Abstract
A procedure based on panfungal PCR and multiplex liquid hybridization was developed for the detection of fungi in tissue specimens. The PCR amplified the fungal internal transcribed spacer (ITS) region (ITS1-5.8S rRNA-ITS2). After capture with specific probes, eight common fungal pathogens (Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Candida krusei, Candida glabrata, Candida parapsilosis, Candida tropicalis, and Cryptococcus neoformans) were identified according to the size of the amplification product on an automated sequencer. The nonhybridized products were identified by sequencing. The performance of the procedure was examined with 12 deep-tissue specimens and 8 polypous tissue biopsies from the paranasal sinuses. A detection level of 0.1 to 1 pg of purified DNA (2 to 20 CFU) was achieved. Of the 20 specimens, PCR was positive for 19 (95%), of which 10 (53%) were hybridization positive. In comparison, 12 (60%) of the specimens were positive by direct microscopy, but only 7 (35%) of the specimens showed fungal growth. Sequencing of the nonhybridized amplification products identified an infecting agent in six specimens, and three specimens yielded only sequences of unknown fungal origin. The procedure provides a rapid (within 2 days) detection of common fungal pathogens in tissue specimens, and it is highly versatile for the identification of other fungal pathogens.
The diagnosis of fungal infections remains a significant problem. The clinical presentation is difficult to interpret, and the findings of noninvasive methods (computed tomographic scanning and X ray) are not specific (24). Culture results are available at the earliest in 2 to 3 days, and blood and deep-tissue sample cultures from infections with focal lesions are frequently negative (5, 30). Direct microscopy and histopathological examination are rapid, but they do not always allow identification of the infecting agent to the species level (24, 30). In contrast, even though the latest generation of monoclonal antibody-based enzyme-linked immunosorbent assays (ELISAs) for circulating Aspergillus and Candida antigens are specific, they lack sensitivity (24). Thus, rapid methods that are sensitive and specific are needed, and PCR has been applied to fulfill these requirements.
A variety of PCR protocols for human samples have been published, including panfungal PCR assays (17, 29) and methods that detect one species (3, 23, 27), members of a fungal family (8, 15, 18, 22, 32), or several species (6, 26). The incidence of individual fungal species in various infections is relatively low, e.g., Candida spp., 10%; Aspergillus spp., 5 to 15%; and Fusarium spp., <2% (5, 9, 24, 30). Identification of the infecting agent to the species level is required to guide appropriate treatment. Therefore, the only efficient and economic approach may be a single protocol that is able to detect and identify many species. However, the protocols for detecting several species (6, 26) use labor-intensive blotting procedures and sequential hybridizations with various radiolabeled probes for differentiation of species, which make these approaches impractical for routine laboratory use.
In this study, we describe a PCR method based on the sequence variation of the fungal internal transcribed spacer (ITS) region (Fig. 1). The method includes multiplex liquid hybridization with species-specific probes, and it uses nonradioactive and automated PCR product detection on a fluorescent automated DNA sequencer (12). Nonhybridized products are easily recognized on the sequencer, and they can be used for identification by sequencing. We show the applicability of this approach to the analysis of tissue samples from deep-seated fungal infections that are frequently negative by culture, and we report the presence of fungi in the majority (88%) of polypous tissue samples of patients with chronic rhinosinusitis (CRS).
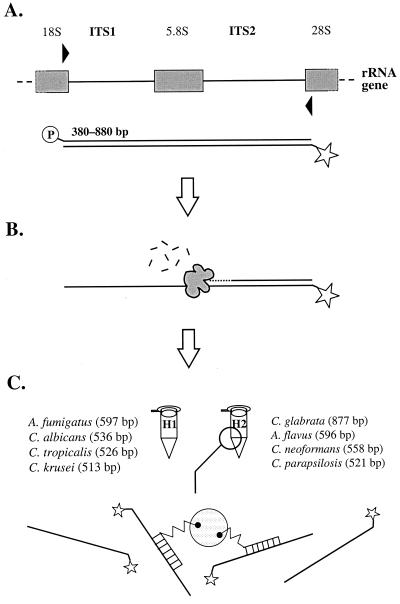
FIG. 1.
Principle of panfungal PCR and multiplex liquid hybridization. (A) The fungal ITS region was amplified with broad-range primers (31) that were labeled with 5′-phosphate and 5′-Cy5, respectively. Depending on the fungal species, PCR products of different sizes were amplified. (B) The amplification products were digested to ssDNA with λ-exonuclease. (C) The amplification products were hybridized simultaneously with four species-specific probes and captured on polystyrene beads for detection on an automated ALFexpress sequencing machine (12).
MATERIALS AND METHODS
Fungal isolates.
The fungal species used in this study were isolated from clinical specimens: Aspergillus fumigatus (five isolates), Aspergillus flavus (three isolates), Candida albicans (three isolates), Candida glabrata, Candida krusei (three isolates), Candida lusitaniae, Candida parapsilosis, Candida tropicalis, and Cryptococcus neoformans. The isolates were grown on Sabouraud's agar with or without penicillin and gentamicin and were identified by standard biochemical and morphological methods (7) and by sequencing of the ITS region (see below). A rat lung positive for Pneumocystis carinii was a gift from Antti Sukura (Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland).
For DNA extraction, a modified QIAamp (QIAGEN, Hilden, Germany) DNA extraction protocol was used. A small amount of culture was taken from the culture plates and boiled for 10 min in 100 μl of freshly prepared 25 mM NaOH–0.5% sodium dodecyl sulfate. After cooling and neutralization with 100 μl of 25 mM HCl, 200 μl of buffer AL (QIAamp kit) was added, and the suspension was boiled again for 10 min. Then, the QIAamp protocol was performed according to kit instructions. DNA from the rat lung was prepared as described for clinical specimens. The amount of DNA was quantitated spectrophotometrically (25), and the DNA was stored in aliquots at −20°C.
PCR.
The fungal ITS region, comprising ITS1, the 5.8S rRNA gene, and ITS2, was amplified with previously described universal primers ITS1 and ITS4 (31; Table 1). A 50-μl reaction mixture contained 1.32 μM ITS1 primer 5′-labeled with phosphate; 0.84 μM ITS4 primer 5′-labeled with Cy-5; 200 μM (each) dATP, dCTP, dGTP, and dTTP; and 1× GeneAmp PCR buffer II (10 mM Tris-HCl [pH 8.3 at 25°C], 50 mM KCl) supplied with 1.5 mM MgCl2 (The Perkin-Elmer Corp., Norwalk, Conn.), 0.5 mM betaine (betaine monohydrate; Sigma Chemical Co., St. Louis, Mo.), 2.5% dimethyl sulfoxide (Amersham Pharmacia Biotech, Uppsala, Sweden), and 2 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer). The reaction profile was as follows: 9 min of initial denaturation at 94°C, 38 cycles of 96°C for 1 min, 55°C for 45 s and 72°C for 1 min and a 10-min final extension at 72°C.
TABLE 1.
Primers and probes used in the fungal ITS PCR liquid hybridization protocol
| Procedure and target organism | Sequence (5′→3′) | Modificationa | Positionb (GenBank accession no.) | Product size (bp)c |
|---|---|---|---|---|
| ITS PCR primers | ||||
| Universal forward (ITS1d) | TCC GTA GGT GAA CCT GCG G | 5′-P | 1769–1787 (M27607) | 848 |
| Universal reverse (ITS4d) | CCT CCG CTT ATT GAT ATG C | 5′-Cy-5 | 820–838 (D89886) | |
| Hybridization probes | ||||
| A. flavus | CGA ACG CAA ATC AAT CTT TT | 5′-SB | 513–532 (AB008415) | 596 |
| A. fumigatus | GAC ACC CAA CTT TAT TTT TC | 5′-SB | 301–320 (U93683) | 597 |
| C. albicans | AGG TCT AAA CTT ACA ACC AA | 5′-SB | 118–137 (X71088) | 536 |
| C. glabrata | ACT CGA CAC TTT CTA ATT AC | 5′-B | 255–274 (L47108) | 877 |
| C. krusei | GTG GAA TAT AGC ATA TAG TC | 5′-SB | 85–104 (L47113) | 513 |
| C. parapsilosis | CGG AGT ATA AAC TAA TGG AT | 5′-B | 397–416 (L47109) | 521 |
| C. tropicalis | CTT ATT TTA AGC GAC TTA GG | 5′-B | 395–414 (L47112) | 526 |
| C. neoformans | GTG ATA ACA ACC ATC TCT TT | 5′-B | 377–396 (L14068) | 558 |
P, phosphate group (PO4); Cy-5, a fluorescent dye; B, biotin; SB, pentaethylene spacer biotin.
Position refers to the specific locations of the primers in the Saccharomyces cerevisiae 18S and ITS sequences and of the probes in the target organism ITS sequences, given in parentheses.
Specific PCR amplification with the ITS1 and ITS4 primers.
Sequences adapted from reference 31.
All reaction mixtures were prepared from prealiquoted reagents in a laminar-flow hood dedicated for PCR, using aerosol-resistant micropipette tips. Each PCR analysis of 20 to 30 samples included a positive control containing 0.5 ng of purified DNA of one of the fungal isolates and at least two blanks with reagents only.
Hybridization.
The oligonucleotide probes were designed using ITS region sequences obtained from the GenBank database as described previously for multiplex PCR primers (11). The probe sequences are listed in Table 1.
Prior to hybridization, the ITS PCR products were purified by using the QIAquick PCR product cleaning kit (QIAGEN) and digested to single-stranded DNA (ssDNA) with 6 to 10 U of λ-exonuclease (Amersham Pharmacia Biotech) in 1× λ-exonuclease buffer (67 mM glycine-KOH [pH 9.3 at 25°C], 2.5 mM MgCl2) at 37°C for 1 h. After inactivation of the nuclease at 96°C for 3.5 min, the hybridization mixture was prepared in a 0.5-ml Eppendorf tube on ice. The mixture comprised the ssDNA product, three or four biotinylated probes (1 pmol each) (Table 1) of 2× SSC (1× SSC is 0.15 M NaCl plus 0.15 M sodium citrate), and 1 M betaine (Sigma) in a volume of 50 μl.
The hybridization was initiated with a denaturation at 98°C for 2 min 30 s. After cooling to 50°C, 1.5 μl of fluoricon avidin-coated polystyrene beads (IDEXX, Westbrook, Maine) was added, and the incubation was continued with three probes and hybridization for 1 h and with four probes and hybridization for 15 min. The tubes were centrifuged at 11,000 × g for 52 s, and the supernatant was removed. The beads were washed in 50 μl of 2× SSC containing 0.5 or 1 M betaine at 50°C for 5 min. After pelleting (as described above) and removal of the washing solution, the beads were suspended in 5 μl of sterile water (Aqua Sterilisata; Amersham Pharmacia Biotech).
The hybridized products were visualized and identified by length on an automated ALFexpress sequencing machine (Amersham Pharmacia Biotech) as described previously for multiplex PCR products (12).
Clinical specimen preparation.
The study included a total of 20 tissue specimens (Table 2). Thirteen samples were from patients with suspected or proven deep-seated fungal infections (six women and seven men, ages 28 to 79 years [mean, 55 years]). The samples were obtained either by fine-needle aspiration or at autopsy, from liver, lung, brain, breastbone, or facial bone. These samples included specimens positive by culture and direct microscopy, specimens positive by direct microscopy only, and specimens negative by both microscopy and culture. Eight samples were preoperative polypoid tissue biopsies of different paranasal sinuses from patients with CRS (three women and four men, ages 32 to 67 years [mean, 51 years]). For culture, the specimens were inoculated on standard agar culture plates, and direct microscopy was performed with the Spot-Test Calcofluor White reagent (Difco Laboratories, Detroit, Mich.) (7). The remainder of the samples was stored frozen at −20°C before processing for PCR. The study protocol was approved by the Ethics Committee of the Helsinki University Central Hospital.
TABLE 2.
Summary of specimen data and results of culture, direct microscopy, ITS PCR-hybridization, and sequencing
| Assay result, no., and sourcea | Culture | Direct microscopyb | Hybridizationc | Signal intensity (% from input) | Sequencingd | Similarity with GenBank sequences |
|---|---|---|---|---|---|---|
| Culture positive | ||||||
| 1 Brain abscess | A. fumigatus | m+ | A. fumigatus | 23.0 | N.D. | |
| 2 CRS | A. fumigatus | N.D. | A. fumigatuse | 22.8 | A. fumigatus | 99.5% over 549 bp |
| 3 Lung biopsy | C. albicans | ym+, yc+++ | C. albicans | 54.1 | N.D. | |
| 4 Lung biopsy | A. fumigatus | mm+ | A. fumigatus | 27.4 | N.D. | |
| 5 Lung | A. fumigatus | m+++ | C. albicans | 34.1 | P. carinii | 99.2% over 479 bp |
| 6 Lung | A. fumigatus | m+ | A. fumigatus | 16.9 | A. fumigatus | 99.5% over 553 bp |
| 7 Liver biopsy | A. fumigatus | m+ | A. fumigatus | 24.3 | N.D. | |
| Direct-microscopy positive | ||||||
| 8 CRS | Bacterial overgrowth | mm+ | A. fumigatus | 8.4 | A. fumigatus | 98.8% over 591 bp |
| 9 Liver biopsy | − | ym++, yc+ | C. albicans | 36.7 | N.D. | |
| 10 Liver | − | yc+ | C. albicans | 28.6 | C. albicans | 99.8% over 493 bp |
| 11 Liver | Bacterial overgrowth | ym+++, yc+++ | − | C. stellatoidea | 100% over 483 bp | |
| 12 Liver biopsy | − | ym+, yc+ | − | 511 bp | B.T.f | |
| 13 Bone | Bacterial overgrowth | mm+ | − | B. graminise | 99.6% over 531 bp | |
| 396 bp | B.T. | |||||
| 591 bp | B.T. | |||||
| Negative | ||||||
| 14 CRS | − | − | − | E. nigrum | 100% over 493 bp | |
| 15 CRS | − | − | − | B. adusta | 99.6% over 561 bp | |
| 16 CRS | − | − | − | 462 bp | B.T. | |
| 17 CRS | − | − | − | Penicillium spp. | 99.8% over 552 bp | |
| 492 bp | B.T. | |||||
| 18 CRS | − | − | − | E. nigrum | 99.6% over 503 bp | |
| 622 bp | B.T. | |||||
| 19 Bone marrow | − | − | − | 337 bp | B.T. | |
| 493 bp | B.T. | |||||
| 501 bp | B.T. | |||||
| 20 CRS | − | − | − |
CRS, polypous tissue from the paranasal sinuses.
Performed with Spot-Test Calcofluor White reagent (Difco Laboratories); −, negative; m, mycelium; mm, mold mycelium; ym, yeast mycelium; yc, yeast cells; +, a few elements; ++, clearly visible structures; +++, crowded samples; N.D., not done.
Hybridization was performed in triplicate with various three-probe combinations or with the following four-probe combinations: A. flavus/C. glabrata/C. parapsilosis/C. neoformans and A. fumigatus/C. albicans/C. krusei/C. tropicalis.
N.D., not done.
The same species was also identified in the sinus secretion obtained by puncture.
B.T., below threshold; similarity to known sequences was less than 98% over the range of 75% of the ITS region.
DNA was extracted with the QIAamp tissue kit (QIAGEN) in a laminar-flow hood. After lysis of 5 to 25 mg of tissue with proteinase K, an incubation step with 25 mM NaOH–0.5% sodium dodecyl sulfate (final concentration) at 95°C for 10 min was added to the protocol, followed by neutralization with HCl. The DNA was eluted from the columns with 225 μl of Aqua Sterilisata (Amersham Pharmacia Biotech), and it was stored in aliquots at −20°C. Twenty microliters of the DNA suspension was used for PCR. Each sample was analyzed, including by hybridization, at least two times on different occasions.
Sequencing of amplification products.
The amplification products from pure fungal cultures and from tissue specimens that yielded one product were purified with the QIAquick protocol (QIAGEN) and sequenced directly with a cycle sequence protocol (20), using primer ITS4 (Table 1). From specimens that yielded two amplification products, the products were separated in agarose gel electrophoresis, purified using standard techniques (25), and reamplified for sequencing. The amplification products from specimens that yielded more than two products were cloned into a plasmid vector using the TOPO cloning kit (Invitrogen, Leek, The Netherlands) and were propagated in Escherichia coli. From an overnight culture, the insert was amplified with primers ITS1 and ITS4 and was processed for sequencing as described above. Sequence similarities were assessed with a search for homology to GenBank sequences with the BLAST search program (1). Sequence similarities higher than 98% over a range of at least 75% of the ITS region were considered significant.
RESULTS
Sensitivity.
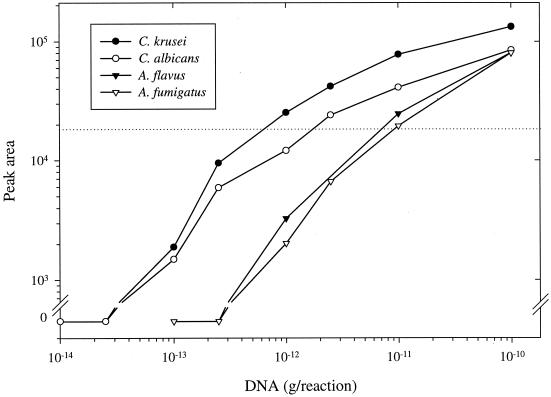
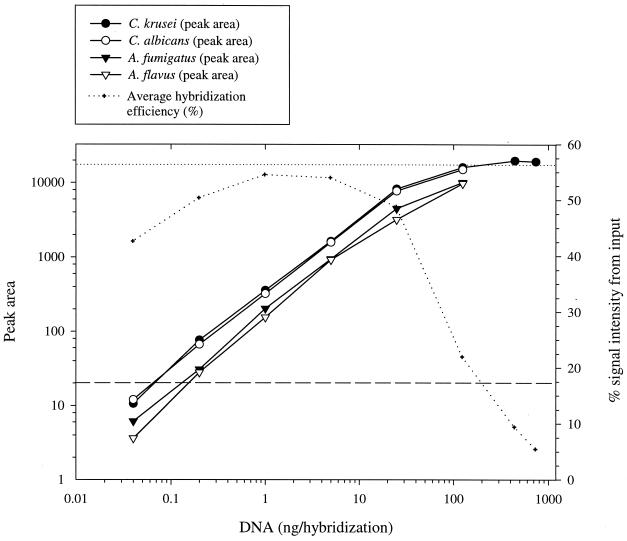
The sensitivity of the ITS PCR was studied with a dilution series of DNA purified from A. flavus, A. fumigatus, C. albicans, and C. krusei. The ability of the PCR to amplify the ITS region from DNA is shown in Figure 2. The detection limit was 100 fg of DNA per reaction for the Candida species and 1 pg for the Aspergillus species. The sensitivity of hybridization was assessed with dilution series of the amplification products, using three probes and hybridization. The amounts of hybridized products are indicated in Figure 3. For all four species, the lowest detected amount of product was 0.2 ng.
FIG. 2.
Sensitivity of the universal ITS PCR. The indicated amount of purified DNA was amplified in the PCR, and the amount of amplification product (peak area) was measured on the ALFexpress sequencer. Each datum point represents the mean of two samples from two replicate experiments and corresponds to 5 μl of a completed reaction mixture. The dotted line is the cutoff value of the linear range of the ALFexpress detector.
FIG. 3.
Hybridization sensitivity and efficiency. The indicated amount of single-stranded ITS PCR amplification product was hybridized in a mixture of three probes. The amount of hybridized product (peak area) was measured on the ALFexpress sequencer, and an average hybridization efficiency (%) was calculated for the four species. Each datum point represents the mean of two replicate experiments. The dashed line is the smallest peak size that can be unequivocally identified, and the dotted line is the cutoff value of the linear range of the ALFexpress detector.
Considering that a volume of up to 25 μl could be used for hybridization, whereas only 5 μl could be run directly in the sequencer, any amount of product that was amplified by PCR could be identified by hybridization. However, without the λ-exonuclease treatment of the amplification product, no hybridization signal was detected (data not shown).
Specificity.
The specificity testing consisted of cross-hybridization experiments with λ-exonuclease-treated amplification products of the eight target species (Table 1), C. lusitaniae (ITS amplification product length, 387 bp) and P. carinii (592 bp). A wider range of fungal species was not evaluated, since falsely hybridized amplification products could be identified by length. Different three- and four-probe combinations were hybridized with various amounts (10 to 100 ng) of an amplification product. When 0.5 M betaine was used in the washing step, the hybridization efficiency (percent hybridization peak area from input peak area) of nonspecific hybridization was on average 0.3% (range, 0 to 1.23%). However, when the concentration of betaine was increased to 1 M, the average hybridization efficiency was reduced to 0.08% (range, 0 to 0.25%). In comparison, the hybridization efficiency of specific hybridizations was 22.0 to 54.3% (Figure 3).
Cross-hybridization experiments were also performed for the multiplex hybridization setting with two or three products simultaneously and for a single probe with various amounts of one or several products, although not systematically. The proportion of hybridized product did not exceed those values obtained in the multiplex system with a single product. This indicated that cooperation was absent in the multiplex system; i.e., the presence of several probes or more than one product in the hybridization mixture did not favor partial hybrids, which would increase the amount of nonspecific hybridization.
Detection of fungal DNA in tissue specimens.
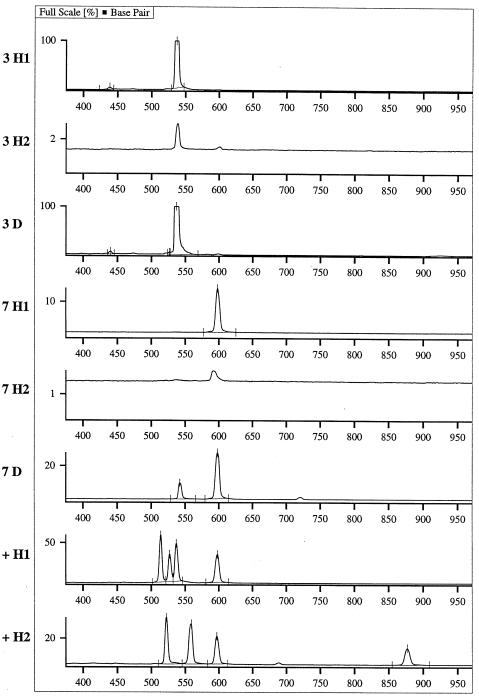
The applicability of the ITS PCR multiplex hybridization protocol was studied with 20 tissue samples from patients with suspected or proven fungal infection. An analytical electropherogram is shown in Figure 4, and Table 2 summarizes the data for all patients. Six (86%) of seven culture-positive specimens were hybridization positive for the indicated organism. One specimen (Table 2, no. 5) was A. fumigatus culture positive, but it tested positive for C. albicans by hybridization. However, this specimen was also Pneumocystis carinii positive by immunofluorescent microscopy examination (data not shown) and by sequencing of the ITS amplification product obtained from the specimen. Hybridization also identified the infecting agent in three of six specimens (50%) that were positive by direct microscopy only.
FIG. 4.
Example of an analytical ALFexpress fragment manager (version 1.2; Amersham Pharmacia Biotech) electropherogram. The specimen number (same as in Table 2) is given beside each lane. The suffix denotes product detection directly after PCR (D) and after hybridization (H1, probe set A. fumigatus, C. albicans, C. krusei, and C. tropicalis; H2, probe set A. flavus, C. glabrata, C. parapsilosis, and C. neoformans). +, positive control. The electropherogram is scaled in base pairs. The ITS PCR product sizes are given in Table 1. Specimen 3, C. albicans, 536 bp; specimen 7, A. fumigatus, 597 bp.
Of the 10 remaining culture- and direct-microscopy-negative specimens, 9 (90%) tested positive in the ITS PCR. However, the amplification products were negative by hybridization; i.e., the hybridization efficiency was less than 0.25%. Specimen 20 was negative by all methods. The amplification products were sequenced to identify the detected species. Direct sequencing was possible for five (56%) of these specimens, whereas four (44%) specimens yielded more than one amplification product, which necessitated cloning prior to sequencing. In addition, four of the hybridization-positive amplification products were sequenced to verify the detection of the target organism. The sequence similarities to known sequences in the GenBank database are also shown in Table 2.
DISCUSSION
Our aim was to develop a molecular diagnostic method for the detection of fungi in tissue specimens. We selected the PCR technique, since it is sensitive and able to detect pathogens that are nonviable or dormant because of antifungal therapy (6). Because various infectious species have been implicated in fungal infections, the ITS region is a good target choice. The length of this region varies among different fungal species (28), and it contains conserved as well as variable domains, which can be exploited for family- or group-specific hybridizations. Moreover, fungi contain a high copy number of the rRNA region (6, 8, 27, 31), providing further amplification of the signal.
We used broad-range fungal primers that amplify the ITS region from most fungal species but not from other eucaryotic or procaryotic organisms (31). The ITS PCR detected 100 fg and 1 pg (2 to 20 CFU) of Candida and Aspergillus DNA, respectively. After hybridization, the sensitivity was preserved. The difference in the sensitivities can be explained by the different GC contents, i.e., 44.8 to 50.6% for Candida spp. and 57.3 to 59.5% for Aspergillus spp. The sensitivity could not be improved by changes in the concentration of secondary-structure-resolving agents (betaine or dimethyl sulfoxide) (data not shown). However, the λ-exonuclease treatment was imperative for gaining any signal in the hybridization. The production of single-stranded DNA by λ-exonuclease has previously been shown to increase the enzyme immunoassay hybridization signal (14), but no influence was observed on a fungal ITS2 PCR-ELISA system (8). It may be concluded that the net effect of the λ-exonuclease treatment is dependent on the amplification product length and specific sequence. The detection level was consistent with those previously reported for other single-round PCR amplifications of fungal rRNA sequences with product detection on agarose gels (6, 17, 27, 32).
The identification of the ITS amplification products relied both on specific hybridization and on assessment of product length. For this reason, extensive specificity testing was not considered necessary. In a study of allergenic fungi, the sequence similarity of the ITS region is less than 84% within a genus and less than 50% between genera (10), whereas intraspecies variation is low or absent (16). Turenne et al. (28) reported that only two pairs of 56 fungal species belonging to 31 genera carry ITS2 regions that are identical in size. In the present study, the cross-hybridization experiments between six members of the Candida family, two Aspergillus species, C. neoformans, and P. carinii showed no false hybridizations in the multiplex system. Nor did the ITS PCR products amplified in the patient samples yield any nonspecific hybridization. The effect of betaine on specificity can be explained by reduction in the formation of partial probe-to-product and product-to-product hybrids caused by GC-rich sequences (13). On the basis of these data, the protocol was considered specific. In other studies to establish specificity, comprehensive cross-hybridization (6, 8, 15, 18, 26) and PCR specificity testing (22, 27, 28, 32) have been required. Our findings suggest that, with proper oligonucleotide design (11), additional fungal species can be incorporated in the multiplex hybridization protocol in a straightforward manner.
In the multiplex liquid hybridization approach, only two simple hybridization mixtures were needed for eight fungal species, and the hybridization was completed in 30 min. The automated amplification product detection on the ALFexpress sequencing machine (12) reduced the operator time considerably compared with previously reported membrane hybridization (6, 15, 23, 26) and ELISA (8) protocols. These techniques necessitate dividing the PCR product among each probe or performing the hybridizations sequentially. Nested-PCR protocols (3, 22, 32) include an additional PCR step. Identification based solely on amplification product length would have been simple and rapid (28), but it would have contained only one criterion for differentiation of species. Our method did not involve radioactivity, and it allowed rapid screening of common fungal pathogens as well as recognition of PCR products that require sequencing for identification.
The ITS PCR amplified fungal DNA in 19 (95%) of 20 tissue samples. To our knowledge, this was the first time that all diagnostic PCR products were identified. In previous studies detecting more than one species or a fungal family, either a panfungal detection probe was used (17, 29), or only certain species were identified by specific hybridization (6, 8, 26). The results demonstrate the superior sensitivity of the ITS PCR when compared with culture and microscopic methods. Importantly, the identification of fungi was not hindered by the presence of bacteria in the specimens. The culture and hybridization results were concordant for six (86%) of seven specimens. This finding is consistent with the results of comparative analysis of tissue specimens with culture methods and PCR, where rRNA gene PCR yielded a sensitivity of 96% for A. fumigatus alone (27) and 80 to 100% for various other fungi (26). The amplification of C. albicans ITS PCR products in a specimen that is positive for A. fumigatus (culture) and P. carinii (immunofluorescent microscopy and sequencing) may indicate that the variation in ITS PCR sensitivity shields certain infecting agents in mixed infections. The extent to which this variation interferes with the detection of some fungi requires further study.
PCR detected fungi in seven (88%) of eight polyposis tissue samples. Considering the body site from which these samples were derived, caution must be pursued in interpreting the findings. Ponikau et al. (21) isolated on average 2.7 fungal species belonging to the common environmental genera in the nasal lavage fluid of both healthy volunteers and CRS patients. Transient contamination of the samples was therefore highly probable. However, the specimens were from CRS patients with long-lasting disease that is resistant to multiple treatments, including surgery. A. fumigatus and Penicillium spp. typically cause fungal sinusitis (2, 4). Two patients tested positive for Epicoccum nigrum. This organism has recently been recognized as a causative agent of allergic fungal sinusitis (19). Both patients had severe polyposis with eosinophils infiltrating the edematous submucosa and peripheral blood eosinophilia, but they were negative for type I (immunoglobulin E-mediated) hypersensitivity (data not shown). Although the presence of a fungus could not be verified by other methods, the results suggest that the possibility that these patients had allergic fungal sinusitis caused by E. nigrum warrants further investigation.
In contrast, the finding of a basidiomycete, Bjerkandera adusta, in one CRS patient and Blumeria graminis, a powdery mildew, in a patient with a destructive facial-bone infection suggests exogenous contamination of the specimens. Neither fungus has been implicated in human disease, and the current knowledge of B. graminis does not support human pathogenicity. Furthermore, fungal ITS sequences that are not found in the GenBank database were amplified in three CRS polypous tissue samples. Whether these fungi are incidental environmental organisms residing on the specimen surface or innocent bystanders of a pathological condition must be the subject of future research. Further PCR studies should be conducted in a follow-up manner with quantitative PCR and analysis of the polypous tissue and nasal secretion simultaneously to elucidate the clinical status of the PCR-detected fungi. Certainly, an involvement in the etiology of sinus disease must be verified by positive direct microscopy and histopathological findings, although the sensitivity of these techniques is poor.
In summary, the ITS PCR hybridization protocol described here provides a sensitive and specific means for the identification of fungi in tissue specimens. The analysis is simple to perform, and it provides results that are easy to interpret. The results are available in 2 working days. Moreover, nonhybridized products may be used for identification by sequencing. The objective of our further study is to include probes for less commonly encountered fungal species and to adapt the protocol for other specimen types such as blood and bronchoalveolar lavage fluid.
ACKNOWLEDGMENTS
We thank Leena Palmunen, Paula Collin-Olkkonen, and Anne Makkonen for expert technical assistance.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blitzer A, Lawson W. Fungal infections of the nose and paranasal sinuses. Otolaryngol Clin N Am. 1993;26:1007–1035. [PubMed] [Google Scholar]
- 3.Chryssanthou E, Andersson B, Petrini B, Löfdahl S, Tollemar J. Detection of Candida albicans DNA in serum by polymerase chain reaction. Scand J Infect Dis. 1994;26:479–485. doi: 10.3109/00365549409008623. [DOI] [PubMed] [Google Scholar]
- 4.DeShazo R D, Chapin K, Swain R E. Fungal sinusitis. N Engl J Med. 1997;337:254–259. doi: 10.1056/NEJM199707243370407. [DOI] [PubMed] [Google Scholar]
- 5.Duthie R, Denning D W. Aspergillus fungemia: report of two cases and review. Clin Infect Dis. 1995;20:598–605. doi: 10.1093/clinids/20.3.598. [DOI] [PubMed] [Google Scholar]
- 6.Einsele H, Hebart H, Roller G, Löffler J, Rothenhöfer I, Müller C A, Bowden R A, van Burik J-A, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans E G V, Richardson M D, editors. Medical mycology: a practical approach. Oxford, United Kingdom: Oxford University Press; 1989. [Google Scholar]
- 8.Fujita S, Lasker B A, Lott T J, Reiss E, Morrison C J. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J Clin Microbiol. 1995;33:962–967. doi: 10.1128/jcm.33.4.962-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamis A, Gudnason T, Giebink G, Ramsay N. Disseminated infection with Fusarium in recipients of bone marrow transplants. Rev Infect Dis. 1991;13:1077–1088. doi: 10.1093/clinids/13.6.1077. [DOI] [PubMed] [Google Scholar]
- 10.Gaskell G J, Carter D A, Britton W J, Tovey E R, Benyon F H L, Løvborg U. Analysis of internal transcribed spacer regions of ribosomal DNA in common airborne allergenic fungi. Electrophoresis. 1997;18:1567–1569. doi: 10.1002/elps.1150180914. [DOI] [PubMed] [Google Scholar]
- 11.Hendolin P H, Markkanen A, Ylikoski J, Wahlfors J J. Use of multiplex PCR for simultaneous detection of four bacterial species in middle ear effusions. J Clin Microbiol. 1997;35:2854–2858. doi: 10.1128/jcm.35.11.2854-2858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendolin P H, Paulin L, Ylikoski J. Clinically applicable multiplex PCR for four middle ear pathogens. J Clin Microbiol. 2000;38:125–132. doi: 10.1128/jcm.38.1.125-132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henke W, Herdel K, Jung K, Schnorr D, Loening S A. Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res. 1997;25:3957–3958. doi: 10.1093/nar/25.19.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway B, Erdman D D, Durigon E L, Murtagh J J., Jr An exonuclease-amplified coupled capture technique improved detection of PCR product. Nucleic Acids Res. 1993;21:3905–3906. doi: 10.1093/nar/21.16.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan J A. PCR identification of four medically important Candida species by using a single primer pair. J Clin Microbiol. 1994;32:2962–2967. doi: 10.1128/jcm.32.12.2962-2967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S B, Taylor J W. Phylogeny of five fungus-like protoctistan Phytophthora species, inferred from the internal transcribed spacers of ribosomal DNA. Mol Biol Evol. 1992;9:636–653. doi: 10.1093/oxfordjournals.molbev.a040750. [DOI] [PubMed] [Google Scholar]
- 17.Makimura K, Murayama S Y, Yamaguchi H. Detection of a wide range of medically important fungi by the polymerase chain reaction. J Med Microbiol. 1994;40:358–364. doi: 10.1099/00222615-40-5-358. [DOI] [PubMed] [Google Scholar]
- 18.Morace G, Sanguinetti M, Posterado B, Cascio G L, Fadda G. Identification of various medically important Candida species in clinical specimens by PCR-restriction enzyme analysis. J Clin Microbiol. 1997;35:667–672. doi: 10.1128/jcm.35.3.667-672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble J A, Crow S A, Ahearn D G, Kuhn F A. Allergic fungal sinusitis in the southeastern USA: involvement of a new agent Epicoccum nigrum Ehrenb ex. Schlect. 1824. J Med Vet Mycol. 1997;35:405–409. [PubMed] [Google Scholar]
- 20.Perkin-Elmer Corp., Applied Biosystems Division. ABI Prism: comparative PCR sequencing. Foster City, Calif: Perkin-Elmer Corp.; 1997. [Google Scholar]
- 21.Ponikau J U, Sherris D A, Kern E B, Homburger H A, Frigas E, Gaffey T A, Roberts G D. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 22.Rand K H, Houck H, Wolff M. Detection of candidemia by polymerase chain reaction. Mol Cell Probes. 1994;8:215–222. doi: 10.1006/mcpr.1994.1030. [DOI] [PubMed] [Google Scholar]
- 23.Reddy L V, Kumar A, Kurup V P. Specific amplification of Aspergillus fumigatus DNA by polymerase chain reaction. Mol Cell Probes. 1993;7:121–126. doi: 10.1006/mcpr.1993.1016. [DOI] [PubMed] [Google Scholar]
- 24.Richardson M D, Kokki M H. New perspectives in the diagnosis of systemic fungal infection. Ann Med. 1999;31:327–335. doi: 10.3109/07853899908995899. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Sandhu G S, Kline B C, Stockman L, Roberts G D. Molecular probes for diagnosis of fungal infections. J Clin Microbiol. 1995;33:2913–2919. doi: 10.1128/jcm.33.11.2913-2919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spreadbury C, Holden D, Aufauvre-Brown A, Bainbridge B, Cohen J. Detection of Aspergillus fumigatus by polymerase chain reaction. J Clin Microbiol. 1993;31:615–621. doi: 10.1128/jcm.31.3.615-621.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turenne C Y, Sanche S E, Hoban D J, Karlowsky J A, Kabani A M. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J Clin Microbiol. 1999;37:1846–1851. doi: 10.1128/jcm.37.6.1846-1851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Burik J-A, Myerson D, Schreckhise R W, Bowden R A. Panfungal PCR assay for detection of fungal infection in human blood specimens. J Clin Microbiol. 1998;36:1169–1175. doi: 10.1128/jcm.36.5.1169-1175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent J L, Anaisiie E, Bruining H, Demajo W, El-Ebiary M, Haber J, Hiramatsu Y, Nitenberg G, Nystrom P O, Pittet D, Rogers T, Sandven P, Sganga G, Shaller M D, Solomkin J. Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med. 1998;24:206–216. doi: 10.1007/s001340050552. [DOI] [PubMed] [Google Scholar]
- 31.White T J, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M A, Gelfand D H, Sninsky J, White T J, editors. PCR protocols, a guide to methods and applications. New York, N.Y: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 32.Yamakami Y, Hashimoto A, Tokimatsu I, Nasu M. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J Clin Microbiol. 1996;34:2464–2468. doi: 10.1128/jcm.34.10.2464-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]