Abstract
Using the cranial window technique, we investigated acute effects of head cooling on cerebral vascular functions in newborn pigs. Head cooling lowered the rectal and extradural brain temperatures to 34.3 ± 0.6°C and 26.1 ± 0.6°C, respectively. During the 3-h hypothermia period, responses of pial arterioles to endothelium-dependent dilators bradykinin and glutamate were reduced, whereas the responses to hypercapnia and an endothelium-independent dilator sodium nitroprusside (SNP) remained intact. All vasodilator responses were restored after rewarming, suggesting that head cooling did not produce endothelial injury. We tested the hypothesis that the cold-sensitive TRPM8 channel is involved in attenuation of cerebrovascular functions. TRPM8 is immunodetected in cerebral vessels and in the brain parenchyma. During normothermia, the TRPM8 agonist icilin produced constriction of pial arterioles that was antagonized by the channel blocker AMTB. Icilin reduced dilation of pial arterioles to bradykinin and glutamate but not to hypercapnia and SNP, thus mimicking the effects of head cooling on vascular functions. AMTB counteracted the impairment of endothelium-dependent vasodilation caused by hypothermia or icilin. Overall, mild hypothermia produced by head cooling leads to acute reversible reduction of selected endothelium-dependent cerebral vasodilator functions via TRPM8 activation, whereas cerebral arteriolar smooth muscle functions are largely preserved.
Keywords: Cerebral circulation, cranial window, hypothermia, icilin, newborn pigs
Introduction
Temperature perception involves the thermosensitive transient receptor potential (TRP) melastatin member 8 (TRPM8). TRPM8 is a calcium-permeable non-selective cation channel that is activated by moderate cold temperatures (<30°C).1–4 TRPM8 was originally identified in a small fraction of sensory neurons in the dorsal root ganglia where it generates nerve impulses in response to cold temperatures (28–8°C) and the cooling agents menthol and icilin. Functional TRPM8 has been also found in tissues that are not exposed to any temperature variations, including prostate, liver, bladder, and kidney.2–6 The role of TRPM8 in numerous pathophysiological disorder and cancer progression has been well established.2,6 A growing body of evidence suggests that TRPM8 is expressed in vascular smooth muscle in large systemic arteries (aorta, femoral, mesenteric, and pulmonary) and contributes to the control of vascular tone at physiological temperature.7–13 In the rodent brain, TRPM8-expressing neurons were detected in the hypothalamus, septal neurons, and the brainstem nuclei involved in temperature control. 14 To date, the presence of functional TRMP8 channels in the cerebral microcirculation has not yet been characterized.
Therapeutic hypothermia, including whole-body cooling and selective head cooling, is a standard method to treat newborns with hypoxic ischemic encephalopathy (HIE)15–17 as well as adult patients with cardiac arrest, ischemic stroke and traumatic brain injury.18–20 Mild or moderate hypothermia that lowers the body temperature to 33–35°C is recommended for perinatal HIE.15–17 Hypothermia produces a reduction in cerebral blood flow up to 30% and causes vasoconstriction of pial arterioles.21–23 Previously, we reported that head cooling prevented long-term cerebral vascular endothelial injury and endothelial dysfunction caused by epileptic seizures in newborn pigs. 23 In spite of frequent use of therapeutic hypothermia in newborns and adults, very little is known about the mechanism by which hypothermia influences cerebral vascular functions related to the cerebral blood flow regulation.
The present study used the in vivo cranial window technique in newborn pigs to test the hypotheses that: 1) selective head cooling leads to acute alterations in cerebral vascular functions, 2) cold-sensing TRPM8 channel is expressed in cerebral resistance arterioles; and 3) TRPM8 activation by head cooling and a cooling mimetic icilin leads to acute changes in selected cerebral vascular functions. Overall, we provide the first evidence that functional TRPM8 channel is expressed in cerebral vessels and participates in modulating endothelium-dependent cerebral vascular functions during hypothermic conditions.
Materials and methods
Animals
Newborn piglets (1–5 days old, 1.5–3.0 kg, either sex, N = 40 animals) were purchased from a commercial breeder. The Animal Care and Use Committee of the University of Tennessee Health Science Center (IACUC) reviewed and approved all procedures involving animals in compliance with National Institutes of Health Office of Laboratory Animal Welfare guidelines. All experiments in the study were conducted according to the ARRIVE guidelines 2.0. 24
Closed cranial windows for intravital microscopy of pial arterioles
The pigs were anesthetized with acepromazine/ketamine/xylazine (3.3/33/2 mg/kg im), intubated via tracheostomies, instrumented with femoral arterial and venous catheters, and ventilated to maintain blood gases in a normal range (PaCO2: 30–40 mm Hg, PaO2: 70–90 mm Hg, and pH 7.3–7.4). Long-term anesthesia was achieved with α-chloralose (50 mg/kg iv initially plus 5 mg/kg maintenance). Cranial windows surgically placed at a left parietal cortex area were used for observation of pial arterioles and topical administration of pharmacological compounds as described previously. 23 The space under the cranial window was filled with artificial cerebrospinal fluid (aCSF) (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3 equilibrated with 6% CO2-6% O2-88% N2 to pH 7.3–7.35 at 37°C. All vasoactive agents were diluted with pre-warmed aCSF before infusion under the cranial window. Pial arterioles were observed with a trinocular intravital microscope. Pial arteriolar diameter was measured with a television camera mounted on the microscope, a video monitor, and a pre-calibrated video microscaler. Several medium-sized pial arterioles (40–80 µm diameter) were selected for observation in each piglet.
Head cooling
For head cooling, a large ice pack was placed on the skull around the cranial window area. Piglets were kept on a servo-controlled heating pad during the experimentation. Rectal and ear temperatures were continuously monitored in all animals during the experiment. A separate group (N = 5 animals) was used to measure brain (extradural) temperature using a thermocouple probe implanted in the parietal bone in contact with the dura mater. 23 The steady state reduction in rectal (34.2 ± 0.4°C), ear (30.8 ± 0.4°C), and brain (26.1 ± 0.6°C) temperatures was achieved within 60 min following ice pack placement. Ice packs were changed approximately every 30–40 minutes throughout the experiment. The total duration of head cooling in all experiments was ∼3 h. After removing head ice packs, complete rewarming was achieved in 60–90 min.
Experimental protocols
Cerebral vascular functions
Pial arterioles are major resistance arterioles that play a key role in cerebral blood flow regulation during physiological and pathophysiological conditions. We evaluated cerebral vascular functions based on the responses of pial arterioles to physiologically relevant vasodilators that act via engaging distinct cellular constituents of the neurovascular unit, including endothelial and vascular smooth muscle cells. Newborn pig cerebral vessels with light-dye-damaged or mechanically-denuded endothelium do not respond to bradykinin, hypercapnia and glutamate.25,26 Thus, we used topical vasodilators bradykinin (10−6 M) and glutamate (10−4 M) to test the endothelium-dependent responses of pial arterioles. To test the endothelium-independent responses of pial arterioles, we used topical SNP (10−5 M), a NO donor that acts on arteriolar smooth muscle to produce cGMP-mediated vasodilation. 27 We also tested the responses of pial arterioles to systemic hypercapnia, a potent physiological regulator of cerebral blood flow. 28 Arterial hypercapnia (PaCO2, 83 ± 7 mm Hg, PaO2, 103 ± 6 mm Hg, pH, 7.01 ± 0.03) was produced by ventilation with 10% CO2/21% O2.
Acute effects of head cooling and rewarming on cerebral vascular functions
Responses of pial arterioles to systemic hypercapnia and topical vasodilators bradykinin (10−6 M), glutamate (10−4 M), and SNP (10−5 M) were repeatedly tested in newborn pigs (N = 9) during: 1) normothermia, 2) head cooling (steady-state mild hypothermia period was achieved in ∼60 min after placing the ice pack), and 3) complete rewarming (steady-state normothermia period was achieved in 90 min after removing the ice pack). In the mock control group (N = 4 animals), the responses of pial arterioles were repeatedly tested three times during the 8 h normothermia period to ensure that repeated administration of the vasodilator stimuli had no lasting effects on vascular responsiveness. In all experiments, responses of pial arterioles to various vasodilator stimuli were tested during the 10-min application period sufficient to achieve a maximal response. The cerebral surface was flushed with aCSF for 15–20 min between the treatments to return to the baseline arteriolar diameter.
The effects of pharmacological modulators of TRPM8 channel on pial arteriolar diameter
We tested the effects of the TRPM8 agonist icilin and a selective channel blocker AMTB (N-(3-Aminopropyl)-2-[(3-methylphenyl)methoxy]-N-(2-thienylmethyl)benzamide hydrochloride) 29–31 on baseline pial arteriolar diameter during normothermia. Icilin (1, 2, 5, and 10 µM) and AMTB (20 and 50 µM), alone or in combination were topically placed under the cranial window at normothermic conditions, and changes in pial arteriolar diameter were recorded (N = 5 pigs).
The involvement of TRPM8 in modulating cerebral vascular functions during head cooling and normothermia
To evaluate the involvement of TRPM8 in modulating cerebral vascular functions, AMTB (50 µM) was continuously infused under the cranial window, and the responses of pial arterioles to hypercapnia, bradykinin (10−6 M), and glutamate (10−4 M) were repeatedly tested in newborn pigs (N = 5) during: 1) normothermia and 2) head cooling (steady-state mild hypothermia was achieved in ∼ 60 min after placing the ice pack).
The effects of icilin and AMTB on cerebral vascular function during normothermia
To evaluate the effects of TRPM8 activation by icilin on cerebral vasodilator functions, we tested the responses of pial arterioles to hypercapnia, bradykinin (10−6 M), glutamate (10−4 M), and SNP (10−5 M) in the absence or presence of topical icilin (5 µM) alone or combined with the TRPM8 blocker AMTB (50 µM) (N = 4 pigs).
Isolation of cerebral vessels and neurovascular cells from the cerebral cortex
Cerebral microvessels (60–300 µm) and astrocyte-enriched fraction of the brain cortex were prepared using differential filtration of the brain cortex homogenates in M199 via 300-, 60-, and 20-µm mesh nylon filters as described previously.32–34 Cerebral vascular endothelial cells were isolated from cerebral microvessels using collagenase-dispase digestion, purified by Percoll density gradient centrifugation, and cultured on Matrigel-coated Costar plates in endothelial growth-supporting media for 5–6 days until confluence. 32 Cerebral vascular smooth muscle cells were outgrown from cerebral microvessels plated on Matrigel-coated Costar plates for 12–15 days until confluence. 33 Cortical astrocytes from the vessel-free brain cortex parenchyma were grown in astrocyte-supporting media for 10–14 days to confluence. 34 Overall, 8 pigs were used for tissue/cell preparations.
TRPM8 immunoblotting
TRPM8 protein was immunodetected in freshly isolated cerebral microvessels, in the astrocyte-enriched fraction of the brain cortex, and in primary cerebral vascular endothelial cells, cerebral vascular smooth muscle cells, and cortical astrocytes from newborn piglets. The samples were solubilized in RIPA buffer, separated on 10% SDS-polyacrylamide gels, and transferred onto PVDF membranes. Membranes were blocked with 5% nonfat milk and probed with rabbit polyclonal TRPM8 antibody (Abcam, Cambridge, MA, 1:1,000 dilution) followed by anti-rabbit IgG-peroxidase antibody (Sigma, Burlington, MA; 1:10,000 dilution). For negative control, the primary TRPM8 antibodies (1:1,000 dilution) were neutralized with TRPM8 antibody blocking peptide (Abcam, Cambridge, MA, 1 µg/ml). For housekeeping protein normalization, the membranes were probed with monoclonal anti-pan-actin antibody (Sigma; 1:5,000 dilution) followed by goat anti-mouse IgG-peroxidase antibodies (Sigma; 1:10,000 dilution). Bands were visualized with the Pierce ECL Western blotting substrate (Thermo Fisher Scientific; Waltham, MA) and quantified using ImageJ software.
Materials
Icilin and AMTB were purchased from Tocris (Minneapolis, MN); other reagents were from Millipore Sigma (St. Louis, MO). Cell culture reagents were purchased from GE Healthcare Life Sciences (Pittsburg, PA). Matrigel was obtained from Corning (Corning, NY).
Statistical analysis
The data were analyzed using Graph Pad Prism version 9.0. Values are presented as means ± SD of absolute values or percentage of control. Individual data points are plotted where applicable. The Kolmogorov–Smirnov test for normality was used to asses data distribution. Normally distributed data were analyzed using Student’s t-test and one-way analysis of variance (ANOVA). Data that did not exhibit a Gaussian distribution were analyzed via a nonparametric equivalent. A probability value of P < 0.05 was considered statistically significant for all tests.
Results
Effects of head cooling on brain and body temperature and systemic parameters
During normothermia, the rectal temperature was 36.9 ± 0.1°C, the ear temperature was 34.6 ± 0.4°C, and the extradural brain temperature was 32.4 ± 0.1°C (Figure 1(a)). Selective head cooling reduced the body temperature to a steady-state hypothermia level in 60 min. During hypothermia conditions, the temperature in the rectum (34.3 ± 0.5°C) and in the ear (30.2 ± 0.4°C) were reduced to mild hypothermia (N = 9 animals). Most dramatic temperature reduction was observed in the brain (26.1 ± 0.6°C; N = 5 animals). During the 2 h rewarming period following the removal of the ice pack and continuous presence of a heating pad, body and brain temperatures greatly increased but remained slightly below the baseline normothermia levels (rectal temperature, 36.2 ± 0.9°C; ear temperature, 33.2 ± 0.9°C; brain temperature, 30.4 ± 0.5°C). Head cooling produced reduction in heart rate and mean arterial blood pressure that was maintained during the cooling period and then returned to baseline normothermia level after ∼90 min of rewarming (Figure 1(b)). Arterial blood gases during an overall 8 h experimental period were maintained at a physiological range (PaCO2: 30–40 mm Hg, arterial PaO2: 70–90 mm Hg, and pH 7.3–7.4).
Figure 1.
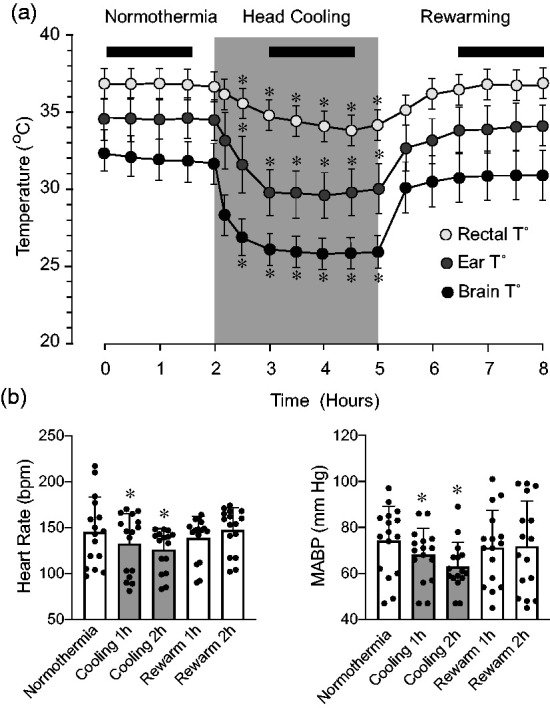
Effects of selective head cooling and rewarming on rectal, ear, and extradural brain temperature (a), heart rate, and mean arterial blood pressure (b) (N = 14 pigs). Black bars indicate the steady-state periods during normothermia, head cooling, and rewarming that have been selected for detection of cerebrovascular function. Values are means ± SD. *P < 0.05, compared with corresponding normothermic values.
Acute effects of head cooling and subsequent rewarming on cerebral vascular functions
Cerebral vasodilator functions were repeatedly tested in the same animals (N = 9 pigs, 29 pial arterioles) during normothermia (Rectal T°, 36.9 ± 0.1°C; Ear T°, 34.6 ± 0.4°C; Brain T°, 32.4 ± 0.1°C), head cooling (Rectal T°, 34.3 ± 0.5°C; Ear T°, 30.2 ± 0.4°C; Brain T°, 26.1 ± 0.6°C), and subsequent rewarming (Rectal T°, 36.2 ± 0.8°C; Ear T°, 33.2 ± 0.9°C; Brain T°, 30.4 ± 0.5°C). All functional tests were conducted after a steady-state temperature was achieved, usually 1–1.5 h after placing or removing head ice packs (Figure 1(a), as indicated by black bars). Endothelium-dependent cerebral vasodilator functions were tested by the responses of pial arterioles to topical vasodilators bradykinin (10−6 M) and glutamate (10−4 M). Endothelium-independent vasodilator function was tested by responses to sodium nitroprusside (10−5 M). We also evaluated the dilator responses of pial arterioles to systemic hypercapnia (PaCO2, 83 ± 7 mm Hg, PaO2, 103 ± 6 mm Hg, pH, 7.01 ± 0.03). During the normothermia period, all topical stimuli and hypercapnia produced strong vasodilator responses (30–40% above baseline) (Figure 2). Control experiments (N = 4 animals) conducted during the normothermic conditions demonstrated that repeated application of the vasodilator stimuli (hypercapnia, bradykinin, glutamate, and SNP) produced no vascular desensitization and had no lasting effects on vascular responsiveness. During the head cooling period, the dilator responses of pial arterioles to topical bradykinin and glutamate were reduced by 30–40% when compared to control normothermia responses (P < 0.05, Figure 2). After complete rewarming, the dilator responses of pial arterioles to topical bradykinin and glutamate were restored to control levels. The vasodilator responses of pial arterioles to systemic hypercapnia and topical SNP were not affected by head cooling and rewarming (Figure 2). These data suggest that head cooling reduces pial arteriolar responses to selected topical endothelium-dependent vasodilators, whereas the dilator responses to systemic hypercapnia as well as general arteriolar smooth muscle function remain unaltered.
Figure 2.
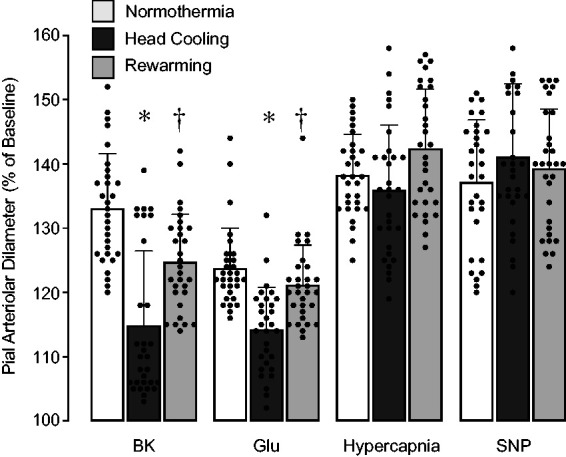
Acute effects of selective head cooling and subsequent rewarming on cerebral vasodilator functions. Vasodilator responses of pial arterioles to topical bradykinin (BK, 10−6 M), glutamate (Glu, 10−4 M), sodium nitroprusside (SNP, 10−5 M), and systemic hypercapnia were repeatedly tested during normothermia, head cooling, and rewarming. N = 9 pigs, 29 arterioles. Values are means ± SD. *P < 0.05 compared with normothermia responses. †P < 0.05 compared with responses during head cooling.
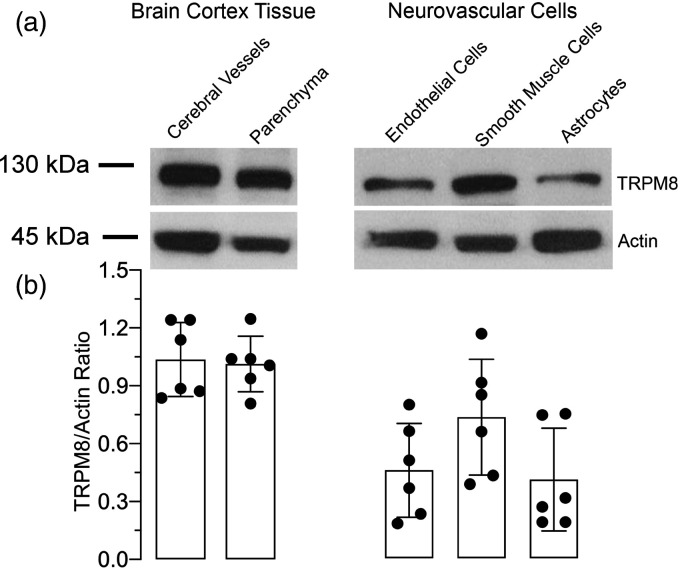
TRPM8 is expressed in cerebral vessels and neurovascular cells
TRPM8 protein is detected by Western immunoblotting in cerebral vessels (60–300 µm) and in astrocyte-enriched brain cortex parenchyma (Figure 3(a) and (b)). TRPM8 protein expression is also detected in primary cerebral vascular endothelial cells, cerebral vascular smooth muscle cells, and cortical astrocytes (Figure 3(a) and (b)). TRPM8 antibodies neutralized with the TRPM8 antibody blocking peptide did not produce any visible bands suggesting antibody specificity (Supplemental Figure 2R1-S).
Figure 3.
TRPM8 expression in the brain cortex tissue and in cerebral vascular cells. Cerebral microvessels (60–300 µm) and the astrocyte-enriched fraction of the brain parenchyma were obtained from the newborn pig brain cortex. Cerebral vascular endothelial and smooth muscle cells and cortical astrocytes were grown in primary cultures (N = 4 independent experiments). (a) Representative blots of TRPM8 expressed in the brain cortex tissue and in neurovascular cells. (b) TRPM8 expression normalized to housekeeping gene actin; Values are means ± SD.
TRPM8 ligands produce distinct cerebrovascular effects during normothermia
We used the TRPM8 ligands icilin (agonist) and AMTB (selective blocker) to investigate the functional properties of TRPM8 in cerebral vessels (Figure 4). During normothermia, icilin infused under the cranial window in consecutively increasing concentrations (1, 2, 5, and 10 µM) caused dose-dependent vasoconstriction of pial arterioles within 10–30% below baseline. Alternatively, topical AMTB (20 and 50 µM) had a moderate vasodilator effect (5–10% above baseline diameter, P < 0.05). Importantly, the vasoconstrictor effect of icilin was completely abolished in the presence of AMTB. The responses of pial arterioles to icilin and AMTB were abolished after a brief infusion of aCSF. These data demonstrate that TRPM8 activation by icilin produces constriction of pial arterioles that is antagonized by the TRPM8 blocker.
Figure 4.
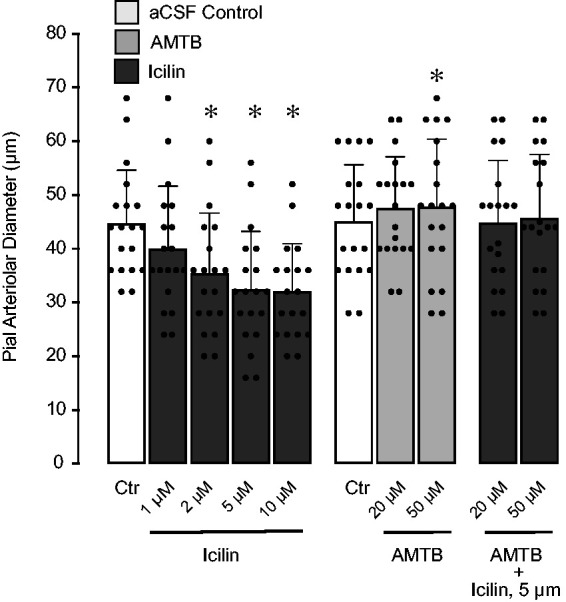
Vasoconstrictor effects of TRPM8 agonist icilin and vasodilator effects of TRPM8 blocker AMTB on baseline pial arteriolar diameter during normothermia. Icilin (1-10 µM) and AMTB (20 and 50 µM), alone or in combination were topically placed under the cranial window at normothermic conditions, and acute changes in pial arteriolar diameter were recorded. N = 5 pigs, 20 arterioles. Values are means ± SD. *P < 0.05 compared with baseline diameter (Ctr).
TRPM8 antagonist AMTB prevents reduction of endothelium-dependent cerebral vascular responses caused by head cooling
We used the TRPM8 blocker AMTB to test the hypothesis that the TRPM8 channel activation contributes to acute reduction of endothelium-dependent vascular function during head cooling. In control experiments, AMTB (50 µM) applied during normothermia did not alter cerebral vasodilator responses to bradykinin (10−6 M), glutamate (10−4 M), and hypercapnia (Figure 5). As in previous experiments (Figure 2), we observed reduction of pial arteriolar responses to topical endothelium-dependent dilators bradykinin (10−6 M) and glutamate (10−4 M), but not to systemic hypercapnia (PaCO2, 85 ± 10 mm Hg, PaO2, 98 ± 13 mm Hg, pH, 7.01 ± 0.04) (Figure 5) (N = 5 pigs, 15 arterioles). AMTB (50 µM) applied during head cooling prevented attenuation of pial arteriolar responses to bradykinin and glutamate (Figure 5). These findings strongly implicate the functional involvement of the TRPM8 channel in the acute reduction of cerebral vascular endothelial function caused by head cooling.
Figure 5.
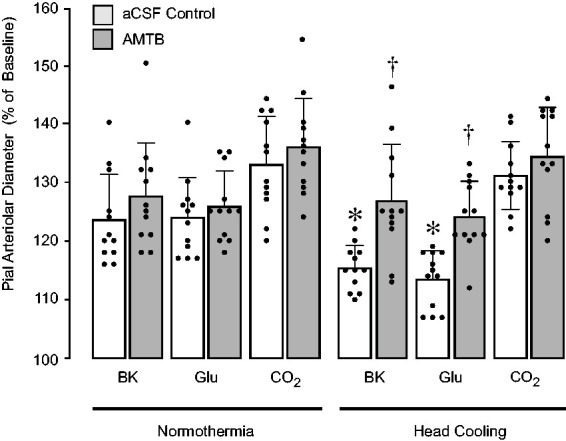
TRPM8 antagonist AMTB prevents reduction of endothelium-dependent cerebral vasodilator functions caused by head cooling. Vasodilator responses of pial arterioles to topical endothelium-dependent vasodilators bradykinin (BK, 10−6 M) and glutamate (Glu, 10−4M), and systemic hypercapnia (CO2) were repeatedly tested during normothermia and head cooling in the absence or presence of topical AMTB (50 µM). N = 5 pigs, 15 arterioles. Values are means ± SD. *P < 0.05 compared with corresponding responses to selected vasodilators during normothermia. †P < 0.05 compared with corresponding control responses to selected vasodilators during head cooling in the absence of AMTB.
TRPM8 agonist icilin attenuates endothelium-dependent cerebral vascular responses during normothermia
We tested the hypothesis that mimicking head cooling by pharmacological activation of the TRPM8 channel during normothermia leads to acute reduction of endothelium-dependent cerebral vascular dilator responses. Icilin (5 µM) topically applied to the cerebral surface during normothermia greatly reduced endothelium-dependent cerebral vasodilator responses to bradykinin (10−6 M) and glutamate (10−4 M), whereas the responses of pial arterioles to systemic hypercapnia and topical SNP were not altered (Figure 6; N = 4 pigs, 16 arterioles). The ability of icilin to attenuate endothelium-dependent cerebral vasodilator responses during the normothermia period was completely abolished in the presence of the TRPM8 antagonist AMTB (50 µM) (Figure 6). These data support our findings on physiological role of TRPM8 in the regulation of cerebral vascular function.
Figure 6.
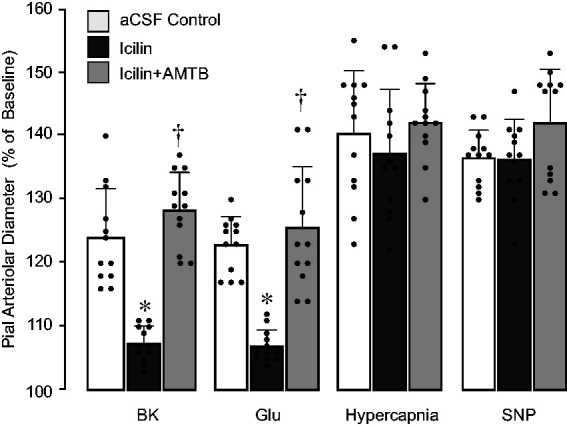
TRPM8 agonist icilin applied during normothermia mimics the effects of head cooling on endothelium-dependent cerebral vasodilator functions. Vasodilator responses of pial arterioles to systemic hypercapnia and topical vasodilators (bradykinin BK, 10−6 M, glutamate Glu, 10−4M) were repeatedly tested during normothermia in the absence or presence of topical icilin (5 µM) alone or combined with the TRPM8 antagonist AMTB (50 µM; N = 4 pigs, 16 arterioles). Values are means ± SD. *P < 0.05 compared with corresponding responses to selected vasodilators during control conditions. †P < 0.05 compared with corresponding control responses to selected vasodilators in the presence of icilin alone.
Discussion
Our novel findings using the in vivo cranial window imaging in healthy newborn pigs demonstrate that head cooling causes acute and reversible attenuation of selected endothelium-dependent vasodilator responses of pial arterioles without producing brain vascular injury. We provide the first evidence that the cold-sensing ion channel TRPM8 is expressed in cerebral vessels and contributes to modulating endothelium-dependent cerebral vascular functions during physiological and hypothermic conditions: 1) during physiological temperature, a super-cooling agonist icilin causes constriction of pial arterioles that is antagonized by the TRPM8 blocker AMTB; 2) head cooling causes reduction of endothelium-dependent dilator responses of pial arterioles that is prevented by AMTB, 3) icilin, at physiological temperature, mimicks the effects of head cooling by attenuating cerebral vasodilator responses.
Detection of endothelium-dependent vasodilator responses using the in vivo cranial window technique allows for assessing the functional integrity of the cerebral vascular endothelium. Brain endothelium is the critical component of the neurovascular unit that contributes to cerebral blood flow regulation, antioxidant defense mechanism, and blood-brain barrier integrity. 35 A variety of endothelium-derived vasoactive factors including prostanoids, nitric oxide, carbon monoxide, and hydrogen sulfide are essential in regulating cerebral blood flow.35,36 Endothelium-produced gaseous mediators carbon monoxide and hydrogen sulfide exhibit antioxidant and cytoprotective properties that contribute to the antioxidant defense mechanism. 36 Intact cerebral endothelium provides blood-brain barrier integrity that is necessary for maintaining brain homeostasis. The cerebral vascular endothelium is most vulnerable to oxidative stress injury caused by hypoxia-asphyxia, brain ischemia, inflammation, and epileptic seizures.23,37 Endothelial damage may lead to cerebrovascular disease and, therefore, endothelial dysfunction is a predictor of cerebrovascular disease.
Our study investigated acute effects of selective head cooling on cerebral vascular functions relevant to cerebral blood flow regulation. We asked whether cerebral resistance arterioles remain dormant or preserve their functional properties during the extended hypothermia period that lowers the brain temperature to 25–26°C while maintaining a mild body hypothermia level (34–35°C). To address this question, we used the in vivo cranial window technique which provides a unique opportunity to evaluate the physiological responses of pial arterioles that play a key role in regulation of cerebral blood flow. 38 We evaluated cerebral vascular functions at normothermia, during 3 h head cooling, and after a complete 2 h rewarming. Endothelium-dependent vasodilator responses were tested using bradykinin, glutamate, and systemic hypercapnia that require the functional contribution of cerebral endothelium,25,26 whereas a NO donor SNP was used to test general arteriolar smooth muscle responsiveness. 27 Lowering the brain temperature to 25–26°C during the 3 h head cooling period did not reduce general arteriolar smooth muscle function and did not affect vasodilation to hypercapnia. In contrast, head cooling produced a dramatic reduction in the responses of pial arterioles to bradykinin and glutamate. Importantly, all vascular responses completely returned to normalcy during rewarming, thus suggesting that no endothelial injury occurred. Extended head cooling causes physiological attenuation of selected endothelium-dependent cerebral vascular functions that is completely reversed during rewarming.
We tested the hypothesis that cold-sensing TRPM8 channel is involved in the mechanism by which head cooling leads to attenuation of cerebral vascular functions. Our findings are the first to demonstrate that TRMP8 protein is expressed in cerebral vessels and in primary neurovascular cells, including endothelium, smooth muscle, and astrocytes. To uncover the functional role of TRPM8 channels in the cerebral circulation of newborn pigs at physiological temperature, we tested the vasoactive effects of the TRPM8 agonist icilin topically administered to pial arterioles. Icilin (2–5 µM) caused dose-dependent vasoconstriction (10–30% below baseline) whereas TRPM8 blocker AMTB (20 µM) produced moderate vasodilation (5–10% above baseline). Furthermore, AMTB completely blocked the vasoconstrictor effects of icilin, thus further suggesting the involvement of TRPM8 channels in reducing baseline pial arteriolar diameter during normothermia. These findings indicate that icilin and AMTB can be used as pharmacological tools to test the TRPM8 functions in cerebral circulation.
We provide evidence that TRPM8 activation contributes to reversible reduction of endothelium-dependent pial arteriolar responses observed during the head cooling period. Head cooling reduced the brain surface temperature to 25–26°C that is purportedly sufficient for endogenous TRPM8 activation.1,3,5,31 When endogenous TRPM8 activation by head cooling was blocked by AMTB, we did not observe any reduction of endothelium-dependent cerebral vascular responses. Consistent with these findings, TRPM8 activation by icilin during normothermia attenuated endothelium-dependent cerebral vasodilation, this mimicking the vascular effects of head cooling. Furthermore, in the presence of AMTB, icilin failed to reduce the responses of pial arterioles to endothelium-dependent dilators. In all experiments, the responses of pial arterioles to hypercapnia and SNP were not affected by head cooling, icilin, or AMTP, alone or in combination. Overall, these data suggest that TRPM8 activation during head cooling is the key factor in the mechanism by which hypothermia acutely and reversibly attenuates cerebral endothelial functions, whereas the general cerebrovascular function is not affected.
The physiological significance of TRPM8 in attenuating physiological functions of cerebral arterioles during head cooling is yet to be investigated. Reducing the neonatal brain temperature for 3 h to 25–26°C and activating TRPM8 by cold reversibly attenuates selected endothelium-dependent cerebral vascular functions but does not produce vascular injury or any other sustained adverse effects in cerebral circulation. Importantly, the reduction of endothelium-dependent vascular functions observed during the head cooling period was completely reversed by rewarming. Previously, we demonstrated that there is no evidence of cerebral vascular damage caused by head cooling as indicated by the absence of apoptotic cells in cerebral vessels and in peripheral blood of newborn pigs subjected to head cooling. 23 Moreover, we demonstrated long-term protective effects of head cooling in the cerebral circulation of epileptic newborn pigs. Head cooling that reduced the brain temperature to 25–26°C while maintaining mild body hypothermia (34–35°C) prevented delayed postictal cerebral endothelial injury caused by brain oxidative stress and inflammation during epileptic seizures. 23
TRPM8 channels expressed in large systemic arteries may contribute to the control of vascular tone at physiological temperature.7–13 Our current findings also suggest that TRPM8 contributes to vascular functions in the neonatal cerebral circulation during normothermia and head cooling. As TRPM8 has been identified as a Ca2+ permeable ion channel,1,3 the channel activation increases intracellular Ca2+ concentrations and would be expected to cause arterial smooth muscle contraction. In cerebral circulation of newborn pigs in vivo, TRPM8 activation by icilin produced substantial vasoconstriction of the cerebral arterioles that was antagonized by the receptor blocker AMTB. However, in the systemic circulation in rats, icilin produced either vasoconstrictor or vasodilator arteriolar responses during distinct experimental conditions, indicative of the contribution of endogenous TRPM8-activating factors.8,39 Indeed, an array of endogenous factors that activate TRPM8 at physiological temperature has been discovered in various tissues (reproductive organs, kidneys, liver, vasculature). These activating factors include arachidonic acid metabolites, phosphatidylinositol 4,5-bisphosphate, lysophosphatidic acid, and several TRP channel-activating proteins (TRP channel-associated factors and phosphoinositide-interacting regulator of TRP).2,6 The role of endogenous factors that may contribute to TRPM8 activation in cerebral circulation during physiological temperature has yet to be elucidated.
Currently, head or total body cooling is approved only for caring for babies with neonatal hypoxic-ischemic insult. Clinical trials demonstrate that mild hypothermia (33–35°C) reduced mortality and prevents neurodevelopmental disabilities in HIE survivors.15–17,40 For complex neonatal heart surgery, cooling the body to moderate hypothermia (28–32°C) or deep hypothermia 32°C (<28°C) is recommended. 41 There are only a few reports on brain temperature during head/body cooling.23,42 As a translational implication of our findings we propose that selective head cooling that reduced the brain temperature to 25–26°C while maintaining mild body hypothermia (34–35°C) does not produce irreversible cerebral vascular damage and has an advantage over whole body cooling because it allows a deeper reduction of brain vs. core body temperature.
Limitations and future research directions
In our experimental newborn pig model, selective head cooling produced by head ice packs reduced core body temperature to the level of moderate hypothermia (34–35°C), while also reducing the brain surface temperature to 25–26°C. These conditions are sufficient for TRPM8 activation that causes reversible attenuation of selected cerebral vascular endothelium-dependent vasodilator functions during head cooling.
Total mild body cooling in babies is frequently used for perinatal HIE. Investigating the effects of total body mild cooling on the dynamics of brain temperature and cerebral vascular functions is needed.
Considering that duration of hypothermia in perinatal HIE is 72 h, extending the head- or total body cooling periods beyond 3 h prior to testing cerebral vascular functions is of clinical relevance.
The in vivo experimental system highly relies on pharmacological approaches and does not allow for detecting the contribution of endothelial-, astrocyte-, and smooth muscle-expressed TRPM8 channels to cerebral vascular functions. The involvement of neuronal TRPM8 in the regulation of cerebral resistance arterioles cannot be excluded. The studies in isolated cerebral vessels and neurovascular cells are needed for using extensive pharmacological, genetic and molecular approaches to characterize the functional significance of TRPM8 channels in the cerebral circulation.
Conclusions
This is the first in vivo study to demonstrate the effects of head cooling and TRPM8 channel in the modulating cerebral vascular functions relevant to cerebral blood flow regulation. We provide evidence that head cooling, via activation of endogenous TRMP8 channels expressed in cerebral circulation leads to reversible attenuation of selected endothelium-dependent cerebral vascular responses and represents a physiological adjustment of cerebral vasculature to hypothermia. Importantly, head cooling does not negatively affect arteriolar smooth muscle function or vasodilator responses to hypercapnia, a powerful physiological regulator of cerebral blood flow.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211018035 for The cold receptor TRPM8 activation leads to attenuation of endothelium-dependent cerebral vascular functions during head cooling by Alex L Fedinec, Jianxiong Liu, Rong Zhang, Mimily Harsono, Massroor Pourcyrous and Helena Parfenova in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We thank Dr. Nathan Tipton for excellent editorial assistance.
Funding: The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health Grants NS101717 and NS105655 (to H. Parfenova). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: AF, HP, MP, MH, RZ, and JL contributed to the experimental concept and design; AF, JL, RZ, and MH performed experiments; AF, HP, MP, MH, RZ, and JL took part in the data analysis; AF, HP, MP, MH, RZ, and JL were involved in the interpretation of result; HP and RZ prepared figures; AF, HP, MP, MH, RZ, and JL edited and approved the final version of the manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416: 52–58. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Mikrani R, He Y, et al. TRPM8 channels: a review of distribution and clinical role. Eur J Pharmacol 2020; 882: 173312. [DOI] [PubMed] [Google Scholar]
- 3.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108: 705–715. [DOI] [PubMed] [Google Scholar]
- 4.Bodding M, Wissenbach U, Flockerzi V. Characterization of TRPM8 as a pharmacophore receptor. Cell Calcium 2007; 42: 618–628. [DOI] [PubMed] [Google Scholar]
- 5.DeFalco J, Duncton MA, Emerling D. TRPM8 biology and medicinal chemistry. Curr Top Med Chem 2011; 11: 2237–2252. [DOI] [PubMed] [Google Scholar]
- 6.Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta 2007; 1772: 937–946. [DOI] [PubMed] [Google Scholar]
- 7.Earley S. Vanilloid and melastatin transient receptor potential channels in vascular smooth muscle. Microcirculation 2010; 17: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson CD, Melanaphy D, Purse A, et al. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 2009; 296: H1868–H1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu YP, Lin DC, Zheng SY, et al. Transient receptor potential melastatin-8 activation induces relaxation of pulmonary artery by inhibition of store-operated calcium entry in normoxic and chronic hypoxic pulmonary hypertensive rats. J Pharmacol Exp Ther 2018; 365: 544–555. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Yang T, Wang P, et al. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/rho kinase pathway. Hypertension 2014; 63: 1354–1363. [DOI] [PubMed] [Google Scholar]
- 11.Yang XR, Lin MJ, McIntosh LS, et al. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 2006; 290: L1267–L1276. [DOI] [PubMed] [Google Scholar]
- 12.Zholos A. Pharmacology of transient receptor potential melastatin channels in the vasculature. Br J Pharmacol 2010; 159: 1559–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zholos A, Johnson C, Burdyga T, et al. TRPM channels in the vasculature. Adv Exp Med Biol 2011; 704: 707–729. [DOI] [PubMed] [Google Scholar]
- 14.Ordás P, Hernández-Ortego P, Vara H, et al. Expression of the cold thermoreceptor TRPM8 in rodent brain thermoregulatory circuits. J Comp Neurol 2021; 529: 234–256. [DOI] [PubMed] [Google Scholar]
- 15.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010; 340: c363–c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx 2006; 3: 154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013; 1: CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris B, Andrews PJ, Murray GD, et al. Systematic review of head cooling in adults after traumatic brain injury and stroke. Health Technol Assess 2012; 16: 1–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olah E, Poto L, Hegyi P, et al. Therapeutic whole-body hypothermia reduces death in severe traumatic brain injury if the cooling index is sufficiently high: meta-analyses of the effect of single cooling parameters and their integrated measure. J Neurotrauma 2018; 35: 2407–2417. [DOI] [PubMed] [Google Scholar]
- 20.Tang XN, Liu L, Yenari MA. Combination therapy with hypothermia for treatment of cerebral ischemia. J Neurotrauma 2009; 26: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busija DW, Leffler CW. Hypothermia reduces cerebral metabolic rate and cerebral blood flow in newborn pigs. Am J Physiol 1987; 253: H869–H873. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Wang B, Normoyle KP, et al. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front Neurosci 2014; 8: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harsono M, Pourcyrous M, Jolly EJ, et al. Selective head cooling during neonatal seizures prevents postictal cerebral vascular dysfunction without reducing epileptiform activity. Am J Physiol Heart Circ Physiol 2016; 311: H1202–H1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar vasomotor responses. Am J Physiol Heart Circ Physiol 2001; 281: H2366–H2377. [DOI] [PubMed] [Google Scholar]
- 26.Fiumana E, Parfenova H, Jaggar JH, et al. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol 2003; 284: H1073–H1079. [DOI] [PubMed] [Google Scholar]
- 27.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 2009; 61: 62–97. [DOI] [PubMed] [Google Scholar]
- 28.Hoiland RL, Fisher JA, Ainslie PN. Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol 2019; 9: 1101–1154. [DOI] [PubMed] [Google Scholar]
- 29.Diver MM, Cheng Y, Julius D. Structural insights into TRPM8 inhibition and desensitization. Science 2019; 365: 1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Muñiz R, Bonache MA, Martín-Escura C, et al. Recent progress in TRPM8 modulation: an update. Int J Mol Sci 2019; 20: 2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mälkiä A, Morenilla-Palao C, Viana F. The emerging pharmacology of TRPM8 channels: hidden therapeutic potential underneath a cold surface. Curr Pharm Biotechnol 2011; 12: 54–67. [DOI] [PubMed] [Google Scholar]
- 32.Parfenova H, Basuroy S, Bhattacharya S, et al. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol 2006; 290: C1399–C1410. [DOI] [PubMed] [Google Scholar]
- 33.Parfenova H, Eidson TH, Leffler CW. Upregulation of COX-2 in cerebral microvascular endothelial cells by smooth muscle cell signals. Am J Physiol Cell Physiol 1997; 273: C277–C288. [DOI] [PubMed] [Google Scholar]
- 34.Parfenova H, Tcheranova D, Basuroy S, et al. Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am J Physiol Heart Circ Physiol 2012; 302: H2257–H2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooper WC, Catravas JD, Heistad DD, et al. Vascular endothelium summary statement I: health promotion and chronic disease prevention. Vascul Pharmacol 2007; 46: 315–317. [DOI] [PubMed] [Google Scholar]
- 36.Leffler CW, Parfenova H, Jaggar JH, et al. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol 2006; 100: 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann A, Leffler CW, Tcheranova D, et al. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol 2007; 293: H2501–H2507. [DOI] [PubMed] [Google Scholar]
- 38.Peterson EC, Wang Z, Britz G. Regulation of cerebral blood flow. Int J Vasc Med 2011; 2011: 823525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melanaphy D, Johnson CD, Kustov MV, et al. Ion channel mechanisms of rat tail artery contraction-relaxation by menthol involving, respectively, TRPM8 activation and L-type Ca2+ channel inhibition. Am J Physiol Heart Circ Physiol 2016; 311: H1416–H1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012; 366: 2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiNardo JA, Shukla AC, McGowan FX. Cardiopulmonary bypass in infants and children: cannulation and circuit considerations. In: David PF, Cladis FP. (eds) Smith's anesthesia for infants and childrens. 9th ed. Amsterdam: Elsevier, 2016, pp.633–698. [Google Scholar]
- 42.Tooley JR, Eagle RC, Satas S, et al. Significant head cooling can be achieved while maintaining normothermia in the newborn piglet. Arch Dis Child Fetal Neonatal Ed 2005; 90: F262–F266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211018035 for The cold receptor TRPM8 activation leads to attenuation of endothelium-dependent cerebral vascular functions during head cooling by Alex L Fedinec, Jianxiong Liu, Rong Zhang, Mimily Harsono, Massroor Pourcyrous and Helena Parfenova in Journal of Cerebral Blood Flow & Metabolism