Abstract
Background
Cerebrovascular disease is a common clinical illness. Many patients with cerebrovascular disease can be accompanied by cognitive impairment. The exosomal microRNA (miRNA)-223-3p is related to vascular endothelial injury, synaptic function, inflammatory response, and other mechanisms. In this study, we investigated the levels of plasma exosomal miRNA-223-3p in patients with cerebral small vessel disease (CSVD), in order to determine whether it could be used as a more accessible potential biomarker for the early diagnosis and treatment of CSVD. This study aimed to explore whether the development of cognitive impairment can be explained by differentially expressed miRNA-223-3p by detecting the level of miRNA-223-3p, which is abundant in peripheral blood exosomes related to cognitive impairment in CSVD.
Methods
The three groups of participants included 40 patients with CSVD cognitive impairment (CSVDCI), 38 patients with CSVD, and 35 normal controls (NC). The real-time polymerase chain reaction (RT-PCR) was used to detect the expression level of blood exosomal miRNA-223-3p. In addition, we also studied the relationship between exosomal miRNA-223-3p and blood Hcy and C-reactive protein (CRP). Receiver-operating characteristic (ROC) curve analysis was used to evaluate the diagnostic efficacy of plasma exosomal miRNA-223-3p.
Results
The expression of exosomal miRNA-223-3p in CSVD increased, and the expression of miRNA-223-3p increased significantly with the occurrence of cognitive impairment. Exosomal miRNA-223-3p was positively correlated with the expression levels of Hcy and CRP in the blood.
Conclusions
The expression of plasma exosomal miRNA-223-3p is associated with the development of cognitive impairment in patients with CSVD. It may be involved in the pathogenesis of CSVD and cognitive impairment, and can be used as a sensitive predictive biomarker.
Keywords: Cognitive impairment caused by cerebral small vessel disease, exosome, miRNA-223-3p, real-time polymerase chain reaction (RT-PCR)
Introduction
Cognitive impairment caused by vascular factors is very common in the elderly, and has become the second largest cause of dementia after Alzheimer’s disease (AD). In addition to stroke with macrovascular disease leading to cognitive decline, multiple studies have shown that cerebral small vessel disease (CSVD) is an important cause of early cognitive decline (1). Due to the improvement of imaging technology, the detection rate of CSVD has also increased in recent years. Imaging findings include lacunes (small cavities resulting from previous infarcts), microinfarcts (small lesions of ischemic origin), microbleeds, enlarged perivascular spaces, white matter injury with changes in connectivity, and brain atrophy. As an example, increased numbers of microbleeds are associated with a greater risk for cognitive decline and dementias. At present, the diagnosis of cognitive impairment is too highly dependent on the scale score. At the same time, due to the objective differences in the accurate use of the neurologic assessment scale by inspectors, the diagnosis of atypical clinical symptoms may be complex and challenging. Therefore, looking for simple and reliable biomarkers in patients with CSVD and cognitive impairment caused by CSVD has become a particular research focus.
Exosomes are small membranous vesicles with uniform size and shape, which are secreted by most cells in the body and carry a variety of signal molecules. Due to the stability of exosomes in plasma, this study will explore the possibility of plasma exosomal microRNA (miRNA) as a biomarker of cognitive impairment caused by CSVD (2-4). At present, the pathogenesis of CSVD cognitive impairment (CSVDCI) is still unclear, and may be related to endothelial dysfunction, hypoperfusion, inflammatory cell reaction, blood-brain barrier (BBB) damage, as well as genetic and other factors (5). Previous studies have confirmed that the exosomal miRNA-223-3p is related to vascular endothelial injury, synaptic function, inflammatory response, and other mechanisms (6). MiRNA-223-3p also plays a role in the regulation of synaptic function, mainly by targeting mannose receptor signal transduction and RhoB, inhibiting antigen endocytosis and presentation, and promoting the tolerance potential of dendritic cells (6). And in previous studies, we can see that miR-223-3p is mainly microglia in the process of participating in inflammatory response. Therefore, we follow this direction and select miR-223-3p for the next research. Notably, exosomal miRNA-223-3p may be related to the occurrence and development of CSVDCI.
In this study, the plasma levels of miRNA-223-3p of three groups [normal control (NC), CSVD, and CSVDCI] were measured. We hypothesized that different levels could exhibit trends as the cognitive impairment progressed. The purpose of this study was to detect the levels of plasma exosomal miRNA-223-3p in patients with CSVDCI to determine whether it could be used more widely as a more accessible potential biomarker for the early diagnosis and treatment of CSVDCI. We present the following article in accordance with the STARD reporting checklist (available at https://dx.doi.org/10.21037/atm-21-6086).
Methods
Participants
This study was conducted in the neurology ward or outpatient department of Mudanjiang Medical University, Affiliated Hongqi Hospital from June 2019 to October 2020. All participants were right-handed Chinese Han, from ChiCTR2000029055. The cognitive functions of all the subjects were assessed by experienced neurologists using the Clinical Dementia Rating Scale (CDR) (7), the Chinese version of the Mini-Mental State Examination (MMSE), and the Beijing version of Montreal Cognitive Assessment (MoCA) (8). Gender- and age-matched healthy controls were recruited simultaneously. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Hongqi Hospital Affiliated to Mudanjiang Medical University (No. 202114). All participants signed the informed consent. At the same time, brain magnetic resonance imaging, venous blood, and cognitive function were evaluated. All testers participated in neuropsychological scale training and passed the test. The images of each patient were reviewed by two radiologists (with 10 years of experience) who were blinded to all patient information.
The inclusion criteria for CSVD were based on the recent research criteria proposed by Chinese guidelines for diagnosis and treatment of cognitive impairment related to small vessel disease (9). Patients eligible for inclusion were diagnosed with cognitive impairment by neuropsychological scale and confirmed to have CSVD by imaging examination. We excluded patients with other diseases causing cognitive impairment, or those that were unwilling or unable to provide written informed consent. MMSE was used to evaluate the overall cognitive function. The scores of the primary school group were less than 21, the middle school and university groups were less than 24, and the MoCA scores were less than 26. The clinical dementia scale (CDR) was less than 1. CSVD group patients did not complain of decreased subjective cognitive function. There were no cognitive impairments in the neuropsychological evaluation, and the CDR score was 0. The inclusion criteria for the NC group including a normal magnetic resonance imaging (MRI) of the head, no complaints of cognitive decline, and no cognitive impairment in the neuropsychological evaluation.
Extraction and identification of plasma exosomes, total RNA extraction, reverse transcription and fluorescence quantitative PCR
Blood sample collection and preservation methods
Venous blood was collected from all subjects on an empty stomach in the morning and centrifuged using a low-temperature centrifuge (4 °C 2,500 r centrifugation for 10 min). The supernatant was plasma. The plasma was transferred to a new 1.5 mL centrifuge tube, labelled, and placed in a refrigerator at −80 °C. The above steps were completed within 2 hours. The exosome extraction and purification kit provided by Shanghai Umibio was used to extract plasma exosomes. The morphology of exosomes was observed using a transmission electron microscope (JEOL, 1230), and the size of the exosomes was observed using the nanoparticle tracking analysis (NTA) method. Fasting venous blood was collected from the three groups. After cell debris was removed by centrifugation, the plasma was transferred to a 1.5-mL centrifuge tube and sent to Shanghai Yumeibo for exosome miRNA sequencing. The total RNA of exosomes was extracted by the miRNA extraction kit provided by Biospin. Cel-mir-39-3p standard RNA was used as external reference. The miRNA the real-time polymerase chain reaction (RT-PCR) starter kit of miDETECT A TrackTM provided by Guangzhou RIBOBIO was used to complete the RT-PCR experiment. The upstream primer of cel-mir-39-3p was designed and provided by Guangzhou RIBOBIO, and the upstream primer sequence of hsa-miRNA-223-3p was (5'-ugucaguugucaaauacccca-3'). All downstream primers, reverse transcription primers, and tailed primers were included in the kit (Ribobio, c10712-2). An ABI7500 Fast PCR was used to analyze the results; each sample was detected three times, and the average cycle threshold (CT) value was taken.
Sequencing results of exosomal miRNA
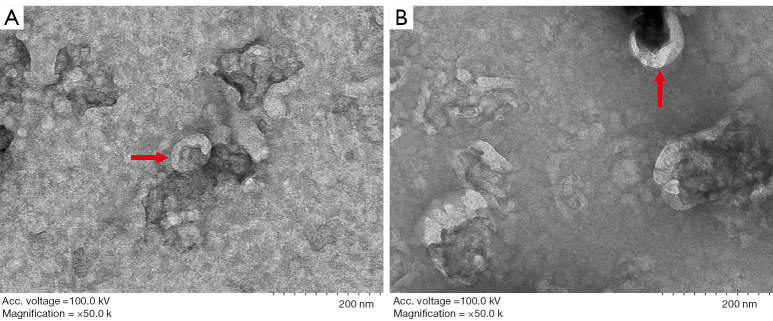
The exosome extraction kit was used in all subjects to extract the plasma exosomes. The diameter of the exosomes ranged from 30 to 100 nm, and the typical shape and size of the exosomes were observed using a transmission electron microscope (Figure 1). Typical exosomes were extracted from all three groups. Following extraction of the exosomes from the plasma of the subjects, the size of the exosomes was approximately 100 nm. The method further verified plasma exosomes. In order to detect the differentially-expressed miRNAs in the exosomes of the three groups, three groups of differentially-expressed genes were screened by high-throughput sequencing. The up-regulated miRNAs were hsa-mir-223-3p, hsa-mir-1290, hsa-mir-1246, hsa-mir-93-5p, and hsa-mir-150-5p, while the down regulated miRNAs were hsa-let-7b-5p, hsa-mir-139-5p, hsa-mir-23a-3p, hsa-mir-126-3p, hsa-mir-139-5p, hsa-miR-199a-3, hsa-miR-221-3p, and hsa-miR-7a-5p (P<0.05). Some studies have shown that there is a correlation between miRNA-223-3p and neurolinear diseases such as Alzheimer’s disease and Parkinson’s disease (10). In order to verify the correlation between miRNA and CSVD (and the cognitive impairment it causes), miRNA-223-3p was selected for RT-PCR to calculate the relative expression of miRNA-223-3p in the three groups.
Figure 1.
Typical exosome morphology. The arrows indicate typical exosomes.
Statistical analysis
Statistical analysis was carried out using Statistical Package for Social Sciences software [solutions statistical package for the social sciences (SPSS), version 21.0]. All statistical tests were two-tailed, and all results were expressed as mean ± standard deviation (SD) (P<0.05). Categorization of demographic variables was assessed using the Chi-square test. Continuous demographic variables were evaluated through analysis of variance (ANOVA). The results of RT-PCR were calculated as fold change (2−ΔCT) divided by the normalized gene expression of the control group (2−ΔCt). Pearson’s test was used for correlation analysis. Receiver-operating characteristic (ROC) curve analysis was used to estimate the potential of miRNA-223-3p as a biomarker of CSVD and cognitive impairment.
Results
Demographic data
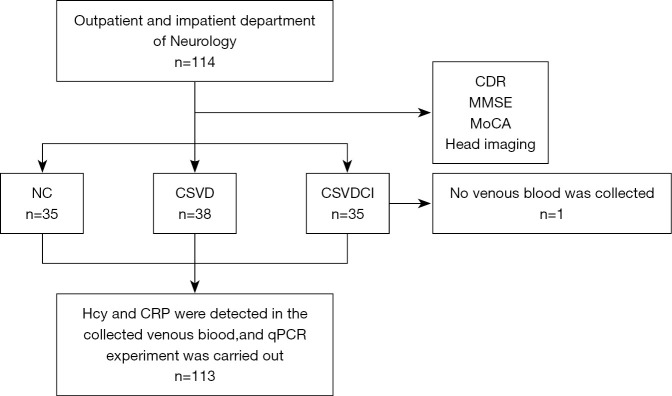
The median age of participants was 68 years (range, 60–75 years), and 114 participants (49.1%) were women. Among the participants, 41 were CSVDCI patients, 38 were CSVD patients, and there were 35 NCs. One patient with CSVDCI was excluded because their venous blood was not collected (Figure 2). There were no significant differences in gender, age, years of education, body mass index (BMI), smoking and drinking history, and previous disease history among NC, CSVD, and CSVDCI groups (P>0.05) (Table 1). The Hamilton Depression Scale (HAMD), the Hamilton Anxiety Scale (HAMA), and the activities of daily living (ADL) scores were 17.23±2.43, 4.65±0.82, and 91.24±11.63, respectively. The cognitive scores of NC group were MMSE score (27.98±0.76) and MOCA score (27.45±0.77). The MMSE and MoCA scores of the CSVD group were 28.05±0.80 and 27.53±0.77, respectively; the MMSE and MoCA scores of the CSVDCI group were 18.68±0.79 and 15.20±1.49. The comparison of the MMSE scores between the NC, CSVD, and CSVDCI groups is shown in Figure 3.
Figure 2.
flow of participants. CDR, Clinical Dementia Rating Scale; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NC, normal controls; CSVD, cerebral small vessel disease; CSVDCI, CSVD cognitive impairment.
Table 1. Basic characteristics of the subjects.
| Patient characteristics | NC (n=35) | CSVD (n=38) | CSVDCI (n=40) |
|---|---|---|---|
| Gender (M/F) | 18/17 | 18/20 | 21/19 |
| Age (y) | 68.00±3.80 | 69.01±4.00 | 67.50±5.96 |
| Education (y) | 9.75±2.32 | 9.60±2.47 | 9.44±2.50 |
| BMI (kg/m2) | 20.38±2.12 | 22.18±2.03 | 21.28±2.08 |
| Smoking | 17 (48.6) | 17 (44.7) | 18 (45.0) |
| Drinking | 15 (42.9) | 14 (36.8) | 16 (40.0) |
| Hypertension | 12 (34.2) | 13 (34.2) | 14 (35.0) |
| Diabetes | 10 (28.6) | 9 (23.68) | 11 (27.5) |
| Coronary heart disease | 8 (20.0) | 7 (17.5) | 10 (25.0) |
NC, normal controls; CSVD, cerebral small vessel disease; CSVDCI, CSVD cognitive impairment; M/F, male/female.
Figure 3.
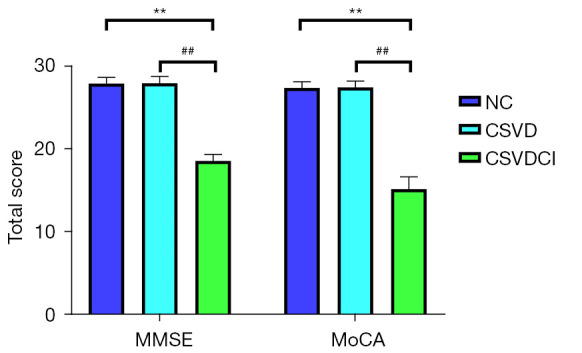
Comparison of total and sub scores of MMSE scale and MOCA scale among each group. **, compared with NC group, P<0.01; ##, compared with CSVD group, P<0.01. NC, normal controls; CSVD, cerebral small vessel disease; CSVDCI, CSVD cognitive impairment.
Quantitative detection of miRNA-223-3p by RT-PCR
The relative expression levels of miRNA-223-3p in the NC, CSVDCI, and CSVD groups were 1.00±0.34, 2.42±0.43, and 1.52±0.38, respectively. Compared with the NC group, the relative expression of miRNA-223-3p in the CSVD and CSVDCI groups was significantly higher (P<0.001). Meanwhile, the relative expression of miRNA-223-3p in the CSVDCI group was significantly higher than that in the CSVD group (P<0.001), and the difference was statistically significant (Table 2 and Figure 4). These results suggest that plasma exosomal miRNA-223-3p may play an important role in CSVD and cognitive impairment.
Table 2. Relative expression of miRNA-223-3p in the exosomes of each group.
| Group | Number of cases | Relative expression of miRNA-223-3p |
|---|---|---|
| NC | 35 | 1.00±0.34 |
| CSVD | 38 | 1.52±0.38 |
| CSVDCI | 40 | 2.42±0.43 |
NC, normal controls; CSVD, cerebral small vessel disease; CSVDCI, CSVD cognitive impairment.
Figure 4.
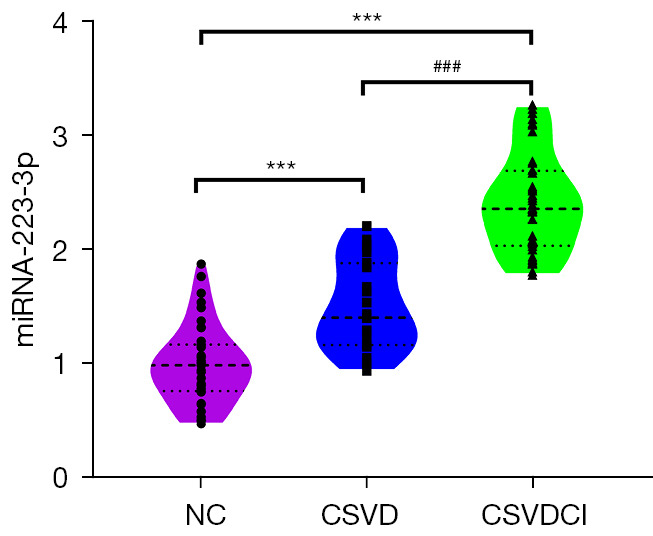
Relative expression of plasma exosomal miRNA-223-3p in each group. ***, compared with NC group, P<0.001; ###, compared with CSVD group, P<0.001. NC, normal controls; CSVD, cerebral small vessel disease; CSVDCI, CSVD cognitive impairment.
The expression levels of homocysteine (Hcy) and C-reactive protein (CRP) in plasma of all groups were compared
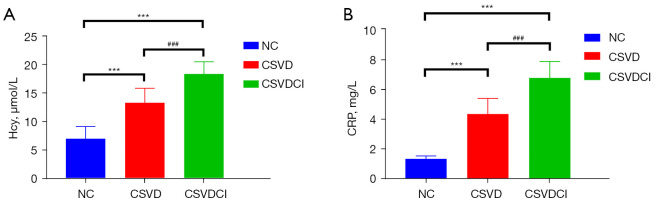
Plasma Hcy and CRP were detected in all subjects. The results showed that the plasma Hcy concentrations in the NC, CSVD, and CSVDCI groups were 7.02±2.14, 13.31±2.53, and 18.35±2.13 µmol/L, respectively. Also, the concentrations of CRP in the three groups were 1.31±0.23, 4.33±1.04 and 6.74±1.10 mg/L, respectively. Compared with the NC group, plasma Hcy and CRP levels in the CSVD and CSVDCI groups were significantly higher (P<0.05). Furthermore, compared to the CSVD group, the levels of Hcy and CRP in the CSVDCI group were markedly higher (P<0.05) (Figure 5A,5B).
Figure 5.
Difference of plasma Hcy and CRP expression among groups. (A) The difference in Hcy expression between the groups. ***, compared with NC group, P<0.001; ###, compared with CSVD group, P<0.001. (B) CRP was expressed differently among the groups. ***, compared with NC group, P<0.001; ###, compared with CSVD group, P<0.001. NC, normal controls; CSVD, cerebral small vessel disease; CSVDCI, CSVD cognitive impairment.
Correlation analysis of plasma exosome miRNA-223-3p with plasma Hcy and CRP
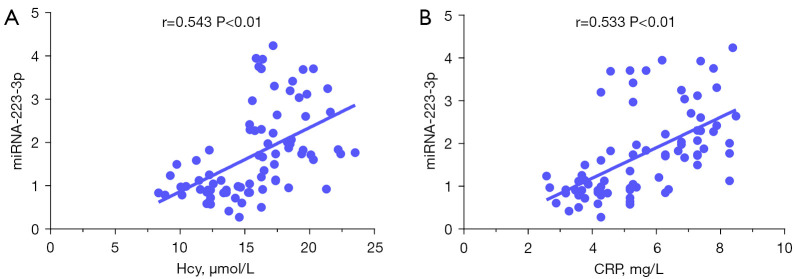
In order to further study the relationship between plasma Hcy and CRP levels (leading to CSVD and its cognitive impairment) and plasma exosomal miRNA-223-3, this study analyzed the correlation between plasma Hcy and CRP levels and plasma exosomal miRNA-223-3p levels in patients with CSVD. As shown in Figure 6A and Figure 6B, in patients with CSVD and cognitive impairment caused by CSVD, the level of plasma secreted miRNA-223-3p was positively correlated with the concentration of plasma Hcy and CRP (r=0.543, 0.533) (P<0.01). These results suggest that plasma exosomal miRNA-223-3p is correlated with plasma Hcy and CRP in patients with CSVD and cognitive impairment caused by CSVD. Thus, plasma exosomal miRNA-223-3p may be involved in the pathogenesis of CSVD and cognitive impairment caused by CSVD.
Figure 6.
Correlation between plasma exosomal miRNA-223-3p and plasma Hcy and CRP concentrations. The scatter plot shows that the level of plasma exosomal miRNA-223-3p was positively correlated with the concentration of Hcy and CRP (A,B). CRP, C-reactive protein.
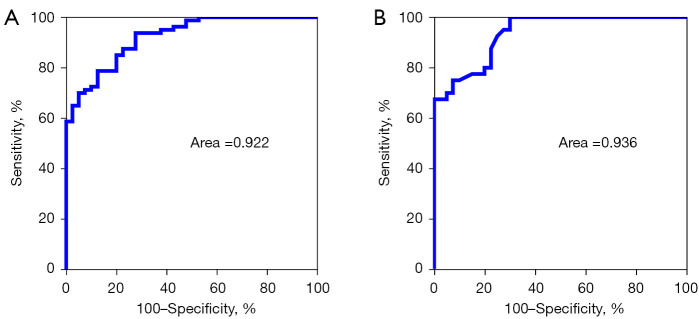
ROC curve analysis of the diagnostic value of plasma exocrine miRNA-223-3p in the diagnosis of CSVD and its cognitive impairment
The ROC curve is a graphical analysis of the relationship between sensitivity and specificity of laboratory tests. Researchers always use ROC curves to analyze diagnostic test performance. In this study, miRNA-223-3p in the plasma exocrine was found to be related to the cognitive impairment of CSVD. In order to further explore its diagnostic value, we developed a ROC curve to evaluate the value of miRNA-223-3p in the plasma exocrine for the diagnosis of CSVD cognitive impairment. The results showed that the area under the curve (AUC) of plasma exosomal miRNA-223-3p distinguishing the NC group from the CSVD group was 0.922 (P<0.01), with good sensitivity and specificity (Figure 7A). The area under the curve (AUC) was 0.936 (P<0.01) in distinguishing the CSVD group and the CSVDCI group, which showed good sensitivity and specificity (Figure 7B). These results suggest that plasma exosomal miRNA-223-3p may be a predictive biomarker for cognitive impairment caused by CSVD.
Figure 7.
A diagnostic value of plasma exosomal miRNA-223-3p level in patients with CSVD; diagnostic value of b-plasma exosomal miRNA-223-3p level in patients with cognitive impairment caused by CSVD. CSVD, cerebral small vessel disease.
Discussion
CSVD disease imaging may change in the early stage, but the clinical symptoms are often not obvious, and gradually become dementia with the development of the disease. An increasing number of studies have shown that CSVD is one of the main causes of cognitive impairment, resulting in huge care and economic burdens to patients, family members, and society (11,12). Clinically, the cognitive impairment caused by CSVD is mainly reflected in the decline of executive function, attention, and information processing speed. Genetic studies have shown that there are numerous reasons for CSVD. At present, some studies believe that endothelial dysfunction and blood-brain barrier destruction are related to the pathogenesis of CSVD. Under the action of inflammation, endothelial damage leads to the destruction of the blood-brain barrier, white matter lesions, inflammatory response, neuronal degeneration and death, ultimately resulting in cognitive impairment (13). Recent studies have found that plasma-secreted miRNA can be used as a biomarker of neurodegenerative diseases (14,15), but there are relatively few studies on plasma-secreted miRNA, CSVD, and cognitive impairment caused by CSVD. This study explored the relationship between plasma-secreted miRNA-related indexes and CSVD and its cognitive impairment, in order to provide more ideas for the diagnosis and treatment of CSVD and its cognitive impairment.
At present, the diagnosis of cognitive impairment is too heavily reliant on scale scores. At the same time, due to the objective differences in the accurate use of neurological assessment scales by examiners, diagnosing cases with atypical clinical symptoms may be complicated and challenging. Therefore, looking for simple and reliable biomarkers in patients with CSVD and cognitive impairment caused by it has become a research hotspot. Due to the stability of exosomes in plasma, this study explored the possibility of plasma exosomal miRNA as a biomarker of CSVD cognitive impairment. Exosomes can freely pass through the blood-brain barrier, as a vehicle in the body, carrying the transportation of miRNA and other signal molecules, and can exist stably in the blood under the protection of a special membrane structure, thereby avoiding the degradation of miRNA. Therefore, exosomal miRNA can reflect the physiological condition of the nervous system without introducing other confounding factors. Shi et al. isolated exosomes specific to the nervous system from plasma, so that the detection of exosome-related components in the blood can also reflect changes in the brain (16). MiRNA is considered to be an important exosome component, which largely determines the effect of exosomes on recipient cells (17). MiRNAs have been found in various body fluids such as blood, cerebrospinal fluid, and urine (18), and can be used as peripheral non-invasive biomarkers for cardiovascular diseases, cancer, and neurodegenerative diseases (19,20). Studies have confirmed that plasma exosomal miRNA can be used as a diagnostic marker for neurodegenerative diseases such as AD and Parkinson’s disease (21,22). Due to the stability of exosomes in plasma, this study explored the possibility of plasma exosomal miRNA as a biomarker for CSVD and cognitive impairment caused by it.
In this study, plasma exosomes were extracted and identified, RNA content and purity were further measured, and genes with differences in expression between groups were searched for by miRNA sequencing technology. It was found that plasma exosomal miRNA-223-3p, miRNA-1290, and miRNA-1246 in CSVD patients were significantly up-regulated compared with the control group. Previous studies have found that miRNA-223-3p is significantly different in dementia patients compared with control patients (22). In this experiment, the exosomal miRNA-223-3p was selected and further verified by RT-PCR. The results showed that compared with the NC group, the relative expression of plasma exosomal miRNA-223-3p in the CSVDCI and CSVD groups was considerably increased, and the difference was statistically significant. Compared with the CSVD group, the relative expression of plasma exosomes miRNA-223-3p in the CSVDCI group was markedly increased, and the result was statistically significant. Wei et al. used dementia patients as the research object to explore the diagnostic value of miRNA-223-3p in dementia, and found that serum exosomal miRNA-223-3p is significantly different between dementia patients and NCs (23). In patients with vascular dementia, serum exosomal miRNA-223-3p is significantly positively correlated with the levels of inflammatory factors interleukin (IL)-1β, IL-6, and C-reactive protein. This study showed that inflammation is more important than AD in vascular cognitive impairment. At the same time, exosomal miRNA-223-3p was shown to have a certain value for the diagnosis of dementia (23). The results of this study are consistent with the results of Wei et al. (23) indicating that exosomal miRNA-223-3p is involved in the pathogenesis of vascular cognitive impairment. As an early pathological change in dementia, inflammation in nerve cells can be considered as a useful signal for the early detection of dementia. Inflammation is also considered to play a potential role in the pathogenesis of CSVD cognitive impairment.
Our results showed that compared with the NC group, the levels of plasma Hcy and CRP in CSVD and CSVDCI were significantly higher, and the difference was statistically significant. Compared with the CSVD group, the levels of plasma Hcy and CRP in the CSVDCI group were significantly higher, and the difference was statistically significant. Plasma Hcy is the product of methionine after transmethylation reaction, which can participate in the immune regulation of nitric oxide synthase. When the expression level of plasma Hcy increases, it can lead to endothelial cell injury by blocking the effect of nitric oxide synthase, resulting in changes in microvascular structure, damage to brain neurons, ultimately leading to cognitive impairment (24). Studies have shown that the total Hcy level is related to the image load of CSVD, especially the loss of lacuna and brain volume (25), which mainly affects the executive function and attention of CSVD patients (26). Plasma CRP is a common sensitive marker of inflammatory reaction. When an inflammatory reaction occurs in the body, vascular endothelial cells are also damaged, which will affects the blood flow perfusion of brain tissue, cause brain tissue damage and cognitive impairment. Some studies have also pointed out that when the plasma Hcy and CRP levels increase, the severity of CSVD cognitive impairment is higher (27). The results of this study are consistent with the above findings, suggesting that plasma Hcy and CRP may be involved in the pathogenesis of CSVD and its cognitive impairment.
This study analyzed the correlation between plasma exosomal miRNA-223-3p and plasma Hcy and CRP. The results showed that the level of plasma-secreted miRNA-223-3p was positively correlated with the concentration of plasma Hcy and CRP in patients with CSVD. Previous studies have shown that miRNA-223 may regulate the immune response and mediate the inflammatory response of the nervous system (28). Microglia plays an important role in the occurrence of the neuroinflammatory response. Studies have shown that secreted miRNA-223-3p can effectively promote the CysLT2R agonist N-methyl-leukotriene C4 (nmltc4)/the nonselective agonist leukotriene D4 (LTD4) to induce harmful M1 microglia to transform into the beneficial M2 phenotype, so as to increase the expression of anti-inflammatory cytokines, reduce the production of pro-inflammatory factors, improve neurological deficit, and enhance learning and memory ability (29). Studies have also confirmed that circulating miRNA-223-3p mainly exists in exosomes, and the content of neuronal exosomal miRNA-223-3p in AD patients is significantly higher than that in control patients (30). In addition, miRNA-223 may play a key role in the induction and persistent inflammation of neuroinflammatory diseases by controlling the activation of the macrophage derived chemokine-induced pathological the importance of T helper 17 (Th17) response, so as to improve neural function (31). Studies have shown that miRNA-223-3p and miRNA-27a-3p are up-regulated in an inflammatory response to mediate a compensatory neuroprotective gene expression program, thus playing a neuroprotective role (32). In CSVD patients, plasma exosomal miRNA-223-3p levels are positively correlated with plasma Hcy and CRP concentrations, which provides support for the correlation between plasma exosomal miRNA-223-3p and CSVD and the pathogenesis of cognitive impairment.
Also, ROC curve analysis in this study showed that plasma-secreted miRNA-223-3p had good sensitivity and specificity in distinguishing the NC group from the CSVD group, and the CSVD group from the CSVDCI group. Due to the specific expression of miRNA-223-3p, it can be used as a biomarker for a variety of diseases (33,34). Studies have confirmed that miRNA-223-3p can mediate the inflammatory response of cardiovascular diseases and has potential as a biomarker for the diagnosis and treatment of cardiovascular diseases (35). Recent studies have shown that the expression level of serum miRNA-223-3p can distinguish mild cognitive impairment, AD, and Parkinson’s disease, suggesting that miRNA-223-3p can be used as a non-invasive biomarker for differential diagnosis and prognosis of these neurodegenerative diseases (36). The results of this study suggest that plasma-secreted miRNA-223-3p may be a predictive diagnostic biomarker of CSVD and its cognitive impairment.
In conclusion, this study provides a direction for the early detection and early intervention of CSVD and cognitive impairment in the future. Also, numerous studies have demonstrated that secreted miRNA-223-3p is related to inflammation, neuronal protection, and other processes. Our study also found that secreted miRNA-223-3p is significantly up-regulated in patients with CSVDCI, and is significantly positively correlated with the levels of plasma Hcy and CRP. These results suggest that plasma-secreted miRNA-223-3p may participate in the regulation of CSVD and its cognitive impairment through the inflammatory response and vascular endothelial injury, however, there are still few studies on the effects of plasma exosomes on CSVD, the exact pathogenesis needs to be further studied.
However, this study had some limitations that should be noted. Firstly, this experiment involved a small sample size and MMSE and MOCA scale scores, which may lead to a bias in the experimental results, and the correlation between peripheral blood plasma exosomes concentration and CSVD was not studied. Secondly, the CSVD classification and the severity of cognitive impairment were not graded. In the next step, we will continue to collect cases to conduct multi-center, large-sample research for confirmation of our findings. In order to further study the exact pathogenesis of plasma-secreted miRNA-223-3p in CSVD and its cognitive impairment, future research also needs to further explore the pathogenesis and pathway of CSVD through in vivo and in vitro experiments. Despite the shortcomings in this study, plasma exosomal miRNA-223-3p will certainly play an important role in the diagnosis and treatment of CSVD cognitive impairment, thus provide new methods and targets for the study of CSVD and the pathogenesis of CSVD-induced cognitive impairment.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81771795); the Foundation of Hongqi (2019HQ-02, 2019HQ-05); and the Basic Scientific Research Expenses and Scientific Research Projects of Provincial Colleges and Universities in Heilongjiang Province (2019-KYYWFMY-0029).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Hongqi Hospital Affiliated to Mudanjiang Medical University (No. 202114). All participants signed the informed consent.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-6086
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-6086
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-6086). The authors have no conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Jokinen H, Koikkalainen J, Laakso HM, et al. Global Burden of Small Vessel Disease-Related Brain Changes on MRI Predicts Cognitive and Functional Decline. Stroke 2020;51:170-8. 10.1161/STROKEAHA.119.026170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Kralingen JC, McFall A, Ord ENJ, et al. Altered Extracellular Vesicle MicroRNA Expression in Ischemic Stroke and Small Vessel Disease. Transl Stroke Res 2019;10:495-508. 10.1007/s12975-018-0682-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijkerk R, Kallenberg MH, Zijlstra LE, et al. Circulating angiopoietin-2 and angiogenic microRNAs associate with cerebral small vessel disease and cognitive decline in older patients reaching end stage renal disease. Nephrol Dial Transplant 2020. [Epub ahead of print]. 10.1093/ndt/gfaa370 [DOI] [PubMed] [Google Scholar]
- 4.Liao Z, Sun H, Chang Y, et al. The expression and clinical significance of miRNA-183 in cerebral ischemia-reperfusion injury patients with cerebral small vessel disease. Ann Transl Med 2020;8:1005. 10.21037/atm-20-5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Zhang L, Geng Y, et al. Long noncoding RNA GAS5 promotes microglial inflammatory response in Parkinson's disease by regulating NLRP3 pathway through sponging miR-223-3p. Int Immunopharmacol 2020;85:106614. 10.1016/j.intimp.2020.106614 [DOI] [PubMed] [Google Scholar]
- 6.Tang HC, Lai YY, Zheng J, et al. miR-223-3p Inhibits Antigen Endocytosis and Presentation and Promotes the Tolerogenic Potential of Dendritic Cells through Targeting Mannose Receptor Signaling and Rhob. J Immunol Res 2020;2020:1379458. 10.1155/2020/1379458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-4. 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Li D, Li F, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 2011;24:184-90. 10.1177/0891988711422528 [DOI] [PubMed] [Google Scholar]
- 9.Geriatric Neurology Group . Clinical practice guideline for cognitive impairment of cerebral small vessel disease of China (2019). Chin J Geriatr 2019;38:345-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand SS, Friedrich MG, Desai D, et al. Reduced Cognitive Assessment Scores Among Individuals With Magnetic Resonance Imaging-Detected Vascular Brain Injury. Stroke 2020;51:1158-65. 10.1161/STROKEAHA.119.028179 [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019;18:684-96. 10.1016/S1474-4422(19)30079-1 [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Navarro SG, Mimenza-Alvarado AJ, Corona-Sevilla I, et al. Cerebral Vascular Reactivity in Frail Older Adults with Vascular Cognitive Impairment. Brain Sci 2019;9:214. 10.3390/brainsci9090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waller R, Narramore R, Simpson JE, et al. Heterogeneity of cellular inflammatory responses in ageing white matter and relationship to Alzheimer's and small vessel disease pathologies. Brain Pathol 2021;31:e12928. 10.1111/bpa.12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lugli G, Cohen AM, Bennett DA, et al. Plasma Exosomal miRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS One 2015;10:e0139233. 10.1371/journal.pone.0139233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheinerman KS, Umansky SR. Circulating cell-free microRNA as biomarkers for screening, diagnosis and monitoring of neurodegenerative diseases and other neurologic pathologies. Front Cell Neurosci 2013;7:150. 10.3389/fncel.2013.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi M, Liu C, Cook TJ, et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol 2014;128:639-50. 10.1007/s00401-014-1314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ailawadi S, Wang X, Gu H, et al. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta 2015;1852:1-11. 10.1016/j.bbadis.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvestro S, Bramanti P, Mazzon E. Role of miRNAs in Alzheimer's Disease and Possible Fields of Application. Int J Mol Sci 2019;20:3979. 10.3390/ijms20163979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Wu Y, Zhang J, et al. Role of miRNA-424 in Cancers. Onco Targets Ther 2020;13:9611-22. 10.2147/OTT.S266541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy PH, Tonk S, Kumar S, et al. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer's disease. Biochem Biophys Res Commun 2017;483:1156-65. 10.1016/j.bbrc.2016.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galimberti D, Villa C, Fenoglio C, et al. Circulating miRNAs as potential biomarkers in Alzheimer's disease. J Alzheimers Dis 2014;42:1261-7. 10.3233/JAD-140756 [DOI] [PubMed] [Google Scholar]
- 22.van den Berg MMJ, Krauskopf J, Ramaekers JG, et al. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog Neurobiol 2020;185:101732. 10.1016/j.pneurobio.2019.101732 [DOI] [PubMed] [Google Scholar]
- 23.Wei H, Xu Y, Xu W, et al. Serum Exosomal miR-223 Serves as a Potential Diagnostic and Prognostic Biomarker for Dementia. Neuroscience 2018;379:167-76. 10.1016/j.neuroscience.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 24.Xie Y, Feng H, Peng S, et al. Association of plasma homocysteine, vitamin B12 and folate levels with cognitive function in Parkinson's disease: A meta-analysis. Neurosci Lett 2017;636:190-5. 10.1016/j.neulet.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Su N, Zhang D, et al. Correlation between total homocysteine and cerebral small vessel disease: A Mendelian randomization study. Eur J Neurol 2021;28:1931-8. 10.1111/ene.14708 [DOI] [PubMed] [Google Scholar]
- 26.Sharma VK, Das SK, Dhar P, et al. Domain specific changes in cognition at high altitude and its correlation with hyperhomocysteinemia. PLoS One 2014;9:e101448. 10.1371/journal.pone.0101448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoda M, Shoji T, Kuwamura Y, et al. Plasma homocysteine and cerebral small vessel disease as possible mediators between kidney and cognitive functions in patients with diabetes mellitus. Sci Rep 2017;7:4382. 10.1038/s41598-017-04515-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz F. The emerging role of miR-223 as novel potential diagnostic and therapeutic target for inflammatory disorders. Cell Immunol 2016;303:1-6. 10.1016/j.cellimm.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Gan Y, Xu G, et al. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci 2020;260:118403. 10.1016/j.lfs.2020.118403 [DOI] [PubMed] [Google Scholar]
- 30.Serpente M, Fenoglio C, D'Anca M, et al. MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer's Disease. Cells 2020;9:1443. 10.3390/cells9061443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ifergan I, Chen S, Zhang B, et al. Cutting Edge: MicroRNA-223 Regulates Myeloid Dendritic Cell-Driven Th17 Responses in Experimental Autoimmune Encephalomyelitis. J Immunol 2016;196:1455-9. 10.4049/jimmunol.1501965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morquette B, Juźwik CA, Drake SS, et al. MicroRNA-223 protects neurons from degeneration in experimental autoimmune encephalomyelitis. Brain 2019;142:2979-95. 10.1093/brain/awz245 [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Hou X, Zhang M, et al. MicroRNA-223-3p modulates dendritic cell function and ameliorates experimental autoimmune myocarditis by targeting the NLRP3 inflammasome. Mol Immunol 2020;117:73-83. 10.1016/j.molimm.2019.10.027 [DOI] [PubMed] [Google Scholar]
- 34.Jimenez Calvente C, Del Pilar H, Tameda M, et al. MicroRNA 223 3p Negatively Regulates the NLRP3 Inflammasome in Acute and Chronic Liver Injury. Mol Ther 2020;28:653-63. 10.1016/j.ymthe.2019.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang MW, Shen YJ, Shi J, et al. MiR-223-3p in Cardiovascular Diseases: A Biomarker and Potential Therapeutic Target. Front Cardiovasc Med 2021;7:610561. 10.3389/fcvm.2020.610561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancuso R, Agostini S, Hernis A, et al. Circulatory miR-223-3p Discriminates Between Parkinson's and Alzheimer's Patients. Sci Rep 2019;9:9393. 10.1038/s41598-019-45687-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as