Abstract
Purpose
Certain patient subgroups who do not respond to induction chemotherapy (IC) show inherent chemoresistance in locally advanced head and neck squamous cell carcinoma (LA-HNSCC). This study aimed to assess the prognostic value of IC, and role of IC in guiding the selection of a definitive locoregional therapy.
Materials and Methods
Out of the 445 patients in multi-institutional LA-HNSCC cohort, 158 (36%) receiving IC were enrolled. The study outcome was to assess overall survival (OS) through IC responsiveness and its role to select subsequent treatments.
Results
Among 135 patients who completed subsequent treatment following IC, 74% responded to IC (complete response in 17% and partial response in 58%). IC-non-responders showed 4.5 times higher risk of mortality than IC-responders (hazard ratio, 4.52; 95% confidence interval, 2.32 to 8.81; p < 0.001). Among IC-responders, 84% subsequently received definitive concurrent chemoradiotherapy (CCRT) and OS was not differed by surgery or CCRT (p=0.960). Regarding IC-non-responders, 54% received CCRT and 46% underwent surgery, and OS was poor in CCRT (24-month survival rate of 38%) or surgery (24-month survival rate of 63%).
Conclusion
Response to IC is a favorable prognostic factor. For IC-responders, either surgery or CCRT achieved similar survival probabilities. For IC-non-responder, multidisciplinary approach was warranted reflecting patients’ preference, morbidity, and prognosis.
Keywords: Locally advanced head and neck squamous cell carcinoma, Induction chemotherapy, Subsequent treatment
Introduction
Locally advanced head and neck squamous cell carcinoma (LA-HNSCC) is a representative tumor with which the greatest treatment outcomes can be achieved through a multi-modal treatment approach [1,2]. Locoregional control has been improved with a tailored surgical technique and concurrent chemoradiotherapy (CCRT) [3,4]. As locoregional control improves, distant metastasis is increasingly being acknowledged as one of the main causes of treatment failure, which suggests that additional systemic chemotherapy aimed at improving distant control might now be important for overall treatment success [5].
However, clinical evidence that systemic chemotherapy delivers survival benefits by suppressing distant metastasis is insufficient [5,6]. Despite decades of investigation, the value of induction chemotherapy (IC) before radiotherapy remains unclear except for the purpose of organ preservation in patients requiring total laryngectomy [7–10].
While no apparent conclusions can be drawn about whether IC before radiotherapy is superior to CCRT in LA-HNSCC, certain patient subgroups who did not respond to IC and showed inherent chemoresistance could serve as guides in the selection of a definitive locoregional therapy [11]. In other words, poor responders to IC are more likely to not respond well to definitive CCRT, and therefore definitive surgery may be a more suitable treatment option. For example, a favorable response to IC indicated a better survival benefit from definitive CCRT, compared to definitive surgery, in the treatment of sinonasal undifferentiated carcinoma of the paranasal sinuses and nasal cavity [12]. In contrast, definitive surgery provided a better chance of disease control and improved survival for patients who did not respond to IC.
From this point of view, we tried to identify the patient populations who stand to gain the most benefit from IC. The purpose of this study was (1) to identify the prognostic value of IC response, and (2) to assess the role of IC for guiding subsequent treatment for LA-HNSCC.
Materials and Methods
1. Study population
This is a post-hoc analytic study of a previously reported large, nationwide cohort of 445 patients with LA-HNSCC between January 2005 and December 2015 at 13 referral hospitals in Korea. All participating hospitals had active multidisciplinary teams available, consisting of a head and neck surgeon, radiation oncologists, medical oncologists, radiologists, and pathologists.
The definition of LA-HNSCC was clinical stage III to IVB according to the American Joint Committee on Cancer Staging 7th edition. The study population included 445 patients ≥ 20 years old with primary squamous cell carcinoma of the oropharynx, oral cavity, hypopharynx, larynx, or nasal cavity, who received IC. IC refers to a type of chemotherapy that facilitates a following definitive treatment including CCRT or surgery. The study patients were divided into a responding (complete response [CR] or partial response [PR]) or non-responding (stable disease [SD] or progressive disease [PD]) group according to their response to IC, in order to compare characteristics and study outcomes. Human papillomavirus (HPV) positivity based on the results from either HPV DNA by real-time PCR or p16 expression by immunohistochemistry.
2. Study outcomes and statistical analysis
The primary study outcome was overall survival (OS) through response to IC in real-world practice for an LA-HNSCC population. OS was defined as the time between the date of HNSCC diagnosis and death from any cause. The secondary outcome was to describe treatment schemes based on IC responsiveness and assess the role of IC in the selection of subsequent treatment for LA-HNSCC. The treatment groups were stratified based on their response to IC and locoregional treatments (i.e., responders to IC treated with definitive CCRT, responders to IC treated with surgery followed by postoperative radiotherapy or CCRT, non-responders to IC treated with definitive CCRT, and non-responders to IC treated with surgery followed by postoperative radiotherapy or CCRT).
Chi-square and independent t test were used to compare the demographics between groups. Survival was assessed using univariate Kaplan-Meier survival analysis based on the log-rank test. Univariate and multivariate Cox regression analysis was performed under proportional hazards assumption to identify significant predictors of outcome using backward elimination until all remaining predictors were significant at the 0.05 level. A two-sided p-value < 0.05 indicates statistical significance. All statistical analyses were performed using the Stata ver. 16.1 software package (Stata Corp LP, College Station, TX).
Results
1. Patient characteristics
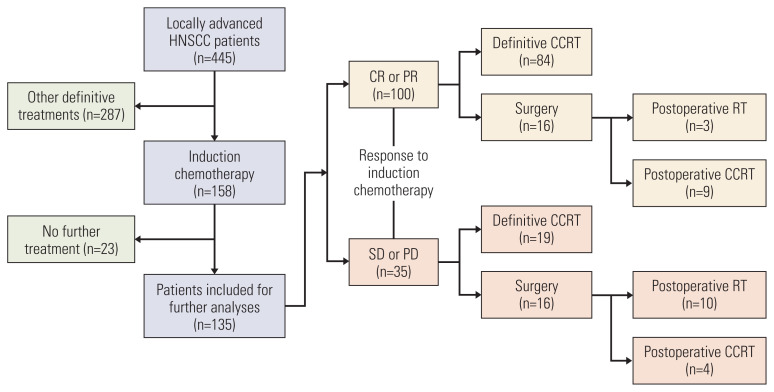
Out of the 445 patients in the LA-HNSCC cohort, 158 (35.5%) received IC and the remaining ones (64.5%) received upfront definitive treatments. Of the 158 patients receiving IC, 135 (85.4%) received further definitive treatment after completion of IC while 23 patients were excluded because they did not receive additional treatment. A total of 135 patients who completed subsequent treatments were included for further analyses (Fig. 1).
Fig. 1.
Flowchart of study population and treatment schemes according to treatment response depicting response to therapy and treatment disposition. CCRT, concurrent chemoradiotherapy; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; HNSCC, head and neck squamous cell carcinoma; IC, induction chemotherapy; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease.
The median age of the study population was 60 years (range, 27 to 76 years). Approximately 86% of the study patients were male. Patients in the IC-non-responder group had the worse performance status compared with the IC-responder group (p=0.004). History of smoking and alcohol use was not different between IC-responders and IC-non-responders. Primary tumor location was the oropharynx in 53%, followed by hypopharynx (19.3%), oral cavity (12.6%), larynx (8.2%), and other sites (7.4%). Others comprised maxillary sinus, nasal cavity, ethmoid sinus and squamous cell carcinoma of unknown primary in the head and neck. The responding group showed a higher proportion in the oropharynx and hypopharynx, and a lower proportion in the oral cavity, which was not statistically significant (p=0.091). Of all patients, 47 (34.8%) were classified as having T4 disease, and 28 (20.7%), 45 (33.3%), 13 (9.6%) as having T3, T2, and T1 disease, respectively. Nodal metastasis was present in 121 (90%) patients. T category was more advanced in the IC-non-responders compared to the IC-responders (p=0.003), whereas N category was similar between both groups (p=0.282) in terms of tumor clinical stage. Study patient characteristics are summarized according to response to IC in Table 1. About 66.7% (90) of study patients was unknown for HPV infection. Of 45 patients who were tested for HPV status, 20.0% (27/45) were positive.
Table 1.
Baseline characteristics of locally advanced head and neck squamous cell carcinoma
| Characteristic | Total (n=135) | Response to IC | p-value | |
|---|---|---|---|---|
| Responding group (n=100, 74%) | Non-responding group (n=35, 26%) | |||
| Age (yr) | 60 (27–76) | 62 (27–75) | 56 (33–76) | 0.341 |
| Sex | ||||
| Female | 19 (14.1) | 13 (13.0) | 6 (17.1) | 0.544 |
| Male | 116 (85.9) | 87 (87.0) | 29 (82.9) | |
| ECOG PS | ||||
| 0 | 29 (21.5) | 21 (21.0) | 8 (22.9) | 0.004 |
| 1 | 90 (66.7) | 73 (73.0) | 17 (48.6) | |
| 2 | 6 (4.4) | 2 (2.0) | 4 (11.4) | |
| Unknown | 10 (7.4) | 4 (4.0) | 6 (17.1) | |
| Smoking history | ||||
| Never | 24 (17.8) | 19 (19.0) | 5 (14.3) | 0.160 |
| Former | 27 (20.0) | 23 (23.0) | 4 (11.4) | |
| Current | 24 (17.8) | 14 (14.0) | 10 (28.6) | |
| Unknown | 60 (44.4) | 44 (44.0) | 16 (45.7) | |
| History of using alcohol | ||||
| Do not drink | 24 (17.8) | 22 (22.0) | 2 (5.7) | 0.095 |
| Drink alcohol | 31 (23.0) | 22 (22.0) | 9 (25.7) | |
| Unknown | 80 (59.3) | 56 (56.0) | 24 (68.6) | |
| Primary tumor location | ||||
| Oropharynx | 71 (52.6) | 56 (56.0) | 15 (42.9) | 0.091 |
| Oral cavity | 17 (12.6) | 8 (8.0) | 9 (25.7) | |
| Hypopharynx | 26 (19.3) | 21 (21.0) | 5 (14.3) | |
| Larynx | 11 (8.2) | 8 (8.0) | 3 (8.6) | |
| Others | 10 (7.4) | 7 (7.0) | 3 (8.6) | |
| Histologic grade | ||||
| Well differentiated | 19 (14.1) | 11 (11.0) | 8 (22.9) | 0.142 |
| Moderate differentiated | 38 (28.2) | 30 (30.0) | 8 (22.9) | |
| Poorly differentiated | 21 (15.6) | 19 (19.0) | 2 (5.7) | |
| Not assessed | 57 (42.2) | 40 (40.0) | 17 (48.6) | |
| T classification | ||||
| T1 | 13 (9.6) | 12 (12.0) | 1 (2.9) | 0.003 |
| T2 | 45 (33.3) | 39 (39.0) | 6 (17.1) | |
| T3 | 28 (20.7) | 22 (22.0) | 6 (17.1) | |
| T4a/T4b | 36/11 (34.8) | 19/6 (25.0) | 17/5 (62.9) | |
| Unknown | 2 (1.5) | 2 (2.0) | 0( | |
| N classification | ||||
| N0 | 14 (10.4) | 8 (8.0) | 6 (17.1) | 0.282 |
| N1 | 19 (14.1) | 13 (13.0) | 6 (17.1) | |
| N2 | 100 (74.1) | 78 (78.0) | 22 (62.9) | |
| N3 | 2 (1.5) | 1 (1.0) | 1 (2.9) | |
| p16/HPV status | ||||
| Negative | 18 (13.3) | 12 (12.0) | 6 (17.1) | 0.047 |
| Positive | 27 (20.0) | 25 (25.0) | 2 (5.7) | |
| Unknown | 90 (66.7) | 63 (63.0) | 27 (77.1) | |
Values are presented as median (range) or number (%). HPV, human papillomavirus; PS, performance status.
2. Induction chemotherapy
The 135 patients included for further analyses received IC with a median of 3 cycles (range, 1 to 5). The mean interval between the date of HNSCC diagnosis and initiation of IC was 13 days (range, 5 to 64 days). Regarding overall response, 74.1% responded to IC (CR in 16.3% and PR in 57.8%) and 25.9% did not respond (SD in 17.8% and PD in 8.2%). In terms of IC regimen, docetaxel and cisplatin was the most preferred (48.9%) regimen, and the triplet regimen combining docetaxel, cisplatin and fluorouracil was the next (28.2%). The choice of chemotherapy was not significantly different between the IC-responders and IC-non-responders (p=0.291) (Table 2). In the absence of unified protocol for IC for each institution, data on the administered dose of chemotherapy could not be retrieved and analyzed.
Table 2.
Characteristics of treatment modalities in patients with LA-HNSCC
| Treatment | Total | Response to induction chemotherapy | p-value | |
|---|---|---|---|---|
| Responding group | Non-responding group | |||
| Induction chemotherapy | 135 (100) | 100 (74.1) | 35 (25.9) | |
| Regimen | ||||
| Docetaxel+cisplatin | 66 (48.9) | 45 (45.0) | 21 (60.0) | 0.291 |
| Docetaxel+cisplatin+fluorouracil | 38 (28.2) | 32 (32.0) | 6 (17.1) | |
| Fluorouracil+cisplatin | 20 (14.8) | 14 (14.0) | 6 (17.1) | |
| Others | 11 (8.2) | 9 (9.0) | 2 (5.7) | |
| No. of cycles | ||||
| Median (range) | 3 (1–5) | 3 (1–4) | 3 (1–5) | 0.188 |
| Best overall response | ||||
| Complete response | 22 (16.3) | 22 (22.0) | 0( | < 0.001 |
| Partial response | 78 (57.8) | 78 (78.0) | 0( | |
| Stable disease | 24 (17.8) | 0( | 24 (68.6) | |
| Progressive disease | 11 (8.2) | 0( | 11 (31.4) | |
Values are presented as number (%). LA-HNSCC, locally advanced head and neck squamous cell carcinoma.
3. Study outcomes
A total of 103 out of the 135 patients (76%) were treated with definitive CCRT, and 32 (24%) underwent surgery after completion of IC. Among the 32 patients receiving surgery, 81% (26 out of 32) were treated with subsequent adjuvant radiation or CCRT. The flow of treatment schemes according to IC responsiveness is depicted in Fig. 1. Among 100 patients who experienced a response to IC, 84% were treated with definitive CCRT. Regarding the other 35 patients who did not experience a response to IC, 54% patients received definitive CCRT and 46% underwent surgery.
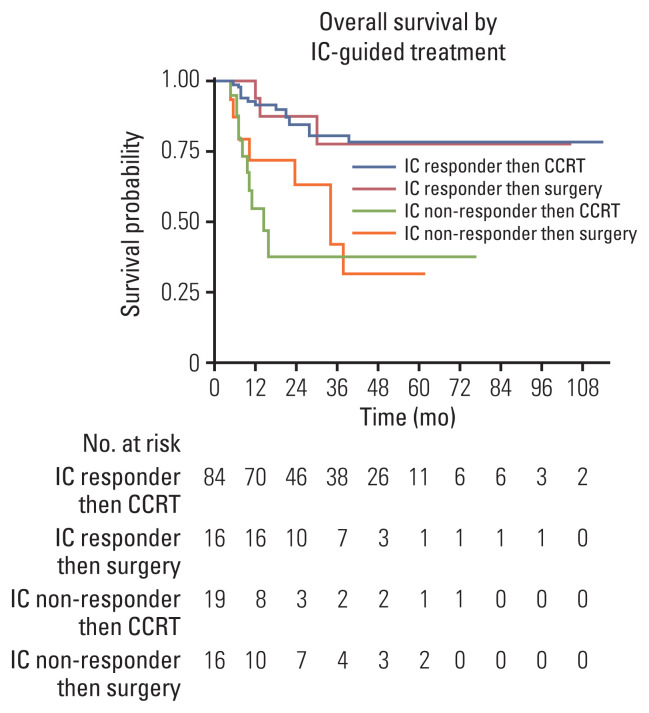
The 12 and 24-month OS probabilities for study population were 86% (95% CI, 79% to 91%), and 77% (95% CI, 68% to 83%), respectively. Fig. 2 summarizes the OS rates among IC-responders and IC-non-responders according to definitive locoregional treatments. The 24-month survival probabilities in patients who experienced a response to IC were 85% (95% CI, 73% to 91%) after subsequent definitive CCRT and 88% (95% CI, 59% to 97%) after subsequent surgery. Among patients who did not show a response to IC, the 24-month survival probabilities were 38% (95% CI, 14% to 62%) in those who were treated with subsequent definitive CCRT, compared to 63% (95% CI, 32% to 83%) in patients who underwent subsequent surgery (p=0.483) (Fig. 2).
Fig. 2.
Overall survival according to induction chemotherapy (IC) and subsequent treatments. 24-Month overall survival probabilities in IC responder followed by concurrent chemoradiotherapy (CCRT): 85% (95% CI, 73 to 91). 24-Month overall survival probabilities in IC responder followed by surgery: 88% (95% CI, 59 to 97). 24-Month overall survival probabilities in IC non-responder followed by CCRT: 38% (95% CI, 14 to 62). 24-Month overall survival probabilities in IC non-responder followed by surgery: 63% (95% CI, 32 to 83).
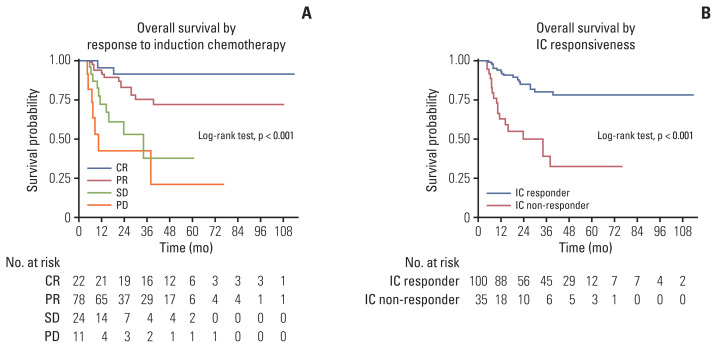
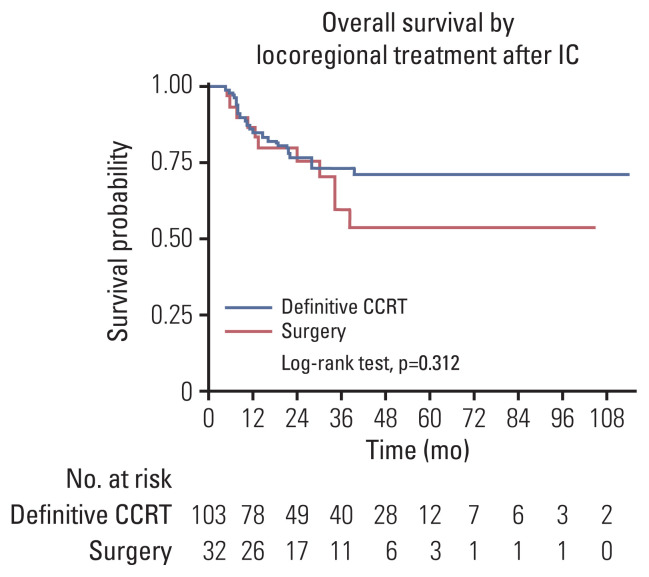
OS were significantly different (p < 0.001) when comparing survival probabilities according to response to IC (Fig. 3A) and IC-non-responders showed 4.5 times higher risk of mortality than IC-responders (hazard ratio [HR], 4.52; 95% CI, 2.32 to 8.81; p < 0.001) (Fig. 3B). The probabilities of survival were not significantly different according to locoregional treatments after IC (HR, 1.44; 95% CI, 0.71 to 2.95; p=0.314) based on an evaluation of the clinical role of locoregional treatments (Fig. 4).
Fig. 3.
(A) Overall survival according to response to induction chemotherapy (IC). (B) Overall survival according to IC responsiveness. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Fig. 4.
Overall survival according to locoregional treatment after induction chemotherapy (IC). Hazard ratio, 1.44; 95% confidence interval, 0.71 to 2.95; p=0.314. CCRT, concurrent chemoradiotherapy.
4. Multivariate analyses for OS
Multivariate analyses of OS showed that primary location in the oral cavity (versus oropharynx), advanced T and N classification (from one unit to the next). IC non-responsiveness were independent poor prognostic factors for OS (Table 3).
Table 3.
Univariate and multivariate analyses for overall survival in 135 LA-HNSCC patients receiving IC
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (yr) | ||||
|
| ||||
| > 60 vs. ≤ 60 | 0.63 (0.32–1.23) | 0.175 | - | - |
|
| ||||
| Sex | ||||
|
| ||||
| Male vs. Female | 1.37 (0.48–3.88) | 0.554 | - | - |
|
| ||||
| ECOG PS | ||||
|
| ||||
| 2 vs. 0–1 | 2.05 (0.62–6.71) | 0.237 | - | - |
|
| ||||
| Smoking history | ||||
|
| ||||
| Current or former vs. never | 1.12 (0.43–2.96) | 0.812 | - | - |
|
| ||||
| Alcohol history | ||||
|
| ||||
| Drink vs. do not drink | 2.38 (0.73–7.74) | 0.150 | - | - |
|
| ||||
| Primary tumor location | ||||
|
| ||||
| Oropharynx | 1 (reference) | 1 (reference) | ||
|
| ||||
| Oral cavity | 4.38 (1.75–10.92) | 0.002 | 3.13 (1.37–7.19) | 0.007 |
|
| ||||
| Hypopharynx | 2.11 (0.85–5.26) | 0.108 | - | - |
|
| ||||
| Larynx | 2.69 (0.93–7.75) | 0.067 | - | - |
|
| ||||
| Others | 1.77 (0.49–6.36) | 0.379 | - | - |
|
| ||||
| T classification | 1.90 (1.31–2.77) | 0.001 | 1.71 (1.14–2.58) | 0.010 |
|
| ||||
| N classification | 1.35 (0.76–2.39) | 0.307 | 1.93 (1.09–3.42) | 0.024 |
|
| ||||
| p16/HPV status | ||||
|
| ||||
| Negative | 1 (reference) | |||
|
| ||||
| Positive | 0.71 (0.31–1.64) | 0.425 | - | - |
|
| ||||
| IC regimen | ||||
|
| ||||
| Fluorouracil+cisplatin | 1 (reference) | |||
|
| ||||
| Docetaxel+cisplatin | 0.51 (0.21–1.21) | 0.128 | - | - |
|
| ||||
| Docetaxel+cisplatin+fluorouracil | 0.65 (0.26–1.59) | 0.066 | - | - |
|
| ||||
| Others | 0.14 (0.02–1.14) | 0.344 | - | - |
|
| ||||
| IC-guided treatment | ||||
|
| ||||
| IC responder+CCRT | 1 (reference) | 1 (reference) | ||
|
| ||||
| IC responder+surgery | 1.03 (0.30–3.60) | 0.960 | - | - |
|
| ||||
| IC non-responder+CCRT | 5.54 (2.43–12.64) | < 0.001 | 4.49 (1.84–10.93) | 0.001 |
|
| ||||
| C non-responder+surgery | 3.75 (1.57–8.94) | 0.003 | 2.60 (1.09–6.20) | 0.030 |
CCRT, concurrent chemoradiotherapy; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; HR, hazard ratio; IC, induction chemotherapy; LA-HNSCC, locally advanced head and neck squamous cell carcinoma.
As for IC-guided treatments, the selection of subsequent treatments did not change the survival outcomes among IC-responders (HR, 1.03; 95% CI, 0.30 to 3.60; p=0.960). Based on the outcomes of IC-responders who subsequently received CCRT as reference, the adjusted HR was 4.49 (95% CI, 1.84 to 10.93; p < 0.001) for IC-non-responders who subsequently received CCRT, and 2.60 (95% CI, 1.09 to 6.20; p=0.030) for IC-non-responders who subsequently underwent surgery. Our finding favored the use of locoregional surgery when patients do not respond to IC.
Discussion
In our study, IC-responders showed better survival outcomes compared to IC-non-responders regardless of the subsequent definitive treatments. IC response was independent favorable prognostic factor for OS. Compared to the IC-responders followed by CCRT, IC-non-responders followed by CCRT showed 4.5 times higher risk of death, whereas IC-non-responders followed by surgery showed less higher risk of death with HR 2.6. Our results indicated that induction chemo-selection approach based on IC-responsiveness is useful for prognostication, and it could be a reasonable strategy to guide subsequent definitive locoregional therapy in LA-HNSCC.
In our study, IC-non-responders did not respond to CCRT as well as systemic chemotherapy. However, even though sensitivity to chemotherapy or radiotherapy is not always in agreement, LA-HNSCC patients who did not respond to IC have a strong propensity toward resistance to radiotherapy. Previous studies reported similar findings that sensitivity to cisplatin-based chemotherapy was correlated with radio-sensitivity in HNSCC [12,13]. Furthermore, a strategy for the selection of patients to receive surgery or radiotherapy according to their response to cisplatin-based chemotherapy suggested a new role for chemotherapy in advanced laryngeal cancer, according to a study conducted by the Veterans Affairs Laryngeal Cancer Study Group [14]. In that study, patients with no response to IC were allocated to the surgery arm, while patients who showed a response to IC subsequently received radiation therapy. This strategy was effective in preserving the larynx without compromising OS. On other words, IC responsiveness could act as a trustworthy predictive factor for response to subsequent CCRT. A recent meta-analysis included seven studies on IC prior to CCRT as definitive treatment for LA-HNSCC and analyzed the likelihood between response to IC and response to subsequent CCRT [11]. The results presented in meta-analysis were similar to our results: patients were more likely to respond to CCRT given previous response to IC and less likely to respond to CCRT if they failed to respond to IC. Thus, we can use our findings further as a helpful guide to choosing further definitive treatment between CCRT and surgery.
On the other hand, IC-non-responders who received definitive surgery showed poorer survival outcomes compared to IC-responders. In our study, 87.5% (14/16) of IC-non-responders who received definitive surgery underwent postoperative radiotherapy or CCRT. These findings suggest that advanced tumor status and insufficient surgical outcome after IC justify additional postoperative treatment. Therefore, a swift change to definitive therapy should be considered patients’ preference, morbidity, marginal operability and poor prognosis.
Despite the several multiple purposes of IC, there are no concordant guidelines regarding the role of IC selection between definitive surgery and CCRT following IC. For example, IC followed by CCRT has a positive effect in terms of organ preservation for laryngeal cancer, thus IC followed by CCRT has been recommended with strong evidence for larynx preservation [15,16]. Furthermore, IC is a useful for making tumor status operable by reducing tumor size in case of oral cavity or oropharyngeal cancer. Despite these advantages, IC followed by CCRT did not prove beneficial in terms of response or survival compared with upfront CCRT in phase III studies of LA-HNSCC [5,17,18]. Therefore, IC is not regarded as a strong option for all patients with LA-HNSCC without any specific objectives such as organ preservation or obtaining operability. From a different perspective, our study suggests that IC responsiveness before definitive treatment is determined could provide a reliable guide to selecting a more beneficial treatment option. Either surgery or CCRT would be fine for the IC responding patients.
Induction chemo-selection approach could be applied in HPV-associated advanced oropharyngeal cancer [19]. In this phase II radiation-deintensification study by Marur et al. [19], the patients with primary-site CR to IC received less dose (54 Gy) of intensity-modulated radiation therapy compared to those (69.3 Gy) with less than CR to IC, and showed 80% of the 2-year progression-free survival (95% CI, 65 to 89). These results encompassed their target 2-year progression-free survival of 85% and radiation dose reduction resulted in improving swallowing and nutritional status. Therefore, this strategy can be utilized to find HPV-associated oropharyngeal cancer patients for reduced radiation dose as a means of sparing late sequelae.
Our data shows that IC-non-responders had more advanced T category and oral cavity cancer. In clinical practice, it is even difficult to have a chance at curative surgery after IC in cases of advance T category such as T4, which invades adjacent structures. Moreover, squamous oral cavity cancer is known to have less response to chemotherapy than other primary sites in head and neck [20]. Therefore, it would be better to consider upfront definitive treatment than IC in cases of patients with advanced T category such as T4 or oral cavity cancer.
Only 28.2% of our patients received a triple regimen of TPF, which is the current evidence-based gold standard for IC [21–23]. The rest of patients received a doublet such as DP or FP. Preference of a doublet over a triple regimen was due to toxicity and compliance among Korean patients [2]. Our analyses revealed that the IC regimen did not affect survival probabilities in our study population. However, further investigation is required to evaluate the role of IC regimen in guiding subsequent treatment in LA-HNSCC.
This study has several limitations. First, it was planned a retrospective design; therefore, exact information regarding treatment toxicities, the reason of choice for subsequent definitive treatment after IC, could not be retrieved. Second, the study population was heterogeneous with various primary sites; therefore, the specific primary sites of cancer must be interpreted with caution. Third, the exclusion of 14.6% of all patients receiving IC could limit the accurate interpretation of our results. Lastly, we could not retrieve some important information regarding patient characteristics such as smoking and HPV status. Future studies with prospective nature will be warranted.
In conclusion, we found that a good response to IC is a favorable prognostic factor for overall survival. For patients who respond to IC, any subsequent treatment either surgery or definitive CCRT would be appropriate without survival differences. On the other hand, patients who do not respond to IC, it is necessary to approach multi-disciplinarily reflecting patients’ preference, morbidity and poor prognosis. Importantly, a multidisciplinary team should share the IC response at an appropriate point in time and discuss further optimal treatments to obtain the best treatment results for each patient.
Acknowledgments
Study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (HA16C0015).
The research was supported (in part) by the Korean Cancer Study Group (KCSG) and KCSG data center (CRA name: Jiyun Mun).
Footnotes
Ethical Statement
The Institutional Review Board for main hospital (IRB-H-1304-089-481) and each participating hospital approved this study. And each Institutional Review Board approved the waiver of written informed consents because of the retrospective nature of this study.
Author Contributions
Conceived and designed the analysis: Lee YG, Kang EJ, Keam B.
Collected the data: Lee YG, Kang EJ, Keam B, Choi JH, Kim JS, Park KU, Lee KE, Kim HJ, Lee KW, Kim MK, Ahn HK, Shin SH, Kim HR, Kim SB, Yun HJ.
Contributed data or analysis tools: Lee YG, Kang EJ, Keam B.
Performed the analysis: Lee YG, Kang EJ, Keam B.
Wrote the paper: Lee YG, Kang EJ, Keam B, Choi JH, Kim JS, Park KU, Lee KE, Kim HJ, Lee KW, Kim MK, Ahn HK, Shin SH, Kim HR, Kim SB, Yun H
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
References
- 1.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386–96. doi: 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Lee YG, Kang EJ, Keam B, Choi JH, Kim JS, Park KU, et al. Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective cohort study (KCSG HN13-01) BMC Cancer. 2020;20:813. doi: 10.1186/s12885-020-07297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon JP, le Maitre A, Maillard E, Bourhis J MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Brizel DM, Prosnitz RG, Hunter S, Fisher SR, Clough RL, Downey MA, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:1418–23. doi: 10.1016/j.ijrobp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–43. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Jiang N, Shi Y, Li S, Wang P, Zhao Y. Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta-analysis. Sci Rep. 2015;5:10798. doi: 10.1038/srep10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddad RI, Posner M, Hitt R, Cohen EE, Schulten J, Lefebvre JL, et al. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck: role, controversy, and future directions. Ann Oncol. 2018;29:1130–40. doi: 10.1093/annonc/mdy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregoire V, Lefebvre JL, Licitra L, Felip E EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v184–6. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 9.Janoray G, Pointreau Y, Garaud P, Chapet S, Alfonsi M, Sire C, et al. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, +/− docetaxel for larynx preservation. J Natl Cancer Inst. 2016;108:djv368. doi: 10.1093/jnci/djv368. [DOI] [PubMed] [Google Scholar]
- 10.Kim R, Hahn S, Shin J, Ock CY, Kim M, Keam B, et al. The effect of induction chemotherapy using docetaxel, cisplatin, and fluorouracil on survival in locally advanced head and neck squamous cell carcinoma: a meta-analysis. Cancer Res Treat. 2016;48:907–16. doi: 10.4143/crt.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiong KL, de Souza NN, Sultana R, Iyer NG. Meta-analysis of induction chemotherapy as a selection marker for chemoradiation in the head and neck. Laryngoscope. 2018;128:1594–601. doi: 10.1002/lary.27011. [DOI] [PubMed] [Google Scholar]
- 12.Ensley JF, Jacobs JR, Weaver A, Kinzie J, Crissman J, Kish JA, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54:811–4. doi: 10.1002/1097-0142(19840901)54:5<811::aid-cncr2820540508>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Hong WK, O’Donoghue GM, Sheetz S, Fofonoff S, Dorman EB, Welch J, et al. Sequential response patterns to chemotherapy and radiotherapy in head and neck cancer: potential impact of treatment in advanced laryngeal cancer. Prog Clin Biol Res. 1985;201:191–7. [PubMed] [Google Scholar]
- 14.Department of Veterans Affairs Laryngeal Cancer Study Group. Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–90. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre JL, Andry G, Chevalier D, Luboinski B, Collette L, Traissac L, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23:2708–14. doi: 10.1093/annonc/mds065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101:498–506. doi: 10.1093/jnci/djp007. [DOI] [PubMed] [Google Scholar]
- 17.Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14:257–64. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 18.Geoffrois L, Martin L, Garaud P, De Raucourt D, Miny J, Maingon P, et al. Induction docetaxel platinum 5-FU (TPF) followed by cetuximab-radiotherapy (cetux-RT) versus concurrent chemo-radiotherapy (CT/RT) in patients with N2b/c-N3 non operated stage III–IV squamous cell cancer of the head and neck (SCCHN): results of the GORTEC 2007-02 phase III randomized trial. J Clin Oncol. 2016;34(15 Suppl):6000. [Google Scholar]
- 19.Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35:490–7. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernani V, Saba NF. Oral cavity cancer: risk factors, pathology, and management. Oncology. 2015;89:187–95. doi: 10.1159/000398801. [DOI] [PubMed] [Google Scholar]
- 21.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 22.Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011;12:153–9. doi: 10.1016/S1470-2045(10)70279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanchard P, Bourhis J, Lacas B, Posner MR, Vermorken JB, Cruz Hernandez JJ, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31:2854–60. doi: 10.1200/JCO.2012.47.7802. [DOI] [PubMed] [Google Scholar]