taylor L, Wright D, Zeitler PS, Barker JM. Congenital adrenal hyperplasia and the second newborn screen. J Pediatr. 2013;163(1):109-13.e1. [DOI] [PubMed] [Google Scholar]
- 248. Pang S, Hotchkiss J, Drash AL, Levine LS, New MI. Microfilter paper method for 17 alpha-hydroxyprogesterone radioimmunoassay: its application for rapid screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1977;45(5):1003-1008. [DOI] [PubMed] [Google Scholar]
- 249. Gonzalez RR, Mäentausta O, Solyom J, Vihko R. Direct solid-phase time-resolved fluoroimmunoassay of 17 alpha-hydroxyprogesterone in serum and dried blood spots on filter paper. Clin Chem. 1990;36(9):1667-1672. [PubMed] [Google Scholar]
- 250. Gidlöf S, Wedell A, Guthenberg C, von Döbeln U, Nordenström A. Nationwide neonatal screening for congenital adrenal hyperplasia in Sweden: a 26-year longitudinal prospective population-based study. JAMA Pediatr. 2014;168(6):567-574. [DOI] [PubMed] [Google Scholar]
- 251. Olgemöller B, Roscher AA, Liebl B, Fingerhut R. Screening for congenital adrenal hyperplasia: adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J Clin Endocrinol Metab. 2003;88(12):5790-5794. [DOI] [PubMed] [Google Scholar]
- 252. Sarafoglou K, Gaviglio A, Hietala A, et al. Comparison of newborn screening protocols for congenital adrenal hyperplasia in preterm infants. J Pediatr. 2014;164(5):1136-1140. [DOI] [PubMed] [Google Scholar]
- 253. Jiang X, Tang F, Feng Y, et al. The adjustment of 17-hydroxyprogesterone cut-off values for congenital adrenal hyperplasia neonatal screening by GSP according to gestational age and age at sampling. J Pediatr Endocrinol Metab. 2019;32(11):1253-1258. [DOI] [PubMed] [Google Scholar]
- 254. Hayashi GY, Carvalho DF, de Miranda MC, et al. Neonatal 17-hydroxyprogesterone levels adjusted according to age at sample collection and birthweight improve the efficacy of congenital adrenal hyperplasia newborn screening. Clin Endocrinol (Oxf). 2017;86(4):480-487. [DOI] [PubMed] [Google Scholar]
- 255. Held PK, Shapira SK, Hinton CF, Jones E, Hannon WH, Ojodu J. Congenital adrenal hyperplasia cases identified by newborn screening in one- and two-screen states. Mol Genet Metab. 2015;116(3):133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Gatelais F, Berthelot J, Beringue F, et al. Effect of single and multiple courses of prenatal corticosteroids on 17-hydroxyprogesterone levels: implication for neonatal screening of congenital adrenal hyperplasia. Pediatr Res. 2004;56(5):701-705. [DOI] [PubMed] [Google Scholar]
- 257. King JL, Naber JM, Hopkin RJ, Repaske DR, Bailey L, Leslie ND. Antenatal corticosteroids and newborn screening for congenital adrenal hyperplasia. Arch Pediatr Adolesc Med. 2001;155(9):1038-1042. [DOI] [PubMed] [Google Scholar]
- 258. Varness TS, Allen DB, Hoffman GL. Newborn screening for congenital adrenal hyperplasia has reduced sensitivity in girls. J Pediatr. 2005;147(4):493-498. [DOI] [PubMed] [Google Scholar]
- 259. van der Linde AAA, Schönbeck Y, van der Kamp HJ, et al. Evaluation of the Dutch neonatal screening for congenital adrenal hyperplasia. Arch Dis Child. 2019;104(7):653-657. [DOI] [PubMed] [Google Scholar]
- 260. Sarafoglou K, Banks K, Kyllo J, Pittock S, Thomas W. Cases of congenital adrenal hyperplasia missed by newborn screening in Minnesota. JAMA. 2012;307(22):2371-2374. [DOI] [PubMed] [Google Scholar]
- 261. Minutti CZ, Lacey JM, Magera MJ, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89(8):3687-3693. [DOI] [PubMed] [Google Scholar]
- 262. Rauh M, Gröschl M, Rascher W, Dörr HG. Automated, fast and sensitive quantification of 17 alpha-hydroxy-progesterone, androstenedione and testosterone by tandem mass spectrometry with on-line extraction. Steroids. 2006;71(6):450-458. [DOI] [PubMed] [Google Scholar]
- 263. Janzen N, Sander S, Terhardt M, et al. Rapid steroid hormone quantification for congenital adrenal hyperplasia (CAH) in dried blood spots using UPLC liquid chromatography-tandem mass spectrometry. Steroids. 2011;76(13):1437-1442. [DOI] [PubMed] [Google Scholar]
- 264. Boelen A, Ruiter AF, Claahsen-van der Grinten HL, Endert E, Ackermans MT. Determination of a steroid profile in heel prick blood using LC-MS/MS. Bioanalysis. 2016;8(5):375-384. [DOI] [PubMed] [Google Scholar]
- 265. Hines JM, Bancos I, Bancos C, et al. High-resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clin Chem. 2017;63(12):1824-1835. [DOI] [PubMed] [Google Scholar]
- 266. Kamrath C, Hartmann MF, Boettcher C, Zimmer KP, Wudy SA. Diagnosis of 21-hydroxylase deficiency by urinary metabolite ratios using gas chromatography-mass spectrometry analysis: reference values for neonates and infants. J Steroid Biochem Mol Biol. 2016;156(2):10-16. [DOI] [PubMed] [Google Scholar]
- 267. Matern D, Tortorelli S, Oglesbee D, Gavrilov D, Rinaldo P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: the Mayo Clinic experience (2004-2007). J Inherit Metab Dis. 2007;30(4):585-592. [DOI] [PubMed] [Google Scholar]
- 268. Lai F, Srinivasan S, Wiley V. Evaluation of a two-tier screening pathway for congenital adrenal hyperplasia in the New South Wales newborn screening programme. Int J Neonatal Screen. 2020;6(3):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Tieh PY, Yee JK, Hicks RA, Mao CS, Lee WN. Utility of a precursor-to-product ratio in the evaluation of presumptive positives in newborn screening of congenital adrenal hyperplasia. J Perinatol. 2017;37(3):283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Schwarz E, Liu A, Randall H, et al. Use of steroid profiling by UPLC-MS/MS as a second tier test in newborn screening for congenital adrenal hyperplasia: the Utah experience. Pediatr Res. 2009;66(2):230-235. [DOI] [PubMed] [Google Scholar]
- 271. Seo JY, Park HD, Kim JW, et al. Steroid profiling for congenital adrenal hyperplasia by tandem mass spectrometry as a second-tier test reduces follow-up burdens in a tertiary care hospital: a retrospective and prospective evaluation. J Perinat Med. 2014;42(1):121-127. [DOI] [PubMed] [Google Scholar]
- 272. Lasarev MR, Bialk ER, Allen DB, Held PK. Application of principal component analysis to newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(8):e2930-e2940. [DOI] [PubMed] [Google Scholar]
- 273. De Jesús VR, Simms DA, Schiffer J, Kennedy M, Mei JV, Hannon WH. Pilot proficiency testing study for second tier congenital adrenal hyperplasia newborn screening. Clin Chim Acta. 2010;411(21-22):1684-1687. [DOI] [PubMed] [Google Scholar]
- 274. Grecsó N, Zádori A, Szécsi I, et al. Storage stability of five steroids and in dried blood spots for newborn screening and retrospective diagnosis of congenital adrenal hyperplasia. PLoS One. 2020;15(5):e0233724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Fitness J, Dixit N, Webster D, et al. Genotyping of CYP21, linked chromosome 6p markers, and a sex-specific gene in neonatal screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1999;84(3):960-966. [DOI] [PubMed] [Google Scholar]
- 276. Kösel S, Burggraf S, Fingerhut R, Dörr HG, Roscher AA, Olgemöller B. Rapid second-tier molecular genetic analysis for congenital adrenal hyperplasia attributable to steroid 21-hydroxylase deficiency. Clin Chem. 2005;51(2):298-304. [DOI] [PubMed] [Google Scholar]
- 277. Németh S, Riedl S, Kriegshäuser G, et al. Reverse-hybridization assay for rapid detection of common CYP21A2 mutations in dried blood spots from newborns with elevated 17-OH progesterone. Clin Chim Acta. 2012;414:211-214. [DOI] [PubMed] [Google Scholar]
- 278. Nordenström A, Thilén A, Hagenfeldt L, Larsson A, Wedell A. Genotyping is a valuable diagnostic complement to neonatal screening for congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1999;84(5):1505-1509. [DOI] [PubMed] [Google Scholar]
- 279. Silveira EL, Elnecave RH, dos Santos EP, et al. Molecular analysis of CYP21A2 can optimize the follow-up of positive results in newborn screening for congenital adrenal hyperplasia. Clin Genet. 2009;76(6):503-510. [DOI] [PubMed] [Google Scholar]
- 280. Marino S, Perez Garrido N, Ramírez P, et al. Molecular analysis of the CYP21A2 gene in dried blood spot samples. Medicina (B Aires). 2020;80(3):197-202. [PubMed] [Google Scholar]
- 281. Wudy SA, Schuler G, Sánchez-Guijo A, Hartmann MF. The art of measuring steroids: principles and practice of current hormonal steroid analysis. J Steroid Biochem Mol Biol. 2018;179(5):88-103. [DOI] [PubMed] [Google Scholar]
- 282. Wudy SA, Wachter UA, Homoki J, Teller WM. 17 alpha-hydroxyprogesterone, 4-androstenedione, and testosterone profiled by routine stable isotope dilution/gas chromatography-mass spectrometry in plasma of children. Pediatr Res. 1995;38(1):76-80. [DOI] [PubMed] [Google Scholar]
- 283. Wong T, Shackleton CH, Covey TR, Ellis G. Identification of the steroids in neonatal plasma that interfere with 17 alpha-hydroxyprogesterone radioimmunoassays. Clin Chem. 1992;38(9):1830-1837. [PubMed] [Google Scholar]
- 284. Lange-Kubini K, Zachmann M, Kempken B, Torresani T. 15 beta-hydroxylated steroids may be diagnostically misleading in confirming congenital adrenal hyperplasia suspected by a newborn screening programme. Early Hum Dev. 1997;49(3):235-236. [DOI] [PubMed] [Google Scholar]
- 285. Han L, Tavakoli NP, Morrissey M, Spink DC, Cao ZT. Liquid chromatography-tandem mass spectrometry analysis of 17-hydroxyprogesterone in dried blood spots revealed matrix effect on immunoassay. Anal Bioanal Chem. 2019;411(2):395-402. [DOI] [PubMed] [Google Scholar]
- 286. al Saedi S, Dean H, Dent W, Stockl E, Cronin C. Screening for congenital adrenal hyperplasia: the Delfia Screening Test overestimates serum 17-hydroxyprogesterone in preterm infants. Pediatrics. 1996;97(1):100-102. [PubMed] [Google Scholar]
- 287. Wang R, Hartmann MF, Tiosano D, Wudy SA. Characterizing the steroidal milieu in amniotic fluid of mid-gestation: a GC-MS study. J Steroid Biochem Mol Biol. 2019;193(10):105412. [DOI] [PubMed] [Google Scholar]
- 288. Akgun S, Ertel NH, Imperato-McGinley J, Sayli BS, Shackleton C. Familial male pseudohermaphroditism due to 5-alpha-reductase deficiency in a Turkish village. Am J Med. 1986;81(2):267-274. [DOI] [PubMed] [Google Scholar]
- 289. Langhammer M, Michaelis M, Hartmann MF, et al. Reproductive performance primarily depends on the female genotype in a two-factorial breeding experiment using high-fertility mouse lines. Reproduction. 2017;153(3):361-368. [DOI] [PubMed] [Google Scholar]
- 290. Greaves RF, Jolly L, Hartmann MF, et al. Harmonisation of serum dihydrotestosterone analysis: establishment of an external quality assurance program. Clin Chem Lab Med. 2017;55(4):522-529. [DOI] [PubMed] [Google Scholar]
- 291. Fanis P, Neocleous V, Kosta K, et al. Late diagnosis of 3β-Hydroxysteroid dehydrogenase deficiency: the pivotal role of gas chromatography-mass spectrometry urinary steroid metabolome analysis and a novel homozygous nonsense mutation in the HSD3B2 gene. J Pediatr Endocrinol Metab. 2021;34(1):131-136. [DOI] [PubMed] [Google Scholar]
- 292. Nguyen HH, Eiden-Plach A, Hannemann F, et al. Phenotypic, metabolic, and molecular genetic characterization of six patients with congenital adrenal hyperplasia caused by novel mutations in the CYP11B1 gene. J Steroid Biochem Mol Biol. 2016;155(Pt A):126-134. [DOI] [PubMed] [Google Scholar]
- 293. Koyama Y, Homma K, Fukami M, et al. Classic and non-classic 21-hydroxylase deficiency can be discriminated from P450 oxidoreductase deficiency in Japanese infants by urinary steroid metabolites. Clin Pediatr Endocrinol. 2016;25(2):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Wudy SA, Hartmann MF, Remer T. Sexual dimorphism in cortisol secretion starts after age 10 in healthy children: urinary cortisol metabolite excretion rates during growth. Am J Physiol Endocrinol Metab. 2007;293(4):E970-E976. [DOI] [PubMed] [Google Scholar]
- 295. Maerker T, van Wijk E, Overlack N, et al. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet. 2008;17(1):71-86. [DOI] [PubMed] [Google Scholar]
- 296. Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF, Wudy SA. The urinary steroidome of treated children with classic 21-hydroxylase deficiency. J Steroid Biochem Mol Biol. 2017;165(Pt B):396-406. [DOI] [PubMed] [Google Scholar]
- 297. Kamrath C, Wettstaedt L, Hartmann MF, Wudy SA. Height velocity defined metabolic control in children with congenital adrenal hyperplasia using urinary GC-MS analysis. J Clin Endocrinol Metab. 2019;104(5):4214-4224. [DOI] [PubMed] [Google Scholar]
- 298. Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF, Wudy SA. Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. 2018;178(4):221-228. [DOI] [PubMed] [Google Scholar]
- 299. Barnard L, Nikolaou N, Louw C, et al. The A-ring reduction of 11-ketotestosterone is efficiently catalysed by AKR1D1 and SRD5A2 but not SRD5A1. J Steroid Biochem Mol Biol. 2020;202(7):105724. [DOI] [PubMed] [Google Scholar]
- 300. Tiosano D, Navon R, Flor O, et al. A steroid metabolomic approach to 17α-hydroxylase/17,20 lyase deficiency. Metabolomics. 2010;6(3):417-426. [Google Scholar]
- 301. Gawlik A, Shmoish M, Hartmann MF, Malecka-Tendera E, Wudy SA, Hochberg Z. Steroid metabolomic disease signature of nonsyndromic childhood obesity. J Clin Endocrinol Metab. 2016;101(11):4329-4337. [DOI] [PubMed] [Google Scholar]
- 302. Vitkin E, Ben-Dor A, Shmoish M, et al. Peer group normalization and urine to blood context in steroid metabolomics: the case of CAH and obesity. Steroids. 2014;88(10):83-89. [DOI] [PubMed] [Google Scholar]
- 303. Kamrath C, Hartmann MF, Pons-Kühnemann J, Wudy SA. Urinary GC-MS steroid metabotyping in treated children with congenital adrenal hyperplasia. Metabolism. 2020;112(9):154354. [DOI] [PubMed] [Google Scholar]
- 304. Sánchez-Guijo A, Oji V, Hartmann MF, Traupe H, Wudy SA. Simultaneous quantification of cholesterol sulfate, androgen sulfates, and progestagen sulfates in human serum by LC-MS/MS. J Lipid Res. 2015;56(9):1843-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Kulle A, Krone N, Holterhus PM, et al. ; EU COST Action . Steroid hormone analysis in diagnosis and treatment of DSD: position paper of EU COST Action BM 1303 ‘DSDnet’. Eur J Endocrinol. 2017;176(5):P1-P9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. White PC, Vitek A, Dupont B, New MI. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1988;85(12):4436-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Ghizzoni L, Cappa M, Vottero A, et al. Relationship of CYP21A2 genotype and serum 17-hydroxyprogesterone and cortisol levels in a large cohort of Italian children with premature pubarche. Eur J Endocrinol. 2011;165(2):307-314. [DOI] [PubMed] [Google Scholar]
- 308. Chan AO, But WM, Ng KL, et al. Molecular analysis of congenital adrenal hyperplasia due to 21-hydroxylase deficiency in Hong Kong Chinese patients. Steroids. 2011;76(10-11):1057-1062. [DOI] [PubMed] [Google Scholar]
- 309. Jang JH, Jin DK, Kim JH, et al. Multiplex ligation-dependent probe amplification assay for diagnosis of congenital adrenal hyperplasia. Ann Clin Lab Sci. 2011;41(1):44-47. [PubMed] [Google Scholar]
- 310. Simpson JL, Rechitsky S. Prenatal genetic testing and treatment for congenital adrenal hyperplasia. Fertil Steril. 2019;111(1):21-23. [DOI] [PubMed] [Google Scholar]
- 311. Forest MG, Dorr HG. Prenatal treatment of congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency: European experience in 223 pregnancies at risk. Ped Res. 1993;33(5):S3. [Google Scholar]
- 312. Frasier SD, Thorneycroft IH, Weiss BA, Horton R. Letter: Elevated amniotic fluid concentration of 17 alpha-hydroxyprogesterone in congenital adrenal hyperplasia. J Pediatr. 1975;86(2):310-312. [DOI] [PubMed] [Google Scholar]
- 313. Jeffcoate TN, Fliegner JR, Russell SH, Davis JC, Wade AP. Diagnosis of the adrenogenital syndrome before birth. Lancet. 1965;2(7412):553-555. [DOI] [PubMed] [Google Scholar]
- 314. Pollack MS, Maurer D, Levine LS, et al. Prenatal diagnosis of congenital adrenal hyperplasia (21-hydroxylase deficiency) by HLA typing. Lancet. 1979;1(8126):1107-1108. [DOI] [PubMed] [Google Scholar]
- 315. Forest MG, Dorr HG. Prenatal therapy in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: retrospective follow-up study of 253 treated pregnancies in 215 families. Endocrinologist. 2003;13(3):252-259. [Google Scholar]
- 316. Salomon LJ, Sotiriadis A, Wulff CB, Odibo A, Akolekar R. Risk of miscarriage following amniocentesis or chorionic villus sampling: systematic review of literature and updated meta-analysis. Ultrasound Obstet Gynecol. 2019;54(4):442-451. [DOI] [PubMed] [Google Scholar]
- 317. Lo YM. Fetal DNA in maternal plasma: biology and diagnostic applications. Clin Chem. 2000;46(12):1903-1906. [PubMed] [Google Scholar]
- 318. Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485-487. [DOI] [PubMed] [Google Scholar]
- 319. Simpson JL, Elias S. Isolating fetal cells from maternal blood. Advances in prenatal diagnosis through molecular technology. JAMA. 1993;270(19):2357-2361. [PubMed] [Google Scholar]
- 320. Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93(2):705-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Devaney SA, Palomaki GE, Scott JA, Bianchi DW. Noninvasive fetal sex determination using cell-free fetal DNA: a systematic review and meta-analysis. JAMA. 2011;306(6):627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Tardy-Guidollet V, Menassa R, Costa JM, et al. New management strategy of pregnancies at risk of congenital adrenal hyperplasia using fetal sex determination in maternal serum: French cohort of 258 cases (2002-2011). J Clin Endocrinol Metab. 2014;99(4):1180-1188. [DOI] [PubMed] [Google Scholar]
- 323. New MI, Tong YK, Yuen T, et al. Noninvasive prenatal diagnosis of congenital adrenal hyperplasia using cell-free fetal DNA in maternal plasma. J Clin Endocrinol Metab. 2014;99(6):E1022-E1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Dondorp W, de Wert G. Refining the ethics of preimplantation genetic diagnosis: a plea for contextualized proportionality. Bioethics. 2019;33(2):294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Simpson JL. Preimplantation genetic diagnosis at 20 years. Prenat Diagn. 2010;30(7):682-695. [DOI] [PubMed] [Google Scholar]
- 326. Clayton PE, Miller WL, Oberfield SE, Ritzén EM, Sippell WG, Speiser PW; ESPE/ LWPES CAH Working Group . Consensus statement on 21-hydroxylase deficiency from the European Society for Paediatric Endocrinology and the Lawson Wilkins Pediatric Endocrine Society. Horm Res. 2002;58(4):188-195. [DOI] [PubMed] [Google Scholar]
- 327. Merke DP, Bornstein SR, Avila NA, Chrousos GP. NIH conference. Future directions in the study and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Ann Intern Med. 2002;136(4):320-334. [DOI] [PubMed] [Google Scholar]
- 328. Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33(1):14-22. [DOI] [PubMed] [Google Scholar]
- 329. Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: a meta-analysis. Stress. 2008;11(3):177-197. [DOI] [PubMed] [Google Scholar]
- 330. Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009;94(9):3477-3480. [DOI] [PubMed] [Google Scholar]
- 331. Merza Z, Rostami-Hodjegan A, Memmott A, et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2006;65(1):45-50. [DOI] [PubMed] [Google Scholar]
- 332. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017(17):10-6736. [DOI] [PubMed] [Google Scholar]
- 333. Lajic S, Nordenström A, Ritzén EM, Wedell A. Prenatal treatment of congenital adrenal hyperplasia. Eur J Endocrinol. 2004;151(Suppl 3):U63-U69. [DOI] [PubMed] [Google Scholar]
- 334. Nordenström A, Marcus C, Axelson M, Wedell A, Ritzén EM. Failure of cortisone acetate treatment in congenital adrenal hyperplasia because of defective 11beta-hydroxysteroid dehydrogenase reductase activity. J Clin Endocrinol Metab. 1999;84(4):1210-1213. [DOI] [PubMed] [Google Scholar]
- 335. Charmandari E, Chrousos GP, Merke DP. Adrenocorticotropin hypersecretion and pituitary microadenoma following bilateral adrenalectomy in a patient with classic 21-hydroxylase deficiency. J Pediatr Endocrinol Metab. 2005;18(1):97-101. [DOI] [PubMed] [Google Scholar]
- 336. Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125-2136. [DOI] [PubMed] [Google Scholar]
- 337. Melin J, Parra-Guillen ZP, Michelet R, et al. Pharmacokinetic/pharmacodynamic evaluation of hydrocortisone therapy in pediatric patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(3):e1729-e1740. [DOI] [PubMed] [Google Scholar]
- 338. German A, Suraiya S, Tenenbaum-Rakover Y, Koren I, Pillar G, Hochberg Z. Control of childhood congenital adrenal hyperplasia and sleep activity and quality with morning or evening glucocorticoid therapy. J Clin Endocrinol Metab. 2008;93(12):4707-4710. [DOI] [PubMed] [Google Scholar]
- 339. Ng SM, Stepien KM, Krishan A. Glucocorticoid replacement regimens for treating congenital adrenal hyperplasia. Cochrane Database Syst Rev. 2020;3:CD012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Auchus RJ, Witchel SF, Leight KR, et al. Guidelines for the development of comprehensive care centers for congenital adrenal hyperplasia: guidance from the CARES foundation initiative. Int J Pediatr Endocrinol. 2010;2010(1):275213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Pijnenburg-Kleizen KJ, Thomas CMG, Otten BJ, Roeleveld N, Claahsen-van der Grinten HL. Long-term follow-up of children with classic congenital adrenal hyperplasia: suggestions for age dependent treatment in childhood and puberty. J Pediatr Endocrinol Metab. 2019;32(10):1055-1063. [DOI] [PubMed] [Google Scholar]
- 342. Barillas JE, Eichner D, Van Wagoner R, Speiser PW. Iatrogenic Cushing syndrome in a child with congenital adrenal hyperplasia: erroneous compounding of hydrocortisone. J Clin Endocrinol Metab. 2018;103(1):7-11. [DOI] [PubMed] [Google Scholar]
- 343. Neumann U, Burau D, Spielmann S, et al. Quality of compounded hydrocortisone capsules used in the treatment of children. Eur J Endocrinol. 2017;177(2):239-242. [DOI] [PubMed] [Google Scholar]
- 344. Merke DP, Cho D, Calis KA, Keil MF, Chrousos GP. Hydrocortisone suspension and hydrocortisone tablets are not bioequivalent in the treatment of children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(1):441-445. [DOI] [PubMed] [Google Scholar]
- 345. Porter J, Withe M, Ross RJ. Immediate-release granule formulation of hydrocortisone, Alkindi®, for treatment of paediatric adrenal insufficiency (Infacort development programme). Expert Rev Endocrinol Metab. 2018;13(3):119-124. [DOI] [PubMed] [Google Scholar]
- 346. Neumann U, Whitaker MJ, Wiegand S, et al. Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clin Endocrinol (Oxf). 2018;88(1):21-29. [DOI] [PubMed] [Google Scholar]
- 347. Frisch H, Battelino T, Schober E, Baumgartner-Parzer S, Nowotny P, Vierhapper H. Salt wasting in simple virilizing congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2001;14(9):1649-1655. [DOI] [PubMed] [Google Scholar]
- 348. Nimkarn S, Lin-Su K, Berglind N, Wilson RC, New MI. Aldosterone-to-renin ratio as a marker for disease severity in 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2007;92(1):137-142. [DOI] [PubMed] [Google Scholar]
- 349. Mullis PE, Hindmarsh PC, Brook CG. Sodium chloride supplement at diagnosis and during infancy in children with salt-losing 21-hydroxylase deficiency. Eur J Pediatr. 1990;150(1):22-25. [DOI] [PubMed] [Google Scholar]
- 350. Bonfig W, Roehl F, Riedl S, et al. Sodium chloride supplementation is not routinely performed in the majority of German and Austrian infants with classic salt-wasting congenital adrenal hyperplasia and has no effect on linear growth and hydrocortisone or fludrocortisone dose. Horm Res Paediatr. 2018;89(1):7-12. [DOI] [PubMed] [Google Scholar]
- 351. Hindmarsh PC, Charmandari E. Variation in absorption and half-life of hydrocortisone influence plasma cortisol concentrations. Clin Endocrinol (Oxf). 2015;82(4):557-561. [DOI] [PubMed] [Google Scholar]
- 352. Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: a re-evaluation of relative potency. J Pediatr. 2003;143(3):402-405. [DOI] [PubMed] [Google Scholar]
- 353. Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: the ability to achieve normal growth. Pediatrics. 2000;106(4):767-773. [DOI] [PubMed] [Google Scholar]
- 354. Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. J Clin Endocrinol Metab. 2007;92(5):1635-1639. [DOI] [PubMed] [Google Scholar]
- 355. Charmandari E, Hindmarsh PC, Johnston A, Brook CG. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: alterations in cortisol pharmacokinetics at puberty. J Clin Endocrinol Metab. 2001;86(6):2701-2708. [DOI] [PubMed] [Google Scholar]
- 356. Gleeson H, Davis J, Jones J, O’Shea E, Clayton PE. The challenge of delivering endocrine care and successful transition to adult services in adolescents with congenital adrenal hyperplasia: experience in a single centre over 18 years. Clin Endocrinol (Oxf). 2013;78(1):23-28. [DOI] [PubMed] [Google Scholar]
- 357. Bachelot A. Transition of care from childhood to adulthood: congenital adrenal hyperplasia. Endocr Dev. 2018;33:17-33. [DOI] [PubMed] [Google Scholar]
- 358. Arlt W, Willis DS, Wild SH, et al. ; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) . Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95(11):5110-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. de Jesus LE, Costa EC, Dekermacher S. Gender dysphoria and XX congenital adrenal hyperplasia: how frequent is it? Is male-sex rearing a good idea? J Pediatr Surg. 2019;54(11):2421-2427. [DOI] [PubMed] [Google Scholar]
- 360. Engberg H, Möller A, Hagenfeldt K, Nordenskjöld A, Frisén L. Identity, sexuality, and parenthood in women with congenital adrenal hyperplasia. J Pediatr Adolesc Gynecol. 2020;33(5):470-476. [DOI] [PubMed] [Google Scholar]
- 361. Jiang X, Xu C, Lei F, et al. MiR-30a targets IL-1α and regulates islet functions as an inflammation buffer and response factor. Sci Rep. 2017;7(1):5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Hughes IA. Congenital adrenal hyperplasia: transitional care. Growth Horm IGF Res. 2004;14(Suppl A):S60-S66. [DOI] [PubMed] [Google Scholar]
- 363. Reisch N. Review of health problems in adult patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Exp Clin Endocrinol Diabetes. 2019;127(2-03):171-177. [DOI] [PubMed] [Google Scholar]
- 364. Paizoni L, Auer MK, Schmidt H, Hübner A, Bidlingmaier M, Reisch N. Effect of androgen excess and glucocorticoid exposure on metabolic risk profiles in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Steroid Biochem Mol Biol. 2020;197(8):105540. [DOI] [PubMed] [Google Scholar]
- 365. Auer MK, Paizoni L, Hofbauer LC, et al. Effects of androgen excess and glucocorticoid exposure on bone health in adult patients with 21-hydroxylase deficiency. J Steroid Biochem Mol Biol. 2020;204(8):105734. [DOI] [PubMed] [Google Scholar]
- 366. Falhammar H, Frisén L, Norrby C, et al. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(12):E2715-E2721. [DOI] [PubMed] [Google Scholar]
- 367. Whittle E, Falhammar H. Glucocorticoid regimens in the treatment of congenital adrenal hyperplasia: a systematic review and meta-analysis. J Endocr Soc. 2019;3(6):1227-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Riepe FG, Krone N, Viemann M, Partsch CJ, Sippell WG. Management of congenital adrenal hyperplasia: results of the ESPE questionnaire. Horm Res. 2002;58(4):196-205. [DOI] [PubMed] [Google Scholar]
- 369. Auchus RJ. Management considerations for the adult with congenital adrenal hyperplasia. Mol Cell Endocrinol. 2015;408(6):190-197. [DOI] [PubMed] [Google Scholar]
- 370. Hagenfeldt K, Janson PO, Holmdahl G, et al. Fertility and pregnancy outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod. 2008;23(7):1607-1613. [DOI] [PubMed] [Google Scholar]
- 371. Han TS, Stimson RH, Rees DA, et al. ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE) . Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2013;78(2):197-203. [DOI] [PubMed] [Google Scholar]
- 372. Xu L, Lin W, Cai L, et al. Efficacy and safety of prenatal dexamethasone treatment in offspring at risk for congenital adrenal hyperplasia due to 21-hydroxylase deficiency: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2020;92(2):109-123. [DOI] [PubMed] [Google Scholar]
- 373. Miller WL. Fetal endocrine therapy for congenital adrenal hyperplasia should not be done. Best Pract Res Clin Endocrinol Metab. 2015;29(3):469-483. [DOI] [PubMed] [Google Scholar]
- 374. New MI, Abraham M, Yuen T, Lekarev O. An update on prenatal diagnosis and treatment of congenital adrenal hyperplasia. Semin Reprod Med. 2012;30(5):396-399. [DOI] [PubMed] [Google Scholar]
- 375. Hirvikoski T, Nordenström A, Wedell A, Ritzén M, Lajic S. Prenatal dexamethasone treatment of children at risk for congenital adrenal hyperplasia: the Swedish experience and standpoint. J Clin Endocrinol Metab. 2012;97(6):1881-1883. [DOI] [PubMed] [Google Scholar]
- 376. Engels M, Span PN, van Herwaarden AE, Sweep FCGJ, Stikkelbroeck NMML, Claahsen-van der Grinten HL. Testicular adrenal rest tumors: current insights on prevalence, characteristics, origin, and treatment. Endocr Rev. 2019;40(4):973-987. [DOI] [PubMed] [Google Scholar]
- 377. Lottspeich C, Müller-Lisse U, Seiler L, Schmitt-Graeff AH, Reincke M, Reisch N. Three cases of testicular adrenal rest tumors in congenital adrenal hyperplasia-a diagnostic and therapeutic challenge. Urology. 2019;129(7):24-28. [DOI] [PubMed] [Google Scholar]
- 378. Johannsson G, Nilsson AG, Bergthorsdottir R, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. 2012;97(2):473-481. [DOI] [PubMed] [Google Scholar]
- 379. Isidori AM, Venneri MA, Graziadio C, et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):173-185. [DOI] [PubMed] [Google Scholar]
- 380. Plat L, Leproult R, L’Hermite-Baleriaux M, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84(9):3082-3092. [DOI] [PubMed] [Google Scholar]
- 381. Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94(5):1548-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Whitaker M, Debono M, Huatan H, Merke D, Arlt W, Ross RJ. An oral multiparticulate, modified-release, hydrocortisone replacement therapy that provides physiological cortisol exposure. Clin Endocrinol (Oxf). 2014;80(4):554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Merke DP, Mallappa A, Arlt W, et al. OR25-02 A Phase 3 study of a modified-release hydrocortisone in the treatment of congenital adrenal hyperplasia. J Endocr Soc. 2020;4(Suppl 1):OR25-02. [Google Scholar]
- 384. Pofi R, Prete A, Thornton-Jones V, et al. Plasma renin measurements are unrelated to mineralocorticoid replacement dose in patients with primary adrenal insufficiency. J Clin Endocrinol Metab. 2020;105(1):314-326. [DOI] [PubMed] [Google Scholar]
- 385. Turcu AF, Rege J, Chomic R, et al. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100(6):2283-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. de Groot MJ, Pijnenburg-Kleizen KJ, Thomas CM, et al. Salivary morning androstenedione and 17α-OH progesterone levels in childhood and puberty in patients with classic congenital adrenal hyperplasia. Clin Chem Lab Med. 2015;53(3):461-468. [DOI] [PubMed] [Google Scholar]
- 387. Bode HH, Rivkees SA, Cowley DM, Pardy K, Johnson S. Home monitoring of 17 hydroxyprogesterone levels in congenital adrenal hyperplasia with filter paper blood samples [see comments]. J Pediatr. 1999;134(2):185-189. [DOI] [PubMed] [Google Scholar]
- 388. Wieacker I, Peter M, Borucki K, Empting S, Roehl FW, Mohnike K. Therapy monitoring in congenital adrenal hyperplasia by dried blood samples. J Pediatr Endocrinol Metab. 2015;28(7-8):867-871. [DOI] [PubMed] [Google Scholar]
- 389. Debono M, Mallappa A, Gounden V, et al. Hormonal circadian rhythms in patients with congenital adrenal hyperplasia: identifying optimal monitoring times and novel disease biomarkers. Eur J Endocrinol. 2015;173(6):727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Bacila I, Adaway J, Hawley J, et al. Measurement of salivary adrenal-specific androgens as biomarkers of therapy control in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2019;104(12):6417-6429. [DOI] [PubMed] [Google Scholar]
- 391. Gaudl A, Kratzsch J, Ceglarek U. Advancement in steroid hormone analysis by LC-MS/MS in clinical routine diagnostics - a three year recap from serum cortisol to dried blood 17α-hydroxyprogesterone. J Steroid Biochem Mol Biol. 2019;192(9):105389. [DOI] [PubMed] [Google Scholar]
- 392. Jenkins-Jones S, Parviainen L, Porter J, et al. Poor compliance and increased mortality, depression and healthcare costs in patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(4):309-320. [DOI] [PubMed] [Google Scholar]
- 393. Hahner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab. 2015;100(2):407-416. [DOI] [PubMed] [Google Scholar]
- 394. Odenwald B, Nennstiel-Ratzel U, Dörr HG, Schmidt H, Wildner M, Bonfig W. Children with classic congenital adrenal hyperplasia experience salt loss and hypoglycemia: evaluation of adrenal crises during the first 6 years of life. Eur J Endocrinol. 2016;174(2):177-186. [DOI] [PubMed] [Google Scholar]
- 395. Reisch N, Willige M, Kohn D, et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(1):35-42. [DOI] [PubMed] [Google Scholar]
- 396. Yang M, White PC. Risk factors for hospitalization of children with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2017;86(5):669-673. [DOI] [PubMed] [Google Scholar]
- 397. Rushworth RL, Falhammar H, Munns CF, Maguire AM, Torpy DJ. Hospital admission patterns in children with CAH: admission rates and adrenal crises decline with age. Int J Endocrinol. 2016;2016(1):5748264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Ali SR, Bryce J, Haghpanahan H, et al. Real-world estimates of adrenal insufficiency-related adverse events in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2021;106(1):e192-e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Tresoldi AS, Sumilo D, Perrins M, et al. Increased infection risk in Addison’s disease and congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(2):418-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. El-Maouche D, Hargreaves CJ, Sinaii N, Mallappa A, Veeraraghavan P, Merke DP. Longitudinal assessment of illnesses, stress dosing, and illness sequelae in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2018;103(6):2336-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Donaldson MD, Thomas PH, Love JG, Murray GD, McNinch AW, Savage DC. Presentation, acute illness, and learning difficulties in salt wasting 21-hydroxylase deficiency. Arch Dis Child. 1994;70(3):214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Merke DP, Chrousos GP, Eisenhofer G, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343(19):1362-1368. [DOI] [PubMed] [Google Scholar]
- 403. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Allolio B. Extensive expertise in endocrinology. Adrenal crisis. Eur J Endocrinol. 2015;172(3):R115-R124. [DOI] [PubMed] [Google Scholar]
- 405. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crisis. N Engl J Med. 2019;381(9):852-861. [DOI] [PubMed] [Google Scholar]
- 406. Aso K, Izawa M, Higuchi A, Kotoh S, Hasegawa Y. Stress doses of glucocorticoids cannot prevent progression of all adrenal crises. Clin Pediatr Endocrinol. 2009;18(1):23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Chrisp GL, Torpy DJ, Maguire AM, et al. The effect of patient-managed stress dosing on electrolytes and blood pressure in acute illness in children with adrenal insufficiency. Clin Endocrinol (Oxf). 2020;93(2):97-103. [DOI] [PubMed] [Google Scholar]
- 408. Prete A, Taylor AE, Bancos I, et al. Prevention of adrenal crisis: cortisol responses to major stress compared to stress dose hydrocortisone delivery. J Clin Endocrinol Metab. 2020;105(7):2262-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Taylor LK, Auchus RJ, Baskin LS, Miller WL. Cortisol response to operative stress with anesthesia in healthy children. J Clin Endocrinol Metab. 2013;98(9):3687-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Bidet M, Bellanné-Chantelot C, Galand-Portier MB, et al. Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. J Clin Endocrinol Metab. 2009;94(5):1570-1578. [DOI] [PubMed] [Google Scholar]
- 411. Nandagopal R, Sinaii N, Avila NA, et al. Phenotypic profiling of parents with cryptic nonclassic congenital adrenal hyperplasia: findings in 145 unrelated families. Eur J Endocrinol. 2011;164(6):977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23(2):167-179. [DOI] [PubMed] [Google Scholar]
- 413. Repping-Wuts HJ, Stikkelbroeck NM, Noordzij A, Kerstens M, Hermus AR. A glucocorticoid education group meeting: an effective strategy for improving self-management to prevent adrenal crisis. Eur J Endocrinol. 2013;169(1):17-22. [DOI] [PubMed] [Google Scholar]
- 414. Dörr HG, Schulze N, Bettendorf M, et al. Genotype-phenotype correlations in children and adolescents with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Mol Cell Pediatr. 2020;7(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. McCann-Crosby B, Chen M, Lyons SK, et al. Non classic CAH; targets of treatment and transition. Pediatr Endocr Rev. 2014;12:224-238. [PubMed] [Google Scholar]
- 416. Speiser PW. Medical treatment of classic and nonclassic congenital adrenal hyperplasia. Adv Exp Med Biol. 2011;707(6):41-45. [DOI] [PubMed] [Google Scholar]
- 417. Witchel SF. Non-classic congenital adrenal hyperplasia. Steroids. 2013;78(8):747-750. [DOI] [PubMed] [Google Scholar]
- 418. New M. Extensive clinical-experience - nonclassical 21-hydroxylase deficiency (vol 91, pg 4205, 2006). J Clin Endocrinol Metabol. 2007;92(1):142. [DOI] [PubMed] [Google Scholar]
- 419. Pijnenburg-Kleizen KJ, Borm GF, Otten BJ, et al. Absence of clinically relevant growth acceleration in untreated children with non-classical congenital adrenal hyperplasia. Horm Res Paediatr. 2012;77(3):164-169. [DOI] [PubMed] [Google Scholar]
- 420. Claahsen-van der Grinten HL, Noordam K, Borm GF, Otten BJ. Absence of increased height velocity in the first year of life in untreated children with simple virilizing congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91(4):1205-1209. [DOI] [PubMed] [Google Scholar]
- 421. Eyal O, Tenenbaum-Rakover Y, Shalitin S, Israel S, Weintrob N. Adult height of subjects with nonclassical 21-hydroxylase deficiency. Acta Paediatr. 2013;102(4):419-423. [DOI] [PubMed] [Google Scholar]
- 422. New MI, Gertner JM, Speiser PW, Del Balzo P. Growth and final height in classical and nonclassical 21-hydroxylase deficiency. J Endocrinol Invest. 1989;12(8 Suppl 3):91-95. [PubMed] [Google Scholar]
- 423. Trinh L, Nimkarn S, New MI, Lin-Su K. Growth and pubertal characteristics in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Pediatr Endocrinol Metab. 2007;20(8):883-891. [DOI] [PubMed] [Google Scholar]
- 424. Claahsen-van der Grinten HL, Stikkelbroeck NM, Otten BJ, Hermus AR. Congenital adrenal hyperplasia – pharmacologic interventions from the prenatal phase to adulthood. Pharmacol Ther. 2011;132(1):1-14. [DOI] [PubMed] [Google Scholar]
- 425. Moran C, Azziz R, Carmina E, et al. 21-Hydroxylase-deficient nonclassic adrenal hyperplasia is a progressive disorder: a multicenter study. Am J Obstet Gynecol. 2000;183(6):1468-1474. [DOI] [PubMed] [Google Scholar]
- 426. Livadas S, Bothou C. Management of the female with non-classical congenital adrenal hyperplasia (NCCAH): a patient-oriented approach. Front Endocrinol (Lausanne). 2019;10(6):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Nordenström A, Falhammar H. MANAGEMENT OF ENDOCRINE DISEASE: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180(3):R127-R145. [DOI] [PubMed] [Google Scholar]
- 428. Buitenwerf E, Links TP, Kema IP, Haadsma ML, Kerstens MN. Congenital adrenal hyperplasia as a cause of adrenal incidentaloma. Neth J Med. 2017;75(7):298-300. [PubMed] [Google Scholar]
- 429. Jaresch S, Kornely E, Kley HK, Schlaghecke R. Adrenal incidentaloma and patients with homozygous or heterozygous congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1992;74(3):685-689. [DOI] [PubMed] [Google Scholar]
- 430. Falhammar H, Torpy DJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency presenting as adrenal incidentaloma: a systematic review and meta-analysis. Endocr Pract. 2016;22(6):736-752. [DOI] [PubMed] [Google Scholar]
- 431. van Zuuren EJ, Fedorowicz Z, Carter B, Pandis N. Interventions for hirsutism (excluding laser and photoepilation therapy alone). Cochrane Database Syst Rev. 2015;4:CD010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Hamzavi I, Tan E, Shapiro J, Lui H. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57(1):54-59. [DOI] [PubMed] [Google Scholar]
- 433. Spritzer P, Billaud L, Thalabard JC, et al. Cyproterone acetate versus hydrocortisone treatment in late-onset adrenal hyperplasia. J Clin Endocrinol Metab. 1990;70(3):642-646. [DOI] [PubMed] [Google Scholar]
- 434. van Zuuren EJ, Fedorowicz Z, Carter B, Andriolo RB, Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2012;5:CD007628. [DOI] [PubMed] [Google Scholar]
- 435. Falhammar H, Nyström HF, Ekström U, Granberg S, Wedell A, Thorén M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2012;166(3):441-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Engels M, Gehrmann K, Falhammar H, et al. ; dsd-LIFE group . Gonadal function in adult male patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(3):285-294. [DOI] [PubMed] [Google Scholar]
- 437. Kocova M, Janevska V, Anastasovska V. Testicular adrenal rest tumors in boys with 21-hydroxylase deficiency, timely diagnosis and follow-up. Endocr Connect. 2018;7(4):544-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Mercado AB, Wilson RC, Cheng KC, Wei JQ, New MI. Prenatal treatment and diagnosis of congenital adrenal hyperplasia owing to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1995;80(7):2014-2020. [DOI] [PubMed] [Google Scholar]
- 439. Mercè Fernández-Balsells M, Muthusamy K, Smushkin G, et al. Prenatal dexamethasone use for the prevention of virilization in pregnancies at risk for classical congenital adrenal hyperplasia because of 21-hydroxylase (CYP21A2) deficiency: a systematic review and meta-analyses. Clin Endocrinol (Oxf). 2010;73(4):436-444. [DOI] [PubMed] [Google Scholar]
- 440. Seckl JR, Miller WL. How safe is long-term prenatal glucocorticoid treatment? JAMA. 1997;277(13):1077-1079. [PubMed] [Google Scholar]
- 441. McCann-Crosby B, Placencia FX, Adeyemi-Fowode O, et al. Challenges in prenatal treatment with dexamethasone. Pediatr Endocrinol Rev. 2018;16(1):186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Witchel SF, Miller WL. Prenatal treatment of congenital adrenal hyperplasia-not standard of care. J Genet Couns. 2012;21(5):615-624. [DOI] [PubMed] [Google Scholar]
- 443. Dreger A, Feder EK, Tamar-Mattis A. Prenatal dexamethasone for congenital adrenal hyperplasia: an ethics canary in the modern medical mine. J Bioeth Inq. 2012;9(3):277-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Heland S, Hewitt JK, McGillivray G, Walker SP. Preventing female virilisation in congenital adrenal hyperplasia: the controversial role of antenatal dexamethasone. Aust N Z J Obstet Gynaecol. 2016;56(3):225-232. [DOI] [PubMed] [Google Scholar]
- 445. Speiser PW, Azziz R, Baskin LS, et al. ; Endocrine Society . Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Ziejewski MK, Solomon HM, Stanislaus D, Clark RL, White TE, Apostoli AR. The potential role for corticosterone in the induction of cleft palate in mice after treatment with a selective NK-1 receptor antagonist, casopitant (GW679769B). Birth Defects Res B Dev Reprod Toxicol. 2012;95(1):54-62. [DOI] [PubMed] [Google Scholar]
- 447. Celsi G, Kistner A, Aizman R, et al. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44(3):317-322. [DOI] [PubMed] [Google Scholar]
- 448. Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R453-R461. [DOI] [PubMed] [Google Scholar]
- 449. Manojlović-Stojanoski MN, Filipović BR, Nestorović NM, et al. Morpho-functional characteristics of rat fetal thyroid gland are affected by prenatal dexamethasone exposure. Steroids. 2014;84(6):22-29. [DOI] [PubMed] [Google Scholar]
- 450. Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27(6):1200-1204. [DOI] [PubMed] [Google Scholar]
- 451. Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101(10):2174-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151(4):1581-1587. [DOI] [PubMed] [Google Scholar]
- 453. de Vries A, Holmes MC, Heijnis A, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117(4):1058-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996;39(11):1299-1305. [DOI] [PubMed] [Google Scholar]
- 455. Holmes MC, Abrahamsen CT, French KL, Paterson JM, Mullins JJ, Seckl JR. The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. J Neurosci. 2006;26(14):3840-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol. 1999;94(2):213-218. [DOI] [PubMed] [Google Scholar]
- 457. Moss TJ, Doherty DA, Nitsos I, Sloboda DM, Harding R, Newnham JP. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am J Obstet Gynecol. 2005;192(1):146-152. [DOI] [PubMed] [Google Scholar]
- 458. Quinlivan JA, Dunlop SA, Newnham JP, Evans SF, Beazley LD. Repeated, but not single, maternal administration of corticosteroids delays myelination in the brain of fetal sheep. Perinat Neonat Med. 1999;4(6):47-45. [Google Scholar]
- 459. Uno H, Lohmiller L, Thieme C, et al. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res. 1990;53(2):157-167. [DOI] [PubMed] [Google Scholar]
- 460. Heberden C, Meffray E, Goustard-Langelier B, Maximin E, Lavialle M. Dexamethasone inhibits the maturation of newly formed neurons and glia supplemented with polyunsaturated fatty acids. J Steroid Biochem Mol Biol. 2013;138(11):395-402. [DOI] [PubMed] [Google Scholar]
- 461. Crudo A, Suderman M, Moisiadis VG, et al. Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology. 2013;154(3):1168-1180. [DOI] [PubMed] [Google Scholar]
- 462. Iqbal M, Moisiadis VG, Kostaki A, Matthews SG. Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function. Endocrinology. 2012;153(7):3295-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Quinn TA, Ratnayake U, Castillo-Melendez M, Moritz KM, Dickinson H, Walker DW. Adrenal steroidogenesis following prenatal dexamethasone exposure in the spiny mouse. J Endocrinol. 2014;221(2):347-362. [DOI] [PubMed] [Google Scholar]
- 464. Crudo A, Petropoulos S, Moisiadis VG, et al. Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: multigenerational effects. Endocrinology. 2012;153(7):3269-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18(1):135-156. [DOI] [PubMed] [Google Scholar]
- 466. Xiao WL, Liu XY, Liu YS, Zhang DZ, Xue LF. The relationship between maternal corticosteroid use and orofacial clefts-a meta-analysis. Reprod Toxicol. 2017;69(4):99-105. [DOI] [PubMed] [Google Scholar]
- 467. Wapner RJ, Sorokin Y, Mele L, et al. ; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357(12):1190-1198. [DOI] [PubMed] [Google Scholar]
- 468. Forest MG, Nicolino M, David M, Morel Y. The virilized female: endocrine background. BJU Int. 2004;93(Suppl 3):35-43. [DOI] [PubMed] [Google Scholar]
- 469. New MI, Carlson A, Obeid J, et al. Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab. 2001;86(12):5651-5657. [DOI] [PubMed] [Google Scholar]
- 470. Lajic S, Wedell A, Bui TH, Ritzén EM, Holst M. Long-term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1998;83(11):3872-3880. [DOI] [PubMed] [Google Scholar]
- 471. Grunt S, Steinlin M, Weisstanner C, Schöning M, Mullis PE, Flück CE. Acute encephalopathy with unilateral cortical-subcortical lesions in two unrelated kindreds treated with glucocorticoids prenatally for congenital adrenal hyperplasia due to 21-hydroxylase deficiency: established facts and novel insight. Horm Res Paediatr. 2013;80(1):57-63. [DOI] [PubMed] [Google Scholar]
- 472. Rijk Y, van Alfen-van der Velden J, Claahsen-van der Grinten HL. Prenatal treatment with dexamethasone in suspected congenital adrenal hyperplasia and orofacial cleft: a case report and review of the literature. Pediatr Endocrinol Rev. 2017;15(1):21-25. [DOI] [PubMed] [Google Scholar]
- 473. Trautman PD, Meyer-Bahlburg HF, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology. 1995;20(4):439-449. [DOI] [PubMed] [Google Scholar]
- 474. Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Cognitive and motor development of children with and without congenital adrenal hyperplasia after early-prenatal dexamethasone. J Clin Endocrinol Metab. 2004;89(2):610-614. [DOI] [PubMed] [Google Scholar]
- 475. Hirvikoski T, Nordenström A, Lindholm T, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92(2):542-548. [DOI] [PubMed] [Google Scholar]
- 476. Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Lajic S. Long-term follow-up of prenatally treated children at risk for congenital adrenal hyperplasia: does dexamethasone cause behavioural problems? Eur J Endocrinol. 2008;159(3):309-316. [DOI] [PubMed] [Google Scholar]
- 477. Hirvikoski T, Lindholm T, Lajic S, Nordenström A. Gender role behaviour in prenatally dexamethasone-treated children at risk for congenital adrenal hyperplasia – a pilot study. Acta Paediatr. 2011;100(9):e112-e119. [DOI] [PubMed] [Google Scholar]
- 478. Meyer-Bahlburg HF, Dolezal C, Haggerty R, Silverman M, New MI. Cognitive outcome of offspring from dexamethasone-treated pregnancies at risk for congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(1):103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Maryniak A, Ginalska-Malinowska M, Bielawska A, Ondruch A. Cognitive and social function in girls with congenital adrenal hyperplasia – influence of prenatally administered dexamethasone. Child Neuropsychol. 2014;20(1):60-70. [DOI] [PubMed] [Google Scholar]
- 480. Wallensteen L, Zimmermann M, Thomsen Sandberg M, et al. Sex-dimorphic effects of prenatal treatment with dexamethasone. J Clin Endocrinol Metab. 2016;101(10):3838-3846. [DOI] [PubMed] [Google Scholar]
- 481. Wallensteen L, Karlsson L, Messina V, et al. Evaluation of behavioral problems after prenatal dexamethasone treatment in Swedish children and adolescents at risk of congenital adrenal hyperplasia. Horm Behav. 2018;98(2):219-224. [DOI] [PubMed] [Google Scholar]
- 482. Karlsson L, Nordenström A, Hirvikoski T, Lajic S. Prenatal dexamethasone treatment in the context of at risk CAH pregnancies: long-term behavioral and cognitive outcome. Psychoneuroendocrinology. 2018;91(5):68-74. [DOI] [PubMed] [Google Scholar]
- 483. Karlsson L, Barbaro M, Ewing E, Gomez-Cabrero D, Lajic S. Epigenetic alterations associated with early prenatal dexamethasone treatment. J Endocr Soc. 2019;3(1):250-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Riveline JP, Baz B, Nguewa JL, et al. Exposure to glucocorticoids in the first part of fetal life is associated with insulin secretory defect in adult humans. J Clin Endocrinol Metab. 2020;105(3):dgz145. [DOI] [PubMed] [Google Scholar]
- 485. Wallensteen L, Karlsson L, Messina V, Nordenstrom A, Lajic S. Perturbed beta-cell function and lipid profile after early prenatal dexamethasone exposure in individuals without CAH. J Clin Endocrinol Metab. 2020;105(7):e2439-e2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Karlsson L, Gezelius A, Nordenström A, Hirvikoski T, Lajic S. Cognitive impairment in adolescents and adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2017;87(6):651-659. [DOI] [PubMed] [Google Scholar]
- 487. Van’t Westeinde A, Karlsson L, Nordenström A, Padilla N, Lajic S. First trimester prenatal dexamethasone treatment is associated with alterations in brain structure at adult age. J Clin Endocrinol Metab. 2020;105(8):2575-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Ilg L, Kirschbaum C, Li SC, Rosenlöcher F, Miller R, Alexander N. Persistent effects of antenatal synthetic glucocorticoids on endocrine stress reactivity from childhood to adolescence. J Clin Endocrinol Metab. 2019;104(3):827-834. [DOI] [PubMed] [Google Scholar]
- 489. Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA. 2020;323(19):1924-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Mallappa A, Sinaii N, Kumar P, et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(3):1137-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Nella AA, Mallappa A, Perritt AF, et al. A phase 2 study of continuous subcutaneous hydrocortisone infusion in adults with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2016;101(12):4690-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Merke DP, Keil MF, Jones JV, Fields J, Hill S, Cutler GB Jr. Flutamide, testolactone, and reduced hydrocortisone dose maintain normal growth velocity and bone maturation despite elevated androgen levels in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85(3):1114-1120. [DOI] [PubMed] [Google Scholar]
- 493. Auchus RJ, Sharifi N. Sex hormones and prostate cancer. Annu Rev Med. 2020;71(1):33-45. [DOI] [PubMed] [Google Scholar]
- 494. Auchus RJ, Osborne E, Hammer GD, et al. Abiraterone acetate added to physiologic hydrocortisone dosing safely controls androgen excess in adult women with classical 21-hydroxylase deficiency. Endocr Rev. 2012;33:SUN-LB2. [Google Scholar]
- 495. Wright C, O’Day P, Alyamani M, Sharifi N, Auchus RJ. Abiraterone acetate treatment lowers 11-oxygenated androgens. Eur J Endocrinol. 2020;182(4):413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496. Turcu AF, Spencer-Segal JL, Farber RH, et al. Single-dose study of a corticotropin-releasing factor receptor-1 antagonist in women with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2016;101(3):1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Gehrand AL, Phillips J, Malott K, Raff H. A long-acting neutralizing monoclonal ACTH antibody blocks corticosterone and adrenal gene responses in neonatal rats. Endocrinology. 2019;160(7):1719-1730. [DOI] [PubMed] [Google Scholar]
- 498. Sanders K, Mol JA, Kooistra HS, Galac S. Melanocortin 2 receptor antagonists in canine pituitary-dependent hypercortisolism: in vitro studies. Vet Res Commun. 2018;42(4):283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Turcu AF, Auchus RJ. Novel treatment strategies in congenital adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Van Wyk JJ, Gunther DF, Ritzén EM, et al. The use of adrenalectomy as a treatment for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1996;81(9):3180-3190. [DOI] [PubMed] [Google Scholar]
- 501. MacKay D, Nordenström A, Falhammar H. Bilateral adrenalectomy in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(5):1767-1778. [DOI] [PubMed] [Google Scholar]
- 502. Crocker MK, Barak S, Millo CM, et al. Use of PET/CT with cosyntropin stimulation to identify and localize adrenal rest tissue following adrenalectomy in a woman with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(11):E2084-E2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Burman P, Falhammar H, Waldenström E, Sundin A, Bitzén U. 11C-metomidate PET/CT detected multiple ectopic adrenal rest tumors in a woman with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;(1):e675-e679. [DOI] [PubMed] [Google Scholar]
- 504. Bry-Gauillard H, Cartes A, Young J. Mitotane for 21-hydroxylase deficiency in an infertile man. N Engl J Med. 2014;371(21):2042-2044. [DOI] [PubMed] [Google Scholar]
- 505. El-Maouche D, Merke DP, Vogiatzi MG, et al. A phase 2, multicenter study of nevanimibe for the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(8):2771-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Quintos JB, Vogiatzi MG, Harbison MD, New MI. Growth hormone therapy alone or in combination with gonadotropin-releasing hormone analog therapy to improve the height deficit in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(4):1511-1517. [DOI] [PubMed] [Google Scholar]
- 507. Lin-Su K, Harbison MD, Lekarev O, Vogiatzi MG, New MI. Final adult height in children with congenital adrenal hyperplasia treated with growth hormone. J Clin Endocrinol Metab. 2011;96(6):1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Lin-Su K, Vogiatzi MG, Marshall I, et al. Treatment with growth hormone and luteinizing hormone releasing hormone analog improves final adult height in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2005;90(6):3318-3325. [DOI] [PubMed] [Google Scholar]
- 509. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. [DOI] [PubMed] [Google Scholar]
- 510. Aghazadeh Y, Nostro MC. Cell therapy for type 1 diabetes: current and future strategies. Curr Diab Rep. 2017;17(6):37. [DOI] [PubMed] [Google Scholar]
- 511. Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29(10):892-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Ruiz-Babot G, Hadjidemetriou I, King PJ, Guasti L. New directions for the treatment of adrenal insufficiency. Front Endocrinol (Lausanne). 2015;6(5):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Ruiz-Babot G, Balyura M, Hadjidemetriou I, et al. Modeling congenital adrenal hyperplasia and testing interventions for adrenal insufficiency using donor-specific reprogrammed cells. Cell Rep. 2018;22(5):1236-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Shiroishi T, Sagai T, Natsuume-Sakai S, Moriwaki K. Lethal deletion of the complement component C4 and steroid 21-hydroxylase genes in the mouse H-2 class III region, caused by meiotic recombination. Proc Natl Acad Sci U S A. 1987;84(9):2819-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Tajima T, Okada T, Ma XM, Ramsey W, Bornstein S, Aguilera G. Restoration of adrenal steroidogenesis by adenovirus-mediated transfer of human cytochromeP450 21-hydroxylase into the adrenal gland of21-hydroxylase-deficient mice. Gene Ther. 1999;6(11):1898-1903. [DOI] [PubMed] [Google Scholar]
- 516. Naiki Y, Miyado M, Horikawa R, et al. Extra-adrenal induction of Cyp21a1 ameliorates systemic steroid metabolism in a mouse model of congenital adrenal hyperplasia. Endocr J. 2016;63(10):897-904. [DOI] [PubMed] [Google Scholar]
- 517. Perdomini M, Dos Santos C, Goumeaux C, Blouin V, Bougnères P. An AAVrh10-CAG-CYP21-HA vector allows persistent correction of 21-hydroxylase deficiency in a Cyp21-/- mouse model. Gene Ther. 2017;24(5):275-281. [DOI] [PubMed] [Google Scholar]
- 518. Markmann S, De BP, Reid J, et al. Biology of the adrenal gland cortex obviates effective use of adeno-associated virus vectors to treat hereditary adrenal disorders. Hum Gene Ther. 2018;29(4):403-412. [DOI] [PubMed] [Google Scholar]
- 519. Jordan-Young RM. Hormones, context, and “brain gender”: a review of evidence from congenital adrenal hyperplasia. Soc Sci Med. 2012;74(11):1738-1744. [DOI] [PubMed] [Google Scholar]
- 520. Hines M. Human gender development. Neurosci Biobehav Rev. 2020;118(7):89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Berenbaum SA. Beyond pink and blue: the complexity of early androgen effects on gender development. Child Dev Perspect. 2018;12(1):58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522. Meyer-Bahlburg HFL. Psychoendocrinology of congenital adrenal hyperplasia. In: New MI, Lekarev O, Parsa A, O’Malley B, Hammer GD, eds. Genetic Steroid Disorders. Elsevier; 2014:285-300. [Google Scholar]
- 523. Stout SA, Litvak M, Robbins NM, Sandberg DE. Congenital adrenal hyperplasia: classification of studies employing psychological endpoints. Int J Pediatr Endocrinol. 2010;2010(10):191520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Stein RE. The 1990s: a decade of change in understanding children with ongoing conditions. Arch Pediatr Adolesc Med. 2011;165(10):880-883. [DOI] [PubMed] [Google Scholar]
- 525. Sandberg DE, Mazur T. A noncategorical approach to the psychosocial care of persons with DSD and their families. In: Kreukels BPC, Steensma TD, de Vries ALC, eds. Gender Dysphoria and Disorders of Sex Development. Springer; 2014:93-114. [Google Scholar]
- 526. Almasri J, Zaiem F, Rodriguez-Gutierrez R, et al. Genital reconstructive surgery in females with congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(11):4089-4096. [DOI] [PubMed] [Google Scholar]
- 527. Cousino MK, Hazen RA. Parenting stress among caregivers of children with chronic illness: a systematic review. J Pediatr Psychol. 2013;38(8):809-828. [DOI] [PubMed] [Google Scholar]
- 528. Driscoll KA, Johnson SB, Barker D, et al. Risk factors associated with depressive symptoms in caregivers of children with type 1 diabetes or cystic fibrosis. J Pediatr Psychol. 2010;35(8):814-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Hansen JA, Weissbrod C, Schwartz DD, Taylor WP. Paternal involvement in pediatric Type 1 diabetes: fathers’ and mothers’ psychological functioning and disease management. Fam Syst Health. 2012;30(1):47-59. [DOI] [PubMed] [Google Scholar]
- 530. Barakat LP, Patterson CA, Daniel LC, Dampier C. Quality of life among adolescents with sickle cell disease: mediation of pain by internalizing symptoms and parenting stress. Health Qual Life Outcomes. 2008;6(8):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Helgeson VS, Becker D, Escobar O, Siminerio L. Families with children with diabetes: implications of parent stress for parent and child health. J Pediatr Psychol. 2012;37(4):467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Colletti CJ, Wolfe-Christensen C, Carpentier MY, et al. The relationship of parental overprotection, perceived vulnerability, and parenting stress to behavioral, emotional, and social adjustment in children with cancer. Pediatr Blood Cancer. 2008;51(2):269-274. [DOI] [PubMed] [Google Scholar]
- 533. Mullins LL, Wolfe-Christensen C, Pai AL, et al. The relationship of parental overprotection, perceived child vulnerability, and parenting stress to uncertainty in youth with chronic illness. J Pediatr Psychol. 2007;32(8):973-982. [DOI] [PubMed] [Google Scholar]
- 534. Tluczek A, McKechnie AC, Brown RL. Factors associated with parental perception of child vulnerability 12 months after abnormal newborn screening results. Res Nurs Health. 2011;34(5):389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Bourdeau TL, Mullins LL, Carpentier MY, Colletti CJM, Wolfe-Christensen C. An examination of parenting variables and child self-care behavior across disease groups. J Dev Phys Disabil. 2007;19(2):125-134. [Google Scholar]
- 536. Litzelman K, Catrine K, Gangnon R, Witt WP. Quality of life among parents of children with cancer or brain tumors: the impact of child characteristics and parental psychosocial factors. Qual Life Res. 2011;20(8):1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Monaghan MC, Hilliard ME, Cogen FR, Streisand R. Nighttime caregiving behaviors among parents of young children with Type 1 diabetes: associations with illness characteristics and parent functioning. Fam Syst Health. 2009;27(1):28-38. [DOI] [PubMed] [Google Scholar]
- 538. Pinquart M. Body image of children and adolescents with chronic illness: a meta-analytic comparison with healthy peers. Body Image. 2013;10(2):141-148. [DOI] [PubMed] [Google Scholar]
- 539. Pinquart M. Self-esteem of children and adolescents with chronic illness: a meta-analysis. Child Care Health Dev. 2013;39(2):153-161. [DOI] [PubMed] [Google Scholar]
- 540. Brady AM, Deighton J, Stansfeld S. Chronic illness in childhood and early adolescence: a longitudinal exploration of co-occurring mental illness. Dev Psychopathol. 2020;4(5):1-14. [DOI] [PubMed] [Google Scholar]
- 541. Sentenac M, Arnaud C, Gavin A, Molcho M, Gabhainn SN, Godeau E. Peer victimization among school-aged children with chronic conditions. Epidemiol Rev. 2012;34(11):120-128. [DOI] [PubMed] [Google Scholar]
- 542. Lum A, Wakefield CE, Donnan B, Burns MA, Fardell JE, Marshall GM. Understanding the school experiences of children and adolescents with serious chronic illness: a systematic meta-review. Child Care Health Dev. 2017;43(5):645-662. [DOI] [PubMed] [Google Scholar]
- 543. Pinquart M, Shen Y. Behavior problems in children and adolescents with chronic physical illness: a meta-analysis. J Pediatr Psychol. 2011;36(9):1003-1016. [DOI] [PubMed] [Google Scholar]
- 544. Secinti E, Thompson EJ, Richards M, Gaysina D. Research review: childhood chronic physical illness and adult emotional health - a systematic review and meta-analysis. J Child Psychol Psychiatry. 2017;58(7):753-769. [DOI] [PubMed] [Google Scholar]
- 545. Engberg H, Butwicka A, Nordenström A, et al. Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: a total population study. Psychoneuroendocrinology. 2015;60(10):195-205. [DOI] [PubMed] [Google Scholar]
- 546. Gray WN, Schaefer MR, Resmini-Rawlinson A, Wagoner ST. Barriers to transition from pediatric to adult care: a systematic review. J Pediatr Psychol. 2018;43(5):488-502. [DOI] [PubMed] [Google Scholar]
- 547. Fleming L, Knafl K, Van Riper M. How the child’s gender matters for families having a child with congenital adrenal hyperplasia. J Fam Nurs. 2017;23(4):516-533. [DOI] [PubMed] [Google Scholar]
- 548. Meyer-Bahlburg HFL, Khuri J, Reyes-Portillo J, New MI. Stigma in medical settings as reported retrospectively by women with congenital adrenal hyperplasia (CAH) for their childhood and adolescence. J Pediatr Psychol. 2017;42(5):496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549. Beatty JE. Career barriers experienced by people with chronic illness: a U.S. study. Empl Responsib Rights J. 2011;24(2):91-110. [Google Scholar]
- 550. Crissman HP, Warner L, Gardner M, et al. Children with disorders of sex development: a qualitative study of early parental experience. Int J Pediatr Endocrinol. 2011;2011(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Meyer-Bahlburg HFL, Khuri J, Reyes-Portillo J, Ehrhardt AA, New MI. Stigma associated with classical congenital adrenal hyperplasia in women’s sexual lives. Arch Sex Behav. 2018;47(4):943-951. [DOI] [PubMed] [Google Scholar]
- 552. Meyer-Bahlburg HFL, Khuri J, Reyes-Portillo J, Ehrhardt AA, New MI. Stigma associated with classical congenital adrenal hyperplasia in women’s sexual lives. Arch Sex Behav. 2017;(5):10-1003. [DOI] [PubMed] [Google Scholar]
- 553. Rolston AM, Gardner M, Vilain E, Sandberg DE. Parental reports of stigma associated with child’s disorder of sex development. Int J Endocrinol. 2015;2015(3):980121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554. Armstrong KL, Henderson C, Hoan NT, Warne GL. Living with congenital adrenal hyperplasia in Vietnam: a survey of parents. J Pediatr Endocrinol Metab. 2006;19(10):1207-1223. [DOI] [PubMed] [Google Scholar]
- 555. Ediati A, Juniarto AZ, Birnie E, et al. Social stigmatisation in late identified patients with disorders of sex development in Indonesia. BMJ Paediatr Open. 2017;1(1):e000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556. de Silva KS, de Zoysa P, Dilanka WM, Dissanayake BS. Psychological impact on parents of children with congenital adrenal hyperplasia: a study from Sri Lanka. J Pediatr Endocrinol Metab. 2014;27(5-6):475-478. [DOI] [PubMed] [Google Scholar]
- 557. Zainuddin AA, Grover SR, Soon CH, et al. A multicenter cross-sectional study of Malaysian females with congenital adrenal hyperplasia: their body image and their perspectives on feminizing surgery. J Pediatr Adolesc Gynecol. 2020;33(5):477-483. [DOI] [PubMed] [Google Scholar]
- 558. Zainuddin AA, Grover SR, Shamsuddin K, Mahdy ZA. Research on quality of life in female patients with congenital adrenal hyperplasia and issues in developing nations. J Pediatr Adolesc Gynecol. 2013;26(6):296-304. [DOI] [PubMed] [Google Scholar]
- 559. Zainuddin AA, Grover SR, Soon CH, et al. Malaysian females with congenital adrenal hyperplasia: surgical outcomes and attitudes. Front Pediatr. 2019;7(4):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Weidler EM, Peterson KE. The impact of culture on disclosure in differences of sex development. Semin Pediatr Surg. 2019;28(5):150840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Qadir F, Khan MM, Medhin G, Prince M. Male gender preference, female gender disadvantage as risk factors for psychological morbidity in Pakistani women of childbearing age - a life course perspective. BMC Public Health. 2011;11(9):745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Schoer MB, Nguyen PN, Merritt DF, Wesevich VG, Hollander AS. The role of patient advocacy and the declining rate of clitoroplasty in 46,XX patients with congenital adrenal hyperplasia. Clin Pediatr (Phila). 2018;57(14):1664-1671. [DOI] [PubMed] [Google Scholar]
- 563. Crocetti D, Arfini EAG, Monro S, Yeadon-Lee T. ‘You’re basically calling doctors torturers’: stakeholder framing issues around naming intersex rights claims as human rights abuses. Sociol Health Illn. 2020;42(4):943-958. [DOI] [PubMed] [Google Scholar]
- 564. Kon AA. Ethical issues in decision-making for infants with disorders of sex development. Horm Metab Res. 2015;47(5):340-343. [DOI] [PubMed] [Google Scholar]
- 565. Chase C. What is the agenda of the intersex patient advocacy movement? Endocrinologist. 2003;13(3):240-242. [Google Scholar]
- 566. Lampalzer U, Briken P, Schweizer K. Dealing with uncertainty and lack of knowledge in diverse sex development: controversies on early surgery and questions of consent. Sex Med. 2020;8(3):472-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567. Lossie AC, Green J. Building trust: the history and ongoing relationships amongst DSD clinicians, researchers, and patient advocacy groups. Horm Metab Res. 2015;47(5):344-350. [DOI] [PubMed] [Google Scholar]
- 568. Austin J, Tamar-Mattis A, Mazur T, Henwood MJ, Rossi WC. Disorders of sex development-when and how to tell the patient. Pediatr Endocrinol Rev. 2011;8(3):213-217; quiz 223. [PubMed] [Google Scholar]
- 569. Roen K. Intersex or diverse sex development: critical review of psychosocial health care research and indications for practice. J Sex Res. 2019;56(4-5):511-528. [DOI] [PubMed] [Google Scholar]
- 570. Lee PA, Houk CP. Review of outcome information in 46,XX patients with congenital adrenal hyperplasia assigned/reared male: what does it say about gender assignment? Int J Pediatr Endocrinol. 2010;2010(12):982025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Lee PA, Houk CP, Husmann DA. Should male gender assignment be considered in the markedly virilized patient With 46,XX and congenital adrenal hyperplasia? J Urol. 2010;184(4 Suppl):1786-1792. [DOI] [PubMed] [Google Scholar]
- 572. Houk CP, Lee PA. Approach to assigning gender in 46,XX congenital adrenal hyperplasia with male external genitalia: replacing dogmatism with pragmatism. J Clin Endocrinol Metab. 2010;95(10):4501-4508. [DOI] [PubMed] [Google Scholar]
- 573. Sandberg DE, Gardner M, Kopec K, et al. Development of a decision support tool in pediatric differences/disorders of sex development. Semin Pediatr Surg. 2019;28(5):150838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574. Bougnères P, Bouvattier C, Cartigny M, Michala L. Deferring surgical treatment of ambiguous genitalia into adolescence in girls with 21-hydroxylase deficiency: a feasibility study. Int J Pediatr Endocrinol. 2017;2017(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Pasterski V, Zucker KJ, Hindmarsh PC, et al. Increased cross-gender identification independent of gender role behavior in girls with congenital adrenal hyperplasia: results from a standardized assessment of 4- to 11-year-old children. Arch Sex Behav. 2015;44(5):1363-1375. [DOI] [PubMed] [Google Scholar]
- 576. Dessens AB, Slijper FM, Drop SL. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Arch Sex Behav. 2005;34(4):389-397. [DOI] [PubMed] [Google Scholar]
- 577. Liao LM, Audi L, Magritte E, Meyer-Bahlburg HF, Quigley CA. Determinant factors of gender identity: a commentary. J Pediatr Urol. 2012;8(6):597-601. [DOI] [PubMed] [Google Scholar]
- 578. Baratz AB, Sharp MK, Sandberg DE. Disorders of sex development peer support. In: Hiort O, Ahmed S, eds. Understanding Differences and Disorders of Sex Development (DSD). Endocrine Development. Karger; 2014:99-112. [DOI] [PubMed] [Google Scholar]
- 579. Lee PA, Fuqua JS, Houk CP, Kogan BA, Mazur T, Caldamone A. Individualized care for patients with intersex (disorders/differences of sex development): part I. J Pediatr Urol. 2020;16(2):230-237. [DOI] [PubMed] [Google Scholar]
- 580. Lin-Su K, Lekarev O, Poppas DP, Vogiatzi MG. Congenital adrenal hyperplasia patient perception of ‘disorders of sex development’ nomenclature. Int J Pediatr Endocrinol. 2015;2015(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581. Johnson EK, Rosoklija I, Finlayson C, et al. Attitudes towards “disorders of sex development” nomenclature among affected individuals. J Pediatr Urol. 2017;13(6):608 e601-608 e608. [DOI] [PubMed] [Google Scholar]
- 582. Bennecke E, Kohler B, Rohle R, et al. Disorders or differences of sex development? Views of affected individuals on DSD terminology. J Sex Res. 2020;(1):1-10. [DOI] [PubMed] [Google Scholar]
- 583. Collaer ML, Hindmarsh PC, Pasterski V, Fane BA, Hines M. Reduced short term memory in congenital adrenal hyperplasia (CAH) and its relationship to spatial and quantitative performance. Psychoneuroendocrinology. 2016;64(2):164-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584. Johannsen TH, Ripa CP, Reinisch JM, Schwartz M, Mortensen EL, Main KM. Impaired cognitive function in women with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91(4):1376-1381. [DOI] [PubMed] [Google Scholar]
- 585. Hamed SA, Metwalley KA, Farghaly HS. Cognitive function in children with classic congenital adrenal hyperplasia. Eur J Pediatr. 2018;177(11):1633-1640. [DOI] [PubMed] [Google Scholar]
- 586. King TF, Lee MC, Williamson EE, Conway GS. Experience in optimizing fertility outcomes in men with congenital adrenal hyperplasia due to 21 hydroxylase deficiency. Clin Endocrinol (Oxf). 2016;84(6):830-836. [DOI] [PubMed] [Google Scholar]
- 587. Reisch N, Flade L, Scherr M, et al. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2009;94(5):1665-1670. [DOI] [PubMed] [Google Scholar]
- 588. Falhammar H, Frisén L, Norrby C, et al. Reduced frequency of biological and increased frequency of adopted children in males with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2017;102(11):4191-4199. [DOI] [PubMed] [Google Scholar]
- 589. Casteràs A, De Silva P, Rumsby G, Conway GS. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin Endocrinol (Oxf). 2009;70(6):833-837. [DOI] [PubMed] [Google Scholar]
- 590. Meyer-Bahlburg HF. What causes low rates of child-bearing in congenital adrenal hyperplasia? [Review] [58 refs]. J Clin Endocrinol Metab. 1999;84(6):1844-1847. [DOI] [PubMed] [Google Scholar]
- 591. Salm N, Yetter E, Tluczek A. Informing parents about positive newborn screen results: parents’ recommendations. J Child Health Care. 2012;16(4):367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592. Mueller SC, Ng P, Sinaii N, et al. Psychiatric characterization of children with genetic causes of hyperandrogenism. Eur J Endocrinol. 2010;163(5):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593. Falhammar H, Nyström H, Thorén M. Quality of life, social situation, and sexual satisfaction, in adult males with congenital adrenal hyperplasia. Endocrine. 2014;(1):1-9. [DOI] [PubMed] [Google Scholar]
- 594. Daae E, Feragen KB, Nermoen I, Falhammar H. Psychological adjustment, quality of life, and self-perceptions of reproductive health in males with congenital adrenal hyperplasia: a systematic review. Endocrine. 2018;62(1):3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595. Falhammar H, Butwicka A, Landén M, et al. Increased psychiatric morbidity in men with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(3):E554-E560. [DOI] [PubMed] [Google Scholar]
- 596. Lee PA, Houk CP, Ahmed SF, Hughes IA; International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology . Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006;118(2):e488-e500. [DOI] [PubMed] [Google Scholar]
- 597. Meyer-Bahlburg HF, Dolezal C, Baker SW, Ehrhardt AA, New MI. Gender development in women with congenital adrenal hyperplasia as a function of disorder severity. Arch Sex Behav. 2006;35(6):667-684. [DOI] [PubMed] [Google Scholar]
- 598. Frisén L, Nordenström A, Falhammar H, et al. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J Clin Endocrinol Metab. 2009;94(9):3432-3439. [DOI] [PubMed] [Google Scholar]
- 599. Nordenström A, Servin A, Bohlin G, Larsson A, Wedell A. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2002;87(11):5119-5124. [DOI] [PubMed] [Google Scholar]
- 600. Costello EJ, Angold A. Developmental epidemiology. In: Cicchetti D, ed. Developmental Psychopathology. Vol. 1. John Wiley & Sons, Inc.; 2016:1-35. [Google Scholar]
- 601. Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe – a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 2005;15(4):357-376. [DOI] [PubMed] [Google Scholar]
- 602. Gustavson K, Knudsen AK, Nesvåg R, Knudsen GP, Vollset SE, Reichborn-Kjennerud T. Prevalence and stability of mental disorders among young adults: findings from a longitudinal study. BMC Psychiatry. 2018;18(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603. Bernal P. Hidden morbidity in pediatric primary care. Pediatr Ann. 2003;32(6):413-428; quiz 421. [DOI] [PubMed] [Google Scholar]
- 604. Centers for Disease Control and Prevention. Mental Health in the United States. 2019. https://www.cdc.gov/workplacehealthpromotion/tools-resources/workplace-health/infographics/mental-health-united-states.html [Google Scholar]
- 605. Sandberg DE, Gardner M, Callens N, Mazur T; DSD-TRN Psychosocial Workgroup, the DSD-TRN Advocacy Advisory Network, and Accord Alliance . Interdisciplinary care in disorders/differences of sex development (DSD): The psychosocial component of the DSD-Translational research network. Am J Med Genet C Semin Med Genet. 2017;175(2):279-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606. Schaeffer TL, Tryggestad JB, Mallappa A, et al. An evidence-based model of multidisciplinary care for patients and families affected by classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Int J Pediatr Endocrinol. 2010;2010(3):692439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607. Witchel SF. The medical home concept and congenital adrenal hyperplasia: a comfortable habitat! Int J Pediatr Endocrinol. 2010;2010(8):561526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608. Law E, Fisher E, Eccleston C, Palermo TM. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2019;3(3):CD009660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609. Pai AL, McGrady M. Systematic review and meta-analysis of psychological interventions to promote treatment adherence in children, adolescents, and young adults with chronic illness. J Pediatr Psychol. 2014;39(8):918-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610. Davis AM, Brown RF, Taylor JL, Epstein RA, McPheeters ML. Transition care for children with special health care needs. Pediatrics. 2014;134(5):900-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611. Stinson J, Kohut SA, Spiegel L, et al. A systematic review of transition readiness and transfer satisfaction measures for adolescents with chronic illness. Int J Adolesc Med Health. 2014;26(2):159-174. [DOI] [PubMed] [Google Scholar]
- 612. van der Zwan YG, Janssen EH, Callens N, et al. ; Dutch Study Group on DSD . Severity of virilization is associated with cosmetic appearance and sexual function in women with congenital adrenal hyperplasia: a cross-sectional study. J Sex Med. 2013;10(3):866-875. [DOI] [PubMed] [Google Scholar]
- 613. Yankovic F, Cherian A, Steven L, Mathur A, Cuckow P. Current practice in feminizing surgery for congenital adrenal hyperplasia; a specialist survey. J Pediatr Urol. 2013;9(6 Pt B):1103-1107. [DOI] [PubMed] [Google Scholar]
- 614. Jesus LE. Feminizing genitoplasties: where are we now? J Pediatr Urol. 2018;14(5):407-415. [DOI] [PubMed] [Google Scholar]
- 615. Binet A, Lardy H, Geslin D, Francois-Fiquet C, Poli-Merol ML. Should we question early feminizing genitoplasty for patients with congenital adrenal hyperplasia and XX karyotype? J Pediatr Surg. 2016;51(3):465-468. [DOI] [PubMed] [Google Scholar]
- 616. Bangalore Krishna K, Kogan BA, Ernst MM, et al. Individualized care for patients with intersex (disorders/differences of sex development): Part 3. J Pediatr Urol. 2020;16(5):598-605. [DOI] [PubMed] [Google Scholar]
- 617. Ernst MM, Kogan BA, Lee PA. Gender identity: a psychosocial primer for providing care to patients with a disorder/difference of sex development and their families [individualized care for patients with intersex (Disorders/differences of sex development): Part 2]. J Pediatr Urol. 2020;16(5):606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618. Braga LH, Pippi Salle JL. Congenital adrenal hyperplasia: a critical appraisal of the evolution of feminizing genitoplasty and the controversies surrounding gender reassignment. Eur J Pediatr Surg. 2009;19(4):203-210. [DOI] [PubMed] [Google Scholar]
- 619. Boehmer U, Timm A, Ozonoff A, Potter J. Applying the female sexual functioning index to sexual minority women. J Womens Health (Larchmt). 2012;21(4):401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620. Crouch NS, Liao LM, Woodhouse CR, Conway GS, Creighton SM. Sexual function and genital sensitivity following feminizing genitoplasty for congenital adrenal hyperplasia. J Urol. 2008;179(2):634-638. [DOI] [PubMed] [Google Scholar]
- 621. Nordenskjöld A, Holmdahl G, Frisén L, et al. Type of mutation and surgical procedure affect long-term quality of life for women with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2008;93(2):380-386. [DOI] [PubMed] [Google Scholar]
- 622. Creighton SM, Minto CL, Steele SJ. Objective cosmetic and anatomical outcomes at adolescence of feminising surgery for ambiguous genitalia done in childhood. Lancet. 2001;358(9276):124-125. [DOI] [PubMed] [Google Scholar]
- 623. Claahsen-van der Grinten HL, Stikkelbroeck N, Falhammar H, Reisch N. MANAGEMENT OF ENDOCRINE DISEASE: gonadal dysfunction in congenital adrenal hyperplasia. Eur J Endocrinol. 2021;184(3):R85-R97. [DOI] [PubMed] [Google Scholar]
- 624. Bouvattier C, Esterle L, Renoult-Pierre P, et al. Clinical outcome, hormonal status, gonadotrope axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French National Survey. J Clin Endocrinol Metab. 2015;100(6):2303-2313. [DOI] [PubMed] [Google Scholar]
- 625. Reisch N, Arlt W. Fine tuning for quality of life: 21st century approach to treatment of Addison’s disease. Endocrinol Metab Clin North Am. 2009;38(2):407-18, ix. [DOI] [PubMed] [Google Scholar]
- 626. Nermoen I, Rørvik J, Holmedal SH, et al. High frequency of adrenal myelolipomas and testicular adrenal rest tumours in adult Norwegian patients with classical congenital adrenal hyperplasia because of 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2011;75(6):753-759. [DOI] [PubMed] [Google Scholar]
- 627. Mouritsen A, Jørgensen N, Main KM, Schwartz M, Juul A. Testicular adrenal rest tumours in boys, adolescents and adult men with congenital adrenal hyperplasia may be associated with the CYP21A2 mutation. Int J Androl. 2010;33(3):521-527. [DOI] [PubMed] [Google Scholar]
- 628. Yılmaz R, Şahin D, Aghayev A, et al. Sonography and magnetic resonance imaging characteristics of testicular adrenal rest tumors. Pol J Radiol. 2017;82(10):583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629. Kim MS, Goodarzian F, Keenan MF, et al. Testicular adrenal rest tumors in boys and young adults with congenital adrenal hyperplasia. J Urol. 2017;197(3 Pt 2):931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630. Lolis E, Juhlin CC, Nordenström A, Falhammar H. Extensive bilateral adrenal rest testicular tumors in a patient with 3β-hydroxysteroid dehydrogenase type 2 deficiency. J Endocr Soc. 2018;2(6):513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631. Avila NA, Shawker TS, Jones JV, Cutler GB Jr, Merke DP. Testicular adrenal rest tissue in congenital adrenal hyperplasia: serial sonographic and clinical findings. AJR Am J Roentgenol. 1999;172(5):1235-1238. [DOI] [PubMed] [Google Scholar]
- 632. Kim JH, Choi JH, Kang E, Kim YM, Lee BH, Yoo HW. Long-term consequences of congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency in adolescents and adults. Exp Clin Endocrinol Diabetes. 2017;125(3):196-201. [DOI] [PubMed] [Google Scholar]
- 633. Bulsari K, Falhammar H. Clinical perspectives in congenital adrenal hyperplasia due to 11β-hydroxylase deficiency. Endocrine. 2017;55(1):19-36. [DOI] [PubMed] [Google Scholar]
- 634. Al Alawi AM, Nordenström A, Falhammar H. Clinical perspectives in congenital adrenal hyperplasia due to 3β-hydroxysteroid dehydrogenase type 2 deficiency. Endocrine. 2019;63(3):407-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635. Claahsen-van der Grinten HL, Sweep FC, Blickman JG, Hermus AR, Otten BJ. Prevalence of testicular adrenal rest tumours in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2007;157(3):339-344. [DOI] [PubMed] [Google Scholar]
- 636. Cabrera MS, Vogiatzi MG, New MI. Long term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(7):3070-3078. [DOI] [PubMed] [Google Scholar]
- 637. Tanaka M, Enatsu N, Chiba K, Fujisawa M. Two cases of reversible male infertility due to congenital adrenal hyperplasia combined with testicular adrenal rest tumor. Reprod Med Biol. 2018;17(1):93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638. Reisch N, Rottenkolber M, Greifenstein A, et al. Testicular adrenal rest tumors develop independently of long-term disease control: a longitudinal analysis of 50 adult men with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2013;98(11):E1820-E1826. [DOI] [PubMed] [Google Scholar]
- 639. Mazzilli R, Stigliano A, Delfino M, et al. The high prevalence of testicular adrenal rest tumors in adult men with congenital adrenal hyperplasia is correlated with ACTH levels. Front Endocrinol (Lausanne). 2019;10(6):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640. Verhees MJM, Kamphuis-Van Ulzen K, Hermus A, Stikkelbroeck N, Mooij CF, Claahsen-van der Grinten HL. Re: Testicular adrenal rest tumors in boys and young adults with congenital adrenal hyperplasia: M. S. Kim, F. Goodarzian, M. F. Keenan, M. E. Geffner, C. M. Koppin, R. E. De Filippo and P. J. Kokorowski J Urol 2017;197:931-936. J Urol. 2018;199(5):1357-1358. [DOI] [PubMed] [Google Scholar]
- 641. Altieri VM, Altieri B, Castellucci R, et al. Leydig cell tumour and giant adrenal myelolipoma associated with adrenogenital syndrome: a case report with a review of the literature. Urologia. 2016;83(1):43-48. [DOI] [PubMed] [Google Scholar]
- 642. Claahsen-van der Grinten HL, Otten BJ, Hermus AR, Sweep FC, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89(3):597-601. [DOI] [PubMed] [Google Scholar]
- 643. Falhammar H, Filipsson Nyström H, Wedell A, Brismar K, Thorén M. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2013;168(3):331-341. [DOI] [PubMed] [Google Scholar]
- 644. Jääskeläinen J, Kiekara O, Hippeläinen M, Voutilainen R. Pituitary gonadal axis and child rate in males with classical 21-hydroxylase deficiency. J Endocrinol Invest. 2000;23(1):23-27. [DOI] [PubMed] [Google Scholar]
- 645. Manoli I, Kanaka-Gantenbein Ch, Voutetakis A, Maniati-Christidi M, Dacou-Voutetakis C. Early growth, pubertal development, body mass index and final height of patients with congenital adrenal hyperplasia: factors influencing the outcome. Clin Endocrinol (Oxf). 2002;57(5):669-676. [DOI] [PubMed] [Google Scholar]
- 646. Kulshreshtha B, Eunice M, Ammini AC. Pubertal development among girls with classical congenital adrenal hyperplasia initiated on treatment at different ages. Indian J Endocrinol Metab. 2012;16(4):599-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647. Bidet M, Bellanné-Chantelot C, Galand-Portier MB, et al. Fertility in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2010;95(3):1182-1190. [DOI] [PubMed] [Google Scholar]
- 648. Carmina E, Dewailly D, Escobar-Morreale HF, et al. Non-classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency revisited: an update with a special focus on adolescent and adult women. Hum Reprod Update. 2017;23(5):580-599. [DOI] [PubMed] [Google Scholar]
- 649. Hague WM, Adams J, Rodda C, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf). 1990;33(4):501-510. [DOI] [PubMed] [Google Scholar]
- 650. Pall M, Azziz R, Beires J, Pignatelli D. The phenotype of hirsute women: a comparison of polycystic ovary syndrome and 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil Steril. 2010;94(2):684-689. [DOI] [PubMed] [Google Scholar]
- 651. Stikkelbroeck NM, Hermus AR, Schouten D, et al. Prevalence of ovarian adrenal rest tumours and polycystic ovaries in females with congenital adrenal hyperplasia: results of ultrasonography and MR imaging. Eur Radiol. 2004;14(10):1802-1806. [DOI] [PubMed] [Google Scholar]
- 652. Kulshreshtha B, Marumudi E, Khurana ML, et al. Fertility among women with classical congenital adrenal hyperplasia: report of seven cases where treatment was started after 9 years of age. Gynecol Endocrinol. 2008;24(5):267-272. [DOI] [PubMed] [Google Scholar]
- 653. van de Grift TC, Kreukels BPC. Breast development and satisfaction in women with disorders/differences of sex development. Hum Reprod. 2019;34(12):2410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654. Jääskeläinen J, Hippeläinen M, Kiekara O, Voutilainen R. Child rate, pregnancy outcome and ovarian function in females with classical 21-hydroxylase deficiency. Acta Obstet Gynecol Scand. 2000;79(8):687-692. [PubMed] [Google Scholar]
- 655. Słowikowska-Hilczer J, Hirschberg AL, Claahsen-van der Grinten H, et al. ; dsd-LIFE Group . Fertility outcome and information on fertility issues in individuals with different forms of disorders of sex development: findings from the dsd-LIFE study. Fertil Steril. 2017;108(5):822-831. [DOI] [PubMed] [Google Scholar]
- 656. Hirschberg AL, Gidlöf S, Falhammar H, et al. Reproductive and perinatal outcomes in women with congenital adrenal hyperplasia: a population-based cohort study. J Clin Endocrinol Metab. 2021;106(2):e957-e965. [DOI] [PubMed] [Google Scholar]
- 657. Leveroni C, Berenbaum SA. Early androgen effects on interest in infants: evidence from children with congenital adrenal hyperplasia. Dev Neuropsychol. 1998;14:321-340. [Google Scholar]
- 658. Helleday J, Siwers B, Ritzén EM, Carlström K. Subnormal androgen and elevated progesterone levels in women treated for congenital virilizing 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1993;76(4):933-936. [DOI] [PubMed] [Google Scholar]
- 659. Holmes-Walker DJ, Conway GS, Honour JW, Rumsby G, Jacobs HS. Menstrual disturbance and hypersecretion of progesterone in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 1995;43(3):291-296. [DOI] [PubMed] [Google Scholar]
- 660. Hoepffner W, Schulze E, Bennek J, Keller E, Willgerodt H. Pregnancies in patients with congenital adrenal hyperplasia with complete or almost complete impairment of 21-hydroxylase activity. Fertil Steril. 2004;81(5):1314-1321. [DOI] [PubMed] [Google Scholar]
- 661. Reichman DE, White PC, New MI, Rosenwaks Z. Fertility in patients with congenital adrenal hyperplasia. Fertil Steril. 2014;101(2):301-309. [DOI] [PubMed] [Google Scholar]
- 662. Reisch N. Pregnancy in congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2019;48(3):619-641. [DOI] [PubMed] [Google Scholar]
- 663. Moran C, Azziz R, Weintrob N, et al. Reproductive outcome of women with 21-hydroxylase-deficient nonclassic adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91(9):3451-3456. [DOI] [PubMed] [Google Scholar]
- 664. Eyal O, Ayalon-Dangur I, Segev-Becker A, Schachter-Davidov A, Israel S, Weintrob N. Pregnancy in women with nonclassic congenital adrenal hyperplasia: Time to conceive and outcome. Clin Endocrinol (Oxf). 2017;87(5):552-556. [DOI] [PubMed] [Google Scholar]
- 665. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275(2):104-115. [DOI] [PubMed] [Google Scholar]
- 666. Bothou C, Anand G, Li D, et al. Current management and outcome of pregnancies in women with adrenal insufficiency: experience from a multicenter survey. J Clin Endocrinol Metab. 2020;105(8):e2853-e2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Krone N, Wachter I, Stefanidou M, Roscher AA, Schwarz HP. Mothers with congenital adrenal hyperplasia and their children: outcome of pregnancy, birth and childhood. Clin Endocrinol (Oxf). 2001;55(4):523-529. [DOI] [PubMed] [Google Scholar]
- 668. Falhammar H, Frisén L, Hirschberg AL, et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2015;100(9):3520-3528. [DOI] [PubMed] [Google Scholar]
- 669. Bachelot A, Golmard JL, Dulon J, et al. Determining clinical and biological indicators for health outcomes in adult patients with childhood onset of congenital adrenal hyperplasia. Eur J Endocrinol. 2015;173(2):175-184. [DOI] [PubMed] [Google Scholar]
- 670. Kim B, Lee MN, Park HD, et al. Dried blood spot testing for seven steroids using liquid chromatography-tandem mass spectrometry with reference interval determination in the Korean population. Ann Lab Med. 2015;35(6):578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Tamhane S, Rodriguez-Gutierrez R, Iqbal AM, et al. Cardiovascular and metabolic outcomes in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(11):4097-4103. [DOI] [PubMed] [Google Scholar]
- 672. Falhammar H, Filipsson H, Holmdahl G, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(1):110-116. [DOI] [PubMed] [Google Scholar]
- 673. Falhammar H, Filipsson Nyström H, Wedell A, Thorén M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2011;164(2):285-293. [DOI] [PubMed] [Google Scholar]
- 674. Mooij CF, Kroese JM, Claahsen-van der Grinten HL, Tack CJ, Hermus AR. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clin Endocrinol (Oxf). 2010;73(2):137-146. [DOI] [PubMed] [Google Scholar]
- 675. Ubertini G, Bizzarri C, Grossi A, et al. Blood pressure and left ventricular characteristics in young patients with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Int J Pediatr Endocrinol. 2009;2009(2):383610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676. Vijayan R, Bhavani N, Pavithran PV, et al. Metabolic profile, cardiovascular risk factors and health-related quality of life in children, adolescents and young adults with congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2019;32(8):871-877. [DOI] [PubMed] [Google Scholar]
- 677. Subbarayan A, Dattani MT, Peters CJ, Hindmarsh PC. Cardiovascular risk factors in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2014;80(4):471-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678. Rosenbaum D, Gallo A, Lethielleux G, et al. ; CARDIOHCS study group . Early central blood pressure elevation in adult patients with 21-hydroxylase deficiency. J Hypertens. 2019;37(1):175-181. [DOI] [PubMed] [Google Scholar]
- 679. Falhammar H, Thorén M. Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine. 2012;41(3):355-373. [DOI] [PubMed] [Google Scholar]
- 680. Sartorato P, Zulian E, Benedini S, et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(3):1015-1018. [DOI] [PubMed] [Google Scholar]
- 681. Marra AM, Improda N, Capalbo D, et al. Cardiovascular abnormalities and impaired exercise performance in adolescents with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(2):644-652. [DOI] [PubMed] [Google Scholar]
- 682. Salem JE, Nguyen LS, Hammoudi N, et al. ; CARDIOHCS Study Group . Complex association of sex hormones on left ventricular systolic function: insight into sexual dimorphism. J Am Soc Echocardiogr. 2018;31(2):231-240.e1. [DOI] [PubMed] [Google Scholar]
- 683. Improda N, Barbieri F, Ciccarelli GP, Capalbo D, Salerno M. Cardiovascular health in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxilase deficiency. Front Endocrinol (Lausanne). 2019;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684. Gomes LG, Mendonca BB, Bachega TASS. Long-term cardio-metabolic outcomes in patients with classical congenital adrenal hyperplasia: is the risk real? Curr Opin Endocrinol Diabetes Obes. 2020;27(3):155-161. [DOI] [PubMed] [Google Scholar]
- 685. Moreira RP, Gomes LG, Mendonca BB, Bachega TA. Impact of glucocorticoid receptor gene polymorphisms on the metabolic profile of adult patients with the classical form of 21-hydroxylase deficiency. PLoS One. 2012;7(9):e44893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686. Bhargava A, Arnold AP, Bangasser DA, et al. Considering sex as a biological variable in basic and clinical studies: an Endocrine Society scientific statement. Endocr Rev. 2021;42(3):219-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687. Ernst M, Maheu FS, Schroth E, et al. Amygdala function in adolescents with congenital adrenal hyperplasia: a model for the study of early steroid abnormalities. Neuropsychologia. 2007;45(9):2104-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688. Webb EA, Elliott L, Carlin D, et al. Quantitative brain MRI in congenital adrenal hyperplasia: in vivo assessment of the cognitive and structural impact of steroid hormones. J Clin Endocrinol Metab. 2018;103(4):1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689. Nass R, Heier L, Moshang T, et al. Magnetic resonance imaging in the congenital adrenal hyperplasia population: increased frequency of white-matter abnormalities and temporal lobe atrophy. J Child Neurol. 1997;12(3):181-186. [DOI] [PubMed] [Google Scholar]
- 690. Mnif MF, Kamoun M, Mnif F, et al. Brain magnetic resonance imaging findings in adult patients with congenital adrenal hyperplasia: Increased frequency of white matter impairment and temporal lobe structures dysgenesis. Indian J Endocrinol Metab. 2013;17(1):121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691. Siegel S, Kirstein CF, Grzywotz A, et al. Neuropsychological functioning in patients with Cushing’s disease and Cushing’s syndrome. Exp Clin Endocrinol Diabetes. 2021;129(3):194-202. [DOI] [PubMed] [Google Scholar]
- 692. Herting MM, Azad A, Kim R, Tyszka JM, Geffner ME, Kim MS. Brain differences in the prefrontal cortex, amygdala, and hippocampus in youth with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(4):1098-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693. Van’t Westeinde A, Karlsson L, Thomsen Sandberg M, Nordenström A, Padilla N, Lajic S. Altered gray matter structure and white matter microstructure in patients with congenital adrenal hyperplasia: relevance for working memory performance. Cereb Cortex. 2020;30(5):2777-2788. [DOI] [PubMed] [Google Scholar]
- 694. Ritchie SJ, Cox SR, Shen X, et al. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex. 2018;28(8):2959-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695. Lotze M, Domin M, Gerlach FH, et al. Novel findings from 2838 adult brains on sex differences in gray matter brain volume. Sci Rep. 2019;9(1):1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696. Ingalhalikar M, Smith A, Parker D, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111(2):823-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697. Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993-1000. [DOI] [PubMed] [Google Scholar]
- 699. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13(10):777-787. [DOI] [PubMed] [Google Scholar]
- 700. Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. 2020;16(8):437-447. [DOI] [PubMed] [Google Scholar]
- 701. Girgis R, Winter JS. The effects of glucocorticoid replacement therapy on growth, bone mineral density, and bone turnover markers in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1997;82(12):3926-3929. [DOI] [PubMed] [Google Scholar]
- 702. Fleischman A, Ringelheim J, Feldman HA, Gordon CM. Bone mineral status in children with congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2007;20(2):227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703. Stikkelbroeck NM, Oyen WJ, van der Wilt GJ, Hermus AR, Otten BJ. Normal bone mineral density and lean body mass, but increased fat mass, in young adult patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003;88(3):1036-1042. [DOI] [PubMed] [Google Scholar]
- 704. Christiansen P, Mølgaard C, Müller J. Normal bone mineral content in young adults with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2004;61(3):133-136. [DOI] [PubMed] [Google Scholar]
- 705. Gussinyé M, Carrascosa A, Potau N, et al. Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics. 1997;100(4):671-674. [DOI] [PubMed] [Google Scholar]
- 706. Guo CY, Weetman AP, Eastell R. Bone turnover and bone mineral density in patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 1996;45(5):535-541. [DOI] [PubMed] [Google Scholar]
- 707. Arisaka O, Hoshi M, Kanazawa S, et al. Preliminary report: effect of adrenal androgen and estrogen on bone maturation and bone mineral density. Metabolism. 2001;50(4):377-379. [DOI] [PubMed] [Google Scholar]
- 708. Zimmermann A, Sido PG, Schulze E, et al. Bone mineral density and bone turnover in Romanian children and young adults with classical 21-hydroxylase deficiency are influenced by glucocorticoid replacement therapy. Clin Endocrinol (Oxf). 2009;71(4):477-484. [DOI] [PubMed] [Google Scholar]
- 709. Cameron FJ, Kaymakci B, Byrt EA, Ebeling PR, Warne GL, Wark JD. Bone mineral density and body composition in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1995;80(7):2238-2243. [DOI] [PubMed] [Google Scholar]
- 710. de Almeida Freire PO, de Lemos-Marini SH, Maciel-Guerra AT, et al. Classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: a cross-sectional study of factors involved in bone mineral density. J Bone Miner Metab. 2003;21(6):396-401. [DOI] [PubMed] [Google Scholar]
- 711. Paganini C, Radetti G, Livieri C, Braga V, Migliavacca D, Adami S. Height, bone mineral density and bone markers in congenital adrenal hyperplasia. Horm Res. 2000;54(4):164-168. [DOI] [PubMed] [Google Scholar]
- 712. Jääskeläinen J, Voutilainen R. Bone mineral density in relation to glucocorticoid substitution therapy in adult patients with 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 1996;45(6):707-713. [DOI] [PubMed] [Google Scholar]
- 713. Sciannamblo M, Russo G, Cuccato D, Chiumello G, Mora S. Reduced bone mineral density and increased bone metabolism rate in young adult patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(11):4453-4458. [DOI] [PubMed] [Google Scholar]
- 714. King JA, Wisniewski AB, Bankowski BJ, Carson KA, Zacur HA, Migeon CJ. Long-term corticosteroid replacement and bone mineral density in adult women with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91(3):865-869. [DOI] [PubMed] [Google Scholar]
- 715. Falhammar H, Filipsson H, Holmdahl G, et al. Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(12):4643-4649. [DOI] [PubMed] [Google Scholar]
- 716. Bachelot A, Plu-Bureau G, Thibaud E, et al. Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2007;67(6):268-276. [DOI] [PubMed] [Google Scholar]
- 717. Koetz KR, Ventz M, Diederich S, Quinkler M. Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J Clin Endocrinol Metab. 2012;97(1):85-92. [DOI] [PubMed] [Google Scholar]
- 718. Ceccato F, Barbot M, Albiger N, et al. Long-term glucocorticoid effect on bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2016;175(2):101-106. [DOI] [PubMed] [Google Scholar]
- 719. Riehl G, Reisch N, Roehle R, Claahsen van der Grinten H, Falhammar H, Quinkler M. Bone mineral density and fractures in congenital adrenal hyperplasia: Findings from the dsd-LIFE study. Clin Endocrinol (Oxf). 2020;92(4):284-294. [DOI] [PubMed] [Google Scholar]
- 720. Kasperk CH, Wakley GK, Hierl T, Ziegler R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J Bone Miner Res. 1997;12(3):464-471. [DOI] [PubMed] [Google Scholar]
- 721. El-Maouche D, Collier S, Prasad M, Reynolds JC, Merke DP. Cortical bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2015;82(3):330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722. Rangaswamaiah S, Gangathimmaiah V, Nordenstrom A, Falhammar H. Bone mineral density in adults with congenital adrenal hyperplasia: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11(7):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723. Nermoen I, Falhammar H. Prevalence and characteristics of adrenal tumors and myelolipomas in congenital adrenal hyperplasia: a systematic review and meta-analysis. Endocr Pract. 2020;26(11):1351-1365. [DOI] [PubMed] [Google Scholar]
- 724. Hamidi O, Raman R, Lazik N, et al. Clinical course of adrenal myelolipoma: A long-term longitudinal follow-up study. Clin Endocrinol (Oxf). 2020;93(1):11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725. Bancos I, Taylor AE, Chortis V, et al. ; ENSAT EURINE-ACT Investigators . Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726. Juniarto AZ, Ulfah M, Ariani MD, Utari A, Faradz SM. Phenotypic variation of 46,XX late identified congenital adrenal hyperplasia among Indonesians. J ASEAN Fed Endocr Soc. 2018;33(1):6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727. Raza J, Mazen I. Achieving diagnostic certainty in resource-limited settings. Endocr Dev. 2014;27(9):257-267. [DOI] [PubMed] [Google Scholar]
- 728. Ediati A, Faradz SM, Juniarto AZ, van der Ende J, Drop SL, Dessens AB. Emotional and behavioral problems in late-identified Indonesian patients with disorders of sex development. J Psychosom Res. 2015;79(1):76-84. [DOI] [PubMed] [Google Scholar]
- 729. Chowdhury TK, Kabir M, Chowdhury MZ, Hutson JM, Banu T. The challenges in diagnosis and gender assignment in disorders of sex development presenting to a pediatric surgical unit in a developing country: the role of laparoscopy and simple tests for gender identity. J Pediatr Urol. 2014;10(6):1255-1260. [DOI] [PubMed] [Google Scholar]
- 730. Warne GL, Raza J. Disorders of sex development (DSDs), their presentation and management in different cultures. Rev Endocr Metab Disord. 2008;9(3):227-236. [DOI] [PubMed] [Google Scholar]
- 731. Ediati A, Maharani N, Utari A. Sociocultural aspects of disorders of sex development. Birth Defects Res C Embryo Today. 2016;108(4):380-383. [DOI] [PubMed] [Google Scholar]
- 732. Ediati A, Juniarto AZ, Birnie E, Drop SL, Faradz SM, Dessens AB. Gender development in Indonesian children, adolescents, and adults with disorders of sex development. Arch Sex Behav. 2015;44(5):1339-1361. [DOI] [PubMed] [Google Scholar]
- 733. Ediati A, Verrips GHW, Juniarto AZ, Faradz SMH, Drop SLS, Dessens AB. Quality of life in late-treated patients with disorders of sex development: insights for patient-centered care. Front Pediatr. 2018;6(6):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734. Boulanger V, Schlemmer M, Rossov S, Seebald A, Gavin P. Establishing patient registries for rare diseases: rationale and challenges. Pharmaceut Med. 2020;34(3):185-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735. Taruscio D, Gentile AE, Evangelista T, Frazzica RG, Bushby K, Montserrat AM. Centres of expertise and European reference networks: key issues in the field of rare diseases. The EUCERD recommendations. Blood Transfus. 2014;12(Suppl 3):s621-s625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736. Kyriakou A, Dessens A, Bryce J, et al. Current models of care for disorders of sex development - results from an International survey of specialist centres. Orphanet J Rare Dis. 2016;11(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737. Rolston AM, Gardner M, van Leeuwen K, et al. ; members of the DSD-TRN Advocacy; Advisory Network Accord Alliance . Disorders of sex development (DSD): clinical service delivery in the United States. Am J Med Genet C Semin Med Genet. 2017;175(2):268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738. Sanders C, Hall J, Dessens A, et al. Involving individuals with disorders of sex development and their parents in exploring new models of shared learning: proceedings from a DSDnet COST action workshop. Sex Dev. 2018;12(10):225-231. [DOI] [PubMed] [Google Scholar]
- 739. Brasil S, Pascoal C, Francisco R, Dos Reis Ferreira V, Videira PA, Valadão AG. Artificial intelligence (AI) in rare diseases: is the future brighter? Genes (Basel). 2019;10(12):978. [DOI] [PMC free article] [PubMed] [Google Scholar]