ABSTRACT
Aims/Introduction
The sodium–glucose cotransporter 2 inhibitor, canagliflozin, reduced kidney failure and cardiovascular events in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial. We carried out a post‐hoc analysis to evaluate the efficacy and safety of canagliflozin in a subgroup of participants in East and South‐East Asian (EA) countries who are at high risk of renal complications.
Materials and Methods
Participants with an estimated glomerular filtration rate of 30 to <90 mL/min/1.73 m2 and urinary albumin‐to‐creatinine ratio of >300–5,000 mg/g were randomized to 100 mg of canagliflozin or a placebo. The effects of canagliflozin treatment on pre‐specified efficacy and safety outcomes were examined using Cox proportional hazards regression between participants from EA countries (China, Japan, Malaysia, the Philippines, South Korea and Taiwan) and the remaining participants.
Results
Of 4,401 participants, 604 (13.7%) were from EA countries; 301 and 303 were assigned to the canagliflozin and placebo groups, respectively. Canagliflozin lowered the risk of primary outcome (composite of end‐stage kidney disease, doubling of serum creatinine level, or renal or cardiovascular death) in EA participants (hazard ratio 0.54, 95% confidence interval 0.35–0.84). The effects of canagliflozin on renal and cardiovascular outcomes in EA participants were generally similar to those of the remaining participants. Safety outcomes were similar between the EA and non‐EA participants.
Conclusions
In the CREDENCE trial, the risk of renal and cardiovascular events was safely reduced in participants from EA countries at high risk of renal events.
Keywords: Canagliflozin, CREDENCE, Nephropathy
A post‐hoc analysis was carried out to evaluate the efficacy and safety of canagliflozin in a subgroup of participants in East and South‐East Asian countries in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial. Canagliflozin lowered the risk of primary composite outcome (composite of end‐stage kidney disease, doubling of serum creatinine level, or death from renal or cardiovascular causes) in East and South‐East Asian participants (hazard ratio 0.54, 95% confidence interval 0.35–0.84). Canagliflozin safely reduced the risk of renal and cardiovascular events in participants from East and South‐East Asian countries at high risk of renal events.

INTRODUCTION
An increasing prevalence of diabetes has occurred worldwide. The Diabetes Atlas, published in 2019, reported that, of the 463 million adult diabetes patients worldwide, 35% reside in the International Diabetes Federation Western Pacific Region, with a significant proportion in East and South‐East Asia (EA) 1 . Diabetes mellitus is a major cause of end‐stage kidney disease (ESKD) 2 , which is often associated with cardiovascular (CV) and renal death 3 , 4 . Presently, treatment options to diminish the development of chronic kidney disease are limited to inhibition of renin–angiotensin–aldosterone systems 5 , 6 .
The high risk of kidney complications in Asian patients with type 2 diabetes mellitus has been observed across many multi‐ethnic studies 7 , 8 , 9 , 10 . Additionally, among 10 countries with a high incidence of treated ESKD, as many as five countries were from the EA region 11 . Thus, it seems very likely that the patients with type 2 diabetes in EA countries are at high risk of ESKD.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial was an international trial set to renal end‐points, showing that the sodium–glucose cotransporter 2 (SGLT2) inhibitor, canagliflozin, safely decreases the risk of renal and CV events in patients with type 2 diabetes mellitus and chronic kidney disease 12 , 13 , 14 , 15 . Based on the data from that study, the US Food and Drug Administration approved canagliflozin in 2019 for lowering the risk of ESKD, doubling of the serum creatinine (DoSC), hospitalization for heart failure (HHF), and CV death in patients with type 2 diabetes and kidney disease with albuminuria 16 . In the CREDENCE study, approximately 20% (877/4,401) of participants were of an Asian race. A subpopulation analysis showed no evidence of difference in the benefit for the primary outcome between the racial subgroups, including the Asian race subgroup. In the present study, we explored whether the effects of canagliflozin on a comprehensive range of renal, cardiovascular and safety outcomes were consistent between the EA participants, who had a high risk of renal complications, and the remaining participants (non‐EA participants). As EA countries, six nations of China, Japan, Malaysia, the Philippines, South Korea and Taiwan were included in the analysis. East and South‐East Asia populations were considered to meet the present study objectives because of the high risk of ESKD and the genetic relatedness of the various ethnic groups 17 , 18 . In contrast, although Indian patients participated in the CREDENCE study, they were excluded from the analysis, because India geographically belongs to South Asia, and the Indian population is genetically distant from East and South‐East Asian populations 17 , 18 .
MATERIALS AND METHODS
Study design
CREDENCE was an international, double‐blind, randomized, multicenter, placebo‐controlled trial, the details of which have been published previously 12 , 19 . The efficacy and safety outcomes were examined in the current post‐hoc analyses in EA participants. Data of the participants reported from the investigators in the EA countries (China, Japan, Malaysia, the Philippines, South Korea and Taiwan) and of the rest of participants were compared.
As this study was a post‐hoc analysis of anonymized data, no ethics committee or institutional review board approvals were required – all such approvals were obtained in the original study (CREDENCE ClinicalTrials.gov number, NCT02065791.)
Study participants
Participants in the CREDENCE study were those with glycated hemoglobin (HbA1c) of 6.5–12.0%, aged ≥30 years, with an estimated glomerular filtration rate (eGFR) of 30 to <90 mL/min/1.73 m2, with a urinary albumin‐to‐creatinine ratio (UACR) of >300 to 5,000 mg/g, and who were being treated for ≥4 weeks with an angiotensin receptor blocker or angiotensin‐converting enzyme inhibitor. An equation of Chronic Kidney Disease Epidemiology Collaboration was used to determine the eGFR. Key exclusion criteria were type 1 diabetes, nondiabetic renal disease, prior immunosuppressive treatment of renal disease, or a history of renal replacement therapy. Table S1 presents detailed inclusion and exclusion criteria.
Study treatment
Participants were stratified by screening eGFR categories (30 to <45, 45 to <60, and 60 to <90 mL/min/1.73 m2) using randomly permuted blocks and were randomized to receive oral canagliflozin 100 mg or corresponding placebo daily. The study treatment was to be ceased at the development of diabetic ketoacidosis, start of dialysis, renal transplant, receipt of disallowed therapy, pregnancy, or study completion. Background treatment intensification based on practice guidelines was recommended for glycemic management and CV protection.
End‐points
The primary outcome was the same as that in the CREDENCE trial for these analyses: the composite of ESKD (chronic dialysis for ≥30 days, kidney transplantation, or eGFR <15 mL/min/1.73 m2 for ≥30 days), DoSC from baseline values sustained for ≥30 days, or renal or CV death. Renal outcomes included DoSC; ESKD; renal death; the composite of ESKD, DoSC, or renal death; the composite of start of renal replacement therapy (start of chronic dialysis or kidney transplantation) or renal death; and the composite of ESKD or renal or CV death. CV efficacy outcomes included the composite of HHF or CV death; the composite of myocardial infarction, CV death, or stroke; CV death; HHF; all‐cause mortality (ACM); and the composite of CV death, myocardial infarction, stroke, HHF, or unstable angina.
This analysis also evaluated the following possible intermediate markers for reduced renal and CV risks: change from baseline in UACR, eGFR, HbA1c, systolic blood pressure, diastolic blood pressure and bodyweight. Additionally, annualized changes in eGFR slope were evaluated as described previously 19 .
Safety events were explored during treatment with canagliflozin including all adverse events (AEs), serious AEs and renal‐related AEs. Renal‐related AEs were defined as the composite of investigator‐reported AEs that were coded as primarily ‘renal’ in accordance with the Medical Dictionary for Regulatory Activities Terminology. Additionally, we analyzed events including acute kidney injury, volume depletion, osmotic diuresis, urinary tract infection, amputation, fracture and genital mycotic infection.
Statistical analysis
Renal, CV and mortality outcomes were analyzed in the intention‐to‐treat principle, based on the stratified Cox proportional hazards regression model according to the treatment effects and the category of eGFR at screening in EA participants and non‐EA participants. The interaction of treatment effects between EA and non‐EA participants was tested by adding regional factor (i.e., EA or non‐EA participants), and a treatment interaction term and regional factor to the model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated for canagliflozin versus placebo. The incidence rates were calculated per 1,000 patient‐years of follow up. The number of patients needed to treat to prevent one event (NNT) for 2.5 years was estimated as the multiplicative inverse of the difference in cumulative incidence between groups, and CIs for the NNT were calculated on HRs and 95% CIs 20 .
For intermediate outcomes in the on‐treatment population over time, a mixed model for repeated measures included the fixed effects of treatment, screening eGFR strata (30 to <45, 45 to <60, 60 to <90 mL/min/1.73 m2), visit, treatment‐by‐visit interaction, baseline value and baseline‐by‐visit interaction (covariance matrix: unstructured). Because the distribution of UACR data was highly skewed, UACR was log transformed to estimate the geometric mean of post‐baseline UACR using a similar model. The changes of the geometric mean of UACR from baseline was used to calculate the reduction in post‐randomization UACR for canagliflozin compared with the placebo. Using a two‐slope model with a knot at week 3, the on‐treatment eGFR slope was estimated as described previously 12 . P‐values are presented for descriptive purposes only, given the post‐hoc nature of analyses.
Safety outcomes were analyzed up to 30 days in all treated participants after the last dose (on‐treatment), except for amputation and fracture events, which were evaluated in the all follow‐up time. HR and 95% CIs for canagliflozin versus placebo, the interaction of treatment effects between EA and non‐EA participants, and annualized incidence rates were calculated by the methods described earlier. All analyses were carried out using SAS (version 9.4; SAS Institute, Cary, NC, USA).
RESULTS
From the 4,401 total participants in the CREDENCE trial, 604 (13.7%) and 3,797 (86.3%) were identified as EA participants and non‐EA participants, respectively. The number of participants by country was 129, 110, 135, 71, 122 and 37 for China, Japan, Malaysia, the Philippines, South Korea and Taiwan, respectively. Compared with the non‐EA participants, EA participants were younger (60.8 vs 63.4 years) and more likely to be male (71.7 vs 65.2%); had a lower body mass index (27.5 vs 31.9 kg/m2) and HbA1c (8.05 vs 8.30%), higher UACR (1054 vs 902 mg/g), and similar diabetes duration, eGFR and blood pressure; were with lower prevalence of heart failure (3.1 vs 16.7%), CV disease (45.5 vs 51.2%) and neuropathy (43.2 vs 49.7%), and greater prevalence of retinopathy (51.2 vs 41.4%); and were less likely to be taking beta‐blockers (25.7 vs 42.5%) and diuretics (27.8 vs 49.7%) at baseline. Baseline characteristics for EA participants were well balanced between the canagliflozin and placebo groups (Table 1).
Table 1.
Key baseline demographic characteristics of participants in East and South‐East Asian and non‐East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE)
| EA participants | Non‐EA participants | |||||
|---|---|---|---|---|---|---|
| Placebo | Canagliflozin | Total | Placebo | Canagliflozin | Total | |
| (n = 303) | (n = 301) | (n = 604) | (n = 1896) | (n = 1901) | (n = 3797) | |
| Age, years (mean ± SD) | 60.9 ± 9.1 | 60.6 ± 9.1 | 60.8 ± 9.1 | 63.5 ± 9.2 | 63.2 ± 9.1 | 63.4 ± 9.2 |
| Male, n (%) | 219 (72.3) | 214 (71.1) | 433 (71.7) | 1248 (65.8) | 1226 (64.5) | 2474 (65.2) |
| Asian race, n (%) | 303 (100.0) | 301 (100.0) | 604 (100.0) | 149 (7.9) | 124 (6.5) | 273 (7.2) |
| Baseline BMI, kg/m2 (mean ± SD) | 27.5 ± 4.3 | 27.6 ± 4.2 | 27.5 ± 4.3 | 31.9 ± 6.2 | 32.0 ± 6.2 | 31.9 ± 6.2 |
| Baseline HbA1c, % (mean ± SD) | 8.03 ± 1.26 | 8.07 ± 1.19 | 8.05 ± 1.22 | 8.30 ± 1.33 | 8.30 ± 1.32 | 8.30 ± 1.32 |
| Duration of diabetes, years (mean ± SD) | 16.39 ± 9.24 | 15.55 ± 8.70 | 15.97 ± 8.98 | 15.96 ± 8.47 | 15.55 ± 8.68 | 15.75 ± 8.57 |
| Screening eGFR, n (%) | ||||||
| 30 to <45 mL/min/1.73 m2 | 85 (28.1) | 84 (27.9) | 169 (28.0) | 571 (30.1) | 573 (30.1) | 1144 (30.1) |
| 45 to <60 mL/min/1.73 m2 | 90 (29.7) | 95 (31.6) | 185 (30.6) | 549 (29.0) | 545 (28.7) | 1094 (28.8) |
| 60 to <90 mL/min/1.73 m2 | 128 (42.2) | 122 (40.5) | 250 (41.4) | 776 (40.9) | 783 (41.2) | 1559 (41.1) |
| Baseline eGFR, mL/min/1.73 m2 (mean ± SD) | 55.4 ± 16.0 | 56.1 ± 17.2 | 55.7 ± 16.6 | 56.1 ± 18.7 | 56.4 ± 18.3 | 56.2 ± 18.5 |
| Median baseline UACR, mg/g (interquartile range) | 1011 (495–2228) | 1067 (525–2001) | 1054 (506–2112) | 906 (467–1786) | 888 (449–1777) | 902 (457–1780) |
| Baseline SBP, mmHg (mean ± SD) | 141.3 ± 15.2 | 138.3 ± 15.7 | 139.8 ± 15.5 | 140.0 ± 15.7 | 140.0 ± 15.6 | 140.0 ± 15.6 |
| Baseline DBP, mmHg (mean ± SD) | 78.2 ± 10.0 | 77.7 ± 10.7 | 78.0 ± 10.3 | 78.4 ± 9.3 | 78.3 ± 9.1 | 78.4 ± 9.2 |
| Medical history, n (%) | ||||||
| Hypertension | 285 (94.1) | 285 (94.7) | 570 (94.4) | 1844 (97.3) | 1846 (97.1) | 3690 (97.2) |
| Heart failure | 11 (3.6) | 8 (2.7) | 19 (3.1) | 312 (16.5) | 321 (16.9) | 633 (16.7) |
| CV disease | 143 (47.2) | 132 (43.9) | 275 (45.5) | 964 (50.8) | 981 (51.6) | 1945 (51.2) |
| Coronary | 73 (24.1) | 61 (20.3) | 134 (22.2) | 587 (31.0) | 592 (31.1) | 1179 (31.1) |
| Cerebrovascular | 67 (22.1) | 72 (23.9) | 139 (23.0) | 291 (15.3) | 270 (14.2) | 561 (14.8) |
| Peripheral vascular | 41 (13.5) | 39 (13.0) | 80 (13.2) | 474 (25.0) | 492 (25.9) | 966 (25.4) |
| Retinopathy | 153 (50.5) | 156 (51.8) | 309 (51.2) | 794 (41.9) | 779 (41.0) | 1573 (41.4) |
| Neuropathy | 135 (44.6) | 126 (41.9) | 261 (43.2) | 935 (49.3) | 951 (50.0) | 1886 (49.7) |
| Medication at baseline, n (%) | ||||||
| Insulin | 190 (62.7) | 185 (61.5) | 375 (62.1) | 1242 (65.5) | 1267 (66.6) | 2509 (66.1) |
| Statin | 225 (74.3) | 226 (75.1) | 451 (74.7) | 1273 (67.1) | 1312 (69.0) | 2585 (68.1) |
| Antithrombotic | 151 (49.8) | 171 (56.8) | 322 (53.3) | 1132 (59.7) | 1170 (61.5) | 2302 (60.6) |
| RAAS inhibitor | 302 (99.7) | 301 (100.0) | 603 (99.8) | 1892 (99.8) | 1900 (99.9) | 3792 (99.9) |
| Beta‐blocker | 76 (25.1) | 79 (26.2) | 155 (25.7) | 811 (42.8) | 804 (42.3) | 1615 (42.5) |
| Diuretic | 88 (29.0) | 80 (26.6) | 168 (27.8) | 943 (49.7) | 946 (49.8) | 1889 (49.7) |
BMI, body mass index; CV, cardiovascular; DBP, diastolic blood pressure; EA participants, participants in East and South‐East Asian countries; eGFR, estimated glomerular filtration rate; non‐EA participants, participants other than East and South‐East Asian participants in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE); HbA1c, glycated hemoglobin; RAAS, renin–angiotensin–aldosterone system; SBP, systolic blood pressure; UACR, urinary albumin (mg)‐to‐creatinine (g) ratio.
As shown in Figure 1, in placebo‐treated participants, most renal events occurred more frequently in EA participants compared with those in non‐EA participants. The renal outcomes were as follows: the composite of ESKD, DoSC or renal death (65.31 vs 36.56 per 1,000 patient‐years; HR 1.92, 95% CI 1.40–2.65); the composite of dialysis, kidney transplantation or renal death (35.57 vs 15.94 per 1,000 patient‐years; HR 2.44, 95% CI 1.57–3.79); DoSC (55.72 vs 30.44 per 1,000 patient‐years; HR 1.97, 95% CI 1.39–2.79); and ESKD (49.58 vs 26.35 per 1,000 patient‐years; HR 2.03, 95% CI 1.40–2.92).
Figure 1.
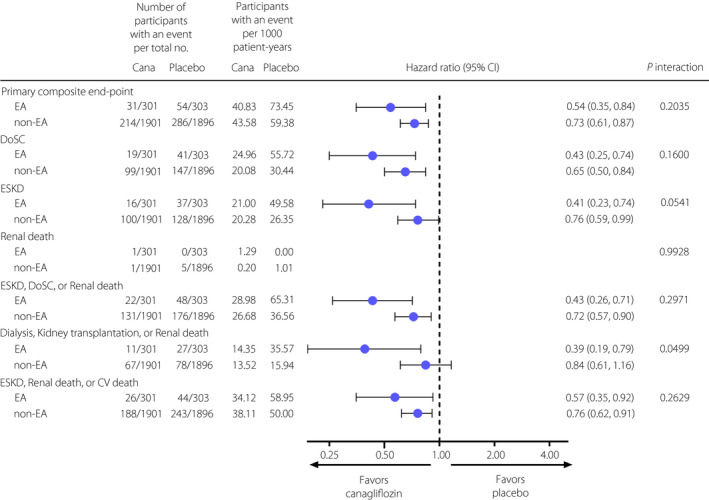
Effects of canagliflozin on renal outcome in participants in East and South‐East Asian and non‐East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE). Cana, canagliflozin; CI, confidence interval; CV death, cardiovascular death; DoSC, doubling of serum creatinine; EA, East and South‐East Asia; ESKD, end‐stage kidney disease; non‐EA, non‐East and South‐East Asia.
Canagliflozin reduced the risk of the primary outcome (composite of ESKD, DoSC, or renal or CV death) compared with the placebo in EA participants (40.83 vs 73.45 per 1,000 patient‐years; HR 0.54, 95% CI 0.35–0.84) with no observed heterogeneity of treatment effect in EA and non‐EA participants (P interaction = 0.2035; Figure 1). Beneficial effects of canagliflozin were observed across other renal outcomes in EA participants, and these effects were generally consistent with those seen in non‐EA participants (all, P interaction > 0.05), except the composite of dialysis, kidney transplantation or renal death (P interaction = 0.0499). The NNTs for 2.5 years for renal outcomes are shown in Table 2. The NNT in EA participants for the primary composite outcome; DoSC; ESKD; the composite of ESKD, DoSC or renal death; the composite of dialysis, kidney transplantation or renal death; and the composite of ESKD, renal death or CV death were 13, 13, 15, 11, 24 and 18, respectively, which were all numerically lower than those in non‐EA participants.
Table 2.
Numbers needed to treat for the renal outcomes of participants in East and South‐East Asian and non‐East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE)
| NNT for 2.5 years (95% CI) | ||
|---|---|---|
| EA participants | Non‐EA participants | |
| Primary composite end‐point | 13 (8–48) | 25 (16–52) |
| DoSC | 13 (8–37) | 35 (23–81) |
| ESKD | 15 (9–63) | 61 (31–1470) |
| ESKD, DoSC or renal death | 11 (7–30) | 37 (23–111) |
| Dialysis, kidney transplantation or renal death | 24 (13–204) | 105 † |
| ESKD, renal death or CV death | 18 (9–551) | 33 (20–96) |
95% confidence interval (CI) for number needed to treat (NNT) is not provided when the 95% CI for absolute risk reduction at 2.5 years includes 0. CV death, cardiovascular death; DoSC, doubling of serum creatinine; EA participants, participants in East and South‐East Asian countries; ESKD, end‐stage kidney disease; non‐EA participants, participants other than East and South‐East Asian participants.
Canagliflozin reduced the risk of CV outcomes, including the composite of CV death or HHF; the composite of CV death, myocardial infarction or stroke; HHF; and the composite of CV death, myocardial infarction, stroke, HHF or unstable angina in EA participants. The results were consistent with those in non‐EA participants (all, P interaction > 0.05; Figure 2). The neutral findings for CV death and ACM in EA participants were also consistent with those seen in non‐EA participants (P interaction = 0.8563 and 0.8986, respectively).
Figure 2.
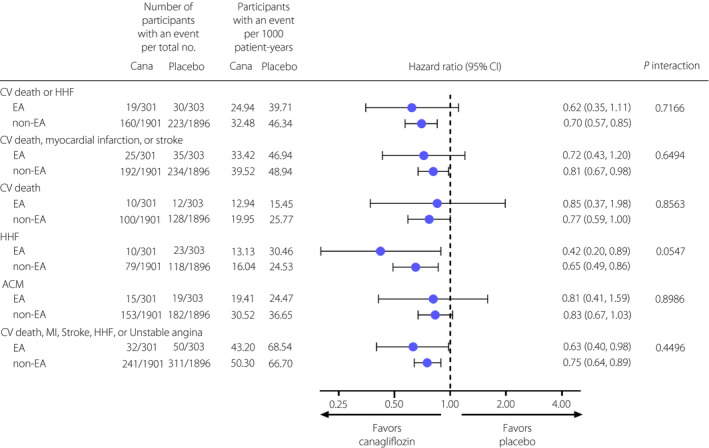
Effects of canagliflozin on cardiovascular and mortality outcomes in participants of East and South‐East Asian and non‐East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE). ACM, all‐cause mortality; Cana, canagliflozin; CI, confidence interval; CV death, cardiovascular death; EA, East and South‐East Asia; HHF, hospitalization for heart failure; MI, myocardial infarction; non‐EA, non‐East and South‐East Asia.
There was only a small difference between the effect of canagliflozin and placebo on HbA1c in EA participants over the course of the study (overall least square [LS] mean difference throughout the trial, −0.29%; 95% CI −0.41 to −0.17; Figure S1). Canagliflozin slightly lowered bodyweight (LS mean difference −0.89 kg; 95% CI −1.15 to −0.62), systolic blood pressure (LS mean difference −4.08 mmHg; 95% CI −5.53 to −2.63) and diastolic blood pressure (LS mean difference −0.69 mmHg; 95% CI −1.56 to 0.18) in EA participants. The geometric mean of UACR change from baseline decreased by 39% (95% CI 31–45; Figure 3a) and 29% (95% CI 25–35) in EA and non‐EA participants, respectively, during the follow‐up period in the canagliflozin groups. The reduction in UACR was comparable between non‐EA and EA participants.
Figure 3.
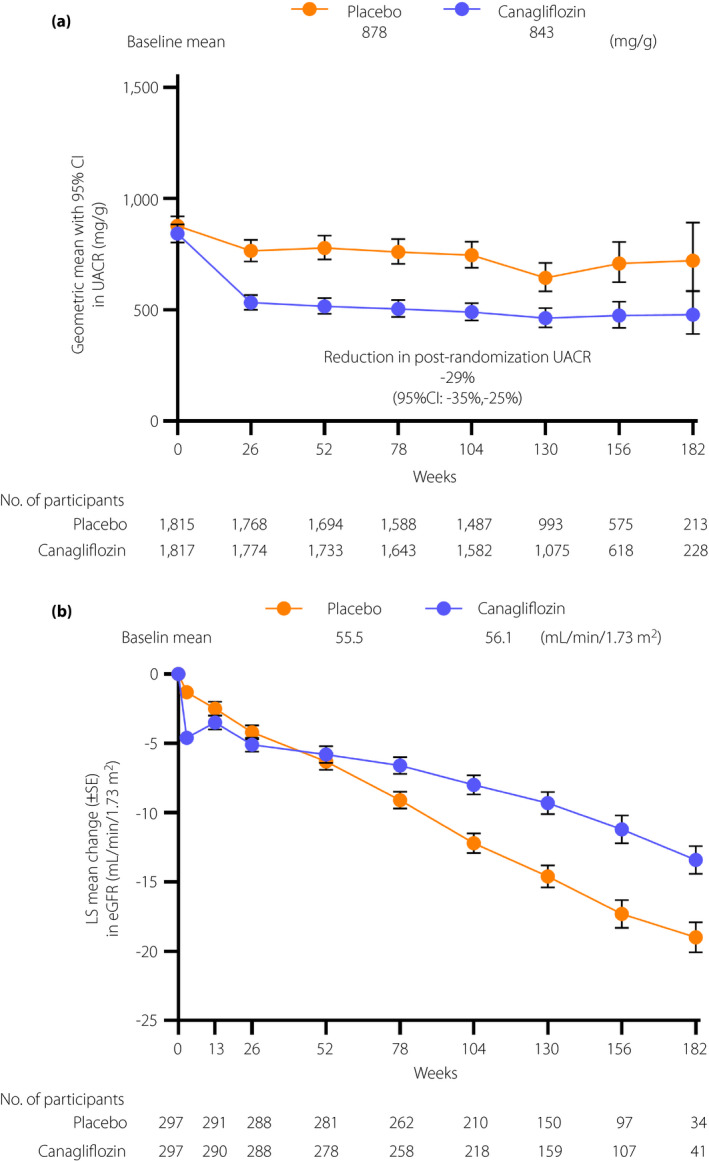
Effects of canagliflozin on intermediate outcomes over time in participants in East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE). Changes in (a) urinary albumin (mg)‐to‐creatinine (g) ratio (UACR) and (b) estimated glomerular filtration rate (eGFR) from baseline. Data are presented as the geometric mean with 95% confidence interval (CI) in (a) and as least square (LS) mean ± standard error (SE).
The mean eGFR in EA participants over the course of the study is shown in Figure 3b. The annual mean slope in eGFR was lower in the canagliflozin group than that in the placebo group (−3.38 vs −5.68 mL/min/1.73 m2/year; placebo‐subtracted difference 2.30 mL/min/1.73 m2/year; 95% CI 1.33 to 3.26). The eGFR decreased from baseline to week 3 by 3.29 mL/min/1.73 m2/3 weeks, and by 0.51 mL/min/1.73 m2/3 weeks in the canagliflozin and placebo groups, respectively (placebo‐subtracted difference −2.78 mL/min/1.73 m2/3 weeks; 95% CI −3.88 to −1.68). From week 3 to the last measurement, the decline in eGFR was slower in the canagliflozin group than that in the placebo group (−2.27 vs −5.63 mL/min/1.73 m2/year; placebo‐subtracted difference 3.35 mL/min/1.73 m2/year; 95% CI 2.40–4.31). Also, the difference in eGFR slopes between the canagliflozin and placebo groups was similar in EA and non‐EA participants (Table S2).
The incidence of AEs and renal‐related AEs was lower in the canagliflozin group than that the placebo group in EA participants (HR 0.83, 95% CI 0.70–0.98 and HR 0.52, 95% CI 0.34–0.79, respectively), and the results were similar between EA and non‐EA participants (P interaction = 0.4738 and 0.1134, respectively; Figure 4). The incidence rates of other AEs, including serious AEs, acute kidney injury, volume depletion, osmotic diuresis, urinary tract infection, genital mycotic infections, amputation and fracture, were not different between the canagliflozin and placebo groups overall, and the results were consistent between the EA and non‐EA subgroups (all, P interaction > 0.05). No unexpected safety signals were observed in EA participants.
Figure 4.
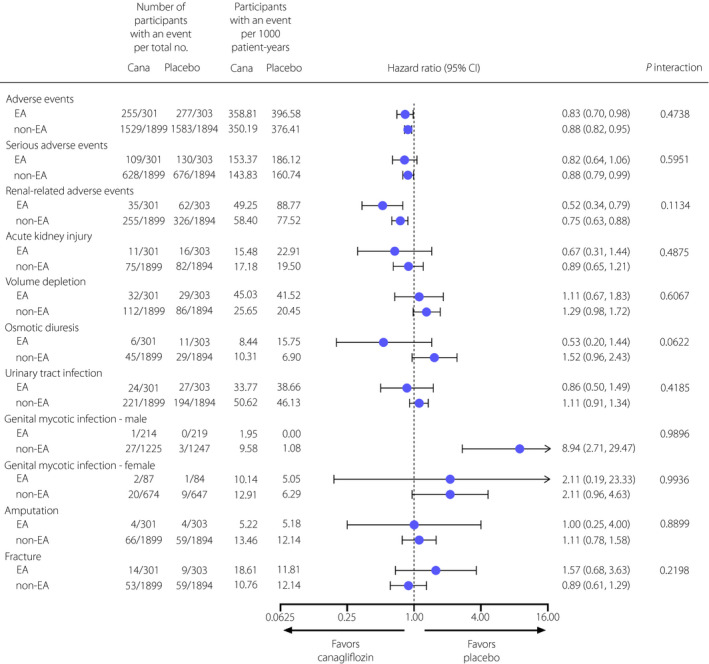
Effects of canagliflozin on safety outcomes in participants in East and South‐East Asian and non‐East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE). Cana, canagliflozin; CI, confidence interval; EA, East and South‐East Asia; non‐EA, non‐East and South‐East Asia.
DISCUSSION
In agreement with the analysis in the overall population of the CREDENCE study 12 , canagliflozin consistently reduced the risk of renal and CV events, including the primary composite outcome of ESKD, DoSC, or renal or CV death, in EA participants. The EA participants represent a high‐risk subpopulation among the overall CREDENCE participants, because the placebo group in EA participants compared with non‐EA participants showed higher incidence of renal outcomes across the renal composite and individual renal outcome components. The efficacy of canagliflozin on the primary outcome was generally consistent between EA and non‐EA participants, as well as with previous subanalysis of all Asians in the CREDENCE study 12 . These results show that canagliflozin reduces the risk of renal and CV events across a diverse group of participants with type 2 diabetes mellitus and albuminuria, including EA participants who are at high risk for renal complications.
The NNT for 2.5 years for renal outcomes ranged from 11 to 24 and 25 to 105 in EA and non‐EA participants, respectively. The CREDENCE study reported NNTs between 22 and 43 for primary and renal outcomes in the overall population 12 . Although the reason for low NNTs in EA participants remains to be determined, it might be relevant to the higher risk at baseline in these participants. Regardless of the underlying mechanism of low NNTs, it is plausible that risk reduction is more robust in EA participants compared with that in non‐EA participants. This result might support the use of canagliflozin, particularly in high‐risk EA participants.
The effects of canagliflozin on intermediate outcomes in the EA subgroup were broadly consistent with those observed in the overall CREDENCE population. A small difference between the effect of canagliflozin and the placebo on HbA1c suggests that at least some renal and CV benefits are derived from a glucose‐independent mechanism(s) of action. Other subanalyses of CREDENCE have also supported the existence of glucose‐independent mechanisms, as similar risk reductions have been shown regardless of baseline HbA1c levels and even in patients for whom glycemic efficacy of canagliflozin was attenuated because of reduced renal function 14 , 21 . The differences in changes in bodyweight, systolic blood pressure and diastolic blood pressure were all modest, and it appears likely that the contributions of these factors to the renal and cardioprotective effects are limited. Among the renal effects of SGLT2 inhibitors, control of glomerular hyperfiltration through the tubuloglomerular feedback is assumed to mediate the renoprotective effects 22 . In the EA subgroup analysis, there was an initial acute drop in eGFR followed by suppression of eGFR decline in the canagliflozin group. However, the relationship between the acute drop of eGFR and the long‐term eGFR trajectories remains unclear. Because albuminuria might cause a direct damage of the glomerulus and the tubule, and ultimately lead to nephron loss 23 , it is possible that UACR reduction by canagliflozin is related to the renoprotective effect. Association of both the initial decrease in UACR and the residual UACR level with renoprotection (decreased risk of renal events) has been reported after canagliflozin administration in the overall analysis of the CREDENCE study 24 . Other potential mechanisms of renal protection by SGLT2 inhibitors are actively being studied 25 , 26 , 27 .
The AE profile of canagliflozin in EA participants was also consistent with that in non‐EA participants. Most notably, the incidence of renal‐related AEs was lower in the canagliflozin group, although it is not clear whether this reduction was relevant to the renoprotective effect of canagliflozin. No increased risk of amputation and fractures was shown with canagliflozin in the present study, as is the case in the overall CREDENCE participants 12 , 28 , although increased risk of these events was reported with canagliflozin in patients at high risk of CV events in the Canagliflozin Cardiovascular Assessment Study 29 , 30 . As aforementioned, no new safety concern of canagliflozin was identified in this population.
The present findings had some limitations. This study used post‐hoc analysis and was not powered to draw definite conclusions for the EA participants. In addition, as the design of the CREDENCE trial was to analyze the effect in patients with type 2 diabetes mellitus and macroalbuminuria, the findings might not be generalized to patients who do not meet the inclusion criteria (e.g., those with normo‐ or micro‐albuminuria). Nevertheless, the present study represents the largest analysis to date dedicated to evaluating the kidney outcomes of an SGLT2 inhibitor in high‐risk patients in EA countries.
In conclusion, canagliflozin decreased the risk of renal and CV events in the subpopulation of EA participants with high risk of renal events in the CREDENCE study, without any additional adverse effects.
DISCLOSURE
TW has received research funding and consulting fees from Takeda Pharmaceutical Company Ltd., Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Kyowa Kirin Co. Ltd., Kissei Pharmaceutical Co. Ltd., Daiichi Sankyo Company Ltd. and Astellas Pharma Inc.; research funding from MSD K.K. and Chugai Pharmaceutical Co. Ltd.; and consulting fees from Taisho Pharma Co. Ltd., Sanwa Chemistry Co. Ltd., Ono Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Kowa Company Ltd., Eli Lilly Japan K.K., Bayer Yakuhin Ltd. and AstraZeneca K.K. MJJ is supported by the Next Generation Clinical Researchers Program Career Development Fellowship funded by Medical Research Future Fund; received research funding from MSD, Gambro, Eli Lilly, CSL, Baxter and Amgen; served on the CREDENCE Steering Committee; has served on advisory boards sponsored by Vifor, MSD, Boehringer Ingelheim, Baxter, Bayer, Astra Zeneca and Akebia; serves on a steering committee sponsored by CSL; and has spoken at scientific meetings sponsored by Vifor, Roche, Janssen and Amgen; with any consultancy, honoraria or travel support paid to her institution. KM‐A, YK, HK, HT, MI and KA are employees of Mitsubishi Tanabe Pharma Corporation
Supporting information
Figure S1 | Effects of canagliflozin on intermediate outcomes over time in participants in East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE).
Table S1 | Inclusion and exclusion criteria.
Table S2 | Annualized estimated glomerular filtration rate slopes in the acute phase, chronic phase, and total period in East and South‐East Asian and non‐East and South‐East Asian participants.
ACKNOWLEDGMENTS
We thank all the participants, investigators and trial teams in the CREDENCE trial for their participation. The present study was carried out by Mitsubishi Tanabe Pharma Corporation, after obtaining permission of Janssen Research & Development, LLC and the Steering Committee of the CREDENCE study. Canagliflozin has been jointly developed by Mitsubishi Tanabe Pharma Corporation and Janssen Research & Development, LLC. Statistical analyses were independently carried out by Takumi Information Technology Inc. (Tokyo, Japan) and confirmed by Mitsubishi Tanabe Pharma Corporation. Editorial assistance was provided by Akira Saito, PhD, of International Medical Translation Service Inc., and was funded by Mitsubishi Tanabe Pharma Corporation. We thank Norman Rosenthal, PhD, George Capuano, PhD, and William Canovatchel, PhD of Janssen Research and Development, LLC for their valuable comments and support in preparation of the manuscript.
J Diabetes Investig 2022; 13: 54–64
Clinical Trial Registry
ClinicalTrials.gov, NCT02065791
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas, 9th edn. Brussels, Belgium: International Diabetes Federation, 2019. https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e‐final‐web.pdf. Accessed May 20, 2021. [Google Scholar]
- 2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12: 2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unsal A, Koc Y, Basturk T, et al. Risk factors for progression of renal disease in patient with diabetic nephropathy. Eur Rev Med Pharmacol Sci 2012; 16: 878–883. [PubMed] [Google Scholar]
- 4. Anders HJ, Huber TB, Isermann B, et al. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018; 14: 361–377. [DOI] [PubMed] [Google Scholar]
- 5. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 6. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 7. Cowie CC, Port FK, Wolfe RA, et al. Disparities in incidence of diabetic end‐stage renal disease according to race and type of diabetes. N Engl J Med 1989; 321: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 8. Stephens GW, Gillaspy JA, Clyne D, et al. Racial differences in the incidence of end‐stage renal disease in types I and II diabetes mellitus. Am J Kidney Dis 1990; 15: 562–567. [DOI] [PubMed] [Google Scholar]
- 9. Chandie Shaw PK, Baboe F, van Es LA, et al. South‐Asian type 2 diabetic patients have higher incidence and faster progression of renal disease compared with Dutch‐European diabetic patients. Diabetes Care 2006; 29: 1383–1385. [DOI] [PubMed] [Google Scholar]
- 10. Parving HH, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006; 69: 2057–2063. [DOI] [PubMed] [Google Scholar]
- 11. Johansen KL, Chertow GM, Foley RN, et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2021; 77: A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 13. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation 2019; 140: 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cannon CP, Perkovic V, Agarwal R, et al. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes mellitus and chronic kidney disease according to baseline HbA1c, including those with HbA1c <7%: results from the CREDENCE trial. Circulation 2020; 141: 407–410. [DOI] [PubMed] [Google Scholar]
- 15. Neuen BL, Ohkuma T, Neal B, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol 2019; 30: 2229–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. INVOKANA (canagliflozin) tablets, for oral use [package insert]. Janssen Pharmaceuticals. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204042s032lbl.pdf. Accessed October 7, 2020. [Google Scholar]
- 17. Li JZ, Absher DM, Tang H, et al. Worldwide human relationships inferred from genome‐wide patterns of variation. Science 2008; 319: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 18. Saitou N. A genetic affinity analysis of human populations. Hum Evol 1995; 10: 17–33. [Google Scholar]
- 19. Jardine MJ, Mahaffey KW, Neal B, et al. The canagliflozin and renal endpoints in diabetes with established nephropathy clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 2017; 46: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Lemos ML. NNT for studies with long‐term follow‐up. CMAJ 2005; 172: 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jardine MJ, Zhou Z, Mahaffey KW, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE Randomized Trial. J Am Soc Nephrol 2020; 31: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 Inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772. [DOI] [PubMed] [Google Scholar]
- 23. Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol 2013; 76: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oshima M, Neuen BL, Li J, et al. Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE Trial. J Am Soc Nephrol 2020; 31: 2925–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heerspink HJL, Kosiborod M, Inzucchi SE, et al. Renoprotective effects of sodium‐glucose cotransporter‐2 inhibitors. Kidney Int 2018; 94: 26–39. [DOI] [PubMed] [Google Scholar]
- 26. Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 2020; 16: 317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 2020; 11: 2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnott C, Huang Y, Neuen B, et al. The effect of canagliflozin on amputation risk in the CANVAS Program and the CREDENCE trial. Diabetes Obes Metab 2020; 22: 1753–1766. [DOI] [PubMed] [Google Scholar]
- 29. Zhou Z, Jardine M, Perkovic V, et al. Canagliflozin and fracture risk in individuals with type 2 diabetes: results from the CANVAS Program. Diabetologia 2019; 62: 1854–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Effects of canagliflozin on intermediate outcomes over time in participants in East and South‐East Asian countries in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE).
Table S1 | Inclusion and exclusion criteria.
Table S2 | Annualized estimated glomerular filtration rate slopes in the acute phase, chronic phase, and total period in East and South‐East Asian and non‐East and South‐East Asian participants.


