Abstract
Previous studies have demonstrated that the four subspecies of the human pathogen Francisella tularensis, despite showing marked variations in their virulence for mammals and originating from different regions in the Northern Hemisphere, display a very close phylogenetic relationship. This property has hampered the development of generally applicable typing methods. To overcome this problem, we evaluated the use of PCR for discrimination of the subspecies using various forms of long arbitrary primers or primers specific for repetitive extragenic palindromic sequences (REP) or enterobacterial repetitive intragenic consensus (ERIC) sequences. Patterns generated by use of REP, ERIC, or long arbitrary primers allowed differentiation at the species level and of the four subspecies of F. tularensis. With each of these three methods, similar or identical clustering of strains was found, and groups of strains of different geographical origins or differing in virulence showed distinct patterns. The discriminatory indices of the methods varied from 0.57 to 0.65; thus, the patterns were not sufficiently discriminatory to distinguish individual strains. The sequence of a fragment generated by amplification with an arbitrary primer was determined, and a region showing interstrain heterogeneity was identified. Specific primers were designed, and a PCR was developed that distinguished strains of F. tularensis subsp. holarctica from strains of other F. tularensis subspecies, including strains of the highly virulent F. tularensis subsp. tularensis. Notably, one European isolate showed the genetic pattern typical of the highly virulent F. tularensis subsp. tularensis, generally believed to exist only in North America. It is proposed that a combination of the specific PCR together with one method generating subspecies-specific patterns is suitable as a rapid and relatively simple strategy for discrimination of Francisella species and subspecies.
Francisella tularensis is one of two recognized species of the genus Francisella, and almost all knowledge of the genus originates from work on this species. It is a virulent, facultative intracellular bacterium and the etiological agent of tularemia, a disease found in rodents, lagomorphs, and humans. The bacterium is widely distributed in nature and has been isolated from about 250 wildlife species (20), many of which can transmit disease to humans. Tularemia is acquired by direct contact with infected animals, through contaminated water or food, or from vectors such as biting insects or ticks. Airborne transmission also occurs, especially during processing of agricultural products. The disease is often epidemic, both in humans and in animals, and clinical manifestations depend on the type of reservoir involved and the means of transmission. F. tularensis is found throughout the Northern Hemisphere, and large outbreaks have been reported in parts of the continental United States, the southern part of the former USSR, and northern Scandinavia.
F. philomiragia, the other species of the genus, is a rarely isolated, opportunistic pathogen closely linked to water (9). The genus also comprises endosymbionts of ticks, although their exact phylogenetic positions are uncertain (15, 16, 24).
All of the four recognized subspecies of F. tularensis (9, 18, 20) have been associated with human tularemia, although they differ drastically in virulence for humans and rabbits. Until recently, isolates of F. tularensis subsp. tularensis were isolated only in North America, but a mite-derived isolate from Slovakia showed the phenotypic characteristics of the subspecies (8). F. tularensis subsp. tularensis isolates in North America are often associated with tick-borne tularemia in lagomorphs and are highly virulent for many mammalian species, including primates. Before the advent of effective antibiotics, mortality in humans ranged from 5 to 30% (3, 4). F. tularensis subsp. holarctica, found in Europe, North America, and Japan, is often associated with lagomorphs in Scandinavia, continental Europe, and Japan, ground voles in the former USSR, and beavers and muskrats in North America. The subspecies causes a less severe form of human illness, often a localized ulceroglandular disease. F. tularensis subsp. mediaasiatica is moderately virulent for rabbits and humans and has been isolated only in the Central Asian republics of the former USSR (18). In 1950, the type strain of F. tularensis subsp. novicida was isolated from a water sample in Utah, and isolates of the subspecies known as “novicida-like” isolates have been linked to human disease on a few occasions (1, 9). Isolates of this subspecies have less stringent growth requirements than representatives of the other F. tularensis subspecies and can therefore be misidentified as belonging to other genera (1).
Isolates of the genus Francisella are readily distinguishable based on a unique set of phenotypic characteristics, including coccoidal morphology, gram negativity, acid but no gas production from a limited number of carbohydrates, a growth requirement for cysteine, and a unique fatty acid composition (20). However, within the genus and particularly within the species F. tularensis, discrimination of strains is not performed conveniently. Due to the contagiousness of Francisella isolates, only limited work has been performed to develop typing methods based on cultivation. Such work is further restricted by the nonfermentative nature of F. tularensis, limiting the number of biochemical tests available for biotyping. To this end, the development of techniques based on analyses of genomic variations is of special interest, since they can be done with killed preparations of the bacterium.
Several molecular methods have been successfully used to discriminate the Francisella species but not the subspecies (5, 11). Repetitive extragenic palindromic sequence (REP)-PCR has been applied to specifically identifying strains of F. tularensis subsp. novicida, but patterns from F. tularensis subsp. holarctica and F. tularensis subsp. tularensis strains were found to be similar (1). A one-base variability in the 16S rRNA sequences of F. tularensis subsp. tularensis and F. tularensis subsp. holarctica has been demonstrated, and on this basis, a PCR that differentiated the two subspecies was developed (5). However, there is not sufficient sequence information available to corroborate that there is a consistency at this position among all isolates of each subspecies (5). A recent study investigated PCR methods for the typing of F. tularensis subsp. holarctica isolates, the majority of which originated from Spain (2). In the present study, we evaluated if PCR-based methods are suitable as generally applicable tools for discrimination of species and subspecies within the genus Francisella.
MATERIALS AND METHODS
Bacteria, media, and growth conditions.
The bacterial strains used in this study are listed in Table 1. The strains belong to the Francisella Strain Collection, which contains more than 200 Francisella strains (5). Each strain has been given a strain collection number, indicated in Table 1. Strains were grown for 3 days at 37°C on modified Thayer-Martin agar plates (19) in 5% CO2 (GasPak; Becton Dickinson, Paramus, N.J.). Cell suspensions of virulent Francisella strains in saline, at a concentration of ∼109 cells/ml, were heat killed at 65°C for 2 h. Debris was removed by centrifugation at 12,000 × g for 5 min. Supernatants were collected and then stored at −20°C until used.
TABLE 1.
Strains included in the study
| Species or subspecies | Source | Strain | Other strain designation(s) | Pattern in:
|
|||
|---|---|---|---|---|---|---|---|
| REP | ERIC | RAPD | Specific PCR | ||||
| F. tularensis subsp. holarctica | Human lymph node, 1926, Japan | FSC017 | Jap S2 | A | a | 1 | I |
| Japan | FSC024 | Yerma | A | a | 1 | I | |
| City water supply, Helena, Mont. | FSC044 | Helena | B | b | 1 | I | |
| North America | FSC056 | Eigelsbach | B | b | 1 | I | |
| Norway | FSC091 | NO 9/15/51 | B | b | 1 | I | |
| Sweden | FSC093 | Sv 43 | B | b | 1 | I | |
| Sweden | FSC094 | Sv 121 | B | b | 1 | I | |
| Sweden | FSC095 | Sv 219 | B | b | 1 | I | |
| Human ulcer, 1981, Sweden | FSC108 | SBL R45/81 | B | b | 1 | I | |
| Human ulcer, 1981, Sweden | FSC109 | SBL R74/81 | B | b | 1 | I | |
| Norway rat, 1988, Russia | FSC150 | 250 | B | b | 1 | I | |
| Russia | FSC155 | ATCC 29684, LVS | B | b | 1 | I | |
| Human blood, 1994, Sweden | FSC157 | CCUG 33270 | B | b | 1 | I | |
| Water, 1995, Sweden | FSC170 | B | b | 1 | I | ||
| Human ulcer, 1995, Sweden | FSC171 | B | b | 1 | I | ||
| Human ulcer, 1995, Sweden | FSC172 | SMI R8/95 | B | b | 1 | I | |
| Ticks, 1995, Czech Republic | FSC180 | T-17 | B | b | 1 | I | |
| Human ulcer, 1996, Sweden | FSC188 | B | b | 1 | I | ||
| Mites, 1988, Slovakia | FSC196 | SE-210/37 | B | b | 1 | I | |
| F. tularensis subsp. tularensis | Squirrel, Georgia | FSC033 | SnMF | C | c | 2 | II |
| Tick, 1935, British Columbia, Canada | FSC041 | Vavenby | C | c | 2 | II | |
| Canada | FSC042 | Utter | C | c | 2 | II | |
| Human ulcer, 1941, Ohio | FSC043 | Schu | C | c | 2 | II | |
| Hare, 1954, Nevada | FSC054 | Nevada 14 | C | c | 2 | II | |
| Human lymph node, 1920, Utah | FSC138 | ATCC 6223 | D | d | 3 | II | |
| Mites, 1988, Slovakia | FSC198 | SE-219/38 | C | c | 2 | II | |
| F. tularensis subsp. mediaasiatica | Hare, 1965, Central Asia | FSC149 | 120 | E | e | 4 | II |
| F. tularensis subsp. novicida | Water, 1950, Utah | FSC040 | ATCC 15482 | F | f | 5 | II |
| Human blood, 1991, Texas | FSC156 | Fx1 | G | g | 6 | II | |
| Human blood, 1995, Texas | FSC159 | Fx2 | H | h | 7 | IIa | |
| F. philomiragia | Water, 1960, Utah | FSC038 | ATCC 25017 | I | i | 8 | None |
| Muskrat, 1959, Utah | FSC144 | ATCC 25015 | J | j | 9 | None | |
| Human abscess, 1982, Sweden | FSC145 | CCUG 12603 | K | k | 10 | None | |
| United States | FSC153 | L | l | 11 | None | ||
| Human blood, 1979, Switzerland | FSC154 | CCUG 13404 | M | m | 12 | None | |
The strain displayed a slightly smaller 0.4-kb amplicon due to a gene truncation.
PCR template preparation.
In an initial evaluation, DNA from bacteria was extracted using a modification (12) of a technique based on the binding of DNA to uniform glass beads (Bio 101, La Jolla, Calif.) in the presence of the chaotropic nuclease inhibitor guanidine isothiocyanate. This method has been previously shown to be superior to three other methods for the extraction of DNA from F. tularensis (21). In an initial evaluation of the REP-PCR and enterobacterial repetitive intragenic consensus sequence (ERIC)-PCR protocols, the usefulness of these DNA preparations as DNA templates was compared to that of heat-killed whole-cell preparations. No significant differences were noted. In subsequent experiments, a supernatant of a heat-killed Francisella suspension was therefore used as a template.
Primer sequences.
The previously described primer pairs REP1R-I–REP2-I and ERIC1R-ERIC2 were used (28). REP consensus primers contained inosine at ambiguous positions. A number of 20-mer oligonucleotides were designed at random, and their usefulness alone or in combination as PCR primers was evaluated using a modified protocol for long-primer randomly amplified polymorphic DNA (RAPD) analysis (LP-RAPD) (6). The primer yielding the pattern with the highest resolution was selected: 5′-GGTAATCGATGAATAAATGA (LP-RAPD2). Oligonucleotides were purchased from Pharmacia Biotech, Uppsala, Sweden.
REP, ERIC, and LP-RAPD amplification.
A 15-μl mixture containing 1 μl of bacterial supernatant, 3 mM MgCl2, and buffer 4 (Advanced Biotechnologies Inc., London, United Kingdom) was heated to 94°C for 10 min. A 10-μl mixture containing (all concentrations per final volume) 2 μM each REP primer, 0.8 μM each ERIC primer, or 0.8 μM LP-RAPD2 primer, 200 μM each deoxynucleoside triphosphate (dNTP) (Pharmacia Biotech), and 1 U of Taq DNA polymerase (Advanced Biotechnologies) was added to the 15-μl mixture. After an additional denaturation at 94°C (REP and ERIC, 10 min; LP-RAPD, 7 min), the reaction mixtures were subjected to 40 cycles of amplification. Each cycle consisted of denaturation at 94°C for 60 s, annealing (REP, 42°C, 120 s; ERIC, 52°C, 60 s; LP-RAPD, 42°C, 60 s), and extension at 72°C for 5 min. The PCR amplification was terminated after the samples were incubated at 72°C for 10 min (REP and ERIC) or 7 min (LP-RAPD). Three microliters of each reaction mixture was loaded onto a 1.5% agarose gel and subjected to electrophoresis, and the amplified products were visualized with UV light after ethidium bromide staining.
Pattern analysis.
PCR patterns were analyzed by visual examination by two individuals. Patterns were regarded as distinct if they repeatedly showed a one-band difference.
Specific PCR amplification.
The degenerate primer 5′- GCCTTAATAGTATGCATACGATT T G T G C TG T T
amplified a distinct PCR product from each F. tularensis strain. The PCR product, approximately 0.7 kb, showed one of two distinct sizes depending on the strain used as the DNA template, with FSC043 exhibiting the larger fragment. The fragment was isolated from a 1.5% SeaKem GTG agarose gel (FMC BioProducts, Rockland, Maine) and was purified by use of a GenElute agarose spin column (Supelco, Bellafonte, Pa.). The fragment was ligated into vector pGEM-T according to the instructions of the manufacturer (Promega Corp., Madison, Wis.) After transformation of Escherichia coli DH5α, recombinant clones were identified by blue-white color screening; plasmid DNA was isolated by the Wizard Plus miniprep procedure (Promega). Inserts were sequenced using pUC/M13 forward and reverse primers and the Big Dye terminator cycle sequencing ready reaction kit (PE Applied Biosystems, Stockholm, Sweden). The sequence was analyzed using an ABI 377 sequencer (PE Applied Biosystems).
Based on the sequence of the 0.7-kb amplicon derived from FSC043, specific primers were designed. A 0.4-kb fragment was amplified from seven strains representing the various Francisella species (FSC024, FSC033, FSC040, FSC056, FSC144, FSC149, and FSC155), and the sequence was determined. Based on the sequence, primers C1 (5′TCCGGTTGGATAGGTGTTGGATT) and C4 (5′GCGCGGATAATTTAAATTTCTCATA) were designed. These primers, yielding an amplicon of approximately 0.3 kb that included the variable region, were used in a multiplex PCR together with the F. tularensis-specific primers, TUL4-435 and TUL4-863. The latter primers generate a 0.4-kb fragment of the gene encoding a 17-kDa lipoprotein shown previously to be useful for the identification of F. tularensis (13, 21, 22).
The multiplex PCR was performed with a reaction mixture containing 1 μl of bacterial supernatant, 0.8 μM each primer, 1 U of DyNAzyme polymerase (Finnzymes OY, Espoo, Finland), and 200 μM each dNTP (Finnzymes OY) in a final volume of 25 μl. After denaturation at 94°C for 5 min, 30 cycles of amplification were performed according to the following protocol: denaturation at 94°C for 30 s, primer annealing at 66°C for 30 s, and primer extension at 72°C for 30 s. After a final extension at 72°C for 5 min, each reaction mixture was subjected to electrophoresis with 3% NuSieve 3:1 agarose gel (FMC BioProducts). After ethidium bromide staining, the DNA products were visualized with UV light.
Nucleotide sequence accession numbers.
The 0.7-kb sequence from strain FSC043 (Schu) has been assigned GenBank accession number AF240631. The GenBank accession numbers of the 0.4-kb fragments are AF247690, (FSC024), AF247689 (FSC033), AF247688 (FSC040), AF247687 (FSC056), AF247686 (FSC144), AF247685 (FSC149), and AF247642 (FSC155).
RESULTS
ERIC-PCR and REP-PCR.
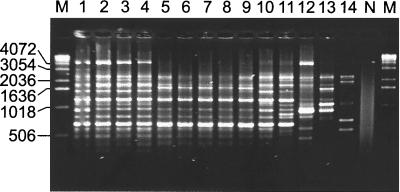
Amplification using REP-PCR revealed similar but distinguishable patterns from strains of each of the three subspecies, F. tularensis subsp. holarctica (19 representatives), F. tularensis subsp. mediaasiatica (1 representative), and F. tularensis subsp. tularensis (7 representatives), whereas patterns from strains of F. tularensis subsp. novicida (3 representatives) and strains of the species F. philomiragia (5 representatives) were unique for each strain. A sample of strains representing each of the species and subspecies is shown in Fig. 1. Clearly visible bands ranged from approximately 0.2 to 4.0 kb. Patterns from the seven isolates of F. tularensis subsp. tularensis were discernible from patterns of the other subspecies and were characterized by prominent bands of approximately 4.0 and 1.0 kb. Notably, FSC198, a Slovakian isolate derived from a mite and reported to show the biochemical characteristics and virulence typical of F. tularensis subsp. tularensis strains (8), also showed this pattern, thereby confirming the first isolation of this subspecies outside North America. The attenuated type strain of the subspecies, ATCC 6223 (FSC138), also demonstrated the typical pattern but with a minor variation in the sizes of the 0.9- and 3.0-kb bands. PCR patterns from F. tularensis subsp. holarctica strains were distinguished by a prominent 1.6-kb band and the absence of a 1.2-kb band. Patterns from the F. tularensis subsp. holarctica strains were identical, with the exception of those from the Japanese strains. The pattern from the F. tularensis subsp. mediaasiatica strain was distinct from those of the other strains but was similar to patterns from F. tularensis subsp. tularensis strains. A summary of the various REP patterns is given in Table 1.
FIG. 1.
PCR amplification of DNA from various Francisella strains using the REP1R-I and REP2-I primers. Samples represent F. tularensis subsp. tularensis strains (FSC138, FSC043, FSC041, and FSC198) (lanes 1 to 4), F. tularensis subsp. holarctica strains (FSC196, FSC155, FSC150, FSC108, and FSC157) (lanes 5 to 9), an F. tularensis subsp. mediaasiatica strain (FSC149) (lane 10), an F. tularensis subsp. holarctica strain from Japan (FSC024) (lane 11), F. tularensis subsp. novicida strains (FSC040 and FSC156) (lanes 12 and 13), and an F. philomiragia strain (FSC 144) (lane 14). Strain designations are indicated in Table 1. Lane N, water used as a negative control. Lane M, molecular markers (sizes in base pairs).
PCR amplification by use of ERIC primers corroborated the clustering of strains observed with REP-PCR, although the patterns were slightly less complex (data not shown). The visualized bands ranged in size from approximately 0.2 to 3.0 kb. A summary of the various ERIC patterns is shown in Table 1. A calculation of the discriminatory power (10) of REP-PCR or ERIC-PCR for typing of the species F. tularensis revealed a discriminatory index of 0.65.
Although the F. tularensis strains displayed very similar patterns after ERIC-PCR or REP-PCR, the patterns were distinct from each of 15 patterns resulting from the amplification of DNA of other clinically relevant species using the same primers (data not shown). Thus, the analyses indicated that patterns generated by the F. tularensis isolates were unique and readily distinguished from those of other clinically relevant species.
ERIC and REP sequences have been reported to be present in a multitude of bacterial species (28), but there is no information confirming their presence in Francisella genomes. To this end, we searched for such sequences in the genome of strain Schu S4 (unpublished data). More than 98% of the genome has been sequenced. No sequences with similarities to REP or ERIC sequences could be identified. Thus, it is likely that the banding patterns obtained from PCR amplification with primers complementary to consensus motifs of REP or ERIC sequences were based on annealing to other types of sequences present in the Francisella genome. It has been reported that ERIC primers can generate relatively complex patterns from eukaryotic and prokaryotic genomes despite a lack of the specific target sequences (7).
PCR amplification with random primers.
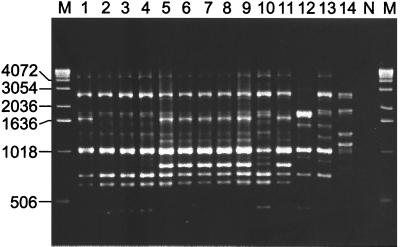
Previously, it was reported that the use of random primers containing 18 to 24 nucleotides results in patterns after PCR-based amplification that are more reproducible than those seen after amplification with shorter random primers (6, 7). PCR amplification based on different combinations of six such random primers was assessed. Only one of the primers gave an amplification pattern of sufficient complexity. The numbers of amplicons observed were similar to the numbers obtained by use of ERIC or REP primers (Fig. 2). The sizes of the amplicons ranged from 0.4 to 5.0 kb. Strains of F. tularensis subsp. tularensis displayed two distinct patterns, one for the avirulent type strain of the subspecies, ATCC 6223 (FSC138), and one for the other six strains. Again, the recently isolated representative from Europe (FSC198) also showed the pattern typical of F. tularensis subsp. tularensis. Patterns from F. tularensis subsp. holarctica strains, including the Japanese ones, were all identical and clearly discernible from those of other subspecies. A distinct pattern was observed for the representative of F. tularensis subsp. mediaasiatica. Patterns from strains of F. philomiragia (five representatives) and F. tularensis subsp. novicida (three representatives) were each unique (Fig. 2; not all strains are shown). Clustering of the strains was thus very similar with LP-RAPD–PCR, ERIC-PCR, and REP-PCR (Table 1). The discriminatory index of LP-RAPD–PCR was 0.57. A summary of the various LP-RAPD patterns is given in Table 1.
FIG. 2.
PCR amplification of DNA from various Francisella strains using the LP-RAPD2 primer. Samples represent F. tularensis subsp. tularensis strains (FSC138, FSC043, FSC041, and FSC198) (lanes 1 to 4), F. tularensis subsp. holarctica strains (FSC196, FSC155, FSC150, FSC108, and FSC157) (lanes 5 to 9), an F. tularensis subsp. mediaasiatica strain (FSC149) (lane 10), an F. tularensis subsp. holarctica strain from Japan (FSC024) (lane 11), F. tularensis subsp. novicida strains (FSC040 and FSC156) (lanes 12 and 13), and an F. philomiragia strain (FSC144) (lane 14). Strain designations are indicated in Table 1. Lane N, water used as a negative control. Lane M, molecular markers (sizes in base pairs).
All the banding patterns produced by REP, ERIC, or LP-RAPD from each of the studied strains were reproducible, but variations in the relative intensities of the bands were observed (data not shown). REP, ERIC, and LP-RAPD reactions were performed at least four times on each strain using templates prepared on three different occasions.
Amplification with F. tularensis-specific primers.
Sequencing of a fragment obtained after amplification with a random primer revealed that a 30-bp sequence was found only in some F. tularensis genomes. By designing F. tularensis-specific primers specific for the region adjacent to this sequence, amplicons of variable length were obtained after PCR. Amplification using these primers was combined with a previously described PCR specific for a gene encoding a 17-kDa lipoprotein conserved in all investigated strains of F. tularensis (22).
The multiplex PCR was designed to generate bands of approximately 0.4 and 0.3 kb (Fig. 3). From all F. tularensis strains, except for the F. tularensis subsp. novicida-like strain FSC159, a fragment of the expected size, 0.4 kb, was amplified from the 17-kDa lipoprotein gene. From strain FSC159, a slightly smaller fragment resulted due to a gene truncation (data not shown). No amplicons were amplified from F. philomiragia strains by use of the primers specific for the 17-kDa lipoprotein gene. From all F. tularensis subsp. holarctica strains, including the two strains from Japan, a 300-bp amplicon was amplified, whereas amplification from all strains of F. tularensis subsp. tularensis and F. tularensis subsp. mediaasiatica and the reference strain of F. tularensis subsp. novicida (FSC 040; ATCC 15482) generated a 330-bp band. The two F. tularensis subsp. novicida-like strains (FSC156 and FSC159) both showed very faint 330-bp bands. A summary of the two patterns for the strains is shown in Table 1.
FIG. 3.
Multiplex PCR amplification using F. tularensis-specific primers. Samples represent F. tularensis subsp. tularensis strains (FSC138, FSC043, FSC041, and FSC198) (lanes 1 to 4), F. tularensis subsp. holarctica strains (FSC196, FSC155, FSC150, FSC108, and FSC157) (lanes 5 to 9), an F. tularensis subsp. mediaasiatica strain (FSC149) (lane 10), an F. tularensis subsp. holarctica strain from Japan (FSC024) (lane 11), F. tularensis subsp. novicida strains (FSC040 and FSC156) (lanes 12 and 13), and an F. philomiragia strain (FSC144) (lane 14). Strain designations are indicated in Table 1. Lane N, water used as a negative control. Lane M, molecular markers (sizes in base pairs).
DISCUSSION
The family Francisellaceae, a member of the γ-subclass of Proteobacteria, comprises closely related organisms within the single genus Francisella (20). Previous studies have revealed that the two recognized species, F. tularensis and F. philomiragia, show a 16S rRNA sequence similarity of ≥98% (5). The close relationship has hampered the development of generally applicable methods for discrimination of F. tularensis subspecies that differ in virulence or geographical origins. PCR amplification of REP and ERIC sequences often enables discrimination of bacterial isolates (17, 26), and it has even been proposed that PCR fingerprinting can be used to elucidate the genetic basis of phenotypic variability for certain species (26). In addition, amplification of bacterial genomes using arbitrary primers has been successfully used for the same purpose.
In the present study, all the protocols based on PCR amplification using specific or arbitrary primers allowed differentiation of strains at the species level. Moreover, the PCR analyses based on the use of ERIC, REP, or long arbitrary primers yielded reproducible banding patterns of similar complexity and allowed the differentiation of strains at the subspecies level. We believe that the significance of this clustering was strengthened by the facts that the fingerprints were reproduced for a rather large number of strains, that each of the three methods supported the same clustering of strains, and that the clusters correlated with the geographical origins of the strains. It was not totally unexpected that the patterns of the subspecies were rather similar, considering that previous analyses of 16S rRNA sequences (5) and recent analyses of three complete 23S rRNA gene sequences, representing three out of the four subspecies of F. tularensis, and seven partial sequences of gyrase B genes (unpublished data) showed them to be very similar and not suitable as a basis for the development of molecular typing methods discriminatory at the subspecies level.
Within all of the four subspecies, with the exception of F. tularensis subsp. novicida, very similar or identical patterns were generated. One notable exception was that F. tularensis subsp. holarctica isolates from Japan were distinguished from other isolates of the subspecies after amplification with REP or ERIC primers. Japanese isolates are also distinguished by lower virulence in experimental animals than F. tularensis subsp. tularensis and F. tularensis subsp. holarctica isolates from Europe and North America, and they cause a relatively mild form of human disease. On this basis, a more detailed analysis of the phenotypic and genotypic properties of Japanese isolates may be warranted to determine whether they should comprise a separate subspecies. Isolates of F. tularensis subsp. mediaasiatica are found only in some areas of the Central Asian republics of the former USSR, and only limited information is available regarding their characteristics. The isolates have been considered to belong to a separate subspecies on the bases of their ability to produce acid from glycerol and degrade ornithine and their low virulence in experimental models of tularemia. This subspecies differentiation was supported by the observation that the isolates showed distinct patterns in each of the three PCR-based methods.
In contrast to isolates of the other subspecies of F. tularensis, F. tularensis subsp. tularensis isolates cause a life-threatening disease, and this high virulence necessitates the availability of methods for their rapid identification. It has long been accepted that isolates of the subspecies exist only in North America (8, 20). Notably, we found that the pattern obtained from a Slovakian strain derived from a mite was identical to those obtained from North American F. tularensis subsp. tularensis strains, thus supporting the recently reported finding that, based on biochemical characteristics and virulence for rabbits, this strain should be classified as a member of F. tularensis subsp. tularensis. This situation is of concern for European reference laboratories, since the highly contagious F. tularensis strains always pose a risk that laboratory staff may acquire tularemia. Although the presented specific PCR does not distinguish among F. tularensis subsp. tularensis, F. tularensis subsp. novicida, and F. tularensis subsp. mediaasiatica strains, it nevertheless could provide useful clinical information by rapidly identifying the highly virulent strains of F. tularensis subsp. tularensis, especially in North America, where these strains, in contrast to strains of the other two subspecies, are relatively common. Moreover, when the specific PCR is combined with one of the described arbitrary-primer PCR methods, isolates of F. tularensis subsp. tularensis are readily distinguished from those of the other subspecies.
A recently published study (2) aimed to identify methods that allowed discrimination of isolates of F. tularensis subsp. holarctica, the majority of which originated from Spain. The study evaluated PCR methods based on REP, ERIC, M13, or T3-T7 primers. It was reported that the discriminatory indices (10) of the methods ranged from 0.14 (REP) to 0.65 (T3-T7), below the recommended value of >0.95 for a method considered suitable for routine typing of individual isolates (23). Our finding of discriminatory indices of ≤0.65 correlates well with those of the Spanish study and shows that the investigated methods do not meet the required minimum criterion for typing of individual isolates. The test population is also an important factor to consider when assessing discriminatory power; the population should reflect the diversity of the particular species as much as possible (23). We believe that our collection of strains, which included strains from the various regions of the Northern Hemisphere where tularemia is endemic and all four subspecies of F. tularensis, is more representative than the Spanish collection for an analysis of the discriminatory indices of the PCR-based methods. It should be noted that even when the results of the four methods analyzed by de la Puente-Redondo et al. (2) were combined, the discriminatory index was 0.90, still below the recommended value of >0.95 (23). Moreover, considering that REP and ERIC sequences do not exist in the Francisella genome, probably all of the methods used are variants of RAPD-PCR, a technique with moderate or low reproducibility (14, 25–27). Although we consider that the investigated methods have discriminatory powers too low to be useful for typing of strains, this conclusion does not exclude, however, the use of one or several of the methods by reference laboratories because a positive finding, i.e., strains showing distinct patterns, may still be of epidemiological interest.
In summary, the relative simplicity and discriminatory power of several of the investigated methods make them useful for clinical laboratories as tools for rapidly identifying and discriminating Francisella species and subspecies but not for discriminating individual strains.
ACKNOWLEDGMENTS
Grant support was obtained from the Swedish Medical Research Council (no. 9485); Samverkansnämnden Norra Sjukvårdsregionen, Umeå, Sweden; and the Medical Faculty, Umeå University, Umeå, Sweden.
REFERENCES
- 1.Clarridge J E, III, Raich T J, Sjöstedt A, Sandström G, Darouiche R O, Shawar R M, Georghiou P R, Osting C, Vo L. Characterization of two unusual clinically significant Francisella strains. J Clin Microbiol. 1996;34:1995–2000. doi: 10.1128/jcm.34.8.1995-2000.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de La Puente-Redondo V A, del Blanco N G, Gutierrez-Martin C B, Garcia-Pena F J, Ferri E F. Comparison of different PCR approaches for typing of Francisella tularensis strains. J Clin Microbiol. 2000;38:1016–1022. doi: 10.1128/jcm.38.3.1016-1022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dienst J, F T. Tularemia—a perusal of three hundred thirty-nine cases. J La State Med Soc. 1963;115:114–127. [PubMed] [Google Scholar]
- 4.Evans M E, Gregory D W, Schaffner W, McGee Z A. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore) 1985;64:251–269. [PubMed] [Google Scholar]
- 5.Forsman M, Sandström G, Sjöstedt A. Analysis of 16S ribosomal DNA sequences of Francisella strains and utilization for determination of the phylogeny of the genus and for identification of strains by PCR. Int J Syst Bacteriol. 1994;44:38–46. doi: 10.1099/00207713-44-1-38. [DOI] [PubMed] [Google Scholar]
- 6.Gillings M, Holley M. Amplification of anonymous DNA fragments using pairs of long primers generates reproducible DNA fingerprints that are sensitive to genetic variation. Electrophoresis. 1997;18:1512–1518. doi: 10.1002/elps.1150180904. [DOI] [PubMed] [Google Scholar]
- 7.Gillings M, Holley M. Repetitive element PCR fingerprinting (rep-PCR) using enterobacterial repetitive intergenic consensus (ERIC) primers is not necessarily directed at ERIC elements. Lett Appl Microbiol. 1997;25:17–21. doi: 10.1046/j.1472-765x.1997.00162.x. [DOI] [PubMed] [Google Scholar]
- 8.Gurycova D. First isolation of Francisella tularensis subsp. tularensis in Europe. Eur J Epidemiol. 1998;14:797–802. doi: 10.1023/a:1007537405242. [DOI] [PubMed] [Google Scholar]
- 9.Hollis D G, Weaver R E, Steigerwalt A G, Wenger J D, Moss C W, Brenner D J. Francisella philomiragia comb. nov. (formerly Yersinia philomiragia) and Francisella tularensis biogroup novicida (formerly Francisella novicida) associated with human disease. J Clin Microbiol. 1989;27:1601–1608. doi: 10.1128/jcm.27.7.1601-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim A, Gerner-Smidt P, Sjöstedt A. Amplification and restriction endonuclease digestion of a large fragment of genes coding for rRNA as a rapid method for discrimination of closely related pathogenic bacteria. J Clin Microbiol. 1996;34:2894–2896. doi: 10.1128/jcm.34.12.2894-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim A, Norlander L, Macellaro A, Sjöstedt A. Specific detection of Coxiella burnetii through partial amplification of 23S rDNA. Eur J Epidemiol. 1997;13:329–334. doi: 10.1023/a:1007385104687. [DOI] [PubMed] [Google Scholar]
- 13.Johansson A, Berglund L, Eriksson U, Göransson I, Wollin R, Forsman M, Tärnvik A, Sjöstedt A. Comparative analysis of PCR versus culture for diagnosis of ulceroglandular tularemia. J Clin Microbiol. 2000;38:22–26. doi: 10.1128/jcm.38.1.22-26.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meunier J R, Grimont P A. Factors affecting reproducibility of random amplified polymorphic DNA fingerprinting. Res Microbiol. 1993;144:373–379. doi: 10.1016/0923-2508(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 15.Niebylski M L, Peacock M G, Fischer E R, Porcella S F, Schwan T G. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl Environ Microbiol. 1997;63:3933–3940. doi: 10.1128/aem.63.10.3933-3940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noda H, Munderloh U G, Kurtti T J. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olive D M, Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37:1661–1669. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsufjev N G, Meshcheryakova I S. Subspecific taxonomy of Francisella tularensis. Int J Syst Bacteriol. 1983;33:872–874. [Google Scholar]
- 19.Sandström G, Tärnvik A, Wolf-Watz H, Löfgren S. Antigen from Francisella tularensis: nonidentity between determinants participating in cell-mediated and humoral reactions. Infect Immun. 1984;45:101–106. doi: 10.1128/iai.45.1.101-106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjöstedt, A. Family XVII. Francisellaceae, genus I. Francisella. In D. J. Brenner (ed.), Bergey's manual of systematic bacteriology, in press. Springer-Verlag, New York, N.Y.
- 21.Sjöstedt A, Eriksson U, Berglund L, Tärnvik A. Detection of Francisella tularensis in ulcers of patients with tularemia by PCR. J Clin Microbiol. 1997;35:1045–1048. doi: 10.1128/jcm.35.5.1045-1048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjöstedt A, Kuoppa K, Johansson T, Sandström G. The 17 kDa lipoprotein and encoding gene of Francisella tularensis LVS are conserved in strains of F. tularensis. Microb Pathog. 1992;13:243–249. doi: 10.1016/0882-4010(92)90025-j. [DOI] [PubMed] [Google Scholar]
- 23.Struelens M J the Members of the European Study Group on Epidemiological Markers (ESGEM) of the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 24.Suitor E C, Jr, Weiss E. Isolation of a rickettsialike microorganism (Wohlbachia persica n. sp.) from Argas persicus (Oken) J Infect Dis. 1961;108:95–106. [Google Scholar]
- 25.Tyler K D, Wang G, Tyler S D, Johnson W M. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J Clin Microbiol. 1997;35:339–346. doi: 10.1128/jcm.35.2.339-346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Belkum A. DNA fingerprinting of medically important microorganisms by use of PCR. Clin Microbiol Rev. 1994;7:174–184. doi: 10.1128/cmr.7.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, et al. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]