Abstract
Aims/Introduction
Very few studies assess the effectiveness of different protocols of intermittent very‐low calorie diet (VLCD) in patients with diabetes. This study was designed to compare the effects of 2 days/week and 4 days/week of intermittent VLCD on glycemic control, diabetes remission, metabolic parameters and quality of life in patients with type 2 diabetes and obesity.
Materials and Methods
Participants with obesity and type 2 diabetes were recruited and randomly assigned to three groups, consisting of control, 2 days/week and 4 days/week of intermittent VLCD. In the intermittent VLCD groups, participants received a 600‐kcal diet per day on restricted days and ad libitum food consumption on non‐restricted days. Glycemic control, rate of diabetes remission, metabolic parameters and quality of life were evaluated at baseline, weeks 2, 10 and 20.
Results
A total of 40 participants were enrolled. The mean body mass index was 30.1 ± 5.9 kg/m2, and the mean glycated hemoglobin was 7.4 ± 1.2%. At week 20, there was an improvement in glycemic control in both intermittent VLCD groups with significant decreases in glycated hemoglobin levels and insulin resistance index throughout the study periods. Diabetes remission without the need for medications was equally found in 29% of participants in both intermittent VLCD groups. Serum triglyceride, bodyweight, body mass index and fat mass were also significantly decreased in both VLCD groups. No serious adverse events were encountered.
Conclusion
Intermittent VLCD was highly effective in achieving optimal glycemic control. The effects of 2 days/week and 4 days/week of intermittent VLCD on diabetes remission were relatively similar.
Keywords: Diabetes, Intermittent very‐low calorie diet, Obesity
Intermittent caloric restriction for 2 and 4 days/week were highly effective in achieving glycemic control without serious adverse events.

Introduction
Type 2 diabetes is a progressive disease with a gradual decrease in β‐cell function over time. Recent studies, however, have shown that inducing negative energy balance can reverse the underlying defects of type 2 diabetes 1 . Very‐low calorie diet (VLCD) has been reported to rapidly improve glycemic control within 1–2 weeks, resulting in diabetes remission 2 , 3 , 4 , 5 . Nevertheless, maintaining the beneficial effects of continuous VLCD is quite challenging, and long‐term diabetes remission is closely related to the ability to maintain long‐term weight loss. Unfortunately, weight regain after discontinuation of VLCD is common, and is detrimental to glycemic and other metabolic effects that have previously been achieved 3 , 6 , 7 , 8 . From the Diabetes Remission Clinical Trial (DiRECT), diabetes remission was closely related to the degree of weight loss maintained at 12 months with the achievement rate of 86% in participants with at least 15 kg weight loss and just 7% of participants who maintained 0–5 kg weight loss 7 . Continuous VLCD also requires careful management of oral hypoglycemic agents to prevent hypoglycemia and carries a risk of long‐term complications, such as micronutrient deficiency 9 .
Intermittent VLCD is one of the modalities proposed to achieve weight loss in overweight and obese patients 10 , 11 , 12 . Theoretically, it provides more flexibility to optimize individual results; however, data are scarce on the effectiveness of intermittent VLCD in patients with type 2 diabetes 2 , 13 , 14 , 15 . In addition, there is no standard definition of “intermittent” VLCD, and no data are available to directly compare different protocols of intermittent VLCD in achieving glycemic control and diabetes remission.
The present study was designed to compare the effects of two intermittent VLCD protocols (2 and 4 days/week) with those of the control group on glycemic control, rate of diabetes remission, metabolic parameters and quality of life in patients with type 2 diabetes and obesity.
Materials and Methods
Study design
This randomized controlled trial utilized an allocation ratio of 1:1:1 to 1 of the three groups (2 days per week, 4 days per week of intermittent VLCD and the control group). Randomization was used to generate an online random number allocation and was not blinded. The study was approved by our institutional research ethics committee (Chulalongkorn University) on 17 November 2016 (certificate of approval number 046/2016). This clinical trial was registered under the Thai Clinical Trials Registry number 20160118001. Reporting has been described in detail with the CONSORT guideline standard. The trial was carried out at the Diabetes, Hormone and Metabolism Excellence Center of King Chulalongkorn Memorial Hospital between January 2016 and June 2018.
Participants
Participants were recruited using advertisements posted in the Hospital. Inclusion criteria were patients aged between 30 and 60 years, and diagnosed with type 2 diabetes within the previous 10 years with a body mass index (BMI) ≥23 kg/m2 and a glycated hemoglobin (HbA1C) level between 6.5 and 10%. Type 2 diabetes was defined as a fasting plasma glucose (FPG) level ≥126 mg/dL or a 2‐h plasma glucose level after a 75‐g oral glucose tolerance test (OGTT) ≥200 mg/dL or use of glucose‐lowering medication(s). Exclusion criteria were fasting C‐peptide level <1 ng/mL, previous use of insulin, previous treatment with a thiazolidinedione or a glucagon‐like peptide‐1 receptor agonist in the past 3 months, serum creatinine more than 1.5 mg/dL and serum alanine aminotransferase more than 2.5‐fold above the upper limit of the reference range.
Interventions
The study protocol was composed of two periods: a 2‐week run‐in period and an 18‐week intermittent caloric restriction period. In the 2‐week run‐in period, participants were tried on VLCD (total calorie intake of 600 kcal/day) for 10 days to assess compliance. In the 18‐week intermittent caloric restriction period, participants received 2 or 4 non‐consecutive days/week of intermittent VLCD. Ad libitum food consumption was allowed on non‐restricted days. A calorie‐restricted diet protocol in the present study consisted of 55% carbohydrate, 15% protein and 30% fat. The calories in our study were divided evenly among the three meals. In some cases, 200 mL of Once‐pro® (Thai Otsuka Pharmaceutical®, Bangkok, Thailand) was provided to replace one meal. Non‐starchy vegetables and other energy‐free beverages were allowed on restricted days. Participants were encouraged to consume a minimum of 2,500 mL of water daily. One daily tablet of multivitamin was provided throughout the study. In the control group, participants received a normal diet of 1,500–2,000 kcal/day throughout the study period and continued to receive usual standard diabetes care. All participants were encouraged to continue their usual physical activities, and were in close contact with an endocrinologist using smartphones to ensure compliance and safety throughout the study periods. Appointments were made with an endocrinologist and a dietitian every 2 weeks for 20 weeks. Blood chemistries, metabolic parameters, bodyweight, body composition and quality of life were evaluated during each study period. Dietary record was used to assess dietary compliance.
Medication protocol
All participants were required to self‐monitor their blood glucose levels by a fingerstick at least twice per week and when necessary to prevent hypoglycemia or hyperglycemia. The records of blood glucose levels were reviewed at each clinical visit. The medical management protocol was developed under the Thai national clinical guideline and an endocrinologist was consulted. At the commencement of VLCD, the dosages of glucose‐lowering medications were reduced by 50%. During the ensuing run‐in period, glucose‐lowering medications were either decreased or discontinued by an endocrinologist based on the glycemic control. The protocol required discontinuation of a sulfonylurea if the baseline HbA1C level was ≤6.5%. If the HbA1C level was >6.5% but <9%, a sulfonylurea was discontinued on the energy restriction days only. During the intervention period, if the mean of all 2‐week blood glucose readings was ≤140 mg/dL, a sulfonylurea was either decreased or discontinued first, followed by an alpha‐glucosidase inhibitor and, finally, metformin. Medications were reinitiated if the mean of all 2‐week blood glucose readings was >140 mg/dL. If the mean level was >200 mg/dL, medications were increased in a reverse order following the Thai national clinical guideline. The medication effect score was used to quantify diabetes medication changes 13 . The medication effect score was calculated as the percentage of the maximum daily dose for each medication multiplied by an adjustment factor. An adjustment factor was the reported median absolute decrease in HbA1C for each medication 16 .A higher score reflects a high use of the medication.
Outcomes and measurements
The primary outcomes were changes in glycemic control (plasma glucose and HbA1C levels) and the rate of diabetes remission, defined as a FPG level <126 mg/dL and a HbA1C level <6.5% in the absence of pharmacological therapy for diabetes, at the end of the study. The secondary outcomes were changes in insulin secretion, insulin sensitivity, anthropometric parameters, cardiovascular risk factors and quality of life.
All outcome data were collected for all participants at baseline, and weeks 2, 10 and 20. The OGTT‐based measurement of insulin secretion, insulin sensitivity and insulin resistance were carried out, in which blood was sampled at 0, 30, 60, 90 and 120 min after a 75‐g OGTT to measure glucose, C‐peptide and insulin concentrations. Homeostasis model assessment of insulin resistance (HOMA‐IR) was calculated using the original equation (fasting plasma insulin [mU/L] × fasting plasma glucose [mmol/L] / 22.5). The Matsuda Index was derived to represent both hepatic and peripheral insulin sensitivity (10,000 / √ [fasting glucose × fasting insulin] [mean glucose × mean insulin]), whereas the insulinogenic index showed the insulin response to a glucose challenge (∆insulin [0–30 min] / ∆glucose [0–30 min]). Finally, the oral disposition index, a composite measure of both insulin secretion and insulin sensitivity, was also determined ([1 / fasting insulin] × [∆insulin (0–30 min) /∆glucose (0–30 min)]). Samples for insulin and C‐peptide measurements were frozen at −20°C for subsequent analysis using a solid phase two‐site chemiluminescence immunoassay kit (Siemens, Erlangen, Germany) with an IMMULITE 1,000 analyzer.
Safety parameters including complete blood count, liver function, renal function, electrolyte and lipid levels were determined in the central laboratory. Anthropometric measurement was collected by use of body composition analysis (Tanita BC‐418, Akita, Japan). Quality of life (QoL) was assessed using the SF‐36 questionnaire, which measured eight health concepts: (i) physical functioning; (ii) role limitations due to physical health problems; (iii) bodily pain; (iv) general health perceptions; (v) vitality, energy or fatigue; (vi) social functioning; (vii) role limitations due to emotional problems; and (viii) general mental health. The eight scaled scores were the weighted sums of the questions in their section. Each scale was directly transformed into a 0–100 scale on the assumption that each question carried equal weight and a higher score indicated a better health status.
Statistical analysis
Power analysis was used to calculate the sample size based on data by Williams et al. 13 A total of 42 participants (14 participants in each group) were required to provide 90% statistical significance to detect differences in an expected proportion of 0.95.
Statistical analyses were carried out using SPSS 17.0 software (SPSS, Chicago, IL, USA). All data are presented as the mean ± standard error of the mean (SEM). The χ2‐test was used to analyze differences between groups at baseline. Analysis of variance (anova) with repeated measures was used to detect changes in metabolic parameters over time during the study periods. Post‐hoc analysis was carried out using the Bonferroni correction. The primary analysis was carried out according to the intention to treat analysis protocol. Sensitivity analysis using the last observation carried forward method assumption was carried out to impute missing data. Analysis was also carried out using a linear mix model to adjust the effects of diabetes medications. Logistic regression analysis was used to determine independent factors associated with the primary outcomes at week 20. A P‐value <0.05 was considered statistically significant.
Results
Participant characteristics
A total of 42 participants with obesity and type 2 diabetes were recruited, but two were excluded due to meeting the exclusion criteria (Figure 1). A total of 40 participants (29 women and 11 men) entered the study with 14 participants in the 2 days/week intermittent VLCD group, 14 participants in the 4 days/week intermittent VLCD group and the remaining 12 participants in the control group. All participants completed the study with no dropouts. Baseline participants’ characteristics are shown in Table 1. The mean age ± SEM was 49.6 ± 7.9 years and the mean BMI ± SEM was 30.1 ± 5.9 kg/m2. The mean duration of diabetes ± SEM was 4.9 ± 3.1 years and the mean HbA1C level ± SEM was 7.4 ± 1.1%. More than half of the study participants had a history of hypertension and dyslipidemia, but none had established cardiovascular diseases. The differences among the three groups were not statistically significant. The majority of participants were prescribed glucose‐lowering medications as monotherapy or dual therapy. A number of glucose‐lowering medications prescribed at baseline were comparable. Metformin was most commonly prescribed (100% in the control group, 79% in the 2 days/week intermittent VLCD group and 93% in the 4 days/week intermittent VLCD group, P = 0.174). The use of sulfonylurea was also not significantly different among the three groups (50% in the control group, 29% in the 2 days/week intermittent VLCD group and 57% in the 4 days/week intermittent VLCD group, P = 0.289). The overall compliance to intermittent VLCD by self‐report dietary records in both groups was excellent (≥95%).
Figure 1.
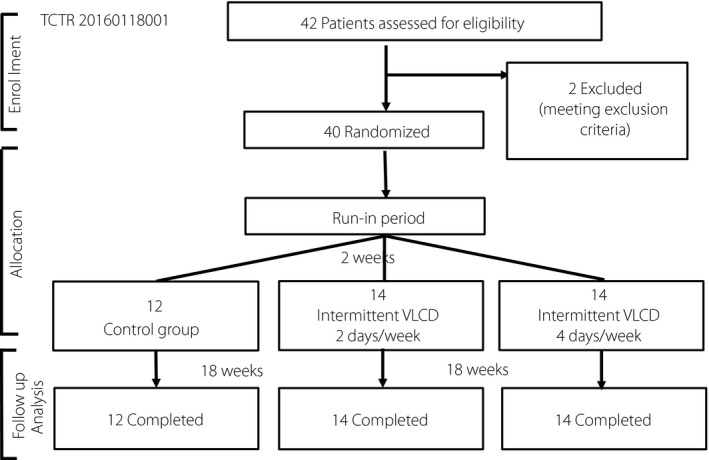
CONSORT flow diagram. TCTR, Thai Clinical Trials Registry; VLCD, very low‐calorie diet.
Table 1.
Baseline characteristics of the participants
| Variable |
Control (n = 12) |
2 days/week intermittent VLCD (n = 14) |
4 days/week intermittent VLCD (n = 14) |
|---|---|---|---|
| Baseline demographics | |||
| Age (years) | 52.0 ± 6.0 | 49.5 ± 7.2 | 47.6 ± 7.9 |
| Female sex (%) | 83.3 | 85.7 | 50.0 |
| Duration of diabetes (years) | 5.2 ± 3.2 | 5.5 ± 3.0 | 3.1 ± 2.8 |
| No. oral diabetes medication (%) | |||
| Diet alone | 0 | 21 | 7 |
| 1 | 42 | 50 | 36 |
| ≥2 | 58 | 29 | 57 |
| Types of oral diabetes medication (%) | |||
| Metformin | 100 | 79 | 93 |
| Sulfonylureas | 50 | 29 | 57 |
| Hypertension (%) | 45.5 | 64.3 | 66.7 |
| Dyslipidemia (%) | 72.7 | 71.4 | 75.0 |
| Glycemic control and indices | |||
| FPG (mg/dL) | 145.1 ± 14.0 | 156.0 ± 13.0 | 159.6 ± 12.8 |
| 2‐h glucose after an OGTT (mg/dL) | 306.7 ± 26.4 | 318.2 ± 24.4 | 349.2 ± 24.4 |
| HbA1C (%) | 6.9 ± 0.3 | 7.5 ± 0.3 | 7.7 ± 0.3 |
| HOMA‐IR | 3.66 ± 1.14 | 4.31 ± 1.06 | 4.52 ± 1.06 |
| Matsuda Index | 5.24 ± 0.96 | 4.94 ± 0.89 | 4.71 ± 0.89 |
| Insulinogenic index | 0.12 ± 0.04 | 0.10 ± 0.03 | 0.14 ± 0.03 |
| Disposition index | 0.44 ± 0.11 | 0.16 ± 0.14 | 0.36 ± 0.10 |
| Metabolic parameters/cardiovascular risk factors | |||
| Total cholesterol (mg/dL) | 188.8 ± 12.5 | 181.1 ± 11.5 | 201.5 ± 11.5 |
| Triglyceride (mg/dL) | 148.2 ± 18.2 | 170.4 ± 16.9 | 139.3 ± 16.9 |
| HDL cholesterol (mg/dL) | 50.3 ± 2.4 | 51.4 ± 2.3 | 43.7 ± 2.2 |
| LDL cholesterol (mg/dL) | 118.1 ± 11.8 | 104.6 ± 10.9 | 135.2 ± 10.9 |
| AST (U/L) | 21.7 ± 3.0 | 19.6 ± 2.8 | 31.1 ± 2.8 |
| ALT (U/L) | 24.5 ± 3.9 | 19.5 ± 3.6 | 32.9 ± 3.6 |
| ALP (IU/L) | 72.0 ± 6.5 | 67.4 ± 6.0 | 71.6 ± 6.0 |
| Albumin (g/dL) | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.3 ± 0.1 |
| Creatinine (mg/dL) | 0.6 ± 0.1 | 0.7 ± 0.04 | 0.7 ± 0.1 |
| Systolic BP (mmHg) | 140.4 ± 5.5 | 122.9 ± 5.1 | 140.9 ± 5.1 |
| Diastolic BP (mmHg) | 80.3 ± 4.3 | 74.9 ± 4.0 | 85.6 ± 4.0 |
| Anthropometric parameters | |||
| Bodyweight (kg) | 73.6 ± 6.0 | 77.2 ± 5.5 | 82.9 ± 5.5 |
| BMI (kg/m2) | 29.1 ± 1.7 | 29.9 ± 1.6 | 31.0 ± 1.6 |
| Waist circumference (cm) | 93.3 ± 3.9 | 94.8 ± 3.6 | 96.2 ± 3.8 |
| %Fat (%) | 36.0 ± 2.2 | 37.7 ± 2.0 | 32.1 ± 2.0 |
| Fat mass (kg) | 26.4 ± 3.3 | 29.7 ± 3.1 | 27.9 ± 3.1 |
| Fat free mass (kg) | 47.2 ± 3.8 | 47.5 ± 3.5 | 55.1 ± 3.5 |
| Muscle mass (kg) | 44.9 ± 3.6 | 45.5 ± 3.4 | 52.4 ± 3.4 |
| Total body water (kg) | 46.9 ± 2.0 | 45.6 ± 1.8 | 47.8 ± 1.8 |
| Quality of life | |||
| SF‐36 (point) | 2,563 ± 163 | 2,444 ± 151 | 2,081 ± 151 |
Data are the mean ± standard error of the mean, unless otherwise specified. ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared) ; BP, blood pressure; FPG, fasting plasma glucose; HbA1C, glycated hemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; OGTT, oral glucose tolerance test; SF‐36, short‐form 36 items (measuring eight health concepts: (i) physical functioning; (ii) role limitations due to physical health problems; (iii) bodily pain; (iv) general health perceptions; (v) vitality, energy or fatigue; (vi) social functioning; (vii) role limitations due to emotional problems; and (viii) general mental health. It consisted of eight scaled scores, which were the weighted sums of the questions in their section. Each scale was directly transformed into a 0–100 scale on the assumption that each question carried equal weight; a higher score indicated a better health status); VLCD, very low‐calorie diet.
Changes in glycemic control and rate of diabetes remission
After VLCD, rapid improvements in FPG and 2‐h plasma glucose levels after an OGTT were observed at week 2 in both of the intermittent VLCD groups compared with those of the control group, and were sustained until week 20, as shown in Figure 2 and Table 2.
Figure 2.
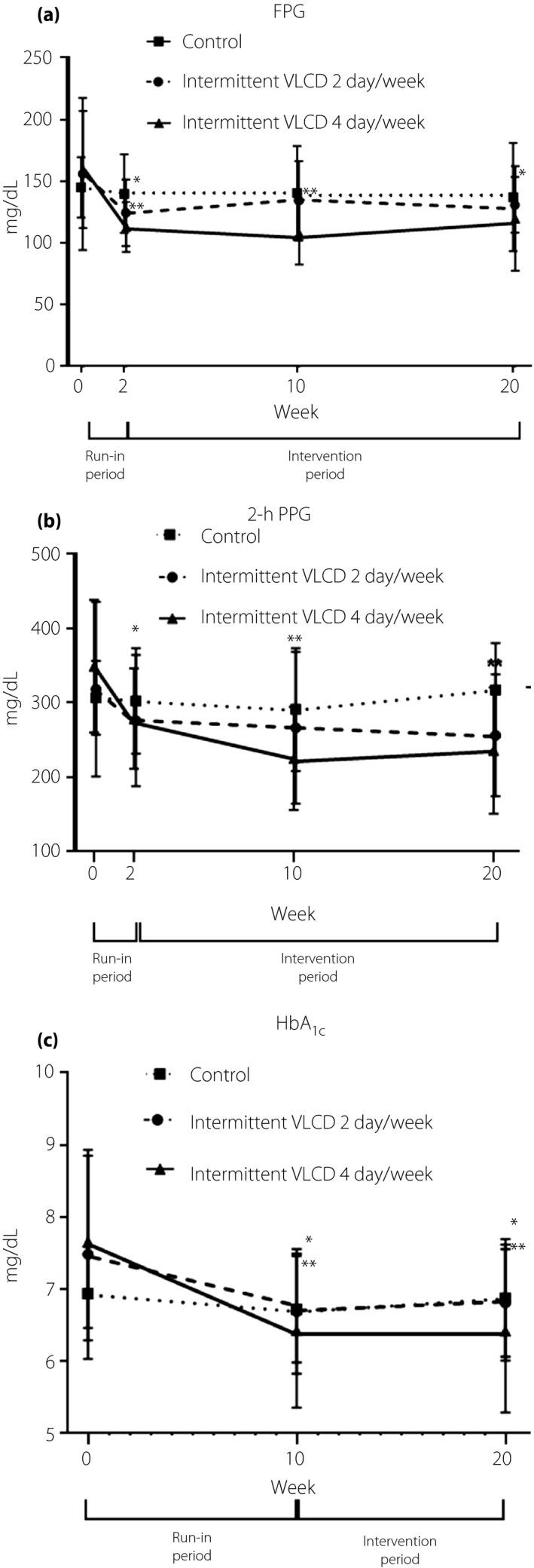
(a) Changes in fasting plasma glucose (FPG), (b) 2‐h plasma glucose after an OGTT (2‐h PPG) and (c) glycated hemoglobin (HbA1C) during the study periods. *P < 0.01, **P < 0.001 compared with values at week 0.
Table 2.
Effects of intermittent very low‐calorie diet on various parameters and mean changes at weeks 10 and 20
| Variable | Week 10 | Week 20 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Control (n = 12) |
2 days/week intermittent VLCD (n = 14) |
4 days/week intermittent VLCD (n = 14) |
Control (n = 12) |
2 days/week intermittent VLCD (n = 14) |
4 days/week intermittent VLCD (n = 14) |
|||||||||
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |||||||||
| Mean difference ± SEM |
P‐value by time |
Mean difference ± SEM |
P ‐value by time |
Mean difference ± SEM |
P ‐value by time |
Mean difference ± SEM |
P ‐value by time |
Mean difference ± SEM |
P ‐value by time |
Mean difference ± SEM |
P ‐value by time |
|||
| Glycemic control and indices | ||||||||||||||
| FPG (mg/dL) | 140.6 ± 9.2 | 134.3 ± 8.5 | 107.9 ± 8.5 | 137.2 ± 10.7 | 130.9 ± 9.9 | 119.9 ± 9.9 | ||||||||
| −4.5 ± 12.8 | 0.728 | −21.7 ± 11.9 | 0.075 | −51.7 ± 11.9 | <0.001 | −7.9 ± 13.5 | 0.560 | −25.1 ± 12.5 | 0.051 | −39.7 ± 12.5 | 0.003 | |||
| 2‐h glucose after OGTT (mg/dL) | 291.0 ± 24.8 | 266.4 ± 23.0 | 225.3 ± 23.0 | 317.3 ± 22.5 | 256.3 ± 20.8 | 235.9 ± 20.8 | ||||||||
| −15.7 ± 25.4 | 0.541 | −51.9 ± 23.5 | 0.033 | −123.9 ± 23.5 | <0.001 | 10.7 ± 23.3 | 0.650 | −61.9 ± 21.6 | 0.007 | −113.4 ± 21.6 | <0.001 | |||
| HbA1C (%) | 6.7 ± 0.3 | 6.7 ± 0.2 | 6.4 ± 0.2 | 6.9 ± 0.3 | 6.8 ± 0.2 | 6.4 ± 0.3 | ||||||||
| −0.2 ± 0.3 | 0.497 | −0.8 ± 0.3 | 0.010 | −1.2 ± 0.3 | <0.001 | −0.1 ± 0.3 | 0.862 | −0.7 ± 0.3 | 0.042 | −1.2 ± 0.3 | <0.001 | |||
| HOMA‐IR | 3.05 ± 0.77 | 2.57 ± 0.71 | 2.54 ± 0.71 | 3.47 ± 0.74 | 2.48 ± 0.68 | 2.39 ± 0.68 | ||||||||
| −0.61 ± 1.00 | 0.546 | −1.74 ± 0.93 | 0.069 | −1.98 ± 0.93 | 0.040 | −0.19 ± 0.94 | 0.837 | −1.83 ± 0.86 | 0.041 | −2.14 ± 0.87 | 0.018 | |||
| Matsuda Index | 5.39 ± 1.48 | 5.37 ± 1.37 | 7.83 ± 1.37 | 6.22 ± 1.40 | 6.24 ± 1.30 | 6.46 ± 1.30 | ||||||||
| 0.15 ± 1.18 | 0.899 | 0.43 ± 1.09 | 0.694 | 3.11 ± 1.09 | 0.007 | 0.98 ± 1.17 | 0.406 | 1.30 ± 1.08 | 0.237 | 1.74 ± 1.08 | 0.115 | |||
| Insulinogenic index | 0.16± 0.05 | 0.10 ± 0.05 | 0.23 ± 0.05 | 0.15 ± 0.06 | 0.13 ± 0.06 | 0.28 ± 0.06 | ||||||||
| 0.04 ± 0.05 | 0.408 | −0.01 ± 0.04 | 0.860 | 0.08 ± 0.04 | 0.067 | 0.04 ± 0.06 | 0.552 | 0.03 ± 0.06 | 0.625 | 0.14 ± 0.06 | 0.019 | |||
| Disposition index | 0.47 ± 0.35 | 0.48 ± 0.33 | 1.03 ± 0.33 | 0.51 ± 0.23 | 0.33 ± 0.21 | 1.00 ± 0.21 | ||||||||
| 0.03 ± 0.37 | 0.938 | 0.33 ± 0.35 | 0.352 | 0.67 ± 0.35 | 0.060 | 0.07 ± 0.24 | 0.765 | 0.17 ± 0.22 | 0.434 | 0.64 ± 0.22 | 0.006 | |||
| Metabolic parameters/cardiovascular risk factors | ||||||||||||||
| Total cholesterol (mg/dL) | 187.6 ± 11.0 | 184.9 ± 10.2 | 195.2 ± 10.2 | 200.0 ± 12.4 | 191.2 ± 11.5 | 205.1 ± 11.5 | ||||||||
| −1.3 ± 12.1 | 0.918 | 3.7 ± 11.2 | 0.742 | −6.3 ± 11.2 | 0.577 | 11.2 ± 13.5 | 0.413 | 10.1 ± 12.5 | 0.425 | 3.6 ± 12.5 | 0.772 | |||
| Triglyceride (mg/dL) | 134.7 ± 21.0 | 131.6 ± 19.4 | 101.8 ± 19.4 | 132.7 ± 13.4 | 126.9 ± 12.4 | 97.8 ± 12.4 | ||||||||
| −13.6 ± 18.5 | 0.468 | −38.9 ± 17.1 | 0.029 | −37.4 ± 17.1 | 0.035 | −15.6 ± 15.6 | 0.325 | −43.5 ± 14.5 | 0.005 | −41.4 ± 14.5 | 0.007 | |||
| HDL cholesterol (mg/dL) | 50.2 ±2.5 | 50.1± 2.3 | 44.0± 2.3 | 51.8 ± 2.5 | 50.4± 2.3 | 46.1± 2.3 | ||||||||
| −0.2±1.7 | 0.923 | −1.3 ± 1.5 | 0.421 | 0.3 ± 1.6 | 0.857 | 1.5 ±2.2 | 0.507 | −0.9 ± 2.1 | 0.657 | 2.4 ± 2.1 | 0.249 | |||
| LDL cholesterol (mg/dL) | 115.0 ± 11.0 | 111.6± 10.7 | 133.6± 10.2 | 127.1 ± 11.6 | 120.2± 10.7 | 143.9± 10.7 | ||||||||
| −3.1 ± 11.5 | 0.790 | 7.0 ± 10.6 | 0.514 | −1.6 ± 10.6 | 0.883 | 9.0 ± 12.7 | 0.483 | 15.6 ± 11.7 | 0.191 | 8.7 ± 11.7 | 0.463 | |||
| AST (U/L) | 19.4 ± 2.6 | 16.4 ± 2.5 | 22.3 ± 2.5 | 19.1 ± 2.7 | 18.8 ± 2.5 | 21.8 ± 2.5 | ||||||||
| −2.3± 3.6 | 0.519 | −3.1 ± 3.3 | 0.349 | −8.8 ± 3.3 | 0.012 | −2.7 ± 3.3 | 0.430 | −0.8 ± 3.1 | 0.801 | −9.3 ± 3.1 | 0.005 | |||
| ALT (U/L) | 19.0 ± 2.3 | 15.1 ± 2.1 | 23.5 ± 2.1 | 20.3 ± 2.2 | 13.7 ± 2.1 | 24.6 ± 2.1 | ||||||||
| −5.5±3.3 | 0.106 | −4.3 ± 3.1 | 0.164 | −9.4 ± 3.1 | 0.004 | −4.2 ± 3.7 | 0.264 | −5.8 ± 3.5 | 0.104 | −8.3 ± 3.5 | 0.022 | |||
| Systolic BP (mmHg) | 133.4 ± 5.0 | 128.3 ± 4.6 | 127.4 ± 4.6 | 125.5 ± 4.0 | 121.7 ± 4.6 | 131.1 ± 3.7 | ||||||||
| −7.0±5.2 | 0.188 | 5.4±4.8 | 0.275 | −13.5±4.8 | 0.008 | −14.9±5.0 | 0.005 | −1.2±4.6 | 0.794 | −9.7 ±4.6 | 0.042 | |||
| Diastolic BP (mmHg) | 78.0 ± 3.4 | 79.2 ± 3.2 | 74.7 ± 3.2 | 73.8 ± 3.3 | 75.0 ± 3.0 | 81.9 ± 3.0 | ||||||||
| −2.3 ± 4.9 | 0.647 | 4.4 ± 4.5 | 0.341 | −10.9 ± 4.5 | 0.021 | −6.5 ± 4.6 | 0.163 | 0.1 ± 4.2 | 0.973 | −3.8 ± 4.2 | 0.377 | |||
| Anthropometric parameters | ||||||||||||||
| Weight (kg) | 68.7 ± 5.6 | 71.7 ± 5.1 | 76.1 ± 5.1 | 68.7 ± 5.7 | 71.7 ± 5.2 | 74.3 ± 5.2 | ||||||||
| −4.9 ± 1.1 | <0.001 | −5.5 ± 1.0 | <0.001 | −6.8 ± 1.0 | <0.001 | −4.9 ± 1.4 | 0.002 | −5.5 ± 1.3 | <0.001 | −8.6 ± 1.3 | <0.001 | |||
| BMI (kg/m2) | 27.2 ± 1.6 | 27.7 ± 1.5 | 28.0 ± 1.5 | 27.1 ± 1.6 | 27.8 ± 1.5 | 27.4 ± 1.5 | ||||||||
| −2.0 ± 0.5 | <0.001 | −2.1 ± 0.4 | <0.001 | −3.0 ± 0.4 | <0.001 | −2.0 ± 0.6 | <0.001 | −2.1 ± 0.5 | 0.001 | −3.6 ± 0.5 | <0.001 | |||
| Fat mass (kg) | 23.0 ± 3.2 | 25.7 ± 3.0 | 23.4 ± 3.0 | 22.6 ± 3.2 | 25.2 ± 3.0 | 22.4 ± 3.0 | ||||||||
| −3.4 ± 0.9 | <0.001 | −4.0 ± 0.8 | <0.001 | −4.5 ± 0.8 | <0.001 | −3.8 ± 1.1 | 0.001 | −4.5 ± 1.0 | <0.001 | −5.4 ± 1.0 | <0.001 | |||
| Fat‐free mass (kg) | 45.7 ± 3.5 | 46.0 ± 3.3 | 52.7 ± 3.3 | 46.1 ± 4.0 | 46.5 ± 3.7 | 49.8 ± 3.7 | ||||||||
| −1.5 ± 0.5 | 0.007 | −1.5 ± 0.5 | 0.005 | −2.3 ± 0.5 | <0.001 | −1.1 ± 2.0 | 0.572 | −1.0 ± 1.8 | 0.603 | −5.2 ± 1.8 | 0.007 | |||
| Quality of life | ||||||||||||||
| SF‐36 (point) | 2,730 ± 116 | 2,785 ± 107 | 2,866 ± 107 | 2,684 ± 127 | 2,757 ± 118 | 2,697 ± 118 | ||||||||
| 166 ± 158 | 0.299 | 341 ± 146 | 0.025 | 784 ± 146 | <0.001 | 120 ± 171 | 0.485 | 313 ± 158 | 0.055 | 615 ± 158 | <0.001 | |||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; FPG, fasting plasma glucose; HbA1C, glycated hemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; OGTT, oral glucose tolerance test; SEM, standard error of the mean; SF‐36, short‐form 36 items; VLCD, very low‐calorie diet.
The three groups did not differ in FPG levels at the end of the study. However, participants in the 4 days/week group were more likely to attain lower FPG, 2‐h plasma glucose and HbA1C levels compared with those in the 2 days/week and control groups (Table 2).
At week 20, the change from baseline in the mean ± SEM FPG level was −39.7 ± 12.5 mg/dL in the 4 days/week group (P = 0.003), −25.1 ± 12.5 mg/dL in the 2 days/week group (P = 0.051) as compared with −7.9 ± 13.5 mg/dL in the control group (P = 0.56), with a mean difference (each of the intermittent VLCD groups vs placebo) of 17.3 ± 14.6 and 6.3 ± 14.6 mg/dL (P = 0.244 and 0.669, respectively). The mean difference in the change in the mean FPG level between the 2 days/week and the 4 days/week intermittent VLCD groups was 10.7 mg/dL (95% confidence interval −10.3 to 33.0, P = 0.439). Similarly, greater improvements in glucose tolerance after an OGTT were observed in both of the intermittent VLCD groups than that in the control group (Table 2).
At week 20, the mean HbA1c ± SEM fell by 1.2 ± 0.3% in the 4 days/week group (P = <0.001), 0.7 ± 0.3% in the 2 days/week group (P = 0.042), and by 0.1 ± 0.3% in the control group (P = 0.862). In addition, the three groups differed in the percentage of patients who attained a HbA1C level of <6.5% at week 20; that is, 10 participants (64%) in the 4 days/week group achieved a HbA1C level of <6.5%, whereas just five patients (29%) in the 2 days/week group and two patients (25%) in the control group, although the difference did not reach statistical significance (P = 0.07).
At the end of week 20, diabetes remission without need for glucose‐lowering medications was found in 29% of participants in both the 2 days/week and the 4 days/week of intermittent VLCD groups compared with none of the participants in the control group (P = 0.117; Table 3, Figure 3). Glucose‐lowering medications were successfully withdrawn in 58% of the control group, 64% of the 2 days/week group and 86% of the 4 days/week group (P = 0.267; Table 3, Figure 3). The total mean ± SEM medication effect score of sulfonylurea and metformin decreased significantly over time and were relatively similar in all three groups (Table 4), suggesting the lower use of the medications.
Table 3.
Rate of diabetes remission and discontinuation of diabetes medications at weeks 10 and 20
| Variable | Time | Group |
Total (n = 40) |
||
|---|---|---|---|---|---|
|
Control (n = 12) |
2 days/week intermittent VLCD (n = 14) |
4 days/week intermittent VLCD (n = 14) |
|||
| Diabetes remission† | Week 10 | 2 (17%) | 3 (21%) | 4 (29%) | 9 (23%) |
| Week 20 | 0 (0%) | 4 (29%) | 4 (29%) | 8 (20%) | |
| Discontinuation of diabetes medications‡ | Week 10 | 7 (58%) | 10 (71%) | 14 (100%) | 31 (78%) |
| Week 20 | 7 (58%) | 9 (64%) | 12 (86%) | 28 (70%) | |
During the run‐in period, a sulfonylurea was discontinued if the baseline glycated hemoglobin level was ≤6.5%. If the glycated hemoglobin level was >6.5% but <9%, a sulfonylurea was discontinued on the energy restriction days only. During the intervention period, if the mean of all 2‐week blood glucose readings was ≤140 mg/dL, a sulfonylurea was either decreased or discontinued first, followed by an alpha‐glucosidase inhibitor and, finally, metformin. Medications were reinitiated if the mean of all 2‐week blood glucose readings was >140 mg/dL. If the mean level was >200 mg/dL, medications were increased in a reverse order. †Diabetes remission was defined as a fasting plasma glucose level <126 mg/dL and glycated hemoglobin level <6.5% in the absence of pharmacological therapy for diabetes, at the end of the study. ‡Diabetes medication protocol. VLCD, very low‐calorie diet.
Figure 3.
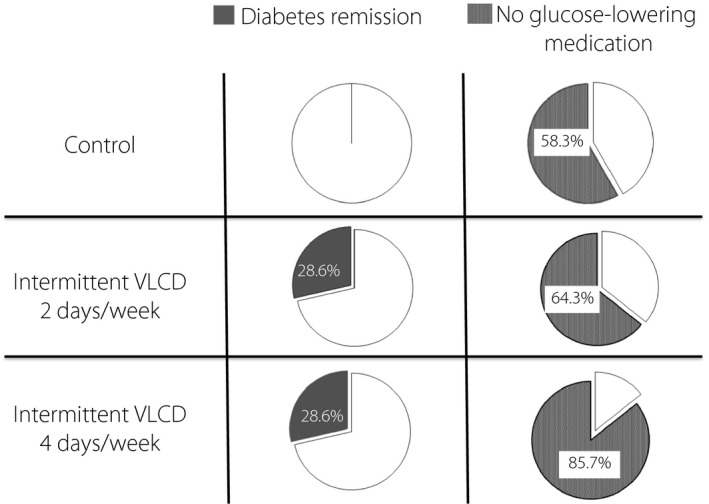
The percentage of diabetes remission at week 20, defined as a fasting plasma glucose (FPG) level <126 mg/dL and glycated hemoglobin <6.5% without the use of glucose‐lowering medications (left panel) and the percentage of participants with no glucose‐lowering medications at week 20 (right panel). VLCD, very‐low calorie diet.
Table 4.
Medication effect score of sulfonylurea and metformin at various time points
| Variable | Groups | Week 0 | Week 10 | Week 20 | |||
|---|---|---|---|---|---|---|---|
| Mean ± SEM | P‐value | Mean ± SEM | P‐value | Mean ± SEM | P‐value | ||
| Medication effect score sulfonylurea |
Control (n = 12) |
0.45 ± 0.15 | – | 3.469E−1018 ± 0.02 | 0.003 | 3.469E−1018 ± 0.02 | 0.003 |
| 2 days/week intermittent VLCD (n = 14) | 0.23 ± 0.13 | – | 0.05 ± 0.02 | 0.202 | 0.05 ± 0.02 | 0.202 | |
|
4 days/week intermittent VLCD (n = 14) |
0.41 ± 0.13 | – | −3.966E−1018 ± 0.02 | 0.004 | −3.966E−1018 ± 0.02 | 0.004 | |
| Medication effect score metformin |
Control (n = 12) |
0.64 ± 0.10 | – | 0.32 ± 0.10 | 0.003 | 0.21 ± 0.07 | <0.001 |
|
2 days/week intermittent VLCD (n = 14) |
0.48 ± 0.10 | – | 0.22 ± 0.10 | 0.07 | 0.18 ± 0.07 | 0.006 | |
|
4 days/week intermittent VLCD (n = 14) |
0.52 ± 0.10 | – | 2.780E−1018 ± 0.10 | <0.001 | 0.02 ± 0.07 | <0.001 | |
Medication effect score (MES = [actual drug dose / maximum drug dose] × drug mean adjustment factor). The MES was calculated as the percentage of the maximum daily dose for each medication multiplied by an adjustment factor. An adjustment factor was the reported median absolute decrease in glycated hemoglobin for each medication. It was used to quantify diabetes medication changes and a higher score reflected a high use of the medication. VLCD, very‐low calorie diet.
After adjusting for different medication use and dosage changes among different participants with a linear mixed model analysis, similar results in plasma glucose levels were obtained. In a stepwise linear regression, no significant effects of age, duration of diabetes, HbA1C level or changes in bodyweight and body composition were observed on diabetes remission.
Changes in insulin resistance/insulin sensitivity and insulin secretion indices
In both intermittent VLCD groups, there were significant improvements in insulin resistance, as reflected in HOMA‐IR at week 20 (Table 2), and the mean difference in changes in HOMA‐IR between the 2 days/week group and the 4 days/week group at week 20 was not significantly different (mean difference 0.1, 95% confidence interval −1.9 to 2.0, P = 0.924). An improvement in the Matsuda Index, an index of insulin sensitivity, was seen only in the 4 days/week group at week 10, but not at week 20. Changes in the insulinogenic index, an index of insulin secretion, showed a significant improvement in the 4 days/week group only at week 20. Finally, significant changes in the disposition index, a composite measure of insulin secretion and insulin sensitivity, were also observed in the 4 days/week group only at week 20 (Table 3).
Changes in bodyweight and body composition
All three groups had significant decreases in weight and BMI at weeks 10 and 20 (Table 2). The average weight loss ± SEM at week 20 was 8.6 ± 1.3 kg (equivalent to 10.4% of participants’ initial bodyweight) in the 4 days/week intermittent VLCD group, 5.5 ± 1.3 kg (equivalent to 7.1% of their initial bodyweight) in the 2 days/week group and 4.9 ± 1.4 kg (equivalent to 6.7% of their initial bodyweight) in the control group. We found no significance differences in changes in bodyweight among the three groups. Similarly, the mean BMI ± SEM decreased by 3.6 ± 0.5 kg/m2 in the 4 days/week group, 2.1 ± 0.5 kg/m2 in the 2 days/week group and 2.0 ± 0.6 kg/m2 in the control group, with no significant differences among the three groups.
Weight loss was predominantly due to fat loss. There were marked decreases in the percentage of fat and fat mass in all groups, and there were no significant differences among the three groups at weeks 10 and 20 (Table 2).
Changes in metabolic parameters
At weeks 10 and 20, the mean serum triglyceride levels were significantly decreased in both the 4 days/week and the 2 days/week groups (Table 2). Changes in serum levels of total cholesterol, low‐density lipoprotein cholesterol and high‐density lipoprotein cholesterol were, however, not statistically significant when compared with their baseline values.
Participants in the 4 days/week intermittent VLCD group also had significant decreases in aspartate transaminase and alanine aminotransferase levels, and systolic blood pressure at weeks 10 and 20 (Table 2). There were no significant differences among the three groups in terms of changes in serum albumin, hemoglobin/hematocrit or creatinine at week 20 (data not shown).
Changes in quality of life
There was a significant improvement in quality of life scores in both intervention groups at week 10 and only in the 4 days/week at week 20 (Table 2), which was primarily due to significantly higher scores in certain domains, such as role limitations due to physical health and health change domains (data not shown).
Safety/side‐effects
During the 20‐week period, no serious adverse events were observed. No severe hypoglycemia was found.
Discussion
To our knowledge, this is the first randomized controlled trial comparing 2 days/week of intermittent VLCD with 4 days/week and the control group in patients with obesity and type 2 diabetes. Our current study showed that either 2 days/week or 4 days/week of intermittent caloric restriction was relatively comparable and highly effective in improving glycemic control. Glucose‐lowering medications could be successfully withdrawn in 64–86% of the intermittent VLCD groups. At the end of the study, diabetes remission was found in almost one‐third of the participants in both of the VLCD groups.
VLCD has been shown to improve glycemic control, resulting in diabetes remission. We and others have previously reported that continuous VLCD is highly effective in inducing short‐term remission of diabetes 6 , 7 , 8 ; however, our long‐term result has shown that only one‐third of participants remained in optimal glycemic control without restarting diabetes medications 12 months after VLCD had ended 6 . The beneficial effects of VLCD seem to diminish after the recurrence of weight increase 3 , 6 , 7 . In this regard, the use of intermittent VLCD might be an interesting option for obese patients who find it difficult to adhere to continuous VLCD to maintain weight loss 17 , as intermittent VLCD provides more flexibility than continuous VLCD 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 .
So far, there have been only a few intermittent VLCD studies carried out in obese patients with type 2 diabetes 2 , 13 , 14 , 15 . The majority of studies have shown that intermittent VLCD could improve glycemic control with the reduction of HbA1C by approximately 0.3–1.5%. The change in HbA1C level in the present study (0.7–1.2%) is comparable to the changes seen in the previous trials. Changes in body composition, such as bodyweight, fat mass and fat free mass, are also similar to what has been reported in the previous trials of obese individuals without type 2 diabetes 15 , 16 , 21 , 26 , 27 , 28 .
Currently, it should be noted that there is no standard definition of “intermittent” caloric restriction/VLCD, and it is extremely difficult to compare various methods of intermittent VLCD among various studies because of the differences in the study populations, the duration of studies and the types of VLCD. Nevertheless, the main result of the present study showed that the beneficial effects of intermittent VLCD could be achieved using only 2 days/week of VLCD and the rate of diabetes remission was comparable to that of 4 days/week, although the beneficial effects in several metabolic parameters were more pronounced in the 4 days/week group.
A recent study comparing intermittent energy restriction (2 days/week of 500–600 kcal/day diet) with continuous energy restriction (1,200–1,500 kcal/day diet, 7 days/week) in patients with type 2 diabetes has shown that glycemic improvement is comparable 16 . At 12 months, the reductions in the mean HbA1C level, weight change, BMI, fat mass and fat‐free mass were relatively similar between the intermittent and the continuous energy restriction groups 15 .
The mechanism by which intermittent energy restriction modulates diabetes remission is not well understood. In the present study, we found a significant reduction in HOMA‐IR, a marker of insulin resistance, but we did not observe a significant change in the Matsuda Index, which represented whole‐body insulin sensitivity. In the 4 days/week intermittent VLCD group, we observed improvements in insulinogenic index and disposition index, suggesting that intermittent VLCD might exert beneficial effects on insulin secretion or β‐cell function. These results are similar to those of our previous study using continuous VLCD for 8 weeks, which has shown improvement in both insulin resistance and β‐cell function 6 .
The present study had certain limitations. First, the sample size was small and was restricted to an Asian population not on insulin therapy only. Second, the slight improvement in the control group might be due to minor differences in baseline data or it could be attributed to some contamination in individuals with intention to lose weight. We observed deliberate weight loss in the control group, which could have affected the outcomes and statistical comparisons between groups. Third, although we provided VLCD and recorded caloric intake on restricted days, we allowed ad libitum intake on non‐restricted days and did not record caloric intake on those days. Therefore, participants might consume less caloric intake on non‐restricted days. Finally, the present study was limited to 20 weeks, and longer‐term follow‐up data are required to evaluate the durability of diabetes remission.
The present study showed that intermittent caloric restriction for 2 days/week and 4 days/week were highly effective in achieving glycemic control without serious adverse events. Improvement in glycemic control was associated with a reduction in insulin resistance, and improvements in insulin secretion, bodyweight, BMI, body composition, cardiovascular risk factors and quality of life. The rate of diabetes remission in individuals using VLCD 2 days/week was comparable to that of 4 days/week, suggesting that this modality of treatment might have great clinical implications for patients with type 2 diabetes and obesity.
Disclosure
MU and WK coauthored a Thai pocketbook with copyright on low calorie menus. MU, PR, SL, WS, KB and WK declare no conflict of interest.
Acknowledgments
This work was supported by National Research Council of Thailand and Heath Systems Research Institute (HRSI). Once‐pro® was provided by Thai Otsuka Pharmaceutical®, Thailand, and glucometer machines were provided by Roche®. The funding sources/sponsors have no role in the study design, collection, analysis and interpretation of data, writing of the manuscript, and decision to submit the article for publication. The authors acknowledge the contribution made by all participants in the study, and wish to express our utmost appreciation to their efforts and dedication.
J Diabetes Investig 2022; 13: 156–166
Clinical Trial Registry
Thai Clinical Trials Registry
20160118001
Contributor Information
Mongkontida Umphonsathien, Email: muijai@hotmail.com.
Weerapan Khovidhunkit, Email: wkhovid@gmail.com.
References
- 1. Taylor R, Barnes AC. Translating aetiological insight into sustainable management of type 2 diabetes. Diabetologia 2018; 61: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wing RR, Blair E, Marcus M, et al. Year‐long weight loss treatment for obese patients with type II diabetes: does including an intermittent very‐low‐calorie diet improve outcome? Am J Med 1994; 97: 354–362. [DOI] [PubMed] [Google Scholar]
- 3. Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011; 54: 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capstick F, Brooks BA, Burns CM, et al. Very low calorie diet (VLCD): a useful alternative in the treatment of the obese NIDDM patient. Diabetes Res Clin Pract 1997; 36: 105–111. [DOI] [PubMed] [Google Scholar]
- 5. Malandrucco I, Pasqualetti P, Giordani I, et al. Very‐low‐calorie diet: a quick therapeutic tool to improve beta cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr 2012; 95: 609–613. [DOI] [PubMed] [Google Scholar]
- 6. Umphonsathien M, Prutanopajai P, Aiam ORJ, et al. Immediate and long‐term effects of a very‐low‐calorie diet on diabetes remission and glycemic control in obese Thai patients with type 2 diabetes mellitus. Food Sci Nutr 2019; 7: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lean ME, Leslie WS, Barnes AC, et al. Primary care‐led weight management for remission of type 2 diabetes (DiRECT): an open‐label, cluster‐randomised trial. Lancet 2018; 391: 541–551. [DOI] [PubMed] [Google Scholar]
- 8. Steven S, Taylor R. Restoring normoglycaemia by use of a very low calorie diet in long‐ and short‐duration Type 2 diabetes. Diabet Med 2015; 32: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 9. Baker S, Jerums G, Proietto J. Effects and clinical potential of very‐low‐calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85: 235–242. [DOI] [PubMed] [Google Scholar]
- 10. Jebeile H, Gow ML, Lister NB, et al. Intermittent energy restriction is a feasible, effective, and acceptable intervention to treat adolescents with obesity. J Nutr 2019; 149: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 11. Stockman MC, Thomas D, Burke J, et al. Intermittent fasting: is the wait worth the weight? Curr Obes Rep 2018; 7: 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev 2018; 19(Suppl 1): 47–60. [DOI] [PubMed] [Google Scholar]
- 13. Williams KV, Mullen ML, Kelley DE, et al. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care 1998; 21: 2–8. [DOI] [PubMed] [Google Scholar]
- 14. Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract 2016; 122: 106–112. [DOI] [PubMed] [Google Scholar]
- 15. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open 2018; 1: e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horne BD, Muhlestein JB, Anderson JL. Health effects of intermittent fasting: hormesis or harm? A systematic review. Am J Clin Nutr 2015; 102: 464–470. [DOI] [PubMed] [Google Scholar]
- 17. Rossner S. Intermittent vs continuous VLCD therapy in obesity treatment. Int J Obes Relat Metab Disord 1998; 22: 190–192. [DOI] [PubMed] [Google Scholar]
- 18. Klempel MC, Bhutani S, Fitzgibbon M, et al. Dietary and physical activity adaptations to alternate day modified fasting: implications for optimal weight loss. Nutr J 2010; 9: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heilbronn LK, Smith SR, Martin CK, et al. Alternate‐day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr 2005; 81: 69–73. [DOI] [PubMed] [Google Scholar]
- 20. Harris L, Hamilton S, Azevedo LB, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta‐analysis. JBI Database System Rev Implement Rep 2018; 16: 507–547. [DOI] [PubMed] [Google Scholar]
- 21. Arguin H, Dionne IJ, Senechal M, et al. Short‐ and long‐term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause 2012; 19: 870–887. [DOI] [PubMed] [Google Scholar]
- 22. Seimon RV, Roekenes JA, Zibellini J, et al. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol 2015; 418(Pt 2): 153–172. [DOI] [PubMed] [Google Scholar]
- 23. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011; 35: 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev 2011; 12: e593–601. [DOI] [PubMed] [Google Scholar]
- 25. Cioffi I, Evangelista A, Ponzo V, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta‐analysis of randomized controlled trials. J Transl Med 2018; 16: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev 2015; 73: 661–674. [DOI] [PubMed] [Google Scholar]
- 27. Headland M, Clifton PM, Carter S, et al. Weight‐loss outcomes: a systematic review and meta‐analysis of intermittent energy restriction trials lasting a minimum of 6 months. Nutrients 2016; 8: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr 2016; 70: 292–299. [DOI] [PubMed] [Google Scholar]


