Abstract
Relapsing fever (RF) is caused by several species of Borrelia; all, except two species, are transmitted to humans by soft (argasid) ticks. The species B. recurrentis is transmitted from one human to another by the body louse, while B. miyamotoi is vectored by hard-bodied ixodid tick species. RF Borrelia have several pathogenic features that facilitate invasion and dissemination in the infected host. In this article we discuss the dynamics of vector acquisition and subsequent transmission of RF Borrelia to their vertebrate hosts. We also review taxonomic challenges for RF Borrelia as new species have been isolated throughout the globe. Moreover, aspects of pathogenesis including symptomology, neurotropism, erythrocyte and platelet adhesion are discussed. We expound on RF Borrelia evasion strategies for innate and adaptive immunity, focusing on the most fundamental pathogenetic attributes, multiphasic antigenic variation. Lastly, we review new and emerging species of RF Borrelia and discuss future directions for this global disease.
Introduction
Relapsing fever (RF), or what we believe to be RF, was historically known by different names including “Febris recurrentis”, “recurrent fever”, “famine fever”, “spirillum fever”, “spirochetal fever”, “saddleback fever”, “vagabond fever”, “fowl nest fever”, “Karlskrona fever”, “gharib ghez”, “Giesinger’s bilious typhoid”, and “kimputu.” RF was recognized as a disease by physicians in ancient Greece at the time of Hippocrates (Felsenfeld, 1971). Later, between 1485 and 1551, five epidemics of “sweating sickness” that swept through England probably included outbreaks of RF. One of the best recorded descriptions of RF came from the physician John Rutty, who kept a detailed diary during his time in Dublin, where he described the weather and illnesses in the area during the mid-1700’s (Rutty, 1770). Interestingly, the fatality rate was very low and most of the affected people did recover after two or three relapses.
RF symptoms also were described in detail by field medics during the 1788 Swedish-Russian war. The Swedish navy conquered the Russian 74-cannon battleship Vladimir and its 783 men crew at a battle in the Finnish bay near the Hogland Island. Following the victory, the ship was brought to Helsinki, but several of the Russian prisoners aboard were sick. The louse-borne RF spread rapidly in the Swedish Fortress Sveaborg and among Helsinki civilians. Five hundred sick soldiers were shipped to Karlskrona, the main Swedish naval base, which then also became heavily plagued by RF (Felsenfeld, 1971; Huldén, 2006). From the documentation, it is possible that many soldiers were afflicted by concomitant infection with Rickettsia prowazekii and possibly other microorganisms. Difficulty in clinically distinguishing RF from other vector-borne infections continues to this day. For example, RF and malaria are often mistaken in areas where both exist. Also, dengue and Colorado Tick Fever, present with similar symptoms to RF such as headache, constitutional symptoms, and “saddleback fever”-like (biphasic fever that may suggest a relapse).
The definite cause of RF was not established until 1868 when the German scientist Otto Obermeier identified a spirochete as the etiologic agent of an epidemic fever outbreak in Berlin. The organism causing this epidemic disease was first named Spirocheta obermeieri but later renamed Borrelia recurrentis. The largest outbreaks occurred during World War I over large areas in Europe, Asia and Africa. The epidemics among the soldiers and civilians were probably due to poor hygienic conditions and the forced migration of refugees (Felsenfeld, 1971).
RF is either tick-borne (TBRF) or louse-borne (LBRF) (Felsenfeld, 1971). LBRF is caused by B. recurrentis, and TBRF is caused by several different Borrelia species. Many of the RF outbreaks described by Rutty and others in Northern Europe were likely louse-borne. These outbreaks occurred when there were optimal conditions for the body louse - the weather was turning cooler and people were adding more layers of clothing, the preferred niche for the human body louse. Also, during wartime, people are more vulnerable to attack by lice because of reduced hygiene, including the lack of clean clothes and bed linen.
Since the initial descriptions of LBRF, there have been millions of human cases in large and small epidemics (Bryceson et al., 1970). However, endemic transmission of the agent continues to persist in the Horn of Africa and adjacent areas (Borgnolo et al., 1993; Mitiku and Mengistu, 2002; Sundnes and Haimanot, 1993). As expected for a disease with this degree of public health importance, there have been numerous clinical, epidemiologic, and entomologic studies of LBRF, its agent, and vector. Many of these reports date to the first half of the last century and provide a wealth of information about the clinical aspects of LBRF.
The reservoir of B. recurrentis is probably restricted to humans, and reports of experimental animal infections with B. recurrentis have been scarce (Barbour and Hayes, 1986). For example, B. recurrentis may cause a spirochetemia in weanling mice, newborn rabbits, and grivet monkeys (Judge et al., 1974a, b, c). More recently, Larsson and coworkers successfully developed a LBRF model in immunodeficient mice (Larsson et al., 2009). The findings demonstrated that, in addition to humoral immunity, innate immune responses could limit the pathogens in the mammalian host.
TBRF is a vector-borne zoonosis found in all regions of the world, apart from Oceania and Antarctica. TBRF accounts for thousands of cases of RF globally each year. Most of the studies on the pathogenesis and immunity of RF have been carried out on TBRF species, whose natural reservoirs are rodents and other mammals. However, the natural reservoir of the RF species B. duttonii has not yet been defined. For the TBRF Borrelia species, mice and rats serve as suitable experimental animal models.
This review by necessity emphasizes studies of TBRF, especially those of two Nearctic species, B. hermsii and B. turicatae, and two Afro-Tropical/Palearctic species, B. duttonii and B. crocidurae. This review also covers important aspects of LBRF infection and its causative agent B. recurrentis, which is descended from the tick-borne species B. duttonii or a close relative (Lescot et al., 2008). Thus, the findings of experimental studies of tick-borne species might be applicable to B. recurrentis and LBRF. Furthermore, this review is an updated version of the publication by Barbour and Guo (2010). We have included a more comprehensive section on vector biology and an update on new and emerging hard tick-transmitted RF species, Borrelia miyamotoi.
Transmission-vector and reservoirs
Harry Hoogstraal, a medical acarologist and parasitologist, suggested that the Borrelia lineage began as symbionts of ticks rather than as parasites of vertebrates (Hoogstraal, 1979). This is an interesting hypothesis but, as Barbour and Hayes stated, “As it is metaphorically true for the chicken and the egg, it may not be known whether Borrelia were originally parasites of arthropods or of vertebrates” (Barbour and Hayes, 1986). Thus, spirochetes are found as endosymbionts in several invertebrates, e.g., termites and mollusks. From that viewpoint, infection of vertebrates is the main role for transmission of the spirochetes to another arthropod host, in this case soft-bodied (argasid) ticks of the genus Ornithodoros (Cooley and Kohls, 1944; Felsenfeld, 1971; Hoogstraal, 1979). On the other hand, one piece of evidence that favors an arthropod origin for the genus Borrelia is the finding of a high frequency of transovarial transmission observed for TBRF species, with between 35 - 100% of offspring, in most but not all species (Burgdorfer and Varma, 1967; Davis, 1943, 1952). When the Borrelia spirochete enters the ovary, it invades the developing oocyte-yolk complex before the impermeable shell is formed around the egg (Aeschlimann, 1958).
TBRF spirochetes, are transmitted either by argasid or ixodid ticks and their life cycles in each vector differ. In argasids, TBRF spirochetes enter the tick when feeding on an infected vertebrate host. While it is not completely clear if replication occurs in the vector, the spirochetes persistently colonize the tick midgut and salivary glands of argasids in the genus Ornithodoros, (Boyle et al., 2014; Krishnavajhala et al., 2017; Policastro et al., 2013; Schwan and Hinnebusch, 1998). Since Ornithodoros ticks are rapid feeders, completing the blood meal within 60 minutes, the population of TBRF spirochetes that colonized the salivary glands enters the host’s skin and blood within seconds of attachment (Boyle et al., 2014; Burgdorfer, 1951). This was demonstrated when Ornithodoros turicata ticks engorged on the ears of mice, and after feeding the bite site was removed and placed into culture medium (Boyle et al., 2014). All the animals became infected, and spirochetes also were cultivated from ear biopsies. These results indicated that, while some spirochetes enter the bloodstream, others that remain at the bite site after feeding. While most species of TBRF are transmitted through the saliva of Ornithodoros ticks, transmission of O. moubata may occur through contamination of the bite site with infected coxal fluid (Varma, 1956).
Recent work with the ixodid tick vector of TBRF spirochetes suggests a slightly different life cycle compared to argasid ticks. Studies with Ixodes scapularis, the vector of B. miyamotoi, indicated that the bacteria persistently colonize the midgut and salivary glands of the tick (Breuner et al., 2017). Upon feeding infected ticks, spirochete DNA was detected in 10% of mice after 24 hours. This indicates that spirochetes in the salivary gland are the first to enter the vertebrate host. Interestingly, by the time ticks had completed feeding, several days later, 73% of the animals were infected. This suggests that unlike argasid-transmitted TBRF spirochetes, the population of B. miyamotoi that colonizes the midgut migrates to the salivary glands then plays a role in vertebrate infection as has been well established for the causative agent of Lyme disease.
The transmission dynamics of LBRF spirochetes are unique compared to the tick-transmitted species. After ingestion by the louse, LBRF spirochetes pass from the midgut to the hemolymph where they multiply and persist for the ~3-week lifespan of the louse. Subsequent transmission of LBRF spirochetes to humans does not occur through the bite or the saliva of the human body louse (Pediculus humanus humanus). LBRF spirochetes are transmitted mechanically and transovarial transmission of B. recurrentis does not occur (Bryceson et al., 1970; Felsenfeld, 1965). Only when the louse is crushed does contaminated hemolymph or feces enter the human (Houhamdi and Raoult, 2005). This occurs through contamination of the conjunctivae, abrasions in the mucous membranes of the mouth, or the louse bite site.
The vertebrate reservoir hosts of the TBRF Borrelia spp. are mostly different species of rodents (Nicolle and Anderson, 1927) but may also include animals like pigs, goats, sheep, rabbits, bats, opossums, armadillos, foxes, cats, dogs, and birds (Felsenfeld, 1971; Hoogstraal, 1985; Pavlovsky, 1963; Thomas et al., 2002). These animals usually become infected during the bite of an Ornithodoros or related soft-bodied tick. Accidental peroral transmission through consumption of livers and brains of infected animals also has been reported (reviewed in Barbour and Hayes, 1986). Transplacental transmission occurs also in mammals and may cause congenital infection with subsequent placental damage and stillbirths (Larsson et al., 2006a). However, the peroral and transplacental routes of RF transmission are rare, and their importance for transmission in nature is probably insufficient for the maintenance of the pathogen. Likewise, for humans, RF is nearly always acquired through contact with a tick or louse carrying the spirochetes. Rarely the disease is acquired through (i) accidental inoculation of infected blood; (ii) contact of abraded or lacerated skin, mucous membranes, or conjunctiva with infected patient or animal blood (Herms and Wheeler, 1935); or (iii) transplacental or perinatal transmission from mother to fetus (Fuchs and Oyama, 1969; Yagupsky and Moses, 1985). Additionally, pregnancy complications connected to RF infection have been reported, especially B. duttonii infections in the sub-Saharan region of Africa (Brasseur, 1985; Dupont et al., 1997; Goubau and Munyangeyo, 1983; Jongen et al., 1997; Melkert and Stel, 1991; van Holten et al., 1997). Importantly, transmission does not occur by aerosols, fomites, saliva, urine, feces, or sexual contact.
Borrelia-arthropod interactions: Life inside the tick!
In the early and mid-twentieth century, there were many studies on the colonization and movement of Borrelia spirochetes in ticks and lice, reviewed in (Barbour and Hayes, 1986; Burgdorfer and Varma, 1967; Felsenfeld, 1965; Felsenfeld, 1971). An example was the comprehensive investigation by Willy Burgdorfer assessing B. duttonii colonization in its tick vector, O. moubata (Burgdorfer, 1951). In that study, he showed that a high density of spirochetes was found around ganglia and in the salivary glands of the tick (Burgdorfer, 1959). Interestingly, Burgdorfer also showed that the transmission of RF Borrelia occurred not only via a tick bite, but also through contamination from infected coxal fluid (Burgdorfer, 1951, 1959).
As efforts have focused on generating spirochete isolates from the field, we are gaining refined insights into vector competence of TBRF spirochetes. For example, multilocus sequencing of ~50 B. hermsii isolates distinguished two genomic groupings, GGI and GGII. A series of tick acquisition studies was performed to determine whether co-infection of GGI and GGII impacted vector colonization and subsequent transmission frequencies from O. hermsi (Policastro et al., 2013). Policastro and colleagues first infected cohorts of ticks with either GGI or GGII spirochetes. After molting, they fed ticks again to allow them to become infected with the opposite genomic group. Finally, in the third tick feeding, individual ticks were assessed to determine the impact of superinfection in transmission. Interestingly, ticks that were first infected with GGII then GGI transmitted only GGII spirochetes. However, prolonging the interval between the second and third feeding resulted in co-transmission of GGI and GGII spirochetes (Policastro et al., 2013). Thus, transmission of different populations of B. hermsii depends upon the order of tick acquisition and the interval between feedings.
Additional studies with B. turicatae further demonstrate that specific populations of TBRF spirochetes colonize disparate environments within the tick. B. turicatae producing GFP established a persistent infection for at least 18 months in the tick midgut and salivary glands (Krishnavajhala et al., 2017). While the population colonizing the salivary glands is the one responsible for transmission to the vertebrate host (Boyle et al., 2014; Schwan and Hinnebusch, 1998), the role of the midgut population remains unclear. Given the rapidity of Ornithodoros feeding and TBRF spirochete transmission, there is an insufficient time for the midgut population to be transmitted through the saliva. There are at least two potential roles for the midgut population. First, it may be involved with spirochete replication in the midgut after infected ticks feed. Alternatively, the midgut population may have a role in transovarial transmission to developing eggs.
Advances in high-throughput methodologies have begun to provide insight into the selective pressures and molecular mechanisms of vector colonization by TBRF spirochetes. Mans and colleagues seminal work produced the first salivary gland transcriptomes of Ornithodoros ticks, reporting the identification of immune regulators that may aid in tick feeding and TBRF spirochete transmission (Mans et al., 2008a; Mans et al., 2008b). Recent studies on the proteome of fed and unfed O. erraticus midguts indicated the production of proteins involved with antioxidant defenses (Oleaga et al., 2015). These findings were expanded further with the salivary gland transcriptome of O. turicata (Bourret et al., 2019). The expression of 57 genes involved in oxidant metabolism or antioxidant defenses were identified. Of these, the genes encoding glutathione peroxidase, thioredoxin peroxidase, manganese superoxide dismutase, copper-zine superoxide dismutase, and catalase were further evaluated in fed and unfed midguts and salivary glands. These findings indicated that the midguts and salivary glands of O. turicata were highly nitrosative and oxidative, respectively. Interestingly, the work also determined that B. turicatae was hyper-resistant to reactive oxygen species compared to Lyme disease (LD)-causing spirochetes (Bourret et al., 2019). Collectively, omics studies have begun to provide insight into TBRF adaptation within the tick vector; however, additional work is needed to understand the mechanisms utilized by the pathogens in the tick-mammalian infectious cycle.
As molecular tools have improved and become available for genetically manipulating TBRF spirochetes, the events of vector colonization are becoming clearer. The most significant advance on the molecular interactions between the arthropod vector and Borrelia was performed with O. hermsi and B. hermsii. Schwan and Hinnebusch began to unravel the intricacies of vector colonization and transmission with the identification of the variable tick protein (Vtp) (previously denoted pIc, VmpC or Vsp33) (Schwan and Hinnebusch, 1998). They demonstrated that Vtp was preferentially produced by spirochetes in the salivary glands of unfed O. hermsi ticks and that its production could be induced in vitro by lowering the incubation temperature to 23° C. Interestingly, they reported that when spirochetes producing Vtp enter a new mouse host from the tick, they reverted back to the mammal-associated variant they last manifested in the mouse host (Schwan and Hinnebusch, 1998). Raffel and co-workers genetically inactivated the vtp locus and it became clear that the protein is involved with preadapting B. hermsii for mammalian entry (Raffel et al., 2014). Mutants lacking Vtp were able to colonize the salivary glands of O. hermsi ticks but failed to establish an infection in mice after tick feeding (Raffel et al., 2014). While the function of Vtp is unclear, the protein is homologous to the Vsp proteins of RF Borrelia spp. (see below) and OspC of Lyme borreliosis species (Carter et al., 1994; Marconi et al., 1993). Interestingly, the finding that the Vtp proteins from different B. hermsii strains are highly polymorphic suggests positive selection involving antibody responses or for the need to survive in different biological niches like rodents, canines, etc. While Vtp is essential in the establishment of early mammalian infection, less is known regarding the molecular mechanism of persistent vector colonization.
Transcriptional studies have been implemented to begin to understand how the TBRF spirochetes establish an infection in the tick vector. Work in B. turicatae suggested that the linear megaplasmid is primarily involved in vector colonization and/or initiating mammalian infection (Wilder et al., 2016). B. turicatae was grown under ambient temperature mimicking the tick and 67% of the genes localized on the linear megaplasmid were upregulated in these conditions compared to spirochetes isolated from infected murine blood. The expression of a cluster of genes on the 3′ end of the plasmid was investigated further in the tick because they were predicted to code for surface proteins. While the results demonstrated up-regulation in infected ticks compared to infected blood, the proteins are of unknown function. With advances in genetic systems for TBRF spirochetes, the identification of genes essential in vector colonization is now possible (Battisti et al., 2008; Lopez et al., 2013b).
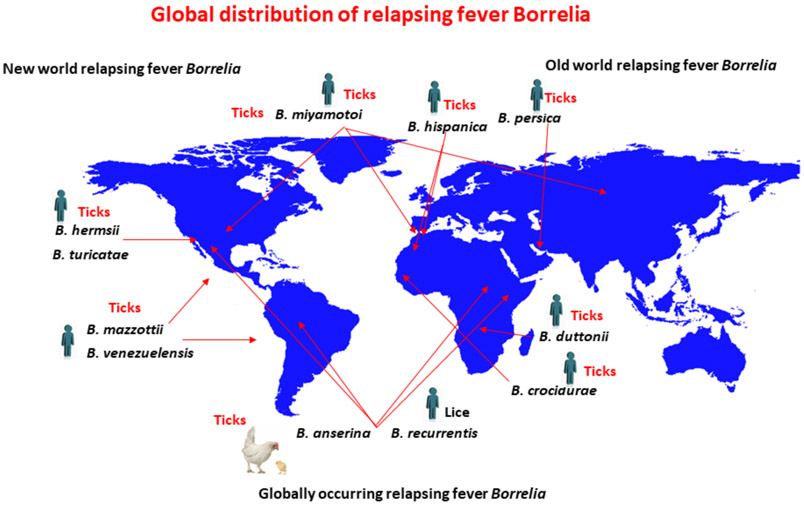
RF Borrelia speciation: Old and New World borreliosis
Currently, there is no systematic way to classify RF Borrelia; nomenclature has been subject to change, and differences exist between the speciation of Old and New World pathogens (Figure 1). A comprehensive description of classified RF Borrelia species is found in the Borrelia chapter of Bergey’s Manual of Systematics of Archaea and Bacteria as well as in the recent review by Talagrand-Reboul (Barbour and Schwan, 2018; Talagrand-Reboul et al., 2018). Well characterized Old World species include B. duttonii and B. crocidurae, which were named after Dr. Joseph Everett Dutton and the vertebrate host in the genus Crocidura, respectively. The Old World species, B. recurrentis (LBRF), was speciated based on its biology in the human host.
Figure 1.
Global distribution of RF Borrelia. Only certain representative RF Borrelia species are included in the figure. A more detailed description of currently defined species is found in (Barbour and Schwan, 2018; Talagrand-Reboul et al., 2018). Drawing was generated by Haitham Elbir. The global map was created using R software version 4.0.1
The speciation of New World RF spirochetes has been somewhat more systematic and primarily based on the species tick vector. For example, B. hermsii, B. parkeri, and B. turicatae were originally speciated according to their argasid vectors, O. hermsi, O. parkeri, and O. turicata. This method of speciation was based on the observed vector specificity of TBRF spirochetes. However, discrepancies do exist with other New World species. For example, B. mazzottii, which was detected in O. talaje from northern Mexico and likely distributed in the southern United States and other regions of Latin America, was named after Dr. Luis Mazzottii (Davis, 1956; Lopez et al., 2016).
Historically, confusion has surrounded the speciation of RF Borrelia and their tick vectors in Central and South America. For example, it was not clear whether O. talaje, O. rudis, or O. venezuelensis were independent species, and this led to the uncertainty in the speciation of their respective RF spirochetes (Davis, 1955). As the ambiguity regarding the tick vectors was clarified, it became apparent that Borrelia neotropicalis was synonymous with B. venezuelensis (Davis, 1955). Within the last 15 years, significant advances have been made toward identifying and speciating Ornithodoros ticks from Central and South America. Venzal and coworkers provided detailed morphological characteristics of larval Ornithodoros ticks and demonstrated the importance in characterizing this developmental stage for speciation (Venzal et al., 2008). Recently, Munoz-Leal and colleagues identified O. rudis in Brazil and this resulted in the first isolation of a TBRF spirochete from the continent (Munoz-Leal et al., 2018). Given the historic association of B. venezuelensis with O. rudis, this novel Borrelia isolate was designated B. venezuelensis. Interestingly, the molecular characterization of this spirochete indicated that it was most closely related to the North American species, B. turicatae.
Speciating TBRF spirochetes in Eurasia has posed similar challenges. This is primarily because a few loci are sequenced for speciation, while genetic, geographical, ecological, and biological factors are not considered when designating a new species of TBRF spirochete. The need for a refined systematic classification of TBRF spirochetes from Eurasia and the Middle East was demonstrated recently with the first isolation of B. caucasica from Ukraine (Filatov et al., 2020). Filatov and co-workers performed a phylogenetic analysis of B. caucasica comparing several single loci. The results indicated that, depending on the locus sequenced (16S rRNA, flagellin, and DNA gyrase), this species could have been designated as B. hispanica or B. persica. However, the spirochete was speciated as B. caucasica given the tick vector from which it was isolated. Moreover, while the intragenic spacer sequence (IGS) was not evaluated in that study, there is accumulating evidence that sequencing this locus may provide the best resolution for speciation of TBRF spirochetes (Bunikis et al., 2004; Cutler et al., 2010; Nieto and Teglas, 2014; Schwan et al., 2007).
Clinical presentation- the crisis phenomenon and the Jarisch-Herxheimer reaction
The clinical hallmark of RF is periods of very high fever spaced by periods of relative well-being, i.e., the recurrent fever episodes. The incubation period is between 5-8 days for LBRF, but varies much more with TBRF (Southern and Sanford, 1969). The reported mortality rates for untreated RF differ (Barbour et al., 2006), with the largest variability seen in LBRF. The high mortality was probably caused by factors associated with wars, famine, and situations within refugee camps, i.e., conditions of malnutrition and insufficient health care. Patients with RF usually complain of headache, backache, muscle pain, arthralgia, and abdominal pain. Sometimes, the clinical examination reveals jaundice and tenderness over the liver and spleen. A gradual decline of general fitness, with extreme weakness and weight loss can occur if successive relapses occur without adequate treatment (Goodman et al., 1969). If death occurs during RF, it is most common during a crisis episode or within hours after the start of antibiotic therapy. The crisis phenomenon results from the rapid disintegration of spirochetes within the blood stream because of the increase of neutralizing antibodies. Antibiotic treatment of spirochetal infections provides a similar effect, which was termed the Jarisch-Herxheimer reaction (J-HR) after its first description by the dermatologists Adolf Jarisch and Karl Herxheimer. They both observed this reaction during antimicrobial treatment with mercury in patients with secondary syphilis. Interestingly, Balfour (1911) observed that chickens experimentally infected with B. anserina also produced an “artificial crisis” after treatment with Salvarsan (arsenic compound 606). Treatment of both LBRF and TBRF with antibiotics was shown to commonly result in the J-HR (Bryceson, 1976; Bryceson et al., 1972; Warrell et al., 1983; Webster et al., 2002). Several studies have tried to resolve if the type of antimicrobial used is important for the generation and severity of J-HR. It is logical to envision that a bactericidal antibiotic is more prone to induce a J-HR response as large amount of cell fragments are released from the bacteria. However, there is still no conclusive result differentiating the J-HR response of a bacteriostatic from a bactericidal antibiotic (Butler, 2017). With the appearance of either neutralizing antibodies or the administration of antibiotics, spirochetes in the blood are lysed, releasing proinflammatory bacterial cell fragments into the circulation. Within a couple of hours after initiation of antibiotic treatment, the patient often presents with acute symptoms, including headache, rigors, malaise, hypotension, and sweating. Subsequently, symptoms might even worsen with an increase of temperature by 1-2° C, tachycardia, and a rise in blood pressure. This period of worsening symptoms occurs over the next couple of hours, followed by diaphoresis, exhaustion, a decline in temperature, hypotension, and leukopenia. This apparent shock-like condition, with dilated vasculature and increased oxygen demands with fever (Warrell et al., 1983), may be exacerbated by myocardial dysfunction if the spirochetes have invaded the heart.
Given the similarity of J-HR to septic shock, early clinical observations predicted endotoxins to be the cause of the J-HR phenomenon (Bryceson et al., 1972). However, Borrelia spirochetes have an outer membrane architecture different from other Gram-negative bacteria and lacking endotoxin, i.e., LPS is not present (Ben-Menachem et al., 2003; Takayama et al., 1987). Instead, spirochetes contains ample amounts of glycolipids (Ben-Menachem et al., 2003; Hossain et al., 2001; Schroder et al., 2008) as well as a large amount of surface located lipoproteins. Another hypothesis about the crisis was that it was attributable to antigen-antibody complexes (Galloway et al., 1977), but changes in complement levels were not detected in patients with LBRF (Warrell et al., 1983). A more likely explanation for J-HR and the crisis is the action of liberated spirochetal lipoproteins on the toll-like 2 receptors (TLR2) of macrophages and other cells (Scragg et al., 2000; Vidal et al., 1998). In patients with RF, the levels of tumor necrosis factor (TNF-α), interleukin (IL)-6, and IL-8 rose several fold over pre-treatment levels during the J-HR in patients treated for LBRF (Negussie et al., 1992). Importantly, in a clinical trial, treatment with anti-TNF antibodies before the penicillin therapy reduced the severity of J-HR (Fekade et al., 1996). In mice experimentally infected with B. turicatae or B. hermsii, serum concentrations of the anti-inflammatory cytokine IL-10 reach high levels (Crowder et al., 2016; Londono et al., 2008). In patients with LBRF, IL-10 was highly elevated, however, treatments with recombinant IL-10 neither prevented J-HR nor inhibited the elevation of TNF, IL-6 or IL-8 (Cooper et al., 2000).
Mammalian infection: RF Borrelia pathogenesis
The different periods of time spent feeding by soft and hard ticks (minutes versus days, respectively) clearly separates the biology of transmission of RF Borrelia and LD Borrelia. Under these very restricted time periods of transmission, a single spirochete may be enough to initiate an RF infection in experimental animals (Beunders and Van Thiel, 1932; Schuhardt and Wilkerson, 1951; Stoenner et al., 1982). The bacteria multiply in the blood at an estimated rate of one cell division every ~6 hours until they number between 106 to 107, sometimes as many as 108, per milliliter of blood at their peak (Figure 2) (Cadavid et al., 1994; Crowder et al., 2016; Schuhardt and Wilkerson, 1951; Stoenner et al., 1982). Cultivable RF species achieve similar generation times in broth medium, such as BSK II (Barbour, 1986). The interval between relapses in experimental infections is usually 2 to 7 days. Untreated LBRF tends to have fewer relapses than does TBRF (Bodman and Stewart, 1948; Borgnolo et al., 1993; Brown et al., 1988; Bryceson et al., 1970; Garnham et al., 1947; Salih et al., 1977; Sundnes and Haimanot, 1993). The reason for this discrepancy in the number of consecutive relapses is not known, but the finding that B. recurrentis have a reduced genome compared to B. duttonii, suggests that the former could have lost genetic traits related to prolonged relapsing (Elbir et al., 2014; Lescot et al., 2008).
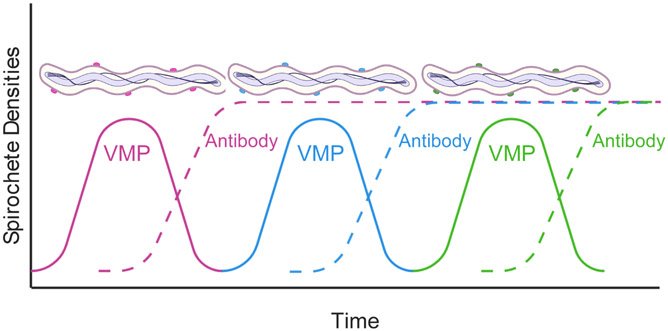
Figure 2.
Antigenic variation and antibody response during RF borreliosis. Show are spirochete densities in the blood over time. As spirochetes replicate in the blood a predominant VMP variant emerges (pink line and spirochetes coated with pink proteins). The antibody response generated against this variant (pink dotted line) clears the population of spirochetes while a new population emerges (blue variant). Again, an antibody response is generated (blue dotted line) to clear the population, and yet another variant (green) emerges. The dynamics between antigenic variation and the host antibody response can continue for months. Drawing was generated using BioRender by Brittany A. Armstrong and Job Lopez.
The incubation period between exposure and onset of fever or other signs of illness is from 3 to 12 days. During relapses, the number of spirochetes in the blood increases again, but usually not to the same density as during the first episode of spirochetemia. The lower concentration of cells in a relapse does not appear to be due to attenuated virulence of the relapse serotypes. Russell described the relapse populations as being as virulent as the “attack” serotype in naïve animals (Russell, 1936). Moreover, Crowder et al. observed that the relapse population of B. hermsii increased more slowly than the serotype that initiated the infection in mice; however, when each serotype was used to infect naïve mice, their growth rates were indistinguishable (Crowder et al., 2016). The existence of this growth retardation was corroborated by the finding that changes in host factors, involving the innate immune responses, could influence the growth rate during infection (Crowder et al., 2016). Further evidence for this was the observation that, during co-infection of experimental animals with a RF Borrelia species and an African trypanosome species, the population growth of the parasite was slower than during single infections (Felsenfeld and Wolf, 1973).
The growth rates and maximum cell densities of Borrelia species vary by species and environmental conditions. Most notably, TBRF species and B. recurrentis differ by a factor of 1,000 or more in the cell densities they achieve in the blood of immunocompetent as well as immunodeficient mice (Barbour and Bundoc, 2001; Crowder et al., 2016; Sadziene et al., 1993). However, during in vitro cultivation in the same type of medium, representative TBRF spirochetes and B. recurrentis have similar growth rates and reach stationary cell densities as high as ~108 per milliliter (Barbour, 1988; Cadavid et al., 1994; Crowder et al., 2016; Pennington et al., 1997). Also, expression profiles analyzed by genome-wide arrays revealed little difference between cells of B. hermsii harvested from BSK II broth medium or in the blood of mice, when the temperature and pH of the broth approximated that of blood, and cell densities at the time of collection were comparable (Zhong and Barbour, 2004). Given these observations, genetic differences in metabolic pathways or other inherent capabilities plausibly account for observed differences between TBRF and LBRF species in peak burdens in the blood. Four pathways that differ between the two groups of species are in the acquisition of glycerol-3-phosphate (Bacon et al., 2004; Schwan et al., 2003; Schwan et al., 1996), pyrimidine biosynthesis (Zhong et al., 2006), purine salvage (Barbour et al., 2005; Lescot et al., 2008; Pettersson et al., 2007), and glutamate-arginine-proline biosynthesis (Lescot et al., 2008).
Greater persistence in the blood presumably increases the fitness of RF Borrelia for transmission from an infected vertebrate to a naïve set of tick vectors (Barbour and Restrepo, 2000). However, RF spirochetes also leave the vascular compartment through the endothelium to invade the central nervous system, the eye, the liver, testes, the placenta, and other organs and tissues (Cadavid and Barbour, 1998; Cadavid et al., 2001; Nordstrand et al., 2001; Shamaei-Tousi et al., 2001). The borreliae survive in these extravascular sites when they have been eliminated from the blood (Cadavid et al., 1993; Cadavid et al., 2006; Kazragis et al., 1996; Larsson et al., 2008). Persistence in extravascular niches may also serve to enhance transmissibility, because invasion of the blood from these sites can recur under conditions of immune suppression (Larsson et al., 2006b). Also, Larsson and coworkers suggest certain immune privileged sites can serve as a location for silent infections that provide a reservoir for reactivation of infection as a result of external signals (Larsson et al., 2006b; Larsson et al., 2008; Lundqvist et al., 2010).
In animal models, tissue invasion appears to be facilitated by recruitment of plasminogen to the borrelial cell surface. Once converted to enzymatically active plasmin, local degradation of extracellular matrix barriers in conjunction with efficient spirochetal motility enable spread to distant organs. Consequently, in plasminogen-deficient mice, B. crocidurae invasion of brain and kidney was delayed (Nordstrand et al., 2001). Another mechanism of tissue invasion occurs indirectly because of erythrocyte rosette formation and microemboli-mediated hemorrhage (Figure 3). In experimental rats, pressure-induced capillary rupture leads to leakage of aggregates of spirochetes and erythrocytes into adjacent tissue (Shamaei-Tousi et al., 2001).
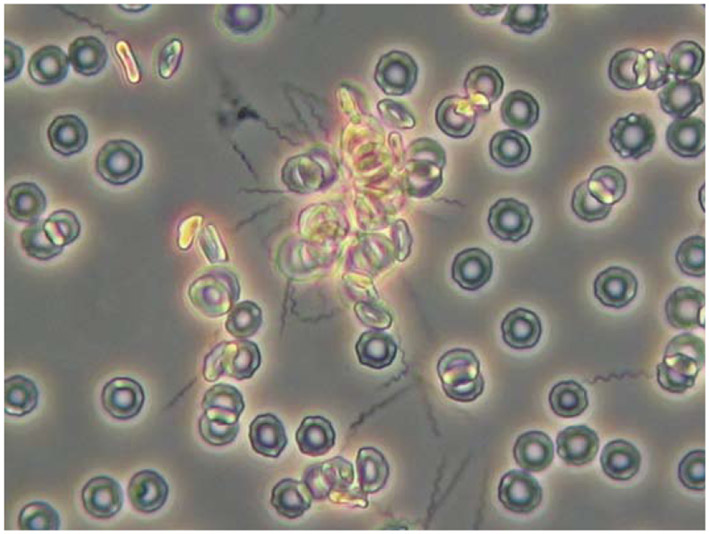
Figure 3.
Phase contrast micrograph of rosette of spirochetes and erythrocytes formed during experimental RF. Blood was examined four days after inoculation of an adult C3H/HeN mouse with Borrelia duttonii. Magnification 400X. Photo courtesy of Marie Andersson, University of Umeå.
When spirochetes are found in tissues, they generally are found in the interstitial spaces between cells, except when they have been engulfed by phagocytes. There is no compelling evidence that RF borreliae are facultative, let alone obligatory, intracellular pathogens. The pathologic findings and subsequent identification of RF spirochetes are mainly from autopsies of fatal human cases and from histologic examinations of experimentally-infected animals (Bryceson et al., 1970; Cadavid and Barbour, 1998; Southern and Sanford, 1969). Extracellular spirochetes were demonstrated in tissues, often in perivascular locations (Cadavid et al., 2001; Duray, 1987; Pennington et al., 1997). The liver and spleen were often enlarged and showed inflammation, with microabscesses in the spleen (Anderson and Zimmerman, 1955; Judge et al., 1974b; Thomas et al., 2002). The microabscesses, areas of necrosis, and petechiae may be the consequence, in part, of microemboli of spirochetes and blood cells, and transendothelial migration of neutrophils (Shamaei-Tousi et al., 2000; Shamaei-Tousi et al., 1999).
Intracranial hemorrhage has been observed in both LBRF and TBRF (Anderson and Zimmerman, 1955; Babes, 1916; Dewar and Walmsley, 1945; Judge et al., 1974d; Thomas et al., 2002). When spirochetes in the brain were noted, they were more common around vessels and adjacent to leptomeninges (Anderson and Zimmerman, 1955; Cadavid et al., 2001; Garcia-Monco et al., 1997). Although cardiac abnormalities are not usually associated with RF, myocarditis with the presence of numerous spirochetes has been demonstrated in experimental infections of both TBRF and LBRF (Breitschwerdt et al., 1996; Cadavid et al., 1994; Judge et al., 1974d; Londono et al., 2005; Wengrower et al., 1984). In experimental B. turicatae infection, the severity of illness correlated with the numbers of spirochetes in the blood (Pennington et al., 1997).
In the blood of infected patients, leukocyte counts are usually in the normal range or only slightly elevated (Parsons, 1947). Leukopenia and platelet counts below 50,000/mm3 may occur during the crisis. Hemorrhagic complications, such as epistaxis, are common in LBRF (Dennis et al., 1976; Perine et al., 1971). The erythrocyte sedimentation rate often is moderately elevated. There may be laboratory evidence of hepatitis, such as elevated serum concentrations of aminotransferases and prolonged prothrombin and partial thromboplastin times (Barbour, 2011). In the cerebrospinal fluid there may be mildly elevated numbers of mononuclear cells and protein levels, but glucose levels are typically normal.
Neurotropism
Both RF and LD Borrelia affect the neurological system, resulting in various neurological and pathognomonic symptoms. LBRF often presents a more severe course, but TBRF, at least for some species of Borrelia, such as B. duttonii and B. turicatae, results in a higher frequency of neurologic complications, including symptoms like facial palsy, weakness, radiculopathy, and, occasionally, stupor or coma (Cadavid and Barbour, 1998). Also, neuropsychiatric symptoms with psychotic events, including confusion, anxiety and hallucination has been described (Aubin et al., 1947). Neurotropism has also been observed experimentally in mammals (Cadavid and Barbour, 1998; Cadavid et al., 1993; Dubois and Pearson, 1939; Kazragis et al., 1996; Larsson et al., 2006b; Shamaei-Tousi et al., 1999; Velu et al., 1930). RF spirochetes are seen in the brain of infected mice as early as the second day after inoculation (Andersson et al., 2007). B. crocidurae cells remain actively growing in the brain and persist there in numbers of ~1000 per mouse brain (Larsson et al., 2006b; Larsson et al., 2008). A similar phenomenon may also occur in the tick vector, as B. duttonii spirochetes accumulate around the central ganglion cells of O. moubata (Burgdorfer, 1951).
In both human cases and experimental infections of rodents, neurotropism is more common following infection by some species than by others (Cadavid and Barbour, 1998). For example, the greater incidence of neurologic involvement in cases of RF due to B. duttonii in Africa and B. turicatae in North America has long been noted (reviewed by Cadavid and Barbour, 1998). Also, B. duttonii appears to cause persistent brain infection that could be reactivated by immune suppression as well as with concomitant infection with malaria (Lundqvist et al., 2010). These findings also present the importance of humans as reservoir for certain RF Borrelia species and a relevant biological signal for reactivation of silent RF borreliosis. A newly described species of RF Borrelia in Spain appears to be particularly neurotropic (Garcia-Monco et al., 1997). This isolate was later taxonomically defined as B. hispanica (Toledo et al., 2010).
Neurotropism also varies between variants of a given strain. Cadavid et al. demonstrated in an experimental animal model that some variants of a B. turicatae strain were present in higher numbers in the brain compared to other variants of the same strain (Cadavid et al., 2001; Cadavid et al., 1997; Cadavid et al., 1994). However, neurotropism is not necessarily associated with greater virulence as is evident from the finding of residual brain infection up to several months for RF Borrelia such as B. duttonii (Larsson et al. 2006). A neuroinvasive variant of B. turicatae had a lower fatality rate among experimentally infected mice than an isogenic variant with a lower potential for invasion of the brain (Cadavid et al., 1994; Pennington et al., 1997). Moreover, the noticeable neurological disease symptoms in certain cases of RF is aggravated by the massive influx of immune cells, including T cells that are important for neuropathogenesis (Liu et al., 2010).
Not only do neurological complications impact treatment, they also can lead to a misdiagnosis of Lyme disease; consequently, caution should be exercised when diagnosing LD in historically nonendemic regions. For example, in Mexico, where B. turicatae is distributed (Cooley and Kohls, 1944; Donaldson et al., 2016), serological responses in patients presenting with neurological symptoms (facial palsy) were screened for LD (Gordillo-Perez et al., 2017; Gordillo-Perez et al., 2018). These tests used whole B. burgdorferi protein lysates or commercially available kits. Unfortunately, given the serological cross reactivity with patients infected with RF and LD, assays should have targeted antigens specific for TBRF species, like glycerophosphodiester phosphodiesterase (GlpQ) or the Borrelia immunogenic protein A (BipA) (Lopez et al., 2010; Schwan et al., 1996). Moreover, there is increasing evidence that serological responses generated during B. turicatae infections cross-react with commercially available LD assays (Gettings et al., 2019). Interestingly, when TBRF-specific antigens are utilized in Mexico, serologic studies point to ongoing circulation of TBRF spirochetes (Vazquez-Guerrero et al., 2019).
Adhesins
Adherence to host cells is an important bacterial virulence mechanism. This enables microbes to gain a foothold on surfaces and resist mechanical removal. Binding of RF Borrelia to cellular and soluble blood components, such as integrins and glycoaminoglycans, has been described (Alugupalli et al., 2001; Magoun et al., 2000). However, in the absence of knock-out mutants, the functional roles of these associations for infectivity and pathogenesis still remain to be confirmed. The function as well as mechanism for these host ligands in mediating adherence of spirochetes to host tissues and cells are likely, and would be consistent with, similar, more established pathogenetic mechanisms of other pathogens, including LD Borrelia (see Radolf and Samuels, 2021).
Some species of RF spirochetes have been noted to form aggregates or rosettes with platelets or erythrocytes in the blood of infected animals (Alugupalli et al., 2003b; Burman et al., 1998; Mooser, 1958). Figure 2 shows a rosette of spirochetes and erythrocytes. Rosettes are best viewed when spirochete density in the blood is at its highest. For B. crocidurae, aggregation of spirochetes and erythrocytes into rosettes appears to delay immune clearance; antibodies appear and rise in the blood more slowly than is observed in experimental infection with Borrelia spp. that do not aggregate erythrocytes (Burman et al., 1998). Erythrocyte and spirochete rosettes also activate endothelial cells and cause microvascular thrombi and emboli that lead in turn to pathologic changes in tissues (Shamaei-Tousi et al., 2000; Shamaei-Tousi et al., 2001; Shamaei-Tousi et al., 1999). Erythrocyte aggregation has also been noted in experimental infections with B. duttonii (Takahashi et al., 2000).
B. hermsii attachment to platelets occurs via the integrin αIIbβ3 (Alugupalli et al., 2001; Alugupalli et al., 2003b), and B. crocidurae binds to erythrocytes via the neolactoglycan lacto-N-neotetraose (Guo et al., 2009). The bacterial ligands for these interactions have not yet been identified for RF species. One possible spirochete ligand is P66, or Oms66, an outer membrane protein porin of both LD and RF Borrelia species (Bunikis et al., 1998; Bunikis et al., 1995; Shang et al., 1998; Skare et al., 1997). P66 of B. burgdorferi has been reported to be an adhesin for the β3-integrin and as such important for dissemination in the infected host (Coburn et al., 1999; Coburn and Cugini, 2003). Two other possible adhesins are products of open reading frames BH0512 and BH0553 of B. hermsii. Both proteins are characterized by multiple repeating domains. Indirect evidence for the adhesin function of these proteins was the finding that antisera to these proteins diminished attachment of B. hermsii to erythrocytes (Guyard et al., 2005). One of the surface lipoproteins of B. turicatae, Vsp1, binds to glycosaminoglycans to a greater degree than other Vsp proteins of the same species or of B. hermsii (Magoun et al., 2000; Zuckert et al., 2004).
Serum, complement, and phagocyte resistance
Early investigators noted that RF Borrelia were susceptible to non-immune sera from some species of animals but resistant to the sera from other species. For example, B. duttonii survived in fresh sera from mice, guinea pigs, rabbits, pigeons, chickens, and horses but not in fresh sera from cow, goat, pig, or sheep (Cuboni, 1929). If the latter sera were first heat-inactivated, viability of the spirochetes was retained, an indication that the alternative complement pathway was involved. This is similar to the differential serum resistance demonstrated by LD Borrelia species (see Radolf and Samuels, 2021). Factor H is a major complement regulator in the serum that prevents C3b formation by accelerating decay of the C3 convertase and by facilitating the irreversible inactivation of C3b. Bacterial acquisition of factor H could enable complement evasion by inactivating C3b or preventing its deposition and, thus, phagocytosis. Some RF Borrelia species, including B. hermsii, but neither B. anserina nor B. coriaceae, display factor H binding activity (McDowell et al., 2003; Meri et al., 2006). This activity was attributed to plasmid-encoded proteins of ~20 kDa called FhbA proteins (Hovis et al., 2004). B. hermsii also binds to factor H-like protein 1, an alternative splice variant of factor H (Hovis et al., 2006; Lopez et al., 2008). FhbA elicits IgM and IgG responses during experimental infection of mice (Colombo and Alugupalli, 2008). The BhCRASP-1 protein of B. hermsii is a 20-kDa outer membrane lipoprotein that is orthologous to the CRASP protein BBA68 of B. burgdorferi. BhCRASP-1 appears to bind factor H as well as plasminogen (Rossmann et al., 2007). Finally, B. duttonii and B. recurrentis are reported to associate with C4b-binding protein in the serum (Meri et al., 2006). Several subsequent studies have identified additional complement factors, including FhbA, HcpA and CihC, which affect different regulators of complement activation. These factors make RF Borrelia resistant to killing by complement and provide other important virulence properties (Brenner et al., 2013; Fine et al., 2014; Grosskinsky et al., 2009; Grosskinsky et al., 2010; Schott et al., 2010; Woodman et al., 2009)
Antigenic variation
Vsp and Vlp proteins
The steady increase of spirochetes in the blood, followed by a rapid and deep drop in their numbers, and then their reappearance in the blood (Figure 2) are the direct result of the bacterium’s unique capacity for antigenic variation. Investigators as early as 1918 noted that each new crop of spirochetes differed antigenically from the population it succeeded and the population to succeed it (Jancso, 1918; Meleney, 1928; Russell, 1936). The individual variants of a given strain are called serotypes. By definition, there is little or no antigenic cross-reactivity between serotypes with polyclonal antisera obtained either from infected patients or immunized animals (Coffey and Eveland, 1967; Stoenner et al., 1982). A serotype that had disappeared from an RF Borrelia population under the force of immunity could reappear again if even a single of the surviving population was transmitted to a naïve animal (Barbour, 1987); thus the hereditary information conferring serotype identity is not lost from a cell. This feature of RF indicated that antigenic variation was not simply an accelerated evolutionary process with selection for a succession of mutants, as typified by influenza A virus.
B. hermsii, a North American species that is most commonly encountered in the mountainous areas of the West (see Radolf and Samuels, 2021), has been the subject of the most extensive studies of antigenic variation (Barbour, 2002). Several serotypes of the HS1 strain of this species were derived from a single cell injected into a mouse (Barbour and Stoenner, 1985; Restrepo et al., 1992; Stoenner et al., 1982). Stoenner raised specific antisera in mice to each of 24 B. hermsii serotypes and used these reagents to study the relapses that occurred during experimental infections (Stoenner et al., 1982). With this battery of antisera in immunofluorescence assays of blood smears from infected animals, he identified the serotypes of ~80-90% of the variants that appeared during relapses in hundreds of mice. Examination of the bulk of B. hermsii chromosomal sequences and plasmids revealed additional vsp and vlp genes for 59 different serotypes (Dai et al., 2006).
Serotype identity is determined by expression of one of a repertoire of polymorphic lipoproteins (Barbour et al., 1982) (see Radolf and Samuels, 2021). Similar to other bacterial lipoproteins, the processed N-terminus is acylated and anchors the protein in the outer membrane (Carter et al., 1994; Scragg et al., 2000). The known set of about 40 proteins, for which expression is documented, is divided about equally between two different families: variable large proteins (Vlp) of about 36 kDa and variable small proteins (Vsp) of about 20 kDa (Barbour et al., 1983; Burman et al., 1990; Carter et al., 1994; Restrepo et al., 1992). These are encoded by vlp and vsp genes, respectively. Full-length Vsp and Vlp proteins may have near-identical N-terminal sequences, but after the conserved signal peptide has been cleaved off, the processed proteins are highly divergent. Vsp and Vlp proteins are orthologs of the OspC proteins and VlsE proteins of LD-causing Borrelia species, respectively (Carter et al., 1994; Marconi et al., 1993; Zhang et al., 1997). Another ortholog of Vsp is the Vtp protein that is expressed in ticks (see above).
Figure 4 represents the structure of a Vsp protein dimer. The Vsp and Vlp proteins are not obviously homologous at the primary sequence level, but they resemble each other at the conformational level, being predominantly α-helical in secondary structure (Burman et al., 1990; Zuckert et al., 2004) and having similar folds (Lawson et al., 2006). Vsp proteins exist as dimers (Zuckert et al., 2001; Zuckert et al., 2004) and in this configuration resemble the deduced structure of a single Vlp protein (Eicken et al., 2002). The C-terminus is not anchored but is close to N-terminus near the cell’s surface for both Vsp and Vlp proteins. The loops between the four α-helices of each monomer are exposed to the environment as ligands or epitopes. The first and fourth alpha helical chains are conserved in sequence, while the second and third chains and loops between the chains comprise four variable regions of the molecule. The Vlp family of proteins is furthered divided into four sub-families with less than 70% sequence identity among them: α, β, γ, and δ (Burman et al., 1990; Dai et al., 2006; Hinnebusch et al., 1998; Restrepo et al., 1992). Different strains of B. hermsii from distant geographic origins in western North America have the same four sub-families (Hinnebusch et al., 1998).
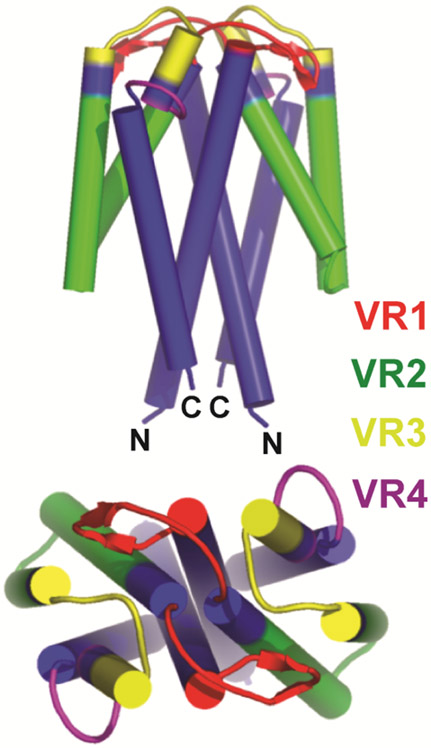
Figure 4.
Schematic representation of structure of a Vsp lipoprotein dimer of B. turicatae. The four major alpha-helical chains in each monomer are depicted by cylinders. The first and fourth alpha-helix (blue) in each Vsp are relatively conserved in sequence between different Vsp proteins of a given species (Dai et al., 2006; Restrepo et al., 1992). The second and third alpha-helical chain and the loops between all the chains are variable in sequence and are divided into variable regions (VR) 1 (red), 2 (green), 3 (yellow), and 4 (purple). The greatest variability is at the top or dome of the protein. Adapted from Lawson et al. (Lawson et al., 2006).
The considerable diversity among the sets of vsp and vlp genes contained in each Borrelia cell is the consequence of multiple rounds of intragenic recombination among the alleles within each cell (Rich et al., 2001b). This process was likely preceded by horizontal transfer of alleles between strains, for otherwise there would not be a fund of heterogeneous sequences upon which recombinational processes could operate. Horizontal transfer could be accomplished by the bacteriophage observed in association with B. hermsii (Barbour and Hayes, 1986), which may be homologous to the 32-kb circular plasmid prophages of B. burgdorferi (Eggers and Samuels, 1999; Stevenson et al., 2000; Zhang and Marconi, 2005). If an ancestor of RF species which possessed a single proto-ospC/vsp gene became merodiploid through acquisition of a second ortholog, then with subsequent rounds of recombination and gene duplications, the number of alleles might increase from two to several within a single lineage.
Genetic mechanisms for antigenic variation
Recognizing the cumulative studies of the biology, biochemistry, and immunology of RF, a modeler of the genetic mechanism of serotype-switching should account for the following features of the phenomenon, reviewed in (Barbour, 2002, 2003) (1) The variation is reversible; a serotype once cleared by an animal can appear again in another animal to whom the pathogen, even a single cell, is passed. (2) It is multiphasic, that is, involving three or more different antigens. (3) Serotype identity is conferred by a set of proteins, only one of which is expressed at a time. (4) Serotype-specific epitopes of the protein are located in different parts of the protein, not just in one region. (5) When there is an antigenic switch in a cell during infection, all or nearly all of the protein changes, not just a limited region of the protein, as in VlsE variation in B. burgdorferi. (6) Antigen switches are effectively random and spontaneous. In other words, they are not an effect of a change in the environment, such as inoculation into a mouse from culture medium or the appearance of neutralizing antibody in the blood (Stoenner et al., 1982).
Reversibility is achieved by archiving the antigen genes at locations where they are silent and, thus, presumably not under direct selection by immune systems and other host factors (Kitten and Barbour, 1990; Meier et al., 1985; Plasterk et al., 1985). For an antigen gene to be expressed, it must be copied and moved to a site where it can be transcribed (Barbour et al., 1991a; Burman et al., 1990; Sohaskey et al., 1999). This site may be altered by subsequent DNA rearrangements, leading to loss of the DNA that was located there, but a copy of that gene remains in the archive for future activation. The archived genes and the expression site for the duplicated gene are located on linear plasmids of 28 to 32 kb in B. hermsii (Ferdows et al., 1996; Kitten and Barbour, 1990; Plasterk et al., 1985) and on plasmids of other sizes in other RF species (Lescot et al., 2008; Penningon et al., 1999; Tabuchi et al., 2002). There are multiple copies of each type of plasmid in each cell with the genomes distributed evenly within the approximately 20 μm long spirochetal cell (Kitten and Barbour, 1992). The number of copies varies due to growth and milieu, but the mean was calculated both in vivo and in vitro to be within the range of 16 to 22 genomes per cell (Kitten and Barbour, 1992).
Sequencing of the linear plasmids that bear vsp and vlp genes in B. hermsii further defined the organization and arrangement of the expression site and the several archival sites (Burman et al., 1990; Dai et al., 2006; Restrepo et al., 1992). There appears to be a complete or nearly complete vsp or vlp gene for each Vsp or Vlp protein that a spirochete is capable of expressing. The report by Dai et al. provides full physical maps of large regions of the plasmids (Dai et al., 2006). In brief, the archived vsp and vlp genes are, with a few exceptions, found in clusters, usually in the same orientation, i.e., head-to-tail, but head-to-head at some locations. Several of the silent genes are full-length, that is, with a start codon and the signal peptide. Most of these complete genes at silent sites also have consensus ribosomal binding sequences and transcriptional start sites, but not discernible promoters. Other archived genes are lacking some sequence at the 5′ end. Some vsp- and vlp-like sequences are likely pseudogenes; they are missing 3′ ends and/or have indels with frameshifts. The expression site for vsp and vlp genes is located near the telomere of a 28-kb plasmid (Kitten and Barbour, 1990); it has a σ70-type promoter (Barbour et al., 1991a) and an upstream T-rich region that appears to enhance transcription (Barbour et al., 1991b; Sohaskey et al., 1999). Similar arrangements of the repertoires of vsp and vlp genes have been found on linear plasmids of various sizes in the RF species B. duttonii and B. recurrentis (Lescot et al., 2008; Tabuchi et al., 2002; Vidal et al., 2002).
In B. hermsii, two mechanisms can account for all the observed features of antigenic variation (Figure 5). The first and most common mechanism is a non-reciprocal recombination between a linear plasmid with an array of silent, archived vsp and vlp genes and another linear plasmid with a transcribed vsp or vlp gene (Kitten and Barbour, 1990; Meier et al., 1985; Plasterk et al., 1985). In B. hermsii, as well as in B. turicatae, what is effectively a gene conversion results in the replacement of the expressed gene for a variable major protein at a site downstream from a promoter at an expression site (Barbour et al., 1991a; Cadavid et al., 1997; Penningon et al., 1999; Pennington et al., 1999). An archived vsp gene can replace an expressed vlp gene or vice versa. The boundaries for the recombination are regions of sequence identity between silent and expression sites around and flanking the extreme 5′ and 3′ ends of the expressed and silent vsp or vlp genes (Barbour et al., 2000; Dai et al., 2006). In some cases, recombination at the expression site occurs within a vsp or vlp gene (Dai et al., 2006; Kitten et al., 1993). However, if the resultant chimeric protein retained one or more specific epitopes of the original protein, it presum ably would still be recognized by circulating antibodies elicited during the preceding spirochetemia (Barstad et al., 1985). In B. hermsii, the longest length of converted sequence that has been observed is about 2 kb (Kitten and Barbour, 1990; Restrepo et al., 1992). In B. turicatae, a more extensive gene conversion involving 10 or more kb downstream of the promoter on the expression-site plasmid occurs (Penningon et al., 1999). The mechanism of intermolecular recombination between plasmids first demonstrated in Nearctic species, like B. hermsii, also seems to occur in Afro-Tropical and Palearctic species. Rearrangements incorporating long lengths of plasmids have also been noted in B. duttonii (Takahashi et al., 2000).
Figure 5.
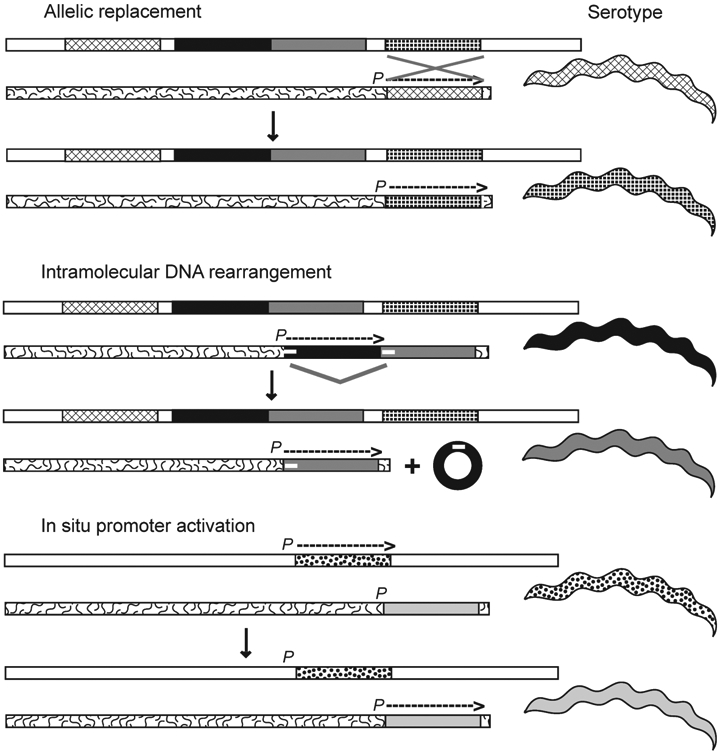
Three mechanisms for serotype switches by B. hermsii: allelic replacement, intramolecular DNA rearrangement, and in situ promoter activation. The different mechanisms are discussed in the text. P indicates the promoter and the location of the expression site near the right telomere of a linear plasmid; an overhead arrow shows whether the gene is active and the direction of transcription. vsp and vlp genes at the expression site location or in archival locations on the same (squiggly line background) or another linear plasmid (white background) are schematically represented by different patterns and grayscale values. The serotype of the cell, similarly indicated by pattern or grayscale, corresponds to vsp or vlp gene, at the expression site. In the second mechanism a deletion by direct repeats (small white horizontal bars) occurs and results in a non-replicative circle. (Courtesy of Alan Barbour, UC Irvine).
The second mechanism for expressing a different variable major protein is an intramolecular DNA rearrangement of the plasmid with the expression site (Figure 5). This mechanism has only been noted when there is another variable major protein gene just downstream of an expressed vlp gene (Restrepo et al., 1994). In such instances, the expressed gene and the non-transcribed downstream gene are in the same orientation. A deletion between short direct repeats at the 5′ ends of each of the tandemly arrayed genes effectively excises the sitting vlp gene, and the downstream gene effectively takes the place of the deleted gene next to the promoter. After this deletion, the newly expressed gene is susceptible to small gene conversions that provide further diversity of variable membrane proteins (Restrepo and Barbour, 1994), but the significance of this phenomenon for further immune avoidance is not known.
The activation of the vtp gene when B. hermsii is in the tick occurs by a mechanism that does not require recombination or transposition. This is a third mechanism: modification of transcripts by expression site switching (Figure 5). The vtp gene is located at another locus on a different plasmid than the expression site. At that location, vtp has its own promoter (Barbour et al., 2000; Carter et al., 1994). There is only one copy of vtp per genome. The mechanism of differential transcription between the vtp locus and the expression site locus is unknown.
Programming of antigenic variation
The process of switching starts in one of the 16 to 22 genome equivalents of the spirochetes, which means that during a certain period the cell is expressing more than one Vmp. This hybrid spirochete would be considered recessive until it becomes homozygous for the new serotype gene because of the antibody response generated against the prior Vmp (Crowder et al., 2017). Moreover, the overall serotype switch rate for B. hermsii is an estimated 10−4 to 10−3 per cell per generation. That is, by the time a given serotype reaches a population size of 1,000 to 10,000 cells in an animal, at least one cell of a different serotype has likely appeared in the population (Barbour and Stoenner, 1985; Stoenner et al., 1982). However, the rates for individual switches, e.g., from serotype 3 to serotype 7 or from serotype 17 to serotype 19, vary over about a thousand-fold range (Barbour et al., 2006; Barbour and Stoenner, 1985). The rates in each direction are not equivalent. For instance, switches from serotype 7 to serotype 19 are much less frequent than a switch from serotype 19 to serotype 7. These differences in switch rates among all possible antigenic variation events could account for the rough ordering of the appearance serotypes during individual infections that has been observed (Coffey and Eveland, 1967; Frank and Barbour, 2006; Stoenner et al., 1982)
The organization of the vsp and vlp genes on the plasmids provided clues to the possible mechanisms for the observed differences in frequencies of serotypes during the first relapse (Dai et al., 2006). The more frequent B. hermsii serotypes had Vsp or Vlp proteins that were encoded by genes with certain characteristics in their silent or archival locations (Barbour et al., 2006). One important characteristic was the extent of identity between the 5′ end of archived vsp or vlp sequence and the 5′ end of the expression site (Figure 6). This region of identity of approximately 50 nucleotides is called the UHS region and is the usual site for the upstream crossover during recombination (Dai et al., 2006). The second major variable was the spatial relationship of a given archived gene to an extragenic repetitive sequence of ~200 nucleotides called the DHS element, which directly follows some but not all archived genes and, when present, is at various distances downstream of a silent allele. The DHS contains the downstream crossover site for the recombination (Dai et al., 2006).
Figure 6.
Organization of the expression plasmid (top) and one archival plasmid (bottom) in B. hermsii. Two examples of vsp or vlp genes are denoted by “X” and “Y” and different fill patterns. There is a single expression site on one plasmid, and silent variants of vsp and vlp genes are found on the same and other plasmids. The direction and extent of transcription of the duplicate gene at the expression site is indicated by the arrow. The UHS element at the expression site comprises 61 nt around the start codon of the variant gene (Barbour et al., 1991a; Dai et al., 2006; Kitten et al., 1993). Silent genes vary in the extent to which their UHS regions are identical to the expression site UHS; this is represented here by the relative length of the UHS block. There is a 214 nt noncoding DHS element downstream from the expression site and adjacent to the plasmid telomere and at various locations on the plasmids. The lengths of vsp/vlp gene examples and the UHS and DHS elements are not to scale. (Courtesy of Alan Barbour, UC Irvine).
The serotypes that were most frequently observed during the first relapse of infection in laboratory mice were specified by vsp or vlp genes which, in their archived locations, had the following properties: (1) a UHS sequence that was identical or near-identical to the UHS at the expression site, and (2) a DHS element 50-200 nucleotides downstream. Archived vsp or vlp alleles that lacked both properties were rarely expressed during the first or early relapses. Silent genes with either property were intermediate in frequency (Barbour et al., 2006). Presumably, possession of both (1) and (2) fostered recombination between the archived sequence and the expression site.
Immunity
Immunity in RF has been the subject of study since the late nineteenth century, as reviewed in (Barbour, 1987; Barbour and Restrepo, 2000). The relapse phenomena observed in experimental animals and in patients with neurosyphilis undergoing fever therapy with RF spirochetes provided evidence for early immunologists, such as Paul Ehrlich, of the fine specificity of the adaptive immune response. The role of immunity in bringing about clearance of spirochetes from the blood was demonstrated by findings that the initial spirochetemia of mice or rats can be prolonged by gamma irradiation (Barbour et al., 1983; Newman and Johnson, 1984), treatment with cyclophosphamide (Newman and Johnson, 1984; Stoenner et al., 1982), the severe combined immunodeficiency (SCID) phenotype (Cadavid et al., 1994), or splenectomy (Meleney, 1928).
Figure 2 shows the appearance of anti-Borrelia antibodies in response to infection and the proliferation of spirochetes. This process was thoroughly described in a study by Crowder and coworkers, which infected <10 cells of B. hermsii in a group of mice and obtained a spirochetal peak of ~108 followed by a clearance of spirochetes that coincides with the rise of variant-specific agglutinating antibodies (Crowder et al., 2016). This first immune response does not prevent the growth of a second wave of spirochetes. As Figure 2 suggests, adaptive immunity to RF species is largely, if not exclusively, variant-specific. If there is an immune response that provides protection against several antigenic variants of a strain or species of RF Borrelia, it has not to our knowledge been reported. This may be one reason why there has not been an effective vaccine against RF, although the agent has been known for over 120 years. Aristowsky and Wainstein immunized humans with one variant of a strain; this provided protection against homologous challenge, but not against heterologous variants (Aristowsky and Wainstein, 1929). The variant-specificity of immunity may also explain the paucity of useful serologic assays for confirmation of the diagnosis of RF or for seroepidemiologic surveys. The most promising subunit serologic assays for RF are based on the GlpQ and BipA proteins, which are found in RF Borrelia spp. but not in LD Borrelia spp. (Lopez et al., 2010; Lopez et al., 2013a; Porcella et al., 2000; Schwan et al., 1996).
In 1896, Gabritchewsky showed the importance of humoral immunity for clearance of spirochetes during RF when he demonstrated the lytic action of immune serum on Borrelia (Gabritchewsky, 1896). Novy and Knapp reported in 1906 that they passively protected animals against infection with immune serum (Novy and Knapp, 1906). Calabi subsequently found that serum taken at the time of clearance reduced the viable count of spirochetes in suspension by up to one million-fold (Calabi, 1959). The lesser importance of cellular immunity for resolution of infection was indicated by the findings that T cell deficient nude mice cleared spirochetemia as effectively as wild-type animals and that infection was cleared following reconstitution of lethally irradiated mice with B cells alone, but not T cells (Barbour and Bundoc, 2001; Newman and Johnson, 1984).
As the foregoing experiments would suggest, IgM antibodies from infected animals are alone sufficient to limit or eliminate infection in the blood (Alugupalli et al., 2003a; Arimitsu and Akama, 1973; Barbour and Bundoc, 2001; Connolly and Benach, 2001; Newman and Johnson, 1981, 1984). Within 24 to 48 hours after the spirochetes reach a peak of 106 to 107 organisms per milliliter of blood, they are undetectable by light microscopy in the blood of an immunocompetent animal. When antibodies are administered intravenously, the spirochetes are cleared from the blood within one hour (Barbour and Bundoc, 2001; Cadavid et al., 1993). As earlier empirical studies had suggested, the neutralizing antibody response is serotype-specific: the animal remains susceptible to other serotypes of the same strain or of different strains (Barbour, 1987; Barbour and Restrepo, 2000). Up to 10 different relapses have been recorded during experimental infections (Felsenfeld, 1965). Whether recovery is due to the development of antibodies to all or most serotypes during the infection’s course or to the eventual rise of antibodies directed against antigens expressed by all serotypes is unknown. In endemic areas, newcomers or visitors appear to be at greater risk of symptomatic TBRF than long-term residents, an indication that long-lasting immunity to the local strain(s) may occur among humans.
Specific antibodies, even Fab monomers, recognizing outer membrane proteins of B. hermsii and other RF species can kill the spirochetes in the absence of complement or phagocytes (Connolly and Benach, 2001; Connolly et al., 2004; Sadziene et al., 1994). The bactericidal action lies in the variable region (LaRocca et al., 2008), as it does for bactericidal antibodies directed to a surface protein of B. burgdorferi (Sadziene et al., 1994). Incubation of the antibody with Borrelia cells leads to bacterial lysis following outer membrane disruption and excessive bleb formation (LaRocca et al., 2008).
B cell memory commonly is dependent on stimulation by helper T cells. The fact that Borrelia clearance is not dependent on T cell function indicated that a different B cell type was responsible for generating bactericidal antibodies. B1b lymphocytes were shown to confer long-lasting immunity independent of a T cell response via rapid generation of Borrelia-specific IgM (Alugupalli et al., 2003a; Alugupalli et al., 2004) . Induction of this response occurs by way of toll-like receptor stimulation and B cell antigen receptor activation (Alugupalli et al., 2007).
During infection, both experimental animals and human patients develop antibody responses against several different spirochetal components, including the periplasmic flagella and heat shock proteins. However, only antibodies directed against variant-specific proteins appear to be effective in controlling infection (Barbour and Bundoc, 2001; Cadavid et al., 1993; Stoenner et al., 1982). Moreover, a host response that eradicates the spirochetes of the predominant serotype from the blood is often not successful in clearing spirochetes from the brain, cerebrospinal fluid, or eye (Barbour, 1987; Cadavid and Barbour, 1998; Larsson et al., 2006b). Unfortunately, given the mechanisms of antigenic variation, targeting the Vmps in a vaccine is not practical.
An alternative approach to generating a vaccine against RF spirochetes would be to neutralize them during early mammalian infection, as they enter the host during tick feeding. Since it takes several generations for antigenic variation to commence, this approach targets more conserved surface proteins that RF spirochetes produce while still colonizing the salivary glands. The efficacy of this approach was demonstrated initially in the B. hermsii model (Krajacich et al., 2015). Groups of mice were immunized with one of two antigenically distinct vtp variants and were subsequently challenged by tick bite (Krajacich et al., 2015). The animals were protected against homologous variant challenge, but not against heterologous challenge. Regardless, this study demonstrated that preventing the establishment of early mammalian infection is a promising approach toward generating a vaccine against RF spirochetes.
Other RF Borrelia species: Emerging diseases
Borrelia miyamotoi is a RF spirochete that, despite its discovery in 1995 (Fukunaga et al., 1995), has only recently been shown to be pathogenic to humans (Platonov et al., 2011). Unlike other RF Borrelia, B. miyamotoi is not transmitted by soft ticks or lice, which mostly reside in the Southern hemisphere, but instead by hard ticks of various Ixodes species that also serve as vectors for B. burgdorferi sensu lato (Wagemakers et al., 2015). The main Ixodes vectors for both Borrelia species live in the temperate climate zones of the Northern hemisphere: Ixodes pacificus and Ixodes scapularis (North-America), Ixodes ricinus (Europe and Asia) and Ixodes pacificus (Russia and Asia). In contrast to B. burgdorferi sensu lato, which is not transmitted from the female adult tick to her offspring (Steere et al., 2016), B. miyamotoi is efficiently transmitted transovarially (Han et al., 2019). Infection rates of nymphs, the Ixodes life stage that is most notorious to bite humans, differ for B. miyamotoi and B. burgdorferi sensu lato, with for instance average infection rates of I. ricinus nymphs with B. burgdorferi sensu lato of approximately 12% (Strnad et al., 2017) compared to approximately 2% for B. miyamotoi in Europe (Cutler et al., 2019; Wagemakers et al., 2015).
Similar to other Borrelia species, B. miyamotoi has a complex genome with one linear chromosome and a variable number of linear and circular plasmids (Hue et al., 2013; Kingry et al., 2017a; Kingry et al., 2017b; Kuleshov et al., 2018). Due to apparent genetic differences between isolates, the broader term B. miyamotoi sensu lato was proposed (Krause et al., 2015). As for other RF Borrelia, multiple plasmids encode different Vmps. Vsps and Vlps belonging to the α, β, γ, and δ sub-families, of which only one Vmp is present and transcribed at the expression site in the linear plasmid p41 in any moment in time in a given RF spirochete (Kuleshov et al., 2020). B. miyamotoi, similar to other RF Borrelia (Barstad et al., 1985; Plasterk et al., 1985) may be able to evade host immune responses by full Vmp gene switching (Wagemakers et al., 2016), in which the initial Vmp is replaced by a different archival full length Vmp gene that is subsequently expressed (Wagemakers et al., 2015). This could explain the occasional spirochetemia relapses in mice experiments (Wagemakers et al., 2016) and in humans (Platonov et al., 2011; Sarksyan et al., 2015a). This could also explain why SCID mice, lacking T and B cells, are able to develop continuous spirochetemia (Wagemakers et al., 2016). Phylogenetic analysis of the available (partial) genomes showed that there are genetic differences between clinical or tick isolates of B. miyamotoi sensu lato from Europe, Asia and North America, presumably related to differences in tick vectors in the respective geographical locations (Kuleshov et al., 2019). Importantly, B. miyamotoi sensu lato is resistant to killing by the human host complement system (Wagemakers et al., 2014). This is, at least in part, mediated through a protein designated complement binding and inhibitory protein A (CbiA), also called Factor H binding protein, that interacts with the complement regulators Factor H, C3, C3b, C4b, C5, and C9 (Rottgerding et al., 2017). CbiA inhibits the activation of the classical pathway and assembly of the terminal complement complex (Rottgerding et al., 2017) and can bind human plasminogen (Nguyen et al., 2018).
Although human exposure to, and infection with, B. miyamotoi appears to occur regularly (Jahfari et al., 2016; Kadkhoda et al., 2017; Krause et al., 2014; Sarksyan et al., 2015b; Sato et al., 2018; Smith et al., 2019; Wroblewski et al., 2017), human B. miyamotoi disease (BMD) is relatively infrequent. Nonetheless, case reports and case series of human BMD have been described in Europe, Asia and North America, with a considerable amount of cases originating from Russia and the United States (Chowdri et al., 2013; Crowder et al., 2014; Hoornstra et al., 2018; Jiang et al., 2018; Jobe et al., 2016; Karan et al., 2018; Koetsveld et al., 2017b; Krause et al., 2013; Krause et al., 2016; Molloy et al., 2015; Platonov et al., 2011; Sarksyan et al., 2015a; Sarksyan et al., 2015b; Sato et al., 2014; Sudhindra et al., 2016; Yamano et al., 2017). Generally, the clinical presentation is rather indistinct, but is characterized by fever a few days to weeks after a tick bite, accompanied by non-specific influenza-like symptoms, including chills, fatigue, headache, myalgia and arthralgia (Platonov et al., 2011). Relapses of fever, well known for other RF Borrelia infections, appear to only occur occasionally (Sarksyan et al., 2015a). Upon examination of blood, abnormalities, such as mild lymphocytopenia or thrombocytopenia and elevated liver enzymes and/or creatinine, can be observed. Thus, based on the clinical presentation and routine blood studies, it is difficult to differentiate BMD from other tick-borne diseases, or even from other non-tick bite related infectious or inflammatory conditions. In immunocompromised individuals, mainly those on B cell depleting therapy (e.g., anti-CD20 monoclonal antibody rituximab), BMD can present as a meningoencephalitis (Boden et al., 2016; Gugliotta et al., 2013; Hovius et al., 2013). However, a B. miyamotoi-induced meningoencephalitis also was described in a seemingly immunocompetent individual in Sweden (Henningsson et al., 2019).
B. miyamotoi disease requires a high level of suspicion among physicians, yet there is little public awareness and diagnostic tools are still experimental and scarcely available. Nonetheless, there are several diagnostic options. Firstly, although most likely of little clinical utility, it is possible to isolate B. miyamotoi from blood of infected individuals (Wagemakers et al., 2014). Secondly, and of greater clinical relevance, DNA from B. miyamotoi sensu lato can be detected by molecular methods in blood (plasma or serum) (Platonov et al., 2011) or cerebrospinal fluid (CSF) (Hovius et al., 2013) and, albeit less sensitive, spirochetes can be directly visualized by dark-field microscopy (Telford et al., 2019). The above-mentioned diagnostic modalities can be used only in febrile episodes, during which spirochetemia occurs. Outside of the spirochetemic episodes, especially with longer lasting symptoms, serology should be considered. Most studies have performed serological tests based on detection of antibodies against the (GlpQ) antigen expressed by RF Borrelia, but not by LD Borrelia (Schwan et al., 1996). Of note, GlpQ is also expressed in other gram-negative bacteria, although with low levels of similarity, and does not discriminate B. miyamotoi from other RF Borrelia. However, due to differences in the geographical spread of hard-bodied Ixodes ticks and soft ticks and their associated RF Borrelia, this is of little concern, except in specific areas such as California, USA (Krause et al., 2018). Another concern with GlpQ as a diagnostic marker is the large variation in the sensitivity to detect B. miyamotoi disease; depending on the population tested, the techniques used to determine seroreactivity results in whether or not patients were treated with antibiotics (Jahfari et al., 2017; Koetsveld et al., 2018a; Krause et al., 2013; Molloy et al., 2015). Therefore, additional antigens, including Vmps, have been investigated (Wagemakers et al., 2016). Interestingly, combining GlpQ with a selection of Vlps and Vsps, results in a high specificity (100% for IgM and 98.3% for IgG) and sensitivity (79% for IgM and 86.7% for IgG) (Koetsveld et al., 2018a). Preliminary and unpublished observations from follow-up studies, which included culture confirmed Lyme borreliosis patients as controls, indicate that Vmps suffer from cross-reactivity (personal communication A.E. Platonov]. Additionally, and vice versa, antibodies against Vmps from BMD patients cross-react with the so-called C6 ELISA (Koetsveld et al., 2020; Molloy et al., 2018), which is based on a conserved peptide within B. burgdorferi sensu lato and is a widely used serodiagnostic test for Lyme borreliosis. Interestingly, when using two-dimensional electrophoresis, several immunogenic B. miyamotoi proteins were identified, including a putative lipoprotein that specifically reacted with mouse anti-B. miyamotoi and not anti-B. burgdorferi antibodies (Harris et al., 2019). Another promising antigen is BipA, which not only can discriminate between LD Borrelia and RF Borrelia (Lopez et al., 2010), but also may differentiate between different RF Borrelia species (Lopez et al., 2013a). Future research will be needed to determine whether BipA can distinguish infection caused by B. miyamotoi and other RF Borrelia.
Based on seroprevalence studies, a considerable number of human B. miyamotoi infections can be asymptomatic. However, when B. miyamotoi disease is suspected or confirmed, antibiotic treatment should be initiated, although in reported cases from Europe and the United States, individuals recovered fully without antibiotic treatment (Hoornstra et al., 2018; Sudhindra et al., 2016). Additionally, in vitro analysis indicated that B. miyamotoi laboratory strains and Russian clinical isolates are susceptible to doxycycline, azithromycin, and ceftriaxone (Koetsveld et al., 2017a; Koetsveld et al., 2018b). In these studies, a (relative) resistance to amoxicillin was shown. Although, this has not been confirmed in vivo and human patients with B. miyamotoi disease have been successfully treated with amoxicillin (Molloy et al., 2015), caution might be warranted using this antibiotic until further studies have been performed. As with LD and RF caused by other Borrelia, when central nervous system involvement is suspected or proven, treatment with intravenous antibiotics, e.g., ceftriaxone, should be considered (Henningsson et al., 2019; Hovius et al., 2013).
Other RF Borrelia species
B. anserina and B. coriaceae are two species transmitted by soft ticks but not known to cause RF in humans. B. anserina, which is the type species of Borrelia, is the agent of avian or fowl spirochetosis. It is transmitted by Argas persicus and related species and is global in distribution in poultry flocks (DaMassa and Adler, 1979; Diab and Soliman, 1977; Zaher et al., 1977). The disease causes severe anemia, weight loss, and high mortality in affected poultry flocks and is the most widespread poultry disease in the world, so is of great economic significance. Heavy spirochetemia of the first episode of illness may extend for 4-6 days in chickens and is prolonged for weeks in bursectomized fowls (Bandopadhyay and Vegad, 1983; DaMassa and Adler, 1979). There are antigenic differences between strains of B. anserina and evidence of strain-specific immunity (DaMassa and Adler, 1979; Pachanian and Smith, 1970; Uppal and Rao, 1966). However, B. anserina has not been known for displaying antigenic variation during infection. The mortality of the early infection is often high, and many experimental infections were terminated after the first spirochetemia (Bandopadhyay and Vegad, 1983). Nevertheless, the occurrence of immune evasion is suggested by Balfour’s report on “Spirochetosis of Sudanese fowls” (Balfour, 1911). He wrote that “in chicks relapses are common, and the disease therefore approaches mammalian spirochetosis in type.” In experimental infections of chicks, Balfour noted that 2-3 day periods of heavy spirochetemia culminated in a “crisis.” This was followed by disappearance of spirochetes from the blood, then reappearance of spirochetes in the blood, sometimes in “large numbers” after an interval of ~4 days. B. anserina has a Vsp-like lipoprotein of 22 kDa (Sambri et al., 1999). Chicks that were passively immunized with antisera to this protein were protected against subsequent homologous challenge with ~105 spirochetes, but antisera to a Vsp-like protein from another strain of B. anserina did not provide protection against heterologous challenge.
B. coriaceae occurs in O. coriaceus soft ticks in California (Hendson and Lane, 2000; Lane and Manweiler, 1988). These ticks feed on deer and cattle, but the role of these large mammals in maintenance of the infection remains to be determined. B. coriaceae was identified by PCR in the blood of a mule deer and a Columbian black-tailed deer in California (Lane et al., 2005; Yabsley et al., 2005). O. coriaceus has been associated with epizootic bovine abortion in California and Nevada (Schmidtmann et al., 1976). B. coriaceae’s etiological role in this disease has been suggested but not proven (Lane et al., 1985).
B. theileri is an RF-like Borrelia species that is transmitted by hard ticks of the metastriate genus Rhipicephalus rather than by argasid ticks. B. theileri produces a microscopically-detectable spirochetemia and weakness, loss of appetite, and anemia in cattle in Africa, Australia, and Europe (Brocklesby et al., 1963; Sharma et al., 2000; Smith et al., 1978). According to Theiler’s original description of the infection, there may be two attacks of fever, suggesting the occurrence of antigenic variation (Theiler, 1902). Transovarial transmission of B. theileri in Rhipicephalus (Boophilus) microplus occurs (Smith et al., 1978). Another Borrelia sp. that clusters with B. theileri was identified in Rhipicephalus (Boophilus) microplus in Brazil (Yparraguirre et al., 2007).
In the last decade, other RF group species that are transmitted by hard rather than soft ticks have been identified. These all cluster with B. theileri in a monophyletic group distinct from species transmitted by soft ticks. One of the newly discovered species is B. lonestari, which was found in the U.S. in Amblyomma americanum, another metastriate tick (Barbour et al., 1996; Rich et al., 2001a). The distribution in the southern and eastern US of A. americanum corresponds with the distribution of an illness characterized by a rash resembling erythema migrans of Lyme borreliosis, which has been called southern tick-associated rash illness or STARI. The clinical association between STARI and B. lonestari came from a positive PCR test from an erythema of a patient with an attached A. americanum, suggesting that this spirochete might be the causative agent of STARI (Campbell et al., 1995). Further clinical and serological studies have clearly separated the erythema skin lesions presented during infection with B. burgdorferi and B. lonestari, respectively (Philipp et al., 2006; Wormser et al., 2005). Interestingly, a subsequent study by the same research group showed that neither B. lonestari nor B. burgdorferi or any other spirochete was the causative agent of an erythema migrans like condition in a specific area in Missouri (Wormser et al., 2005). The etiology of the erythema migrans like disease found in that area is still unclear. Recent reports on cases of STARI infections in other areas in the US will aid in the understanding of the epidemiology of B. lonestari (Feder et al., 2011). Also, the successful culture isolation of B. lonestari will promote the understanding of this spirochete (Varela et al., 2004). To further study the infection biology of B. lonestari (Moyer et al., 2006) used a low-passage culture isolate to infect white-tailed deer (Odocoileus virginianus), a common host of A. americanum ticks, as well as dogs, mice, and cattle. Only deer developed spirochetemia, as evidenced by blood smear and PCR; the spirochetemia lasted for 6-9 days. Yabsley et al. subsequently found that raccoons were refractory to infection with B. lonestari. (Yabsley et al., 2008) The failure of B. lonestari to infect mice is attributable to the bactericidal activity of naive mouse serum for this species (Grigery et al., 2005).
Future Directions
Many other species within the family of Borreliaceae probably have reservoir host-vector cycles that remain vague and their role in human infection is unknown. Consequently, these organisms have escaped attention. Of importance for the development of therapeutic, diagnostic, and preventative measures is the ability to isolate spirochetes. While isolation in culture medium can be challenging for RF spirochetes, once bacterial isolates are obtained the available tool-box of multiple omics techniques will enable further exploration to the unique life of these microorganisms. Importantly, with the increasing number of tools for genetic manipulation of RF Borrelia, we will further unravel mechanisms of pathogenesis. Also, the accumulation of knowledge on RF Borrelia pathogenesis will aid in development of anti-virulence drugs that might alter the use of broad-spectrum antibiotics and, as such, decrease the burden on the pandemic of antibiotic resistance.
As additional species of Borrelia are identified, they may include organisms that, like B. miyamotoi and B. lonestari, share features with both the RF species and LD species or have other novel features. New species may not include features linking these two groups of spirochetes, but they could be useful for further defining features distinguishing the pathogenesis of RF and LD. Finally, especially for those RF Borrelia species with relatively short-lived and low levels of spirochetemia, e.g., B. miyamotoi, novel molecular and serological diagnostic antigens are warranted. Ideally those would be able to discriminate between spirochetes belonging to the B. burgdorferi sensu lato group and those that belong to RF Borrelia and even amongst different RF Borrelia species.
Acknowledgement
This article was based on a previous publication by Alan Barbour and Betty Guo, whose contributions are the foundation of the current review. JL was supported by the NIH grant AI137412,, JWH was supported by the European Union’s regional development fund (INTERREG) as part of the NorthTick project and SB from the Swedish Research Council (VR-MH 01885).
References
- Aeschlimann A (1958). Développement embryonnaire d'Ornithodorus moubata (Murray) et transmission transovarienne de Borrelia duttoni. Acta Tropica 15, 15–64. [PubMed] [Google Scholar]
- Alugupalli KR, Akira S, Lien E, and Leong JM (2007). MyD88- and Bruton's tyrosine kinase-mediated signals are essential for T cell-independent pathogen-specific IgM responses. J Immunol 178, 3740–3749. doi: 10.4049/jimmunol.178.6.3740 [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, and Leong JM (2003a). The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol 170, 3819–3827. doi: 10.4049/jimmunol.170.7.3819 [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, and Gerstein RM (2004). B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21, 379–390. doi: 10.1016/j.immuni.2004.06.019 [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, Michelson AD, Barnard MR, Robbins D, Coburn J, Baker EK, Ginsberg MH, Schwan TG, and Leong JM (2001). Platelet activation by a relapsing fever spirochaete results in enhanced bacterium-platelet interaction via integrin alphaIIbbeta3 activation. Mol Microbiol 39, 330–340. doi: 10.1046/j.1365-2958.2001.02201.x [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, Michelson AD, Joris I, Schwan TG, Hodivala-Dilke K, Hynes RO, and Leong JM (2003b). Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 102, 2843–2850. doi: 10.1182/blood-2003-02-0426 [DOI] [PubMed] [Google Scholar]
- Anderson TR, and Zimmerman LE (1955). Relapsing fever in Korea; a clinicopathologic study of eleven fatal cases with special attention to association with Salmonella infections. Am J Pathol 31, 1083–1109. [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Nordstrand A, Shamaei-Tousi A, Jansson A, Bergstrom S, and Guo BP (2007). In situ immune response in brain and kidney during early relapsing fever borreliosis. J Neuroimmunol 183, 26–32. doi: 10.1016/j.jneuroim.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Arimitsu Y, and Akama K (1973). Characterization of protective antibodies produced in mice infected with Borrelia duttonii. Jpn J Med Sci Biol 26, 229–237. doi: 10.7883/yoken1952.26.229 [DOI] [PubMed] [Google Scholar]
- Aristowsky WM, and Wainstein AB (1929). Rekurrens-Schutzimpfungsversuche am Menschen. I. Zeitschrift für Immunitaetsforschung und Experimetelle Therapie 61, 296–308. [Google Scholar]
- Aubin H, Gachkel, and et al. (1947). Les troubles mentaux dans la fievre recurrente. Alger Medicale 50, 408–416. [PubMed] [Google Scholar]
- Babes V (1916). Hemorragies meningees et autres manifestations hemorragiques dans la fievre recurrente. Comptes Rendus des Seances Societe de Biologie (Paris) 79, 855–857. [Google Scholar]
- Bacon RM, Pilgard MA, Johnson BJ, Raffel SJ, and Schwan TG (2004). Glycerophosphodiester phosphodiesterase gene (glpQ) of Borrelia lonestari identified as a target for differentiating Borrelia species associated with hard ticks (Acari:Ixodidae). J Clin Microbiol 42, 2326–2328. doi: 10.1128/JCM.42.5.2326-2328.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour A (1911). Fourth Report of the Wellcome Tropical Research Laboratories at the Gordon Memorial College Khartoum (London: Wellcome Tropical Research Laboratories; ), pp. 76–107. [Google Scholar]
- Bandopadhyay AC, and Vegad JL (1983). Observations on the pathology of experimental avian spirochaetosis. Res Vet Sci 35, 138–144. [PubMed] [Google Scholar]
- Barbour AG (1986). Cultivation of Borrelia: a historical overview. Zentralbl Bakteriol Mikrobiol Hyg A 263, 11–14. doi: 10.1016/s0176-6724(86)80095-5 [DOI] [PubMed] [Google Scholar]
- Barbour AG (1987). Immunobiology of relapsing fever. Contrib Microbiol Immunol 8, 125–137. [PubMed] [Google Scholar]
- Barbour AG (1988). Laboratory aspects of Lyme borreliosis. Clin Microbiol Rev 1, 399–414. doi: 10.1128/CMR.1.4.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG (2002). Antigenic variation of relapsing fever Borrelia spp. and other bacterial pathogens. In Mobile DNA II, Craig NL, Craigie R, Gellert M, and A. Lambowitz, eds. (Washington, D.C.: American Society for Microbiology; ), pp. 972–994. doi: 10.1128/9781555817954.ch41 [DOI] [Google Scholar]
- Barbour AG (2003). Antigenic variation in Borrelia: relapsing fever and Lyme borreliosis. In Antigenic Variation, Craig A, and Scherf A, eds. (London: Academic Press; ), pp. 319–356. doi: 10.1016/B978-012194851-1/50040-8 [DOI] [Google Scholar]
- Barbour AG (2011). Relapsing fever and other Borrelia diseases. In Tropical Infectious Diseases Principles, Pathogens, & Practice, Guerrant RL, Walker DH, and Weller PF, eds. (Philadelphia: WB Saunders Co Ltd; ), pp. 295–302. doi: 10.1016/B978-0-7020-3935-5.00044-6 [DOI] [Google Scholar]
- Barbour AG, Barrera O, and Judd RC (1983). Structural analysis of the variable major proteins of Borrelia hermsii. J Exp Med 158, 2127–2140. doi: 10.1084/jem.158.6.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, and Bundoc V (2001). In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect Immun 69, 1009–1015. doi: 10.1128/IAI.69.2.1009-1015.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Burman N, Carter CJ, Kitten T, and Bergstrom S (1991a). Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol 5, 489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x [DOI] [PubMed] [Google Scholar]
- Barbour AG, Carter CJ, Burman N, Freitag CS, Garon CF, and Bergstrom S (1991b). Tandem insertion sequence-like elements define the expression site for variable antigen genes of Borrelia hermsii. Infect Immun 59, 390–397. doi: 10.1128/IAI.59.1.390-397.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Carter CJ, and Sohaskey CD (2000). Surface protein variation by expression site switching in the relapsing fever agent Borrelia hermsii. Infect Immun 68, 7114–7121. doi: 10.1128/iai.68.12.7114-7121.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Dai Q, Restrepo BI, Stoenner HG, and Frank SA (2006). Pathogen escape from host immunity by a genome program for antigenic variation. Proc Natl Acad Sci U S A 103, 18290–18295. doi: 10.1073/pnas.0605302103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, and Guo BP (2010). Borrelia: Molecular Biology, Host Interaction and Pathogenesis (Caister Academic Press; ). [Google Scholar]
- Barbour AG, and Hayes SF (1986). Biology of Borrelia species. Microbiol Rev 50, 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, and Piesman J (1996). Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis 173, 403–409. doi: 10.1093/infdis/173.2.403 [DOI] [PubMed] [Google Scholar]
- Barbour AG, Putteet-Driver AD, and Bunikis J (2005). Horizontally acquired genes for purine salvage in Borrelia spp. causing relapsing fever. Infect Immun 73, 6165–6168. doi: 10.1128/IAI.73.9.6165-6168.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, and Restrepo BI (2000). Antigenic variation in vector-borne pathogens. Emerging Infectious Diseases 6, 449–457. doi: 10.3201/eid0605.000502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, and Schwan TG (2018). Borrelia. In Bergey's Manual of Systematics of Archaea and Bacteria, Trujillo ME, Dedysh S, DeVos P, Hedlund B, Kämpfer P, Rainey FA, and Whitman WB, eds. (John Wiley & Sons, Inc., in association with Bergey's Manual Trust; ), pp. 1–22. doi: 10.1002/9781118960608.gbm01246.pub2 [DOI] [Google Scholar]
- Barbour AG, and Stoenner HG (1985). Antigenic variation of Borrelia hermsii. In Genome Rearrangement, Simon MI, and Herskowitz I, eds. (New York: Alan R. Liss, Inc.), pp. 123–135. [Google Scholar]
- Barbour AG, Tessier SL, and Stoenner HG (1982). Variable major proteins of Borrellia hermsii. J Exp Med 156, 1312–1324. doi: 10.1084/jem.156.5.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstad PA, Coligan JE, Raum MG, and Barbour AG (1985). Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med 161, 1302–1314. doi: 10.1084/jem.161.6.1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti JM, Raffel SJ, and Schwan TG (2008). A system for site-specific genetic manipulation of the relapsing fever spirochete Borrelia hermsii. Methods Mol Biol 431, 69–84. doi: 10.1007/978-1-60327-032-8_6 [DOI] [PubMed] [Google Scholar]
- Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, and Schneerson R (2003). A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci U S A 100, 7913–7918. doi: 10.1073/pnas.1232451100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beunders B, and Van Thiel P (1932). Untersuchungen ueber die persistenz von Spirochaeta duttoni in mausegehirnen bei experimenteller febris recurrens. Zeitschrift fuer Hygiene und Infektionskrankheiten 114, 568–583. [Google Scholar]
- Boden K, Lobenstein S, Hermann B, Margos G, and Fingerle V (2016). Borrelia miyamotoi-Associated Neuroborreliosis in Immunocompromised Person. Emerging Infectious Diseases 22, 1617–1620. doi: 10.3201/eid2209.152034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodman RI, and Stewart IS (1948). Louse-borne relapsing fever in Persia. Br Med J 1, 291–293. doi: 10.1136/bmj.1.4545.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgnolo G, Hailu B, Ciancarelli A, Almaviva M, and Woldemariam T (1993). Louse-borne relapsing fever. A clinical and an epidemiological study of 389 patients in Asella Hospital, Ethiopia. Trop Geogr Med 45, 66–69. [PubMed] [Google Scholar]
- Bourret TJ, Boyle WK, Zalud AK, Valenzuela JG, Oliveira F, and Lopez JE (2019). The relapsing fever spirochete Borrelia turicatae persists in the highly oxidative environment of its soft-bodied tick vector. Cell Microbiol 21, e12987. doi: 10.1111/cmi.12987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WK, Wilder HK, Lawrence AM, and Lopez JE (2014). Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS neglected tropical diseases 8, e2767. doi: 10.1371/journal.pntd.0002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur D (1985). Tick-borne relapsing fever in a premature infant. Ann Trop Paediatr 5, 161–162. doi: 10.1080/02724936.1985.11748384 [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Geoly FJ, Meuten DJ, Levine JF, Howard P, Hegarty BC, and Stafford LC (1996). Myocarditis in mice and guinea pigs experimentally infected with a canine-origin Borrelia isolate from Florida. Am J Vet Res 57, 505–511. [PubMed] [Google Scholar]
- Brenner C, Bomans K, Habicht J, Simon MM, and Wallich R (2013). Mapping the ligand-binding region of Borrelia hermsii fibronectin-binding protein. PLoS One 8, e63437. doi: 10.1371/journal.pone.0063437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner NE, Dolan MC, Replogle AJ, Sexton C, Hojgaard A, Boegler KA, Clark RJ, and Eisen L (2017). Transmission of Borrelia miyamotoi sensu lato relapsing fever group spirochetes in relation to duration of attachment by Ixodes scapularis nymphs. Ticks Tick Borne Dis 8, 677–681. doi: 10.1016/j.ttbdis.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklesby DW, Scott GR, and Rampton CS (1963). Borrelia theileri and transient fevers in cattle. Vet Rec 75, 103–104. [Google Scholar]
- Brown V, Larouze B, Desve G, Rousset JJ, Thibon M, Fourrier A, and Schwoebel V (1988). Clinical Presentation of Louse-Borne Relapsing Fever among Ethiopian Refugees in Northern Somalia. Ann Trop Med Parasit 82, 499–502. doi: 10.1080/00034983.1988.11812282 [DOI] [PubMed] [Google Scholar]
- Bryceson A, Parry E, Perine P, Warrel D, Vukotich D, and Leithead C (1970). Louse-borne relapsing fever: A clinical and laboratory study of 62 cases in Ethiopia and a reconsideration of the literature. Quarterly Journal of Medicine 153, 129–170. [PubMed] [Google Scholar]
- Bryceson AD (1976). Clinical pathology of the Jarisch-Herxheimer reaction. J Infect Dis 133, 696–704. doi: 10.1093/infdis/133.6.696 [DOI] [PubMed] [Google Scholar]
- Bryceson AD, Cooper KE, Warrell DA, Perine PL, and Parry EH (1972). Studies of the mechanism of the Jarisch-Herxheimer reaction in louse-borne relapsing fever: evidence for the presence of circulating Borrelia endotoxin. Clin Sci 43, 343–354. doi: 10.1042/cs0430343 [DOI] [PubMed] [Google Scholar]
- Bunikis J, Luke CJ, Bunikiene E, Bergstrom S, and Barbour AG (1998). A surface-exposed region of a novel outer membrane protein (P66) of Borrelia spp. is variable in size and sequence. J Bacteriol 180, 1618–1623. doi: 10.1128/JB.180.7.1618-1623.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Noppa L, and Bergstrom S (1995). Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett 131, 139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x [DOI] [PubMed] [Google Scholar]
- Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, and Barbour AG (2004). Typing of Borrelia relapsing fever group strains. Emerging Infectious Diseases 10, 1661–1664. doi: 10.3201/eid1009.040236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W (1951). Analyse des Infektionsverlaufes bei Ornithodorus moubata und der natürlichen Uebertragung von Spirochaeta duttoni. Acta Tropica 8, 196–262. [PubMed] [Google Scholar]
- Burgdorfer W (1959). Zum organotropismus der Spirochaete B. duttoni gegenuber der ubertragenden zecke. Acta Tropica 16, 242–243. [Google Scholar]
- Burgdorfer W, and Varma MG (1967). Trans-stadial and transovarial development of disease agents in arthropods. Annu Rev Entomol 12, 347–376. doi: 10.1146/annurev.en.12.010167.002023 [DOI] [PubMed] [Google Scholar]
- Burman N, Bergstrom S, Restrepo BI, and Barbour AG (1990). The variable antigens Vmp7 and Vmp21 of the relapsing fever bacterium Borrelia hermsii are structurally analogous to the VSG proteins of the African trypanosome. Mol Microbiol 4, 1715–1726. doi: 10.1111/j.1365-2958.1990.tb00549.x [DOI] [PubMed] [Google Scholar]
- Burman N, Shamaei-Tousi A, and Bergstrom S (1998). The spirochete Borrelia crocidurae causes erythrocyte rosetting during relapsing fever. Infect Immun 66, 815–819. doi: 10.1128/IAI.66.2.815-819.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T (2017). The Jarisch-Herxheimer Reaction After Antibiotic Treatment of Spirochetal Infections: A Review of Recent Cases and Our Understanding of Pathogenesis. Am J Trop Med Hyg 96, 46–52. doi: 10.4269/ajtmh.16-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, and Barbour AG (1998). Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin Infect Dis 26, 151–164. doi: 10.1086/516276 [DOI] [PubMed] [Google Scholar]
- Cadavid D, Bundoc V, and Barbour AG (1993). Experimental infection of the mouse brain by a relapsing fever Borrelia species: a molecular analysis. J Infect Dis 168, 143–151. doi: 10.1093/infdis/168.1.143 [DOI] [PubMed] [Google Scholar]
- Cadavid D, Pachner AR, Estanislao L, Patalapati R, and Barbour AG (2001). Isogenic serotypes of Borrelia turicatae show different localization in the brain and skin of mice. Infect Immun 69, 3389–3397. doi: 10.1128/IAI.69.5.3389-3397.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Pennington PM, Kerentseva TA, Bergstrom S, and Barbour AG (1997). Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun 65, 3352–3360. doi: 10.1128/IAI.65.8.3352-3360.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Sondey M, Garcia E, and Lawson CL (2006). Residual brain infection in relapsing-fever borreliosis. J Infect Dis 193, 1451–1458. doi: 10.1086/503367 [DOI] [PubMed] [Google Scholar]
- Cadavid D, Thomas DD, Crawley R, and Barbour AG (1994). Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med 179, 631–642. doi: 10.1084/jem.179.2.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabi O (1959). The presence of plasma inhibitors during the crisis phenomenon in experimental relapsing fever (Borrelia novyi). J Exp Med 110, 811–825. doi: 10.1084/jem.110.5.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GL, Paul WS, Schriefer ME, Craven RB, Robbins KE, and Dennis DT (1995). Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990-1993. J Infect Dis 172, 470–480. doi: 10.1093/infdis/172.2.470 [DOI] [PubMed] [Google Scholar]
- Carter CJ, Bergstrom S, Norris SJ, and Barbour AG (1994). A family of surface-exposed proteins of 20 kilodaltons in the genus Borrelia. Infect Immun 62, 2792–2799. doi: 10.1128/IAI.62.7.2792-2799.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ., Sterling SL, and Telford SR (2013). Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med 159, 21–27. doi: 10.7326/0003-4819-159-1-201307020-00005 [DOI] [PubMed] [Google Scholar]
- Coburn J, Chege W, Magoun L, Bodary SC, and Leong JM (1999). Characterization of a candidate Borrelia burgdorferi beta3-chain integrin ligand identified using a phage display library. Mol Microbiol 34, 926–940. doi: 10.1046/j.1365-2958.1999.01654.x [DOI] [PubMed] [Google Scholar]
- Coburn J, and Cugini C (2003). Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin alphavbeta3. Proc Natl Acad Sci U S A 100, 7301–7306. doi: 10.1073/pnas.1131117100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey EM, and Eveland WC (1967). Experimental relapsing fever initiated by Borrelia hermsi. II. Sequential appearance of major serotypes in the rat. J Infect Dis 117, 29–34. doi: 10.1093/infdis/117.1.29 [DOI] [PubMed] [Google Scholar]
- Colombo MJ, and Alugupalli KR (2008). Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol 180, 4858–4864. doi: 10.4049/jimmunol.180.7.4858 [DOI] [PubMed] [Google Scholar]
- Connolly SE, and Benach JL (2001). Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J Immunol 167, 3029–3032. doi: 10.4049/jimmunol.167.6.3029 [DOI] [PubMed] [Google Scholar]
- Connolly SE, Thanassi DG, and Benach JL (2004). Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J Immunol 172, 1191–1197. doi: 10.4049/jimmunol.172.2.1191 [DOI] [PubMed] [Google Scholar]
- Cooley RA, and Kohls GM (1944). The Argasidae of North America, Central America, and Cuba (South Bend: Notre Dame University Press; ). doi: 10.5962/bhl.title.4511 [DOI] [Google Scholar]
- Cooper PJ, Fekade D, Remick DG, Grint P, Wherry J, and Griffin GE (2000). Recombinant human interleukin-10 fails to alter proinflammatory cytokine production or physiologic changes associated with the Jarisch-Herxheimer reaction. The Journal of infectious diseases 181, 203–209. doi: 10.1086/315183 [DOI] [PubMed] [Google Scholar]
- Crowder CD, Carolan HE, Rounds MA, Honig V, Mothes B, Haag H, Nolte O, Luft BJ, Grubhoffer L, Ecker DJ, et al. (2014). Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerging Infectious Diseases 20, 1678–1682. doi: 10.3201/eid2010.131583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder CD, Denny RL, and Barbour AG (2017). Segregation Lag in Polyploid Cells of the Pathogen Genus Borrelia: Implications for Antigenic Variation. Yale J Biol Med 90, 195–218. [PMC free article] [PubMed] [Google Scholar]
- Crowder CD, Ghalyanchi Langeroudi A, Shojaee Estabragh A, Lewis ERG, Marcsisin RA, and Barbour AG (2016). Pathogen and Host Response Dynamics in a Mouse Model of Borrelia hermsii Relapsing Fever. Vet Sci 3. doi: 10.3390/vetsci3030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuboni E (1929). Sul potere spirocheticida del siero di sangue di alcuni animali. Bol Inst Sieroterap Milan 8, 813–817. [Google Scholar]
- Cutler S, Vayssier-Taussat M, Estrada-Pena A, Potkonjak A, Mihalca AD, and Zeller H (2019). A new Borrelia on the block: Borrelia miyamotoi - a human health risk? Euro Surveill 24. doi: 10.2807/1560-7917.ES.2019.24.18.1800170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SJ, Bonilla EM, and Singh RJ (2010). Population structure of East African relapsing fever Borrelia spp. Emerging Infectious Diseases 16, 1076–1080. doi: 10.3201/eid1607.091085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Restrepo BI, Porcella SF, Raffel SJ, Schwan TG, and Barbour AG (2006). Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol Microbiol 60, 1329–1343. doi: 10.1111/j.1365-2958.2006.05177.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaMassa AJ, and Adler HE (1979). Avian spirochetosis: natural transmission by Argas (Persicargas) sanchezi (Ixodoidea: argasidae) and existence of different serologic and immunologic types of Borrelia anserina in the United States. Am J Vet Res 40, 154–157. [PubMed] [Google Scholar]
- Davis GE (1943). Relapsing fever: the tick Ornithodoros turicata as a spirochetal reservoir. Pub Health Rep 58, 839–842. [Google Scholar]
- Davis GE (1952). Biology as an aid to the identification of two closely related species of ticks of the genus Ornithodoros. J Parasitol 38, 477–480. [PubMed] [Google Scholar]
- Davis GE (1955). Relapsing Fever Spirochetes - the Present Status of Borrelia-Venezuelensis Brumpt and Borrelia-Neotropicalis Bates-and-St-John. Int B Bact Nomencl T 5, 107–109. doi: 10.1099/0096266X-5-3-107 [DOI] [Google Scholar]
- Davis GE (1956). A relapsing fever spirochete, Borrelia mazzottii (sp. nov.) from Ornithodoros talaje from Mexico. American journal of hygiene 63, 13–17. doi: 10.1093/oxfordjournals.aje.a119787 [DOI] [PubMed] [Google Scholar]
- Dennis DT, Awoke S, Doberstyn EB, and Fresh JW (1976). Bleeding in louse-borne relapsing fever in Ethiopia; clinical and laboratory features in 29 patients. East Afr Med J 53, 220–225. [PubMed] [Google Scholar]
- Dewar HA, and Walmsley R (1945). Relapsing fever with nephritis and subarachnoid haemorrhage. Lancet 2, 630. doi: 10.1016/s0140-6736(45)90763-x [DOI] [PubMed] [Google Scholar]
- Diab FM, and Soliman ZR (1977). An experimental study of Borrelia anserina in four species of Argas ticks. 1. Spirochete localization and densities. Z Parasitenkd 53, 201–212. doi: 10.1007/BF00380465 [DOI] [PubMed] [Google Scholar]
- Donaldson TG, Perez de Leon AA, Li AY, Castro-Arellano I, Wozniak E, Boyle WK, Hargrove R, Wilder HK, Kim HJ, Teel PD, et al. (2016). Assessment of the Geographic Distribution of Ornithodoros turicata (Argasidae): Climate Variation and Host Diversity. PLoS neglected tropical diseases 10, e0004383. doi: 10.1371/journal.pntd.0004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois A, and Pearson Y (1939). Variabilite du neurotropisme d'une souche de Spirochaeta dutoni, immunite en l'abscence de neurotropisme. Comptes Rendus des Seances Societe de Biologie (Paris) 130, 1379–1381. [Google Scholar]
- Dupont HT, La Scola B, Williams R, and Raoult D (1997). A focus of tick-borne relapsing fever in southern Zaire. Clin Infect Dis 25, 139–144. doi: 10.1086/514496 [DOI] [PubMed] [Google Scholar]
- Duray PH (1987). The surgical pathology of human Lyme disease. An enlarging picture. Am J Surg Pathol 11 Suppl 1, 47–60. doi: 10.1097/00000478-198700111-00005 [DOI] [PubMed] [Google Scholar]
- Eggers CH, and Samuels DS (1999). Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J Bacteriol 181, 7308–7313. doi: 10.1128/JB.181.23.7308-7313.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, and Sacchettini JC (2002). Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem 277, 21691–21696. doi: 10.1074/jbc.M201547200 [DOI] [PubMed] [Google Scholar]
- Elbir H, Abi-Rached L, Pontarotti P, Yoosuf N, and Drancourt M (2014). African relapsing Fever borreliae genomospecies revealed by comparative genomics. Front Public Health 2, 43. doi: 10.3389/fpubh.2014.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HM Jr., Hoss DM, Zemel L, Telford SR 3rd, Dias F, and Wormser GP (2011). Southern Tick-Associated Rash Illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis 53, e142–146. doi: 10.1093/cid/cir553 [DOI] [PubMed] [Google Scholar]
- Fekade D, Knox K, Hussein K, Melka A, Lalloo DG, Coxon RE, and Warrell DA (1996). Prevention of Jarisch-Herxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N Engl J Med 335, 311–315. doi: 10.1056/NEJM199608013350503 [DOI] [PubMed] [Google Scholar]
- Felsenfeld O (1965). Borrelia, human relapsing fever, and parasite-vector-host relationships. Bacteriological Reviews 29, 46–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld O (1971). Borrelia. Strains, Vectors, Human and Animal Borreliosis (St. Louis, MO: Warren H. Greene, Inc.). doi: 10.7326/0003-4819-75-5-822_4 [DOI] [Google Scholar]
- Felsenfeld O, and Wolf RH (1973). Interference between borreliae and trypanosomes. I. Antigenic analysis, fluorescent antibody and immobilisine studies of Borrelia turicatae and Trypanosoma cruzi. Ann Trop Med Parasitol 67, 335–340. [PubMed] [Google Scholar]
- Ferdows MS, Serwer P, Griess GA, Norris SJ, and Barbour AG (1996). Conversion of a linear to a circular plasmid in the relapsing fever agent Borrelia hermsii. J Bacteriol 178, 793–800. doi: 10.1128/jb.178.3.793-800.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov S, Krishnavajhala A, Armstrong BA, Kneubehl AR, Nieto NC, Perez De Leon AA, and Lopez JE (2020). Isolation and Molecular Characterization of Tick-Borne Relapsing Fever Borrelia Infecting Ornithodoros (Pavlovskyella) verrucosus Ticks Collected in Ukraine. J Infect Dis 221, 804–811. doi: 10.1093/infdis/jiz500 [DOI] [PubMed] [Google Scholar]
- Fine LM, Miller DP, Mallory KL, Tegels BK, Earnhart CG, and Marconi RT (2014). The Borrelia hermsii factor H binding protein FhbA is not required for infectivity in mice or for resistance to human complement in vitro. Infect Immun 82, 3324–3332. doi: 10.1128/IAI.01892-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA, and Barbour AG (2006). Within-host dynamics of antigenic variation. Infect Genet Evol 6, 141–146. doi: 10.1016/j.meegid.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Fuchs PC, and Oyama AA (1969). Neonatal relapsing fever due to transplacental transmission of Borrelia. JAMA : the journal of the American Medical Association 208, 690–692. [PubMed] [Google Scholar]
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, and Nakao M (1995). Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. International Journal of Systematic Bacteriology 45, 804–810. doi: 10.1099/00207713-45-4-804 [DOI] [PubMed] [Google Scholar]
- Gabritchewsky GN (1896). Les bases de la sérothérapie de la fievre récurrente. Annales Institut Pasteur 10, 630–653. [Google Scholar]
- Galloway RE, Levin J, Butler T, Naff GB, Goldsmith GH, Saito H, Awoke S, and Wallace CK (1977). Activation of protein mediators of inflammation and evidence for endotoxemia in Borrelia recurrentis infection. Am J Med 63, 933–938. doi: 10.1016/0002-9343(77)90548-4 [DOI] [PubMed] [Google Scholar]
- Garcia-Monco JC, Miller NS, Backenson PB, Anda P, and Benach JL (1997). A mouse model of Borrelia meningitis after intradermal injection. J Infect Dis 175, 1243–1245. doi: 10.1086/593681 [DOI] [PubMed] [Google Scholar]
- Garnham P, Davis C, Heisch R, and Timms G (1947). An epidemic of louse-borne relapsing fever in Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 41, 141–170. doi: 10.1016/s0035-9203(47)90209-5 [DOI] [PubMed] [Google Scholar]
- Gettings JR, Lopez JE, Krishnavahjala A, Armstrong BA, Thompson AT, and Yabsley MJ (2019). Antibodies to Borrelia turicatae in Experimentally Infected Dogs Cross-React with Borrelia burgdorferi Serologic Assays. J Clin Microbiol 57. doi: 10.1128/JCM. 00628–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Arndt KA, and Steigbigel NH (1969). Borrelia in Boston. JAMA : the journal of the American Medical Association 210, 722–724. doi: 10.1001/jama.1969.03160300062020 [DOI] [PubMed] [Google Scholar]
- Gordillo-Perez G, Garcia-Juarez I, Solorzano-Santos F, Corrales-Zuniga L, Munoz-Hernandez O, and Torres-Lopez J (2017). Serological Evidence of Borrelia Burgdorferi Infection in Mexican Patients with Facial Palsy. Rev Invest Clin 69, 344–348. doi: 10.24875/RIC.17002344 [DOI] [PubMed] [Google Scholar]
- Gordillo-Perez G, Solorzano F, Cervantes-Castillo A, Sanchez-Vaca G, Garcia-Ramirez R, Diaz AM, Munoz O, and Torres J (2018). Lyme Neuroborreliosis is a Severe and Frequent Neurological Disease in Mexico. Arch Med Res 49, 399–404. doi: 10.1016/j.arcmed.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Goubau PF, and Munyangeyo C (1983). Fièvre récurrente congénitale à Borrelia duttoni. A propos d'un cas au Rwanda [Congenital relapsing fever caused by Borrelia duttoni. Apropos of a case in Rwanda]. Ann Soc Belg Med Trop 63, 367–369. [PubMed] [Google Scholar]
- Grigery CN, Moyer P, Little SE, and Masters EJ (2005). Bacteriocidal activity of lizard and mouse serum for Borrelia lonestari, putative agent of a Lyme-like illness (AKA STARI or Masters disease) in Missouri. Mo Med 102, 442–446. [PubMed] [Google Scholar]
- Grosskinsky S, Schott M, Brenner C, Cutler SJ, Kraiczy P, Zipfel PF, Simon MM, and Wallich R (2009). Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS One 4, e4858. doi: 10.1371/journal.pone.0004858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskinsky S, Schott M, Brenner C, Cutler SJ, Simon MM, and Wallich R (2010). Human complement regulators C4b-binding protein and C1 esterase inhibitor interact with a novel outer surface protein of Borrelia recurrentis. PLoS Negl Trop Dis 4, e698. doi: 10.1371/journal.pntd.0000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugliotta JL, Goethert HK, Berardi VP, and Telford SR 3rd (2013). Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 368, 240–245. doi: 10.1056/NEJMoa1209039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BP, Teneberg S, Munch R, Terunuma D, Hatano K, Matsuoka K, Angstrom J, Boren T, and Bergstrom S (2009). Relapsing fever Borrelia binds to neolacto glycans and mediates rosetting of human erythrocytes. Proc Natl Acad Sci U S A 106, 19280–19285. doi: 10.1073/pnas.0905470106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyard C, Chester EM, Raffel SJ, Schrumpf ME, Policastro PF, Porcella SF, Leong JM, and Schwan TG (2005). Relapsing fever spirochetes contain chromosomal genes with unique direct tandemly repeated sequences. Infect Immun 73, 3025–3037. doi: 10.1128/IAI.73.5.3025-3037.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lubelczyk C, Hickling GJ, Belperron AA, Bockenstedt LK, and Tsao JI (2019). Vertical transmission rates of Borrelia miyamotoi in Ixodes scapularis collected from white-tailed deer. Ticks Tick Borne Dis 10, 682–689. doi: 10.1016/j.ttbdis.2019.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EK, Harton MR, de Mello Marques MA, Belisle JT, Molins CR, Breuner N, Wormser GP, and Gilmore RD (2019). Immunoproteomic analysis of Borrelia miyamotoi for the identification of serodiagnostic antigens. Sci Rep 9, 16808. doi: 10.1038/s41598-019-53248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendson M, and Lane RS (2000). Genetic characteristics of Borrelia coriaceae isolates from the soft tick Ornithodoros coriaceus (Acari: Argasidae). J Clin Microbiol 38, 2678–2682. doi: 10.1128/JCM.38.7.2678-2682.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsson AJ, Asgeirsson H, Hammas B, Karlsson E, Parke A, Hoornstra D, Wilhelmsson P, and Hovius JW (2019). Two Cases of Borrelia miyamotoi Meningitis, Sweden, 2018. Emerging Infectious Diseases 25, 1965–1968. doi: 10.3201/eid2510.190416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms WB, and Wheeler CM (1935). Tick transmission of California relapsing fever. J Econ Entom 28, 846–855. doi: 10.1093/jee/28.6.846 [DOI] [Google Scholar]
- Hinnebusch BJ, Barbour AG, Restrepo BI, and Schwan TG (1998). Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun 66, 432–440. doi: 10.1128/IAI.66.2.432-440.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H (1979). Ticks and spirochetes. Acta Trop 36, 133–136. [PubMed] [Google Scholar]
- Hoogstraal H (1985). Argasid and nuttalliellid ticks as parasites and vectors. Adv Parasitol 24, 135–238. doi: 10.1016/s0065-308x(08)60563-1 [DOI] [PubMed] [Google Scholar]
- Hoornstra D, Koetsveld J, Sprong H, Platonov AE, and Hovius JW (2018). Borrelia miyamotoi Disease in an Immunocompetent Patient, Western Europe. Emerging Infectious Diseases 24, 1770–1772. doi: 10.3201/eid2409.180806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain H, Wellensiek HJ, Geyer R, and Lochnit G (2001). Structural analysis of glycolipids from Borrelia burgdorferi. Biochimie 83, 683–692. doi: 10.1016/s0300-9084(01)01296-2 [DOI] [PubMed] [Google Scholar]
- Houhamdi L, and Raoult D (2005). Excretion of living Borrelia recurrentis in feces of infected human body lice. The Journal of infectious diseases 191, 1898–1906. doi: 10.1086/429920 [DOI] [PubMed] [Google Scholar]
- Hovis KM, McDowell JV, Griffin L, and Marconi RT (2004). Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J Bacteriol 186, 2612–2618. doi: 10.1128/jb.186.9.2612-2618.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovis KM, Schriefer ME, Bahlani S, and Marconi RT (2006). Immunological and molecular analyses of the Borrelia hermsii factor H and factor H-like protein 1 binding protein, FhbA: demonstration of its utility as a diagnostic marker and epidemiological tool for tick-borne relapsing fever. Infect Immun 74, 4519–4529. doi: 10.1128/IAI.00377-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, et al. (2013). A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382, 658. doi: 10.1016/S0140-6736(13)61644-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue F, Ghalyanchi Langeroudi A, and Barbour AG (2013). Chromosome Sequence of Borrelia miyamotoi, an Uncultivable Tick-Borne Agent of Human Infection. Genome Announc 1. doi: 10.1128/genomeA.00713-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huldén L (2006). Den sexbente fienden fienden: leddjurens inverkan på västerländsk krigsföring (Helsingfors, Finland: Schildt; ). [Google Scholar]
- Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, and Sprong H (2016). Molecular Detection of Tick-Borne Pathogens in Humans with Tick Bites and Erythema Migrans, in the Netherlands. PLoS neglected tropical diseases 10, e0005042. doi: 10.1371/journal.pntd.0005042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Sarksyan DS, Kolyasnikova NM, Hovius JW, Sprong H, and Platonov AE (2017). Evaluation of a serological test for the diagnosis of Borrelia miyamotoi disease in Europe. J Microbiol Methods 136, 11–16. doi: 10.1016/j.mimet.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Jancso N (1918). Experimentelle Untersuchung bezüglich der Pathogenese der Rezidive des Rückfallfiebers. Zentbl Bakt Orig 81, 457–474. [Google Scholar]
- Jiang BG, Jia N, Jiang JF, Zheng YC, Chu YL, Jiang RR, Wang YW, Liu HB, Wei R, Zhang WH, et al. (2018). Borrelia miyamotoi Infections in Humans and Ticks, Northeastern China. Emerging Infectious Diseases 24, 236–241. doi: 10.3201/eid2402.160378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe DA, Lovrich SD, Oldenburg DG, Kowalski TJ, and Callister SM (2016). Borrelia miyamotoi Infection in Patients from Upper Midwestern United States, 2014-2015. Emerging Infectious Diseases 22, 1471–1473. doi: 10.3201/eid2208.151878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen VH, van Roosmalen J, Tiems J, Van Holten J, and Wetsteyn JC (1997). Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstet Gynecol Scand 76, 834–838. doi: 10.3109/00016349709024361 [DOI] [PubMed] [Google Scholar]
- Judge DM, La Croix JT, and Perine PL. (1974a). Experimental louse-borne relapsing fever in the grivet monkey, Cercopithecus aethiops. I. Clinical course. The American journal of tropical medicine and hygiene 23, 957–961. doi: 10.4269/ajtmh.1974.23.957 [DOI] [PubMed] [Google Scholar]
- Judge DM, La Croix JT, and Perine PL. (1974b). Experimental louse-borne relapsing fever in the grivet monkey, Cercopithecus aethiops. II. Pathology. The American journal of tropical medicine and hygiene 23, 962–968. doi: 10.4269/ajtmh.1974.23.962 [DOI] [PubMed] [Google Scholar]
- Judge DM, La Croix JT, and Perine PL. (1974c). Experimental louse-borne relapsing fever in the grivet monkey, Cercopithecus aethiops. III. Crisis following therapy. The American journal of tropical medicine and hygiene 23, 969–973. doi: 10.4269/ajtmh.1974.23.969 [DOI] [PubMed] [Google Scholar]
- Judge DM, Samuel I, Perine PL, Vukotic D, and Ababa A (1974d). Louse-borne relapsing fever in man. Arch Pathol 97, 136–170. [PubMed] [Google Scholar]
- Kadkhoda K, Dumouchel C, Brancato J, Gretchen A, and Krause PJ. (2017). Human seroprevalence of Borrelia miyamotoi in Manitoba, Canada, in 2011-2014: a cross-sectional study. CMAJ Open 5, E690–E693. doi: 10.9778/cmajo.20170070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan L, Makenov M, Kolyasnikova N, Stukolova O, Toporkova M, and Olenkova O (2018). Dynamics of Spirochetemia and Early PCR Detection of Borrelia miyamotoi. Emerging Infectious Diseases 24, 860–867. doi: 10.3201/eid2405.170829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazragis RJ, Dever LL, Jorgensen JH, and Barbour AG (1996). In vivo activities of ceftriaxone and vancomycin against Borrelia spp. in the mouse brain and other sites. Antimicrob Agents Chemother 40, 2632–2636. doi: 10.1128/AAC.40.11.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingry LC, Replogle A, Batra D, Rowe LA, Sexton C, Dolan M, Connally N, Petersen JM, and Schriefer ME (2017a). Toward a Complete North American Borrelia miyamotoi Genome. Genome Announc 5. doi: 10.1128/genomeA.01557-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingry LC, Replogle A, Dolan M, Sexton C, Padgett KA, and Schriefer ME (2017b). Chromosome and Large Linear Plasmid Sequences of a Borrelia miyamotoi Strain Isolated from Ixodes pacificus Ticks from California. Genome Announc 5. doi: 10.1128/genomeA.00960-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitten T, and Barbour AG (1990). Juxtaposition of expressed variable antigen genes with a conserved telomere in the bacterium Borrelia hermsii. Proc Natl Acad Sci U S A 87, 6077–6081. doi: 10.1073/pnas.87.16.6077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitten T, and Barbour AG (1992). The relapsing fever agent Borrelia hermsii has multiple copies of its chromosome and linear plasmids. Genetics 132, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitten T, Barrera AV, and Barbour AG (1993). Intragenic recombination and a chimeric outer membrane protein in the relapsing fever agent Borrelia hermsii. J Bacteriol 175, 2516–2522. doi: 10.1128/jb.175.9.2516-2522.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetsveld J, Draga ROP, Wagemakers A, Manger A, Oei A, Visser CE, and Hovius JW (2017a). In Vitro Susceptibility of the Relapsing-Fever Spirochete Borrelia miyamotoi to Antimicrobial Agents. Antimicrob Agents Chemother 61. doi: 10.1128/AAC.00535-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetsveld J, Kolyasnikova NM, Wagemakers A, Stukolova OA, Hoornstra D, Sarksyan DS, Toporkova MG, Henningsson AJ, Hvidsten D, Ang W, et al. (2018a). Serodiagnosis of Borrelia miyamotoi disease by measuring antibodies against GlpQ and variable major proteins. Clin Microbiol Infect 24, 1338 e1331–1338 e1337. doi: 10.1016/j.cmi.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Koetsveld J, Kolyasnikova NM, Wagemakers A, Toporkova MG, Sarksyan DS, Oei A, Platonov AE, and Hovius JW (2017b). Development and optimization of an in vitro cultivation protocol allows for isolation of Borrelia miyamotoi from patients with hard tick-borne relapsing fever. Clin Microbiol Infect 23, 480–484. doi: 10.1016/j.cmi.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Koetsveld J, Manger A, Hoornstra D, Draga RO, Oei A, Kolyasnikova NM, Toporkova MG, Sarksyan DS, Wagemakers A, Platonov AE, et al. (2018b). In Vitro Antimicrobial Susceptibility of Clinical Isolates of Borrelia miyamotoi. Antimicrob Agents Chemother 62. doi: 10.1128/AAC.00419-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetsveld J, Platonov AE, Kuleshov K, Wagemakers A, Hoornstra D, Ang W, Szekeres S, van Duijvendijk GLA, Fikrig E, Embers ME, et al. (2020). Borrelia miyamotoi infection leads to cross-reactive antibodies to the C6 peptide in mice and men. Clin Microbiol Infect 26, 513 e511–513 e516. doi: 10.1016/j.cmi.2019.07.026 [DOI] [PubMed] [Google Scholar]
- Krajacich BJ, Lopez JE, Raffel SJ, and Schwan TG. (2015). Vaccination with the variable tick protein of the relapsing fever spirochete Borrelia hermsii protects mice from infection by tick-bite. Parasit Vectors 8, 546. doi: 10.1186/s13071-015-1170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Carroll M, Fedorova N, Brancato J, Dumouchel C, Akosa F, Narasimhan S, Fikrig E, and Lane RS (2018). Human Borrelia miyamotoi infection in California: Serodiagnosis is complicated by multiple endemic Borrelia species. PLoS One 13, e0191725. doi: 10.1371/journal.pone.0191725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Fish D, Narasimhan S, and Barbour AG (2015). Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect 21, 631–639. doi: 10.1016/j.cmi.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, et al. (2014). Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerging Infectious Diseases 20, 1183–1190. doi: 10.3201/eid2007.131587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, and Fish D (2013). Human Borrelia miyamotoi infection in the United States. N Engl J Med 368, 291–293. doi: 10.1056/NEJMc1215469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Schwab J, Narasimhan S, Brancato J, Xu G, and Rich SM (2016). Hard Tick Relapsing Fever Caused by Borrelia miyamotoi in a Child. Pediatr Infect Dis J 35, 1352–1354. doi: 10.1097/INF.0000000000001330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnavajhala A, Wilder HK, Boyle WK, Damania A, Thornton JA, Perez de Leon AA, Teel PD, and Lopez JE (2017). Imaging of Borrelia turicatae producing the green fluorescent protein reveals persistent colonization of the Ornithodoros turicata midgut and salivary glands from nymphal acquisition through transmission. Appl Environ Microbiol 83. doi: 10.1128/AEM.02503-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov KV, Hoornstra D, Sprong H, Platonov AE, and Hovius JW (2019). Draft Whole-Genome Sequences of Two Western European Borrelia miyamotoi Isolates. Microbiol Resour Announc 8. doi: 10.1128/MRA.01314-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov KV, Koetsveld J, Goptar IA, Markelov ML, Kolyasnikova NM, Sarksyan DS, Toporkova MG, Kirdyashkina NP, Shipulin GA, Hovius JW, et al. (2018). Whole-Genome Sequencing of Six Borrelia miyamotoi Clinical Strains Isolated in Russia. Genome Announc 6. doi: 10.1128/genomeA.01424-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov KV, Margos G, Fingerle V, Koetsveld J, Goptar IA, Markelov ML, Kolyasnikova NM, Sarksyan DS, Kirdyashkina NP, Shipulin GA, et al. (2020). Whole genome sequencing of Borrelia miyamotoi isolate Izh-4: reference for a complex bacterial genome. BMC Genomics 21, 16. doi: 10.1186/s12864-019-6388-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Burgdorfer W, Hayes SF, and Barbour AG (1985). Isolation of a spirochete from the soft tick, Ornithodoros coriaceus: a possible agent of epizootic bovine abortion. Science 230, 85–87. doi: 10.1126/science.3898367 [DOI] [PubMed] [Google Scholar]
- Lane RS, and Manweiler SA (1988). Borrelia coriaceae in its tick vector, Ornithodoros coriaceus (Acari: Argasidae), with emphasis on transstadial and transovarial infection. J Med Entomol 25, 172–177. doi: 10.1093/jmedent/25.3.172 [DOI] [PubMed] [Google Scholar]
- Lane RS, Mun J, Parker JM, and White M (2005). Columbian black-tailed deer (Odocoileus hemionus columbianus) as hosts for Borrelia spp. in northern California. J Wildl Dis 41, 115–125. doi: 10.7589/0090-3558-41.1.115 [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Katona LI, Thanassi DG, and Benach JL (2008). Bactericidal action of a complement-independent antibody against relapsing fever Borrelia resides in its variable region. J Immunol 180, 6222–6228. doi: 10.4049/jimmunol.180.9.6222 [DOI] [PubMed] [Google Scholar]
- Larsson C, Andersson M, Guo BP, Nordstrand A, Hagerstrand I, Carlsson S, and Bergstrom S (2006a). Complications of pregnancy and transplacental transmission of relapsing-fever borreliosis. J Infect Dis 194, 1367–1374. doi: 10.1086/508425 [DOI] [PubMed] [Google Scholar]
- Larsson C, Andersson M, Pelkonen J, Guo BP, Nordstrand A, and Bergstrom S (2006b). Persistent brain infection and disease reactivation in relapsing fever borreliosis. Microbes Infect 8, 2213–2219. doi: 10.1016/j.micinf.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Larsson C, Lundqvist J, and Bergstrom S (2008). Residual brain infection in murine relapsing fever borreliosis can be successfully treated with ceftriaxone. Microb Pathog 44, 262–264. doi: 10.1016/j.micpath.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Larsson C, Lundqvist J, van Rooijen N, and Bergstrom S (2009). A novel animal model of Borrelia recurrentis louse-borne relapsing fever borreliosis using immunodeficient mice. PLoS neglected tropical diseases 3, e522. doi: 10.1371/journal.pntd.0000522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson CL, Yung BH, Barbour AG, and Zuckert WR (2006). Crystal structure of neurotropism-associated variable surface protein 1 (Vsp1) of Borrelia turicatae. J Bacteriol 188, 4522–4530. doi: 10.1128/JB.00028-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, Wincker P, Couloux A, Claverie JM, Raoult D, et al. (2008). The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet 4, e1000185. doi: 10.1371/journal.pgen.1000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fitzgerald D, Gran B, Leong JM, and Alugupalli KR (2010). Induction of distinct neurologic disease manifestations during relapsing fever requires T lymphocytes. J Immunol 184, 5859–5864. doi: 10.4049/jimmunol.0902737 [DOI] [PubMed] [Google Scholar]
- Londono D, Bai Y, Zuckert WR, Gelderblom H, and Cadavid D (2005). Cardiac apoptosis in severe relapsing fever borreliosis. Infect Immun 73, 7669–7676. doi: 10.1128/IAI.73.11.7669-7676.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono D, Marques A, Hornung RL, and Cadavid D (2008). IL-10 helps control pathogen load during high-level bacteremia. J Immunol 181, 2076–2083. doi: 10.4049/jimmunol.181.3.2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JE, Krishnavahjala A, Garcia MN, and Bermudez SE (2016). Tick-borne relapsing fever spirochetes in the Americas. Vet Sci 3, 1–18. doi: 10.3390/vetsci3030016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JE, Schrumpf ME, Nagarajan V, Raffel SJ, McCoy BN, and Schwan TG (2010). A novel surface antigen of relapsing fever spirochetes can discriminate between relapsing fever and Lyme borreliosis. Clin Vaccine Immunol 17, 564–571. doi: 10.1128/CVI.00518-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JE, Schrumpf ME, Raffel SJ, Policastro PF, Porcella SF, and Schwan TG. (2008). Relapsing fever spirochetes retain infectivity after prolonged in vitro cultivation. Vector Borne Zoonotic Dis 8, 813–820. doi: 10.1089/vbz.2008.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JE, Wilder HK, Boyle W, Drumheller LB, Thornton JA, Willeford B, Morgan TW, and Varela-Stokes A (2013a). Sequence analysis and serological responses against Borrelia turicatae BipA, a putative species-specific antigen. PLoS neglected tropical diseases 7, e2454. doi: 10.1371/journal.pntd.0002454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JE, Wilder HK, Hargrove R, Brooks CP, Peterson KE, Beare PA, Sturdevant DE, Nagarajan V, Raffel SJ, and Schwan TG (2013b). Development of genetic system to inactivate a Borrelia turicatae surface protein selectively produced within the salivary glands of the arthropod vector. PLoS neglected tropical diseases 7, e2514. doi: 10.1371/journal.pntd.0002514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist J, Larsson C, Nelson M, Andersson M, Bergstrom S, and Persson C (2010). Concomitant infection decreases the malaria burden but escalates relapsing fever borreliosis. Infect Immun 78, 1924–1930. doi: 10.1128/IAI.01082-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoun L, Zuckert WR, Robbins D, Parveen N, Alugupalli KR, Schwan TG, Barbour AG, and Leong JM (2000). Variable small protein (Vsp)-dependent and Vsp-independent pathways for glycosaminoglycan recognition by relapsing fever spirochaetes. Mol Microbiol 36, 886–897. doi: 10.1046/j.1365-2958.2000.01906.x [DOI] [PubMed] [Google Scholar]
- Mans BJ, Andersen JF, Francischetti IM, Valenzuela JG, Schwan TG, Pham VM, Garfield MK, Hammer CH, and Ribeiro JM (2008a). Comparative sialomics between hard and soft ticks: implications for the evolution of blood-feeding behavior. Insect biochemistry and molecular biology 38, 42–58. doi: 10.1016/j.ibmb.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Andersen JF, Schwan TG, and Ribeiro JM (2008b). Characterization of anti-hemostatic factors in the argasid, Argas monolakensis: implications for the evolution of blood-feeding in the soft tick family. Insect Biochem Mol Biol 38, 22–41. doi: 10.1016/j.ibmb.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi RT, Samuels DS, Schwan TG, and Garon CF (1993). Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J Clin Microbiol 31, 2577–2583. doi: 10.1128/JCM.31.10.2577-2583.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Tran E, Hamilton D, Wolfgang J, Miller K, and Marconi RT (2003). Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave c3b. J Clin Microbiol 41, 3905–3910. doi: 10.1128/jcm.41.8.3905-3910.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JT, Simon MI, and Barbour AG (1985). Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell 41, 403–409. doi: 10.1016/s0092-8674(85)80013-1 [DOI] [PubMed] [Google Scholar]
- Meleney HE (1928). Relapse Phenomena of Spironema Recurrentis. J Exp Med 48, 65–82. doi: 10.1084/jem.48.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkert PW, and Stel HV (1991). Neonatal Borrelia infections (relapsing fever): report of 5 cases and review of the literature. East Afr Med J 68, 999–1005. [PubMed] [Google Scholar]
- Meri T, Cutler SJ, Blom AM, Meri S, and Jokiranta TS (2006).. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect Immun 74, 4157–4163. doi: 10.1128/IAI.00007-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitiku K, and Mengistu G (2002). Relapsing fever in Gondar, Ethiopia. East Afr Med J 79, 85–87. doi: 10.4314/eamj.v79i2.8908 [DOI] [PubMed] [Google Scholar]
- Molloy PJ, Telford SR 3rd, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, and Berardi VP (2015). Borrelia miyamotoi Disease in the Northeastern United States: A Case Series. Ann Intern Med 163, 91–98. doi: 10.7326/M15-0333 [DOI] [PubMed] [Google Scholar]
- Molloy PJ, Weeks KE, Todd B, and Wormser GP (2018). Seroreactivity to the C6 Peptide in Borrelia miyamotoi Infections Occurring in the Northeastern United States. Clin Infect Dis 66, 1407–1410. doi: 10.1093/cid/cix1023 [DOI] [PubMed] [Google Scholar]
- Mooser H (1958). Erytrozyten-adhäsion und hämagglomeration durch rückfallfieber-spirochäten. Tropenmedizin und Parasitologie 9, 93–111. [PubMed] [Google Scholar]
- Moyer PL, Varela AS, Luttrell MP, Moore V.A.t., Stallknecht DE, and Little SE (2006). White-tailed deer (Odocoileus virginianus) develop spirochetemia following experimental infection with Borrelia lonestari. Vet Microbiol 115, 229–236. doi: 10.1016/j.vetmic.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Munoz-Leal S, Faccini-Martinez AA, Costa FB, Marcili A, Mesquita E, Marques EP Jr., and Labruna MB (2018). Isolation and molecular characterization of a relapsing fever Borrelia recovered from Ornithodoros rudis in Brazil. Ticks Tick Borne Dis 9, 864–871. doi: 10.1016/j.ttbdis.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Negussie Y, Remick DG, DeForge LE, Kunkel SL, Eynon A, and Griffin GE (1992). Detection of plasma tumor necrosis factor, interleukins 6, and 8 during the Jarisch-Herxheimer Reaction of relapsing fever. J Exp Med 175, 1207–1212. doi: 10.1084/jem.175.5.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman K Jr., and Johnson RC (1981). In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect Immun 31, 465–469. doi: 10.1128/IAI.31.1.465-469.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman K Jr., and Johnson RC (1984). T-cell-independent elimination of Borrelia turicatae. Infect Immun 45, 572–576. doi: 10.1128/IAI.45.3.572-576.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NTT, Rottgerding F, Devraj G, Lin YP, Koenigs A, and Kraiczy P (2018). The Complement Binding and Inhibitory Protein CbiA of Borrelia miyamotoi Degrades Extracellular Matrix Components by Interacting with Plasmin(ogen). Frontiers in cellular and infection microbiology 8, 23. doi: 10.3389/fcimb.2018.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle C, and Anderson C (1927). Etude compartive de quelques viru recurrents, pathogenes pour l'homme. Arch Inst Past de Tunis 16, 123–206. [Google Scholar]
- Nieto NC, and Teglas MB (2014). Relapsing fever group Borrelia in Southern California rodents. J Med Entomol 51, 1029–1034. doi: 10.1603/me14021 [DOI] [PubMed] [Google Scholar]
- Nordstrand A, Shamaei-Tousi A, Ny A, and Bergstrom S (2001). Delayed invasion of the kidney and brain by Borrelia crocidurae in plasminogen-deficient mice. Infect Immun 69, 5832–5839. doi: 10.1128/iai.69.9.5832-5839.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy FG, and Knapp RE (1906). Studies on Spirillum obermeieri and related organisms. J Infect Dis 3, 291–293. [Google Scholar]
- Oleaga A, Obolo-Mvoulouga P, Manzano-Roman R, and Perez-Sanchez R (2015). Midgut proteome of an argasid tick, Ornithodoros erraticus: a comparison between unfed and engorged females. Parasit Vectors 8, 525. doi: 10.1186/s13071-015-1148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachanian A, and Smith JB (1970). Immunization of chicks against avian spirochetosis with vaccines of Borrelia anserina. Tex Rep Biol Med 28, 287–301. [PubMed] [Google Scholar]
- Parsons L (1947). Relapsing fever at Lake Tahoe, California-Nevada. Am J Clin Pathol 17, 388–392. [DOI] [PubMed] [Google Scholar]
- Pavlovsky YN. (1963). Tick-borne relapsing fever. In Human Disease with Natural Foci, Pavlovsky YN, ed. (Moscow: Foreign Languages Publishing House; ), pp. 138–184. [Google Scholar]
- Penningon PM, Cadavid D, Bunikis J, Norris SJ, and Barbour AG (1999). Extensive interplasmidic duplications change the virulence phenotype of the relapsing fever agent Borrelia turicatae. Mol Microbiol 34, 1120–1132. doi: 10.1046/j.1365-2958.1999.01675.x [DOI] [PubMed] [Google Scholar]
- Pennington PM, Allred CD, West CS, Alvarez R, and Barbour AG (1997). Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun 65, 285–292. doi: 10.1128/IAI.65.1.285-292.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington PM, Cadavid D, and Barbour AG (1999). Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect Immun 67, 4637–4645. doi: 10.1128/IAI.67.9.4637-4645.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perine PL, Parry EHO, Vukotich D, Warrell DA, and Bryceson ADM (1971). Bleeding in louse-borne relapsing fever I. Clinical studies in 37 patients. Transactions of the Royal Society of Tropical Medicine and Hygiene 65, 776–781. doi: 10.1016/0035-9203(71)90091-5 [DOI] [PubMed] [Google Scholar]
- Pettersson J, Schrumpf ME, Raffel SJ, Porcella SF, Guyard C, Lawrence K, Gherardini FC, and Schwan TG. (2007). Purine salvage pathways among Borrelia species. Infect Immun 75, 3877–3884. doi: 10.1128/IAI.00199-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp MT, Masters E, Wormser GP, Hogrefe W, and Martin D (2006). Serologic evaluation of patients from Missouri with erythema migrans-like skin lesions with the C6 Lyme test. Clin Vaccine Immunol0 13, 1170–1171. doi: 10.1128/CVI.00238-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk RH, Simon MI, and Barbour AG (1985). Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature 318, 257–263. doi: 10.1038/318257a0 [DOI] [PubMed] [Google Scholar]
- Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, and Krause PJ. (2011). Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerging Infectious Diseases 17, 1816–1823. doi: 10.3201/eid1710.101474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policastro PF, Raffel SJ, and Schwan TG (2013). Borrelia hermsii acquisition order in superinfected ticks determines transmission efficiency. Infect Immun 81, 2899–2908. doi: 10.1128/IAI.00542-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcella SF, Raffel SJ, Schrumpf ME, Schriefer ME, Dennis DT, and Schwan TG. (2000). Serodiagnosis of Louse-Borne relapsing fever with glycerophosphodiester phosphodiesterase (GlpQ) from Borrelia recurrentis. J Clin Microbiol 38, 3561–3571. doi: 10.1128/JCM.38.10.3561-3571.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD and Samuels DS (2021). Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions and Disease Pathogenesis (Norfolk, UK: Caister Academic Press; ). 10.21775/9781913652616 [DOI] [Google Scholar]
- Raffel SJ, Battisti JM, Fischer RJ, and Schwan TG. (2014). Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS pathogens 10, e1004056. doi: 10.1371/journal.ppat.1004056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo BI, and Barbour AG (1994). Antigen Diversity in the Bacterium B-Hermsii through Somatic Mutations in Rearranged Vmp Genes. Cell 78, 867–876. doi: 10.1016/S0092-8674(94)90642-4 [DOI] [PubMed] [Google Scholar]
- Restrepo BI, Carter CJ, and Barbour AG (1994). Activation of a vmp pseudogene in Borrelia hermsii: an alternate mechanism of antigenic variation during relapsing fever. Mol Microbiol 13, 287–299. doi: 10.1111/j.1365-2958.1994.tb00423.x [DOI] [PubMed] [Google Scholar]
- Restrepo BI, Kitten T, Carter CJ, Infante D, and Barbour AG (1992). Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol Microbiol 6, 3299–3311. doi: 10.1111/j.1365-2958.1992.tb02198.x [DOI] [PubMed] [Google Scholar]
- Rich SM, Armstrong PM, Smith RD, and Telford SR 3rd (2001a). Lone star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J Clin Microbiol 39, 494–497. doi: 10.1128/JCM.39.2.494-497.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich SM, Sawyer SA, and Barbour AG (2001b). Antigen polymorphism in Borrelia hermsii, a clonal pathogenic bacterium. Proc Natl Acad Sci U S A 98, 15038–15043. doi: 10.1073/pnas.071042098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann E, Kraiczy P, Herzberger P, Skerka C, Kirschfink M, Simon MM, Zipfel PF, and Wallich R (2007). Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J Immunol 178, 7292–7301. doi: 10.4049/jimmunol.178.11.7292 [DOI] [PubMed] [Google Scholar]
- Rottgerding F, Wagemakers A, Koetsveld J, Fingerle V, Kirschfink M, Hovius JW, Zipfel PF, Wallich R, and Kraiczy P (2017). Immune evasion of Borrelia miyamotoi: CbiA, a novel outer surface protein exhibiting complement binding and inactivating properties. Sci Rep 7, 303. doi: 10.1038/s41598-017-00412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell H (1936). Observations on immunity in relapsing fever and trypanosomiasis. Trans R Soc Trop Med Hyg 30, 179–190. doi: 10.1016/S0035-9203(36)90086-X [DOI] [Google Scholar]
- Rutty J (1770). A Chronological History of the Weather and Seasons, and of the Prevailing Diseases in Dublin: With the Various Periods, Successions, and Revolutions, During the Space of Forty Years : with a Comparative View of the Difference of the Irish Climate and Diseases, and Those of England and Other Countries (Robinson and Roberts; ). [Google Scholar]
- Sadziene A, Barbour AG, Rosa PA, and Thomas DD (1993). An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun 61, 3590–3596. doi: 10.1128/IAI.61.9.3590-3596.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Jonsson M, Bergstrom S, Bright RK, Kennedy RC, and Barbour AG (1994). A Bactericidal Antibody to Borrelia-Burgdorferi Is Directed against a Variable Region of the Ospb Protein. Infection and Immunity 62, 2037–2045. doi: 10.1128/Iai.62.5.2037-2045.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih SY, Mustafa D, Abdel Wahab SM, Ahmed MA, and Omer A (1977). Louse-borne relapsing fever: I. A clinical and laboratory study of 363 cases in the Sudan. Trans R Soc Trop Med Hyg 71, 43–48. doi: 10.1016/0035-9203(77)90206-1 [DOI] [PubMed] [Google Scholar]
- Sambri V, Marangoni A, Olmo A, Storni E, Montagnani M, Fabbi M, and Cevenini R (1999). Specific antibodies reactive with the 22-kilodalton major outer surface protein of Borrelia anserina Ni-NL protect chicks from infection. Infect Immun 67, 2633–2637. doi: 10.1128/IAI.67.5.2633-2637.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarksyan DS, Maleev VV, Platonov AE, Platonova OV, and Karan LS (2015a). [Relapsing (recurrent) disease caused by Borrelia miyamotoi]. Ter Arkh 87, 18–25. doi: 10.17116/terarkh2015871118-25 [DOI] [PubMed] [Google Scholar]
- Sarksyan DS, Platonov AE, Karan LS, Shipulin GA, Sprong H, and Hovius JW (2015b). Probability of Spirochete Borrelia miyamotoi Transmission from Ticks to Humans. Emerging Infectious Diseases 21, 2273–2274. doi: 10.3201/eid2112.151097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sakakibara K, Masuzawa T, Ohnishi M, and Kawabata H (2018). Case control study: Serological evidence that Borrelia miyamotoi disease occurs nationwide in Japan. J Infect Chemother 24, 828–833. doi: 10.1016/j.jiac.2018.06.017 [DOI] [PubMed] [Google Scholar]
- Sato K, Takano A, Konnai S, Nakao M, Ito T, Koyama K, Kaneko M, Ohnishi M, and Kawabata H (2014). Human infections with Borrelia miyamotoi, Japan. Emerging Infectious Diseases 20, 1391–1393. doi: 10.3201/eid2008.131761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmann ET, Bushnell RB, Loomis EC, Oliver MN, and Theis JH (1976). Experimental and epizootiologic evidence associating Ornithodoros coriaceus (Acari:Agrasidae) with the exposure of cattle to epizootic bovine abortion in California. J Med Entomol 13, 292–299. doi: 10.1093/jmedent/13.3.292 [DOI] [PubMed] [Google Scholar]
- Schott M, Grosskinsky S, Brenner C, Kraiczy P, and Wallich R (2010). Molecular characterization of the interaction of Borrelia parkeri and Borrelia turicatae with human complement regulators. Infect Immun 78, 2199–2208. doi: 10.1128/IAI.00089-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder NW, Eckert J, Stubs G, and Schumann RR (2008). Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology 213, 329–340. doi: 10.1016/j.imbio.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Schuhardt VT, and Wilkerson M (1951). Relapse phenomena in rats infected with single spirochetes (Borrelia recurrenis var. turicatae). J Bacteriol 62, 215–219. doi: 10.1128/JB.62.2.215-219.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Battisti JM, Porcella SF, Raffel SJ, Schrumpf ME, Fischer ER, Carroll JA, Stewart PE, Rosa P, and Somerville GA (2003). Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J Bacteriol 185, 1346–1356. doi: 10.1128/jb.185.4.1346-1356.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, and Hinnebusch BJ (1998). Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 280, 1938–1940. doi: 10.1126/science.280.5371.1938 [DOI] [PubMed] [Google Scholar]
- Schwan TG, Raffel SJ, Schrumpf ME, and Porcella SF (2007). Diversity and distribution of Borrelia hermsii. Emerg Infect Dis 13, 436–442. doi: 10.3201/eid1303.060958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE Jr., and Konkel ME (1996). GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol 34, 2483–2492. doi: 10.1128/JCM.34.10.2483-2492.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scragg IG, Kwiatkowski D, Vidal V, Reason A, Paxton T, Panico M, Dell A, and Morris H (2000). Structural characterization of the inflammatory moiety of a variable major lipoprotein of Borrelia recurrentis. J Biol Chem 275, 937–941. doi: 10.1074/jbc.275.2.937 [DOI] [PubMed] [Google Scholar]
- Shamaei-Tousi A, Burns MJ, Benach JL, Furie MB, Gergel EI, and Bergstrom S (2000). The relapsing fever spirochaete, Borrelia crocidurae, activates human endothelial cells and promotes the transendothelial migration of neutrophils. Cell Microbiol 2, 591–599. doi: 10.1046/j.1462-5822.2000.00083.X [DOI] [PubMed] [Google Scholar]
- Shamaei-Tousi A, Collin O, Bergh A, and Bergstrom S (2001). Testicular damage by microcirculatory disruption and colonization of an immune-privileged site during Borrelia crocidurae infection. J Exp Med 193, 995–1004. doi: 10.1084/jem.193.9.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamaei-Tousi A, Martin P, Bergh A, Burman N, Brännström T, and Bergström S (1999). Erythrocyte-aggregating relapsing fever spirochete Borrelia crocidurae induces formation of microemboli. J Infect Dis 180, 1929–1938. doi: 10.1086/315118 [DOI] [PubMed] [Google Scholar]
- Shang ES, Skare JT, Exner MM, Blanco DR, Kagan BL, Miller JN, and Lovett MA (1998). Isolation and characterization of the outer membrane of Borrelia hermsii. Infect Immun 66, 1082–1091. doi: 10.1128/IAI.66.3.1082-1091.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SP, Amanfu W, and Losho TC. (2000). Bovine borreliosis in Botswana. Onderstepoort J Vet Res 67, 221–223. [PubMed] [Google Scholar]
- Skare JT, Mirzabekov TA, Shang ES, Blanco DR, Erdjument-Bromage H, Bunikis J, Bergström S, Tempst P, Kagan BL, Miller JN, et al. (1997). The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun 65, 3654–3661. doi: 10.1128/IAI.65.9.3654-3661.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Brener J, Osorno M, and Ristic M (1978). Pathobiology of Borrelia theileri in the tropical cattle tick, Boophilus microplus. J Invertebr Pathol 32, 182–190. doi: 10.1016/0022-2011(78)90028-9 [DOI] [PubMed] [Google Scholar]
- Smith RP Jr., Elias SP, Cavanaugh CE, Lubelczyk CB, Lacombe EH, Brancato J, Doyle H, Rand PW, Ebel GD, and Krause PJ (2019). Seroprevalence of Borrelia burgdorferi, B. miyamotoi, and Powassan Virus in Residents Bitten by Ixodes Ticks, Maine, USA. Emerging Infectious Diseases 25, 804–807. doi: 10.3201/eid2504.180202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaskey CD, Zuckert WR, and Barbour AG (1999). The extended promoters for two outer membrane lipoprotein genes of Borrelia spp. uniquely include a T-rich region. Mol Microbiol 33, 41–51. doi: 10.1046/j.1365-2958.1999.01443.x [DOI] [PubMed] [Google Scholar]
- Southern P, and Sanford J (1969). Relapsing fever. A clinical and microbiological review. Medicine 48, 129–150. [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Brands JA, Hovius JW, Li X, and Mead PS. (2016). Lyme borreliosis. Nat Rev Dis Primers 2, 16090. doi: 10.1038/nrdp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Porcella SF, Oie KL, Fitzpatrick CA, Raffel SJ, Lubke L, Schrumpf ME, and Schwan TG. (2000). The relapsing fever spirochete Borrelia hermsii contains multiple, antigen-encoding circular plasmids that are homologous to the cp32 plasmids of Lyme disease spirochetes. Infect Immun 68, 3900–3908. doi: 10.1128/iai.68.7.3900-3908.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoenner HG, Dodd T, and Larsen C (1982). Antigenic variation of Borrelia hermsii. J Exp Med 156, 1297–1311. doi: 10.1084/jem.156.5.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad M, Honig V, Ruzek D, Grubhoffer L, and Rego ROM (2017). Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl Environ Microbiol 83. doi: 10.1128/AEM.00609-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhindra P, Wang G, Schriefer ME, McKenna D, Zhuge J, Krause PJ, Marques AR, and Wormser GP (2016). Insights into Borrelia miyamotoi infection from an untreated case demonstrating relapsing fever, monocytosis and a positive C6 Lyme serology. Diagn Microbiol Infect Dis 86, 93–96. doi: 10.1016/j.diagmicrobio.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundnes KO, and Haimanot AT (1993). Epidemic of louse-borne relapsing fever in Ethiopia. Lancet 342, 1213–1215. doi: 10.1016/0140-6736(93)92190-5 [DOI] [PubMed] [Google Scholar]
- Tabuchi N, Mitani H, Seino S, and Fukunaga M (2002). The 44-kb linear plasmid molecule in the relapsing fever agent Borrelia duttonii strain Ly serve as a preservation of vmp genes. Microbiol Immunol 46, 159–165. doi: 10.1111/j.1348-0421.2002.tb02681.x [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Cutler SJ, and Fukunaga M (2000). Size conversion of a linear plasmid in the relapsing fever agent Borrelia duttonii. Microbiol Immunol 44, 1071–1074. doi: 10.1111/j.1348-0421.2000.tb02605.x [DOI] [PubMed] [Google Scholar]
- Takayama K, Rothenberg RJ, and Barbour AG (1987). Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 55, 2311–2313. doi: 10.1128/IAI.55.9.2311-2313.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talagrand-Reboul E, Boyer PH, Bergström S, Vial L, and Boulanger N (2018). Relapsing Fevers: Neglected Tick-Borne Diseases. Frontiers in cellular and infection microbiology 8, 98. doi: 10.3389/fcimb.2018.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, Goethert HK, Molloy PJ, and Berardi V (2019). Blood Smears Have Poor Sensitivity for Confirming Borrelia miyamotoi Disease. J Clin Microbiol 57. doi: 10.1128/JCM.01468-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler AJ (1902). Spirillosis of cattle. Compar Pathol Ther 17, 47–55. doi: 10.1016/S0368-1742(04)80003-1 [DOI] [Google Scholar]
- Thomas NJ, Bunikis J, Barbour AG, and Wolcott MJ (2002). Fatal spirochetosis due to a relapsing fever-like Borrelia sp. in a northern spotted owl. J Wildl Dis 38, 187–193. doi: 10.7589/0090-3558-38.1.187 [DOI] [PubMed] [Google Scholar]
- Toledo A, Anda P, Escudero R, Larsson C, Bergstrom S, and Benach JL (2010). Phylogenetic analysis of a virulent Borrelia species isolated from patients with relapsing fever. J Clin Microbiol 48, 2484–2489. doi: 10.1128/JCM.00541-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal DR, and Rao SB (1966). Studies on the preparation of a spirochaete vaccine. I. Indian Vet J 43, 191–195. [PubMed] [Google Scholar]
- van Holten J, Tiems J, and Jongen VH. (1997). Neonatal Borrelia duttoni infection: a report of three cases. Trop Doct 27, 115–116. doi: 10.1177/004947559702700229 [DOI] [PubMed] [Google Scholar]
- Varela AS, Luttrell MP, Howerth EW, Moore VA, Davidson WR, Stallknecht DE, and Little SE (2004). First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol 42, 1163–1169. doi: 10.1128/jcm.42.3.1163-1169.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma MGR (1956). Infections of Ornithodoros ticks with relapsing fever spirochaetes, and the mechanisms of their transmission. Ann Trop Med Parasitol 50, 18–31. doi: 10.1080/00034983.1956.11685735 [DOI] [PubMed] [Google Scholar]
- Vazquez-Guerrero E, Adan-Bante NP, Mercado-Uribe MC, Hernandez-Rodriguez C, Villa-Tanaca L, Lopez JE, and Ibarra JA (2019). Case report: A retrospective serological analysis indicating human exposure to tick-borne relapsing fever spirochetes in Sonora, Mexico. PLoS Negl Trop Dis 13, e0007215. doi: 10.1371/journal.pntd.0007215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu H, Balozet L, and Zottner G (1930). Neurotropisme de Spirochaeta hispanicum (souche de Mansouriah) pour le lapin' forme nouvelle de l'infection inapparente. Comptes Rendus des Seances Societe de Biologie (Paris) 103, 381–383. [Google Scholar]
- Venzal JM, Estrada-Pena A, Mangold AJ, Gonzalez-Acuna D, and Guglielmone AA (2008). The Ornithodoros (Alectorobius) talaje species group (Acari: Ixodida: Argasidae): description of Ornithodoros (Alectorobius) rioplatensis n. sp. from southern South America. J Med Entomol 45, 832–840. doi: 10.1603/0022-2585(2008)45X32:toatsgR.0.co;2 [DOI] [PubMed] [Google Scholar]
- Vidal V, Cutler S, Scragg IG, Wright DJ, and Kwiatkowski D (2002). Characterisation of silent and active genes for a variable large protein of Borrelia recurrentis. BMC Infect Dis 2, 25. doi: 10.1186/1471-2334-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal V, Scragg IG, Cutler SJ, Rockett KA, Fekade D, Warrell DA, Wright DJ, and Kwiatkowski D (1998). Variable major lipoprotein is a principal TNF-inducing factor of louse- borne relapsing fever. Nat Med 4, 1416–1420. doi: 10.1038/4007 [DOI] [PubMed] [Google Scholar]
- Wagemakers A, Koetsveld J, Narasimhan S, Wickel M, Deponte K, Bleijlevens B, Jahfari S, Sprong H, Karan LS, Sarksyan DS, et al. (2016). Variable Major Proteins as Targets for Specific Antibodies against Borrelia miyamotoi. J Immunol 196, 4185–4195. doi: 10.4049/jimmunol.1600014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemakers A, Oei A, Fikrig MM, Miellet WR, and Hovius JW (2014). The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit Vectors 7, 418. doi: 10.1186/1756-3305-7-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemakers A, Staarink PJ, Sprong H, and Hovius JW (2015). Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 31, 260–269. doi: 10.1016/j.pt.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Warrell DA, Perine PL, Krause DW, Bing DH, and MacDougal SJ (1983). Pathophysiology and immunology of the Jarisch-Herxheimer-like reaction in louse-borne relapsing fever: comparison of tetracycline and slow-release penicillin. J Infect Dis 147, 898–909. doi: 10.1093/infdis/147.5.898 [DOI] [PubMed] [Google Scholar]
- Webster G, Schiffman JD, Dosanjh AS, Amieva MR, Gans HA, and Sectish TC (2002). Jarisch-Herxheimer reaction associated with ciprofloxacin administration for tick-borne relapsing fever. Pediatr Infect Dis J 21, 571–573. doi: 10.1097/00006454-200206000-00020 [DOI] [PubMed] [Google Scholar]
- Wengrower D, Knobler H, Gillis S, and Chajek-Shaul T (1984). Myocarditis in tick-borne relapsing fever. J Infect Dis 149, 1033. doi: 10.1093/infdis/149.6.1033 [DOI] [PubMed] [Google Scholar]
- Wilder HK, Raffel SJ, Barbour AG, Porcella SF, Sturdevant DE, Vaisvil B, Kapatral V, Schmitt DP, Schwan TG, and Lopez JE (2016). Transcriptional Profiling the 150 kb Linear Megaplasmid of Borrelia turicatae Suggests a Role in Vector Colonization and Initiating Mammalian Infection. PLoS One 11, e0147707. doi: 10.1371/journal.pone.0147707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman ME, Cooley AE, Avdiushko R, Bowman A, Botto M, Wooten RM, van Rooijen N, Cohen DA, and Stevenson B (2009). Roles for phagocytic cells and complement in controlling relapsing fever infection. J Leukoc Biol 86, 727–736. doi: 10.1189/jlb.0309169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Masters E, Liveris D, Nowakowski J, Nadelman RB, Holmgren D, Bittker S, Cooper D, Wang G, and Schwartz I (2005). Microbiologic evaluation of patients from Missouri with erythema migrans. Clin Infect Dis 40, 423–428. doi: 10.1086/427289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski D, Gebhardt L, Prusinski MA, Meehan LJ, Halse TA, and Musser KA (2017). Detection of Borrelia miyamotoi and other tick-borne pathogens in human clinical specimens and Ixodes scapularis ticks in New York State, 2012-2015. Ticks Tick Borne Dis 8, 407–411. doi: 10.1016/j.ttbdis.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Davidson WR, Stallknecht DE, Varela AS, Swift PK, Devos JC Jr., and Dubay SA (2005). Evidence of tick-borne organisms in mule deer (Odocoileus hemionus) from the western United States. Vector Borne Zoonotic Dis 5, 351–362. doi: 10.1089/vbz.2005.5.351 [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Murphy SM, Luttrell MP, Little SE, Massung RF, Stallknecht DE, Conti LA, Blackmore CG, and Durden LA (2008). Experimental and field studies on the suitability of raccoons (Procyon lotor) as hosts for tick-borne pathogens. Vector Borne Zoonotic Dis 8, 491–503. doi: 10.1089/vbz.2007.0240 [DOI] [PubMed] [Google Scholar]
- Yagupsky P, and Moses S (1985). Neonatal Borrelia species infection (relapsing fever). Am J Dis Child 139, 74–76. doi: 10.1001/archpedi.1985.02140030076034 [DOI] [PubMed] [Google Scholar]
- Yamano K, Ito T, Kiyanagi K, Yamazaki H, Sugawara M, Saito T, Ohashi N, Zamoto-Niikura A, Sato K, and Kawabata H (2017). Case Report: Clinical Features of a Case of Suspected Borrelia miyamotoi Disease in Hokkaido, Japan. Am J Trop Med Hyg 97, 84–87. doi: 10.4269/ajtmh.16-0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yparraguirre LA, Machado-Ferreira E, Ullmann AJ, Piesman J, Zeidner NS, and Soares CA (2007). A hard tick relapsing fever group spirochete in a Brazilian Rhipicephalus (Boophilus) microplus. Vector Borne Zoonotic Dis 7, 717–721. doi: 10.1089/vbz.2007.0144 [DOI] [PubMed] [Google Scholar]
- Zaher MA, Soliman ZR, and Diab FM (1977). An experimental study of Borrelia anserina in four species of Argas ticks. 2. Transstadial survival and transovarial transmission. Z Parasitenkd 53, 213–223. doi: 10.1007/BF00380466 [DOI] [PubMed] [Google Scholar]
- Zhang H, and Marconi RT (2005). Demonstration of cotranscription and 1-methyl-3-nitroso-nitroguanidine induction of a 30-gene operon of Borrelia burgdorferi: evidence that the 32-kilobase circular plasmids are prophages. J Bacteriol 187, 7985–7995. doi: 10.1128/JB.187.23.7985-7995.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Hardham JM, Barbour AG, and Norris SJ (1997). Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89, 275–285. doi: 10.1016/s0092-8674(00)80206-8 [DOI] [PubMed] [Google Scholar]
- Zhong J, and Barbour AG (2004). Cross-species hybridization of a Borrelia burgdorferi DNA array reveals infection- and culture associated genes of the unsequenced genome of the relapsing fever agent Borrelia hermsii. Mol Microbiol 51, 729–748. doi: 10.1046/j.1365-2958.2003.03849.X [DOI] [PubMed] [Google Scholar]
- Zhong J, Skouloubris S, Dai Q, Myllykallio H, and Barbour AG (2006). Function and evolution of plasmid-borne genes for pyrimidine biosynthesis in Borrelia spp. J Bacteriol 188, 909–918. doi: 10.1128/JB.188.3.909-918.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckert WR, Kerentseva TA, Lawson CL, and Barbour AG (2001). Structural conservation of neurotropism-associated VspA within the variable Borrelia Vsp-OspC lipoprotein family. J Biol Chem 276, 457–463. doi: 10.1074/jbc.M008449200 [DOI] [PubMed] [Google Scholar]
- Zuckert WR, Lloyd JE, Stewart PE, Rosa PA, and Barbour AG (2004). Cross-species surface display of functional spirochetal lipoproteins by recombinant Borrelia burgdorferi. Infect Immun 72, 1463–1469. doi: 10.1128/iai.72.3.1463-1469.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]