Abstract
Purpose
Current World Health Organization/International Society of Urological Pathology (2004 WHO/ISUP) grading of bladder urothelial carcinoma relies on the highest pathologic grade of the specimen and does not reflect the inherent qualitative and quantitative heterogeneity of disease.
Materials and Methods
We retrospectively studied consecutive urothelial high-grade cT1 (cT1HG) carcinomas submitted to adjuvant bacille Calmette–Guérin between 2008 and 2015 to evaluate the prognostic potential of grade 3 (presence or predominance) according to the 1973 WHO system concerning disease progression and cancer-specific death.
Results
Among 253 patients, grading distribution was 34.4% 1+2, 7.5% 2+1, 20.2% 2+2, 19.0% 2+3, 5.1% 3+2, and 13.8% 3+3. Recurrence was diagnosed in 115 (45.5%), progression in 83 (32.8%), and cancer-specific death in 50 patients (19.8%). Mean time to recurrence, progression, and death from disease were 35.9±31.7, 47.6±44.5, and 51.2±50.4 months, respectively. Grade 3 presence (2+3, 3+2, or 3+3) occurred in 96 (37.9%) and independently predicted time to progression (p<0.001; hazard ratio [HR], 3.11; 95% confidence interval [CI], 1.88–5.14). Grade 3 predominance (3+2 or 3+3) occurred in 48 (18.9%) and independently predicted time to disease-specific death.
Conclusions
Grade 3 presence and predominance are independent predictors of progression and disease-specific death and occur in about 40% and 20% of cT1HG, respectively. Describing qualitative and quantitative heterogeneity in urothelial carcinoma grading might improve the stratification of patients. This gives three prognostic high-grade groups based on WHO/ISUP 1973: prognostic grade group I (grade 3 absence), prognostic grade group II (grade 3 presence), and prognostic grade group III (grade 3 predominance).
Keywords: Histology, Prognosis, Urinary bladder neoplasms
Graphical Abstract

INTRODUCTION
Urothelial bladder carcinoma is a global health problem and the second most frequent malignant tumor and cause of death in patients with genitourinary cancer [1]. It is the fourth most common neoplasm in men and the ninth in women [2]. At the time of diagnosis, between 75% and 85% of the tumors are non-muscle-invasive bladder carcinoma (NMIBC), and half of patients will experience recurrence within 1 year after transurethral resection–exclusive treatment, which significantly decreases by adjuvant intravesical bacille Calmette–Guérin (BCG) instillation [3]. NMIBC has been shown to be a heterogeneous and complex tumor [4] that varies in clinical and biological behavior, mainly according to stage and grade [5].
Until 1998, the World Health Organization and the International Society of Urological Pathology (WHO/ISUP 1973) classified urothelial carcinomas in three grades: 1, 2, and 3. Since 2004, histological grade 1 is considered low-grade, and most of grade 2 and all of grade 3 are high-grade (WHO/ISUP 2004). This classification does not distinguish grade 2 from 3 and does not include the amounts of each grade in the high-grade specimen [6].
Considering the heterogeneity of high-grade NMIBC, we intended to investigate the prognostic value regarding progression and cancer-specific death of a modified classification system that accounts for high-grade type and quantity in urothelial high-grade cT1 (cT1HG) cancer, as proposed by Billis et al. [7] in 2001.
MATERIALS AND METHODS
After Institutional Ethics Committee approval (approval number: 570.640), we retrospectively analyzed 312 consecutive NMIBCs that underwent transurethral bladder resection (TURB) and adjuvant BCG between 2008 and 2015. Written informed consent was waived by the board.
Patients with variant histology, upper tract disease, cTa, low-grade cT1, not submitted to repeat TURB owing to muscle-missing sample, and incomplete BCG protocol were excluded. Among 253 consecutive patients with cT1HG disease, demography, histology, recurrence, progression, and cancer-specific mortality were evaluated.
To account for high-grade type and quantity, grade was classified as grade 2 or 3 according to the 1973 WHO/ISUP system and reported in a method of two numbers referring to the primary (predominant) and secondary (second most common) histological grade, respectively, by an expert uropathologist (AB). The number was repeated when only one grade was seen [7].
All patients underwent staging abdominopelvic computed tomography, and adjuvant treatment with intravesical BCG started 2 to 3 weeks after TURB in the following protocol: 6 weekly, 12 monthly, 4 trimonthly, and 2 half-yearly doses, for a total of 24 doses [8]. The cystoscopy follow-up protocol [9] was accompanied by urine cytology and biopsies when warranted in intervals of 4 months for 2 years, 6 months thereafter, and then annually after 5 years.
Recurrence was defined as the same or inferior previous grade or stage and treated by repeat induction BCG for those not choosing cystectomy. Progression was defined as higher-stage disease and these patients were offered cystectomy.
We used a Cox proportional hazards ratio model to evaluate clinical outcomes such as progression and cancer-specific death concerning the abovementioned classification corrected for age, sex, multiplicity, size, and concomitant carcinoma in situ (CIS) as confounders and the independent risk factors found by multivariate stepwise selection criteria. We used Kaplan–Meier curves to analyze the time to progression and cancer-specific death between groups. All analyses had a 5% level of significance. Statistical analyses were performed using SAS ver. 9.4. (SAS Institute, Cary, NC, USA).
RESULTS
The median age of the study population was 67 years (range, 31–83 y), with 192 (75.9%) male patients and 61 (24.1%) female, 212 (83.7%) white, and 41 (16.2%) nonwhite. CIS occurred in 36 (14.2%). The median tobacco consumption was 28.5 cigarettes/month, ranging from 0 to 440, and 220 patients (86.9%) had no occupational risk exposure (Table 1).
Table 1. Patient demographics and tumor characteristics (n=253).
| Characteristic | Value | |
|---|---|---|
| Age (y) | 66.6±10.4 | |
| Sex | ||
| Male | 192 (75.9) | |
| Female | 61 (24.1) | |
| Human race | ||
| White | 212 (83.7) | |
| Nonwhite | 41 (16.2) | |
| Cigarettes/month | 28.5 (0–440) | |
| Occupational risk | ||
| No | 220 (86.9) | |
| Yes | 33 (13.0) | |
| Tumor size (cm) | 3.9±2.3 | |
| Lesion | ||
| Multiple | 119 (47.0) | |
| Single | 134 (53.0) | |
| CIS presence | ||
| No | 217 (85.8) | |
| Yes | 36 (14.2) | |
| Lymphovascular invasion | ||
| No | 210 (83.0) | |
| Yes | 43 (17.0) | |
| Perineural invasion | ||
| No | 230 (90.9) | |
| Yes | 23 (9.1) | |
| Follow-up (mo) | 69 (11–156) | |
| Recurrence | ||
| No | 138 (54.5) | |
| Yes | 115 (45.5) | |
| Progression | ||
| No | 170 (67.2) | |
| Yes | 83 (32.8) | |
| Cancer-specific death | ||
| No | 203 (80.2) | |
| Yes | 50 (19.8) | |
| Cystectomy | ||
| No | 165 (65.2) | |
| Yes | 88 (34.8) | |
Values are presented as mean±standard deviation, number (%), or median (range).
CIS, carcinoma in situ.
Recurrence was diagnosed in 115 (45.5%), progression in 83 (32.8%), and cancer-specific death in 50 patients (19.8%). The mean time to disease recurrence, progression, and disease-specific death were 35.9±31.7, 47.6±44.5, and 51.2±50.4 months, respectively. During a median follow-up of 69 months (range, 11–156), 88 patients (34.8%) underwent cystectomy.
Table 2 shows the grading distribution using combined numbers. There were no significant differences regarding recurrence.
Table 2. Score distribution of 253 patients with cT1HG disease.
| WHO/ISUP1973/2004 | Score | Frequency | Percentage |
|---|---|---|---|
| 2/HG | 1+2 | 87 | 34.4 |
| 2/HG | 2+1 | 19 | 7.5 |
| 2/HG | 2+2 | 51 | 20.2 |
| 3/HG | 2+3 | 48 | 19.0 |
| 3/HG | 3+2 | 13 | 5.1 |
| 3/HG | 3+3 | 35 | 13.8 |
WHO, World Health Organization; ISUP, International Society of Urological Pathology; HG, high-grade.
Among high-grade groups, progression rates were significantly higher in grades 2+3 (p=0.0036; hazard ratio [HR], 4.404; 95% confidence interval [CI], 1.622–11.956), 3+2 (p=0.0012; HR, 5.453; 95% CI, 1.956–15.200), and 3+3 (p=0.0003; HR, 8.314; 95% CI, 2.629–26.290), and disease-specific mortality was significantly higher in grades 3+2 (p=0.0031; HR, 5.451; 95% CI, 1.774–16.752) and 3+3 (p=0.0040; HR, 6.401; 95% CI, 1.805–22.696), as shown in Table 3.
Table 3. Disease progression and survival Cox regression hazard ratio.
| Score | p-value | Hazard ratio | 95% confidence interval | |
|---|---|---|---|---|
| Disease progression | ||||
| 2+3 | 0.0036 | 4.404 | 1.622–11.956 | |
| 3+2 | 0.0012 | 5.453 | 1.956–15.200 | |
| 3+3 | 0.0003 | 8.314 | 2.629–26.290 | |
| Disease-specific survival | ||||
| 2+3 | 0.1782 | 2.282 | 0.687–7.584 | |
| 3+2 | 0.0031 | 5.451 | 1.774–16.752 | |
| 3+3 | 0.0040 | 6.401 | 1.805–22.696 | |
Compared to high-grade in the absence of grade 3 (1+2, 2+1, 2+2).
When corrected for age, sex, multiplicity, size, concomitant lymphovascular invasion, and CIS as confounders, grade 3 presence (2+3, 3+2, or 3+3: n=96, 37.9%) was the only independent predictor of progression (p=0.020), whereas grade 3 predominance (3+2 or 3+3: n=48, 18.9%) independently predicted disease-specific death (p=0.035). The reference groups in the model were grade 3 absence (n=157, 62.1%) and non-predominance (n=205, 81.0%), respectively. This resulted in three prognostic high-grade groups based on the WHO/ISUP 1973 system: grade 3 absence, presence, or predominance.
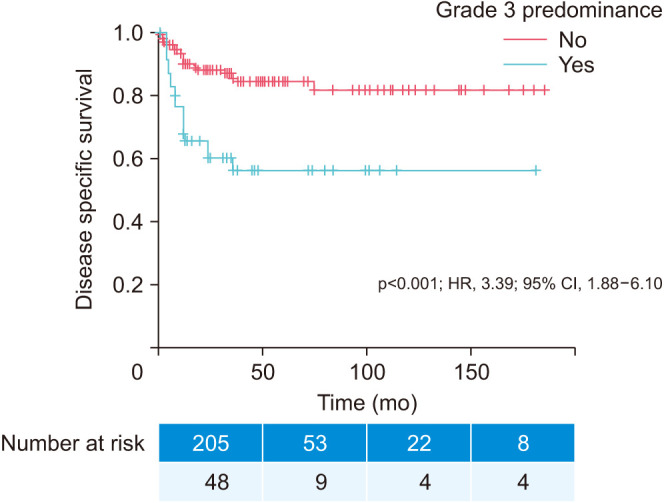
Kaplan–Meier curves in Fig. 1 and Fig. 2 illustrate the impact of grade 3 presence (prognostic grade group II) on time to progression (p<0.001; HR, 3.11; 95% CI, 1.88–5.14) and grade 3 predominance (prognostic grade group III) on time to cancer-specific death (p<0.001; HR, 3.39; 95% CI, 1.88–6.10), respectively.
Fig. 1. Kaplan–Meier time to progression curve. Grade 3 presence: no (n=157; 62.1%) versus yes (n=96; 37.9%). HR, hazard ratio; CI, confidence interval.
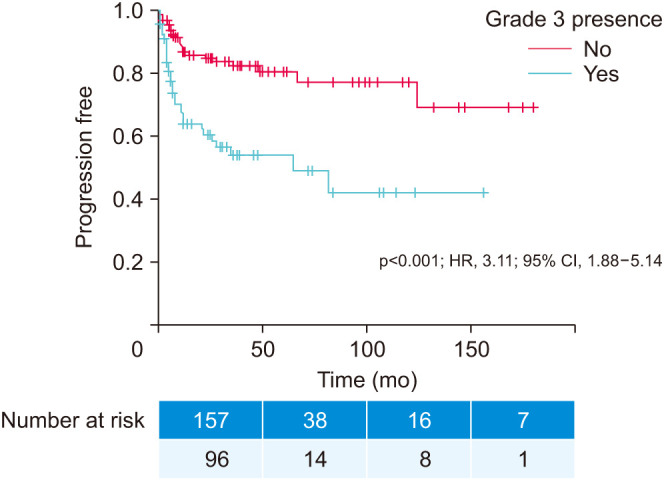
Fig. 2. Kaplan–Meier time to disease-specific death curve. Grade 3 predominance: no (n=205; 81.0%) versus yes (n=48; 18.9%). HR, hazard ratio; CI, confidence interval.
DISCUSSION
The current pathological classification of bladder cancer fails in the characterization and quantification of high-grade phenotypes, mainly cT1HG, one of the most important prognostic factors so far [10,11,12,13,14,15]. To address these shortcomings among high-grade patients, using combined grades based on the Billis et al.’s study [7], we propose three practical and accurate prognostic grade groups defined by grade 3 (1973 WHO/ISUP) absence, presence, or predominance. Grade 3 presence (2+3, 3+2, or 3+3) occurred in 96 patients (37.9%) and independently predicted time to progression, whereas grade 3 predominance (3+2 or 3+3) occurred in 48 patients (18.9%) and independently predicted time to disease-specific death. Our proposal is original and further categorizes cT1 high-grade disease grounded on solid endpoints, progression, and death. By contrast, the 2004 WHO/ISUP classification gives almost no prognostic information for these patients, nearly all of whom have disease classified as high-grade.
Previous studies that recognized grade 3 as the most relevant predictive factor for tumor progression, especially for the most challenging cT1 stage that is currently reduced to a one-tier system [16,17,18], support our results. Also in line with our proposal, van Rhijn et al. [19] reported the superiority of the WHO 1973 grading over the 2004 grading for both Ta and T1 bladder cancers in a large international series of NMIBC.
A systematic literature search analyzing more than 3,500 studies of bladder tumors compared the reproducibility and performance of these two classifications (1973 vs. 2004) and showed that the 1973 classification can identify the more aggressive tumors, and the 2004 system showed more concordance within and between observers [20]. In a comprehensive assessment, those methods do not seem to overcome each other.
Although the combined numbers method proposed herein might raise reproducibility concerns, it utilizes the foundation of an established grading system (1973 WHO/ISUP: grade 1, well-differentiated; grade 2, intermediate differentiation; grade 3, poorly differentiated), allowing enough information to qualify and quantify the inherent urothelial tumor heterogeneity that is missed in the high-grade group in the 2004 WHO/ISUP classification, ultimately impeding the necessary cT1HG subclassifications [21].
The guidelines of the European Association of Urology (EAU) suggest the simultaneous use of both (1973 and 2004) classification systems, and the National Institute for Health and Care Excellence (NICE) guidelines use the oldest classification system, whereas the American Urological Association (AUA)/Society of Urologic Oncology (SUO) and National Comprehensive Cancer Network (NCCN) adopt the most recent classification scheme [21,22].
Also, in a validated prognostic tool by the European Organization for Research and Treatment of Cancer (EORTC) and Urological Club of Oncology Treatment (CUETO), the 1973 WHO/ISUP grades 1, 2, or 3 add prognostic information when considered in conjunction with other clinical data [15]. Furthermore, the largest cT1 cohort published in 2018 did not identify any prognosticators for progression other than the 1973 WHO/ISUP grade system [23], which was confirmed and refined by current data. Combined with the 1973 or both WHO/ISUP classifications, the present proposal might optimize the timing of radical treatment for NMIBC. Early cystectomy should be considered in case of grade 3 predominance and intensive surveillance in case of grade 3 presence.
The grade 3 histological phenotype demonstrates extreme nuclear abnormalities, disordered architecture, loss of polarity, and frequent mitotic activity. Besides, the difference between grade 3 and combined grades 1 and 2 (1973 WHO/ISUP) in terms of gene expression amounts to 2,256 different genes (16.1%), compared with 741 genes (5.3%) between low- and high-grade (2004 WHO/ISUP) tumors (p<0.001). Moreover, between grades 1 and 2, only 53 genes differ significantly, whereas 1,269 genes differ between grade 2 and 3 tumors (0.4% vs. 9.1%, respectively, of 14,000 assayed genes). Stressing the difference between grades 2 and 3 and the similarity between grades 1 and 2, a marker of favorable prognosis, FGFR3 mutation, occurred in 75%, 61%, and 28% of grades 1, 2, and 3, respectively [24].
Although the current study presents limitations as a single-institution retrospective data analysis and needs future evaluation of interobserver variation, it is original and improves understanding of cT1HG bladder tumors. By individualizing treatment on the basis of risk stratification, it is possible to limit over- or undertreatment and enhance outcomes and patient benefits.
The current study also qualifies some additional aspects of treatment, including re-TURB and standard first-line adjuvant treatment with BCG and the maintenance schedule. The second point concerns the grading attribution that was prospectively and simultaneously assigned at the time of diagnosis, according to both WHO/ISUP classification systems (1973 and 2004) by a single uropathologist (AB), reproducing routine practice.
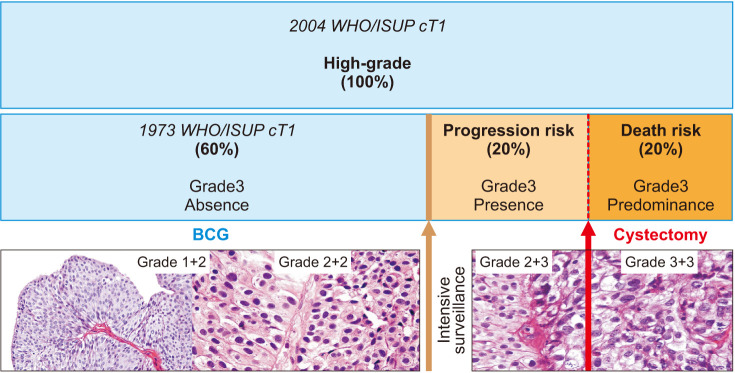
Our results on reproducibility and therapeutic impact should be confirmed in future prospective studies. Considering the neglected heterogeneity of the bladder tumor, for every 10 patients equally classified as high-grade on 2004 WHO/ISUP, the proposed classification will identify 4 (40%) at significantly higher risk for disease progression (grade 3 presence=prognostic grade group II), of whom 2 (50%) will be at higher risk for disease-specific death (grade 3 predominance=prognostic grade group III), as shown in Fig. 3.
Fig. 3. Prognostic impact and treatment decision of cT1HG based on World Health Organization/International Society of Urological Pathology (WHO/ISUP) 2004 versus 1973 grade 3 absence (prognostic grade group I), presence (prognostic grade group II), or predominance (prognostic grade group III). Grade 3: intense pleomorphism and disorganization with frequent mitoses. Grade 2: moderate pleomorphism and disorganization with hyperchromatic nuclei. Grade 1: uniform and orderly distributed nuclei without hyperchromasia or mitotic figures. BCG, bacille Calmette–Guérin.
CONCLUSIONS
Histological grade 3 presence and predominance (1973 WHO) are independent predictors of progression and death from disease and occur in about 40% and 20% of cT1HG, respectively. Describing the qualitative and quantitative heterogeneity in urothelial carcinoma grading might improve patients’ stratification. As such, we propose three prognostic high-grade groups based on WHO/ISUP 1973: prognostic grade group I (grade 3 absence), prognostic grade group II (grade 3 presence), and prognostic grade group III (grade 3 predominance).
ACKNOWLEDGMENTS
We acknowledge the involved institution(s), the patients, and those that provided and cared for study patients.
CAPES, Brazil - grant: BEX 14679/13-2
CNPq Research Productivity, Brazil - grants: 302622/2015-2 and 304747/2018-1
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Leonardo Oliveira Reis.
- Data acquisition: Gustavo B. de Mendonça.
- Statistical analysis: Luciana S. B. Dal Col and Diego M. Capibaribe.
- Data analysis and interpretation: Leonardo Oliveira Reis, Fernandes Denardi, and Athanase Billis.
- Drafting of the manuscript: Leonardo Oliveira Reis.
- Critical revision of the manuscript: All.
- Obtaining funding: Leonardo Oliveira Reis.
- Administrative, technical, or material support: Leonardo Oliveira Reis.
- Supervision: Leonardo Oliveira Reis.
- Approval of the final manuscript: All.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Schwaibold HE, Sivalingam S, May F, Hartung R. The value of a second transurethral resection for T1 bladder cancer. BJU Int. 2006;97:1199–1201. doi: 10.1111/j.1464-410X.2006.06144.x. [DOI] [PubMed] [Google Scholar]
- 4.Andrade DL, Moretti TBC, Neto WA, Benedetti J, Reis LO. Smoke load prognostic impact on bacillus Calmette-Guérin (BCG) treated non-muscle invasive bladder cancer. Int Urol Nephrol. 2020;52:1471–1476. doi: 10.1007/s11255-020-02438-6. [DOI] [PubMed] [Google Scholar]
- 5.Bryan RT, Zeegers MP, James ND, Wallace DM, Cheng KK. Biomarkers in bladder cancer. BJU Int. 2010;105:608–613. doi: 10.1111/j.1464-410X.2009.08880.x. [DOI] [PubMed] [Google Scholar]
- 6.Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol. 2005;13:143–153. doi: 10.1177/106689690501300203. [DOI] [PubMed] [Google Scholar]
- 7.Billis A, Carvalho RB, Mattos AC, Negretti F, Nogueira CR, Oliveira MC, et al. Tumor grade heterogeneity in urothelial bladder carcinoma--proposal of a system using combined numbers. Scand J Urol Nephrol. 2001;35:275–279. doi: 10.1080/003655901750425837. [DOI] [PubMed] [Google Scholar]
- 8.Carneiro BDB, Sanches BCF, Andrade DL, Voris BRI, Reis LO. Moreau strain bacillus Calmette-Guérin low versus standard dose in the treatment of high-grade T1 bladder cancer: a retrospective observational cohort study. Clin Genitourin Cancer. 2019;17:e779–e783. doi: 10.1016/j.clgc.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Datovo JCF, Neto WA, Mendonça GB, Andrade DL, Reis LO. Prognostic impact of non-adherence to follow-up cystoscopy in non-muscle-invasive bladder cancer (NMIBC) World J Urol. 2019;37:2067–2071. doi: 10.1007/s00345-019-02697-8. [DOI] [PubMed] [Google Scholar]
- 10.Lapham RL, Grignon D, Ro JY. Pathologic prognostic parameters in bladder urothelial biopsy, transurethral resection, and cystectomy specimens. Semin Diagn Pathol. 1997;14:109–122. [PubMed] [Google Scholar]
- 11.Jordan AM, Weingarten J, Murphy WM. Transitional cell neoplasms of the urinary bladder. Can biologic potential be predicted from histologic grading? Cancer. 1987;60:2766–2774. doi: 10.1002/1097-0142(19871201)60:11<2766::aid-cncr2820601129>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Mostofi FK, Sobin LH, Torloni H. Histological typing of urinary bladder tumours. Geneva: World Health Organization; 1973. pp. 15–19. [Google Scholar]
- 13.Murphy WM, Beckwith JB, Farrow GM. Tumors of the kidney, bladder, and related urinary structures. Washington DC: Armed Forces Institute of Pathology; 1994. pp. 211–219. [Google Scholar]
- 14.Eble JN, Sauter G, Epstein J, Sesterhenn I. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press; 2004. [Google Scholar]
- 15.Reis LO, Taheri D, Chaux A, Guner G, Mendoza Rodriguez MA, Bivalacqua TJ, et al. Significance of a minor high-grade component in a low-grade noninvasive papillary urothelial carcinoma of bladder. Hum Pathol. 2016;47:20–25. doi: 10.1016/j.humpath.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Lokeshwar SD, Ruiz-Cordero R, Hupe MC, Jorda M, Soloway MS. Impact of 2004 ISUP/WHO classification on bladder cancer grading. World J Urol. 2015;33:1929–1936. doi: 10.1007/s00345-015-1548-x. [DOI] [PubMed] [Google Scholar]
- 17.Otto W, Denzinger S, Fritsche HM, Burger M, Wieland WF, Hofstädter F, et al. The WHO classification of 1973 is more suitable than the WHO classification of 2004 for predicting survival in pT1 urothelial bladder cancer. BJU Int. 2011;107:404–408. doi: 10.1111/j.1464-410X.2010.09515.x. [DOI] [PubMed] [Google Scholar]
- 18.Pellucchi F, Freschi M, Moschini M, Rocchini L, Maccagnano C, Nazareno S, et al. Oncological predictive value of the 2004 World Health Organisation grading classification in primary T1 non-muscle-invasive bladder cancer. A step forward or back? BJU Int. 2015;115:267–273. doi: 10.1111/bju.12666. [DOI] [PubMed] [Google Scholar]
- 19.van Rhijn BWG, Hentschel AE, Bründl J, Compérat EM, Hernández V, Čapoun O, et al. Multi-center EAU Non-Muscle-Invasive Bladder Cancer Guidelines Panel Study Consortium on the WHO1973 WHO 2004 2016 Classification Systems for Grade Prognostic value of the WHO1973 and WHO2004/2016 classification systems for grade in primary Ta/T1 non-muscle-invasive bladder cancer: a multicenter European Association of Urology non-muscle-invasive bladder cancer guidelines panel study. Eur Urol Oncol. 2021;4:182–191. doi: 10.1016/j.euo.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Soukup V, Čapoun O, Cohen D, Hernández V, Babjuk M, Burger M, et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification systems in non-muscle-invasive bladder cancer: a European Association of Urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur Urol. 2017;72:801–813. doi: 10.1016/j.eururo.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. 2017;119:371–380. doi: 10.1111/bju.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis LO, Moro JC, Ribeiro LF, Voris BR, Sadi MV. Are we following the guidelines on non-muscle invasive bladder cancer? Int Braz J Urol. 2016;42:22–28. doi: 10.1590/S1677-5538.IBJU.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Putte EEF, Bosschieter J, van der Kwast TH, Bertz S, Denzinger S, Manach Q, et al. The World Health Organization 1973 classification system for grade is an important prognosticator in T1 non-muscle-invasive bladder cancer. BJU Int. 2018;122:978–985. doi: 10.1111/bju.14238. [DOI] [PubMed] [Google Scholar]
- 24.Liedberg F, Lauss M, Patschan O, Aine M, Chebil G, Cwikiel M, et al. Lund Bladder Cancer Group. The importance of being grade 3: WHO 1999 versus WHO 2004 pathologic grading. Eur Urol. 2012;62:620–623. doi: 10.1016/j.eururo.2012.05.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.