Abstract
Acute pancreatitis (AP) is a common gastrointestinal disease that affects 1 million individuals worldwide. Inflammation and apoptosis are considered to be important pathogenic mechanisms of AP, and high mobility group box 1 (HMGB1) has been shown to play a particularly important role in the etiology of this disease. MicroRNAs (miRs) are emerging as critical regulators of gene expression and, as such, they represent a promising area of therapeutic target identification and development for a variety of diseases, including AP. Using the online database query (microRNA.org), the current study identified a site in the 3' untranslated region of HMGB1 mRNA that was a viable target for miR-340-5p. The present study aimed to investigate the association between miR-340-5p and HMGB1 expression in pancreatic acinar cells following lipopolysaccharide (LPS) treatment by performing luciferase, western blotting and reverse transcription-quantitative PCR assays. The results suggest that miR-340-5p attenuates the induction of HMGB1 by LPS, thereby inhibiting inflammation and apoptosis via blunted activation of Toll-like receptor 4 and enhanced AKT signaling. Thus, the therapeutic application of miR-340-5p may be a useful strategy in AP via upregulation of HMGB1 and subsequent promotion of inflammation and apoptosis.
Keywords: microRNA-340-5p, high mobility group box 1, pancreatic acinar cells, apoptosis, inflammation
Introduction
Acute pancreatitis (AP) is a common gastrointestinal disease caused by a variety of factors, such as excessive drinking, drug misuse and surgery. In total, 10-15% of patients with AP develop severe AP (SAP) (1), a condition that has a 10-30% worldwide mortality rate and is a major risk factor for pancreatic cancer (2,3). The pathogenesis of AP is complicated and its mechanisms have not been fully elucidated, a fact that has caused concern in the medical community. Although there is abundant literature on the pathogenesis of AP, the availability of effective evidence-based treatment methods remains limited (4). In recent years, research has revealed a new treatment method that alleviates the inflammatory symptoms of the disease via the transfection of foreign DNA into the cells of the gastrointestinal tract to produce small RNA (5).
A major development in gene regulation has been the discovery of microRNA (miRNA/miR), a non-coding, highly-conserved, single-stranded small RNA averaging 22 bp, which is involved in the regulation of post-transcriptional gene expression (6). The first confirmed miRNAs were lin-4 and let-7, both found in C. elegans, which can inhibit protein translation by complementarily binding to the 3'non-coding region of the target mRNAs, thereby preventing their expression (7). Subsequently, numerous research teams have identified >30,000 miRNAs in >200 organisms (8-10), most of which are highly conserved and differentially expressed (11). Under normal conditions, miRNA binding to the complementary sequence of the 3'untranslated region (UTR) of the target mRNA leads to transcript degradation and abrogated expression (12). With the rapid development of miRNA mass spectrometry technology, evidence the regulatory role of miRNAs in a variety of diseases has emerged in the literature. In the context of inflammatory diseases, Liu et al (13) showed that miR-381 could target HMGB1 expression to alleviate the inflammatory response of macrophages in polymyositis. Moreover, Wei et al (14) reported that miR-198 could act on PTEN in retinoblastoma cells by activating the PI3K/AKT signaling pathway, while Zhang et al (15) demonstrated that TGF-β could induce the production of miR-216a to increase the expression of TGF-β receptor 1 and phosphorylated (p)-AKT via the downregulation of SMAD7 and PTEN.
Recent evidence has suggested HMGB1 involvement in the development and progression of AP (16). HMGB1 was first discovered by Goodwin and John in 1973 who extracted and identified a group of highly conserved nuclear proteins from the bovine thymus, which displayed rapid migration during electrophoresis (17). In the HMGB family, HMGB1 is the most abundant and widely distributed member among tissues in humans (16). Research has shown that intracellular HMGB1 inhibits the development of pancreatitis (18-21), while extracellular HMGB1 likely promotes the progression of AP from local inflammation to systemic inflammatory response syndrome and sepsis (22). In the early stage of AP, pancreatic acinar cells and peritoneal macrophages successively release inflammatory factors, such as IL-1, TNFα and NF-κB (23-25). These early inflammatory mediators destroy the pancreas and surrounding tissues, stimulate the secretion of HMGB1 and subsequently aggravate pancreatitis (20,26,27). Inflammation is not only part of the early phase of pancreatitis but also occurs throughout its duration (28). In addition to causing tissue damage via cell death, these inflammatory factors excessively activate granulocytes, leading to lysosome release and additional cytokines/chemokines that increase oxidative stress, injure vascular endothelium and ultimately cause apoptosis (29). Furthermore, HMGB1 is prone to binding other pro-inflammatory molecules, including DNA, RNA, histones, nucleosomes, lipopolysaccharide (LPS), stromal cell-derived factor 1, IL-1α and IL-1β, amongst others. These complexes act synergistically to aggravate pancreatitis via cognate receptors to the HMGB1-partner molecules. Although the list of reported HMGB1 receptors is fairly extensive, only two receptor systems, MOK protein kinase and Toll-like receptor (TLR)4, have been fully confirmed to act as genuine HMGB1 receptors (30). TLR4-deficient mice have been shown to succumb to endotoxemia in the presence of increased levels of HMGB1, while caspase11-deficient mice survive (31).
The PI3K/AKT signaling pathway plays a key role in the regulation of cell apoptosis and proliferation. Activated (i.e. phosphorylated) AKT promotes cell proliferation and inhibits apoptosis, leading to both blunted and exacerbated inflammatory responses, depending on the context (32).
Based on prior reports and an online search regarding the association between miR-340-5p and HMGB1 expression in pancreatic acinar cells, we hypothesized that the upregulation of miR-340-5p could cause the downregulation of HMGB1, in turn inhibiting the activation of TLR4 and restraining cellular inflammation and apoptosis (Fig. S1).
Materials and methods
Primary culture of pancreatic acinar cells and treatment
Pancreatic acinar cells were isolated from healthy adult male 4-6 weeks-old C57BL/6J mice (weight, 25-30 g; Beijing Vital River Laboratory Animal Technology Co., Ltd.). Animals were housed in specific pathogen-free conditions under a standard temperature (22±1˚C), humidity (50-60%) and light cycle (12 h light/dark cycle), with ad libitum access to food and water. The animal experiments were approved by the Ethics Committee (approval no. 2021-1523) of Xi'an Jiaotong University (Xi'an, China). The number of animals used was 20. Mouse death was confirmed by the stopping of the heartbeat. Preparation of mouse pancreatic acinar cells was carried out by using previously described methods (33). Briefly, mice were sacrificed by exsanguination under deep anesthesia (sodium pentobarbital intraperitoneal injection, 50 mg/kg), the pancreas was immediately removed from the sacrificed mouse and incubated in buffer solution (Thermo Scientific Fisher, Inc.) at 37˚C for 10 min in a shaking bath (100 cycles/min). The buffer solution contained: 130 mM NaCl; 4.7 mM KCl; 1.3 mM CaCl2; 1 mM MgCl2; 1.2 mM KH2PO4; 10 mM glucose; 10 mM HEPES; 0.01% trypsin inhibitor (soybean) and 0.2% BSA (pH adjusted to 7.4 with NaOH, Sigma-Aldrich; Merck KGaA). Then, the cell suspension was centrifuged at 335 x g for 5 min at 4˚C. Next, the acinar cell pellets were resuspended in HEPES buffer without collagenase and centrifuged at 335 x g for 5 min at 4˚C, following which the supernatant was removed. Primary pancreatic cells were maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 1% antibiotics (penicillin and streptomycin; Gibco; Thermo Fisher Scientific, Inc.), 2 mM L-glutamine and 25 µg/ml gentamicin at 37˚C with 5% CO2. LPS (100 ng/ml; Sigma-Aldrich; Merck KGaA) was added to cells for 0, 2, 6, 12, 24, 48 h at 37˚C.
Cell viability analysis
Pancreatic acinar cells (1x104) were cultured on a 96-well plate and treated with increasing concentrations of LPS (0-200 ng/ml) for 48 h at 37˚C. Cell viability was then measured using a Cell Counting Kit (CCK)-8 assay for 1 h (Beyotime Institute of Biotechnology) according to the manufacturer's instructions.
Western blotting
Briefly, cell pellets were lysed in the ice-cold RIPA buffer (Xi'an Hat Biotechnology Co., Ltd.). Proteins were extracted from the pancreatic cells and quantified using a BCA protein kit (Thermo Fisher Scientific, Inc.). Proteins (30 µg) were loaded on 12% SDS-PAGE gels per lane, separated via electrophoresis and transferred to PVDF membranes (MilliporeSigma). The membranes were blocked with 5% non-fat milk diluted with TBS-0.1% Tween-20 (TBST) buffer at room temperature for 1 h and then incubated with primary antibodies overnight at 4˚C. Antibodies against HMGB1 (1:500; Abcam; cat. no. ab18256), TLR4 (1:1,000; Abcam; cat. no. ab13556), pan-AKT (1:1,000; Abcam; cat. no. ab8805), p-AKT (1:500; Abcam; cat. no. ab38449), Bcl2 (1:500; Abcam; cat. no. ab182858), cleaved-caspase3 (1:500; Invitrogen; cat. no. PA5-114687; Thermo Fisher Scientific, Inc.) and β-actin (1:2,000; Invitrogen; cat. no. MA5-15739-HRP; Thermo Fisher Scientific, Inc.) were used. On the following day, the PVDF membranes were washed with TBST buffer and then incubated with HRP-conjugated secondary antibody (1:10,000; cat. no. TA130004; OriGene Technologies, Inc.) at room temperature for 2 h. Western blots were developed using an ECL reagent (MilliporeSigma) plus a western blotting detection system (Bio-Rad Laboratories, Inc.). Densitometry was performed using Universal Hood III software (version no. 731BR03155; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative (RT-q) PCR analysis
Total RNA was extracted using TRIzol® reagent (MilliporeSigma) following the manufacturer's instructions. cDNA was synthesized by using a FastKing RT kit (Qiagen China Co., Ltd.) in accordance with the manufacturer's protocol. RT-qPCR were carried out with a SuperReal Premix Plus kit (Vazyme Biotech Co., Ltd.). The thermocycling conditions for qPCR were as follows: 15 min at 95˚C to activate the chemically modified hot-start Taq DNA polymerase, followed by 40 cycles of duration for 15 sec at 95˚C and 30 sec of annealing and extension at 60˚C. The primer sequences were as follows: HMGB1 forward (F), 5'-CGCGGAGGAAAATCAACTAA-3' and reverse (R), 5'-TCATAACGAGCCTTGTCAGC-3'; TNFα F, 5'-TCCCAGGTTCTCTTCAAGGGA-3' and R, 5'-GGTGAGGAGCACGTAGTCGG-3'; IL-1β F, 5'-TGGAAAAGCGGTTTGTCTTC-3' and R, 5'-TACCAGTTGGGGAACTCTGC-3'; IL-6 F, 5'-GCTGGTGACAACCACGGCCT-3' and R, 5'-AGCCTCCGACTTGTGAAGTGGT-3'; and GAPDH F, 5'-CGTCCCGTAGACAAAATGGT-3' and R, 5'-TTGATGGCAACAATCTCCAC-3'. GAPDH was amplified as the internal control. The miR-340-5p primer was the Bulge-Loop™ miRNA RT-PCR primer (Guangzhou RiboBio Co., Ltd.), with U6 snRNA (forward, 5'-CCGCCCGCCGCCAGGCCCC-3' and reverse, 5'-ATATGGAACGCTTCACGAATT-3') as the miRNA quantitative internal reference. The original Ct values of the sample (cycle of the threshold) were adjusted to internal control and relative transcript levels were analyzed using the 2-ΔΔCq method (34).
Luciferase assay and LPS treatment
A lentivirus vector (pGCL-GFP; Promega Corporation) containing a U6 promoter and a green fluorescent protein (GFP) reporter was used for cloning HMGB1 short hairpin RNAs (shRNAs/shR). The shRNA sequences were as follows: shR-HMGB1, 5'-GGCTCGTTATGAAAGAGAAAT-3'; and shR-control, 5'-GTTCTCCGAACGTGTCACGT-3'. 293T cells were inoculated in T75 culture flasks (Nalge Nunc International) at a density of 2x106 cells and were left to reach 70-80% confluence the day prior to infection. The lentiviral plasmid PGCL-GFP (6 µg) and packaging plasmids (pHelper 1.0 4.5 µg and pHelper 2.0; 2.4 µg; all Invitrogen; Thermo Fisher Scientific Inc.) were transfected into 293T cells using the X-tremeGENE™ HP DNA Transfection Reagent (Roche Diagnostics GmbH) for 16 h at 37˚C in 5% CO2, according to the manufacturer's protocol. Following 48-72 h, supernatants containing lentiviral particles were harvested and filtered through a 0.45-µm filter (EMD Millipore) to remove cell debris. The supernatants were concentrated by ultracentrifugation at 50,000 x g at 4˚C for 90 min, after which the lentiviral particle pellet was resuspended in 100% FBS and stored at -80˚C. The viral titers of concentrated lentiviral particles were measured by infecting 293T cells that were seeded at a density of 1x105 cells/well in a 12-well plate with viral serial dilutions (10-10-8). After 3 days, GFP expression was detected using flow cytometry and the viral titer was calculated using the following equation: Viral titer (Tu/µl)=(% GFP + cells x number of cells transduced)/virus volume. For lentiviral transduction, Pancreatic acinar cells were seeded at 1x106 cells/ml in six-well plates and infected with lentivirus at a multiplicity of infection of 10 for 24 h at 37˚C and 5% CO2. After 2 days of culture, the cells were collected, and the expression of GFP was detected using fluorescence microscopy. Then, cells were treated with 100 ng/ml LPS for 24 h at 37˚C, and then lysates were harvested and analyzed with a Luciferase Reporter Assay system (Promega Corporation).
Construction of luciferase reporter gene vector and dual-luciferase reporter gene assay
The miR-340-5p mimics and negative control duplex were synthesized by Shanghai GenePharma Co., Ltd. The sequence of the miR-340-5p mimic was 5'-UUAGUCAGAGUAACGAAAUAUU-3'; and that of the NC mimic was 5'-GCCUGAGUCUGGCAUCCGGGGC-3'. The microRNA.org online (http://www.targetscan.org/cgibin/targetscan/vert_72/targetscan.cgi?species=Mouse&gid=HMGB1&mir_sc=&mir_c=&mir_nc=&mir_vnc=&mirg=miR-340-5p) target gene prediction tool predicted the association between miR-340-5p and the 3'UTR of HMGB1 mRNA. HMGB1 3'UTR full length fragment and mutation-containing fragments were cloned into the luciferase reporter plasmid (pSiCHECK2 vectors; Promega Corporation) to transform DH5α competent cells (Sangon Biotech Co., Ltd.; cat. no. B528413). The efficient plasmids were sequenced, screened and named wild-type (WT)-HMGB1 and mutated (MUT)-HMGB1. The sequences were as follows: WT-HMGB1, 5'-AUACAUUUGCUUUUUCUUUAUAA-3'; and MUT-HMGB1, 5'-AUACAUUUGCUUUUUGAAAUAUU-3'. The WT-HMGB1 or MUT-HMGB1 and miR-340-5p mimics were co-transfected into 293T cells using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. 293T cells were seeded into 6-well plates at a density of 105 cells/well. A total of 10 µl transfection reagent was mixed with 100 µl serum-free culture. Meanwhile, 10 µl miR-340-5p mimics, WT-HMGB1 and MUT-HMGB1 was mixed as aforementioned. Next, the two mixtures were mixed and incubated at room temperature for 10 min. Subsequently, the mixture was added to the 6-well plate at a final concentration of 20 nM. The reporter luciferase activities were detected via a thermoplate reader (Rayto Life and Analytical Science Co., Ltd.) after 48 h. Firefly luciferase activity was then normalized to that of Renilla luciferase. All the transfection experiments were performed in triplicate and repeated at least three times independently.
Statistical analysis
All data are presented as the mean ± SD, and were analyzed using SPSS statistical software version 18.0 (SPSS, Inc.). Each experiment was repeated in triplicate and the statistical difference was analyzed using an unpaired Student's t-test for comparisons of two groups or one-way ANOVA for two factor experiments or one-way ANOVA followed by Tukey's test for comparisons of multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
LPS-induced acute inflammation and apoptosis parallels miR-340-5p suppression and HMGB1 elevation in pancreatic acinar cells
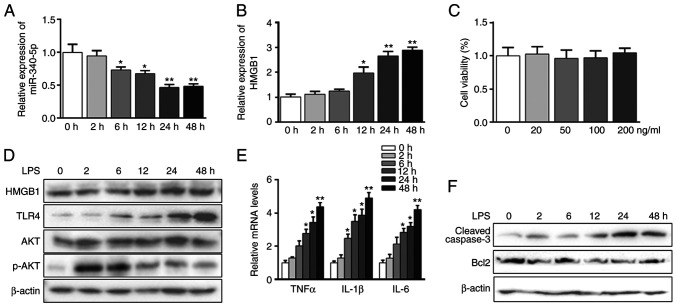
The pancreatic acinar cell model induced by LPS was used for the inflammation study (35-38). It was found that LPS inhibited the expression of miR-340-5p and upregulated HMGB1 mRNA expression in a time-dependent manner (Fig. 1A and B). The cytotoxic effects of LPS were evaluated using a CCK-8 assay, which showed no obvious cell death at up to 200 ng/ml LPS treatment for 48 h (Fig. 1C). Western blot analysis confirmed that HMGB1 protein expression was increased in the pancreatic acinar cells in a time-dependent manner following LPS treatment (Fig. 1D). Additionally, LPS increased the expression level of TLR4, as well as enhanced those of TNFα, IL-1β and IL-6 in a time-dependent manner (Fig. 1D and E). p-AKT expression showed a significant increase when LPS stimulated pancreatic acinar cells within 2 h, and then this slowly decreased (Figs. 1D and S2A). Moreover, LPS treatment significantly enhanced the expression of cleaved-caspase3 and downregulated Bcl2 expression over time (Fig. 1F).
Figure 1.
LPS-induced acute inflammation and apoptosis parallels with miR-340-5p suppression and HMGB1 elevation in pancreatic acinar cells. (A) Pancreatic acinar cells were treated with LPS (100 ng/ml) for different durations. The expression level of miR-340-5p was assessed via RT-qPCR. (B) Pancreatic acinar cells were treated with of LPS (100 ng/ml) for different durations. The expression level of HMGB1 was assayed via RT-qPCR. (C) Cell viability assay results: pancreatic acinar cells were treated with various doses of LPS for 48 h. (D) Pancreatic acinar cells were treated with LPS for different durations. The protein expression levels of HMGB1, TLR4, AKT and p-AKT were assayed via western blotting. β-actin was used as a control. (E) Pancreatic acinar cells were treated with LPS for different durations. The mRNA expression levels of TNFα, IL-1β and IL-6 were assayed via RT-qPCR. (F) Pancreatic acinar cells were treated with LPS for different durations. The expression levels of cleaved-caspase3 and Bcl2 were examined via western blotting. β-actin was used as a control. Data are expressed as the mean ± SD. *P<0.05 and **P<0.01 vs. 0 h. RT-qPCR, reverse transcription-quantitative PCR; TLR, Toll-like receptor; LPS, lipopolysaccharide; HMGB1, high mobility group box 1; p-, phosphorylated; miR, microRNA.
miR-340-5p inhibits HMGB1 expression
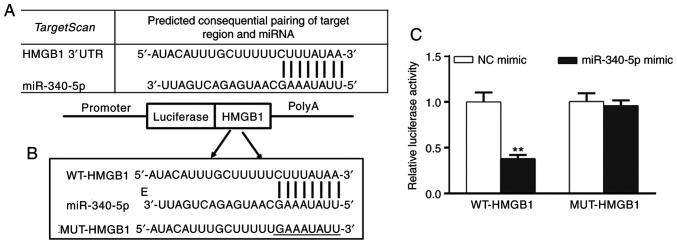
A query of the microRNA.org online target gene database predicted that there was a targeted binding site for miR-340-5p on the 3'UTR of HMGB1 mRNA (Fig. 2A and B). Furthermore, transfection of miR-340-5p mimics significantly reduced the relative luciferase activity of HMGB1 compared with NC mimic, suggesting that miR-340-5p was capable of inhibiting the expression of HMGB1 (Fig. 2C).
Figure 2.
miR-340-5p inhibits HMGB1 expression. (A) miR-340-5p binding site on the 3'UTR of HMGB1 mRNA. (B) miR-340-5p may target the 3'UTR region of HMGB1 as determined using a (C) dual luciferase reporter assay. **P<0.01 vs. NC mimic. miR-340-5p, microRNA-340-5p; HMGB1, high mobility group box 1; 3'UTR, 3'untranslated region; NC, negative control; WT, wild-type; MUT, mutated.
miR-340-5p inhibits inflammation and apoptosis in pancreatic acinar cells following LPS treatment
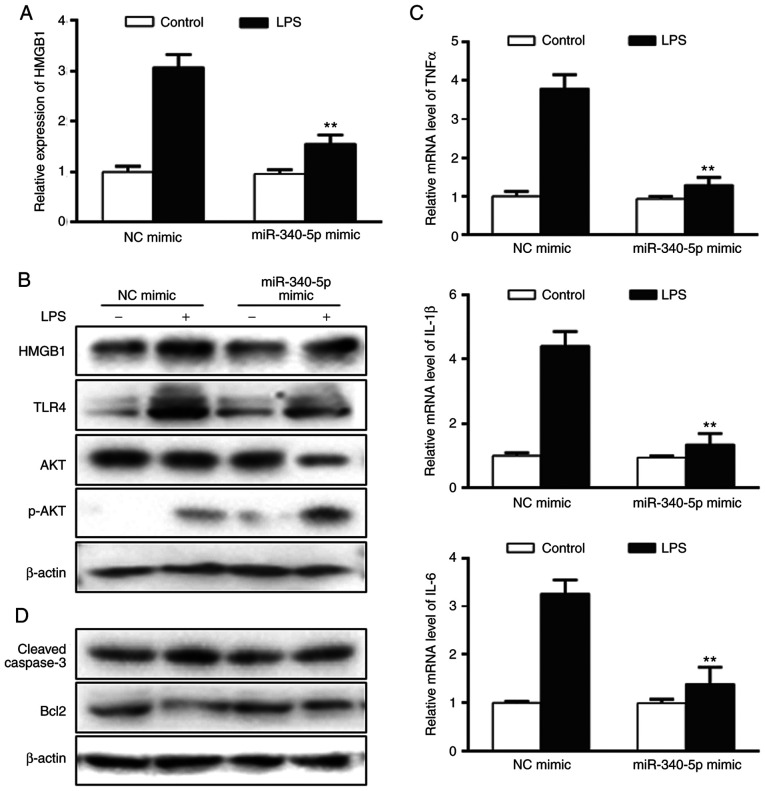
To further investigate the role of miR-340-5p in the current model, it was determined whether miR-340-5p exerted anti-inflammatory and anti-apoptotic effects in pancreatic acinar cells treated with LPS. Cells were transfected with miR-340-5p mimics or NC mimics, subjected to LPS 24 h and then the aforementioned endpoints were examined. miR-340-5p expression was upregulated in cells transfected with miR-340-5p mimics (Fig. S2C). Transfection with miR-340-5p mimics led to decreased HMGB1, TLR4 and AKT upregulation following LPS (Fig. 3A and B), as well as enhanced p-AKT expression (Fig. 3B). In addition, LPS-induced expression of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were significantly decreased compared with NC mimics (Fig. 3C). The LPS-induced expression levels of cleaved-caspase3 were notably inhibited, while anti-apoptotic Bcl2 expression was markedly enhanced in cells transfected with miR-340-5p mimics compared with NC mimics (Fig. 3D).
Figure 3.
miR-340-5p overexpression can inhibit HMGB1 expression, as well as inflammation and apoptosis in pancreatic acinar cells with LPS. (A) HMGB1 expression in pancreatic acinar cells transfected with miR-340-5p mimics was assayed via RT-qPCR. (B) Expression levels of HMGB1, TLR4, AKT and p-AKT in pancreatic acinar cells with miR-340-5p mimic were assayed via western blotting. (C) mRNA expression levels of TNFα, IL-1β and IL-6 in pancreatic acinar cells transfected with miR-340-5p mimics were examined via RT-qPCR. (D) Expression level of cleaved-caspase3 and Bcl2 in pancreatic acinar cells with miR-340-5p mimic were assayed via western blotting. Data are expressed as the mean ± SD. **P<0.01 vs. control. miR-340-5p, microRNA-340-5p; NC, negative control; RT-qPCR, reverse transcription-quantitative PCR; TLR, Toll-like receptor; HMGB1, high mobility group box 1; p-, phosphorylated; LPS, lipopolysaccharide.
miR-340-5p could inhibit inflammation and apoptosis via HMGB1 targeting in pancreatic acinar cells following LPS treatment
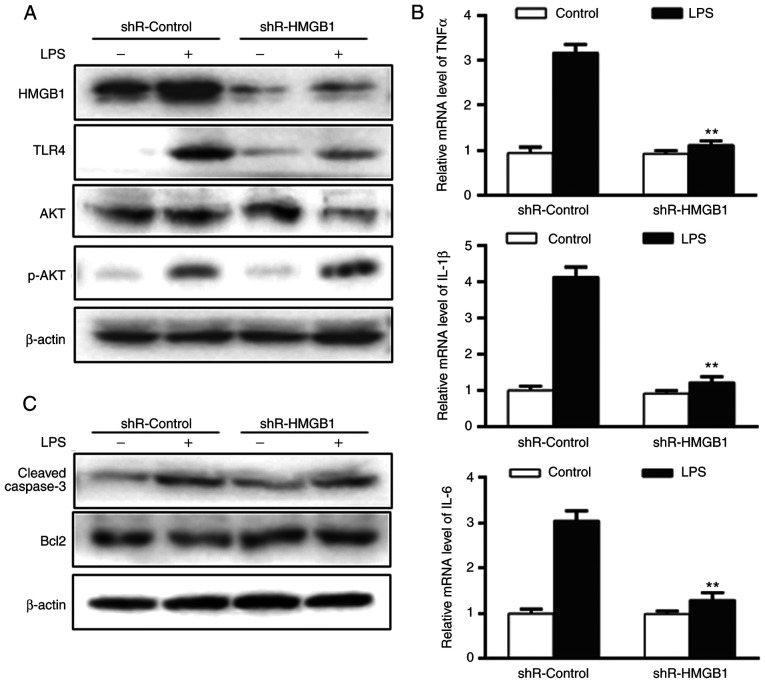
To further examine the role of HMGB1, it was determined whether the miR-340-5p-mediated anti-inflammatory and anti-apoptotic effects in pancreatic acinar cells following LPS treatment were mediated by HMGB1. Cells were transfected with either non-specific shRNA (shR-control) or shR-HMGB1, subjected to LPS for 24 h and then the same endpoints were examined. The expression level of HMGB1 was significantly decreased in cells transfected with shR-HMGB1 (Figs. 4A and S2B). HMGB1 knockdown caused marked decreases in TLR4 and AKT upregulation following LPS (Fig. 4A), and enhanced p-AKT expression (Fig. 4A). Additionally, cells transfected with shR-HMGB1 had significantly lower expression levels of pro-inflammatory cytokines (Fig. 4B), decreased cleaved-caspase3 and increased Bcl2 compared with shR-control (Fig. 4C) following LPS treatment.
Figure 4.
miR-340-5p can inhibit inflammation and apoptosis by targeting HMGB1 in pancreatic acinar cells with LPS. (A) Expression levels of TLR4, AKT and p-AKT in pancreatic acinar cells with HMGB1 knockdown were assayed via western blotting. (B) mRNA expression levels of TNFα, IL-1β and IL-6 in pancreatic acinar cells with HMGB1 knockdown were assayed via reverse transcription-quantitative PCR. (C) Expression levels of cleaved-caspase3 and Bcl2 in pancreatic acinar cells with HMGB1 knockdown were assayed via western blotting. Data are expressed as the mean ± SD. **P<0.01 vs. control. shR, short hairpin RNA; TLR, Toll-like receptor; HMGB1, high mobility group box 1; p-, phosphorylated; LPS, lipopolysaccharide; miR-340-5p, microRNA-340-5p.
Discussion
A critical component in the pathophysiology of AP is inflammation (39,40). The present study demonstrated the protective effect of miR-340-5p on LPS-induced inflammation and apoptosis in pancreatic acinar cells. miRNAs have emerged as important post-transcriptional regulatory factors in recent years (10). The current results demonstrated that miR-340-5p attenuated LPS-induced upregulation of HMGB1, decreased TLR4 activation and promoted the activation of AKT, subsequently leading to decreased inflammatory and apoptotic signaling. TLR4 is widely expressed in the pancreatic tissues and plays an important role in pancreatitis (41). A previous study has shown that TLR4 participates in the early stage of SAP and may also be involved in its progression (42). The PI3K/AKT signaling pathway plays an important role in the activation of pancreatic trypsinogen, a causal factor in the onset and aggravation of AP (35). Moreover, increasing evidence suggests that PI3K/AKT signaling is involved in the pathogenesis of inflammatory diseases, such as AP (43). Pharmacological activation of the PI3K/AKT pathway may therefore represent a promising new direction in therapeutics to limit inflammation in SAP (44), as recent studies suggest (45).
HMGB1 is an important mediator of damage-associated molecular pattern (DAMP) signaling. DAMPs can activate pattern recognition receptors such as TLRs and NOD-like receptors. Among them, TLRs are considered to be ‘gateway’ proteins that initiate the inflammatory response. TLR4 has been reported to bind HMGB1 secreted by macrophages and neutrophils to recruit myeloid differentiation protein 88 and IL-1 receptor-related kinases, thereby causing the downstream regulator TRAF to activate the MAPK pathway and nuclear (46,47) transcription factors to induce the inflammatory response. In addition to TLR4, extracellular HMGB1 can interact with TLR2 and RAGE (48). TLR4 activation promotes the degradation of IκB, leading to NF-κB p65 nuclear translocation and subsequent expression of pro-inflammatory cytokines/chemokines and other inflammatory mediators (49-54), ultimately leading to intestinal damage. In addition to its deleterious effects, at low levels HMGB1 can promote tissue repair. Diener et al (55) reported that HMGB1, as an ‘alarmin’ secreted by damaged tissue, can induce stem cells or primitive cells to migrate to the damaged area to aid in its repair and replacement. Biscetti et al (56) also showed that HMGB1 plays a role in protecting and repairing myocardial tissue following myocardial infarction. However, information regarding the threshold and mechanism via which HMGB1 promotes the pro-inflammatory and deleterious effects, as compared with the protective effects in tissues, remains to be determined. Recent experimental studies have shown that early inflammatory factors reach their peak quickly after AP model induction (e.g. LPS exposure), and then rapidly decline to normal level, although the damage to the pancreas persists (23,35). A good systemic indicator of organ damage is the serum levels of HMGB1. In a mouse model of acute necrotizing pancreatitis, serum HMGB1 increased significantly with disease onset and correlated positively with disease severity (57). In this regard, some investigators have proposed using the serum HMGB1 level as a biomarker of AP severity in patients and there are existing reports to support its use in this manner (58). Compared with early inflammation, HMGB1 appears late, has a long action time and forms an inflammatory positive feedback loop. As such, it plays a key role in regulating inflammation in the context of AP. Inhibition of extracellular HMGB1 has also been proposed to be a therapeutic target for AP treatment (57).
miRNAs are known to regulate a wide variety of physiological and pathological processes, and rapidly accumulating evidence is suggesting that they are key regulators of numerous diseases (9,11,59). However, regulation of target genes by miRNAs is complicated and difficult to model and study. For example, while one single miRNA may be capable of regulating expression of multiple genes, the combination of several miRNAs may be necessary to fine-tune the expression of a single gene. Most of the evidence to date suggests that the main role of miRNA is to downregulate and, in rare cases, upregulate target gene expression (9). miRNA sequences are complementary to the target gene mRNA, usually inhibiting the translation of the target mRNA and thereby acting similarly to RNA interference (59). From a clinical perspective, miRNA-based diagnostic and treatment methods have exciting and have a broad potential for the future. They may serve in a gene therapy capacity, where adenovirus vectors carrying target miRNAs may be used to regulate the expression of target genes (60,61). With respect to miR-340-5p, a prior study reported that this miRNA was downregulated in hearts with ischemia-reperfusion injury. Overexpression of miR-340-5p inhibited ischemia-reperfusion-induced apoptosis in H9C2 cardiomyoblasts (62). Overexpression of miR-340-5p was also shown to significantly promote the activation of the PI3K/AKT signaling pathway to alleviate neuronal cell damage (63). However, to the best of our knowledge, a role for miR-340-5p in AP has not been previous examined. The current study is important as it identified that miR-340-5p was capable of targeting HMGB1 and inhibiting its expression (Fig. S1), although the precise mechanism of action requires further investigation.
In conclusion, the presented study demonstrated that miR-340-5p effectively attenuated the severity of LPS-induced inflammation and apoptosis in pancreatic acinar cells, and this effect was likely mediated via the suppression of HMGB1 and activation of PI3K/AKT signaling. Collectively, these findings support the notion that miR-340-5p may be a promising target for new AP therapies, and they also support a need for additional mechanistic studies that examine a role for HMGB1 in AP progression.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: This research study was approved and financially supported by Shaanxi Provincial Science and Technology Research Subject of Traditional Chinese Medicine (grant no. 2019-ZZ-JC031), Fund for Free Exploration Project of The Second Affiliated Hospital of Xi'an Jiaotong University [grant no. 2020YJ(ZYTS)359], the Natural Science Basic Research Plan in Shaanxi Province of China (grant no. 2021JM-284) and the Health Research Projects of Shaanxi Province (grant no. 2021A010).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors contributed to this work. LP and YazhouG made substantial contributions to the conception and design of the study, and were involved in the drafting of the manuscript. YazhouG, LW, JS and YanxiaG made substantial contributions to data acquisition. ZN, HF and JL were responsible for the development of the study methodology, analysis and interpretation of the data. LP and YazhouG confirm the authenticity of the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The use of animals and all related procedures were in accordance with the Institutional Animal Care Committee of Xi'an Jiaotong University. The animal experiments were approved by the Ethics Committee of Xi'an Jiaotong University (Xi'an, China). All efforts were made to reduce number of animals and to lower their sufferings.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ji L, Lv JC, Song ZF, Jiang MT, Li L, Sun B. Risk factors of infected pancreatic necrosis secondary to severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2016;15:428–433. doi: 10.1016/s1499-3872(15)60043-1. [DOI] [PubMed] [Google Scholar]
- 2.Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J Gastroenterol. 2016;35:153–166. doi: 10.1007/s12664-016-0647-y. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 4.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: Bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Yamato E, Miyazaki J. Intravenous delivery of naked plasmid DNA for in vivo cytokine expression. Biochem Biophys Res Commun. 2001;289:1088–1092. doi: 10.1006/bbrc.2001.6100. [DOI] [PubMed] [Google Scholar]
- 6.Shimomura A, Shiino S, Kawauchi J, Takizawa S, Sakamoto H, Matsuzaki J, Ono M, Takeshita F, Niida S, Shimizu C, et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016;107:326–334. doi: 10.1111/cas.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M. Emerging roles of microRNAs in ischemic stroke: As possible therapeutic agents. J Stroke. 2017;19:166–187. doi: 10.5853/jos.2016.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malumbres M. miRNAs and cancer: An epigenetics view. Mol Aspects Med. 2013;34:863–874. doi: 10.1016/j.mam.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matts J, Jagadeeswaran G, Roe BA, Sunkar R. Identification of microRNAs and their targets in switchgrass, a model biofuel plant species. J Plant Physiol. 2010;167:896–904. doi: 10.1016/j.jplph.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Qu B, Shen N. miRNAs in the pathogenesis of systemic lupus erythematosus. Int J Mol Sci. 2015;16:9557–9572. doi: 10.3390/ijms16059557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Dai LX, Zhang S, Yang Y, Yan N, Fan P, Dai L, Tian HW, Cheng L, Zhang XM, et al. Regulation of epidermal growth factor receptor signaling by plasmid-based microRNA-7 inhibits human malignant gliomas growth and metastasis in vivo. Neoplasma. 2013;60:274–283. doi: 10.4149/neo_2013_036. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Gao Y, Yang J, Shi C, Wang Y, Xu Y. MicroRNA-381 reduces inflammation and infiltration of macrophages in polymyositis via downregulating HMGB1. Int J Oncol. 2018;53:1332–1342. doi: 10.3892/ijo.2018.4463. [DOI] [PubMed] [Google Scholar]
- 14.Wei D, Miao Y, Yu L, Wang D, Wang Y. Downregulation of microRNA-198 suppresses cell proliferation and invasion in retinoblastoma by directly targeting PTEN. Mol Med Rep. 2018;18:595–602. doi: 10.3892/mmr.2018.8979. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Ning X, Cui W, Bi M, Zhang D, Zhang J. Transforming growth factor (TGF)-β-induced microRNA-216a promotes acute pancreatitis via Akt and TGF-β pathway in mice. Dig Dis Sci. 2015;60:127–135. doi: 10.1007/s10620-014-3261-9. [DOI] [PubMed] [Google Scholar]
- 16.Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, et al. Epidemiology of sepsis in Germany: Results from a national prospective multicenter study. Intensive Care Med. 2007;33:606–618. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Li R, Pei LG, Wei ZH, Kang LN, Wang L, Xie J, Xu B. Emerging role of high mobility group box-1 in thrombosis-related diseases. Cell Physiol Biochem. 2018;47:1319–1337. doi: 10.1159/000490818. [DOI] [PubMed] [Google Scholar]
- 18.Gao N, Yan C, Zhang G. Changes of serum procalcitonin (PCT), C-reactive protein (CRP), interleukin-17 (IL-17), interleukin-6 (IL-6), high mobility group protein-B1 (HMGB1) and D-dimer in patients with severe acute pancreatitis treated with continuous renal replacement therapy (CRRT) and its clinical significance. Med Sci Monit. 2018;24:5881–5886. doi: 10.12659/MSM.910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S, Yang J, Liu T, Zeng J, Mi L, Xiang K. Dexamethasone inhibits NF-κBp65 and HMGB1 expression in the pancreas of rats with severe acute pancreatitis. Mol Med Rep. 2018;18:5345–5352. doi: 10.3892/mmr.2018.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q, et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097–1107. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen TK, Zhu YF, Wu L. Antioxidant inhibits HMGB1 expression and reduces pancreas injury in rats with severe acute pancreatitis. Dig Dis Sci. 2010;55:2529–2536. doi: 10.1007/s10620-009-1073-0. [DOI] [PubMed] [Google Scholar]
- 22.Kang R, Lotze MT, Zeh HJ, Billiar TR, Tang D. Cell death and DAMPs in acute pancreatitis. Mol Med. 2014;20:466–477. doi: 10.2119/molmed.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saluja A, Dudeja V, Dawra R, Sah RP. Early intra-acinar events in pathogenesis of pancreatitis. Gastroenterology. 2019;156:1979–1993. doi: 10.1053/j.gastro.2019.01.268. [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Luo Z, Xiang K, Ren J, Huang Z, Tang L, Tian F. Clinical significance of serum triglyceride elevation at early stage of acute biliary pancreatitis. BMC Gastroenterol. 2015;15(19) doi: 10.1186/s12876-015-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sit M, Aktas G, Yilmaz EE, Alcelik A, Terzi EH, Tosun M. Effects of the inflammatory response on serum omentin levels in early acute and chronic pancreatitis. Clin Ter. 2014;165:e148–e152. doi: 10.7471/CT.2014.1699. [DOI] [PubMed] [Google Scholar]
- 26.Schneider L, Jabrailova B, Strobel O, Hackert T, Werner J. Inflammatory profiling of early experimental necrotizing pancreatitis. Life Sci. 2015;126:76–80. doi: 10.1016/j.lfs.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Tenhunen J, Tonnessen TI. HMGB1 and histones play a significant role in inducing systemic inflammation and multiple organ dysfunctions in severe acute pancreatitis. Int J Inflam. 2017;2017(1817564) doi: 10.1155/2017/1817564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol. 2015;31:395–399. doi: 10.1097/MOG.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Ni HM, Chao X, Wang H, Bridges B, Kumer S, Schmitt T, Mareninova O, Gukovskaya A, De Lisle RC, et al. Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy. 2019;15:1954–1969. doi: 10.1080/15548627.2019.1596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xin-Peng Z, Yong-Hua H, Yong L, Jing-Jing W, Guang-Hua W, Ren-Jie W, Min Z. A high-mobility group box 1 that binds to DNA, enhances pro-inflammatory activity, and acts as an anti-infection molecule in black rockfish, Sebastes schlegelii. Fish Shellfish Immunol. 2016;56:402–409. doi: 10.1016/j.fsi.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 31.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 32.Keppler-Noreuil KM, Parker VE, Darling TN, Martinez-Agosto JA. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C Semin Med Genet. 2016;172:402–421. doi: 10.1002/ajmg.c.31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estaras M, Ameur FZ, Roncero V, Fernandez-Bermejo M, Blanco G, Lopez D, Mateos JM, Salido GM, Gonzalez A. The melatonin receptor antagonist luzindole induces Ca2+ mobilization, reactive oxygen species generation and impairs trypsin secretion in mouse pancreatic acinar cells. Biochim Biophys Acta Gen Subj. 2019;1863(129407) doi: 10.1016/j.bbagen.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Q, Zhang H, Wu J, Lv X, Jin X, Hu J. Melatonin inhibits the endoplasmic reticulum stress-induced, C/EBP homologous protein-mediated pathway in acute pancreatitis. Mol Med Rep. 2020;22:1647–1655. doi: 10.3892/mmr.2020.11219. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Yang L, Chen KL, Zhou B, Yan H, Zhou ZG, Li Y. Knockdown of GRP78 promotes apoptosis in pancreatic acinar cells and attenuates the severity of cerulein and LPS induced pancreatic inflammation. PLoS One. 2014;9(e92389) doi: 10.1371/journal.pone.0092389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Xu C, Tao Y, Liang Y, Liang Q, Li J, Li R, Ye H. Anisodamine alleviates lipopolysaccharide-induced pancreatic acinar cell injury through NLRP3 inflammasome and NF-κB signaling pathway. J Recept Signal Transduct Res. 2020;40:58–66. doi: 10.1080/10799893.2020.1713808. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Li YY, Wu XZ. Effect of Tetrandrine on LPS-induced NF-kappaB activation in isolated pancreatic acinar cells of rat. World J Gastroenterol. 2006;12:4232–4236. doi: 10.3748/wjg.v12.i26.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343–351. doi: 10.2174/1568010023344517. [DOI] [PubMed] [Google Scholar]
- 40.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Hong YP, Yu J, Su YR, Mei FC, Li M, Zhao KL, Zhao L, Deng WH, Chen C, Wang WX. High-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxid Med Cell Longev. 2020;2020(8172714) doi: 10.1155/2020/8172714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Zhu C, Wu D, Jiang X. Possible role of toll-like receptor 4 in acute pancreatitis. Pancreas. 2010;39:819–824. doi: 10.1097/MPA.0b013e3181ca065c. [DOI] [PubMed] [Google Scholar]
- 43.Abliz A, Deng W, Sun R, Guo W, Zhao L, Wang W. Wortmannin, PI3K/Akt signaling pathway inhibitor, attenuates thyroid injury associated with severe acute pancreatitis in rats. Int J Clin Exp Pathol. 2015;8:13821–13833. [PMC free article] [PubMed] [Google Scholar]
- 44.Xu P, Wang J, Yang ZW, Lou XL, Chen C. Regulatory roles of the PI3K/Akt signaling pathway in rats with severe acute pancreatitis. PLoS One. 2013;8(e81767) doi: 10.1371/journal.pone.0081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Liao R, Qiang Z, Zhang C. Pro-inflammatory cytokine-driven PI3K/Akt/Sp1 signalling and H2S production facilitates the pathogenesis of severe acute pancreatitis. Biosci Rep. 2017;37(BSR20160483) doi: 10.1042/BSR20160483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: Role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 47.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 48.Tian X, Sun L, Feng D, Sun Q, Dou Y, Liu C, Zhou F, Li H, Shen H, Wang Z, Chen G. HMGB1 promotes neurovascular remodeling via Rage in the late phase of subarachnoid hemorrhage. Brain Res. 2017;1670:135–145. doi: 10.1016/j.brainres.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 50.Xu GF, Guo M, Tian ZQ, Wu GZ, Zou XP, Zhang WJ. Increased of serum high-mobility group box chromosomal protein 1 correlated with intestinal mucosal barrier injury in patients with severe acute pancreatitis. World J Emerg Surg. 2014;9(61) doi: 10.1186/1749-7922-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kylänpää ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol. 2010;16:2867–2872. doi: 10.3748/wjg.v16.i23.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J, Shi S, Wang Q, Yu K, Wang R. Continuous hemodiafiltration therapy reduces damage of multi-organs by ameliorating of HMGB1/TLR4/NFκB in a dog sepsis model. Int J Clin Exp Pathol. 2015;8:1555–1564. [PMC free article] [PubMed] [Google Scholar]
- 53.Todorova J, Pasheva E. High mobility group B1 protein interacts with its receptor RAGE in tumor cells but not in normal tissues. Oncol Lett. 2012;3:214–218. doi: 10.3892/ol.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diener KR, Al-Dasooqi N, Lousberg EL, Hayball JD. The multifunctional alarmin HMGB1 with roles in the pathophysiology of sepsis and cancer. Immunol Cell Biol. 2013;91:443–450. doi: 10.1038/icb.2013.25. [DOI] [PubMed] [Google Scholar]
- 56.Biscetti F, Ghirlanda G, Flex A. Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration. Curr Vasc Pharmacol. 2011;9:677–681. doi: 10.2174/157016111797484125. [DOI] [PubMed] [Google Scholar]
- 57.Kanakoudi-Tsakalidou F, Farmaki E, Tzimouli V, Taparkou A, Paterakis G, Trachana M, Pratsidou-Gertsi P, Nalbanti P, Papachristou F. Simultaneous changes in serum HMGB1 and IFN-α levels and in LAIR-1 expression on plasmatoid dendritic cells of patients with juvenile SLE. New therapeutic options? Lupus. 2014;23:305–312. doi: 10.1177/0961203313519157. [DOI] [PubMed] [Google Scholar]
- 58.Gu H, Werner J, Bergmann F, Whitcomb DC, Büchler MW, Fortunato F. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis. 2013;4(e816) doi: 10.1038/cddis.2013.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samanta S, Balasubramanian S, Rajasingh S, Patel U, Dhanasekaran A, Dawn B, Rajasingh J. MicroRNA: A new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc Med. 2016;26:407–419. doi: 10.1016/j.tcm.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther. 2007;15:255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]
- 61.Wang AY, Peng PD, Ehrhardt A, Storm TA, Kay MA. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther. 2004;15:405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 62.Li D, Zhou J, Yang B, Yu Y. MicroRNA-340-5p inhibits hypoxia/reoxygenation-induced apoptosis and oxidative stress in cardiomyocytes by regulating the Act1/NF-κB pathway. J Cell Biochem. 2019;120:14618–14627. doi: 10.1002/jcb.28723. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, Zhao P, Lian Y, Li S, Chen Y, Li L. MiR-340-5p alleviates oxygen-glucose deprivation/reoxygenation-induced neuronal injury via PI3K/Akt activation by targeting PDCD4. Neurochem Int. 2020;134(104650) doi: 10.1016/j.neuint.2019.104650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.