Abstract

Selective hydrogenation plays an important role in the chemical industry and has a wide range of applications, including the production of fine chemicals and petrochemicals, pharmaceutical synthesis, healthcare product development, and the synthesis of agrochemicals. Pd-based catalysts have been widely applied for selective hydrogenation due to their unique electronic structure and ability to adsorb and activate hydrogen and unsaturated substrates. However, the exclusive and comprehensive summarization of the size, composition, and surface and interface effect of metal Pd on the performance for selective hydrogenation is still lacking. In this perspective, the research progress on selective hydrogenation using Pd-based catalysts is summarized. The strategies for improving the catalytic hydrogenation performance over Pd-based catalysts are investigated. Specifically, the effects of the size, composition, and surface and interfacial structure of Pd-based catalysts, which could influence the dissociation mode of hydrogen, the adsorption, and the reaction mode of the catalytic substrate, on the performance have been systemically reviewed. Then, the progress on Pd-based catalysts for selective hydrogenation of unsaturated alkynes, aldehydes, ketones, and nitroaromatic hydrocarbons is revealed based on the fundamental principles of selective hydrogenation. Finally, perspectives on the further development of strategies for chemical selective hydrogenation are provided. It is hoped that this perspective would provide an instructive guideline for constructing efficient heterogeneous Pd-based catalysts for various selective hydrogenation reactions.
1. Introduction
Catalysis in the 20th century was focused primarily on the activity and turnover rate to produce more molecules per unit time; however, due to increased processing costs and the negative ecological impacts of undesirable byproducts, the present focus and future direction of catalysis science pursue high selectivity in all catalyst-based chemical processes.1 With the development of societies, the waste and depletion of natural resources have prompted the synthesis of many artificial chemicals to meet human needs. This situation requires improved catalytic selectivity and a decrease in the formation of byproducts in accordance with the principles of green chemistry.2 Hydrogenation is the core of many industrial processes.3 To address the low-carbon environmental protection, selective hydrogenation, which refers to a reaction in which only one target functional group is reduced while all other functional groups are still unsaturated in the substrate, is arguably the most important.4 For instance, during fine chemical synthesis, various functional groups, such as −C≡C, −C=O, −NO2, −C≡N, −COOR, and −CONH2, can be selectively reduced by clean and cheap H2 to their corresponding alkenes, alcohols, and amines, as shown in Figure 1, which are key intermediates for fine chemicals, agrochemicals, polymers, and pharmaceuticals.5 More specifically, the hydrogenation of acetylene and propyne in petroleum cracking gases and the conversion of alkynes to olefins can be achieved by selectively hydrogenating C≡C to C=C for producing high-purity ethylene and propylene.6,7 Notably, the crucial point is to promote the hydrogenation reaction of C≡C into C=C while inhibiting the overhydrogenation to form C–C compounds. Heterogeneous catalysts using metal nanoparticles as the main active components are more favorable in the chemical industry due to their high activity, reusability, and stability. However, traditional heterogeneous catalysts often lead to overhydrogenation, resulting in low selectivity. Thus, improving the product selectivity remains a major challenge for the hydrogenation reactions.
Figure 1.

Selective hydrogenation of unsaturated bonds by palladium-based catalysts.
To achieve selective hydrogenation, a suitable catalyst must be chosen to obtain the target products. Pd-based catalysts have been widely used in metal-organic chemistry, and they present fantastic performance in a variety of organic chemistry reactions such as selective hydrogenation, oxidative dehydrogenation, coupling, and cycloaddition.8−10 Due to their unique electronic structures and their ability to adsorb and activate hydrogen and unsaturated substrates, Pd-based catalysts are also widely used in selective hydrogenation. In the past decades, many efforts have been made to design highly selective and active Pd-based catalysts for selective hydrogenation.11−17 For instance, Grabovskii et al. have described the recent advances in palladium catalysts deposited on various inorganic and organic for C=C bond hydrogenation.18 Also, Sajiki and co-workers have also outlined the effect of support, such as chelate resin, ceramic, spherically shaped activated carbon, 3 Å molecular sieves, and boron nitride, on the selective hydrogenation performance over heterogeneous palladium catalysts.19 Moreover, McCue et al. have introduced the selective hydrogenation of acetylene using Pd-containing catalysts.20 These works strongly indicate that Pd-based catalysts play a crucial role in the field of selective hydrogenation reactions. However, the exclusive summarization of the size, composition, and surface and interface effect of metal Pd, which could significantly affect the performance for selective hydrogenation, is still lacking. Moreover, a comprehensive overview on the recent progress of different selective hydrogenation reactions over Pd-based catalysts is also unavailable.
In this context, we present such a timely perspective on recent advances in Pd-based catalysts for selective hydrogenation reactions. We first introduce the advantages of Pd-based catalysts for selective hydrogenation and the effects of the size, composition, and surface and interfacial structure on influencing their catalytic performance. Then, the progress on Pd-based catalysts for selective hydrogenation of unsaturated alkynes, aldehydes, ketones, and nitroaromatic hydrocarbons is revealed based on the fundamental principles of selective hydrogenation. Finally, on the basis of the current progress in this area, future perspectives in the aspects of synthesizing efficient Pd-based catalysts have been proposed. It is hoped that this perspective would provide an instructive guideline for constructing efficient heterogeneous Pd-based catalysts toward various selective hydrogenation reactions.
2. Advantages of Pd-Based Catalysts and the Factors Influencing Their Catalytic Performance
2.1. Advantages of Pd-Based Catalysts
Commonly used selective hydrogenation catalysts are composed of noble metals such as Pd, Pt, Ru, and Rh and a base metal such as Fe or Ni. Although Fe-based catalysts are inexpensive, their activity is low, and the resulting iron mud is difficult to treat and easily causes pollution. Ni-based catalysts generally exhibit low selectivities with poor stability and require harsh reaction conditions. The precious metal palladium (Pd) is widely used in metal-organic chemistry due to its excellent catalytic performance.21 This is mainly because of the unfilled d-electron orbital of Pd, which easily adsorbs reactants on its surface. Pd0 (zero-valent Pd) is a good nucleophile and could be easily oxidized to Pd1+ or Pd2+. Based on the nucleophilic addition of Pd0 to various organic groups, Pd0 has been widely studied for chemical synthesis. Pd1+ and Pd2+ are good electrophiles, are air-stable, and can be dissolved in a variety of common organic solvents. Pd2+ shows strong electrophilic properties toward electron-rich compounds such as alkenes and alkynes. Pd1+ and Pd2+ compounds can react with alkenes or alkynes to form unstable intermediates that are then attacked and released by nucleophilic reagents to carry out catalytic reactions.22,23 These characteristics have resulted in the wide use of Pd in catalytic reactions.
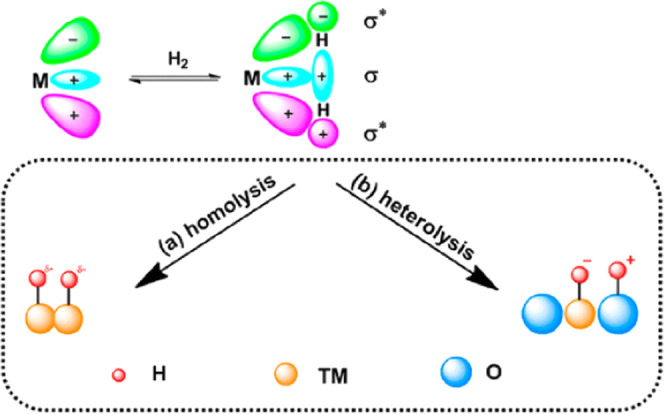
2.2. Homolytic and Heterolytic Dissociation of H2
The activation manner and the types of H species (i.e., H+, H–) also profoundly influence the selectivity during hydrogenation. During this reaction, H2 dissociates on the surface of a catalyst and then interacts with the functional groups to be hydrogenated to obtain the target product.24 H2 on Pd sites usually dissociates in two ways: homolysis and heterolysis (Figure 2). The partially occupied d-orbitals of Pd can accept the σ electrons of H2, and d-electrons can be donated to the σ* antibonding orbital of H2. Therefore, the H–H bond is weakened and cracked to form two hydrides via H2 dissociation. Generally, increasing the electron density of Pd sites promotes the homogeneous dissociation of H2.25 Different to homolytic dissociation, the heterolysis of H2 does not involve charge transfer. H2 is cleaved to H+ and H– when it is activated on the surface of Pd and other coordination heteroatoms. Then, it combines with heteroatoms and Pd, respectively. In general, heterolysis more easily reduces polar groups, and H+ and H– can be directly transferred to substrates. Due to the noncompetitive adsorption of the substrate and H2, there is sufficient space for the activation of H2. Besides, H+/H– pairs tend to kinetically reduce polar groups, which greatly improves the chemical selectivity of hydrogenation.26 For example, Zheng’s group found that the Pd/Cu2O catalyst showed excellent performance for semihydrogenation of terminal alkynes. This was mainly because the unique interface structure of Pd/Cu2O and Pd1+ promoted the heterolysis of H2 at Pd–O–Cu sites. This formed Pd–Hδ− and O–Hδ+, which promoted the hydrogenation of alkynes. Since the hydrogenation energy barrier of olefin intermediates is higher than its desorption energy barrier at the active site with isomeric hydrogen species, the formation of alkanes is inhibited; thus, olefin products can be obtained with high selectivity.27
Figure 2.
Heterolysis of H2.28 Reproduced with permission from ref (28). Copyright 2007 American Association for the Advancement of Science.
2.3. Factors Affecting the Catalytic Performance of Pd-Based Catalysts
The selective hydrogenation performance of Pd-based catalysts is related to the size, composition, and surface–interface structure (Figure 3). First, when the particle size of Pd-based catalysts is decreased, their morphology and the spatial distribution of components change, resulting in significant differences in their activity and selectivity. Second, the composition of Pd-based catalysts significantly impacts their selective hydrogenation ability (for example, different Pd-based alloy catalysts like PdCu, PdAg, PdAu, PdZn, etc.). The arrangement of surface atoms and the coordination structure of Pd-based catalysts also influence the catalytic activity; thus, the addition of appropriate ligands, modifiers, and carriers plays an important role in improving the catalyst performance. Moreover, the different synthesis methods of Pd-based catalysts are also related to the selective hydrogenation performance of the catalysts; therefore, the main goal of selective hydrogenation research is to clarify the structure–activity relationship of Pd-based catalysts and prepare Pd catalysts with high activity, high selectivity, and high stability.29−31
Figure 3.

(A) Size effect, (B) composition effect, and (C) surface and interface effect over Pd-based catalysts.
2.3.1. Size Effects
Surface-coordinated unsaturated atoms often interact with other atoms. Decreasing the size of Pd-based catalysts introduces surface atomic coordination structures, increases the specific surface area, as well as introduces unsaturated coordination sites.32 When the particle size decreases, the defects on the catalyst surface increase. Decreasing the particle size also increases the number of defects on the catalyst surface, and terrace easily forms, which forms catalytic active sites that are different from planar sites.33 Abdollahi’s group calculated the activation energy of acetylene hydrogenation to ethylene on small Pd2 by density functional theory (DFT), which was 23.96 kJ/mol lower than that of Pd12 nanoclusters. The ethylene adsorption energy on Pd2 was lower than that on Pd12, so ethane was more difficult to synthesize using Pd2 compared with Pd12; therefore, decreasing the size of nanoparticles improved the hydrogenation selectivity.34
Selectivity depends on the adsorption strength and configuration of reactants or intermediates on a catalyst surface. The atom located at the edge, corner, and terrace of the nanoparticle shows different coordination environments and electronic structures. The absorption strength and configuration depend on the electronic and geometric structure of active sites. For example, depending on the assemblies of Pd atoms, ethylene has three adsorption modes: the ethylidyne mode on threefold Pd sites, the di-σ mode on bridged Pd dimers, and the π-bonded mode on isolated Pd atoms. The adsorption strength decreases in the order ethylidyne > di-σ > π-bonded.35,36 When two or more functional groups that can be adsorbed on the catalyst simultaneously exist in the substrate, multiple absorption modes will also exist; thus, various byproducts are produced, leading to poor chemical selectivity. To achieve excellent chemical selectivity, the catalytic active sites should have uniform geometric and electronic structures so that the target functional groups can be adsorbed on the catalyst and avoid multiple absorption modes. If the size of nanoparticles is reduced to a minimum, i.e., atomic dispersion, then the metal atoms will directly interact with heteroatoms in the carrier. Single-atom catalysts have unique geometric and electronic structures that can isolate the adsorption sites and prevent multisite adsorption, which effectively controls the catalyst selectivity.37−39 Single-atom catalysts also reduce the noble metal loading. Therefore, controlling the size of Pd-based catalysts and changing the geometric and electronic structures of catalysts help improve their catalytic activity and selectivity.
2.3.2. Composition Effects
The composition of a catalyst changes its absorption capacity for substrates and intermediates, which produces different catalytic activities and selectivities. The incorporation of other metals into Pd catalysts reduces costs and also changes the electronic structure of the surface atomic coordination structure due to changes in the chemical composition and microstructure.40 The selective hydrogenation ability of a catalyst can be improved through synergistic and electronic effects. For gas-phase acetylene hydrogenation, Pd–Ag, Pd–Au, Pd–Bi, Pd–Cu, Pd–Ga, Pd–Sn, and Pd–Zn are often used in the industry.41−44 For liquid-phase alkyne hydrogenation, Pd/CaCO3 (Lindlar catalyst) whose surface has been poisoned by Pb and quinoline is generally used. By doping with other metals, Pd sites separated by intermetallic compounds are formed, which changes the adsorption energy of the substrate and intermediate products and further improves the selectivity. For instance, the selectivity of PdCu/CNT nanoparticles synthesized by a one-pot method reported by the Godard group was >90% toward ethylene during acetylene hydrogenation. This was attributed to the electronic and geometric effects of Cu, which promoted the dissociation of H2 on Pd and resulted in the weak adsorption of ethylene on Pd. Ethylene displayed good selectivity even at a high acetylene conversion rate. This method has already been extended to the preparation of colloidal and carbon-supported single-metal Cu catalysts, whose catalytic performance is much higher than that of ordinary acetylene semihydrogenation catalysts.45 Besides, constructing Pd intermetallic to make the continuous Pd ensembles separated or even totally isolated can largely enhance the hydrogenation selectivity. Single-atom alloy (SAA) catalysts are promising candidates for selective hydrogenation because the active metal atoms are exclusively surrounded by the second metal atoms at proper compositions and the interaction between each component leads to high thermal stability and structural integrity under reaction conditions.37,46
2.3.3. Surface and Interface Effects
The interface structure of a catalyst affects its selectivity. A fine surface interfacial structure includes the arrangement of surface atoms and the coordination structure of surface atoms. During the preparation of Pd-based catalysts, the introduction of suitable ligands produces catalysts with good morphology and appropriate size and the ligands adsorbed on the surface of the catalysts change the adsorption mode of the substrate and intermediate products, which significantly improves the catalytic selectivity.47−49 Common organic ligands include surfactants and polymers, e.g., oil amines, oleic acid, trioctylphosphine, dodecyl mercaptan, cetyltrimethylammonium bromide, poly(vinylpyrrolidone) (PVP), poly(vinyl alcohol) (PVA), and polyamidoamine (Table 1).
Table 1. Partial Pd-Based Catalysts Modified by Ligands.
| ligand | metal precursor | reducing agent | size (nm) | ref |
|---|---|---|---|---|
| ethylene glycol | PdCl2 | 2.4 ± 0.4 | (50) | |
| ethylene glycol | Na2PdCl4 | 14.9 ± 2.3 | (51) | |
| trisodium citrate (Na3C6H5O7) | Na2PdCl4 | alcohol | 15 | (52) |
| diethylene glycol | Na2PdCl4 | 19 | (53) | |
| oleylamine (OAM) | Pd(acac)2 | borane tributylamine complex | 4.5 | (54) |
| poly(N-vinyl-2-pyrrolidone) (PVP) | Na2PdCl4 | acetaldehyde | 18 | (55) |
| poly(vinyl alcohol) (PVA) | Na2PdCl4·2H2O | 3.1 ± 0.7 | (56) | |
| dendritic phosphine ligand | Pd(acac)2 | H2 | 3.2–5.0 | (57) |
| n-didocosyl sulfide | Pd(CH3CN)2Cl2 | NaBH4 | 4–6 | (58) |
| thiol | Pd(acac)2 | CO | 5 | (59) |
For example, poly(vinylpyrrolidone) (PVP) is a polymer end-capping ligand, in which an O atom is firmly bonded to the (100) surface of Pd.60 During the growth of PVP-protected Pd nanoparticles, Pd atoms must first bond to the (111) surface and then the attached atoms migrate to the surface edge, extending to form nanocubes on the (100) surface. A capping agent acts as a suitable ligand to form a complex with the metal precursor. For example, during the synthesis of Pd nanocrystals in OAM, Pd(acac)2 first reacted with OAM to form an intermediate complex, Pd(ACAC)x(OAM)y. Then, formaldehyde was added, and the rate of catalysis decreased upon increasing the amount of OAM.61 Although traditional studies suggest that the presence of ligands affects the catalytic activity, more recent studies have shown that appropriate ligand modification affects the spatial arrangement and coordination structure of the exposed atoms on the surface, which positively impacts the adsorption of substrates and intermediate products, thus improving the product selectivity.
On the one hand, the surface coordination bond structure is closely related to the types of coordination atoms and metals, and the arrangement structure between ligands is often affected by the peripheral noncoordination groups or the surface curvature of nanomaterials, thereby affecting the enrichment process and catalytic reaction kinetics of different reaction species on the metal surface. On the other hand, the coordination of organic molecules with surface metal atoms will also change the electronic structure of surface metal atoms and adjust the adsorption strength of different types of reaction substrates and intermediates on the metal surface. In addition, the coordination of organic ligands with metal atoms also segments the continuous arrangement of metal atoms into smaller active sites, thus affecting the adsorption form and reaction process of different reaction groups on the surface. More importantly, surface organic matter can also form an active interface with metal atoms, changing the catalytic reaction pathway. For example, Medlin’s group modified thiol molecules with different degrees of steric hindrance on the surface of Pd nanoparticles, which improved the selectivity of furan alcohols and methyl furans during furfural hydrogenation, as well as cinnamyl alcohol during cinnamaldehyde hydrogenation.62 They believed that the self-assembled monolayers (SAMs) formed from thiols and metals through covalent binding of sulfur groups changed the adsorption mode of the substrate and intermediate products to provide selectivity.
Understanding the catalytic mechanism at the molecular level is the key to improving catalyst performance. So, the well-defined catalysts need to be prepared to regulate the active sites. As we know, clusters have accurate atomic structure and can be characterized by mass spectrometry, nuclear magnetic resonance (NMR), and other characterization methods. Using metal nanoclusters with clear compositions and structures as model catalysts helps to understand the structure–activity relationship behind the catalytic reaction of surface ligands.63,64 Besides, we can first synthesize clean-surface nanoparticles with clear structures and then modify ligands on them to improve their selectivity.
Pd nanoparticles are often loaded on supports as catalysts. Common supports include acidic or alkaline oxides, natural minerals (TiO2, pumice, etc.), silica gel, and various types of carbon materials (activated carbon, graphene, silicon carbide), etc. The use of a carrier improves the dispersion of Pd-based catalysts, changes the electronic structure and spatial distribution of Pd, and forms strong metal–support interactions.65 This affects the arrangement and coordination structure of the surface atoms of the catalyst, which permits the selective adsorption of functional groups of the substrate and changes the adsorption energy of the substrate and intermediate.66−69 In recent years, a large number of studies have shown that the catalytic performance of supported metal catalysts is highly dependent on the support. Therefore, it is particularly important to fabricate model catalysts with well-defined and abundant metal–support interfacial sites for their involvement in hydrogenation. Several strategies have been developed to enhance the performance: (1) decorating catalytic metal particles with species having similar composition to the supports to create abundant metal–support interfaces, (2) designing reverse structure which reducible metal oxide supports metal catalysts adopted in metal catalysts so that the interfacial O2–/OH– species can involve hydrogenation, and (3) developing atomically dispersed metal catalysts (ADCs) for revealing the molecular functions of supports in hydrogenation reactions.
Moreover, when metal nanoparticles are confined to porous materials (such as metal-organic frameworks (MOFs), zeolites, etc.), the functional groups of the substrate can be selectively adsorbed at metal active centers by adjusting the pore size to selectivity obtain the target product. Gong et al. reported a Pd nanocluster catalyst confined by a periclase (sodalite (SOD)) zeolite, named Pd@SOD. In the reaction, H2 dissociated on Pd@SOD to form H species (H+, H–), which overflowed to the surface of SOD, and the ethylene selectivity reached 94.5%. Other studies have shown that the main reason for such high selectivity is that H2 can enter the pores, activate Pd, and prevent deep hydrogenation of ethylene.21
3. Selective Hydrogenation Using Pd-Based Catalysts
In both bulk and fine chemical industries, selective hydrogenation plays an important role. As the most widely used hydrogenation catalysts, Pd-based catalysts require high activity and stability as well as high selectivity.
3.1. Application of Pd-Based Catalysts in Selective Hydrogenation of Alkynes
The selective hydrogenation of C≡C to C=C is an important industrial process for the production of fine chemical intermediates.70 Industrial olefin feedstock often contains a small amount of unsaturated alkynes, and ethylene doping in the feed affects the synthesis and product quality; therefore, it is necessary to selectively hydrogenate acetylene mixed in the ethylene flow to ethylene and remove it to the ppm level to meet quality requirements. Among the various methods to eliminate alkynes, semihydrogenation of alkynes to alkenes has proven to be the most efficient one. A variety of catalysts have been explored for the selective hydrogenation of alkynes, among which Pd-based catalysts have attracted the most attention due to their high intrinsic activity.71 The performance of some Pd-based catalysts in selective hydrogenation of alkynes is shown in Table 2.
Table 2. Activity and Selectivity of Selective Hydrogenation of Some Alkynes.
| substrate | product | catalyst | sel./conv. (%) | ref |
|---|---|---|---|---|
| acetylene | ethylene | Pd4S/CNF | 94/100 | (72) |
| acetylene | ethylene | Pd | >91/99 | (73) |
| acetylene | ethylene | AgPd0.005/SiO2 | 92.6/93.6 | (37) |
| acetylene | ethylene | Pd/ND@C | 90/100 | (74) |
| phenylacetylene | styrene | Pd–Au | >80/100 | (65) |
| phenylacetylene | styrene | Pd@Ag | 99/99 | (75) |
| phenylacetylene | styrene | Pd-Cu2O | 98/99.2 | (76) |
| 2-butyne-1,4-diol | (Z)-2-butene-1,4-diol | Pd/Boehmite | >60/100 | (77) |
| 3-hexyn-1-ol | 3-hexen-1-ol | Pd/TiO2 | 88/100 | (78) |
| 1-hexyne | 1-hexene | PdAu | 85/100 | (79) |
| butyne | butenes | PdS4/C | 98/100 | (80) |
| propyne | propene | Pd/Al2O3 | 97/97 | (81) |
| diphenylacetylene | stilbene | PdNP | 95/99 | (82) |
| diphenylacetylene | stilbene | Pd + PEI@HSS | 94/100 | (83) |
| diphenylacetylene | stilbene | FFSienPd | 94/100 | (31) |
| 1-phenyl-1-propyne | 1-phenyl-1-propene | PdS | 97/100 | (59) |
The C≡C bonds contain one σ bond and two π bonds. The electrons of the π bonds are mobile and tend to attack electrophilic reagents. When a π bond is broken, C is added to the active hydrogen species to form an olefin containing a σ bond and a π bond to complete the addition reaction; however, olefins can also add to saturated alkanes. Semihydrogenation of alkynes generally follows the Horiuti–Polanyi (H–P) mechanism in which H2 is first adsorbed and dissociated on the catalyst and then an alkyne is adsorbed and two hydrides are continuously added to the unsaturated bond. In the hydrogenation reaction following the H–P mechanism, the adsorption mode of the acetylene substrate on the catalyst surface plays a decisive role in product selectivity. For example, when controlling the hydrogenation of acetylene to ethylene, the desorption energy barrier of the π bond of ethylene is lower than that of hydrogenation, and ethylene is desorbed from the surface, which avoids excessive hydrogenation to ethane. Zhang’s research group prepared a PdZn/ZnO catalyst, which achieved >90% conversion and selectivity of ethylene. This was mainly attributed to the special active sites of Pd–Zn–Pd (Figure 4A). Initially, ethylene is adsorbed on isolated Pd sites by weak π bonds, which allows it to easily desorb from the PdZn surface, thus preventing its further hydrogenation to ethane. Second, single Pd sites on the Pd–Zn–Pd provide two adjacent isolated Pd sites for the σ absorption of acetylene, which activates C≡C through an ethylene-like intermediate to efficiently convert C≡C to ethylene. The Pd active sites are also favorable for H2 activation. Therefore, the PdZn catalyst showed superior activity and selectivity during acetylene hydrogenation due to its good thermodynamics and kinetics for the conversion of acetylene to ethylene.84
Figure 4.
(A) PdZn intermetallic nanostructure with Pd–Zn–Pd ensembles are both highly active and selective for semihydrogenation of acetylene to ethylene; (B) high-resolution transmission electron microscopy (HRTEM) image of Pd/ZnO sample reduced at 400 °C; (C) conversion and selectivity with time on stream in absence of ethylene over PdZn;84 (D) thiol treatment is demonstrated as a highly effective strategy for promoting the catalytic selectivity of Pd nanocatalysts in the hydrogenation of internal alkynes to alkenes; (E) energy barriers of transition states of PhC≡CCH3 hydrogenation on Pd4S@SPhF2, Pd3S@SPhF2, and Pd(111); (F) catalytic stability and selectivity of the Pd@SPhF2(1:1) obtained;59 (G) a defective nanodiamond-graphene (ND@G) to prepare an atomically dispersed metal catalyst which is used for selective hydrogenation; (H) high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images of Pd1/ND@G at high magnifications, (I) durability test on Pd1/ND@G at 180 °C for 30 h. (Reaction conditions: 1% C2H2, 10% H2, 20% C2H4 gas mix balanced with He; gas hourly space velocity (GHSV) = 60 000 h–1.).74 (A–C) Reproduced with permission from ref (84). Copyright 2016 American Chemical Society. (D–F) Reproduced with permission from ref (59). Copyright 2018 Elsevier. (G–I) Reproduced with permission from ref (74). Copyright 2018 American Chemical Society.
The alkynyl hydrogenation selectivity can be improved by doping other metals into Pd to form an alloy, which segments the surface Pd sites. In addition, the doping of main group nonmetals into the surface and lattice of Pd-based catalysts can also regulate the coordination and electronic structure of Pd sites on the surface. Besides, the steric hindrance induced by an ordered array of surface ligands can also manipulate the binding of hydrogenation intermediates to improve selectivity. For example, Zheng’s research group used thiol-treated ultrathin Pd nanosheets as a model catalyst and demonstrated the development of stable, efficient, and selective Pd catalysts for semihydrogenation of internal alkynes. In the hydrogenation of 1-phenyl-1-propyne, thiol-treated Pd nanosheets exhibited excellent catalytic selectivity (>97%) toward 1-phenyl-1-propene and no obvious decay in the activity and selectivity after 10 cycles (Figure 4B). It was found that a mercaptan dissociated on the surface of Pd nanosheets, the C–S bond was broken, and then S diffused into Pd nanosheets to form palladium sulfide. The unique Pd-sulfide/thiolate interface created by the thiol treatment was crucial to semihydrogenation. The electronic effect produced by PdSx and the steric hindrance produced by thiol caused the thiol-modified palladium nanosheets to reduce the reaction energy barrier of the first step in the hydrogenation of phenylpropyne and increase the reaction energy barrier of the third step of hydrogenation. This made the subsequent hydrogenation energy barrier of olefins much higher than that of alkyne hydrogenation and the olefin surface desorption energy barrier, which finally realized the selective hydrogenation of alkynes to olefins. More importantly, this thiol treatment strategy is applicable to commercial Pd/C catalysts for semihydrogenation of internal alkynes.59
It has been well documented that the adsorption and dissociation of H2 are closely related to the electronic structure and coordination environment of catalytic metal centers.85 The catalytic metal center with a low valence state and an unsaturated coordination structure is conducive to the activation of H2, thus showing good hydrogenation activity.86,87 The supported atomically dispersed catalysts where all active metal atoms are exposed have been fabricated for selective hydrogenation of alkynes. Professor Martin prepared an atomically dispersed metal catalyst Pd/nanodiamond-graphene (ND@G) by an impregnation–reduction method by controlling the pH. Acetylene could be reduced by a mixture of H2 and He in 30 min with 100% conversion and 90% ethylene selectivity. Compared with Pd foils, the X-ray absorption near-edge spectroscopy (XANES) peaks of Pd1/ND@G and Pdn/ND@G shifted to a higher valence state, which demonstrated the existence of slightly positively charged Pd species. The absorption peak of Pd1/ND@G was located between those of Pdn/ND@G and PdO, suggesting that the interactions of atomically dispersed Pd species and ND@G were stronger than those of Pd in Pdn/ND@G. Extended X-ray absorption fine structure (EXAFS) contained peaks of Pd–C or Pd–O at 1.5 Å, which provided key evidence for the coordination environments of Pd atoms anchored on ND@G. The Pd–Pd coordination signal at 2.4 Å in Pdn/ND@G confirmed the existence of Pd nanoclusters (Figure 4C). According to DFT calculations, acetylene gas is preferentially adsorbed on the Pd atoms of Pd1@Gr. The adsorption energy of acetylene on Pd atoms of Pd1@Gr (−0.61 eV) was weaker than that on the Pd(111) surface (−1.79 eV). Then, hydrogen underwent heterolytic dissociation, in which one atom combined with a C atom and the other combined with a Pd atom. The energetic barrier of further hydrogenation of the adsorbed C2H4 intermediate to ethane (1.17 eV) was much higher than the desorption energy of surface C2H4 atoms. The atomically dispersed Pd atoms blocked the formation of unselective subsurface hydrogen species and promoted the facile desorption of ethylene, which prevented its overhydrogenation to ethane.74
In short, the selective hydrogenation of alkynes is of great significance to both basic research and industrial applications. Based on the understanding of the adsorption behavior of olefins and alkynes onto catalyst surfaces, some strategies have been developed to improve the selectivity of catalysts. For example, other metal atoms can be incorporated into Pd, forming alloys or intermetallic compounds, to isolate Pd active sites and change the adsorption behavior of intermediate products, which effectively inhibits side reactions. Another efficient strategy involves modifying the Pd surface with thiols, amines, phosphines, or other ligands, which selectively poisons active sites and induces electronic and steric effects, thus changing the adsorption mode of substrates or intermediates. To maximize the efficiency for metal utilization, various monoatomic catalysts, including catalysts anchored on the supports or alloyed with the second metal, have been prepared, showing good activity that is superior to nanoparticle catalysts in the semihydrogenation reactions in gas or liquid phases. In addition, the structure–activity relationship of the catalyst can be clearly analyzed by spherical differential electron microscopy and EXAFS when the size of the catalyst is reduced to the single-atom level.
3.2. Selective Hydrogenation of Aldehydes and Ketones
The selective hydrogenation of unsaturated aldehydes/ketones (C=O and C=C coexisting in one molecule) usually requires a long reaction time and harsh conditions,24 such as high temperatures and high pressures; therefore, new efficient systems are needed to realize the selective conversion of aldehydes and ketones. Because C=C hydrogenation is thermodynamically favored over C=O hydrogenation, more efforts were made to selective hydrogenation of C=O to unsaturated alcohols (UOLs). The rational design of heterogeneous catalysts is a crucial step toward high selectivity. Some strategies like reducing the size to the atomic level, changing the electronic properties, and forming a steric effect have been used to design a catalyst. Pd-based catalysts are widely studied for selective hydrogenation of aldehydes and ketones, and the performance of selected Pd catalysts is listed in Table 3.
Table 3. Activity and Selectivity of Selective Hydrogenation of Some Unsaturated Aldehydes and Ketones.
| substrate | product | catalyst | sel./conv. (%) | ref |
|---|---|---|---|---|
| cinnamaldehyde | 3-phenyl-1-propanal | Na2[Pd(HSS)] | 92.5/100 | (88) |
| cinnamaldehyde | phenylpropionaldehyde | PdAu | 90/100 | (89) |
| 1-phenyl-1-propanone | 1-phenyl-1-propanol | Pd/TiO2 | 99.7/100 | (90) |
| cinnamaldehyde | hydrocinnamaldehyde | PdZn | 70/90 | (91) |
| cinnamaldehyde | hydrocinnamaldehyde | Pd-NMC | 93/100 | (92) |
| benzaldehyde | benzyl alcohol | Pd/MIL-101(Fe)-NH2 | 77/100 | (93) |
| 5-hydroxymethylfurfural | 2,5-dimethylfuran | Pd–Co9S8/S-CNT | 83.7/96 | (94) |
| furfural | furfuryl | Pd/Cu | 96.5/96.4 | (95) |
| chalcone | dihydrochalcone | Pd | 99/98 | (96) |
| cinnamaldehyde | cinnamyl alcohol | Pd/Al2O3 | 90/100 | (62) |
When the size of Pd decreases, especially to the single-atom level, its catalytic performance changes, and the catalytic effect is improved after being dispersed on a support. In the target C=O group, the C-terminal is positively charged and the O-terminal is electron-rich, so the positive center in the catalyst can promote the polarization and activation of C=O bonds.97 So, the objectives of catalyst design should be preferentially to adsorb C=O bonds and to prevent the C=C bonds from approaching the metal surface. A Pd1+NPs/TiO2 catalyst was synthesized by Li’s research group to explore the performance of ketone/aldehyde hydrogenation catalyzed by a synergic catalyst (Figure 5A). They found that the catalytic activity of Pd1+NPs/TiO2 was 3.2 times higher than that of the commercial Pd/C (5.2 wt %). Under 0.3 MPa H2 and 40 °C, Pd1+NPs/TiO2 showed excellent catalytic activity with 100% conversion of 4-methylacetophenone within 20 min and an alcohol selectivity up to 98%. EXAFS analysis showed that Pd1 and Pdn had a synergistic effect (Figure 5B). Compared with Pd1/TiO2 and PdNPs/TiO2 catalysts, Pd1+NPs/TiO2 catalysts synthesized by a simple large-scale spray pyrolysis method combined the advantages of Pd1 and PdNPs on mesoporous TiO2 supports. By exploiting the synergistic effect of Pd1 and PdNPs, some Pd1 sites contributed dispersion sites to activate C=O groups, while the PdNPs sites promoted the dissociation of H2 molecules to H atoms. According to DFT results, H2 was preferentially dissociated on metal PdNPs sites, while ketones and aldehydes were adsorbed at Pd1 sites. Pd1 dispersed around PdNPs helped to improve the hydrogenation activity and selectivity of acrolein. The synergistic effect of Pd1 and PdNPs on TiO2 improved the reaction activity. A large number of Pd1 sites activated C=O groups, and PdNPs sites promoted the dissociation of H2.90
Figure 5.
(A) HAADF-STEM energy-dispersive spectrometry (EDS) mapping of Pd1+NPs/TiO2 and AC-HAADF-STEM image of Pd1+NPs/TiO2, (B) R-spaced Pd K-edge Fourier transform (FT)-EXAFS spectra of Pd1+NPs/TiO2 (green line) referred to bulk Pd foil (black line) and PdO (magenta line),90 (C) cinnamaldehyde hydrogenation pathways, and (D) cinnamaldehyde hydrogenation performance profile using Pd–Au catalysts.98 (A, B) Reproduced with permission from ref (90). Copyright 2020 Springer Nature. (C, D) Reproduced with permission from ref (98). Copyright 2006 Wiley-VCH.
Reducing the size of a catalyst to the single-atom level changes the adsorption energy of the C=O bond and improves the catalyst selectivity. Changing the catalyst composition by constructing a bimetallic catalyst also changes the selectivity of the catalyst through geometric and electronic effects. The Au–Pd alloy catalyst synthesized by Pârvulescu’s research group improved the selectivity of cinnamaldehyde hydrogenation into cinnamic alcohol.98 A series of bimetallic Au–Pd colloidal nanoparticles with different Au/Pd molar ratios were synthesized by a sol–gel method by adjusting the concentration of metal ions in an aqueous solution. On monometallic Pd- and Au-embedded colloids, 3-phenylpropanal and 3-phenyl-1-propanol were found to be the predominant reaction products. Alloying of the two nanometer-sized metals in colloids led to a very important enhancement of the selectivity, with cinnamyl alcohol being the major product (Figure 5C,D). Besides the influence of the Au–Pd ratio on the catalytic performance, other factors also affect the selectivity. For example, increasing the H2 pressure can improve the gas concentration in the reaction medium, thereby enhancing the addition of H2 to functional groups. The solvent also affects the catalyst. For instance, a polar solvent can promote the hydrogenation of carbonyl groups.99 It is clear from these results that Au, although totally inactive when it is present alone, plays an important role when alloyed with Pd, increasing the catalytic activity and selectivity with respect to those of monometallic Pd.
In addition, modifying a catalyst by adding proper ligands can change the sites that adsorb functional groups during catalytic reactions, thus affecting the reaction pathway during selective hydrogenation. At present, researchers have used organic ligands such as mercaptans,100 amines,101 and organic phosphines102 to modify the surface of a catalyst to introduce steric and electronic effects, which affect the adsorption mode of functional groups and change the catalyst selectivity. When thiols are exposed to the metal surface, they tend to occupy the terrace sites, forming densely stacked monolayers, thus covering continuous metal sites and leaving discontinuous corner/edge sites. Due to the decrease of the assemblies of accessible metal atoms, the adsorption mode of the reactants is limited, thus improving the selectivity. This is called the “active-site selection” strategy (Figure 6).103 Medlin’s group reported a SAM of thiols with varying surface densities to tune the selectivity of supported Pd catalysts. As confirmed by vibrational spectroscopy, while 1-adamantanethiol (AT) prefers to coordinate to corner sites of Pd nanoparticles, octadecanethiol (C18) preferentially coordinates with terrace sites. In the hydrogenation of furfural in a fixed-bed reactor at 190 °C and 0.1 MPa H2, the selectivity to furfural alcohol was below 5% over unmodified and AT-modified Pd/Al2O3, while over octadecanethiol-modified Pd/Al2O3, the selectivity was increased to above 70% (Figure 6B).
Figure 6.
Illustrations of approaches for tuning the selectivity of nanocatalysts in hydrogenation of α,β-unsaturated aldehydes using organic modifiers. (A) Proposed schematic depicting active-site selection, (B) product selectivity for furfural hydrogenation over uncoated and alkanethiolate SAM-coated Pd/Al2O3 catalysts, (C) thiol SAMs used to coat the Pt/Al2O3 surface, and (D) selectivity to cinnamyl alcohol for the hydrogenation of cinnamaldehyde over Pt/Al2O3 catalysts. (A, B) Reproduced with permission from ref (103). Copyright 2013 Springer Nature. (C, D) Reproduced with permission from ref (104). Copyright 2014 American Chemical Society.
It has been shown previously that binding in a horizontal configuration favors C=C hydrogenation, while binding in a vertical orientation favors C=O hydrogenation. In cinnamaldehyde hydrogenation, the authors chose thiol molecules with a tail structure similar to that of the substrate. While linear alkyl ligands are not expected to interact preferentially with a particular region of the cinnamaldehyde reactant, phenylated ligands can interact with cinnamaldehyde’s phenyl group through aromatic π–π stacking. The cinnamic alcohol selectivity reached 90% when 3-phenylpropanethiol was used as the modifier during selective hydrogenation of cinnamaldehyde under conditions of 50 °C and 4 MPa H2. The cinnamyl alcohol selectivity was very poor when a long-chain alkyl mercaptan or short-chain 2-phenylethyl thiol and long-chain 4-phenylbutanthiol were used. The selectivity was mainly due to π–π stacking between cinnamyl aldehyde and 3-phenyl thiol.104
Selective hydrogenation of unsaturated aldehydes/ketones C=O to unsaturated alcohols is an important reaction in the pharmaceutical industry, but it is still challenging because C=C groups are more easily saturated. Among them, selective poisoning of a catalyst is used to modify thiols, amines, and other organic ligands on a catalyst’s surface to promote selective adsorption of C=O. Selective hydrogenation of C=O can be achieved by reducing the catalytic active components to the single-atom scale and inhibiting the adsorption of C=C. Similarly, the selectivity can also be increased by constructing bimetallic nanoparticles by exploiting electronic effects and by changing the substrate and intermediate adsorption energy. Besides, more investigations on the effect of the valence state of transition-metal cations and the solvent on the adsorption strength of the carbonyl group also need to be done.
3.3. Selective Hydrogenation of Nitroaromatic Compounds
Selective hydrogenation of nitroarenes is a simple, economic, and effective way to synthesize aromatic amines.105 At present, high H2 pressures and high temperatures are often used in the industry, and the process is mainly realized by reducing agents or organic solvents that are environmentally unfriendly. Although Pt-based catalysts have a good effect on nitro hydrogenation, research into Pd-based catalysts is still challenging and has focused on the development of Pd-based catalysts with high catalytic activity, good sustainability, and environmental friendliness. When using Pd-based catalysts, it is necessary to ensure that other functional groups are not destroyed during −NO2 reduction. Taking nitrostyrene as an example, C=C is more easily reduced than −NO2. It has been pointed out that the adsorption of C=C is very sensitive to the catalyst structure, and −NO2 preferentially undergoes monodentate adsorption on the catalyst surface; therefore, researchers have studied the geometry and electronic structure of a catalyst to regulate the adsorption mode of C=C and −NO2 to preferentially adsorb nitro groups for selective hydrogenation. Table 4 shows the performance of some Pd-based catalysts for selective hydrogenation of nitro and nitrile groups.
Table 4. Activity and Selectivity of Selective Hydrogenation of Some Nitro and Nitrile Groups.
| substrate | product | catalyst | sel./conv. (%) | ref |
|---|---|---|---|---|
| 4-nitrophenol | 4-aminophenol | Pd/CeO2 | 99.9/100 | (106) |
| nitrobenzene | azoxybenzene | Pd(acac)2 | 96.8/100 | (107) |
| nitrobenzene | azoxybenzene | Pd | 87.6/100 | (108) |
| 2-nitroaniline | 2-phenylenediamine | VPY-NVP-Pd | 98/100 | (109) |
| nitrobenzenes | aniline | SiO2-BisILS[Cl]R-Pd | 100/100 | (110) |
| nitrophenol | aminophenol | Pd/GO | 99/100 | (111) |
| 2-methylnitrobenzene | 2-methylaniline | Pd | 100/100 | (112) |
| 4-chloronitrobenzene | 4-chloroaminobenzene | Pd/B-MCM-41 | 100/100 | (113) |
| 1-chloro-4-nitrobenzene | 4-chloro-aniline | PdFe | 99/100 | (114) |
| nitrobenzene | aminobenzene | Pd/PBA | 98/100 | (115) |
| nitroarenes | azoxybenzene | Pd/SiO2 | 92/100 | (116) |
| 4-vinyl nitrobenzene | 4-vinylaminobenzene | Pd/CSs | 100/100 | (117) |
| 4-chloronitrobenzene | 4-chloroaniline | Pd-BNNS | >99/100 | (118) |
| mandelonitrile | phenylethylamine | Pd/C | 87/100 | (119) |
| benzonitrile | benzylamine | Pd/C | 90/99 | (120) |
| benzonitrile | benzylamine | PdNi | 94/100 | (121) |
| 4-nitrostyrene | 4-aminostyrene | PdNPs/MAX | 93/100 | (122) |
Taking the hydrogenation of acetonitrile to ethyl amine as an example, the Pd(111) surface provides multiple adsorption sites. The acetonitrile reaction substrate and hydrogen are coadsorbed at the active sites of the catalyst. If the dissociated H is used exclusively for conversion to the intermediate of the desired amine product, the selectivity can be improved. The imine intermediate CH3CH=NH is crucial for the formation of secondary and tertiary amines. If there are few dissociated hydrogen atoms on the surface or if the active sites are difficult to access, these strongly bound intermediates may poison the catalyst’s surface, which reduces the selectivity.123 Pd in the catalyst mainly determines the activity of the catalyst. When an alloy is formed by doping other metals in a catalyst, changes in its chemical composition and microstructure will change its catalytic performance. Single-atom Pd1 is prepared by atomic layer deposition (ALD) on the surface of SiO2 loaded with Ni nanoparticles. Quasi-atomically dispersed Pd was formed on the outer layer of Ni nanoparticles, and a core–shell quasi-PdNi single-atom structure was formed by adjusting the Pd coverage on NiNPs (Figure 7A). The yield of dibenzylamine increased sharply from 5 to about 97% under H2/0.6 MPa and 80 °C. Studies have shown that using Ni to isolate Pd destroys the strong metal selectivity during the hydrogenation of −C≡N and promotes the yield of secondary amines to >94% using a wide range of −C≡N groups (Figure 7B). More importantly, the obtained material also showed excellent recyclability and completely inhibited the formation of hydrolysate byproducts, demonstrating its potential in practical applications.121
Figure 7.
(A) Schematic diagram for synthesis of the Pd–Ni/SiO2 bimetallic catalyst and (B) Pd/SiO2, Pt/SiO2, and PdNi/SiO2 catalytic performance diagram.121 Reproduced with permission from ref (121). Copyright 2019 Springer Nature.
The presence of a surface and an interface during a catalytic reaction directly affects a catalyst’s performance. The arrangement of surface atoms and coordination structures directly affects the adsorption mode of the substrate, thus affecting the activity and selectivity of a catalyst. Qu’s group synthesized a graphene oxide-supported Pd nanocatalyst (Pd/GO) by a one-step method. This Pd nanocatalyst, in which Pd nanoparticles were uniformly dispersed in GO, was cost-effective and environmentally friendly and efficiently reduced nitroaromatic compounds. Pd/GO nanocatalysts in aqueous solution converted nitroaromatic compounds into corresponding amino-aromatic compounds with up to 99% yield (Figure 8).111 Researchers from the University of Drexel reported the preparation of Ti3SiC2, Ti3AlC2, and Ti2AlC at 1500 °C with trace Pd doping. Ti3SiC2 without Pd loading and Ti3SiC2 loaded with Pd by an impregnation method were mixed mechanically to achieve more selective reduction. After optimizing the amount of Pd, the results showed that when the Pd content was 130 ppm, the conversion of 4-nitroacetylene reached 100%, and the selectivity was 93%. The well-dispersed Pd on the support and its support composition, surface, and metal–support interactions all played important roles in the reaction. The high chemoselectivity of Pd/MAX was attributed to the synergistic effect between the Pd nanoparticle size and dispersion and the non-Ti-containing oxides formed on the MAX phase, which preferentially activated the nitro group. H2 activation was a key step in chemical selective hydrogenation, and the role of Pd in the catalytic reaction was mainly to cleave and activate H2 molecules. They speculated that due to its low content, Pd may exist as single atoms, which enabled it to serve as a hydride receptor to activate H2, which is very effective for selective hydrogenation.122
Figure 8.

Pd loading supported on MAX phases for chemoselective hydrogenation.122 Reproduced with permission from ref (122). Copyright 2020 American Chemical Society.
In summary, for selective hydrogenation of nitro compounds, based on the current understanding of the reaction mechanism, the activity and selectivity of catalysts can be regulated from several aspects. First, since the hydrogenation of C=C is structurally sensitive and the hydrogenation of NO2 is not, the reaction of C=C can be inhibited by reducing the aggregation of metal atoms, such as by poisoning or modifying with organic ligands, forming alloys, or enhancing the selectivity of catalysts through strong metal–support interactions. Second, C=C is electron-rich, while −NO2 is electron-deficient; thus, we can construct nucleophilic sites on catalysts that strongly and selectively adsorb NO2 and weakly adsorb other groups by using oxygen-rich supports or by modifying the support surface with −NH2. Third, when the catalyst is decreased to the single-atom scale, hydrogen undergoes heterolytic cleavage, which allows it to more easily adsorb nitro groups to achieve selective hydrogenation.
4. Summary and Perspectives
Selective hydrogenation is a very important hydrogenation reaction that is widely used to synthesize fine chemicals, drugs, healthcare products, and agricultural chemicals. Typical reactions include partial hydrogenation of alkynes to alkenes, hydrogenation of aldehydes and ketones to unsaturated alcohols, and hydrogenation of nitro groups to amino groups. The grim state of the environment and the need to achieve “peak carbon dioxide emission, carbon neutralization” as soon as possible put forward unprecedented challenges for catalytic materials. Catalysts must have high chemical reactivity and high stability to save energy and high selectivity to achieve atom economy and reduce pollution and byproducts generated to achieve green chemical processes.
The performance of catalytic hydrogenation mainly depends on the interaction and reaction between H2 and metal unsaturated substrates. As pointed out by the Sabatier principle, the high activity requires that the interaction between the substrate and the metal site is neither too strong nor too weak.124 In addition, for substrates containing multiple unsaturated groups, the scaling relationship between the relevant adsorption groups on similar active metal sites often leads to inevitable overhydrogenation.125 To prevent overhydrogenation, it is important to understand the dissociation mode of H2 on the catalyst surface and the adsorption mode and reaction mechanism of the substrate at active sites during hydrogenation. With the progress of various characterization techniques and the guidance of DFT simulations, the structure of catalysts has become clearer; therefore, the rational design of the electronic and coordination structure is essential to optimize their hydrogenation performance.
First, the use of atomically dispersed catalysts reduces metal loading, and the separation of active sites determines the substrate’s adsorption mode, which prevents the occurrence of side reactions and is closer to achieving ″green catalysis″. The coordination environments of those atomically dispersed metal species, including the central metal atom and its oxidation state, coordination number and geometry, play together to induce steric and electronic effects to determine the overall catalytic performance. Moreover, atomically dispersed catalysts are desirable because the active metal atoms are 100% exposed and accessible to reactants and thus greatly improve the atom utilization efficiency of noble metal catalysts.
Second, changing the composition of the catalyst, forming alloys or intermetallic compounds, creating electronic and geometric ensemble effects that control the binding energetics of reactants or hydrogenated intermediates on metal surfaces can also be used to improve selectivity. Among these, single-atom alloy (SAA) catalysts are an ideal system because the electronic structure of the isolated catalytic sites tailored by the host metal can also regulate the adsorption structure of reactants or intermediates, therefore impacting both the activity and selectivity during hydrogenation.
Third, organic ligands containing N, P, and S atoms were used to modify the catalyst surface to change the atomic arrangement and coordination of the active elements to produce electronic or build up a confinement space generating steric hindrance effects that affected the substrate adsorption and increased the catalyst’s selectivity. Besides, the supported metal catalysts constructing metal–support interfaces not only make the heterogeneous activation of H2 possible to enhance the hydrogenation of polar unsaturated bonds but also make it easy to generate unsaturated low-valence metal sites on the reducible supports. Finally, catalyst selectivity can also be improved by confining metal atoms to a porous structure, forming strong metal–support interactions, and adjusting the reaction parameters.
Although these strategies for chemical selective hydrogenation have made exciting and encouraging progress, there are still some challenges to be overcome in fundamental research and practical applications. (1) Successful preparation of well-defined catalysts and accurate characterization of its active sites: The key to preparing atomically dispersed catalysts is that the supports need to provide atoms to coordinate with the catalytic single atoms. So, different supports such as ionic supports, metallic supports, and MOFs should be chosen according to metal valence. (2) Achieving both high activity and selectivity: In most situations, high chemoselectivity is often accompanied by a certain loss of activity by means of surface modifiers or doping other elements or oxides. Therefore, to achieve high chemical selectivity without affecting the activity, a promising way to develop in this direction is to construct well-defined catalysts with an adjustable coordination environment. (3) Mechanism understanding at the molecular level: The key factors including metal size, support, and ligand modifiers determine the performance of heterogeneous metal nanocatalysts, which manipulate the activation path of H2 and the interaction between the activated hydrogen species and the substrate on the surface of catalysts. The atomic dispersion feature can be identified by aberration-corrected TEM-STEM, and the average coordination structure can be identified by EXAFS. Precise in situ local characterization techniques such as Mössbauer spectroscopy, temperature-programmed desorption-mass spectrometry (TPD-MS), and nuclear magnetic resonance (NMR) should be applied for chemical environments of active sites in different structures. Combining DFT calculations, constructing a structure model reflecting its active coordination structure, and providing experimental evidence of the intermediates or labeled products expected from the proposed mechanism can help to incisively understand the catalytic mechanism.
In conclusion, rational design of the electronic and coordination structure of Pd-based catalysts, understanding the structure–activity relationships of Pd-based catalysts, and expanding large-scale application prospects for industrial applications are efficient ways to achieve green industrial hydrogenation techniques.
Acknowledgments
This work was supported by the NSF of China (21802085, 21805164), the General Project of the NSF of Fujian Province (2019J01730, 2020J01776, 2019J01735), and the Innovation and Entrepreneurship Projects for High-Level Talents of Quanzhou (2017Z028).
The authors declare no competing financial interest.
References
- Somorjai G. A.; Rioux R. M. High technology catalysts towards 100% selectivity fabrication, characterization and reaction studies. Catal. Today 2005, 100, 201–215. 10.1016/j.cattod.2004.07.059. [DOI] [Google Scholar]
- Noyori R. Synthesizing our future. Nat. Chem. 2009, 1, 5–6. 10.1038/nchem.143. [DOI] [PubMed] [Google Scholar]
- Vilé G.; Albani D.; Almora-Barrios N.; Lopez N.; Perez-Ramirez J. Advances in the Design of Nanostructured Catalysts for Selective Hydrogenation. ChemCatChem 2016, 8, 21–33. 10.1002/cctc.201501269. [DOI] [Google Scholar]
- Blaser H. U.; Malan C.; Pugin B.; Spindler F.; Steiner H.; Studer M. Selective hydrogenation for fine chemicals: Recent trends and new developments. Adv. Syn. Catal. 2003, 345, 103–151. 10.1002/adsc.200390000. [DOI] [Google Scholar]
- Meemken F.; Baiker A. Recent Progress in Heterogeneous Asymmetric Hydrogenation of C=O and C=C Bonds on Supported Noble Metal Catalysts. Chem. Rev. 2017, 117, 11522–11569. 10.1021/acs.chemrev.7b00272. [DOI] [PubMed] [Google Scholar]
- Sankar M.; Dimitratos N.; Miedziak P. J.; Wells P. P.; Kiely C. J.; Hutchings G. J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. 10.1039/c2cs35296f. [DOI] [PubMed] [Google Scholar]
- Michaelides I. N.; Dixon D. J. Catalytic stereoselective semihydrogenation of alkynes to E-alkenes. Angew. Chem., Int. Ed. 2013, 52, 806–808. 10.1002/anie.201208120. [DOI] [PubMed] [Google Scholar]
- Seifert W. K.; Condit P. C. Selective Catalytic Hydrogenation of Nitroölefins. J. Org. Chem. 1963, 28, 265–267. 10.1021/jo01036a536. [DOI] [Google Scholar]
- Chernichenko K.; Madarász Á.; Pápai I.; Nieger M.; Leskelä M.; Repo T. A frustrated-Lewis-pair approach to catalytic reduction of alkynes to cis-alkenes. Nat. Chem. 2013, 5, 718–723. 10.1038/nchem.1693. [DOI] [PubMed] [Google Scholar]
- Furukawa S.; Komatsu T. Selective Hydrogenation of Functionalized Alkynes to (E)-Alkenes, Using Ordered Alloys as Catalysts. ACS Catal. 2016, 6, 2121–2125. 10.1021/acscatal.5b02953. [DOI] [Google Scholar]
- Chen X.; Engle K. M.; Wang D.-H.; Yu J.-Q. Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo I. E. M. d. S.; de Sousa S. A. A.; Pereira L. N. d. S.; Oliveira J. M.; Castro K. P. R.; Costa J. C. S.; de Moura E. M.; de Moura C. V. R.; Garcia M. A. S. Au–Pd Selectivity-switchable Alcohol-oxidation Catalyst: Controlling the Duality of the Mechanism using a Multivariate Approach. ChemCatChem 2019, 11, 3022–3034. 10.1002/cctc.201900512. [DOI] [Google Scholar]
- Tessonnier J.-P.; Pesant L.; Ehret G.; Ledoux M. J.; Pham-Huu C. Pd nanoparticles introduced inside multi-walled carbon nanotubes for selective hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. Appl. Catal., A 2005, 288, 203–210. 10.1016/j.apcata.2005.04.034. [DOI] [Google Scholar]
- Schoenbaum C. A.; Schwartz D. K.; Medlin J. W. Controlling the Surface Environment of Heterogeneous Catalysts Using Self-Assembled Monolayers. Acc. Chem. Res. 2014, 47, 1438–1445. 10.1021/ar500029y. [DOI] [PubMed] [Google Scholar]
- Vilé G.; Almora-Barrios N.; Mitchell S.; Lopez N.; Perez-Ramirez J. From the Lindlar Catalyst to Supported Ligand-Modified Palladium Nanoparticles: Selectivity Patterns and Accessibility Constraints in the Continuous-Flow Three-Phase Hydrogenation of Acetylenic Compounds. Chem.—Eur. J. 2014, 20, 5926–5937. 10.1002/chem.201304795. [DOI] [PubMed] [Google Scholar]
- Niu W.; Gao Y.; Zhang W.; Yan N.; Lu X. Pd-Pb Alloy Nanocrystals with Tailored Composition for Semihydrogenation: Taking Advantage of Catalyst Poisoning. Angew. Chem., Int. Ed. 2015, 54, 8271–8274. 10.1002/anie.201503148. [DOI] [PubMed] [Google Scholar]
- Liu K.; Qin R.; Zheng N. Insights into the Interfacial Effects in Heterogeneous Metal Nanocatalysts toward Selective Hydrogenation. J. Am. Chem. Soc. 2021, 143, 4483–4499. 10.1021/jacs.0c13185. [DOI] [PubMed] [Google Scholar]
- Grabovskii S. A.; Akchurin T. I.; Dokichev V. A. Heterogeneous Palladium Catalysts in the Hydrogenation of the Carbon-carbon Double Bond. Curr. Org. Chem. 2021, 25, 315–329. 10.2174/1385272824999201202084812. [DOI] [Google Scholar]
- Monguchi Y.; Ichikawa T.; Sajiki H. Recent Development of Palladium-Supported Catalysts for Chemoselective Hydrogenation. Chem. Pharm. Bull. 2017, 65, 2–9. 10.1248/cpb.c16-00153. [DOI] [PubMed] [Google Scholar]
- McCue A. J.; Anderson J. A. Recent advances in selective acetylene hydrogenation using palladium containing catalysts. Front. Chem. Sci. Eng. 2015, 9, 142–153. 10.1007/s11705-015-1516-4. [DOI] [Google Scholar]
- Wang S.; Zhao Z. J.; Chang X.; Zhao J.; Tian H.; Yang C.; Li M.; Fu Q.; Mu R.; Gong J. Activation and Spillover of Hydrogen on Sub-1 nm Palladium Nanoclusters Confined within Sodalite Zeolite for the Semi-Hydrogenation of Alkynes. Angew. Chem., Int. Ed. 2019, 58, 7668–7672. 10.1002/anie.201903827. [DOI] [PubMed] [Google Scholar]
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]
- Teschner D.; Borsodi J.; Wootsch A.; Revay Z.; Havecker M.; Knop-Gericke A.; Jackson S. D.; Schlogl R. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 2008, 320, 86–89. 10.1126/science.1155200. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhou M.; Wang A.; Zhang T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2020, 120, 683–733. 10.1021/acs.chemrev.9b00230. [DOI] [PubMed] [Google Scholar]
- Alshakova I. D.; Gabidullin B.; Nikonov G. I. Ru-Catalyzed Transfer Hydrogenation of Nitriles, Aromatics, Olefins, Alkynes and Esters. ChemCatChem 2018, 10, 4860–4869. 10.1002/cctc.201801039. [DOI] [Google Scholar]
- Jagtap S. A.; Bhanage B. M. Ligand Assisted Rhodium Catalyzed Selective Semi-hydrogenation of Alkynes Using Syngas and Molecular Hydrogen. ChemistrySelect 2018, 3, 713–718. 10.1002/slct.201702976. [DOI] [Google Scholar]
- Liu K.; Qin R.; Zhou L.; Liu P.; Zhang Q.; Jing W.; Ruan P.; Gu L.; Fu G.; Zheng N. Cu2O-Supported Atomically Dispersed Pd Catalysts for Semihydrogenation of Terminal Alkynes: Critical Role of Oxide Supports. CCS Chem. 2019, 1, 207–214. 10.31635/ccschem.019.20190008. [DOI] [Google Scholar]
- Frey G. D.; Lavallo V.; Donnadieu B.; Schoeller W. W.; Bertrand G. Facile Splitting of Hydrogen and Ammonia by Nucleophilic Activation at a Single Carbon Center. Science 2007, 316, 439–441. 10.1126/science.1141474. [DOI] [PubMed] [Google Scholar]
- An K.; Somorjai G. A. Size and Shape Control of Metal Nanoparticles for Reaction Selectivity in Catalysis. ChemCatChem 2012, 4, 1512–1524. 10.1002/cctc.201200229. [DOI] [Google Scholar]
- Roldan Cuenya B.; Behafarid F. Nanocatalysis: size- and shape-dependent chemisorption and catalytic reactivity. Surf. Sci. Rep. 2015, 70, 135–187. 10.1016/j.surfrep.2015.01.001. [DOI] [Google Scholar]
- da Silva F. P.; Fiorio J. L.; Rossi L. M. Tuning the Catalytic Activity and Selectivity of Pd Nanoparticles Using Ligand-Modified Supports and Surfaces. ACS Omega 2017, 2, 6014–6022. 10.1021/acsomega.7b00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N.; Zhang T. Preface: single-atom catalysts as a new generation of heterogeneous catalysts. Natl. Sci. Rev. 2018, 5, 625. 10.1093/nsr/nwy095. [DOI] [Google Scholar]
- Li Z.; Ji S.; Liu Y.; Cao X.; Tian S.; Chen Y.; Niu Z.; Li Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. 10.1021/acs.chemrev.9b00311. [DOI] [PubMed] [Google Scholar]
- Abdollahi T.; Farmanzadeh D. Selective hydrogenation of acetylene in the presence of ethylene on palladium nanocluster surfaces: A DFT study. Appl. Surf. Sci. 2018, 433, 513–529. 10.1016/j.apsusc.2017.10.085. [DOI] [Google Scholar]
- Li M.; Shen J. Microcalorimetric studies of O2 and C2H4 adsorption on Pd/SiO2 catalysts modi ed by Cu and Ag. Thermochim. Acta 2001, 379, 45–50. 10.1016/S0040-6031(01)00600-1. [DOI] [Google Scholar]
- Hamm G.; Schmidt T.; Breitbach J.; Franke D.; Becker C.; Wandelt K. The Adsorption of Ethene on Pd(111) and Ordered Sn/Pd(111) Surface Alloys. Surf. Sci. 2004, 562, 170–182. 10.1016/j.susc.2004.05.119. [DOI] [Google Scholar]
- Pei G. X.; Liu X. Y.; Wang A.; Lee A. F.; Isaacs M. A.; Li L.; Pan X.; Yang X.; Wang X.; Tai Z.; Wilson K.; Zhang T. Ag Alloyed Pd Single-Atom Catalysts for Efficient Selective Hydrogenation of Acetylene to Ethylene in Excess Ethylene. ACS Catal. 2015, 5, 3717–3725. 10.1021/acscatal.5b00700. [DOI] [Google Scholar]
- Vilé G.; Albani D.; Nachtegaal M.; Chen Z.; Dontsova D.; Antonietti M.; Lopez N.; Perez-Ramirez J. A stable single-site palladium catalyst for hydrogenations. Angew. Chem., Int. Ed. 2015, 54, 11265–11269. 10.1002/anie.201505073. [DOI] [PubMed] [Google Scholar]
- Kyriakou G.; Boucher M. B.; Jewell A. D.; Lewis E. A.; Lawton T. J.; Baber A. E.; Tierney H. L.; Flytzani-Stephanopoulos M.; Sykes E. C. H. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335, 1209–1212. 10.1126/science.1215864. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Xu W.; Xu L.; Shao Q.; Huang X. Concavity Tuning of Intermetallic Pd–Pb Nanocubes for Selective Semihydrogenation Catalysis. Chem. Mater. 2018, 30, 6338–6345. 10.1021/acs.chemmater.8b02337. [DOI] [Google Scholar]
- Saranya A.; Vivekanandan G.; Thirunavukkarasu K.; Krishnamurthy K. R.; Viswanathan B. Studies on palladium based bimetallic catalysts Pd-M/TiO2 (M = Cu, Ag & Au): I-Selective hydrogenation of 1-heptyne. Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem. 2019, 58, 271–280. [Google Scholar]
- Zhang R. G.; Xue M. F.; Wang B. J.; Ling L. X.; Fan M. H. C2H2 Selective Hydrogenation over the M@Pd and M@Cu (M = Au, Ag, Cu, and Pd) Core-Shell Nanocluster Catalysts: The Effects of Composition and Nanocluster Size on Catalytic Activity and Selectivity. J. Phys. Chem. C 2019, 123, 16107–16117. 10.1021/acs.jpcc.9b01757. [DOI] [Google Scholar]
- Yang J. Y.; Fan Y. P.; Li Z. L.; Peng Z. K.; Yang J. H.; Liu B. Z.; Liu Z. Y. Bimetallic Pd-M (M= Pt, Ni, Cu, Co) nanoparticles catalysts with strong electrostatic metal-support interaction for hydrogenation of toluene and benzene. Mol. Catal. 2020, 492, 110992. 10.1016/j.mcat.2020.110992. [DOI] [Google Scholar]
- Cao X. X.; Mirjalili A.; Xie W. T.; Jang W. L. Selective hydrogenation of acetylene in ethylene over Cu-Pd catalysts. Abstr. Pap. Am. Chem. Soc. 2016, 251. [Google Scholar]
- Lomelí-Rosales D. A.; Delgado J. A.; Diaz de Los Bernardos M.; Perez-Rodriguez S.; Gual A.; Claver C.; Godard C. A General One-Pot Methodology for the Preparation of Mono- and Bimetallic Nanoparticles Supported on Carbon Nanotubes: Application in the Semi-hydrogenation of Alkynes and Acetylene. Chem.—Eur. J. 2019, 25, 8321–8331. 10.1002/chem.201901041. [DOI] [PubMed] [Google Scholar]
- Pei G. X.; Liu X. Y.; Yang X.; Zhang L.; Wang A.; Li L.; Wang H.; Wang X.; Zhang T. Performance of Cu-Alloyed Pd Single-Atom Catalyst for Semihydrogenation of Acetylene under Simulated Front-End Conditions. ACS Catal. 2017, 7, 1491–1500. 10.1021/acscatal.6b03293. [DOI] [Google Scholar]
- Dong Z.; Dong C.; Liu Y.; Le X.; Jin Z.; Ma J. Hydrodechlorination and further hydrogenation of 4-chlorophenol to cyclohexanone in water over Pd nanoparticles modified N-doped mesoporous carbon microspheres. Chem. Eng. J. 2015, 270, 215–222. 10.1016/j.cej.2015.02.045. [DOI] [Google Scholar]
- Ding S.; Zhang C.; Liu Y.; Jiang H.; Chen R. Selective hydrogenation of phenol to cyclohexanone in water over Pd@N-doped carbons derived from ZIF-67: Role of dicyandiamide. Appl. Surf. Sci. 2017, 425, 484–491. 10.1016/j.apsusc.2017.07.068. [DOI] [Google Scholar]
- Zhang C.; Zhang J.; Shao Y.; Jiang H.; Chen R.; Xing W. Controllable Synthesis of 1D Pd@N-CNFs with High Catalytic Performance for Phenol Hydrogenation. Catal. Lett. 2021, 151, 1013. 10.1007/s10562-020-03374-x. [DOI] [Google Scholar]
- Chen L. J.; Wan C. C.; Wang Y. Y. Chemical preparation of Pd nanoparticles in room temperature ethylene glycol system and its application to electroless copper deposition. J. Colloid Interface Sci. 2006, 297, 143–150. 10.1016/j.jcis.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Wang X.; Choi S. I.; Roling L. T.; Luo M.; Ma C.; Zhang L.; Chi M.; Liu J.; Xie Z.; Herron J. A.; Mavrikakis M.; Xia Y. Palladium-platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 7594 10.1038/ncomms8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Vara M.; Luo M.; Huang H.; Ruditskiy A.; Park J.; Bao S.; Liu J.; Howe J.; Chi M.; Xie Z.; Xia Y. Pd@Pt Core–Shell Concave Decahedra: A Class of Catalysts for the Oxygen Reduction Reaction with Enhanced Activity and Durability. J. Am. Chem. Soc. 2015, 137, 15036–15042. 10.1021/jacs.5b10059. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zheng S.; Wan N.; Zhang L.; Wang Q.; He N.; Huang Y. Synthesis of a Au-on-Pd Heteronanostructure Stabilized by Citrate and its Catalytic Application. Part. Part. Syst. Charact. 2013, 30, 905–910. 10.1002/ppsc.201300152. [DOI] [Google Scholar]
- Effenberger F. B.; Sulca M. A.; Machini M. T.; Couto R. A.; Kiyohara P. K.; Machado G.; Rossi L. M. Copper nanoparticles synthesized by thermal decomposition in liquid phase: the influence of capping ligands on the synthesis and bactericidal activity. J. Nanopart. Res. 2014, 16, 2588 10.1007/s11051-014-2588-7. [DOI] [Google Scholar]
- Crespo-Quesada M.; Yarulin A.; Jin M.; Xia Y.; Kiwi-Minsker L. Structure sensitivity of alkynol hydrogenation on shape- and size-controlled palladium nanocrystals: which sites are most active and selective?. J. Am. Chem. Soc. 2011, 133, 12787–12794. 10.1021/ja204557m. [DOI] [PubMed] [Google Scholar]
- Chan-Thaw C. E.; Villa A.; Wang D.; Dal Santo V.; Biroli A. O.; Veith G. M.; Thomas A.; Prati L. PdHx Entrapped in a Covalent Triazine Framework Modulates Selectivity in Glycerol Oxidation. ChemCatChem 2015, 7, 2149–2154. 10.1002/cctc.201500055. [DOI] [Google Scholar]
- Wu L.; Li Z.-W.; Zhang F.; He Y.-M.; Fan Q.-H. Air-Stable and Highly Active Dendritic Phosphine Oxide- Stabilized Palladium Nanoparticles: Preparation, Characterization and Applications in the Carbon-Carbon Bond Formation and Hydrogenation Reactions. Adv. Synth. Catal. 2008, 350, 846–862. 10.1002/adsc.200700441. [DOI] [Google Scholar]
- Kumar S.; Rao G. K.; Kumar A.; Singh M. P.; Saleem F.; Singh A. K. Efficient catalytic activation of Suzuki–Miyaura C–C coupling reactions with recyclable palladium nanoparticles tailored with sterically demanding di-n-alkyl sulfides. RSC Adv. 2015, 5, 20081–20089. 10.1039/C5RA00441A. [DOI] [Google Scholar]
- Zhao X.; Zhou L.; Zhang W.; Hu C.; Dai L.; Ren L.; Wu B.; Fu G.; Zheng N. Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes. Chem 2018, 4, 1080–1091. 10.1016/j.chempr.2018.02.011. [DOI] [Google Scholar]
- Jin M.; Zhang H.; Xie Z.; Xia Y. Palladium nanocrystals enclosed by {100} and {111} facets in controlled proportions and their catalytic activities for formic acid oxidation. Energy Environ. Sci. 2012, 5, 6352–6357. 10.1039/C2EE02866B. [DOI] [Google Scholar]
- Rossi L. M.; Fiorio J. L.; Garcia M. A. S.; Ferraz C. P. The role and fate of capping ligands in colloidally prepared metal nanoparticle catalysts. Dalton Trans. 2018, 47, 5889–5915. 10.1039/C7DT04728B. [DOI] [PubMed] [Google Scholar]
- Kahsar K. R.; Johnson S.; Schwartz D. K.; Medlin J. W. Hydrogenation of Cinnamaldehyde over Pd/Al2O3 Catalysts Modified with Thiol Monolayers. Top. Catal. 2014, 57, 1505–1511. 10.1007/s11244-014-0325-1. [DOI] [Google Scholar]
- Wang Y.; Wan X. K.; Ren L. T.; Su H. F.; Li G.; Malola S.; Lin S. C.; Tang Z. C.; Hakkinen H.; Teo B. K.; Wang Q. M.; Zheng N. F. Atomically Precise Alkynyl-Protected Metal Nanoclusters as a Model Catalyst: Observation of Promoting Effect of Surface Ligands on Catalysis by Metal Nanoparticles. J. Am. Chem. Soc. 2016, 138, 3278–3281. 10.1021/jacs.5b12730. [DOI] [PubMed] [Google Scholar]
- Sun C.; Mammen N.; Kaappa S.; Yuan P.; Deng G.; Zhao C.; Yan J.; Malola S.; Honkala K.; Häkkinen H.; Teo B. K.; Zheng N. Atomically Precise, Thiolated Copper–Hydride Nanoclusters as Single-Site Hydrogenation Catalysts for Ketones in Mild Conditions. ACS Nano 2019, 13, 5975–5986. 10.1021/acsnano.9b02052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Xin Z.; Huang X.; Yu W.; Niu S.; Shao L. Nanosized Pd-Au bimetallic phases on carbon nanotubes for selective phenylacetylene hydrogenation. Phys. Chem. Chem. Phys. 2017, 19, 6164–6168. 10.1039/C6CP08805H. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Ding Y.; Wu K. H.; Niu Y.; Luo J.; Yang X.; Zhang B.; Su D. Pd@C core-shell nanoparticles on carbon nanotubes as highly stable and selective catalysts for hydrogenation of acetylene to ethylene. Nanoscale 2017, 9, 14317–14321. 10.1039/C7NR04992G. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Shang L.; Zhao Y.; Shi R.; Waterhouse G. I. N.; Huang Y. C.; Zheng L.; Zhang T. Pd Single-Atom Catalysts on Nitrogen-Doped Graphene for the Highly Selective Photothermal Hydrogenation of Acetylene to Ethylene. Adv. Mater. 2019, 31, 1900509. 10.1002/adma.201900509. [DOI] [PubMed] [Google Scholar]
- Khouya A. A.; Ba H.; Baaziz W.; Nhut J.-M.; Rossin A.; Zafeiratos S.; Ersen O.; Giambastiani G.; Ritleng V.; Pham-Huu C. Palladium Nanosheet-Carbon Black Powder Composites for Selective Hydrogenation of Alkynes to Alkenes. ACS Appl. Nano Mater. 2021, 4, 2265–2277. 10.1021/acsanm.1c00002. [DOI] [Google Scholar]
- Lee H.; Nguyen-Huy C.; Jeong Jang E.; Lee J.; Yang E.; Lee M. S.; Kwak J. H.; An K. Interfacial effect of Pd supported on mesoporous oxide for catalytic furfural hydrogenation. Catal. Today 2021, 365, 291–300. 10.1016/j.cattod.2020.02.035. [DOI] [Google Scholar]
- Crespo-Quesada M.; Cardenas-Lizana F.; Dessimoz A.-L.; Kiwi-Minsker L. Modern Trends in Catalyst and Process Design for Alkyne Hydrogenations. ACS Catal. 2012, 2, 1773–1786. 10.1021/cs300284r. [DOI] [Google Scholar]
- McCue A. J.; Anderson J. A. Recent advances in selective acetylene hydrogenation using palladium containing catalysts. Front. Chem. Sci. Eng. 2015, 9, 142–153. 10.1007/s11705-015-1516-4. [DOI] [Google Scholar]
- McCue A. J.; Guerrero-Ruiz A.; Rodríguez-Ramos I.; Anderson J. A. Palladium sulphide – A highly selective catalyst for the gas phase hydrogenation of alkynes to alkenes. J. Catal. 2016, 340, 10–16. 10.1016/j.jcat.2016.05.002. [DOI] [Google Scholar]
- Wei S.; Li A.; Liu J. C.; Li Z.; Chen W.; Gong Y.; Zhang Q.; Cheong W. C.; Wang Y.; Zheng L.; Xiao H.; Chen C.; Wang D.; Peng Q.; Gu L.; Han X.; Li J.; Li Y. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 2018, 13, 856–861. 10.1038/s41565-018-0197-9. [DOI] [PubMed] [Google Scholar]
- Huang F.; Deng Y.; Chen Y.; Cai X.; Peng M.; Jia Z.; Ren P.; Xiao D.; Wen X.; Wang N.; Liu H.; Ma D. Atomically Dispersed Pd on Nanodiamond/Graphene Hybrid for Selective Hydrogenation of Acetylene. J. Am. Chem. Soc. 2018, 140, 13142–13146. 10.1021/jacs.8b07476. [DOI] [PubMed] [Google Scholar]
- Mitsudome T.; Urayama T.; Yamazaki K.; Maehara Y.; Yamasaki J.; Gohara K.; Maeno Z.; Mizugaki T.; Jitsukawa K.; Kaneda K. Design of Core-Pd/Shell-Ag Nanocomposite Catalyst for Selective Semihydrogenation of Alkynes. ACS Catal. 2016, 6, 666–670. 10.1021/acscatal.5b02518. [DOI] [Google Scholar]
- Yang S.; Cao C.; Peng L.; Zhang J.; Han B.; Song W. A Pd-Cu2O nanocomposite as an effective synergistic catalyst for selective semi-hydrogenation of the terminal alkynes only. Chem. Commun. 2016, 52, 3627–3630. 10.1039/C6CC00143B. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Calcio Gaudino E.; Rotolo L.; Medlock J.; Bonrath W.; Cravotto G. Efficient partial hydrogenation of 2-butyne-1,4-diol and other alkynes under microwave irradiation. Chem. Eng. Process. 2016, 110, 220–224. 10.1016/j.cep.2016.10.016. [DOI] [Google Scholar]
- Sittikun J.; Boonyongmaneerat Y.; Weerachawanasak P.; Praserthdam P.; Panpranot J. Pd/TiO2 catalysts prepared by electroless deposition with and without SnCl2 sensitization for the liquid-phase hydrogenation of 3-hexyn-1-ol. React. Kinet., Mech. Catal. 2014, 111, 123–135. 10.1007/s11144-013-0634-6. [DOI] [Google Scholar]
- Liu J.; Uhlman M. B.; Montemore M. M.; Trimpalis A.; Giannakakis G.; Shan J.; Cao S.; Hannagan R. T.; Sykes E. C. H.; Flytzani-Stephanopoulos M. Integrated Catalysis-Surface Science-Theory Approach to Understand Selectivity in the Hydrogenation of 1-Hexyne to 1-Hexene on PdAu Single-Atom Alloy Catalysts. ACS Catal. 2019, 9, 8757–8765. 10.1021/acscatal.9b00491. [DOI] [Google Scholar]
- Liu Y.; Li Y.; Anderson J. A.; Feng J.; Guerrero-Ruiz A.; Rodríguez-Ramos I.; McCue A. J.; Li D. Comparison of Pd and Pd4S based catalysts for partial hydrogenation of external and internal butynes. J. Catal. 2020, 383, 51–59. 10.1016/j.jcat.2020.01.010. [DOI] [Google Scholar]
- Friedrich M. F.; Lucas M.; Claus P. Selective hydrogenation of propyne on a solid Pd/Al2O3 catalyst modified with ionic liquid layer (SCILL). Catal. Commun. 2017, 88, 73–76. 10.1016/j.catcom.2016.09.036. [DOI] [Google Scholar]
- Lu Y.; Feng X.; Takale B. S.; Yamamoto Y.; Zhang W.; Bao M. Highly Selective Semihydrogenation of Alkynes to Alkenes by Using an Unsupported Nanoporous Palladium Catalyst: No Leaching of Palladium into the Reaction Mixture. ACS Catal. 2017, 7, 8296–8303. 10.1021/acscatal.7b02915. [DOI] [Google Scholar]
- Kuwahara Y.; Kango H.; Yamashita H. Pd Nanoparticles and Aminopolymers Confined in Hollow Silica Spheres as Efficient and Reusable Heterogeneous Catalysts for Semihydrogenation of Alkynes. ACS Catal. 2019, 9, 1993–2006. 10.1021/acscatal.8b04653. [DOI] [Google Scholar]
- Zhou H.; Yang X.; Li L.; Liu X.; Huang Y.; Pan X.; Wang A.; Li J.; Zhang T. PdZn Intermetallic Nanostructure with Pd–Zn–Pd Ensembles for Highly Active and Chemoselective Semi-Hydrogenation of Acetylene. ACS Catal. 2016, 6, 1054–1061. 10.1021/acscatal.5b01933. [DOI] [Google Scholar]
- Liu P.; Zheng N. Coordination chemistry of atomically dispersed catalysts. Natl. Sci. Rev. 2018, 5, 636–638. 10.1093/nsr/nwy051. [DOI] [Google Scholar]
- Rossell M. D.; Caparrós F. J.; Angurell I.; Muller G.; Llorca J.; Seco M.; Rossell O. Magnetite-supported palladium single-atoms do not catalyse the hydrogenation of alkenes but small clusters do. Catal. Sci. Technol. 2016, 6, 4081–4085. 10.1039/C6CY00596A. [DOI] [Google Scholar]
- Vorobyeva E.; Chen Z.; Mitchell S.; Leary R. K.; Midgley P.; Thomas J. M.; Hauert R.; Fako E.; López N.; Pérez-Ramírez J. Tailoring the framework composition of carbon nitride to improve the catalytic efficiency of the stabilised palladium atoms. J. Mater. Chem. A 2017, 5, 16393–16403. 10.1039/C7TA04607C. [DOI] [Google Scholar]
- Gombos R.; Nagyházi B.; Joó F. Hydrogenation of α,β-unsaturated aldehydes in aqueous media with a water-soluble Pd(II)-sulfosalan complex catalyst. React. Kinet., Mech. Catal. 2019, 126, 439–451. 10.1007/s11144-018-1488-8. [DOI] [Google Scholar]
- Zhang Y.; Yang X.; Zhou Y.; Li G.; Li Z.; Liu C.; Bao M.; Shen W. Selective hydrogenation of the C=C bond in α,β-unsaturated aldehydes and ketones over ultra-small Pd–Au clusters. Nanoscale 2016, 8, 18626–18629. 10.1039/C6NR07013B. [DOI] [PubMed] [Google Scholar]
- Kuai L.; Chen Z.; Liu S.; Kan E.; Yu N.; Ren Y.; Fang C.; Li X.; Li Y.; Geng B. Titania supported synergistic palladium single atoms and nanoparticles for room temperature ketone and aldehydes hydrogenation. Nat. Commun. 2020, 11, 48 10.1038/s41467-019-13941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S.-i.; Mitani H.; Zhang C.; Li K.; Zhao F.; Arai M. Pd and PdZn supported on ZnO as catalysts for the hydrogenation of cinnamaldehyde to hydrocinnamyl alcohol. Mol. Catal. 2017, 442, 12–19. 10.1016/j.mcat.2017.08.018. [DOI] [Google Scholar]
- Nagpure A. S.; Gurrala L.; Gogoi P.; Chilukuri S. V. Hydrogenation of cinnamaldehyde to hydrocinnamaldehyde over Pd nanoparticles deposited on nitrogen-doped mesoporous carbon. RSC Adv. 2016, 6, 44333–44340. 10.1039/C6RA04154J. [DOI] [Google Scholar]
- Dong S.; Liu Z.; Liu R.; Chen L.; Chen J.; Xu Y. Visible-Light-Induced Catalytic Transfer Hydrogenation of Aromatic Aldehydes by Palladium Immobilized on Amine-Functionalized Iron-Based Metal–Organic Frameworks. ACS Appl. Nano. Mater. 2018, 1, 4247–4257. 10.1021/acsanm.8b01039. [DOI] [Google Scholar]
- Liao W.; Zhu Z.; Chen N.; Su T.; Deng C.; Zhao Y.; Ren W.; Lü H. Highly active bifunctional Pd-Co9S8/S-CNT catalysts for selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran. Mol. Catal. 2020, 482, 110756 10.1016/j.mcat.2019.110756. [DOI] [Google Scholar]
- Deng Q.; Wen X.; Zhang P. Pd/Cu-MOF as a highly efficient catalyst for synthesis of cyclopentanone compounds from biomass-derived furanic aldehydes. Catal. Commun. 2019, 126, 5–9. 10.1016/j.catcom.2019.04.008. [DOI] [Google Scholar]
- Chen P.; Li W.; Wang Y. Atmospheric hydrogenation of α, β-unsaturated ketones catalyzed by highly efficient and recyclable Pd nanocatalyst. Catal. Commun. 2019, 125, 10–14. 10.1016/j.catcom.2019.03.008. [DOI] [Google Scholar]
- Han J.; Kim Y.-H.; Jang H.-S.; Hwang S.-Y.; Jegal J.; Kim J. W.; Lee Y.-S. Heterogeneous zirconia-supported ruthenium catalyst for highly selective hydrogenation of 5-hydroxymethyl-2-furaldehyde to 2,5-bis(hydroxymethyl)furans in various n-alcohol solvents. RSC Adv. 2016, 6, 93394–93397. 10.1039/C6RA18016G. [DOI] [Google Scholar]
- Pârvulescu V. I.; Pârvulescu V.; Endruschat U.; Filoti G.; Wagner F. E.; Kübel C.; Richards R. Characterization and Catalytic-Hydrogenation Behavior of SiO2-Embedded Nanoscopic Pd, Au, and Pd–Au Alloy Colloids. Chem.—Eur. J. 2006, 12, 2343–2357. 10.1002/chem.200500971. [DOI] [PubMed] [Google Scholar]
- Cattaneo S.; Freakley S. J.; Morgan D. J.; Sankar M.; Dimitratos N.; Hutchings G. J. Cinnamaldehyde hydrogenation using Au–Pd catalysts prepared by sol immobilisation. Catal. Sci. Technol. 2018, 8, 1677–1685. 10.1039/C7CY02556D. [DOI] [Google Scholar]
- Schoenbaum C. A.; Schwartz D. K.; Medlin J. W. Controlling the surface environment of heterogeneous catalysts using self-assembled monolayers. Acc. Chem. Res. 2014, 47, 1438–1445. 10.1021/ar500029y. [DOI] [PubMed] [Google Scholar]
- Wu B.; Huang H.; Yang J.; Zheng N.; Fu G. Selective Hydrogenation of α,β-Unsaturated Aldehydes Catalyzed by Amine-Capped Platinum-Cobalt Nanocrystals. Angew. Chem., Int. Ed. 2012, 51, 3440–3443. 10.1002/anie.201108593. [DOI] [PubMed] [Google Scholar]
- Guo M.; Li H.; Ren Y.; Ren X.; Yang Q.; Li C. Improving Catalytic Hydrogenation Performance of Pd Nanoparticles by Electronic Modulation Using Phosphine Ligands. ACS Catal. 2018, 8, 6476–6485. 10.1021/acscatal.8b00872. [DOI] [Google Scholar]
- Pang S. H.; Schoenbaum C. A.; Schwartz D. K.; Medlin J. W. Directing reaction pathways by catalyst active-site selection using self-assembled monolayers. Nat. Commun. 2013, 4, 2448 10.1038/ncomms3448. [DOI] [PubMed] [Google Scholar]
- Kahsar K. R.; Schwartz D. K.; Medlin J. W. Control of Metal Catalyst Selectivity through Specific Noncovalent Molecular Interactions. J. Am. Chem. Soc. 2014, 136, 520–526. 10.1021/ja411973p. [DOI] [PubMed] [Google Scholar]
- Alexander A.-M.; Hargreaves J. S. J. Alternative catalytic materials: carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev. 2010, 39, 4388–4401. 10.1039/b916787k. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Chang C. R.; Huang Z. Q.; Li J.; Wu Z.; Ma Y.; Zhang Z.; Wang Y.; Qu Y. High Catalytic Activity and Chemoselectivity of Sub-nanometric Pd Clusters on Porous Nanorods of CeO2 for Hydrogenation of Nitroarenes. J. Am. Chem. Soc. 2016, 138, 2629–2637. 10.1021/jacs.5b11413. [DOI] [PubMed] [Google Scholar]
- Wang J.; Hu L.; Cao X.; Lu J.; Li X.; Gu H. Catalysis by Pd nanoclusters generated in situ of high-efficiency synthesis of aromatic azo compounds from nitroaromatics under H2 atmosphere. RSC Adv. 2013, 3, 4899. 10.1039/c3ra23004j. [DOI] [Google Scholar]
- Hu L.; Cao X.; Shi L.; Qi F.; Guo Z.; Lu J.; Gu H. A Highly Active Nano-Palladium Catalyst for the Preparation of Aromatic Azos under Mild Conditions. Org. Lett. 2011, 13, 5640–5643. 10.1021/ol202362f. [DOI] [PubMed] [Google Scholar]
- Xu S.; Xi X.; Shi J.; Cao S. A homogeneous catalyst made of poly 4-vinylpyridine-co-N-vinylpyrrolidone -Pd 0 complex for hydrogenation of aromatic nitro compounds. J. Mol. Catal. A: Chem. 2000, 160, 287–292. 10.1016/S1381-1169(00)00263-6. [DOI] [Google Scholar]
- Li J.; Shi X.-Y.; Bi Y.-Y.; Wei J.-F.; Chen Z.-G. Pd Nanoparticles in Ionic Liquid Brush: A Highly Active and Reusable Heterogeneous Catalytic Assembly for Solvent-Free or On-Water Hydrogenation of Nitroarene under Mild Conditions. ACS Catal. 2011, 1, 657–664. 10.1021/cs200105u. [DOI] [Google Scholar]
- Zhang K.; Hong K.; Suh J. M.; Lee T. H.; Kwon O.; Shokouhimehr M.; Jang H. W. Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res. Chem. Intermed. 2019, 45, 599–611. 10.1007/s11164-018-3621-8. [DOI] [Google Scholar]
- Arai N.; Onodera N.; Dekita A.; Hori J.; Ohkuma T. Hydrogenation of nitroarenes with palladium nanoparticles stabilized by alkyne derivatives in homogeneous phase. Tetrahedron Lett. 2015, 56, 3913–3915. 10.1016/j.tetlet.2015.04.116. [DOI] [Google Scholar]
- Chatterjee M.; Ishizaka T.; Suzuki T.; Suzuki A.; Kawanami H. In situ synthesized Pd nanoparticles supported on B-MCM-41: an efficient catalyst for hydrogenation of nitroaromatics in supercritical carbon dioxide. Green Chem. 2012, 14, 3415. 10.1039/c2gc36160d. [DOI] [Google Scholar]
- Gabriel C. M.; Parmentier M.; Riegert C.; Lanz M.; Handa S.; Lipshutz B. H.; Gallou F. Sustainable and Scalable Fe/ppm Pd Nanoparticle Nitro Group Reductions in Water at Room Temperature. Org. Process Res. Dev. 2017, 21, 247–252. 10.1021/acs.oprd.6b00410. [DOI] [Google Scholar]
- Zhang K.; Cha J. H.; Jeon S. Y.; Kirlikovali K. O.; Ostadhassan M.; Rasouli V.; Farha O. K.; Jang H. W.; Varma R. S.; Shokouhimehr M. Pd modified Prussian blue frameworks: Multiple electron transfer pathways for improving catalytic activity toward hydrogenation of nitroaromatics. Mol. Catal. 2020, 492, 110967. 10.1016/j.mcat.2020.110967. [DOI] [Google Scholar]
- Lakshminarayana B.; Manna A. K.; Satyanarayana G.; Subrahmanyam C. Palladium Nanoparticles on Silica Nanospheres for Switchable Reductive Coupling of Nitroarenes. Catal. Lett. 2020, 150, 2309–2321. 10.1007/s10562-020-03127-w. [DOI] [Google Scholar]
- Lu Y.-M.; Zhu H.-Z.; Li W.-G.; Hu B.; Yu S.-H. Size-controllable palladium nanoparticles immobilized on carbon nanospheres for nitroaromatic hydrogenation. J. Mater. Chem. A 2013, 1, 3783. 10.1039/c3ta00159h. [DOI] [Google Scholar]
- Sun W.; Meng Y.; Fu Q.; Wang F.; Wang G.; Gao W.; Huang X.; Lu F. High-Yield Production of Boron Nitride Nanosheets and Its Uses as a Catalyst Support for Hydrogenation of Nitroaromatics. ACS Appl. Mater. Interfaces 2016, 8, 9881–9888. 10.1021/acsami.6b01008. [DOI] [PubMed] [Google Scholar]
- McAllister M. I.; Boulho C.; Brennan C.; Parker S. F.; Lennon D. Toward Sustained Product Formation in the Liquid-Phase Hydrogenation of Mandelonitrile over a Pd/C Catalyst. Org. Process Res. Dev. 2020, 24, 1112–1123. 10.1021/acs.oprd.0c00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedűs L.; Máthé T. Selective heterogeneous catalytic hydrogenation of nitriles to primary amines in liquid phase. Appl. Catal., A 2005, 296, 209–215. 10.1016/j.apcata.2005.08.024. [DOI] [Google Scholar]
- Wang H.; Luo Q.; Liu W.; Lin Y.; Guan Q.; Zheng X.; Pan H.; Zhu J.; Sun Z.; Wei S.; Yang J.; Lu J. Quasi Pd1Ni single-atom surface alloy catalyst enables hydrogenation of nitriles to secondary amines. Nat. Commun. 2019, 10, 4998 10.1038/s41467-019-12993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trandafir M. M.; Neatu F.; Chirica I. M.; Neatu Ş.; Kuncser A. C.; Cucolea E. I.; Natu V.; Barsoum M. W.; Florea M. Highly Efficient Ultralow Pd Loading Supported on MAX Phases for Chemoselective Hydrogenation. ACS Catal. 2020, 10, 5899–5908. 10.1021/acscatal.0c00082. [DOI] [Google Scholar]
- Adamczyk A. J. First-principles analysis of acetonitrile reaction pathways to primary, secondary, and tertiary amines on Pd(111). Surf. Sci. 2019, 682, 84–98. 10.1016/j.susc.2018.09.006. [DOI] [Google Scholar]
- Roduner E. Understanding catalysis. Chem. Soc. Rev. 2014, 43, 8226–8239. 10.1039/C4CS00210E. [DOI] [PubMed] [Google Scholar]
- Pérez-Ramírez J.; López N. Strategies to break linear scaling relationships. Nat. Catal. 2019, 2, 971–976. 10.1038/s41929-019-0376-6. [DOI] [Google Scholar]






