Abstract
Cavernous angiomas with symptomatic hemorrhage (CASH) have a high risk of rebleeding, and hence an accurate diagnosis is needed. With blood flow and vascular leak as established mechanisms, we analyzed perfusion and permeability derivations of dynamic contrast-enhanced quantitative perfusion (DCEQP) MRI in 745 lesions of 205 consecutive patients. Thirteen respective derivations of lesional perfusion and permeability were compared between lesions that bled within a year prior to imaging (N = 86), versus non-CASH (N = 659) using machine learning and univariate analyses. Based on logistic regression and minimizing the Bayesian information criterion (BIC), the best diagnostic biomarker of CASH within the prior year included brainstem lesion location, sporadic genotype, perfusion skewness, and high-perfusion cluster area (BIC = 414.9, sensitivity = 74%, specificity = 87%). Adding a diagnostic plasma protein biomarker enhanced sensitivity to 100% and specificity to 85%. A slightly modified derivation achieved similar accuracy (BIC = 321.6, sensitivity = 80%, specificity = 82%) in the cohort where CASH occurred 3–12 months prior to imaging after signs of hemorrhage would have disappeared on conventional MRI sequences. Adding the same plasma biomarker enhanced sensitivity to 100% and specificity to 87%. Lesional blood flow on DCEQP may distinguish CASH after hemorrhagic signs on conventional MRI have disappeared and are enhanced in combination with a plasma biomarker.
Keywords: Cavernous angioma with symptomatic hemorrhage, biomarkers, MR permeability, MR perfusion, machine learning
Introduction
Cavernous angiomas (CAs), or cerebral cavernous malformations, are clusters of abnormal capillaries surrounded by a monolayer of dysregulated endothelium without mature angioarchitecture. 1 CA disease affects 0.3–0.9% of Americans. 1 CAs exist as either a familial/multifocal (20-30% of cases) or sporadic/solitary disease (70-80%). 2 Familial/multifocal patients harbor an autosomal dominant germline mutation in one of three documented CA genes (CCM1/KRIT1, CCM2/Malcavernin, CCM3/PDCD10) and biallelic somatic mutations in numerous lesions throughout the brain and spinal cord. Sporadic patients develop a solitary CA as a result of somatic mutations in the same genes in the absence of the germline mutations.1,3–11 The CA genes maintain endothelial barrier integrity by inhibiting RhoA kinase.12,13 CAs may arise from increased RhoA kinase activity that lead to dysregulated tight junctions and hyperpermeability. 13 Furthermore, hypoperfusion through low fluid shear stress was recently shown in vitro to upregulate RhoA kinase, inflammation, oxidative stress, and key pathways associated to CA pathogenesis, 14 such as Kruppel-like factors 2 and 4. 15 Recent studies in zebrafish highlighted trapped blood flow as a critical factor in developing cavernous malformation lesions. 16 These findings suggest a complex interplay between permeability, 13 perfusion,14,16 inflammation,14,17 and angiogenesis in the CA disease.18,19
While most CA patients are asymptomatic, 1 50% of symptomatic patients have seizures, 25% have focal neurological deficits, and 25% have CA with symptomatic hemorrhage (CASH). 4 Diagnosis of CASH requires evidence of lesional bleeding on magnetic resonance imaging (MRI) associated with clinical symptoms attributable to the lesion.1,4 The risk for an initial CASH is low and estimated to be around 0.08% per patient-year.4,20 However, the risk for recurrent hemorrhage increases ten-fold in the subsequent five-year period, reaching up to 42%.3,4,21 Subclinical hemorrhage, lesional growth, or new lesion formation have been associated with prior and recurrent CASH. 22 Repurposed drugs such as atorvastatin, aimed at impacting lesional permeability, are under investigation in clinical trials to reduce the risk of recurrent CASH (clinicaltrials.gov, NCT02603328). 23 Another drug, propranolol, is also being investigated (clinicaltrials.gov, NCT03589014), in part based on effects on lesional perfusion. 16 A Trial Readiness initiative (NCT03652181) is ongoing in parallel to assess the efficacy of imaging biomarkers in monitoring CASH. 24
Surgical intervention carries high risks of morbidity and mortality, especially when lesions are located in eloquent structures such as the brainstem. 1 Confirming a CASH compels the consideration of such an aggressive intervention, and allows the stratification of higher risk patients in clinical trials. However, diagnosis of CASH is often confused by nonspecific symptoms (e.g. headaches, seizures) and in cases who present more than three months after a suspected hemorrhage, as signatures of bleeding would have resolved on conventional imaging. In other cases, clinical symptoms may be confusing. Hence, there is a need for a diagnostic biomarker of CASH.
T1-weighted dynamic contrast-enhanced quantitative perfusion (DCEQP) MRI, which measures vascular permeability and perfusion, has been proposed as a potential MRI biomarker of CAs and CASH.25,26 Previous in vivo studies have reported higher permeability in non-lesional brain parenchyma in familial/multifocal CA subjects using DCEQP while mean lesional permeability was comparable between sporadic/solitary and familial/multifocal subjects. 26 Mean lesional permeability was also shown to be increased after symptomatic lesional bleeding or growth. 25 However, there has not been any study on aspects of CA permeability other than mean lesional value nor analysis of lesional perfusion reflecting low shear stress 14 or altered hemodynamics. 27 It remains unclear whether various derivations of lesional permeability or perfusion on DCEQP can diagnose a CASH.
We have recently reported that a panel of inflammatory and angiogenic proteins assessed in peripheral plasma diagnosed CASH. 28 A linear weighted combination of plasma levels of soluble CD14, C-reactive protein, vascular endothelial growth factor, and interleukin-10 diagnosed CASH in the prior year with 76% sensitivity and 80% specificity. 28 Its imperfect sensitivity and specificity suggest potentially unaccounted factors. It is unknown if DCEQP permeability or perfusion are more efficacious at diagnosing CASH than plasma protein biomarkers and if they enhance/complement each other’s diagnostic associations.
This study aimed to investigate whether a panel of lesional permeability and perfusion derivations measured by DCEQP can diagnose CASH that occurred in the prior year. We further hypothesize that lesional permeability and perfusion can be used as diagnostic biomarkers of CASH even when a lesion bled more than 3 months prior to the MRI, when imaging signatures of bleed would have resolved on conventional sequences. We also hypothesize that combinations of permeability and perfusion derivations can complement and/or enhance the diagnostic associations identified with the previously published plasma protein biomarker of CASH. 28
Material and methods
Participants
This case-control study included 205 consecutively enrolled patients with a diagnosis of CA confirmed via MRI by a neuroradiologist at a single referral center (http://www.uchicagomedicine.org/ccm). CA patients who underwent a DCEQP MRI sequence in conjunction with conventional MRI between July 2012 and December 2019 were included in the study. Patients with multiple CAs throughout the brain on MR susceptibility weighted imaging (SWI), a documented CCM1, CCM2, or CCM3 germline mutation, and/or first-degree relative with a history of CA were classified as familial/multifocal. Patients were otherwise classified as sporadic/solitary, typically harboring a single lesion on SWI, or a cluster of CAs associated with a developmental venous anomaly. Patients with partial or complete resection of CA or any prior brain irradiation were excluded. All patients provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the University of Chicago Institutional Review Board (Study No. 12-0204). The ethical principles guiding the Institutional Review Board adhered to The Belmont Report and the Federal Policy for the Protection of Human Subjects (56 FR 28,003).
Classification of cavernous angioma lesions
The CA lesions included for analysis were ≥5 mm at maximum diameter on axial T2-weighted sequences and with satisfactory DCEQP data acquisition. Each lesion was classified as either sporadic/solitary or familial/multifocal based on the patient’s phenotype (as defined above). A CASH event required evidence of new bleeding on imaging studies acquired at the time of the suspected ictus and new clinical symptoms attributable to the lesional bleed as per published and widely accepted evidence-based criteria.3,24,29 Imaging evidence included acute or subacute bleeding on computed tomography or MRI, new fluid-attenuated inversion recovery signal on MRI, and/or lesion expansion in any diameter by ≥3 mm on comparable T1- or T2-weighted sequences.25,29 Such imaging evidence of hemorrhage had often resolved at the time the DCEQP MRI was performed within the following year, hence imaging studies contemporaneous with the clinical ictus were used for establishing a CASH diagnosis. A symptomatic hemorrhage within a year prior was further categorized either as having bled (1) less than 3 months or (2) between 3–12 months prior to the DCEQP MRI. CAs without symptomatic hemorrhage in the year prior to the DCEQP MRI were classified as non-CASH. The senior author (IAA) prospectively adjudicated each lesion’s clinical status and classified each lesion into the aforementioned criteria, blinded to any DCEQP data.
Imaging acquisition and postprocessing
Vascular permeability and perfusion imaging were assessed using T1-weighted, gadolinium contrast-based DCEQP sequence on a 3.0-Tesla Philips Achieva systems as described in established protocols.25,26,30–32 Data were postprocessed into permeability and perfusion maps using MATLAB (MathWorks) by imaging scientists (RG & NH). 25 Thirteen permeability and 13 perfusion derivations were calculated using MATLAB: mean, median, upper and lower terciles, coefficient of variation (CV), skewness, kurtosis, entropy, lesion area, high-value cluster mean and area, as well as low-value cluster mean and area (Supplementary Table 1). High- or low-value cluster areas were regions of largest diameter where all values were ≥1 standard deviation (SD) above mean or ≥1 SD below mean, respectively. The selected derivations allowed for maximizing the diversity in which permeability and perfusion were characterized. Mean, median, and terciles reflected central tendency. Entropy, CV, skewness, and kurtosis represented intralesional heterogeneity. 33 High- and low-value clusters reflected how “extreme” values were spatially distributed relative to each other, as points of shear stress14,34 and altered hemodynamics 27 have been associated with vascular pathologies including CAs 14 and intracranial hemorrhage. For more information on imaging acquisition, please refer to Supplemental Methods.
Machine learning, Bayesian modeling, and statistical analyses
Demographics between CASH versus non-CASH lesions within a year before DCEQP imaging were compared with independent samples t-test, χ2-test, or Fisher’s exact test. Post hoc analyses for demographics with more than two categories were conducted by comparing adjusted standardized residuals and calculating Bonferroni corrections. Continuous variables were tested for normality using Shapiro-Wilk test and collinearity using Spearman’s ρ. Permeability and perfusion derivation values of CASH lesions within a year prior and 3–12 months prior to imaging were compared to non-CASH lesions using Mann-Whitney U-test. Outliers with absolute studentized residuals ≥3 were excluded. Supervised machine learning methods, including Random Forest and Gradient Boosting Machine, were performed to identify the outperformed model initially assessed to identify derivations and demographics with weighted contributions in distinguishing CASH within a year prior and 3–12 months prior versus non-CASH.
Multivariate analyses were conducted using logistic regression: in which coefficients of models were reported with 95% confidence intervals (CIs). McFadden R2 was used to measure each model’s explanation of variance in the data.35,36 The best combinations of non-correlated permeability and perfusion derivations of interest from exploratory analyses (p < 0.1) and demographics (age, sex, sporadic/familial, brainstem lesion location) were selected by minimizing the Bayesian information criterion (BIC). 37 The predicted probability values calculated from the logistic regression models were used as canonical biomarker scores which were compared between CASH and non-CASH using Mann-Whitney U-test.
Receiver operating characteristic (ROC) curves and area-under-the-curve (AUC) values were then generated. Optimal sensitivity and specificity for each ROC curve were calculated using the Youden method. 38 Models were internally validated by re-deriving them in the first 2/3 of group-matched lesions sequentially by scan date and validating their sensitivities and specificities in the subsequent 1/3 of lesions. Monte Carlo analyses were conducted to validate the efficacy of the combined permeability, perfusion, and plasma markers in 1000-fold increased simulated populations after studentized outliers were excluded. 39 All p-values were considered statistically significant at α < 0.05. The p-values were considered to have statistical trends at α < 0.1. The statistical analyses were performed using SPSS v22.0 (IBM, Armonk, NY), Prism v7.0 (GraphPad, San Diego, CA), and R statistical platform (R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/). For more information on statistical analyses and machine learning methods, please refer to Supplementary Methods.
Results
Classification of cavernous angioma lesions
Among 205 patients enrolled in the study (Supplementary Results and Supplementary Tables 2 and 3), there were 745 CAs with DCEQP image acquisition (Figure 1). Eighty-six of the 745 CAs had a symptomatic hemorrhage within the prior year (Table 1), with 55 having a symptomatic hemorrhage 3–12 months prior to DCEQP imaging. Among the 659 non-CASH lesions with DCEQP image acquisition, one permeability and 5 perfusion scans were discarded due to postprocessing errors. There were ultimately 658 with valid permeability scans, 654 with valid perfusion scans, and 653 with both. Comparison of demographics and clinical features between CA patients included in our study and those evaluated at our center during the same time epoch who did not receive DCEQP imaging (excluded from this study) is presented in Supplementary Table 2.
Figure 1.

Consort diagram of patients with cavernous angiomas (CAs) who underwent DCEQP imaging. Two hundred and five patients were consecutively enrolled during their standard clinical visits at the University of Chicago Center of Excellence for Cerebral Cavernous Malformations and underwent dynamic contrast-enhanced quantitative perfusion (DCEQP) magnetic resonance imaging. A total of 745 CAs were scanned with DCEQP imaging. There were 86 CAs with symptomatic hemorrhage (CASH) that bled within a year prior, of which 55 had bled 3–12 months prior to DCEQP imaging. In the subset of patients who also had plasma collected, seven had CASH within a year prior, and six had CASH 3–12 months prior to DCEQP imaging.
Table 1.
Demographic characteristics of cavernous angioma with symptomatic hemorrhage (CASH) and non-CASH lesions analyzed by DCEQP.
| Demographics | CASH (N = 86) | Non-CASH (N = 659) | p-Value | |
|---|---|---|---|---|
| Age Groups, N (%) | 0.0003 | |||
| <30 years old | 25 (29.1%) | 300 (45.5%) | 0.004‡ | |
| 30–50 years old | 44 (51.2%) | 196 (29.7%) | <0.0001‡ | |
| >50 years old | 17 (19.8%) | 163 (24.7%) | 0.31 | |
| Female, N (%) | 54 (62.8%) | 471 (71.5%) | 0.10 | |
| Ethnicity/race, N (%)* | 0.0005† | |||
| African American | 7 (8.2%) | 18 (2.7%) | 0.008 | |
| Ashkenazi Jewish | 0 (0.0%) | 7 (1.1%) | 0.34 | |
| Asian | 1 (1.2%) | 7 (1.1%) | 0.93 | |
| Hispanic of Mexican descent | 5 (5.9%) | 3 (0.5%) | <0.0001‡ | |
| Hispanic of other descent | 1 (1.2%) | 33 (5.0%) | 0.11 | |
| White/Caucasian | 69 (81.2%) | 580 (88.1%) | 0.068 | |
| Other | 2 (2.4%) | 10 (1.5%) | 0.57 | |
| Sporadic, N (%) | 49 (57.0%) | 102 (15.5%) | <0.0001 | |
| Familial genotype, N (%) | <0.0001† | |||
| CCM1 | 13 (15.1%) | 106 (16.1%) | 0.016 | |
| CCM2 | 5 (5.8%) | 24 (3.6%) | 0.012 | |
| CCM3 | 8 (9.3%) | 396 (60.1%) | <0.0001‡ | |
| Multifocal unknown | 11 (12.8%) | 31 (4.7%) | <0.0001‡ | |
| Brainstem lesion, N (%) | 31 (36.0%) | 37 (5.6%) | <0.0001 | |
*One patient declined to provide information on their ethnicity/race.
†Ethnicity/race and genotype were compared using Fisher’s exact test.
‡These post hoc comparisons remained significant after respective Bonferroni corrections.
The proportions of CASH and non-CASH lesions were significantly different in age and race/ethnicity groups overall (both p < 0.05; Table 1), in which higher proportions of CASH lesions were harbored by middle-aged (30–50 years) patients and Hispanics of Mexican descent (both p < 0.05). In addition, higher proportions of CASH lesions were sporadic or located in the brainstem (both p < 0.05). Finally, a greater proportion of familial/multifocal CASH lesions was defined as multifocal unknown genotype, while more non-CASH lesions were CCM3 (both p < 0.05). No effect of sex was observed between CASH and non-CASH lesions.
Machine learning, univariate analyses, and Bayesian modeling identified common perfusion and permeability derivations to distinguish cavernous angioma with symptomatic hemorrhage within a year prior to imaging
Perfusion imaging analysis. Exploratory supervised machine learning analyses using a penalized regression approach were first deployed to assess whether perfusion derivations may be diagnostic biomarkers of CASH within the prior year to DCEQP imaging. Analyses in the training dataset of 80% total lesions identified brainstem lesion location, sporadic genotype, sex, age, and 7 perfusion derivations as having the highest weighted contributions in distinguishing CASH (Supplementary Figure 1). This outperformed model distinguished CASH within a year prior in the testing dataset of 20% total lesions with 82% sensitivity and 71% specificity. Univariate analyses showed that perfusion entropy, lesion area, high-perfusion cluster area, skewness, and kurtosis were significantly increased (all p < 0.05), along with higher trend in high-perfusion cluster mean (p = 0.068), in CASH lesions compared to non-CASH lesions (Supplementary Figure 2). Furthermore, low-perfusion cluster mean and coefficient of variation (CV) were decreased (both p < 0.05) in CASH lesions.
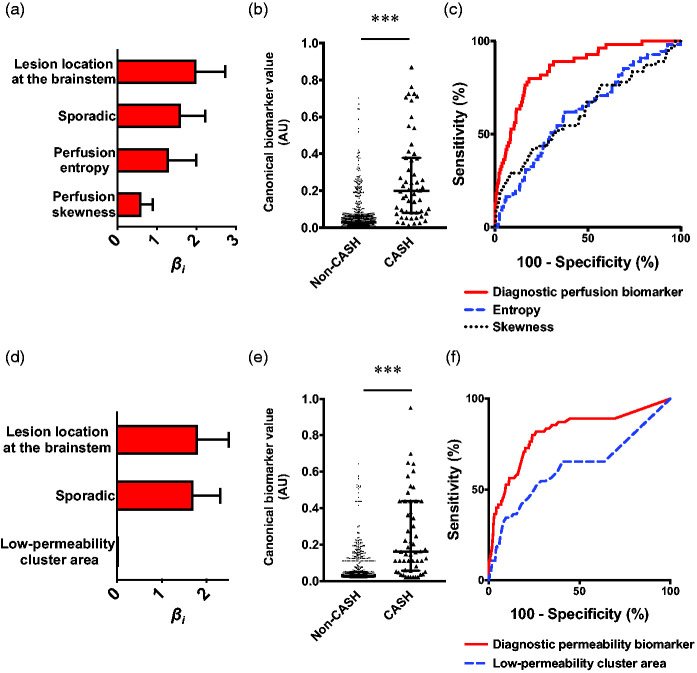
After testing the perfusion derivations for collinearity (Supplementary Figure 3), Bayesian model selection showed that the best perfusion model of CASH within a year prior (BIC = 414.9, McFadden R2 = 0.28) included brainstem lesion location, sporadic genotype, skewness, and high-perfusion cluster area (all covariates p < 0.05; Figure 2(a); Supplementary Table 4), formulated as:
Figure 2.
The best diagnostic perfusion and permeability biomarkers of cavernous angioma with symptomatic hemorrhage (CASH) within a year prior to imaging. (a) Weighted βi coefficients of covariates in the perfusion biomarker of CASH (Bayesian information criterion [BIC]=414.9, McFadden R2=0.28); error bars are 95% confidence intervals (CI). (b) Canonical score of the perfusion biomarker was higher (p < 0.001) in CASH (median [interquartile range (IQR)]=0.28 [0.14–0.56]) than non-CASH lesions (0.04 [0.03–0.08]). (c) The perfusion biomarker had better efficacy in distinguishing CASH within a year prior to imaging (area under the curve (AUC) [95% CI]=86% [82–90%], p < 0.0001) than high-perfusion cluster area (73% [67–79%], p < 0.0001) and skewness individually (64% [57–71%], p < 0.0001). (d) Weighted βi (95% CI) coefficients of covariates in the permeability biomarker (BIC = 438.4, McFadden R2=0.25). (e) Canonical score of the permeability biomarker was higher (p < 0.001) in CASH (median [IQR]=0.29 [0.13–0.47]) than non-CASH lesions (0.04 [0.03–0.09]). (f) The permeability biomarker had better efficacy in distinguishing CASH within a year prior (AUC [95% CI]=84% [79–89%], p < 0.0001) than high-permeability cluster area (71% [65–77%], p < 0.0001), entropy (69% [63–75%], p < 0.0001), and skewness individually (56% [49–62%], p = 0.081). *** p < 0.001. AU: arbitrary unit.
This model showed higher canonical scores for CASH lesions (p < 0.001) and distinguished CASH with 74% sensitivity and 87% specificity (AUC = 86%, p < 0.0001; Figure 2(b) and (c)). Internal validation yielded the same covariates and similar sensitivity and specificity (Supplementary Figure 4).
Permeability imaging analysis. The same diagnostic hypothesis was tested in permeability imaging. Supervised exploratory machine learning showed the same demographics as in perfusion analyses and 6 permeability derivations with the highest weighted contributions in distinguishing CASH (Supplementary Figure 5). This outperformed model yielded 82% sensitivity and 71% specificity in the testing dataset.
In parallel, univariate analyses showed that mean, median, and upper and lower tercile permeability, high-permeability cluster mean, entropy, lesion area, as well as high- and low-permeability cluster areas were increased (all p < 0.05) in CASH relative to non-CASH lesions (Supplementary Figure 6). Skewness showed a higher trend in CASH (p = 0.088).
After testing the permeability derivations for collinearity (Supplementary Figure 7), the best permeability model of CASH within a year prior (BIC = 438.4, McFadden R2 = 0.25) included a combination of brainstem lesion location, sporadic genotype, entropy, skewness, and high-permeability cluster area (all covariates p < 0.05; Figure 2(d); Supplementary Table 5). The model was formulated as:
This model showed higher canonical scores for CASH (p < 0.001) and distinguished CASH with 81% sensitivity and 77% specificity (AUC = 84%, p < 0.0001; Figure 2(e) and (f)). Internal validation included the same derivations, excepting high-permeability cluster area, and yielded similar sensitivity and specificity (Supplementary Figure 8).
Combined imaging analysis. Further machine learning analyses showed that a combination of perfusion and permeability derivations resulted in similar sensitivity and specificity of 84% and 70%, respectively (Supplementary Figure 9). However, after testing for collinearity between perfusion and permeability derivations (Supplementary Figure 10), univariate analyses and Bayesian model selection showed that adding permeability derivations to the perfusion model of CASH within a year prior did not improve the diagnostic efficacy nor change covariates.
Machine learning and univariate analyses identified common perfusion and permeability derivations to distinguish cavernous angioma with symptomatic hemorrhage 3–12 months prior to imaging
Perfusion imaging analysis. Further analyses were performed on the cohort of CASH 3–12 months prior to DCEQP imaging. Exploratory machine learning analyses using Random Forest were first deployed to assess whether perfusion derivations can diagnose CASH 3–12 months prior to DCEQP imaging. Machine learning analyses identified brainstem lesion location, sporadic genotype, and 8 perfusion derivations as having the highest weighted contributions in distinguishing CASH (Supplementary Figure 11). This outperformed model distinguished CASH 3–12 months prior from non-CASH in the testing dataset with 69% sensitivity and 86% specificity.
The univariate analyses showed greater perfusion entropy, lesion area, high-perfusion cluster area, skewness, and kurtosis, as well as lower CV in CASH 3–12 months prior to imaging compared to non-CASH (all p < 0.05; Supplementary Figure 12).
Bayesian model selection showed that the best perfusion model of CASH 3–12 months prior to imaging (BIC = 321.6, McFadden R2 = 0.25) included brainstem lesion location, sporadic genotype, entropy, and skewness (all covariates p < 0.05; Figure 3(a); Supplementary Table 6) formulated as:
Figure 3.
The best diagnostic perfusion and permeability biomarkers of cavernous angioma with symptomatic hemorrhage (CASH) 3–12 months prior to imaging. (a) Weighted βi coefficients of covariates in the perfusion biomarker of CASH 3–12 months prior to imaging (Bayesian information criterion [BIC]=321.6); error bars are 95% confidence intervals (CI). (b) Canonical score of the perfusion biomarker was higher (p < 0.001) in CASH (median [interquartile range (IQR)]=0.20 [0.08–0.38]) than non-CASH lesions (0.03 [0.01–0.06]). (c) The perfusion biomarker had better efficacy in distinguishing CASH 3–12 months prior to imaging (area under the curve (AUC) [95% CI]=86% [81–91%], p < 0.0001) than entropy (62% [54–70%], p = 0.003) or skewness alone (62% [54–71%], p = 0.003). (d) Weighted βi coefficients of covariates in the permeability biomarker (BIC = 331.9); error bars are 95% CI. (e) Canonical score of the permeability biomarker was higher (p < 0.001) in CASH (median [IQR]=0.16 [0.06–0.44]) than non-CASH lesions (0.03 [0.02–0.05]). (f) The permeability biomarker had better efficacy (AUC [95% CI]=81% [74–88%], p < 0.0001) than low-permeability cluster area alone (62% [53–71%], p = 0.003). *** p < 0.001. AU: arbitrary unit.
This model showed higher canonical scores for CASH lesions (p < 0.001; Figure 3(b)) and distinguished CASH 3–12 months prior to imaging from non-CASH with 80% sensitivity and 82% specificity (AUC = 86%, p < 0.0001; Figure 3(c)). Internal validation yielded the same covariates and similar sensitivity and specificity (Supplementary Figure 13).
Permeability imaging analysis. The same diagnostic hypothesis was tested for permeability derivations. Supervised exploratory machine learning using Random Forest showed the same demographic covariates as in perfusion analyses and 7 permeability derivations with the highest weighted contributions (Supplementary Figure 14). A combination of demographic covariates and permeability derivations distinguished CASH 3–12 months prior with 92% sensitivity and 87% specificity in the testing dataset. Univariate analyses showed greater permeability entropy, high-permeability cluster mean, as well as whole lesion, high-permeability cluster, and low-permeability cluster areas in CASH 3–12 months prior to imaging compared to non-CASH (all p < 0.05; Supplementary Figure 15).
The best permeability model of CASH 3–12 months prior to imaging (BIC = 331.9, McFadden R2=0.21) was a combination including brainstem lesion location, sporadic genotype, and low-permeability cluster area (all covariates p < 0.05; Figure 3(d), Supplementary Table 7). This model was formulated as:
Further analyses showed higher canonical scores in CASH compared to non-CASH (p < 0.001; Figure 3(e)). This permeability biomarker had 80% sensitivity and 76% specificity (AUC = 81%, p < 0.0001; Figure 3(f)). Internal validation resulted in the same covariates and similar sensitivity and specificity (Supplementary Figure 16).
Combined imaging analysis. Additional machine learning analyses using Gradient Boosting Machine showed that a combination of perfusion and permeability derivatives using supervised resulted in similar sensitivity and specificity of 92% and 85%, respectively (Supplementary Figure 17). Univariate analyses and Bayesian model selection showed that adding permeability derivations to the perfusion biomarker of CASH 3–12 months prior did not improve the efficacy nor change covariates.
Combining the best diagnostic perfusion and permeability imaging biomarkers with a plasma protein biomarker enhanced their sensitivities and specificities
Integrative analysis of CASH within a year prior to imaging. Finally, we assessed whether previously published plasma CASH biomarkers can enhance the diagnostic associations of the DCEQP imaging biomarkers. This integrative analysis initially included a subset of 7 patients with CASH within a year prior and 49 non-CASH patients with both plasma and DCEQP imaging biomarker data (46 with perfusion scans). The integrated perfusion and plasma biomarker (sensitivity = 100%, specificity = 85%, AUC = 95%, p = 0.0001) distinguished CASH patients better than perfusion (sensitivity = 86%, specificity = 89%, AUC = 89%, p = 0.001) or plasma alone (sensitivity = 100%, specificity = 52%, AUC = 78%, p = 0.018) (Figure 4(a) and (b)). Monte Carlo simulation of 6,990 CASH and 48,832 non-CASH patients yielded similar results in which the integrated model outperformed perfusion or plasma separately (Supplementary Figure 18a).
Figure 4.
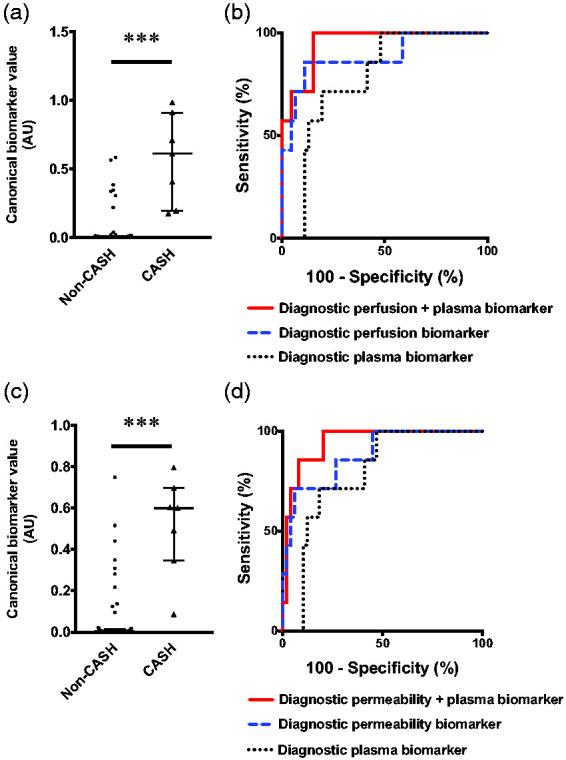
Integrated perfusion, permeability, and plasma protein biomarkers of cavernous angioma with symptomatic hemorrhage (CASH) within a year prior to imaging. (a) Canonical score of the integrated perfusion and plasma biomarker was higher (p < 0.001) in CASH (median [interquartile range (IQR)]=0.61 [0.20–0.91]) than non-CASH patients (0.005 [0.001–0.01]). (b) The integrated biomarker (100%, sensitivity, 85% specificity, area under the curve (AUC) [95% confidence interval (CI)]=95% [89–100%], p = 0.0001) distinguished CASH patients (N = 7) from non-CASH patients (N = 46) than perfusion (86% sensitivity, 89% specificity, AUC [95% CI]=89% [73–100%], p = 0.001) or plasma individually (100% sensitivity, 52% specificity, AUC [95% CI]=78% [63–92%], p = 0.018). (c) Similarly, the canonical score of the integrated permeability and plasma biomarker was higher (p < 0.001) in CASH (median [IQR]=0.60 [0.35–0.70]) than non-CASH patients (0.004 [0.001–0.02]). (d) The integrated biomarker (100% sensitivity, 80% specificity, AUC [95% CI]=95% [88–100%], p = 0.0002) distinguished CASH (N = 7) from non-CASH patients (N = 49) better than permeability (71% sensitivity, 94% specificity, AUC [95% CI]=88% [75–100%], p = 0.001) or plasma alone (100% sensitivity, 53% specificity, AUC [95% CI]=79% [65–93%], p = 0.015). *** p < 0.001. AU: arbitrary unit.
The same analysis with the diagnostic permeability biomarker of CASH within a year prior similarly showed that the integrated model had better sensitivity and specificity than permeability imaging or plasma alone (Figure 4(c) and (d)). The integrated biomarker had 100% sensitivity and 80% specificity (AUC = 95%, p = 0.0002), permeability had 71% sensitivity and 94% specificity (AUC = 88%, p = 0.001), and plasma had 100% sensitivity and 53% specificity (AUC = 79%, p = 0.015). Monte Carlo simulation showed that the integrated model similarly outperformed permeability or plasma separately (Supplementary Figure 18b).
Integrative analysis of CASH 3–12 months prior to imaging. Further integrative analysis was performed in a subset of 6 patients with CASH 3–12 months prior and the same 49 non-CASH patients with both plasma and DCEQP imaging biomarker data (46 non-CASH with perfusion scans as above). The integrated perfusion and plasma biomarker (sensitivity = 100%, specificity = 87%, AUC = 95%, p = 0.0004) was better than perfusion (sensitivity = 100%, specificity = 78%, AUC = 91%, p = 0.001) or plasma alone (sensitivity = 100%, specificity = 52%, AUC = 76%, p = 0.037) at distinguishing patients with CASH 3–12 months prior to DCEQP imaging (Figure 5(a) and (b)). Monte Carlo simulation of 5,988 CASH and 48,834 non-CASH patients yielded similar results in which the integrated biomarker outperformed perfusion imaging or plasma separately (Supplementary Figure 19a).
Figure 5.
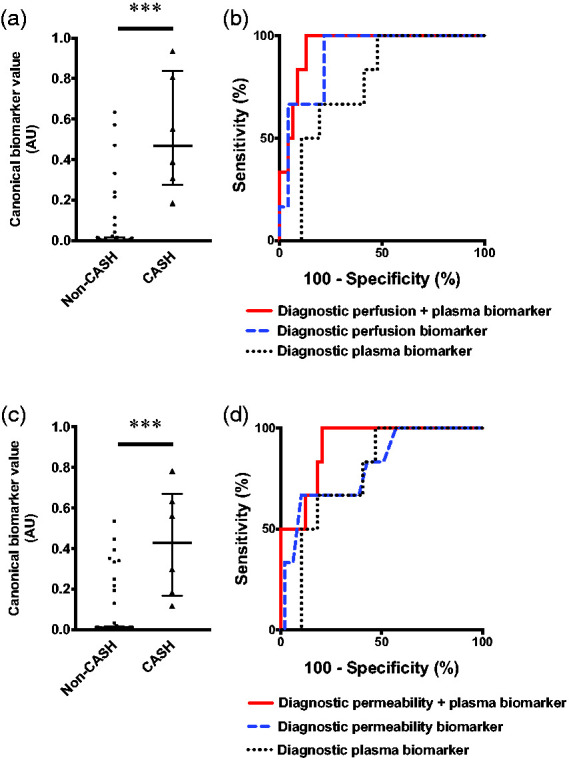
Integrated perfusion, permeability, and plasma protein biomarkers of cavernous angioma with symptomatic hemorrhage (CASH) 3–12 months prior to imaging. (a) Canonical score of the combined diagnostic perfusion and plasma biomarker was higher (p < 0.001) in CASH (median [interquartile range (IQR)]=0.47 [0.28–0.84]) versus non-CASH patients (0.002 [0.0005–0.015]). (b) The combined perfusion and plasma biomarker (100% sensitivity, 87% specificity, area under the curve (AUC) [95% confidence interval (CI)]=95% [88–100%], p = 0.0004) was better than perfusion (100% sensitivity, 78% specificity, AUC [95% CI]=91% [81–100%], p = 0.001) or plasma alone (100% sensitivity, 52% specificity, AUC [95% CI]=76% [61–92%], p = 0.037). (c) Canonical score of the integrated permeability and plasma biomarker was higher (p < 0.001) in CASH (median [IQR]=0.43 [0.17–0.67]) than non-CASH patients (0.007 [0.002–0.02]). (d) The integrated biomarker (100% sensitivity, 80% specificity, AUC [95% CI]=92% [83–100%], p = 0.001) distinguished CASH patients better than permeability (67% sensitivity, 90% specificity, AUC [95% CI]=80% [62–99%], p = 0.016) or plasma alone (100% sensitivity, 53% specificity, AUC [95% CI]=77% [62–93%], p = 0.031). *** p < 0.001. AU: arbitrary unit.
The same analyses with the diagnostic permeability biomarker of CASH 3–12 months prior to DCEQP imaging also showed that the integrated permeability and plasma biomarker (sensitivity = 100%, specificity = 80%, AUC = 92%, p = 0.001) distinguished CASH 3–12 months prior better than permeability (sensitivity = 67%, specificity = 90%, AUC = 80%, p = 0.016) or plasma separately (sensitivity = 100%, specificity = 53%, AUC = 77%, p = 0.031) (Figure 5(c) and (d)). Monte Carlo simulation supported that the integrated model performed better than permeability or plasma alone (Supplementary Figure 19b).
Discussion
In this study, perfusion and permeability imaging derivations measured by DCEQP MRI were investigated as potential diagnostic biomarkers of prior CASH using a Bayesian approach complemented with supervised machine learning. The weighted contributions suggest that perfusion (lesional blood flow) derivations, in addition to known clinical features such as brainstem lesion location and sporadic genotype, substantially add diagnostic efficacy in distinguishing CASH lesions within a year prior, including cases in which hemorrhagic signs have resolved on conventional MRI. Lesional perfusion imaging had not been previously studied in CAs. Permeability derivations (vascular leak), previously studied in this disease, also distinguish CASH lesions but do not complement nor enhance the diagnostic associations of perfusion derivations with CASH. Furthermore, combining the perfusion biomarker with a plasma protein biomarker 28 enhances the respective diagnostic associations compared to perfusion or plasma proteins alone.
These findings advance the potential to accurately diagnose a lesional bleed. As such, the diagnostic biomarkers may provide a solution to detecting a CASH more than three months (up to a year) after the hemorrhagic onset when conventional MRI is considered to be no longer diagnostic, as well as in cases in which clinical symptoms are nonspecific. Because the risk of recurrent hemorrhage persists for several years after a symptomatic hemorrhage, 4 these biomarkers may facilitate the consideration of aggressive intervention for patients with delayed CASH presentation. The published protocols of DCEQP MRI25,26,30–32 suggest that this imaging may be acquired and postprocessed in conjunction with other standard clinical MRI sequences, hence minimizing its added labor intensity and enhancing its cost-effectiveness as a diagnostic tool. Additionally, in clinical trials, higher-risk patients may be further stratified when investigating potential alternative treatments, such as atorvastatin 23 or propranolol, 16 which rely on statistical power calculations, chance of rebleeding, and accurate characterization of baseline characteristics. Enhanced diagnostic accuracy may aid risk-benefit analyses of aggressive therapies, such as surgical resection, which entail potentially high risks. 1 Lastly, the enhanced efficacy of combining perfusion and permeability imaging with a plasma protein biomarker suggests that an integrative approach may be necessary in large multi-site investigations and multimodal tests warranted in clinical settings to accurately diagnose CASH. Integrating these findings into management guidelines 3 may ultimately improve clinical decision-making regarding CAs and CASH and decrease the morbidity and mortality of patients who suffer from CA.
Within the biomarker models, higher odds of prior CASH in brainstem and sporadic lesions contributed significant weighted contributions (high βi coefficients of covariates). These factors may reflect a detection bias or differential biological mechanisms. The brainstem is an eloquent structure which may trigger observable symptoms after a slight bleed. 1 Thus, patients with brainstem CA hemorrhage may be more likely to manifest clinically than those with bleeding in other lesion locations. Conversely, it is possible that brainstem lesions may have different properties that further predispose them to bleeding. Sporadic and familial CAs have comparable lesional permeability 26 and the same molecular mechanisms that drive the disease. 2 However, patients with a solitary sporadic lesion may be more likely to present upon new clinical symptoms, while familial patients have CAs that are more often found and monitored after genotyping and clinic visits. 40 Future multisite studies with larger cohorts should query potential biological risks of hemorrhage in these subgroups.
Permeability25,26 had been mechanistically postulated, and perfusion is an emerging topic in investigations on CAs and CASH. The transcriptome of neurovascular units within CASH lesions reveals differentially expressed genes encoding angiogenic and inflammatory proteins which modulate vascular perfusion and permeability. 28 For example, VEGFA was a differentially expressed gene in CASH neurovascular units, and vascular endothelial growth factor was a component of the diagnostic plasma biomarker. 28 Vascular endothelial growth factor is involved in angiogenesis, perfusion, and blood-brain barrier permeability, 41 especially in a state of hypoperfusion that can often follow hemorrhagic stroke. 42 The imperfect sensitivities and specificities of perfusion and permeability biomarkers and their low-weighted βi coefficients of covariates suggest that unexplored biological markers may improve the diagnostic associations.28,43,44 The integration of plasma biomarkers of CASH, likely reflecting complementary biologic mechanisms, indeed, optimizes the sensitivity (to 100%) and enhances the specificity of the diagnostic biomarkers.
This study adds insight about changes in perfusion and permeability in a CA lesion after a symptomatic hemorrhage. Features other than mean lesional perfusion and permeability (skewness, entropy and high-perfusion cluster area) likely reflect inhomogeneous dysregulated processes associated with lesional bleed. Admittedly, the array of DCEQP derivations chosen may have biased or limited the biomarkers’ sensitivities and specificities. Unsupervised deep learning45,46 may be used to identify potentially more efficacious derivations and models of perfusion and permeability patterns reflective of hemorrhage.
The diagnostic associations may have been limited by the study design in which no period beyond one year after the hemorrhagic onset was examined. Nevertheless, this time frame is most clinically applicable to current CA management guidelines 3 as well as clinical trials 16,23 and Trial Readiness initiatives 24 of CASH. Because the study assessed the prevalence of symptomatic hemorrhage within the prior year, the prospective risk of hemorrhage after a prior bleed3,4,21 was not addressed. However, the prevalence of CASH is similar to that reported at other referral centers and thus remain representative of the population. 47 Other limitations of this study include the small sample sizes and low statistical power, particularly in the cohorts of internal validation and integrated biomarkers of perfusion, permeability, and plasma proteins. Selection biases of enrolled cases could have influenced biomarker correlations. Prospective validation in an independent cohort is still needed. Still, this represents the largest study of its kind in such a rare disease. With preliminary feasibility and proof of concept, a multi-site study with larger and more diverse cohorts is warranted. In future studies, both supervised machine learning pipelines, 48 including Random Forest 49 and Elastic Net, 50 and deep learning methods45,46 could be utilized in an integrative approach to develop complex multimodal diagnostic and prognostic biomarkers of CASH which include perfusion and permeability imaging derivations, plasma proteins, and microRNAs.
Future investigation should address whether perfusion and permeability derivations may be prognostic biomarkers of CASH and whether their combination with a published plasma protein biomarker28,43 complements and/or enhances the prognostic association with CASH. This prognostic plasma biomarker was similar to the diagnostic biomarker in which a weighted combination of soluble CD14, vascular endothelial growth factor, interleukin-1β, and soluble roundabout guidance receptor 4 predicted CASH within a year after sampling with 83% sensitivity and 93% specificity.28,43 As with the diagnostic biomarker herein, it is also possible that potentially dysregulated permeability and perfusion in DCEQP scans could predict a future bleed and enhance the prognostic power of plasma biomarkers. A prognostic CASH biomarker that would predict an impending CA hemorrhage or lesion growth would ultimately enhance the findings of this study and influence another compelling clinical context of use and patient stratification in clinical trials.
Conclusion
We have shown that perfusion imaging may diagnose CASH even after evidence of hemorrhage has disappeared on conventional MRI. The addition of plasma protein biomarkers to perfusion and permeability derivations enhances the diagnostic associations of the integrated biomarkers.
Data sharing
Data will be shared on reasonable request through direct contact with the corresponding author.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211020587 for Perfusion and permeability as diagnostic biomarkers of cavernous angioma with symptomatic hemorrhage by Je Yeong Sone, Yan Li, Nicholas Hobson, Sharbel G Romanos, Abhinav Srinath, Seán B Lyne, Abdallah Shkoukani, Julián Carrión-Penagos, Agnieszka Stadnik, Kristina Piedad, Rhonda Lightle, Thomas Moore, Ying Li, Dehua Bi, Robert Shenkar, Timothy Carroll, Yuan Ji, Romuald Girard and Issam A Awad in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by grants from the NIH (R21NS087328, 5U01NS104157-02, 1R01NS107887-01), William and Judith Davis Fund in Neurovascular Research to IAA, by the Safadi Translational Fellowship to RG, and the Burroughs Wellcome Fund and the University of Chicago Pritzker School of Medicine to JYS. Funding sources played no role in the formulation of research questions nor the interpretation of results.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: IAA and RG designed and conceptualized the study, oversaw data analyses and edited the final manuscript. JYS, NH, RG and YaL helped optimize the study design. NH, RG, ASh, JCP, ASt, KP, RL, TM and TC acquired the data. JYS, YaL, NH, SGR, ASr, SBL, YiL, DB, RS, YJ, RG and IAA analyzed and interpreted the data. All authors drafted the work, critically revised it for essential intellectual content, gave final approval of the published work, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity are appropriately investigated and resolved.
Supplementary material: Supplemental material for this article is available online.
ORCID iD: Robert Shenkar https://orcid.org/0000-0002-1300-6845
References
- 1.Awad IA, Polster SP. Cavernous angiomas: deconstructing a neurosurgical disease. J Neurosurg 2019; 131: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald DA, Shi C, Shenkar R, et al. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: evidence for a common biochemical pathway for CCM pathogenesis. Hum Mol Genet 2014; 23: 4357–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akers A, Al-Shahi Salman R, A Awad I, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery 2017; 80: 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shahi Salman R, Hall JM, Horne MA, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 2012; 11: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergametti F, Denier C, Labauge P, et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet 2005; 76: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denier C, Goutagny S, Labauge P, et al. Mutations within the MGC4607 gene cause cerebral cavernous malformations. Am J Hum Genet 2004; 74: 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guclu B, Ozturk AK, Pricola KL, et al. Mutations in apoptosis-related gene, PDCD10, cause cerebral cavernous malformation 3. Neurosurgery 2005; 57: 1008–1013. [DOI] [PubMed] [Google Scholar]
- 8.Laberge-Le Couteulx S, Jung HH, Labauge P, et al. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet 1999; 23: 189–193. [DOI] [PubMed] [Google Scholar]
- 9.Liquori CL, Berg MJ, Siegel AM, et al. Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. Am J Hum Genet 2003; 73: 1459–1464. 2003/11/19. DOI: 10.1086/380314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahoo T, Johnson EW, Thomas JW, et al. Mutations in the gene encoding KRIT1, a krev-1/rap1a binding protein, cause cerebral cavernous malformations (CCM1). Hum Mol Genet 1999; 8: 2325–2333. [DOI] [PubMed] [Google Scholar]
- 11.Riant F, Bergametti F, Ayrignac X, et al. Recent insights into cerebral cavernous malformations: the molecular genetics of CCM. FEBS J 2010; 277: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 12.Stamatovic SM, Sladojevic N, Keep RF, et al. PDCD10 (CCM3) regulates brain endothelial barrier integrity in cerebral cavernous malformation type 3: role of CCM3-ERK1/2-cortactin cross-talk. Acta Neuropathol 2015; 130: 731–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockton RA, Shenkar R, Awad IA, et al. Cerebral cavernous malformations proteins inhibit rho kinase to stabilize vascular integrity. J Exp Med 2010; 207: 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Zhao Y, Coleman P, et al. Low fluid shear stress conditions contribute to activation of cerebral cavernous malformation signalling pathways. Biochim Biophys Acta Mol Basis Dis 2019; 1865: 165519–165508. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Tang AT, Wong WY, et al. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature 2016; 532: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Shenkar R, Detter MR, et al. Propranolol inhibits cavernous vascular malformations by beta1 adrenergic receptor antagonism in animal models. J Clin Invest 131(3): e144893, 10.1172/JCI144893 (2020, accessed 18 May 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang AT, Choi JP, Kotzin JJ, et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 2017; 545: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wustehube J, Bartol A, Liebler SS, et al. Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating Delta-NOTCH signaling. Proc Natl Acad Sci U S A 2010; 107: 12640–12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galie PA, Nguyen DH, Choi CK, et al. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A 2014; 111: 7968–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore SA, Brown RD, Jr, Christianson TJ, et al. Long-term natural history of incidentally discovered cavernous malformations in a single-center cohort. J Neurosurg 2014; 120: 1188–1192. [DOI] [PubMed] [Google Scholar]
- 21.Horne MA, Flemming KD, Su IC, et al. Clinical course of untreated cerebral cavernous malformations: a Meta-analysis of individual patient data. Lancet Neurol 2016; 15: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrion-Penagos J, Zeineddine HA, Polster SP, et al. Subclinical imaging changes in cerebral cavernous angiomas during prospective surveillance. J Neurosurg. Epub ahead of print 3 April 2020. DOI: 10.3171/2020.1.JNS193479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polster SP, Stadnik A, Akers AL, et al. Atorvastatin treatment of cavernous angiomas with symptomatic hemorrhage exploratory proof of concept (at CASH EPOC) trial. Neurosurgery 2019; 85: 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polster SP, Cao Y, Carroll T, et al. Trial readiness in cavernous angiomas with symptomatic hemorrhage (CASH). Neurosurgery 2019; 84: 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard R, Fam MD, Zeineddine HA, et al. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg 2017; 127: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikati AG, Khanna O, Zhang L, et al. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab 2015; 35: 1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A, Zipfel GJ, Hildebolt C, et al. Hemodynamic effects of developmental venous anomalies with and without cavernous malformations. AJNR Am J Neuroradiol 2013; 34: 1746–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyne SB, Girard R, Koskimaki J, et al. Biomarkers of cavernous angioma with symptomatic hemorrhage. JCI Insight 4(12): e128577, 10.1172/jci.insight.128577 (2019, accessed 18 May 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Shahi Salman R, Berg MJ, Morrison L, et al. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma alliance scientific advisory board. Stroke 2008; 39: 3222–3230. [DOI] [PubMed] [Google Scholar]
- 30.Larsson HB, Hansen AE, Berg HK, et al. Dynamic contrast-enhanced quantitative perfusion measurement of the brain using T1-weighted MRI at 3T. J Magn Reson Imaging 2008; 27: 754–762. [DOI] [PubMed] [Google Scholar]
- 31.Larsson HB, Courivaud F, Rostrup E, et al. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med 2009; 62: 1270–1281. [DOI] [PubMed] [Google Scholar]
- 32.Hobson N, Polster SP, Cao Y, et al. Phantom validation of quantitative susceptibility and dynamic contrast-enhanced permeability MR sequences across instruments and sites. J Magn Reson Imaging 2019; 51: 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Li W, Hu F, et al. MR texture analysis: potential imaging biomarker for predicting the chemotherapeutic response of patients with colorectal liver metastases. Abdom Radiol (NY) 2019; 44: 65–71. [DOI] [PubMed] [Google Scholar]
- 34.Boyd AJ, Kuhn DC, Lozowy RJ, et al. Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J Vasc Surg 2016; 63: 1613–1619. [DOI] [PubMed] [Google Scholar]
- 35.Domencich TA, McFadden DL. Urban travel demand: a behavioral analysis. Oxford, UK: North-Holland Publishing Company Limited, 1975. [Google Scholar]
- 36.McFadden DL. Quantitative methods for analyzing travel behaviour of individuals: some recent developments. In: Hensher D, Stopher P. (eds) Behavioural travel modelling. London, UK: Croom Helm, 1977, pp.279–318. [Google Scholar]
- 37.Schwarz G. Estimating the dimension of a model. Ann Stat 1978; 6: 461–464. [Google Scholar]
- 38.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–35. [DOI] [PubMed] [Google Scholar]
- 39.BFJ M. Randomization, bootstrap, and Monte Carlo methods in biology. London, UK: Chapman & Hall, 1997. [Google Scholar]
- 40.Angioma Alliance. Angioma alliance, www.angioma.org/ (2020, accessed 13 May 2021).
- 41.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000; 106: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prabhakaran S, Naidech AM. Ischemic brain injury after intracerebral hemorrhage: a critical review. Stroke 2012; 43: 2258–2263. [DOI] [PubMed] [Google Scholar]
- 43.Girard R, Zeineddine HA, Koskimaki J, et al. Plasma biomarkers of inflammation and angiogenesis predict cerebral cavernous malformation symptomatic hemorrhage or lesional growth. Circ Res 2018; 122: 1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polster SP, Sharma A, Tanes C, et al. Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma. Nat Commun 2020; 11: 2659–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer 2018; 18: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu G, Jiang B, Tong L, et al. Applications of deep learning to Neuro-Imaging techniques. Front Neurol 2019; 10: 869–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H, Flemming K, Nelson J, et al. Baseline characteristics of patients with cerebral cavernous angiomas with symptomatic hemorrhage in a multisite trial readiness study. Stroke 2021; 52: AP46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wernick MN, Yang Y, Brankov JG, et al. Machine learning in medical imaging. IEEE Signal Process Mag 2010; 27: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parmar C, Grossmann P, Bussink J, et al. Machine learning methods for quantitative radiomic biomarkers. Sci Rep 2015; 5: 13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpraga S, Alvarez-Jimenez R, Mansvelder HD, et al. EEG machine learning for accurate detection of cholinergic intervention and Alzheimer’s disease. Sci Rep 2017; 7: 5775–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211020587 for Perfusion and permeability as diagnostic biomarkers of cavernous angioma with symptomatic hemorrhage by Je Yeong Sone, Yan Li, Nicholas Hobson, Sharbel G Romanos, Abhinav Srinath, Seán B Lyne, Abdallah Shkoukani, Julián Carrión-Penagos, Agnieszka Stadnik, Kristina Piedad, Rhonda Lightle, Thomas Moore, Ying Li, Dehua Bi, Robert Shenkar, Timothy Carroll, Yuan Ji, Romuald Girard and Issam A Awad in Journal of Cerebral Blood Flow & Metabolism