Abstract
Aneurysmal subarachnoid hemorrhage (aSAH) patients develop delayed cerebral ischemia and delayed deficits (DCI) within 2 weeks of aneurysm rupture at a rate of approximately 30%. DCI is a major contributor to morbidity and mortality after SAH. The cause of DCI is multi-factorial with contributions from microthrombi, blood vessel constriction, inflammation, and cortical spreading depolarizations. Platelets play central roles in hemostasis, inflammation, and vascular function. Within this review, we examine the potential roles of platelets in microthrombi formation, large artery vasospasm, microvessel constriction, inflammation, and cortical spreading depolarization. Evidence from experimental and clinical studies is provided to support the role(s) of platelets in each pathophysiology which contributes to DCI. The review concludes with a suggestion for future therapeutic targets to prevent DCI after aSAH.
Keywords: Subarachnoid hemorrhage, aneurysm, delayed deficits, delayed cerebral ischemia, platelets
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) has a high initial mortality rate, and approximately 30% of aSAH patients develop delayed cerebral ischemia and neurological deficits (DCI).1,2 Furthermore, roughly half of aSAH survivors will sustain persistent neurological deficits. 1 Not only is DCI a critical contributor to morbidity and mortality during the first few weeks following aSAH, it is also the main cause of long-term poor outcomes in aSAH patients. 1 The progression of DCI usually begins between the 4th and 7th day after aSAH and is characterized by either a new ischemic lesion or neurological decline. 3 It is currently accepted that DCI is multi-factorial and the mechanisms include microthrombi formation, blood vessel constriction, inflammation, and cortical spreading depolarization.1,2,4,5 However, it is not well-known if these processes are independent 6 or if there is a crucial mediator which induces these pathological events. This review will examine the potential role(s) of platelets in each of the pathological events of DCI after aSAH (Figure 1) and discuss future therapeutic targets.
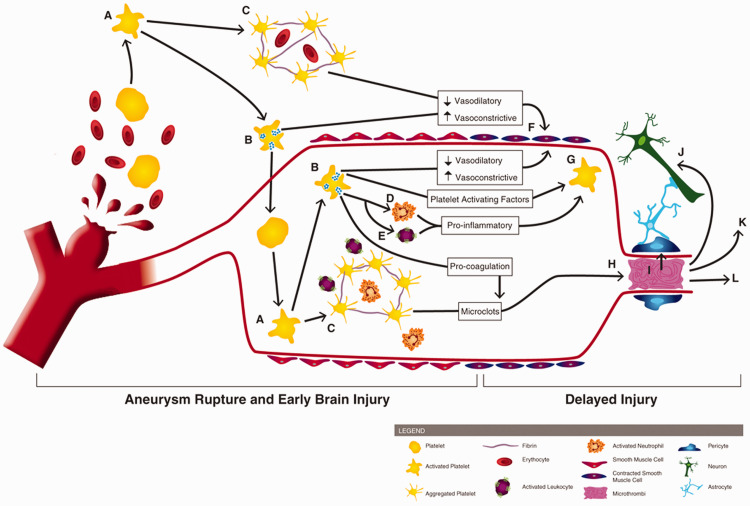
Figure 1.
Involvement of platelets in early brain injury and the pathogenesis of delayed cerebral ischemia. Following aneurysm rupture, erythrocytes and platelets are spilled into the subarachnoid space. Upon exposure to the extracellular space (and its matrix proteins, collagen and laminin), platelets become activated (A) Activated platelets degranulate (B) and form aggregates which can contain erythrocytes and immune cells (C). Platelet degranulation can activate intraluminal platelets (thereby propagating platelet activation, degranulation, and aggregation), neutrophils (D) and leukocytes (E). Activated platelets reduce their vasodilation mechanisms and release vasoconstrictive molecules, promoting large artery vasospasm (F). Degranulating platelets, along with activated leukocytes/neutrophils, exacerbate platelet activation (G). Pro-coagulant factors, released by platelets, promotes microclot formation which can deposit within brain microvessels (H). Platelet aggregates and microthrombi in the microvessels can induce pericyte constriction (I), cause neurotoxicity (J), initiate cortical spreading depolarization (K), and occlude blood flow, thereby reducing cerebral blood flow and causing development of delayed infarctions (L).
Platelets are critical mediators of hemostasis, inflammation, and vascular tone. 7 Platelets promote clotting via self-aggregation and interactions with fibrin(ogen) and endothelial cells, participate in inflammation through the release of chemokines/cytokines and by interactions with immune cells, and initiate blood vessel constriction via the release of vasoconstricting factors.
Delayed platelet activation
In the days following aneurysm securement, aSAH patients remain hypercoagulable which contributes to DCI. 8 More specifically, platelet reactivity is increased in aSAH patients who go on to develop DCI compared to aSAH patients who did not develop DCI. 9
Platelets coursing through the vasculature are subject to numerous activation signals released during in response to SAH (Figure 2). In humans, aSAH leads to an increase in serum inflammatory cytokines/chemokines/lipid-mediators (CXCL12, 10 platelet activating factor (PAF) 11 ), von Willebrand factor (vWF),11,12 as well as platelet-derived factors (thrombin,11,13 thromboxane14,15), all of which have roles in platelet activation (Figure 2). Additionally, experimental SAH studies have observed platelet activation via direct interactions with activated leukocytes (via P-selectin and PSGL-1 interactions) 16 and disrupted endothelial cells (by a yet unknown mechanism). 17
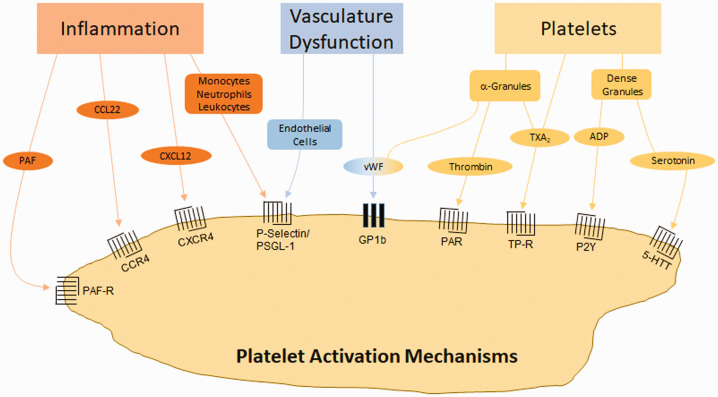
Figure 2.
Mechanisms of platelet activation by SAH. Inflammation can induce platelet activation through receptors which respond to inflammatory cytokines (PAF, CCL22, CXCL12) or inflammatory cell binding (monocytes, neutrophils, leukocytes). Vascular dysfunction can induce platelet activation via endothelial cell adherence or through vWF. Platelets are also activated through molecules released from α-granules and dense granules. CCL22 (CC chemokine ligand 22): (ligand of CCR4 (CC chemokine receptor 4)), CXCL12 (CXC motif chemokine ligand 12): (ligand of CXCR4 (CXC motif chemokine receptor 4)). 5-HTT: serotonin receptor; ADP: adenosine diphosphate; GP: glycoprotein; P2Y: purinergic receptor; PAF: platelet activating factor; PAF-R: PAF receptor; PAR: protease activated receptor; PSGL-1: P-selectin glycoprotein ligand-1; TP-R: thromboxane receptor; TXA2: thromboxane A2; vWF: von Willebrand factor.
Upon activation, platelets degranulate, releasing platelet activating factors (thromboxane A2 (TXA2), adenosine diphosphate (ADP), thrombin, vWF, serotonin) and increasing platelet expression of activation and aggregation receptors (glycoprotein (GP) Ib, GPVI, integrins), thus propagating platelet activation. Additionally, activated platelets release extracellular vesicles stimulating coagulation,18,19 inflammation, and activating neighboring platelets (Figure 3), 7 although this has not been explicitly shown for SAH.
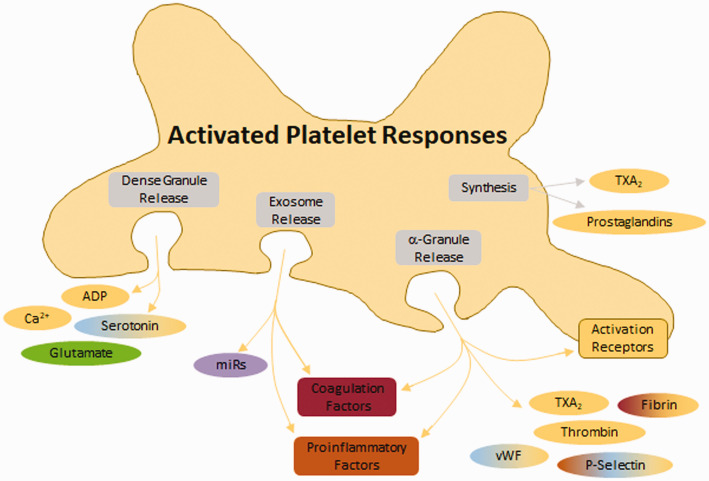
Figure 3.
Response of platelets to activation. Following activation, platelets release several molecules from dense granules, exosomes, α-granules, and via synthesis. Dense granules release small molecules. Exosomes release miRs which have functions in coagulation, inflammation, and vasoconstriction. α-granules release proteins which promote coagulation, inflammation, and vasoconstriction. Additionally, α-granules deposit several platelet activation/aggregation receptors on the membrane surface (including glycoprotein (GP)VI, GP1b, P-selectin, platelet-endothelial cell adhesion molecule 1 (PECAM), and integrins).
ADP: adenosine diphosphate; TXA2: thromboxane A2; vWF: von Willebrand factor.
Platelets cause microthrombi formation
Following SAH, platelets are subject to brief adhesion events to the aggravated vasculature tissue (including in distal microvessels)16,20 which leads to aggregation and microthrombi formation. 20 Deposition of platelet aggregates/microthrombi (which can contain leukocytes) within the microvasculature reduces cerebral blood flow. 20 Consequently, lower blood flow propagates intravascular microthrombosis and capillary obstruction either directly 21 or by reduced platelet inhibition, 22 and prolonged lowering of cerebral blood flow leads to the development of DCI.
In humans, microthrombi have been reported as an associative cause of DCI and worse neurological outcome.23,24 In aSAH autopsy studies, microthrombi (which are diffuse throughout the brain) accompany regions of infarct23,24 and develop with a timing similar to DCI. 25 Analysis of the cerebral microthrombi composition in aSAH patients indicates that the microthrombi consist of aggregated platelets and fibrin, sometimes mixed with leukocytes. 24
In animals subjected to SAH, microthrombi are observed in brain vasculature both locally and distally from the insult,26–32 as well as in both hemispheres,33–38 and microthrombi and occluded blood vessels occur throughout the brain from 2 days 32 to 7 days post-SAH.33,35,37,39,40 Furthermore, after experimental SAH, microthrombi count positively correlates with the number of apoptotic neurons, 32 infarction volume, 34 and delayed deficits. 34
A potential mechanism which platelets lead to microthrombosis is via P-selectin and nitric oxide (NO). Sabri et al. reported that mice with SAH had increased expression of membrane P-selectin on endothelial cells and that microthrombi co-localized with P-selectin staining. 32 Additionally, it was reported that P-selectin negatively correlates with brain NO. 32 As NO is a potent inhibitor of platelet activation, and P-selectin is involved in platelet-cell interactions, the authors concluded that the increased P-selectin and decreased NO may drive platelets towards microthrombosis. 32
Platelets induce vessel constriction
Platelets/microthrombi are found near arteries experiencing vasospasm.41–43 Upon adhering to the intraluminal surface of blood vessel endothelial cells, platelets rapidly change the local environment by releasing vasoconstricting agents (i.e. thromboxane A2 (TXA2),44–46 prostaglandin endoperoxides (e.g. PGG2, PGH2),47,48 platelet-derived growth factor (PDGF)-β,49,50 and serotonin51,52).
Large artery vasospasm
In aSAH patients with large artery vasospasm, platelets are hyper-active and have higher aggregability than in patients not experiencing vasospasm.53,54 Experimentally, platelets are reported to cause prolonged cerebral vasospasm,52,55–58 and several studies have even suggested that platelet activation precedes vasospasm after aSAH.9,49,50,53,54,59,60 The mechanism by which platelets induce large artery vasospasm may be via elevated levels of the vasoconstricting factors, TXA2 and PDGF-β.
A number of clinical studies have highlighted TXA2 as being involved in the development of vasospasm and pathogenesis of DCI.53,54,61–63 In a rabbit model of SAH, platelets were shown to cause contraction of the basilar artery which was effectively prevented when platelets were pre-incubated with a thromboxane synthetase inhibitor. 60 The study by Tanaka et al. provides evidence that platelets are a cause of large artery vasospasm. 60
PDGF-β has also been reported to positively correlate with incidence and severity of vasospasm after aSAH,49,50 and in animals models.55,58 Experimentally, vasospasm increases as plasma levels of PDGF-β rise 55 and use of a PDGF antagonist can prevent vasospasm. 58 However, the evidence for PDGF-β being a possible mechanism is correlational and does not provide insight into causality. Furthermore, the source of PDGF-β was not reported.
Three additional preclinical studies which further support platelets as a major contributor to vasospasm following SAH were those of Pisapia et al. and Sonobe and Suzuki. Pisapia et al. utilized a vasodilating drug and a microthrombi antagonist after SAH in mice and found that the vasodilating drug had no effect on microthrombi count. 35 Unfortunately the study did not report on vasospasm, so it is unclear if the microthrombi antagonist had any effect on vasospasm. However, this study highlights two important ideas: (1) large artery vasospasm does not cause microthrombi, and (2) the events (microthrombi and vasospasm) may either be distinct pathophysiological mechanisms or platelets have a causal relationship with vasospasm. Direct evidence of platelets causing vasospasm after SAH was provided by Sonobe and Suzuki; the authors applied blood directly to feline basilar arteries and concluded that the observed vasospasm was due to platelet-derived serotonin 57 which has been supported by another study. 52
Taken together, these clinical and experimental models argue that platelets play a significant role in the cause of large artery vasospasm. While the mechanism remains unknown, platelets may be a therapeutic target which can attenuate the burden of large artery vasospasm after aSAH.
Microvessel constriction
Direct observation of pial arterioles after SAH in mice led to a very prominent correlation between platelet aggregates and microvessel constriction,27,32,64 which lead to cerebral blood flow deficits. 64 Studies have shown that microvessel constriction and formation of platelet aggregates/clots began within seconds after SAH 27 and that platelet aggregates were observed within the most constricted microvessels, 27 leading to a striking correlation between platelet aggregates/clots and microvessel constriction. 27 In the study by Friedrich et al., it was concluded that the microvessels containing platelet aggregates have perfusion deficits, and that these platelet aggregates may initiate or propagate local microvessel constriction. 64 In a follow-up study, the supplemental video shows that after the microthrombus forms the microvessel constricts even further, as well as a new constriction beginning near the microthrombi. 27 Additional imaging studies are needed since antagonists for either platelets or vasospasm were not administer, so it is not possible to determine a causal relationship.
Two studies allude to mechanisms by which platelets are linked to microvessel constriction, however causality cannot be determined. First, Sabri et al. reported that decreased NO and increased P-selectin on arteriole endothelium may be a mechanism for microthrombosis and microvessel constriction. 32 Of note, Sabri et al. observed microthrombi within microvessels experiencing constriction. Increased P-selectin on the endothelium promotes platelet binding and subsequent thrombi formation, whereas decreased NO will not be able to inhibit platelets. In the second study, Li et al. observed an elevated expression of the PDGF-β receptor on capillary pericytes. 65 Although PDGF-β levels were not measured by Li et al., since others report that PDGF-β is elevated following SAH and that PDGF-β positively correlates with DCI,49,50 this may be a mechanism of platelet-induced microvessel constriction. The role of PDGF-β in platelet-mediated microvessel constriction is a yet unexplored area.
Although the mechanisms by which platelets cause microvessel constrictions remains unknown, there is evidence that platelets play a role,6,13 possibly through release of vasoactive factors, 23 or P-selectin binding. 32 Additional studies are needed before a causal relationship can be confirmed.
Platelets promote inflammation
The inflammatory role of platelets is well-documented; platelets can activate or propagate inflammation via release of pro-inflammatory factors and through direct interactions with leukocytes and neutrophils. 7
After SAH, although a causal relationship cannot be determined, both platelets and inflammation are associated with worse early brain injury, DCI, and poor functional outcomes.66–69 The work of Frontera et al. in aSAH patients found that brain injury severity correlated with more platelet activation and inflammation (e.g. C-reactive peptide), and that both platelet activation and C-reactive peptide were associated with worse 3-month functional outcomes.66,67 Since platelet reactivity and C-reactive peptide increased concomitantly, a causal relationship could not be identified.66,67 However, in aSAH studies by Ray et al., platelet activation (e.g. coated platelets) correlated with DCI,68,69 and an increase in platelet activation preceded an increase in the number of circulating neutrophils. 68 Ray et al. only reported one inflammatory cell measurement (i.e. inflammatory cytokines were not measured), so it is not possible to say definitively that platelet activation preceded inflammation after SAH. However, the evidence is clear that platelet activation correlated with a delayed increase in the number of neutrophils. More studies are needed to attempt to unveil a causal relationship between inflammation and platelets following SAH.
Using intravital microscopy, Ishikawa et al. observed significant platelet-leukocyte interactions after SAH in mice within 24 h after injury. 16 Use of a platelet antagonist (P-selectin antibody) prevented platelet-leukocyte interactions, as well as reduced leukocyte adherence to endothelium, suggesting that platelets can induce leukocyte activation after SAH. Although general inflammation (such as cytokine levels) was not assessed, the finding that a platelet antagonist can reduce leukocyte activation provides some evidence of a causal relationship.
Can platelets cause cortical spreading depolarization/ischemia?
Cortical spreading depolarizations/ischemia have been correlated with DCI. 70 Evidence is limited on platelets directly causing cortical spreading depolarization/ischemia. However, microthrombi, vasospasm, and microvessel constriction each promote cortical spreading depolarization/ischemia. 71
A mechanism by which platelets induce cortical spreading depolarization/ischemia may be via glutamate. Glutamate levels are high after aSAH in patients, 72 and excessive amounts of glutamate cause excitotoxicity, spreading depolarizations, and neuronal death. 73 Interestingly, platelets induce glutamate-mediated neuronal toxicity and death. Bell et al. showed that activated platelets are very potent sources of glutamate (which can exceed 300 µm in vitro), and the concentration of glutamate released by activated platelets is neurotoxic in cell culture and after SAH in rats. 29 Further, seven days post-SAH, there was a strong correlation between the proximity of microthrombi and the reduction of glutamate receptors on neurons, suggesting platelets can induce neuronal dysfunction and death. Although cortical spreading depolarizations/ischemia were not examined, this study warrants investigation into platelets as an initiator of cortical spreading depolarizations/ischemia after SAH.
Platelets as a therapeutic target to prevent DCI
Within the above sections, platelets were shown to have potential roles in the pathological events which contribute to DCI after SAH. Thus, platelets may be a therapeutic target to prevent DCI. One reservation of using anti-platelet therapies in aSAH patients is the potential of new bleeds (i.e. rebleeding or microbleeds). However, recent aSAH clinical trials have shown that the risk of new bleeds for anti-platelet drugs is minimal. 74 Of course, caution in patients with intraventricular drains or those in need of surgical intervention is the rule,75,76 it should be noted that there exists a patient population which receives anti-platelet drugs following coil embolization with minimal complications.77–80 So why are anti-platelets not more widely used for aSAH patients? Despite their safety in use, trials of anti-platelet agents have not shown a clear clinical benefit. Two reasons may account for this: (1) only a portion of patients with SAH will develop DCI and so far we are unable to predict this cohort and (2) we have limited understanding of the mechanism for DCI including the most potent targets, as well as the timing and duration of pharmacologic treatment. Another possible reason anti-platelets are not widely used or studied for aSAH is that there are many different targets on platelets (i.e. inhibiting activation, inhibiting aggregation, inhibiting interactions with other cells) and no one knows which target to choose.
To date, the use of anti-platelet therapies have had marginal success in clinical trials. In the 1970s, a clinical study investigated the potential of serotonin antagonists for treating aSAH patients. The study had small sample sizes (32 treated patients, 99 untreated patients), but the results suggested that serotonin antagonists may be useful in preventing delayed vasospasm, temporary deficits, and permanent deficits. 81 Despite promising findings, serotonin antagonists were not investigated in large, randomized clinical trials.
Trials using classical anti-platelet therapies (inhibitors of cyclooxygenase (acetylsalicylic acid),82–84 thromboxane synthetase,85,86 phosphodiesterase 3, 87 and P2Y12 88 ) had a tendency towards less secondary cerebral ischemia and better outcome, however the studies were generally under-powered. 89 Only one study examining ticlopidine observed significantly improved outcome for aSAH patients treated with an anti-platelet. 88 A meta-analysis of these 7 anti-platelet trials indicated that there was no conclusive evidence that single anti-platelet therapy was beneficial for preventing DCI after aSAH. 89 More recent clinical trials have focused on investigating the potential for dual anti-platelet therapy in SAH. The data from studies using dual anti-platelet therapy have been promising for reducing DCI. 90 Below we discuss other potential targets for therapeutic design.
Alternative anti-platelet targets
Platelet activation receptors
Several platelet activation receptors (protease activated receptors (PAR), thromboxane receptors, purinergic receptors (P2Y)) have been tested as therapeutic targets for aSAH.74,78,91–95 Two activation receptors which may be promising are the GPIb-IX-V complex and GPVI. The GPIb-IX-V complex interacts with vWF and thrombin, and is crucial in platelet adhesion to endothelial cells and stabilizing microclots. GPVI interacts with fibrinogen and is important in platelet-platelet interaction/aggregation. To date, neither of these platelet activation receptors have been tested for experimental nor clinical SAH.
Endogenous platelet inhibition
Platelets have endogenous inhibitory mechanisms, so augmenting these mechanisms may have therapeutic potential. Prostacyclin (acts via prostaglandin I2 receptor) and NO, which are platelet inhibitors and vasodilators, 7 were promising in recent clinical studies.96–98 In a small study with 90 aSAH patients, prostacyclin lowered the incidence of DCI (21%–23% for prostacyclin treatment vs 38% for placebo) although this was not statistically significant since the study was not powered to investigate a difference in DCI incidence. 96 Two studies using a NO donor, molsidomine, observed a significantly lower incidence of DCI (13.8% molsidomine plus nimodipine vs 48% nimodipine only, p < 0.01), improved 3 month outcome, and lower mortality in aSAH patients receiving the NO donor plus nimodipine.97,98 Large, randomized trials should be considered to determine if either of these agents can prevent DCI and improve outcome after aSAH.
Another endogenous inhibitory mechanism, glucagon-like peptide 1, was tested in a preclinical SAH study. The experimental study investigated the therapeutic benefit of glucagon-like peptide 1 on early brain injury after SAH. 99 The results suggested that glucagon-like peptide 1 is promising for early brain injury, but its benefit in the delayed phase is not known.
Platelet aggregation
Although there has not yet been a large, randomized clinical trial to prevent platelet aggregation, a number of small clinical studies and case reports have shown that antagonizing platelet aggregation via GP IIb/IIIa may be therapeutically beneficial.100–109 Overall, the conclusions from these studies indicate that GP IIb/IIIa antagonists are safe and effective, with minimal risk of hemorrhagic complications. Large, randomized trials need to be performed to determine if GP IIb/IIIa antagonists can reduce DCI and improve outcome after SAH.
Other targets
Targeting either P-selectin or its receptor P-selectin glycoprotein ligand 1 would limit platelet adherence to leukocytes and endothelial cells. As demonstrated in experimental SAH models, P-selectin has some therapeutic potential in preventing microthrombi, 32 platelet-endothelial binding, 32 and platelet-leukocyte interactions. 16 Additional experimental studies would need to investigate the efficacy of P-selectin or its receptor in the context of delayed injury after experimental SAH prior to thinking about clinical translation. vWF is free in the blood, released by activated platelets and endothelial cells, and expressed on endothelial cells.7,110 Upon activation and release of the Weibel-Palade bodies, endothelial cells rapidly increase free vWF (promoting platelet-platelet binding) and their surface vWF (promoting platelet adhesion). Thus, targeting either vWF or its receptors are potential therapeutic targets. Support for targeting vWF comes from experimental SAH studies which found that ADAMTS13 (which cleaves multimers of vWF) effectively reduces the thrombotic potential of vWF. Additionally, ADAMTS13 is a thrombolytic. 111 Several experimental SAH studies have reported that recombinant ADAMTS13 reduces microthrombi,33,112–114 improves short-term functional outcome, 33 attenuates delayed microthrombi, 112 and reduces delayed neuronal death/ischemia. 112 Additionally, in mice, ADAMTS13 treatment did not increase the amount of subarachnoid hematoma. 112 Thus, the dual function of ADAMTS13 makes it an attractive option as a therapy for SAH and it may be time to investigate ADAMTS13 in small clinical studies.
Summary
Platelets are a key player in the pathogenesis of DCI following SAH since platelets are major contributors to microthrombosis, vasospasm, microvessel constriction, and inflammation after SAH, and may also be important initiators of cortical spreading depolarization/ischemia and neurotoxcitiy. Although early clinical trials on anti-platelet therapies were inconclusive, newer anti-platelets and alternative targets on platelets should be pursued and tested for preventing DCI after SAH. At this time, GP IIb/IIIa antagonists, prostacyclin, and NO are ready for large, randomized clinical trials, while ADAMTS13 should be considered for small clinical studies.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding support was provided by the Brain Aneurysm Foundation (DWM) and NIH (SLB).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Budohoski KP, Guilfoyle M, Helmy A, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2014; 85: 1343–1353. [DOI] [PubMed] [Google Scholar]
- 2.Durrant JC, Hinson HE. Rescue therapy for refractory vasospasm after subarachnoid hemorrhage. Curr Neurol Neurosci Rep 2015; 15: 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41: 2391–2395. [DOI] [PubMed] [Google Scholar]
- 4.Foreman B, Albers D, Schmidt JM, et al. Intracortical electrophysiological correlates of blood flow after severe SAH: a multimodality monitoring study. J Cereb Blood Flow Metab 2018; 38: 506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rots ML, van Putten MJ, Hoedemaekers CW, et al. Continuous EEG monitoring for early detection of delayed cerebral ischemia in subarachnoid hemorrhage: a pilot study. Neurocrit Care 2016; 24: 207–216. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol 2007; 3: 256–263. [DOI] [PubMed] [Google Scholar]
- 7.Michelson A, Cattaneo M, Frelinger A, et al. (eds) Platelets . 4th ed. New York: Academic Press, 2019.
- 8.Baranich AI, Polupan AA, Sychev AA, et al. Thromboelastometry as a comprehensive assessment of hypercoagulation after aneurysmal subarachnoid hemorrhage: a case report and literature review. In: Martin RD, Boling W, Chen G, et al. (eds) Subarachnoid hemorrhage: neurological care and protection. Cham: Springer International Publishing, 2020, pp.165–169. [DOI] [PubMed] [Google Scholar]
- 9.Perez P, Lukaszewicz A-C, Lenck S, et al. Platelet activation and aggregation after aneurysmal subarachnoid hemorrhage. BMC Neurol 2018; 18: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan D-S, Yan M, Hassan M, et al. Elevation of serum CXC chemokine ligand-12 levels predicts poor outcome after aneurysmal subarachnoid hemorrhage. J Neurol Sci 2016; 362: 53–58. [DOI] [PubMed] [Google Scholar]
- 11.Boluijt J, Meijers JC, Rinkel GJ, et al. Hemostasis and fibrinolysis in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a systematic review. J Cereb Blood Flow Metab 2015; 35: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergouwen MD, Bakhtiari K, van Geloven N, et al. Reduced ADAMTS13 activity in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2009; 29: 1734–1741. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, Kudo A, Otawara Y, et al. Extrinsic pathway of blood coagulation and thrombin in the cerebrospinal fluid after subarachnoid hemorrhage. Neurosurgery 1999; 44: 487–493. [DOI] [PubMed] [Google Scholar]
- 14.Hirashima Y, Hamada H, Kurimoto M, et al. Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 2005; 102: 882–887. [DOI] [PubMed] [Google Scholar]
- 15.Vinge E, Brandt L, Ljunggren B, et al. Thromboxane B2 levels in serum during continuous administration of nimodipine to patients with aneurysmal subarachnoid hemorrhage. Stroke 1988; 19: 644–647. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa M, Kusaka G, Yamaguchi N, et al. Platelet and leukocyte adhesion in the microvasculature at the cerebral surface immediately after subarachnoid hemorrhage. Neurosurgery 2009; 64: 546–553. [DOI] [PubMed] [Google Scholar]
- 17.Ohkuma H, Ogane K, Fujita S, et al. Impairment of anti-platelet-aggregating activity of endothelial cells after experimental subarachnoid hemorrhage. Stroke 1993; 24: 1541–1545. [DOI] [PubMed] [Google Scholar]
- 18.Morel O, Morel N, Freyssinet JM, et al. Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses. Platelets 2008; 19: 9–23. [DOI] [PubMed] [Google Scholar]
- 19.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med 2003; 197: 1585–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa M, Kajimura M, Morikawa T, et al. Leukocyte plugging and cortical capillary flow after subarachnoid hemorrhage. Acta Neurochir (Wien) 2016; 158: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 21.Ames A, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol 1968; 52: 437–453. [PMC free article] [PubMed] [Google Scholar]
- 22.Konidala S, Gutterman DD. Coronary vasospasm and the regulation of coronary blood flow. Prog Cardiovasc Dis 2004; 46: 349–373. [DOI] [PubMed] [Google Scholar]
- 23.Stein SC, Browne KD, Chen XH, et al. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery 2006; 59: 781–788. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Kimura M, Souma M, et al. Cerebral microthrombosis in symptomatic cerebral vasospasm–a quantitative histological study in autopsy cases. Neurol Med Chir (Tokyo) 1990; 30: 309–316. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58. [DOI] [PubMed] [Google Scholar]
- 26.Sabri M, Ai J, Lakovic K, et al. Mechanisms of microthrombosis and microcirculatory constriction after experimental subarachnoid hemorrhage. Acta Neurochir Suppl 2013; 115: 185–192. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich B, Müller F, Feiler S, et al. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab 2012; 32: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabri M, Ai J, Knight B, et al. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 2011; 31: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell JD, Thomas TC, Lass E, et al. Platelet-mediated changes to neuronal glutamate receptor expression at sites of microthrombosis following experimental subarachnoid hemorrhage. J Neurosurg 2014; 121: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 30.Liu ZW, Gu H, Zhang BF, et al. Rapidly increased vasopressin promotes acute platelet aggregation and early brain injury after experimental subarachnoid hemorrhage in a rat model. Brain Res 2016; 1639: 108–119. [DOI] [PubMed] [Google Scholar]
- 31.Boettinger S, Kolk F, Broessner G, et al. Behavioral characterization of the anterior injection model of subarachnoid hemorrhage. Behav Brain Res 2017; 323: 154–161. [DOI] [PubMed] [Google Scholar]
- 32.Sabri M, Ai J, Lakovic K, et al. Mechanisms of microthrombi formation after experimental subarachnoid hemorrhage. Neuroscience 2012; 224: 26–37. [DOI] [PubMed] [Google Scholar]
- 33.Muroi C, Fujioka M, Mishima K, et al. Effect of ADAMTS-13 on cerebrovascular microthrombosis and neuronal injury after experimental subarachnoid hemorrhage. J Thromb Haemost 2014; 12: 505–514. [DOI] [PubMed] [Google Scholar]
- 34.Dienel A, Matsumura K, Veettil RA, et al. Microthrombi correlates with infarction and delayed neurological deficits after subarachnoid hemorrhage in mice. Stroke 2020; 51: 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisapia JM, Xu X, Kelly J, et al. Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp Neurol 2012; 233: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G, Tariq A, Ai J, et al. Different effects of clazosentan on consequences of subarachnoid hemorrhage in rats. Brain Res 2011; 1392: 132–139. [DOI] [PubMed] [Google Scholar]
- 37.Sehba FA, Mostafa G, Friedrich V, Jr, et al. Acute microvascular platelet aggregation after subarachnoid hemorrhage. J Neurosurg 2005; 102: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 38.Andereggen L, Neuschmelting V, von Gunten M, et al. The role of microclot formation in an acute subarachnoid hemorrhage model in the rabbit. BioMed Res Int 2014; 2014: 161702–161708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Chen G, Zhu WW, et al. Influence of simvastatin on microthrombosis in the brain after subarachnoid hemorrhage in rats: a preliminary study. Ann Clin Lab Sci 2010; 40: 32–42. [PubMed] [Google Scholar]
- 40.Herz DA, Baez S, Shulman K. Pial microcirculation in subarachnoid hemorrhage. Stroke 1975; 6: 417–424. [DOI] [PubMed] [Google Scholar]
- 41.Alksne JF, Branson PJ. Pathogenesis of cerebral vasospasm. Neurol Res 1980; 2: 273–282. [DOI] [PubMed] [Google Scholar]
- 42.Fein JM, Flor WJ, Cohan SL, et al. Sequential changes of vascular ultrastructure in experimental cerebral vasospasm. Myonecrosis of subarachnoid arteries. J Neurosurg 1974; 41: 49–58. [DOI] [PubMed] [Google Scholar]
- 43.Mizukami M, Kin H, Araki G, et al. Is angiographic spasm real spasm? Acta Neurochir (Wien) 1976; 34: 247–259. [DOI] [PubMed] [Google Scholar]
- 44.Michibayashi T. [ Prostaglandins released from the isolated perfused arterial segment and vasocontractile response–influence of plasma constituents and platelets. Nihon Heikatsukin Gakkai Zasshi 1989; 25: 31–38.] [DOI] [PubMed] [Google Scholar]
- 45.Michibayashi T. Vaso-contractile responsiveness of perfused arterial segments to platelet-derived thromboxane A2. J Smooth Muscle Res 1992; 28: 25–33. [DOI] [PubMed] [Google Scholar]
- 46.Michibayashi T. Platelet aggregating response to platelet activating factor participates in activation of the 12-lipoxygenase pathway in platelets from rabbits. Int Angiology 2002; 21: 260–267. [PubMed] [Google Scholar]
- 47.Svensson J, Hamberg M, Samuelsson B. Prostaglandin endoperoxides IX. Characterization of rabbit aorta contracting substance (RCS) from guinea pig lung and human platelets. Acta Physiol Scand 1975; 94: 222–228. [DOI] [PubMed] [Google Scholar]
- 48.Henson PM. Bioassay of platelet-activating factor by release of [3H]serotonin. Methods Enzymol 1990; 187: 130–134. [DOI] [PubMed] [Google Scholar]
- 49.Gaetani P, Tancioni F, Grignani G, et al. Platelet derived growth factor and subarachnoid haemorrhage: a study on cisternal cerebrospinal fluid. Acta Neurochir 1997; 139: 319–324. [DOI] [PubMed] [Google Scholar]
- 50.Ghali MGZ, Srinivasan VM, Johnson J, et al. Therapeutically targeting platelet-derived growth factor-mediated signaling underlying the pathogenesis of subarachnoid hemorrhage-related vasospasm. J Stroke Cerebrovasc Dis 2018; 27: 2289–2295. [DOI] [PubMed] [Google Scholar]
- 51.Voldby B, Engbaek F, Enevoldsen EM. CSF serotonin concentrations and cerebral arterial spasm in patients with ruptured intracranial aneurysm. Stroke 1982; 13: 184–189. [DOI] [PubMed] [Google Scholar]
- 52.Satoh S, Suzuki Y, Harada T, et al. The role of platelets in the development of cerebral vasospasm. Brain Res Bull 1991; 27: 663–668. [DOI] [PubMed] [Google Scholar]
- 53.Juvela S, Ohman J, Servo A, et al. Angiographic vasospasm and release of platelet thromboxane after subarachnoid hemorrhage. Stroke 1991; 22: 451–455. [DOI] [PubMed] [Google Scholar]
- 54.Ohkuma H, Suzuki S, Kimura M, et al. Role of platelet function in symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1991; 22: 854–859. [DOI] [PubMed] [Google Scholar]
- 55.Cui HK, Yan RF, Ding XL, et al. Platelet-derived growth factor-beta expression in rabbit models of cerebral vasospasm following subarachnoid hemorrhage. Mol Med Rep 2014; 10: 1416–1422. [DOI] [PubMed] [Google Scholar]
- 56.Okwuasaba F, Cook D, Weir B. Changes in vasoactive properties of blood products with time and attempted identification of the spasmogens. Stroke 1981; 12: 775–780. [DOI] [PubMed] [Google Scholar]
- 57.Sonobe M, Suzuki J. Vasospasmogenic substance produced following subarachnoid haemorrhage, and its fate. Acta Neurochir (Wien) 1978; 44: 97–106. [DOI] [PubMed] [Google Scholar]
- 58.Zhang ZW, Yanamoto H, Nagata I, et al. Platelet-derived growth factor-induced severe and chronic vasoconstriction of cerebral arteries: proposed growth factor explanation of cerebral vasospasm. Neurosurgery 2010; 66: 728–735; discussion 735. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal A, Salunke P, Singh H, et al. Vasospasm following aneurysmal subarachnoid hemorrhage: thrombocytopenia a marker. J Neurosci Rural Prac 2013; 4: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka Y, Kassell NF, Torner JC. Effects of subarachnoid hemorrhage on platelet-derived vasoconstriction of rabbit basilar artery. Surg Neurol 1989; 32: 439–444. [DOI] [PubMed] [Google Scholar]
- 61.Juvela S, Kaste M, Hillbom M. Effect of nimodipine on platelet function in patients with subarachnoid hemorrhage. Stroke 1990; 21: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 62.Juvela S, Kaste M, Hillbom M. Platelet thromboxane release after subarachnoid hemorrhage and surgery. Stroke 1990; 21: 566–571. [DOI] [PubMed] [Google Scholar]
- 63.Moncada S, Vane JR. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med 1979; 300: 1142–1147. [DOI] [PubMed] [Google Scholar]
- 64.Friedrich V, Flores R, Muller A, et al. Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage. Brain Res 2010; 1354: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q, Chen Y, Li B, et al. Hemoglobin induced NO/cGMP suppression deteriorate microcirculation via pericyte phenotype transformation after subarachnoid hemorrhage in rats. Sci Rep 2016; 6: 22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frontera JA, Aledort L, Gordon E, et al. Early platelet activation, inflammation and acute brain injury after a subarachnoid hemorrhage: a pilot study. J Thromb Haemost 2012; 10: 711–713. [DOI] [PubMed] [Google Scholar]
- 67.Frontera JA, Provencio JJ, Sehba FA, et al. The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care 2017; 26: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ray B, Hollabaugh K, Ford L, et al. Abstract TP547: periperhal blood indices for platelet reactivity and systemic inflammation correlate with coated-platelet trends after aneurysmal subarachnoid hemorrhage. Stroke 2019; 50: ATP547. [Google Scholar]
- 69.Ray B, Pandav VM, Mathews EA, et al. Coated-platelet trends predict short-term clinical outcome after subarachnoid hemorrhage. Transl Stroke Res 2018; 9: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foreman B. The pathophysiology of delayed cerebral ischemia. J Clin Neurophysiol 2016; 33: 174–182. [DOI] [PubMed] [Google Scholar]
- 71.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 72.Nilsson OG, Säveland H, Boris-Möller F, et al. Increased levels of glutamate in patients with subarachnoid haemorrhage as measured by intracerebral microdialysis. Acta Neurochir Suppl 1996; 67: 45–47. [DOI] [PubMed] [Google Scholar]
- 73.Hinzman JM, DiNapoli VA, Mahoney EJ, et al. Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Exp Neurol 2015; 267: 243–253. [DOI] [PubMed] [Google Scholar]
- 74.Darkwah Oppong M, Gembruch O, Pierscianek D, et al. Post-treatment antiplatelet therapy reduces risk for delayed cerebral ischemia due to aneurysmal subarachnoid hemorrhage. Neurosurgery 2019; 85: 827–833. [DOI] [PubMed] [Google Scholar]
- 75.Bruder M, Schuss P, Konczalla J, et al. Ventriculostomy-related hemorrhage after treatment of acutely ruptured aneurysms: the influence of anticoagulation and antiplatelet treatment. World Neurosurg 2015; 84: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 76.Mahaney KB, Chalouhi N, Viljoen S, et al. Risk of hemorrhagic complication associated with ventriculoperitoneal shunt placement in aneurysmal subarachnoid hemorrhage patients on dual antiplatelet therapy. J Neurosurg 2013; 119: 937–942. [DOI] [PubMed] [Google Scholar]
- 77.Chalouhi N, Jabbour P, Kung D, et al. Safety and efficacy of tirofiban in stent-assisted coil embolization of intracranial aneurysms. Neurosurgery 2012; 71: 710–714; discussion 714. [DOI] [PubMed] [Google Scholar]
- 78.Entezami P, Holden DN, Boulos AS, et al. Cangrelor dose titration using platelet function testing during cerebrovascular stent placement. Intervent Neuroradiol. Epub ahead of print 1 July 2020. DOI: 10.1177/1591019920936923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsumoto Y, Iko M, Tsutsumi M, et al. The safety and efficacy of triple antiplatelet therapy after intracranial stent-assisted coil embolization. J Stroke Cerebrovasc Dis 2015; 24: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 80.Rossen JD, Chalouhi N, Wassef SN, et al. Incidence of cerebral ischemic events after discontinuation of clopidogrel in patients with intracranial aneurysms treated with stent-assisted techniques. J Neurosurg 2012; 117: 929–933. [DOI] [PubMed] [Google Scholar]
- 81.Knuckey NW, Stokes BA. Medical management of patients following a ruptured cerebral aneurysm, with epsilon-aminocaproic acid, kanamycin, and reserpine. Surg Neurol 1982; 17: 181–185. [DOI] [PubMed] [Google Scholar]
- 82.Hop JW, Rinkel GJ, Algra A, et al. Randomized pilot trial of postoperative aspirin in subarachnoid hemorrhage. Neurology 2000; 54: 872–878. [DOI] [PubMed] [Google Scholar]
- 83.Mendelow AD, Stockdill G, Steers AJ, et al. Double-blind trial of aspirin in patient receiving tranexamic acid for subarachnoid hemorrhage. Acta Neurochir (Wien) 1982; 62: 195–202. [DOI] [PubMed] [Google Scholar]
- 84.van den Bergh WM, Algra A, Dorhout Mees SM, MASH Study Group et al. Randomized controlled trial of acetylsalicylic acid in aneurysmal subarachnoid hemorrhage: the MASH study. Stroke 2006; 37: 2326–2330. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki S, Sano K, Handa H, et al. Clinical study of OKY-046, a thromboxane synthetase inhibitor, in prevention of cerebral vasospasms and delayed cerebral ischaemic symptoms after subarachnoid haemorrhage due to aneurysmal rupture: a randomized double-blind study. Neurol Res 1989; 11: 79–88. [DOI] [PubMed] [Google Scholar]
- 86.Tokiyoshi K, Ohnishi T, Nii Y. Efficacy and toxicity of thromboxane synthetase inhibitor for cerebral vasospasm after subarachnoid hemorrhage. Surg Neurol 1991; 36: 112–118. [DOI] [PubMed] [Google Scholar]
- 87.Shaw MD, Foy PM, Conway M, et al. Dipyridamole and postoperative ischemic deficits in aneurysmal subarachnoid hemorrhage. J Neurosurg 1985; 63: 699–703. [DOI] [PubMed] [Google Scholar]
- 88.Ono H, Mizukami M, Kitamura K, et al. Subarachnoid hemorrhage. Agents Actions Supp 1984; 15: 259–272. [PubMed] [Google Scholar]
- 89.Dorhout Mees SM, van den Bergh WM, Algra A, et al. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. The Cochrane Database of Systematic Reviews 2007: Cd006184. [DOI] [PMC free article] [PubMed]
- 90.Clarke JV, Suggs JM, Diwan D, et al. Microvascular platelet aggregation and thrombosis after subarachnoid hemorrhage: a review and synthesis. J Cereb Blood Flow Metab 2020; 40: 1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benken ST, Tesoro EP, Kim KS, et al. Treatment outcomes of heparin-induced thrombocytopenia in subarachnoid hemorrhage patients: a 4-year, retrospective single-center review. Neurocrit Care 2012; 17: 177–182. [DOI] [PubMed] [Google Scholar]
- 92.Linfante I, Ravipati K, Starosciak A, et al. Intravenous cangrelor and oral ticagrelor as an alternative to clopidogrel in acute intervention. J Neurointervent Surg 2021; 13: 30–32. [DOI] [PubMed] [Google Scholar]
- 93.Nagahama Y, Allan L, Nakagawa D, et al. Dual antiplatelet therapy in aneurysmal subarachnoid hemorrhage: association with reduced risk of clinical vasospasm and delayed cerebral ischemia. J Neurosurg 2018; 129: 702–710. [DOI] [PubMed] [Google Scholar]
- 94.Fujita K, Yamashita H, Masumura M, et al. The effects of ticlopidine and nicardipine on the prevention of symptomatic vasospasm after aneurysmal rupture. No Shinkei Geka Neurological Geka 1988; 16: 741–746. [PubMed] [Google Scholar]
- 95.Kobayashi S, Sugita K, Tanizaki Y, et al. Mortality study of patients with subarachnoid haemorrhage at university hospitals and their affiliated hospitals in Japan. Acta Neurochir (Wien) 1982; 63: 175–183. [DOI] [PubMed] [Google Scholar]
- 96.Rasmussen R, Wetterslev J, Stavngaard T, et al. Effects of prostacyclin on cerebral blood flow and vasospasm after subarachnoid hemorrhage: randomized, pilot trial. Stroke 2015; 46: 37–41. [DOI] [PubMed] [Google Scholar]
- 97.Ehlert A, Schmidt C, Wölfer J, et al. Molsidomine for the prevention of vasospasm-related delayed ischemic neurological deficits and delayed brain infarction and the improvement of clinical outcome after subarachnoid hemorrhage: a single-center clinical observational study. J Neurosurg 2016; 124: 51–58. [DOI] [PubMed] [Google Scholar]
- 98.Ehlert A, Starekova J, Manthei G, et al. Nitric oxide-based treatment of poor-grade patients after severe aneurysmal subarachnoid hemorrhage. Neurocrit Care 2020; 32: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie Z, Enkhjargal B, Wu L, et al. Exendin-4 attenuates neuronal death via GLP-1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology 2018; 128: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cavalcanti DD, Abla AA, Martirosyan NL, et al. Endovascular management of distal ACA aneurysms: single-institution clinical experience in 22 consecutive patients and literature review. AJNR Am J Neuroradiol 2013; 34: 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dababneh H, Guerrero W, Mehta S, et al. Possible role of eptifibatide drip in-patient with aneurysmal subarachnoid hemorrhage in vasospasm prevention. J Vasc Intervent Neurol 2014; 7: 8–13. [PMC free article] [PubMed] [Google Scholar]
- 102.Dandapat S, Zanaty M, Nakagawa D, et al. Abstract TP545: continuous intravenous tirofiban in patient with aneurysmal subarachnoid hemorrhage requiring external ventricular drain and ventriculoperitoneal shunts. Stroke 2019; 50: ATP545. [Google Scholar]
- 103.Gentric JC, Brisson J, Batista AL, et al. Safety of abciximab injection during endovascular treatment of ruptured aneurysms. Interv Neuroradiol 2015; 21: 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Katsaridis V, Papagiannaki C, Skoulios N, et al. Local intra-arterial eptifibatide for intraoperative vessel thrombosis during aneurysm coiling. AJNR Am J Neuroradiol 2008; 29: 1414–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levrier O, Stordeur JM, Bruder N, et al. Postoperative intracranial thrombolysis and angioplasty. Interv Neuroradiol 2001; 7: 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang XD, Wang ZL, Li TX, et al. Safety and efficacy of a new prophylactic tirofiban protocol without oral intraoperative antiplatelet therapy for endovascular treatment of ruptured intracranial aneurysms. J Neurointerv Surg 2016; 8: 1148–1153. [DOI] [PubMed] [Google Scholar]
- 107.Park JH, Kim JE, Sheen SH, et al. Intraarterial abciximab for treatment of thromboembolism during coil embolization of intracranial aneurysms: outcome and fatal hemorrhagic complications. J Neurosurg 2008; 108: 450–457. [DOI] [PubMed] [Google Scholar]
- 108.Samaniego EA, Gibson E, Nakagawa D, et al. Safety of tirofiban and dual antiplatelet therapy in treating intracranial aneurysms. Stroke Vasc Neurol 2019; 4: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zanaty M, Osorno-Cruz C, Byer S, et al. Tirofiban protocol protects against delayed cerebral ischemia: a case-series study. Neurosurgery 2020; 87: E552–E556. [DOI] [PubMed] [Google Scholar]
- 110.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol 2005; 25: 1321–1324. [DOI] [PubMed] [Google Scholar]
- 111.Crescente M, Thomas GM, Demers M, et al. ADAMTS13 exerts a thrombolytic effect in microcirculation. Thromb Haemost 2012; 108: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fujioka M, Muroi C, Tsuboi A, et al. Neuroprotective effects of ADAMTS13 against delayed brain ischemia after aneurysmal subarachnoid hemorrhage. J Neurol Sci 2017; 381: 504. [Google Scholar]
- 113.Vergouwen MD, Knaup VL, Roelofs JJ, et al. Effect of recombinant ADAMTS-13 on microthrombosis and brain injury after experimental subarachnoid hemorrhage. J Thromb Haemost 2014; 12: 943–947. [DOI] [PubMed] [Google Scholar]
- 114.Wan H, Wang Y, Ai J, et al. Role of von Willebrand factor and ADAMTS-13 in early brain injury after experimental subarachnoid hemorrhage. J Thromb Haemost 2018; 16: 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]