Abstract
The present retrospective study aimed to identify factors associated with an increased risk of acute kidney injury in Japanese patients with type 2 diabetes treated with sodium–glucose cotransporter 2 inhibitors. We identified 171,622 patients with type 2 diabetes treated with sodium–glucose cotransporter 2 inhibitors; among them, 476 (0.3%) patients developed acute kidney injury. The hazard ratio for acute kidney injury occurrence risk was analyzed using a Cox proportional hazards model adjusted for patient characteristics at baseline and use of concomitant agents. In the adjusted model, patients who developed acute kidney injury were mostly men, aged ≥65 years, had lower body mass index, had a history of heart failure and used diuretics more frequently than those who did not. These findings suggest that associated clinical risk factors should be thoroughly evaluated before administering sodium–glucose cotransporter 2 inhibitors to minimize acute kidney injury onset.
Keywords: Acute kidney injury, Risk factors, Sodium–glucose cotransporter 2 inhibitors
BLURB FOR ETOC: AKI risk associated with SGLT2 inhibitors

INTRODUCTION
Among hypoglycemic agents, sodium–glucose cotransporter 2 inhibitors (SGLT2is) exert a hypoglycemic effect by promoting glucose excretion 1 . According to multiple meta‐analyses and real‐world data, SGLT2is reduce the risk of acute kidney injury (AKI) 2 , 3 , 4 , 5 , 6 . However, SGLT2is might cause AKI as a result of hypovolemia caused by osmotic diuresis 7 . Whether SGLT2i affects high‐risk clinical situations of AKI is unknown. Therefore, we aimed to investigate the various factors linked to the risk of AKI events in type 2 diabetes treated with SGLT2is.
MATERIALS AND METHODS
This was a real‐world, retrospective cohort study using the Medical Data Vision (MDV) administrative claims database, which is a nationwide database consisting of data from Japanese acute care hospitals that utilize the Diagnosis Procedure Combination system; treatments for approximately 31 million cumulative patients are detailed therein. Patients were censored at the time of AKI diagnosis, SGLT2i discontinuation, death or loss of insurance coverage. The observation start date was set to the date when SGLT2i was first prescribed. The minimum observation period was 1 day after SGLT2i initiation. The maximum observation period was 365 days after SGLT2i initiation.
Study population
Data on patients diagnosed with type 2 diabetes (International Classification of Diseases, 10th revision [ICD‐10] codes: E10‐E14) and registered from 1 April 2008 to 31 October 2019 in the MDV database were extracted. The exclusion criteria were: (i) diagnosis of type 1 diabetes (ICD‐10 code: E10), both type 1 and type 2 diabetes, and diabetes other than type 1 and type 2 diabetes; (ii) age <18 years; and (iii) non‐prescription of SGLT2is.
Identification of comorbidities and concomitant drugs
Diagnoses of AKI, chronic heart failure, cardiovascular disease, peripheral artery disease and stroke in the MDV database were made using the relevant ICD‐10 codes (Table S1). The concomitant drugs were angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, diuretics and non‐steroidal anti‐inflammatory drugs (NSAIDs). These drugs increase the AKI risk according to an article published by the Japan Diabetes Society 8 . Concomitant drugs and comorbidities were confirmed to have been administered and diagnosed 30 days before initiating SGLT2is administration.
Patient characteristics
Age, sex, body mass index (BMI), glycohemoglobin (HbA1c) and estimated glomerular filtration rate (eGFR) were the baseline characteristics identified using data in the claim record within 30 days of the initial date of observation. Obesity was defined as BMI ≥25 kg/m2 according to Japanese standards 9 .
Statistical analysis
Normally distributed data (age, BMI, HbA1c and eGFR) are expressed as the mean ± standard deviation. Continuous variables were analyzed using a one‐tailed unpaired t‐test. Categorical variables were analyzed using a χ2‐test and expressed as absolute numbers or percentages. Hazard ratios (HRs) for AKI occurrence were analyzed using a Cox proportional hazards model adjusted for sex, age, BMI (cut‐off value 25 kg/m2), type of SGLT2i, chronic heart failure, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, diuretics and NSAIDs. In the supplemental analysis, the HbA1c and eGFR values were added. All statistical analyses were carried out using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). Differences were regarded as significant when P < 0.05.
Ethical considerations
The present study was carried out in accordance with the ethical guidelines for human medical and health research. This study was approved by the Kitasato University Ethics Committee (control number: B19‐285).
RESULTS
We identified 171,622 type 2 diabetes patients treated with SGLT2is (Figure 1). Table 1 shows the clinical characteristics of all patients in the present study according to their AKI status.
Figure 1.
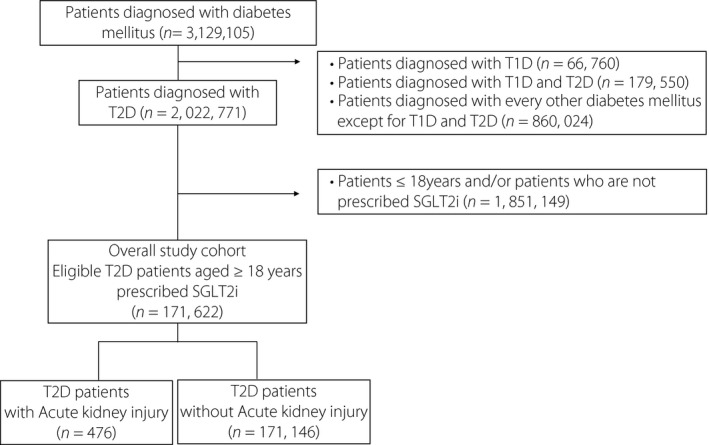
We extracted data of 3,129,105 patients diagnosed with diabetes mellitus (International Classification of Diseases, 10th revision [ICD‐10] code: E11‐E14) registered between 1 April 2008 and 31 October 2019 in the Medical Data Vision database. We scrutinized the data of patients diagnosed with type 2 (T2D) diabetes (ICD‐10 code: E11 or E14) based on the following exclusion criteria: (i) diagnosed with type 1 diabetes (T1D; ICD‐10 code: E10); (ii) diagnosed with both type 1 and type 2 diabetes; and (iii) diagnosed with any other type of diabetes mellitus except type 1 and type 2 diabetes. Subsequently, adult patients diagnosed with type 2 diabetes registered between 1 December 2018 and 31 October 2019 in the Medical Data Vision database were selected based on the following exclusion criteria: age <18 years. Finally, 171,622 adult patients with type 2 diabetes were identified and divided into two groups based on the presence or absence of acute kidney injury. SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Table 1.
Baseline patient characteristics of patients with type 2 diabetes using sodium–glucose cotransporter 2 inhibitors
| Overall (n = 171,622) | Acute kidney injury (+) (n = 476) | Acute kidney injury (−) (n = 171,146) | P‐value (acute kidney injury [+] vs acute kidney injury [−]) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | n (%) | Mean ± SD | n (%) | Mean ± SD | n (%) | ||
| Sex, n (%) | |||||||
| Male | 112,301 (65.4) | 348 (73.1) | 112,173 (65.5) | <0.001 | |||
| Female | 59,321 (34.6) | 128 (26.9) | 58,973 (34.5) | ||||
| Age (years) | |||||||
| Mean (years) | 62.4 ± 13.5 | 67.8 ± 12.7 | 62.4 ± 13.5 | <0.001 | |||
| Distribution, n (%) | |||||||
| <25 | 884 (0.5) | 0 (0) | 884 (0.5) | <0.001 | |||
| 25–<44 | 17,136 (10.0) | 29 (6.1) | 17,107 (10.0) | ||||
| 45–<64 | 69,543 (40.5) | 124 (26.1) | 69,419 (40.6) | ||||
| ≥65 | 84,059 (49.0) | 323 (67.8) | 83,736 (48.9) | ||||
| BMI (kg/m2) | |||||||
| Mean (kg/m2) | 27.1 ± 5.9 | 26.2 ± 5.3 | 27.1 ± 5.9 | 0.017 | |||
| Distribution, n (%) | |||||||
| <25 | 65,256 (38.0) | 236 (49.6) | 65,020 (38.0) | <0.001 | |||
| ≥25 | 106,366 (62.0) | 240 (50.4) | 106,126 (62.0) | ||||
| Comorbidities | |||||||
| Chronic heart failure, n (%) | 44,516 (25.9) | 259 (54.4) | 44,257 (25.9) | <0.001 | |||
| Cardiovascular disease, n (%) | 26,981 (15.7) | 80 (16.8) | 26,901 (15.7) | 0.515 | |||
| Stroke, n (%) | 5,287 (3.1) | 27 (5.7) | 5,260 (3.1) | 0.001 | |||
| Peripheral artery disease, n (%) | 942 (0.5) | 4 (0.8) | 938 (0.5) | 0.389 | |||
| SGLT2is | |||||||
| Canagliflozin | 26,460 (15.4) | 63 (13.2) | 26,397 (15.4) | <0.001 | |||
| Dapagliflozin | 34,579 (20.1) | 89 (18.7) | 34,490 (20.2) | <0.001 | |||
| Empagliflozin | 50,368 (29.3) | 174 (36.6) | 50,194 (29.3) | <0.001 | |||
| Ipragliflozin | 33,194 (19.3) | 85 (17.8) | 33,109 (19.3) | <0.001 | |||
| Luseogliflozin | 10,800 (6.3) | 26 (5.5) | 10,774 (6.3) | <0.001 | |||
| Tofogliflozin | 16,221 (9.6) | 39 (8.2) | 16,182 (9.5) | <0.001 | |||
| Baseline use of antidiabetic medications | |||||||
| α‐GIs | 20,283 (11.8) | 55 (11.6) | 20,228 (11.8) | 0.858 | |||
| DPP‐4 inhibitors | 97,717 (56.9) | 258 (54.2) | 97,459 (56.9) | 0.227 | |||
| Glinides | 10,290 (6.0) | 32 (6.7) | 10,258 (6.0) | 0.504 | |||
| GLP‐1 agonists | 7,901 (4.6) | 12 (2.5) | 7,889 (4.6) | 0.030 | |||
| Insulin | 34,915 (20.3) | 141 (29.6) | 34,774 (20.3) | <0.001 | |||
| Metformin | 73,083 (42.6) | 117 (24.6) | 72,966 (42.6) | <0.001 | |||
| Sulfonylureas | 34,903 (20.3) | 65 (13.7) | 34,838 (20.4) | <0.001 | |||
| Thiazolidinediones | 12,996 (7.6) | 19 (4.0) | 12,977 (7.6) | 0.003 | |||
| Concomitant drug | |||||||
| ACEis/ARBs | 94,088 (54.8) | 307 (64.5) | 93,781 (54.8) | <0.001 | |||
| Diuretics | 34,132 (19.9) | 250 (52.5) | 33,882 (19.8) | <0.001 | |||
| NSAIDs | 24,345 (14.2) | 69 (14.5) | 24,276 (14.2) | 0.846 | |||
α‐GIs, alpha‐glucosidase inhibitor; ACEis, angiotensin‐converting enzyme inhibitors; Acute kidney injury (–), patients without acute kidney injury events; Acute kidney injury (+), patients with acute kidney injury events; ARBs, angiotensin receptor blockers; BMI, body mass index; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitor; GLP‐1 agonists, glucagon‐like peptide‐1 receptor agonist; NSAIDs, non‐steroidal anti‐inflammatory drugs; SD, standard deviation; SGLT2is, sodium–glucose cotransporter 2 inhibitors.
In the adjusted model (Table 2), the HR was higher in men (1.43‐fold) and in patients aged ≥65 years (1.58‐fold), 1.41‐fold higher in patients with BMI <25 kg/m2, 1.71‐fold higher in patients diagnosed with chronic heart failure, and 1.78‐fold higher in patients diagnosed with stroke. Considering the concomitant medications, diuretic users had an elevated AKI risk (HR 2.80). No significant differences were observed in the HR calculated based on the types of SGLT2is.
Table 2.
Hazard ratio of acute kidney injury in patients with type 2 diabetes using sodium–glucose cotransporter 2 inhibitors
| Outcome | Acute kidney injury (+) (n = 476) | Crude model | Adjusted model | ||||
|---|---|---|---|---|---|---|---|
| n (%) | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Sex | |||||||
| Female | 128 (26.9) | Reference | – | – | Reference | – | – |
| Male | 348 (73.1) | 1.43 | 1.17–1.75 | 0.001 | 1.43 | 1.16–1.75 | 0.001 |
| Age (years) | |||||||
| <65 | 153 (32.2) | Reference | – | – | Reference | – | – |
| ≥65 | 323 (67.8) | 2.20 | 1.82–2.67 | <0.001 | 1.58 | 1.29–1.93 | <0.001 |
| BMI (kg/m2) | |||||||
| ≥25 | 236 (49.6) | Reference | – | – | Reference | – | – |
| <25 | 240 (50.4) | 1.60 | 1.34–1.92 | <0.001 | 1.41 | 1.16–1.64 | <0.001 |
| Comorbidities | |||||||
| Chronic heart failure | 259 (54.4) | 3.42 | 2.85–4.09 | <0.001 | 1.71 | 1.38–2.11 | <0.001 |
| Cardiovascular disease | 80 (16.8) | 1.08 | 0.85–1.38 | 0.515 | 1.25 | 0.98–1.53 | 0.073 |
| Stroke | 27 (5.7) | 1.98 | 1.29–2.80 | 0.001 | 1.78 | 1.20–2.61 | 0.004 |
| Peripheral artery disease | 4 (0.8) | 1.54 | 0.57–4.11 | 0.392 | 1.33 | 0.50–3.56 | 0.57 |
| SGLT2is | |||||||
| Canagliflozin |
63 (13.2) |
Reference | – | – | Reference | – | – |
| Dapagliflozin | 89 (18.7) | 1.08 | 0.78–1.49 | 0.636 | 1.20 | 0.87–1.65 | 0.328 |
| Empagliflozin | 174 (36.6) | 1.45 | 1.09–1.94 | 0.011 | 1.25 | 0.93–1.67 | 0.134 |
| Ipragliflozin | 85 (17.8) | 1.08 | 0.78–1.49 | 0.662 | 1.22 | 0.84–1.70 | 0.351 |
| Luseogliflozin | 26 (5.5) | 1.01 | 0.64–1.60 | 0.963 | 1.08 | 0.70–1.57 | 0.451 |
| Tofogliflozin | 39 (8.2) | 1.01 | 0.68–1.51 | 0.96 | 1.23 | 0.81–1.82 | 0.535 |
| Concomitant drugs | |||||||
| ACEis/ARBs | 307 (64.5) | 1.50 | 1.24–1.81 | <0.001 | 1.04 | 0.86–1.27 | 0.678 |
| Diuretics | 250 (52.5) | 4.47 | 3.73–5.35 | <0.001 | 2.80 | 2.26–3.46 | <0.001 |
| NSAIDs | 69 (14.5) | 1.03 | 0.80–1.32 | 0.846 | 1.10 | 0.85–1.43 | 0.459 |
Adjusted for sex, age, body mass index (BMI; cut‐off value 25 kg/m2), sodium–glucose cotransporter 2 inhibitors (SGLT2i), chronic heart failure, angiotensin‐converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs), diuretics and non‐steroidal anti‐inflammatory drugs (NSAIDs).
Acute kidney injury (+), patients with acute kidney injury event; BMI, body mass index; CI, confidence interval; HR, hazard ratio.
We investigated the risk factors associated with AKI in patients for whom eGFR and HbA1c values were available (n = 16,690; Table S2). In the adjusted model (Table S3), the HR was higher in patients aged ≥65 years or HbA1c <7.0, and in those with renal dysfunction, indicating an elevated AKI risk.
The mean duration from SGLT2i treatment initiation until AKI onset was 146.3 ± 104.4 days. The highest AKI incidence was observed in the first 30 days of SGLT2i initiation, with a general downward trend thereafter (Figure 2).
Figure 2.
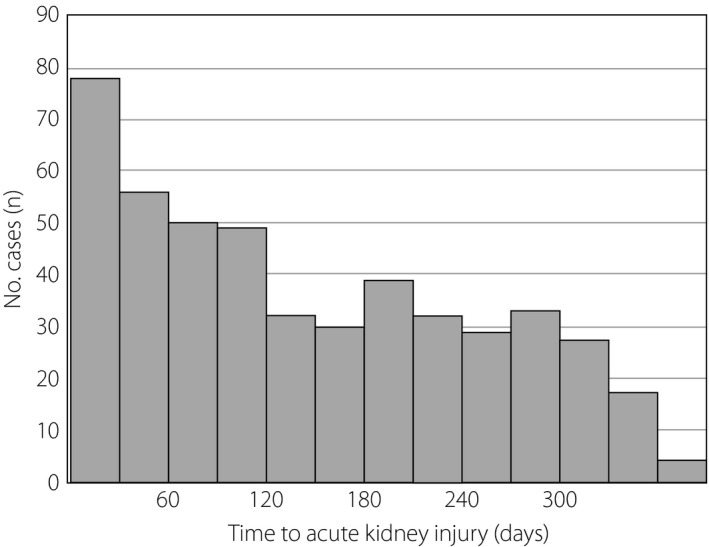
Duration from initiation of treatment with sodium–glucose cotransporter 2 inhibitors to the onset of acute kidney injury.
DISCUSSION
We found that among type 2 diabetes patients treated with SGLT2is, men, elderly patients, those with low BMI and chronic heart failure, and those with concomitant diuretic use were at an increased AKI risk. In a few cases, lower eGFR and lower HbA1c had a strong effect on increased AKI risk. AKI incidence in previous studies was approximately 0.5–3.0 2 , 3 , 6 , and 0.3% in the present study. Although the observation period and patient background in this study were different from those in previous studies, the frequency of AKI onset was almost similar. AKI as a result of SGLT2i use has been attributed to a high‐risk patient population, and might not be related to SGLT2i‐specific nephrotoxicity 5 . The risk factors for AKI according to the present study are consistent with previously reported patient demographics 10 . Among other risk factors, the concomitant use of diuretics with SGLT2is increased AKI risk remarkably. In a post‐marketing surveillance of SGLT2is, pollakiuria and polyuria in the early stages of administration were found to be the most common side‐effects. The specified drug use results survey of ipragliflozin treatment in elderly type 2 diabetic patients (STELLA‐ELDER study), which was limited to elderly patients who received ipragliflozin, reported that the risk of dehydration in patients who received loop diuretics was 2.1‐fold higher than those who did not. 11 The diuretic effect was presumed to be enhanced by the concomitant action of the diuretic and SGLT2i, and the AKI risk was increased by progressive dehydration. Excessive dehydration can cause not only AKI, but also an excessive decrease in blood pressure and the development of cerebral infarction. In the present study, the frequency of AKI onset was high in the early stages of SGLT2i administration; thus, caution is required during this period. Furthermore, although the increase in AKI risk due to NSAIDs is well known, no increase in AKI risk was observed. Previously, it was reported that NSAIDs alone did not increase AKI risk, but triple combination therapy consisting of diuretics, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and NSAIDs did. 12 In the present study, approximately 2% of the patients received triple therapy, showing that the use of NSAIDs did not significantly increase AKI risk. Only with stroke was significantly increased AKI risk for comorbidity due to arteriosclerosis observed. Caution must be exercised, as the use of SGLT2is might increase the AKI risk, depending on the severity of arteriosclerosis.
The present study had several limitations. First, the MDV database includes data on patients undergoing treatment at hospitals that utilize the Diagnosis Procedure Combination system. This means that not all patients with type 2 diabetes treated in Japan are included in the MDV database; therefore, the results of the present study might not be applicable to all type 2 diabetes patients. However, considering that the proportion of type 2 diabetes patients reported in the MDV database and the Japanese Health and Nutrition Examination Survey 13 was similar, the results of this the present might be applied to most type 2 diabetes patients. Second, information on certain confounding factors for AKI (e.g., HbA1c and eGFR) was available only for a few patients. Therefore, it is necessary to accumulate the number of patients and then consider research methods that include multiple factors. Finally, AKI could not be precisely defined by considering factors, such as increased creatinine, due to the lack of laboratory data. This might have led us to overestimate or underestimate the onset of AKI.
In conclusion, type 2 diabetes patients with heart failure or severe renal impairment are likely to possess the risk factors for AKI. SGLT2is reduce cardiovascular and renal disease events; thus, the frequency of SGLT2i administration to type 2 diabetes patients with impaired renal or cardiac function might increase. To minimize AKI onset, clinical features should be thoroughly evaluated before initiating SGLT2i administration. We hope to investigate other drugs expected to increase the AKI risk when used concomitantly with SGLT2is.
ACKNOWLEDGMENTS
No specific funding or grants were received for this work.
DISCLOSURE
AS has received lecture fees from Astellas Pharma Inc., Eli Lilly Japan K.K., Novo Nordisk Pharma Inc. and Sanofi. The other authors declare no conflict of interest.
Supporting information
Table S1. | International Classification of Diseases, 10th revision codes used to identify comorbidities
Table S2. | Patient characteristics including glycohemoglobin and estimated glomerular filtration rate at baseline of the patients with type 2 diabetes using sodium–glucose cotransporter 2 inhibitors
Table S3. | Hazard ratio of acute kidney injury including glycohemoglobin and estimated glomerular filtration rate in patients with type 2 diabetes using sodium–glucose cotransporter 2 inhibitors
J Diabetes Investig 2022; 13: 42–46
REFERENCES
- 1. Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium‐dependent glucose cotransporter 2 inhibitors on cardio‐renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 2017; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zinman B, Wanner C, Lachin JM, et al. EMPA‐REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 4. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injuryin patients on SGLT2 inhibitors: a propensity‐matched analysis. Diabetes Care 2017; 40: 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cahn A, Raz I, Bonaca M, et al. Safety of dapagliflozin in a broad population of patients with type 2 diabetes – analyses from the DECLARE‐TIMI 58 study. Diabetes Obes Metab 2020; 22: 1357–1368. [DOI] [PubMed] [Google Scholar]
- 6. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2019; 7: 845–854. [DOI] [PubMed] [Google Scholar]
- 7. Menne J, Dumann E, Haller H, et al. Acute kidney injury and adverse renal events in patients receiving SGLT2‐inhibitors: a systematic review and meta‐analysis. PLoS Med 2019; 16: e1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Committee on the Proper Use of SGLT2 Inhibitors . Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig 2020; 11: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Examination Committee of Criteria for 'Obesity Disease' in Japan, Japan Society for the Study of Obesity . New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, James MT. Acute kidney injury. Ann Intern Med 2018; 168: 837. [DOI] [PubMed] [Google Scholar]
- 11. Yokote K, Terauchi Y, Nakamura I, et al. Real‐world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA‐ELDER): final results of a post‐marketing surveillance study. Expert Opin Pharmacother 2016; 17: 1995–2003. [DOI] [PubMed] [Google Scholar]
- 12. Lapi F, Azoulay L, Yin H, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non‐steroidal anti‐inflammatory drugs and risk of acute kidney injury: nested case‐control study. BMJ 2013; 346: e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ministry of Health, Labour and Welfare . [National Health and Nutrition Survey; 2017]. [Cited March 2020]. Available from: https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. | International Classification of Diseases, 10th revision codes used to identify comorbidities
Table S2. | Patient characteristics including glycohemoglobin and estimated glomerular filtration rate at baseline of the patients with type 2 diabetes using sodium–glucose cotransporter 2 inhibitors
Table S3. | Hazard ratio of acute kidney injury including glycohemoglobin and estimated glomerular filtration rate in patients with type 2 diabetes using sodium–glucose cotransporter 2 inhibitors


