Abstract
Major depressive disorder (MDD) is one of the most common psychiatric illnesses in the general population. In mental disorders, the activation of inflammatory pathways in the brain is a major producer of excitotoxicity and an inducer of oxidative stress. The occurrence of these 2 events is partly responsible for the neuronal damage inherent in patients with mental disorders. In the case of MDD, the release of hormone and increase in pro-inflammatory cytokines in plasma and indicators of oxidative stress have been identified as consequences of this event. The most important affectations in patients with MDD are changes in their cognitive and executive functions due to brain inflammation. Hence, these biomarkers can serve as diagnostic and severity classification tools and treatment. In this work, we described the communication pathway between the immune and neuroendocrine systems in MDD and suggested possible therapeutic options for the disease.
Keywords: Depressive disorder, immune response, inflammatory process, monoamines, oxidative stress, review
MAJOR DEPRESSIVE DISORDER
Major depressive disorder (MDD) is one of the most common psychiatric illnesses in the world. Within the psychiatric entities, it is one of the main causes of mortality and disability in the general population. MDD is a psychiatric problem characterized by a set of symptoms such as low mood, irritability, lack of sleep, anhedonia, fatigue, attention deficit, anorexia, chronic pain, guilty feeling, and overexpression of sadness in practically all situations of life (Perales and Loredo, 2015). Currently, the diagnosis of MDD is by DSM V criteria (American Psychiatric Association, 2013). The mean age of patients with this disorder is 30.4 years, and the prevalence is twice higher in women than in men (Pérez et al., 2017). It is considered an important cause of suicide and morbidity in adolescents and young adults; hence, its diagnosis and care are extremely important in these age groups (Perales and Loredo, 2015).
Etiology
The causes of MDD are very complex and encompass multiple factors, such as genetic, immune system, and endocrine factors, triggered by stress-related psychosocial conditions. In fact, it has been observed that acute stress and major depression share the same pathophysiological alterations (inflammatory state, metabolic alterations, and prothrombotic state) (Gold et al., 2015). Neuroimaging studies have reported increased activity of the amygdala, ventral stratum, and medial prefrontal cortex in response to adverse emotional stimuli (Richter and Xu, 2018). In addition, the influence of genetic and epigenetic factors in the development and evolution of MDD has been observed. To date, almost 200 genes involved in MDD have been reported (Shadrina et al., 2018). Seven of these genes (5HTTP/SLC6A4, APOE, DRD4, GNB3, HTR1A, MTHFR, and SLC6A3) are reported to be the most important (Yong-Ku et al., 2019). Moreover, mutations in the genetic regions of the FKBP5 allele involving abnormal function of the hypothalamic-pituitary-adrenal (HPA) axis, associated with elevated blood cortisol and increased adrenocorticotropic hormone (ACTH) in plasma, have been observed (Hennings et al., 2019). The presence of elevated cortisol levels and failure of its inhibitory mechanism alter communication between the HPA axis and/or the central nervous system with the immune system (Ramírez et al., 2018), thus, establishing an inflammatory process (Maes et al., 2015).
Activation of inflammatory pathways in the brain is considered to be an important producer of excitotoxicity and oxidative stress inducer that contributes to the neuronal damage seen in the disorder. This activation is mainly due to the presence of pro-inflammatory cytokines. These biomolecules activate the tryptophan-kynurenine (KP) pathway in microglial cells and astrocytes. The effect of this activation is structural changes in the brain manifested by decreased volume and thickness of the prefrontal cortex, cingulate cortex, amygdala, and hippocampus in patients, practically at the onset of the disease (Ramírez et al., 2018; Castanheira et al., 2019).
INFLAMMATORY RESPONSE AND IMMUNE SYSTEM ASSOCIATED WITH MDD
The HPA axis regulates and integrates endocrine and neurological responses to stressful stimuli. In addition, it is responsible for establishing direct communication with the immune system through specific receptors and ligands present on the surface of its cellular components, thus giving rise to an efficient 2-way communication (Kamimura et al., 2020).
The mechanism behind these actions is described as follows: under stressful stimuli, the paraventricular neurosecretory cells of the hypothalamus release corticotrophin releasing factor and arginine vasopressin in the pituitary gland. This gland secretes and releases ACTH into the circulation. ACTH stimulates the adrenal cortex to release cortisol. This response is mediated by specific cortisol elevation receptors. There are 2 types of these receptors: type 1 are the high-density mineralocorticoids located in the central nervous system, and type 2 are the low-density glucocorticoids that are systemically found and distributed in various tissues (Ceruso et al., 2020). The elevated level of cortisol exerts an inhibitory feedback mechanism on its receptors in the hippocampus and hypothalamus, thereby stopping the stimulation of these structures to restore balance (Wittenborn et al., 2016). When this balance is disrupted, hypercortisolemia directly stimulates the extrahepatic enzyme 2,3-indolimine dioxygenase (IDO) located in various tissues (intestine, placenta, liver, and brain) and in cells of the immune system (macrophages and dendritic cells) (Savitz, 2020). One of the different pathways of relationship between the immune system and the HPA is the IDO. The stimulation of interleukins (IL) and interferons (IFN) increases its activity by 1000 times. Elevation of IDO activities causes the metabolism of 99% of available tryptophan in the KP pathway, thus substantially reducing serotonin synthesis (Hestad et al., 2017). The elevation of KP leads to the production of reactive oxygen species (ROS) and nitrogen radicals. In addition, the high level of KP triggers glutamatergic systems promoted by kynurenic acid. In this form, the excitotoxicity generated produces tissue lesions and activates the inflammatory response (Cruzblanca et al., 2016).
The proinflammatory interleukins (IL-1γ, IL-6, tumor necrosis factor [TNF]-α, IFN) released in body’s perifery by fibroblasts, endothelial and epithelial cells are the key factors in the inflammatory response. In addition, the proinflammatory interleukins are also released by microglial cells, neurons, and astrocytes from brain regions such as the hypothalamus, hippocampus, cerebellum, basal ganglia, and brainstem nucleus (Tonhajzerova et al., 2020).
The regulation of peripheral levels of proinflammatory IL is carried out by afferent and efferent terminals of the vagus nerves through the release of acetylcholine (Ach), an inhibitor of cytokine release through the sympathetic-cholinergic-anti-inflammatory pathway (Breit et al., 2018). Any alteration in the levels of these cytokines detected by the peripheral afferent terminals of the vagus nerve—connected to the brainstem, hypothalamus, and forebrain nuclei (Breit et al., 2018)—induces an increase in IL-1β in the brain and an elevation of ACTH in the anterior pituitary. In this way, a vicious circuit is established due to the elevation of cortisol. This event triggers neuroinflammation (Figure 1).
Figure 1.
Stimulation/inhibition mechanisms of hypothalamic-pituitary-adrenal gland (HPA) axis by cortisol. Naturally, there is an inhibitory mechanism carried out by cortisol receptors in the hypothalamus. Nevertheless, in patients with major depressive disorder, this mechanism is inhibited, thus, favoring a hypercortisolemia state.
Neuroinflammation
Cytokine transport to the brain is possible due to its free passage through certain regions of the blood-brain barrier. Also, cytokines can permeate the blood-brain barrier by stimulating extracellular membrane receptors, such as toll-like receptors (TLRs) in perivascular macrophages, endothelial cells of brain venules and ventricular organs (Kealy et al., 2020). Just like the TLRs, the formation of inflammasomes plays an immunomodulatory function to express membrane receptors capable of acquiring inflammatory actions called M1 or an alternative inflammatory action termed M2. The M1 phase has the ability to release inflammatory mediators accompanied by an increase in helper lymphocyte (Th) and inflammatory mediators (IF-γ, IL-2, and TNF) (Martínez et al., 2018). The activated alternative, the M2 pathway, promotes anti-inflammatory action through the release of cellular protective factors and IL-10 and induction of energy, thus limiting neuroinflammation (Singhal and Baune, 2017). In addition, cytokine can gain entrance into the brain by binding to cytokine receptors associated with peripheral afferent nerve fibers (vagus nerve) or through the delivery of signals of inflammatory stimulus to relevant brain regions, including the nucleus of the solitary tract and the hypothalamus (Breit et al., 2018).
Once in the brain, proinflammatory cytokines activate microglia, astrocytes, and oligodendroglia through the nuclear factor-kappa-beta (NF-κB) pathway in the same way as in peripheral system. Activation of TLR4 stimulates the beta subunit of NF. Consequently, NF triggers nuclear synthesis of the nucleotide-binding domain, a family that contains leucine-rich inflammasome of the family 3 that contains pyrin domain. This synthesis of the nucleotide-binding domain gives rise to the generation of caspase 1 and the activation and release of pro-inflammatory interleukins IL-1b and IL-18 (Suárez and Buelvas, 2015). These biomolecules stimulate the hypothalamus to produce inflammatory prostaglandin E2, which binds to receptors present in neurons and glial cells and alters the structure of these receptors through a neurotoxicity mechanism (Ceylan et al., 2020). The culmination of this pathway is the release of ACTH, which binds to nicotinic alpha-7 receptors (α-7nAchR). Alpha-7 nAChR is a cholinergic receptor. It is known that this receptor regulates the ascending cholinergic inflammatory pathway, in which there is a decrease in inflammation through the vagus nerve with decreased activity of peripheral macrophages (Yaoyu et al., 2021).
One study on depressive phenotype by deletion of the nicotinic receptor α7 nAChR knockout (KO) in mice showed that the animals displayed a depression-like anhedonia. Likewise, TNF-α and IL-1β are indicators of proinflammatory cytokines (Ji-Chun et al., 2016). In this section and in line with the above where the immune system is involved through pro-inflammatory cytokines, we would like to address another factor, the intestinal microbiota, associated with the immune system and MDD. In an experimental study with mice, it was observed that treatment with probiotic bacteria can interfere with the HPA axis, suggesting a connection between the intestinal microbiota and the stress response through the HPA (Naseribafrouei et al., 2014). Some studies also indicate that fecal transplantation of microbiota in mice with depression phenotype modulates depression-like behavior in these animals in the absence of these germs (Barandouzi et al., 2020). In another experimental study with alpha-7 nAChR KO mice treated with antibiotics, it was found to cause phenotypes similar to depression and systemic inflammation compared with control mice treated with only fecal matter transplant, thus showing a relationship with the brain-intestinal axis (Yaoyu et al., 2021).
Neuroprogression
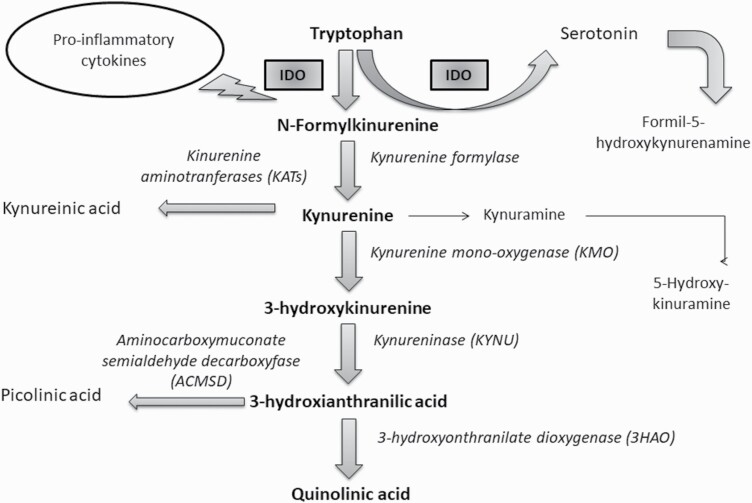
Neuroprogression is considered a potentially aggressive state of neuronal impairment that includes apoptosis and reduced neurogenesis and plasticity, all of which are associated with an increase in the inflammatory immune response (Labra et al., 2018). The initial step towards neurotoxic metabolism is the formation of KP, which, by itself, is not neuroactive. Kynurenic acid is metabolized by the enzyme ortho-3-hydroxyquinurerin to quinolinic acid (QUIN) by oxidation reaction (Garrison et al., 2018). QUIN modulates dopaminergic, nicotinergic, and glutamatergic neurotransmission. In addition, it activates the N-methyl-D-aspartate (NMDA) receptors that modulate the synthesis of IL-1b and IL-6. The presence of these ILs inhibits glutamate reuptake in synaptic spaces, thus leading to an overstimulation of ionotropic calcium channels (Ca2+) and, in this way, facilitating the entry of calcium/sodium ions and the exit of potassium. The glutamatergic system impairs the production of trophic factors essential for brain plasticity and facilitates the generation of oxygen and nitrogen free radicals (FR), thereby, establishing oxidative stress (OS) damage (Kindler et al., 2020). Several types of neurons and glial elements associated with the regulation of mood in different regions of the brain are severely damaged (van Velzen et al., 2017). The sustained elevation of these ILs maintains the dysfunctional state of the HPA axis and promotes IDO hypersensitivity that absorbs available serotonins and degrades them to formyl-5-hydroxy-kynurenine through an oxidation reaction (Figure 2) (Maurya et al., 2016).
Figure 2.
Kynurenine pathway. The induction of indolamine 2,3-dioxygenase enzyme through proinflammatory cytokines to catalyze the insertion of 2 oxygen atoms in the pyrrole ring of various indolamines, which include tryptophan, 5-hydroxytriptophan, and serotonin among others. The degradation of serotonin by indolamine 2,3-deoxygenase, called kynurenine derivation, produces formyl-5-hydroxykynurenamine detected in brain of patients with major depressive disorder.
In response to sustained inflammatory stimuli, microglia undergo quantitative, functional, and morphological changes. The consequences are alterations in the density of neurotransmitter packaging; decreased neurogenesis; and decreased number and size of glial cells in hippocampus, amygdala, prefrontal cortex, anterior cingulate, and basal ganglia (Yong-Ku and Eunsoo, 2017). These alterations are consistent with the neuroanatomical findings that characterize MDD in imaging studies (Chun-Hong et al., 2019). Microglia directly influence neurotransmission by synthesizing and regulating immunomodulators, such as TNF and transforming growth factor and by responding to the inducing effect of IFN. IFN regulates the expression of biomolecules, such as NO (nitric oxide), associated with intercellular communication (Farooq et al., 2017). This radical depends on the use of tetrahydrobiopterin, which is an enzymatic cofactor of tyrosine hydroxylase that converts tyrosine into L-3,4Hydroxyphenylalanine (L-DOPA). This bioamine, L-DOPA, is the limiting factor in the synthesis of dopamine (DA), whose final process is its transformation into noradrenaline by the enzyme DA-hydroxylase. Reduced DA is associated with anhedonia, loss of interest, and less motivation and incentive, and the decrease of noradrenaline is related to alterations in motivation, sleep-wake state, level of consciousness, appetite regulation, sexual behavior, and neuromodulation in learning and memory mechanisms (Cosci and Chouinard, 2019). Once the tetrahydrobiopterin cofactor is activated to synthesize NO in the microglia, the glutamatergic system is activated, thus triggering neuronal apoptosis and degeneration with accelerated cellular aging (Leonard, 2010).
INFLAMMATORY PROCESS AND OXIDATIVE STRESS
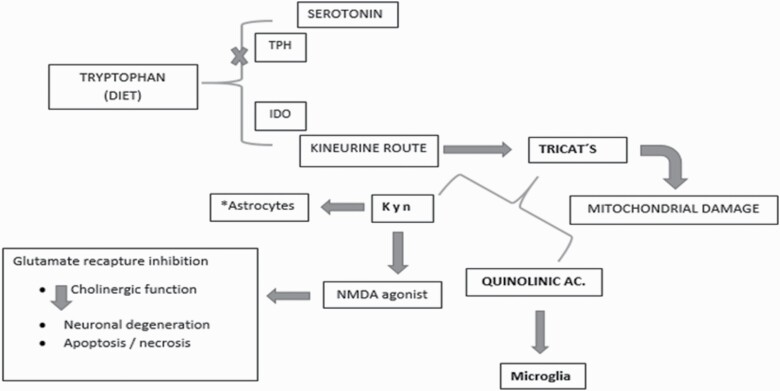
The proposed mechanism between the linkage of the pro-inflammatory process and oxidants points to the activation of the KP pathway triggered by the activation of the glial enzyme IDO. This leads to the generation of neuroactive metabolites (Lindqvist et al., 2017). Some of these neuroactive metabolites (KA, picolinic acid, NAD+) have neuroprotective functions whereas others (QUIN and 3-HK) have neurotoxic effects. Therefore, an imbalance in the local release of cytokines by the microglia, together with an increased function of astrocytes, generates catalytic activation of the IDO enzyme and facilitates uncontrolled production of neuroactive metabolites (Figure 3).
Figure 3.
Simplified scheme of the induction of indolamine 2,3-dioxygenase enzyme and the use of tryptophan towards the synthesis of kynurenine with consequent excitotoxicity.
It is considered that the interaction of 3-HK with xanthine oxidase produces ROS, superoxide (O2−), and hydrogen peroxide (H2O2) radicals. In this way, the nuclear DNA cleavage is induced and apoptosis is favored. The ROS generated may compromise the activation of G protein-coupled membrane receptors. This promotes lipoperoxidation and reduces catecholaminergic and serotonergic receptors. On its part, QUIN, an NMDA receptor agonist, stimulates glutamatergic receptors, which favors the entry of Ca2+ ion, thus contributing to a greater generation of ROS, FR, lipoperoxidation, and neuronal damage due to OS (da Silva et al., 2017). Nerve cells naturally counteract this damage by activating nuclear factor-erythroid 2 related factor 2 (Nrf2), which is an inactive cytosol protein. In the presence of OS, it moves to the cell nucleus to join the genes responsible for activating the antioxidant response element. Through the gene expression of the antioxidant response element, inflammation and OS damage are regulated. This event decreases the IM activity of macrophages and glial cells as well as the production of pro-inflammatory cytokines by inhibiting synthase or inducing catalase to reduce ROS and nitrous radicals (NR) production. The neuroprotective functions of Nrf2 include the release of antioxidant and detoxifying agents (glutathione and glucuronidation enzymes) and the activation of endogenous antioxidant enzymes (catalase, superoxide dismutase, and peroxiredoxins).
Nrf2 is related to antioxidant regulation and anti-inflammatory response. Wei et al. (2016) reported low levels of BNF in a model with Nrf2 KO mice where depression was induced by lipopolysaccharides and chronic social defeat stress, which is different from the result obtained with TrkB agonist subtype antidepressant (Wei et al., 2021). In addition, not only was the anti-inflammatory response observed, but also Nrf2 was reported to bind to enzymatic elements and proteins, inducing an antioxidant response with the reduction of BNF, which is another element that supports oxidative stress in MDD. On the other hand, coupled with the anti-inflammatory response of Nrf2, it was observed that the action of ketamine has a rapid and long-lasting antidepressant action in Nrf2 KO mice (Younge et al., 2021). In the absence of OS, Nrf2 is trapped in a protein complex in the cytosol (Tonelli et al., 2018). Despite the effort to maintain a balance, the brain is particularly vulnerable to oxidative damage due to its lipid nature, weakness in the antioxidant defense, and for the generation of FR from the enormous consumption of oxygen for its metabolism (van Velzen et al., 2017).
Role of Biomarkers in MDD
Currently, there is a lack of quantitative and non-invasive testing for the diagnosis of MDD. However, in clinical practice, the diagnosis is made through the criteria provided by the DSMV. The diagnosis can be complemented with the analysis of hormones such as cortisol in nail and hair samples as well as in saliva, urine, blood, and cerebrospinal fluid. The evidence of all the biochemical processes involved in the pathology, which have already been mentioned in this work, and the quantitative means to identify them are available and offer all the tools suggested as possible biomarkers (Strawbridge et al., 2017). The biochemical indicators recently evaluated are the profiles of cytokines and other pro-inflammatory proteins that trigger the oxidative stress process (Morera et al., 2019).
Elevations of IL-1, IL-6, and TNF-α show that there is resistance in the modulation of glucocorticoids, maintenance of the increased activity of the HPA axis (Cassano et al., 2017), and affectation of neurogenesis in hippocampus due to the reduction of brain-derived neurotrophic factor. Increased IL-1 is associated with manifestations of confusion, disappointment, and drowsiness in patients. TNF-α was found to be increased in the dorsal-anterior cingulate cortex and in the anterior insula of postmortem patients who showed social rejection and increased anxiety (Lovelace et al., 2017). Hormone screening includes cortisol as an indicator of HPA axis hyperactivity; thyroid hormones, which have been a successful therapeutic target in antidepressant treatment; serum and plasma levels of leptin and ghrelin that show a decrease during chronic stress and are associated with a lack of appetite and energy in patients; and an increase in plasma neopterin level associated with melancholic symptoms (Ellis et al., 2020).
The determination of growth factors in serum and plasma such as brain-derived neurotrophic factor, vascular endothelial growth factor, and insulin-like growth factor that are involved in plasticity and synapse processes are reported to be decreased in patients. Their levels can be reversed with antidepressant treatment (Hacimusalar and Esel, 2018). MDA is an indicator of damage to cell membranes and is responsible for the conversion of the O2 radical into H2O2 and O2. It is a therapeutic target to control cellular ROS levels (Sarandol et al., 2007). The presence in urine and blood of elevated levels of 8-hydroxy-deoxyguanosine, an indicator of oxidative damage in DNA (Forlenza and Miller, 2006), shows an increase in isoprostanes that confirms an association of depressive state. Furthermore, superoxide dismutase is decreased (Chung et al., 2013). Exploration of the genetic area continues to determine the connection of treatment response between candidate genes and pharmacodynamics (Jha and Trivedi, 2018).
Finally, imaging studies that show direct evaluation of the functional metabolic state and changes in brain structures (reduction in size and volume of hippocampus, basal ganglia, anterior cingulate cortex, frontal orbital cortex, dorsolateral prefrontal cortex, decrease in grey matter, and deterioration of the integrity of the white matter) help to predict the course of the pathology (Lai, 2019). These measurable indicators or biomarkers are used as diagnostic tools and as the basis for classifying the level of MDD and evaluating the evolution of the disease (Gururajan et al., 2016). The presence of a high number of indicators is associated with a high suicide rate, greater functional deterioration, and poor response to drug treatment in patients with MDD (Table 1).
Table 1.
Oxidative Stress Serum Parameters in Patients With Major Depressive Disorder
| Frequent parameters | Indicator |
|---|---|
| 8-Hydroxydesoxyguanosin | One of the most recognized biomarkers of oxidative DNA damage; generated after repairement of damage induced by Reactive oxygen Species (ROS); can be determined in urine and blood |
| F2-Isoprostanes | Inflammation; arachidonic acid peroxidation; can be determined in urine and blood |
| Peroxidase | |
| Malondialdehyde | Lipid peroxidation; serum |
| Superoxide dismutase | Antioxidant; determination in blood |
| Glutathione | Antioxidant; determination in blood |
| Glutathione peroxidase | Antioxidant damage by H2O2 |
| Nitric oxide | Accelerated production of superoxide anion |
| Xanthine oxidase | Production of superoxide and H2O2 determination in blood |
TREATMENT IN MDD
Several psychoactive serotonin reuptake inhibitors, noradrenergic antagonists and specific serotonin receptor antagonists, and selective serotonin and norepinephrine inhibitors have frequently been used for the treatment of depression (Minen et al., 2016). For decades, this type of treatment has offered a better quality of life to patients. Unfortunately, there is a time lapse of 2 to 4 weeks between the pharmacological effects of the administration and the long-term therapeutic effect. This situation is critical for the patient. It can lead to a possible worsening of the mood, which can trigger suicide attempts due to apparently ineffective therapy and the adverse effects of these drugs. Studies on available antidepressant drugs targeting these pathways report 40% resistance, even with multiple mixed treatments, and 8% persistence without response to any conventional treatment (Magnani et al., 2016).
In an attempt to achieve efficacy and safety, other drug options have been tried. These treatments include the use of a variety of non-antidepressant adjunctive agents such as lithium, pinodol, omega-3 fatty acids, 5-ademosyl-L-methionine, methylfolate, lamotrigine, sex steroids (testosterone for men and estrogen for women), and thyroid hormones, among others (Tundo et al., 2015). However, these tests have shown to be insufficient or contradictory with respect to their results, with the exception of NMDA receptor antagonist compounds and cyclooxygenase inhibitors (COX-1 and COX-2). An example of this is the use of ketamine, which when administered in single or multiple doses of i.v. subanesthetic induces an improvement with a rapid response in 100% of cases and a remission rate of 72% in treatment-resistant depressed patients (Kiraly et al., 2007). In the last decade, the use of ketamine as an antidepressant has been proposed. However, despite various studies, the main mechanism of its action has not yet been established. It was proposed that the antidepressant effect of ketamine is mediated by the blockade of NMDA receptors, located in the inhibitory neurons of γ-aminobutyric acid, and this results in increased glutamate. Ketamine is composed of 2 enantiomers—the esketamine ((S) -ketamine) and the arketamine ((R) -ketamine)—both of which have higher affinity for the NMDA S-ketamine receptor, with R-ketamine having a greater antidepressant efficacy (Jelen et al., 2021). An excitability response in pyramidal cells of mouse brain sections has also been demonstrated with enantiomers (R, S) –ketamine (Widman and McMahon, 2018), which through indirect action reduce synaptic inhibition of γ-aminobutyric acid.
However, in recent years, the therapeutic targets and the development of new drugs have shifted towards the pathways of inflammatory and oxidative stress (Maes et al., 2012). Anti-inflammatory drugs, particularly piroxicam and celecoxib, have been shown to reduce anhedonia and to increase norepinephrine and serotonin in the hippocampus of some patients with major depression (Miller and Raison, 2016). This discovery has placed the pro-inflammatory response as a possible therapeutic target.
PERSPECTIVES
MDD is a complex pathology in which physiological imbalances affect different areas of the body. The involvement of the immune system and the pro-inflammatory process in the development of MDD appears to take place in the context of an intense and long-lasting “fictitious” inflammatory response, together with a poor resolution process in the stress cascade. Inflammation is a physiological mechanism present in chronic diseases. It is defined as a non-specific response mediated by soluble agents to destroy some harmful agents, as well as to repair affected tissues. Many of these reactions involve the participation of pro-inflammatory cytokines, mostly secreted by monocytes, microglia, and Th 1 lymphocytes. These reactions are also accompanied by increased levels of acute phase reactants, such as C-reactive protein and complement factors. As mentioned above, the pathophysiological process of MDD appears to be associated with an increase in pro-inflammatory cytokines. This increase can trigger the induction of cyclooxygenase enzymes that participate in the production of prostaglandins. Non-steroidal anti-inflammatory drugs show promising results in clinical trials in depression despite the fact that there are studies related to antifungal therapy. Further studies including patients with inflammatory markers in plasma or cerebrospinal fluid are needed to demonstrate the relationship of symptoms and effectiveness of treatment.
Cytokines are important in brain development because they promote neuronal integrity, neurogenesis, and synaptic remodeling that confer them with the ability to intervene in various circuits and in the neurotransmitter system.
Different mechanisms have been proposed in the interactions of cytokines with the HPA axis and with the IDO enzyme to explain the participation of neuroactive substances such as quinolinic acid present in the plasma and cerebrospinal fluid of patients with major depression and with those who have suffered suicide attempts. The contribution of neuroinflammation appears to occur through the interaction of these pro-inflammatory cytokines with their TLR types, particularly TLR3 and TLR4 that are present in the limbic system. This situation leads to changes in the cognitive and executive function of patients. Therefore, it is considered of great importance to determine the inflammatory biomarkers and associated pain in the management of these patients.
To date, the response to treatment is insufficient and ineffective. Therefore, it is of utmost importance to create and test new neuropharmacological strategies targeting the immune system. This would open up a wide range of therapeutic possibilities that can improve the mental health and the quality of life of the patients.
Acknowledgments
We thank Dr Cyril Ndidi Nwoye Nnamezie, an expert translator whose native language is English, for his help in preparing this manuscript. The authors express their profound gratitude to the National Institute of Pediatrics (INP) for the support in the publication of this article, derived from the project 027/2018 of the Program A022, authorized by the Ethics and Investigation Committee of this Institute and following format PRISMA.
This research received no specific grant from any funding agency in the public, commercial, private, or not-for-profit sectors.
Interest Statement
The authors declare that they have no conflicts of interest.
References
- American Psychiatric Association (2013) Diagnostic and statics manual of mental disorders fifth edition (DSM V), pp. 104–106. American Psychiatric Publishing. [Google Scholar]
- Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS (2020) Altered composition of gut microbiota in depression: a systematic review. Front Psychiatry 11:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, Hasler G (2018) Vagus nerve as modulator of the brain-gut-axis in psychiatric and inflammatory disorders. Front Psychiatry 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano P, Bui E, Rogers AH, et al. (2017) Inflammatory cytokines in major depressive disorder: a case–control study. Aust N Z J Psychiatry 51:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanheira L, Silva C, Cheniaux E, Telles-Correia D (2019) Neuroimaging correlates of depression-implications to clinical practice. Front Psychiatry 10:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceruso A, Martínez-Cengotitabengoa M, Peters-Corbett A, Diaz-Gutierrez MJ, Martínez-Cengotitabengoa M (2020) Alterations of the HPA axis observed in patients with major depressive disorder and their relation to early life stress: a systematic review. Neuropsychobiology 79:417–427. [DOI] [PubMed] [Google Scholar]
- Ceylan D, Yılmaz S, Tuna G, Kant M, Er A, Ildız A, Verim B, Akış M, Akan P, İşlekel H, Veldic M, Frye M, Özerdem A (2020) Alterations in levels of 8-Oxo-2’-deoxyguanosine and 8-Oxoguanine DNA glycosylase 1 during a current episode and after remission in unipolar and bipolar depression. Psychoneuroendocrinology 114:104600. [DOI] [PubMed] [Google Scholar]
- Chung CP, Schmidt D, Stein CM, Morrow JD, Salomon RM (2013) Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res 206:213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosci F, Chouinard G (2019) The monoamine hypothesis of depression revisited: could it mechanistically novel antidepressant strategies? In: Neurobiology of depression: road to novel therapeutics (Quevedo J, Carvalho AF, Zarate CA, eds), pp 63–73. London, UK: Elsevier. [Google Scholar]
- Cruzblanca-Hernández H, Lupercio-Coronel P, Collas-Aguilar J, Castro-Rodríguez E (2016) Neurobiology of major depression and its pharmacological treatment. Salud Ment 39:47–58. (In Spanish) [Google Scholar]
- da Silva e Carvalho M, Miyagui Yonamine C, Dal Mas C, Silva Nunes DF, Furuie Hayashi MA (2017) Tryptophan metabolism in mental disorders: a focus on schizophrenia. Vitalle-Revista de Ciencias da Saúde 29:44–56. (In Portuguese) [Google Scholar]
- Ellis RJ, Letendre SL, Atkinson JH, Clifford D, Collier AC, Gelman BB, Marra C, McCutchan JA, Morgello S, Sacktor N, Tang B, Heaton RK (2020) Higher levels of plasma inflammation biomarkers are associated with depressed mood and quality of life in aging, virally suppressed men, but not women, with HIV. Brain Behav Immun Health 7:100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq RK, Asghar K, Kanwal S, Zulqernain A (2017) Role of inflammatory cytokines in depression: focus on interleukin-1β. Biomed Rep 6:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza MJ, Miller GE (2006) Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom Med 68:1–7. [DOI] [PubMed] [Google Scholar]
- Garrison AM, Parrott JM, Tuñon A, Delgado J, Redus L, O’Connor JC (2018) Kynurenine pathway metabolic balance influences microglia activity: targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology 94:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Machado-Vieira R, Pavlatou MG (2015) Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast 2015:581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan A, Clarke G, Dinan TG, Cryan JF (2016) Molecular biomarkers of depression. Neurosci Biobehav Rev 64:101–133. [DOI] [PubMed] [Google Scholar]
- Hacimusalar Y, Esel E (2018) Suggested biomarkers for major depressive disorder. Noro Psikiyatr Ars 55:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings JM, Kohli MA, Uhr M, Holsboer F, Ising M, Lucae S (2019) Polymorphisms in the BDNF and BDNFOS genes are associated with hypothalamus-pituitary axis regulation in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 95:109686. [DOI] [PubMed] [Google Scholar]
- Hestad KA, Engedal K, Whist JE, Farup PG (2017) The relationship among tryptophan, kynurenine, indoleamine 2,3 dioxygenase, depression and neuropsycological performance. Front Psychol 8:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelen LA, Young AH, Stone JM (2021) Ketamine: a tale of two enantiomers. J Psychopharmacol 35:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Trivedi MH (2018) Pharmacogenomics and biomarkers of depression, pp. 101–113. In: Antidepressants. Handbook of experimental pharmacology (Macaluso M, Preskorn S, eds), Vol. 250. Cham, Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Tanaka Y, Hasebe R, Murakami M (2020) Bidirectional communication between neural and immune systems. Int Immunol 32:693–701. [DOI] [PubMed] [Google Scholar]
- Kealy J, Greene C, Campbell M (2020) Blood-brain barrier regulation in psychiatric disorder. Neurosci Lett 726:133664. [DOI] [PubMed] [Google Scholar]
- Kim YK, Ham BJ, Han KM (2019) Interactive effects of genetic polymorphisms and childhood adversity on brain morphologic changes in depression. Prog Neuro-Psychopharmacol Biol Psychiatry 91:4–13. [DOI] [PubMed] [Google Scholar]
- Kim YK, Won E (2017) The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive. Behav Brain Res 329:6–11. [DOI] [PubMed] [Google Scholar]
- Kindler J, Lim CK, Weickert CS, et al. (2020) Dysregulation of kynurenine metabolism is related to proinflammatory cytokines attention and prefrontal cortex volume in schizophrenia. Mol Psychiatry 25:2860–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Horn SR, Von Dam NT, et al. (2007) Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry 7:e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labra Ruiz NA, Santamaría del Ángel D, Juárez-Olguín H, Lindoro Silva M (2018) Neuroprogression: the hidden mechanism of depression. Neuropsychol Dis Treat 4:2837–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH (2019) Promising neuroimagin biomarkers in depression. Psychiatry Invest 16:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE (2010) The concept of depression as a dysfunction of the immune system. Curr Immunol Rev 6:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, Reus VI, Verhoeven JE, Epel ES, Mahan L, Rosser R, Wolkowitz OM, Mellon SH (2017) Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 76:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Zhang GZ, Bin Li B, et al. (2019) Role of inflammation in depression relapse. J Neuroinflammation 16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K, Guillemin GJ, Brew BJ (2017) Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 112:373–388. [DOI] [PubMed] [Google Scholar]
- Maes M, Fisar Z, Medina M, Scapagnini G, Nowak G, Berk M (2012) New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant and neuroprogresive pathways. Inflamopharmacology 20:27–150. [DOI] [PubMed] [Google Scholar]
- Maes M, Noto C, Brietzke E (2015) Omics-based depression and inflammation research. Braz J Psychiatry 37:1–2. [DOI] [PubMed] [Google Scholar]
- Magnani M, Sasdelli A, Bellinis S, et al. (2016) Treating depression: what patients want, findings from a randomized controlled trial in primary care. Psycosomatics 57:616–623. [DOI] [PubMed] [Google Scholar]
- Martínez-Tapia RJ, Estrada-Rojo F, Hernández-Chávez AA, Barajas-Martínez A, Islas Escoto SL, Navarro L, Chavarría A (2018) Neuroinflammation: the ying-yang of neuroimmunology. Rev Fac Med 61:44–53. (In Spanish) [Google Scholar]
- Maurya PK, Noto C, Rizzo LB, et al. (2016) The role of oxidative and nitrosative stress in accelerated aging in major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 65:134–144. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minen MT, Begasse De Dhaem O, et al. (2016) Migraine and it’s psychiatric comorbidities. J Neurol Neurosur Psychiatry 87:741–749. [DOI] [PubMed] [Google Scholar]
- Morera LP, Tempesti TC, Pérez E, Medrano LA (2019) Biomarkers in stress measurement: a systematic review. Ansiedad Estrés 25:49–58. (In Spanish) [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, Rudi K (2014) Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26:1155–1162. [DOI] [PubMed] [Google Scholar]
- Perales-Blum MTL, Loredo L (2015) Family dysfunction and suicide in adolescents with major depressive disorder. Salud Ment 38:195–200. (In Spanish) [Google Scholar]
- Pérez-Padilla EA, Cervantes-Ramírez VM, Hijuelos-García NA, Pineda-Cortes JC, Salgado-Burgos H (2017) Prevalence cases and treatment of major depression. Revista Biomédica 28:89–115. (in Spanish) [Google Scholar]
- Pu Y, Tan Y, Qu Y, et al. (2021) A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after faecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun 94:318–326. [DOI] [PubMed] [Google Scholar]
- Qu Y, Shan J, Wang S, et al. (2021) Rapid-acting and long-lasting antidepressant-like action of (R) -ketamine in Nrf2 knock-out mice: a role of TrkB signaling. Eur Arch Psychiatry Clin Neurosci 271:439–446. [DOI] [PubMed] [Google Scholar]
- Ramírez LA, Pérez-Padilla EA, García-Oscos F, Salgado H, Atzori M, Pineda JC (2018) New theory about depression: a balance of mood between the nervous and immune systems, with regulation of serotonin-quinurenine and the hypothalamus-pituitary-adrenal axis. Biomédica 38:437–450. (In Spanish) [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Xu L (2018) How could stress lead to major depressive disorder? IBRO Rep 4:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S (2007) Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol 22:67–73. [DOI] [PubMed] [Google Scholar]
- Savitz J (2020) The kynurenine pathway a finger in every pie. Mol Psychiatry 25:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadrina M, Bondarenko EA, Slominsky PA (2018) Genetics factors in major depression disease. Front Psychiatry 9:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal G, Baune BT (2017) Microglia: an interface between the loss of neuroplasticity and depression. Front Cell Neurosci 11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Young AH, Cleare AJ (2017) Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr Dis Treat 13:1245–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez R, Buelvas N (2015) Inflammasome: activation mechanisms. Investig Clin 56:77–99. [PubMed] [Google Scholar]
- Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid Redox Signal 29:1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonhajzerova I, Sekaninova N, Bona Olexova L, Visnovcova Z (2020) Novel insight into neuroimmune regulatory mechanisms and biomarkers linking major depression and vascular diseases: the dilemma continues. Int J Mol Sci 21:2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tundo A, de Filippis R, Proietti L (2015) Pharmacologic approaches to treatment resistant depression: evidences and personal experience. World J Psychiatry 5:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen LS, Wijdeveld M, Black CN, et al. (2017) Oxidative stress and brain morphology in individuals with depression and healthy controls. Prog Neuro-Psychopharmacol Biol Psychiatry 76:140–144. [DOI] [PubMed] [Google Scholar]
- Yao W, Zhang JC, Ishima T, et al. (2016) Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep 6:30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Lin S, Su J, et al. (2021) Activation of BDNF by transcription factor Nrf2 contributes to antidepressant-like actions in rodents. Transl Psychiatry 11:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widman AJ, McMahon LL (2018) Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A 115:E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenborn AK, Rahmandad H, Rick J, Hosseinichimeh N (2016) Depression as a systemic syndrome: mapping the feedback loops of major depressive disorder. Psychol Med 46:551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Ren Q, et al. (2016) Depression-like phenotype by deletion of α7 nicotinic acetylcholine receptor: role of BDNF-TrkB in nucleus accumbens. Sci Rep 6:36705. [DOI] [PMC free article] [PubMed] [Google Scholar]