Summary
Polygenic risk scores (PRSs) are heralded as useful tools for risk stratification and personalized preventive care, but they are clinically useful only if they can be translated into action. The risk information conveyed by a PRS must be contextualized to enable this. Best practices are evolving but are likely to involve integrating a PRS into an absolute risk model and using guideline-driven care linked to a specific threshold of risk. Because this approach is not currently available for most diseases, it may be necessary to use different methods of presenting risk and linking it to appropriate clinical action. We discuss the trade-offs of each strategy and argue for transparent communication to providers and patients of the imprecision in both risk estimates and action thresholds for PRSs.
Information in polygenic risk scores must be contextualized to enable clinical action. There are several possible approaches to this contextualization, each of which has trade-offs relevant for clinical actionability. The imprecision in both risk estimates and thresholds for action must be communicated transparently to providers and patients.
Main text
Computational and methodological advances have renewed the excitement over polygenic risk scores (PRSs) and their potential applications to preventive medicine.1,2 Broadening the scope of genomic risk assessment beyond monogenic diseases, PRSs combine information from hundreds or even millions of genetic loci, each with a very small effect size on the risk of common diseases. The result is a continuous quantitative risk factor for the known genetic susceptibility to conditions such as coronary artery disease, type 2 diabetes, and breast cancer. Compared to rarer monogenic disease variants, PRSs might have greater transformative potential for preventive medicine in their ability to identify much larger proportions of the population at elevated risk for disease.
At the interface of PRS development and clinical application is a set of considerations that will affect the clinical impact of PRSs (Figure 1). After the generation of a PRS, there are choices about how risk is reported and contextualized for clinical decision-making. Early examples of PRS reports reflect this diversity of options.3, 4, 5, 6 The disease or phenotype in question, combined with how clinicians already think about risk management for that phenotype, will determine the most appropriate approach to PRS reporting. Here we outline the major choices for how PRS risk is contextualized at two key steps: how the risk itself is represented and how it is linked to action. We highlight some known limitations of PRSs and show how these limitations and the selected risk contextualization approach impact downstream clinical actionability.
Figure 1.
Contextualizing the risk information conveyed by a PRS to enable clinical action
A PRS may need to be adjusted to ensure that the distribution of score values is independent of genetic ancestry. A first contextualization step is to present the resultant score. A second contextualization step is to frame this risk alongside a threshold for clinical action.
Limitations of PRSs that affect clinical actionability
The robustness and replicability of PRSs as disease risk factors have been established, but limitations still impede their ability to inform medical decision-making. PRSs reflect only known genetic risk from genome-wide association studies and do not account for the effects of environment or lifestyle, which, unlike PRSs, might be modifiable targets for disease prevention. As a result, even the most predictive PRSs explain a low proportion of the overall variance of a trait in a population for most common conditions.7 Indeed, it is difficult to describe exactly what effects PRSs do measure.8
Moreover, the predictive performance of PRSs varies by ancestry, with more of the variance in a trait explained in European-ancestry populations.7,9 Poor performance in non-European-ancestry populations has led some companies to offer their tests only to patients of European ancestry.3,4 This differential performance, which mostly stems from the over-representation of those with European ancestry in the underlying data, is rightly framed as an equity issue;9 it also generates implementation challenges. First, the distribution of PRSs can look very different for populations of different ancestries.10 These differences in mean values and standard deviations are not expected to reflect underlying differences in trait distribution10 and yet would have a determinative effect on PRS interpretation if not suitably controlled for early in the clinical pipeline (Figure 1).11 Second, as discussed below, additional challenges arise in reporting these results to individuals from different populations.
These limitations need not halt the clinical implementation of PRSs, but they do require careful attention in PRS reporting and use for patient care.
Three approaches to risk representation: percentile, relative effect, and absolute risk
There are three main options for representing the risk information a PRS conveys (Figure 2): framing it as a percentile within a given population, framing it as a metric of relative effect (such as a relative risk or odds ratio), and translating it to an absolute risk (such as a 10-year or lifetime risk for developing a condition). These approaches are not mutually exclusive, and each has a potential clinical role. Some of the considerations for deciding on the appropriate reporting strategy in a given context are outlined below and summarized in Table 1. We emphasize that there is a range of ethical, legal, and social issues associated with the clinical reporting of PRSs that we have addressed elsewhere.12
Figure 2.
Three options for representing risk information from a PRS
The change in risk estimate for an individual for a given condition can be represented as: a percentile rank, within a suitably chosen population (top); some measure of relative effect compared to a suitably chosen population, for example a relative risk or odds ratio (middle); or a measure of absolute risk, such as 5-year risk or lifetime-remaining risk for developing a condition (bottom).
Table 1.
Three approaches to PRS risk contextualization
| Approach | Advantages | Disadvantages | Link to action | Example application |
|---|---|---|---|---|
| Percentile rank of PRS | Information may be more readily understood by patients and can be visualized on a normal distribution | Rank gives no indication of how much of the genetic or overall disease risk is explained by the PRS | Rank compared to others may motivate adherence to generally applicable recommendations | Lifestyle recommendations for those with a high type 2 diabetes PRS percentile rank |
| Measure of relative effect (e.g., relative risk, odds ratio) | Measure gives an indication of the magnitude of the change in risk | Measures of relative effect can be misleading, particularly if the prevalence of the condition is low | Clinicians may justifiably recommend the same actions as those recommended by guidelines for equivalent levels of relative risk from traditional risk factors | Earlier screening for patients with a colorectal cancer PRS relative risk equivalent to the relative risk of having a first-degree family member with the condition |
| Effect can be compared to those of other risk factors (e.g., family history) | Measures of relative effect vary by population (e.g., ancestry) | |||
| Integration into an overall clinical model for absolute risk (e.g., lifetime risk, 5-year risk) | Absolute risk estimates account both for relative risk and underlying disease prevalence/incidence | Professional guidelines endorse absolute risk models for only a handful of conditions | Clinicians may justifiably recommend the same actions as those recommended by guidelines for equivalent levels of absolute risk from models with traditional risk factors alone | Statin initiation for ASCVD prevention based on absolute risk estimate from model integrating PRS into Pooled Cohort Equations |
| Absolute risks can convey PRS risk in the context of other established risk factors (e.g., lifestyle, environment) | Differential performance by population (e.g., ancestry) remains a limitation |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; PRS, polygenic risk.
The first option, a percentile rank, compares an individual’s PRS to the distribution of PRSs within a chosen population (Figure 2, top). For example, someone at the 95th percentile has a PRS that is higher than 95 out of every 100 people in a chosen population. This approach has been taken, for example, by investigators constructing a PRS report for coronary artery disease.6 The image of a normal distribution is widely understood and can be used to visualize an individual’s position along that distribution. However, the percentile alone is limited and does not enable sufficient clinical risk contextualization, because it gives no indication of how much of the genetic or overall disease risk the PRS explains. As a result, even a percentile rank at the extreme tail of the distribution might confer modest risk.
The second option, framing a PRS as a measure of relative effect, provides the context that, for example, an individual has three-fold risk (relative risk) or odds (odds ratio) of developing a condition compared with a member of the general population (Figure 2, middle). This approach has been taken, for example, by Ambry Genetics for their prostate cancer report for affected men.3 Any measure of relative effect will be sensitive to how disease cases and controls were chosen and ascertained and the additional covariates included in the matching or adjustment. This contextual dependency is both a strength and a limitation of using measures of relative effects. On one hand, and unlike percentile ranks, they can be used to summarize PRS-associated risk after accounting for other non-genetic risk factors, provided those were measured and accounted for in the original analyses. On the other hand, that single risk summary belies the significant intra-individual variation in risk associated with a PRS and would not apply to a patient whose characteristics were not represented in the population used to derive the risk estimate. Because, as summarized above, the relative effect of PRSs varies by ancestry, reporting an appropriate risk estimate for an individual patient is challenging. To address this, laboratories can either use a single PRS for a disease and report different risk estimates for specific populations, such as ancestries, or they can implement entirely different scores developed for specific populations.13 In either case, the practice of matching an individual patient to an appropriate reference population in clinical care is difficult to implement at best and ethically fraught at worst. Finally, communicating measures of relative effect comes with the standard caution that these can be notoriously misleading.14
The third option is to incorporate PRSs into an estimate of absolute risk (Figure 2, bottom). One simple approach is to multiply the incidence rate in a subpopulation (e.g., a specific stratum of age and reported ethnicity) with the relative risk from a PRS, as is done by 23andMe for their type 2 diabetes report.5 Another approach incorporates a PRS into an existing prediction model of absolute risk. For example, the Myriad Genetics riskScore incorporates a PRS into the commonly used Tyrer-Cuzick model for breast cancer risk, which itself includes clinical risk predictors such as obstetric history and prior hormone replacement therapy.4 This approach follows standard practice for risk prediction: the use of a validated model that incorporates multiple risk factors to generate an absolute risk estimate for a clinically important outcome over a clinically important time period.15 Several such models exist and are endorsed by multiple professional societies, including the Pooled Cohort Equations (PCE) to estimate 10-year atherosclerotic cardiovascular disease (ASCVD) risk16 and the Gail Model to estimate a woman’s 5-year and lifetime risks of invasive breast cancer.17 A prediction model is evaluated by its performance (i.e., discrimination and calibration) both in the original population in which it was developed and in independent, ideally diverse, populations. Rich training and validating data are needed, as data on all the risk factors incorporated into the model must be available. Integration of PRSs into such models has the advantage of accurately reflecting the role of PRSs as one of several risk factors in complex disease. Despite representing standard practice, limitations to this approach exist. It is notable that professional societies have endorsed absolute-risk models for only a handful of diseases. And even where these models do exist, they inherit the same differential performance by subpopulation as relative risks. We note, for example, that the Tyrer-Cuzick model may overpredict breast cancer risk for Hispanic women,18 and the PCE may overpredict ASCVD risk for Asian Americans.19 How to deal with differential performance by race for such models is currently an actively debated topic in medicine.20,21 The output of such models (e.g., an estimated 7.6% 10-year ASCVD risk for a 54-year-old self-reported African American man with specific blood pressure and lipid measurements) gives the illusion of precision. There is a danger that the inclusion of genetics leads to even greater faith being placed in these models, compounding this illusion, when PRSs capture only a small fraction of even the genetic variance of a trait and have variable predictive accuracy by population. To counter this, the communication of how a PRS affects risk estimation should incorporate this imprecision. This will involve reflecting on which of several approaches to calculating uncertainty intervals is appropriate,22,23 as well as how best this uncertainty should be communicated.24,25
Linking risk with clinical action
Even with appropriate risk representation, patients and physicians can translate a risk estimate into an improved health outcome only if it changes clinical action. For example, the clinical value of the PCE and the Gail Model depend substantively on their links to recommendations on whether a patient should take a statin to lower ASCVD risk or initiate early mammography for breast cancer screening, respectively. Every day clinicians use thresholds to translate continuous risk into binary action, either explicitly through guideline-driven care or implicitly through mental models of risk. As illustrated in Figure 3, a continuous measure of polygenic risk—be it a percentile, a measure of relative effect, or a measure of absolute risk—must ultimately inform a binary action. As there are different considerations depending on which continuous measure of risk is being used, we consider each of the three in turn below.
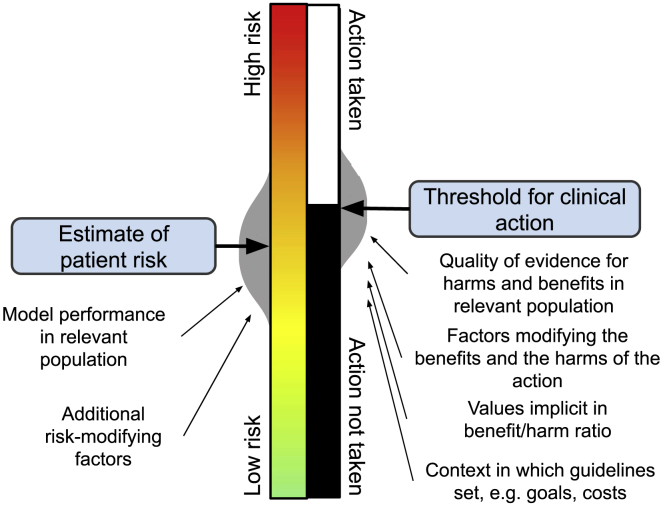
Figure 3.
Conceptual model of the relationship between continuous risk prediction and binary preventive action
Although risk lies on a quantitative continuum, its clinical value is its ability to inform a binary choice about a clinical action. For the individual patient, both assessments (where they lie on the continuum of risk and whether they lie below or above a threshold for action) have inherent imprecisions and limitations. These gray areas allow physicians and patients to use more than an algorithmic comparison of two numbers to make medical decisions.
If a percentile alone has been chosen to represent polygenic risk, it is not clear that this information can justifiably be used to change clinical decision-making beyond recommending that the patient adhere to lifestyle and screening guidelines suitable for the general population. There may, however, be an educational role for percentiles in helping the patient visualize and personalize the concept of risk and motivating their pursuit of risk reduction measures. If a measure of relative effect is chosen, clinicians might reasonably contextualize this information compared to the magnitude of effect of other risk factors that already have established guidelines. For example, clinical guidance for colorectal cancer suggests earlier screening in individuals with an affected first-degree relative.26 On average, those with a family history of colorectal cancer are at two-fold increased risk of developing the condition themselves, compared to those without.26 The same two-fold increase in risk from a PRS might reasonably be linked to the same recommendation for enhanced screening, although prospective clinical trials would be needed to support such an approach more conclusively.
For an absolute risk model, the ideal approach would be centered on an evidence-based risk threshold for clinical action already endorsed by professional societies and used in current practice. Best practice would show improved performance of a version of the absolute risk model that incorporated the PRS around this threshold, using some measure of reclassification. In contrast to the common practice of reporting odds ratios or incremental improvements in areas under the receiver operating characteristic curves, performance metrics leveraging action thresholds have greater clinical meaning.27 For example, in one study, integrating a PRS for coronary artery disease into the PCE correctly reclassified 4% of individuals around the 10-year ASCVD risk threshold of ≥7.5% generally recommended by the 2018 Multisociety Guideline on the Management of Blood Cholesterol for statin initiation.28,29
Even with comprehensive absolute-risk models, some guidelines suggest a different clinical action threshold if strong risk factors not included in the clinical prediction model are present. For example, the 2018 Multisociety Guideline recommends statin initiation at a 10-year ASCVD risk threshold of ≥5% instead of ≥7.5% if a risk-enhancing factor such as HIV infection or a high-sensitivity C-reactive protein (hsCRP) level ≥ 2.0 mg/L is present.29 A similar approach might be reasonably used for a well-performing PRS, based on some estimation of the additional risk it confers to the standard clinical model.
Like risk estimates, action thresholds—even if evidence based and guideline recommended—give a false sense of precision. For example, National Comprehensive Cancer Network (NCCN) guidelines suggest that women aged 35–39 years with ≥1.7% 5-year breast cancer risk should have annual mammograms.30 Why not 1.8% or 5%? The committee chose 1.7% because of its equivalency to the estimated risk of an average white woman aged 60–64 years (calculated using data and models of the time to be 1.66%), one inclusion criterion for the 1992 Breast Cancer Prevention Trial.31 This threshold has since been included in several breast cancer prevention guidelines. Similarly, the 2018 Multisociety Guideline statin initiation threshold of ≥7.5% 10-year ASCVD risk was chosen because it was thought to approximate the inclusion criteria for the relevant statin trials for ASCVD prevention.16 Moreover, the guideline panel judged that at 7.5% ASCVD risk, the number needed to treat to benefit from high-intensity statin use (n = 33) counterbalanced the number needed to harm (n = 30) with statin-induced diabetes. The recommendation to lower the action threshold to 5% in the case of risk-enhancing factors, and the choice of thresholds for continuous risk factors such as hsCRP themselves, rests on less conclusive evidence still. Thus, even in this best-practice scenario, action thresholds are limited not only by the imprecision of the prediction models on which they are based but also by well-intentioned but somewhat arbitrary methods, including historical precedent and panelist values on what constitutes appropriate harm-to-benefit and cost-to-benefit ratios at the population level. Variation in the benefit an individual experiences from the intervention further compounds this imprecision. Given these issues, it is not surprising that different bodies recommend different action thresholds. For example, the US Preventive Service Task Force generally recommends a statin initiation threshold of 10% ASCVD risk over 10 years.32
A decision to act on a risk estimate ultimately requires the addition of patient values. It is important for the uncertainty in these action thresholds—in addition to the uncertainties in the risk estimate—to be communicated to the patient in order for a decision to be made in the context of those values. For example, a patient who is told that her ASCVD risk is 8.2% and statin initiation is recommended at 7.5% risk might make a different but better-informed decision if she is told her risk falls between 7% and 9% and the threshold for statin initiation is between 5% and 10%. Evidence suggests that communicating uncertainty does not affect patient trust in the information provided.33 Communicating this imprecision to patients is challenging but paramount.
Conclusions
Suggested approaches for linking PRS-based risk estimation to clinical action all have limitations, including the potential for poor communication about the modest contribution of PRSs to risk, the imprecision with which that risk is estimated, and how that risk compares to current clinical models. We recommend transparent communication of the imprecision both in risk estimates and in the thresholds for clinical action. Contrary to the increasing formalization of much of medicine through algorithms and decision tools, acting on risk will always be more than the algorithmic comparison of two numbers. The gray areas in ostensibly black and white decisions give patients and their providers the space for decision-making tuned to the individual patient.
Acknowledgments
This work was supported by NIH/NHGRI grant R35 HG010706. The authors thank Peter W.F. Wilson, MD, for his input on an early version of this manuscript.
Declaration of interests
R.C.G. has received compensation for advising the following companies: AIA, Genomic Life, Grail, Humanity, Kneed Media, Plumcare, UnitedHealth, Verily, and VibrentHealth, and is co-founder of Genome Medical, Inc. All other authors declare no competing interests.
References
- 1.Khera A.V., Chaffin M., Aragam K.G., Haas M.E., Roselli C., Choi S.H., Natarajan P., Lander E.S., Lubitz S.A., Ellinor P.T., Kathiresan S. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torkamani A., Wineinger N.E., Topol E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018;19:581–590. doi: 10.1038/s41576-018-0018-x. [DOI] [PubMed] [Google Scholar]
- 3.Ambry Genetics. Cancer Genetic Testing. AmbryScore. Health Risk Tests. http://www.ambrygen.com/providers/ambryscore.
- 4.Myriad Publications. riskScore. Myriad MyRisk. https://myriadmyrisk.com/riskscore/.
- 5.23andMe Blog . 2019. 23andMe Offers New Genetic Report on Type 2 Diabetes.https://blog.23andme.com/health-traits/type-2-diabetes/ [Google Scholar]
- 6.Brockman D.G., Petronio L., Dron J.S., Kwon B.C., Vosburg T., Nip L., Tang A., O’Reilly M., Lennon N., et al. Design and user experience testing of a polygenic score report: a qualitative study of prospective users. medRxiv. 2021 doi: 10.1101/2021.04.14.21255397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan L., Shen H., Gelaye B., Meijsen J., Ressler K., Feldman M., Peterson R., Domingue B. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 2019;10:3328. doi: 10.1038/s41467-019-11112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young A.I., Benonisdottir S., Przeworski M., Kong A. Deconstructing the sources of genotype-phenotype associations in humans. Science. 2019;365:1396–1400. doi: 10.1126/science.aax3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin A.R., Kanai M., Kamatani Y., Okada Y., Neale B.M., Daly M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019;51:584–591. doi: 10.1038/s41588-019-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin A.R., Gignoux C.R., Walters R.K., Wojcik G.L., Neale B.M., Gravel S., Daly M.J., Bustamante C.D., Kenny E.E. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am. J. Hum. Genet. 2017;100:635–649. doi: 10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera A.V., Chaffin M., Zekavat S.M., Collins R.L., Roselli C., Natarajan P., Lichtman J.H., D’Onofrio G., Mattera J., Dreyer R., et al. Whole-Genome Sequencing to Characterize Monogenic and Polygenic Contributions in Patients Hospitalized With Early-Onset Myocardial Infarction. Circulation. 2019;139:1593–1602. doi: 10.1161/CIRCULATIONAHA.118.035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis A.C.F., Green R.C. Polygenic risk scores in the clinic: new perspectives needed on familiar ethical issues. Genome Med. 2021;13:14. doi: 10.1186/s13073-021-00829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shieh Y., Fejerman L., Lott P.C., Marker K., Sawyer S.D., Hu D., Huntsman S., Torres J., Echeverry M., Bohorquez M.E., et al. A polygenic risk score for breast cancer in U.S. Latinas and Latin-American women. bioRxiv. 2019 doi: 10.1101/598730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagerlin A., Zikmund-Fisher B.J., Ubel P.A. Helping patients decide: ten steps to better risk communication. J. Natl. Cancer Inst. 2011;103:1436–1443. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steyerberg E. Springer-Verlag; 2009. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. [Google Scholar]
- 16.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., Goldberg A.C., Gordon D., Levy D., Lloyd-Jones D.M., et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/
- 18.Kurian A.W., Hughes E., Bernhisel R., Probst B., Lanchbury J., Wagner S., Gutin A., Caswell-Jin J.L., Rohan T.E., Shadyab A.H., et al. Performance of the IBIS/Tyrer-Cuzick (TC) Model by race/ethnicity in the Women’s Health Initiative. J. Clin. Oncol. 2020;38:1503. [Google Scholar]
- 19.Rodriguez F., Chung S., Blum M.R., Coulet A., Basu S., Palaniappan L.P. Atherosclerotic Cardiovascular Disease Risk Prediction in Disaggregated Asian and Hispanic Subgroups Using Electronic Health Records. J. Am. Heart Assoc. 2019;8:e011874. doi: 10.1161/JAHA.118.011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyas D.A., Eisenstein L.G., Jones D.S. Hidden in Plain Sight - Reconsidering the Use of Race Correction in Clinical Algorithms. N. Engl. J. Med. 2020;383:874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 21.Borrell L.N., Elhawary J.R., Fuentes-Afflick E., Witonsky J., Bhakta N., Wu A.H.B., Bibbins-Domingo K., Rodríguez-Santana J.R., Lenoir M.A., Gavin J.R., 3rd., et al. Race and Genetic Ancestry in Medicine — A Time for Reckoning with Racism. N. Engl. J. Med. 2021;384:474–480. doi: 10.1056/NEJMms2029562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petousis P., Naeim A., Mosleh A., Hsu W. Evaluating the Impact of Uncertainty on Risk Prediction: Towards More Robust Prediction Models. AMIA Annu. Symp. Proc. 2018;2018:1461–1470. [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Y., Hou K., Burch K.S., Lapinska S., Privé F., Vilhjálmsson B., Sankararaman S., Pasaniuc B. Large uncertainty in individual PRS estimation impacts PRS-based risk stratification. bioRxiv. 2021 doi: 10.1101/2020.11.30.403188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Bles A.M., van der Linden S., Freeman A.L.J., Mitchell J., Galvao A.B., Zaval L., Spiegelhalter D.J. Communicating uncertainty about facts, numbers and science. R. Soc. Open Sci. 2019;6:181870. doi: 10.1098/rsos.181870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lautenbach D.M., Christensen K.D., Sparks J.A., Green R.C. Communicating genetic risk information for common disorders in the era of genomic medicine. Annu. Rev. Genomics Hum. Genet. 2013;14:491–513. doi: 10.1146/annurev-genom-092010-110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaukat A., Kahi C.J., Burke C.A., Rabeneck L., Sauer B.G., Rex D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 27.Steyerberg E.W., Vickers A.J., Cook N.R., Gerds T., Gonen M., Obuchowski N., Pencina M.J., Kattan M.W. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott J., Bodinier B., Bond T.A., Chadeau-Hyam M., Evangelou E., Moons K.G.M., Dehghan A., Muller D.C., Elliott P., Tzoulaki I. Predictive Accuracy of a Polygenic Risk Score-Enhanced Prediction Model vs a Clinical Risk Score for Coronary Artery Disease. JAMA. 2020;323:636–645. doi: 10.1001/jama.2019.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Screening and Diagnosis. Version 1. 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed May 6, 2021.
- 31.Anderson S.J., Ahnn S., Duff K. NSABP Breast Cancer Prevention Trial risk assessment program, Version 2. Department of Biostatistics, University of Pittsburgh; Pittsburgh (PA): 1992. NSABP Biostatistical Center Technical Report. [Google Scholar]
- 32.Chou R., Dana T., Blazina I., Daeges M., Jeanne T.L. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;316:2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 33.van der Bles A.M., van der Linden S., Freeman A.L.J., Spiegelhalter D.J. The effects of communicating uncertainty on public trust in facts and numbers. Proc. Natl. Acad. Sci. USA. 2020;117:7672–7683. doi: 10.1073/pnas.1913678117. [DOI] [PMC free article] [PubMed] [Google Scholar]