Abstract
Fragile X syndrome (FXS), the most common inherited cause of intellectual disability, learning disability, and autism spectrum disorder, is associated with an increased prevalence of certain medical conditions including seizures. The goal of this study was to better understand seizures in individuals with FXS using the Fragile X Online Registry with Accessible Research Database, a multisite observational study initiated in 2012 involving FXS clinics in the Fragile X Clinic and Research Consortium. Seizure data were available for 1,607 participants, mostly male (77%) and white (74.5%). The overall prevalence of at least one seizure was 12%, with this rate being significantly higher in males than females (13.7 vs. 6.2%, p < 0.001). As compared to individuals with FXS without seizures, those with seizures were more likely to have autism spectrum disorder, current sleep apnea, later acquisition of expressive language, more severe intellectual disability, hyperactivity, irritability, and stereotyped movements. The mean age of seizure onset was 6.4 (SD 6.1) years of age with the great majority (>80%) having onset of seizures which was before 10. For those with epilepsy, about half (52%) had seizures for more than 3 years. This group was found to have greater cognitive and language impairment, but not behavioral disruptions, compared with those with seizures for <3 years. Antiepileptic drugs were more often used in males (60.6%) than females (34.8%), and females more often required more than one medication. The most commonly used anticonvulsants were oxcarbazepine, valproic acid, lamotrigine, and levetiracetam. The current study is the largest and first longitudinal study ever conducted to describe seizures in FXS. Overall, this study confirms previous reports of seizures in FXS and extends previous findings by further defining the cognitive and behavioral phenotype of those with epilepsy in FXS. Future studies should further investigate the natural history of seizures in FXS and the characteristics of seizures in FXS in adulthood.
Keywords: Fragile X syndrome, seizures, epilepsy, longitudinal, autism spectrum disorder
Introduction
Fragile X syndrome (FXS) is the most common known inherited cause of intellectual disability, learning disability, and autism spectrum disorder (ASD), with an estimated prevalence of about 1/4,000 (1). Individuals with FXS display variable physical features such as large ears, long face, macrocephaly, macroorchidism, and variable levels of functioning, with a fairly stereotyped cognitive profile and behavioral features characterized by hyperactivity, anxiety, and socialization difficulties. Additionally, certain medical problems are more common in FXS, such as strabismus, otitis media, gastroesophageal reflux, loose stools, sleep apnea, and seizures (2).
FXS results from a trinucleotide repeat (CGG) expansion mutation of >200 repeats (full mutation) in the promoter of FMR1 (fragile X mental retardation 1) gene (3) which leads to transcriptional silencing of FMR1 and loss or significant reduction of expression of the gene product, FMRP (fragile X mental retardation protein) (4). FMRP is an RNA-binding protein that appears to function as a dendritic translational repressor that modulates receptor-activated dendritic protein synthesis and regulates multiple ion channels (5–7). In the absence of FMRP in the Fmr1 knockout (k/o) mouse model of FXS, there is immature dendritic spine morphology and region- and cell-dependent deficits in synaptic plasticity (8). Accordingly, these synaptic abnormalities result in abnormal epileptiform discharges (9) and a high frequency of audiogenic seizures in the Fmr1 k/o mouse (10), presumably modeling the increased risk of seizures in humans with FXS.
Past reports have identified seizures in 4.4%-40% of males with FXS (2, 11–19) with a lower frequency of 4.4%-18% in larger cohorts with less referral bias (2, 13, 15–19). Many children with FXS also have abnormal electroencephalograms (EEGs) without overt epileptic seizures (15, 17, 19), frequently with a pattern of centrotemporal spikes (similar to benign focal epilepsy of childhood) (13, 15, 19, 20). Types of seizures reported in FXS vary widely, and clinical and EEG findings consistent with Panayiotopoulos syndrome, a benign autonomic epilepsy syndrome, have also been seen in FXS (21). Complex partial (focal onset with impaired awareness) seizures were reported as most common in FXS in several series (13, 15, 18), although simple partial (focal onset without impaired awareness) and generalized tonic-clonic seizures occur often, and status epilepticus has also been observed (22). Seizures are reported to be easily controlled in most cases (15, 17, 18) and resolve during childhood in the majority of individuals with FXS, although in a study of hospital encounters for adolescents and adults with FXS, seizures represented an identifiable reason for emergency room presentation and hospitalization in FXS (23). Occasional patients with FXS and intractable seizures due to mesial temporal sclerosis have been described, suggesting a secondary etiology amenable to surgical management (24). A small case series describing three females with FXS and severe developmental impairment and medically refractory focal epilepsy also suggests that severe epilepsy in FXS may signal a secondary genetic condition (25).
Studies investigating the association of seizures with other disease features in FXS include a small cohort which showed a trend toward an increased rate of seizures in individuals who were also diagnosed with ASD (26) and a second small case series of 11 patients which suggested an association between epilepsy and attention problems (27). In the largest investigation of seizures in FXS involving 1,394 individuals assessed by a survey and 352 individuals assessed in clinic (19), 14% of males and 6% of females reported seizures. Seizures tended to be infrequent and more often focal, had onset between 4 and 10 years of age, were generally easily treated, and were associated with ASD, but not academic achievement. A clinic-based study of 135 patients with FXS seen in clinic (17) suggested that many patients described spells such as staring, but most of these were without EEG correlate, and only 4.4% of the cohort were actually diagnosed with seizures, emphasizing the importance of clinical confirmation of seizure diagnoses in persons with FXS. A large study of ASD in FXS based on data from 547 participants with FXS in the Fragile X Online Registry and Accessible Research Database (FORWARD) natural history study (28) showed that having a diagnosis of ASD was associated with an approximately three-fold increased risk of seizures both in younger children and in adolescent/young adults (29).
The Fragile X Clinic and Research Consortium (FXCRC) initial database collected data from 260 participants with FXS from 9 FXS Clinics from 2005 to 2011 and showed a seizure frequency of 10% (12% in males) (2). Longitudinal data about seizures from participants with FXS have subsequently been collected as part of the FORWARD project (28), from 2012 to 2021. In the present report, we use these FORWARD data to investigate seizure prevalence and characteristics, including gender differences, age of onset and resolution, duration and associated comorbid conditions, and other features (e.g., use of anticonvulsants), in the largest and first longitudinal study ever conducted to describe seizures in FXS. Specific goals of these analyses are to update information on seizure frequency and co-occurring problems associated with seizures in FXS in a larger group than previously reported, study the onset and trajectory of seizures experienced by those with FXS across time with longitudinal data, and understand factors associated with more severe epilepsy in FXS.
Materials and Methods
Data analyzed for this report were derived from FORWARD. As described previously (28), FORWARD is a multisite observational study initiated in 2012. The study collects data yearly on a Registry form, Parent Report form, and Clinician Report form, as well as standardized questionnaires including the Aberrant Behavior Checklist-Community Edition (ABC-C) (29), the Social Communication Questionnaire (SCQ) (30), and the Social Responsiveness Scale – Second Edition (SRS-2) (31). The analyses for this report were performed using baseline and longitudinal data collected during follow-up visits from FORWARD Version 5, with data obtained from 1,607 individuals with FXS evaluated between 2012 and 2020, who had information available about seizures derived from the Clinician Report form. There were 1,607 baseline visits and 1,945 follow-up visits (occurring after the baseline visit) for 803 participants with at least 1 year of follow-up. The study was approved by the Institutional Review Board for each participating FXS Clinic where data were collected, and written informed consent was obtained from primary caregivers or adult patients who were their own guardians.
Demographic variables including age, sex, and ethnicity were collected on the Registry form. Data from the Clinician Report form included variables related to seizures including presence or absence of seizures currently or in the past, age (in years and months) at seizure onset, age when seizures resolved if the participant had been 2 years without seizures, type of seizures (focal, generalized, febrile), whether antiepileptic medication was used for seizures at the time of the visit, and, if so, which antiepileptic medication(s) the participant was taking. Antiepileptic medication use was tracked at all visits and was reported in this paper based on use described at any visit over the course of follow-up. Other data from the Clinician Report form about problems that could potentially be associated with seizures were also utilized, including level of intellectual disability (ID), presence of ASD, presence of sleep apnea, severity of behaviors on the ABC-C adjusted for FXS (ABCFX) (32), and severity of ASD symptoms on the SCQ and SRS-2.
Statistical Analysis
Frequency tabulations and proportions for categorical variables, and means and standard deviations for continuous variables, were used for the descriptive analyses. The chi-square test or Fisher's exact test for categorical variables, and analysis of variance (ANOVA) for continuous variables were used to compare characteristics of those with and without seizure experience and to compare males and females with seizures. Using longitudinal data, subjects were divided into three groups based on seizure experience. Participants who reported no seizures ever and who were observed at least once at or over the age of 15 comprised one group; participants who reported having seizures but only for a period <3 years and were followed at least 2 years after the last reported seizure comprised a second group; and participants who reported seizures over a period >3 years comprised the third group. Comparisons of demographic and clinical variables between these three groups were made using chi-square tests or Fisher's exact tests, for categorical variables, and Wilcoxon rank-sum test and Student's t-tests for continuous variables, as appropriate.
Kaplan–Meier survival estimates were produced to model and plot time to first reported seizure for the full sample. Mixed-effect logistic regression models were fit to estimate the rate of current seizures for age groups, using the longitudinal data, across the full sample. This model featured random intercepts for subject.
Analyses were performed using SPSS version 26 and SAS version 9.4. In each analysis reported in this paper, data were used for all individuals who had valid values for the variables used in that analysis. Statistical significance level was set at 5%.
Results
Characteristics of the 1,607 FORWARD participants with available seizure data used for this study are shown in Table 1: 77% were male, 74.5% were White, 7.7% were African American/Black, 3.4% were Asian, and 12.9% were Hispanic. The mean age at the baseline evaluation for the cohort was 13.8 years. A history of current or past seizures at any evaluation was reported for 193 patients (170 males and 23 females) or 12% of the sample. Approximately half had moderate (38.7%) or severe/profound (7.5%) ID, ~39.5% were diagnosed with ASD by a clinician, and sleep apnea was present in 16.9% of the cohort. Severity of ID, ASD symptom severity by SRS-2, and percent of patients with an ASD diagnosis by SCQ were all lower in females with FXS relative to males.
Table 1.
Demographics of the FORWARD cohort.
| Characteristic (N)* | |
|---|---|
| Sex (N = 1607) | 77% male |
| Race/ethnicity (N = 1607) | 74.5% White, 7.7% African American, 3.4% Asian, 12.9% Hispanic, 1.4% Other |
| Age at first visit (years, N = 1607) | 26.3% age 0–5, 27.1% age 6–10, 21.0% age 11–15, 11.4% age 16–20, 14.2% age 21+ |
| Number of visits (N = 1607) | 1–804, 2–329, 3–191, 4–103, 5–73, 6–39, 7–39, 8–28, 9–1 |
| ASD by clinician (DSM5, N = 1592) | 39.5% |
| ASD by SCQ (N = 1187) | 61% no ASD, 39% ASD |
| ASD by SRS (N = 1061) | 16% no ASD, 42% mild ASD, 42% severe ASD |
| Intellectual disability (N = 1536) | 7.7% normal, 15.4% DD, 7.4% borderline, 23.2% mild, 38.7% mod, 7.2% severe, 0.3% profound |
| Current or past seizures (N = 1607) | 12% |
| Current or past sleep apnea (N = 1323) |
16.9% |
N is less than total based on missing data for some items, and the SCQ and SRS were filled out separately from the Clinician forms and not collected on all individuals.
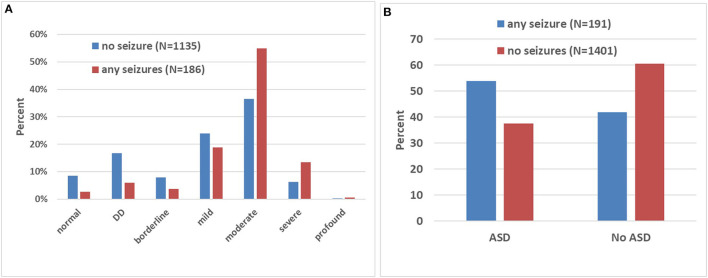
In the entire cohort, there were a higher proportion of males with FXS relative to females who had current or past seizures at any time prior to baseline or during follow-up, 13.7 and 6.2%, respectively (Fisher's exact p < 0.0001). Seizures occurred more often in severely affected patients, with 68.7% of patients with seizures having moderate, severe, or profound ID, while only 43% of patients without seizures had this level of ID (chi-square p < 0.0001 for distribution of ID within the seizure and no seizure groups, Table 2, Figure 1A). Those with seizures had later onset of spoken language (age 2.37 vs. 2.02 years, ANOVA p = 0.03). Those with seizures were more likely to have an ASD diagnosis compared to those without seizures (53.9 vs. 41.9%, chi-square p < 0.0001, Table 2, Figure 1B). The group with seizures had a trend to have an increased likelihood of a diagnosis of ASD by SCQ (score of over 15) at the baseline FORWARD visit (45.9 vs. 38.3%, Fisher's exact p = 0.087), although not statistically significant, and increased severity of ASD symptoms by SRS-2 (9.2 vs. 16.4% absence of ASD, 53.8 vs. 40.5% severe ASD, chi-square p = 0.008). Current symptoms of obstructive sleep apnea were seen more often in those with seizures (4.2 vs. 2.5%) which led to a chi-square of p = 0.001 for the distribution of sleep apnea category between those with and without seizures. However, if one considered past history of sleep apnea, the distribution of seizures was similar in those with and without sleep apnea, making it unclear whether there is a true association. There were no effects of race/ethnicity, place of residence, household income, or level of education of parents on the likelihood of patients manifesting seizures at any time.
Table 2.
Association of cohort characteristics with seizures.
| Characteristic | Seizures (N = 193) | No seizures (N = 1,414) | p-value** |
|---|---|---|---|
| N (% of total with seizures or no seizures) | |||
| Sex | <0.0001 | ||
| Male | 170 (88.1) | 1067 (75.4) | |
| Female | 23 (11.9) | 347 (24.6) | |
| Age at first visit* | 13.7 (8.7) | 11.6 (9.4) | 0.004 |
| Age at last visit* | 16.1 (8.9) | 13.5 (9.8) | <0.00001 |
| Race/ethnicity | |||
| White non-Hispanic | 139 (72.0) | 1061 (75.0) | 0.6 |
| Black non-Hispanic | 17 (8.8) | 106 (7.5) | |
| Asian | 9 (4.7) | 45 (3.2) | |
| Hispanic | 24 (12.4) | 184 (13.0) | |
| Other | 4 (2.1) | 18 (1.3) | |
| ASD diagnosis | <0.00001 | ||
| No | 80 (41.9) | 847 (60.5) | |
| Yes | 103 (53.9) | 526 (37.5) | |
| ASD by SCQ | 0.087 | ||
| No | 80 (54.1) | 641 (61.7) | |
| Yes | 68 (45.9) | 398 (38.3) | |
| Unknown | 8 (4.2) | 28 (2) | |
| ASD by SRS-2 | 0.008 | ||
| Absence of ASD | 12 (9.2) | 153 (16.4) | |
| Mild ASD | 48 (36.9) | 401 (43.1) | |
| Severe ASD | 70 (48) | 377 (40.5) | |
| Level of ID | <0.00001 | ||
| None | 5 (2.7) | 114 (3.0) | |
| Devel Delay | 11 (5.9) | 226 (16.7) | |
| Borderline | 7 (3.8) | 107 (7.9) | |
| Mild | 35 (18.8) | 322 (23.9) | |
| Moderate | 102 (54.8) | 493 (36.5) | |
| Severe | 25 (13.4) | 85 (6.3) | |
| Profound | 1 (0.5) | 3 (0.2) | |
| Sleep apnea | 0.001 | ||
| Currently | 7 (4.2) | 29 (2.5) | |
| Past | 7 (4.2) | 69 (6.0) | |
| No | 130 (78.8) | 1000 (86.4) | |
| Don't know | 21 (12.7) | 60 (5.2) | |
Values reported are mean (SD).
Significance (p-value) is reported for distribution of categories (chi-squared) in characteristic and indicates whether these are differently distributed between the group with seizures and those without.
Figure 1.
Association between intellectual disability (ID), autism spectrum disorder (ASD), and seizures in FXS. (A) The bar graph depicts the proportion (in percentages) of individuals with and without seizures at each level of ID. Note the higher proportion of patients with seizures at the higher levels of ID severity. DD corresponds to developmental delay in younger individuals. (B) The graph shows the proportion (in percentages) of individuals with and without seizures diagnosed (or not) with ASD. Note in the group with ASD, a higher proportion of patients with seizures.
Patients were more likely to have reported seizures if they were older at the first and last evaluations (chi-square p < 0.0001 for age distribution between those with and without seizures), thought likely to be related to being past the typical age of onset of seizures in FXS. There was no effect of the number of evaluations in FORWARD.
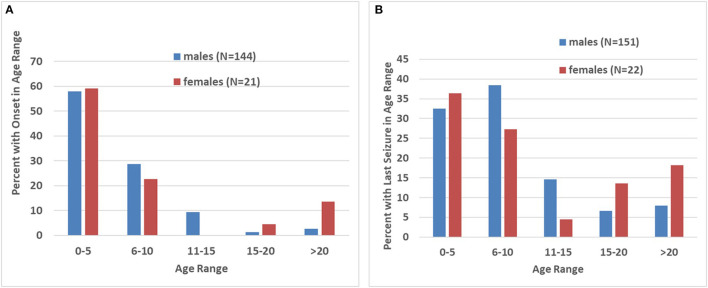
In the group of 193 patients with seizures (Table 3), the mean age at the baseline visit was 13.7 ± 8.7 and the number of evaluations during follow-up was 2.5 ± 1.8. The mean age of seizure onset was 6.4 ± 6.1, and the mean time elapsed since the last seizure was 6.4 ± 7.7 years. In most cases, seizure onset occurred before the age of 10, including 86.7 and 81.8% of males and females, respectively (Figure 2A). For males (N = 170), 96% had the first seizure by age 15, with only 4% presenting after age 15. For females (N = 23), 18.2% had their first seizure after age 15, a trend in age distribution with borderline significance (p = 0.058). The age of the last seizure followed a similar distribution to age of onset of seizures with 70.9% and 63.6% of seizures resolving by age 10 in males and females, respectively (Figure 2B). Only 7.9% of males and 18.2% of females had seizures continuing after age 20; this sex difference, based on a low number of females, was not significant.
Table 3.
Characteristics of the FXS FORWARD participants with seizures.
| Sex | # of evaluations (clinician form) | Age at first evaluation | Age of seizure onset | SCQ Total Score | Total SRS T Scores | # of unique medications reported across all visits | Years from last seizure onset to last evaluation | |
|---|---|---|---|---|---|---|---|---|
| 1 Male | Mean | 2.48 | 13.39 | 6.22 | 16.17 | 76.12 | 0.95 | 6.42 |
| N | 170 | 170 | 150 | 131 | 114 | 170 | 151 | |
| SD | 1.860 | 8.748 | 5.690 | 6.450 | 11.161 | 1.010 | 7.647 | |
| 2 Female | Mean | 2.35 | 15.83 | 7.36 | 11.94* | 75.69 | 0.61 | 6.23 |
| N | 23 | 23 | 22 | 17 | 16 | 23 | 22 | |
| SD | 1.402 | 8.072 | 8.255 | 4.905 | 13.489 | 0.941 | 7.994 | |
| Total | Mean | 2.47 | 13.68 | 6.37 | 15.68 | 76.07 | 0.91 | 6.39 |
| N | 193 | 193 | 172 | 148 | 130 | 193 | 173 | |
| SD | 1.809 | 8.686 | 6.060 | 6.422 | 11.415 | 1.006 | 7.668 | |
p = 0.01, no other characteristics are significantly different between males and females.
Figure 2.
Age of onset and resolution of seizures. (A) Bar graph showing the proportion (in percentages) of individuals, divided by sex, with onset of seizures at different 5 year bins. Note that, with exception of a subset of females with onset after age 15, most patients with FXS displayed an onset before age 10. (B) Bar graph showing the proportion (in percentages) of individuals, divided by sex, experiencing last seizures at different 5 year bins. As for seizure onset, most patients had their last seizure before age 15, with exception of a subset of females who has seizure resolution after this age.
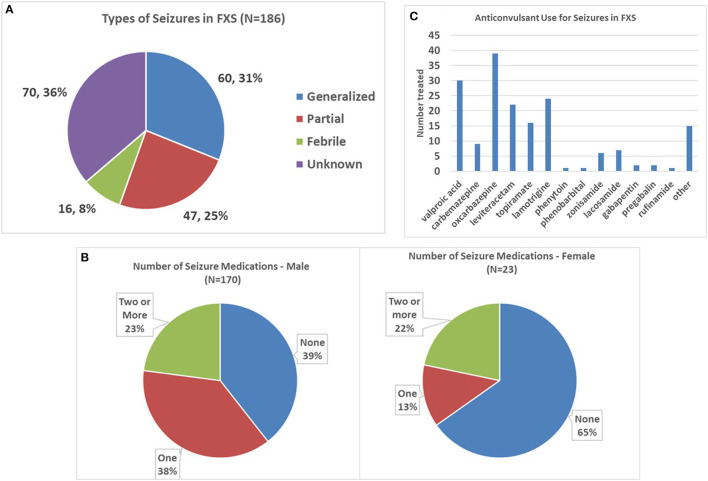
Partial seizures were reported in 25% and generalized seizures in 31% of patients, with febrile seizures in 8% and the remainder of seizures being of unknown type (Figure 3A). Males and females did not show a different distribution of seizure types.
Figure 3.
Seizure types and anticonvulsant use in FXS. (A) Pie graph depicting the proportion of each seizure type in the entire FXS cohort. (B) Graphs showing the number of anticonvulsants per patient in males and females, respectively. Note the higher proportion of males on anticonvulsants. (C) Number of patients with FXS using the most common anticonvulsants. Oxcarbazepine, valproic acid, and lamotrigine were the three most commonly prescribed anticonvulsants.
The average number of anticonvulsants used across the entire group with seizures was 0.91 ± 1.01. Anticonvulsant use was reported in 60.6% of males with seizures at any evaluation vs. 34.8% of females (Fisher's exact p = 0.024, Figure 3B), with 37.6% of males vs. 13.0% of females requiring a single medication (chi-square p = 0.035), and 23 and 22%, respectively, requiring two or more medications. The most commonly used anticonvulsant was oxcarbazepine, followed by valproic acid, lamotrigine, and levetiracetam (Figure 3C).
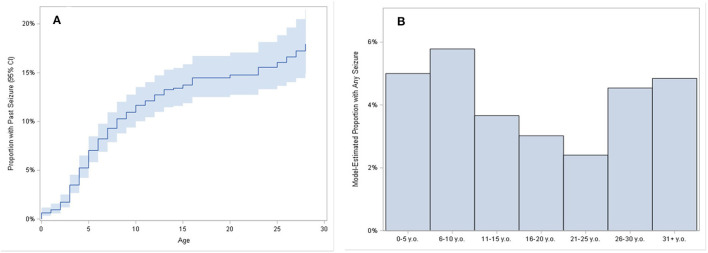
During the follow-up period, 26 incident seizures were reported in 20 males and 6 females in 1945 patient-years of follow-up, for a calculated rate of 0.013 new seizures per patient year of follow-up. Incident seizures had an age and sex distribution resembling that for age and sex distribution of seizures at baseline. Twelve males experienced a change in seizure type in 283 patient years of follow-up, indicating that seizure type changes about 4.2% of time in follow-up. Figure 4A shows a Kaplan–Meier plot of age of seizure onset in the FXS cohort, which reached a plateau by age 15 years. There was also an apparent increase in seizure onset after age 25, despite the low actual numbers of adults with seizures. This is most likely because the Kaplan–Meier model estimated the proportion based only on the subjects observed until later ages, even though many of the younger subjects would have likely reached age 25 years without having a seizure. For that reason, the Kaplan–Meier estimate of the total proportion experiencing seizures (about 17%) is greater than the raw calculation of the proportion reporting seizures (12% as noted above). Figure 4B shows the proportion of patients having seizures separated into 5 year groups. As expected, the proportions are higher in the youngest groups, tapering off until reaching the 26–30 year age group.
Figure 4.
Plot of time to first seizure. (A) Reverse Kaplan–Meier estimates show the estimated proportion of subjects who had their first seizure onset reported, over ages 0 to 30. Band shows 95% confidence intervals for estimates. For example, approximately 7% of subjects had first seizure onset by age 5, approximately 12% of subjects had first seizure onset by age 10, and approximately 14% of subjects had first seizure onset by age 15. Bar chart of the model-estimated proportion of having any seizures during selected age periods (B). Proportion estimates are derived from mixed-effect logistic regression models fit for the odds of having experienced a seizure, with categorized age as the predictor, and random intercepts for subject.
Patients with persistent seizures, operationally defined as those with seizures lasting over 3 years (N = 75), were compared with those with seizures lasting <3 years (N = 70) and those without seizures who had reached age 15 (N = 514) (Table 4). The group without seizures was limited to those over 15 years to ensure that the comparator group had a very low likelihood of developing seizures. Those without seizures were older at the first and last evaluation than the other groups; however, this was expected given the age limitation on this group. There was no significant difference in age at evaluation between the two seizure groups. Relative to those with seizures <3 years, those with seizures lasting >3 years were on more anticonvulsants (p < 0.0001 both first and last evaluations), were more likely to have partial seizures and less likely to have febrile seizures (p = 0.005 at first evaluation and 0.03 at last evaluation), made less language progress during follow-up, had more severe language impairment at the most recent visit (p = 0.03), and had a higher proportion of severe/profound ID (although this was not statistically significant, p = 0.14), but did not have higher prevalence of ASD, sleep apnea, or severity of behavioral issues on any subscale of the ABCFX. The lack of language progress in the group with seizures lasting >3 years was remarkable, with 25% non-verbal at the initial visit and 21.4% remaining non-verbal at the last visit compared to 14.4% non-verbal at the initial visit and 5.7% at the last visit for those with seizures lasting <3 years. Underscoring the phenotypical severity associated with having seizures regardless of duration, those with no seizures had significantly less severe ID than the <3 year seizure group (p = 0.003), less severe language impairment at the first but not the last visit for those with seizures <3 years (p = 0.038 and 0.15, respectively), less severe language impairment than those with seizures >3 years at both visits (p = 0.0004 and 0.0062, respectively), a higher percent with sustained conversation ability at both visits than those with seizures <3 years at both visits (p = 0.0003 and 0.01, respectively), a lower proportion with ASD diagnosis than both seizure groups at both visits (p = 0.009 for both groups at the last visit), and lower scores on the ABCFX Hyperactivity, Irritability, and Stereotypy subscales than both seizure groups at both visits (p-values ranging from < 0.0001 to 0.029).
Table 4.
Comparison of characteristics between groups with no seizures, seizures reported for < 3 years, and seizures reported for > 3 years*.
| Overall (N = 659) | No Seizures (N = 514) | Seizures < 3 years (N = 70) | Seizures > 3 years (N = 75) | p -values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % or Mean (SD) | n | % or Mean (SD) | n | % or Mean (SD) | n | % or Mean (SD) | Overall | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 |
| Number of evaluations | 659 | 2.8 (2.0) | 514 | 2.8 (2.0) | 70 | 2.6 (1.7) | 75 | 2.8 (2.1) | 0.7533 | 0.4488 | 0.8982 | 0.6178 |
| Number of evaluations [Min, Max] | 659 | (1, 9) | 514 | (1, 9) | 70 | (1, 8) | 75 | (1, 8) | ||||
| Number of evaluations–binary | 0.5789 | 0.8537 | 0.3185 | 0.3821 | ||||||||
| 1 | 235 | 35.7% | 180 | 35.0% | 24 | 34.3% | 31 | 41.3% | ||||
| 2+ | 424 | 64.3% | 334 | 65.0% | 46 | 65.7% | 44 | 58.7% | ||||
| Gender | 0.0535 | 0.0537 | 0.1033 | 0.7522 | ||||||||
| Male | 520 | 79.0% | 395 | 77.0% | 61 | 87.1% | 64 | 85.3% | ||||
| Female | 138 | 21.0% | 118 | 23.0% | 9 | 12.9% | 11 | 14.7% | ||||
| Age at first evaluation | 659 | 19.9 (9.6) | 514 | 21.1 (9.4) | 70 | 14.7 (8.3) | 75 | 16.4 (9.5) | <0.0001 | <0.0001 | <0.0001 | 0.2669 |
| Age at last evaluation | 659 | 22.6 (9.3) | 514 | 23.8 (9.0) | 70 | 17.6 (8.4) | 75 | 18.9 (9.6) | <0.0001 | <0.0001 | <0.0001 | 0.3685 |
| Years between first and last evaluation | 419 | 4.3 (2.2) | 330 | 4.3 (2.3) | 46 | 4.3 (2.1) | 43 | 4.4 (2.2) | 0.9798 | 0.8835 | 0.4309 | 0.4973 |
| Age of onset | 135 | 6.7 (6.6) | N/A | 66 | 5.9 (5.3) | 69 | 7.5 (7.6) | N/A | N/A | N/A | 0.1625 | |
| Mean inter-evaluation period | 423 | 1.8 (1.2) | 333 | 1.8 (1.2) | 46 | 2.0 (1.2) | 44 | 1.6 (1.0) | 0.3655 | 0.3427 | 0.3576 | 0.1326 |
| Number of AEDs first evaluation | 659 | 0.1 (0.5) | 514 | 0.0 (0.0) | 70 | 0.3 (0.5) | 75 | 1.1 (1.0) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Number of AEDs first evaluation [Median (IQR)] | 659 | 0.0 (0.0-0.0) | 514 | 0.0 (0.0-0.0) | 70 | 0.0 (0.0-0.0) | 75 | 1.0 (0.0-2.0) | ||||
| Number of AEDs last evaluation | 659 | 0.2 (0.5) | 514 | 0.0 (0.0) | 70 | 0.3 (0.5) | 75 | 1.3 (1.0) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Number of AEDs last evaluation [Median (IQR)] | 659 | 0.0 (0.0-0.0) | 514 | 0.0 (0.0-0.0) | 70 | 0.0 (0.0-0.0) | 75 | 1.0 (1.0-2.0) | ||||
| Seizure type first seizure evaluation | N/A | N/A | N/A | 0.0051 | ||||||||
| Generalized | 61 | 45.5% | N/A | 28 | 43.8% | 33 | 47.1% | |||||
| Partial | 46 | 34.3% | N/A | 16 | 25.0% | 30 | 42.9% | |||||
| Febrile | 11 | 8.2% | N/A | 10 | 15.6% | 1 | 1.4% | |||||
| Unknown | 16 | 11.9% | N/A | 10 | 15.6% | 6 | 8.6% | |||||
| Seizure type last seizure evaluation | N/A | N/A | N/A | 0.0363 | ||||||||
| Generalized | 39 | 45.9% | N/A | 19 | 42.2% | 20 | 50.0% | |||||
| Partial | 25 | 29.4% | N/A | 10 | 22.2% | 15 | 37.5% | |||||
| Febrile | 11 | 12.9% | N/A | 10 | 22.2% | 1 | 2.5% | |||||
| Unknown | 10 | 11.8% | N/A | 6 | 13.3% | 4 | 10.0% | |||||
| Intellectual disability Dx last evaluation | 0.0001 | 0.0003 | 0.0735 | 0.1380 | ||||||||
| No ID | 35 | 5.5% | 31 | 6.3% | 4 | 5.8% | 0 | 0.0% | ||||
| Delayed development | 3 | 0.5% | 0 | 0.0% | 3 | 4.3% | 0 | 0.0% | ||||
| Borderline | 35 | 5.5% | 30 | 6.1% | 2 | 2.9% | 3 | 4.3% | ||||
| Mild | 146 | 23.0% | 119 | 24.0% | 11 | 15.9% | 16 | 22.9% | ||||
| Moderate | 356 | 56.2% | 274 | 55.4% | 42 | 60.9% | 40 | 57.1% | ||||
| Severe | 57 | 9.0% | 40 | 8.1% | 7 | 10.1% | 10 | 14.3% | ||||
| Profound | 2 | 0.3% | 1 | 0.2% | 0 | 0.0% | 1 | 1.4% | ||||
| Language milestone (Q24) first evaluation | 0.0049 | 0.0378 | 0.0004 | 0.6416 | ||||||||
| Non-verbal | 12 | 1.8% | 6 | 1.2% | 2 | 2.9% | 4 | 5.3% | ||||
| Signing | 7 | 1.1% | 5 | 1.0% | 0 | 0.0% | 2 | 2.7% | ||||
| Babbling | 2 | 0.3% | 0 | 0.0% | 1 | 1.4% | 1 | 1.3% | ||||
| Few words | 52 | 8.0% | 34 | 6.7% | 7 | 10.1% | 11 | 14.7% | ||||
| Word combinations | 64 | 9.9% | 47 | 9.3% | 9 | 13.0% | 8 | 10.7% | ||||
| Phrases | 512 | 78.9% | 413 | 81.8% | 50 | 72.5% | 49 | 65.3% | ||||
| Sustained conversation (Q26) first evaluation | 0.0009 | 0.0774 | 0.0003 | 0.5314 | ||||||||
| Non-verbal | 26 | 4.1% | 15 | 3.0% | 4 | 6.0% | 7 | 9.6% | ||||
| Yes, sustained conversion | 385 | 60.3% | 320 | 64.1% | 34 | 50.7% | 31 | 42.5% | ||||
| No sustained conversion | 228 | 35.7% | 164 | 32.9% | 29 | 43.3% | 35 | 47.9% | ||||
| Language milestone (Q24) last evaluation | 0.0034 | 0.1536 | 0.0062 | 0.0358 | ||||||||
| Non-verbal | 8 | 1.2% | 5 | 1.0% | 1 | 1.4% | 2 | 2.7% | ||||
| Signing | 8 | 1.2% | 5 | 1.0% | 2 | 2.9% | 1 | 1.3% | ||||
| Babbling | 4 | 0.6% | 2 | 0.4% | 0 | 0.0% | 2 | 2.7% | ||||
| Few words | 40 | 6.1% | 28 | 5.5% | 1 | 1.4% | 11 | 14.7% | ||||
| Word combinations | 53 | 8.1% | 37 | 7.3% | 10 | 14.3% | 6 | 8.0% | ||||
| Phrases | 538 | 82.6% | 429 | 84.8% | 56 | 80.0% | 53 | 70.7% | ||||
| Sustained conversation (Q26) last evaluation | 0.0236 | 0.2432 | 0.0122 | 0.1287 | ||||||||
| Non-verbal | 12 | 2.0% | 9 | 1.9% | 0 | 0.0% | 3 | 4.5% | ||||
| Yes, sustained conversion | 404 | 65.8% | 329 | 68.4% | 41 | 62.1% | 34 | 50.7% | ||||
| No sustained conversion | 198 | 32.2% | 143 | 29.7% | 25 | 37.9% | 30 | 44.8% | ||||
| Autism Dx ever | 0.0003 | 0.0005 | 0.0246 | 0.1952 | ||||||||
| No | 321 | 48.9% | 273 | 53.2% | 20 | 28.6% | 28 | 37.8% | ||||
| Yes | 328 | 49.9% | 234 | 45.6% | 48 | 68.6% | 46 | 62.2% | ||||
| Autism Dx current (last evaluation) | 0.0022 | 0.0095 | 0.0094 | 0.3945 | ||||||||
| Yes | 276 | 43.0% | 196 | 39.0% | 39 | 57.4% | 41 | 57.7% | ||||
| No | 331 | 51.6% | 277 | 55.1% | 28 | 41.2% | 26 | 36.6% | ||||
| Don't know | 35 | 5.5% | 30 | 6.0% | 1 | 1.5% | 4 | 5.6% | ||||
| Sleep apnea last evaluation | 0.1434 | 0.3075 | 0.0555 | 0.6080 | ||||||||
| No | 378 | 97.2% | 295 | 98.0% | 43 | 95.6% | 40 | 93.0% | ||||
| Yes | 11 | 2.8% | 6 | 2.0% | 2 | 4.4% | 3 | 7.0% | ||||
| ABC hyperactivity first evaluation | 506 | 18.8 (7.4) | 393 | 17.9 (6.9) | 58 | 23.3 (8.1) | 55 | 20.6 (8.0) | <0.0001 | <0.0001 | 0.0076 | 0.0795 |
| ABC inappropriate Speech first evaluation | 506 | 8.4 (3.4) | 393 | 8.3 (3.4) | 58 | 9.1 (3.6) | 55 | 8.1 (3.3) | 0.2196 | 0.1187 | 0.5732 | 0.1172 |
| ABC irritability first evaluation | 506 | 31.0 (11.9) | 393 | 29.9 (11.4) | 58 | 35.8 (14.1) | 55 | 33.2 (11.8) | 0.0006 | 0.0004 | 0.0473 | 0.2830 |
| ABC social avoidance first evaluation | 506 | 7.7 (3.4) | 393 | 7.7 (3.4) | 58 | 7.6 (3.4) | 55 | 7.4 (3.4) | 0.7930 | 0.8768 | 0.4987 | 0.6881 |
| ABC social unresponsiveness first evaluation | 506 | 18.8 (5.7) | 393 | 18.5 (5.9) | 58 | 19.9 (5.3) | 55 | 19.6 (5.3) | 0.1154 | 0.0811 | 0.1916 | 0.7393 |
| ABC stereotypy first evaluation |
506 | 10.9 (4.8) | 393 | 10.5 (4.6) | 58 | 12.7 (5.4) | 55 | 11.9 (5.1) | 0.0011 | 0.0010 | 0.0296 | 0.4655 |
| ABC hyperactivity last evaluation |
504 | 17.5 (6.9) | 382 | 16.4 (6.2) | 58 | 21.5 (7.2) | 64 | 20.1 (8.3) | <0.0001 | <0.0001 | <0.0001 | 0.3237 |
| ABC inappropriate Speech last evaluation |
504 | 8.0 (3.4) | 382 | 7.8 (3.4) | 58 | 8.7 (3.5) | 64 | 8.4 (3.4) | 0.1310 | 0.0789 | 0.2204 | 0.6524 |
| ABC irritability last evaluation | 504 | 30.0 (11.7) | 382 | 28.8 (11.1) | 58 | 33.5 (12.3) | 64 | 34.1 (13.0) | 0.0002 | 0.0032 | 0.0007 | 0.8072 |
| ABC social avoidance last evaluation | 504 | 7.5 (3.4) | 382 | 7.4 (3.3) | 58 | 8.1 (3.8) | 64 | 7.2 (3.3) | 0.2760 | 0.1532 | 0.6003 | 0.1568 |
| ABC social unresponsiveness last evaluation | 504 | 18.4 (5.7) | 382 | 18.2 (5.8) | 58 | 19.0 (5.1) | 64 | 19.5 (5.1) | 0.1634 | 0.3221 | 0.0856 | 0.5669 |
| ABC stereotypy last evaluation | 504 | 10.5 (4.6) | 382 | 10.1 (4.3) | 58 | 11.8 (5.0) | 64 | 12.0 (5.0) | 0.0006 | 0.0061 | 0.0016 | 0.8347 |
for the p-values listed for group comparisons, 1 = no seizures, 2 = seizures for <3 years, 3 = seizures for > 3 years.
Discussion
In this study, we characterized seizures in the largest clinically evaluated cohort of individuals with FXS. In addition, this is the only longitudinal study of seizures to date in FXS and thus the only study ever to evaluate incident onset of seizures prospectively in this syndrome. In addition to confirming previously reported characteristics of individuals with FXS and seizures, such as prevalence, sex distribution (13.7% males, 6.2% females), and association with ASD (19, 26, 33), we identified an association with current sleep apnea and additional features. Those with seizures were also more likely to have more severe ID and later onset of expressive language, findings which have not been reported before, likely due to lack of sufficient numbers of subjects (10–15, 26) or lack of clinical assessment or evaluation of levels of ID and language (19, 33) in prior studies. The group with seizures was also more likely to have more severe behavioral problems, as measured by the ABCFX (Hyperactivity, Irritability, and Stereotypy subscales). Availability of longitudinal data allowed the delineation of key aspects in the evolution of seizures in FXS and their association with other features of the disorder. A longer course of epilepsy in FXS was associated with greater overall cognitive and language impairments, but not more severe behavioral problems.
In this FORWARD cohort, individuals with FXS and seizures were more likely to have ASD diagnosed by the clinician based on DSM5 criteria. The association between seizures and a clinical diagnosis of ASD in children with FXS is supported by the finding that this subset has more severe ASD symptoms on the SCQ and SRS-2. Consistent with our findings, previous studies have found an association between the diagnosis of ASD and seizures and children with ASD with seizures have been found to have more severe ASD symptoms (34, 35). However, although previous studies suggested that children with ASD and genetic disorders have high rates of epilepsy (36), this study suggests that the rate of seizures in FXS is rather moderate, at 12%, comparable to the 12.1% rate of epilepsy in individuals with idiopathic ASD reported in a recent meta-analysis (37). Additionally, studies on children with ASD also demonstrate that many have subclinical epileptiform discharges in a centrotemporal pattern (38, 39) and occipital regions (40), similar to EEG profiles reported in FXS (13, 15, 19–21). Children with ASD and EEGs with subclinical epileptiform discharges are found to be more severely impaired (35, 41) but may show improvement in behavior and cognition with treatments targeted to reduced epileptiform discharges (35, 38, 42). Although EEG data were not collected in the FORWARD study, children with FXS and an abnormal EEG are a subgroup of FXS patients that may warrant closer study.
Onset of seizures in FXS was typically (>80%) below age 10, with a very low proportion of males having seizure onset after age 15 years. A higher percent of females had seizure onset after age 15 years, which suggests a slightly different pattern for seizure onset in females, although females with first seizure at age >15 represented only four patients. Thus, the marginally significant chi-square (p = 0.058) for a different male–female distribution of age of seizure onset may be due to the relatively small number of females with seizures in the cohort. Consequently, a larger cohort would be needed to confirm that females with FXS have a larger proportion of late-onset seizures than males. There is a new concern in adolescents and adults with idiopathic ASD of having new-onset seizures (43), especially in those with ID (44), which appears to be linked to increased morbidity (45, 46). Interestingly, we did not find this trend in those with FXS, making this less of a concern in this genetic disorder.
As with idiopathic ASD and many other ID syndromes, seizure type was variable between patients. However, the proportions of generalized and partial seizures are in general agreement with the literature (13–15, 19). Overall, the number of anticonvulsants required for those with seizures was low, consistent with past observations (15, 19). As might be expected considering that males with FXS are more affected than females, males with seizures were more likely to be on anticonvulsants, including multiple drugs, and to be followed in clinic and in FORWARD longer than females with seizures. The anticonvulsants used in the FXS population were consistent with recommendations in the field (2, 19, 47) and were those typically employed in any pediatric seizure population, and similar perhaps with somewhat less use of levetiracetam, most likely due to concerns about aggravating behavior.
For all participants with follow-up visits in FORWARD, new (i.e., incident) seizures were rare, with risk being only approximately 1% per patient year of follow-up in FORWARD. Risk of change in seizure type was only approximately 4% per patient year. The Kaplan–Meier plot (Figure 4) shows risk of having seizures at any given age across the lifespan. It is clear that most seizures are present early in life. After being stable through the 15–25 year age period with fewer patients having any seizure during this time period, the proportion of seizures goes up after age 25, most likely due to the small number of adults >25 years old included in FORWARD to date. There is also a likely referral bias such that patients are more likely to remain in care at an FXS clinic if they have ongoing seizures or be referred to an FXS clinic if they have new-onset seizures. This may be particularly the case for clinics run by pediatric neurologists, who tend to see adults with FXS more readily. Since the FORWARD project is now focused on increasing enrollment of adults, it is expected that more definitive estimates can be obtained for seizure prevalence and onset in adults upon future analysis of a larger cohort.
When patients with longer duration of seizures (>3 years) were compared with those with shorter duration (<3 years), only a few differences emerged, including increased use of anticonvulsants, higher prevalence of partial seizures, and lower prevalence of febrile seizures and, most importantly, more severe language outcomes despite lack of differences in behavioral abnormalities (including ASD) and non-significant increases in ID severity. This suggests that a longer course of seizures in FXS is an index of severity linked to more severe outcome in this some functional domains. It is possible that the division of the groups at 3 years of persistent seizures did not isolate those with the most severe phenotypes and a 5 year cutoff might have been better to more fully explore this concept. Nonetheless, there were not enough patients in FORWARD with 5 years of longitudinal data at present to support such an analysis.
Strengths of this study are the size of the subject sample, very large for rare disease standards, and the ability to examine longitudinal data. Weaknesses include lack of availability of follow-up visits into the late teenage years in about half the cohort, lack of complete evaluations of seizure type (many were undefined), and a relatively small population of adults in the current FORWARD database. Additionally, as participants with FXS were not a randomly selected, sampling biases inherent to clinic-based samples cannot be discounted.
Based on the data from this study, the prototypical individual with FXS and seizures would be male, with moderate or severe ID and expressive language delay, with a DSM-5 diagnosis of ASD and a corresponding SCQ >15 and SRS-2 in the severe range, who had a first evaluation at a FORWARD (perhaps any FXS) clinic after age 5. Females with FXS and seizures would show similar characteristics; however, among females a subgroup with a distinctive but uncommon profile of onset of seizures after age 15 is identified. It appears that this subgroup has greater cognitive impairment than other females with or without seizures and had a first evaluation at a FORWARD clinic after age 15. The duration of seizures also seems to be a parameter associated with worse outcomes, particularly in cognitive function.
FMRP has been linked to seizures in FXS through excessive mGluR5 signaling of dendritic translation, based on reversal of audiogenic seizures by mGluR5 blockers in the fmr1 knockout mouse (48). FMRP also operates presynaptically and may cause seizures through interruption of a direct interaction of FMRP with a presynaptic BK channel subunit (49). Seizures are associated with mutations in CYFIP2, which interacts with FMRP (50), and FMRP appears to underlie enhanced mLTD in adult rats triggered by early-life seizures (51). Thus, absence of FMRP may potentiate seizure via multiple neural mechanisms which are likely to vary between patients, thus resulting in variable penetrance and severity of seizures in FXS. However, likely the presence of seizures is a signal of more problematic neural and synaptic dysfunction related to variation in FMRP deficits in cells, and variation in these multiple interacting pathways. The present study adds to the understanding of the characteristics, risk factors, and course of seizures in FXS and provides a basis for anticipatory guidance for clinicians and families. The study also defines gaps and additional areas of investigation that could answer further questions about seizures, their neural underpinnings, and their evolution across the lifespan in FXS.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional IRBs at all FORWARD academic sites. Written informed consent to participate in this study was provided by the participant's legal guardian/next of kin.
Forward Consortium
Elizabeth Berry-Kravis (Rush University Medical Center), Milen Velinov (Rutgers Robert Wood Johnson Medical School), Amy L. Talboy (Emory University School of Medicine), Stephanie L. Sherman (Emory University School of Medicine), Walter E. Kaufmann (Emory University School of Medicine), Marcy Schuster (Elwyn Inc), Nicole Tartaglia (Children's Hospital Colorado), Robyn A. Filipink (University of Pittsburgh School of Medicine), Dejan B. Budimirovic (Kennedy Krieger Institute, Johns Hopkins Medical Institutions), Deborah Barbouth (University of Miami, Miller School of Medicine), Amy Lightbody (Stanford University School of Medicine), Allan Reiss (Stanford University School of Medicine), Carol M. Delahunty (The Metrohealth System, Cleveland), Randi J. Hagerman (MIND Institute, University of California Davis Medical Center), David Hessl (MIND Institute, University of California Davis Medical Center), Craig A. Erickson (Cincinnati Children's Hospital Medical Center), Gary Feldman (Miller Children's & Woman's Hospital), Jonathan D. Picker (Boston Children's Hospital), Ave M. Lachiewicz (Duke Health Center, Lenox Baker Children's Hospital), Holly K. Harris (Baylor College of Medicine & Meyer Center for Developmental Pediatrics), Amy Esler (University of Minnesota), Richard E. Frye (Barrow Neurological Institute at Phoenix Children's Hospital), Patricia A. Evans (University of Texas Southwestern Medical Center), Mary Ann Morris (University of Texas Southwestern Medical Center), Barbara A. Haas-Givler (Geisinger's Autism & Developmental Medicine Institute), Andrea L. Gropman (Children's National Medical Center), Ryan S. Uy (Children's National Medical Center), Reymundo Lozano (Icahn School of Medicine at Mount Sinai), Carrie Buchanan (Greenwood Genetic Center), Jean A. Frazier (Eunice Kennedy Shriver Center, University of Massachusetts Medical School), Stephanie M. Morris (Washington University in St. Louis).
Author Contributions
EB-K, RFi, RFr, SG, SM, HA, T-HC, and WK: study concept and design, acquisition, analysis, and interpretation of data, drafting and critical revision of the manuscript, and have approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
Work on this publication was supported by cooperative agreements (U01DD000231, U19DD000753, and U01DD001189), funded by the Centers for Disease Control and Prevention.
Author Disclaimer
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the participating FXS Clinics and the families for their efforts in the collection of the FORWARD data.
Contributor Information
The FORWARD Consortium:
Elizabeth Berry-Kravis, Milen Velinov, Amy L. Talboy, Stephanie L. Sherman, Walter E. Kaufmann, Marcy Schuster, Nicole Tartaglia, Robyn A. Filipink, Dejan B. Budimirovic, Deborah Barbouth, Amy Lightbody, Allan Reiss, Carol M. Delahunty, Randi J. Hagerman, David Hessl, Craig A. Erickson, Gary Feldman, Jonathan D. Picker, Ave M. Lachiewicz, Holly K. Harris, Amy Esler, Richard E. Frye, Patricia A. Evans, Mary Ann Morris, Barbara A. Haas-Givler, Andrea L. Gropman, Ryan S. Uy, Reymundo Lozano, Carrie Buchanan, Jean A. Frazier, and Stephanie M. Morris
References
- 1.Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. (2009) 85:503–14. 10.1016/j.ajhg.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidd SA, Lachiewicz A, Barbouth D, Blitz RK, Delahunty C, McBrien D, et al. Fragile X syndrome: a review of associated medical problems. Pediatrics. (2014) 134:995–1005. 10.1542/peds.2013-4301 [DOI] [PubMed] [Google Scholar]
- 3.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. (1991) 65:905–14. 10.1016/0092-8674(91)90397-H [DOI] [PubMed] [Google Scholar]
- 4.Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. (1993) 4:335–40. 10.1038/ng0893-335 [DOI] [PubMed] [Google Scholar]
- 5.Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Mutiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. (2007) 43:11624–34. 10.1523/JNEUROSCI.2266-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee A, Ifrim MF, Valdez AN, Raj N, Bassell GJ. Aberrant RNA translation in fragile X syndrome: from FMRP mechanisms to emerging therapeutic strategies. Brain Res. (2018) 1693:24–36. 10.1016/j.brainres.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross C, Hoffmann A, Bassell GJ, Berry-Kravis EM. Therapeutic strategies in fragile X syndrome: from bench to bedside and back. Neurotherapeutics. (2015) 12:584–608. 10.1007/s13311-015-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He CX, Portera-Cailliau C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience. (2013) 251:120–8. 10.1016/j.neuroscience.2012.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang SC, Zhao W, Bauschwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. (2005) 25:8048–55. 10.1523/JNEUROSCI.1777-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, et al. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. (2000) 41:19–23. 10.1111/j.1528-1157.2000.tb01499.x [DOI] [PubMed] [Google Scholar]
- 11.Kluger G, Bohm I, Laub MC, Waldenmaier C. Epilepsy and fragile X gene mutations. Pediatr Neurol. (1996) 15:358–60. 10.1016/S0887-8994(96)00251-2 [DOI] [PubMed] [Google Scholar]
- 12.Musumeci SA, Ferri R, Elia M, Colognola RM, Bergonzi P, Tassinari CA. Epilepsy and fragile X syndrome: a follow-up study. Am J Med Genet. (1991) 38:511–13. 10.1002/ajmg.1320380276 [DOI] [PubMed] [Google Scholar]
- 13.Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, et al. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. (1999) 40:1092–99. 10.1111/j.1528-1157.1999.tb00824.x [DOI] [PubMed] [Google Scholar]
- 14.Sabaratnam M, Vroegop PG, Gangadharan SK. Epilepsy and EEG findings in 18 males with fragile X syndrome. Seizure. (2001) 10:60–3. 10.1053/seiz.2000.0492 [DOI] [PubMed] [Google Scholar]
- 15.Berry-Kravis E. Epilepsy in fragile X syndrome. Dev Med Child Neurol. (2002) 44:724–28. 10.1111/j.1469-8749.2002.tb00277.x [DOI] [PubMed] [Google Scholar]
- 16.Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet. (2008) 146A:2060–69. 10.1002/ajmg.a.32439 [DOI] [PubMed] [Google Scholar]
- 17.Heard TT, Ramgopal S, Picker J, Lincoln SA, Rotenberg A, Kothare SV, et al. Abnormalities and seizures in genetically diagnosed fragile X syndrome. Int J Dev Neurosci. (2014) 38:155–60. 10.1016/j.ijdevneu.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 18.Haessler F, Gaese F, Huss M, Kretschmar C, Brinkman M, Peters H, et al. Characterization, treatment patterns, and patient-related outcomes of patients with Fragile X syndrome in Germany: final results of the observational EXPLAIN-FXS study. BMC Psychiatry. (2016) 16:318. 10.1186/s12888-016-1020-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey D. Seizures in fragile X syndrome: characteristics and co-morbid diagnoses. Am J Intellect Dev Disabil. (2010) 115:461–72. 10.1352/1944-7558-115.6.461 [DOI] [PubMed] [Google Scholar]
- 20.Rees M, Diebold U, Parker K, Doose H, Gardiner RM, Whitehouse WP. Benign childhood epilepsy with centrotemporal spikes and the focal sharp wave trait is not linked to the fragile X region. Neuropediatrics. (1993) 24:211–13. 10.1055/s-2008-1071542 [DOI] [PubMed] [Google Scholar]
- 21.Bonanni P, Casellato S, Fabbro F, Negrin S. Epilepsy in fragile-X-syndrome mimicking panayiotopoulos syndrome: description of three patients. Am J Med Genet A. (2017) 173:2753–57. 10.1002/ajmg.a.38373 [DOI] [PubMed] [Google Scholar]
- 22.Gauthey M, Poloni CB, Ramelli GP, Roulet-Perez E, Korff CM. Status epilepticus in fragile X syndrome. Epilepsia. (2010) 51:2470–3. 10.1111/j.1528-1167.2010.02761.x [DOI] [PubMed] [Google Scholar]
- 23.McDermott S, Hardin JW, Royer JA, Mann JR, Tong X, Ozturk OD, et al. Emergency department and inpatient hospitalizations for young people with fragile X syndrome. Am J Intellect Dev Disabil. (2015) 120:230–43. 10.1352/1944-7558-120.3.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenmuir C, Richardson M, Ghearing G. Surgical treatment for medically refractory focal epilepsy in a patient with fragile X syndrome. Brain Dev. (2015) 37:916–8. 10.1016/j.braindev.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 25.Myers KA, van 't Hof FNG, Sadleir LG, Legault G, Simard-Tremblay E, Amor DJ, et al. Fragile females: case series of epilepsy in girls with FMR1 disruption. Pediatrics. (2019) 144:e20190599. 10.1542/peds.2019-0599 [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Nonell C, Ratera ER, Harris SW, Hessl D, Ono MY, Tartaglia N, et al. Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. Am J Med Genet. (2008) 146A:1911–16. 10.1002/ajmg.a.32290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowley B, Kirjanen S, Partanen J, Castrén ML. Epileptic electroencephalography profile associates with attention problems in children with fragile X syndrome: review and case series. Front Hum Neurosci. (2016) 10:353. 10.3389/fnhum.2016.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman SL, Kidd SA, Berry-Kravis E, Andrews H, Lincoln S, Swanson M, et al. Fragile X online registry with accessible research database (FORWARD): experience from the fragile X clinical and research consortium to study the natural history fragile X syndrome. Pediatrics. (2017) 139:S183–93. 10.1542/peds.2016-1159E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. (1985) 89:485–91. 10.1037/t10453-000 [DOI] [PubMed] [Google Scholar]
- 30.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire: Manual. Los Angeles, CA: Western Psychological Services; (2003). [Google Scholar]
- 31.Constantino JN, Gruber CP. The Social Responsiveness Scale-Second Edition. Los Angeles, CA: Western Psychological Services; (2012). [Google Scholar]
- 32.Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufmann WE, et al. Psychometric study of the aberrant behavior checklist in fragile X syndrome and implications for targeted treatment. J Autism Dev Disord. (2012) 42:1377–92. 10.1007/s10803-011-1370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, et al. Autism spectrum disorder in fragile X syndrome: characterization using FORWARD. Pediatrics. (2017) 139:S194–206. 10.1542/peds.2016-1159F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Achkar CM, Spence SJ. Clinical characteristics of children and young adults with co-occurring autism spectrum disorder and epilepsy. Epilepsy Behav. (2015) 47:183–90. 10.1016/j.yebeh.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 35.Frye RE. Prevalence, significance and clinical characteristics of seizures, epilepsy and subclinical electrical activity in autism. N A J Med Sci. (2015) 8:113–22. [Google Scholar]
- 36.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. (2008) 9:341–55. 10.1038/nrg2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukmanji S, Manji SA, Kadhim S, Sauro KM, Wirrell EC, Kwon CS, et al. The co-occurrence of epilepsy and autism: a systematic review. Epilepsy Behav. (2019) 98:238–48. 10.1016/j.yebeh.2019.07.037 [DOI] [PubMed] [Google Scholar]
- 38.Lewine JD, Andrews R, Chez M, Patil AA, Devinsky O, Smith M, et al. Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics. (1999) 104:405–18. 10.1542/peds.104.3.405 [DOI] [PubMed] [Google Scholar]
- 39.Rossi PG, Parmeggiani A, Bach V, Santucci M, Visconti P. EEG features and epilepsy in patients with autism. Brain Dev. (1995) 17:169–74. 10.1016/0387-7604(95)00019-8 [DOI] [PubMed] [Google Scholar]
- 40.Nass R, Gross A, Devinsky O. Autism and autistic epileptiform regression with occipital spikes. Dev Med Child Neurol. (1998) 40:453–8. 10.1111/j.1469-8749.1998.tb15395.x [DOI] [PubMed] [Google Scholar]
- 41.Capal JK, Carosella C, Corbin E, Horn PS, Caine R, Manning-Courtney P, et al. Endophenotypes in autism spectrum disorder. Epilepsy Behav. (2018) 88:341–8. 10.1016/j.yebeh.2018.09.036 [DOI] [PubMed] [Google Scholar]
- 42.Frye RE, Butler I, Strickland D, Castillo E, Papanicolaou A. Electroencephalogram discharges in atypical cognitive development. J Child Neurol. (2010) 25:556–66. 10.1177/0883073809344743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuchman R, Cuccaro M, Alessandri M. Autism and epilepsy: historical perspective. Brain Dev. (2010) 32:709–18. 10.1016/j.braindev.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 44.Bishop L, McLean KJ, Rubenstein E. Epilepsy in adulthood: prevalence, incidence, and associated antiepileptic drug use in autistic adults in a state Medicaid system. Autism. (2021) 25:831–9. 10.1177/1362361320942982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pickett J, Xiu E, Tuchman R, Dawson G, Lajonchere C. Mortality in individuals with autism, with and without epilepsy. J Child Neurol. (2011) 26:932–9. 10.1177/0883073811402203 [DOI] [PubMed] [Google Scholar]
- 46.Hirvikoski T, Mittendorfer-Rutz E, Boman M, Larsson H, Lichtenstein P, Bölte S. Premature mortality in autism spectrum disorder. Br J Psychiatry. (2016) 208:232–8. 10.1192/bjp.bp.114.160192 [DOI] [PubMed] [Google Scholar]
- 47.Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, et al. Advances in the treatment of fragile X syndrome. J Pediatr. (2009) 123:378–90. 10.1542/peds.2008-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. (2005) 49:1053–66. 10.1016/j.neuropharm.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 49.Myrick LK, Deng PY, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, et al. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci U S A. (2015) 112:949–56. 10.1073/pnas.1423094112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zweier M, Begemann A, McWalter K, Cho MT, Abela L, Banka S, et al. Spatially clustering de novo variants in CYFIP2, encoding the cytoplasmic FMRP interacting protein 2, cause intellectual disability and seizures. Eur J Hum Genet. (2019) 27:747–59. 10.1038/s41431-018-0331-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernard PB, Castano AM, O'Leary H, Simpson K, Browning MD, Benke TA. Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures. Neurobiol Dis. (2013) 59:1–17. 10.1016/j.nbd.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.