Abstract
Introduction
Fear of cancer recurrence (FCR) is a common condition among cancer survivors that can lead to significant levels of distress, anxiety and depression. Online mindfulness programmes may provide the mechanism to support cancer survivors manage FCR and distress, and improve people’s well-being over the short, medium and long term. The primary aim of this study is to determine the potential efficacy of MindOnLine, a 9 session mindfulness-based programme for survivors of breast, prostate and colorectal cancer. A formal economic programme will also be conducted.
Methods and analysis
A single-blind randomised controlled trial to determine the efficacy and cost-efficacy of a MindOnLine programme for cancer survivors. A total of 400 people living with cancer will be recruited via online advertisements on social media platforms, peak consumer advocacy groups or through outpatient services at healthcare providers across Victoria, Australia. People will be randomly allocated to either the MindOnLine programme (n=200) or waitlist control (n=200). Participant assessments will occur at baseline, at 9 weeks and 9-month follow-up. The primary outcome is change in Fear of Recurrence Index Score total score between baseline and 9 weeks; secondary outcomes are changes in depression and anxiety, quality of life and mindfulness. The economic analysis comprises a cost-consequences analysis where all outcomes will be compared with costs.
Ethics and dissemination
Ethics approval was obtained from the Peter MacCallum Cancer Centre (20-53) and Deakin University (2020-284). All participants will be required to provide written informed consent. Findings will be disseminated in peer reviewed journals and among key stakeholder organisations including hospitals, cancer and community organisations and Government. If successful the project will be rolled out nationally with a formal implementation plan.
Trial registration number
Australian New Zealand Clinical Trials Registry (12620000645954); Pre-results. Registered 6 June 2020, https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379520&isReview=true.
Keywords: oncology, health economics, world wide web technology
Strengths and limitations of this study.
Strengths of our randomised controlled trial include the assessment of both the efficacy and cost-effectiveness of the MindOnLine programme, and the involvement of consumer advocacy groups to support recruitment, interpretation of results, dissemination and translation.
Incorporating an economic evaluation into the study design will complement clinical findings and support decision-making processes for potential scaling.
Advances in social platforms, smartphone technology and web-based programming can change substantially in a short period and, while this may affect the actual online platform used, measures are in place to maintain the same intervention during the study period, so we do not believe that this will influence the programme content or delivery mechanisms.
Recruitment primarily through social media platforms means we cannot accurately assess reach of the intervention, as we will not be able to identify the number of eligible people exposed to our advertisements.
Participants will need access to the internet, which will result in some people unable to take part in the study.
Introduction
Over one million Australians are cancer survivors, and this population is expected to grow substantially due to an ageing population and improved community-based screening programmes and treatments.1 A cancer diagnosis can cause people to confront their own mortality, often for the first time,2 so it may be unsurprising that three-quarters of cancer survivors experience fear of cancer recurrence (FCR) and 49% report moderate to high levels of fear,3 as well as high levels of clinical depression3 and anxiety.4
FCR is a highly prevalent and persistent condition that can result in significant levels of anxiety and depression across the disease trajectory.5 It is imperative to address this issue and our recent work into early psychosocial support indicates it may be possible to significantly reduce FCR through an online mindfulness programme.6 Mindfulness is a state of mind in which one pays attention to the present experience in a nonjudgmental, curious and accepting way. Some studies have shown mindfulness is associated with improved mental health outcomes and management of the emotional consequences of cancer,7 8 while other have found no effect.9
Mindfulness-based interventions consist of regular informal and formal mindfulness meditation practices and are supported by educational principles that are person and relationship-centred.10 Research into the use of mindfulness has mostly evaluated face-to-face programmes which are time-intensive, of limited accessibility and costly.11 Online mindfulness programmes represent a potentially cost-effective mechanism to help people with physical health conditions.12 For cancer survivors, there is evidence that online mindfulness programmes may help manage FCR and distress, and improve mental well-being over the short, medium and long term.2
There is also some evidence that online mindfulness-based cognitive therapy (MBCT) can improve psychological outcomes. A recent study compared an online programme to face-to-face MBCT which showed improved outcomes;13 however, the sample comprised of mainly breast cancer survivors and it is unclear whether the programme would asist with other cancer types.13 Although this intervention was found to be as effective as a face-to-face MBCT in reducing psychological distress and FCR in patients with cancer,13 there is a lack of robust evidence assessing the effectiveness of a general online mindfulness programme for cancer survivors, limiting capacity for implementation and dissemination.14 15
The aim of this study is to conduct a randomised controlled trial of MindOnLine, an online 9 session mindfulness-based intervention, for survivors of breast, prostate and colorectal cancer (CRC), the most common solid tumours among men and women in Australia,1 to determine the effectiveness and cost-effectiveness of the programme.
Preliminary work
To inform the development of MindOnLine, we undertook a systematic review of methodologies for internet-based mindfulness interventions.16 This review showed a dearth of studies with long-term follow-up periods. Our team also conducted an exploratory study on the knowledge of, attitudes toward and behaviours regarding meditation among patients with melanoma.17 Our study of 291 cancer survivors recruited at a single tertiary cancer hospital found that a key barrier to engaging with meditation was a lack of knowledge about its practice. Findings also indicated interest in an online meditation-based intervention once informed about possible benefits of meditation for people with cancer. Those interested in an online meditation-based programme reported higher perceived stress, indicating a need for such a programme.
MindOnline was initially developed as a 6-week online mindfulness-based intervention and follows the Framework for mindfulness-based programme described by Crane et al.10 The programme promoted awareness and acceptance of thoughts and emotions, and empowered participants to address their distressing thoughts and emotions in more adaptive ways. Through this action, participants learn to manage anxious and depressive moods. These moods are triggered by unhelpful and intrusive thoughts, which are strongly associated with moderate to high levels of FCR.18 A pilot study was conducted to assess the potential impact of a 6-week mindfulness programme and explore whether the intervention impacted on FCR, worry and perceived stress compared with usual care. Details of the pilot study are published elsewhere.6 Briefly, 69 melanoma survivors agreed to participate, and 46 participants were randomised into the intervention group (2:1). Scores on all FCR Inventory (FCRI) subscales reduced in the intervention group, with the severity subscale decreasing significantly compared with the control group (−2.6, 95% CI −4.4 to –0.7; p=0.008) after 6 weeks. The total FCRI score also showed a decrease although non-significant (−6.2, 95% CI −13.12 to 0.68, p=0.07). Previous studies have indicated that a 4.1 point decrease on the severity scale is a clinically important change.19
Based on participant feedback from the pilot study6 regarding the benefits of mindfulness practice and the suggestion of a maintenance period to enhance sustainability of the effects, MindOnLine was expanded to a 9-week programme with the last 3 weeks revisiting concepts already explored in the programme and supporting regular practice. The structure of MindOnLine reflects the mindfulness-based stress reduction approach by incorporating characteristics typical of mindfulness-based programmes, namely educational component, and formal and informal mindfulness practices. Keeping in line with Crane et al’s,10 Framework for adaptation of mindfulness-based programmes, MindOnLine adapted the delivery of the programme to an online version to facilitate access and convenience of use.
Methods and analysis
Aims and hypotheses
The aims of this study are to determine the effect of MindOnLine on FCR, anxiety and depression in cancer survivors. The specific aims are:
Aim 1
To evaluate the impact of the MindOnLine intervention on the primary outcome (FCR), measured using the FCRI total score20 at the end of the 9-week intervention period. Hypothesis 1: participants receiving the intervention will report lower average FCRI total scores at 9 weeks, compared with the waitlist group.
Aim 2
To evaluate the impact of MindOnLine on secondary outcomes at 9 weeks: (1) anxiety and depression, using the Patient Health Questionnaire (PHQ-9)21 and Generalised Anxiety Disorder (GAD-7) Scale;22 (2) Quality of Life (QoL) measured by Assessment of Quality of Life (AQOL-4D);23 and (3) mindfulness, using Cognitive and Affective Mindfulness Scale-Revised (CAMS-R).24 Hypothesis 2: compared with the waitlist group, participants in the intervention group will report improvement in all of the secondary outcomes at 9 weeks.
Aim 3
To assess if the effect of the intervention on the primary and secondary outcomes, relative to usual care, is sustained at the 9-month follow-up. Hypothesis 3: compared with the waitlist group, participants in the intervention group will report sustained improvement in primary and secondary outcomes at 9 months.
Aim 4
To assess, from a health sector and broader societal perspective, the cost-effectiveness of MindOnLine. Hypothesis 4: compared with the waitlist group, MindOnLine will be cost-effective with an incremental cost-effectiveness ratio likely to fall below the commonly used threshold of US$28 000–US$50 000/QoL Year.
Study design
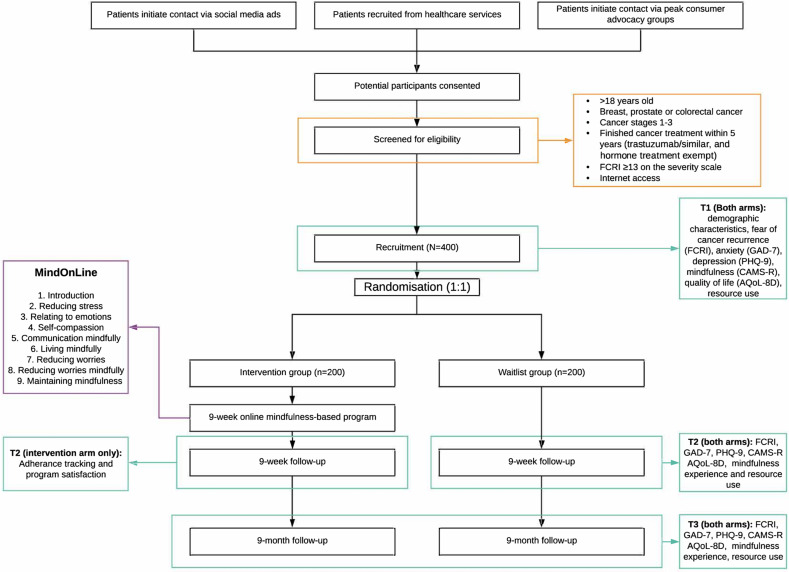
This study is a randomised controlled trial, to test the effectiveness and cost-effectiveness of MindOnLine compared with usual care on FCR, anxiety, depression and QoL among people diagnosed with breast, prostate or CRC. Overall, 400 patients will be recruited with 200 patients randomly allocated to the intervention group and 200 to the waitlist group (usual care only). The intervention group will receive usual care and the online mindfulness programme. Primary and secondary outcomes will be collected at baseline, 9 weeks and 9 months post randomisation. Nine months corresponds to approximately 6 months following the end of the intervention period. Following completion of the study (9 months), participants in the waitlist group will be offered the MindOnLine intervention (figure 1).
Figure 1.
Study flow chart. AQoL-4D, Assessment of Quality of Life-4 Dimensions; CAMS-R, Cognitive and Affective Mindfulness Scale-Revised; FCRI, Fear of Cancer Recurrence Inventory; GAD-7, General Anxiety and Distress Scale; PHQ-9, Patient Health Questionnaire.
Participants
People with a diagnosis of breast, prostate or CRC will be recruited via online advertisements on social media platforms, peak consumer advocacy groups for each cancer Breast Cancer Network Australia (BCNA), or Prostate Cancer Foundation Australia (PCFA), Bowel Cancer Australia social media platforms and CRC support groups or through outpatient services at healthcare providers across Victoria; see figure 1.
Inclusion criteria
Adults, aged 18 years of older, living in Australia, who completed active treatment for stage 1–3 breast, prostate or CRC, (trastuzumab/similar and hormone treatment exempt) within the past 5 years and have no evidence of disease; have internet access and an FCRI severity score ≥13, indicating clinically significant FCR.19 Our pilot study showed 74% of participants with melanoma were identified as having clinically significant FCR.6
Exclusion criteria
Insufficient English language skills to understand videos presented in English, complete surveys in English or living with advanced cancer (stage IV disease with less than a 12-month prognosis of survival).
Recruitment procedures
Multiple methods will be applied to recruit people to the study:
Online through MindOnLine social media pages including Facebook, Instagram, Twitter, Reddit and LinkedIn.
Sharing recruitment flyers through BCNA and PCFA social media platforms, and Australian-based cancer groups.
Email invitations sent to BCNA and PCFA supporters (and CRC support groups) and cancer registries.
Paid Facebook and Instagram advertising.
Through outpatient clinics, chemotherapy and radiotherapy units and rooms of oncologists and surgeons at cancer treatment centres.
Online recruitment procedure
(1) The MindOnLine social media pages will be shared among social networks and will allow people to post questions about the project. (2) A recruitment flyer will be distributed by BCNA, PCFA, Bowel Cancer Australia and CRC Facebook support groups using their existing social media platforms. (3) Study invitations will be sent to supporters registered with BCNA and PCFA who have indicated an interest in learning about research projects. (4) Paid advertisements will run throughout the recruitment period on Facebook, Instagram and Twitter to distribute the project details to a wider audience. The use of paid advertisements in health research is becoming popular and a systematic review has shown this to be an effective recruitment strategy.25
In all online recruitment methods, people will have access to the recruitment flyer, which will provide a brief overview of the study, the link to the MindOnLine registration page and the contact number of the project manager.
Health service recruitment procedures
If recruitment across social media platforms, advertisements and peak consumer advocacy groups does not generate sufficient participation levels, participating health services oncologists or surgeons involved in the project, will support the recruitment process. The research assistant (RA) at each site will screen patients and confirm eligibility of patients with treating clinicians or with nurses working in the outpatient units. RAs will then contact patients by phone and interested patients will be emailed the study details with a link to the study webpage and registration page. If there is no response from patients, a message will be left on their phone. Two further attempts to reach patients will be made (a week apart), and after a third unsuccessful attempt no further contact will be made. If patients have not enrolled in the study within 2 weeks, one follow-up phone call will be made to answer any queries patients may have about the study and to assist with registration. We have used similar screening and recruitment approaches in previous studies and they were found to be acceptable and successful.6 We anticipate a recruitment period of 18 months.
Consent and screening
Once directed to the MindOnLine registration page, participants will be presented with the plain language statement and then asked to provide consent (online supplemental file 1). Potential participants will be asked to provide basic demographic and disease information allowing screening to ensure they meet study eligibility criteria. Potential participants will also complete the severity subscale of the FCRI to allow those with scores ≥13 to be screening into the study. Those screened into the study will provide their email address and contact number, and directed to the baseline questionnaires. People who are not eligible will receive an online message thanking them for their interest in the study and referring them to local support services provided by leading cancer charities should they require support.
bmjopen-2021-057212supp001.pdf (928.8KB, pdf)
Randomisation
Eligible participants will be allocated to treatment groups using random sequences embedded in the online survey platform Qualtrics, ensuring allocation concealment. Randomisation (using stratified random permuted blocks) will be conducted in blocks of size 2, stratified by cancer type (breast, prostate, CRC) and age (<60; ≥60 years old). Participants will be unblinded to group assignment, while researchers and data analysts will be blinded to the group condition.
Waitlist control group
Participants allocated to the waitlist group will receive usual care. Following randomisation, they will receive an email with a list of services they may contact for information and support. They will be informed that they will be granted access to MindOnLine intervention in 9-month’s time, when intervention participants have completed the final survey.
Intervention group—MindOnLine program
Participants allocated to the intervention will be provided with the link to MindOnLine, which comprises three main components:
an educational component to increase participants’ knowledge about the science and practice of mindfulness and how it may benefit them in everyday life;
a formal mindfulness meditation practice to improve awareness and emotion regulation; and
an informal practice to teach participants how to bring mindfulness to daily activities.
A new theme is introduced each week, with a new meditation practice which participants will be encouraged to undertake every day The MindOnLine programme is detailed in table 1.
Table 1.
Weekly content of the MindOnLine programme
| Week | Theme | Meditation | Daily practice |
| 1 | Introduction to mindfulness | Breath | Being present with the experience |
| 2 | Reducing stress | Body scan | Notice how the body responds to stress |
| 3 | Relating to emotions | Working mindfully with emotions | Noticing the cycle of emotions |
| 4 | Self-compassion | Self-compassion | Notice self-criticism |
| 5 | Communicating mindfully | Listening/sound meditations | Bringing attention back to the conversation |
| 6 | Living mindfully | Practising with gentleness and patience | Pause throughout the day |
| 7 | Reducing worries | Mindfully working with worries and fears | Notice when caught up overthinking |
| 8 | Reducing worries mindfully | Loving kindness meditation | Notice acts of kindness |
| 9 | Maintaining mindfulness | Silence with bells | Notice when distracted from being present |
Each module’s theme will be explained through a short 5–10 min video. At the end of each week, participants will receive an email with a link to the video introducing the theme for the upcoming week. The transcripts for the videos will be available for downloading and saving or printing in a pdf format so that participants can keep a copy for later reference. At the end of each module, participants will receive an automatically generated email reminding them to continue daily meditation practice (formal practice) and given specific everyday mindfulness exercises to apply during daily activities (informal practice).
To enhance adherence and retention to the 9-week programme and deepen their mindfulness experience, participants will have access to additional programme features. The features are guided by a framework proposed by Abraham and Michie26 to facilitate behaviour change in interventions:
Two times per day reminders to complete mindfulness practice. Adapted from the pilot study, emails containing a link to a short, guided meditation audio file will be sent to participants two times per day. These emails will serve as reminders to meditate and will provide easy access to the meditation practice of the week.
Progress tracking. Participants will be able to monitor their own mindfulness practice each day by reviewing how many times they have used each section of the programme, and the duration of use. Embedded usage data tracking systems records each login and provides real time representation of programme use.
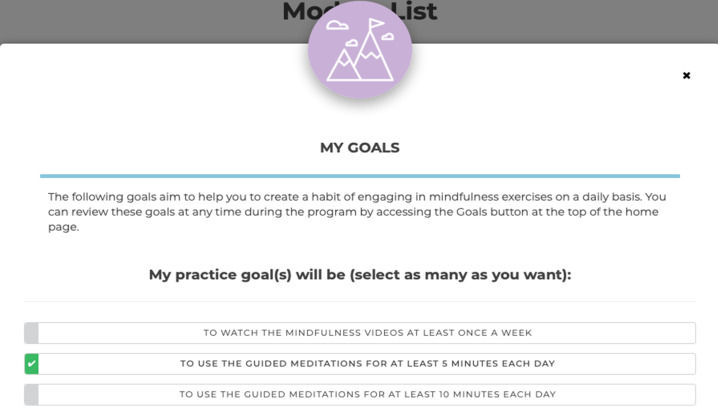
Goal setting. When enrolled in the programme, participants will have the opportunity to set goals for their mindfulness practice (figure 2). Goals are linked to usage data tracking to provide participants with feedback about whether they are reaching their goals. Goals may be practice orientated, for example, to practise mindfulness for 5 min each day, or may be specific to each person’s situation for example, I would like to manage my worries leading up to my oncologist appointment.
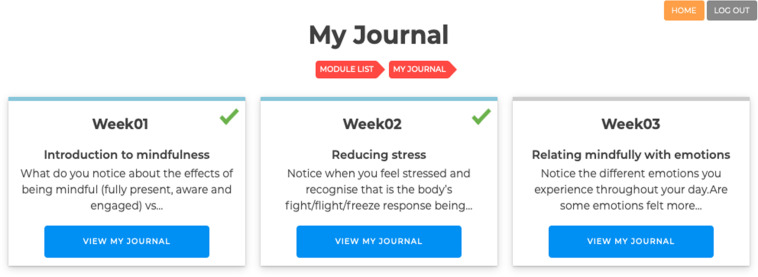
Reflective journaling. Participants will have the opportunity to journal their experiences during the mindfulness programme by using the ‘My Journal’ functionality (figure 3). Each week’s content will have a journal section, which will include prompts related to mindfulness programme content, participants will be able to enter and save their responses within the programme for future review. Prompts will be developed specifically for the study.
Figure 2.
My Goal functionality in MindOnLine.
Figure 3.
My Journal-guided self-reflection practise in MindOnLine.
The mindfulness programme can be accessed at any time via direct login to the website or via the hyperlink sent to participants in the daily emails.
Data collection
Table 2 illustrates the overall schedule for trial participants in both groups. All assessments will be performed online. The questionnaires at baseline, at 9 weeks including the satisfaction survey for those in the intervention group and at 9 months, will be sent via Qualtrics through an automatically generated schedule. Participants who do not complete questionnaires will be followed up by telephone at each data collection point. At baseline, participants’ demographic information (ie, gender, age, marital status, current employment status, highest level of education, postcode, type of cancer, date of diagnosis, time since end of last treatment, type of treatment and previous meditation experience) will be collected.
Table 2.
Schedule of enrolment, interventions and assessments
| Timepoint |
Study period | |||||||||||
| Enrolment | Allocation | Post allocation | Post intervention | |||||||||
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | 9 months | |||
| Enrolment | ||||||||||||
| Eligibility screen | X | |||||||||||
| Informed consent | X | |||||||||||
| Allocation | X | |||||||||||
| Interventions | ||||||||||||
| Immediate access to MindOnLine |

|
|||||||||||
| Waitlist group | ||||||||||||
| Assessments (both groups) | ||||||||||||
| Demographic characteristics | X | |||||||||||
| FCRI 18 | X | X | X | |||||||||
| GAD-7 20 | X | X | X | |||||||||
| PHQ-9 19 | X | X | X | |||||||||
| CAMS-R 22 | X | X | X | |||||||||
| AQoL-4D 21 | X | X | X | |||||||||
| Mindfulness experience | X | X | X | |||||||||
| Resource use | X | X | X | |||||||||
| COVID-19 measures | X | X | X | |||||||||
| Assessments (intervention group only) | ||||||||||||
| Adherence tracking and meditation log | X | X | X | X | X | X | X | X | X | X | ||
| Programme satisfaction | X | X | ||||||||||
AQOL-4D, Assessment of Quality of Life; CAMS-R, Cognitive and Affective Mindfulness Scale-Revised; FCRI, Fear of Cancer Recurrence Inventory; GAD-7, Generalised Anxiety Disorder; PHQ-9, Patient Health Questionnaire.
Outcome measures
Primary outcome
Fear of Cancer Recurrence Inventory
The 42-item FCRI is a multidimensional FCR scale intended for use with all patients with cancer. Items were developed on the basis of a cognitive–behavioural formulation of FCR (range: 0–168).19 The FCRI consists of seven domains: triggers, severity, psychological distress, functional impairment, reassurance, insight and coping strategies (scoring range: 0–36). It has shown high internal consistency, good construct and criterion validity in adults with different cancer types.20
Secondary outcomes
Anxiety and depression
The GAD-7 Scale22 is a valid and efficient tool for assessing generalised anxiety symptoms and assessing severity in clinical practice and research. The seven items assess the frequency of core symptoms of GAD within the past 2 weeks (scoring range: 0–21).22
The PHQ-920 parallels the nine diagnostic symptom criteria that define the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) major depressive disorders (fourth edition). At only 9 items (scoring range: 0–27), the PHQ-9 is shorter than most depression tools. Unlike most other measures of depression, the PHQ-9 was developed, tested and refined for use with medical patients.21
The PHQ-9 and GAD-7 are recommended for use among cancer survivors in the American Society of Clinical Oncology Guidelines.27
Mindfulness
Trait mindfulness is measured using the CAMS-R,24 a 10-item self-report questionnaire. This scale uses everyday language appropriate for those with little meditation experience and is designed to capture mindfulness as a general daily experience. The questionnaire comprises four domains of mindfulness (attention, present-focus, awareness and acceptance/non-judgement). Participants are asked to rate on a 4-point Likert scale how much they relate to each statement (scoring range: 4–40). Compared with other measures of mindfulness, the CAMS-R is unique in that it is related to psychological distress,28 which is highly relevant to the current study population.29
Other outcome measures
Mindfulness experience
In order to control for access to external mindfulness-based programmes particularly in the waitlist group, all participants will be asked whether they have enrolled in a mindfulness-based programme in the period between surveys and/or used other supportive care services (eg, peer support, psychologists, psychotherapy, counsellors, yoga and meditation).
Program satisfaction
Participants in the intervention group will be asked to provide feedback about the MindOnLine programme. Quantitative and qualitative data using open-ended questions will be collected in relation to satisfaction with programme content, the helpfulness of the programme, usability and areas for improvement. The satisfaction questionnaire has been adapted from the satisfaction questionnaire used in the pilot study.6
Economic outcomes
AQoL 4D23 is a health-related QoL utility measure. It is generally used in economic evaluations. The Resource Use Questionnaire covers general healthcare services usage (self-reported), use of other welfare services and impacts on work force participation. The questionnaire has been successfully used in cancer psychosocial intervention studies.30
The surveys will take approximately 20 min to complete.
Adherence tracking and meditation log
The software package used to run MindOnLine was developed at Deakin University and has inbuilt functionality to collect and clean usage data. Google Analytics has been incorporated into the platform to allow for validation of findings. Both software will track participants’ online activity, including login date/times, navigation patterns, page views and duration and features used (video, audio, goals and reflective journaling).
Impact of COVID-19
To control for potential environmental impacts on mental well-being outcomes, participants will be asked whether COVID-19 has had an impact on their mental well-being in the 2 weeks prior to baseline, 9-week and 9-month assessments.
Sample size calculations
Power calculations are conservative, that is, the detectable differences reported below are possibly larger than the true detectable differences, because they are based on two-group comparison of change while the main analysis (see Analysis plan) will adjust for baseline values of the outcome and for factors used in the stratified randomisation.31 The statistical software PASS V.14.0.9 (NCSS, LLC) was used for all calculations (α=0.05; two-sided tests).
Primary outcome
Change in FCRI total score between baseline and 9 weeks. The target sample size (200 participants per arm) achieves 94% (80%) power to detect a mean difference between arms of 10 points (8 points) change in FCRI score or 80% power to detect a difference of 8 points (SD: 23.532) SD estimate obtained from Butow et al,32 as their study included a heterogenous sample of patients with cancer while our pilot study only included patients with melanoma stage 2–3. The selected effect sizes correspond to Cohen’s d of 0.43 (moderate/large) and 0.34 (small/moderate), respectively. Currently, there is no definition of a clinically significant improvement for FCRI score; however, the proposed effect size is comparable to that described in other studies.32
Secondary outcomes
The target sample size (200 participants per arm) achieves 80% power to detect an intervention effect of size 0.34 (Cohen’s f, small/moderate) at 9 weeks for any of the outcomes. This effect size corresponds to mean differences between groups of: (1) 1.5 point in PHQ-9 depression score (SD=4.5, maximum SD reported in patients with breast, colorectal and prostate cancer;33 PHQ-9 minimal clinically important difference (MCID) for patients with cancer: 2.6–5);34 (2) 1.4 point in GAD-7 anxiety score (SD=4.1, maximum SD reported in patients with breast, colorectal and prostate cancer;35 MCID=1.95);33 and mean differences between group in change from baseline to 9 weeks of: (3) 1.2 points in FCRI severity score (SD=3.6, pilot study effect: 2.6);6 (4) 1.1 points in CAMS-R score (SD=3.4); (5) 2.5 points in Worry score (SD=7.3); (6) 3.0 points in PSS score (SD=8.9); the last three reflecting moderate Cohen’s d effect sizes (<0.35).
To meet the sample size needs of our desired statistical power, we will recruit 400 participants. In our pilot study, six participants (13%) withdrew in the intervention group and none in the control group. Assuming a conservative 30% attrition rate at 9 months, we expect to have complete data for approximately 280 participants (140 per group).
Analysis plan
All statistical analysis will be conducted on an intention-to-treat basis whereby all randomised participants with at least one postbaseline measurement will be analysed by original treatment assignment regardless of adherence. Baseline characteristics will be described using summary measures selected based on variable distribution. The main analysis will adjust for baseline values of the outcome and for factors used in the stratified randomisation.31
Aims 1 and 2: the effect of the intervention on each of the outcomes, defined as change from baseline to 9 weeks, will be assessed using linear models including group and the stratification factors. Aim 3: the effect of the intervention across the three measurement times will be estimated using linear mixed models, including study group, time (categorical: 9 weeks, 9 months) interaction group×time and the stratification factors as fixed effects and participant as a random effect. If there is a positive intervention effect on mental health outcomes, exploratory mediation analyses will be conducted to determine whether improvements are mediated by increases in mindfulness.36 For outcomes where it is a plausible assumption that missing data are completely at random, we will use complete case analysis; if not plausible, we will use multiple imputation. Subgroup analysis: we will explore whether age or gender modify the effect of the intervention on FCR, depression and anxiety at 9 weeks.
Aim 4: this study will also comprise a cost-consequences analysis where incremental costs of the intervention will be compared with the full spectrum of outcomes included in the study. A series of cost-effectiveness ratios can be determined which have been shown to be useful for decision-makers. Inclusion of the AQoL 4D will also enable a cost-utility analysis to be undertaken, thereby allowing practical judgements to be made regarding value for money credentials of the intervention. Nevertheless, the economic analysis will be primarily from the perspective of the healthcare sector and a secondary analysis from the broader societal perspective will also be undertaken. A detailed costing of the intervention will be undertaken and the evaluation will first measure and value any change to the use of healthcare resources over the period of the study between the two arms of the trial and then compare any additional costs to the additional outcomes achieved. Standardised economic evaluation techniques will be used including incremental analysis of mean differences and bootstrapping to determine CIs along with a net monetary analysis to determine the cost-effectiveness of the intervention for different value for money threshold criteria. The costs of routine roll-out will be estimated.
MindOnLine usage data by the intervention group will be reported using descriptive statistics. Linear mixed models, with random intercept and slope for each person, will be fitted to estimate time trends in usage.
Data management
Data will be exported from Qualtrics on a monthly basis and crossed checked during exportation to ensure accuracy in results. All identifying participant information will be removed from data sets. Documents containing sensitive information will be saved as password protected files and stored within the Deakin University One Drive.
Monitoring
Data
The adherence data will be monitored by the programme developer. The programme developer does not have any competing interests. Other project data will be monitored by the project steering committee with regular meetings and progress updates. No interim analysis will be performed during the trial.
Patient and public involvement
Representatives from three consumer organisations have been involved in the design and implementation of the project since its inception. Their contribution has included development of the intervention and its content, wording on recruitment material, and provided advice on recruitment strategies. Representatives from each consumer organisation have contributed to project steering meetings.
Ethics and dissemination
Harms
All participants will be required to provide written informed consent. In the event that a participant reports distress to the project manager they will be advised to seek assistance from the regular medical professionals and provided with additional referrals to lifeline.org.au. Ethics approval was obtained from the Peter MacCallum Cancer Centre (20-53) and Deakin University (2020-284). Any adverse events will be reported to the ethics committees.
Auditing
The trial may be audited by the governing Human Research Ethics Committees.
Protocol amendments
Protocol amendments will be approved by the governing Human Research Ethics Committees. Any relevant changes will be submitted as a modification to the Australian and New Zealand Clinical Trial Registry.
Dissemination
The findings of this study will be written by study authors and published in peer reviewed journals project steering committee. All identifying participant information will be removed prior to publication.
Discussion
One of the most significant changes across society is the use of web-based technology. Online mindfulness-based interventions circumvent problems with traditional face-to-face delivery of the programme, impacted by work commitments, caring responsibilities, geographical isolation and pandemics.37 38
This study will rigorously evaluate the efficacy of a self-directed online mindfulness programme in reducing FCR in breast, prostate and CRC survivors. The study will advance the literature regarding the benefits of mindfulness for cancer survivors by representing one of few large well controlled trials of a self-directed mindfulness-based programme, involving smartphone technology, aimed at reducing FCR. Including a health economic evaluation of the programme adds to the utility of the trial with the study providing information that budget holders and policy-makers need when considering recommendations and support for supportive care programmes. This trial will fill a gap in knowledge regarding the potential impact of an online mindfulness programme in supporting cancer survivors.7 Extensive pilot work in identifying the type of programme cancer survivors is interested in involving consumers in designing the content and length of the programme and providing reminders and practice tips increase the likelihood of participants engaging with the programme and the intervention having a positive impact.
The study is being conducted in partnership with health services and cancer advocacy groups. As partners in the study, they will ensure the intervention can be rolled out to cancer survivors if shown to be effective. In addition to consumer advocacy groups, the study is being conducted in partnership with government. As we expect the MindOnLine intervention to improve health outcomes, reduce the fear and distress in cancer survivorship and reduce health service and community costs our partnership with government will ensure that policy-makers are informed of the study’s findings particularly cost-effectiveness findings.
The study has a number of strengths and weaknesses. Development of the intervention through a review of the literature, input from consumers and findings from a pilot study and involvement of consumer advocacy groups and government are study strengths ensuring translation of the programme into practice if shown to be effective. For example, consumer advocacy groups have contributed to the design of the intervention programme, recruitment of eligible patients, and will provide advice on the interpretation of results, dissemination and translation. Incorporating an economic evaluation into the study design is a strength as it will complement clinical findings and support decision-making processes for potential implementation.
However, several methodological limitations also need to be acknowledged. Recruitment through social media platforms means we cannot accurately assess uptake of the intervention, as we will not be able to identify the number of eligible people exposed to our advertisements. This may limit our ability to determine reach of the programme. However, recording the time taken for recruitment and accessing google analytic data on internet traffic and page visits may provide some information in this area. Participants will need access to the internet to participate. While this may mean some people will be excluded from the study, we believe this will have minimal impact on the study. We envisage that the study will take approximately 4 years to complete. Advances in social platforms, technology and app-based programming can change substantially in a short period. While this may affect the actual online platform used for the programme, we do not consider this will influence the programme content or delivery mechanisms. As technology advances will likely increase interest in self-directed support programmes for cancer survivors, it is essential that cancer survivors access programmes with demonstrated effectiveness.
Supplementary Material
Footnotes
Twitter: @Afaf_Girgis, @cancer_K2A
Contributors: PML, LR, LO, NW, MJ, AG, DA, EO, CM, AU, RC, JP-N, DH, MB, BR, KW, MF, ABS, KP, MS, DC and VW contributed to the conception of the program or design of the study. EO designed the web platform and analytics with input from LR, NW, RC, DA, AW, PML. CM designed the economic component of the study. PML, LR, LO, NW, MJ, AG, DA, EO, CM, AU, RC, JP-N, DH, MB, BR, KW, MF, ABS, KP, SS, AW, KG, MS, DC, BP and VW provided substantial input into the development of the protocol or revising it critically for important intellectual content. PML, LR, NW, LO and VW drafted the manuscript with contributions from MJ, AG, DA, EO, CM, AU, RC, JP-N, DH, MB, BR, KW, MF, ABS, KP, SS, AW, KG, MS, DC, BP. Each of the authors contributed to, read and approved the final manuscript. Each of the co-authors are on the steering committee, will oversee implementation of the study and data collection and will contribute to the acquisition, analysis or interpretation of the data.
Funding: This study is funded by the National Health and Medical Research Council (NHMRC) Partnership Grant ID APP1179317. The funder supported the cost of undertaking the project.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Australian Institute of Health and Welbeing . Australia’s health in brief 2020. Canberra: AIHW, 2020. [Google Scholar]
- 2.Carlson LE. Mindfulness-based interventions for coping with cancer. Ann N Y Acad Sci 2016;1373:5–12. 10.1111/nyas.13029 [DOI] [PubMed] [Google Scholar]
- 3.Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv 2013;7:300–22. 10.1007/s11764-013-0272-z [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AJ. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol 2007;25:4670–81. 10.1200/JCO.2006.10.0438 [DOI] [PubMed] [Google Scholar]
- 5.Savard J, Ivers H. The evolution of fear of cancer recurrence during the cancer care trajectory and its relationship with cancer characteristics. J Psychosom Res 2013;74:354–60. 10.1016/j.jpsychores.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 6.Russell L, Ugalde A, Orellana L, et al. A pilot randomised controlled trial of an online mindfulness-based program for people diagnosed with melanoma. Support Care Cancer 2019;27:2735–46. 10.1007/s00520-018-4574-6 [DOI] [PubMed] [Google Scholar]
- 7.Keng S-L, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clin Psychol Rev 2011;31:1041–56. 10.1016/j.cpr.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piet J, Würtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta-analysis. J Consult Clin Psychol 2012;80:1007–20. 10.1037/a0028329 [DOI] [PubMed] [Google Scholar]
- 9.Chambers SK, Occhipinti S, Foley E, et al. Mindfulness-Based cognitive therapy in advanced prostate cancer: a randomized controlled trial. JCO 2017;35:291–7. 10.1200/JCO.2016.68.8788 [DOI] [PubMed] [Google Scholar]
- 10.Crane RS, Brewer J, Feldman C, et al. What defines mindfulness-based programs? the warp and the weft. Psychol Med 2017;47:990–9. 10.1017/S0033291716003317 [DOI] [PubMed] [Google Scholar]
- 11.van Emmerik AAP, Berings F, Lancee J. Efficacy of a Mindfulness-Based mobile application: a randomized waiting-list controlled trial. Mindfulness 2018;9:187–98. 10.1007/s12671-017-0761-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toivonen KI, Zernicke K, Carlson LE. Web-Based mindfulness interventions for people with physical health conditions: systematic review. J Med Internet Res 2017;19:e303. 10.2196/jmir.7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compen F, Bisseling E, Schellekens M, et al. Face-to-face and internet-based mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol 2018;36:2413–21. 10.1200/JCO.2017.76.5669 [DOI] [PubMed] [Google Scholar]
- 14.Zernicke KA, Campbell TS, Speca M, et al. The eCALM trial: eTherapy for cancer applying mindfulness. exploratory analyses of the associations between online Mindfulness-Based cancer recovery participation and changes in mood, stress symptoms, mindfulness, posttraumatic growth, and spirituality. Mindfulness 2016;7:1071–81. 10.1007/s12671-016-0545-5 [DOI] [Google Scholar]
- 15.Bruggeman-Everts FZ, Wolvers MDJ, van de Schoot R, et al. Effectiveness of two web-based interventions for chronic cancer-related fatigue compared to an active control condition: results of the "fitter na kanker" randomized controlled trial. J Med Internet Res 2017;19:e336. 10.2196/jmir.7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell L, Ugalde A, Milne D, et al. Digital characteristics and dissemination indicators to optimize delivery of Internet-Supported Mindfulness-Based interventions for people with a chronic condition: systematic review. JMIR Ment Health 2018;5:e53. 10.2196/mental.9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell L, Orellana L, Ugalde A, et al. Exploring knowledge, attitudes, and practice associated with meditation among patients with melanoma. Integr Cancer Ther 2018;17:237–47. 10.1177/1534735417699514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharpe L, Turner J, Fardell JE, et al. Psychological intervention (ConquerFear) for treating fear of cancer recurrence: mediators and moderators of treatment efficacy. J Cancer Surviv 2019;13:695–702. 10.1007/s11764-019-00788-4 [DOI] [PubMed] [Google Scholar]
- 19.Smith Allan 'Ben', Thewes B, Turner J, et al. Pilot of a theoretically grounded psychologist-delivered intervention for fear of cancer recurrence (conquer fear). Psychooncology 2015;24:967–70. 10.1002/pon.3775 [DOI] [PubMed] [Google Scholar]
- 20.Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv 2015;9:481–91. 10.1007/s11764-015-0424-4 [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care 2008;46:266–74. 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- 23.Richardson J, Iezzi A, Khan MA, et al. Validity and reliability of the assessment of quality of life (AQoL)-8D multi-attribute utility instrument. Patient 2014;7:85–96. 10.1007/s40271-013-0036-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman G, Hayes A, Kumar S, et al. Mindfulness and emotion regulation: the development and initial validation of the cognitive and affective mindfulness Scale-Revised (CAMS-R). J Psychopathol Behav Assess 2007;29:177–90. 10.1007/s10862-006-9035-8 [DOI] [Google Scholar]
- 25.Whitaker C, Stevelink S, Fear N. The use of Facebook in recruiting participants for health research purposes: a systematic review. J Med Internet Res 2017;19:e290–e90. 10.2196/jmir.7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379–87. 10.1037/0278-6133.27.3.379 [DOI] [PubMed] [Google Scholar]
- 27.Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of clinical oncology guideline adaptation. J Clin Oncol 2014;32:1605–19. 10.1200/JCO.2013.52.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergomi C, Tschacher W, Kupper Z. The assessment of mindfulness with self-report measures: existing scales and open issues. Mindfulness 2013;4:191–202. 10.1007/s12671-012-0110-9 [DOI] [Google Scholar]
- 29.Kasparian NA, McLoone JK, Butow PN. Psychological responses and coping strategies among patients with malignant melanoma: a systematic review of the literature. Arch Dermatol 2009;145:1415–27. 10.1001/archdermatol.2009.308 [DOI] [PubMed] [Google Scholar]
- 30.Livingston PM, Heckel L, Orellana L, et al. Outcomes of a randomized controlled trial assessing a smartphone application to reduce unmet needs among people diagnosed with cancer (ACE). Cancer Med 2020;9:507–16. 10.1002/cam4.2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Tsiatis AA. Efficiency study of estimators for a treatment effect in a pretest–posttest trial. Am Stat 2001;55:314–21. 10.1198/000313001753272466 [DOI] [Google Scholar]
- 32.Butow PN, Turner J, Gilchrist J, et al. Randomized trial of ConquerFear: a novel, theoretically based psychosocial intervention for fear of cancer recurrence. J Clin Oncol 2017;35:4066–77. 10.1200/JCO.2017.73.1257 [DOI] [PubMed] [Google Scholar]
- 33.Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer 2017;72:46–53. 10.1016/j.ejca.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 34.Scott EL, Kroenke K, Wu J, et al. Beneficial effects of improvement in depression, pain Catastrophizing, and anxiety on pain outcomes: a 12-month longitudinal analysis. J Pain 2016;17:215–22. 10.1016/j.jpain.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 35.Faller H, Brähler E, Härter M, et al. Unmet needs for information and psychosocial support in relation to quality of life and emotional distress: a comparison between gynecological and breast cancer patients. Patient Educ Couns 2017;100:1934–42. 10.1016/j.pec.2017.05.031 [DOI] [PubMed] [Google Scholar]
- 36.Labelle LE, Campbell TS, Carlson LE. Mindfulness-Based stress reduction in oncology: evaluating mindfulness and Rumination as mediators of change in depressive symptoms. Mindfulness 2010;1:28–40. 10.1007/s12671-010-0005-6 [DOI] [Google Scholar]
- 37.Simard S, Savard J. Fear of cancer recurrence inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer 2009;17:241–51. 10.1007/s00520-008-0444-y [DOI] [PubMed] [Google Scholar]
- 38.Behan C. The benefits of meditation and mindfulness practices during times of crisis such as COVID-19. Ir J Psychol Med 2020;37:256–8. 10.1017/ipm.2020.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057212supp001.pdf (928.8KB, pdf)