Summary
Aim
Low- and middle-income countries (LMICs) are currently experiencing increasing cardiovascular disease (CVD) rates. Green leafy vegetables (GLV), which are abundant in these countries, are known to be particularly rich in cardioprotective nutrients. This study sought to determine the specific effect of GLV intake on the incidence of CVD.
Methods
Previously published cohort studies on GLV intake and incidence of CVD were retrieved through a systematic search of Google Scholar, EMBASE, MEDLINE, HINARI and Cochrane Library. A methodological evaluation of studies was carried out using the network of Ottawa scale, and a fixed-effect meta-analysis was applied to estimate pooled relative risk (RR) and 95% confidence interval (CI). Heterogeneity was determined using the I2 statistic. Sensitivity analysis was done using the leave-one-study-out technique. All statistical analysis was carried out at p < 0.05 using RevMan 5.4.
Results
The pooled RR (95% CI) of incident CVD events from 17 studies was 0.93 (0.92–0.95). Specifically, GLV intake was inversely related with incident cerebral infarction (RR: 0.92; 95% CI: 0.88–0.96), heart disease (RR: 0.93; 95% CI: 0.87–0.99) and other CVD events (RR: 0.95; 95% CI: 0.93–0.98).
Conclusion
GLV intake was associated with a lower incidence of CVD, and may be a promising primary-prevention strategy against CVD events. The findings are especially important in LMICs where the burden of CVD remains high.
Keywords: green leafy vegetables, cardiovascular diseases, cerebral infarction, coronary heart disease, heart disease, metaanalysis
Cardiovascular diseases (CVD) account for about 17.9 million deaths annually1 and a huge burden of health expenditure worldwide.2,3 Although CVD rates appear to be declining globally,1,2,4-6 populations in low- and middle-income countries (LMIC)6,7 continue to experience increasing CVD rates. CVD are preventable and efforts are currently being mobilised to achieve a 25% reduction in mortality rate attributable to CVD by 2025.8,9
A promising preventative strategy for CVD is diet.10-13 However, studies on the potential association of diet and CVD events have focused on the effect of red meat,14,15 salt intake,16 alcohol,17 saturated fats/oils and dairy products.18 Prior reviews and metaanalyses19-24 investigating the effect of fruit and vegetables on the risk profile for CVD have focused on broad categories of the nutritional modalities. For example, Deng et al.19 and Kwok et al.24 in two reviews of meta-analyses assessed the effect of fruit and vegetable intake, in general, on the burden of diseases and all-cause mortality without providing information on the specific effect(s) of green leafy vegetables (GLV) on the incidence of distinct CVD events.
The information provided by individual studies on the effect of GLV intake remains inconclusive. While some studies reported a reduction in the incidence of CVD events with higher consumption of GLV,10,25,26 others observed statistically insignificant relationships.27,28 The pooled effect of GLV intake on incident CVD is currently unknown.
GLV are widely available in LMIC.29 The vegetables are rich in phytochemicals and micronutrients known to be essential for health.13,30-32 Also, GLV contain folic acid, vitamins A, C, E and K, as well as high amounts of calcium, iron, potassium, phosphorous and zinc,33,34 which may be protectively associated with CVD risk.35 This systematic review and meta-analysis investigated the pooled effect of GLV intake on incident CVD events.
Methods
The systematic review was registered in the international prospective register of systematic reviews and is accessible via https://www.crd.york.ac.uk/prospero/display_record. php?ID=CRD42020181050. Google Scholar, EMBASE, MEDLINE, HINARI and Cochrane Library were searched (in December 2020 using specific search terms independent of language and publication dates) for previously published epidemiological reports on consumption of GLV and CVD. The following search terms were used.
EMBASE, Google Scholar and Cochrane Library search terms: ‘vegetables’ OR ‘chlorophyll-containing vegetables’ OR ‘green leafy vegetables’ OR ‘broccoli’ OR ‘cabbage’ OR ‘celery’ OR ‘collard green’ OR ‘green pea’ OR ‘lettuce’ OR ‘spinach’ OR ‘swiss chard’ OR ‘turnip green’ AND ‘cardiovascular disease’ OR ‘cerebrovascular disease’ OR ‘cerebral infarction’ OR ‘cerebral haemorrhage’ OR ‘coronary heart disease’ OR ‘heart failure’ OR ‘subarachnoid haemorrhage’.
MEDLINE and HINARI search terms using PubMed interphases: ‘vegetables (Title/Abstract)’ OR ‘green leaves (Title/ Abstract)’ OR ‘edible green leaves (Title/Abstract)’ OR ‘green vegetables (Title/Abstract)’ OR ‘leafy vegetables (Title/Abstract)’ OR ‘green leafy vegetables (Title/Abstract)’ OR ‘chlorophyllcontaining vegetables (Title/Abstract)’ OR ‘broccoli (Title/ Abstract)’ OR ‘cabbage (Title/Abstract)’ OR ‘celery (Title/ Abstract)’ OR ‘collard green (Title/Abstract)’ OR ‘green pea (Title/Abstract)’ OR ‘lettuce (Title/Abstract)’ OR ‘spinach (Title/ Abstract)’ OR ‘swiss chard (Title/Abstract)’ OR ‘turnip green (Title/Abstract)’ AND ‘stroke (MesH terms)’ OR ‘transient ischemic attack (MeSH terms)’ OR ‘haemorrhagic stroke (MeSH terms)’ OR ‘ischaemic stroke (MeSH terms)’ OR ‘cardiovascular disease (MeSH terms)’ OR ‘cerebrovascular disease (MeSH terms)’ OR ‘cerebral infarction (MeSH terms)’ OR ‘cerebral haemorrhage (MeSH terms)’ OR ‘coronary heart disease (MeSH terms)’ OR ‘heart failure (MeSH terms)’ OR ‘subarachnoid haemorrhage (MeSH terms)’. Details of the literature search are in the PRISMA flow chart (Fig. 1).
Fig. 1.
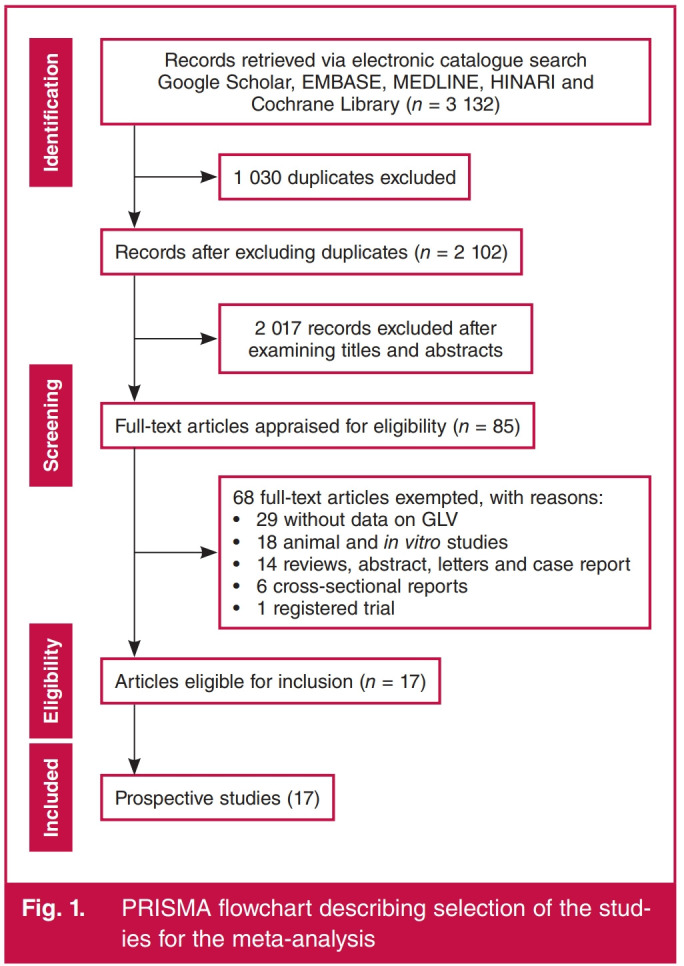
PRISMA flowchart describing selection of the studies for the meta-analysis.
Study assessment for inclusion and exclusion criteria and data extraction were conducted by two independent assessors (AO and APO) based on the descriptions in the original article. Only studies with usable data and appropriate analytical techniques were included in the meta-analysis. The following information was extracted from each included study: first author name, publication year, sample size, average follow-up time, the incidence of CVD, adjusted relative risk (RR)/hazard ratio and 95% confidence interval (CI), etc.
Studies included in this meta-analysis were prospective cohort reports (where the primary exposure was GLV consumption and outcomes were CVD events) only. Where there are significant levels of data overlap among published studies, the study with complete evidence was included in the quantitative synthesis.
A methodological assessment for risk of bias of included studies was conducted (independently by two members of the review team) using the Newcastle–Ottawa scale for quality assessment of observational reports36 following the Cochrane Collaboration guidelines.37
Statistical analysis
Using the RR and 95% CI for highest quintile/category of GLV consumption compared to the lowest quintile/category of GLV consumption (as reference) for the incidence of CVD events reported in the included studies, we computed the log of RR and the matching standard error for the overall pooled RR (95% CI) for the incidence of CVD events and by subgroup stratification [cerebral infarction, cerebral haemorrhage, coronary heart disease (CHD), etc.] using an inverse-of-variance method for weighting in all quantitative estimations for dichotomous outcomes.
The degree of heterogeneity was assessed using I2 statistics assuming a fixed-effect model (where I2 < 50%) or a randomeffect meta-analysis model if I2 > 50%. The fixed-effect model presupposes the effect size is likely relatively similar across studies in the meta-analysis.37,38 However, a random-effect model ideates the difference in effect estimates across studies are valid but follows a normal distribution. Publication bias for the likely effect estimate of GLV intake on CVD events was tested using funnel plots.
The constancy of the pooled RR (95% CI) was tested using the leave-one-study-out method (carrying out the meta-analysis several times, excluding a study at a time). All quantitative analyses were conducted at p < 0.05 using the RevMan 5.4 software.39
Results
Over 3 000 records were retrieved from the literature search in Google Scholar, EMBASE, MEDLINE, HINARI and Cochrane Library but 1 021 duplicates were excluded. Also, 2 011 records were excluded after screening the titles and abstracts (Fig. 1). On full-text assessment, 65 records were excluded and 17 prospective reports (five reports on composite CVD events,10,25-27,40 five reports on coronary heart disease,28,41-44 one report on heart failure45 and six reports on stroke46-51) were included in the meta-analysis.
Studies on this subject (Table 1) were published over 12 years (1995–2017). Most reports assessed GLV intakes using the foodfrequency questionnaire, but limited studies42,45,50 adjusted for total energy intakes (and other dietary confounding factors) in the multivariate analysis of GLV and CVD outcomes.
Table 1. Characteristics of prospective reports included in the meta-analysis.
| Study | characteristics | Baselineloutcomes evaluation | ||||||
| First author | Year | Country | GLV intake | Incidence | Total | CVD event(s) | Assessment | Ascertainment |
| Gaziano JM | 1995 | United States | < 1 s/d* VS 1 s/d | 161 | 1 299 | CVD | Relative-reported deaths | Not reported |
| Joshipura KJ | 1999 | United States | Increment of 1 s/d³ | 366" 204M | 75 596W 38 683M | Ischaemic stroke | Self/relative report | National Stroke Soci- ety (NSS) criteria |
| Joshipura KJ | 2001 | United States | Increment of 1 s/d2,3 | 1 127" 1 063M | 84 251" 42 148M | CHD | Self/relative report | World Health Organ- isation (WHO) criteria |
| Johnsen SP | 2003 | Denmark | 1.4 g/d* VS 28.00 g/d | 266 | 54 506 | Ischaemic stroke | Self/relative report | WHO criteria |
| Sauvaget C | 2003 | Japan | < 1 s/week* VS 1 s/d² | 1 926 | 40 349 | Stroke | Stroke mortality | WHO criteria |
| Hung HC | 2004 | United States | Increment of 1 s/d³ | 3864 | 109 635 | CVD | Self/relative report | NSS criteria |
| Takachi R | 2007 | Japan | Not reported | 1 386 | 77 891 | CVD | MI or stroke diagnosis using CT scan/MRI | WHO and NSS criteria |
| Joshipura KJ | 2009 | United States | Not reported | 1852" 2040M | 70 870w 38 918M | Ischaemic CVD | Self/relative report | WHO and NSS criteria |
| Bendinelli B | 2010 | Italy | < 17.60 g/d* VS > 50.80 g/d¹ | 144 | 29 689 | CHD | Self/relative report | Minnesota Code |
| Oude Griep LM | 2011A | Netherlands | 34 g/d* VS 105 g/d2,3 | 233 | 20 069 | Stroke | Population and hospital discharge register | Dutch guidelines |
| Oude Griep LM | 2011B | Netherlands | 34 g/d* VS 105 g/d2,3 | 245 | 20 069 | CHD | Population and hospital discharge register | WHO criteria |
| Larsson S | 2013 | Sweden | < 2.3 s/d* VS >6.0 s/d1,2,3 | 4 089 | 74 961 | Stroke | Self report | Not reported |
| Bhupathiraju SN | 2013 | United States | 0.22 s/d* VS 1.50 s/day1,2 | 6189 | 71 141 | CHD | Self/relative report | WHO criteria |
| Rautiainen S | 2015 | Sweden | < 0.2 s/d* VS > 1 s/d1,2,3 | 3051 | 34 319 | Heart failure | Heart failure diagnosis and related deaths | ESC criteria |
| Wang JB | 2016 | China | Increment of twice/week | 355 | 2 445 | Stroke | Case, pathology, cytology, X-rays, biochemical, ultrasound, endos- copy and surgery reports | Team of reviewers |
| Buil-Cosiales P | 2016 | Spain | 32-16g/d* VS 113.00 g/d¹ | 342 | 7216 | CVD | Self/relative report | Team of reviewers |
| Blekkenhorst LC | 2017 | Australia | Intake per 10 g/d | 238 | 1 226 | CHD | CHD diagnosis and related death+ | Not reported |
*Reference group for comparison; 1energy-adjusted dietary intakes of GLV; 2additionally adjusted for other intakes, etc; 3using median values of quintiles; Mmen; Wwomen; cMI events, coronary revascularisation, or both not preceded by any other CHD event; ‡authenticated via vital statistics or medical records or designated registry;
†validated death certificate. g/d – grams per day; s/d – servings per day; GLV – green leafy vegetables; ESC – European Society of Cardiology; CVD – cardiovascular disease; CHD – coronary heart disease; CT – computed tomography; MI – myocardial infarction; MRI – magnetic resonance imaging.
More than half of the studies included in this report presented a low risk of bias (Table 2). In all, methodological assessment of included reports revealed no evidence of high risk of bias in most studies included in the meta-analysis.
Table 2. Methodological assessment of prospective studies using the Newcastle–Ottawa scale.
| Selection | Compa- rability | Outcome | Total | Risk of bias of included | |||||||
| Study | Year | SI | S2 | S3 | S4 | C1 | 01 | 02 | 03 | Scores | studies |
| Gaziano et al. | 1995 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | High | ||
| Joshipura et al. | 1999 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low | |
| Joshipura et al. | 2001 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Moderate | ||
| Johnsen et al. | 2003 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Moderate | ||
| Sauvaget et al. | 2003 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | Moderate | |
| Hung et al. | 2004 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low |
| Takachi et al. | 2007 | 1 | 1 | 2 | 1 | 1 | 7 | Moderate | |||
| Joshipura et al. | 2008 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Moderate | ||
| Bendinelli et al. | 2010 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low | |
| Oude Griep et al. | 2011A | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low | |
| Oude Griep et al. | 2011B | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low |
| Larsson et al. | 2013 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low | |
| Bhupathiraju et al. | 2013 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low |
| Rautiainen et al. | 2014 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low |
| Buil-Cosiales et al. | 2016 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low | |
| Wang et al. | 2016 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | Moderate | |
| Blekkenhorst et al. | 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Low |
Risk of bias of included studies: high risk of bias: ≤ 6; moderate risk of bias: 7–8; low risk of bias: 9 and empty cells indicate a score of 0. S1 – representativeness of the exposed cohort; S2 – selection of the non-exposed cohort; S3 – ascertainment of exposure; S4 – demonstration that outcome of interest was absent at the start of the study; C1 – comparability of the cohort based on the design or analysis; O1 – assessment of outcome; O2 – was follow up long enough for outcomes to occur?; O3 – adequacy of follow up of cohorts.
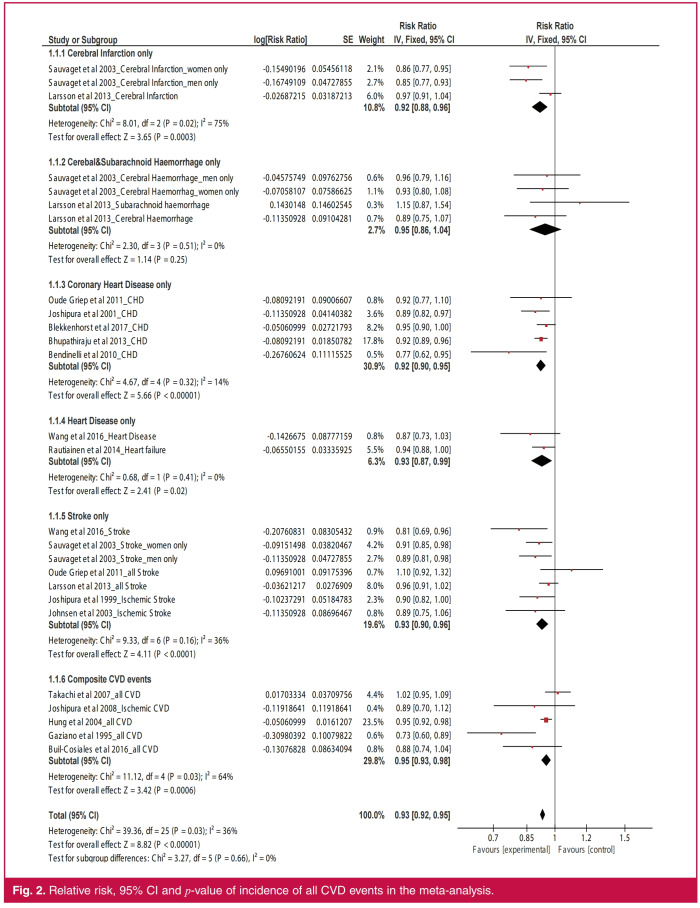
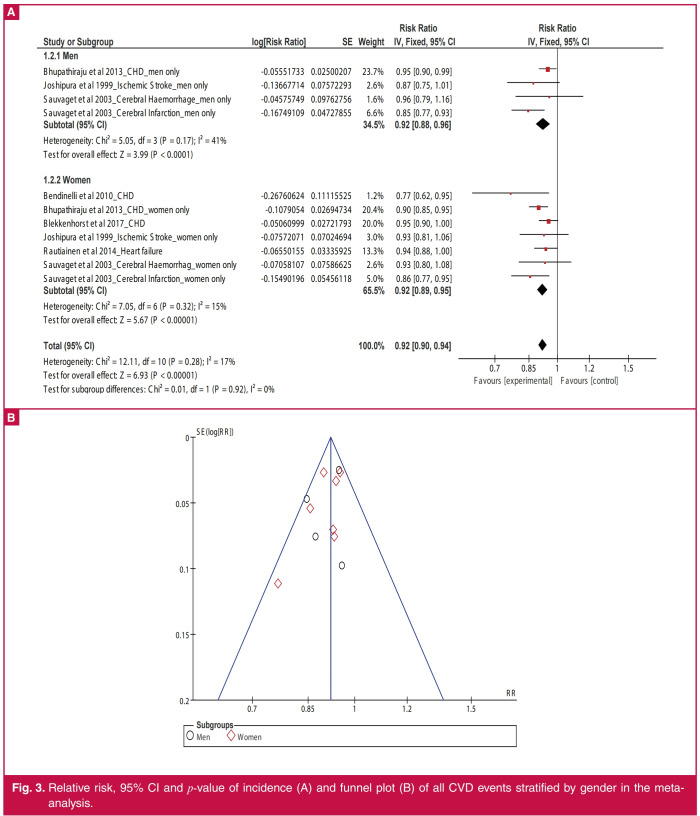
Overall, higher intake of GLV (Fig. 2) was associated with reduced incidence of all CVD events by 7% (RR: 0.93; 95% CI: 0.92–0.95; p < 0.00001). Similarly, higher GLV intake was inversely related to the incidence of cerebral infarction (RR: 0.92; 95% CI: 0.88–0.96; p = 0.0003), CHD (RR: 0.92; 95% CI: 0.90–0.95; p < 0.00001), heart disease (RR: 0.93; 95% CI: 0.87–0.99; p = 0.02) and stroke (RR: 0.93; 95% CI: 0.90–0.96; p < 0.0001). The result remained unchanged after stratifying the studies by gender of respondents (Fig. 3A); men (RR: 0.92; 95% CI: 0.88–0.96; p < 0.0001) and women (RR: 0.92; 95% CI: 0.89–0.95; p < 0.00001).
Fig. 2.
Relative risk, 95% CI and p-value of incidence of all CVD events in the meta-analysis.
Fig. 3.
Relative risk, 95% CI and p-value of incidence (A) and funnel plot (B) of all CVD events stratified by gender in the metaanalysis.
Statistical heterogeneity (Fig. 1) was low for studies on heart disease only (I2 = 0%), CHD only (I2 = 14%), and stroke only (I2 = 36%) but not among studies on cerebral infarction only (I2 = 75%).
Funnel plots (Figs 3B, 4) suggested no evidence of publication bias and no sole study exerted a significant effect on the sensitivity of the overall findings of the meta-analysis (Tables 3, 4 ).
Fig. 4.
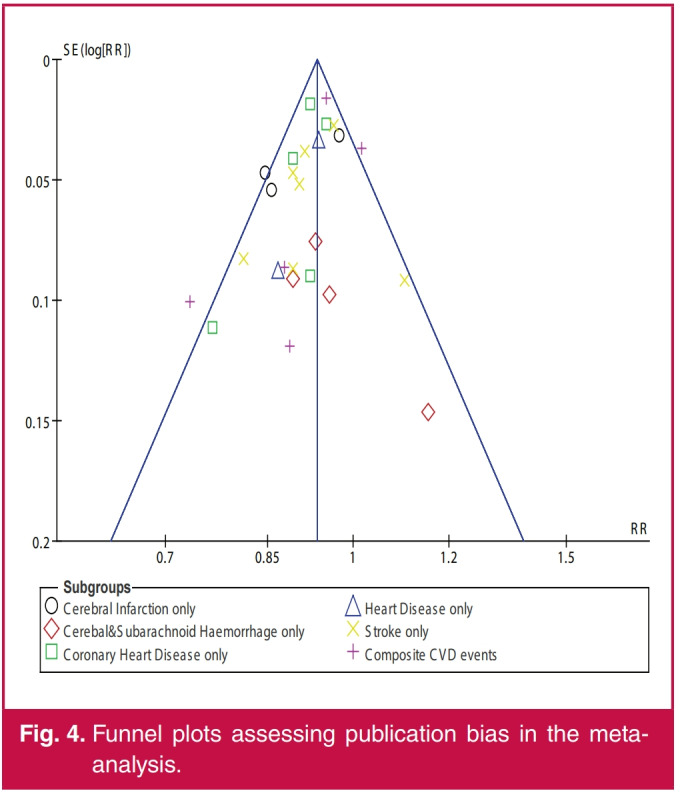
Funnel plots assessing publication bias in the metaanalysis.
Table 3. Sensitivity analysis of pooled RR stratified by categories of CVD events in the meta-analysis.
| Studies in the meta-analysis | I² (%) | Pooled RR (95% CI) | p-value |
| All studies | 36 | 0.93 (0.92-0.95) | < 0.00001 |
| Cerebral infarction only | 28 | 0.94 (0.92-0.95) | < 0.00001 |
| Cerebal and subarachnoid haemor- rhage only | 43 | 0.93 (0.92-0.95) | < 0.00001 |
| Coronary heart disease only | 41 | 0.94 (0.92-0.96) | < 0.00001 |
| Heart disease only | 40 | 0.93 (0.92-0.95) | < 0.00001 |
| Stroke only | 40 | 0.93 (0.92-0.95) | < 0.00001 |
| Composite CVD events | 22 | 0.93 (0.91-0.94) | < 0.00001 |
Table 4. Sensitivity analysis of pooled RR of all cohort studies included in the meta-analysis.
| I² | Pooled RR | ||
| Studies in the meta-analysis | (%) | (95% CI) | p-value |
| Cerebral infarction only | |||
| All studies | 75 | 0.92 (0.88-0.96) | 0.0003 |
| Larsson et al. 2013_Cerebral infarction | 0 | 0.85 (0.79-0.91) | < 0.00001 |
| Sauvaget et al. 2003_Cerebral infarc- tion_men only | 76 | 0.94 (0.89-0.99) | 0.03 |
| Sauvaget et al. 2003_Cerebral infarc- ion_women only | 84 | 0.93 (0.88-0.98) | 0.007 |
| Cerebal and subarachnoid haemorrhage only | |||
| All studies | 0 | 0.95 (0.86-1.04) | 0.25 |
| Larsson et al. 2013_Cerebral haemor- rhage | 0 | 0.97 (0.87-1.08) | 0.57 |
| Larsson et al. 2013_Subarachnoid haemorrhage | 0 | 0.93 (0.84-1.02) | 0.12 |
| Sauvaget et al. 2003_Cerebral haemor- rhage_women only | 0 | 0.96 (0.85-1.08) | 0.48 |
| Sauvaget et al. 2003_Cerebral haemor- rhage_men only | 13 | 0.95 (0.85-1.05) | 0.30 |
| Coronary heart disease only | |||
| All studies | 14 | 0.92 (0.90-0.95) | <0.00001 |
| Bendinelli et al. 2010_CHD | 0 | 0.93 (0.90-0.95) | <0.00001 |
| Bhupathiraju et al. 2013_CHD | 36 | 0.93 (0.89-0.97) | 0.0003 |
| Blekkenhorst et al. 2017_CHD | 4 | 0.91 (0.88-0.94) | < 0.00001 |
| Joshipura et al. 2001_CHD | 23 | 0.93 (0.90-0.96) | < 0.00001 |
| Oude Griep et al. 2011_CHD | 36 | 0.92 (0.90-0.95) | < 0.00001 |
| Heart disease only | |||
| All studies | 0 | 0.93 (0.87-0.99) | 0.02 |
| Rautiainen et al. 2014_Heart failure | - | 0.87 (0.73-1.03) | 0.10 |
| Wang et al. 2016_Heart disease | - | 0.94 (0.88-1.00) | 0.05 |
| Stroke only | |||
| All studies | 36 | 0.93 (0.90-0.96) | < 0.0001 |
| Johnsen et al. 2003_Ischemic stroke | 45 | 0.93 (0.90-0.97) | < 0.0001 |
| Joshipura et al. 1999_Ischemic stroke | 44 | 0.93 (0.90-0.97) | 0.0003 |
| Larsson et al. 2013_all stroke | 22 | 0.91 (0.87-0.95) | < 0.0001 |
| Oude Griep et al. 2011_all stroke | 14 | 0.92 (0.89-0.96) | < 0.0001 |
| Sauvaget et al. 2003_Stroke_men only | 41 | 0.94 (0.90-0.97) | 0.0005 |
| Sauvaget et al. 2003_Stroke_women only | 45 | 0.93 (0.90-0.97) | 0.0007 |
| Wang et al. 2016_Stroke | 24 | 0.94 (0.90-0.97) | 0.0003 |
| Composite CVD events | |||
| All studies | 64 | 0.95 (0.93-0.98) | 0.0006 |
| Buil-Cosiales et al. 2016_all CVD | 75 | 0.95 (0.93-0.98) | 0.001 |
| Gaziano et al. 1995_all CVD | 30 | 0.96 (0.93-0.98) | 0.003 |
| Hung et al. 2004_all CVD | 73 | 0.96 (0.90-1.02) | 0.17 |
| Joshipura et al. 2008_Ischemic CVD | 72 | 0.95 (0.93-0.98) | 0.0009 |
| Takachi et al. 2007_all CVD | 59 | 0.94 (0.91-0.97) | < 0.0001 |
Discussion
In this study, higher intake of GLV was linked to reduced incidence of all CVD events by 7% and, in particular, it was inversely related to the incidence of cerebral infarction, CHD, heart disease and stroke. These findings may suggest a potential role of GLV intake as a primary-prevention strategy in the management of CVD.
Similar to our findings, the largest study on stroke among Africans [the Stroke Investigative Research and Educational Network (SIREN) study] reported a strong protective dose– response association such that daily consumption of GLV was more protective against stroke [odds ratio (OR): 0.27; 95% CI: 0.19–0.38] than weekly consumption (OR: 0.70; 95% CI: 0.52– 0.95), compared to no consumption.52 Earlier systematic reviews and meta-analyses were broadly focused and generally combined fruit and vegetables in investigating the effect of these nutritional modalities on incident CVD events.11,19,20,22,53-58 The uniqueness of our study is therefore in the deconstruction of the specific contribution of GLV on CVD. Also, our approach offered vital insights into the potential roles of GLV in the occurrence of CVD subtypes.
Although the exact mechanism of the protective effect of GLV is not well understood, some constituents of GLV are likely to confer small-to-moderate but clinically important protection against CVD.25 For example, Vitamin B9, micronutrients and other constituents of GLV are known to promote optimal health and protect against several diseases.29,59 The fibre component of GLV is also known for its cholesterol-lowering effects.60 Similarly, folic acid (a constituent of GLV) intake is inversely associated with homocysteinaemia,61,62 a known risk factor for atherosclerosis and ischaemic stroke.63-65 Furthermore, micronutrients in GLV may promote cardiovascular integrity, haemostasis (Vitamin K content), neuronal transmission (calcium content), antioxidant activity (vitamins C and E content)32,66 and vasodilatory effects (nitrates content).67,68
There are existing gaps in the literature on the effect of GLV on CVD outcomes not covered by the present systematic review and meta-analysis. For example, the mode of preparation and preservation of GLV on CVD outcomes remains unclear. Similarly, the underlying molecular mechanisms mediating the protective effect of GLV remains incomplete. These gaps in our understanding of the relationship between GLV and CVD could be the basis of future cohort studies and clinical trials.
Limitations, strengths and recommendations
GLV are not consumed singly in diets. Similarly, higher GLV consumption in the presence of exposure to traditional risk factors of CVD (such as smoking, alcohol intake, low physical activity) does not imply less CVD risk. Our study considered populations exposed to higher GLV intakes in their overall diet only, independent of the magnitude of consumption of other food items.
This systematic review and meta-analysis has other limitations. First, this meta-analysis did not investigate the relationship between GLV and CVD outcomes according to ethnic background and country of study due to the limited number of studies on the subject. Most studies were from the United States. There were limited studies from populations of African and Asian ancestry. This hindered us from performing subgroup analyses by region and ethnicity as indicated in the study protocol.
Second, there were methodological differences in the estimation of GLV intake among studies included in this systematic review and meta-analysis. However, these differences are likely insignificant given the consistent direction and strength of the relationship in our reported pooled-effect estimate after stratifying the meta-analysis across several subgroups. However, it is necessary to establish models that can uniformly quantitate GLV consumption across different populations.
Third, our search for grey literature was limited to informal requests for unpublished data and reports on the effect of GLV on CVD from local specialists in human nutritional research. This strategy did not result in the retrieval of additional primary data suitable for our meta-analysis objectives.
A key strength of our study is that it may be the first to summarise data on the association between GLV intake and not only incident CVD events in general but also subtypes of these outcomes.
Conclusion
Our meta-analysis demonstrated that a higher intake of GLV was associated with a lower incidence of CVD events, independent of subtypes of CVD manifestation. Promoting the consumption of GLV may be useful for the management and prevention of CVD. Also, dietary strategies that incorporate GLV consumption may be encouraged and promoted. Further studies are necessary to determine the underlying mechanism(s) and the significance of duration of exposure on the magnitude of the effect of GLV on CVD events. In particular, a future multicentre cohort study with uniform quantification of GLV consumption and duration between exposure and CVD events would be desirable to confirm these findings.
Acknowledgments
The SIREN (U54HG007479) and SIBS genomics (R01NS107900) studies were funded by the National Institutes of Health under the H3Africa initiative. Investigators are further supported by NIH grant SIBS Gen Gen R01NS107900- 02S1; ARISES R01NS115944-01; H3Africa CVD Supplement 3U24HG009780- 03S5 and PINGS 2 R01HL152188. OMA and APO received partial funding from the Postgraduate College, University of Ibadan, Nigeria. APO received support from the Brain Pool Program through the National Research Foundation of Korea, funded by the Ministry of Science & ICT (2020H103A104081265). The funders had no role in study design, data collection, analysis, interpretation, decision to publish or preparation of the manuscript. A Ojagbemi and AP Okekunle contributed equally to the manuscript and are joint first authors.
Contributor Information
Akin Ojagbemi, Department of Psychiatry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Akinkunmi Paul Okekunle, Email: akinokekunle@gmail.com.
Mayowa Owolabi, Email: mayowaowolabi@yahoo.com.
Akinkunmi Paul Okekunle, Epidemiology Laboratory, Department of Food and Nutrition, College of Human Ecology, Seoul National University, Seoul, Korea.
Paul Olowoyo, College of Medicine and Health Sciences, Afe Babalola University; Department of Medicine, Federal Teaching Hospital, Ado-Ekiti, Nigeria.
Mayowa Owolabi, Email: mayowaowolabi@yahoo.com.
Bruce Ovbiagele, Weill Institute for Neurosciences; School of Medicine, University of California, San Francisco, California, USA.
Mayowa Owolabi, Email: mayowaowolabi@yahoo.com, Department of Medicine, College of Medicine, University of Ibadan; University College Hospital; Blossom Specialist Medical Centre, Ibadan, Nigeria.
References
- 1.WHO. Cardiovascular diseases (CVDs): WHO; 2017 [cited 2020 03.08.2020]. Available from: https://www.who.int/en/news-room/factsheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB. et al. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD. et al. Forecasting the future of cardiovascular disease in the United States. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S. et al. Heart disease and stroke statistics – 2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M. et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 6.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP. et al. Heart disease and stroke statistics – 2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 7.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA. et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leong DP, Joseph PG, McKee M, Anand SS, Teo KK, Schwalm J-D. Reducing the global burden of cardiovascular disease, Part 2. et al. Circ Res. 2017;121(6):695–710. doi: 10.1161/CIRCRESAHA.117.311849. [DOI] [PubMed] [Google Scholar]
- 9.Joseph P, Leong D, McKee M, Anand SS, Schwalm J-D, Teo K. et al. Reducing the global burden of cardiovascular disease, Part 1. Circ Res. 2017;121(6):677–694. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 10.Buil-Cosiales P, Toledo E, Salas-Salvado J, Zazpe I, Farras M, Basterra- Gortari FJ. et al. Association between dietary fibre intake and fruit, vegetable or whole-grain consumption and the risk of CVD: results from the PREvencion con DIeta MEDiterranea (PREDIMED) trial. Br J Nutr. 2016;116(3):534–546. doi: 10.1017/S0007114516002099. [DOI] [PubMed] [Google Scholar]
- 11.Rees K, Takeda A, Martin N, Ellis L, Wijesekara D, Vepa A. et al. Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2019;3 doi: 10.1002/14651858.CD009825.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A. et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD009874.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R. et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71(22):2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Kaluza J, Wolk A, Larsson SC. Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43(10):2556–2560. doi: 10.1161/STROKEAHA.112.663286. [DOI] [PubMed] [Google Scholar]
- 15.Yang C, Pan L, Sun C, Xi Y, Wang L, Li D. Red meat consumption and the risk of stroke: a dose–response meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. 2016;25(5):1177–1186. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. Br Med J. 2009;339:4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renaud SC. Diet and stroke. J Nutr Health Aging. 2001;5(3):167–172. [PubMed] [Google Scholar]
- 18.Spence JD. Diet for stroke prevention. Stroke Vasc Neurol. 2018;3(2):44–50. doi: 10.1136/svn-2017-000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng C, Lu Q, Gong B, Li L, Chang L, Fu L. et al. Stroke and food groups: an overview of systematic reviews and meta-analyses. Public Health Nutr. 2017;21(4):766–776. doi: 10.1017/S1368980017003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mytton OT, Nnoaham K, Eyles H, Scarborough P, Ni Mhurchu C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health. 2014;14(1):886. doi: 10.1186/1471-2458-14-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock RL. The effect of green leafy and cruciferous vegetable intake on the incidence of cardiovascular disease: A meta-analysis. JRSM Cardiovasc Dis. 2016;5:2048004016661435. doi: 10.1177/2048004016661435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yip CSC, Chan W, Fielding R. The associations of fruit and vegetable intakes with burden of diseases: a systematic review of meta-analyses. J Acad Nutr Diet. 2019;119(3):464–481. doi: 10.1016/j.jand.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T. et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality – a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029– 1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok CS, Gulati M, Michos ED, Potts J, Wu P, Watson L. et al. Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur J Prevent Cardiol. 2019;26(13):1415–1429. doi: 10.1177/2047487319843667. [DOI] [PubMed] [Google Scholar]
- 25.Gaziano JM, Manson JE, Branch LG, Colditz GA, Willett WC, Buring JE. A prospective study of consumption of carotenoids in fruits and vegetables and decreased cardiovascular mortality in the elderly. Ann Epidemiol. 1995;5(4):255–260. doi: 10.1016/1047-2797(94)00090-g. [DOI] [PubMed] [Google Scholar]
- 26.Hung H-C, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA. et al. Fruit and vegetable intake and risk of major chronic disease. J Nat Cancer Instit. 2004;96(21):1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 27.Takachi R, Inoue M, Ishihara J, Kurahashi N, Iwasaki M, Sasazuki S. et al. Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan public health center-based prospective study. Am J Epidemiol. 2007;167(1):59–70. doi: 10.1093/aje/kwm263. [DOI] [PubMed] [Google Scholar]
- 28.Oude Griep LM, Monique Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Colours of fruit and vegetables and 10-year incidence of CHD. Br J Nutr. 2011;106(10):1562–1569. doi: 10.1017/S0007114511001942. [DOI] [PubMed] [Google Scholar]
- 29.Moyo SM, Serem JC, Bester MJ, Mavumengwana V, Kayitesi E. African Green leafy vegetables: health benefits beyond nutrition. Food Rev Int. 2020:1–18. [Google Scholar]
- 30.Gertsch J. The metabolic plant feedback hypothesis: how plant secondary metabolites nonspecifically impact human health. Planta Med. 2016;82(11/12):920–929. doi: 10.1055/s-0042-108340. [DOI] [PubMed] [Google Scholar]
- 31.Fang X, Liang C, Li M, Montgomery S, Fall K, Aaseth J. et al. Dose– response relationship between dietary magnesium intake and cardiovascular mortality: A systematic review and dose-based meta-regression analysis of prospective studies. J Trace Elem Med Biol. 2016;38:64–73. doi: 10.1016/j.jtemb.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez-Aguilar DM, Grusak MA. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J Food Compost Anal. 2017;58:33–39. [Google Scholar]
- 33.Ejoh SI, Wireko-Manu FD, Page D, Mgc Renard C. Traditional green leafy vegetables as underutilised sources of micronutrients in a rural farming community in south-west Nigeria II: consumption pattern and potential contribution to micronutrient requirements. S Afr J Clin Nutr. 2019:1–6. [Google Scholar]
- 34.Ejoh SI, Wireko-Manu FD, Page D, Renard CMGC. Traditional green leafy vegetables as underutilised sources of micronutrients in a rural farming community in south-west Nigeria I: estimation of vitamin C, carotenoids and mineral contents. S Afr J Clin Nutr. 2019:1–6. [Google Scholar]
- 35.Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ. et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98(12):1198–1204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 36.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M. et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Canada: Ottawa Hospital Research Institute. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp ; 2014 [updated 2014. Available from: http://www.ohri.ca/programs/ clinical_epidemiology/oxford.asp . [Google Scholar]
- 37.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration. 2011 Available from www.cochrane-handbook.org . [Google Scholar]
- 38.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.Review Manager (RevMan) [Computer program]. Version 5.3.Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2014 [Google Scholar]
- 40.Joshipura KJ, Hung H-C, Li TY, Hu FB, Rimm EB, Stampfer MJ. et al. Intakes of fruits, vegetables and carbohydrate and the risk of CVD. Public Health Nutr. 2009;12(1):115–121. doi: 10.1017/S1368980008002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bendinelli B, Masala G, Saieva C, Salvini S, Calonico C, Sacerdote C. et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: the EPICOR Study. Am J Clin Nutr. 2011;93(2):275–283. doi: 10.3945/ajcn.110.000521. [DOI] [PubMed] [Google Scholar]
- 42.Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, Willett WC. et al. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr. 2013;98(6):1514–1523. doi: 10.3945/ajcn.113.066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE. et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134(12):1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 44.Blekkenhorst LC, Bondonno CP, Lewis JR, Devine A, Zhu K, Lim WH. et al. Cruciferous and allium vegetable intakes are inversely associated with atherosclerotic vascular disease deaths in older adult women. J Am Heart Assoc. 2017;6(10):e006558. doi: 10.1161/JAHA.117.006558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rautiainen S, Levitan EB, Mittleman MA, Wolk A. Fruit and vegetable intake and rate of heart failure: a population-based prospective cohort of women. Eur J Heart Fail. 2015;17(1):20–26. doi: 10.1002/ejhf.191. [DOI] [PubMed] [Google Scholar]
- 46.Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE. et al. Fruit and vegetable intake in relation to risk of ischemic stroke. J Am Med Assoc. 1999;282(13):1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 47.Johnsen SP, Overvad K, Stripp C, Tjonneland A, Husted SE, Sorensen HT. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am J Clin Nutr. 2003;78(1):57–64. doi: 10.1093/ajcn/78.1.57. [DOI] [PubMed] [Google Scholar]
- 48.Sauvaget C, Nagano J, Allen N, Kodama K. Vegetable and fruit intake and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Stroke. 2003;34(10):2355–2360. doi: 10.1161/01.STR.0000089293.29739.97. [DOI] [PubMed] [Google Scholar]
- 49.Griep LMO, Verschuren WMM, Kromhout D, Ocke MC, Geleijnse JM. Colors of fruit and vegetables and 10-year incidence of stroke. Stroke. 2011;42(11):3190–3195.. doi: 10.1161/STROKEAHA.110.611152. [DOI] [PubMed] [Google Scholar]
- 50.Larsson SC, Virtamo J, Wolk A. Total and specific fruit and vegetable consumption and risk of stroke: A prospective study. Atherosclerosis. 2013;227(1):147–152. doi: 10.1016/j.atherosclerosis.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 51.Wang J-B, Fan J-H, Dawsey SM, Sinha R, Freedman ND, Taylor PR. et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep. 2016;6:22619. doi: 10.1038/srep22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owolabi MO, Sarfo F, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A. et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case–control study. Lancet Glob Health. 2018;6(4):e436–e46. doi: 10.1016/S2214-109X(18)30002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angelino D, Godos J, Ghelfi F, Tieri M, Titta L, Lafranconi A. et al. Fruit and vegetable consumption and health outcomes: an umbrella review of observational studies. Int J Food Sci Nutr. 2019;70(6):652–667. doi: 10.1080/09637486.2019.1571021. [DOI] [PubMed] [Google Scholar]
- 54.Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5(6):492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 55.Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleova H. et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. et al. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tada N, Maruyama C, Koba S, Tanaka H, Birou S, Teramoto T. et al. Japanese dietary lifestyle and cardiovascular disease. J Atheroscler Thromb. 2011;18(9):723–734. doi: 10.5551/jat.8193. [DOI] [PubMed] [Google Scholar]
- 58.Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M. et al. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(8):CD009825. doi: 10.1002/14651858.CD009825.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Roberts JL, Moreau R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016;7(8):3337–3353. doi: 10.1039/c6fo00051g. [DOI] [PubMed] [Google Scholar]
- 60.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69(1):30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 61.He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC. et al. Folate, Vitamin B6 and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35(1):169–174. doi: 10.1161/01.STR.0000106762.55994.86. [DOI] [PubMed] [Google Scholar]
- 62.Verly-Jr E, Steluti J, Fisberg RM, Marchioni DML. A quantile regression approach can reveal the effect of fruit and vegetable consumption on plasma homocysteine levels. PLoS One. 2014;9(11):e111619. doi: 10.1371/journal.pone.0111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han L, Wu Q, Wang C, Hao Y, Zhao J, Zhang L. et al. Homocysteine, ischemic stroke, and coronary heart disease in hypertensive patients. Stroke. 2015;46(7):1777–1786. doi: 10.1161/STROKEAHA.115.009111. [DOI] [PubMed] [Google Scholar]
- 64.Rita C, Nagaraja D, Shankar SK. Homocysteine and cerebral stroke in developing countries. Curr Med Chem. 2007;14(22):2393–2401. doi: 10.2174/092986707781745613. [DOI] [PubMed] [Google Scholar]
- 65.Modi M, Prabhakar S, Majumdar S, Khullar M, Lal V, Das C. Hyperhomocysteinemia as a risk factor for ischemic stroke: An Indian scenario. Neurol India. 2005;53(3):297–301. doi: 10.4103/0028-3886.16927. [DOI] [PubMed] [Google Scholar]
- 66.Oboh G, Raddatz H, Henle T. Antioxidant properties of polar and nonpolar extracts of some tropical green leafy vegetables. J Sci Food Agric. 2008;88(14):2486–2492. [Google Scholar]
- 67.Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate–nitrite–nitric oxide pathway. Br J Clin Pharmacol. 2013;75(3):677–696. doi: 10.1111/j.1365-2125.2012.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashworth A, Mitchell K, Blackwell JR, Vanhatalo A, Jones AM. Highnitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public Health Nutr. 2015;18(14):2669–2678. doi: 10.1017/S1368980015000038. [DOI] [PMC free article] [PubMed] [Google Scholar]