Abstract
Purpose
To compare hospital readmissions, biochemical recurrence rates, incidence of metastasis, and cancer-specific and overall mortality for prostate cancer patients undergoing radiotherapy vs. radical prostatectomy. The secondary outcome was to identify patient and disease characteristics affecting physician’s choice of either therapy.
Materials and Methods
A total of 297 patients diagnosed with prostate cancer between 2008 and 2014 were identified from a single academic center’s cancer database. Clinical information including age, ethnicity, comorbidities, prostate-specific antigen, Gleason score, stage, National Comprehensive Cancer Network (NCCN) risk group, biochemical recurrence, hospital readmissions, and survival outcomes were gathered and analyzed from ambulatory medical records until 2018.
Results
Patients selected for radiotherapy were older and had more comorbidities and NCCN high-risk disease. Biochemical recurrence was higher after radical prostatectomy for locally advanced disease, 59.3% vs. 20.0% (p<0.001), favoring radiotherapy. Hospital readmission was higher for patients with locally advanced disease undergoing radiotherapy, 48.6% vs. 18.5% (p=0.002), and 35.2% vs. 19.7% (p=0.044) for those with localized disease, with most of these readmissions occurring 24 months after the initial therapy. Radiation proctitis and colitis were the most common complications after radiotherapy and accounted for 46.3% of readmissions.
Conclusions
Selection of patients for radiotherapy instead of surgery was influenced by age, significant comorbidities, and NCCN high-risk disease. The incidence of treatment- or cancer-related hospital readmissions was significantly higher for patients undergoing radiotherapy compared with radical prostatectomy, especially for those with locally advanced prostate cancer. This information may be useful in guiding a patient’s choice of therapy.
Keywords: Hospital readmission, Prostate cancer, Prostatectomy, Radiation therapy
INTRODUCTION
In the post-ProtecT trial [1] era, we now know that prostate cancer–specific and overall survival at 10 years are equivalent for both surgery and radiotherapy. Choosing between these therapies has become more nuanced and the almost equivalent outcomes with watchful waiting for low-risk prostate cancer [2] has reinforced the risks of overtreatment and the morbidity of the therapies that we recommend. It is the task of the managing physician to guide patients toward an informed choice between radiation and surgery for those with intermediate, high-risk, and locally advanced disease. In this regard, patient factors, disease factors, and the adverse effect profiles of each therapy are important considerations [3].
Patient-reported quality of life data with a 6-year follow-up have been reported from the cohort of men recruited for the ProtecT trial [4]. The findings of this study suggested that sexual function and urinary continence were worse in men undergoing prostatectomy and that bowel function was worse in men undergoing radiotherapy with no significant difference in general health-related or cancer-related quality of life between treatment choices. Although these data do provide a broad overview of the adverse effects that patients can expect from either therapy, they do not provide insight into the severe complications with either therapy. Also, this study was performed in a heavily prostate-specific antigen (PSA)-screened population and included only localized prostate cancer. It is currently unanswered whether the adverse effects of either therapy are different for locally advanced prostate cancer, the incidence of which is higher in many Asian countries that do not perform population-based screening. Severe complications requiring hospital admission have also not been previously compared between radiotherapy and surgery.
In this academic, single-center retrospective cohort study of patients with prostate cancer, we compared outcomes between surgery and radiotherapy for patients with localized or and locally advanced prostate cancer, in terms of biochemical recurrence (BCR) rates, hospital readmission rates, incidence of metastases, prostate cancer–specific mortality, and overall mortality. We also sought to identify the patient and disease characteristics that were the primary determinants of a physician’s choice between radiotherapy and surgery.
MATERIALS AND METHODS
The National University Hospital Cancer Registry Database was used to identify patients who were diagnosed, treated, and followed up for prostate cancer at National University Hospital (Singapore) between January 1, 2008 and December 31, 2014. Demographic details and clinical information including age, ethnicity, comorbidities, serum PSA, Gleason score, histopathologic stage, National Comprehensive Cancer Network (NCCN) risk group [5], BCR, hospital readmissions, and survival outcomes were gathered from ambulatory medical records up until June 2018. As this was a study performed retrospectively from a database without patient contact, our institution ethics board allowed for a waiver of consent for this particular study and the study was ethics approved. It would be inaccurate for us to state that informed consent was obtained from all participants. The study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB Reference: 2018/00360).
Our primary outcomes were BCR rates, hospital readmission rates, incidence of metastasis, prostate cancer–specific mortality, and overall mortality arising from the choice of primary intervention. Secondary outcomes were identifying patient demographics and disease characteristics affecting the choice of therapy.
We identified a total of 297 patients with clinically localized (clinical stage ≤T2c) or locally advanced (clinical stage T3/T4 or presence of nodal disease) prostate cancer between 2008 and 2014 who underwent either radiation therapy or radical prostatectomy. Patients who underwent active surveillance, underwent watchful waiting, or had metastasis at the time of presentation were excluded.
We defined BCR according to the criteria set out by the EAU-ESTRO-ESUR-SIOG guidelines 2018 [6] on prostate cancer as two consecutive PSA values of >0.2 ng/mL and a rising PSA trend after radical prostatectomy or a PSA increase of ≥2 ng/mL above the PSA nadir after radiation therapy. Metastatic progression was defined as new metastatic disease detected on either technetium-99 bone scan or on cross-sectional imaging.
1. Radical prostatectomy and radiotherapy methods
Three different surgical techniques were used: open, laparoscopic, and robot-assisted radical prostatectomy, all via the retropubic approach described by Walsh [7]. A nerve-sparing operation was done at the discretion of the individual surgeons. The decision to undertake a minimally invasive or an open approach was at the discretion of the individual surgeons. Bilateral pelvic lymphadenectomy of the obturator, internal iliac, and external iliac nodes was performed for all patients with NCCN high-risk or intermediate-risk disease with a Briganti score of more than 5%. Histopathologic staging was performed on all resected prostate specimens to determine surgical margins, extraprostatic extension of the tumor, and the presence of positive lymph nodes. All radical prostatectomy samples were centrally reviewed at our institution’s multidisciplinary tumor board.
Two different types of radiotherapy treatments were used in the management of prostate cancer: external beam radiotherapy (EBRT) alone or EBRT with high-dose-rate (HDR) brachytherapy boost. EBRT monotherapy was delivered using three-dimensional (3D)-conformal radiotherapy to the prostate. The radiation dose received in standard EBRT was 78 Gray (Gy) and was administered in 2-Gy fractions over 39 cycles.
Patients who received EBRT with HDR brachytherapy boost received 45 to 50 Gy in 20 cycles via 3D-conformal radiotherapy and 19 Gy over 2 fractions of HDR brachytherapy via transperineal sheath insertion into the prostate with administration of iridium-192. Patients with intermediate-risk disease were concurrently treated with 6 months of adjuvant androgen-deprivation therapy (ADT) and those with high-risk disease were treated with 3 years of ADT.
2. Statistical methods
Continuous data are expressed as median (interquartile range [IQR]) whereas discrete data are presented as a number and percent. The Mann–Whitney test was used for continuous variables and γ2 or Fisher’s exact test for categorical variables to investigate differences in distributions of patient and tumor characteristics by treatment groups. Cox proportional hazard regression models were used to determine cancer-specific and overall survival. Adjustment for potential confounders of mortality such as age, NCCN risk group, clinical stage, and Charlson Comorbidity score were adjusted for by use of a proportional hazards model. Overall survival and disease-free progression for each treatment group and the statistical significance was determined with the log-rank test. All statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 22 (IBM Corp., Armonk, NY, USA). Statistical significance was set at a p-value of <0.05.
RESULTS
Of the 297 men included in the study, 120 (40.4%) underwent surgery and 177 (59.6%) underwent radiation therapy. The mean age at diagnosis was 67.6 years. The median follow-up duration was 5.1 years (range, 3.4–9.8 y). Table 1 presents the demographic and disease characteristics of the men in each treatment group. The proportion of men with locally advanced disease in our cohort was 30.0%, compared with 16.3% in a contemporary series [8] from the West, probably because of the lower rate of PSA screening. Patients who received radiation therapy were older (median 70.0 years vs. 64.0 years, p<0.001), had more comorbidities with Charlson Comorbidity Index >2 (p=0.02), and were more likely to have NCCN high-risk disease (p<0.01).
Table 1. Baseline patient demographics and disease characteristics stratified by stage of disease and treatment type.
| Characteristic | Locally advanced disease | Localized disease | |||||
|---|---|---|---|---|---|---|---|
| Surgery (n=54) | Radiotherapy (n=35) | p-value | Surgery (n=66) | Radiotherapy (n=142) | p-value | ||
| Age (y) | 64.0 (60.0–69.0) | 70.0 (63.0–76.5) | <0.001 | 64.0 (61.0–68.8) | 70.5 (65.0–75.0) | <0.001 | |
| Ethnicity | 0.177 | 0.426 | |||||
| Chinese | 43 (79.6) | 25 (71.4) | 54 (81.8) | 119 (83.8) | |||
| Others | 11 (20.4) | 10 (28.6) | 12 (18.2) | 23 (16.2) | |||
| Charlson Comorbidity Index | 0.02 | 0.004 | |||||
| ≤10 | 29 (53.7) | 10 (28.6) | 30 (45.5) | 36 (25.4) | |||
| >2 | 25 (46.3) | 25 (71.4) | 36 (54.5) | 106 (74.6) | |||
| NCCN risk group | >0.999 | <0.01 | |||||
| Low | 0 (0.0) | 0 (0.0) | 12 (18.2) | 15 (10.6) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 9 (13.6) | 18 (12.7) | |||
| High | 54 (100.0) | 35 (100.0) | 45 (68.2) | 109 (76.8) | |||
| Gleason score | 0.006 | <0.001 | |||||
| <6 | 3 (5.6) | 5 (14.3) | 18 (27.3) | 35 (24.6) | |||
| 7 | 37 (68.5) | 12 (34.3) | 46 (69.7) | 59 (41.5) | |||
| 8–10 | 14 (25.9) | 18 (51.4) | 2 (3.0) | 48 (33.8) | |||
| Initial PSA (ng/mL) | 0.181 | <0.001 | |||||
| <10 | 21 (38.9) | 11 (31.4) | 47 (71.2) | 41 (28.9) | |||
| 10–20 | 17 (31.5) | 7 (20.0) | 13 (19.7) | 43 (30.3) | |||
| >20 | 16 (29.6) | 17 (48.6) | 6 (9.1) | 58 (40.8) | |||
| Radical prostatectomy | - | - | |||||
| Open | 28 (51.9) | NA | 17 (25.8) | NA | |||
| Laparoscopic | 2 (3.7) | NA | 2 (3.0) | NA | |||
| Robotic | 24 (44.4) | NA | 47 (71.2) | NA | |||
| Radiation therapy | - | - | |||||
| EBRT | NA | 26 (74.3) | NA | 104 (73.2) | |||
| Brachytherapy | NA | 5 (14.3) | NA | 27 (19.0) | |||
| EBRT+brachytherapy | NA | 4 (11.4) | NA | 11 (7.7) | |||
Values are presented as median (range) or number (%).
NCCN, National Comprehensive Cancer Network; PSA, prostate-specific antigen; EBRT, external beam radiotherapy; NA, not available.
With a median follow-up duration of 5.1 years, death due to prostate cancer was rare, occurring in 2.4% of patients (Table 2). There was no significant difference in prostate cancer–specific mortality between radiation and surgery (hazard ratio [HR], 0.18; 95% confidence interval [CI], 0.03–3.94; p=0.32). All-cause mortality was higher in the radiotherapy group than in the surgery group (Table 2). However, after adjustment for the confounding covariates of age and comorbidities, this effect was not statistically significant (HR, 0.36; 95% CI, 0.12–1.14; p=0.08). There was no significant difference in the incidence of metastatic disease between the groups (locally advanced disease, p=0.275; localized disease, p=0.409).
Table 2. Cancer-specific mortality, overall mortality, BCR, metastatic disease, and hospital readmissions by stage and treatment group.
| Variable | Locally advanced disease | Clinically localized disease | |||||
|---|---|---|---|---|---|---|---|
| Surgery (n=54) | Radiotherapy (n=35) | p-value | Surgery (n=66) | Radiotherapy (n=142) | p-value | ||
| Cancer-specific mortality | 0.082 | 0.597 | |||||
| Total person-years in follow-up | 254 | 168 | 344 | 768 | |||
| Number of deaths due to prostate cancer | 0 | 2 | 1 | 4 | |||
| Prostate cancer deaths per 1,000 person-years | 0.0 (0.0–14.4) | 11.9 (1.3–43.0) | 2.9 (0.0–16.2) | 5.2 (1.4–13.3) | |||
| Overall mortality | 0.003 | 0.019 | |||||
| Total person-years in follow-up | 254 | 168 | 344 | 768 | |||
| Number of deaths due to any cause | 1 | 8 | 4 | 29 | |||
| All-cause deaths per 1,000 person-years | 3.9 (0.1–21.9) | 47.6 (20.5–93.8) | 11.6 (3.1–29.8) | 37.8 (25.3–54.2) | |||
| Incidence of BCR | <0.001 | 0.897 | |||||
| Person-years in follow-up free of BCR | 155 | 150 | 297 | 699 | |||
| Number of men with BCR | 32 | 7 | 12 | 27 | |||
| BCR per 1,000 person-years | 207.0 (141.0–292.0) | 46.7 (18.7–96.2) | 40.4 (20.9–70.6) | 38.6 (25.5–56.2) | |||
| Incidence of metastatic disease | 0.275 | 0.409 | |||||
| Person-years in follow-up free of metastatic disease | 240 | 154 | 338 | 744 | |||
| Number of men with metastatic disease | 6 | 7 | 4 | 14 | |||
| Metastatic disease per 1,000 person-years | 25.0 (9.1–54.4) | 45.5 (18.2–93.7) | 11.8 (3.2–30.3) | 18.8 (10.3–31.6) | |||
| Incidence of hospital readmissions | 0.002 | 0.044 | |||||
| Number of men with hospital readmissions | 10 | 17 | 13 | 50 | |||
| Proportion of men with readmissions | 18.5 | 48.6 | 19.7 | 35.2 | |||
| Hospital readmissions per 1,000 person-years | 46.3 (22.2–85.1) | 146.0 (85.3–235.0) | 54.2 | (30.3–89.3) | 82.8 (61.8–109.0) | ||
Values are presented as number only, hazard ratio (95% confidence interval), or percentage only.
BCR, biochemical recurrence.
The incidence of BCR was higher after radical prostatectomy for men with locally advanced disease. The BCR rate per 1,000 person-years was 207.0 after surgery compared with 46.7 after radiation (p<0.001). In the cohort with clinically localized disease, there was no significant difference in the incidence of BCR (p=0.897) between the radiotherapy and surgery groups.
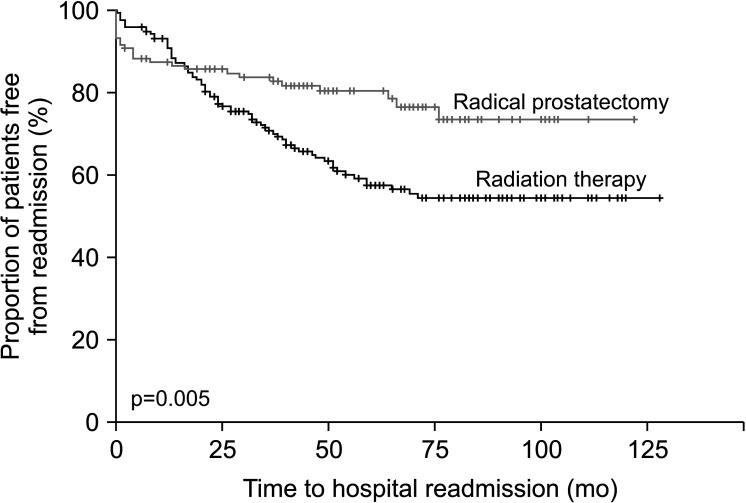
A total of 30.3% of patients were readmitted for prostate cancer and treatment-related complications over the median 5.1 years of follow-up. The proportion of patients readmitted to the hospital was higher for patients undergoing radiotherapy than surgery, 48.6% vs. 18.5% (p=0.002) for those with locally advanced disease and 35.2% vs. 19.7% (p=0.044) for those with localized disease, respectively. Radiation proctitis and colitis were the most common complications after radiotherapy, occurring in 17.5% of all patients and accounting for 46.3% of readmissions (Table 3). Urethral strictures were the most common complication after radical prostatectomy, occurring in 5.0% of all patients and accounting for 26.1% of readmissions (Table 4). In the first 24 months after treatment, more hospital readmissions were seen in the radical prostatectomy group than in the radiation therapy group, but this trend reversed with late hospital readmissions being significantly greater in the radiation therapy group (p=0.005) (Fig. 1). In the first 12 months after primary therapy, the hospital readmission rate after radical prostatectomy was 12.5% compared with 3.4% after radiotherapy.
Table 3. Reasons for hospital readmission after radiotherapy.
| Admission reason | Number of cases | Proportion of total readmissions, % (n=67) | Proportion of total patients, % (n=177) |
|---|---|---|---|
| Total readmissions | 67 | - | 37.9 |
| Radiation proctitis/colitis (RTOG grade 2/3/4) | 31 | 46.3 | 17.5 |
| Radiation cystitis (RTOG grade 3/4) | 12 | 17.9 | 6.8 |
| Urethral stricture | 8 | 11.9 | 4.5 |
| Urinary retention | 8 | 11.9 | 4.5 |
| Metastasis-related symptoms | 4 | 6.0 | 2.3 |
| Others | 4 | 6.0 | 2.3 |
RTOG, Radiation Therapy Oncology Group.
Table 4. Reasons for hospital readmission after radical prostatectomy.
| Admission reason | Number of cases | Proportion of total readmissions, % (n=23) | Proportion of total patients, % (n=120) |
|---|---|---|---|
| Total readmissions | 23 | - | 19.2 |
| Urethral stricture | 6 | 26.1 | 5.0 |
| Infection | 5 | 21.7 | 4.2 |
| Metastasis-related symptoms | 3 | 13.0 | 2.5 |
| Lymphocele | 2 | 8.7 | 1.7 |
| Urinary retention | 1 | 4.3 | 0.8 |
| Others | 6 | 26.1 | 5.0 |
Fig. 1. Readmissions over time.
DISCUSSION
Consistent with demographic trends in other observational prostate cancer studies [9,10,11], patients who were selected for radiation therapy were older, had more comorbidities, and had a greater proportion of NCCN high-risk disease. These seem to be the major factors that influence a physician’s choice of treatment between the two treatment modalities.
There was a statistically significant difference in BCR in men with locally advanced disease, favoring radiation over surgery. This difference was not seen in men with localized disease. It is more common for men with locally advanced disease undergoing radical prostatectomy to have positive surgical margins, which might be the initial focus for local recurrence. Although not captured in our data, a proportion of patients initially thought to have intermediate localized risk disease on the basis of initial clinical staging might not have undergone lymph node dissection at the time of prostatectomy. These patients may be pathologically upstaged to T3a or higher after prostatectomy. Micro-metastatic nodal disease might be an explanation for this BCR compared with patients presenting upfront as clinical T3a or higher, in whom EBRT to the prostate and pelvic nodes would be more likely to cover all malignant cells within the radiation field.
Of the 54 patients in our study with pathologically staged locally advanced prostate cancer, 28 (51.9%) were clinically staged to have localized disease initially and were upstaged after surgery. Twenty-four of these patients had clinical staging by magnetic resonance imaging of the prostate and 4 by computed tomography imaging. Comparing pathological stage T3a or higher after prostatectomy with clinical stage T3a or higher with radiation therapy would seem an intuitive limitation, because radiologically evident T3 disease might imply a higher disease burden. However, there is no evidence that there is a difference in prognosis between macroscopic and microscopic T3a prostate cancer. Furthermore, in this series, patients treated with radiotherapy had a longer time to BCR than did those who underwent surgery.
One potential criticism for the difference in BCR was that 88.1% of men in the radiotherapy group had received adjuvant ADT, which may have masked early cancer progression or cancer persistence. However, this alone is an insufficient explanation because the maximum duration of ADT was 3 years for men with NCCN high-risk prostate cancer. The duration of our study’s median follow-up was 5.1 years (range, 3.4–9.8 y), meaning that all patients were outside this ADT window at the time of analysis. Also, there was no statistically significant difference in BCR among men with localized prostate cancer even though 76.8% had NCCN high-risk disease and had also received 3 years of adjuvant ADT with radiotherapy.
The difference in BCR did not translate into a difference in prostate cancer–specific survival or overall survival in our study. The increased overall mortality seen in the radiation therapy group initially was not found to be statistically significant after adjustment for the covariates of age and comorbidities. Given the long natural history of prostate cancer, meaningful differences in cancer-specific and overall survival are unlikely to be seen with the short 5.1-year follow-up duration of our study, which we recognize as one of its limitations. Even studies with longer follow-up such as the ProtecT trial [1] failed to show a significant difference in overall or cancer-specific mortality after 10 years. At least 15 to 20 years of follow-up data is probably required to show differences in oncologic survival, if any, between radiotherapy and surgery for prostate cancer. At present, only the adverse effects of therapy, age, comorbidities, and the technical likelihood of complete resection can be used to guide the decision between radiotherapy and surgery.
The 5-year hospital readmission rate was higher for patients undergoing radiotherapy than for those undergoing radical prostatectomy, which suggests that severe adverse effects are more common among patients treated with radiotherapy. Concerning readmission, 48.6% vs. 18.5% with locally advanced cancer were readmitted and 35.2% vs. 19.7% with localized cancer were readmitted after radiotherapy compared with radical prostatectomy, respectively. Our finding of radiation-induced bowel complications as one of the most common causes of morbidity after radiotherapy, occurring in 17.5% of patients and accounting for 46.3% of all readmissions, is in keeping with the broader literature. Radiation Therapy Oncology Group (RTOG) bowel complications of grade 2 or higher are reported with an incidence of 15% [12].
Of the patients treated with radical prostatectomy, 9 of 66 patients with localized disease and 26 of 54 patients with locally advanced disease had salvage or adjuvant radiotherapy in our series. This could be a confounder in determining the cause of readmission in these patients. In our detailed analysis of the causes of readmission in radical prostatectomy patients, only urethral strictures and urinary retention were attributable to either surgery or radiotherapy. Of the six patients in this group (see Table 4) who developed urethral strictures, five did not receive radiotherapy and one developed a stricture before salvage radiotherapy. The one patient who developed urinary retention in the prostatectomy series did not receive radiotherapy. Only one of the patients in this series who developed BCR after primary radiotherapy underwent a salvage prostatectomy and had no hospital readmissions.
The decision to admit a patient for treatment-related complications was at the discretion of individual physicians. Also, this study reflects the practices of a single institution. These limitations may affect the generalizability of our results.
In our study, we anticipated and found baseline differences in median age (70.0 vs. 64.0 y) and the number of comorbidities in patients undergoing radiation therapy compared with surgery. To mitigate the potential impact of these confounders on hospital readmissions, the study was designed to include only readmissions strictly related to cancer or cancer therapy.
One of the largest studies on quality of life in men with localized prostate cancer after prostate cancer treatment reported worse sexual function and urinary continence in men undergoing prostatectomy and worse bowel function in men undergoing radiotherapy with no overall differences in quality of life between treatments [4]. This study included only localized prostate cancer patients and thus it is hard to extrapolate the findings to those presenting with locally advanced disease. Singapore, like many other countries in Asia, does not practice population-based PSA screening and the proportion of men with locally advanced disease in our cohort, at 30.0%, is comparatively higher than in many Western populations [8]. We found that cancer-related readmission rates for patients with locally advanced cancer undergoing radiotherapy are significantly higher than for those undergoing surgery. The increased radiation field to these patients to cover the pelvic lymph nodes may contribute to increased bowel and bladder adverse effects.
In the first 24 months after therapy, more hospital readmissions were seen in the radical prostatectomy group than in the radiation therapy group, with this trend reversing after 24 months. The majority of surgical complications requiring readmission, such as infection or lymphocele, tend to present early. Urethral or anastomotic strictures are also more likely to develop within the first 2 years after surgery. This is in contrast to the complications of radiation therapy such as radiation cystitis or proctitis, which may not develop until many years after surgery. The long latent “silent interval” before the adverse effects of radiotherapy manifest typically ranges from months to several years. It is thought to be due to the target cell hypothesis, which postulates that irreversible cellular damage expresses itself in due course as radiated cells attempt mitosis, experience mitotic death, and subsequently compromise organ function over time [13]. The higher delayed readmissions seen in patients who underwent radiotherapy in this study demonstrate this.
One limitation of our study is that the 85.7% of our patients who underwent radiotherapy received EBRT using 3D-conformal radiotherapy as this was standard of care at the time. In the last decade, many centers have transitioned to the use of intensity-modulated radiotherapy, which has been shown in some series to reduce gastrointestinal toxicity with a reported reduction in incidence of 4.9 vs. 6.5 per 100 person-years (adjusted HR, 0.66) [14]. Thus, the reported frequency of radiotherapy adverse effects could be overestimated in our series compared with a more contemporary cohort.
Urology department audits often focus on complications and readmissions related to surgery. It becomes easy to underestimate the frequency and severity of complications when they happen years after the conclusion of therapy or when the most common reason for hospitalization, radiation enteritis, leads to admission under the colorectal department. This study puts into perspective the proportion of patients affected and the potential severity of radiotherapy adverse effects.
CONCLUSIONS
Selection of patients for radiotherapy instead of surgery was influenced by age, significant comorbidities, and NCCN high-risk disease. The incidence of BCR was significantly lower for patients with locally advanced prostate cancer undergoing radiotherapy compared with radical prostatectomy, but this did not translate into a difference in overall or cancer-specific survival at a median follow-up of 5 years. The incidence of treatment- or cancer-related hospital readmissions was significantly higher for patients undergoing radiotherapy, especially for those with locally advanced prostate cancer. The majority of readmissions occurred after 24 months from the initial therapy, most commonly from radiation proctitis or colitis. This information may be useful in guiding a patient’s choice of therapy.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose
- Research conception and design: Arshvin Kesavan and Kesavan Esuvaranathan.
- Data acquisition: Keeran Vickneson.
- Statistical analysis: Keeran Vickneson.
- Data analysis and interpretation: Arshvin Kesavan.
- Drafting of the manuscript: Arshvin Kesavan and Keeran Vickneson.
- Critical revision of the manuscript: Kesavan Esuvaranathan.
- Supervision: Kesavan Esuvaranathan.
- Approval of the final manuscript: Kesavan Esuvaranathan.
References
- 1.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 2.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–1793. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Tomaszewski JE, et al. The combination of preoperative prostate specific antigen and postoperative pathological findings to predict prostate specific antigen outcome in clinically localized prostate cancer. J Urol. 1998;160(6 Pt 1):2096–2101. doi: 10.1097/00005392-199812010-00041. [DOI] [PubMed] [Google Scholar]
- 6.Mottet N, van den Bergh RCN, Briers E, Bourke L, Cornford P, De Santis M, et al. EAU–ESTRO–ESUR–SIOG guidelines on prostate cancer. Arnhem: European Association of Urology; 2018. [Google Scholar]
- 7.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160(6 Pt 2):2418–2424. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 8.Falchook AD, Martin NE, Basak R, Smith AB, Milowsky MI, Chen RC. Stage at presentation and survival outcomes of patients with Gleason 8-10 prostate cancer and low prostate-specific antigen. Urol Oncol. 2016;34:119.e19–119.e26. doi: 10.1016/j.urolonc.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Sooriakumaran P, Nyberg T, Akre O, Haendler L, Heus I, Olsson M, et al. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. BMJ. 2014;348:g1502. doi: 10.1136/bmj.g1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, Horwitz EM, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–2891. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmid MP, Pötter R, Bombosch V, Sljivic S, Kirisits C, Dörr W, et al. Late gastrointestinal and urogenital side-effects after radiotherapy--incidence and prevalence. Subgroup-analysis within the prospective Austrian-German phase II multicenter trial for localized prostate cancer. Radiother Oncol. 2012;104:114–118. doi: 10.1016/j.radonc.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 14.Sujenthiran A, Nossiter J, Charman SC, Parry M, Dasgupta P, van der Meulen J, et al. National population-based study comparing treatment-related toxicity in men who received intensity modulated versus 3-dimensional conformal radical radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2017;99:1253–1260. doi: 10.1016/j.ijrobp.2017.07.040. [DOI] [PubMed] [Google Scholar]