Abstract
Background
Movement disorders can be associated with anti-neuronal antibodies.
Methods
We conducted a systematic review of cases with documented anti-neuronal antibodies in serum and/or cerebrospinal fluid published in PubMed before April 1, 2020. Only patients with at least one movement disorder were included. We used random forests for variable selection and recursive partitioning and regression trees for the creation of a data-driven decision algorithm, integrated with expert’s clinical feedback.
Results
Three hundred and seventy-seven studies met eligibility criteria, totaling 844 patients and 13 antibodies: amphiphysin, GAD, GlyR, mGluR1, ANNA-2/Ri, Yo/PCA-1, Caspr2, NMDAR, LGI-1, CRMP5/CV2, ANNA-1/Hu, IgLON5, and DPPX. Stiffness/rigidity/spasm spectrum symptoms were more frequently associated with amphiphysin, GAD, and GlyR; ataxia with mGluR1, ANNA-2/Ri, Yo/PCA-1, Caspr2, and ANNA-1/Hu; dyskinesia with NMDAR and paroxysmal movement with LGI1; chorea/choreoathetosis with CRMP5/CV2, IgLON5, and NMDAR; myoclonus with GlyR and DPPX; tremors with ANNA2/Ri and anti-DPPX; and parkinsonism with IgLON5 and NMDAR. Data-driven classification analysis determined the following diagnostic predictions (with probability selection): psychiatric symptoms and dyskinesia predicted NMDAR (71% and 87%, respectively); stiffness/rigidity/spasm and ataxia, GAD (67% and 47%, respectively); ataxia and opsoclonus, ANNA-2/Ri (68%); chorea/choreoathetosis, CRMP5/CV2 (41%). These symptoms remained the top predictors in random forests analysis. The integration with an expert opinion analysis refined the precision of the approach. Breast and lung tumors were the most common tumors. On neuroimaging, cerebellar involvement was associated with GAD and Yo/PCA-1; temporal involvement with Caspr2, LGI-1, ANNA-1/Hu.
Conclusion
Selected movement disorders are associated with specific anti-neuronal antibodies. The combination of data-driven and expert opinion approach to the diagnosis may assist early management efforts.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-021-10934-7.
Keywords: Movement disorders, Neuronal antibodies, Systematic review
Introduction
The presence of anti-neuronal antibodies is responsible for a clinically heterogeneous range of neurological disorders [1]. Autoimmune-induced movement disorders are often present, either at presentation or throughout the disease course [2]. The targeting of specific synaptic proteins from regions involved in motor control such as the cortex, basal ganglia, and brainstem [3] provide the rationale for the pathogenicity of anti-neuronal antibodies and the range of their associated symptoms and signs.
The detection of anti-neuronal antibodies is a key step in the diagnosis and management of these conditions. Over the past several years, the number of antigenic targets for such antibodies has greatly expanded, making the laboratory diagnostic work up challenging. The identification of the associated antibody is a critical step also in determining the management given the potential response to immunomodulatory therapies and the associated comorbidities, such as tumors [4, 5].
Movement disorders can be a useful diagnostic clue to orient the diagnosis towards specific antibodies. High index of suspicion for a specific antibody expedites the diagnostic process, improves the clinical outcomes, and may reduce the health care costs. Toward this aim, we conducted a systematic review of clinical reports of patients with movement disorders associated with autoantibodies in order to generate a specific approach for the identification of specific pathogenic antibodies in the setting of specific abnormal movements.
Methods
We conducted a systematic review and single-patient meta-analysis using random forest analysis method to ascertain the associated tumors, neuroimaging abnormalities, and temporal sequence of symptoms in patients with autoimmune movement disorders. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Observational Studies in Epidemiology (MOOSE) guidelines [6, 7], searching PubMed for studies published between 1967 and April 1, 2020. The search strategy used both clinical nosology and antibodies (full MeSH [Medical Subject Headings] terms; Supplementary material 1). Abstracts and full-text articles were independently reviewed for eligibility criteria by four authors (BG, PB, KD, JAV). The reference list of each included article was searched to screen for additional studies. Only studies in human subjects and published in English were considered; no other restrictions were applied.
Inclusion/exclusion criteria
We only included studies with confirmed presence of antibodies, defined as those in whom an antibody was identified in blood and/or cerebrospinal fluid (CSF), and with at least one movement disorder. Studies with aggregated rather than individualized data or of subjects with more than one antibody or with associated conditions that could have affected the clinical presentation were excluded, except for PCA-2/MAP1B, given their common association with other antibodies [8]. No age restriction was applied. Only antibodies with at least 15 cases were included [9].
Data extraction
A data collection form was used to extract the variables of interest from eligible studies by two neurologists trained in movement disorders (AS and LM). Any discrepancies in data collection were resolved by a senior neurologist (AJE) and an expert in autoimmune encephalitis (MG). Variables extracted included the year of publication, study type, demographics, antibody, clinical and magnetic resonance imaging (MRI) data. We used a standardized data collection form to extract data from the included studies. We also documented whether abnormal movements appeared at the onset or at the nadir or recovery phases. We divided the symptoms according to: 1) movement disorders nosology: muscle hyperactivity (stiffness/rigidity/spasm), dyskinesia, opsoclonus, myoclonus/jerking, cerebellar ataxia (both gait and/or appendicular), chorea/choreoathetosis, dystonia, parkinsonism, startle reaction, tremors, stereotypies, opisthotonus; and 2) non-movement disorders nosology: oculomotor abnormalities other than opsoclonus (e.g. nystagmus, diplopia, ophthalmoplegia, ptosis), sensory, alteration in consciousness, seizure, dysphagia, autonomic, cognitive, and psychiatric symptoms. We considered opsoclonus as a movement nosology given its association with myoclonus. Symptoms were analyzed independently from the pathogenic mechanism or if part of a syndromic condition. When a patient was reported in multiple studies only one study was selected.
Statistical analysis
Data were summarized with either mean or median and standard deviation (SD) or frequency and proportion as per the type and distribution of variables. Toward the creation of a diagnostic algorithm, we used two sequential approaches, (1) unsupervised and (2) supervised classification. In both steps, we applied the random forests analysis (a type of recursive partitioning method) for variable selection utilizing R packages “randomForest”. The results of unsupervised learning were validated with supervised learning approaches. An unsupervised learning approach was used to develop two models: one included only the movement and non-movement disorders symptoms while the second also included the sociodemographic characteristics to further optimize classifications. We then conducted supervised analysis of the symptoms with higher discriminative value and compared antibodies with the highest prevalence of each symptom to validate the most discriminative symptoms. For the supervised analysis, a priori, stiffness/rigidity/spasm, ataxia, dystonia, dyskinesia, chorea/choreoathetosis, myoclonus/jerking, Parkinsonism and tremors were used for classifying considered antibodies. To develop random forest models, we used 5000 trees with 5 variables used in each classification. A mean decrease accuracy plot was used to select the variables of importance to predict different types of anti-neuronal antibody. We developed multiple models and finalized models with least out of bag (OOB) error among all alternatives. The predictive accuracies of each variable were summarized with their relative importance; important variables were selected from the random forest models with a predictive relative importance score of at least 40%.
Results
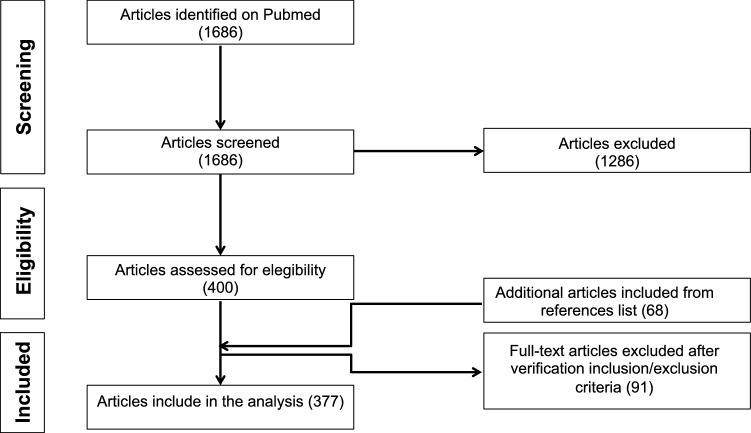
Out of 1686 eligible articles on PubMed, 377 met the eligibility criteria and were included in the systematic review for a total of 844 patients (Fig. 1; Supplementary material 2). Data were reported if the antibody was associated with at least 15 cases [9]. Thirteen antibodies against the following targets were available for this analysis: glutamic acid decarboxylase (GAD) (n = 259), N-methyl-d-aspartate receptor (NMDAR) (n = 255), glycine receptor (GlyR) (n = 57), leucine-rich glioma-inactivated protein 1 (LGI-1) (n = 49), antineuronal nuclear autoantibody type 2 (ANNA-2/Ri) (n = 40), immunoglobulin-like cell adhesion molecule 5 (IgLON5) (n = 33), dipeptidyl-peptidase-like protein-6 (DPPX) (n = 27), anti-collapsing response-mediator protein-5 (CRMP5/CV2) (n = 25), amphiphysin (n = 23), contactin-associated protein-like 2 (Caspr2) (n = 21), metabotropic glutamate receptor 1 (mGluR1) (n = 19), Purkinje cell cytoplasmic antibody type 1 (Yo/PCA-1) (n = 19), antineuronal nuclear antibody-type 1 (ANNA-1/Hu) (n = 17).
Fig. 1.
Systematic review flowchart
Descriptive analysis
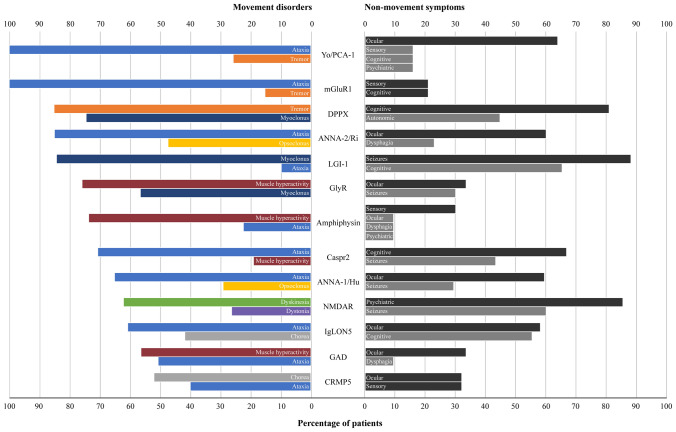
Ataxia, stiffness/rigidity/spasm, dyskinesia, myoclonus and dystonia were the most prevalent disorders (Fig. 2). Ataxia was most commonly observed with mGluR1, Yo/PCA-1 (both 100%), and ANNA-2/Ri (85%); stiffness/rigidity/spasm in amphiphysin (74%), GlyR (77%), and GAD (57%); myoclonus/jerking in LGI-1 (84%), DPPX (74%) and GlyR (58%). High prevalence of dyskinesia (62%) was only documented in NMDAR, whereas high prevalence of tremors only in DPPX (85%). The commonest non-movement features were psychiatric (> 80%; NMDAR), cognitive abnormalities (> 60%; DPPX, Caspr, and LGI-1), seizures (60%, NMDAR; > 80%, LG1-1) and ocular abnormalities (~ 60%; ANNA-1/Hu, ANNA-2/Ri, Yo/PCA-1, and IgLON5). Older ages at diagnosis were documented in amphiphysin, Anna2/Ri, LGI-1, CRMP5, and IgLON5 (mean age range: 62.0–63.5 years); NMDAR stood at the youngest age at diagnosis (mean age: 17.6) (Table 1). A female predominance was noted for amphiphysin, GAD, Anna2/Ri, Yo/PCA-1, and NMDAR; male predominance for Anti-Caspr2, LGI-1, GlyR, and DPPX. Some antibodies, namely GlyR, NMDAR, LGI-1, IgLON5, and DPPX exhibited many non-movement disorders features with high prevalence (Supplementary material 3).
Fig. 2.
Prevalence of movement disorders and non-movement symptoms according to the autoantibody. Absolute number of patients for each movement disorder: ataxia (n = 320); stiffness/rigidity/spasm (n = 243); dyskinesia (n = 168); myoclonus/jerking (n = 189); dystonia (n = 101); chorea/choreoathetosis (n = 106); tremors (n = 83); parkinsonism (n = 59); opsoclonus (n = 33); startle reaction (n = 32); stereotypies (n = 14); and opisthotonus (n = 19). Opsoclonus was associated with myoclonus in 67% of the cases (22/33); in particular with ANNA-2/Ri (10/19), and ANNA-1/Hu (3/5)
Table 1.
Age at diagnosis, disease duration, and sex distribution per autoantibody
| Antibodies | Age at diagnosis Mean (SD); median [IQR] |
Disease duration Mean months (SD); median [IQR] |
Sex Male:female |
|---|---|---|---|
| Amphiphysin | 63 (8.7); 66.0 [55.0–69.0] | 0.39 (1.50); 0 [0–0] | 8:15 |
| GAD | 52.9 (15.5); 55.0 [44.0–63.0] | 21.95 (48.54); 0 [0–24] | 67:145 |
| GlyR | 45.4 (19.2); 49 [37.0–58.0] | 0.81 (3.17); 0 [0–0.2] | 32:25 |
| mGluR1 | 43.6 (15.6); 49.5 [33.0–50.5] | 3.18 (5.65); 1.5 [1.5–1.5] | 7:12 |
| Anna2/Ri | 63.5 (8.9); 63.5 [58.0–70.0] | 1.38 (4.52); 0 [0–0] | 9:31 |
| Yo/PCA-1 | 59.1 (16.2); 62.0 [51.0–71.0] | 5.77 (9.71); 2 [0–6] | 4:15 |
| Anti-Caspr2 | 57.2 (14.5); 60.0 [53.0–66.0] | 3.05 (6.33); 0 [0–2.3] | 18:3 |
| NMDAR | 17.6 (14.4); 15.0 [7.0–24.0] | 0.88 (5.51); 0 [0–0.2] | 66:183 |
| LGI-1 | 62.0 (14.5); 64.0 [57.0–72.0] | 3.45 (6.21); 1.5 [0.5–4] | 33:11 |
| CRMP5/CV2 | 62.9 (11.3); 65 [52.0–71.0] | 1.13 (4.80); 0 [0–0] | 12:13 |
| Anna-1/Hu | 44.2 (31.1); 60.0 [9.0–67.0] | 6.55 (14.48); 0 [0–1] | 5:12 |
| IgLON5 | 62.4 (6.5); 62.5 [58.0–67.0] | 16.79 (37.91); 0 (0–2) | 9:17 |
| DPPX | 48.2 (15.9); 50.5 [38.0–59.5] | 1.18 (4.08); 0 [0–0] | 11:5 |
GAD glutamic acid decarboxylase, GlyR glycine receptor, mGluR1 mGluR1 anti-metabotropic glutamate receptor 1, ANNA-2/Ri anti-neuronal nuclear autoantibody type 2, Yo/PCA-1 Purkinje cell cytoplasmic antibody type 1, Caspr2 contactin-associated protein-like 2, NMDAR Anti-N-methyl-D-aspartate receptor, LGI-1 leucine-rich glioma-inactivated 1, CRMP5/CV2 collapsing response-mediator protein-5, ANNA-1/Hu anti-neuronal nuclear autoantibody type 2, IgLON5 immunoglobulin-like cell adhesion molecule 5, DPPX dipeptidyl-peptidase–like protein 6, SD standard deviation, IQR interquartile range
Relative importance of each predictor for antibodies
Unsupervised analysis. In the symptoms-based unsupervised model, stiffness/rigidity/spasm (57.4%), psychiatric (100%), chorea/choreoathetosis (61.9%), dyskinesia (60%), ataxia (53.2%), opsoclonus (55.1%), dystonia (56.4%), myoclonus/jerking (65.1%), and seizure (61.5%) were most predictive of antibodies (Table 2). In particular, the presence of psychiatric symptoms and dyskinesia predicted NMDAR antibodies with a probability of 71% and 87%, respectively; stiffness/rigidity/spasm and ataxia, GAD (67% and 47%, respectively); ataxia with opsoclonus, ANNA-2/Ri (68%); and chorea/choreoathetosis, CRMP5/CV2 (41%) (Table 3). After adding demographic data, the random forest analysis identified stiffness/rigidity/spasm (57.5%), psychiatric (74%), chorea/choreoathetosis (57.1%), opsoclonus (52.7%), myoclonus/jerking (54.3%), cognitive (49.2%), seizure (50.4%) in addition with disease duration (75.9%) and age at the examination (100%) as the most predictive for differentiating antibodies (Table 2). stiffness/rigidity/spasm predicted GAD with different probabilities according to the age at examination: age ≥ 37 years, 65% vs. age < 37, 76%. Psychiatric symptoms also predicted NMDAR with different probabilities also according to the age at examination: age ≥ 37 years, 38% vs. age < 37, 58%. Psychiatric symptoms also predicted LGI-1 among those ≥ 37 years with 47% probability. The presence of chorea/choreoathetosis predicted IgLON5 among patients ≥ 37 years with 42% (Table 3).
Table 2.
Relative importance of each predictor for antibodies (unsupervised method)
| Variables | Clinical features only | Clinical features + demographic characteristics | ||
|---|---|---|---|---|
| Mean decrease accuracy | Variable importance (%) | Mean decrease accuracy | Variable importance (%) | |
| Age at examination | 204.6 | 100.0 | ||
| Disease duration | 155.3 | 75.9 | ||
| Gender | 59.3 | 29.0 | ||
| Dystonia | 119.7 | 56.4 | 75.5 | 36.9 |
| SPS spectrum | 122.0 | 57.4 | 117.6 | 57.5 |
| Psychiatric | 212.5 | 100.0 | 151.4 | 74.0 |
| Chorea/choreoathetosis | 131.7 | 61.9 | 116.8 | 57.1 |
| Tremors | 94.0 | 44.2 | 82.3 | 40.2 |
| Ataxia | 113.2 | 53.2 | 89.2 | 43.6 |
| Dyskinesia | 127.4 | 60.0 | 83.6 | 40.9 |
| Opsoclonus | 117.0 | 55.1 | 107.9 | 52.7 |
| Parkinsonism | 35.5 | 16.7 | 42.26 | 20.6 |
| Autonomic | 68.9 | 32.4 | 63.8 | 31.2 |
| Cognitive | 100.5 | 47.3 | 100.6 | 49.2 |
| Seizure | 130.7 | 61.5 | 103.2 | 50.4 |
| Consciousness | 62.3 | 29.3 | 55.2 | 27.0 |
| Dysphagia | 57.8 | 27.2 | 36.4 | 17.8 |
| Sensory | 61.5 | 28.9 | 57.6 | 28.1 |
| Startle reaction | 66.6 | 31.3 | 45.7 | 22.3 |
| Myoclonus/jerking | 146.2 | 68.8 | 111.2 | 54.3 |
| Stereotypies | 39.7 | 18.7 | 19.2 | 9.4 |
| Myorhythmia | 3.4 | 1.6 | 2.4 | 1.2 |
| Opisthotonus | 6.0 | 2.8 | 1.6 | 0.8 |
Table 3.
Decision tree based on various methods, clinical features and demographic characteristics
| Decision tree | Conditional attribute | Predicted antibody (probability) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Psychiatric | SPS spectrum | Ataxia | Dyskinesia | Opsoclonus | Chorea/choreoathetosis | Dystonia | Age | Disease duration | ||
| Unsupervised method based on clinical feature only | 0 | 0 | 1 | 0 | GAD (0.47) | |||||
| 0 | 1 | GAD (0.67) | ||||||||
| 0 | 0 | 1 | 1 | ANNA-2/Ri (0.68) | ||||||
| 1 | NMDAR (0.71) | |||||||||
| 0 | 0 | 0 | 1 | NMDAR (0.87) | ||||||
| 0 | 0 | 0 | 0 | 1 | CRMP5/CV2 (0.41) | |||||
| Supervised method based on clinical features only | 0 | 1 | 0 | 0 | GAD (0.42) | |||||
| 1 | 0 | GAD (0.61) | ||||||||
| 0 | 1 | 0 | 1 | ANNA-2/Ri (0.40) | ||||||
| 1 | NMDAR (0.92) | |||||||||
| Unsupervised method based on clinical features and demographic characteristics | 1 | ≥ 37 | GAD (0.65) | |||||||
| 0 | 1 | < 37 | GAD (0.76) | |||||||
| 1 | 0 | 0 | ≥ 37 | < 0.23 | NMDAR (0.38) | |||||
| 1 | 1 | < 37 | NMDAR (0.58) | |||||||
| 1 | 0 | 0 | ≥ 37 | ≥ 0.23 | LGI-1 (0.47) | |||||
| 0 | 1 | ≥ 37 | IgLON5 (0.42) | |||||||
| Supervised method based on clinical features and demographic characteristics | 0 | 1 | 0 | ≥ 37 | GAD (0.44) | |||||
| 1 | ≥ 37 | GAD (0.65) | ||||||||
| 0 | 1 | < 37 | GAD (0.76) | |||||||
| 1 | 1 | < 37 | NMDAR (0.52) | |||||||
| 0 | 0 | 1 | ≥ 37 | < 0.05 | CRMP5/CV2 (0.53) | |||||
| 0 | 1 | 1 | ≥ 37 | IgLON5 (0.67) | ||||||
Supervised analysis. The supervised analysis was based on a priori selection of variables based on their distributions. Random forest analyses confirmed the significant predictors as obtained in the unsupervised analysis (Table 4). Variables selected according to the relative importance (> 40%) of each variable in a recursive tree analysis further validated the findings obtained in unsupervised analysis. In the symptoms-based supervised model, stiffness/rigidity/spasm (86%), psychiatric (100%), ataxia (76.4%), myoclonus/jerking (85.1%), dyskinesia (63%), and chorea/choreoathetosis (61.1%) were found to be most predictive of antibodies (Table 4). After adding demographic data, the random forest analysis identified stiffness/rigidity/spasm (59.1%), psychiatric (65.1%), chorea/choreoathetosis (53.4%), and myoclonus/jerking (54.3%), in addition with disease duration (73.2%) and age at the examination (100%) as the most discriminating of antibodies (Table 4). Accordingly, ataxia was confirmed as highly predictive of ANNA-2/Ri, stiffness/rigidity/spasm and ataxia of GAD, dyskinesia of NMDAR, psychiatric symptoms in young subjects (< 37 years) of NMDAR, and chorea/choreoathetosis of CRMP5/CV2 and IGLON5 (Table 3).
Table 4.
Relative importance of each predictor for antibodies (supervised method)
| Variables | Clinical features only | Clinical features + demographic characteristics | ||
|---|---|---|---|---|
| Mean decrease accuracy | Variable importance (%) | Mean decrease accuracy | Variable importance (%) | |
| Age at examination | 281.2 | 100.0 | ||
| Dystonia | 154.0 | 60.8 | 82.1 | 29.2 |
| SPS symptoms | 217.7 | 86.0 | 163.5 | 58.1 |
| Psychiatric | 253.2 | 100.0 | 183.1 | 65.1 |
| Disease duration | 206.0 | 73.2 | ||
| Ataxia | 193.5 | 76.4 | 103.9 | 36.9 |
| Chorea/choreoathetosis | 154.8 | 61.1 | 150.2 | 53.4 |
| Dyskinesia | 159.7 | 63.0 | 89.6 | 31.9 |
| Parkinsonism | 64.6 | 25.5 | 64.7 | 23.0 |
| Gender | 77.2 | 27.4 | ||
| Opsoclonus | 152.1 | 60.0 | 141.6 | 50.3 |
| Myoclonus/jerking | 215.5 | 85.1 | 152.8 | 54.3 |
| Total tremors | 123.8 | 48.9 | 110.6 | 39.3 |
Expert opinion approach
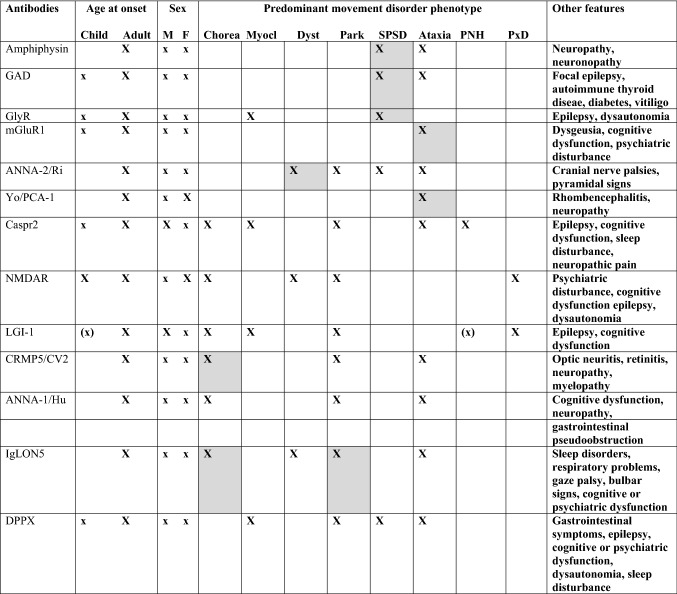
The “one phenotype-one antibody” data-driven approach, in part due to the bias introduced by the single-case reports on which this analysis is based, is not congruent with the clinical approach taken by experts. To render the material adaptable for use at the bedside, according to a practice similar to that of neurogenetics, which has shifted laboratory requisitions from single-antibody testing to antibody panels, we created a table that combines data-driven and expert input in the prediction of the pathogenic antibody according to the age at onset, sex, and predominant movement disorder phenotype (Table 5).
Table 5.
Data driven and expert opinion approach
Based on the 13 antibodies identified in the systematic review, the table presents associations identified by the data-driven algorithm (grey cells) and expanded panels based on previous literature reviews and expert opinion. It starts with the main movement disorders presentation (chorea, myoclonus, dystonia, parkinsonism, stiff person spectrum disorder [SPSD; includes presentations with prominent myoclonus, such as progressive encephalomyelitis with rigidity an myoclonus], ataxia, peripheral nerve hyperexcitability [PNH], or paroxysmal dyskinesia [PxD]). Antibody test selection takes into consideration the age at onset (for example, NMDAR antibodies are more prevalent in children and young adults, while Caspr2 and IgLON5 antibodies occur later in life) and sex (for example, Caspr2 antibodies occur much more frequently in males). Other associated features may help to narrow the differential diagnosis. Of note, the spectrum of antibodies associated with movement disorders includes antibodies mainly reported in aggregated data (e.g., anti-Ma2 related parkinsonism) and other, rarer antibodies not discussed here. The table is based on typical manifestations, and does not account for the rare occurrence of atypical presentations regarding phenomenology or epidemiology. Opsoclonus-myoclonus syndrome is not included here because there is no syndrome-specific antibody; similarly, tremor is a non-specific finding in antibody-related syndromes
GAD glutamic acid decarboxylase, GlyR glycine receptor, mGluR1 mGluR1 anti-metabotropic glutamate receptor 1, ANNA-2/Ri anti-neuronal nuclear autoantibody type 2, Yo/PCA-1 Purkinje cell cytoplasmic antibody type 1, Caspr2 contactin-associated protein-like 2, NMDAR Anti-N-methyl-D-aspartate receptor, LGI-1 leucine-rich glioma-inactivated 1, CRMP5/CV2 collapsing response-mediator protein-5, ANNA-1/Hu anti-neuronal nuclear autoantibody type 2, IgLON5 immunoglobulin-like cell adhesion molecule 5, DPPX dipeptidyl-peptidase–like protein 6
Secondary analyses
Temporal sequence. Most movement disorders appeared in the nadir/recovery phase. However, ataxia was present at the onset, except in GAD and Caspr2, where its appearance was more common in the nadir/recovery phase. Opsoclonus and myoclonus were present at the onset when associated with ANNA-1/Hu (Supplementary material 4).
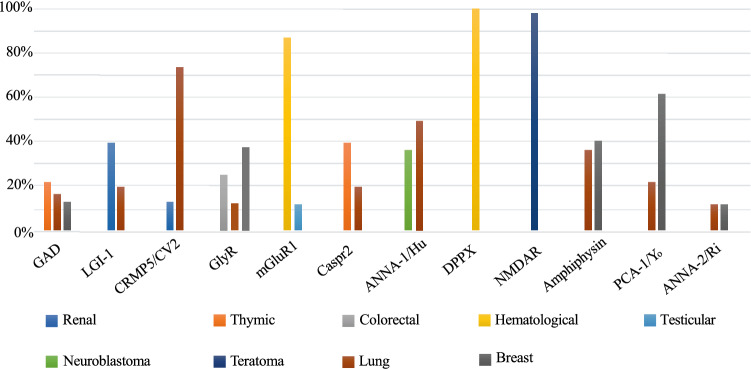
Associated tumors. Breast and lung tumor were the most common tumors across all antibodies, except for mGluR1, NMDAR, IgLON5, and DPPX (Fig. 3, Supplementary material 3). No tumors were found in anti IgLON5 and only 2 cases in DPPX subjects (all hematological).
Fig. 3.
Distribution of the most common tumors for each antibody. Percentage was calculated considering only subjects with tumor
MRI features. When present, abnormalities were largely restricted to cerebellar abnormalities in anti-GAD (21%) and anti-Yo/PCA-1 (36%) and temporal involvement in Caspr2 (25%), LGI-1 (29%), and ANNA-1/Hu (27%). In anti-NMDAR the brain MRI was mostly normal; if abnormal, it was associated with diffuse and non-regional specific abnormalities (Supplementary material 3).
Discussion
To our knowledge, this is the first systematic evaluation of autoimmune disorders associated with abnormal movements aiming to identify the most antibody-discriminative symptoms and building a tentative data-driven algorithm enriched with expert feedback to assist the clinician in the diagnostic approach. Recognizable associations were observed between selected movement disorders and anti-neuronal antibodies, with a diagnostic approach facilitated by other demographic features. Stiffness/rigidity/spasm, psychiatric, chorea/choreoathetosis, dyskinesia, ataxia, opsoclonus, dystonia, and myoclonus/jerking symptoms emerged as the most discriminative movement disorders for the systematic approach to a differential diagnosis. In particular, stiffness/rigidity/spasms and ataxia predicted GAD; psychiatric symptoms NMDAR in young individuals, LGI-1 in older ones; dyskinesia, NMDAR; ataxia and opsoclonus, ANNA-2/Ri; chorea/choreoathetosis, CRMP5/CV2 and IgLON5 in those older than 37 years. Among demographic data, disease duration and age were the most predictive for differentiating antibodies. We acknowledge that, as intrathecal antibody production could not be determined in many cases, GAD antibodies may not necessarily correlate with symptoms other than stiffness and rigidity.
Most non-movement features were highly prevalent in antibodies with wide distribution in the nervous system, such as GlyR [10], NMDAR [11], DPPX [12], and LGI-1 [13, 14]. For instance, GlyR are highly diffuse in the brain, particularly in areas associated with autonomic function, such as the locus coeruleus, nucleus solitarius, and the rostral ventrolateral medulla, and the pathogenic mechanism seems to be associated with the internalization or direct blockage of GlyR [15]; NMDAR, in particular the subunit NR1, are widely expressed in the cortex; DPPX is localized in the neuronal dendrites and soma of both central and peripheral nervous system; and LGI-1 is a neuronal protein that binds pre- and postsynaptic receptors: ADAM23 and ADAM22, respectively, widely distributed [16, 17]. The high association between certain symptoms and selected antibodies confirmed the presence of well-known syndromic conditions such as faciobrachial dystonic seizures in LGI-1 [18], and opsoclonus and myoclonus (opsoclonus–myoclonus syndrome) in ANNA-2/Ri [19].
Breast and/or lung were the most common tumors across the autoimmune disorders. However, other tumors were associated with specific antibodies, such as thymic neoplasia with GAD and Caspr2 and neuroblastoma with ANNA-1/Hu, as has been previously reported [20–22]. Teratoma was almost exclusively associated with NMDAR, in line with previous studies [11], Conversely, there were no tumors reported in subjects with IgLON5 antibodies [23]. MRI data confirmed that, in general, autoimmune movement disorders are not associated with imaging abnormalities, although cerebellar involvement was relatively common in GAD [24] and Yo/PCA-1[25], and temporal abnormalities in Caspr2, LGI-1, and ANNA-1/Hu. This distribution likely reflects the tropism of specific antibodies. For instance, anti-GAD and anti-Yo/PCA-1 antibodies exhibit selective tropism for cerebellar regions, LGI-1 for the hippocampus[26]; NMDAR antibodies are, conversely, non-selective, with widely distributed targets.
Some limitations are worth noting. Lacking a standardized approach between studies, we had to rely on potentially different classification of symptoms in different studies. To facilitate its use by neurologists without expertise in the field of autoimmune disorders, we divided presentations by symptoms rather than by clinical syndromes, independently from their pathogenesis. For example, spasms in Caspr2 are classified as neuronal hyperexcitability, whereas in GAD within the stiff-person-syndrome spectrum. Second, not all the studies examined the whole spectrum of available antibodies, potentially biasing the movement-antibody associations and the assessments of prevalence. Some studies had a relatively short follow-up, giving rise to the possibility that selected late-onset symptoms were overlooked. The single-case reports serving as the source of data for this analysis may have overrepresented unusual manifestations and generated a “one phenotype-one antibody” pitfall, which is different from the more nuanced approach undertaken by expert clinicians. Finally, important symptoms such as cognitive impairment may be underestimated by this analysis due to its lower ascertainment and the reporting of frequencies of certain antibodies may appear different than seen in clinical experience (for instance, anti-GAD antibodies are most frequent in both ataxia and stiffness but seemed lower for the former). Mitigating these sources of biases, we excluded studies with more than one antibody in order to minimize counfounders on the clinical presentation; however, autoimmune comorbidity is not rare in practice. One source of bias is also that aggregated data was not included in the analysis, which may have led to an under representation of classic antibody associations. This analysis cannot be viewed as representing a generalizable measure of the prevalence of autoimmune movement disorders, but a tentative guide for the movement disorders specialist in cases where the presence of anti-neuronal antibodies is suspected. Besides predicted frequencies, another consideration guiding antibody testing in practice is that some rarer antibodies have particular implications. For example, in a patient with stiffness/rigidity/spasm, GAD antibodies are more likely, but the presence of amphiphysin antibodies would warrant a tumour search. The analysis of larger databases could enhance the clinical flowchart for antibody selection proposed here.
In sum, this systematic review of published reports provides a tentative approach to patients with autoimmune movement disorders. Prompt identification of pathogenic antibodies stands to assist in the clinical management and early identification of associated comorbidities, such as tumors. Future studies with larger databases will help refine a data driven diagnostic differential at the bedside.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplementary Material 1. MeSH terms (DOCX 13 kb)
Supplementary file2 Supplementary Material 2. List of the studies analyzed (DOCX 77 kb)
Supplementary file3 Supplementary material 3. Clinical, tumors, and MRI features of each antibody (DOCX 31 kb)
Supplementary file4 Supplementary Material 4. Temporal sequence of symptoms (DOCX 28 kb)
Acknowledgements
The authors thank Prof. Josep Dalmau for his review and feedback on this manuscript.
Author contributions
AS: research project: conception, organization, execution; statistical analysis: review and critique; manuscript preparation: writing of the first draft, review and critique. AKD: research project: execution; statistical analysis: design, execution, review and critique; manuscript preparation: review and critique. MG: research project: conception, organization, execution; statistical analysis: review and critique; manuscript preparation: review and critique. MBG: research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique. PB: research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique. KRD: research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique. JAV: research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique. EA: research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique. NW: research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique. LM: research project: conception, organization; research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique. AJE: research project: conception, organization; research project: execution; statistical analysis: review and critique; manuscript preparation: review and critique.
Funding
None.
Data availability
Dr. Espay had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the conduct of the research. He has the right to publish any and all data, separate and apart from the guidance of any sponsor.
Declarations
Conflicts of interest
MBG, PB, KRD, JAV, EA, NW, and BB have nothing to report. AS is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies. AKD is supported as a co-investigator by the NIH (1 R21 HL143030-01) and (R21 AI133207) grants. He is also currently serving as a statistician in CPRIT funded studies (PP200006, PP190058, PP180003, and PP170068). MG has received honoraria from Roche. LM has received honoraria from the International Association of Parkinsonism and Related Disorders (IAPRD) Society. He serves on the Editorial board of the Journal of Clinical Movement Disorders. AJE: has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Neuroderm, Neurocrine, Amneal, Adamas, Acadia, Acorda, Kyowa Kirin, Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Acadia, and Sunovion. He serves on the editorial boards of the Journal of Parkinson’s Disease, European Journal of Neurology, and JAMA Neurology. He is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies.
Ethical approval
Not required.
Consent to participate
Not required.
References
- 1.Damato V, Balint B, Kienzler AK, Irani SR. The clinical features, underlying immunology, and treatment of autoantibody-mediated movement disorders. Mov Dis Off J Mov Dis Soc. 2018;33(9):1376–1389. doi: 10.1002/mds.27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balint B, Vincent A, Meinck HM, Irani SR, Bhatia KP. Movement disorders with neuronal antibodies: syndromic approach, genetic parallels and pathophysiology. Brain J Neurol. 2018;141(1):13–36. doi: 10.1093/brain/awx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley BP, Patel SC, Marin HL, Corrigan JJ, Mitsias PD, Griffith B. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070–1078. doi: 10.3174/ajnr.A5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol (Seoul, Korea) 2016;12(1):1–13. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirra M, Marsili L, Gallerini S, Keeling EG, Marconi R, Colosimo C. Paraneoplastic movement disorders: phenomenology, diagnosis, and treatment. Eur J Intern Med. 2019;67:14–23. doi: 10.1016/j.ejim.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Gadoth A, Kryzer TJ, Fryer J, McKeon A, Lennon VA, Pittock SJ. Microtubule-associated protein 1B: novel paraneoplastic biomarker. Ann Neurol. 2017;81(2):266–277. doi: 10.1002/ana.24872. [DOI] [PubMed] [Google Scholar]
- 9.Marsili L, Vizcarra JA, Sturchio A, Dwivedi AK, Keeling EG, Patel D, Mishra M, Farooqi A, Merola A, Fasano A, Mata IF, Kauffman MA, Espay AJ. When does postural instability appear in monogenic parkinsonisms? An individual-patient meta-analysis. J Neurol. 2020;268(9):3203–3211. doi: 10.1007/s00415-020-09892-3. [DOI] [PubMed] [Google Scholar]
- 10.Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, Lang B, Pettingill P, Carr A, Sheerin UM, Press R, Lunn MP, Lim M, Maddison P, Meinck HM, Vandenberghe W, Vincent A. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain J Neurol. 2014;137(Pt 8):2178–2192. doi: 10.1093/brain/awu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boronat A, Gelfand JM, Gresa-Arribas N, Jeong HY, Walsh M, Roberts K, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R, Graus F, Rudy B, Dalmau J. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol. 2013;73(1):120–128. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain J Neurol. 2010;133(9):2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crisp SJ, Dixon CL, Jacobson L, Chabrol E, Irani SR, Leite MI, Leschziner G, Slaght SJ, Vincent A, Kullmann DM. Glycine receptor autoantibodies disrupt inhibitory neurotransmission. Brain J Neurol. 2019;142(11):3398–3410. doi: 10.1093/brain/awz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science (New York, NY) 2006;313(5794):1792–1795. doi: 10.1126/science.1129947. [DOI] [PubMed] [Google Scholar]
- 17.Petit-Pedrol M, Sell J, Planagumà J, Mannara F, Radosevic M, Haselmann H, Ceanga M, Sabater L, Spatola M, Soto D, Gasull X, Dalmau J, Geis C. LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain J Neurol. 2018;141(11):3144–3159. doi: 10.1093/brain/awy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irani SR, Stagg CJ, Schott JM, Rosenthal CR, Schneider SA, Pettingill P, Pettingill R, Waters P, Thomas A, Voets NL, Cardoso MJ, Cash DM, Manning EN, Lang B, Smith SJ, Vincent A, Johnson MR. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain J Neurol. 2013;136(Pt 10):3151–3162. doi: 10.1093/brain/awt212. [DOI] [PubMed] [Google Scholar]
- 19.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53(5):580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 20.Ariño H, Höftberger R, Gresa-Arribas N, Martínez-Hernández E, Armangue T, Kruer MC, Arpa J, Domingo J, Rojc B, Bataller L, Saiz A, Dalmau J, Graus F. Paraneoplastic neurological syndromes and glutamic acid decarboxylase antibodies. JAMA Neurol. 2015;72(8):874–881. doi: 10.1001/jamaneurol.2015.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyko M, Au K, Casault C, de Robles P, Pfeffer G. Systematic review of the clinical spectrum of CASPR2 antibody syndrome. J Neurol. 2020;267(4):1137–1146. doi: 10.1007/s00415-019-09686-2. [DOI] [PubMed] [Google Scholar]
- 22.Dalmau J, Graus F, Cheung NK, Rosenblum MK, Ho A, Cañete A, Delattre JY, Thompson SJ, Posner JB. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995;75(1):99–109. doi: 10.1002/1097-0142(19950101)75:1<99::aid-cncr2820750117>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Gaig C, Graus F, Compta Y, Högl B, Bataller L, Brüggemann N, Giordana C, Heidbreder A, Kotschet K, Lewerenz J, Macher S, Martí MJ, Montojo T, Pérez-Pérez J, Puertas I, Seitz C, Simabukuro M, Téllez N, Wandinger KP, Iranzo A, Dalmau J. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88(18):1736–1743. doi: 10.1212/WNL.0000000000003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R, Ramió-Torrentà L, Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain J Neurol. 2008;131(Pt 10):2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 25.Venkatraman A, Opal P. Paraneoplastic cerebellar degeneration with anti-Yo antibodies—a review. Ann Clin Transl Neurol. 2016;3(8):655–663. doi: 10.1002/acn3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplementary Material 1. MeSH terms (DOCX 13 kb)
Supplementary file2 Supplementary Material 2. List of the studies analyzed (DOCX 77 kb)
Supplementary file3 Supplementary material 3. Clinical, tumors, and MRI features of each antibody (DOCX 31 kb)
Supplementary file4 Supplementary Material 4. Temporal sequence of symptoms (DOCX 28 kb)
Data Availability Statement
Dr. Espay had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the conduct of the research. He has the right to publish any and all data, separate and apart from the guidance of any sponsor.