Abstract
Helicobacter pylori (H. pylori) is known to be a major pathogen causing gastric diseases through its direct localization in gastric epithelium cells. H. pylori releases outer membrane vesicles (OMVs) throughout the growth process. The content, function, and mechanism of H. pylori OMVs in gastric epithelial cells remain unclear. In this study, we extracted and characterized H. pylori OMVs of two strains (standard strain NCTC11637 and clinical strain Hp-400) and analyzed the specific content by proteomic technology. We identified more than 400 proteins in H. pylori OMVs. In addition, we investigated the impact of H. pylori OMVs on cellular functions by detecting proteomic changes in GES1 cells. GES1 cells cocultured with increasing concentrations of H. pylori OMVs were subjected to quantitative proteomic analyses using label-free methods for relative quantitation. The results showed that a total of 4261 proteins were verified, 153 of which were significantly altered in abundance when cocultured with NCTC11637 OMVs, and a total of 4234 proteins in Hp-400 OMVs, 390 of which were significantly altered. Gene ontology analysis and Kyoto encyclopedia of genes and genomes pathway mapping identified significantly altered inflammatory and cancer signaling pathways, including metabolic pathways and the PI3K-Akt signaling pathway. Furthermore, we explored the proteomic changes in GES1 cells induced by H. pylori. Bioinformatics analysis showed that changes in multiple pathways coincided with OMV-mediated proteomic changes. Based on these results, H. pylori induced pathogenicity in epithelial cells at least partially by secreting OMVs that mediated dramatic and specific proteomic changes in host cells. Data are available via ProteomeXchange with identifiers PXD025216, PXD025259, and PXD025281.
Introduction
Helicobacter pylori (H. pylori) is a spiral, microaerophilic, and Gram-negative bacterium that primarily colonizes the human stomach.1H. pylori persists in the human stomach lifelong and is predicted to have infected approximately half of the global population to cause multiple diseases, such as chronic gastritis, peptic ulcer, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer. H. pylori was also identified as a type I carcinogen by the WHO (World Health Organization) and contributes to a higher occurrence of gastric carcinoma.
Outer membrane vesicles (OMVs) are nanosized particles derived from the outer membrane of Gram-negative bacteria and play central roles in initiating and regulating pathogenesis in the host. OMVs generally have a diameter of 20–250 nm and are secreted under all environmental conditions and during all growth phases.2,3 Originally considered as artifacts of the cell wall, OMVs are now accepted as a general secretion system.4 OMVs carry a large amount of cargo from their parent bacterium, including virulence factors and toxins, such as outer membrane proteins, adhesins, invasions, proteases, and lipopolysaccharide (LPS),5,6 illustrating that OMV secretion is an additional virulence mechanism of pathogens. The cargo may either be located in the vesicle lumen or integrated into the vesicle membrane.7,8 Compared to other secretion systems, OMVs protect their contents from the external environment and transport their cargo over a long distance.3,9
Similar to other Gram-negative bacteria, H. pylori spontaneously secretes OMVs that play important roles in the pathogen–host interaction mechanism.10 Several studies showed that the secreted H. pylori OMVs are internalized by gastric epithelial cells.11−13 After internalization, OMVs regulate gastric epithelial cell proliferation, facilitate the secretion of inflammatory factors, and induce apoptosis.12,14 In addition, H. pylori OMVs cause genomic instability in epithelial cells, as assessed using the cytokinesis-block micronuclei assay.15 Furthermore, H. pylori OMVs induce human eosinophil degranulation.16 Based on these results, we speculated that OMVs derived from H. pylori contributed to the H. pylori-induced pathogenic effects on the stomach.
In this study, we purified and identified proteins in H. pylori-derived OMVs. We detected the protein contents of OMVs, including cagA, vacA, ureB, outer membrane proteins, and other virulence factors. We also found that H. pylori OMVs promoted the secretion of inflammatory cytokines, consistent with their parental bacteria. Furthermore, we identified proteomic changes in GES1 cells in response to OMVs or their parental bacteria. The bioinformatics analysis showed that multiple pathways overlapped, suggesting that OMVs contain most of the contents from their parental bacteria. Therefore, we highlight that H. pylori secretes and delivers gastric pathogenic virulence factors mostly via outer membrane vesicles.
Results
Purification and Characterization of the H. pylori OMVs
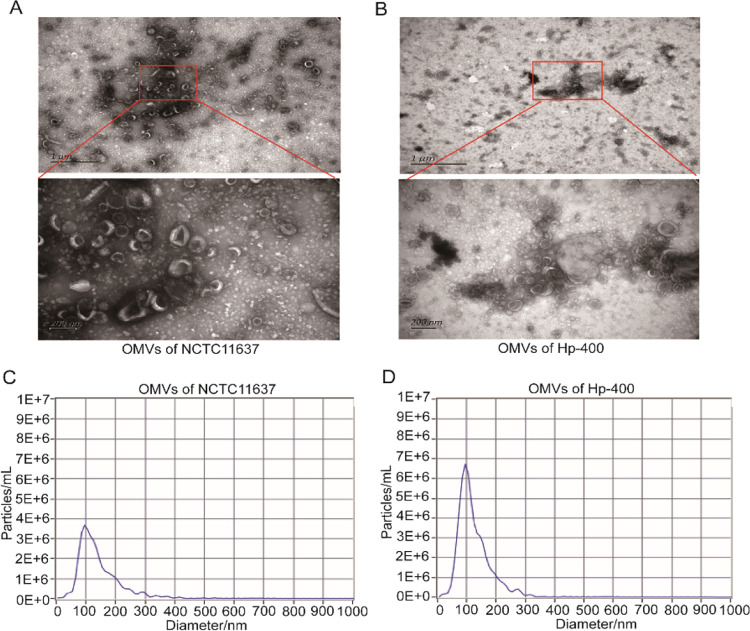
H. pylori continuously secretes OMVs into the extracellular environment during growth. We collected a conditioned medium from NCTC11637 or Hp-400 and isolated OMVs after culturing for 72 h. Then, the H. pylori OMVs were characterized using nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). TEM images revealed that the vesicles showed a spherical, bilayered morphology and a typical cup-shaped structure (Figure 1A,B). Additionally, NTA results showed that the size distribution of the OMVs ranged from 50 to 250 nm in diameter (Figure 1C,D), which is the typical size of OMVs produced by Gram-negative bacteria. Taken together, we successfully purified H. pylori OMVs.
Figure 1.
Purification and characterization of the H. pylori OMVs. (A) Representative TEM images of OMVs secreted by NCTC11637. (B) Representative TEM images of OMVs secreted by Hp-400. (C) NTA analysis of the size distributions and numbers of OMVs derived from NCTC11637. (D) NTA analysis of the size distributions and numbers of OMVs derived from Hp-400.
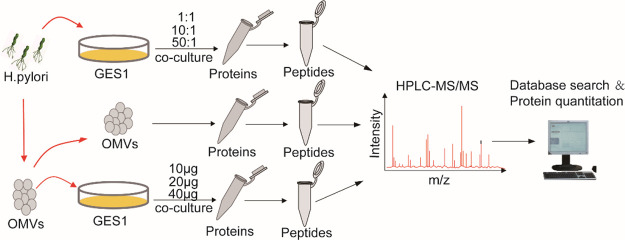
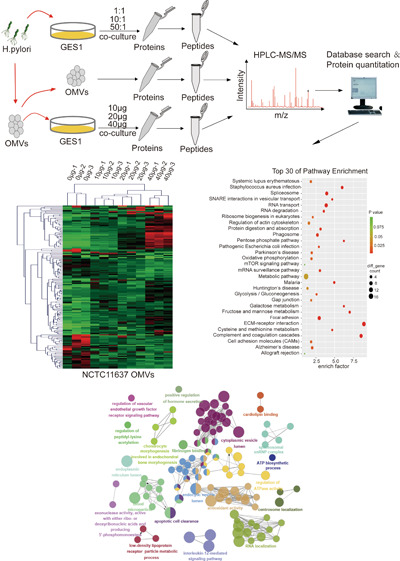
Subsequently, in order to detect the proteomic changes in GES1 cells cocultured with gradually increasing concentrations of OMVs and H. pylori as well as to reveal the influence of OMVs and H. pylori on host gastric epithelial cells, an experimental scheme focused on the HPLC-MS/MS method was adopted and its workflow is shown in Figure 2.
Figure 2.
Schematic experimental workflow: First, the contents of purified OMVs were examined using HPLC-MS/MS. Then, GES1 cells cocultured with gradually increasing concentrations of OMVs and H. pylori were subjected to quantitative proteomic analysis. Finally, bioinformatics analysis was performed on the above data, on which basis, the exact pathways and proteins that changed in the infected GES1 cell proteome were characterized. The MS analysis of each sample was performed in triplicate, and data analysis was performed with the software PD2.2.
Identification of the Protein Contents of H. pylori OMVs
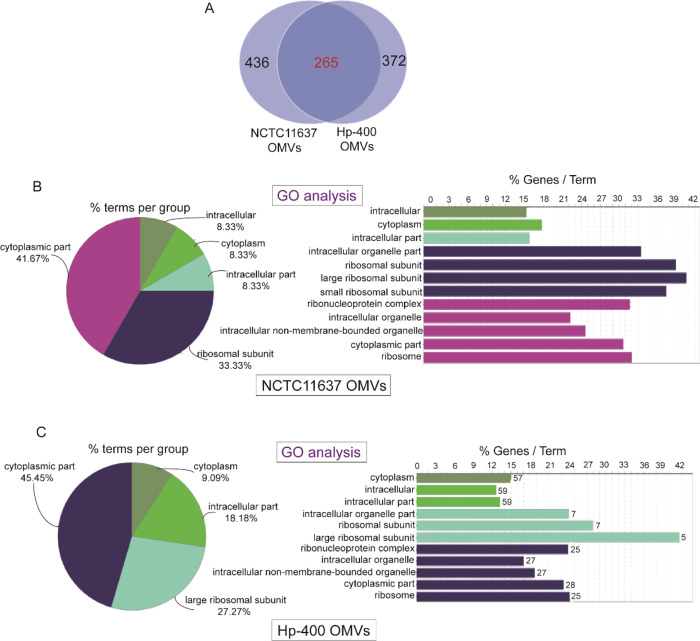
Next, we determined the protein contents of H. pylori OMVs to evaluate the mechanisms by which these components confer their immunomodulatory and cytotoxic activities to host cells, as these disease-associated activities are also transferred by the bacterium from which the vesicles are derived. We detected the protein contents of OMVs using HPLC-MS/MS analysis, and 436 proteins were found in NCTC11637 OMVs (Table 1) and 372 proteins in Hp-400 OMVs (Table 2). Although a significant overlap in the proteins identified between NCTC11637 and Hp-400 was observed, not all proteins appeared to be shared, suggesting that these two H. pylori strains have different genotypes (Figure 3A). The proteomic analysis illustrated the enrichment of membrane proteins, adhesins, porins, and several proteins known to regulate cell proliferation, cytokine secretion, and other host cellular processes in H. pylori OMVs. In addition, the OMVs contained the previously documented toxins cagA, vacA, and several OMV components possessing immunological activity, including urease, HpaA, OMP18, peptidyl-prolyl-cis-trans-isomerase, and gamma-glutamyl transpeptidase (Tables 1 and 2). Furthermore, the GO analysis revealed similar cellular components for the OMV contents in the two strains, mainly including the cytoplasmic part, such as the cytoplasm and cytomembrane (Figure 3B,C). Taken together, the H. pylori OMVs are equipped with the molecules required to interact with host cells in a manner similar to the intact pathogen.
Table 1. Protein Contents in NCTC11637 OMVs.
| number | accession | gene symbol | number | accession | gene symbol |
|---|---|---|---|---|---|
| 1 | P42383 | groL | 2 | P56003 | tuf |
| 3 | P69996 | ureB | 4 | P0A0S1 | flaA |
| 5 | P77872 | katA | 6 | O26107 | HP_1588 |
| 7 | P56002 | fusA | 8 | P55980 | cagA |
| 9 | O25905 | HP_1350 | 10 | P55994 | dnaK |
| 11 | P55987 | atpA | 12 | P56418 | acnB |
| 13 | O25242 | dnaN | 14 | P56063 | icd |
| 15 | P56112 | HP_0175 | 16 | P56185 | rnj |
| 17 | P55975 | tsf | 18 | P94845 | glnA |
| 19 | P55988 | atpD | 20 | P55969 | hpaA |
| 21 | O25806 | rpoBC | 22 | O25743 | Ggt |
| 23 | O25294 | pepA | 24 | O25017 | HP_0231 |
| 25 | P56116 | htpG | 26 | P55981 | vacA |
| 27 | O25284 | HP_0558 | 28 | P21762 | ahpC |
| 29 | P52093 | ftnA | 30 | P14916 | ureA |
| 31 | P71404 | clpB | 32 | O25011 | msrAB |
| 33 | P56149 | aspA | 34 | P0A0V0 | lpp20 |
| 35 | O25556 | Omp19 | 36 | P56088 | guaB |
| 37 | P56008 | rpsA | 38 | G2J5T2 | hp1018/19 |
| 39 | O25286 | FabG | 40 | P50610 | flgE |
| 41 | O25948 | Ald | 42 | Q07911 | flaB |
| 43 | O25750 | Omp18 | 44 | O06913 | frdA |
| 45 | O25325 | hemE | 46 | O25318 | HP_0596 |
| 47 | P43313 | dps | 48 | O25751 | tolB |
| 49 | O25349 | HydB | 50 | O25739 | HP_1111 |
| 51 | P56420 | tig | 52 | O26102 | pdxJ |
| 53 | O25883 | fumC | 54 | O25786 | GlnH |
| 55 | O25656 | PqqE | 56 | O25997 | HP_1461 |
| 57 | P56030 | rplB | 58 | P56036 | rplJ |
| 59 | O25749 | HP_1124 | 60 | O25546 | HP_0879 |
| 61 | O25321 | HylB | 62 | O25371 | YmxG |
| 63 | O24854 | ribH | 64 | P56126 | lysS |
| 65 | O24897 | PutA | 66 | O25570 | Omp20 |
| 67 | O25015 | Omp6 | 68 | O24993 | plsX |
| 69 | P56070 | ppsA | 70 | O25326 | HP_0605 |
| 71 | O25135 | HP_0371 | 72 | O25216 | PepF |
| 73 | O06914 | frdB | 74 | O25736 | HP_1108 |
| 75 | P56001 | rpoA | 76 | O25668 | CbpA |
| 77 | A0A0M3KL20 | C694_06140 | 78 | O26082 | CeuE |
| 79 | P56033 | rplE | 80 | O25311 | HP_0589 |
| 81 | P56062 | gltA | 82 | O24923 | HP_0097 |
| 83 | O24944 | HP_0130 | 84 | O25225 | typA |
| 85 | O25534 | pgbB | 86 | O25254 | hslU |
| 87 | O25995 | HP_1457 | 88 | P96786 | fliD |
| 89 | O26084 | HP_1564 | 90 | O25993 | HP_1454 |
| 91 | O25158 | HP_0397 | 92 | P56114 | gatA |
| 93 | P56109 | fba | 94 | O25825 | HP_1227 |
| 95 | O25856 | NQO3 | 96 | O34523 | Omp29 |
| 97 | O25927 | lpxA | 98 | O25134 | HP_0370 |
| 99 | O25399 | HP_0690 | 100 | P56029 | rplA |
| 101 | O25738 | HP_1110 | 102 | O25414 | HP_0710 |
| 103 | O25423 | HP_0721 | 104 | O25347 | HP_0630 |
| 105 | O25715 | HP_1083 | 106 | P56456 | ileS |
| 107 | P56145 | pheT | 108 | P56031 | rplC |
| 109 | O24914 | HP_0087 | 110 | O25732 | Cad |
| 111 | O25116 | pyrG | 112 | O25383 | HP_0672 |
| 113 | O25787 | HP_1173 | 114 | O25166 | HP_0410 |
| 115 | O25052 | AddB | 116 | O25465 | HP_0773 |
| 117 | O25008 | iscS | 118 | O25067 | amiE |
| 119 | P56060 | kdsA | 120 | O25658 | HdhA |
| 121 | O25936 | fbp | 122 | P55982 | nrdA |
| 123 | O25089 | HP_0322 | 124 | P66928 | trxA |
| 125 | P56047 | rplV | 126 | O24990 | fabI |
| 127 | P0A0R3 | groS | 128 | O26104 | FlgG |
| 129 | O25560 | hypB | 130 | O25571 | Omp21 |
| 131 | O25873 | HP_1286 | 132 | P56078 | rplY |
| 133 | O25608 | rdxA | 134 | O25410 | Omp15 |
| 135 | O24913 | mqo | 136 | O25312 | HP_0590 |
| 137 | P56004 | efp | 138 | O25925 | mreB |
| 139 | P56431 | trxB | 140 | O25327 | MtrC |
| 141 | P25177 | glmM | 142 | O25147 | HP_0385 |
| 143 | P56111 | edd | 144 | P56146 | pheS |
| 145 | O25731 | glk | 146 | O25820 | Dld |
| 147 | O25372 | gatB | 148 | P56034 | rplF |
| 149 | P42445 | recA | 150 | P56460 | metK |
| 151 | P56071 | thrS | 152 | O25088 | tatA |
| 153 | P56154 | pgk | 154 | P56458 | serS |
| 155 | O24922 | HP_0096 | 156 | O25009 | HP_0221 |
| 157 | P56032 | rplD | 158 | O24925 | TlpA |
| 159 | O24911 | TlpC | 160 | O26004 | ilvE |
| 161 | O25373 | HP_0659 | 162 | O24870 | Omp2 |
| 163 | Q09066 | ureG | 164 | O25776 | fldA |
| 165 | O25720 | TktA | 166 | O25079 | HP_0309 |
| 167 | O24924 | thrC | 168 | P56082 | atpG |
| 169 | O25503 | speE | 170 | P55972 | infB |
| 171 | P56457 | leuS | 172 | O25313 | HP_0591 |
| 173 | O25034 | Omp7 | 174 | P56155 | pyrF |
| 175 | P64655 | HP_0135 | 176 | P55995 | lon |
| 177 | O25872 | HP_1285 | 178 | O26075 | yajC |
| 179 | P66609 | rpsG | 180 | P94851 | HP_1488 |
| 181 | O25744 | HAP1 | 182 | O25140 | DsbC |
| 183 | O24947 | HP_0134 | 184 | Q48248 | cdh |
| 185 | O25151 | tpx | 186 | O25779 | TrxB |
| 187 | O25046 | HP_0267 | 188 | P71408 | ftsH |
| 189 | P55834 | rplL | 190 | O25018 | HP_0232 |
| 191 | P56046 | rplU | 192 | O25341 | AspB |
| 193 | P66328 | rpsJ | 194 | P56035 | rplI |
| 195 | O25597 | dadA | 196 | O25369 | bamA |
| 197 | O25389 | HP_0678 | 198 | P96551 | gltX1 |
| 199 | O25684 | HP_1043 | 200 | O24886 | fcl |
| 201 | O25671 | fur | 202 | P66572 | rpsE |
| 203 | P56069 | metB | 204 | O25607 | HP_0953 |
| 205 | O25029 | rhpA | 206 | O25756 | AtpH |
| 207 | O25530 | RfaD | 208 | P48285 | eno |
| 209 | P66052 | rplK | 210 | O25625 | HP_0973 |
| 211 | O25728 | hcpC | 212 | P56089 | glyA |
| 213 | O25729 | HP_1099 | 214 | O24976 | HP_0170 |
| 215 | P56007 | scoB | 216 | O25249 | pgbA |
| 217 | O25762 | HP_1143 | 218 | P56106 | pyrH |
| 219 | O25998 | HP_1462 | 220 | P56459 | aspS |
| 221 | O25068 | Fla | 222 | O24951 | HP_0139 |
| 223 | P66637 | rpsI | 224 | O25036 | Omp8 |
| 225 | P56191 | ddl | 226 | P56052 | rpmC |
| 227 | O25087 | hugZ | 228 | P48370 | gyrA |
| 229 | O25080 | pgdA | 230 | O25276 | Cag22 |
| 231 | O25157 | HP_0396 | 232 | O25773 | proC |
| 233 | O25996 | HP_1458 | 234 | O25424 | ansA |
| 235 | P56020 | rpsM | 236 | O25283 | accA |
| 237 | O25342 | ispG | 238 | O25442 | HP_0746 |
| 239 | P56038 | rplM | 240 | P56009 | rpsB |
| 241 | O25771 | Omp25 | 242 | P56011 | rpsD |
| 243 | O25001 | hcpA | 244 | P56041 | rplP |
| 245 | O24996 | HP_0204 | 246 | P66449 | rpsQ |
| 247 | O25164 | HP_0408 | 248 | O25681 | HP_1037 |
| 249 | Q48255 | aroQ | 250 | P56018 | rpsK |
| 251 | P0A0X4 | rpsL | 252 | O25791 | Omp27 |
| 253 | P56417 | tyrS | 254 | O25999 | HP_1463 |
| 255 | P56010 | rpsC | 256 | O25250 | GlcD |
| 257 | O25572 | HP_0914 | 258 | P56006 | scoA |
| 259 | O25176 | HP_0422 | 260 | O24999 | mrp |
| 261 | P56156 | clpP | 262 | O25360 | gltX2 |
| 263 | O26037 | HP_1507 | 264 | O25413 | HP_0709 |
| 265 | O25229 | HP_0485 | 266 | O25781 | pgi |
| 267 | O25564 | HP_0906 | 268 | P56039 | rplN |
| 269 | P56084 | atpC | 270 | O25673 | HP_1029 |
| 271 | O24949 | HP_0137 | 272 | O25452 | HP_0757 |
| 273 | O25553 | HP_0893 | 274 | O24865 | HP_0020 |
| 275 | O26035 | RibG | 276 | O25949 | HP_1399 |
| 277 | O25255 | HP_0518 | 278 | O26083 | CeuE |
| 279 | O25213 | HP_0466 | 280 | O25253 | hslV |
| 281 | O25006 | HP_0218 | 282 | P55992 | gyrB |
| 283 | O24950 | HP_0138 | 284 | O25280 | HP_0554 |
| 285 | P56141 | trpA | 286 | P56110 | zwf |
| 287 | O25076 | HP_0305 | 288 | O25926 | clpX |
| 289 | O25930 | bamD | 290 | P56045 | rplT |
| 291 | P56097 | ftsZ | 292 | O25899 | tonB |
| 293 | P56128 | argS | 294 | O25489 | HP_0809 |
| 295 | O25664 | ispDF | 296 | O25234 | HP_0492 |
| 297 | O25511 | pseB | 298 | O25737 | HP_1109 |
| 299 | P64653 | HP_0122 | 300 | O25516 | thiM |
| 301 | O25990 | HP_1451 | 302 | O25801 | asd |
| 303 | O25310 | HP_0588 | 304 | O24934 | HP_0112 |
| 305 | O25030 | HP_0248 | 306 | O24943 | HP_0129 |
| 307 | O24941 | Omp4 | 308 | P56086 | atpF |
| 309 | P56455 | hisS | 310 | P55970 | grpE |
| 311 | O25566 | HP_0908 | 312 | P56044 | rplS |
| 313 | O25524 | YheS | 314 | O25257 | Cag1 |
| 315 | O25982 | ppiA | 316 | O25470 | HP_0781 |
| 317 | O25992 | HP_1453 | 318 | O25931 | TyrA |
| 319 | P66185 | rpmE | 320 | P56162 | pyrE |
| 321 | O25853 | nuoD | 322 | P56067 | cysM |
| 323 | O24884 | HP_0043 | 324 | O25510 | OmpP1 |
| 325 | O25421 | HP_0719 | 326 | P56075 | ndk |
| 327 | P55976 | nusG | 328 | O24991 | lpxD |
| 329 | P56396 | trpS | 330 | O25019 | HP_0233 |
| 331 | O25529 | hldE | 332 | O25614 | gpsA |
| 333 | P43312 | sodB | 334 | O26067 | HP_1542 |
| 335 | P56021 | rpsZ | 336 | O25565 | FlgD |
| 337 | O25858 | nuoI | 338 | O25759 | Soj |
| 339 | O25584 | surE | 340 | P66119 | rplW |
| 341 | P56000 | valS | 342 | P55971 | gapA |
| 343 | O25362 | Slt | 344 | P55985 | truD |
| 345 | O25758 | parB | 346 | O25782 | HP_1167 |
| 347 | O25343 | dapD | 348 | O25032 | OppD |
| 349 | O25956 | bioB | 350 | O26094 | RibC |
| 351 | O25686 | acsA | 352 | O25748 | slyD |
| 353 | O25953 | HsdM | 354 | P66621 | rpsH |
| 355 | Q59465 | cadA | 356 | O25277 | Cag24 |
| 357 | O25171 | Cfa | 358 | P56124 | proS |
| 359 | P56040 | rplO | 360 | P55979 | bcp |
| 361 | P56195 | deoB | 362 | O25913 | HP_1359 |
| 363 | O25521 | HsdM | 364 | O25132 | HP_0368 |
| 365 | O25757 | AtpF′ | 366 | O25896 | HP_1338 |
| 367 | O25525 | guaC | 368 | O25121 | dxs |
| 369 | O25549 | ruvA | 370 | P56104 | adk |
| 371 | O25293 | ychF | 372 | O25595 | alr |
| 373 | P56737 | trpD | 374 | O26103 | pdxA |
| 375 | P56137 | purA | 376 | P56452 | alaS |
| 377 | P94842 | ybgC | 378 | O24885 | gmd |
| 379 | O24864 | CheV | 380 | P56184 | prs |
| 381 | O25475 | secA | 382 | O25477 | HP_0788 |
| 383 | O24973 | OmpR | 384 | O26096 | metN |
| 385 | P56157 | dnaE | 386 | O24890 | HP_0049 |
| 387 | O26064 | HP_1539 | 388 | O25533 | coaX |
| 389 | P56115 | hemL | 390 | O25577 | carB |
| 391 | P56176 | nnr | 392 | O25335 | HP_0614 |
| 393 | P56153 | ppa | 394 | O25469 | HP_0780 |
| 395 | O25624 | HP_0971 | 396 | O25376 | hemN |
| 397 | O25929 | fliW2 | 398 | O25281 | HP_0555 |
| 399 | O25233 | HP_0490 | 400 | O25398 | HP_0689 |
| 401 | O25435 | Gpt | 402 | O24994 | fabH |
| 403 | O25136 | dcd | 404 | P56022 | rpsO |
| 405 | O26074 | secD | 406 | P56074 | hemB |
| 407 | O25382 | Omp14 | 408 | O25991 | mnmE |
| 409 | O25945 | Omp30 | 410 | P55990 | gdhA |
| 411 | P56028 | rpsU | 412 | O25430 | HP_0730 |
| 413 | O25696 | HP_1056 | 414 | O25122 | lepA |
| 415 | O25390 | WbpB | 416 | P56127 | metG |
| 417 | O25348 | HydA | 418 | P56142 | trpB |
| 419 | O25902 | Gap | 420 | P56122 | aroC |
| 421 | O25849 | cobB | 422 | O24956 | FixO |
| 423 | O25308 | HP_0586 | 424 | O25484 | ribB |
| 425 | O25594 | YckK | 426 | O25817 | purD |
| 427 | O25500 | Lex2B | 428 | P56131 | miaB |
| 429 | O25055 | GppA | 430 | O25195 | HP_0447 |
| 431 | O25912 | HP_1358 | 432 | O25142 | HP_0379 |
| 433 | O25363 | HP_0646 | 434 | O25082 | HP_0312 |
| 435 | O25509 | HP_0838 | 436 | O25278 | Cag25 |
Table 2. Protein Contents in Hp-400 OMVs.
| number | accession | gene symbol | number | accession | gene symbol |
|---|---|---|---|---|---|
| 1 | P42383 | groL | 2 | P56003 | tuf |
| 3 | P69996 | ureB | 4 | P77872 | katA |
| 5 | O25806 | rpoBC | 6 | G2J5T2 | hp1018/19 |
| 7 | O26107 | HP_1588 | 8 | O24870 | Omp2 |
| 9 | P55987 | atpA | 10 | O25743 | HP_1118 |
| 11 | P56418 | acnB | 12 | P55975 | tsf |
| 13 | P56063 | icd | 14 | O25011 | msrAB |
| 15 | P0A0V0 | lpp20 | 16 | P71404 | clpB |
| 17 | P55969 | hpaA | 18 | P55994 | dnaK |
| 19 | O25905 | HP_1350 | 20 | O25286 | HP_0561 |
| 21 | O25751 | tolB | 22 | P94845 | glnA |
| 23 | O25242 | dnaN | 24 | P55988 | atpD |
| 25 | O25791 | Omp27 | 26 | A0A0M3KL20 | C694_06140 |
| 27 | P56002 | fusA | 28 | O25017 | HP_0231 |
| 29 | O25311 | HP_0589 | 30 | O25825 | HP_1227 |
| 31 | O25321 | HP_0599 | 32 | O25294 | pepA |
| 33 | O26083 | HP_1562 | 34 | O25423 | HP_0721 |
| 35 | P14916 | ureA | 36 | O06913 | frdA |
| 37 | O25732 | HP_1104 | 38 | O25284 | HP_0558 |
| 39 | O25052 | AddB | 40 | P56008 | rpsA |
| 41 | O25015 | Omp6 | 42 | O25786 | GlnH |
| 43 | O24925 | TlpA | 44 | P56112 | HP_0175 |
| 45 | O25749 | HP_1124 | 46 | O26084 | HP_1564 |
| 47 | P56456 | ileS | 48 | P50610 | flgE |
| 49 | O25840 | Omp28 | 50 | P21762 | ahpC |
| 51 | P55981 | vacA | 52 | P52093 | ftnA |
| 53 | P56036 | rplJ | 54 | O25993 | HP_1454 |
| 55 | P56149 | aspA | 56 | O25312 | HP_0590 |
| 57 | O25883 | fumC | 58 | O25216 | PepF |
| 59 | O25147 | HP_0385 | 60 | O25656 | PqqE |
| 61 | O25997 | HP_1461 | 62 | P56062 | gltA |
| 63 | O25738 | HP_1110 | 64 | O25414 | HP_0710 |
| 65 | P56185 | rnj | 66 | O25736 | HP_1108 |
| 67 | P56145 | pheT | 68 | O26082 | CeuE |
| 69 | P56155 | pyrF | 70 | O24968 | pyrF |
| 71 | O26102 | pdxJ | 72 | O25995 | HP_1457 |
| 73 | O25927 | lpxA | 74 | O25750 | Omp18 |
| 75 | O24944 | HP_0130 | 76 | O25157 | HP_0396 |
| 77 | O24923 | HP_0097 | 78 | O25872 | HP_1285 |
| 79 | O25046 | HP_0267 | 80 | P56116 | htpG |
| 81 | O24922 | HP_0096 | 82 | O25729 | Eda |
| 83 | O25992 | HP_1453 | 84 | O25158 | HP_0397 |
| 85 | O25597 | dadA | 86 | O24993 | plsX |
| 87 | O25055 | GppA | 88 | O25510 | OmpP1 |
| 89 | O25229 | HP_0485 | 90 | P55982 | nrdA |
| 91 | O25556 | Omp19 | 92 | P66928 | trxA |
| 93 | P55993 | rpoD | 94 | O25402 | HyuA |
| 95 | O25135 | HP_0371 | 96 | O25349 | HydB |
| 97 | O25728 | hcpC | 98 | P0A0R3 | groS |
| 99 | P43313 | dps | 100 | P56070 | ppsA |
| 101 | O25757 | AtpF′ | 102 | O26042 | FrpB |
| 103 | O25739 | HP_1111 | 104 | O25326 | HP_0605 |
| 105 | O25371 | YmxG | 106 | O25225 | typA |
| 107 | O06914 | frdB | 108 | P56030 | rplB |
| 109 | O25088 | tatA | 110 | P56060 | kdsA |
| 111 | P56047 | rplV | 112 | P55980 | cagA |
| 113 | P56111 | edd | 114 | O25313 | HP_0591 |
| 115 | O25045 | pyrC′ | 116 | P56420 | tig |
| 117 | O25776 | fldA | 118 | O25257 | Cag1 |
| 119 | P56088 | guaB | 120 | O25771 | Omp25 |
| 121 | O25936 | fbp | 122 | O25176 | HP_0422 |
| 123 | P56007 | scoB | 124 | O25948 | Ald |
| 125 | P56078 | rplY | 126 | P56431 | trxB |
| 127 | O25570 | Omp20 | 128 | O25756 | AtpH |
| 129 | O25399 | FadA | 130 | P25177 | glmM |
| 131 | O25715 | HP_1083 | 132 | O25787 | HP_1173 |
| 133 | P56034 | rplF | 134 | O25658 | HdhA |
| 135 | O25607 | HP_0953 | 136 | O25369 | bamA |
| 137 | P56110 | zwf | 138 | O25318 | HP_0596 |
| 139 | O25562 | HP_0902 | 140 | O25076 | HP_0305 |
| 141 | O26071 | HP_1546 | 142 | O25625 | HP_0973 |
| 143 | O24996 | HP_0204 | 144 | P56001 | rpoA |
| 145 | O25410 | Omp15 | 146 | O25325 | hemE |
| 147 | O25781 | pgi | 148 | O25153 | CheA |
| 149 | O26067 | HP_1542 | 150 | O24913 | mqo |
| 151 | O24947 | HP_0134 | 152 | O25403 | HP_0696 |
| 153 | P56154 | pgk | 154 | O25465 | HP_0773 |
| 155 | P42445 | recA | 156 | O24914 | HP_0087 |
| 157 | O24911 | TlpC | 158 | O24897 | PutA |
| 159 | O25327 | MtrC | 160 | O25534 | pgbB |
| 161 | P56146 | pheS | 162 | O25442 | HP_0746 |
| 163 | O25773 | proC | 164 | O25742 | HP_1117 |
| 165 | O25503 | speE | 166 | O24881 | HP_0040 |
| 167 | P56458 | serS | 168 | O25372 | gatB |
| 169 | O24950 | HP_0138 | 170 | O25373 | HP_0659 |
| 171 | O25998 | HP_1462 | 172 | P56114 | gatA |
| 173 | O25546 | HP_0879 | 174 | O25668 | CbpA |
| 175 | P56067 | cysM | 176 | O25469 | HP_0780 |
| 177 | O25249 | pgbA | 178 | O25069 | DppA |
| 179 | O25873 | HP_1286 | 180 | O25230 | HP_0486 |
| 181 | O24909 | HP_0080 | 182 | P56106 | pyrH |
| 183 | O25470 | HP_0781 | 184 | P56046 | rplU |
| 185 | P56126 | lysS | 186 | O25458 | ftsY |
| 187 | P56082 | atpG | 188 | P56075 | ndk |
| 189 | O25273 | Cag19 | 190 | P56006 | scoA |
| 191 | O25140 | DsbC | 192 | O25926 | clpX |
| 193 | P56109 | fba | 194 | P56460 | metK |
| 195 | O25134 | HP_0370 | 196 | O25213 | HP_0466 |
| 197 | Q48248 | cdh | 198 | P56127 | metG |
| 199 | P64655 | HP_0135 | 200 | P56052 | rpmC |
| 201 | O26091 | rlpA | 202 | P56031 | rplC |
| 203 | Q09066 | ureG | 204 | O25018 | HP_0232 |
| 205 | O25902 | Gap | 206 | P56457 | leuS |
| 207 | O24929 | TlpB | 208 | O25009 | NifU |
| 209 | O25424 | ansA | 210 | O26031 | Omp32 |
| 211 | O25564 | HP_0906 | 212 | P56104 | adk |
| 213 | O25477 | HP_0788 | 214 | O25475 | secA |
| 215 | P56035 | rplI | 216 | O25474 | lolA |
| 217 | O25165 | guaA | 218 | O25283 | accA |
| 219 | P56468 | purB | 220 | O25574 | FrpB |
| 221 | O24924 | thrC | 222 | O25572 | HP_0914 |
| 223 | P56004 | efp | 224 | P56032 | rplD |
| 225 | O25452 | HP_0757 | 226 | P56455 | hisS |
| 227 | O25508 | HP_0837 | 228 | P56137 | purA |
| 229 | O25036 | Omp8 | 230 | O25925 | mreB |
| 231 | O25594 | YckK | 232 | P56459 | aspS |
| 233 | O24949 | HP_0137 | 234 | O25999 | HP_1463 |
| 235 | P56018 | rpsK | 236 | O25820 | Dld |
| 237 | P56029 | rplA | 238 | O25255 | HP_0518 |
| 239 | O24863 | HP_0018 | 240 | O25087 | hugZ |
| 241 | O26037 | HP_1507 | 242 | P56084 | atpC |
| 243 | O24930 | CpdB | 244 | P56020 | rpsM |
| 245 | O25368 | mqnE | 246 | P55970 | grpE |
| 247 | O25218 | Omp11 | 248 | P64653 | HP_0122 |
| 249 | O25234 | HP_0492 | 250 | O25383 | HP_0672 |
| 251 | O25762 | HP_1143 | 252 | P66637 | HP_1143 |
| 253 | O26039 | plsY | 254 | O24864 | CheV |
| 255 | O26052 | HP_1524 | 256 | O25073 | DppF |
| 257 | O24999 | mrp | 258 | O25684 | HP_1043 |
| 259 | P56041 | rplP | 260 | O25288 | HP_0564 |
| 261 | P56039 | rplN | 262 | O25930 | BamD |
| 263 | O24951 | HP_0139 | 264 | O25089 | HP_0322 |
| 265 | O25426 | HP_0726 | 266 | O25116 | pyrG |
| 267 | O25256 | HP_0519 | 268 | P94844 | dapB |
| 269 | P66052 | rplK | 270 | O25573 | FrpB |
| 271 | O25856 | NQO3 | 272 | O25713 | HP_1081 |
| 273 | O25276 | Cag22 | 274 | O25362 | Slt |
| 275 | O25355 | Omp13 | 276 | O25090 | Nuc |
| 277 | O25472 | HP_0783 | 278 | P56089 | glyA |
| 279 | P64649 | HP_0031 | 280 | P56033 | rplE |
| 281 | O24854 | ribH | 282 | O24941 | Omp4 |
| 283 | O34523 | Omp29 | 284 | O25770 | murG |
| 285 | O25595 | alr | 286 | O25336 | ligA |
| 287 | O25489 | HP_0809 | 288 | P56044 | rplS |
| 289 | O26004 | ilvE | 290 | P55971 | gapA |
| 291 | P56069 | metB | 292 | O25289 | HP_0565 |
| 293 | P56086 | atpF | 294 | P48285 | eno |
| 295 | O25152 | CheW | 296 | O24946 | SdaC |
| 297 | P56197 | aroA | 298 | O25297 | HP_0573 |
| 299 | O25397 | HP_0688 | 300 | O25990 | HP_1451 |
| 301 | O24865 | HP_0020 | 302 | O25343 | dapD |
| 303 | O87326 | trl | 304 | O25507 | HP_0836 |
| 305 | O25171 | Cfa | 306 | O25681 | HP_1037 |
| 307 | O24871 | HP_0028 | 308 | O25161 | HP_0405 |
| 309 | P94851 | HP_1488 | 310 | O25072 | DppD |
| 311 | O25029 | rhpA | 312 | O25571 | Omp21 |
| 313 | O25530 | RfaD | 314 | O25696 | HP_1056 |
| 315 | O25671 | fur | 316 | O25144 | YJR117W |
| 317 | P55995 | lon | 318 | O25079 | HP_0309 |
| 319 | O25560 | hypB | 320 | O26096 | metN |
| 321 | O25509 | HP_0838 | 322 | O25612 | HP_0958 |
| 323 | P66119 | rplW | 324 | O25512 | CoaBC |
| 325 | O25296 | apt | 326 | O25032 | OppD |
| 327 | P56061 | panC | 328 | O25281 | HP_0555 |
| 329 | O25748 | slyD | 330 | O25484 | ribB |
| 331 | P66328 | rpsJ | 332 | O25250 | GlcD |
| 333 | O25337 | CheV | 334 | O25584 | surE |
| 335 | P56467 | folD | 336 | O24886 | fcl |
| 337 | O26075 | yajC | 338 | P56072 | sdaA |
| 339 | P56011 | rpsD | 340 | O25772 | Omp26 |
| 341 | O24991 | lpxD | 342 | O25166 | HP_0410 |
| 343 | O25039 | xseA | 344 | P55972 | infB |
| 345 | O25382 | Omp14 | 346 | O25348 | HydA |
| 347 | P56191 | ddl | 348 | P56153 | ppa |
| 349 | O25413 | HP_0709 | 350 | O25714 | MsbA |
| 351 | O25673 | HP_1029 | 352 | O25991 | mnmE |
| 353 | P66572 | rpsE | 354 | P55834 | rplL |
| 355 | P56000 | valS | 356 | P55992 | gyrB |
| 357 | P56040 | rplO | 358 | O25945 | Omp30 |
| 359 | O25535 | HP_0864 | 360 | O25008 | iscS |
| 361 | P55976 | nusG | 362 | P55986 | HP_1459 |
| 363 | O25347 | Mda66 | 364 | P56038 | rplM |
| 365 | O25274 | Cag20 | 366 | O25151 | tpx |
| 367 | O25852 | HP_1262 | 368 | P56022 | rpsO |
| 369 | P56156 | clpP | 370 | O25931 | TyrA |
| 371 | O25614 | gpsA | 372 | P56097 | ftsZ |
Figure 3.
Identification of protein contents of H. pylori OMVs. (A) Proteins in NCTC11637 and Hp-400 detected by HPLC-MS/MS. (B) Cellular components of NCTC11637 OMV contents revealed by GO analysis. (C) Cellular components of Hp-400 OMV contents revealed by GO analysis.
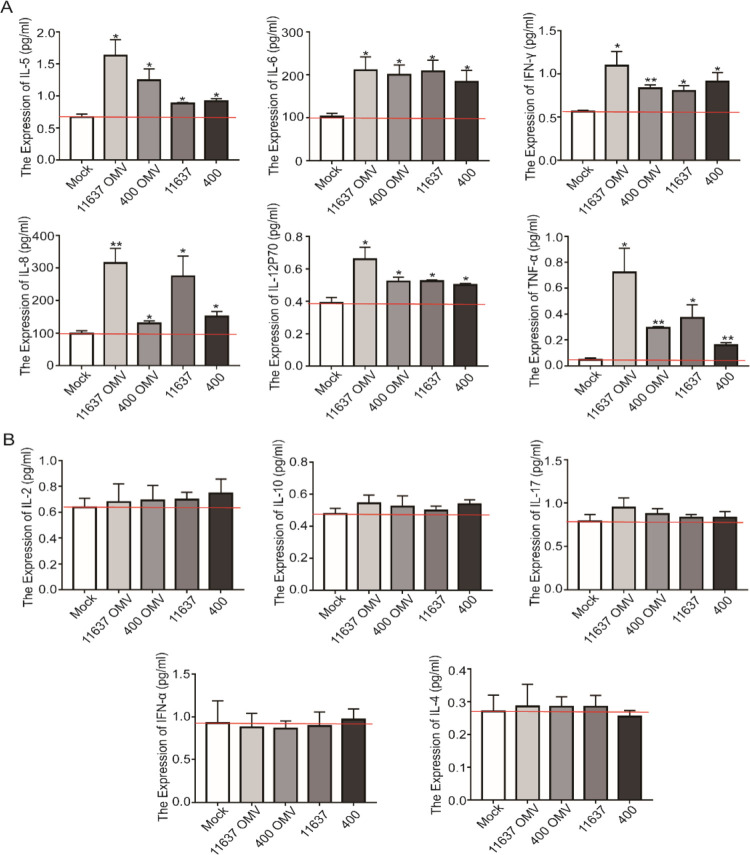
H. pylori OMVs Promoted the Secretion of Inflammatory Factors of GES1 Cells
H. pylori colonizes the gastric mucosa and causes acute and chronic gastritis accompanied by a chronic pro-inflammatory environment, and thus the inflammatory response is the main characteristic of H. pylori infection. As shown above, OMVs contain a variety of virulence factors; therefore, we hypothesized that OMVs induce inflammation similar to the bacterium from which they are derived. Inflammatory factors were detected in the cultured supernatant of GES1 cells cocultured with OMVs (40 μg) or H. pylori (50:1). The levels of secreted IL-5, IL-6, IFN-γ, IL-8, IL-12P70, and TNF-α were significantly increased when cells were cocultured with either OMVs or H. pylori (Figure 4A). In particular, IL-6, IL-8, and TNF-α play an important role in the activation of neutrophils and lymphocytes and the induction of T cell activation, proliferation, and differentiation. The levels of other inflammatory factors, IL-2, IL-10, and IL-17, were also slightly increased (Figure 4B), although the difference was not significant. Additionally, we did not observe a difference in cytokine levels between H. pylori-treated cells and OMV-treated cells, suggesting that H. pylori induced an inflammatory response mainly through OMVs.
Figure 4.
H. pylori and OMVs induced secretion of inflammatory factors. (A) Inflammatory factors, IL-5, IL-6, IFN-γ, IL-8, IL-12P70, and TNF-α, in the cultural supernatant were detected by flow cytometry. (B) Inflammatory factors, IL-2, IL-10, IL-17, IFN-α, and IL-4, the in cultural supernatant were detected by flow cytometry. *p < 0.05, **p < 0.01.
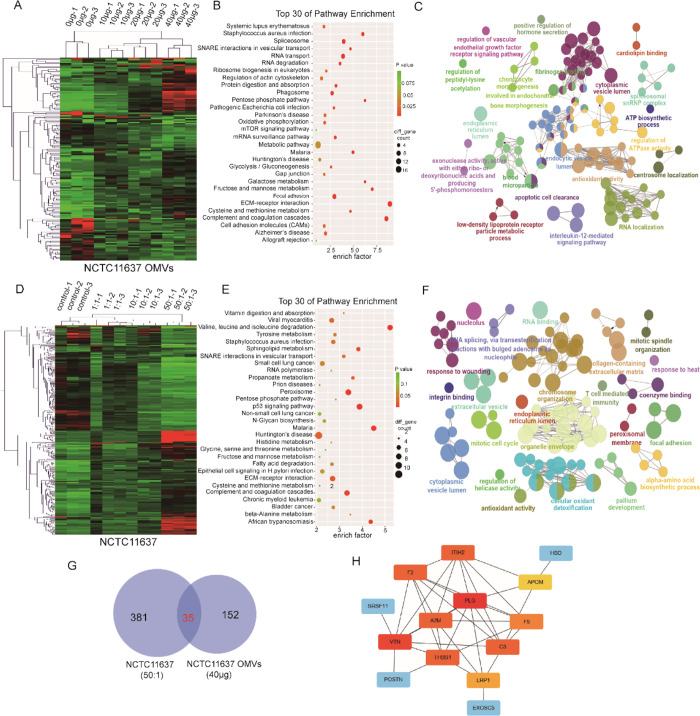
The Proteomic Changes in GES1 Cells Cocultured with NCTC11637 OMVs Were Consistent with Those of GES1 Cells Cocultured with the NCTC11637 Strain
GES1 cells were cocultured with increasing concentrations of OMVs (0, 10, 20, or 40 μg) or bacteria (control, 1:1, 10:1, or 50:1) and subjected to quantitative proteomic analyses using label-free methods for relative and absolute quantitation to further define the effects of NCTC11637 OMVs and the parental strain on gastric epithelial cells. A total of 4261 proteins were quantified in GES1 cells cocultured with OMVs, 79, 128, and 153 of which were markedly changed (|fold change| > 2) in abundance after treatment with 10, 20, and 40 μg of OMVs, respectively, compared to control samples (Figure 5A and Supporting information Table S1); we described the difference in the proteome of cells treated with OMVs (40 μg). KEGG and GO analyses were next used to find biologically relevant canonical signaling pathways that were significantly altered by OMVs. In the KEGG pathway analysis, RNA transport and degradation, oxidative phosphorylation, metabolism, tight junctions, cytoskeleton, and extracellular matrix signaling were significantly altered (Figure 5B). In the GO analysis, including biological processes, cellular components and molecular functions, IL-12 signaling pathways, VEGF receptor pathway, antioxidant activity, apoptosis, and other terms were dramatically altered (Figure 5C). These pathways are related to immune regulation and carcinogenesis.
Figure 5.
Proteomic changes of GES1 infected by NCTC11637 OMVs and bacteria. (A) Heat map showing differentially expressed proteins in different groups of GES1 cocultured with increasing NCTC11637 OMVs (0, 10, 20, and 40 μg/well). (B) KEGG analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 40 μg of NCTC11637 OMVs. (C) GO analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 40 μg of NCTC11637 OMVs. (D) Heat map showing differentially expressed proteins in different groups of GES1 cocultured with increasing NCTC11637 (0, 1:1, 10:1, and 50:1). (E) KEGG analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 50:1 NCTC11637. (F) GO analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 50:1 NCTC11637. (G) Venn diagram revealed the overlapped proteins between differentially expressed proteins in GES1 infected by NCTC11637 and OMVs. (H) Top 10 hub genes of the overlapped proteins in panel G.
Similarly, we identified 4360 proteins in GES1 cells cocultured with the NCTC11637 strain; 53, 165, and 367 proteins displayed significantly altered abundance (|fold change| > 2) after infection with the bacteria at multiplicities of infection (MOIs) of 1:1, 10:1, and 50:1, respectively, compared to uninfected samples (Figure 5D and Supporting information Table S2). Therefore, we analyzed the proteome of infected GES1 cells in the 50:1 group. The KEGG analysis showed significant changes in amino acid metabolism, p53 signaling pathway, ECM-receptor interaction, and epithelial cell signaling in response to the H. pylori infection (Figure 5E). The GO analysis revealed that T cell-mediated immunity, integrin binding, mitotic cell cycle, antioxidant activity, and chromosome organization were dramatically altered (Figure 5F) in response to NCTC11637 infection.
In addition, 35 proteins overlapped between the proteomes of GES1 cells infected with NCTC11637 and OMVs (Figure 5G). Furthermore, the top 10 hub genes were screened (Figure 5H). Taken together, these results revealed that NCTC11637 OMVs led to changes in the GES1 cell proteome and the altered pathways mapped to the donor bacteria.
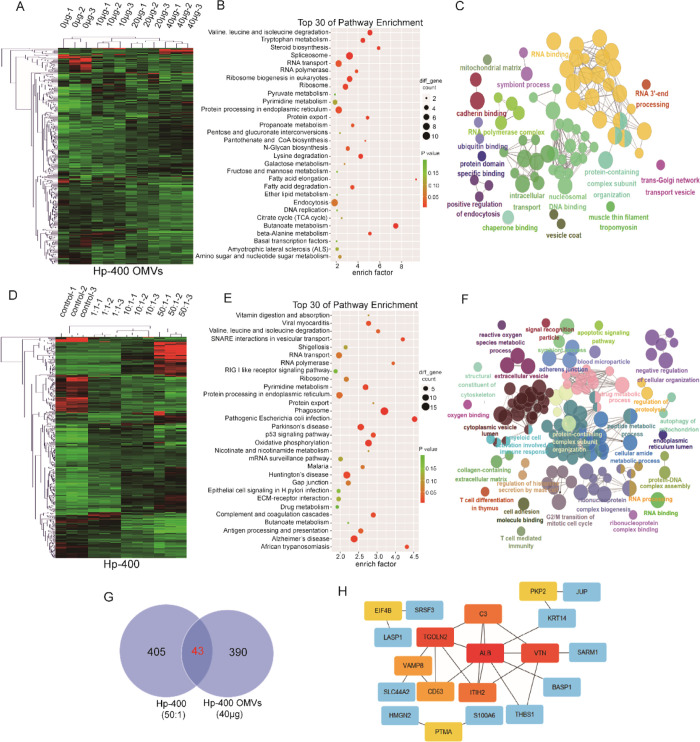
The Proteomic Changes in GES1 Cells Cocultured with Hp-400 OMVs Were in Accordance with Those of GES1 Cells Cocultured with Hp-400
Another H. pylori strain, Hp-400, was used to detect proteomic changes in cells cocultured with OMVs or the H. pylori strain and to further confirm our hypothesis that OMVs play vital roles in H. pylori-treated GES1 cells. Hp-400 is a clinical strain isolated from northern China, where the incidence of gastric cancer is high. Consistent with our hypothesis, the quantitative proteomic analysis verified a total of 4234 proteins in GES1 cells cocultured with Hp-400 OMVs, 303, 236, and 390 of which exhibited significantly altered (|fold change| > 1.5) abundance following infection with 10, 20, and 40 μg of Hp-400 OMVs, respectively, compared to uninfected samples (Figure 6A and Supporting information Table S3). Consistent with the aforementioned findings, we described the GES1 proteomic change induced by OMVs (40 μg). The KEGG analysis revealed marked changes in several pathways, including amino acid metabolism, spliceosome, RNA process, and protein exporting (Figure 6B). The GO analysis showed significant changes in cadherin binding, endocytosis, mitochondrial matrix, and ubiquitin binding pathways in GES1 cells cultured with 40 μg of OMVs (Figure 6C).
Figure 6.
Proteomic changes of GES1 infected by Hp-400 OMVs and bacteria. (A) Heat map showing differentially expressed proteins in different groups of GES1 cocultured with increasing Hp-400 OMVs (0, 10, 20, and 40 μg/well). (B) KEGG analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 40 μg of Hp-400 OMVs. (C) GO analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 40 μg of Hp-400 OMVs. (D) Heat map showing differentially expressed proteins in different groups of GES1 cocultured with increasing Hp-400 (0, 1:1, 10:1, and 50:1). (E) KEGG analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 50:1 Hp-400. (F) GO analysis of differentially expressed proteins in GES1 control and GES1 cocultured with 50:1 Hp-400. (G) Venn diagram revealed the overlapped proteins between differentially expressed proteins in GES1 infected by Hp-400 and OMVs. (H) Top 10 hub genes of the overlapped proteins in panel G.
We also detected proteomic changes in GES1 cells cocultured with Hp-400 cells. A total of 4406 proteins were identified, and 243, 307, and 405 proteins were significantly changed (|fold change| > 2) in abundance after infection with Hp-400 at MOIs of 1:1, 10:1, and 50:1, respectively, compared to uninfected samples (Figure 6D and Supporting information Table S4). We characterized changes in the GES1 cell proteome after infection with 50:1 Hp-400. The KEGG analysis showed significant changes in oxidative phosphorylation, phagosome, pyrimidine metabolism, and p53 signaling pathways (Figure 6E). In addition, the GO analysis showed that T cell-mediated immunity, apoptosis, protein–DNA complex assembly, and the cell cycle were altered (Figure 6F).
Certain pathways altered by Hp-400 OMVs were also changed in response to Hp-400, including adhesion molecules, RNA polymerase, protein processing in the endoplasmic reticulum, and RNA processing. Forty-three proteins overlapped between Hp-400 OMV- and Hp-400-infected GES1 cells (Figure 6G), of which the top 10 hub genes were screened (Figure 6H). These results further indicated that H. pylori affected the proteomes of gastric epithelial cells partially by secreting OMVs.
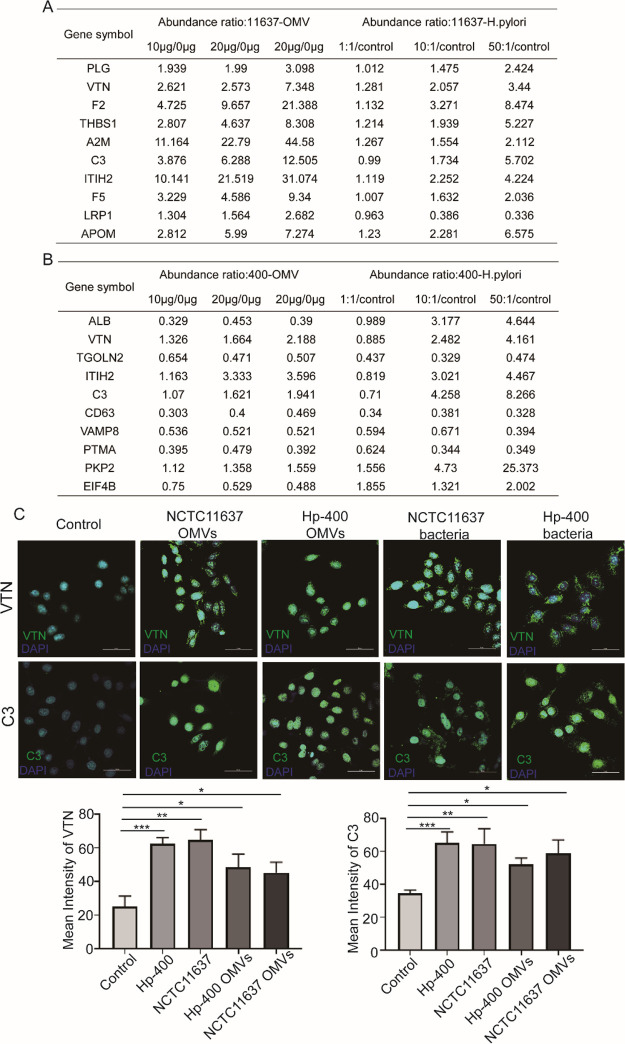
OMVs and H. pylori Mediated the Upregulation of VTN and C3 in GES1 Cells
We integrated the top 10 hub genes shown in Figures 5H and 6H to confirm the common markers of H. pylori and OMV infection. Furthermore, we screened the expression abundance of these proteins and found that the levels of most proteins increased progressively with the increase in the concentrations of OMVs or H. pylori (Figure 7A,B). Among these proteins, VTN and C3 were both elevated in response to treatments with OMVs and H. pylori strains. Hence, we detected the expression of VTN and C3 using laser scanning confocal microscopy (LSCM). Both OMVs and H. pylori promoted VTN and C3 expressions (Figure 7C). Taken together, we revealed that VTN and C3 were the pathogenic targets of H. pylori on gastric epithelium cells by secreting OMVs.
Figure 7.
Screening and verification of the hub genes altered both by H. pylori and OMVs. (A) Actual expression of hub genes in NCTC11637 and OMV proteomic data. (B) Actual expression of hub genes in Hp-400 and OMV proteomic data. (C) Immunofluorescence analysis of VTN and C3 in GES1 infected by NCTC11637 or Hp-400 or their OMVs. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
In the study, we aimed to demonstrate the pathogenicity of H. pylori primarily through secretion of OMVs. OMVs are released by kinds of Gram-negative bacteria and contain proteins, DNA, toxins, peptidoglycan, and lipids, which play roles in the infection process, including helping to build a colonization niche22 and the delivery of virulence factors and toxins to host cells.23 We isolated OMVs using size exclusion chromatography (SEC) and identified 436 proteins in NCTC11637 OMVs and 372 proteins in Hp-400 OMVs. The global proteomic analysis of H. pylori OMVs illustrated that there were a variety of proteins in OMVs, including well-known toxin proteins of H. pylori, which further emphasized the crucial contribution of OMVs to mediate pathogenesis in the host. Several main toxin factors were detected in H. pylori OMVs, such as vacA and cagA. The vacA gene is conserved among all H. pylori strains, which has the ability to induce cell vacuolation. CagA is a strain-specific H. pylori gene that is considered a marker for strains that lead to a high risk of gastric cancer. The delivery of Cag A protein is mainly through the bacterial type four secretion system, which causes a direct effect on epithelial cells, including disrupting cell signaling pathways and cell polarity.24−26
H. pylori is reported to have a high degree of genomic diversity because of high frequencies of mutation and recombination.27−30 Recently, Furuta et al. reported multi-locus sequence typing and whole genome sequence analyses of very closely related H. pylori strains from the same family members consisting of parents and children in Japan, suggesting adaptation to a new host through mutations in virulence-related genes, restriction-modification genes, and OMP genes.31,32 In our study, NCTC11637 was the standard strain, and Hp-400 was isolated from gastric tissues from patients in Hebei Province, an area with a high incidence of gastric cancer. These strains induced similar but not identical proteomic changes in GES1 cells, indicating that different strains have different pathogenic mechanisms. The results suggested that precise individualized treatment is necessary in clinical applications.
OMVs serve as vehicles for toxin delivery into host cells to promote bacterial pathogenicity and induce an inflammatory response. In this study, we mimicked the in vivo interaction between H. pylori or OMVs and the gastric mucosa through the coculture of GES1 cells and H. pylori or OMVs. Inflammatory factors were detected using flow cytometry; we demonstrated that OMVs contribute, at least in part, to driving a robust inflammatory response in gastric epithelial cells. A number of cytokines are elevated when infected by OMVs and H. pylori. For example, IL-8 is a potential neutrophil chemoattractant and activating factor that mediates strong pro-inflammatory responses. IL-8 levels are increased by H. pylori infection in a cag-dependent manner,33 and polymorphisms in IL-8 are associated with increased risks of chronic atrophic gastritis and gastric cancer.34,35 IL-6 is a significant mediator of inflammation that promotes a Th17-mediated inflammatory response. IL-6 expression is associated with the disease status among patients with H. pylori-associated gastritis36 and gastric cancer.37 TNF-α is a cytokine involved in systemic inflammation and the Th1 response, and TNF levels are increased in patients with H. pylori-associated gastritis.36
In addition to altering inflammatory signaling pathways, H. pylori has also been shown to disrupt cellular junctional complexes38 and induce cytoskeletal rearrangements that are suggestive of the uncontrolled growth induced by growth factors.39H. pylori has also been shown to disrupt the balance between gastric epithelial cell proliferation and apoptosis.40 However, the molecular mechanism of virulence factor delivery via OMVs has been unclear. We speculated that the main function of OMVs is to mimic parental pathogens and induce pathological damage. In addition to the well-established secretion systems, OMVs have been recently considered a new independent secretion system. Many “well-known” virulence factors and toxins have been identified that use OMVs as an alternative secretory pathway. OMVs provide unique advantages compare to other secretion systems by transporting high concentrations of proteins and delivering them to target destinations over long distances. Transmission of bacterial proteins by OMVs into host cells appears to be an important aspect in pathogens. In our study, disease pathways and networks induced by OMVs are directly related to gastrointestinal injury, disease, and development of cancer. These described pathways and networks will allow future functional analyses of specific proteomic targets that have been previously uncharacterized with response to either H. pylori infection or gastric carcinogenesis but now may play an important role in the development of gastric injury and cancer. A more thorough understanding of these networks will enable the exploitation of targetable pathways and effectors for clinical benefits and disease prevention.
VTN and complement C3 are two proteins that were detected through label-free mapping and were upregulated upon treatments with H. pylori and OMVs from both NCTC11637 and Hp-400. These targets were validated by LSCM, and the data were consistent with the HPLC-MS/MS results. VTN has not been previously identified to be associated with H. pylori infection. However, it has been previously shown to promote gastric cancer cell growth and motility in vitro and in vivo. In addition, VTN was also identified as a factor contributing to a poor prognosis of gastric cancer.41 In contrast, complement C3 has been reported to be activated directly by H. pylori,(42) and overexpression of complement C3 correlates with gastric cancer progression by activating the JAK2/STAT3 pathway.43
In conclusion, by utilizing proteomic approaches and pathway analyses, we were able to define proteomic changes in GES1 cells in response to H. pylori or OMVs infection. These data mirrored alterations observed among humans infected with H. pylori, further validating our conjecture that H. pylori delivers pathogenic factors by secreting OMVs. Importantly, this technique and approach facilitated the identification and validation of novel protein targets that play important roles in H. pylori-induced gastric diseases in individuals at a high risk of infection. Indeed, this technique and approach prospectively accelerates the identification of novel biomarkers that arise in the early inflammatory and carcinogenic cascade and are conductive to therapeutic intervention and disease prevention.
Materials and Methods
H. pylori Culture
Two kinds of H. pylori were used in the article. The standard strain NCTC11637 was donated by the Shijiazhuang Center for Disease Control and Prevention. The well-characterized clinically isolated H. pylori 400 strain was separated from gastric tissues obtained from patients in Hebei Province, which has a high incidence of gastric cancer, and was preserved in the China General Microbiological Culture Collection Center (CGMCC 15126). H. pylori was cultured for 72 h in a Columbia blood plate medium under microaerobic conditions. H. pylori used for OMV isolation was cultured in brain heart infusion broth (BHI, Oxoid) supplemented with 10% fetal bovine serum (BI) and 2% antibiotics for 72 h at 37 °C under microaerobic conditions and with constant rotation (150 rpm).
OMV Preparation and Purification
OMVs were isolated using size exclusion chromatography (SEC).17 Briefly, after 72 h of incubation, the broth cultures were centrifugated (3000g, 15 min) to remove bacteria. The culture supernatants were then filtered via a 0.22 μm filter (Millipore, USA) to eliminate contaminating particles. The filtered supernatant was condensed to 1 mL using Amicon Ultra-15 centrifugal filter units (Millipore, USA) for use in Exosupur columns in accordance with the manufacturer’s instructions (Echo Biotech, China). OMVs were collected and condensed to an appropriate volume by centrifugation through Amicon Ultra-4 centrifugal filter units (Millipore, USA). The morphology was characterized using transmission electron microscopy (TEM, JEOL2100F). The particle size distribution and concentration of the OMVs were measured using nanoparticle tracking analysis (NTA).
Cell Culture
The immortalized gastric epithelial cell line, GES1, was obtained from Procell Life Science & Technology (Wuhan, China), which was cultured in RPMI 1640 (Gibco, UA), supplemented with 10% fetal calf serum (BI, Israel), penicillin, and streptomycin (Invitrogen, UA), and incubated at 37 °C with 5% CO2.
Cytokine Detection
GES1 cells (1 × 105) were seeded in 6-well plates and cultured for 24 h before OMVs and H. pylori were added. Forty micrograms of total OMVs or 5 × 106H. pylori were added to each well. After 48 h of coculture, the cellular supernatant was collected for cytokine detection, including IL-2, IL-5, IFN-α, IL-10, IL-6, IFN-γ, IL-8, IL-17, IL-4, IL-12P70, and TNF-α, using flow cytometry in accordance with the manufacturer’s instructions (RAISE CARE).
Mass Spectrometry-Based Proteome Profiling
Protein Extraction and Digestion
RIPA buffer was added into the H. pylori or OMVs cocultured GES1 cells and purified OMVs for protein extraction and then sonicated for 5 s on and 5 s off with a total of six cycles. The proteins were then denatured at 95 °C for 2 min. The insoluble fragment was removed by centrifugation at 12,000g for 10 min, and the supernatant was used for the proteomic experiment. The protein concentration was measured using a BCA kit (Thermo).
A filter-aided sample preparation (FASP) procedure was used for protein digestion. Briefly, proteins were loaded in 10 kDa centrifugal filter tubes (Thermo, 88513), the disulfide bond was cleaved with 50 mM DTT in 300 μL UA buffer (8 M urea in 0.1 M Tris–HCl, pH 8.5) for 30 min in 37 °C, alkylated with 50 mM IAA in 300 μL of UA buffer for 30 min in the dark, washed thrice with 300 μL of UA buffer, and then washed twice with 300 μL of 50 mM NH4HCO3. All the above steps were centrifuged at 12,000g at 25 °C. Proteins were digested at 37 °C for 18 h with trypsin (Promega) at a concentration of 1:100 (w/w) in 50 mM NH4HCO3. After digestion, peptides were eluted by centrifugation. Subsequently, peptides were purified and extracted using homemade C18 tips (Empore) in 80% ACN and 2% TFA. Peptides were lyophilized and acidified in 0.1% FA. The peptide concentration was determined by the BCA peptide quantification kit (Thermo).
Proteomic Analysis
For proteomic analysis, the peptides (∼1 μg of each sample) were loaded on a nanoflow HPLC Easy-nLC1200 system (Thermo Fisher Scientific), using a 90 min LC gradient at 300 nL/min. Buffer A consisted of 0.1% (v/v) FA in H2O and buffer B consisted of 0.1% (v/v) FA in 80% ACN. The gradient was set as follows: 2–8% B in 1 min, 8–28% B in 60 min, 28–37% B in 14 min, 37–100% B in 5 min, and 100% B in 10 min. Proteomic analyses were performed on a Q Exactive HF mass spectrometer (Thermo Fisher Scientific). The spray voltage was set at 2100 V in a positive ion mode, and the ion transfer tube temperature was set at 320 °C. Data-dependent acquisition was performed using Xcalibur software in a profile spectrum data type. The MS1 full scan was set at a resolution of 60,000 at m/z 200, AGC target 3e6 and maximum IT 20 ms by an orbitrap mass analyzer (350–1500 m/z), followed by “top 20” MS2 scans generated by higher energy collisional dissociation (HCD) fragmentation at a resolution of 15,000 at m/z 200, AGC target 1e5 and maximum IT 45 ms. The fixed first mass of the MS2 spectrum was set 110.0 m/z. An isolation window was set at 1.6 m/z. The normalized collision energy (NCE) was set at NCE 27%, and the dynamic exclusion time was 45 s. Precursors with charges 1, 8, and >8 were excluded for MS2 analysis.
Database Searching of MS Data
All preliminary data processing was performed in Proteome Discoverer 2.2 using an ion currently-based label-free quantification method or basic protein identification similar to that previously described.18 Identification of peptides was performed with Sequest HT using a maximum 10 ppm mass tolerance for the parent ion and a 0.02 Da fragment tolerance for tandem mass spectrometry. All data were searched against the UniProtSwissProt Human canonical database (downloaded on Uniprot, 2019) or UniProtSwissProt H. Pylori database (downloaded on Uniprot, 2019). Carbamido methylation of cysteines was considered as a static modification; acetylation of the protein N-termini and oxidation of methionine were applied as potential variable modification. Multiple testing corrections were performed using false discovery rate calculations, as previously described.19 A 1% false discovery rate cutoff was applied to both the peptide spectral matches (calculated using Percolator20) and peptide group levels. Quantification ratios for each peptide were determined via pairwise analysis of individual peptides and then averaged for peptide group and protein levels. Significance was then determined by analysis of variance based on the peptide background at both the peptide group and protein levels.21
The criterion for differentially expressed proteins was |fold change| > 2. For enrichment analyses, gene ontology (GO) was analyzed using ClueGo of Cytoscape, and the enrichment terms with a p value less than 0.05 was reported. Kyoto Encyclopedia of Genes and Genomes (KEGG) was analyzed online (http://enrich.shbio.com) and the top 30 of enriched pathways were presented in the figures along with the p-value.
Immunofluorescence Assay
GES1 cells were seeded on the glass placed in the 24-well plate in advance, treated with OMVs and H. pylori for 24 h. Then, they were fixed with methanol for 6 h at 4 °C and permeabilized by 0.1% Triton X-100. The cells were blocked with sheep serum and incubated with primary antibodies overnight at 4 °C, VTN (A1667, ABclonal) and C3 (A13283, ABclonal). The protein signals were detected by anti-rabbit IgG Fab2 conjugated with Alexa Fluor 488 (Cell Signaling Technology, USA). Finally, the cells were incubated with DAPI for 15 min and visualized by a laser confocal microscope (Nikon).
Statistical Analysis
All statistical analyses were performed using SPSS version 13.0 software. All data are presented as the mean ± standard deviation from three independent experiments that were each measured in triplicate. One-way analysis of variance and the student’s t test were performed for comparison as described. A chi-square test was used to analyze categorical variables. A p value of less than 0.05 was considered statistically significant (*p value < 0.05), and all statistical tests were two-tailed.
Acknowledgments
This study was supported by the Natural Science Foundation of China (nos. 81772550, 81673642, 81502032, 81973520, and 81902798), the Outstanding Youth Foundation of Hebei Province, China (H2019206697), and the Natural Science Foundation of Hebei Province (H2020206131).
Glossary
Abbreviations
- H. pylori
Helicobacter pylori
- OMVs
outer membrane vesicles
- HPLC-MS/MS
high-performance liquid chromatography–tandem mass spectrometry
- GO
gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- MALT
mucosa-associated lymphoid tissue
- LPS
lipopolysaccharide
- BHI
brain heart infusion
- CGMCC
China General Microbiological Culture Collection Center
- FBS
fetal bovine serum
- SEC
size exclusion chromatography
- FASP
filter-aided sample preparation
- IAA
indole acetic acid
- DTT
dithiothreitol
- UA
urea
- TFA
trifluoroacetic acid
- FA
formic acid
- ACN
acetonitrile
- NCE
normalized collision energy
- PD
proteome discoverer
- TEM
transmission electron microscopy
- NTA
nanoparticle tracking analysis
- C3
complement C3
- VTN
vitronectin
- LSCM
laser scanning confocal microscope
- VEGF
vascular endothelial growth factor
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04549.
Detailed data of differentially expressed proteins in GES1 cocultured with increasing concentrations of OMVs (0, 10, 20, or 40 μg) and H. pylori (1:1, 10:1, or 50:1) in 11,637 and 400 (PDF)
Author Contributions
# S.W. and X.L. contributed equally to this study.
The authors declare no competing financial interest.
Notes
Mass spectrometry experimental data have been deposited on ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD025216, PXD025259, and PXD025281.
Supplementary Material
References
- Marshall B. J.; Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 323, 1311–1315. 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Ellis T. N.; Kuehn M. J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnington K. E.; Kuehn M. J. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta 2014, 1843, 1612–1619. 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Mandujano A.; Hernández-Cortez C.; Ibarra J. A.; Castro-Escarpulli G. The outer membrane vesicles: Secretion system type zero. Traffic 2017, 18, 425–432. 10.1111/tra.12488. [DOI] [PubMed] [Google Scholar]
- Rompikuntal P. K.; Vdovikova S.; Duperthuy M.; Johnson T. L.; Ahlund M.; Lundmark R.; Oscarsson J.; Sandkvist M.; Uhlin B. E.; Wai S. N. Outer Membrane Vesicle-Mediated Export of Processed PrtV Protease from Vibrio cholerae. PLoS One 2015, 10, e0134098 10.1371/journal.pone.0134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaja S. K.; Russo A. J.; Behl B.; Banerjee I.; Yankova M.; Deshmukh S. D.; Rathinam V. A. K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. 10.1016/j.cell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielaszewska M.; Rüter C.; Bauwens A.; Greune L.; Jarosch K. A.; Steil D.; Zhang W.; He X.; Lloubes R.; Fruth A.; Kim K. S.; Schmidt M. A.; Dobrindt U.; Mellmann A.; Karch H. Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: Intracellular delivery, trafficking and mechanisms of cell injury. PLoS Pathog. 2017, 13, e1006159 10.1371/journal.ppat.1006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsmann L.; Ruter C.; Bauwens A.; Greune L.; Gluder M.; Kemper B.; Fruth A.; Wai S. N.; He X.; Lloubes R.; Schmidt M. A.; Dobrindt U.; Mellmann A.; Karch H.; Bielaszewska M. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci. Rep. 2015, 5, 13252. 10.1038/srep13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomberger J. M.; Maceachran D. P.; Coutermarsh B. A.; Ye S.; O’Toole G. A.; Stanton B. A. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009, 5, e1000382 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L.; Bitto N. J.; Steer D. L.; Lo C.; D’Costa K.; Ramm G.; Shambrook M.; Hill A. F.; Ferrero R. L.; Kaparakis-Liaskos M. Helicobacter pylori Outer Membrane Vesicle Size Determines Their Mechanisms of Host Cell Entry and Protein Content. Front Immunol. 2018, 9, 1466. 10.3389/fimmu.2018.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H.; Chitcholtan K.; Hampton M. B.; Keenan J. I. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 2010, 78, 5054–5061. 10.1128/IAI.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaparakis M.; Turnbull L.; Carneiro L.; Firth S.; Coleman H. A.; Parkington H. C.; Le Bourhis L.; Karrar A.; Viala J.; Mak J.; Hutton M. L.; Davies J. K.; Crack P. J.; Hertzog P. J.; Philpott D. J.; Girardin S. E.; Whitchurch C. B.; Ferrero R. L. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 2010, 12, 372–385. 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- Fiocca R.; Necchi V.; Sommi P.; Ricci V.; Telford J.; Cover T. L.; Solcia E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 1999, 188, 220–226. . [DOI] [PubMed] [Google Scholar]
- Ismail S.; Hampton M. B.; Keenan J. I. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect. Immun. 2003, 71, 5670–5675. 10.1128/IAI.71.10.5670-5675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitcholtan K.; Hampton M. B.; Keenan J. I. Outer membrane vesicles enhance the carcinogenic potential of Helicobacter pylori. Carcinogenesis 2008, 29, 2400–2405. 10.1093/carcin/bgn218. [DOI] [PubMed] [Google Scholar]
- Ko S. H.; Jeon J. I.; Kim Y. J.; Yoon H. J.; Kim H.; Kim N.; Kim J. S.; Kim J. M. Helicobacter pylori outer membrane vesicle proteins induce human eosinophil degranulation via a β2 Integrin CD11/CD18- and ICAM-1-dependent mechanism. Mediators Inflammation 2015, 2015, 301716. 10.1155/2015/301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann D.; Kirchner B.; Hermann S.; Märte M.; Wurmser C.; Brandes F.; Kotschote S.; Bonin M.; Steinlein O. K.; Pfaffl M. W.; Schelling G.; Reithmair M. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J. Extracell. Vesicles 2018, 7, 1481321. 10.1080/20013078.2018.1481321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.; Shen S.; Li J.; Hu Q.; Nie L.; Tu C.; Wang X.; Orsburn B.; Wang J.; Qu J. An IonStar Experimental Strategy for MS1 Ion Current-Based Quantification Using Ultrahigh-Field Orbitrap: Reproducible, In-Depth, and Accurate Protein Measurement in Large Cohorts. J. Proteome Res. 2017, 16, 2445–2456. 10.1021/acs.jproteome.7b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The M.; Tasnim A.; Kall L. How to talk about protein-level false discovery rates in shotgun proteomics. Proteomics 2016, 16, 2461–2469. 10.1002/pmic.201500431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall L.; Canterbury J. D.; Weston J.; Noble W. S.; MacCoss M. J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. 10.1038/nmeth1113. [DOI] [PubMed] [Google Scholar]
- Oberg A. L.; Vitek O. Statistical design of quantitative mass spectrometry-based proteomic experiments. J. Proteome Res. 2009, 8, 2144–2156. 10.1021/pr8010099. [DOI] [PubMed] [Google Scholar]
- Li Z.; Clarke A. J.; Beveridge T. J. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 1998, 180, 5478–5483. 10.1128/JB.180.20.5478-5483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesty N. C.; Mason K. M.; Reedy M.; Miller S. E.; Kuehn M. J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J 2004, 23, 4538–4549. 10.1038/sj.emboj.7600471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M. Pathogenicity island-dependent effects of Helicobacter pylori on intracellular signal transduction in epithelial cells. Int. J. Med. Microbiol. 2005, 295, 335–341. 10.1016/j.ijmm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bebb J. R.; Leach L.; Zaitoun A.; Hand N.; Letley D. P.; Thomas R.; Atherton J. C. Effects of Helicobacter pylori on the cadherin-catenin complex. J. Clin. Pathol. 2006, 59, 1261–1266. 10.1136/jcp.2006.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat I.; Higashi H.; Obuse C.; Umeda M.; Murata-Kamiya N.; Saito Y.; Lu H.; Ohnishi N.; Azuma T.; Suzuki A.; Ohno S.; Hatakeyama M. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 2007, 447, 330–333. 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- Odenbreit S.; Haas R. Helicobacter pylori: impact of gene transfer and the role of the cag pathogenicity island for host adaptation and virulence. Curr. Top Microbiol. Immunol. 2002, 264, 1–22. [PubMed] [Google Scholar]
- Falush D.; Kraft C.; Taylor N. S.; Correa P.; Fox J. G.; Achtman M.; Suerbaum S. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 15056–15061. 10.1073/pnas.251396098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara K.; Kawai M.; Furuta Y.; Takahashi N.; Handa N.; Tsuru T.; Oshima K.; Yoshida M.; Azuma T.; Hattori M.; Uchiyama I.; Kobayashi I. Genome-wide survey of mutual homologous recombination in a highly sexual bacterial species. Genome Biol. Evol. 2012, 4, 628–640. 10.1093/gbe/evs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm R. A.; Ling L. S.; Moir D. T.; King B. L.; Brown E. D.; Doig P. C.; Smith D. R.; Noonan B.; Guild B. C.; deJonge B. L.; Carmel G.; Tummino P. J.; Caruso A.; Uria-Nickelsen M.; Mills D. M.; Ives C.; Gibson R.; Merberg D.; Mills S. D.; Jiang Q.; Taylor D. E.; Vovis G. F.; Trust T. J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 1999, 397, 176–180. 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- Furuta Y.; Konno M.; Osaki T.; Yonezawa H.; Ishige T.; Imai M.; Shiwa Y.; Shibata-Hatta M.; Kanesaki Y.; Yoshikawa H.; Kamiya S.; Kobayashi I. Microevolution of Virulence-Related Genes in Helicobacter pylori Familial Infection. PLoS One 2015, 10, e0127197 10.1371/journal.pone.0127197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki T.; Konno M.; Yonezawa H.; Hojo F.; Zaman C.; Takahashi M.; Fujiwara S.; Kamiya S. Analysis of intra-familial transmission of Helicobacter pylori in Japanese families. J. Med. Microbiol. 2015, 64, 67–73. 10.1099/jmm.0.080507-0. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E.; Farmery S. M.; Lindley I. J.; Figura N.; Peichl P.; Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J.;Clin. Pathol. 1994, 47, 945–950. 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira J. G.; Rossi A. F.; Nizato D. M.; Cadamuro A. C.; Jorge Y. C.; Valsechi M. C.; Venancio L. P.; Rahal P.; Pavarino E. C.; Goloni-Bertollo E. M.; Silva A. E. Influence of functional polymorphisms in TNF-alpha, IL-8, and IL-10 cytokine genes on mRNA expression levels and risk of gastric cancer. Tumour Biol. 2015, 36, 9159–9170. 10.1007/s13277-015-3593-x. [DOI] [PubMed] [Google Scholar]
- Wang Y. M.; Li Z. X.; Tang F. B.; Zhang Y.; Zhou T.; Zhang L.; Ma J. L.; You W. C.; Pan K. F. Association of genetic polymorphisms of interleukins with gastric cancer and precancerous gastric lesions in a high-risk Chinese population. Tumour Biol. 2016, 37, 2233–2242. 10.1007/s13277-015-4022-x. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E.; Shallcross T. M.; Heatley R. V.; Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 1991, 32, 1473–1477. 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W.; Wang S. R.; Chao M. F.; Wu T. C.; Lui W. Y.; P’Eng F K.; Chi C. W. Serum interleukin-6 levels reflect disease status of gastric cancer. Am. J. Gastroenterol. 1996, 91, 1417–1422. [PubMed] [Google Scholar]
- Backert S.; Schmidt T. P.; Harrer A.; Wessler S. Exploiting the Gastric Epithelial Barrier: Helicobacter pylori’s Attack on Tight and Adherens Junctions. Curr. Top Microbiol. Immunol. 2017, 400, 195–226. 10.1007/978-3-319-50520-6_9. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer N.; Neddermann M.; Asche C. I.; Backert S. Subversion of host kinases: a key network in cellular signaling hijacked by Helicobacter pylori CagA. Mol. Microbiol. 2017, 105, 358–372. 10.1111/mmi.13707. [DOI] [PubMed] [Google Scholar]
- Meng W.; Bai B.; Sheng L.; Li Y.; Yue P.; Li X.; Qiao L. Role of Helicobacter pylori in gastric cancer: advances and controversies. Discov. Med. 2015, 20, 285–293. [PubMed] [Google Scholar]
- Lian L.; Li X. L.; Xu M. D.; Li X. M.; Wu M. Y.; Zhang Y.; Tao M.; Li W.; Shen X. M.; Zhou C.; Jiang M. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer 2019, 19, 183. 10.1186/s12885-019-5322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstad A. E.; Hogasen K.; Bukholm G.; Moran A. P.; Brandtzaeg P. Complement activation directly induced by Helicobacter pylori. Gastroenterology 2001, 120, 1108–1116. 10.1053/gast.2001.23248. [DOI] [PubMed] [Google Scholar]
- Yuan K.; Ye J.; Liu Z.; Ren Y.; He W.; Xu J.; He Y.; Yuan Y. Complement C3 overexpression activates JAK2/STAT3 pathway and correlates with gastric cancer progression. J. Exp. Clin. Cancer Res. 2020, 39, 9. 10.1186/s13046-019-1514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.