Abstract
Thirty-two Rhipicephalus sanguineus females collected from eight free-roaming dogs in Okinawa Island, Japan, were examined for ehrlichial DNA by 16S rRNA-based PCR and subsequent sequencing. Partial sequences of Ehrlichia platys 16S rRNA (678 to 679 bp) were detected in three ticks (9.4%) from two dogs. This is the first report of detection of E. platys in Japan, and also the first report of detection in ticks.
The ehrlichioses are important emerging tick-borne diseases in both humans and animals. Four species, Ehrlichia muris (6, 7), Ehrlichia detected from Ixodes ovatus (13), Ehrlichia sennetsu (11), and the Stellantchasmus falcatus agent (3), have been found in the main cluster of islands of Japan. Okinawa Island is located 1,500 km southwest of the capital, Tokyo. It has a subtropical climate and a different natural fauna from the main islands. Rhipicephalus sanguineus is the most prevalent tick species in Okinawa Island, and 14% of free-roaming dogs were found to have antibodies to Ehrlichia canis (5). After World War II, a U.S. Army base was established on Okinawa Island and many dogs were consequently introduced. Okinawa Island is geographically close to Taiwan, where canine cases of Ehrlichia platys infection have been reported recently (1). Despite this epidemiological situation, there have been no clinical cases in dogs and little information on canine ehrlichiosis is available in Okinawa.
Thirty-two ticks were collected from eight free-roaming dogs that were caught on Okinawa Island in August 1997. Collected ticks were immersed in 70% ethanol and stored at room temperature. All of them were identified as semiengorged R. sanguineus females by morphological observation. The ticks were taken from the 70% ethanol solution, air dried, and cut into small pieces for DNA extraction. DNA of each tick was extracted by the QIAamp tissue kit procedure (Qiagen GmbH, Hilden, Germany). Finally, DNA from each tick was extracted in 200 μl of Tris-EDTA buffer and stored at −20°C until used.
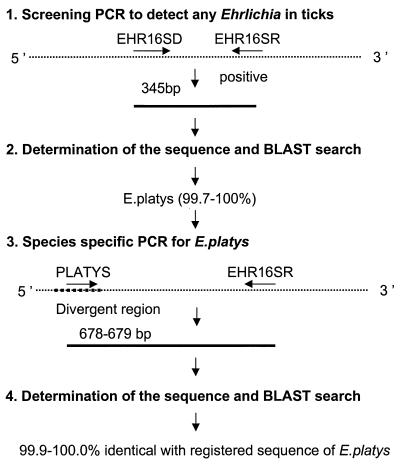
The amplification of Ehrlichia DNA was performed using a genus-specific set of primers of the 16S rRNA gene (12). Briefly, the primer set EHR16SD (5′-GGT-ACC-YAC-AGA-AGA-AGT-CC-3′) and EHR16SR (5′-TAG-CAC-TCA-TCG-TTT-ACA-GC-3′) was used for screening the ehrlichia agents (Fig. 1). The set of primers can amplify various species, including E. canis, E. chaffeensis, E. muris, Ehrlichia detected from I. ovatus ticks, Cowdria ruminantium, the agent of human granulocytic ehrlichiosis, E. equi, E. phagocytophila, E. platys, Anaplasma marginale, Anaplasma centrale, Wolbachia pipientis, E. sennetsu, E. risticii, and Neorickettsia helminthoeca (12; H. Inokuma et al., unpublished data). For the amplification of Ehrlichia DNA, each reaction mixture contained 12.5 pmol of each primer, 0.75 U of Taq DNA polymerase, 20 mM concentrations of each deoxynucleoside triphosphate, 10 mM Tris-HCl, 50 mM KCl, 1.6 mM MgCl2, and 7.5 μl of template tick DNA with a final volume of 25 μl. The amplification was performed in a Peltier model PTC-200 thermal cycler (MJ Research, Inc., Watertown, Mass.) with the following program: an initial 5-min denaturation at 95°C; 34 repeated cycles of denaturation (95°C for 30 s), annealing (55°C for 30 s), and extension (72°C for 90 s); and a 5-min extension at 72°C. In each test, distilled water and DNA of human granulocytic ehrlichia were included as a negative and a positive control, respectively. The amplification products were visualized on a 1 to 2% agarose gel after electrophoretic migration of 8 μl of amplified material. The positive amplification products with 345 bp were then extracted with a QIA PCR purification kit (Qiagen GmbH) for sequence analysis.
FIG. 1.
PCR and sequencing strategy for detection of E. platys DNA from ticks.
To confirm the screening PCR and sequence data, a new PCR method was performed for the positive samples with the screening PCR (Fig. 1). The E. platys specific forward primer (PLATYS; 5′-GAT-TTT-TGT-CGT-AGC-TTG-CTA-TG-3′) was combined with the reverse primer EHR16SR. Five microliters of each sample was used as the template DNA in a final volume of 25 μl, and the rest of the conditions were the same as for the screening PCR except that the number of cycles was 40.
The PCR products used for DNA sequencing were purified with QIAquick PCR purification kits (Qiagen GmbH). For DNA sequencing reactions, fluorescence-labeled dideoxynucleotide technology was used (Perkin-Elmer, Applied Biosystems Division). The sequencing fragments were separated, and data were collected on an ABI 310 automated DNA sequencer (Perkin-Elmer, Applied Biosystems Division). The collected sequences were assembled and edited with the AutoAssembler version 1.4 (Perkin-Elmer). The sequence data of the PCR products in both screening and confirmation were analyzed by the BLAST 2.0 program (National Center for Biotechnology Information [http://www.ncbi.nlm.gov/BLAST/]) for homology.
Seven ticks from four dogs were positive in the screening PCR (Table 1). Analysis of the sequence of 345 bp PCR products showed that three ticks (RS3 and RS5 from dog 1 and RS21 from dog 4) were closely related to E. platys (99.7 and 100.0%, respectively) and that another four ticks (three from dog 3 and another from dog 8) were related to Wolbachia (93.0 and 99.7%, respectively). To confirm the result of screening PCR, E. platys-specific PCR was performed for those three ticks. The 678- or 679-bp nucleotide sequences excluding the primer region obtained from the PCR amplification were all identified as part of the 16S rRNA gene of E. platys with high homology, 100.0% identical for RS3 and RS5 and 99.9% identical (1 nucleotide absent) for RS21, to the sequence of E. platys found in China that is registered in GenBank (AF156784) (Table 2).
TABLE 1.
Detection of partial 16S rRNA gene of E. platys from females of R. sanguineus collected from dogs on Okinawa Island
| Dog | No. of ticks
|
Sequence results | |
|---|---|---|---|
| Examined | Positive in screening PCR | ||
| 1 | 5 | 2 | E. platys |
| 2 | 4 | 0 | |
| 3 | 7 | 3 | |
| 4 | 5 | 1 | E. platys |
| 5 | 3 | 0 | |
| 6 | 3 | 0 | |
| 7 | 2 | 0 | |
| 8 | 2 | 1 | |
TABLE 2.
Comparison of nucleotide sequence of partial 16S rRNA gene detected from R. sanguineus with those of E. platys and similar species registered in GenBank
E. platys parasitizes circulating platelets of dogs and was first reported in 1978 (4). The agent causes canine infectious cyclic thrombocytopenia, an infection which is usually mild or asymptomatic but which may prove fatal when infected dogs hemorrhage after accidents or during surgery (10). E. platys belongs to the group 4 ehrlichiae, closely related to the human granulocytic ehrlichia and Anaplasma, which cause tick-borne transmitted ehrlichioses. Although the vector was previously unknown in Japan, the finding of E. platys on Okinawa Island is not surprising. In fact, one may suggest that E. platys was introduced with dogs transferred from the United States or by the frequent exchange with Taiwan, where the disease has already been reported (2, 9, 10). Although there have been no clinical cases of E. platys infection reported yet in Japan, veterinary clinicians need to be aware of canine infectious cyclic thrombocytopenia. This is the first detection of E. platys in R. sanguineus ticks. Unfortunately, all the ticks we examined were semiengorged and the host dogs were not examined for Ehrlichia agents. As Simpson et al. (14) reported that R. sanguineus might not transmit E. platys infection, it would be premature to assume that the tick is a vector of E. platys. However, the fact that only some of the semiengorged ticks collected on the same dog contained E. platys DNA suggests that the R. sanguineus may be a vector of E. platys. Consequently, examination of unengorged ticks and carrier dogs should be performed to clarify this. Although R. sanguineus is the dominant tick species found on dogs, and canine antibodies against E. canis have been reported in the Okinawa Prefecture (5, 8), no DNA of E. canis was detected in the present study and the reason is unknown. More epidemiological study is needed for a better understanding of the phenomenon. PCR detection of possible canine Ehrlichia spp. such as E. platys and E. canis from dogs in the area is worth trying.
In addition, the two-step procedure used in this study was demonstrated to be useful in detecting DNA from ticks. The first PCR is able to detect a 345-bp 16S rRNA gene of any Ehrlichia. After sequencing analysis of this PCR product, the second species-specific PCR is able to establish the identification of the causative Ehrlichia.
Nucleotide sequence accession numbers.
The sequences derived from the positive ticks have been deposited in GenBank under accession numbers AF288135 (RS3) and AF288136 (RS21).
Acknowledgments
This work was supported in part by grant-in-aid no. 10839019 for Scientific Research, from the Ministry of Education, Science, Sports and Culture, Japan, and a grant from the EGIDE, France.
We thank J. S. Dumler, Johns Hopkins University, for English review of the manuscript.
REFERENCES
- 1.Chang A C H, Chang W L, Lin C T, Pan M J, Lee S C. Canine infectious cyclic thrombocytopenia found in Taiwan. J Vet Med Sci. 1996;58:473–476. doi: 10.1292/jvms.58.473. [DOI] [PubMed] [Google Scholar]
- 2.Chang W L, Pan M J. Specific amplification of Ehrlichia platys DNA from blood specimens by two-step PCR. J Clin Microbiol. 1996;34:3142–3146. doi: 10.1128/jcm.34.12.3142-3146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda T, Sasahara T, Kitao T. Studies on the causative agent of “Hyuganetsu disease.” XI. Vector. J Jpn Assoc Infect Dis. 1972;36:235–241. [Google Scholar]
- 4.Harvey J W, Simpson C F, Gaskin J M. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J Infect Dis. 1978;137:182–188. doi: 10.1093/infdis/137.2.182. [DOI] [PubMed] [Google Scholar]
- 5.Inokuma H, Yamamoto S, Tanahara N, Kiyuna T, Ohshiro S. Surveys for tick infestation and tick-borne disease infection of dogs in Okinawa-island. J Jpn Vet Med Assoc. 1998;51:361–364. [Google Scholar]
- 6.Kawahara M, Suto C, Rikihisa Y, Yamamoto S, Tsuboi Y. Characterization of ehrlichial organisms isolated from a wild mouse. J Clin Microbiol. 1993;31:89–96. doi: 10.1128/jcm.31.1.89-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawahara M, Ito T, Suto C, Shibata S, Rikihisa Y, Hata K, Hirai K. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J Clin Microbiol. 1999;37:1123–1129. doi: 10.1128/jcm.37.4.1123-1129.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keefe T J, Holland C J, Salyer P E, Ristic M. Distribution of Ehrlichia canis among military working dogs in the world and selected civilian dogs in the United States. J Am Vet Med Assoc. 1982;181:236–238. [PubMed] [Google Scholar]
- 9.Kordick S K, Breitschwerdt E B, Hegarty B C, Southwick K L, Colitz C M, Hancock S I, Bradley J M, Rumbough R, McPherson J T, MacCormack J N. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew J S, Ewing S A, Murphy G L, Kocan K M, Corstvet R E, Fox J C. Characterization of a new isolate of Ehrlichia platys (Order Rickettsiales) using electron microscopy and polymerase chain reaction. Vet Parasitol. 1997;68:1–10. doi: 10.1016/s0304-4017(96)01052-7. [DOI] [PubMed] [Google Scholar]
- 11.Misao T, Kobayashi Y. Studies on infectious mononucleosis (glandular fever). I. Isolation of etiologic agent from blood, bone marrow and lymph node of a patient with infectious mononucleosis by using mice. Kyushu J Med Sci. 1955;6:145–152. [Google Scholar]
- 12.Parola, P., V. Roux, J.-L. Camicas, I. Baradji, P. Brouqui, and D. Raoult. Detection of ehrlichiae in African ticks by PCR. Trans. R. Soc. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 13.Shibata S-I, Kawahara M, Rikihisa Y, Fujita H, Watanabe Y, Suto C, Ito T. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J Clin Microbiol. 2000;38:1331–1338. doi: 10.1128/jcm.38.4.1331-1338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson R M, Gaunt S D, Hair J A, Kocan K M, Henk W G, Casey H W. Evaluation of Rhipicephalus sanguineus as a potential biological vector of Ehrlichia platys. Am J Vet Res. 1991;52:1537–1541. [PubMed] [Google Scholar]