Abstract
Objective
Rheumatoid arthritis (RA) is often complicated with chronic lung diseases (CLD), including interstitial lung disease (ILD) and airway disease, which occur as extra-articular manifestations. CLD in RA have been associated with the production of rheumatoid factor (RF), anti-citrullinated peptide antibody (ACPA), or anti-carbamylated protein (CarP) antibody. However, few validation studies have been performed thus far. In the present study, we investigated the association of RF, ACPA, and anti-CarP antibodies with RA complicated with CLD.
Methods
Sera from RA patients with or without CLD were collected. The levels of serum RF, RF immunoglobulin A (IgA), ACPA IgG, ACPA IgA, and ACPA secretory component (SC) were measured using enzyme-linked immunosorbent assay.
Results
The comparison of RA patients with and without CLD showed that RF IgA was associated with ILD (mean ± standard deviation: 206.6 ± 400.5 vs. 95.0 ± 523.1 U/ml, respectively, P = 1.13 × 10− 8), particularly usual interstitial pneumonia (UIP) (263.5 ± 502.0 U/ml, P = 1.00 × 10− 7). ACPA SC was associated with RA complicated with ILD (mean ± standard deviation: 8.6 ± 25.1 vs. 2.3 ± 3.4 U/ml, respectively, P = 0.0003), particularly nonspecific interstitial pneumonia (NSIP) (10.7 ± 31.5 U/ml, P = 0.0017). Anti-CarP antibodies were associated with RA complicated with ILD (0.042 ± 0.285 vs. 0.003 ± 0.011 U/ml, respectively, P = 1.04X10− 11).
Conclusion
RF IgA and ACPA SC in RA were associated with UIP and NSIP, respectively, suggesting different specificities in patients with RA. Anti-CarP antibodies were associated with ILD in RA. These results may help elucidate the different pathogeneses of UIP and NSIP in RA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-021-04985-0.
Keywords: Rheumatoid arthritis, Interstitial lung disease, Anti-citrullinated peptide antibody, Secretory component, Rheumatoid factor, Usual interstitial pneumonia, Nonspecific interstitial pneumonia, IgA
Introduction
Rheumatoid arthritis (RA), a systemic autoimmune disease affecting synovial joints, is often complicated with chronic lung diseases (CLD), including interstitial lung disease (ILD) and airway disease (AD). RA patients complicated with ILD or AD are associated with a poor prognosis [1–5]. Thus, it is necessary to clarify the pathological conditions of ILD and AD in RA.
Rheumatoid factors (RFs) are autoantibodies against immunoglobulin G (IgG) Fc fragments; most RFs belong to the IgM class. The serum levels of RFs are linked to RA-associated ILD (RA-ILD) [6, 7]. The levels of RF IgA are also related to RA-ILD [8, 9]. Anti-citrullinated peptide antibodies (ACPAs) are autoantibodies against citrullinated peptides. Citrullinated peptides are generated through posttranslational modification of arginine residues to citrulline by peptidylarginine deiminases. The specificity of ACPA for RA is higher than that of RFs, and the serum levels of ACPA IgG are associated with RA-ILD [6, 9, 10]. Notably, idiopathic pulmonary fibrosis is associated with ACPA IgA [8, 11]. Moreover, the serum levels of ACPAs with secretory component (SC) have been linked to RA-ILD [12]. SC attaches to IgA and IgM to form secretory IgA and IgM, respectively; these secretory Igs are subsequently transported to the mucosa. Small amounts of secretory Igs have also been found in sera [13, 14]. Studies have revealed that the major portion of serum ACPA SC is composed of IgM [15]. Anti-carbamylated protein (CarP) antibodies recognize homo-citrullinated peptides generated by posttranslational modification of lysine residues. Anti-CarP antibodies were reported to be associated with ILD in RA [16]. However, few validation studies on those associations of autoantibodies with RA-ILD have been performed thus far. In the present study, we investigated the association of RF, ACPA, and anti-CarP antibodies with RA-ILD in Japan.
Materials and methods
Patients and sera
A total of 453 patients with RA, for whom chest computed tomography data were available, were recruited at Sagamihara National Hospital, Himeji Medical Center, Miyakonojo Medical Center, Nagoya Medical Center, and Nagasaki Medical Center from 2010 to 2017. These patients fulfilled the American College of Rheumatology criteria for RA [17] or Rheumatoid Arthritis Classification Criteria [18]. Sera from the patients were collected and analyzed for the production of autoantibodies. Based on the predominant findings of chest computed tomography, the patients were categorized as follows, usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), AD, emphysema, or no CLD [19]. The study was reviewed and approved by the National Hospital Organization Central Institutional Review Board, Research Ethics Committees of Sagamihara National Hospital and Tokyo National Hospital. Written informed consent was provided by all the participants. This study was conducted in accordance with the principles stipulated in the Declaration of Helsinki.
Autoantibody detection
RF was detected using an N-latex RF kit (Siemens Healthcare Diagnostics, München, Germany), and the cut-off value set by manufacturer was 15 U/ml in the kit. RF IgA was detected using a Rheumatoid factor IgA kit (Organtech Diagnostika, Mainz, Germany). Based on the 98th percentile among 52 healthy controls, the cut-off value for positivity was set to 14.572. ACPA IgG was measured using the Mesacup-2 test cyclic citrullinated peptide, and the cut-off value set by manufacturer was 4.5 U/ml in the kit (Medical & Biological Laboratories, Nagoya, Japan). ACPA IgA was detected with the Mesacup-2 test CCP and peroxidase conjugated goat anti-human IgA alpha chain antibodies (Jackson ImmunoResearch, West Grove, PA, USA). Sera and secondary antibodies were diluted (1:100 and 1:10,000, respectively) using the dilution buffer included in the kit. The levels of ACPA IgA in pooled sera from three patients with known high levels of ACPA IgA were designated to be 27 U/ml. The pooled sera were serially diluted to be used as the calibrator, and the results were presented as arbitrary units (U/ml). Based on the 98th percentile among 52 healthy controls, the cut-off value for positivity was set to 0.944. ACPA SC was detected using Immunoscan CCPlus (Svar Life Science, Malmö, Sweden) and peroxidase conjugated goat anti-human SC antibodies (Nordic-MUbio, Susteren, Netherlands). Sera and secondary antibodies were diluted (1:22 and 1:2000, respectively) using the dilution buffer included in the kit. The levels of ACPA SC in pooled sera from three patients with known high levels of ACPA SC were designated to be 27 U/ml. The pooled sera were serially diluted to be used as the calibrator, and the results were presented as arbitrary units (U/ml). Based on the 98th percentile among 52 healthy controls, the cut-off value for positivity was set to 0.966. Anti-CarP antibodies were detected using human anti-CarP ELISA kit (Wuhan Fine Biotech Co., Ltd., Wuhan, China). Sera were diluted (1:25) using the dilution buffer included in the kit. The levels of anti-CarP antibodies in the standard were designated to be 1 U/ml. The results were presented as arbitrary units (U/ml). Based on the 98th percentile among 52 healthy controls, the cut-off value for positivity was set to 0. The presence of RF and ACPA IgG autoantibodies in patients with RA has been reported in previous studies [19, 20]. Krebs von den lungen-6 (KL-6) was detected using a Picolumi KL-6 Electrochemiluminescence immunoassay system (EIDIA Co., Ltd., Tokyo, Japan) and the cut-off value set by manufacturer was 500 U/ml. Surfactant protein-D (SP-D) was detected using the SP-D kit “Yamasa” EIA II (Yamasa Corporation, Choshi, Japan) and the cut-off value for positivity set by manufacturer was 110 ng/ml. Steinbrocker stages were measured as previously described [19, 21].
Statistical analysis
The demographic features of RA patients and autoantibody productions were compared with those of RA patients without CLD by Fisher’s exact test using 2 × 2 contingency tables or the Mann–Whitney U test. Receiver operator characteristic (ROC) curves for RF or ACPA were generated to compare RA patients with and without CLD. The area under the curve (AUC) values of the ROC curves with 95% confidence intervals were estimated. In addition, the optimized cut-off levels with specificities and sensitivities conditional on the highest Youden’s index were calculated. A P < 0.05 value denoted statistically significant difference.
Results
Clinical features of patients with RA
Patient characteristics are presented in Table 1. The mean age, age at onset, KL-6 levels, and SP-D levels were increased in RA patients with ILD versus those without CLD. The percentage of smokers or former smokers and KL-6 levels were higher in RA patients with AD. Furthermore, Steinbrocker stage, percentage of smokers or former smokers, KL-6 levels, and SP-D levels in RA patients with emphysema were also increased.
Table 1.
Characteristics of patients with RA
| ILD | UIP | NSIP | AD | Emphysema | CLD(+) | CLD(−) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | P | P | P | P | P | ||||||||
| Number, n | 115 | 46 | 69 | 112 | 37 | 264 | 189 | ||||||
| Mean age, years (SD) | 67.2 (8.3) | 0.0009 | 67.1 (9.1) | 0.0137 | 67.3 (7.8) | 0.0061 | 65.6 (9.8) | 0.0553 | 66.3 (8.1) | 0.1247 | 66.4 (9.0) | 0.0013 | 62.8 (11.1) |
| Male, n (%) | 27 (23.5) | *0.3076 | 16 (34.8) | *0.0267 | 11 (15.9) | *0.7155 | 15 (13.4) | *0.2667 | 22 (59.5) | *1.06 × 10−6 | 64 (24.2) | *0.1668 | 35 (18.5) |
| Age at onset, years (SD) | 54.1 (12.9) | 0.0018 | 54.0 (14.3) | 0.0336 | 54.2 (12.0) | 0.0067 | 50.5 (14.6) | 0.2257 | 56.5 (11.2) | 0.0012 | 52.9 (13.6) | 0.0014 | 49.2 (13.2) |
| Steinbrocker stages III and IV, n (%) | 53 (46.1) | *0.0773 | 25 (54.3) | *0.8687 | 28 (40.6) | *0.0248 | 65 (58.6) | *0.8092 | 13 (35.1) | *0.0194 | 131 (49.8) | *0.1812 | 107 (56.6) |
| Smoker or former smoker, n (%) | 42 (38.5) | *0.1186 | 18 (40.9) | *0.1471 | 24 (36.9) | *0.2730 | 41 (41.0) | *0.0469 | 28 (84.8) | *1.98 × 10−9 | 111 (45.9) | *0.0005 | 51 (29.0) |
| KL-6, U/ml (SD) | 726.6 (639.4) | 1.11 × 10−15 | 749.9 (652.6) | 1.77 × 10− 9 | 710.5 (635.1) | 3.10 × 10−12 | 383.2 (330.0) | 0.0012 | 585.5 (466.0) | 1.92 × 10−6 | 585.3 (546.4) | 2.60 × 10−13 | 293.6 (294.4) |
| SP-D, ng/ml (SD) | 122.3 (144.9) | 1.95 × 10−9 | 128.6 (92.6) | 2.43 × 10− 7 | 117.9 (173.8) | 2.27 × 10−6 | 67.2 (70.9) | 0.1588 | 93.2 (69.5) | 0.0003 | 99.2 (117.4) | 4.58 × 10−7 | 51.1 (40.9) |
AD Airway disease, CLD Chronic lung disease, CLD(+), with CLD, CLD(−), without CLD, KL-6 Krebs von den lungen-6, NSIP Nonspecific interstitial pneumonia, RA Rheumatoid arthritis, SP-D Surfactant protein-D, UIP Usual interstitial pneumonia
ILD group includes UIP and NSIP groups. CLD(+) group includes UIP, NSIP, AD, and emphysema groups
Data are presented as the mean value or number of each group. Standard deviations or percentages are shown in parentheses. Statistical differences were tested in comparison with the CLD(−) population by Fisher’s exact test using 2 × 2 contingency tables or the Mann–Whitney U test. *Fisher’s exact test
RF, ACPA, and anti-CarP antibodies in patients with RA
The production of RF and ACPA was analyzed in the sera of RA patients with and without CLD (Table 2, Fig. 1). RF was associated with ILD (mean ± standard deviation: 510.9 ± 1213.6 vs. 235.69 ± 569.9 U/ml, respectively, P = 0.0025), AD (233.6 ± 362.6 U/ml, P = 0.0149), emphysema (871.4 ± 1993.6 U/ml, P = 0.0002), and CLD (443.8 ± 1133.3 U/ml, P = 9.58 × 10− 5). RF IgA was associated with ILD (206.6 ± 400.5 vs. 95.0 ± 523.1 U/ml, respectively, P = 1.13 × 10− 8), particularly UIP (263.5 ± 502.0 U/ml, P = 1.00 × 10− 7). RF IgA was also associated with emphysema (293.0 ± 687.4, P = 8.64 × 10− 6) and CLD (195.1 ± 601.9 U/ml, P = 7.21 × 10− 7). ACPA IgG was associated with RA with emphysema (445.6 ± 400.6 vs. 270.7 ± 308.3 U/ml, respectively, P = 0.0033). ACPA IgA was associated with RA with ILD (11.8 ± 42.7 vs. 4.1 ± 6.7 U/ml, respectively, P = 0.0159), AD (9.2 ± 34.4 U/ml, P = 0.0468), emphysema (14.0 ± 29.7 U/ml, P = 0.0020), and CLD (11.0 ± 37.6 U/ml, P = 0.0015). ACPA SC was associated with RA complicated with ILD (8.6 ± 25.1 vs. 2.3 ± 3.4 U/ml, respectively, P = 0.0003), particularly NSIP (10.7 ± 31.5 U/ml, P = 0.0017). ACPA SC was also associated with emphysema (7.9 ± 8.6 U/ml, P = 3.89 × 10− 7) and CLD (6.8 ± 18.7 U/ml, P = 3.05 × 10− 5). Anti-CarP antibodies were associated with RA complicated with ILD (0.042 ± 0.285 vs. 0.003 ± 0.011 U/ml, respectively, P = 1.04X10− 11). Anti-CarP antibodies were also associated with CLD (0.021 ± 0.189 U/ml, P = 4.75 × 10− 5). The positivity for RF, ACPA, and anti-CarP antibodies was also analyzed in RA patients with or without CLD (Supplementary Table S1). Although similar results were obtained, the association of the positivity of ACPA SC with NSIP in RA was not stronger than that observed for the levels of ACPA SC (Supplementary Table S1, Fig. 1E). This may be attributed to the extremely higher expression levels of ACPA SC in some RA patients with NSIP. Thus, RF IgA in RA was associated with ILD (particularly UIP), while ACPA SC in RA was associated with ILD (particularly NSIP). Additionally, anti-CarP antibodies were associated with ILD in RA.
Table 2.
RF, ACPA, and anti-CarP Ab in patients with RA
| ILD | UIP | NSIP | AD | emphysema | CLD(+) | CLD(−) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | P | P | P | P | P | ||||||||
| RF, U/ml (SD) | 510.9 (1213.6) | 0.0025 | 501.2 (992.0) | 0.0071 | 517.5 (1348.2) | 0.0320 | 233.6 (362.6) | 0.0149 | 871.4 (1993.6) | 0.0002 | 443.8 (1133.3) | 9.58X10−5 | 235.6 (569.9) |
| RF IgA, U/ml (SD) | 206.6 (400.5) | 1.13X10−8 | 263.5 (502.0) | 1.00X10−7 | 168.6 (313.8) | 0.0001 | 151.1 (731.5) | 0.1945 | 293.0 (687.4) | 8.64X10−6 | 195.1 (601.9) | 7.21X10−7 | 95.0 (523.1) |
| ACPA IgG, U/ml (SD) | 363.8 (776.1) | 0.5244 | 282.5 (302.2) | 0.5943 | 415.7 (962.9) | 0.6334 | 306.4 (388.6) | 0.5285 | 445.6 (400.6) | 0.0033 | 350.8 (589.1) | 0.1439 | 270.7 (308.3) |
| ACPA IgA, U/ml (SD) | 11.8 (42.7) | 0.0159 | 8.5 (21.4) | 0.0198 | 14.0 (52.3) | 0.1172 | 9.2 (34.4) | 0.0468 | 14.0 (29.7) | 0.0020 | 11.0 (37.6) | 0.0015 | 4.1 (6.7) |
| ACPA sc, U/ml (SD) | 8.6 (25.1) | 0.0003 | 5.4 (9.3) | 0.0136 | 10.7 (31.5) | 0.0017 | 4.6 (12.1) | 0.0999 | 7.9 (8.6) | 3.89X10−7 | 6.8 (18.7) | 3.05X10−5 | 2.3 (3.4) |
| Anti-CarPAb, U/ml (SD) | 0.042 (0.285) | 1.04X10−11 | 0.012 (0.016) | 1.22X10−9 | 0.063 (0.367) | 1.02X10−7 | 0.004 (0.012) | 0.7585 | 0.010 (0.032) | 0.1229 | 0.021 (0.189) | 4.75X10−5 | 0.003 (0.011) |
ACPA Anti-citrullinated peptide antibody, AD Airway disease, CLD Chronic lung disease, CLD(+) with CLD, CLD(−) Without CLD, ILD Interstitial lung disease, NSIP, Nonspecific interstitial pneumonia, RF Rheumatoid factor, RA Rheumatoid arthritis, SC Secretory component, UIP Usual interstitial pneumonia
The ILD group includes the UIP and NSIP groups. The CLD(+) group includes the UIP, NSIP, AD, and emphysema groups
Data are presented as the mean value of each group; standard deviations are shown in parentheses
Statistical difference was tested in comparison with the CLD(−) population using the Mann–Whitney U test
Fig. 1.
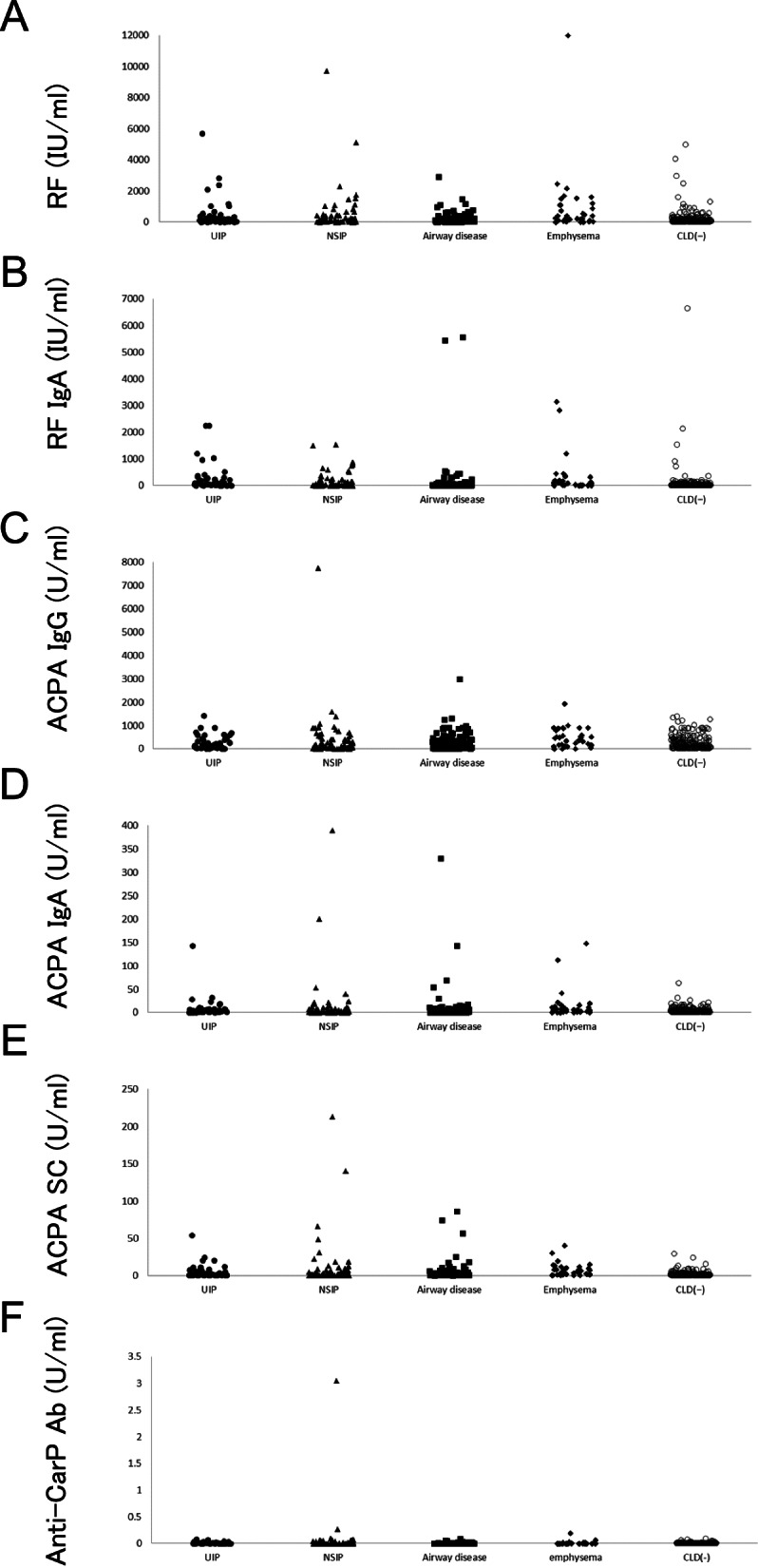
Evaluation of the RF or ACPA levels in patients with RA. Distribution of RF (A), RF IgA (B), ACPA IgG (C), ACPA IgA (D), ACPA SC (E), and anti-CarP Ab (F) levels. The filled circle, filled triangle, filled square, filled diamond, and empty circle represent RA with UIP, RA with NSIP, RA with airway disease, RA with emphysema, and RA without CLD, respectively. ACPA: anti-cyclic citrullinated peptide antibody, CLD: chronic lung disease, CLD(−): without CLD Ig immunoglobulin, NSIP: nonspecific interstitial pneumonia, RA: rheumatoid arthritis, RF: rheumatoid factor, SC: secretory component, UIP: usual interstitial pneumonia, CarP: carbamylated protein, Ab: antibody
ROC curves for RF, ACPA, and anti-CarP antibodies were generated to compare RA patients with and without CLD (Supplementary Fig. S1). The AUC values of the ROC curves with 95% confidence intervals were calculated. However, AUC values of these ROC curves were < 0.7. These data indicated that RF, ACPA, and anti-CarP antibodies are not sufficiently strong biomarkers for the diagnosis of CLD.
Discussion
In the present study, RF IgA was associated with RA-ILD (particularly UIP), while ACPA SC was associated with RA complicated with ILD (particularly NSIP). Anti-CarP antibodies were associated with ILD in RA. The association of RF IgA with RA-ILD was previously reported [8, 9]. Although this association was confirmed in this study, the stronger association with UIP was not observed. The association of ACPA SC with RA-ILD was also previously reported [12], and a stronger association with NSIP was found in the present study. Thus, the present results suggested different specificities of RF IgA for UIP and ACPA SC for NSIP in patients with RA. Furthermore, the evidence suggests the involvement of these autoantibodies in the development of UIP or NSIP in RA.
The data obtained from this study indicates that RF, ACPA, and anti-CarP antibodies are not good biomarkers for the diagnosis of ILD or CLD compared with the levels of KL-6 or SP-D (Tables 1 and 2, Supplementary Fig. S1). However, the association of RF IgA with UIP may elucidate the pathogenesis of UIP in RA. Analogically, the association of ACPA SC with NSIP in RA may explain the pathophysiology of NSIP in RA. Autoantibody levels in RA with AD were lower (Table 2), suggesting the heterogeneity of CLD in RA. In contrast, the expression levels of RF and ACPA were elevated in RA patients with emphysema; notably, the percentages of males and ever smokers were higher in this group of patients than in other groups. It is established that smoking increases the expression of peptidylarginine deiminase 2 and generates citrullinated autoantigens in the lung [22, 23]. These data suggest that smoking affects the autoantibody production and the development of RA; nevertheless, it was difficult to determine the roles of autoantibodies in the development of emphysema in RA. The origin of ACPA SC remains unknown and may be the lung, gastrointestinal tract, or oral cavity. In RA patients with emphysema, the origin of these antibodies may be the lung. CarPs were detected in the synovial tissues of RA patients and lung tissues from smokers [24, 25]. Although anti-CarP antibodies were not increased in RA with emphysema (Table 2), they might react with homo-citrullinated peptides in lung and causes inflammatory responses leading to ILD, but not to emphysema. Some studies reported on the associations of autoantibody productions and RA-ILD, namely anti-citrullinated alpha-enolase peptide-1 antibodies [26, 27], anti-citrullinated heat shock protein 90 antibodies [28], and anti-malondialdehyde-acetaldehyde antibodies [29]. These results suggested the involvement of several autoantibodies on the pathogenesis of ILD in RA. Epitope spread against citrullinated peptides may contribute to the development of RA and RA-ILD [30, 31]; the citrullinated autoantigens of ACPA SC in RA-ILD should be validated in future investigations.
To the best of our knowledge, this is the first study to report the different specificities of RF IgA for UIP and ACPA SC for NSIP in patients with RA. The sample size of the present study was small. Therefore, additional large-scale studies on RF IgA for UIP and ACPA SC for NSIP should be conducted to validate the present findings. Serum autoantibodies with SC have also been detected in other collagen vascular disease-associated ILD than RA. Serum anti-proteinase 3 autoantibodies with SC were detected in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis [32]. Autoantibody profiles in patients with other collagen vascular disease-associated ILD than RA or idiopathic interstitial pneumonia were not analyzed in the present study. Future investigations should analyse RF IgA, ACPA SC, and anti-CarP antibodies in these patients.
Supplementary Information
Authors’ contributions
HF and ST conceived and designed the experiments. SO, TH, and HF performed the experiments. SO and HF analyzed the data. HF, KS, AO, AH, AK, KS, NY, MK, TM, NF, KM, and ST contributed reagents/materials/analysis tools. SO, HF, and ST contributed to the writing of the manuscript. All authors read and approved the final version of the manuscript.
Funding
The work was supported by Grants-in-Aid for Scientific Research (B, C) (26293123, 22591090, 15 K09543, 18 K08402) and for Young Scientists (B) (24791018) from the Japan Society for the Promotion of Science, Health and Labour Science Research Grants from the Ministry of Health, Labour, and Welfare of Japan, Grants-in-Aid of the Practical Research Project for Allergic Diseases and Immunology (Research on Allergic Diseases and Immunology) from Japan Agency for Medical Research and Development, Grants-in-Aid for Clinical Research from National Hospital Organization, Research Grants from Daiwa Securities Health Foundation, Research Grants from Japan Research Foundation for Clinical Pharmacology, Research Grants from The Nakatomi Foundation, Research Grants from Takeda Science Foundation, Research Grants from Mitsui Sumitomo Insurance Welfare Foundation, and Bristol-Myers K.K. RA Clinical Investigation Grant from Bristol-Myers Squibb Co. Research grants were also received from the following pharmaceutical companies: Abbott Japan Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Merck Sharp and Dohme Inc., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited, and Teijin Pharma Limited. The funders had no role in the study design, data collection and analysis, decision to publish the data, or preparation of the manuscript.
Declarations
Competing interests
HF has the following conflicts, and the following funders are supported wholly or in part by the indicated pharmaceutical companies. The Japan Research Foundation for Clinical Pharmacology is run by Daiichi Sankyo, the Takeda Science Foundation is supported by an endowment from Takeda Pharmaceutical Company and the Nakatomi Foundation was established by Hisamitsu Pharmaceutical Co., Inc. The Daiwa Securities Health Foundation was established by Daiwa Securities Group Inc. and Mitsui Sumitomo Insurance Welfare Foundation was established by Mitsui Sumitomo Insurance Co., Ltd. HF was supported by research grants from Bristol-Myers Squibb Co. HF received honoraria from Ajinomoto Co., Inc., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Pfizer Japan Inc., and Takeda Pharmaceutical Company, Luminex Japan Corporation Ltd., and Ayumi Pharmaceutical Corporation. ST was supported by research grants from nine pharmaceutical companies: Abbott Japan Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Merck Sharp and Dohme Inc., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited, and Teijin Pharma Limited. ST received honoraria from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., AbbVie GK., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Pfizer Japan Inc. The other authors have no financial or commercial conflict of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hakala M. Poor prognosis in patients with rheumatoid arthritis hospitalized for interstitial lung fibrosis. Chest. 1988;93(1):114–118. doi: 10.1378/chest.93.1.114. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2004;33(2):65–72. doi: 10.1080/03009740310004621. [DOI] [PubMed] [Google Scholar]
- 3.Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, Dixey J, Gough A, Prouse P, Winfield J, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology. 2010;49(8):1483–1489. doi: 10.1093/rheumatology/keq035. [DOI] [PubMed] [Google Scholar]
- 4.Vergnenegre A, Pugnere N, Antonini MT, Arnaud M, Melloni B, Treves R, Bonnaud F. Airway obstruction and rheumatoid arthritis. Eur Respir J. 1997;10(5):1072–1078. doi: 10.1183/09031936.97.10051072. [DOI] [PubMed] [Google Scholar]
- 5.Swinson DR, Symmons D, Suresh U, Jones M, Booth J. Decreased survival in patients with co-existent rheumatoid arthritis and bronchiectasis. Br J Rheumatol. 1997;36(6):689–691. doi: 10.1093/rheumatology/36.6.689. [DOI] [PubMed] [Google Scholar]
- 6.Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106(11):1591–1599. doi: 10.1016/j.rmed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Kakutani T, Hashimoto A, Tominaga A, Kodama K, Nogi S, Tsuno H, Ogihara H, Nunokawa T, Komiya A, Furukawa H, et al. Related factors, increased mortality and causes of death in patients with rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. 2020;30(3):458–464. doi: 10.1080/14397595.2019.1621462. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein EJ, Barr RG, Austin JHM, Kawut SM, Raghu G, Sell JL, Hoffman EA, Newell JD, Jr, Watts JR, Jr, Nath PH, et al. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the multi-ethnic study of atherosclerosis. Thorax. 2016;71(12):1082–1090. doi: 10.1136/thoraxjnl-2016-208932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshua V, Hensvold AH, Reynisdottir G, Hansson M, Cornillet M, Nogueira L, Serre G, Nyren S, Karimi R, Eklund A, et al. Association between number and type of different ACPA fine specificities with lung abnormalities in early, untreated rheumatoid arthritis. RMD Open. 2020;6(2):2020–001278. doi: 10.1136/rmdopen-2020-001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Zhou Y, Chen X, Li J. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol. 2014;41(7):1282–1289. doi: 10.3899/jrheum.131341. [DOI] [PubMed] [Google Scholar]
- 11.Solomon JJ, Matson S, Kelmenson LB, Chung JH, Hobbs SB, Rosas IO, Dellaripa PF, Doyle TJ, Poli S, Esposito AJ, et al. IgA antibodies directed against Citrullinated protein antigens are elevated in patients with idiopathic pulmonary fibrosis. Chest. 2020;157(6):1513–1521. doi: 10.1016/j.chest.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos Ljungberg K, Joshua V, Skogh T, Eklund A, Sköld CM, Karimi R, Nyrén S, Svärd A, Catrina AI, Kastbom A. Secretory anti-citrullinated protein antibodies in serum associate with lung involvement in early rheumatoid arthritis. Rheumatology (Oxford) 2020;59(4):852–859. doi: 10.1093/rheumatology/kez377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldman RH, Mach JP, Stella MM, Rowe DS. Secretory IgA in human serum. J Immunol. 1970;105(1):43–47. [PubMed] [Google Scholar]
- 14.Eijgenraam JW, Oortwijn BD, Kamerling SW, de Fijter JW, van den Wall Bake AW, Daha MR, van Kooten C. Secretory immunoglobulin a (IgA) responses in IgA nephropathy patients after mucosal immunization, as part of a polymeric IgA response. Clin Exp Immunol. 2008;152(2):227–232. doi: 10.1111/j.1365-2249.2008.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Delft MAM, van der Woude D, Toes REM, Trouw LA. Secretory form of rheumatoid arthritis-associated autoantibodies in serum are mainly of the IgM isotype, suggesting a continuous reactivation of autoantibody responses at mucosal surfaces. Ann Rheum Dis. 2019;78(1):146–148. doi: 10.1136/annrheumdis-2018-213724. [DOI] [PubMed] [Google Scholar]
- 16.Castellanos-Moreira R, Rodríguez-García SC, Gomara MJ, Ruiz-Esquide V, Cuervo A, Casafont-Solé I, Ramírez J, Holgado S, Gómez-Puerta JA, Cañete JD, et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann Rheum Dis. 2020;79(5):587–594. doi: 10.1136/annrheumdis-2019-216709. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 19.Oka S, Furukawa H, Shimada K, Sugii S, Hashimoto A, Komiya A, Fukui N, Suda A, Tsunoda S, Ito S, et al. Association of Human Leukocyte Antigen Alleles with chronic lung diseases in rheumatoid arthritis. Rheumatology (Oxford) 2016;55(7):1301–1307. doi: 10.1093/rheumatology/kew025. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa H, Oka S, Shimada K, Okamoto A, Hashimoto A, Komiya A, Saisho K, Yoshikawa N, Katayama M, Matsui T, et al. Serum Metabolomic profiling in rheumatoid arthritis patients with interstitial lung disease: a case-control study. Front Med (Lausanne) 2020;7(599794):599794. doi: 10.3389/fmed.2020.599794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc. 1949;140(8):659–662. doi: 10.1001/jama.1949.02900430001001. [DOI] [PubMed] [Google Scholar]
- 22.Klareskog L, Stolt P, Lundberg K, Källberg H, Bengtsson C, Grunewald J, Rönnelid J, Harris HE, Ulfgren AK, Rantapää-Dahlqvist S, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 23.Källberg H, Ding B, Padyukov L, Bengtsson C, Rönnelid J, Klareskog L, Alfredsson L. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70(3):508–511. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turunen S, Huhtakangas J, Nousiainen T, Valkealahti M, Melkko J, Risteli J, Lehenkari P. Rheumatoid arthritis antigens homocitrulline and citrulline are generated by local myeloperoxidase and peptidyl arginine deiminases 2, 3 and 4 in rheumatoid nodule and synovial tissue. Arthritis Res Ther. 2016;18(1):239. doi: 10.1186/s13075-016-1140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugli EB, Correia RE, Fischer R, Lundberg K, Bracke KR, Montgomery AB, Kessler BM, Brusselle GG, Venables PJ. Expression of citrulline and homocitrulline residues in the lungs of non-smokers and smokers: implications for autoimmunity in rheumatoid arthritis. Arthritis Res Ther. 2015;17(1):9. doi: 10.1186/s13075-015-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alunno A, Bistoni O, Pratesi F, La Paglia GMC, Puxeddu I, Migliorini P, Gerli R. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology (Oxford) 2018;57(5):850–855. doi: 10.1093/rheumatology/kex520. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Liu C, Li L, Zhang F, Li Y, Zhang S. High levels of antibodies to citrullinated α-enolase peptide-1 (CEP-1) identify erosions and interstitial lung disease (ILD) in a Chinese rheumatoid arthritis cohort. Clin Immunol. 2019;200:10–15. doi: 10.1016/j.clim.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, Oddis CV, Ascherman DP. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869–879. doi: 10.1002/art.37881. [DOI] [PubMed] [Google Scholar]
- 29.England BR, Duryee MJ, Roul P, Mahajan TD, Singh N, Poole JA, Ascherman DP, Caplan L, Demoruelle MK, Deane KD, et al. Malondialdehyde-acetaldehyde adducts and antibody responses in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 2019;71(9):1483–1493. doi: 10.1002/art.40900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. Plos One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, Siegelman S, Connors G, Robinson WH, Bathon JM. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. 2014;73(8):1487–1494. doi: 10.1136/annrheumdis-2012-203160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandin C, Eriksson P, Segelmark M, Skogh T, Kastbom A. IgA- and SIgA anti-PR3 antibodies in serum versus organ involvement and disease activity in PR3-ANCA-associated vasculitis. Clin Exp Immunol. 2016;184(2):208–215. doi: 10.1111/cei.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


