Abstract
Background
Energy drinks (EDs) reduce sleepiness and fatigue and improve driving performance whereas alcohol does just the opposite. Although it is a trendy combination among young people, the effects of alcohol mixed with EDs on driving performance have been poorly studied. The aim was to assess if there is an interaction between the effects of both drinks on driving-related skills as well as perceptions about driving ability.
Methods
We conducted a randomized, double-blind, and placebo-controlled 4-way crossover clinical trial. Participants were 16 healthy volunteers. Interventions of 60 g of ethanol and 750 mL of Red Bull (RB) were administered in 2 separated doses. Conditions were alcohol + RB placebo, alcohol + RB, alcohol placebo + RB, and both placebos. Objective performance was assessed using a tracking test and simple reaction time, N-Back, and movement estimation tasks. Additionally, willingness to drive, other subjective effects, and ethanol and caffeine blood concentrations were also measured.
Results
Alcohol increased the time outside the road in the tracking test and increased simple reaction time, but the addition of RB had no main or interaction effects on performance. Nonetheless, driving-related skills after alcohol + RB were better than after alcohol alone. Willingness to drive increased with the combination of drinks. RB also reduced alcohol-induced sedation whereas drunkenness did not change. These effects were seen even though alcohol + RB increased alcohol (14.8%) and caffeine plasma concentrations (17.6%).
Conclusions
Mixing EDs with alcohol predisposes consumers to drive under alcohol influence, perhaps in part because EDs counteract its detrimental effects on driving-related skills.
Keywords: Alcohol, energy drinks, caffeine, interaction, driving-related skills, pharmacokinetics, addiction
Significance Statement.
Consumption of energy drinks (EDs) with alcohol has become trendy among young people. The stimulant effects of caffeine in these drinks may predispose users to underestimate the extent of their alcohol-induced impairment. In this study, mixing 3 ED with alcohol reduced sedation and increased willingness to drive compared with alcohol alone. Alcohol impaired driving performance more than the combination of alcohol + EDs. Altogether these results suggest that consumers are at risk when driving under their influence. Furthermore, a modest increase in caffeine and alcohol concentrations was observed with the combination of EDs with alcohol. Mechanisms involved should be further investigated.
Introduction
For most individuals, alcohol is the most consumed drug in the last month (Global Drug Survey, 2019), and acute alcohol impairment still remains a societal problem with severe consequences to the user and those around them. In addition to traffic accidents, alcohol consumption can turn into alcoholism and is a risk factor for mental and behavioral disorders, liver cirrhosis, some cancers, and cardiovascular diseases (World Health Organization, 2018). Almost one-half of students in Europe report alcohol current use (ESPAD, 2019).
Energy drinks (EDs) contain principally caffeine, but also taurine, vitamin B complex, vegetal extracts (guarana, ginseng), sugar, and artificial sweeteners. ED consumers use these drinks to bring them energy, reduce tiredness and fatigue, and increase intellectual capacity (Ravelo et al., 2013). EDs are commonly used as a source of dietary supplements, but there is less knowledge of their health-related harms, such as the potential relationship with cardiovascular diseases (Mangi et al., 2017), compared with other substances (Pacifici et al., 2016). In a national survey conducted in the United States (Mitchell et al., 2014), the greatest prevalence of ED consumption was found among 13– to 24–year–olds. In Europe, 13% of daily caffeine intake among adolescent ED consumers came from these drinks (Zucconi et al., 2013).
Consumption of alcohol with EDs (AmED) is popular among young people, either as a mixed cocktail or in the same drinking session (Arria et al., 2016). Surveys conducted in Europe report that 48% of university students using EDs had consumed them with alcohol (Oteri et al., 2007), and 1 in 4 youth in the United States and Canada consumed AmED during the last year (Martz et al., 2015; Wilson et al., 2018). Furthermore, adolescents who regularly drink AmED more often binge drink (O’Brien et al., 2008).
Alcohol produces perception-sensorium impairments like visual deficits, loss of perception in movements, reduced capacity of attention, false security, and impulsivity. Drivers under the influence of alcohol are more susceptible to be cited for traffic violations and to have traffic accidents (Directorate-General for Traffic, 2016). This problem is particularly important in the young population, where there are many erroneous beliefs about actions to mitigate the effects of alcohol on driving (Olivera et al., 2002).
On the other hand, studies have demonstrated that consuming EDs before driving would have positive effects, reducing somnolence in long travels and augmenting alertness (Mets et al., 2011; Ronen et al., 2014). Consumers of AmED report use to reduce drunkenness, but only 1 study showed a reduction of subjective intoxication (Marczinski et al., 2006) and other studies failed to show a masking effect of EDs (Benson et al., 2014; Verster et al., 2018).
Consumers of AmED have been reported to reach a state that has been called “wide-awake drunkenness” (Pennay et al., 2015). They report greater prolonged sessions of heavy alcohol consumption and engagement in risk-taking behaviors, showing a greater likelihood of alcohol-related unsafe driving (Martz et al., 2015). Their higher rates of impaired driving could be explained by an erroneous perception of increased driving ability that contradicts objective performance outcomes (Marczinski et al., 2018). Experimental data, however, do not clearly support those surveys (Peacock et al., 2014; Verster et al., 2018).
Regarding impact on cognition, some studies have shown that EDs counteract some alcohol-induced cognitive deficits, depending on the alcohol dose, concentration, and limb of the blood alcohol time course (T-C) curve, but these effects are partial or disappear with complex tasks (McKetin et al., 2015; Lalanne et al., 2017).
Few studies have assessed effects of caffeinated beverages on driving performance. In experimental studies, an ED (80 mg caffeine) significantly improved driving performance and reduced driver sleepiness during prolonged highway driving (Mets et al., 2011), and 2 EDs facilitated lower-lane position deviations and reduced steering wheel deviations in truck drivers (Ronen et al., 2014). Mixed with alcohol, caffeine capsules (200 or 400 mg) partially counteracted alcohol-induced impairment in brake latency, but performance remained more impaired when alcohol was present (0.6 g/kg of alcohol) (Liguori and Robinson, 2001). Another study with caffeinated and un-caffeinated beer (average caffeine and alcohol doses of 383 mg and 1 g/kg, respectively) showed no improvement of driving performance (speed, lane position, and crashes) with the addition of caffeine (Howland et al., 2011). Finally, EDs decreased alcohol induced-impairment in a tracking test, mainly when alcohol concentrations were in the descending limb of the blood alcohol T-C curve (Peacock et al., 2015). Those studies matched performance with breath alcohol concentrations (BrAC).
The main aim of the study was to assess if there is an interaction between the effects of alcohol and EDs on driving-related skills.
MATERIALS AND METHODS
Participants
Participants needed to be healthy and consumers of at least 1 Standard Unit (SU)/d (10 g of alcohol in Spain), with several previous acute drunkenness experiences (≥once monthly), with a body mass index between 19 and 27 kg/m2 and weight between 50 and 100 kg. A minimum consumption of 5 drinks/wk containing methylxanthines and a driving license were also required. See exclusion criteria in supplementary Materials.
Treatments
Alcohol was administered as Vodka Absolut (40º alcohol) at a dose of 30 g (94 mL of vodka), and its placebo was Fontvella water. The ED used was lime-flavor Red Bull (RB) at a dose of 375 mL (1.5 cans), and its placebo was Seven-Up. Every can of RB contained 80 mg of caffeine and 1 g of taurine. The flavor and the content of carbohydrates (every 100 mL contains 11 g) of RB and its placebo were similar. Two doses were administered (1 hour of separation between them), so participants received a total of 60 g of alcohol (188 mL of vodka) and 750 mL of RB (3 cans, 240 mg of caffeine). Every dose was served cold and distributed in 3 opaque cups and ingested in a period of 15 minutes (5 min/cup). The total doses selected in the study correspond to 6 SU in Spain, 7.5 SU in England, and a bit more than 4 SU in the United States. Randomization and treatments were prepared by a person not involved in the experimental sessions. The fact that alcohol was quite diluted, the mixer was very sweet, the lime flavor, the cold and the opaque cups contributed to mask the treatment conditions.
Study Design
The study was double-blind and placebo-controlled. Individuals participated in four 8-hour experimental sessions with a minimum 3-day washout period. Treatments were randomly assigned using a balanced 4 × 4 Latin-square design: (a) alcohol + RB placebo, (b) alcohol + RB, (c) alcohol placebo + RB, and (d) alcohol placebo + RB placebo.
Alcohol, drinks with methylxanthines, and meals rich in taurine were prohibited 72 hours before until 24 hours after administration. Every session day, participants arrived to the Parc de Salut Mar Clinical Research Unit at 07:45 am after an overnight fast. An indwelling intravenous catheter was inserted into a subcutaneous vein of the non-dominant arm to obtain blood samples. Doses were administered at 8:30 and 9:30 am. Participants remained in beds in a calm environment and psychomotor tests were performed in a separate room. Urine generated was collected until 8 hours after administration. Breakfast was ingested at 3 hours and consisted of a turkey sandwich with 150 mL of water and the meal included pasta, chicken with salad, an apple, and 330 mL of water at 6 hours.
Driving-Related Skills
The battery included a Tracking Test (TT), Simple Reaction Time task (SRT), N-Back task (NB), and Movement Estimation task (MET). These tests were performed along 20 minutes at baseline and 0.5 (between doses), 2, and 4 hours after administration of the first dose, using a laptop and the Cambridge Neuropsychological Test Automated Battery. Participants were trained at screening.
The TT used is an adaptation of the classic Critical Tracking Task (Jex et al., 1966), very similar to the Visuomotor Bimanual Coordination Test used in Spain to obtain the driving license (Gombao et al., 2006). It is an interactive task of 290 seconds that requires individuals to maintain 2 yellow vehicles circulating in the middle of 2 roads. Each vehicle is controlled by 1 hand with a joystick. It allows registering TimeOut (the total time when a vehicle is outside of the road), the number of errors (times that a car goes off the road more than 0.4 seconds) and the number of gyres (changes in joystick direction). The main outcome of the study was the change in the TimeOut. Detailed information of tests is included in supplementary Materials.
Subjective Effects Rating Scales
Several visual analog scales (VAS), Biphasic Alcohol Effects Scale (BAES) (Martin et al., 1993), and the Spanish validated version of the short-form Addiction Research Center Inventory (ARCI) were used (Lamas et al., 1994).
VAS (0–100 mm) marked at opposite end with “not at all” and “extremely” included assessment of drunkenness, dizziness, drowsiness, palpitations, anxiety, and headache. Additionally capability to drive and willingness to drive under various emotional and rewarding circumstances (“taking an ill child to the hospital,” “an indisposed friend home,” and “a friend to a party”) were measured at baseline and 0.5, 2, 4, 6, and 8 hours after first administration (Ménétrey et al., 2005).
BAES evaluates subjective ratings of activation (BAES-A) and sedation (BAES-S) using a 14-adjective rating scale (7 adjectives for stimulation and 7 for sedation). A Likert scale ranging from 0 (no at all) to 10 (extremely) is used, and stimulation and sedation scores were summed separately (score subscale range = 0–70).
ARCI is a true/false 49-item questionnaire used to assess drug effects with 5 subscales: PCAG (pentobarbital-chlorpromazine-alcohol group, a measure of sedation), MBG (morphine-benzedrine group, a measure of euphoria), LSD (lysergic acid diethylamide group, a measure of dysphoria and somatic symptoms), BG (benzedrine group, a stimulant subscale related to intellectual efficiency and energy), and A (amphetamine, a measure of d-amphetamine effects).
Concentrations
BrAC were measured at baseline and 0.75, 1, 1.5, 1.75, 2, 2.25, 2.5, 3, 4, and 8 hours (Dräger Breathalyzer 7410 Plus, Denmark). Blood alcohol concentrations (BAC) were measured additionally at 0.25, 0.50, and 6 hours. Caffeine and taurine plasma concentrations were measured at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, and 8 hours. Caffeine and caffeine metabolite concentrations were measured in urine.
Vital Signs and Adverse Events
Safety and tolerability of drinks were assessed. Systolic and diastolic blood pressure, heart rate, and oral temperature were measured at baseline and 0.5, 1, 1.5, 2, 2.5, 4, 6, and 8 hours. Adverse events were also recorded.
Analytical Assays
Alcohol concentrations were determined using the enzymatic assay DRI Ethyl Alcohol Assay (Thermo Fisher, Fremont, CA, USA) with a cut-off point of 10 mg/dL. Taurine and caffeine were simultaneously analyzed in plasma by Liquid Chromatography with tandem Mass Spectrometry (LC-MS-MS). Caffeine, paraxanthine, theobromine, and theophylline were analyzed in urine by Gas Chromatography-Mass Spectrometry (GC/MS). Details on the method are in supplementary Materials.
Ethical Aspects
The study protocol and information sheet were approved by the Clinical Research Ethics Committee of Parc de Salut Mar (CEIm-Parc de Salut Mar approval no. 2015/6361/I). The study was conducted according to the Declaration of Helsinki and each participant signed an informed consent prior to participation. The study was registered in clinicaltrials.gov. Participants were compensated with 625 Euros for their participation.
Statistical Analysis
Differences to baseline and the following pharmacokinetic parameters were calculated for all outcomes: area under the curve of the concentrations and effects (AUC), the time needed to reach the maximum concentration and effect (tmax) and the maximum concentration (Cmax) and effect (peak or Emax).
AUC and Emax were compared using a 2 (alcohol: active, placebo) × 2 (RB: active, placebo) factorial repeated-measures ANOVA. The interaction term was used as indicative of the capacity of RB to affect the effect of alcohol. When the alcohol × RB interaction was statistically significant, multiple post-hoc comparisons were performed among the 4 conditions using the Tukey’s test. In addition, these models were used to estimate the substance effects of alcohol and RB (vs placebo), respectively. For tmax, the Friedman’s test and Wilcoxon’s test were used. Concentrations were compared by a paired Student’s t test (AUC and Cmax) and a Wilcoxon’s test (tmax). Furthermore, a detailed comparison of T-C of effects was conducted using repeated-measures 2-way ANOVA, with treatment condition and time as factors. When the treatment condition × time interaction was statistically significant, multiple Tukey post-hoc comparisons between the 4 conditions were performed at each time point. Repeated-measures correlations were calculated to assess the within-individual associations between alcohol concentrations and effects at the different points (Bakdash and Marusich, 2017).
Statistical analysis was performed using PAWS Statistics version 18 (SPSS Inc., Chicago, IL, USA) and R Statistical Software (Vienna, Austria; https://www.r-project.org/), version 4.0.2. A value of P <.05 was considered statistically significant.
RESULTS
Study Participants
A total of 16 healthy male participants were included in the study (see supplemental Figure 1). Participants mean and SD age was 24.0 ± 5.2 years (range 19–29 years), weighed 72.6 ± 10.1 kg, and their body mass index was 23.4 ± 2.3 kg/m2. All but 4 participants were non-smokers. Their average alcohol and methylxanthine consumption was 1.8 ± 0.8 SU/d, and 1.9 ± 0.8 drinks/d, respectively. Their average consumption of EDs was 9.9 ± 15.4 cans in the last year and 37.7 ± 42.7 cans throughout life. Taking into account participants’ weight, average alcohol and caffeine doses administered were 0.8 g/kg and 3.4 mg/kg, respectively.
Driving-Related Skills
Main results are shown in Figure 1. Table 1 shows pharmacodynamic parameters.
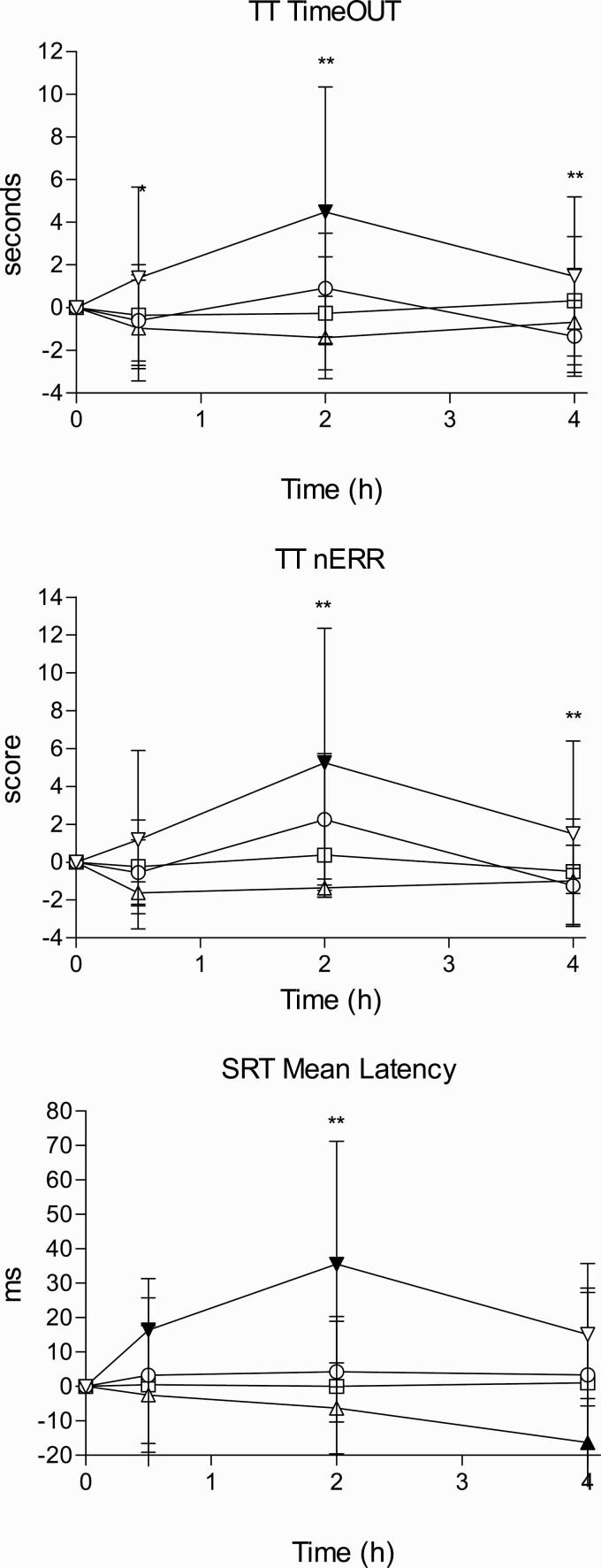
Figure 1.
Time course of effects for driving-related skills (differences to baseline). Data points and error bars represent mean and SD values for 16 participants. Conditions: alcohol (60 g); alcohol (60 g) + RB (240 mg caffeine); RB (240 mg caffeine); placebo. * P < .05 and ** P < .01 indicate alcohol significant differences with A/RB. Filled symbols indicate a significant difference from placebo (P < .05). The significance is only reported for the comparison of more interest (A vs A/RB) and also between all conditions and placebo. Other comparisons are not included in the figure to make it easy to understand.
Table 1.
Driving-Related Skills Pharmacodynamic Parameters’ Statistical Analysis
| Outcomes | A | A/RB | RB | P | Parameter | ES (95% CI) | ES (95% CI) | Interaction |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Alcohol | RB | |||
| Time out | 3.81 ± 6.37 | −0.34 ± 3.25 | −1.52 ± 2.93 | 0.86 ± 3.51 | Peak | 2.9 (−0.4 to 6.3) N.S |
−2.4 (−5.7 to 0.9) N.S. |
N.S. |
| (TT) s | 10.54 ± 16.49 | −0.36 ± 5.60 | −4.10 ± 7.41 | −0.49 ± 9.03 | AUC0-4h | 11.0 (2.8 to 19.2)** |
−3.6 (11.8 to 4.5) N.S. |
N.S. |
| Errors | 5.25 ± 7.50 | 0.88 ± 4.46 | −1.75 ± 3.00 | −0.31 ± 3.50 | Peak | 5.6 (1.8 to 9.3)** |
−1.4 (−5.2 to 2.3) N.S. |
N.S. |
| (TT) | 15.45 ± 21.53 | 2.13 ± 7.43 | −5.03 ± 6.73 | −0.09 ± 6.93 | AUC0-4h | 15.5 (6.0 to 25.1) ** |
−4.9 (−14.4 to 4.6) N.S. |
N.S. |
| Gyre | 67 ± 99.57 | 20.31 ± 122.90 | −12.00 ± 88.64 | 4.38 ± 102.74 | Peak | 62.5 (−15.2 to 140.5) N.S |
−16.4 (−94.2 to 61.5) N.S. |
N.S. |
| (TT) | 179.31 ± 263.40 | 65.06 ± 299.4 | 25.08 ± 200.81 | 91.44 ± 213.24 | AUC0-4h | 87.9 (−92.4 to 268.1) N.S. |
−66.4 (−246.6 to 113.9) N.S. |
N.S. |
| Latency | 43.21 ± 34.20 | 8.89 ± 28.41 | −17.19 ± 18.84 | −0.04 ± 30.40 | Peak | 43.3 (22.4 to 64.1)*** |
−17.2 (−38.0 to 3.7) N.S. |
N.S. |
| (SRT) ms | 93.73 ± 71.49 | 14.26 ± 52.57 | −29.83 ± 45.96 | 1.73 ± 72.94 | AUC 0-4h | 92.0 (45.1 to 138.9)*** |
−31.6 (−78.5 to 15.4) N.S. |
N.S. |
| Latency SD | 42.63 ± 37.70 | 15.89 ± 24.70 | −6.86 ± 26.64 | 2.48 ± 28.50 | Peak | 40.1 (18.3 to 62.0)*** |
−9.3 (−31.2 to 12.5) N.S. |
N.S. |
| (SRT) | 98.26 ± 81.81 | 33.43 ± 52.08 | −17.18 ± 58.32 | 2.18 ± 66.22 | AUC0-4h | 96.1 (44.9 to 147.4)*** |
−19.3 (−70.6 to 31.9) N.S. |
N.S. |
| Absolute error | 0.06 ± 0.23 | −0.05 ± 0.33 | −0.03 ± 0.26 | −0.03 ± 0.29 | Peak | 0.1 (−0.1 to 0.3) N.S. |
0.0 (−0.2 to 0.2) N.S. |
N.S |
| Time (MET) s | 0.11 ± 0.45 | −0.19 ± 0.76 | −0.10 ± 0.61 | −0.01 ± 0.66 | AUC0-4h | 0.1 (−0.4 to 0.6) N.S. |
−0.1 (−0.6 to 0.4) N.S. |
N.S. |
| Missings | 0.13 ± 1.96 | 0.19 ± 1.05 | 0.00 ± 0.97 | 0.38 ± 1.50 | Peak | −0.3 (−0.9 to 0.8) N.S. |
−0.4 (−1.5 to 0.7) N.S. |
N.S |
| (MET) | −0.84 ± 4.92 | 0.23 ± 2.80 | −0.50 ± 2.40 | 2.34 ± 3.35 | AUC0-4h | −3.2 (−5.9 to −0.5)* |
−2.8 (−5.6 to −0.1)* |
* |
| Hits | −0.88 ± 5.70 | −1.50 ± 6.38 | −1.88 ± 3.96 | −0.69 ± 5.10 | Peak | −0.2 (−4.0 to 3.7) N.S. |
−1.2 (2.7 to 0.7) N.S. |
N.S. |
| (NB) | −0.42 ± 13.00 | −1.48 ± 11.92 | −1.84 ± 6.95 | 0.80 ± 10.37 | AUC0-4h | −1.2 (−9.5 to 7.1) N.S. |
−2.6 (−10.9 to 5.6) N.S. |
N.S. |
| Errors | 0.19 ± 5.75 | 1.31 ± 6.43 | 0.44 ± 3.46 | −0.13 ± 4.90 | Peak | 0.3 (−3.2 to 3.7) N.S. |
0.6 (−2.9 to 4.0) N.S. |
N.S. |
| (NB) | −1.22 ± 14.14 | 0.91 ± 12.00 | 0.72 ± 5.92 | −1.94 ± 10.24 | AUC0-4h | 0.7 (−7.1 to 8.5) N.S. |
2.7 (−5.1 to 10.4) N.S. |
N.S. |
| Reaction time | −0.02 ± 0.11 | 0.00 ± 0.99 | −0.03 ± 0.10 | −0.04 ± 0.14 | Peak | 0.0 (−0.1 to 0.1) N.S. |
0.0 (−0.1 to 0.1) N.S. |
N.S. |
| (NB) s | 0.06 ± 0.17 | −0.05 ± 0.17 | −0.05 ± 0.20 | −0.03 ± 0.24 | AUC0-4h | 0.1 (−0.1 to 0.3) N.S. |
−0.1 (−0.3 to 0.10) N.S. |
N.S |
Abbreviations: A, alcohol + placebo energy drink; A/RB, alcohol + Red Bull; AUC, area under the curve; CI, confidence interval; ES, estimated substance effect; MET, movement estimation task; ms, millisecond, NB, N-Back task; P, placebo alcohol + placebo Red Bull; RB, Red Bull + placebo alcohol; SD, standard deviation; s,second; TimeOut, time outside the road; TT, tracking test; SRT, simple reaction time. N.S. mean not significant. * P < .05; ** P < .01; *** P < .001.
TT.
—Alcohol significantly increased TimeOut while RB had no main or interaction effects (peak and AUC). In the T-C alcohol increased TimeOut in comparison with placebo at all time points while differences among both conditions with alcohol were found at 2 hours. The combination of alcohol + RB reduced TimeOut compared with alcohol alone. The same results were found for the number of errors.
SRT.
—A main effect of alcohol was found in mean latency, which increased with alcohol consumption, while RB had no main or interaction effects (peak and AUC). In the T-C, RB counteracted alcohol effects at 2 hours (see Figure 1). The maximum effects were 43.2 ± 34.2 ms for alcohol and 8.9 ± 28.4 ms for A/RB. Differences in the same direction were found for latency SD. Commission errors were 2.6, 2.2, 0.6, and 1.2 for alcohol, A/RB, RB, and placebo, respectively, 2 hours after administration (not significant). Also no differences were found in the number of omission errors and correct responses.
NB.
—One volunteer was excluded due to outlying scores. No statistically significant differences were found between all conditions in this task.
MET.
—Absolute error time increased 60 ms with alcohol and was reduced 50, 30, and 30 ms for A/RB, RB, and placebo conditions, respectively. When the absolute error was calculated taking into account the sign (anticipation or delay), placebo error time was +180 ms while it was −70, −100, and −50 ms, for alcohol, A/RB, and RB, respectively (not statistically significant).
Subjective Effects
Results of pharmacodynamic parameters are shown in Table 2. Data about T-C for selected outcomes is included in Figure 2. In general terms, subjective effects reached their peak 1.5-2.5 hours after administration and returned to baseline from 4 to 8 hours later.
Table 2.
Subjective Effects Pharmacodynamics Parameters’ Statistical Analysis
| Outcomes | A | A/RB | RB | P | Parameter | ES (95% CI) |
ES (95% CI) |
Interaction | A vs A/RB |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | MEAN ± SD | Mean ± SD | Alcohol | RB | ||||
| Drunkenness | 51.81 ± 25.37 | 40.31 ± 24.72 | 0.00 ± 0.00 | 3.81 ± 8.14 | Peak | 48 (33.8 to 62.2)*** | −3.8 (−18.0 to 10.4) N.S. | N.S. | |
| mm | 132.72 ± 83.27 | 105.14 ± 84.83 | 0.00 ± 0.00 | 4.75 ± 10.30 | AUC0-8h | 127.9 (83.1 to 172.8)*** | −4.8 (−49.6 to 40.1) N.S. | N.S. | |
| Drowsiness | 50.25 ± 28.79 | 25.31 ± 26.86 | 6.94 ± 21.74 | 12.25 ± 20.07 | Peak | 38.0 (23.6 to 52.4)*** | −5.3 (−19.7 to 9.1) N.S. | * | ** |
| mm | 170.63 ± 131.86 | 56.97 ± 81.69 | 19.36 ± 69.22 | 36.67 ± 80.36 | AUC0-8h | 134.0 (92.2 to 175.7)*** | −17.3 (−59.1 to 24.4) N.S. | ** | *** |
| Dizziness | 23.63 ± 24.72 | 15.63 ± 18.59 | 5.19 ± 18.96 | 4.31 ± 15.46 | Peak | 19.3 (8.5 to 30.1)*** | 0.9 (−9.9 to 11.7) N.S. | N.S | |
| mm | 70.47 ± 101.31 | 33.02 ± 49.57 | 7.41 ± 28.71 | 6.44 ± 23.81 | AUC0-8h | 64.0 (28.6 to 99.4)*** | 1 (−34.5 to 36.4) N.S. | N.S | |
| Palpitations | 3.69 ± 8.93 | 4.13 ± 8.92 | 5.75 ± 20.41 | 1.19 ± 4.75 | Peak | 2.5 (−4.2 to 9.2) N.S. | 4.6 (−2.2 to 11.3) N.S. | N.S. | |
| mm | 15.83 ± 50.17 | 11.78 ± 28.12 | 9.38 ± 35.01 | 4.69 ± 18.75 | AUC0-8h | 11.1 (−1.8 to 24.1) N.S. | 4.7 (−8.3 to 17.6) N.S. | N.S. | |
| Anxiety | 9.13 ± 17.24 | 5.31 ± 9.39 | 6.69 ± 22.35 | 2.94 ± 9.62 | Peak | 6.2 (−1.4 to 13.7) N.S. | 3.8 (−3.8 to 11.3) N.S. | N.S. | |
| mm | 23.42 ± 62.26 | 13.70 ± 29.38 | 13.22 ± 48.53 | 9.00 ± 34.69 | AUC0-8h | 14.4 (−0.1 to 28.9) N.S. | 4.3 (−10.3 to 18.7) N.S. | N.S | |
| Headache | 11.94 ± 17.60 | 5.00 ± 10.98 | 0.13 ± 0.50 | 3.13 ± 6.36 | Peak | 8.8 (0.8 to 16.9)* | −3.0 (−11.1 to 5.0) N.S. | N.S. | |
| mm | 35.31 ± 63.71 | 9.13 ± 24.58 | 0.13 ± 0.50 | 6.98 ± 15.85 | AUC0-8h | 28.3 (1.3 to 55.4)* | −6.8(−33.9 to 20.2) N.S. | N.S. | |
| EAVc1 | 58.31 ± 25.73 | 41.00 ± 28.55 | 2.81 ± 11.25 | 3.69 ± 8.14 | Peak | 54.6 (39.5 to 39.5)*** | −0.9 (−16.0 to 14.3) N.S. | N.S. | |
| mm | 148.72 ± 212.44 | 100.48 ± 76.08 | 5.59 ± 22.38 | 7.34 ± 16.36 | AUC0-4h | 141.4 (100.5 to 182.3)*** | −1.8 (−42.7 to 39.2) N.S. | N.S. | |
| mm | 72.96 ± 96.31 | 85.41 ± 60.82 | 17.06 ± 48.26 | 19.16 ± 35.47 | AUC0-8h | 193.3 (135.1 to 251.4)*** | −2.0 (−60.3 to 56.1) N.S. | ** | *** |
| EAVc2 | −61.94 ± 30.94 | −41.56 ± 34.62 | −3.00 ± 12.00 | −5.81 ± 13.43 | Peak | −56.1 (−71.7 to −40.5)*** | 2.8 (−128 to 18.4) N.S. | N.S. | |
| mm | −177.64 ± 116.40 | −112.36 ± 103.41 | −7.56 ± 30.25 | −10.69 ± 24.31 | AUC0-4h | −167.0 (−220.7 to −113.2)*** | 3.1 (−50.7 to 56.9) N.S. | N.S. | |
| mm | −247.09 ± 150.72 | −107.28 ± 123.28 | −21.13 ± 59.76 | −24.47 ± 54.86 | AUC0-8h | −222.6 (−313.9 to −131.3)*** | 3.3 (−87.9 to 94.6) N.S. | * | ** |
| EAVc3 | −69.50 ± 31.16 | −50.44 ± 37.42 | −2.19 ± 8.75 | −6.38 ± 13.81 | Peak | −63.1 (−80.7 to −45.6)*** | 4.2 (−13.4 to 21.7) N.S. | N.S. | |
| mm | −204.81 ± 121.55 | −139.78 ± 118.95 | −4.73 ± 18.94 | −11.61 ± 27.05 | AUC0-4h | −193.2 (−253.6 to −132.8)*** | 6.8 (−53.5 to 67.3) N.S. | N.S. | |
| mm | −284.00 ± 139.82 | −127.84 ± 146.47 | −13.84 ± 39.16 | −23.81 ± 44.10 | AUC0-8h | −260.2 (−361.6 to −158.8)*** | 9.9 (−91.4 to 111.4) N.S. | * | ** |
| EAVc4 | −78.50 ± 28.00 | −60.13 ± 35.50 | −1.56 ± 6.25 | −7.81 ± 17.78 | Peak | −70.7 (−88.4 to −52.9)*** | 6.3 (−11.5 to 24.0) N.S. | N.S. | |
| mm | −234.06 ± 105.57 | −175.38 ± 116.47 | −5.06 ± 20.25 | −16.38 ± 40.12 | AUC0-4h | −217.7 (−276.4 to −159.1)*** | 11.3 (−47.3 to 70.0) N.S. | N.S. | |
| mm | −334.69 ± 143.12 | −166.91 ± 168.22 | −13.25 ± 37.48 | −34.56 ± 69.77 | AUC0-8h | −300.1 (−411.6 to −188.7)*** | 21.3 (−90.1 to 132.8) N.S. | * | ** |
| BAES-A | 14.75 ± 9.07 | 22.69 ± 15.87 | 8.75 ± 11.47 | 3.13 ± 7.07 | Peak | 11.6 (4.2 to 19.1)** | 5.6 (−1.8 to 13.1) N.S. | N.S. | |
| score | 37.73 ± 32.98 | 60.34 ± 49.64 | 18.68 ± 28.53 | 7.81 ± 25.59 | AUC0-8h | 29.9 (11.7 to 48.2)** | 10.9 (−7.4 to 29.1) N.S. | N.S. | |
| BAES-S | 21.19 ± 13.83 | 12.63 ± 11.84 | 3.63 ± 12.68 | 5.63 ± 14.01 | Peak | 15.6 (9.2 to 21.9)*** | −2.0 (−8.4 to 4.4) N.S. | N.S. | |
| score | 83.23 ± 81.40 | 44.27 ± 61.83 | 20.56 ± 77.85 | 26.33 ± 78.28 | AUC0-8h | 56.9 (32.5 to 81.3)*** | −5.7 (−30.2 to 18.7) N.S. | * | ** |
| ARCI-PCAG | 4.81 ± 3.75 | 1.75 ± 3.92 | −0.63 ± 2.87 | 0.94 ± 2.70 | Peak | 3.9 (1.7 to 6.0)*** | −1.6 (−3.7 to 0.6) N.S. | N.S. | |
| score | 25.03 ± 21.73 | 6.22 ± 15.81 | −0.53 ± 14.62 | 5.19 ± 11.24 | AUC 0-8h | 19.8 (10.6 to 29.1)*** | −5.7 (−15.0 to 3.5) N.S. | * | *** |
| ARCI-MBG | 5.41 ± 8.97 | 5.19 ± 4.05 | 1.00 ± 1.32 | 0.75 ± 1.53 | Peak | 4.7 (0.7 to 8.6)* | 0.3 (−3.7 to 4.2) N.S. | N.S. | |
| score | 5.20 ± 5.77 | 14.50 ± 12.69 | 3.09 ± 4.21 | 1.23 ± 4.57 | AUC0-8h | 4.0 (−1.4 to 9.3) N.S. | 1.9 (−3.5 to 7.2) N.S | * | ** |
| ARCI-LSD | 1.38 ± 2.55 | 0.81 ± 2.43 | 0.44 ± 2.03 | 0.25 ± 2.08 | Peak | 1.1 (0.1 to 2.1)* | 0.2 (−0.8 to 1.2) N.S. | N.S. | |
| score | 4.00 ± 13.42 | 3.69 ± 11.67 | 3.13 ± 13.04 | 2.14 ± 11.90 | AUC0-8h | 1.9 (−1.1 to 4.8) N.S. | 1.0 (−2.0 to 3.9) N.S | N.S | |
| ARCI-BG | −1.25 ± 2.32 | 1.88 ± 2.16 | 0.81 ± 0.91 | 0.25 ± 1.61 | Peak | −1.5 (−2.9 to −0.1)* | 0.6 (−0.9 to 2.0) N.S | ** | *** |
| score | −6.64 ± 8.74 | 5.59 ± 7.36 | 2.14 ± 4.76 | −0.38 ± 3.71 | AUC0-8h | −6.3 (−11.0 to −1.5)** | 2.5 (−2.3 to 7.3) N.S | ** | *** |
| ARCI-A | 1.93 ± 1.57 | 3.69 ± 2.12 | 1.00 ± 1.41 | 0.88 ± 1.59 | Peak | 1.0 (−0.1 to 2.2) N.S. | 0.1 (.1.0 to 1.2) N.S. | * | ** |
| score | 6.56 ± 6.93 | 13.52 ± 9.84 | 3.97 ± 6.95 | 3.14 ± 7.90 | AUC0-8h | 3.4 (−0.8 to 7.6) N.S | 0.8 (−3.4 to 5.1) N.S | * | ** |
Abbreviations: A, alcohol + placebo Red Bull; A/RB, alcohol + Red Bull; ARCI, Addiction Research Center Inventory; ARCI-A, amphetamine; ARCI-LSD, lysergic acid dyethylamide scale; ARCI-MG, benzedrine group, ARCI-MBG, morphine-benzedrine group; ARCI-PCAG, pentobarbital-chlorpromazine-alcohol group; AUC, area under the curve; BAES-A, Biphasic alcohol effects scale activation, BAES-S, biphasic alcohol effects scale sedation; CI, confidence interval; EAVc1, driving capability; EAVc2, willingness to drive an ill child to hospital; EAVc3, willingness to drive a sick friend home; EAVc4, willingness to drive a friend to a party; ES, estimated substance effect; RB, Red Bull + placebo alcohol; P, placebo alcohol + placebo Red Bull. N.S. mean not significant; * P < .05; ** P < .01; *** P < .001.
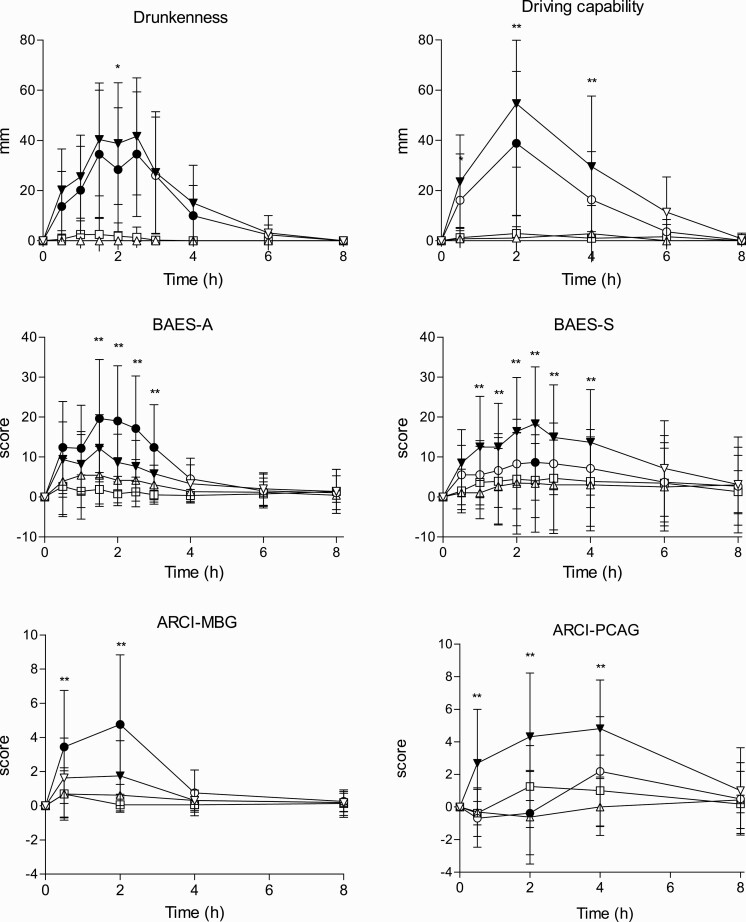
Figure 2.
Time course of subjective effects (differences to baseline). Data points and error bars represent mean and SD values for 16 participants. Conditions: alcohol (60 g); alcohol (60 g) + RB (240 mg caffeine), RB (240 mg caffeine), placebo. *P < .05 and **P < .01 indicate alcohol significant differences with alcohol + RB. Filled symbols indicate a significant difference from placebo (P < .05). The significance is only reported for the comparison of more interest (A vs A/RB) and between all conditions and placebo. Other comparisons are not included in the figure to make it easy to understand.
Subjective Intoxication and Other Subjective Outcomes (VAS).
—A total 60 g of alcohol with or without RB produced mild-moderate drunkenness. Drunkenness sensation was similar among both alcoholic conditions, being slightly lower with A/RB at 2 hours. Furthermore, no interaction effects of RB and alcohol were found in peak or AUC values. Alcohol induced moderate drowsiness compared with other treatments, whereas A/RB reduced it to one-half. In this outcome, an interaction effect of RB with alcohol was found (peak and AUC). The 2 alcohol conditions produced slight dizziness whereas none of the beverages administered produced significant palpitations, anxiety, or headache.
Capability to Drive and Willingness to Drive.
—Data were obtained until 4 hours for the first 8 volunteers and until 8 hours for the last 8 volunteers. Alcohol and A/RB reduced subjective capability to drive and reduced willingness to drive in all the driving situations proposed. Participants were more willing to drive under A/RB compared with alcohol alone. There was an alcohol main effect for all these outcomes, and an interaction effect for RB was found in AUC. In the T-C, differences among both conditions with alcohol appeared until 4 hours after administration (n = 8).
BAES.
—In the first 3 hours, participants rated stimulation and sedation and from then only rated sedation. A main effect of alcohol was found for both BAES subscales. RB reduced alcohol-induced sedation (interaction effect found in AUC). In the T-C, differences between both alcoholic conditions appeared from 1.5 to 3 hours for stimulation and from 1 to 4 hours for sedation.
ARCI.
—Alcohol increased the scores of the PCAG (sedation) scale compared with other conditions, partially counteracted by RB. In the T-C, it was also observed that A/RB produced greater euphoric effects (ARCI-MBG), stimulant effects (ARCI-BG), and amphetamine-like effects (ARCI-A) than the other 3 conditions the first 2 hours. Interaction effects of RB with alcohol were found in all previous subscales (AUC) whereas RB had no main effects.
Concentrations
Pharmacokinetic parameters are summarized in Table 3 and T-C of concentrations in Figure 3.
Table 3.
Alcohol and Caffeine Pharmacokinetic Parameters and Statistical Analysis for Comparison Between Conditions with Ethanol (A/RB vs A) and Conditions with Caffeine (A/RB vs RB)
| Outcome | Parameters | Values | ||
|---|---|---|---|---|
| A/RB | Alcohol | P | ||
| Alcohol plasma | Cmax (µmol/mL) | 18.2 ± 3.7 | 15.3 ± 2.2 | ** |
| tmax (h) | 2.0 (1.5–2.5) | 1.8 (1.5–2.5) | N.S. | |
| AUC0-8h (µmol.h/mL) | 64.1 ± 17.1 | 54.6 ± 17.6 | ** | |
| Elimination rate (µmol/mL/h) | 2.6 ± 0.7 | 2.7 ± 0.7 | N.S. | |
| A/RB | RB | P | ||
| Caffeine plasma | Cmax (nmol/mL) | 20.4 ± 2.9 | 17.6 ± 3.4 | * |
| tmax (h) | 2.0 (1.5–6.0) | 2.0 (1.5–4.0) | N.S. | |
| AUC0-8h (nmol.h/mL) | 111.9 ± 21.8 | 90.3 ± 20.2 | ** | |
| t 1/2 (h) | 5.8 ± 2.8 | 5.4 ± 2.3 | N.S. | |
| Caffeineu | Excretion 0–8h (nmol) | 32449.7 ± 10012.5 | 21568.6 ± 7262.1 | ** |
| Paraxanthineu | Excretion 0–8h (nmol) | 22531.8 ± 11004.6 | 40048.8 ± 16722.5 | ** |
| Theobromineu | Excretion 0–8h (nmol) | 13734.4 ± 4436.5 | 10785.3 ± 5123.6 | N.S. |
| Theophyllineu | Excretion 0–8h (nmol) | 405.0 ± 237.0 | 1460.2 ± 1587.0 | * |
| 17X/137X ratiou | Excretion 0–4h | 0.3 ± 0.1 | 0.9 ± 0.6 | *** |
| 17X/137X ratiou | Excretion 4–8h | 4.2 ± 2.3 | 6.4 ± 3.5 | *** |
| 17X/137X ratiou | Excretion 0–8h | 0.7 ± 0.4 | 2.0 ± 1.0 | *** |
Abbreviations: Alcohol, alcohol + placebo RB; A/RB, alcohol + Red Bull; AUC, area under the curve; Cmax, peak concentration; P, placebo alcohol + placebo RB; RB, Red Bull + placebo alcohol; tmax, time to reach peak concentrations; t1/2, half-life; U, urine; 17X/137X, paraxanthine/caffeine ratio. Values are mean ± SD except for tmax (median, minimum and maximum). Paired Student’s t test: N.S. mean not significant; * P < .05; ** P < .01; *** P < .001. Amount of 18.2 µmol/mL of alcohol corresponds to 83.5 mg/dL and 15.3 µmol/mL to 70.5 mg/dL.
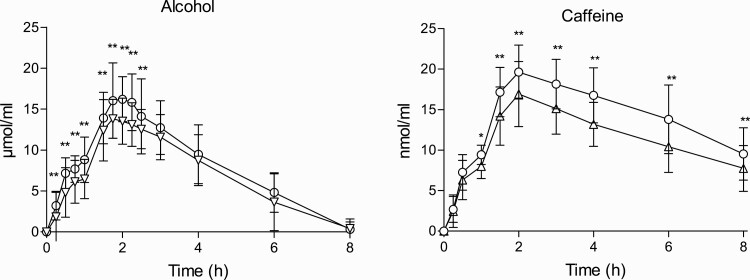
Figure 3.
Time course of plasma concentrations of alcohol and caffeine. Data points and error bars represent mean and SD values for 16 participants. Amount of alcohol of 10 μmol/mL corresponds to 46.07 mg/dL and 20 μmol/mL to 92.1 mg/dL. Conditions: alcohol (60 g); alcohol (60 g) + RB (240 mg caffeine), RB (240 mg caffeine), placebo. *P < .05 and **P < .01 indicate significant differences among both conditions.
Alcohol.
—BACs were detected during 8 hours. Cmax was reached around 2 hours after the first administration. Peak BAC with alcohol alone was 0.7 g/L with the multiple dose (30 g + 30 g), while theoretical BAC from a single dose of 60 g for a 73 kg participant is 1.17g/L. Alcohol concentrations were higher with A/RB. Mean increases of 14.8% and 13.2% were found in AUC and Cmax, respectively. Differences were mainly observed from 0.25 to 2.5 hours. Driving related-skills assessments were conducted with a mean BAC of 0.22 g/L (0.5 hour), 0.60 g/L (2 hours), and 0.40 g/L (4 hours).
Regarding BrAC, similar peak values were obtained (0.38 mg/L for alcohol and 0.40 mg/L for A/RB), and no differences were found among conditions. The T-C for BrAC was very similar to BAC, and concentrations in both matrices were strongly correlated (correlation coefficients of 0.96, see Table 5).
Table 5.
Correlations Between Alcohol Concentrations and Effects. Data are Expressed as r (95% CI)
| Outcomes | BAC | |
|---|---|---|
| A | A/RB | |
| Drunkenness | 0.58 (0.45 to 0.68) | 0.64 (0.53 to 0.73) |
| Drowsiness | 0.61 (0.50 to 0.70) | 0.26 (0.10 to 0.40) |
| Dizziness | 0.44 (0.30 to 0.56) | 0.37 (0.22 to 0.50) |
| EAVc1 | 0.75 (0.62 to 0.84) | 0.70 (0.55 to 0.80) |
| SRT mean latency | 0.50 (0.25 to 0.69) | 0.13 (−0.16 to 0.40) |
| TT time out | 0.54 (0.30 to 0.72) | 0.20 (−0.09 to 0.46) |
| TT errors | 0.53 (0.29 to 0.71) | 0.38 (0.11 to 0.60) |
| BAES-A | 0.29 (0.13 to 0.43) | 0.52 (0.39 to 0.63) |
| BAES-S | 0.63 (0.52 to 0.72) | 0.38 (0.23 to 0.51) |
| ARCI-A | 0.55 (0.35 to 0.70) | 0.72 (0.57 to 0.82) |
| ARCI-BG | −0.33 (−0.54 to −0.03) | 0.48 (0.27 to 0.65) |
| ARCI-LSD | 0.18 (−0.07 to 0,41) | 0.27 (0.02 to 0.48) |
| ARCI-MBG | 0.41 (0.18 to 0.60) | 0.61 (0.42 to 0.74) |
| ARCI-PCAG | 0.64 (0.47 to 0.77) | −0.04 (−0.29 to −0.21) |
| BrAC | 0.96 (0.94 to 0.97) | 0.96 (0.93 to 0.97) |
Abbreviations: A, alcohol + placebo Red Bull; A/RB, alcohol + Red Bull; ARCI, Addiction Research Center Inventory; ARCI-A, amphetamine; ARCI-LSD, lysergic acid dyethylamide scale; ARCI-MG, benzedrine group, ARCI-MBG, morphine-benzedrine group; ARCI-PCAG, pentobarbital-chlorpromazine-alcohol group; BAES-A, Biphasic alcohol effects scale activation, BAES-S, biphasic alcohol effects scale sedation; BAC, blood alcohol concentrations; CI, confidence interval; BrAC, breath alcohol concentrarions; RB, Red Bull + placebo alcohol; EAVc1, driving capability; SRT, simple reaction time; TT, tracking test.
Caffeine and Taurine.
—Caffeine concentrations in blood were higher when alcohol was present, from 1 to 8 hours. A mean increase of 17.6% was found in AUC0-8h and of 12.7% in Cmax. No differences in taurine concentrations were observed between both RB conditions.
Caffeine and Caffeine Metabolites in Urine.
—Data from 1 volunteer were excluded due to outlying concentrations. Results showed higher recoveries of paraxanthine and lower recoveries of caffeine with RB than with A/RB from 0 to 8 hours. Furthermore, higher recoveries of theophylline were found in the absence of ethanol. The volume of urine generated was higher with A/RB (1981.9 ± 349.5 mL) than in the other experimental conditions (1642.5 ± 368.5 mL for alcohol, 1581.6 ± 338.5 mL for RB, and 1420.0 ± 358.3 mL for placebo).
Vital Signs and Adverse Events
Results are shown in Table 4. Systolic blood pressure increased mainly with RB conditions in the first 2.5 hours, but no main or interaction effects were found (peak and AUC). RB increased diastolic blood pressure the first 2.5 hours (RB main effects in peak and AUC values). In the T-C, blood pressure with A/RB was significantly higher than with alcohol (1– 2.5 hours). Regarding heart rate, T-C showed an increase with both alcoholic conditions across 8 hours with no differences among them. A main effect of alcohol was found (peak and AUC). No differences were found in oral temperature.
Table 4.
Vital Signs Pharmacodynamics Parameters’ Statistical Analysis
| Outcomes | A | A/RB | RB | P | Parameter | ES (95% CI) |
ES (95% CI) |
Interaction |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Alcohol | RB | |||
| SBP | 5.06 ± 16.61 | 9.25 ± 13.69 | 7.50 ± 11.56 | −0.56 ± 11.57 | Peak | 5.6 (−3.1 to 14.4) N.S. | 8.1(−0.7 to 16.8) N.S. | N.S. |
| mmHg | −8.94 ± 46.98 | 16.53 ± 50.70 | 18.20 ± 44.57 | −8.13 ± 36.83 | AUC0-8h | −0.8 (29.0 to 27.3) N.S. | 26.3 (−1.8 to 54.5) N.S. | N.S. |
| DBP | −6.31 ± 12.86 | −0.75 ± 14.72 | 7.13 ± 9.18 | −1.00 ± 10.66 | Peak | −5.3 (−12.8 to 2.2) N.S. | 8.1 (0.6 to 15.6)* | N.S. |
| mmHg | −36.72 ± 48.46 | −13.05 ± 51.88 | 17.42 ± 33.75 | −14.92 ± 35.84 | AUC0-8h | −21.8 (−48.1 to 4.6) N.S. | 32.3 (6.0 to 58.7)* | N.S. |
| Heart rate | 15.06 ± 11.13 | 11.75 ± 12.21 | 0.38 ± 10.51 | 4.38 ± 13.51 | Peak | 10.7 (3.1 to 18.3)** | −4.0 (−11.6 to 3.6) N.S. | N.S. |
| bpm | 58.34 ± 45.45 | 53.13 ± 63.88 | −6.58 ± 37.09 | 6.48 ± 52.05 | AUC0-8h | 51.9 (17.5 to 86.2)** | −13.1 (−47.5 to 21.3) N.S. | N.S. |
| Temperature | −0.34 ± 0.49 | 0.03 ± 0.64 | 0.02 ± 0.72 | −0.33 ± 0.55 | Peak | −0.0 (−0.4 to 0.4) N.S. | 0.4 (−0.0 to 0.7) N.S. | N.S. |
| ºC | −0.83 ± 1.66 | 0.51 ± 1.99 | 0.88 ± 2.56 | −0.84 ± 2.15 | AUC0-8h | 0.0 (−1.4 to 1.5) N.S. | 1.7 (0.3 to 3.2)* | N.S. |
Abbreviations: A, alcohol + placebo Red Bull; A/RB, alcohol + Red Bull; AUC, area under the curve; CI, confidence interval; DBP, diastolic blood pressure; ES, estimated substance effect; P, placebo alcohol + placebo Red Bull; RB, Red Bull+ placebo alcohol; SBP, systolic blood pressure. N.S, not significant, * P < .05; ** P < .01; *** P < .001.
A total of 12 nonserious adverse events were registered in 7 volunteers, and 8 adverse events were considered possibly or probably related to treatment (headache and gastrointestinal symptoms).
Relationship Between Concentrations and Effects
Acute tolerance in TimeOut, SRT mean latency, drunkenness, and driving capability was not observed (similar effects in the ascending and descending limb of BAC). A strong association was found between BAC and driving capability in both alcoholic conditions whereas it was moderate with alcohol alone for drunkenness, drowsiness, SRT mean latency, TT time out/number of errors, BAES-S, ARCI-A, and ARCI-PCAG (see Table 5). Correlation turned weak when RB was present for most of them.
Discussion
In this study, alcohol impaired performance whereas EDs had no main or interaction effects with alcohol on driving-related skills. Although ED co-treatment did not fully reverse or antagonize the effects of alcohol, for many tasks, performance after the combination was significantly better than performance for alcohol alone at several time points.
EDs increased perceived capability to drive and willingness to drive under certain circumstances. Furthermore, EDs reduced alcohol-induced sedation and increased stimulation at several time points but not unmask drunkenness feelings. Additionally, higher caffeine and alcohol concentrations were found with A/RB.
The reduction in the time outside the road with the tracking test was 20% 2 hours after administration when RB was combined with alcohol compared with alcohol alone (absolute difference of 3.58 s). Other studies administering alcohol and caffeine showed mixed results. Caffeine capsules (2.93–5.87 mg/kg) partially offset alcohol-induced impairment measured with the CTT (Moskowitz and Burns, 1981) whereas no effect was found for decaffeinated coffee fortified with 200, 250, or 500 mg of caffeine (Nuotto et al., 1982). In another study using a Bimanual Visuomotor Task, coffee did not counteract alcohol-induced (BAC 0.6 g/L) errors (Buela-Casal and Caballo, 1990). On the other hand, EDs reduced dose dependently alcohol impairment in the CTT in the descending limb of alcohol concentrations (Peacock et al., 2015). Our results are in agreement with those observed with simulated driving with caffeinated beer (383 mg of caffeine and 1 g/kg of alcohol), where the medium driving performance (speed, lane position, and crashes) did not improve compared with non-caffeinated beer (Howland et al., 2011). However, also with simulated driving, 200–400 mg of caffeine counteracted alcohol (0.6 g/kg)-induced increases in braking latency (Liguori and Robinson, 2001). The correlation between performance in a tracking task and SD of lateral position measured with a driving simulator exists but is modest (Verster and Roth, 2012); therefore, discrepancies between studies might easily exist due to the variability in the measurement instruments. The doses administered and previous tolerance to effects must also be taken into account.
A reduction of 12% in mean latency of SRT task was found with A/RB at 2 hours compared with alcohol (absolute difference of 31.3 ms). In most studies where caffeine/EDs were mixed with alcohol, no improvement was found (McKetin et al., 2015) with the exception of another trial conducted in a similar setting (Azcona et al.,1995). Alford and colleagues (Alford et al., 2012) also showed a trend (P < .1) with an ED to compensate the increase in recognition reaction time produced by alcohol (participants received 2 alcohol doses to achieve 0.046 and 0.087% BrAC). With more potent psychostimulants, whereas cocaine reduced the alcohol-induced increase of SRT (Farré et al., 1993), MDMA tended to produce more impairment (Hernández-López et al., 2002).
Results in the NB task were congruent with a study where no significant differences were found between control and alcohol groups (BAC of 0.02 and 0.08%) in response accuracy or reaction time (Gundersen et al., 2008). Another study (n = 32), however, showed an increase in omission and commission errors with heavy memory load (2-back) (Casbon et al., 2003). In the case of movement estimation, we did not find a reduction of the impairment produced by alcohol when RB was added. Similar results were found in 30 volunteers using another test of movement estimation and a coffee (Buela-Casal and Caballo, 1990).
In summary, our results on driving related-skills suggest that driving would improve when EDs are mixed with alcohol compared with alcohol alone. However, to better assess the real impact, a driving simulator should be used.
In relation with perceived impairment, A/RB improved the subjective driving capability measured with VAS at several time points. Most studies have not shown an effect of EDs/caffeine in these outcomes (McKetin et al., 2015). Discrepancies could be explained by a reduced acute tolerance in our subjects.
In our study, perceived intoxication (drunkenness feelings) was similar with A/RB when compared to alcohol alone. Other studies also did not demonstrate a clear reduction in drunkenness (Benson et al., 2014; Verster et al., 2018), even with more potent psychostimulants (Farré et al., 1993; Hernández-López et al., 2002). A possible explanation is that drunkenness refers to negative behavior and physical effects of alcohol and only some symptoms can improve with psychostimulants.
Furthermore, a reduction in drowsiness/sedation produced by alcohol was observed with A/RB, as in previous studies (Ferreira et al., 2006; McKetin et al., 2015). The consumption of EDs allows subjects to stay awake for longer on a party night and could lead to faster (Marczkinski et al., 2017) and possibly higher alcohol consumption than expected.
Interestingly it was observed that A/RB consumption produces more pleasant effects than alcohol alone (more euphoria and amphetamine-like effects in ARCI), although maximum scores were mild in comparison other psychostimulants (Farré et al., 1993; Hernández-López et al., 2002). It has been proposed that caffeine antagonizes unwanted effects of alcohol through changes in adenosine neurotransmission. Caffeine is an adenosine receptor antagonist more effective when adenosine activity is high and the individual is feeling sleepy or sedated, both circumstances produced by alcohol ingestion (Ferré et al., 2011). In addition, caffeine enhances the reward experienced from alcohol because it reduces adenosine effects on dopamine neurotransmission (activation of A2A receptors inhibits dopamine transmission) and contributes to the continued alcohol consumption (Marczinski, 2014). These results are in the same line of studies in rats (Roldán et al., 2018). Furthermore, taurine also increases dopamine levels in nucleus accumbens (Ericson et al., 2006). Altogether these data suggest that co-administration of both drinks could increase the risk of development of alcohol use disorders, an hypothesis that should be tested in future studies.
The pharmacodynamic changes do not seem to be explained due to kinetic mechanisms as both alcohol and caffeine concentrations increased with A/RB. Regarding pharmacokinetics, an inhibition of caffeine elimination by alcohol was previously reported (Mitchell et al., 1983). Alcohol intake significantly prolonged the caffeine half-life and diminished the caffeine clearance (George et al., 1986), and the AUC for caffeine was also significantly higher when caffeine was administered with 0.8 g/kg of alcohol (Azcona et al., 1995). Authors proposed that alcohol inhibition of caffeine metabolism could be related with alterations in membrane function, NADPH levels or inhibition of drug binding to cytochromes (Mitchell et al., 1983; George et al., 1986). In our study caffeine half-life did not increase but it was calculated with data only till 8-h.
Our results in urine showed less paraxanthine concentrations with A/RB, in comparison with RB, supporting caffeine metabolism inhibition through CYP1A2 by alcohol (Gazzaz et al., 2018). The increase in alcohol concentrations with the mix however, was not expected. Most studies have found no differences (McKetin et al., 2015) or just the opposite one (Peacock et al., 2015), and also with other psychostimulants (Farré et al., 1993; Hernández-López et al., 2002). Increases observed in our study were mild in magnitude but found in 14 volunteers. Cmax also was higher with A/RB, so it seems that a component in RB increased alcohol absorption. Both mixers (Red Bull and Seven-Up) have natural sweeteners, gas, and nearly the same carbohydrate and energy content, so these components also do not seem to play a role. Caffeine also does not affect gastric myoelectrical activity or gastric emptying (Boekema et al., 1999; Jonderko et al., 2014). Nevertheless, effects on alcohol absorption of other differential ingredients could not be discounted (Huang et al., 2011). Additionally, a small fraction of alcohol could be using a common metabolic pathway with caffeine, such as CYP1A2 (Salmela et al., 1998). Regardless of the explanation, consumption of both beverages in less-tolerant individuals could favor higher intoxication levels. Future studies should include different ED brands (or compositions) and study separately the effects of each ingredient on alcohol pharmacokinetics.
Blood pressure was higher with A/RB than with alcohol at several time points. These differences can be explained by caffeine peripheral vasoconstriction effects (Fletcher et al., 2017). Additionally, both alcoholic conditions increased heart rate, explained by sympathetic nervous system activation with alcohol (Spaak et al., 2010). Both increases are modest but should be taken into account in subjects with previous cardiovascular diseases like arterial hypertension or arrhythmias. In fact, reported cases of atrial fibrillation after EDs consumption in healthy participants suggest that EDs can be arrhythmogenic (Di Rocco et al., 2011). Furthermore, a greater volume of urine was generated with A/RB because alcohol and caffeine have both diuretic properties, increasing the risk of dehydration (Stookey, 1999; O’Brien et al., 2008).
The main strength of our study is the complete factorial interaction design. Furthermore, the cross-over design allows each subject to be its own control avoiding bias due to variability in previous alcohol and caffeine tolerance. Moreover, the study was double-blind to reduce observer and participant expectancy effects.
In this study a multiple dose was administered mimicking the recreational consumption of alcohol and EDs during a party night. Furthermore we used the most consumed brand of ED (RB), but results may not extrapolate to all of them.
The sample size was small. Power calculation was based on the primary endpoint and may not have allowed to find differences in some secondary endpoints (e.g., NB). Furthermore, women were not included so the results cannot be extrapolated to them. We enrolled only males for avoiding potential sex differences in ethanol pharmacokinetics and subjective effects, mainly due to a lower volume of distribution, reduced metabolism and a decreased tolerability to ethanol in women (Mumenthaler et al., 1999; Baraona et al., 2001).
In our study a tracking task similar to the one used to obtain the driving license in Spain was used. Although the test has not been validated to assess the effects of acute alcohol consumption, a dose-response effect has been found with our own data (after the first administration the effects were lower than after the second one). Otherwise, the results in the tracking task were congruent with those obtained in SRT. Future studies should be conducted to confirm our results using a driving simulator, including women and testing different doses of alcohol and EDs.
Conclusions
This interaction study shows that alcohol impairs driving-related skills; the addition of EDs reduced, but did not eliminate, the effects of alcohol at several times and in several tasks. The reduction of the alcohol-induced sedation using EDs could favor the use of alcohol for longer periods of time and an underestimation of alcohol-induced impairment. At the pharmacokinetic level, alcohol mixed with EDs increased modestly caffeine and alcohol concentrations. Mechanisms involved should be further investigated.
Supplementary Material
Acknowledgments
We want to thank Esther Menoyo, Soraya Martín, Yan Zhang, Dídac Prat and Mohammed Ezzel Din their contribution in the conduct of the study, Jordi Serna for his contribution in biological samples analysis. Laura Blanco-Hinojo and Dídac Macià contributed with driving tests programming.
This work was supported by a grant from Ministerio del Interior, Dirección General de Tráfico-DGT (SPSIP2015-01798), Red de Trastornos Adictivos-RTA (SPIP2015-01798), Red de Trastornos Adictivos-RTA (RD16/0017/0010), Plan Nacional Sobre Drogas (PNSD 2018I037). Clara Pérez-Mañá and Esther Papaseit benefited from Juan Rodés fellowships (ISCIII, JR15/00005 and JR16/00020, respectively). The author’s work was independent from sources of funding.
Statement of Interest
None.
References
- Alford C, Hamilton-Morris J, Verster JC (2012) The effects of energy drink in combination with alcohol on performance and subjective awareness. Psychopharmacology 222:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, Caldeira KM, Bugbee BA, Vincent KB, O’Grady KE (2016) Energy drink use patterns among young adults: associations with drunk driving. Alcohol Clin Exp Res 40:2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcona O, Barbanoj MJ, Torrent J, Jané F (1995) Evaluation of the central effects of alcohol and caffeine interaction. Br J Clin Pharmacol 40:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakdash JZ, Marusich LR (2017) Repeated measures correlation. Front Psychol 8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS (2001) Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res 25:502–507. [PubMed] [Google Scholar]
- Benson S, Verster JC, Alford C, Scholey A (2014) Effects of mixing alcohol with caffeinated beverages on subjective intoxication: a systematic review and meta-analysis. Neurosci Biobehav Rev 47:16–21. [DOI] [PubMed] [Google Scholar]
- Boekema PJ, Samsom M, van Berge Henegouwen GP, Smout AJ (1999) Coffee and gastrointestinal function: facts and fiction. A review. Scand J Gastroenterol Suppl 230:35–39. [DOI] [PubMed] [Google Scholar]
- Buela-Casal G, Caballo VE (1990) Effects of alcohol and coffee consumption on driving: importance of alcohol tolerance level. MAPFRE seguridad Nº46, 2nd trimester. https://www.fundacionmapfre.org/documentacion/publico/es/catalogo_imagenes/grupo.cmd?path=1010904. Accessed October 19, 2020.
- Casbon TS, Curtin JJ, Lang AR, Patrick CJ (2003) Deleterious effects of alcohol intoxication: diminished cognitive control and its behavioral consequences. J Abnorm Psychol 112:476–487. [DOI] [PubMed] [Google Scholar]
- Di Rocco JR, During A, Morelli PJ, Heyden M, Biancaniello TA (2011) Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: two case reports. J Med Case Rep 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Directorate-General for Traffic (2016) El alcohol y la conducción. http://www.dgt.es/PEVI/documentos/catalogo_recursos/didacticos/did_adultas/alcohol.pdf. Accessed October 19, 2020.
- Ericson M, Molander A, Stomberg R, Söderpalm B (2006) Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine. Eur J Neurosci 23:3225–3229. [DOI] [PubMed] [Google Scholar]
- European School Survey Project on Alcohol and Other Drugs (2019) ESPAD Report 2019. Results from the European School Survey Project on alcohol and other drugs. http://espad.org/sites/espad.org/files/2020.3878_EN_04.pdf. Accessed March 11, 2021.
- Farré M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Camí J (1993) Alcohol and cocaine interactions in humans. J Pharmacol Exp Ther 266:1364–1373. [PubMed] [Google Scholar]
- Ferré S, O’Brien MC (2011) Alcohol and caffeine: the perfect storm. J Caffeine Res 1:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SE, de Mello MT, Pompéia S, de Souza-Formigoni ML (2006) Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res 30:598–605. [DOI] [PubMed] [Google Scholar]
- Fletcher EA, Lacey CS, Aaron M, Kolasa M, Occiano A, Shah SA (2017) Randomized controlled trial of high-volume energy drink versus caffeine consumption on ecg and hemodynamic parameters. J Am Heart Assoc 6:e004448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaz M, Kinzig M, Schaeffeler E, Jübner M, Hsin CH, Li X, Taubert M, Trueck C, Iltgen-Breburda J, Kraus D, Queckenberg C, Stoffel M, Schwab M, Sörgel F, Fuhr U (2018) Drinking ethanol has few acute effects on CYP2C9, CYP2C19, NAT2, and P-glycoprotein activities but somewhat inhibits CYP1A2, CYP2D6, and intestinal CYP3A: so what? Clin Pharmacol Ther 104:1249–1259. [DOI] [PubMed] [Google Scholar]
- George J, Murphy T, Roberts R, Cooksley WG, Halliday JW, Powell LW (1986) Influence of alcohol and caffeine consumption on caffeine elimination. Clin Exp Pharmacol Physiol 13:731–736. [DOI] [PubMed] [Google Scholar]
- Global Drug Survey (2019) https://www.globaldrugsurvey.com/gds-2018/. Accessed October 19, 2020.
- Gombao JC, Muñoz A, Monterde H (2006) El Reconocimiento Psicológico Oficial para la Licencia de Armas y Carnet de Conducir con el Equipo LND. Madrid, Spain: LNDeter SA. [Google Scholar]
- Gundersen H, Grüner R, Specht K, Hugdahl K (2008) The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimag J 2:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-López C, Farré M, Roset PN, Menoyo E, Pizarro N, Ortuño J, Torrens M, Camí J, de La Torre R (2002) 3,4-Methylenedioxymethamphetamine (ecstasy) and alcohol interactions in humans: psychomotor performance, subjective effects, and pharmacokinetics. J Pharmacol Exp Ther 300:236–244. [DOI] [PubMed] [Google Scholar]
- Howland J, Rohsenow DJ, Arnedt JT, Bliss CA, Hunt SK, Calise TV, Heeren T, Winter M, Littlefield C, Gottlieb DJ (2011) The acute effects of caffeinated versus non-caffeinated alcoholic beverage on driving performance and attention/reaction time. Addiction 106:335–341. [DOI] [PubMed] [Google Scholar]
- Huang KH, Chang CC, Ho JD, Lu RH, Tsai LH (2011) Role of taurine on acid secretion in the rat stomach. J Biomed Sci 18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jex HR, McDonnell JD, Phatak AV (1966) A “Critical’’ tracking task for manual control research. IEEE Trans Hum Fact Elect 7:138–145. [Google Scholar]
- Jonderko K, Kwiecień J, Kasicka-Jonderko A, Buschhaus M (2014) The effect of drugs and stimulants on gastric myoelectrical activity. Prz Gastroenterol 9:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne L, Lutz PE, Paille F (2017) Acute impact of caffeinated alcoholic beverages on cognition: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 76:188–194. [DOI] [PubMed] [Google Scholar]
- Lamas X, Farré M, Llorente M, Camí J (1994) Spanish version of the 49-item short form of the Addiction Research Center Inventory (ARCI). Drug Alcohol Depend 35:203–209. [DOI] [PubMed] [Google Scholar]
- Liguori A, Robinson JH (2001) Caffeine antagonism of alcohol-induced driving impairment. Drug Alcohol Depend 63:123–129. [DOI] [PubMed] [Google Scholar]
- Mangi MA, Rehman H, Rafique M, Illovsky M (2017) Energy drinks and the risk of cardiovascular disease: a review of current literature. Cureus 9:e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA (2014) Combined alcohol and energy drink use: hedonistic motives, adenosine, and alcohol dependence. Alcohol Clin Exp Res 38:1822–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT (2006) Clubgoers and their trendy cocktails: implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Exp Clin Psychopharmacol 14:450–458. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Stamates AL, Maloney SF (2018) Differential development of acute tolerance may explain heightened rates of impaired driving after consumption of alcohol mixed with energy drinks versus alcohol alone. Exp Clin Psychopharmacol 26:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993) Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17:140–146. [DOI] [PubMed] [Google Scholar]
- Martz ME, Patrick ME, Schulenberg JE (2015) Alcohol mixed with energy drink use among u.s. 12th-grade students: prevalence, correlates, and associations with unsafe driving. J Adolesc Health 56:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Coen A, Kaye S (2015) A comprehensive review of the effects of mixing caffeinated energy drinks with alcohol. Drug Alcohol Depend 151:15–30. [DOI] [PubMed] [Google Scholar]
- Ménétrey A, Augsburger M, Favrat B, Pin MA, Rothuizen LE, Appenzeller M, Buclin T, Mangin P, Giroud C (2005) Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoids levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Delta9-THC. J Anal Toxicol 29:327–338. [DOI] [PubMed] [Google Scholar]
- Mets MA, Ketzer S, Blom C, van Gerven MH, van Willigenburg GM, Olivier B, Verster JC (2011) Positive effects of Red Bull® Energy Drink on driving performance during prolonged driving. Psychopharmacology 214:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ (2014) Beverage caffeine intakes in the U.S. Food Chem Toxicol 63:136–142. [DOI] [PubMed] [Google Scholar]
- Mitchell MC, Hoyumpa AM, Schenker S, Johnson RF, Nichols S, Patwardhan RV (1983) Inhibition of caffeine elimination by short-term ethanol administration. J Lab Clin Med 101:826–834. [PubMed] [Google Scholar]
- Moskowitz H, Burns M (1981) The effects of alcohol and caffeine, alone and in combination, on skills performance. In: Alcohol, drugs and traffic safety,Vol. 3 (Goldberg L, ed), pp. 969–983. Stockholm, Sweden: Almqvist & Wiksell. [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA (1999) Gender differences in moderate drinking effects. Alcohol Res Health 23:55–64. [PMC free article] [PubMed] [Google Scholar]
- Nuotto E, Mattila MJ, Seppälä T, Konno K (1982) Coffee and caffeine and alcohol effects on psychomotor function. Clin Pharmacol Ther 31:68–76. [DOI] [PubMed] [Google Scholar]
- O’Brien MC, McCoy TP, Rhodes SD, Wagoner A, Wolfson M (2008) Caffeinated cocktails: energy drink consumption, high-risk drinking, and alcohol-related consequences among college students. Acad Emerg Med 15:453–460. [DOI] [PubMed] [Google Scholar]
- Olivera C, Planes M, Cunill Olivas M, Gras ME (2002) Efectos del alcohol y conducción de vehículos: creencias y conductas de los jóvenes. Rev Esp Drogodepend 27:66–80. [Google Scholar]
- Oteri A, Salvo F, Caputi AP, Calapai G (2007) Intake of energy drinks in association with alcoholic beverages in a cohort of students of the School of Medicine of the University of Messina. Alcohol Clin Exp Res 31:1677–1680. [DOI] [PubMed] [Google Scholar]
- Pacifici R, Palmi I, Vian P, Andreotti A, Mortali C, Berretta P, Mastrobattista L, Pichini S (2016) Emerging trends in consuming behaviours for non-controlled substances by Italian urban youth: a cross sectional study. Ann Ist Super Sanita 52:104–113. [DOI] [PubMed] [Google Scholar]
- Peacock A, Pennay A, Droste N, Bruno R, Lubman DI (2014) ‘High’ risk? A systematic review of the acute outcomes of mixing alcohol with energy drinks. Addiction 109:1612–1633. [DOI] [PubMed] [Google Scholar]
- Peacock A, Cash C, Bruno R (2015) Cognitive impairment following consumption of alcohol with and without energy drinks. Alcohol Clin Exp Res 39:733–742. [DOI] [PubMed] [Google Scholar]
- Pennay A, Miller P, Busija L, Jenkinson R, Droste N, Quinn B, Jones SC, Lubman DI (2015) ‘Wide-awake drunkenness’? Investigating the association between alcohol intoxication and stimulant use in the night-time economy. Addiction 110:356–365. [DOI] [PubMed] [Google Scholar]
- Ravelo A, Rubio C, Soler A, Casas C, Casas E, Gutierrez AJ (2013) Consumption of energy drink on college. Rev Esp Nutr Comunitaria 19:201–206. [Google Scholar]
- Roldán M, Echeverry-Alzate V, Bühler KM, Sánchez-Diez IJ, Calleja-Conde J, Olmos P, Boehm SL, Maldonado R, Rodríguez de Fonseca F, Santiago C, Gómez-Gallego F, Giné E, López-Moreno JA (2018) Red Bull® energy drink increases consumption of higher concentrations of alcohol. Addict Biol 23:1094–1105. [DOI] [PubMed] [Google Scholar]
- Ronen A, Oron-Gilad T, Gershon P (2014) The combination of short rest and energy drink consumption as fatigue countermeasures during a prolonged drive of professional truck drivers. J Safety Res 49:39–43. [DOI] [PubMed] [Google Scholar]
- Salmela KS, Kessova IG, Tsyrlov IB, Lieber CS (1998) Respective roles of human cytochrome P-4502E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol Clin Exp Res 22:2125–2132. [PubMed] [Google Scholar]
- Simple Reaction Time . (2021) Cambridge Cognition. https://www.cambridgecognition.com/cantab/cognitive-tests/simple-reaction-time-srt/. Accessed October 19, 2020.
- Spaak J, Tomlinson G, McGowan CL, Soleas GJ, Morris BL, Picton P, Notarius CF, Floras JS (2010) Dose-related effects of red wine and alcohol on heart rate variability. Am J Physiol Heart Circ Physiol 298:H2226–H2231. [DOI] [PubMed] [Google Scholar]
- Stookey JD (1999) The diuretic effects of alcohol and caffeine and total water intake misclassification. Eur J Epidemiol 15:181–188. [DOI] [PubMed] [Google Scholar]
- Verster JC, Benson S, Johnson SJ, Alford C, Godefroy SB, Scholey A (2018) Alcohol mixed with energy drink (AMED): a critical review and meta-analysis. Hum Psychopharmacol 33:e2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Roth T (2012) Predicting psychopharmacological drug effects on actual driving performance (SDLP) from psychometric tests measuring driving-related skills. Psychopharmacology 220:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MN, Cumming T, Burkhalter R, Langille DB, Ogilvie R, Asbridge M (2018) Driving under the influence behaviours among high school students who mix alcohol with energy drinks. Prev Med 111:402–409. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2018) Alcohol. https://www.who.int/es/news-room/fact-sheets/detail/alcohol. Accessed November 20, 2019.
- Zucconi S, Volpato C, Adinolfi F, Gandini E, Gentile E, Loi A (2013) Gathering consumption data on specific consumer groups of energy drinks. External scientific report 2013. Supporting Publications; EN-394, [190pp]. https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/sp.efsa.2013.EN-394. Accessed October 19, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.