Abstract
Organoids are three-dimensional (3D) self-renewing and self-organizing clusters of cells that imitate an organ’s structure and function, making them an important tool in various fields ranging from regenerative medicine to drug discovery. Organoids can be developed ex vivo by isolating adult stem cells from an organ-specific tissue (e.g., intestine, brain, and lung) and allowing the stem cells to grow and differentiate in an appropriate growth media with some structural support elements. A 3D extracellular matrix (ECM) hydrogel, a network of highly hydrophilic cross-linked polymer chains, provides essential support and cues for ex vivo organoid growth. Commercially available hydrogel matrices (for example, Matrigel and collagen) are primarily derived from animal tissues. Notably, these animal-derived hydrogel matrices are not suitable for controlled modifications and pose risks of immunogen and pathogen transfer, thus diminishing their clinical application. These limitations of animal-derived hydrogel matrices can, however, be overcome using synthetic hydrogel matrices based on polymers such as polyethylene glycol, nanocellulose, alginate, hyaluronic acid, and polylactic-co-glycolic acid. This review highlights some of the current approaches and advantages of developing synthetic ECM-mimic hydrogels, focusing primarily on intestinal organoid culture.
1. Introduction
Recently, three-dimensional (3D) ex vivo organoid culture has garnered considerable attention due to the limitations of 2D cell culture in mimicking the exact physiological intricacy of tissues and the higher costs coupled with time constraints of animal studies.1 Depending on the tissue of origin, organoids containing different cell types can be developed from adult pluripotent stem cells when cultured with an appropriate stem cell growth medium. These organoids exhibit a nearly identical physiological tissue organization and function to their in vivo tissue counterparts. Notably, by modulating media composition, the differentiation of the organoids can be manipulated in order to obtain a specific cell lineage of interest. In concert, these features promote the use of organoids as a model to study infectious diseases, host–pathogen interaction, genetic diseases, gene function, regenerative medicine, developmental biology, and pharmaceutical research.2
In addition to appropriate stem cell growth media, ex vivo organoid culture also requires an extracellular matrix (ECM) hydrogel, which provides biochemical cues and structural support to the organoids during their development. The ECM is a complex network of hydrated macromolecular proteins and sugars. Using a commercially available ECM, Clevers’ laboratory introduced first a murine intestinal organoid culture from leucine-rich repeat-containing G-protein-coupled receptor 5 positive (Lgr5+) intestinal stem cells (ISCs) originating from intestinal crypts.3 Most commercially available ECMs have been engineered using animal-tissue-derived matrices based on the notion that tissue-derived biomaterial could possibly facilitate the integration of host tissue during organoid culture. Nearly every tissue type has been decellularized and subsequently processed for reuse as tissue-derived ECM protein implants and scaffolds. The ultimate goal is to promote healing and integration with host tissues while avoiding rejection to the foreign body. Currently, there is no consensus for defining potential ECM sources or their compositions, most complementary to decellularized biomaterials in order to establish this standard. Moreover, detrimental artifacts including but not limited to residual ECM-derived DNA, endotoxins, and inflammatory proteins may interfere with organoid growth and reproducibility, highlighting the limitations of commercially available animal-derived ECMs.4
The development of a synthetic ECM hydrogel, designed with physicochemical properties in a controlled manner, would limit variability between subsequent lot preparations and ideally lead to standardization of organoid cultures by reduction or elimination of detrimental artifacts, which result from current methods for organoid culture. Synthetic ECMs are conducive to physical or biological manipulation and have led to chemically defined, highly tunable, and reproducible alternatives. Moreover, the development of synthetic ECMs is more time and economically efficient in comparison to animal-derived ECMs. Additionally, various properties (water content, porosity, rigidity, and stiffness of the synthetic polymers) can be controlled as required. Functional chemical groups such as bioactive molecules can be introduced into the synthetic ECM due to the abundance of available groups in the polymer. Moreover, polymer-based synthetic ECMs will be less likely to trigger immunogenic or pathogenic responses in comparison to animal-derived Matrigel.5
2. Results
2.1. Anatomy of the Native Intestine
The epithelial lining found in the small intestine is organized into invaginations called crypts and finger-like protrusions called villi (Figure 1).3 Crypts contain intestinal stem cells that differentiate to form transiently amplifying (TA) cells, which migrate upward toward the villi, giving rise to enterocytes, goblet cells, and enteroendocrine cells; on the contrary, Paneth cells, after being differentiated from stem cells, migrate from the stem cell zone downward toward the base of crypts (Figure 1).3 Enterocytes play an important role in the digestion of food and tolerating microbial antigens found in the intestine, whereas goblet cells are responsible for mucus production. Enteroendocrine cells are vital sensors of gut microbiota and/or microbial metabolites, whereas Paneth cells regulate microbial density in the small intestine and protect the nearby stem cells.6
Figure 1.
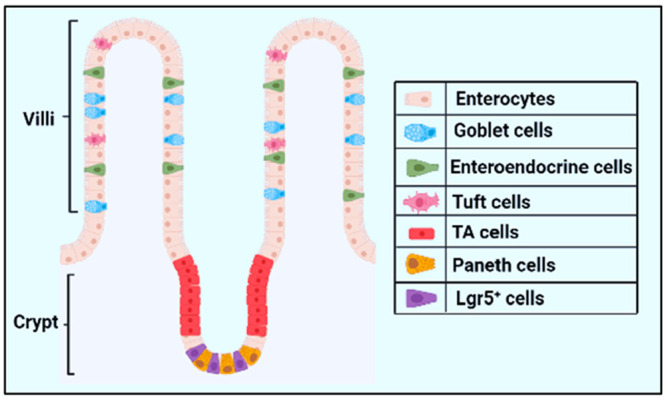
Structure of intestinal epithelial layer. The epithelial layer is organized into crypt and villi, where a single villus is surrounded by multiple crypts. The crypts generate a continuous stream of cells of various types that differentiate and migrate upward toward the tip of the villus (e.g., transiently amplifying cells, enterocytes, and goblet cells) as well as at the base of crypts (e.g., Paneth cells). Created with the BioRender.com.
Crypts contain two main types of intestinal stem cells (ISCs). Rapidly proliferating radiosensitive columnar Lgr5-expressing stem cells reside at the base of crypts, and quiescent or slowly proliferating radio-resistant Bmi1 (B-cell-specific Moloney murine leukemia virus integration site 1)-expressing stem cells that are located at positions 3–6 above the crypt base (Figure 1). The Lgr5-expressing stem cells are intercalated with Paneth cells. Lgr5+ cells are primarily responsible for maintaining intestinal homeostasis under a steady-state condition, whereas the Bmi1-expressing stem cells trigger intestinal recovery upon intestinal damage.7
2.2. Intestinal Organoids
The intestinal organoid is a complex structure resulting from the growth of cell masses, often derived from a single Lgr5+ intestinal stem cell. This stem cell can self-renew and behave like a mature cell to form the intestinal epithelial tissue, which has the maximum self-renewing capacity in the mammalian body, holding a 4–5 day turnover in mice.8
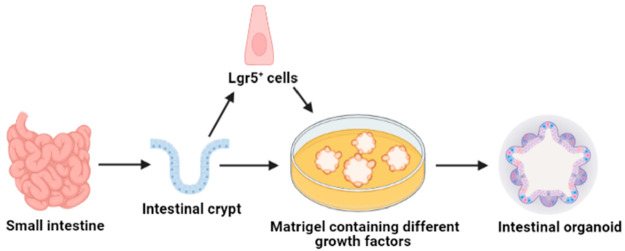
Intestinal organoids grow when ISCs are cultured in the presence of ECM hydrogel with an appropriate stem cell growth media containing a cocktail of epidermal growth factor (EGF), R-spondin-1, and Wnt3A (Figure 2).9 Under aforementioned favorable conditions, the ISCs are eventually organized into organoids and enteroids. Organoids are derived from intestinal pluripotent stem cells and contain mesenchymal cells, whereas enteroids are derived from intestinal stem cells and contain epithelial cells. These organoids are also termed “mini-guts” due to their complexity and ability to exhibit vital functions of the normal intestine.
Figure 2.
Schematic representation of human intestinal organoid culture from a Lgr5+ intestinal stem cell or from a intestinal crypt isolated from the small intestine. Created with the BioRender.com.
The major components of the ECM include fibronectin, laminin, integrin, and collagen. Notably, ECM is a dynamic structure and can undergo remodeling. Due to its complexity and biological origins, it has variable compositions, but it is expensive to procure and is not conducive to all desired laboratory modifications. Most importantly, ECM poses the threat of immunogenic and/or pathogenic reactions, making it undesirable for organoid expansion for large-scale clinical utilization.10 In this regard, the development of novel synthetic ECM holds great promise for the field of tissue engineering.
2.3. Animal-Derived ECMs and Intestinal Organoid Culture
Animal tissues are made up of cells and extracellular spaces that are filled by the ECM. The matrix is composed of a variety of proteins such as collagen, elastin, fibronectin, laminin, as well as polysaccharides such as glycosaminoglycans and mucopolysaccharides.11 The negatively charged glycosaminoglycans consisting of repeating disaccharide units remain covalently linked to proteins in the form of proteoglycans that are secreted by cells. Animal-derived commercially available Matrigel and collagen have been used as ECM for intestinal organoid growth for the past several decades. The Matrigel is a gelatinous protein mixture secreted from mouse Engelbreth–Holm–Swarm (EHS) sarcoma cells containing laminin, entactin, collagen IV, and proteoglycan with growth factors to facilitate organoid development.11 The first establishment of 3D intestinal organoids cultured from Lgr5+ stem cells was described to form crypt–villus structures.11 These organoids were grown in Matrigel in the presence of R-spondin, a Wnt agonist, EGF (epidermal growth factor), and Noggin, a BMP (bone morphogenetic protein) inhibitor.11
Animal-derived ECMs not only promote intestinal organoid growth but also can be used as a valuable tool for in vivo organoid delivery, which may offer a strategy to heal the damaged gut in clinics. However, Matrigel’s intricate composition and variable efficacy between the batches reduce the reproducibility of organoid growth. Collagen is another common animal-derived ECM mainly found in connective tissue of higher organisms.12a These ECMs have been used in various organoid growth experimentation, including intestinal organoids.12b The limitations of extra expense combined with lower performance in comparison to Matrigel in the culture of organoids is a driving factor to develop an alternative support structure for intestinal organoid growth.12b
2.4. Synthetic ECMs and Intestinal Organoid Culture
Synthetic polymers are inert in nature, thus eliminating the risk of eliciting immunogenic or pathogenic reactions posed by animal-derived polymers. Synthetic polymers also contain cell-adhesive sites which can be easily degraded by cellular protease enzymes separating organoids from the polymers as needed. In addition, synthetic polymers can easily be functionalized in order to maximize organoid growth. Finally, it is economical in comparison to animal-derived ECMs. The common synthetic polymers for organoid culture are polyethylene glycol (PEG), nanocellulose, and other polymer matrices such as polylactic-co-glycolic acid (PLGA) and alginate.13a The hydrogel formation using these polymers mainly occurs based on the principle that polymers are cross-linked with suitable cross-linkers. The cross-linking process is the stabilization of polymers with covalent or noncovalent bonds, which leads to the multidimensional extension of polymer chains, thereby forming a stable network-like structure which differs from the native polymers. The cross-linking can be done both by physical cross-linking or chemical cross-linking methods.13b
2.4.1. PEG-Based Hydrogels
In the late 1970s, various laboratories started using PEG-based hydrogel matrices for cell culture and intestinal organoid formation.14 PEG is a petroleum-derived thermoresponsive injectable polymer that becomes hydrogel when it is cross-linked or heated to body temperature. This soft and hydrated polyethylene compound mimics the basic physical properties of Matrigel without contributing any biochemical signals. A comprehensive study by Gjorevski et al. showed that PEG can only promote ISC survival and proliferation when ECM components (fibronectin, laminin-111, collagen IV, hyaluronic acid, and perlecan) are added to the PEG, suggesting that biochemical cues play a crucial role in organoid growth.15 Gjorevski et al. also reported that PEG functionalized with arginylglycylaspartic acid (RGD) and laminin-111-based matrices are well-defined, tunable, and reproducible for mouse intestinal organoid growth.16 Cruz-Acuña et al. reported in vitro growth and the expansion of human intestinal organoids on a four-armed maleimide-terminated PEG macromer, which exhibited high cytocompatibility.17 It has been hypothesized that hydrogels are prepared by enzymatically cross-linking between four-armed PEG precursors with glutamine and lysine-containing peptides and could be used as injection vehicles to deliver stem cells into injured intestinal mucosa for grafting human intestinal organoids and repairing the damaged gut.16,18 Additionally, the authors modified the hydrogel with an RGD peptide, a smaller fibronectin derivative. This peptide was chosen for modification due to its ability to support the growth of epithelial cells and cyst formation in the hydrogel. The addition of RGD peptides increases the cell adhesion to the matrix, increases the interaction between the biomolecule and the matrices, and lowers cell apoptosis. The RGD peptide also decreases the stiffness and enhances elastic properties of any hydrogel matrices, and thus it promotes organoid growth.17 In addition, the effects of adding collagen IV, HA, and perlecan in PEG matrices were studied for the ability to enhance ISC survival and proliferation.17
The procedure for preparing PEG-based synthetic hydrogel was described elsewhere.16 Briefly, two forms of PEG (vinyl-sulfonated PEG and acrylate-functionalized PEG) was reacted with transglutaminase FXIIIa peptides in the presence of triethanolamine. Then the product was dialyzed against ultrapure water and lyophilized. The different concentrations of stock solutions of these samples were prepared in deionized water. The hydrogel was formed by mixing thrombin and FXIIIa with 13.33% w/v stock in the presence of Tris-buffered saline (pH 7.6) and 50 mM of CaCl2. Then the sample was functionalized with different proteins such as fibronectin, laminin-111, collagen IV, and perlecan. PEG precursors are functionalized with proteins simply by combining various concentrations of proteins into the sample while incubating it at 37 °C for approximately 15 min.16
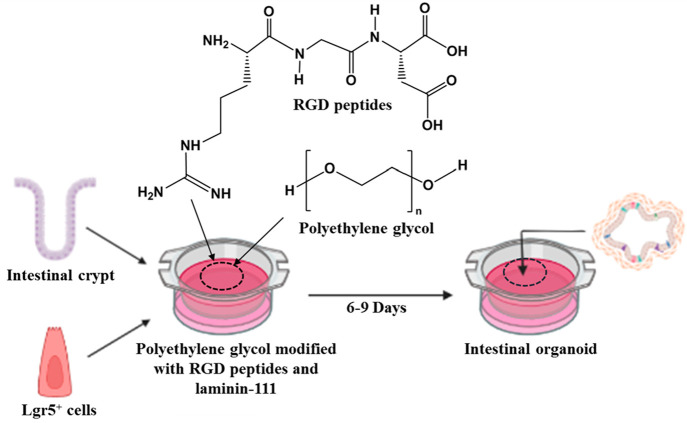
The formation of this organoid based on functionalized PEG with RGD and laminin-111 is schematically shown in Figure 3. First, mouse intestinal crypts or Lgr5+ ISCs are added into the functionalized PEG at 37 °C in the presence of organoid growth media. Hydrogel formation for the ISC expansion or organoid formation takes 2–3 h, and the fully matured organoid is formed within 6–9 days.16
Figure 3.
Schematic representation of intestinal organoid growth from a Lgr5+ ISC or a crypt using PEG-based hydrogel functionalized with arginyl-glycyl-aspartic acid and laminin-111. Created with the BioRender.com.
2.4.2. Nanocellulose-Based Hydrogels
Nanocellulose hydrogels are highly hydrated, soft, and porous. They contain a significant amount of −OH (hydroxyl) groups and hence possess excellent binding capacity, hydrophilicity, and biocompatibility. Considering each of these beneficial attributes, it is feasible to speculate that nanocellulose could be an excellent synthetic ECM for intestinal organoid growth. Nanocellulose is divided into mainly three types based on source and characteristics: cellulose nanocrystals, cellulose nanofibers, and bacterial nanocellulose. The diameter of nanocellulose is approximately 100 nm, and it is a few micrometers in length.
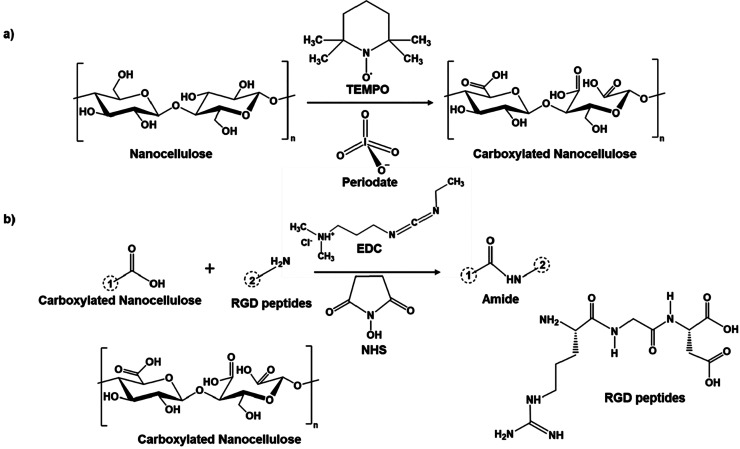
Curvello et al. reported utilization of plant-based nanocellulose for the growth of mouse small intestinal organoids.19 The carboxylated nanocellulose was functionalized with cell adhesive peptides and cross-linked with cations such as Ca2+ and Mg2+ to form hydrogel matrices for a 3D cell culture. The procedure for the nanocellulose-based ECM hydrogel for organoid growth has been described elsewhere.19,20 Briefly, the carboxylation or oxidation of nanocellulose was done by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) in the presence of periodate. Then it was functionalized with fibronectin or RGD peptides in the presence of 1-ethyl-3,3-diethylaminopropylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS). RGD peptides are amino-terminated peptides that contain arginine, glycine, and aspartate amino acids. Thus, the formed RGD-functionalized TEMPO-periodate-oxidized nanocellulose hydrogel was neutralized with sodium hydroxide and maintained at pH 7. The reaction scheme for the carboxylation of nanocellulose and its functionalization with RGD peptides is shown in Figure 4.
Figure 4.
Steps involved in the formation of nanocellulose carboxylation and their further modification. (a) Carboxylation of nanocellulose by TEMPO and periodate oxidation. (b) Modification of carboxylated nanocellulose with RGD peptides in the presence of EDC and NHS.
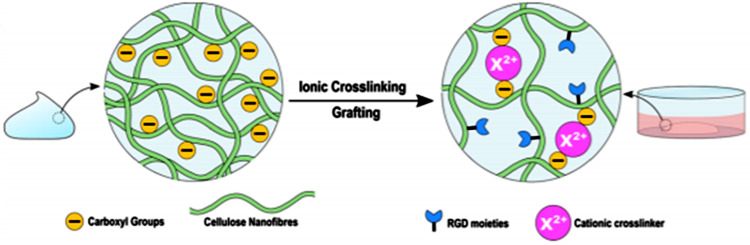
For intestinal organoid culture, well plates are initially coated with a divalent cationic solution such as CaCl2 and MgCl2. Nanocellulose hydrogel is then layered, ionically cross-linked with cations (Ca2+ and Mg2+), and finally, crypts are embedded in the hydrogel. The grafting of carboxylation of nanocellulose with RGD peptides and ionic cross-linking in the presence of Ca2+ and Mg2+ is shown in Figure 5.
Figure 5.
Schematic representation of cationic cross-linking of RGD peptides functionalized carboxylated nanocellulose with Ca2+ and Mg2+ ions. Reprinted from ref (19). Copyright 2021 American Chemical Society.
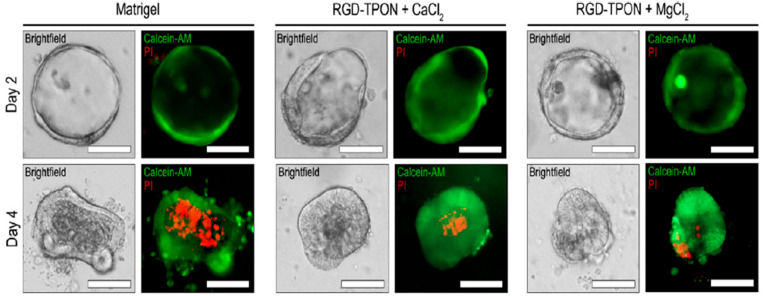
Curvello et al. used this hydrogel matrix as an ECM for an intestinal organoid experimentation. After 4 days, organoids grown in an ionic cross-linked nanocellulose hydrogel were stained with calcein-AM (acetoxymethyl derivative of calcein) and continued to grow until a fully matured mouse intestinal organoid developed, as shown in Figure 6.19
Figure 6.
Photomicrographs showing intestinal organoids grown in Matrigel and RGD-functionalized carboxylated nanocellulose (RGD-TPON) hydrogel cross-linked with CaCl2 and MgCl2. Cystic glands were grown on day 2 (top panels), and organoids began to develop on day 4 (bottom panels) as shown by bright-field and fluorescent microscopy. Cystic glands and organoids were stained with calcein-AM (green) and propidium iodide (PI, red) to readily distinguish living from dead cells. Viable cells produce a strong green fluorescence resulting from the conversion of calcein-AM to calcein, whereas nonviable cells exhibit strong red fluorescence due to the presence of propidium iodide. Reprinted from ref (19). Copyright 2021 American Chemical Society.
The organoids developed in a nanocellulose-based hydrogel produce similar results compared to organoids grown in Matrigel, mainly due to similar mechanical properties of functionalized hydrogel and animal-derived matrices or Matrigel. The storage modulus of Matrigel is higher than that of RGD-TON hydrogel, but it is less than that of the RGD-TPON hydrogel. The light transmittance of Matrigel is between the RGD-TPON and RGD-TON. The transmittance of RGD-TPON, RGD-TON, and Matrigel is 100, 90, and 70%, respectively. The intestinal organoids grown in RGD-TPON within 4 days of culture show single prominent budding, whereas RGD-TON exhibits smaller budding.19 Therefore, nanocellulose-based hydrogel can provide the ideal microenvironment (biochemical and physical properties) for intestinal organoid growth and recovery by enhancing the adhesion between the cellulosic surface and intestinal organoid.
Curvello’s team reported the development of intestinal organoids on nanocellulose blended with collagen.20 The nanocellulose blended with collagen is thermoresponsive in nature. Nanocellulose improves collagen’s poor mechanical properties and provides a workable, affordable, and sustainable matrix for organoid growth due to the fact that the sol–gel transition and the viscoelastic profile of nanocellulose grafted with collagen type I is quite similar to those of the standard animal-based matrices.20 Therefore, a hybrid matrix comprising nanocellulose grafted with collagen could be a viable alternative for organoid culture. Essentially, the efficacy of functionalized nanocellulose in facilitating human intestinal organoid growth is yet to be established.
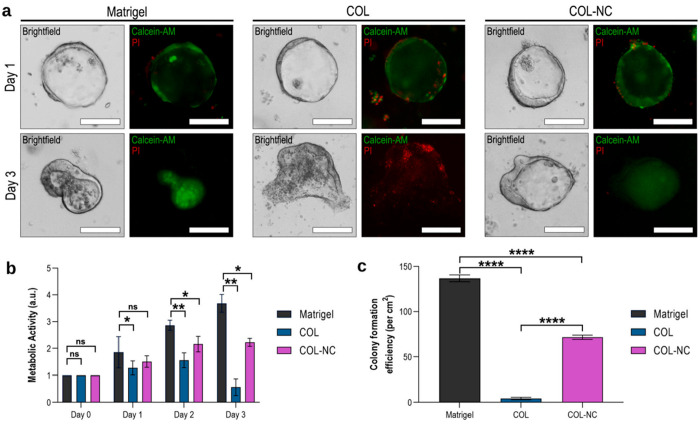
A study on the comparative efficacy of Matrigel, type I collagen (COL), and collagen–nanocellulose (COL-NC) hydrogels by Curvello showed that COL is less conducive for promoting mouse intestinal organoid growth than Matrigel and COL-NC (Figure 7).20 The authors demonstrate that compared to Matrigel or COL-NC, COL hydrogel induces apoptosis, resulting in loss of structural integrity of organoids by day 3 (Figure 7a). Moreover, this study showed that COL hydrogel results in a significant decrease in metabolic activity in organoids, as compared to that with Matrigel or COL-NC, which could be due to enhanced apoptosis caused by COL hydrogel (Figure 7b). Finally, the authors exhibit that COL hydrogel does not support the colony-forming ability of the intestinal organoid (Figure 7c). These data clearly demonstrate that the combination of collagen with nanocellulose facilitates intestinal organoid growth.
Figure 7.
Photomicrographs showing growth of intestinal organoid in various matrices. (a) Intestinal organoid grown in Matrigel, collagen (COL), and collagen–nanocellulose (COL-NC) hydrogels, as shown by bright-field and fluorescent microscopy. Organoids were stained with calcein-AM (green) and propidium iodide (PI, red) to distinguish cell viability. Live cells produce a strong green fluorescence resulting from the conversion of calcein-AM to calcein, whereas dead cells have a strong red fluorescence due to the presence of propidium iodide. (b) Metabolic activity detected in the organoids embedded in the 3 hydrogels. (c) Colony formation efficiency in three hydrogels. Reprinted with permission from ref (20). Copyright 2021 Elsevier.
2.4.3. Other Polymer Matrices
2.4.3.1. Alginate
Alginate is an FDA-approved polysaccharide derived from algae which consists of 1,4-β-d-mannuronic acid (M) and 1,4-α-l-guluronic acid (G) monomers with a homogeneous (poly-G, poly-M) or heterogeneous (MG) block composition. It is cost-effective and biocompatible and has gelation and viscoelastic properties.
Capeling et al. hypothesized that nonadhesive alginate-based hydrogel supports the growth of intestinal organoids.21 The gelation of alginate can be adjusted by ionically cross-linking with calcium chloride solution. Because unmodified alginate does not possess cell-adhesive properties, it is not an efficient matrix for providing mechanical support optimum for intestinal organoid growth. It was found that human intestinal organoids grown in alginate-based gel resemble the organoids grown in Matrigel within 28 days.21 The colony-forming ability of alginate is similar to that of PEG, but when combined with Matrigel, alginate yields more favorable results than PEG. In addition, the degree of maturation of human intestinal organoids (such as epithelial patterning, mesenchyme formation, polarization, and fully differentiated intestinal cell types, including enterocytes, goblet cells, and enteroendocrine cells) in alginate and Matrigel is almost equal.21
2.4.3.2. Hyaluronic Acid
Hyaluronic acid (HA) is another significant matrix for intestinal organoid development.22a HA is a fully defined, biodegradable designer matrix comprising N-acetylglucosamine and glucuronic acid. Hunt et al. were productive in their effort of culturing human intestinal organoids from a single dissociated cell on an HA-based hydrogel.22b Briefly, the HA-based hydrogel was prepared by a formation of a hydrazone bond intermediate of the chemically modified hyaluronate and modified benzaldehyde biopolymers. The organoid formation at 10 days on this HA gel was close in comparison to that with Matrigel.22b
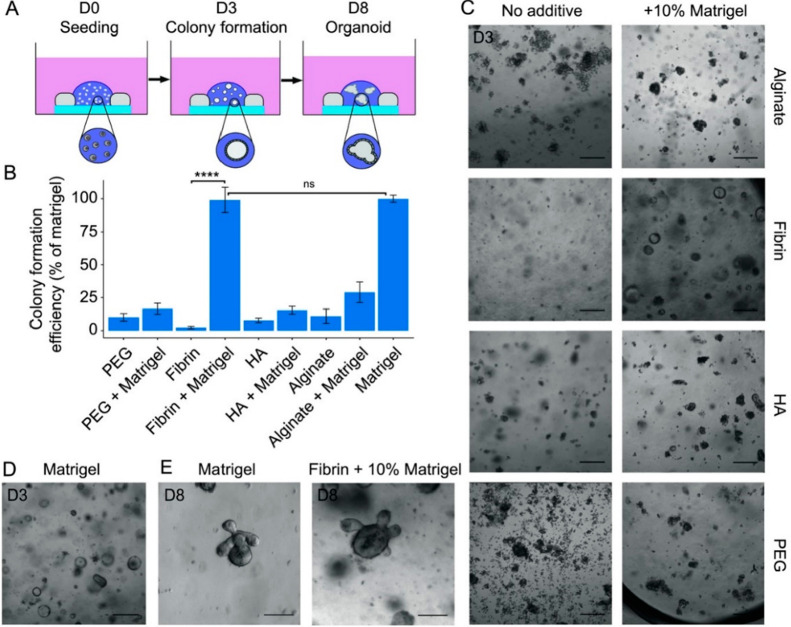
Figure 8 illustrates the development of mouse intestinal organoids on PEG, PEG + Matrigel, fibrin, fibrin + Matrigel, HA, HA + Matrigel, alginate, alginate + Matrigel, and Matrigel. As the name implies, fibrin is a fibrous, nonglobular protein formed by thrombin-mediated cleavage of fibrinogen. The colony formation efficiency of individual HA is less efficient in comparison with that of PEG. However, when combined with Matrigel, the efficiency appears virtually equivalent. Individually, fibrin protein exhibits low colony formation efficiency, yet when combined with Matrigel, its efficiency is equal to that of Matrigel. The organoid formation at 8 days on the fibrin-functionalized Matrigel is similar to the formation of an organoid grown in Matrigel for an equal time. Therefore, fibrin-functionalized Matrigel forms the better matrices for mouse intestinal organoid growth.23
Figure 8.
(A) Mouse intestinal stem cells. (B) Quantification of colony-forming ability of mouse intestinal stem cells on different hydrogels. (C,D) Bright-field image of organoid on hydrogels and Matrigel. (E) Organoid in fibrin + 10% fibrinogen. Reprinted with permission from ref (23). Copyright 2018 Wiley.
2.4.3.3. Polylactic-co-Glycolic Acid
Polylactic-co-glycolic acid is the FDA-approved biodegradable polymer which is a copolymer of polylactic acid and polyglycolic acid. PLGA contains −COOH (carboxylic acid) and −OH functional groups, which help to make it hydrophilic, biodegradable, and easily functionalized with proteins and peptides. These studies suggest that PLGA shows favorable potential to support organoid growth.
Several laboratories have shown the effects of PLGA and have demonstrated that Matrigel containing PLGA nanoparticles does not adversely affect organoid growth.24 These nanoparticles can easily be digested in the intestinal lumen and also facilitate drug delivery to inflammatory regions of organoids.24
The dependency on animal models for biological studies can be expensive and time-consuming. Considering the time and ease of fabrications, new approaches such as studies on organoids, which mimic the structure and function of the actual organs, could be highly useful in generating a reliable biological response and designing novel drug molecules. For organoid growth, ECMs play an important role. However, most of the commercially available ECMs are derived from animal tissue. The use of these animal-derived hydrogels in matrices is not suitable for controlled modifications as needed. In addition, animal-derived hydrogels pose risks of immunogen and pathogen transfer, which diminishes their clinical applicability. The current review has highlighted the potentials and advantages of using different types of synthetic ECMs, specifically for intestinal organoid growth. The polymers that these different synthetic ECMs were based on are listed in Table 1. An important aspect of using ECM for organoid culture is its hydrophilicity and ability to provide biochemical cues and structural support for organoid growth. Table 1 gives a quick visual of each of the polymer’s hydrophilicity, biocompatibility, and potential to be modified as necessary due to the types of functional groups available in each polymer.
Table 1. Polymers Discussed for the Fabrication of Synthetic ECMs with Their Respective Functional Groups.
| polymer name | functional groups |
|---|---|
| polyethylene glycol | –OH |
| nanocellulose | –OH |
| alginate | –OH |
| –COOH | |
| hyaluronic acid | –OH |
| –COOH | |
| –CONHR | |
| Polylactic-co-glycolic acid | –OH |
| –COOH |
Hydrogels based on synthetic polymer matrices such as PEG and nanocellulose were shown to play an important role in intestinal organoid growth. Scientists already proved that intestinal organoids, including human intestinal organoids, can be successfully grown on the proteins or peptides functionalized with PEG and nanocellulose-based hydrogel despite there being some challenges in delivering the organoid. As PEG, nanocellulose, and other polymers contain several −OH groups, they need to be oxidized in order to be functionalized with the RGD peptides or other fibronectin proteins. The functionalization is done in the presence of EDC and NHS. Then, they should ionically react with some salts such as calcium chloride or magnesium chloride. Hydrogel is formed, and this can be used as an ECM or culture medium for organoid formation. Based on the above result, matured organoids can be developed after 8–10 days in PEG and nanocellulose-based matrices.
Hydrogels based on other polymers such as alginate, HA, and PLGA were also shown to play a crucial role in intestinal organoid growth. These biocompatible, biodegradable, and tunable polymers can be easily functionalized with peptides and proteins, making them ideal components for organoid growth matrices. Researchers have already proved that intestinal organoids, including mouse and human intestinal organoids, can be successfully grown in the alginate, HA, and PLGA-based hydrogel matrices. A summary of these synthetic matrices based on the polymers discussed above can be found in Table 2.
Table 2. Summary of the Synthetic ECMs Based on the Polymers.
| polymer basis | fabrication of the ECMs |
|---|---|
| polyethylene glycol | functionalized with arginylglycylaspartic acid and laminin-111 |
| nanocellulose | carboxylated and functionalized with cell-adhesive peptides and then cross-linked with cations (such as Ca2+ and Mg2+) |
| blended with type I collagen as a hybrid matrix | |
| alginate | gelated by cross-linking with Ca2+ |
| hyaluronic acid | formation of hydrazone bond between modified HA and modified benzaldehyde biopolymers |
| polylactic-co-glycolic acid | PLGA nanoparticles blended with Matrigel as a hybrid matrix |
The stiffness and elastic moduli of the synthetic hydrogel matrices also play a crucial role in the developmental trajectory in organoid formation. Soft hydrogel matrices increase the efficiency of the formation of large sized cysts.25 Therefore, hydrogel matrices having low stiffness and low elastic moduli, such as those based on the polymers discussed throughout, favor the formation of intestinal organoids.
Despite the fact that synthetic ECMs are an alternative to the Matrigel for intestinal organoid formation, they have some challenges related to cell culture, regenerative medicine, and overall organoid formation. Some synthetic polymer matrices show poor biocompatibility and nonbiodegradability. The one-size-fits-all approach cannot apply in the development of synthetic scaffolds. It is rather time-consuming and difficult to recapitulate the complexity of native tissues. Additionally, creating scaffolds by the easy way for all end-users to employ is another challenge. Despite there being some challenges associated with synthetic ECM, with more research and development, hydrogel based on the above-mentioned polymeric materials and others could play an important role in the formation and delivery of human intestinal organoids.
3. Conclusion
Polymer matrices and hydrogels based on polymers have been developed to mimic the composition and structure of native intestinal tissue in 3D in vitro models. They are used as a tissue-like matrix to support the cell functions, cell behaviors, and tissue morphogenesis necessary for developing tissue and organ-like structures. The formation of organoids using synthetic hydrogels based on polymeric materials including PEG, nanocellulose, alginate, HA, and PLGA is easy and economical compared to animal-derived matrices like Matrigel and collagen. In the case of growing mouse intestinal organoids, using the polymer-based hydrogels has shown results of organoid viability and metabolic activity that are comparable and competitive to those with the traditional Matrigel. These results have changed the face of commercial biological research. Continued research in this field to develop and improve the fabrication of synthetic and alternative ECM will help pave the way toward scaffolds for organoid growth that are superior to the commercially used animal-derived matrices. Likewise, as more research is performed, further knowledge will be gained about the fundamental mechanisms involved in ECM fabrication and organoid culture, creating possibilities for the growth of better quality organoids than before. The advancements discussed here and those to come will permit the development of a model that represents the complexity of the intestine and will create the opportunity for real progress in developing new therapeutic treatments for intestinal diseases.
Acknowledgments
A.G. and R.P. would like to thank AR INBRE Grant No. GR013696, which was supported by the National Institute of General Medical Sciences (NIGMS) under Grant No. 2P20GM103429-19. This study was also partly supported by P20GM109005 from NIGMS (R.P.). The authors would like to thank BioRender.com for the software used to create the figures.
Biographies

Professor Anindya Ghosh received his Ph.D. degree in 2004 from Carnegie Mellon University, Pittsburgh, USA. His research interest focuses on the development and applications of novel homogeneous and heterogeneous catalysts. In addition, his research focuses on developing novel nanomaterials for different biomedical applications. Dr. Ghosh is a professor and chair of the Chemistry Department, University of Arkansas at Little Rock, Little Rock, USA.

Professor Rupak Pathak obtained his Ph.D. degree in 2007 from Kalyani University, India. His research interests focus on the role of stromal cells in intestinal repair following injury, specifically under radiation injury. Dr. Pathak is an Assistant Professor in the Department of Pharmaceutical Sciences, University of Arkansas for Medical Sciences (UAMS), Little Rock, USA.

Humendra Poudel received his master’s degree in 2014 from Tribhuvan University, Nepal. He is a doctoral student at the University of Arkansas at Little Rock, USA, working under the supervision of Dr. Anindya Ghosh. His research interest is to synthesize novel materials and their application in biomedical research.

Karie Sanford received her undergraduate degree in 2020 from the University of Arkansas at Little Rock, USA. She is a Ph.D. student at UA Little Rock working in Dr. Anindya Ghosh’s group. Her research interest is to synthesize novel catalysts for small molecule activations and green chemistry research.

Peter K. Szwedo is a Ph.D. student at the University of Arkansas at Little Rock, USA, working under the supervision of Dr. Anindya Ghosh. His research interest lies in developing pincer catalysts and their application in C–N and C–C coupling reactions.
Author Contributions
A.G. and R.P. conceived the idea of developing the manuscript. H.P. wrote the manuscript and collected all relevant references. K.S., P.K.S., A.G., and R.P. all contributed in writing and reviewed the manuscript. All authors have approved the final version of the manuscript.
The authors declare no competing financial interest.
References
- Kapałczyńska M.; Kolenda T.; Przybyła W.; Zaja̧czkowska M.; Teresiak A.; Filas V.; Ibbs M.; Bliźniak R.; Łuczewski Ł.; Lamperska K. 2D and 3D Cell Cultures – a Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14 (4), 910–919. 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.; Duan X.; Yang L.; Nilsson-Payant B. E.; Wang P.; Duan F.; Tang X.; Yaron T. M.; Zhang T.; Uhl S.; et al. Identification of SARS-CoV-2 Inhibitors Using Lung and Colonic Organoids. Nature 2021, 589, 270–275. 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. The Intestinal Crypt, a Prototype Stem Cell Compartment. Cell 2013, 154 (2), 274–284. 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Urbischek M.; Rannikmae H.; Foets T.; Ravn K.; Hyvonen M.; de la Roche M. Organoid Culture Media Containing Growth Factors of Defined Cellular Activity. Sci. Rep. 2019, 9, 6193. 10.1038/s41598-019-42604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenbrey E. A.; Murphy W. L. Synthetic Alternatives to Matrigel. Nat. Rev. Mater. 2020, 5 (7), 539–551. 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble F. M.; Reimann F. Function and Mechanisms of Enteroendocrine Cells and Gut Hormones in Metabolism. Nat. Rev. Endocrinol. 2019, 15 (4), 226–237. 10.1038/s41574-019-0168-8. [DOI] [PubMed] [Google Scholar]
- Yan K. S.; Chia L. A.; Li X.; Ootani A.; Su J.; Lee J. Y.; Su N.; Luo Y.; Heilshorn S. C.; Amieva M. R.; et al. The Intestinal Stem Cell Markers Bmi1 and Lgr5 Identify Two Functionally Distinct Populations. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (2), 466–471. 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N.; Tsukamoto Y.; Kujala P.; Peters P. J.; Clevers H. Intestinal Epithelial Organoids Fuse to Form Self-Organizing Tubes in Floating Collagen Gels. Development 2017, 144 (6), 1107–1112. 10.1242/dev.143933. [DOI] [PubMed] [Google Scholar]
- Kretzschmar K.; Clevers H. Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev. Cell 2016, 38 (6), 590–600. 10.1016/j.devcel.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Vukicevic S.; Kleinman H. K.; Luyten F. P.; Roberts A. B.; Roche N. S.; Reddi A. H. Identification of Multiple Active Growth Factors in Basement Membrane Matrigel Suggests Caution in Interpretation of Cellular Activity Related to Extracellular Matrix Components. Exp. Cell Res. 1992, 202 (1), 1–8. 10.1016/0014-4827(92)90397-Q. [DOI] [PubMed] [Google Scholar]
- Corrò C.; Novellasdemunt L.; Li V. S. W. A Brief History of Organoids. Am. J. Physiol. - Cell Physiol. 2020, 319 (1), C151–C165. 10.1152/ajpcell.00120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Babu I. R.; Ganesh K. N. Enhanced Triple Helix Stability of Collagen Peptides with 4R-Aminoprolyl (Amp) Residues: Relative Roles of Electrostatic and Hydrogen Bonding Effects. J. Am. Chem. Soc. 2001, 123 (9), 2079–2080. 10.1021/ja002165d. [DOI] [PubMed] [Google Scholar]; b Jee J. H.; Lee D. H.; Ko J.; Hahn S.; Jeong S. Y.; Kim H. K.; Park E.; Choi S. Y.; Jeong S.; Lee J. W.; et al. Development of Collagen-Based 3D Matrix for Gastrointestinal Tract-Derived Organoid Culture. Stem Cells Int. 2019, 2019, 1–15. 10.1155/2019/8472712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Magno V.; Meinhardt A.; Werner C. Polymer Hydrogels to Guide Organotypic and Organoid Cultures. Adv. Funct. Mater. 2020, 30, 2000097. 10.1002/adfm.202000097. [DOI] [Google Scholar]; b Lu L.; Yuan S.; Wang J.; Shen Y.; Deng S.; Xie L.; Yang Q. The Formation of Mechanism of Hydrogels. Curr. Stem Cell Res. Ther. 2018, 13 (7), 490–496. 10.2174/1574888X12666170612102706. [DOI] [PubMed] [Google Scholar]
- Davis F. F. The Origin of Pegnology. Adv. Drug Delivery Rev. 2002, 54 (4), 457–458. 10.1016/S0169-409X(02)00021-2. [DOI] [PubMed] [Google Scholar]
- Gjorevski N.; Sachs N.; Manfrin A.; Giger S.; Bragina M. E.; Ordóñez-Morán P.; Clevers H.; Lutolf M. P. Designer Matrices for Intestinal Stem Cell and Organoid Culture. Nature 2016, 539 (7630), 560–564. 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- Gjorevski N.; Lutolf M. P. Synthesis and Characterization of Well-defined Hydrogel Matrices and their Application to Intestinal Stem Cell and Organoid Culture. Nat. Protoc. 2017, 12 (11), 2263–2274. 10.1038/nprot.2017.095. [DOI] [PubMed] [Google Scholar]
- Cruz-Acuña R.; Quirós M.; Huang S.; Siuda D.; Spence J. R.; Nusrat A.; García A. J. PEG-4MAL Hydrogels for Human Organoid Generation, Culture, and in Vivo Delivery. Nat. Protoc. 2018, 13 (9), 2102–2119. 10.1038/s41596-018-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuña R.; Quirós M.; Farkas A. E.; Dedhia P. H.; Huang S.; Siuda D.; García-Hernández V.; Miller A. J.; Spence J. R.; Nusrat A.; et al. Synthetic Hydrogels for Human Intestinal Organoid Generation and Colonic Wound Repair. Nat. Cell Biol. 2017, 19 (11), 1326–1335. 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curvello R.; Garnier G. Cationic Cross-Linked Nanocellulose-Based Matrices for the Growth and Recovery of Intestinal Organoids. Biomacromolecules 2021, 22 (2), 701–709. 10.1021/acs.biomac.0c01510. [DOI] [PubMed] [Google Scholar]
- Curvello R.; Alves D.; Abud H. E.; Garnier G. A. Thermo-Responsive Collagen-Nanocellulose Hydrogel for the Growth of Intestinal Organoids. Mater. Sci. Eng., C 2021, 124, 112051. 10.1016/j.msec.2021.112051. [DOI] [PubMed] [Google Scholar]
- Capeling M. M.; Czerwinski M.; Huang S.; Tsai Y. H.; Wu A.; Nagy M. S.; Juliar B.; Sundaram N.; Song Y.; Han W. M.; et al. Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep. 2019, 12 (2), 381–394. 10.1016/j.stemcr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bayer I. S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25 (11), 2649. 10.3390/molecules25112649. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hunt D. R.; Klett K. C.; Mascharak S.; Wang H.; Gong D.; Lou J.; Li X.; Cai P. C.; Suhar R. A.; Co J. Y.; et al. Engineered Matrices Enable the Culture of Human Patient-Derived Intestinal Organoids. Adv. Sci. 2021, 8, 2004705. 10.1002/advs.202004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broguiere N.; Isenmann L.; Hirt C.; Ringel T.; Placzek S.; Cavalli E.; Ringnalda F.; Villiger L.; Zullig R.; Lehmann R.; Rogler G.; Heim M. H.; Schuler J.; Zenobi-Wong M.; Schwank G. Growth of Epithelial Organoids in a Defined Hydrogel. Adv. Mater. 2018, 30 (43), 1801621. 10.1002/adma.201801621. [DOI] [PubMed] [Google Scholar]
- Davoudi Z.; Peroutka-Bigus N.; Bellaire B.; Wannemuehler M.; Barrett T. A.; Narasimhan B.; Wang Q. Intestinal Organoids Containing Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of Inflammatory Bowel Diseases. J. Biomed. Mater. Res., Part A 2018, 106 (4), 876–886. 10.1002/jbm.a.36305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfak A.; Bouzhir L.; Gontran E.; Minier N.; Dupuis-Williams P.; Gobaa S. Biophysical Control of Bile Duct Epithelial Morphogenesis in Natural and Synthetic Scaffolds. Front. Bioeng. Biotechnol. 2019, 7, 417. 10.3389/fbioe.2019.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]