Summary
Background
Human immunodeficiency virus (HIV) infection and highly active antiretroviral therapy (HAART) are implicated in cardiovascular diseases. The objective of this study was to evaluate the clinical and echocardiographic findings in HIV-infected adults.
Methods
One hundred HIV subjects on HAART, 100 HAART-naïve patients and 100 controls were recruited in this cross-sectional study.
Results
Mean CD4 cell count was significantly higher in the HAART-exposed (408.43 ± 221.62) than the HAART-naïve groups (250.06 ± 154.26) (p < 0.001). Weight loss (49%), skin lesions (14%), body weakness (24%), oral thrush (10%) and lymphadenopathy (10%) were more prevalent in HAARTnaïve patients (p < 0.05). Dimensions of aortic root (2.71 cm), left atrium (3.27 cm) and left ventricular mass index (79.95) were significantly higher in HIV-positive subjects on HAART (p < 0.05).
Conclusion
Clinical features of HIV and the CD4 nadir were more prevalent in the HIV-positive, HAART-naïve subjects. Dimensions of the aortic root, left atrium and left ventricle were relatively larger in the HAART-exposed patients while wall thickness and ejection fraction were higher in the HAART-naïve subjects.
Keywords: human immunodeficiency virus, antiretroviral therapy, CD4 cell, cardiovascular, echocardiography, dimension
Despite the decreasing national prevalence of human immunodeficiency virus (HIV) infection in Nigeria (1.4%)1 and some other developing countries, the challenge of managing the burden of HIV infection still remains high due to the estimated 1.9 million1 and 36.9 million2 people in Nigeria and the world, respectively, living with HIV infection. These figures will likely increase with time, because of improved longevity arising from the availability of more potent antiretroviral and other antimicrobial agents used in treating affected individuals.3,4 This challenge is compounded by cardiovascular diseases, which occur in this group of people due to the effect of both the HI virus and the antiretroviral medications employed in its treatment.
The effects of HIV on the cardiovascular system are many and related to immunosuppression, with the occurrence of myocarditis, pericarditis, opportunistic infections and tumours.5 Antiretroviral therapy used in treating HIV has been identified to cause metabolic disorders. Highly active antiretroviral therapy (HAART), especially protease inhibitors (PI), have been found to induce disorders of lipid metabolism such as diabetes and dyslipidaemia, which have been implicated in the increased incidence of cardiovascular disease in this patient population.6,7
A few echocardiographic studies have evaluated the effect of antiretroviral therapy on cardiac function in children.8,9 While some other similar studies in adults compared findings in HIV-positive patients as a group, with controls,10-16 studies evaluating findings in HIV-positive HAART-naive, HIV-positive HAART-exposed and control subjects are few and therefore underscore the need for more studies in cohorts of HIV-positive subjects taking antiretroviral therapy. This study evaluated the clinical and echocardiographic findings in two groups of HIV-infected adults and non-infected controls in Enugu, southeast Nigeria.
Methods
We carried out a cross-sectional study between November 2010 and November 2011 at the University of Nigeria Teaching Hospital (UNTH), Enugu, Nigeria, and adhered to ethical standards of the Helsinki Declaration17 (1964, amended 2008) of the World Medical Association. Approval for the study was given by the ethics committee of the UNTH, Enugu, and written consent was given by the subjects. Information obtained was made anonymous.
The inclusion criteria were: adult Nigerians who were aged 18 years and above, in addition to confirmed HIV-positive serology. Enzyme-linked immunoassay (ELISA) was the method of HIV screening while confirmation was by Western blot electrophoresis. Flow cytometry was used to quantify CD4 T-lymphocytes.
Fisher’s formula was used to calculate the sample size:18
where n = minimum sample size; z = 95% confidence level, i.e. 1.96; d = level of precision (0.075);19 p = maximum prevalence reported in a study of a similar population20 (13.6%); and q = 1–p.
A sample size of 100 HIV-positive, HAART-naïve patients was recruited consecutively. One hundred age- and gender-matched HIV-positive patients on HAART for at least three months and 100 controls with HIV-negative serology were recruited for comparison. Antiretroviral therapy was commenced according to Nigerian guidelines for HIV and AIDS treatment and care.21 The controls were recruited from subjects being screened for marriage, blood donation and insurance purposes.
We excluded patients in end-stage AIDS disease, classified as category C by the Centre for Disease Control, 1993.22 Also excluded were those less than 18 years of age and subjects with a history or laboratory evidence of arterial hypertension, coronary artery or ischaemic heart disease, congestive heart failure, cardiomyopathy, peripheral or cerebrovascular disease and diabetes mellitus. In addition, pregnant women or those in pueperium, those with a significant history of tobacco and/or alcohol use, as well as those who used drugs known to affect the cardiovascular system were excluded.
Clinical evaluation was carried out on every subject. Their anthropometric parameters such as height (m) and weight (kg) were measured, while body mass index (BMI) (kg/m2) and body surface area (m2) were calculated.
A resting 12-lead surface electrocardiogram (ECG) was done on all recruited subjects in the supine position, at a speed of 25 mm/s, using a two-channel automated Techmel ECG machine (USA), ECG-1101 model. Analysis of the ECG tracings from each participant was done in the standard fashion, and long-lead II tracing was used as the rhythm strip. Parameters analysed were heart rate, rhythm, P wave (duration, shape), height (paroxysmal atrial complexes), PR interval, QRS wave (duration, shape, height, axis), paroxysmal ventricular complexes, QT interval, QTc, Q wave, T wave (shape), ST-segment (shape), and R and S waves for ventricular hypertrophy.
Resting two-dimensional echocardiography was carried out on all subjects using the SonoScape SS1-5000 machine and transducer of frequency 3.5 MHz. M-mode, two-dimensional, pulsed-wave, continuous-wave, tissue Doppler imaging and colour Doppler assessments were carried out on each subject in the left lateral decubitus position. Measzurements were taken (in cm) using the American Society of Echocardiography guidelines (leading-edge methodology).23
Statistical analysis
Data were analysed using EPI INFO version 6 software. Association between categorical variables was done using the chi-squared test. The Student’s t-test was used to compare means of normally distributed continuous variables while the Mann– Whitney U-test was used to compare median of skewed data. The means ± standard deviations of parameters across the three groups were compared using one-way ANOVA, and the Duncan post hoc multiple comparisons test was done to indicate means for groups in homogenous subsets (means not significantly different). A p-value < 0.05 was taken as statistically significant.
Results
There was a significant difference in the mean weight, height, BMI and heart rate among the study groups (p < 0.05) (Table 1). The mean age for HIV-positive, HAART-naive patients was 34.43 ± 9.49 years with a range of 18–59, while that for HIV-positive patients on HAART was 35.85 ± 8.94 years with a range of 23–59, and that for the controls was 35.76 ± 9.74 years with a range of 18–57 years. The highest number of subjects (70) was from the age bracket 31–35 years, while the least number (21) was from the age group above 50 years (Table 2, Fig. 1). There were 51 HIV-positive patients on HAART, 48 HIV-positive, HAART-naive patients and 52 controls (p > 0.841).
Table 1. Comparison of some demographic parameters across the three groups using one-way ANOVA.
| Parameters | HIV+ on HAART | HIV+ HAART-naïve | Controls | F-value | p-value |
| Age | 35.85 + 8.94 | 34.43 + 9.49 | 35.76 + 9.74 | 0.716 | 0.490 |
| Weight | 65.77 + 13.92* | 62.40 + 12.45 | 68.69 + 8.67* | 7.007 | 0.001 |
| BMI | 24.14 + 4.55* | 22.47 + 3.65 | 24.18 + 3.32* | 6.301 | 0.002 |
| HR | 82.92 + 14.08 * | 84.28 + 16.79 * | 68.77 + 8.02 | 40.232 | < 0.001 |
*Duncan’s post hoc multiple comparisons test indicating means for groups in homogenous subsets (means not significantly different). BMI: body mass index; HR: heart rate.
Fig. 1.
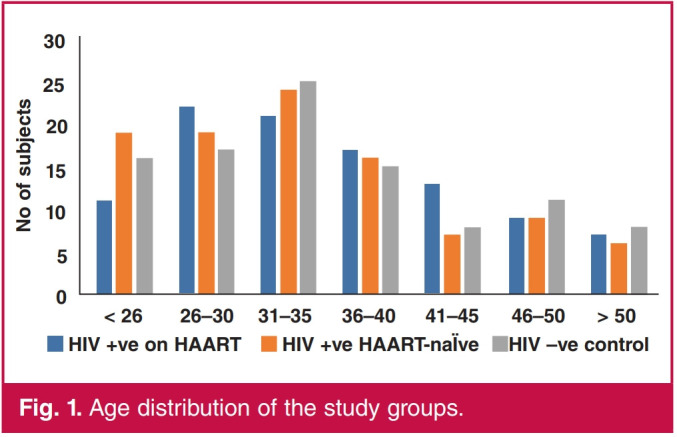
Age distribution of the study groups.
Table 2. Demographic characteristics of the study participants.
| Parameters | HIV+ on HAART | HIV+ HAART-naïve | Control | F-value | p-value |
| Gender Male | 51 | 48 | 52 | 0.347 | 0.841 |
| Female | 49 | 52 | 48 | ||
| Age group, years | |||||
| < 26 | 11 | 19 | 16 | 6.058 | 0.913 |
| 26-30 | 22 | 19 | 17 | ||
| 31-35 | 21 | 24 | 25 | ||
| 36-40 | 17 | 16 | 15 | ||
| 41-45 | 13 | 7 | 8 | ||
| 46-50 | 9 | 9 | 11 | ||
| > 50 | 7 | 6 | 8 |
The median time from diagnosis of HIV for HIV-positive, HAART-naive patients was one year with minimum and maximum durations of one and 21 years, respectively, while that for HIV-positive patients on HAART was three years with minimum and maximum durations of one and 13 years, respectively. This was statistically significant (U = 2 144, p < 0.001).
The types of HAART received by the treated group were the non-nucleoside reverse-transcriptase inhibitors (NNRTIs), namely Nevirapine (NVP) 200 mg BD, Efavirenz (EFV) 600 mg OD; nucleoside reverse-transcriptase inhibitors (NRTIs), namely Zidovudine (ZDV) 250 mg BD, Lamivudine (3TC) 150 mg BD, Stavudine (d4T) 30 mg BD, Tenofovir (TDF) 300 mg OD; and the protease inhibitors, namely Retinovir (RTV) 100 mg BD,
Lopinavir-ritonavir (LPV/r) 400/100 mg BD. They were given in proportion according to the Nigerian guidelines21 (Table 3). Other medications received were mainly Co-trimoxazole 960 mg, Artemether/Lumefantrine 80 mg/480 mg, Clotrimazole cream, Fluconazole 200 mg, Diphenoxylate 5 mg, Metronidazole 400 mg, Mebendazole 400 mg, Fesolate 200 mg, paracetamol and multivitamins.
Table 3. Proportions of HAART received by the treated group.
| First-line drugs | Second-line drugs |
| ZDV + 3TC + EFV | TDF + 3TC or FTC + ATV/r or LPVr |
| OR | OR |
| If d4T or AZT used in first-line therapy | AZT + 3TC + ATV/r or LPVr. |
| ZDV + 3TC + NVP | If TDF used in first-line therapy |
ZDV, Zidovudine; 3TC, Lamivudine; EFV, Efavirenz; NVP, Nevirapine; TDF,
Tenofovir; FTC, Emtricitabine; ATV/r, Atazanavir/ritonavir; LPV/r, Lopinavir/ ritonavir; d4T, Stavudine; AZT, Zidovudine.
For the patients on HAART, the mean duration of HAART medication was 4.0 ± 2.4 years with a minimum and maximum duration of one and 10 years, respectively. Seven per cent of these patients were on a PI-containing HAART regimen while 93% were on a non-PI regimen. Those on a PI regimen received it for less than six months.
Between the HIV-positive patients on HAART and HIV-positive, HAART-naive groups, there was a significant difference in the number of subjects with weight loss, skin lesions, body weakness, oral thrush and peripheral lymphadenopathy (p < 0.05) (Table 4). Forty-nine per cent of HIV-positive, HAART-naive patients had weight loss compared to 12% in the group of HIV-positive patients on HAART. Similarly, 14% of HIV-positive, HAART-naive patients had dermatological lesions, compared to only 2% of the group of HIV-positive patients on HAART. Twenty-four per cent of HIV-positive, HAART-naive patients had generalised body weakness compared to 5% of patients in the HIV-positive group on HAART. Similarly, 10% of HIV-positive, HAART-naive patients had oral thrush and peripheral lymphadenopathy, respectively, compared to 1% in the HIV-positive group on HAART.
Table 4. Clinical features in the study population.
| Parameters | HIV+ on HAART (n) | HIV+ HAART- naïve (n) 2 | X2 | p-value |
| Weight loss | 12 | 49 | 32.292 | < 0.001 |
| Skin lesion | 2 | 14 | 9.783 | 0.002 |
| Pruritus | 1 | 0 | 1.005 | 0.316 |
| Hepatomegally | 1 | 0 | 1.005 | 0.316 |
| Palpitation | 5 | 8 | 0.740 | 0.390 |
| Breathlessness | 3 | 3 | 0.000 | 1.000 |
| Weakness | 5 | 35 | 14.559 | < 0.001 |
| Fever | 4 | 8 | 1.418 | 0.234 |
| Diarrhoea | 3 | 7 | 1.684 | 0.194 |
| Cough | 2 | 6 | 2.083 | 0.149 |
| Oral thrush | 1 | 10 | 7.792 | 0.005 |
| Peripheral lymph | 1 | 10 | 7.792 | 0.005 |
The mean CD4 cell count for the HIV-positive patients on HAART was 408.43 ± 221.62 cells/mm3, while that of the HIV-positive, HAART-naive group was 250.06 ± 154.26 cells/ mm3. There was a significant difference between the means of the CD4 cell counts between HIV-positive patients on HAART and HIV-positive, HAART-naive patients (t = 5.865, p < 0.001). There was also a significant difference in the number of subjects with CD4 cell counts < 200 cells/mm3 and those with CD4 cell counts ≥ 200 cells/mm3 in both HIV-positive patients on HAART and HIV-positive, HAART-naive groups (χ2 = 16.095, p < 0.001).
There were more ECG abnormalities in HIV patients on HAART compared to HAART-naive patients and the controls (Table 5). T-wave inversion in leads VI–VIII occurred in 44% of the HIV-positive patients on HAART, 22% of HIV-positive, HAART-naive patients and 8% of the controls (χ2 = 24.682, p < 0.001). Left ventricular hypertrophy (LVH) was found in only the HIV-positive, HAART-naive patients (p < 0.001).
Table 5. ECG abnormalities in the study groups and controls.
| HIV+ on HAART | HIV+ HAART-naïve | ||||
| ECG abnormalities | Controls | X2 | p-value | ||
| LAD | 15 | 10 | 8 | 2.656 | 0.265 |
| T-wave inversion leads VI-VIII | 44 | 22 | 14 | 24.682 | < 0.001 |
| Low-voltage complex | 1 | 0 | 0 | 2.007 | 0.367 |
| 1st-degree heart block | 3 | 1 | 2 | 1.020 | 0.600 |
| T-wave inversion | 4.811 | 0.090 | |||
| leads II, III aVF (inferior leads) | 2 | 1 | 6 | ||
| VEB | 0 | 1 | 0 | 2.007 | 0.367 |
| T-wave inversion leads I, avL, V5-V6 | 0 | 2 | 2 | 2.027 | 0.363 |
| LBBB | 1 | 0 | 0 | 2.007 | 0.367 |
| RBBB | 1 | 0 | 2 | 2.020 | 0.364 |
| LVH | 0 | 8 | 0 | 16.438 | < 0.001 |
| Tachycardia | 0 | 1 | 2 | 2.020 | 0.364 |
| ST-segment | 0 | 2 | 0 | 4.027 | 0.134 |
| elevation | |||||
| Bradycardia | 0 | 0 | 12 | 25.000 | < 0.001 |
| Mean QTc, mean + SD | 0.42 + 0.04 | 0.41 + 0.04 | 0.39 + 0.03 | ||
| Prolonged QTc, n (%) | 17 (18.2) | 12 (16.4) | 4 (10.5) | 8.784 | 0.012 |
| Total, n (%) | 93 (100) | 73 (100) | 38 (100) |
For QTc, F = 15.779; p < 0.001. Duncan’s post hoc multiple comparisons test showed all significantly different.
LAD: left-axis deviation; VEB: ventricular ectopic beat; LBBB: left bundle branch block; RBBB: right bundle branch block; LVH: left ventricular hypertrophy.
Comparing some echocardiographic parameters measured across the groups using one-way ANOVA, there was a significant difference in the mean aorta, left atrium, end-diastolic diameter, interventricular septum and ejection fraction, respectively, among the study groups (p < 0.05) (Table 6).
Table 6. Comparison of echocardiographic parameters measured across the groups using one-way ANOVA.
| Parameters | HIV+ on HAART | HIV+ HAART- naïve | Controls | F-value | p-value |
| AO (cm) | 2.71 + 0.40* | 2.41 + 0.37 | 2.74 + 0.42* | 21.363 | < 0.001 |
| LA (cm) | 3.27 + 0.62 | 2.68 + 0.51 | 3.11 + 0.47 | 31.385 | < 0.001 |
| EDD (cm) | 4.73 + 0.70* | 4.41 + 0.55 | 4.75 + 0.42* | 11.240 | < 0.001 |
| ESD (cm) | 3.01 + 0.51 | 2.84 + 0.57 | 2.92 + 0.43 | 2.616 | 0.075 |
| IVS (cm) | 0.77 + 0.17* | 0.85 + 0.17 | 0.78 + 0.15* | 6.098 | 0.003 |
| PW (cm) | 0.82 + 0.16 | 0.87 + 0.17 | 0.82 + 0.13 | 2.878 | 0.058 |
| EF (%) | 68.95 + 12.43* | 72.81 + 11.70 | 67.36 + 9.04* | 6.223 | 0.002 |
| FS (%) | 36.77 + 9.81 | 36.51 + 8.64 | 37.77 + 6.53 | 0.623 | 0.537 |
| LVM (g) | 141.94 + 49.75 | 138.61 + 48.53 | 131.26 + 31.55 | 1.540 | 0.216 |
| LVMI | 79.95 + 26.25 | 77.55 + 25.91 | 72.37 + 16.52 | 2.760 | 0.065 |
*Duncan’s post hoc multiple comparisons test indicating means for groups in homogenous subsets (means not significantly different).
AO: aorta; LA: left atrium; EDD: end-diastolic diameter of left ventricle; ESD: end-systolic diameter of left ventricle; IVS: interventricular septum; PW: posterior wall of left ventricle; EF: ejection fraction; FS: fractional shortening; LVM: left ventricular mass; LVMI: left ventricular mass index.
Discussion
The mean ages of the three groups were 34.43 ± 9.49 years (HIV-positive, HAART-naive), 35.85 ± 8.94 years (HIV-positive patients on HAART) and 35.76 ± 9.74 years (controls). Similar findings have been documented in a recent study done in a similar population24 and in other related studies.12,25 A higher number of HIV-infected patients (70) were within the age group of 31–35 years and this represents the global age distribution in which most of the people infected with HIV/AIDS are within the sexually active age bracket of 15–35 years.26
Clinical features of HIV21,27 and immunosuppression were more prevalent in the HIV-positive, HAART-naive group compared to the group of HIV-positive patients on HAART in this study. It has been documented in other studies that HAART is effective in increasing CD4 cell count and decreasing the viral load, with an associated decrease in morbidity and mortality among HIV-infected individuals.28,29 For the same reason, opportunistic infection was seen less often in the HIV-positive patients on HAART.10,30-32
The mean weight and BMI were higher in the HIV-positive patients on HAART than in the HAART-naive group. There was no significant difference in the mean weight and BMI between the HIV-positive patients on HAART and the controls (Table 1).
Weight loss is a feature of HIV infection, and weight gain in the HIV patients on HAART could have been due to a HAARTinduced decrease in viral burden,33,34 as well as the metabolic side effects of HAART. In addition, the use of antiretrovirals, such as NRTIs and PIs, has been found to cause an increase in weight and mean BMI of subjects on HAART.35,36 They are also associated with the metabolic abnormalities of diabetes, dyslipidaemia, altered body fat distribution, especially HIV lipodystrophy syndrome, as well as mitochondrial abnormalities.37 Weight and BMI of HIV patients on HAART in this study therefore increased and became comparable to that of the controls. A similar finding was documented in a related study between a cohort of HIV/AIDS patients and normal controls,12,16 but there was no separation, as in our study, of the HIV patients into those on HAART and HAART-naive groups. In our study, antimicrobials and multivitamins received by the group on HAART helped to control opportunistic infections and diarrhoeal diseases, and improved appetite, weight gain and over-all well-being of the subjects.
The mean CD4 cell count in the HIV subjects was higher in the group on HAART (408.43 ± 221.62 cells/mm3) compared to the HAART-naive group (250.06 ± 154.26 cells/mm3) (Fig. 2), underscoring the benefits of HAART in this patient population.28 The mean CD4 cell count for the HAART-naive group was relatively high in this study because HIV patients with clinical features of end-stage AIDS, classified as category C by CDC 1993,22 were excluded.
Fig. 2.
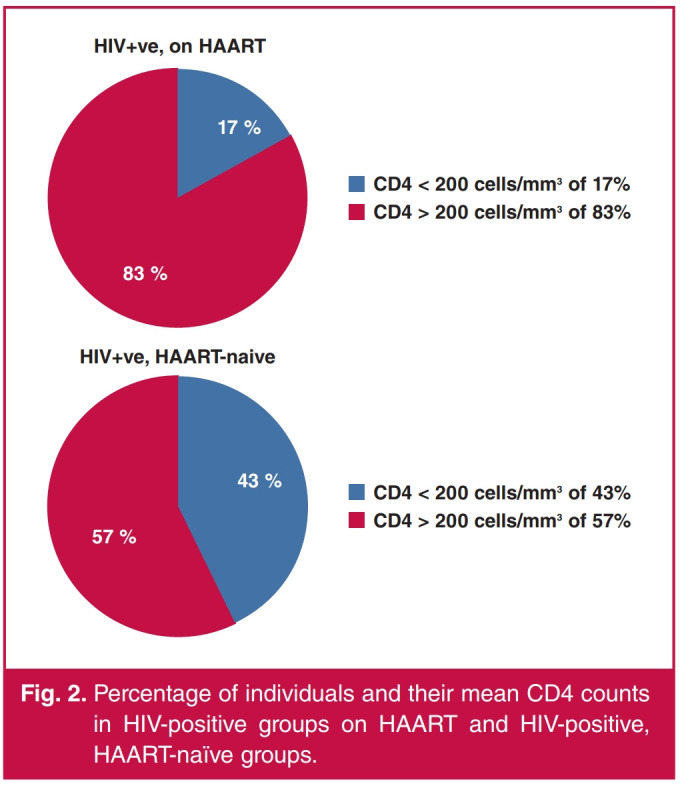
Percentage of individuals and their mean CD4 counts in HIV-positive groups on HAART and HIV-positive, HAART-naïve groups.
Cardiac complications of HIV infection such as left ventricular dysfunction and dilated cardiomyopathy have been found to occur more often in patients with low CD4 counts.38 In a study carried out in the pre-HAART era, global left ventricular hypokinesia was found to be associated with lower CD4 counts.11 The dimensions of the aortic root, left atrium and left ventricle were within the normal range in the study groups. Although comparable to the controls, they were relatively higher in the HIV-positive patients on HAART than in the HIV-positive, HAART-naive subjects (Table 6).
HAART has been found to induce myocardial toxicity and was believed to be the cause of right ventricular wall impairment and dilatation in a related study in children.39 In a similar study by Ajala et al.,24 in which the mean duration of HAART was not stated, no difference in chamber dimension was reported (Table 7). However, HIV disease has been found in some studies to be independently associated with increased left atrial volume in addition to increased left ventricular mass index,8,12 while other studies have shown no difference.13
Table 7. Comparison of main findings with other similar studies.
| Studies | ECG abnormalities | Echocardiography: wall thickness | Echocardiography: mean chamber dimensions and LVEF |
| Index study | ECG abnormalities were higher in HAART- exposed (93%), and HAART-naïve (73%), compared to controls (38%). | Mean thickness of IV septum was higher in the HAART-naïve but similar between HAART-exposed and controls. | Aortic root, LA and LV were slightly higher in HAART-exposed than HAART-naïve and controls. LVEF was higher in cases (HAART-naïve, 72.8%, and |
| LVH was found in 11% of HAART-naïve cases but none in the HAART-exposed and controls | LVM and LVMI were higher in cases than controls | HAART-exposed, 68.9%) compared to the controls (67.3%) | |
| Ajala et al.24 | ECG abnormalities were higher in cases (49%) than controls (42%) | IV septum was slightly higher in controls than cases but LVM and LVMI were higher in cases than controls | LA was slightly higher in cases than controls Aortic root was slightly higher in controls than cases. The mean LVEF was higher in the cases (71.9%) compared to controls (68.9%) |
| Ogunmodede et al. 12 | ECG abnormalities were more in HIV-positive patients (55.3%) than controls (2.7%) LVH was higher in cases (17.3%), than controls (4%) | IV septum, LVM and LVMI were higher in cases than controls | LV was slightly higher in cases than controls. LA was slightly higher in controls. LVEF not stated |
| Uwanuruochi et al. 16 | Not stated | IV septum and LVMI were higher in cases than controls. PW slightly higher in controls than cases | LV was slightly higher in controls. LVEF was higher in cases (60.3%) than controls (57.9%) |
| Danbauchi et al. 20 | Not stated | IV septum, PW, LVM and LVMI were higher in cases than controls | Aortic root, LA and LV were higher in cases The LVEF was the same in both the cases and controls (66%) |
| Reinsch et al. 14 | Not stated | IV septum and PW were higher in 18 and 11% of cases, respectively | LV was higher in 10.% of cases LVEF was 57.5% |
LVH, left ventricular hypertrophy; IV, interventricular; LA, left atrium; LV, left ventricle; LVM, left ventricular mass; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; PW, posterior wall.
Although the mean thickness of the interventricular septum was within normal limits in all the groups in our study, it was higher in the HIV-positive, HAART-naive subjects but similar between the HIV-positive, HAART-exposed subjects and the controls (Table 6). Similarly, the posterior wall was higher in this group compared to the HAART-exposed or control groups. However, no difference in wall thickness was found in a recent study done in a similar population.24 Disparate results have been reported, indicating possible HAART-induced increases in left ventricular wall thickness and mass in some studies,8,14 while others showed increased thickness of the interventricular septum and posterior wall,40 and eccentric patterns of LVH, with an increase in left ventricular cavity size in HIV-infected persons who were not on HAART41,42 (Table 7).
Possible causes of a thicker interventricular septum and posterior wall in the HAART-naive group were higher HIV viraemia and its toxic effect on the myocardial cells, causing local release of cytokines and other factors, leading to subclinical inflammation and myocarditis.43 This is corroborated by the postulation of immune dysfunction, as measured by the CD4 nadir, which is an independent risk factor for increased left ventricular mass, LVH and dysfunction.44-46 The HAART-naive group consisted of more immunosuppressed subjects with a CD4 nadir and HIV viraemia, compared to the HAART-exposed subjects (Fig. 2). ECG findings in this study showed a relatively high prevalence of LVH in the HIV-positive, HAART-naive patients, compared to it not being seen in subjects on HAART (Table 5), as assessed by the voltage criteria of Sokolow and Lyon, and Araoye.47,48
Heart rate and left ventricular ejection fraction (LVEF) were higher in the HIV-positive, HAART-naive subjects compared to the controls and HIV-positive, HAART-exposed subjects in this study (Tables 1, 6). This could have been due to increased prevalence of opportunistic infections, fever, anaemia, diarrhoea and dehydration, which would drive increased sympathetic activity and cardiac contractility in many of the individuals in this study group.16 Hyperdynamic left ventricular performance with enhanced contractility was reported in this subgroup of patients by Lipshultz et al.49 In a recent study done in a similar population, there was no statistically significant difference in LVEF in the two HIV-positive groups but LVEF was slightly higher in subjects who were on PI-based HAART.24 However, duration of PI use and clinical characteristics of the study population were not stated.
A limitation of the study is the relatively short duration of the use of PI and HAART, which have been found to cause diabetes mellitus and dyslipidaemia, which in turn have been identified to cause cardiovascular disease among HIV patients.
Conclusion
In this study, clinical features of HIV infection, immunosuppression and reduced CD4 cell count were more prevalent in HIV-positive patients who were HAART-naive than in HIV-positive patients on HAART. The dimensions of the aortic root, left atrium and left ventricle were within normal limits but relatively larger in the HIV-positive, HAART-exposed group, while the wall thickness and LVEF were higher in the HIV-positive, HAART-naive subjects. A longitudinal study would help identify possible links in the use of antiretroviral therapy.
Acknowledgments
We acknowledge the staff of the HIV clinic at the University of Nigeria Teaching Hospital, Enugu, for their support during the study. We thank the patients and control subjects for volunteering in the study.
References
- 1.UNAIDS. Press release: new survey results indicate that Nigeria has an HIV prevalence of 1.4%. Available at: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2019/march/20190314_nigeria . [Google Scholar]
- 2.The United States President’s Emergency Plan for AIDS Relief 2019 Annual Report to Congress. https://www.state.gov/wp-content/ uploads/2019/09/PEPFAR2019ARC.pdf. [Google Scholar]
- 3.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM. et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 4.Hooshyar D, Hanson DL, Wolfe M, Selik RM, Buskin SE, McNaghten AD. et al. Trends in perimortal conditions and mortality rates among HIV-infected patients. AIDS. 2007;21:2093–2100. doi: 10.1097/QAD.0b013e3282e9a664. [DOI] [PubMed] [Google Scholar]
- 5.Ntsekhe M, Hakim J. Impact of human immunodeficiency virus infection on cardiovascular disease in Africa. Circulation. 2005;112:3602–3607. doi: 10.1161/CIRCULATIONAHA.105.549220. [DOI] [PubMed] [Google Scholar]
- 6.Thiebaut R, Dabis F, Malvy D, Jacqmin-Gadda H, Mercie P, Valentin VD. Serum triglycerides, HIV infection, and highly active antiretroviral therapy, Aquitaine Cohort, France, 1996 to 1998. Groupe d’Epidemiologie Clinique du Sida en Aquitaine (GECSA). J Acquir Immune Defic Syndr. 2000;23:261–265. doi: 10.1097/00126334-200003010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Henry K, Melroe H, Huebsch J, Hermundson J, Levine C, Swensen L. Severe premature coronary artery disease with protease inhibitor. Lancet. 1998;351:13–28. doi: 10.1016/S0140-6736(05)79053-X. [DOI] [PubMed] [Google Scholar]
- 8.Animasahun BA, Diaku-Akinwumi IN, Ubuane PO, Ibitoye E. Cardiac size and systolic function of HIV-infected Lagos children accessing routine care: a pilot study. J Xiangya Med. 2018;3:14. [Google Scholar]
- 9.Idris NS, Cheung MMH, Grobbee DE, Burgner D, Kurniati N. Uiterwaal CSPM. Cardiac effects of antiretroviral-naive versus antiretroviral- exposed HIV infection in children. PLoS One. 2016;11(1):e0146753. doi: 10.1371/journal.pone.0146753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R. et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3(1):132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herskowitz A, Vlahov D, Willoughby S, Chaisson RE, Schulman SP, Neumann DA. et al. Prevalence and incidence of left ventricular dysfunction in patients with human immunodeficiency virus infection. Am J Cardiol. 1993;71(11):955–958. doi: 10.1016/0002-9149(93)90913-w. [DOI] [PubMed] [Google Scholar]
- 12.Ogunmodede JA, Kolo PM, Katibi IA, Salami AK, Omotoso A. Structural echocardiographic abnormalities seen in HIV/AIDS patients are independent of cd4 count. Niger J Clin Pract. 2017;20:716–723. doi: 10.4103/1119-3077.208954. [DOI] [PubMed] [Google Scholar]
- 13.Meng Q, Lima JA, Lai H, Vlahov D, Celentano DD, Strathdee S. et al. Use of HIV protease inhibitors is associated with left ventricular morphologic changes and diastolic dysfunction . J AIDS. 2002;30:306–310. doi: 10.1097/00126334-200207010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Reinsch N, Kahlert P, Esser S, Sundermeyer A, Neuhaus A, Brockmeyer N. et al. Echocardiographic findings and abnormalities in HIV-infected patients: results from a large, prospective, multicenter HIV-heart study. Am J Cardiovasc Dis. 2011;1(2):176–184. [PMC free article] [PubMed] [Google Scholar]
- 15.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 16.Uwanuruochi K, Onwubere BJ, Anisiuba BC. Echocardiographic study of left ventricular function in HIV-infected Nigerians. West Afr J Radiol. 2015;22:27–31. [Google Scholar]
- 17.WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subject. www.wma.net/policies-post/wmadeclaration- of-helsinki-ethical-principles-for-medical-research-involving- human-subjects/ [PubMed] [Google Scholar]
- 18.Araoye MO. Ilorin: Nathadex. Reseach Methodology with Statistics for Health and Social Sciences, 2003. :117–121. [Google Scholar]
- 19.Sani MU, Okeahialam BN, Ukoli CO. Electrocardiographic abnormalities in Nigerian AIDS patients. Cardiologie Tropicale. 2004;30:3–6. [Google Scholar]
- 20.Danbauchi SS, Sani BG, Alhassan AM, Oyati AI. Echocardiographic features of HIV/AIDS subjects on 1–2 years of ARV drugs in Nigeria. Available at http://www2.umdnj.edu/shindler/hivecho.html. [Google Scholar]
- 21.Federal Ministry Of Health Abuja – Nigeria: [October 2010]. National Guidelines for HIV and AIDS Treatment and Care in Adolescents and Adults. pp. 16–28. https://www.who.int/hiv/pub/guidelines/nigeria_art.pdf. [Google Scholar]
- 22.HIV/AIDS surveillance report, Atlanta: 1993. Centre for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. pp. 1–23. [PubMed] [Google Scholar]
- 23.Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL. et al. Report of the American Society of Echocardiography committee on nomenclature and standards in 2-D echocardiography. Circulation. 1980;62:212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 24.Ajala AO, Akpa MR, Dodiyi-Manuel S. Pattern of electrocardiographic and echocardiographic abnormalities among HIV patients in Port Harcourt, Nigeria. Int J HIV/AIDS Prevent Edu Behav Sci. 2020;6(1):15–24. [Google Scholar]
- 25.Akinyemi JO, Ogunbosi BO, Fayemiwo AS, Adesina OA, Obaro M, Kuti MA. et al. Demographic and epidemiological characteristics of HIV opportunistic infections among older adults in Nigeria. Afr Health Sci. 2017;17(2):315–321. doi: 10.4314/ahs.v17i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walensky RP, Paltiel AD, Losina E, Mercincacavage LM, Schackman BR, Sax PE. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 27.WHO case definitions of HIV for surveillance and revised Clinical Staging and Immunological Classification of HIV-related disease in adults and children: 12–28. https://Apps.Who.Int/Iris/Handle/10665/43699. [Google Scholar]
- 28.Nogueras M, Navarro G, Anton E, Sala M, Cervantes M, Amengual M. et al. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis. 2006;6:159. doi: 10.1186/1471-2334-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez JL, Moore RD. Greater effect of highly active antiretroviral therapy on survival in people aged ≥ 50 years compared to younger people in an urban observational cohort. Clin Infect Dis. 2003;36:212–218. doi: 10.1086/345669. [DOI] [PubMed] [Google Scholar]
- 30.Prasitsuebsai W, Kariminia A, Puthanakit T, Lumbiganon P, Hansudewechakul R, Siew Moy F. et al. Impact of antiretroviral therapy on opportunistic infections of HIV-infected children in the therapeutic research, education and AIDS training: Asia pediatric HIV observational database. Pediatr Infect Dis J. 2014;33(7):747–752. doi: 10.1097/INF.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H. et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. J Am Med Assoc. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 32.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I. et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 33.Shikuma CM, Zackin R, Sattler F, Mildvan D, Nyangweso P, Alston B. Changes in weight and lean body mass during highly active antiretroviral therapy. Clin Infect Dis. 2004;39:1223–1230. doi: 10.1086/424665. [DOI] [PubMed] [Google Scholar]
- 34.Pernerstorfer-Schoen H, Schindler K, Parschalk B. Beneficial effects of protease inhibitors on body composition and energy expenditure: a comparison between HIV-infected and AIDS patients. AIDS. 1999;13:2389–239. doi: 10.1097/00002030-199912030-00010. [DOI] [PubMed] [Google Scholar]
- 35.McDermott AY, Shevitz A, Knox T, Roubenoff R, Kehayias J, Gorbach S. Effect of highly active antiretroviral therapy on fat, lean, and bone mass in HIV-seropositive men and women. Am J Clin Nutr. 2001;74(5):679–686. doi: 10.1093/ajcn/74.5.679. [DOI] [PubMed] [Google Scholar]
- 36.Engelson ES, Kotler DP, Tan Y, Agin D, Wang J, Pierson RN. Fat distribution in HIV-infected patients reporting truncal enlargement quantified by whole-body magnetic resonance imaging. Am J Clin Nutr. 1999;69:1162–1169. doi: 10.1093/ajcn/69.6.1162. [DOI] [PubMed] [Google Scholar]
- 37.Sweet DE. Metabolic complications of antiretroviral therapy. Top HIV Med. 2005;13:70–74. [PubMed] [Google Scholar]
- 38.Dau B, Holodniy M. The relationship between HIV infection and cardiovascular disease. Curr Cardiol Rev. 2008;4(3):203–218. doi: 10.2174/157340308785160589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallilo NG, Durigon GS, Lianza AC, de Fatima RD, Shiraishi S, Karen S. Echocardiographic follow-up of perinatally HIV-infected children and adolescents. Pediat Infect Dis J. 2020;39(6):526–532. doi: 10.1097/INF.0000000000002628. [DOI] [PubMed] [Google Scholar]
- 40.Williams PL, Correia K, Karalius B, van Dyke RB, Wilkinson JD, Shearer WT. Pediatric HIV/AIDS Cohort Study. Cardiac status of perinatally HIV-infected children: assessing combination antiretroviral regimens in observational studies. AIDS. 2018;32:2337–2346. doi: 10.1097/QAD.0000000000001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin EJ, Levy D. Why is left ventricular hypertrophy so predictive of morbidity and mortality? Am J Med Sci. 1999;317:168–175. doi: 10.1097/00000441-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Ather M, Elizabeth TG, Jack D, Kathryn A, Robert CK, Jason M. The association of HIV infection with left ventricular mass/hypertrophy. AIDS Res Human Retrovirus. 2009;25(5):475–481. doi: 10.1089/aid.2008.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okeke NL, Alenezi F, Bloomfield GS, Dunning A, Clement ME, Shah SH. et al. Determinants of left ventricular hypertrophy and diastolic dysfunction in an HIV clinical cohort. J Cardiac Fail. 2018;24(8):496–503. doi: 10.1016/j.cardfail.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima–media thickness in patients with HIV infection. Circulation. 2004;1:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 46.Ho JE, Scherzer R, Hecht FM, Maka K, Selby V, Martin JN. et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS. 2012;2:1115–1120. doi: 10.1097/QAD.0b013e328352ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Njoku PO, Ejim EC, Anisiuba BC, Ike SO, Onwubere BJC. Electrocardiographic findings in a cross-sectional study of human immunodeficiency virus (HIV) patients in Enugu, south-east Nigeria. Cardiovasc J Afr. 2016;27(4):252. doi: 10.5830/CVJA-2016-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araoye MA. Basic Electrocardiography. 2nd edn. Ibadan: Spectrum Books: 2012. pp. 102–103. [Google Scholar]
- 49.Lipshultz SE, Chanock S, Sanders SP, Colan SD, Perez-Atayde A, McIntosh K. Cardiovascular manifestations of HIV infection in infants and children. Am J Cardiol. 1989;63:1489–1497. doi: 10.1016/0002-9149(89)90014-3. [DOI] [PubMed] [Google Scholar]


