Abstract
Purpose
There remains controversy about relationship between obesity and gastric cancer. We aimed to examine the association using obesity-persistence.
Materials and Methods
We analyzed a nationwide population-based cohort which underwent health check-up between 2009 and 2012. Among them, those who had annual examinations during the last 5 years were selected. Gastric cancer risk was compared between those without obesity during the 5 years (never-obesity group) and those with obesity diagnosis during the 5 years (non-persistent obesity group; persistent obesity group).
Results
Among 2,757,017 individuals, 13,441 developed gastric cancer after median 6.78 years of follow-up. Gastric cancer risk was the highest in persistent obesity group (incidence rate [IR], 0.89/1,000 person-years; hazard ratio [HR], 1.197; 95% confidence interval [CI], 1.117 to 1.284), followed by non-persistent obesity group (IR, 0.83/1,000 person-years; HR, 1.113; 95% CI, 1.056 to 1.172) compared with never-obesity group. In subgroup analysis, this positive relationship was true among those < 65 years old and male. Among heavy-drinkers, the impact of obesity-persistence on the gastric cancer risk far increased (non-persistent obesity: HR, 1.297; 95% CI, 1.094 to 1.538; persistent obesity: HR, 1.351; 95% CI, 1.076 to 1.698).
Conclusion
Obesity-persistence is associated with increased risk of gastric cancer in a dose-response manner, especially among male < 65 years old. The risk raising effect was much stronger among heavy-drinkers.
Keywords: Obesity, Stomach neoplasms, Dose-response relationship
Introduction
Despite the decreasing incidence and mortality, gastric cancer still remains the fifth most common cancer and the third most common cause of cancer-related death in the world [1]. Since Helicobacter pylori has been established as class I carcinogen for gastric cancer by the World Health Organization [2], there have been many studies evaluating risk factors for gastric cancer. Aside from H. pylori, following factors have been revealed so far: advanced age [3], male sex [1], smoking [4], alcohol [5], low consumption of fresh vegetables [6], and high salt intake [7]. Also, low income is highly associated with H. pylori prevalence [8] which is the single most important risk factor for gastric cancer.
In contrast, there have been mixed evidence regarding the relationship between overweight/obesity and gastric cancer risk [9]. A previous meta-analysis has shown that body mass index (BMI) is highly related with gastric cardia cancer but not with non-cardia cancer [10]. Meanwhile, a recent study suggested that obesity is associated with early gastric cancer and dysplasia under the adjustment of H. pylori infection [11]. Also a study reported positive relationship between overweight and gastric cancer among non-Asians, while such relationship was not shown among Asians [12]. However, most of the previous studies have dealt with obesity status only in a certain time point and have not concentrated on the persistence of obesity. As BMI is rather dynamic compared with other above mentioned risk factors, it can be easily changed. Also, reverse causality is often a problem in case-control studies: BMI tends to decrease when an individual has malignancy [13]. Persistent obesity may be more influential to the development of gastric cancer than temporary obesity.
From this background, we aimed to investigate the relationship between the persistence of obesity and the risk of gastric cancer using a population-based prospective cohort in Korean population.
Materials and Methods
1. Study population
We used the 5-year data of a population-based prospective cohort which underwent health check-up provided by the National Health Insurance Corporation (NHIC) between 2008 and 2012 to identify individuals according to the obesity-persistence. The NHIC covers almost 97% of Korean population and offers a standardized health check-up examination at least biennially.
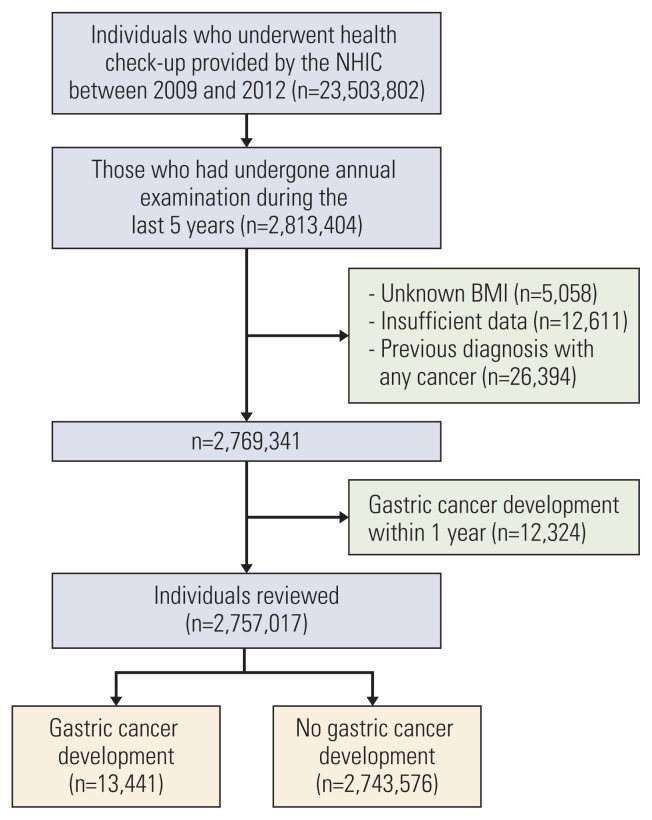
Among the total 23,503,802 individuals, 2,813,404 subjects who had undergone five annual examinations during the last 5 years were selected. Index date was defined as the last examination date among the five times. Thus we used the last health check-up data during the 5 years for the analysis of all the variables. Among this population, those with unknown BMI (n=5,058), those with other insufficient data (n=12,611), those who had diagnosis with any cancer before the index date (n=26,394), and those who had gastric cancer development within one year (n=12,324) were excluded. Finally, the remaining 2,757,017 individuals were included in this study and followed up until 2017 (Fig. 1).
Fig. 1.
Study flow chart showing patient enrollment. BMI, body mass index; NHIC, National Health Insurance Corporation.
Cancer diagnosis was identified from the National Health Insurance Service claim data using the International Classification of Diseases, 10th revision (ICD-10) codes.
2. Clinical parameters and biochemical analysis
We used answers to the standardized self-reporting questionnaire which was obtained at the time of health check-up examination. Following parameters thereof were analyzed: age, sex, residency (rural or urban), yearly income (low: lowest quartile range among those under NHIS and the income of those under Medical Aid which is for the recipients of National Basic Livelihood Security Program vs. normal to high: income of the remaining), smoking status (never, former, or current), alcohol consumption (none, mild: < 30 g/day, or heavy: ≥ 30 g/day), and regular exercise (high-intensity activity ≥ 3 times/week or moderate-intensity activity ≥ 5 times/wk vs. not). BMI, waist circumference (WC, cm), and systolic/diastolic blood pressure (mmHg) were measured on the day of health check-up examination. Plasma glucose level and total cholesterol level were measured from each individuals’ blood sample taken after at least 8 hours of fasting. All questionnaire data and the measurement data used in this study were taken from the examination of the index date.
3. Definitions
Diabetes mellitus was defined as a fasting plasma glucose level ≥ 126 mg/dL, taking oral hypoglycemic agents/insulin, or having the ICD-10 code of E11–14. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg; taking antihypertensive medication; or having the ICD-10 code, I10–13. Dyslipidemia was defined as fasting total cholesterol ≥ 240 mg/dL, taking lipid lowering medication, or having the ICD-10 code E78. Obesity was defined as BMI ≥ 25 kg/m2 based on the criteria for the Asia-Pacific region [14] and abdominal obesity was defined as WC ≥ 90 cm among male and WC ≥ 85 cm among female [15].
According to the number of diagnoses with obesity during the 5 years, individuals were grouped as followings: never-obesity group (those who had never diagnosed with obesity during the 5 years) and ever-obesity group (those who had diagnosed with obesity at least once during the 5 years). Also ever-obesity group was subdivided as follows: non-persistent obesity group (those who had been diagnosed with obesity 1–4 times during the 5 years) and persistent obesity group (those who had been diagnosed with obesity 5 times during the 5 years).
4. Statistical analysis
Continuous variables were presented as mean±standard deviation for normally distributed one, and otherwise presented as medians with ranges. Categorical variables were presented as numbers with proportions. To compare continuous variables, Student t test or analysis of variance was used. For categorical variables, chi-square test was used for analysis. Continuous variables with non-normal distribution were analyzed after log transformations. Clinically important variables with p < 0.05 in univariable analyses were taken for multivariable analyses.
Incidence rates were presented as the number of events per 1,000 person-years at risk. For independent risk evaluation, Cox proportional hazard model was adopted under adjustment with clinically important variables.
All the statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC) and R ver. 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). All tests were two-sided and p-values < 0.05 were considered statistically significant.
The data that support the findings of this study are available from the NHIC but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the NHIC.
Results
1. Baseline characteristics of the study population
Table 1 shows the baseline characteristics of the study population according to the obesity status. Based on the definition of obesity as an ever diagnosis with obesity, 1,613,198 individuals (58.5%) had never been diagnosed with obesity during the 5 years and 1,143,819 individuals (41.5%) had been diagnosed with obesity at least once during the 5 years. Ever-obesity group were older and had larger proportion of male than never-obesity group, and the mean age linearly increased as the number of diagnoses with obesity increases (p < 0.001). The final BMI and WC showed increasing tendencies in dose-response manner according to the number of diagnoses with obesity. However, even among ever-obesity group, 0.03% were underweight at the time of index date. Ever-obesity group had larger proportion of individuals with low income and those with smoking or alcohol consumption habit, and these traits also showed similar proportional distribution according to the cumulative number of obesity diagnoses (p < 0.001). Interestingly, ever-obesity group was more likely to do regular exercise than never-obesity group, and among the ever-obesity group, those with greater obesity-persistence were more likely to do regular exercise (p < 0.001). The proportions of diabetes, hypertension, and dyslipidemia were all greater among ever-obesity group than among never-obesity group, and showed increasing tendencies as the obesity-persistence increases.
Table 1.
The lowest quartile range of yearly income of those under National Health Insurance Service and the income of those under Medical Aid which is for the recipients of National Basic Livelihood Security Program
| Ever-obesitya) | Times of obesity diagnosis during the continuous 5 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| No (n=1,613,198) | Yes (n=1,143,819) | p-value | 1 (n=175,721) | 2 (n=124,172) | 3 (n=120,688) | 4 (n=153,739) | 5 (n=569,499) | p-value | |
| Age (yr) | 41.9±10.6 | 43.5±10.2 | < 0.001 | 43.0±10.6 | 43.1±10.4 | 43.2±10.4 | 43.6±10.3 | 43.9±9.9 | < 0.001 |
|
| |||||||||
| 20–39 | 755,323 (46.8) | 469,483 (41.1) | < 0.001 | 75,922 (43.2) | 53,313 (42.9) | 51,382 (42.6) | 63,270 (41.2) | 225,596 (39.6) | < 0.001 |
|
| |||||||||
| 40–64 | 767,454 (47.6) | 598,233 (52.3) | 88,039 (50.1) | 62,709 (50.5) | 61,365 (50.9) | 79,632 (51.8) | 306,488 (53.8) | ||
|
| |||||||||
| ≥ 65 | 90,421 (5.6) | 76,103 (6.7) | 11,760 (6.7) | 8,150 (6.6) | 7,941 (6.6) | 10,837 (7.1) | 37,415 (6.6) | ||
|
| |||||||||
| Male sex | 1,086,886 (67.4) | 953,807 (83.4) | < 0.001 | 136,107 (77.5) | 100,415 (80.9) | 98,996 (82.0) | 127,951 (83.2) | 490,338 (86.1) | < 0.001 |
|
| |||||||||
| BMI (kg/m 2 ) | 21.8±1.9 | 26.6±2.3 | < 0.001 | 24.4±1.3 | 25.0±1.1 | 25.4±1.2 | 26.0±1.4 | 28.0±2.1 | < 0.001 |
|
| |||||||||
| < 18.5 | 95,080 (5.9) | 397 (0.03) | < 0.001 | 270 (0.2) | 35 (0.03) | 29 (0.02) | 63 (0.04) | 0 | < 0.001 |
|
| |||||||||
| 18.5–23 | 1,036,096 (64.2) | 2,604 (2.3) | 17,672 (10.1) | 4,418 (3.6) | 2,278 (1.9) | 1,679 (1.1) | 0 | ||
|
| |||||||||
| 23–25 | 482,022 (29.9) | 223,972 (19.6) | 101,395 (57.7) | 55,997 (45.1) | 38,327 (31.8) | 28,253 (18.4) | 0 | ||
|
| |||||||||
| 25–30 | 0 | 803,186 (70.2) | 56,005 (31.9) | 63,553 (51.2) | 79,697 (66.0) | 121,830 (79.2) | 482,101 (84.7) | ||
|
| |||||||||
| ≥ 30 | 0 | 90,217 (7.9) | 379 (0.2) | 169 (0.1) | 357 (0.3) | 1,914 (1.2) | 87,398 (15.4) | ||
|
| |||||||||
| WC (cm) | 76.2±7.0 | 87.0±7.0 | < 0.001 | 82.2±5.8 | 83.6±5.5 | 84.5±5.5 | 85.9±5.6 | 90.1±6.6 | < 0.001 |
|
| |||||||||
| Rural residence | 909,449 (56.4) | 659,387 (57.7) | < 0.001 | 100,973 (57.5) | 71,635 (57.7) | 69,720 (57.8) | 88,019 (57.3) | 329,040 (57.8) | < 0.001 |
|
| |||||||||
| Low income b) | 296,126 (18.4) | 223,388 (19.5) | < 0.001 | 34,209 (19.5) | 24,365 (19.6) | 23,637 (19.6) | 30,995 (20.2) | 11,082 (19.4) | < 0.001 |
|
| |||||||||
| Smoking | |||||||||
|
| |||||||||
| Never | 813,106 (50.4) | 442,672 (38.7) | < 0.001 | 76,087 (43.3) | 50,534 (40.7) | 48,098 (39.9) | 59,883 (39.0) | 208,070 (36.5) | < 0.001 |
|
| |||||||||
| Former | 283,182 (17.6) | 274,738 (24.0) | 39,605 (22.5) | 29,665 (23.9) | 28,937 (24.0) | 37,449 (24.4) | 139,082 (24.4) | ||
|
| |||||||||
| Current | 516,910 (32.0) | 426,409 (37.3) | 60,029 (34.2) | 43,973 (35.4) | 43,653 (36.2) | 56,407 (36.7) | 222,347 (39.0) | ||
|
| |||||||||
| Alcohol | |||||||||
|
| |||||||||
| None | 661,031 (41.0) | 393,159 (34.4) | < 0.001 | 65,135 (37.1) | 44,013 (35.5) | 42,077 (34.9) | 53,599 (34.9) | 188,335 (33.1) | < 0.001 |
|
| |||||||||
| Mildc | 849,087 (52.6) | 638,080 (55.8) | 95,969 (54.6) | 69,520 (56.0) | 65,525 (56.0) | 85,777 (55.8) | 319,287 (56.1) | ||
|
| |||||||||
| Heavyd) | 103,080 (6.4) | 112,580 (9.8) | 14,617 (8.3) | 10,639 (8.6) | 11,084 (9.2) | 14,363 (9.3) | 61,877 (10.9) | ||
|
| |||||||||
| Regular exercise | 971,031 (60.2) | 758,148 (66.3) | < 0.001 | 112,436 (64.0) | 81,303 (65.5) | 79,368 (65.8) | 102,096 (66.4) | 382,945 (67.2) | < 0.001 |
|
| |||||||||
| Diabetes | 68,504 (4.3) | 106,608 (9.3) | < 0.001 | 11,374 (6.5) | 8,832 (7.1) | 9,300 (7.7) | 13,362 (8.7) | 63,740 (11.2) | < 0.001 |
|
| |||||||||
| Hypertension | 211,457 (13.1) | 319,950 (28.0) | < 0.001 | 35,072 (20.0) | 26,835 (21.6) | 28,249 (23.4) | 39,769 (25.9) | 190,025 (33.4) | < 0.001 |
|
| |||||||||
| Dyslipidemia | 174,977 (10.9) | 241,345 (21.1) | < 0.001 | 29,456 (16.8) | 22,228 (17.9) | 22,578 (18.7) | 31,285 (20.4) | 135,798 (23.9) | < 0.001 |
|
| |||||||||
| Follow-up duration, median (Q1–Q3) | 6.76 (5.33–7.35) | 6.82 (5.4–7.39) | < 0.001 | 6.77 (5.37–7.37) | 6.74 (5.35–7.35) | 6.74 (5.36–7.35) | 6.75 (5.35–7.35) | 6.76 (5.30–7.35) | < 0.001 |
Values are presented as mean±standard deviation or numbers (percentage excluding missing data). Data were from the final health check-up of each individual. BMI, body mass index; WC, waist circumference.
Diagnosis of obesity at least once during the five continuous annual health check-ups,
The lowest quartile range of yearly income of those under National Health Insurance Service and the income of those under Medical Aid which is for the recipients of National Basic Livelihood Security Program,
Alcohol consumption < 30 g/day,
Alcohol consumption ≥ 30 g/day.
Additionally, we have analyzed baseline characteristics of those who were excluded due to unknown BMI (n=5,058) (S1 Table). They showed similar characteristics to the ever-obesity group. Their mean WC was greater than that of the ever-obesity group. Furthermore, the portions of smokers and drinkers, or those with diabetes, hypertension, and dyslipidemia in those with missing BMI data were also largely comparable to those in the ever-obesity group.
2. Degree of obesity and the risk for gastric cancer
The adjusted HRs (aHRs) for gastric cancer were calculated according to the degree of obesity at the index date, adjusted with age, sex, smoking (never, former, or current), alcohol consumption, regular exercise, income, diabetes, hypertension, and dyslipidemia (Table 2). Stratification was made with BMI or WC. Under five-level stratification with BMI (< 18.5; 18.5–23; 23–25; 25–30; and ≥ 30 kg/m2), underweight individuals (BMI < 18.5 kg/m2) showed an increased risk for gastric cancer compared with normal BMI group (18.5 kg/m2 ≤ BMI < 23 kg/m2). Meanwhile, the other three groups failed to show any risk with statistical significance.
Table 2.
Degree of obesity and the adjusted risk for gastric cancer
| Gastric cancer | Duration (person-years) | Incidence rate (per 1,000 person-years) | aHR (95% CI)a) | |
|---|---|---|---|---|
| BMI b) | ||||
| < 18.5 | 314 | 602,029.76 | 0.52 | 1.152 (1.027–1.292) |
| 18.5–23 | 4,634 | 6,769,453.49 | 0.68 | 1 (reference) |
| 23–25 | 3,744 | 4,520,560.49 | 0.83 | 0.977 (0.935–1.020) |
| 25–30 | 4,403 | 5,108,345.76 | 0.86 | 1.027 (0.985–1.072) |
| ≥ 30 | 346 | 560,217.27 | 0.62 | 0.985 (0.881–1.100) |
| BMI | ||||
| < 25 | 8,692 | 11,892,043.75 | 0.73 | 1 (reference) |
| ≥ 25 | 4,749 | 5,668,563.03 | 0.84 | 1.031 (0.994–1.069) |
| WC, M/F (cm) b) | ||||
| < 80/< 75 | 3,623 | 6,607,430.37 | 0.55 | 0.951 (0.906–0.998) |
| 80–85/75–80 | 3,685 | 4,578,966.61 | 0.80 | 0.966 (0.922–1.013) |
| 85–90/80–85 | 3,308 | 3,496,236.26 | 0.95 | 1 (reference) |
| 90–95/85–90 | 1,832 | 1,821,759.93 | 1.01 | 0.997 (0.942–1.056) |
| 95–100/90–95 | 693 | 708,149.86 | 0.98 | 0.997 (0.928–1.082) |
| ≥ 105/≥ 100 | 300 | 348,063.75 | 0.86 | 1.004 (0.892–1.131) |
| WC, M/F (cm) | ||||
| < 90/< 85 | 10,342 | 14,139,457.95 | 0.73 | 1 (reference) |
| ≥ 90/≥ 85 | 3,099 | 3,421,148.83 | 0.91 | 1.008 (0.967–1.050) |
aHR, adjusted hazard ratio; BMI, body mass index; CI, confidence interval; F, female; M, male; WC, waist circumference.
Adjusted for age, sex, smoking (never, former, or current smoker), alcohol consumption (none, < 30 g/day, or ≥ 30 g/day), regular exercise, income (low, normal-high), diabetes, hypertension, and dyslipidemia,
WC and BMI were from the last examination in the 5-year period.
When divided into two groups with the cutoff value of BMI 25 which is the cutoff for obesity in the Asian population, those with BMI ≥ 25 kg/m2 showed a greater incidence rate of gastric cancer compared with those with BMI < 25 kg/m2 (0.73 vs. 0.84 per 1,000 person-years), which did not reach a statistical significance.
In regard with WC, when stratified into six levels (< 80/< 75; 80–85/75–80; 85–90/80–85; 90–95/85–90; 95–100/90–95; and ≥ 105/≥ 100 cm for male/female, respectively), WC < 80/< 75 cm group showed slightly decreased risk for gastric cancer with reference to the group with normal WC (male/female: 85–90/80–85 cm) and the other groups did not show any significant risk for gastric cancer. When divided into two groups with the cutoff value of WC 90 cm for male and 80 cm for female which are the cutoff for abdominal obesity, those with abdominal obesity did not show any difference in the risk for gastric cancer from those without abdominal obesity.
3. Obesity-persistence and the risk for gastric cancer
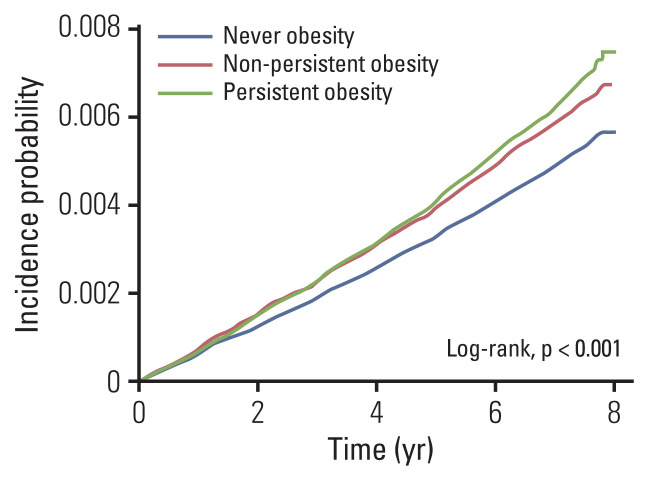
To evaluate the effect of obesity-persistence on the risk for gastric cancer, the aHRs for gastric cancer were calculated according to the number of obesity diagnoses under adjustment with age, sex, smoking (never or former, current), alcohol consumption (none, < 30 g/day, or ≥ 30 g/day), regular exercise, income (low, normal to high), diabetes, hypertension, dyslipidemia, and BMI (Table 3). According to the obesity-persistence, there were 1,613,198 individuals in the never-obesity group, 574,320 in the non-persistent obesity group, and 569,499 in the persistent obesity group. Compared with never-obesity group, both non-persistent obesity group and the persistent obesity group showed an increased risk for gastric cancer (aHR, 1.113; 95% confidence interval [CI], 1.056 to 1.172 and aHR, 1.197; 95% CI, 1.117 to 1.284, respectively) (Fig. 2).
Table 3.
Obesity-persistence and adjusted risk for gastric cancer
| No. of diagnosis with obesitya) | Gastric cancer | Duration (person-years) | Incidence rate (per 1,000 person-years) | HR (95% CI)b) |
|---|---|---|---|---|
| 0 | 7,187 | 10,292,257.46 | 0.70 | 1 (reference) |
| 1–4 | 3,043 | 3,653,452.65 | 0.83 | 1.113 (1.056–1.172) |
| 5 | 3,211 | 3,614,896.68 | 0.89 | 1.197 (1.117–1.284) |
CI, confidence interval; HR, hazard ratio.
Number of obesity diagnoses during the five continuous annual health check-ups,
Adjusted for age, sex, smoking (never/former, or current), alcohol consumption (none, < 30 g/day, or ≥ 30 g/day), regular exercise, income (low, normal-high), diabetes, hypertension, dyslipidemia, and body mass index.
Fig. 2.
Kaplan-Meier curves depicting the incidence probability for gastric cancer in never-obesity, non-persistent obesity and persistent obesity group.
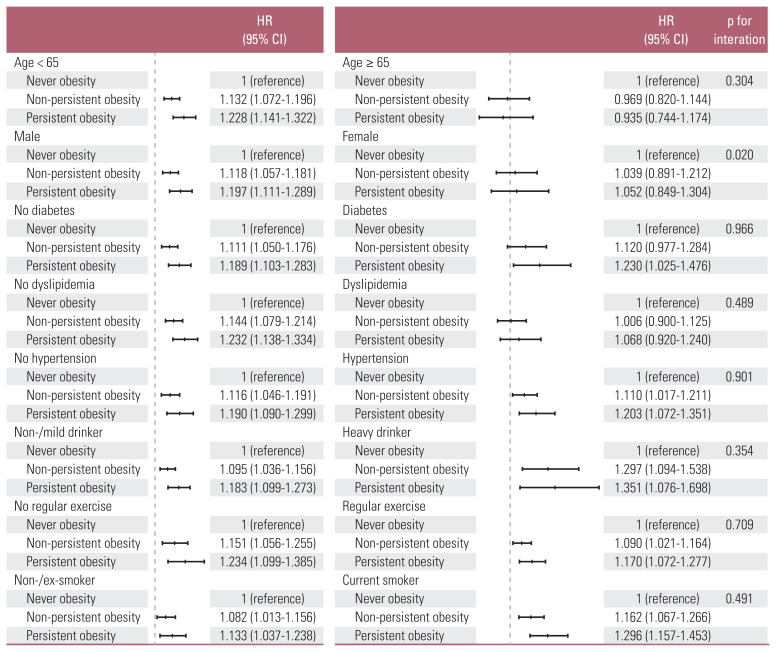
4. Subgroup analyses according to age, sex, diabetes, dyslipidemia, hypertension, alcohol consumption, regular exercise, and smoking status
We performed subgroup analyses according to age, sex, diabetes, dyslipidemia, hypertension, alcohol consumption, regular exercise, and smoking status to know which subgroup is more under the effect of obesity-persistence on the risk of gastric cancer. Fig. 3 shows the aHRs and p for interaction of each subgroup under adjustment with age, sex, diabetes, dyslipidemia, hypertension, alcohol consumption (none, mild < 30 g/day, or heavy ≥ 30 g/day), regular exercise, smoking (never, former, or current), income (low, normal-high), and BMI.
Fig. 3.
The impact of obesity-persistence on the risk of gastric cancer in different subgroups. Forest plots of hazard ratios (HRs; middle markings on the bars) and 95% confidence intervals (CI; error bars) under adjustment with age, sex, diabetes, dyslipidemia, hypertension, alcohol consumption (none, < 30 g/day vs. ≥ 30 g/day), regular exercise, and smoking (never, former vs. current) were illustrated. The vertical dotted lines mean hazard ratio 1.
Among those < 65 years old, the relationship between obesity-persistence and the risk of gastric cancer was strongly positive, while those ≥ 65 years old did not show significant relationship. According to the sex, only male showed positive relationship between obesity-persistence and the risk for gastric cancer. Male sex also showed significant interaction with obesity-persistence on the risk for gastric cancer (p for interaction=0.020). In terms of underlying chronic metabolic conditions, those with diabetes or dyslipidemia failed to show consistent association between obesity-persistence and gastric cancer risk. On the other hand, the positive association was not affected by hypertension. The risk raising effect of obesity-persistence on gastric cancer was consistently positive regardless of alcohol consumption, regular exercise, or smoking status. However, the effects were greater among heavy-drinkers, those without regular exercise, and current smokers than the counterparts. Especially among heavy-drinkers, the HRs for non-persistent and persistent obesity group were as high as 1.297 and 1.351, respectively.
Discussion
We found that obesity-persistence is positively associated with the risk of gastric cancer. The risk for gastric cancer increased by approximately 11% in non-persistent obesity group and 20% in persistent obesity group compared with never-obesity group showing a dose-response relationship. Especially, the positive relationship was true among those who are young, male, and without diabetes or dyslipidemia. Furthermore, the positive relationship was more prominent among heavy-drinkers, those without regular exercise, and current smokers.
Obesity is established as an obvious risk factor for several types of cancers. Based on the International Agency for Research on Cancer Working Group, convincing evidences support that excessive body weight is associated with increased risk for following cancers: endometrial, esophageal, renal, colorectal, breast, ovarian, gallbladder, thyroid, and pancreatic cancer; hepatocellular carcinoma; meningioma; and multiple myeloma [16]. However the evidence for the relationship between obesity and gastric cancer has been conflicting. A previous meta-analysis reported 36% and 21% increment of gastric cancer risk in regard to obesity and overweight, respectively [12]. Meanwhile, later meta-analyses including more prospective studies revealed no statistical relationship between obesity and gastric cancer except for non-cardia one [10,17]. However, these studies were mostly from Western countries where gastric cancer incidence is relatively low compared with East Asian countries, and the prevalence of gastric cardia cancer is significantly growing. We assumed that a large-scale study in an area where gastric cancer is highly prevalent may elucidate the true association between obesity and gastric cancer.
In current study, the analysis using BMI or WC measured at a single time point failed to reveal any risk raising effect for gastric cancer. Rather the underweight population showed an increased risk for gastric cancer. The reason for this phenomenon is unclear, however, considering that those with lowest level of WC showed slightly decreased risk for gastric cancer, it might be a phenomenon of chance. Divided into two groups with the cutoff for obesity using BMI or WC, those with obesity or abdominal obesity were slightly more likely to develop gastric cancer, although without statistical significance. In epidemiological studies, reverse causality is often a problem; patients with gastric cancer tend to lose weight (cancer cachexia), which underestimates the impact of obesity on gastric cancer [13]. Also, it might be because of the fluctuation of the obesity status. Therefore we focused on obesity-persistence using five-year cumulative data. In this regard, the degree of obesity-persistence or the cumulative exposure to obesity demonstrated a consistent risk increasing effect on gastric cancer development, even after controlling for potential confounders.
The mechanism by which obesity promotes gastric cancer is not clear. However, this can be assumed similar with other obesity-related cancers. According to previous literatures, the abnormal fat deposition may cause molecular changes by hyperinsulinemia, increase of insulin-like growth factors, adipocytokine imbalance, and increased estrogen, affecting DNA repair, as well as cell proliferation and malignant transformation [18,19]. Also, ectopic accumulation of adipose tissue exerts pro-inflammatory effect through inflammatory cytokines such as tumor necrosis factor and interleukin-6, leading to chronic subclinical inflammatory state subject to oxidative stress which is a well-known promoter of carcinogenesis [20–22].
In the subgroup analysis, most of the subgroups consistently showed positive relationship between obesity-persistence and the risk of gastric cancer. However, which was not maintained among old age and females. One of the probable reason is that the number of individuals included in those subgroups were relatively small. Also, in most cases the rate of progression of gastric cancer among elderly is slower than among young people [23]. Therefore the median 6.78 years of follow-up period might be insufficient to see the development. In terms of sex disparity, similar phenomenon has been seen in colorectal cancer showing stronger relationship among male [24]. Considering the fact that male are more prone to visceral adiposity which is the key determinant of insulin resistance, female having more subcutaneous fat than men would less likely be affected by obesity simply defined with BMI. Also, like in colon cancer, estrogen might have protective effect on gastric carcinogenesis [25,26].
Current smoking and heavy alcohol consumption played a role in enhancing the linkage between obesity and gastric cancer. Especially among heavy-drinkers, the effect of obesity on gastric cancer susceptibility was highly increased by up to 19%, inferring certain synergistic effect. In previous researches, similar synergistic effect between smoking or alcohol consumption and obesity has been reported in regard to cardiovascular disease [27,28]. This might be because of their cumulative pro-inflammatory function, promoting neoplastic transformation. Also, regular exercise attenuated the degree of gastric cancer susceptibility induced by obesity-persistence. Therefore the combination of smoking/alcohol cessation, regular exercise, and sustainable weight loss may better reduce the risk of gastric cancer.
In this study, the HR of gastric cancer in persistent obesity group was slightly higher than in the non-obesity group. This might be because the duration of 5 years, which was used for the definition of persistent obesity in this study, was not enough to show considerable risk increment. For example, there might be some people who had lost weight after 5 years. However, the relationship shown here cannot be made by chance just because of large sample size, since the various subgroup analyses have shown robust results confirming the positive association. Also, the persistence of obesity showed an increased risk for gastric cancer with dose-response manner with statistical significance. Therefore, the association between obesity-persistence and gastric cancer risk might be true. However, more well-designed long-term follow-up studies are necessary to confirm our findings.
There are several limitations in this study. First, the H. pylori infection status could not be evaluated. As H. pylori is a major risk factor for gastric cancer, this needs to be analyzed in future studies. However, considering that H. pylori has been reported to have reverse relationship with obesity in previous studies [29,30], it is thought current positive association between obesity-persistence and gastric cancer risk will not be changed, or rather can be enhanced, by adjustment with H. pylori status. Also, a previous study has shown positive relationship between obesity and early gastric cancer under adjustment with H. pylori status [11]. Secondly, we could not achieve detailed information about gastric cancer such as stage, pathologic type, and whether it is cardia or non-cardia cancer. Also, the study participants may not well represent general population. Since we enrolled only those who had taken annual health check-ups during the 5 years, the majority must be non-manual workers. To overcome this selection bias, further study including large scale of general population is warranted. Also, those who were excluded because of unknown BMI showed similar baseline characteristics to the ever-obesity group (S1 Table). Some of them might have refused to measure their weight to hide that they were obese. However, considering that they were only 0.44% of the ever-obesity group, this may not have substantially affected the outcomes. Another limitation is the validity issue on the identification of gastric cancer with the NHIC data. However, if any, there must be very few cases of missing or false registration due to gastric cancer-mimicking lesions, because the identification of gastric cancer in this research was defined as those who achieved the health insurance benefit coverage for cancer patients. Lastly, there is possibility of incorrect onset time of gastric cancer development. However, we believe the 1 year lag applied to the selection of study population may have the power to overcome such bias.
In conclusion, current study confirmed that obesity-persistence increases the risk of gastric cancer in a manner of dose-response, especially among male younger than 65 years. However, the effect size varied according to behavioral factors such as smoking, alcohol consumption, and regular exercise. The present study demonstrated that sustained obesity longer than 5 years among male ≤ 65 years can be a good surrogate marker for gastric cancer development. Sustainable weight loss along with smoking/drinking cessation and regular exercise may have good primary preventive effect on gastric cancer.
Supplementary Information
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, epublic of Korea (grant number: HI18C1140).
Footnotes
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Ethical Statement
This study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB No. X-1608/360-906) and complied with the 1964 Declaration of Helsinki. Patient consent was waived due to the retrospective nature of this study using nationwide database, and which was approved by the Institutional Review Board.
Author Contributions
Conceived and designed the analysis: Lim JH, Shin CM, Han KD.
Performed the analysis: Lim JH, Shin CM, Han KD.
Wrote the paper: Lim JH.
Critical revision: Lee SW, Jin EH, Choi YJ, Yoon H, Park YS, Kim N, Lee DH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; Lyon. 7–14 June 1994; 1994. pp. 1–241. [PMC free article] [PubMed] [Google Scholar]
- 3.Hisamichi S, Sasaki R, Sugawara N, Yanbo T, Yamagata S. Stomach cancer in various age groups (Japan) as detected by gastric mass survey. J Am Geriatr Soc. 1979;27:439–43. doi: 10.1111/j.1532-5415.1979.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 4.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 5.Sung NY, Choi KS, Park EC, Park K, Lee SY, Lee AK, et al. Smoking, alcohol and gastric cancer risk in Korean men: the National Health Insurance Corporation Study. Br J Cancer. 2007;97:700–4. doi: 10.1038/sj.bjc.6603893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCullough ML, Robertson AS, Jacobs EJ, Chao A, Calle EE, Thun MJ. A prospective study of diet and stomach cancer mortality in United States men and women. Cancer Epidemiol Biomarkers Prev. 2001;10:1201–5. [PubMed] [Google Scholar]
- 7.Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 8.Buckley MJ, O’Shea J, Grace A, English L, Keane C, Hourihan D, et al. A community-based study of the epidemiology of Helicobacter pylori infection and associated asymptomatic gastroduodenal pathology. Eur J Gastroenterol Hepatol. 1998;10:375–9. doi: 10.1097/00042737-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. 2020;42:e2020004. doi: 10.4178/epih.e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Liu L, Wang X, Wang J, Yan Z, Cheng J, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev. 2013;22:1395–408. doi: 10.1158/1055-9965.EPI-13-0042. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Kim N, Kim HY, Lee HS, Yoon H, Shin CM, et al. Relationship between body mass index and the risk of early gastric cancer and dysplasia regardless of Helicobacter pylori infection. Gastric Cancer. 2015;18:762–73. doi: 10.1007/s10120-014-0429-0. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–73. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 14.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–6. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer: viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. 2013;24:609–17. doi: 10.1093/annonc/mds244. [DOI] [PubMed] [Google Scholar]
- 18.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317–32. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 19.Chaves J, Saif MW. IGF system in cancer: from bench to clinic. Anticancer Drugs. 2011;22:206–12. doi: 10.1097/CAD.0b013e32834258a1. [DOI] [PubMed] [Google Scholar]
- 20.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 21.Dalamaga M, Christodoulatos GS, Mantzoros CS. The role of extracellular and intracellular Nicotinamide phosphoribosyl-transferase in cancer: diagnostic and therapeutic perspectives and challenges. Metabolism. 2018;82:72–87. doi: 10.1016/j.metabol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–94. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan WL, Yuan LP, Yan XL, Yang DJ, Qiu MZ. More attention should be paid to adult gastric cancer patients younger than 35 years old: extremely poor prognosis was found. J Cancer. 2019;10:472–8. doi: 10.7150/jca.27517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv210. djv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, Luo W, Guo Z, Wu M, Zhou Z. The impact of interaction between alcohol consumption and obesity on incident hypertension. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49:728–32. [PubMed] [Google Scholar]
- 28.Luo WS, Chen F, Ji JM, Guo ZR. Interaction of tobacco smoking and alcohol consumption with obesity on cardiovascular disease in a Chinese cohort. Coron Artery Dis. 2020;31:372–7. doi: 10.1097/MCA.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 29.La Vecchia C. Hypothesis: is the fall in Helicobacter pylori related to the global rise in body mass index? Eur J Cancer Prev. 2011;20:556. doi: 10.1097/CEJ.0b013e32834a8018. [DOI] [PubMed] [Google Scholar]
- 30.Kamada T, Hata J, Kusunoki H, Ito M, Tanaka S, Kawamura Y, et al. Eradication of Helicobacter pylori increases the incidence of hyperlipidaemia and obesity in peptic ulcer patients. Dig Liver Dis. 2005;37:39–43. doi: 10.1016/j.dld.2004.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.