Abstract
A Ru(II)-catalyzed regioselective direct ortho-amidation of 2-aryl benzo[d]thiazoles employing acyl azides as a nitrogen source has been accomplished. This approach utilizes the efficiency of benzothiazole as a directing group and the role of acyl azide as an effective amidating agent toward C–N bond formation, thereby evading the general Curtius rearrangement. The protocol highlights significant functional group tolerance, single-step, and external oxidant-free conditions, with the release of only innocuous molecular nitrogen as the byproduct. The reaction mechanism and the intermediates associated with this selective Ru-catalyzed reaction have been investigated using ESI-MS. The protocol also aided in the construction of ortho-amidated β-carbolines, unveiling another class of fluorescent molecules.
Introduction
C–H functionalization has proved to be a sustainable alternative approach in synthetic chemistry. This includes introducing functional groups (C, O, N, S, and halides),1a bi-aryl coupling,1b,1c or cyclizations1d,1e with superior chemo-, regio-, and stereoselectivity. Transition metal-catalyzed C–H functionalization has occupied a prominent position in modern synthetic chemistry due to its extreme precision in constructing carbon–heteroatom bonds in an atom-economic way. Over the past few decades, various C–X (X = O, S, and N)2 bond formation reactions have been studied and utilized for diverse synthetic metamorphosis. Among them, the C–N bond has garnered considerable attention from organic chemists due to its presence in various naturally and pharmaceutically important molecules such as capsaicin, penicillin, and paracetamol. Utilization of a directing group acts as a promising strategy in achieving high selectivity due to coordination of the transition metal with the heteroatom of the directing group, which leads to the activation of the proximal C–H bond via chelation assistance.3
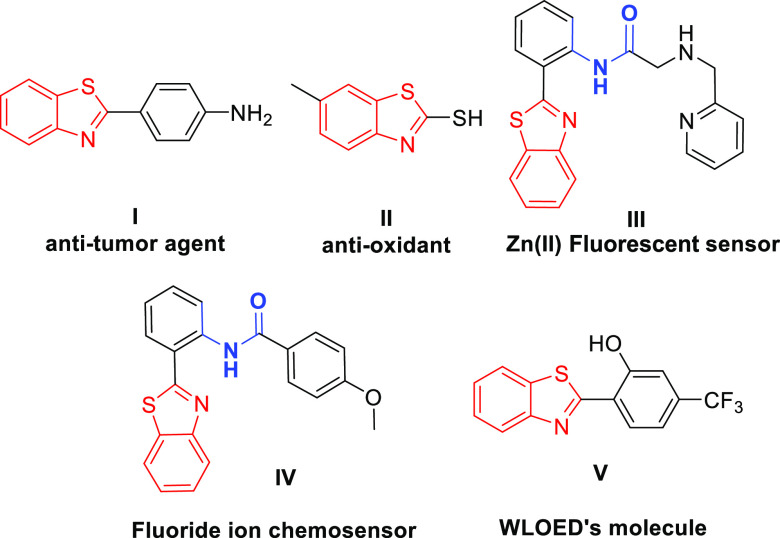
Over time, most of the reports on functionality derived reactions such as ortho-acylation, alkenylation, arylation, cyanation, hydroxylation, sulfonation, nitration, acetoxylation, halogenation, and amidation have delineated the use of benzothiazoles as an effective directing group.4 The prevalence of benzothiazole scaffolds in various natural products with eminent pharmacological activity and efficiency as a directing group has placed them at the forefront of chemical research. They possess vivid biological activities such as anti-tumor (I),5 anti-hepatitis C,6 anti-Alzheimer,7 anti-inflammatory, analgesic,8 and anti-oxidant (II).9 The adept fluorescent nature of benzothiazole-based molecules represents a class of heterocycles with efficient white light emission10 and chemosensor11 (III and IV) ability (Figure 1). In recent times, white organic light-emitting diodes (WOLEDs) have drawn considerable attention with prominent application in the field of flat panel displays, lighting equipment, and so on.12 There are many competent methods described for white light emission.13 A newer addition to this category is the use of single organic molecules as white light emitters with added advantages over the others, such as good reproducibility, simple fabrication process, and improved stability. Benzothiazole-based molecules have been well established as significant molecules in the field of WOLEDs (V), making them a moiety of particular interest (Figure 1).
Figure 1.
Benzothiazole-containing molecules.
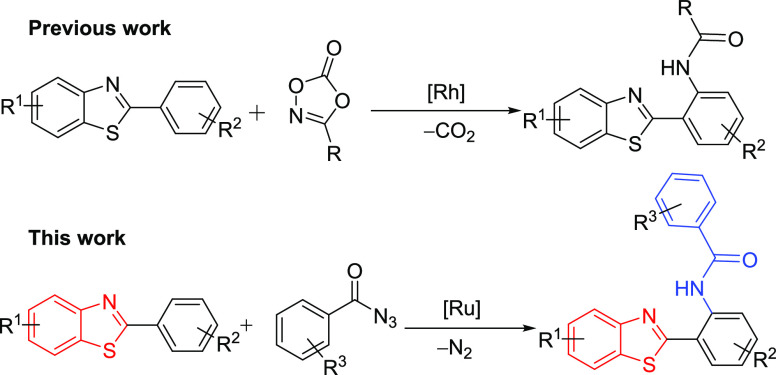
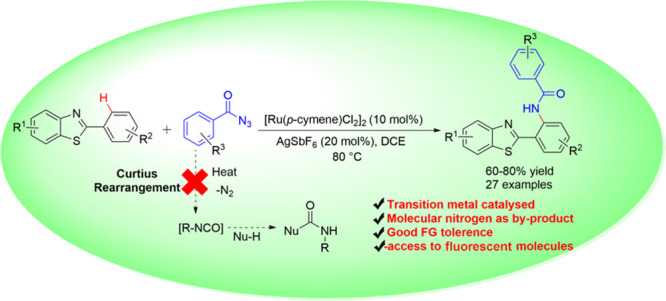
Therefore, efficient synthetic approaches for the functionalized benzothiazoles are highly desired to explore their potential applications. An extensive abundance of carbamide functionality in biologically important molecules advocates the importance of amide linkage. Amide linkages enhance the molecule’s interaction with an increased probability of hydrogen bonding when compared in the absence of an amide bond. Synthesis of bioactive molecules by amide incorporation has been one of the most significant organic manipulations undertaken by researchers globally.14 C–N bond formation aided by transition metal15 has efficiently assisted the need for synthetic methodologies for selective synthesis of carbamide-containing molecules. Various substrates such as N-tosylates,16 dioxazolones,17 amides,18 carbamates,19 and others20 have been successfully employed as amidating reagents. Ding et al. reported Rh-catalyzed 2-arylbenzo[d]thiazole C–H amidation using dioxazolone as an amidating agent17c (Scheme 1). On the other hand, acyl azides have been employed in the facile formation of C–C bonds in various moieties21 via in situ generation of isocyanates. Chang et al. explored the dual reactivity of acyl azide to facilitate C–N and C–C amidation based on the catalytic system.22 This was followed by acyl azide-mediated C–N amide formation utilizing Cu,23a,23b Ir,23c,23d Ru,23e and Co23f transition metal catalysts. Acyl azides are readily prepared, and their broad-spectrum utilization in selective amidation in the presence of less-expensive catalysts is highly desirable. With only handful of such results, acyl azides can be an interesting approach toward amidation strategy. Therefore, in continuation of our efforts24 toward the development of transition metal-catalyzed C–H activation, herein, we report a Ru(II)-catalyzed regioselective construction of the C–N bond on benzothiazoles using acyl azide as an amidating agent (Scheme 1).
Scheme 1. Methods for o-Amidation of 2-Aryl Benzothiazoles.
Results and Discussion
The key substrates 1a–o and 2a–l were primarily synthesized according to the previous reports.25 The reaction was commenced with a model reaction between 2-phenylbenzo[d]thiazole (1a) and acyl azide (2a) as an amidating agent employing [Ru(p-cymene)Cl2]2 (5 mol %) and AgSbF6 (20 mol %) as additives in DCE at 80 °C. To our delight, the desired product 3a was obtained in 50% yield (entry 1, Table 1). Encouraged by the desired result, we further aimed at enhancing the yield of product 3a by scrutinizing different additives such as Cu(OAc)2, Ag(OAc)2, TBAI, O2, Ag2CO3, and AgOTf (entries 2–7, Table 1). Additionally, a different combination of additive Cu(OAc)2 with toluene as a solvent at higher temperature was also examined, but disappointingly, no increase in product yield was observed (entry 8, Table 1). This prompted us to shift our focus to the solvents used in the reaction. We screened the reaction in various solvent systems such as toluene, 1,4-dioxane, ACN, and EtOH and realized that DCE was the best for the reaction (entries 9–12, Table 1). An increase in the catalyst loading from 5 to 10 mol % invariably enhanced the product 3a yield to 70% (entry 13, Table 1). The formation of a trace amount of product 3a in the absence of AgSbF6 indicated the significance of the additive for the reaction (entry 14, Table 1). A change in the catalytic system employing Pd(OAc)2 and CoCl2 with Cu(OAc)2 and PIDA as additives, respectively, failed to produce the desired product 3a (entries 15–16, Table 1). The reaction system in the presence of the Rh(III) catalyst with AgSbF6 as an additive delivered only 20% yield of the desired product (entry 17, Table 1).
Table 1. Optimization of the Reaction Conditions for C–H Activationa.
| entry | catalyst (5 mol %) | additive | solvent | yield (%) |
|---|---|---|---|---|
| 1 | [Ru(p-cymene)Cl2]2 | AgSbF6 | DCE | 50 |
| 2 | [Ru(p-cymene)Cl2]2 | Cu(OAc)2 | DCE | 20 |
| 3 | [Ru(p-cymene)Cl2]2 | Ag(OAc)2 | DCE | 30 |
| 4 | [Ru(p-cymene)Cl2]2 | TBAI | DCE | NR |
| 5 | [Ru(p-cymene)Cl2]2 | O2 | DCE | trace |
| 6 | [Ru(p-cymene)Cl2]2 | Ag2CO3 | DCE | NR |
| 7 | [Ru(p-cymene)Cl2]2 | AgOTf | DCE | 25 |
| 8 | [Ru(p-cymene)Cl2]2 | Cu(OAc)2 | Toluene | 10b |
| 9 | [Ru(p-cymene)Cl2]2 | AgSbF6 | Toluene | 45b |
| 10 | [Ru(p-cymene)Cl2]2 | AgSbF6 | 1,4-dioxane | 35c |
| 11 | [Ru(p-cymene)Cl2]2 | AgSbF6 | CH3CN | 10 |
| 12 | [Ru(p-cymene)Cl2]2 | AgSbF6 | EtOH | NR |
| 13 | [Ru(p-cymene)Cl2]2 | AgSbF6 | DCE | 70d |
| 14 | [Ru(p-cymene)Cl2]2 | DCE | trace | |
| 15 | Pd(OAc)2 | Cu(OAc)2 | CH3CN | NR |
| 16 | CoCl2 | PIDA | DCE | NR |
| 17 | [RhCp*Cl2]2 | AgSbF6 | DCE | 20 |
Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), [Ru(p-cymene)Cl2]2 (5 mol %), additive (20 mol %), solvent (2 mL), 80 °C, and 5 h in the pressure tube.
At 110 °C.
At 100 °C.
Catalyst (10 mol %). NR = no reaction.
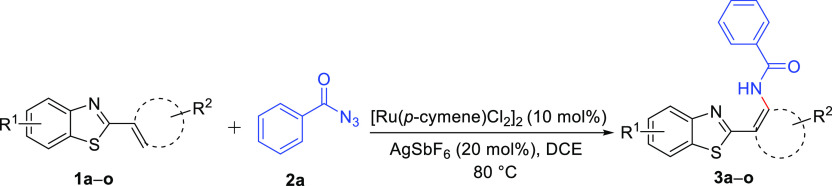
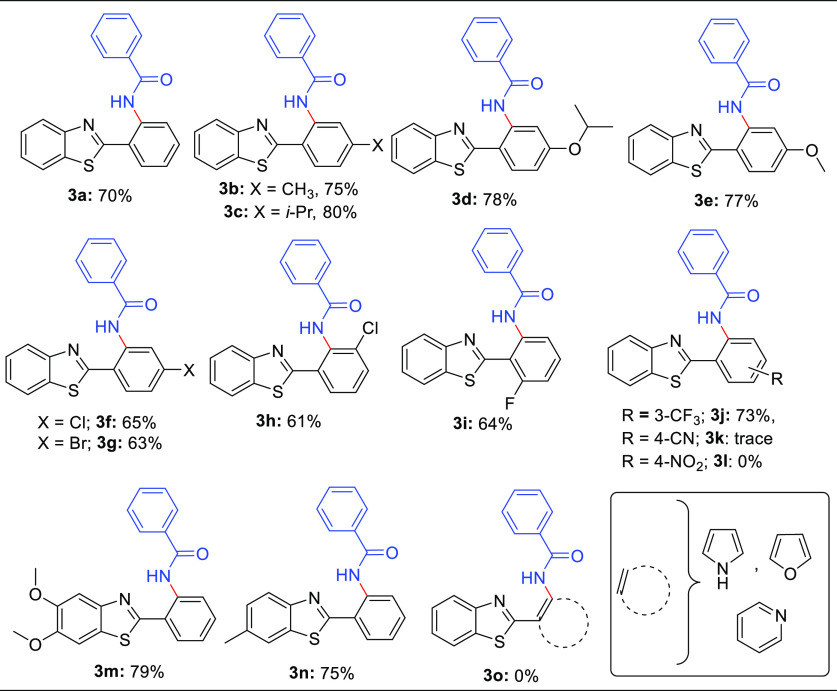
With the optimized reaction conditions, we further investigated the substrate scope of the protocol using various substituted 2-arylbenzo[d]thiazoles to explore the suitability of the protocol. All the substrates proved to produce the desired ortho-amidated products 3a–j and 3m, n in moderate to good yields. Substrates with substitution at the para-position (methyl, isopropyl, isopropoxy, and methoxy) of 1b–e yielded the expected products 3b–e in good yields (75–80%, Table 2). The para-halogenated substrates (chloro- and bromo-) 1f and 1g also afforded the desired products 3f and 3g in 65 and 63% yield, respectively. We also studied the effect of the substitution position and gratifyingly found that the meta- (chloro-) 1h and ortho-position (fluoro) 1i did not deter the product formation and resulted in 3h and 3i, respectively, in moderate yields (61 and 64%, respectively, Table 2). For unveiling the effect of EWG on the reaction results, we subjected meta-substituted trifluoromethyl and 4-CN- and 4-NO2-substituted substrates and realized that 3-CF3 delivered amidated product 3j in 73% yield. Disappointingly, 4-CN showed only a trace amount of product 3k formation, whereas the 4-NO2 substitution failed to produce the desired product 3l. Various substitution patterns on the benzene ring of benzothiazoles were found to be compatible with the reaction conditions and therefore afforded 3m and 3n in moderate yields (79 and 75%, respectively, Table 2). Disappointingly, replacing the 2-phenyl ring with the hetero-aromatic system failed to produce the ortho-amidated product 3o, limiting the set protocol.
Table 2. Ru-Catalyzed Regioselective o-Amidation of 2-Arylbenzo[d]thiazolesa.
Reaction conditions: 1 (1 equiv), 2a (1.5 equiv), [Ru(p-cymene)Cl2]2 (10 mol %), AgSbF6 (20 mol %), DCE (2 mL), 80 °C, and 5–7 h in the pressure tube.
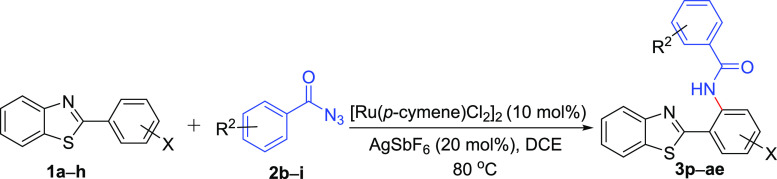
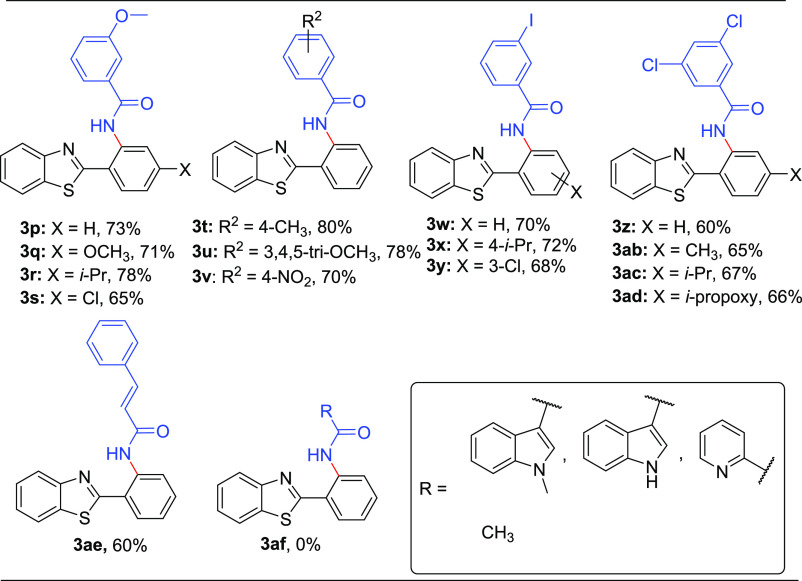
Subsequently, we shifted our attention toward exploring the protocol for C–H amidation of 2-aryl benzo[d]thiazoles with substituted acyl azides. Delightfully, the optimized condition utilizing substituted acyl azides as the amide source favored the formation of the desired products 3p–ae in moderate to good yields. The study about the effect of electron-donating substitutions (4-OCH3 and 4-CH3) on acyl azides coupled with substituted 2-aryl benzothiazoles satisfactorily furnished the desired products 3p–t (65–80%, Table 3).
Table 3. Ru-Catalyzed Regioselective o-Amidation of 2-Arylbenzo[d]thiazoles with Substituted Acyl Azidesa.
Reaction conditions: 1a (1 equiv), 2 (1.5 equiv), [Ru(p-cymene)Cl2]2 (10 mol %), AgSbF6 (20 mol %), DCE (2 mL), 80 °C, and 5–7 h in the pressure tube.
The trimethoxy-substituted acyl azides also underwent the reaction smoothly and produced 3u in 78% yield. The electron-withdrawing acyl azide (4-NO2) did not affect the reaction results and delivered 3v in 70% yield. Furthermore, a study with mono- and di-halogenated acyl azides (3-I and 3,5-diCl) also enabled access to the amidated products 3w–ad in moderate yields (60–72%, Table 3). Additionally, an understanding of the substrate scope revealed the utility of the optimized condition in employing cinnamoyl azide to afford the target molecule 3ae in a moderate yield of 60%. In our attempt to further decipher the scope of acyl azide, we employed hetero-aromatics such as 3-indole acyl azide, N-methyl 3-indole acyl azide, and picolinic acyl azide; however, it was observed that these acyl azides along with the aliphatic acyl azide (acetyl azide) failed to construct the desired molecules of interest (3af, Table 3).
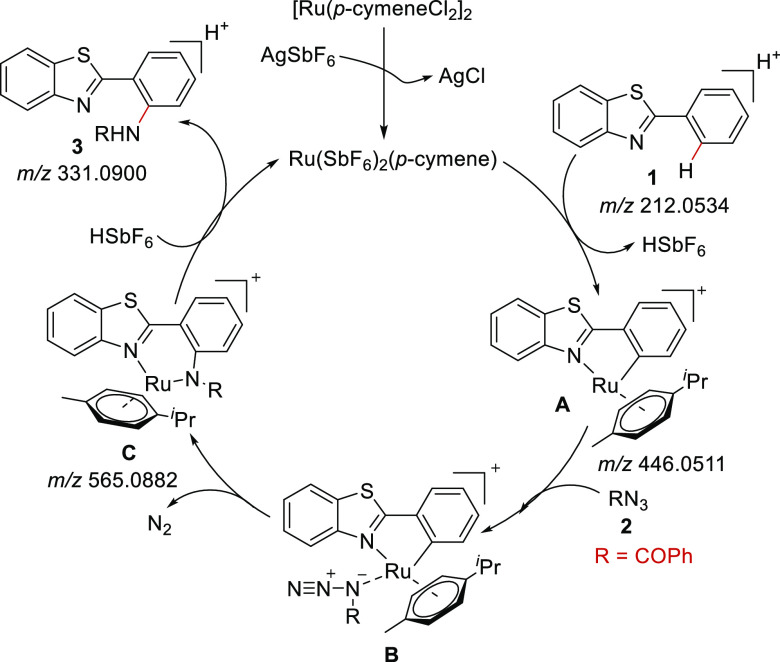
Based on the previous reports26 and with assistance of the ESI-MS technique wherein the intermediates were captured at varied time intervals to compile the MS data, a probable pathway for the reaction mechanism was determined. Figure 2a displays an ESI-MS spectrum of the reaction mixture after mixing 1a and 2a, the catalyst, and the additive in the reaction media, wherein m/z 212.0535 represents [1a + H]+. Furthermore, the reaction progress was observed after 5 min with the capturing of the five-membered ruthenacycle A with [A]+ at a m/z of 446.0511 (Figure 2b). After 30 min of reaction in the pressure tube, a peak at a m/z of 565.0849 appeared for C with [C]+, the transition metal complex characterized by the isotopic peak. A small peak accompanied this at a m/z of 331.0867, indicative of the product formation (Figure 2c). A further assessment of the reaction after 3 h revealed an increase in the intensity of the product peak compared to the substrate (Figure 2d).
Figure 2.
ESI-QTOF-MS monitoring of acyl azide-mediated amidation of 1a at varied time intervals from 0.0 min to 3 h.
With the aid of a previous report from the Punniyamurthy group23e and the ionization species identified from the abovementioned experiment, a probable mechanism is sketched in Scheme 2. The neutral precursor [Ru(p-cymeneCl2)]2 is activated to form the active cationic Ru(II) species in the presence of AgSbF6. Next, a five-membered ruthenacycle species A is formed via C–H activation. The co-ordination of acyl azide (2) to intermediate A leads to ruthenium intermediate B. Furthermore, six-membered ruthenacycle species C formation is prompted by insertion of an amide moiety into the ruthenacycle followed by extrusion of the N2 gas. Finally, the desired product 3 is generated by protonolysis along with active cationic ruthenium species regeneration.
Scheme 2. Plausible Reaction Mechanism.
Furthermore, we also performed an intermolecular competition experiment to compare the reactivity of varied azides, that is, 2d (3,4,5-tri-OMe) and 2e (4-NO2) with 1a. The results unveiled faster reaction of the 2e azide than the electron-rich 2d azide, which can be attributed to the greater stability of the electron-deficient acyl azide (Scheme 3).
Scheme 3. Intermolecular Competition Experiment: EDG vs EWG.
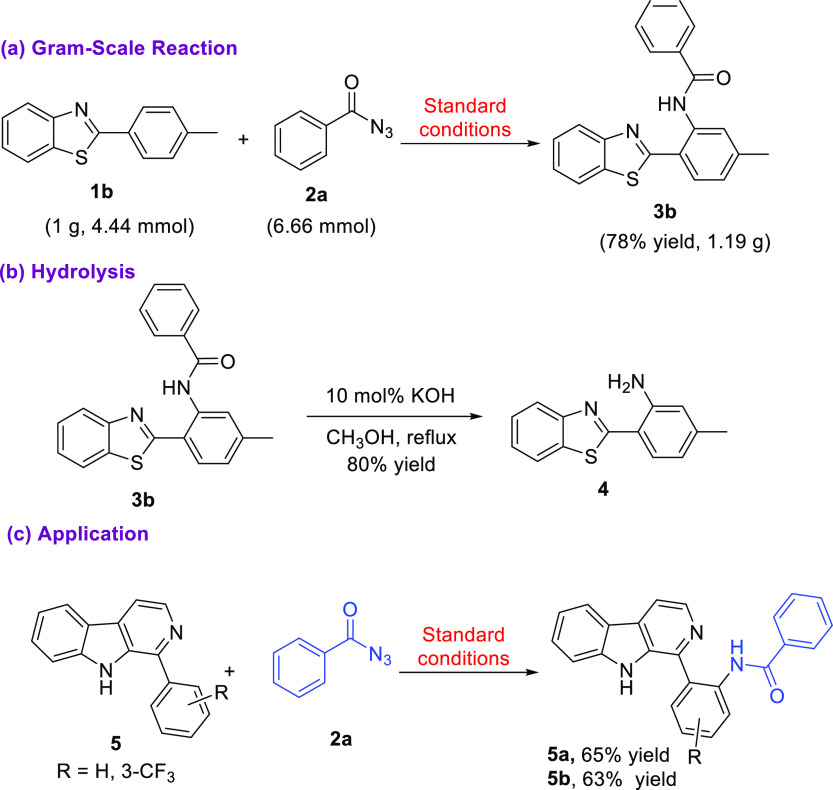
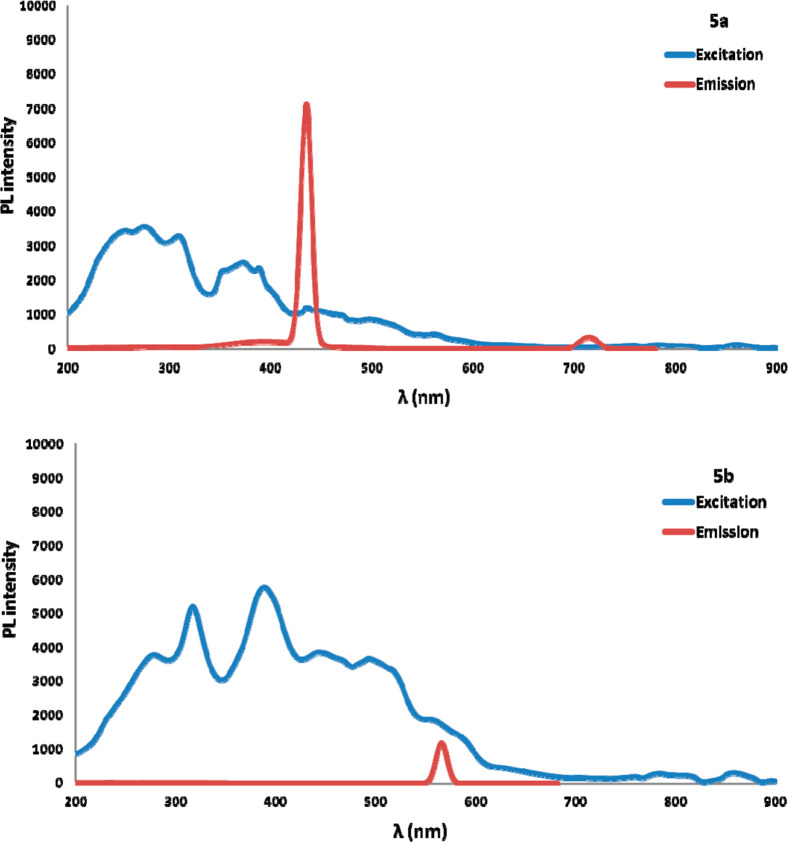
To reveal the efficiency of the reaction at the gram-scale level, we coupled 1b (4.44 mmol) with 2a (6.66 mmol) and obtained amidated product 3b in 78% yield. Further hydrolysis of 3b afforded the free amine (4, 80% yield) at the ortho-position, which can be employed in synthetic transformations such as in the synthesis of N-benzylated benzothiazoles established as chemosensors for Hg2+ ions27a or the benzothiazole-tetraoxocalix[2]arene[2]triazine conjugates enabling cyanate detection27b (Scheme 4). Fascinatingly, in our pursuit to reconnoiter the application of our protocol, we strategized the development of amide linkage on biologically relevant β-carbolines 5. To our delight, the established protocol encouragingly yielded ortho-amidated β-carbolines (5a,b) in 65 and 63% yield, respectively. The amidated β-carbolines exhibited florescent property28 as depicted in Figure 3. Compound 5a and 5b demonstrated λmax (excitation) at 275 nm and λmax (emission) at 435 nm and λmax (excitation) at 385 nm and λmax (emission) at 566 nm, respectively.
Scheme 4. Gram-Scale, Hydrolysis Experiment, and Application.
Figure 3.
Absorption and emission spectra of 5a and 5b.
Conclusions
In summary, a protocol for ortho-amidation of 2-aryl benzothiazole in the presence of a Ru(II) catalyst via C–H activation was accomplished. The utility of acyl azide for the formation of a C–N bond was successfully explored, overcoming the intervention with isocyanate formation that might have led to a mixture of C–N and C–C amidated products. The mild reaction conditions proved to be efficient in the presence of functional diversity, yielding products in good to moderate yields. The release of molecular nitrogen as the only byproduct paves the way to an environmentally benign protocol. This method provides a single-step construction for amidated molecules, which are well established for their use in different ion detections and as WOLED molecules.
Experimental Section
General Information
Commercially available reagents and solvents were used without further purification. The 1H NMR spectra were recorded on a NMR instrument operated at 500 MHz. Chemical shifts are reported in ppm with the solvent resonance as the internal standard (CDCl3: δ = 7.25 ppm and DMSO-d6: δ = 2.50 ppm). 13C NMR spectra were recorded on a NMR instrument operated at 125 MHz with complete proton decoupling. Chemical shifts are reported in ppm with the solvent resonance as the internal standard (CDCl3: δ = 77.16 ppm and DMSO-d6: δ = 39.52 ppm). The following abbreviations were used for 1H NMR spectra to indicate the signal multiplicity: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (doublet of doublet), and dt (doublet of triplet). HRMS was measured in an ESI-MS mass spectrophotometer. Thin-layer chromatography was performed on MERCK precoated silica gel 60F-254 (0.5 mm) aluminum plates and visualized under UV light at 254 nm. Column chromatography was performed using silica gel 60–120 and 100–200 meshes.
Caution: The acyl azides are explosive in nature and hence should be stored below room temperature and the reactions should be handled carefully in sophisticated fume hoods. Precautions should be taken while using the rotary evaporator during the purification process wherein the temperature of the water bath should not exceed 30 °C.
General Procedure for Ru(II)-Catalyzed Ortho-Selective C–H Amidation of 2-Arylbenzothiazoles (3)
2-Arylbenzothiazoles 1 (1.0 equiv), benzoyl azides 2 (1.5 equiv), [Ru(p-cymene)Cl2]2 (10 mol %), AgSbF6 (20 mol %), and DCE (2 mL) were stirred at 80 °C for 5–7 h in a sealed tube. After completion of the reaction (monitored by TLC), the reaction mixture was quenched with water and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on a silica gel using 2–5% ethyl acetate in hexane as the eluent to give the desired product 3. All the synthesized compounds were thoroughly characterized by IR, 1H and 13C NMR, and HRMS (ESI).
N-(2-(Benzo[d]thiazol-2-yl)phenyl)benzamide (3a)
(76.3 mg), 70% yield, white solid, mp: 186–189 °C, FT-IR (cm)−1: 3062, 1672, 1615, 1543, 1458; 1H NMR (500 MHz, CDCl3): δ 13.40 (s, 1H), 9.09 (d, J = 8.3 Hz, 1H), 8.27 (d, J = 5.6 Hz, 2H), 8.04 (d, J = 8.0 Hz, 1H), 7.96 (dd, J = 10.9, 8.0 Hz, 2H), 7.64 (d, J = 5.7 Hz, 3H), 7.58 (t, J = 7.6 Hz, 2H), 7.48 (t, J = 7.5 Hz, 1H), 7.24 (t, J = 7.5 Hz, 1H) ppm; 13C{1H} (125 MHz, CDCl3): δ 169.1, 166.3, 152.8, 138.5, 135.7, 133.4, 132.2, 131.9, 129.9, 128.7, 127.8, 126.7, 125.9, 123.3, 122.3, 121.6, 120.9, 119.4 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H15N2OS, 331.0900; found, 331.0899.
N-(2-(Benzo[d]thiazol-2-yl)-5-methylphenyl)benzamide (3b)
(80.2 mg), 75% yield, white solid, mp: 162–165 °C, FT-IR (cm)−1: 3023, 1691, 1615, 1598, 1511; 1H NMR (500 MHz, DMSO-d6): δ 13.07 (s, 1H), 8.70 (s, 1H), 8.21 (d, J = 7.6 Hz, 1H), 8.18–8.15 (m, 2H), 8.06 (d, J = 8.0 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.75–7.71 (m, 3H), 7.67–7.61 (m, 1H), 7.57–7.52 (m, 1H), 7.16 (dd, J = 8.0, 0.9 Hz, 1H), 2.44 (s, 3H) ppm; 13C{1H} NMR (125 MHz, DMSO-d6): δ 168.7, 165.7, 152.4, 142.9, 137.8, 134.8, 132.8, 132.6, 130.6, 129.2, 127.2, 126.7, 125.3, 122.7, 122.1, 121.7, 117.1, 21.5 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H17N2OS, 345.1056; found, 345.1050.
N-(2-(Benzo[d]thiazol-2-yl)-5-isopropylphenyl)benzamide (3c)
(81.6 mg), 80% yield, white solid; mp: 180–183 °C; FT-IR (cm)−1: 3089, 2961, 1671, 1618, 1578; 1H NMR (500 MHz, CDCl3): δ 13.38 (s, 1H), 8.99 (d, J = 1.6 Hz, 1H), 8.25 (m, 2H), 7.98 (d, J = 8.1 Hz, 1H), 7.92 (d, J = 7.6 Hz, 1H), 7.84 (d, J = 8.1 Hz, 1H), 7.63–7.57 (m, 3H), 7.56–7.49 (m, 1H), 7.45–7.39 (m, 1H), 7.08 (dd, J = 8.1, 1.6 Hz, 1H), 3.09–2.99 (m, 1H), 1.35 (s, 3H), 1.34 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.1, 166.4, 153.8, 152.5, 138.9, 135.4, 133.0, 131.8, 129.9, 128.4, 128.0, 126.5, 125.7, 121.9, 121.3, 118.3, 117.0, 34.8, 23.7 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C23H21N2OS, 373.1369; found, 373.1360.
N-(2-(Benzo[d]thiazol-2-yl)-5-isopropoxyphenyl)benzamide (3d)
(78.9 mg), 78% yield, white solid; mp: 184–187 °C; FT-IR (cm)−1: 3062, 1671, 1625, 1583, 1413; 1H NMR (500 MHz, CDCl3): δ 13.52 (s, 1H), 8.74 (d, J = 2.5 Hz, 1H), 8.25 (dd, J = 7.5, 2.1 Hz, 2H), 7.92 (dd, J = 22.5, 7.8 Hz, 2H), 7.78 (d, J = 8.8 Hz, 1H), 7.65–7.58 (m, 3H), 7.55–7.48 (m, 1H), 7.43–7.35 (m, 1H), 6.71 (dd, J = 8.8, 2.6 Hz, 1H), 4.78 (dt, J = 12.1, 6.1 Hz, 1H), 1.43 (s, 3H), 1.42 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 168.9, 166.5, 161.2, 152.7, 140.3, 135.6, 133.0, 131.9, 131.3, 128.7, 127.9, 126.6, 125.3, 121.7, 121.4, 112.2, 105.9, 70.5, 22.0 ppm. HRMS (ESI-QTOF): m/z [M + H]+ calcd for C23H20N2O2S, 389.1318; found, 389.1357.
N-(2-(Benzo[d]thiazol-2-yl)-5-methoxyphenyl)benzamide (3e)
(80.5 mg), 77% yield, white solid, mp: 170–173 °C, FT-IR (cm)−1: 3061, 2922, 1671, 1620, 1585; 1H NMR (500 MHz, CDCl3): δ 13.57 (s, 1H), 8.75 (d, J = 2.6 Hz, 1H), 8.25–8.24 (m, 2H), 7.91 (dd, J = 21.5, 7.8 Hz, 2H), 7.79 (d, J = 8.8 Hz, 1H), 7.64–7.60 (m, 3H), 7.51 (dd, J = 7.6, 6.6 Hz, 1H), 7.42–7.38 (m, 1H), 6.74 (dd, J = 8.8, 2.6 Hz, 1H), 3.95 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3): δ 169.0, 166.6, 162.8, 152.6, 140.3, 135.5, 132.9, 131.9, 131.1, 128.6, 127.8, 126.5, 125.4, 121.8, 121.4, 112.5, 110.9, 104.4, 55.6 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H17N2O2S, 361.1005; found, 361.1010.
N-(2-(Benzo[d]thiazol-2-yl)-5-chlorophenyl)benzamide (3f)
(67.7 mg), 65% yield, white solid, mp: 148–150 °C, FT-IR (cm)−1: 3112, 3055, 1673, 1620, 1580; 1H NMR (500 MHz, CDCl3): δ 13.40 (s, 1H), 9.10 (d, J = 2.0 Hz, 1H), 8.16 (d, J = 8.0 Hz, 2H), 7.94 (d, J = 8.1 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.76 (d, J = 8.5 Hz, 1H), 7.62–7.52 (m, 3H), 7.49 (t, J = 9.5 Hz, 1H), 7.40 (t, J = 7.6 Hz, 1H), 7.11 (dd, J = 8.4, 2.1 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 166.2, 152.4, 139.2, 138.3, 135.2, 132.1, 130.7, 128.7, 127.8, 127.0, 126.1, 123.4, 122.3, 121.6, 120.8, 117.8 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H14ClN2OS, 365.0510; found, 365.0519.
N-(2-(Benzo[d]thiazol-2-yl)-5-bromophenyl)benzamide (3g)
(62.2 mg), 63% yield, white solid, mp: 150–153 °C; FT-IR (cm)−1: 2960, 1672, 1616, 1577, 698; 1H NMR (500 MHz, CDCl3): δ 13.44 (s, 1H), 9.32 (d, J = 2.0 Hz, 1H), 8.24–8.21 (m, 2H), 8.01 (d, J = 8.1 Hz, 1H), 7.96–7.93 (m, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.64–7.59 (m, 3H), 7.58–7.54 (m, 1H), 7.49–7.45 (m, 1H), 7.33 (dd, J = 8.4, 2.0 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 168.1, 165.9, 152.3, 139.1, 135.6, 132.8, 132.0, 130.6, 129.0, 128.4, 127.0, 126.7, 126.5, 126.1, 123.7, 122.3, 121.6, 118.1 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H14BrN2OS, 409.0005; found, 409.0010.
N-(2-(Benzo[d]thiazol-2-yl)-6-chlorophenyl)benzamide (3h)
(63.4 mg), 61% yield, white solid, mp: 144–147 °C, FT-IR (cm)−1: 3112, 1687, 1612, 1589, 745; 1H NMR (500 MHz, CDCl3): δ 13.31 (s, 1H), 9.04 (d, J = 9.0 Hz, 1H), 8.21 (dd, J = 7.9, 1.7 Hz, 2H), 8.01 (d, J = 8.1 Hz, 1H), 7.95 (d, J = 8.4 Hz, 1H), 7.86 (d, J = 2.4 Hz, 1H), 7.65–7.53 (m, 4H), 7.50–7.44 (m, 2H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 167.5, 166.2, 152.5, 137.0, 135.2, 133.3, 132.1, 131.9, 129.2, 128.7, 128.2, 127.8, 127.0, 126.3, 126.3, 122.5, 122.3, 121.7, 120.7 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H14ClN2OS, 365.0510; found, 365.0513.
N-(2-(Benzo[d]thiazol-2-yl)-3-fluorophenyl)benzamide (3i)
(66.6 mg), 64% yield, white solid; mp: 150–152 °C FT-IR (cm)−1: 3020, 1679, 1660, 1593, 1314; 1H NMR (500 MHz, DMSO-d6): δ 13.68 (s, 1H), 8.77 (d, J = 8.4 Hz, 1H), 8.27 (d, J = 7.9 Hz, 1H), 8.15 (dd, J = 5.9, 2.5 Hz, 2H), 8.10 (d, J = 8.1 Hz, 1H), 7.73 (dd, J = 6.1, 2.7 Hz, 3H), 7.71–7.64 (m, 2H), 7.62–7.55 (m, 1H), 7.28 (m, 1H) ppm; 13C{1H} NMR (125 MHz, DMSO-d6): δ 166.8, 161.8, 160.0, 150.2, 140.0, 135.2, 133.8, 133.6, 133.5, 132.9, 129.5, 127.8, 126.8, 122.7, 122.3, 117.0, 111.1, 111.0, 109.0, 108.9 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H13FN2OS, 349.0805; found, 349.0799.
N-(2-(Benzo[d]thiazol-2-yl)-5-(trifluoromethyl)phenyl)benzamide (3j)
(72.8 mg), 73% yield, white solid, mp: 179–181 °C; FT-IR (cm)−1: 3011, 1688, 1716, 1596, 1300; 1H NMR (500 MHz, DMSO-d6): δ 13.56 (s, 1H), 9.21 (d, J = 8.8 Hz, 1H), 8.25–8.21 (m, 2H), 8.12 (s, 1H), 8.00 (dd, J = 26.1, 8.0 Hz, 2H), 7.76 (d, J = 7.6 Hz, 1H), 7.67–7.56 (m, 4H), 7.49 (t, J = 7.5 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, DMSO-d6): δ 162.9, 161.8, 147.8, 136.4, 130.3, 128.6, 127.6, 124.0, 123.1, 122.3, 122.0, 121.7, 117.7, 117.0, 116.3, 114.5 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H14F3N2OS, 399.0773; found, 399.0794.
N-(2-(5,6-Dimethoxybenzo[d]thiazol-2-yl)phenyl)benzamide (3m)
(79.5 mg), 79% yield, white solid, mp: 190–193 °C; FT-IR (cm)−1: 3011, 2981, 1699, 1578, 1500; 1H NMR (500 MHz, CDCl3): δ 13.39 (s, 1H), 9.03 (d, J = 8.4 Hz, 1H), 8.25–8.23 (m, 2H), 7.84 (dd, J = 7.9, 1.3 Hz, 1H), 7.61–7.55 (m, 3H), 7.53–7.48 (m, 1H), 7.45 (s, 1H), 7.33 (s, 1H), 7.20–7.16 (m, 1H), 4.02 (s, 3H), 4.00 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 167.2, 166.3, 149.8, 149.3, 147.0, 138.0, 135.9, 131.8, 131.5, 129.3, 128.5, 127.9, 125.7, 123.3, 120.8, 119.8, 119.75, 111.4, 103.8, 102.4, 56.4, 56.1 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C22H19N2O3S, 391.1111; found, 391.1158.
N-(2-(5-Methylbenzo[d]thiazol-2-yl)phenyl)benzamide (3n)
(80.6 mg), 75% yield, white solid, mp: 168–171 °C: FT-IR (cm)−1: 3016, 1699, 1612, 1577, 1485; 1H NMR (500 MHz, CDCl3): δ 13.40 (s, 1H), 9.05 (dd, J = 8.5, 1.0 Hz, 1H), 8.25–8.22 (m, 2H), 7.91–7.86 (m, 2H), 7.73 (s, 1H), 7.62–7.58 (m, 3H), 7.55–7.51 (m, 1H), 7.35 (dd, J = 8.3, 1.2 Hz, 1H), 7.20 (m, 1H), 2.52 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 163.2, 161.5, 146.1, 133.6, 131.4, 130.9, 128.8, 127.2, 127.1, 125.0, 123.9, 123.5, 123.1, 118.5, 117.0, 116.5, 116.1, 114.8, 16.9 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H17N2OS, 345.1056; found, 345.1085.
N-(2-(Benzo[d]thiazol-2-yl)phenyl)-3-methoxybenzamide (3p)
(87.2 mg), 73% yield, white solid, mp: 160–163 °C; FT-IR (cm)−1: 3169, 3001, 1669, 1613, 1538, 1487; 1H NMR (500 MHz, DMSO-d6): δ 13.03 (s, 1H), 8.81 (d, J = 8.4 Hz, 1H), 8.23 (d, J = 8.0 Hz, 1H), 8.10–8.05 (m, 2H), 7.76 (d, J = 7.7 Hz, 1H), 7.68–7.62 (m, 4H), 7.55 (m, 1H), 7.36–7.32 (m, 1H), 7.30 (dd, J = 8.2, 2.6 Hz, 1H), 3.90 (s, 3H) ppm; 13C{1H} NMR (125 MHz, DMSO-d6): δ 168.7, 165.5, 160.1, 152.5, 137.9, 136.6, 133.4, 132.7, 130.7, 130.7, 127.7, 126.7, 124.6, 122.8, 122.5, 121.1, 120.1, 119.7, 117.9, 113.8, 55.9 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H17N2O2S, 361.1005; found, 361.1006.
N-(2-(Benzo[d]thiazol-2-yl)-5-methoxyphenyl)-3-methoxybenzamide (3q)
(80.4 mg), 71% yield, white solid; mp: 182–185 °C; FT-IR (cm)−1: 2965, 2838, 1678, 1625, 1544; 1H NMR (500 MHz, CDCl3): δ 13.57 (s, 1H), 8.74 (d, J = 2.6 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.86 (ddd, J = 4.7, 2.3, 0.6 Hz, 2H), 7.80–7.78 (m, 2H), 7.54–7.49 (m, 2H), 7.40 (dd, J = 8.0, 1.0 Hz, 1H), 7.15 (m, 1H), 6.74 (dd, J = 8.8, 2.6 Hz, 1H), 3.95 (s, 3H), 3.93 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.0, 166.4, 162.7, 160.1, 152.8, 140.3, 137.0, 132.9, 131.2, 129.6, 126.6, 125.4, 121.9, 121.4, 119.8, 117.8, 113.5, 112.6, 111.0, 104.4, 55.5 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C22H19N2O3S, 391.1111; found, 391.1112.
N-(2-(Benzo[d]thiazol-2-yl)-5-isopropylphenyl)-3-methoxybenzamide (3r)
(86.7 mg), 78% yield, white solid, mp: 160–163 °C; FT-IR (cm)−1: 3003, 2960, 2836, 1681, 1618; 1H NMR (500 MHz, CDCl3): δ 13.37 (s, 1H), 8.98 (d, J = 1.6 Hz, 1H), 8.05 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 7.7 Hz, 1H), 7.84 (dd, J = 8.1, 5.4 Hz, 2H), 7.82–7.77 (m, 1H), 7.56–7.48 (m, 2H), 7.46–7.38 (m, 1H), 7.15 (m, 1H), 7.08 (dd, J = 8.1, 1.6 Hz, 1H), 3.93 (s, 3H), 3.03 (m, 1H), 1.35 (s, 3H), 1.34 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.0, 166.2, 160.0, 154.2, 152.6, 138.4, 136.9, 133.0, 129.6, 129.6, 126.6, 125.6, 122.3, 121.6, 121.5, 119.7, 118.9, 117.2, 117.2, 113.5, 55.7, 34.5, 23.7 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C24H23N2O2S, 403.1475; found, 403.1474.
N-(2-(Benzo[d]thiazol-2-yl)-5-chlorophenyl)-3-methoxybenzamide (3s)
(73.1 mg), 65% yield, white solid, mp: 180–182 °C; FT-IR (cm)−1: 2960, 2831, 1682, 1618, 1581; 1H NMR (500 MHz, CDCl3): δ 13.44 (s, 1H), 9.14 (d, J = 1.9 Hz, 1H), 8.05 (d, J = 8.1 Hz, 1H), 7.92 (d, J = 7.9 Hz, 1H), 7.80 (dd, J = 7.9, 3.0 Hz, 2H), 7.77 (s, 1H), 7.53 (dt, J = 14.3, 7.9 Hz, 2H), 7.45 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 8.4 Hz, 2H), 3.93 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 168.0, 166.2, 160.2, 152.4, 139.2, 138.2, 136.6, 133.1, 130.7, 129.7, 126.9, 126.1, 123.4, 122.5, 121.5, 120.7, 119.7, 117.8, 117.7, 113.7, 55.5 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H16ClN2O2S, 395.0616; found, 395.0623.
N-(2-(Benzo[d]thiazol-2-yl)phenyl)-4-methylbenzamide (3t)
(91.7 mg), 80% yield, white solid, mp: 198–201 °C; FT-IR (cm)−1: 3021, 1689, 1614, 1578, 1512; 1H NMR (500 MHz, CDCl3): δ 13.21 (s, 1H), 8.99 (d, J = 8.4 Hz, 1H), 8.07 (d, J = 8.2 Hz, 2H), 7.95 (d, J = 8.1 Hz, 1H), 7.90–7.80 (m, 2H), 7.55–7.42 (m, 2H), 7.39 (dd, J = 11.6, 4.6 Hz, 1H), 7.33 (d, J = 8.0 Hz, 2H), 7.13 (dd, J = 11.6, 4.7 Hz, 1H), 2.42 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 168.2, 165.2, 151.7, 141.2, 137.5, 132.4, 131.7, 131.2, 128.9, 128.3, 126.9, 125.7, 124.8, 122.1, 121.3, 120.6, 119.9, 118.3, 20.3 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H17N2OS, 345.1056; found, 345.1052.
N-(2-(Benzo[d]thiazol-2-yl)phenyl)-3,4,5-trimethoxybenzamide (3u)
(109.0 mg), 78% yield, white solid, mp: 140–144 °C; FT-IR (cm)−1: 3096, 2945, 1687, 1612, 1566; 1H NMR (500 MHz, CDCl3): δ 13.18 (s, 1H), 8.99 (d, J = 8.4 Hz, 1H), 8.09–7.75 (m, 3H), 7.58–7.49 (m, 2H), 7.47–7.41 (m, 1H), 7.39 (s, 2H), 7.22 (m, 1H), 3.98 (s, 3H), 3.96 (s, 6H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.1, 166.4, 153.4, 152.8, 138.4, 133.3, 132.2, 131.5, 130.0, 126.7, 125.9, 123.3, 122.3, 121.6, 120.8, 119.4, 106.1, 61.0, 56.8 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C23H21N2O4S, 421.1217; found, 421.1262.
N-(2-(Benzo[d]thiazol-2-yl)phenyl)-4-nitrobenzamide (3v)
(86.8 mg) 70% yield, yellow solid, mp: 170–172 °C; 1H NMR (500 MHz, CDCl3): δ 13.61 (s, 1H), 9.01 (d, J = 8.3 Hz, 1H), 8.43 (dd, J = 30.4, 8.6 Hz, 4H), 7.96 (t, J = 7.9 Hz, 3H), 7.63–7.55 (m, 2H), 7.49 (t, J = 7.6 Hz, 1H), 7.28 (s, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.1, 164.0, 152.5, 141.2, 137.8, 133.2, 132.4, 130.0, 128.9, 127.1, 126.2, 124.1, 123.9, 122.1, 121.8, 120.9, 119.6 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H14N3O3S, 376.0750; found, 376.0756.
N-(2-(Benzo[d]thiazol-2-yl)phenyl)-3-iodobenzamide (3w)
(106.3 mg), 70% yield, white solid, mp: 160–163 °C; FT-IR (cm)−1: 3058, 1675, 1612, 1588, 1489; 1H NMR (500 MHz, CDCl3): δ 13.35 (s, 1H), 9.02 (dd, J = 8.5, 0.9 Hz, 1H), 8.54 (t, J = 1.6 Hz, 1H), 8.23 (d, J = 8.1 Hz, 1H), 8.19 (dd, J = 8.0, 1.3 Hz, 1H), 7.99–7.88 (m, 3H), 7.60–7.51 (m, 2H), 7.46 (m, 1H), 7.34 (t, J = 7.8 Hz, 1H), 7.22 (m, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.0, 164.5, 152.7, 140.8, 138.0, 137.5, 136.3, 133.2, 132.2, 130.4, 129.8, 127.4, 126.8, 126.0, 123.6, 123.0, 121.5, 120.9, 119.5, 94.3 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H14IN2OS, 456.9866; found, 456.9867.
N-(2-(Benzo[d]thiazol-2-yl)-5-isopropylphenyl)-4-iodobenzamide (3x)
(99.5 mg), 72% yield, white solid, mp: 180–183 °C; FT-IR (cm)−1: 2959, 2868, 1672.40, 1617, 1578; 1H NMR (500 MHz, CDCl3): δ 13.36 (s, 1H), 8.95 (d, J = 1.5 Hz, 1H), 8.56 (t, J = 1.6 Hz, 1H), 8.21 (dd, J = 11.0, 4.5 Hz, 2H), 7.94 (t, J = 8.5 Hz, 2H), 7.85 (d, J = 8.1 Hz, 1H), 7.58–7.50 (m, 1H), 7.47–7.41 (m, 1H), 7.34 (t, J = 7.8 Hz, 1H), 7.10 (dd, J = 8.1, 1.6 Hz, 1H), 3.04 (m, 1H), 1.35 (s, 3H), 1.34 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.0, 164.6, 154.0, 152.6, 140.6, 138.1, 137.4, 136.2, 133.1, 130.5, 130.0, 127.4, 126.7, 125.8, 122.8, 121.9, 121.5, 119.0, 117.4, 94.3, 34.5, 23.7 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C23H20IN2OS, 499.0336; found, 499.0335.
N-(2-(Benzo[d]thiazol-2-yl)-6-chlorophenyl)-4-iodobenzamide (3y)
(95.5 mg), 68% yield, white solid, mp: 178–180 °C; FT-IR (cm)−1: 3011, 2977, 1691, 1655, 1596; 1H NMR (500 MHz, CDCl3): δ 13.28 (s, 1H), 9.00 (d, J = 9.0 Hz, 1H), 8.51 (t, J = 1.6 Hz, 1H), 8.25 (d, J = 8.1 Hz, 1H), 8.18–8.15 (m, 1H), 7.97–7.93 (m, 2H), 7.86 (d, J = 2.4 Hz, 1H), 7.61–7.55 (m, 1H), 7.52–7.46 (m, 2H), 7.33 (t, J = 7.8 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 167.3, 164.5, 152.6, 140.9, 137.2, 136.7, 136.2, 133.2, 131.9, 130.5, 129.2, 128.5, 127.4, 127.0, 126.4, 123.2, 122.4, 121.6, 120.8, 94.3 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H13ClIN2OS, 490.9476; found, 490.9480.
N-(2-(Benzo[d]thiazol-2-yl)phenyl)-3,5-dichlorobenzamide (3z)
(79.2 mg), 60% yield, white solid, mp: 210–213 °C; FT-IR (cm)−1: 3071, 2917, 1677, 1620.74, 1547; 1H NMR (500 MHz, CDCl3): δ 13.46 (s, 1H), 9.00 (d, J = 8.1 Hz, 1H), 8.23 (d, J = 7.8 Hz, 1H), 8.15 (s, 2H), 8.00–7.88 (m, 2H), 7.57 (dd, J = 20.4, 11.6 Hz, 3H), 7.47 (t, J = 7.2 Hz, 1H), 7.23 (d, J = 7.5 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 168.6, 163.4, 152.8, 138.3, 137.8, 135.6, 135.1, 133.2, 132.3, 131.7, 129.9, 126.9, 126.4, 126.1, 123.9, 122.6, 121.5, 120.9, 119.5 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C20H12Cl2N2OS, 399.0120; found, 399.0121.
N-(2-(Benzo[d]thiazol-2-yl)-5-methylphenyl)-3,5-dichlorobenzamide (3ab)
(83.6 mg), 65% yield, white solid, mp: 170–174 °C, FT-IR (cm)−1: 2914, 1678, 1623, 1591, 1566; 1H NMR (500 MHz, CDCl3): δ 13.40 (s, 1H), 8.83 (s, 1H), 8.20 (d, J = 8.0 Hz, 1H), 8.14 (s, 2H), 7.92 (d, J = 7.8 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.59 (s, 1H), 7.55 (t, J = 7.6 Hz, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.04 (d, J = 7.9 Hz, 1H), 2.47 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.1, 163.5, 153.0, 143.4, 138.7, 137.9, 135.7, 133.1, 131.4, 130.2, 126.8, 126.4, 125.8, 124.8, 122.4, 121.4, 121.2, 117.1, 22.0 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C21H15Cl2N2OS, 413.0277; found, 413.0279.
N-(2-(Benzo[d]thiazol-2-yl)-5-isopropylphenyl)-3,5-dichlorobenzamide (3ac)
(81.8 mg), 67% yield, white solid, mp: 170–173 °C; FT-IR (cm)−1: 3081, 2965, 1679, 1619, 1577; 1H NMR (500 MHz, CDCl3): δ 13.43 (s, 1H), 8.92 (d, J = 1.7 Hz, 1H), 8.20 (d, J = 8.1 Hz, 1H), 8.15 (d, J = 1.9 Hz, 2H), 7.91 (d, J = 8.0 Hz, 1H), 7.84 (d, J = 8.1 Hz, 1H), 7.59 (t, J = 1.9 Hz, 1H), 7.57–7.51 (m, 1H), 7.46–7.41 (m, 1H), 7.10 (m, 1H), 3.04 (m, 1H), 1.35 (s, 3H), 1.34 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 168.9, 163.2, 153.8, 152.3, 138.1, 138.0, 135.2, 132.8, 131.7, 129.9, 126.8, 126.8, 125.8, 122.5, 122.1, 121.4, 118.9, 117.4, 34.6, 23.6 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C23H18Cl2N2OS, 441.0590; found, 441.0592.
N-(2-(Benzo[d]thiazol-2-yl)-5-isopropoxyphenyl)-3,5-dichlorobenzamide (3ad)
(78.5 mg), 66% yield, white solid, mp: 169–172 °C; FT-IR (cm)−1: 2978, 1682, 1625, 1583, 1478; 1H NMR (500 MHz, CDCl3): δ 13.53 (s, 1H), 8.63 (d, J = 2.4 Hz, 1H), 8.14 (s, 1H), 8.12 (d, J = 1.7 Hz, 2H), 7.86 (d, J = 7.9 Hz, 1H), 7.75 (d, J = 8.8 Hz, 1H), 7.57 (s, 1H), 7.50 (t, J = 7.4 Hz, 1H), 7.38 (t, J = 7.4 Hz, 1H), 6.70 (dd, J = 8.8, 2.5 Hz, 1H), 4.74 (m, 1H), 1.43 (s, 3H), 1.42 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 169.0, 163.4, 161.1, 152.6, 139.5, 138.2, 135.6, 132.7, 131.6, 131.3, 126.7, 126.4, 125.4, 122.1, 121.3, 112.4, 112.2, 106.1, 70.4, 29.7, 22.0 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C23H19Cl2N2O2S, 457.0539; found, 457.0533.
N-(2-(Benzo[d]thiazol-2-yl)phenyl)cinnamamide (3ae)
(70.0 mg), 60% yield, white solid, mp: 190–193 °C; FT-IR (cm)−1: 2986, 2563, 1675, 1586, 998; 1H NMR (500 MHz, CDCl3): δ 12.76 (s, 1H), 8.90 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.1 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.83 (d, J = 8.8 Hz, 1H), 7.75 (d, J = 15.7 Hz, 1H), 7.57 (d, J = 7.0 Hz, 2H), 7.50 (t, J = 7.7 Hz, 1H), 7.45 (t, J = 7.3 Hz, 1H), 7.42–7.32 (m, 4H), 7.12 (t, J = 7.6 Hz, 1H), 6.68 (d, J = 15.7 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 168.9, 165.0, 153.0, 141.6, 138.2, 135.1, 132.2, 130.0, 129.0, 128.0, 126.7, 125.9, 123.3, 122.8, 122.6, 121.6, 121.0, 119.2 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C22H17N2OS, 357.1056; found, 357.1050.
Competition Experiment Using Acyl Azides 2d and 2e
2-Phenyl benzthiazole 1a (1 equiv), 2d (1.5 equiv), 2e (1.5 equiv), [Ru(p-cymene)Cl2]2 (10 mol %), and AgSbF6 (20 mol %) in DCE (2.0 mL) were subjected to the standard reaction conditions described in the general procedure for 2 h to produce 3u and 3v in 10 and 30% yields, respectively.
Gram-Scale Reaction
To demonstrate the scalability of the regioselective amidation, a gram-scale synthesis was performed using 1b (1.5 g, 4.44 mmol) and 2a (6.66 mmol) under the optimized conditions, as discussed in the general procedure. The reaction proceeded efficiently to give the desired product 3b (1.19 g) in 78% yield.
General Procedure for Hydrolysis of N-(2-(Benzo[d]thiazol-2-yl)-5-methylphenyl)benzamide (4)
N-(2-(Benzo[d]thiazol-2-yl)-5-methylphenyl)benzamide 3b (0.2 mmol) was refluxed in methanol in the presence of 10 mol % KOH. After completion of the reaction (monitored by TLC), the residual solvent was removed under reduced pressure. Further reaction mixture was extracted using ethyl acetate and water. The combined organic layers were dried over sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on a silica gel using 20% ethyl acetate in hexane as the eluent to give the desired 2-(benzo[d]thiazol-2-yl)methylaniline (4) (39 mg), 80% yield, white solid, mp: 210–220 °C; FT-IR (cm)−1: 3400, 3320, 1677, 1578, 1300; 1H NMR (500 MHz, DMSO-d6): δ 8.07 (d, J = 7.9 Hz, 1H), 7.98 (d, J = 8.1 Hz, 1H), 7.51 (dd, J = 17.5, 7.7 Hz, 2H), 7.41 (t, J = 7.6 Hz, 1H), 7.28 (s, 2H), 6.70 (s, 1H), 6.50 (d, J = 8.0 Hz, 1H), 2.24 (s, 3H) ppm; 13C NMR (125 MHz, DMSO-d6): δ 169.3, 153.8, 148.1, 142.2, 132.7, 130.4, 126.8, 125.4, 122.3, 122.1, 117.6, 116.9, 111.5, 21.7 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C14H12N2S, 241.0794; found, 241.0794.
General Procedure for ortho-Amidation of 2-Phenyl-Substituted β-Carbolines (5a and b)
The 2-phenyl-substituted β-carbolines24a5 (1 equiv), benzoyl azides 2 (1.5 equiv), [Ru(p-cymene)Cl2]2 (10 mol %), AgSbF6 (20 mol %), and DCE (2 mL) were stirred at 80 °C for 12 h in a sealed tube. After completion of the reaction (monitored by TLC), the reaction mixture was quenched with water and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on a silica gel using 20% ethyl acetate in hexane as the eluent to give the desired product 5a and 5b.
N-(2-(9H-Pyrido[3,4-b]indol-1-yl)phenyl)benzamide (5a)
(67.68 mg), 65% yield white solid, mp: 222–225 °C; 1H NMR (500 MHz, DMSO-d6): δ 12.47 (s, 1H), 11.67 (s, 1H), 8.61 (d, J = 5.2 Hz, 1H), 8.57 (d, J = 8.2 Hz, 1H), 8.31 (d, J = 7.9 Hz, 1H), 8.26 (d, J = 5.2 Hz, 1H), 8.05 (dd, J = 7.7, 1.4 Hz, 1H), 7.90–7.86 (m, 2H), 7.67 (d, J = 8.2 Hz, 1H), 7.61–7.54 (m, 5H), 7.44 (td, J = 7.5, 1.2 Hz, 1H), 7.30 (t, J = 7.2 Hz, 1H) ppm; 13C NMR (125 MHz, DMSO-d6): δ 164.6, 141.8, 141.7, 137.4, 137.1, 135.0, 134.0, 132.4, 130.7, 130.2, 129.8, 129.4, 129.3, 129.1, 127.3, 126.47, 124.7, 122.4, 122.3, 122.2, 121.2, 120.3, 118.6, 115.1, 113.0 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C24H18N3O, 364.1444; found, 364.1457.
N-(2-(9H-Pyrido[3,4-b]indol-1-yl)-3-(trifluoromethyl)phenyl)benzamide (5b)
(60.92 mg), 63% yield, white solid mp: 226–229 °C; 1H NMR (500 MHz, DMSO-d6): δ 12.34 (s, 1H), 11.83 (s, 1H), 8.76 (d, J = 8.6 Hz, 1H), 8.63 (d, J = 5.2 Hz, 1H), 8.32 (dd, J = 12.4, 6.5 Hz, 2H), 8.26 (s, 1H), 7.95 (d, J = 8.5 Hz, 1H), 7.86 (d, J = 7.2 Hz, 2H), 7.68–7.53 (m, 5H), 7.31 (t, J = 7.4 Hz, 1H) ppm; 13C NMR (125 MHz, DMSO-d6): δ 165.04, 141.85, 140.47, 140.24, 137.67, 134.56, 132.7, 131.1, 129.4, 129.3, 127.5, 127.1, 127.0, 122.9, 122.4, 121.3, 120.4, 115.7, 112.9 ppm; HRMS (ESI-QTOF): m/z [M + H]+ calcd for C25H17N3F3O, 432.1318; found, 432.1323.
Acknowledgments
Authors are thankful to the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India, New Delhi, for the award of NIPER fellowship NIPER-H. Manuscript Communication no.: NIPER-H/2021/189.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05910.
Copies of 1H and 13C{1H} NMR spectra for the isolated final products 3a–3ae, 4, 5a, and 5b (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Borpatra P. J.; Deka B.; Deb M. L.; Baruah P. K. Recent advances in intramolecular C–O/C–N/C–S bond formation via C–H functionalization. Org. Chem. Front. 2019, 6, 3445–3489. 10.1039/c9qo00863b. [DOI] [Google Scholar]; b Ito M.; Kubo H.; Itani I.; Morimoto K.; Dohi T.; Kita Y. Organocatalytic C-H/C-H’ cross-biaryl-coupling: C-selective arylation of sulfonanilides with aromatic hydrocarbons. J. Am. Chem. Soc. 2013, 135, 14078–14081. 10.1021/ja407944p. [DOI] [PubMed] [Google Scholar]; c Xiao B.; Fu Y.; Xu J.; Gong T.-J.; Dai J.-J.; Yi J.; Liu L. Pd(II)-catalyzed C-H activation/aryl-aryl coupling of phenol esters. J. Am. Chem. Soc. 2010, 132, 468–469. 10.1021/ja909818n. [DOI] [PubMed] [Google Scholar]; d Zhu C.; Wang R.; Falck J. R. Amide-directed tandem C-C/C-N bond formation through C-H activation. Chem.–Asian J. 2012, 7, 1502–1514. 10.1002/asia.201200035. [DOI] [PubMed] [Google Scholar]; e Zhang X.; Liu B.; Shu X.; Gao Y.; Lv H.; Zhu J. Silver-mediated C–H activation: oxidative coupling/cyclization of N-arylimines and alkynes for the synthesis of quinolines. J. Org. Chem. 2012, 77, 501–510. 10.1021/jo202087j. [DOI] [PubMed] [Google Scholar]
- For reviews; a Song G.; Wang F.; Li X. C–C, C–O and C–N bond formation via rhodium(III)-catalyzed oxidative C–H activation. Chem. Soc. Rev. 2012, 41, 3651–3678. 10.1039/c2cs15281a. [DOI] [PubMed] [Google Scholar]; b Shen C.; Zhang P.; Sun Q.; Bai S.; Hor T. S. A.; Liu X. Recent advances in C–S bond formation via C-H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. 10.1039/c4cs00239c. [DOI] [PubMed] [Google Scholar]; c Rao W.-H.; Shi B.-F. Recent advances in copper-mediated chelation-assisted functionalization of unactivated C–H bonds. Org. Chem. Front. 2016, 3, 1028–1047. 10.1039/c6qo00156d. [DOI] [Google Scholar]
- For review; a Sambiagio C.; Schönbauer D.; Blieck R.; Dao-Huy T.; Pototschnig G.; Schaaf P.; Wiesinger T.; Zia M. F.; Wencel-Delord J.; Besset T.; Maes B. U. W.; Schnürch M. A comprehensive overview of directing groups applied in metal-catalyzed C–H functionalization chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. 10.1039/c8cs00201k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rasheed O. K.; Sun B. Advances in development of C-H activation/functionalization using a catalytic directing group. ChemistrySelect 2018, 3, 5689–5708. 10.1002/slct.201801097. [DOI] [Google Scholar]
- Sunny S.; John S. E.; Shankaraiah N. Exploration of C-H activation strategies in construction of functionalized 2-aryl benzoazoles: a decisive review. Asian J. Org. Chem. 2021, 10, 1986–2009. 10.1002/ajoc.202100297. [DOI] [Google Scholar]
- a Leong C.-O.; Gaskell M.; Martin E. A.; Heydon R. T.; Farmer P. B.; Bibby M. C.; Cooper P. A.; Double J. A.; Bradshaw T. D.; Stevens M. F. G. Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. Br. J. Cancer 2003, 88, 470–477. 10.1038/sj.bjc.6600719. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bradshaw T.; Wrigley S.; Shi D.-F.; Schultz R.; Paull K.; Stevens M. 2- (4-Aminophenyl)benzothiazoles: novel agents with selective profiles of in vitro antitumour activity. Br. J. Cancer 1998, 77, 745–752. 10.1038/bjc.1998.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Arasappan A.; Bennett F.; Girijavallabhan V.; Huang Y.; Huelgas R.; Alvarez C.; Chen L.; Gavalas S.; Kim S.-H.; Kosinski A.; Pinto P.; Rizvi R.; Rossman R.; Shankar B.; Tong L.; Velazquez F.; Venkatraman S.; Verma V. A.; Kozlowski J.; Shih N.-Y.; Piwinski J. J.; MacCoss M.; Kwong C. D.; Clark J. L.; Fowler A. T.; Geng F.; Kezar H. S.; Roychowdhury A.; Reynolds R. C.; Maddry J. A.; Ananthan S.; Secrist J. A.; Li C.; Chase R.; Curry S.; Huang H.-C.; Tong X.; Njoroge F. G. 5-Benzothiazole substituted pyrimidine derivatives as HCV replication (replicase) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3229–3234. 10.1016/j.bmcl.2012.03.036. [DOI] [PubMed] [Google Scholar]; b Girijavallabhan V. M.; Alvarez C.; Bennett F.; Chen L.; Gavalas S.; Huang Y.; Kim S.-H.; Kosinski A.; Pinto P.; Rizvi R.; Rossman R.; Shankar B.; Tong L.; Velazquez F.; Venkatraman S.; Verma V. A.; Kozlowski J.; Shih N.-Y.; Piwinski J. J.; MacCoss M.; Kwong C. D.; Bansal N.; Clark J. L.; Fowler A. T.; Kezar H. S.; Valiyaveettil J.; Reynolds R. C.; Maddry J. A.; Ananthan S.; Secrist J. A.; Li C.; Chase R.; Curry S.; Huang H.-C.; Tong X.; Njoroge F. G.; Arasappan A. Synthesis and SAR of pyridothiazole substituted pyrimidine derived HCV replication inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5652–5657. 10.1016/j.bmcl.2012.06.099. [DOI] [PubMed] [Google Scholar]
- a Ono M.; Hayashi S.; Kimura H.; Kawashima H.; Nakayama M.; Saji H. Push-pull benzothiazole derivatives as probes for detecting β-amyloid plaques in Alzheimer’s brains. Bioorg. Med. Chem. 2009, 17, 7002–7007. 10.1016/j.bmc.2009.08.032. [DOI] [PubMed] [Google Scholar]; b Zheng M.-Q.; Yin D.-Z.; Qiao J.-P.; Zhang L.; Wang Y.-X. Syntheses and evaluation of fluorinated benzothiazole anilines as potential tracers for β-amyloid plaques in Alzheimer’s disease. J. Fluorine Chem. 2008, 129, 210–216. 10.1016/j.jfluchem.2007.11.005. [DOI] [Google Scholar]
- a Kaur H.; Kumar S.; Singh I.; Saxena K. K.; Kumar A. Synthesis, characterization and biological activity of various substituted benzothiazole derivatives. Dig. J. Nanomater. Biostructures 2010, 5, 67–76. [Google Scholar]; b Shafi S.; Mahboob Alam M.; Mulakayala N.; Mulakayala C.; Vanaja G.; Kalle A. M.; Pallu R.; Alam M. S. Synthesis of novel 2-mercapto benzothiazole and 1,2,3-triazole based bis-heterocycles: their anti-inflammatory and antinociceptive activities. Eur. J. Med. Chem. 2012, 49, 324–333. 10.1016/j.ejmech.2012.01.032. [DOI] [PubMed] [Google Scholar]
- a Tzanova T.; Gerova M.; Petrov O.; Karaivanova M.; Bagrel D. Synthesis and antioxidant potential of novel synthetic benzophenone analogues. Eur. J. Med. Chem. 2009, 44, 2724–2730. 10.1016/j.ejmech.2008.09.010. [DOI] [PubMed] [Google Scholar]; b Cressier D.; Prouillac C.; Hernandez P.; Amourette C.; Diserbo M.; Lion C.; Rima G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. 10.1016/j.bmc.2009.05.039. [DOI] [PubMed] [Google Scholar]
- a Duarte L. G. T. A.; Germino J. C.; Berbigier J. F.; Barboza C. A.; Faleiros M. M.; de Alencar Simoni D.; Galante M. T.; de Holanda M. S.; Rodembusch F. S.; Atvars T. D. Z. White-light generation from all-solution-processed OLEDs using a benzothiazole–salophen derivative reactive to the ESIPT process. Phys. Chem. Chem. Phys. 2019, 21, 1172–1182. 10.1039/c8cp06485g. [DOI] [PubMed] [Google Scholar]; b Lu F.; Hu R.; Wang S.; Guo X.; Yang G. Luminescent properties of benzothiazole derivatives and their application in white light emission. RSC Adv. 2017, 7, 4196–4202. 10.1039/c6ra25369e. [DOI] [Google Scholar]
- a Dhaka G.; Singh J.; Kaur N. Benzothiazole possessing reversible and reusable selective chemosensor for fluoride detection based on inhibition of excited state intramolecular proton transfer. Inorg. Chim. Acta 2016, 450, 380–385. 10.1016/j.ica.2016.06.032. [DOI] [Google Scholar]; b Tang L.; Dai X.; Zhong K.; Wen X.; Wu D. A Phenylbenzothiazole derived fluorescent sensor for Zn(II) recognition in aqueous solution through “Turn-On” excited-state intramolecular protontransfer Emission. J. Fluoresc. 2014, 24, 1487–1493. 10.1007/s10895-014-1434-8. [DOI] [PubMed] [Google Scholar]
- a Li D.; Hu W.; Wang J.; Zhang Q.; Cao X.-M.; Ma X.; Tian H. White-light emission from a single organic compound with unique self-folded conformation and multistimuli responsiveness. Chem. Sci. 2018, 9, 5709–5715. 10.1039/c8sc01915k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wu Z.; Ma D. Recent advances in white organic light emitting diodes. Mater. Sci. Eng. 2016, 107, 1–42. 10.1016/j.mser.2016.06.001. [DOI] [Google Scholar]
- a Yan X.; Cook T. R.; Wang P.; Huang F.; Stang P. J. Highly emissive platinum(II) metallacages. Nat. Chem. 2015, 7, 342–348. 10.1038/nchem.2201. [DOI] [PubMed] [Google Scholar]; b Park M. J.; Son Y. H.; Yang H. I.; Kim S. K.; Lampande R.; Kwon J. H. Optical design and optimization of highly efficient sunlight-like three-stacked warm white organic light emitting diodes. ACS Photonics 2018, 5, 655–662. 10.1021/acsphotonics.7b01379. [DOI] [Google Scholar]
- a Jadala C.; Sathish M.; Reddy T. S.; Reddy V. G.; Tokala R.; Bhargava S. K.; Shankaraiah N.; Nagesh N.; Kamal A. Synthesis and in vitro cytotoxicity evaluation of β-carboline-combretastatin carboxamides as apoptosis inducing agents: DNA intercalation and topoisomerase-II inhibition. Bioorg. Med. Chem. 2019, 27, 3285–3298. 10.1016/j.bmc.2019.06.007. [DOI] [PubMed] [Google Scholar]; b Jadala C.; Sathish M.; Anchi P.; Tokala R.; Lakshmi U. J.; Reddy V. G.; Shankaraiah N.; Godugu C.; Kamal A. Synthesis of combretastatin-A4 carboxamides mimicking with sulfonyl piperazines by a molecular hybridization approach: In vitro cytotoxicity evaluation and tubulin polymerization inhibition. ChemMedChem 2019, 14, 2052–2060. 10.1002/cmdc.201900541. [DOI] [PubMed] [Google Scholar]
- a Park Y.; Kim Y.; Chang S. Transition metal-catalyzed C–H amination: Scope, mechanism, and applications. Chem. Rev. 2017, 117, 9247–9301. 10.1021/acs.chemrev.6b00644. [DOI] [PubMed] [Google Scholar]; b Wippich J.; Truchan N.; Bach T. Rhodium-catalyzed N-tert-butoxycarbonyl (boc) amination by directed C-H bond activation. Adv. Synth. Catal. 2016, 358, 2083–2087. 10.1002/adsc.201600410. [DOI] [Google Scholar]; c Ali M. A.; Yao X.; Li G.; Lu H. Rhodium-catalyzed selective mono and diamination of arenes with single directing site “on water”. Org. Lett. 2016, 18, 1386–1389. 10.1021/acs.orglett.6b00318. [DOI] [PubMed] [Google Scholar]; d Feng Y.-L.; Shi B.-F. Recent Advances in Base Metal (Copper, Cobalt and Nickel)-Catalyzed Directed C—H Amination. Chin. J. Org. Chem. 2021, 41, 3753–3770. 10.6023/cjoc202104004. [DOI] [Google Scholar]
- a Yu S.; Wan B.; Li X. Rhodium(III)-catalyzed C-H activation and amidation of arenes using N-arenesulfonated imides as amidating reagents. Org. Lett. 2013, 15, 3706–3709. 10.1021/ol401569u. [DOI] [PubMed] [Google Scholar]; b Thirunavukkarasu V. S.; Kozhushkov S. I.; Ackermann L. C–H nitrogenation and oxygenation by ruthenium catalysis. Chem. Commun. 2014, 50, 29–39. 10.1039/c3cc47028h. [DOI] [PubMed] [Google Scholar]
- a Huang D.-Y.; Yao Q.-J.; Zhang S.; Xu X.-T.; Zhang K.; Shi B.-F. Amide-directed cobalt(III)-catalyzed C–H amidation of ferrocenes. Org. Lett. 2019, 21, 951–954. 10.1021/acs.orglett.8b03938. [DOI] [PubMed] [Google Scholar]; b Samanta S.; Mondal S.; Ghosh D.; Hajra A. Rhodium-catalyzed directed C–H amidation of imidazoheterocycles with dioxazolones. Org. Lett. 2019, 21, 4905–4909. 10.1021/acs.orglett.9b01832. [DOI] [PubMed] [Google Scholar]; c Liu D.; Ding Q.; Fu Y.; Song Z.; Peng Y. Rh-catalyzed C–H amidation of 2-arylbenzo[d]thiazoles: an approach to single organic molecule white light emitters in the solid state. Org. Lett. 2019, 21, 2523–2527. 10.1021/acs.orglett.9b00115. [DOI] [PubMed] [Google Scholar]; d Ghosh P.; Samanta S.; Hajra A. Rhodium(III)-catalyzed ortho-C–H amidation of 2-arylindazoles with dioxazolone as an amidating reagent. Org. Biomol. Chem. 2020, 18, 1728–1732. 10.1039/c9ob02756d. [DOI] [PubMed] [Google Scholar]
- a Shang M.; Sun S.-Z.; Dai H.-X.; Yu J.-Q. Cu(II)-Mediated C–H amidation and amination of arenes: exceptional compatibility with heterocycles. J. Am. Chem. Soc. 2014, 136, 3354–3357. 10.1021/ja412880r. [DOI] [PubMed] [Google Scholar]; b Song C.; Wang T.; Yu T.; Cui D.-M.; Zhang C. 2,4-Diamino-1,3,5-triazine-enabled Cu-catalyzed direct sulfonamidation of aromatic C-H bonds. Org. Biomol. Chem. 2017, 15, 7212–7217. 10.1039/c7ob01872j. [DOI] [PubMed] [Google Scholar]; c Ju G.; Li G.; Qian G.; Zhang J.; Zhao Y. Rh(III)-Catalyzed C–H Amidation of Arenes with N-Methoxyamide as an Amidating Reagent. Org. Lett. 2019, 21, 7333–7336. 10.1021/acs.orglett.9b02625. [DOI] [PubMed] [Google Scholar]
- a Ng K.-H.; Ng F.-N.; Yu W.-Y. A convenient synthesis of anthranilic acids by Pd-catalyzed direct intermolecular ortho-C–H amidation of benzoic acids. Chem. Commun. 2012, 48, 11680–11682. 10.1039/c2cc36502b. [DOI] [PubMed] [Google Scholar]; b Zhou B.; Du J.; Yang Y.; Feng H.; Li Y. Rh(III)-catalyzed C–H amidation with N-hydroxycarbamates: a new entry to N-carbamate-protected arylamines. Org. Lett. 2014, 16, 592–595. 10.1021/ol403477w. [DOI] [PubMed] [Google Scholar]
- a Hu X.-H.; Yang X.-F.; Loh T.-P. Chelation-assisted Rhodium-catalyzed direct amidation with amidobenziodoxolones: C(sp2)–H, C(sp3)–H, and late-stage functionalizations. ACS Catal. 2016, 6, 5930–5934. 10.1021/acscatal.6b02015. [DOI] [Google Scholar]; b Kim H.; Park G.; Park J.; Chang S. A facile access to primary alkylamines and anilines via Ir(III)-catalyzed C-H amidation by using azidoformates. ACS Catal. 2016, 6, 5922–5929. 10.1021/acscatal.6b01869. [DOI] [Google Scholar]; c Zhou X.; Luo P.; Long L.; Ouyang M.; Sang X.; Ding Q. Ru-catalyzed direct C-H amidation of 2-arylbenzo[d]thiazoles with sulfonyl azides. Tetrahedron 2014, 70, 6742–6748. 10.1016/j.tet.2014.07.076. [DOI] [Google Scholar]
- a De Sarkar S.; Ackermann L. Ruthenium(II)-catalyzed C-H activation with isocyanates: a versatile route to phthalimides. Chem.—Eur. J. 2014, 20, 13932. 10.1002/chem.201404261. [DOI] [PubMed] [Google Scholar]; b Hummel J. R.; Ellman J. A. Cobalt(III)-catalyzed C–H bond amidation with isocyanates. Org. Lett. 2015, 17, 2400–2403. 10.1021/acs.orglett.5b00910. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Li J.; Ackermann L. Cobalt(III)-catalyzed aryl and alkenyl C-H aminocarbonylation with isocyanates and acyl azides. Angew. Chem., Int. Ed. 2015, 54, 8551. 10.1002/anie.201501926. [DOI] [PubMed] [Google Scholar]
- Shin K.; Ryu J.; Chang S. Orthogonal reactivity of acyl azides in C–H activation: dichotomy between C–C and C–N amidations based on catalyst systems. Org. Lett. 2014, 16, 2022–2025. 10.1021/ol500602b. [DOI] [PubMed] [Google Scholar]
- a Liu H.; Zhou Y.; Yan X.; Chen C.; Liu Q.; Xi C. Copper-mediated amidation of alkenyl zirconocenes with acyl azides:formation of enamides. Org. Lett. 2013, 15, 5174–5177. 10.1021/ol402212g. [DOI] [PubMed] [Google Scholar]; b Ghosh T.; Maity P.; Ranu B. C. Cu(OAc)2 promoted ortho C(sp2)–H amidation of 8-aminoquinoline benzamide with acyl azide: selective formation of aroyl or acetyl amide based on catalyst loading. J. Org. Chem. 2018, 83, 11758–11767. 10.1021/acs.joc.8b01654. [DOI] [PubMed] [Google Scholar]; c Ryu J.; Kwak J.; Shin K.; Lee D.; Chang S. Ir(III)-catalyzed mild C–H amidation of arenes and alkenes: an efficient usage of acyl azides as the nitrogen source. J. Am. Chem. Soc. 2013, 135, 12861–12868. 10.1021/ja406383h. [DOI] [PubMed] [Google Scholar]; d Li W.-H.; Dong L. Synthesis of 7-azaindole amidated derivatives: an efficient usage of acyl azides as the nitrogen source. Adv. Synth. Catal. 2018, 360, 1104–1110. 10.1002/adsc.201701336. [DOI] [Google Scholar]; e Banerjee S.; De P. B.; Pradhan S.; Shah T. A.; Punniyamurthy T. Ru(II)-catalyzed regioselective C-N bond formation of indolines and carbazole with acyl azides. Eur. J. Org. Chem. 2019, 2019, 1677–1684. 10.1002/ejoc.201801829. [DOI] [PubMed] [Google Scholar]; f Shah T. A.; De P. B.; Pradhan S.; Banerjee S.; Punniyamurthy T. Cp*Co(III)-catalyzed regioselective C2-amidation of indoles using acyl azides. J. Org. Chem. 2019, 84, 16278–16285. 10.1021/acs.joc.9b02244. [DOI] [PubMed] [Google Scholar]
- a Tokala R.; Bora D.; Sana S.; Nachtigall F. M.; Santos L. S.; Shankaraiah N. Ru(II)-catalyzed regioselective hydroxymethylation of β-carbolines and isoquinolines via C–H functionalization: probing the mechanism by online ESI-MS/MS screening. J. Org. Chem. 2019, 84, 5504–5513. 10.1021/acs.joc.9b00454. [DOI] [PubMed] [Google Scholar]; b Bora D.; Tokala R.; John S. E.; Prasanth B.; Shankaraiah N. β-Carboline directed regioselective hydroxylation by employing Cu(OAc)2 and mechanistic investigation by ESI-MS. Org. Biomol. Chem. 2020, 18, 2307–2311. 10.1039/d0ob00250j. [DOI] [PubMed] [Google Scholar]
- a Hu R.; Li X.; Tong Y.; Dazhuang M.; Qiang P.; Jiang Z.; Gan H.; Han S. Catalyst-Free synthesis of 2-Arylbenzothiazoles in an air/DMSO Oxidant System. Synlett 2016, 27, 1387–1390. 10.1055/s-0035-1561575. [DOI] [Google Scholar]; b Che X.; Jiang J.; Xiao F.; Huang H.; Deng G.-J. Assembly of 2-arylbenzothiazoles through three-component oxidative annulation under transition-metal-free conditions. Org. Lett. 2017, 19, 4576–4579. 10.1021/acs.orglett.7b02168. [DOI] [PubMed] [Google Scholar]; c Weekes A. A.; Dix M. C.; Bagley M. C.; Westwell A. D. Rapid and convenient thermal or microwave-assisted synthesis of substituted 2-phenylbenzothiazoles. Synth. Commun. 2010, 40, 3027–3032. 10.1080/00397910903353739. [DOI] [Google Scholar]; d Metin B. Acyl Azides: Versatile compounds in the synthesis of various heterocycles. Synthesis 2018, 50, 1373–1401. 10.1055/s-0036-1589527. [DOI] [Google Scholar]
- Liu J.; Mandel S.; Hadad C. M.; Platz M. S. A comparison of acetyl- and methoxycarbonylnitrenes by computational methods and a laser flash photolysis study of benzoylnitrene. J. Org. Chem. 2004, 69, 8583–8593. 10.1021/jo048433y. [DOI] [PubMed] [Google Scholar]
- a Dhaka G.; Singh J.; Kaur N. Benzothiazole based chemosensors having appended amino group(s): Selective binding of Hg2+ ions by three related receptors. Inorg. Chim. Acta 2017, 462, 152–157. 10.1016/j.ica.2017.03.030. [DOI] [Google Scholar]; b Bozkurt S.; Halay E. Synthesis, application and AIE properties of novel fluorescent tetraoxocalix[2]arene[2]triazine: The detection of a hazardous anion, cyanate. Tetrahedron 2020, 76, 131647. 10.1016/j.tet.2020.131647. [DOI] [Google Scholar]
- Bora D.; Reddy Dannarm S.; Elza John S.; Sana S.; Sonti R.; Shankaraiah N. Regioselective ortho-sulfonamidation: Exploration of intrinsic directing property of β-Carbolines and their photophysical studies. Asian J. Org. Chem. 2021, 10, 3384. 10.1002/ajoc.202100602. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.