Abstract
MDMA is increasingly used in clinical research, but no cGMP process has yet been reported. We describe here the first fully validated cGMP synthesis of up to 5 kg (≈30 000 patient doses) of MDMA in a four-step process beginning with a noncontrolled starting material. The overall yield was acceptable (41–53%, over four steps), and the chemical purity of the final product was excellent, exceeding 99.9% of the peak area by HPLC in each of the four validation trials. The availability of cGMP-compliant MDMA will facilitate ongoing clinical trials and provide for future therapeutic use, if encouraging results lead to FDA approval.
Introduction
Interest in the clinical utility of psychedelic compounds has increased dramatically in recent years. Although medical usage of these substances, in tandem with psychotherapy, was briefly—and controversially1—explored, in the 1950s and 1960s,2 increased regulatory oversight and social disapprobation effectively eliminated such research until the late 1990s, when tentative efforts to revive it commenced.3 Promising early results very slowly stimulated additional engagement, both experimentally and culturally, provoking recent regulatory shifts that have further stimulated engagement by making research chemicals more accessible and expanding the permissible scope of clinical studies.4
This second wave of so-called psychedelic studies more expansively includes compounds like entactogen 3,4-methylenedioxymethamphetamine (MDMA). Like traditional psychedelics, MDMA had previously enjoyed a brief period of encouraging early-stage exploration, in the 1970s and 1980s, which was similarly curtailed by social and regulatory backlash. In contrast to psychedelics like LSD and psilocybin, however, the addition of MDMA to the U.S. Drug Enforcement Administration’s (DEA) Schedule I appeared to be largely related to MDMA’s popularity as an illicit “party drug,”5 rather than to significant concerns regarding either contemporary research efforts or its therapeutic utility.6 Indeed, in clinical trials conducted since the U.S. Food and Drug Administration (FDA) and DEA first granted research approval, in 2004,7 MDMA has shown promise as a psychotherapeutic aid for patients suffering from PTSD,8 autism-related social anxiety,9 and alcoholism.10
As the research environment grows steadily more supportive of clinical exploration, and as successful clinical trials open the door for fully approved treatments, the need for pharmaceutically acceptable MDMA continues to expand. To ensure that patients receive safe, effective drugs, the manufacture of pharmaceutical substances is closely regulated by the FDA, under a structure called Current Good Manufacturing Practice (cGMP).11 These rules delineate standards for every aspect of the manufacturing process, including facility design, establishment and documentation of operating procedures, process monitoring, and chemical analysis. Because only small samples of each pharmaceutical batch are submitted for (destructive) quality control testing, a well-controlled manufacturing process is the best-known way to ensure that all drugs distributed to consumers are of predictably high quality, consistency, and efficacy. cGMP-compliant synthetic processes are typically developed for drug candidates in tandem with progressing clinical trials.12
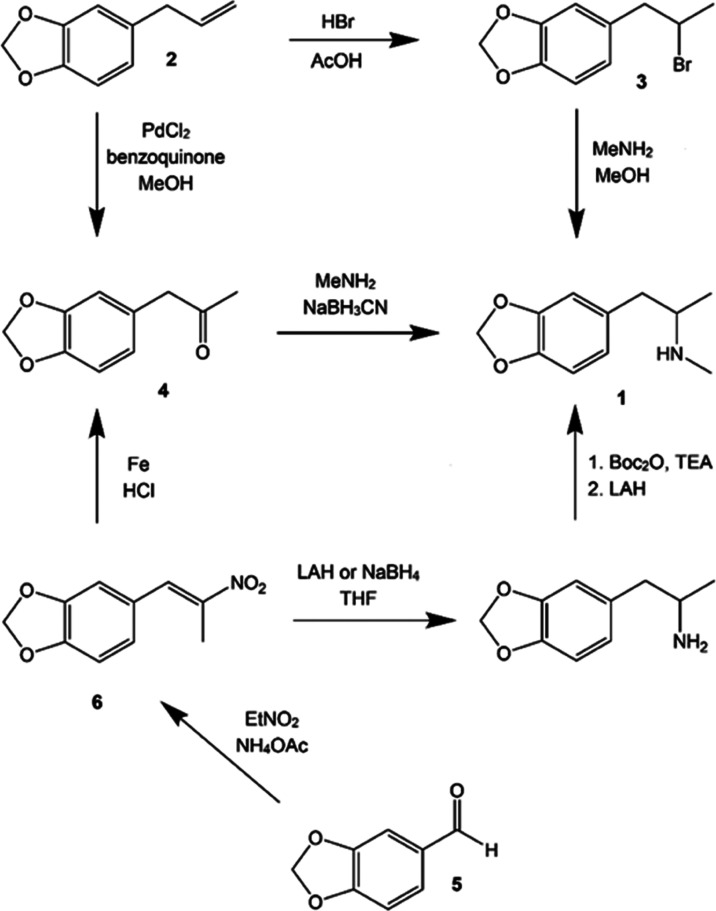
Unlike many drug candidates, MDMA (1) enjoyed a robust synthetic history prior to receiving any serious consideration as a pharmaceutical substance. MDMA was first synthesized by Merck, in 1912, as an intermediate to the styptic compound methylhydrastitine.13 Scientists periodically explored its pharmacological effects over the intervening half-century, both at Merck and in the United States Army,14 but MDMA does not appear in either the patent or the chemical literature again until 1960, when Biniecki and Krajewski published a synthesis identical to Merck’s in Poloniae Pharmaceutica15 (it is unlikely that they were aware of the Merck patent). This synthetic route proceeded via hydrobromination of the natural product safrole (2), yielding the Markovnikov adduct 3, which was then converted to MDMA using methylamine in methanol. A variety of synthetic approaches from methyl piperonyl ketone (4), which was commercially available at the time, and which can be easily prepared either from safrole—typically via Wacker oxidation—or piperonal (5)—typically by reducing its nitroalkene derivative 6 with iron in hydrochloric acid—were summarized by Shulgin, in 1986.16 A novel approach from piperonal, via Curtius rearrangement, was reported by Schulze, in 2010,17 and a handful of asymmetric syntheses of (S)-MDMA, some relying on alternate starting materials, have also appeared in the literature (Scheme 1).18
Scheme 1. Common Synthetic Approaches to MDMA.
Clandestine chemists preparing MDMA for the black market have additionally developed a number of synthetic routes from readily available starting materials like catechol (7),19 eugenol (8),19 isosafrole (9),20 and piperine (10),21 though most still approach MDMA through a safrole22 or (less frequently) piperonal21 intermediate. These synthetic methods often rely on chemicals readily available to ordinary consumers, in an effort to circumvent controlled substance precursor regulations. Most of these clandestine syntheses are well-documented, both by anonymous chemists, in online forums, and by forensic scientists, who often identify clandestine production methods by their distinct impurity profiles.20
To date, none of the synthetic explorations into MDMA appear to have considered cGMPs. While intended for pharmaceutical production, Merck’s early investigations occurred less than a decade after the FDA was founded, and well before its cGMP rules were developed. Some clandestine labs reliably produce large quantities of high-quality MDMA;23 however, these facilities necessarily operate outside of regulatory frameworks and certainly do not report or document cGMP-compliant procedures. Most other synthetic explorations of MDMA have been geared toward the production of MDMA as a research chemical, usually for small-scale studies in animals or for forensic analysis (Figure 1).
Figure 1.
Less-used MDMA precursors.
Indeed, prior to a recently completed Phase 3 trial for PTSD,8 it is likely that few even contemplated a need for cGMP-compliant MDMA. As a Schedule I substance, it officially had no recognized medical utility up until now. As a well-known compound with a lengthy history in the public domain and a short treatment regimen, it also had little apparent commercial value.24
Results
We report here the first cGMP synthesis of MDMA and its hydrochloride salt (MDMA·HCl), which is used in pharmaceutical formulations. In this fully validated, four-stage process, up to 5 kg of MDMA·HCl was reproducibly synthesized, with an overall yield of 41.8–54.6% and a minimum purity of 99.4% (w/w) by HPLC assay. Over a minimum of four consecutive trials, for each stage, the established targets for yields and impurity profiles were achieved—and, in most cases, exceeded. Chemical impurities in the final product (MDMA·HCl) averaged 0.04% of the total peak area, by HPLC, and no single impurity ever exceeded 0.03% of the total peak area. Of all of the organic solvents used in the production process, only isopropanol (Class 3, 409–509 ppm), tetrahydrofuran (Class 2, <7 ppm), methanol (Class 2, <6 ppm), and n-heptane (Class 3, <67 ppm) were detected in the final product—all in concentrations well below the permitted daily exposure (PDE) per FDA guidance.25 The scale and reliability of this cGMP process will improve access to MDMA for ongoing and future clinical trials—and potentially for licensed therapeutic use, pending FDA approval.
Discussion
Increased demand for pharmaceutical-grade MDMA encouraged us to develop a cGMP-compliant production process, both to supply our own Phase III clinical trials, for PTSD, and to ameliorate existing supply constraints for the broader research community. While large-scale clandestine production is common, to the best of our knowledge, no multi-kilogram synthesis of pharmaceutical-grade MDMA has yet been reported in the literature. We therefore needed to develop a practicable synthetic route while simultaneously addressing cGMP requirements.
MDMA is not a particularly complex molecule, and many synthetic pathways have been reported. Most begin from either safrole or piperonal, which are highly regulated and consequently difficult to obtain; for the sake of convenience and efficiency, we elected to avoid these. We identified 5-bromo-1,3-benzodioxole (11), which does not appear on any geopolitical entity’s list of controlled substance precursors, as a useful starting material for our synthesis. The 1,3-benzodioxole moiety appears in a variety of natural products, including oils,26 spices,27 and traditional plant-based medicines.28 Many compounds containing this structural feature are known to interact with cytochrome P450 enzymes in mammals, producing a range of clinically notable effects, both pharmacologically useful and neurotoxic.29 Compound 11 is synthesized via the bromination of benzodioxole with NBS; analysis of multiple batches, from a range of suppliers, indicated that the only significant impurities present in the batch are 5,6-dibromo-1,3-benzodioxole and succinimide, which is insoluble in Compound 11 and consequently present in only very trace amounts. We additionally screen for the presence of 4-bromo-1,3-benzodioxole, which would likely present separation challenges during production, but we have never observed this isomer in the starting material. At the levels observed, neither of the two significant impurities interfered with the downstream chemistry.
Compound 11 has been previously used in at least two (reported) approaches to MDMA: as a starting material in the asymmetric synthesis of (S)-MDMA, through a protected aziridine intermediate,30 and as a precursor to safrole, via Grignard reaction with allyl bromide (Scheme 2).19,31
Scheme 2. Synthesis of cGMP MDMA·HCl.
Instead of approaching MDMA conventionally, via safrole, we elected to generate a 2-propanol substituent via ring-opening addition between the same aryl Grignard reagent used to synthesize safrole, above, and 1,2-propylene oxide (12), which is both inexpensive and readily available. Reactions of Grignard reagents and epoxides are well-known,32 but—to the best of our knowledge—this particular synthetic pathway has not previously been used to produce MDMA. Our familiarity with this type of reaction made us optimistic that scale-up would proceed smoothly—and it did. Although Grignard formation is slow, the bulk reaction can be expedited via initiation with a small amount of previously prepared Grignard reagent. The 5,6-dibromo-1,3-benzodioxole impurity present in the starting material does not undergo Grignard formation and is removed during workup as part of the organic layer. The workup at this stage was quite efficient, and distillation via a wiped-film evaporator (two to three passes) yielded 1-(3,4-methylenedioxy-phenyl)-2-propanol (13) in excess of 96% chemical purity by HPLC. The adjusted yield, based on HPLC assay, was 79.22–87.39% (w/w) over five trials.
The next three steps relied on well-known synthetic transformations. 13 was oxidized to methyl piperonyl ketone (4) with a biphasic (DCM/H2O) TEMPO/KBr/bleach reagent system, which was followed by aqueous workup and filtration to remove remaining solids. The solvent was removed via a rotatory evaporator, and the crude product was of sufficient purity to proceed to the next process stage, without an additional purification step (100.2–108.2% yield over four trials; 84.98–90.01% w/w by HPLC assay). Stage 3, reductive amination of 4, was accomplished with aqueous methylamine and NaOH/NaBH4. Workup was somewhat complex, using an acid/base treatment to remove the vast majority of impurities, followed by acidification with HCl in isopropanol which yielded 71.6–75.8% MDMA·HCl (14), over eight trials, with chemical purity exceeding 99.26% of peak area, by HPLC. Recrystallization in isopropanol (Stage 4) yielded 85.5–86.2% of a white, crystalline solid, with a minimum purity of 99.95% by HPLC and a minimum assay of 99.40% (w/w), also by HPLC (Table 1).
Table 1. Results from Stage 4 Validation Trials for the Synthesis of cGMP MDMA·HCl.
| trial | yield (%) | purity (% peak area by HPLC) | assay (% w/w by HPLC) |
|---|---|---|---|
| 1 | 85.5 | 99.95 | 99.64 |
| 2 | 85.9 | 99.96 | 99.40 |
| 3 | 86.2 | 99.99 | 99.77 |
| 4 | 86.1 | 99.95 | 99.76 |
MDMA·HCl was previously known to form one major crystal form (Form I) and at least four hydrates that incorporate 0.25–1 waters of hydration.16 Our polymorphic screening process identified two new anhydrous crystal forms (Forms II and III) and established Form I as the most stable of the three. Form II can be reproducibly produced from a variety of alcoholic solvents, as well as in the presence of ethyl acetate and an ethereal antisolvent. Unlike Form III, which spontaneously converted to Form I after 2.5 weeks at ambient conditions, and could not be reproduced, Form II is shelf-stable, though it will convert to Form I under competitive equilibration conditions. Interestingly, both Form I and Form II reversibly convert into the known monohydrate; upon dehydration, the monohydrate formed from Form I will revert back to Form I, and the monohydrate formed from Form II will revert back to Form II. If crystallized from a concentrated aqueous solution with no form memory, the monohydrate will thermally dehydrate exclusively into Form I. X-ray powder diffraction spectra for all three forms are shown in Figure 2.
Figure 2.
XRPD spectra for MDMA·HCl forms I–III and MDMA·HCl monohydrate.
To maintain compliance with cGMP regulations, all reagents were visually inspected and tested, prior to use. Conformance to established specification(s) was documented, reagents were labeled with identifying raw material numbers, and these identifying numbers were recorded whenever a reagent was used, in-process. Organic reagents were typically confirmed by FT-IR, as well as by other methods specific to their chemical identity and various process needs (e.g., Karl–Fischer titration to establish water content, etc.), in accordance with established procedures. Inorganic reagents were confirmed by appropriate chemical identification tests. Reagents that failed to meet all established specifications were not used at any stage of the process.
Another concern for cGMP manufacturing is the presence of residual solvents, which must be below solvent-specific concentration thresholds defined in USP <467>. The limits set for residual solvent concentrations are based on anticipated daily exposure to a pharmaceutical product. In clinical use, MDMA is never recommended for daily—or even regular—consumption; instead, it is ingested during a small number of therapy sessions, spread over weeks or months. Nevertheless, our monograph utilizes the USP <467> PDE limits as acceptance criteria—and our process yielded residual solvent concentrations significantly below these limits, over four consecutive validation trials (Table 2). The limit of detection for all tested solvents was 1 ppm; solvents detected in concentrations below the quantitation limit were reported as such.
Table 2. Residual Solvent Profile of cGMP MDMA·HCl.
| solvent | acceptance criteria (ppm) | highest level found (ppm) |
|---|---|---|
| THF | 720 | <7 |
| tert-Butyl methyl ether (TBME) | 5000 | not detected |
| n-Heptane | 5000 | <67 |
| methanol | 3000 | <6 |
| 2-propanol | 5000 | 509 |
| dichloromethane (DCM) | 600 | not detected |
In addition to meeting residual solvent concentration limits, cGMP pharmaceuticals must have acceptable impurity profiles. Any single impurity exceeding 0.1% must be both characterized and quantified. Over four trials, our process yielded MDMA·HCl with chemical purity in excess of 99.9% of peak area by HPLC; no single impurity ever exceeded 0.05% of the total peak area. While impurity characterization was consequently not required, we routinely screened for two known impurities (Figure 3), both of which were generated via low-level electrophilic addition during the Stage 2 oxidation of 13. Chlorination was only significant when the bleach was overcharged, and the reaction conditions used in Stage 2 prevent this. Bromination, which also increased with excess bleach, was a more significant side reaction, but it was successfully minimized using KBr in catalytic, rather than stoichiometric, quantities. Neither impurity was ever found in excess of 0.03% of the total peak area, by HPLC, in any of the four Stage 4 validation trials.
Figure 3.
Known impurities in MDMA·HCl.
Heavy metal impurities in finished pharmaceutical products are also an area of potential concern. As with residual solvents, cGMP-compliant limits are established with the assumption that a medication will be consumed on a daily basis, a usage pattern that we do not anticipate will ever be in effect for clinically administered MDMA. Nevertheless, we used the oral daily dose PDEs from USP ⟨232⟩ when determining acceptability parameters. As shown in Table 3, the greatest quantifiable amount of any heavy metal impurity was 97% less than the permissible daily intake limit—and most were well below that level.
Table 3. Heavy Metal Impurities found in cGMP MDMA·HCl.
| element | concentration limit (μg/g) | highest value found in product (μg/g) |
|---|---|---|
| cadmium | 5 | <0.1 |
| lead | 5 | <0.1 |
| arsenic | 15 | <0.1 |
| mercury | 30 | 0.7 |
| cobalt | 50 | <0.1 |
| vanadium | 100 | 0.2 |
| nickel | 200 | 1.1 |
| copper | 3000 | 3.3 |
To validate this cGMP process, each stage was successfully completed at the 8 kg scale (based on the starting charge of benzodioxole) at least four consecutive times, in accordance with the documented procedures. All reagents, products, intermediates, common impurities, and (as required) reaction end points were validated using cGMP-compliant analytical methods, some of which were specifically developed for this synthetic process. Any deviations from the documented procedures or parameters were noted, and the anticipated impact on the final product—if any—was characterized. No documented deviation appeared to affect either the final product or the outcome of the Stage 4 recrystallization step, which yielded remarkably consistent results throughout the validation process (Table 1). We are confident that our cGMP protocols are sufficient to reliably produce enough pharmaceutically acceptable MDMA to meet expanding research and therapeutic needs.
Experimental Section
General
Reactions were performed using commercially available raw materials and solvents. Unless otherwise stated, all commercially obtained reagents were qualified prior to use and then used as received. Reactions were conducted in a 50 L reaction vessel. The small-scale production of the Grignard reagent, used to initiate the bulk reaction, was conducted in a 2 L reactor fitted with a reflux condenser. A Huber Unistat was used for temperature control and logging. In-process analysis was conducted by HPLC, with supplemental 1H NMR analysis used to quantify residual solvent content during evaporation steps. A wiped-film evaporator was used for distillation. All processes were conducted under nitrogen (target: <5% O2). Residual solvent testing was performed on an Agilent J&W DB-624 HRGC column (60 m × 0.32 mm, 1.80 μm film thickness).
Stage 1—Synthesis of 1-(3,4-Methylenedioxyphenyl)-2-propanol
Grignard Formation
Into a 2 L vessel was charged 16.6 g of magnesium turnings (0.68 mol, 1.1 equiv), followed by 500 mL of THF (181 ppm H2O by KF titration) at 20 °C. Stirring was initiated after the introduction of THF, and the vessel was heated to a gentle reflux. To the vessel was then charged 125 g of 5-bromo-1,3-benzodioxole (0.62 mol, 1 equivalent chemical purity >98.70% by HPLC) in two unequal portions. The first portion weighed 6.3 g and was stirred for 12 h at a gentle reflux until an exotherm was observed. Following this initiation step, the remaining 118.4 g was added, dropwise, to the reaction vessel, and the resultant Grignard solution was stirred at reflux for 40 min and then cooled to 25 °C.
Into a separate 50 L reaction vessel was charged 1.06 kg of magnesium turnings (44 mol; 1.1 equiv) and 32 L of THF. The suspension was stirred and then heated to a gentle reflux. To the reaction vessel was then added 400.0 g of 5-bromo-1,3-benxodioxole (2.0 mol), followed by 400 mL of the small-batch Grignard solution described above. Reflux was maintained. After 5 min, a significant increase in the reflux rate was observed in the glass condenser, indicating initiation. While maintaining reflux, 7.6 kg of 5-bromo-1,3-benxodioxole (38 mol) was then added to the reaction vessel, using a dropping funnel, and the batch was stirred at a gentle reflux for 40 min.
Addition to Propylene Oxide
The bulk Grignard solution was cooled to 10 °C, and 128.8 g of copper iodide (1.5 mol, 0.4 equiv) was added to the 50 L vessel. A solution of 2.5 L (±)-propylene oxide (37 mol, 0.93 equiv) in 2.5 L of THF was then added to the reaction vessel while maintaining the temperature at 0–10 °C. The container and dropping funnel were rinsed with an additional 800 mL of THF, which was then added to the 50 L reaction vessel. The batch was stirred for 40 min at 5–20 °C, forming a dark brown solution and a crystalline suspension. Completion analysis performed by HPLC confirmed the reaction end point (0.30% 5-bromo-1,3-benzodioxole; target limit was ≤1%).
The batch was then divided into two 20.4 L portions for workup. For each portion, 5.45 L of a 10% (w/w) sodium chloride solution, followed by 1.37 L of acetic acid, was added to the 50 L reaction vessel while maintaining the temperature at 10–25 °C. The half-batch portion was then transferred from carboy into the reaction vessel while ensuring that the temperature remained below 40 °C. Following this addition, the half-batch portion was stirred at 30–40 °C for 45 min; then, the pH was adjusted to <5 by sequential addition of three 200 mL of aliquots of acetic acid. The batch was allowed to settle, and the aqueous layer was removed. 8.2 L of n-heptane were then charged into the 50 L reaction vessel, followed by an additional 8 L of the sodium chloride solution. The batch was stirred and allowed to settle, and the aqueous layer was again removed. The dark brown organic layer was filtered over a vacuum, using a plate filter with a 11 μm filter mesh. Following workup, the two half-batches were combined, and the solvent was removed in a 20 L rotatory evaporator. The crude yield was 7442.7 g and analysis by 1H NMR revealed 2.5% residual THF (n-heptane not detected; target limit is ≤10% total amount of both solvents).
The crude product was charged with 1488.5 g of PEG400 (0.2 equiv w/w) and mixed to ensure homogeneity. This mixture was then distilled at 150–185 °C and 0.1–1.5 mbar, using a wiped-film evaporator. Two passes yielded 6293.2 g of a pale yellow oil (94.2% yield; 96.44% area, 89.78% w/w by HPLC).
Stage 2—Oxidation to 1-(3,4-Methylenedioxyphenyl)-propan-2-one
A 50 L reaction vessel was charged with 2760.1 g of crude 1-(3,4-methylenedioxyphenyl)-2-propanol from Stage 1 (88.56% w/w by HPLC assay; active charge is 2444.3 g, 13.6 mol, 1 equivalent) and 9780 mL of dichloromethane at 10–25 °C. Stirring was initiated, and 178.5 g of potassium bromide was added, followed by 233.2 g of TEMPO (0.11 equiv). The batch was cooled to 0 °C, and 7280 mL (60%) of a solution of sodium hydrogen carbonate (0.25 equiv) in 12120 mL of bleach (1.6 equiv, diluted to 12.5% w/v) was added, dropwise, while stirring efficiently and maintaining the temperature at −10 to 10 °C. A 1 mL of sample was then removed, for analysis by HPLC, and four additional 610 mL (5%) of aliquots of the NaHCO3/bleach solution were then added, dropwise, to the reaction vessel. A sample was collected after each addition, and HPLC analysis was used to monitor the reaction progress. After the fourth aliquot was added, 1.61% of Stage 1 starting material remained (target limit is ≤5%). Stirring was halted, and the layers were allowed to settle. The layers were separated, and the organic layer was returned to the 50 L vessel.
For workup, the organic layer was cooled to 0 °C, and 4890 mL of a 12% (w/w) solution of aqueous sodium hydrosulfite was added while maintaining the temperature at 0–10 °C. The reaction mixture was then warmed to 19.5 °C and stirred for 15 min. The layers were separated, and the organic layer was returned to the 50 L reaction vessel. Then, 4900 mL of freshly prepared 0.5 M aqueous NaOH was added, and the reaction mixture was stirred for 15 min. The layers were separated, and the brown organic layer was returned to the 50 L reaction vessel. To this were added 4900 mL of 11% (w/w) aqueous NaCl, followed by 98 mL of concentrated HCl 36% w/w aqueous solution. After stirring for 15 min at 18.5 °C, the layers were separated, and the organic layer was returned to the reaction vessel. Two more washes—the first with another 4900 mL of the 11% NaCl solution, the second with 4900 mL of a saturated NaCl solution—were completed, following the same procedure. The organic layer was filtered over a Buchner funnel fitted with a filter cloth rinsing with 500 mL of DCM and then transferred to a 20 L rotatory evaporator. The solvent was removed under vacuum, yielding 2442.1 g of a yellow-to-brown oil (101.0% crude yield; 94.52% peak area, 89.80 w/w by HPLC).
Stage 3—Reductive Amination to MDMA·HCl
Next, 2963.9 g of crude 1-(3,4-methylenedioxyphenyl)-propan-2-one from Step 2 (13.7 mol, 81.26% w/w by HPLC) was added to a 50 L reaction vessel with 31170 mL of methanol (Kimia, confirmed by FT-IR), and the temperature was lowered to 5 °C. Then, 3520 mL of 40% (w/w) aqueous methylamine (102 mol, 7.5 equiv) was added, dropwise, and the batch was then cooled to −10 °C. To the reaction vessel was added 40.4 g of NaOH (1 mol) and 286.4 g of NaBH4 (7.6 mol, 0.5 equiv) in 630 mL of purified water, over the course of 120 min. The clear brown solution was then warmed to 3.9 °C and stirred for 25 min. A sample was removed and submitted for completion analysis by HPLC; the peak area for the product was 81.04%, and the starting material was undetected, which met the completion threshold of ≤1%. Then, 9640 mL of purified water was added to the reaction vessel, portionwise, while maintaining the temperature at 0–10 °C. The mixture was transferred to a 20 L rotatory evaporator, and methanol was removed, under vacuum. A sample was submitted for analysis, and 1H NMR indicated ≤10% residual methanol, which met the specification.
The crude product was returned to the 50 L reaction vessel and then stirred with 12 100 mL TBME for 15 min at 18.6 °C. The layers were then separated, and the aqueous layer was washed with an additional 2400 mL of TBME. The organic layers were then combined in the 50 L reaction vessel; 12 000 mL of 2.0 M HCl was added portionwise, and the mixture was stirred for 20 min at 15–30 °C. At this point, the pH was 1 (target is 1–2), and layers were again separated. The lower, aqueous layer was returned to the 50 L flask, washed with 12000 mL of TBME and then stirred for 15 min with 6000 mL of 5.4 M aqueous NaOH. Another 12 000 mL of TBME was then added, along with 1589.6 g of Rochelle Salt, and the mixture was stirred for 120 min. The pale brown/orange organic layer was separated from the aqueous layer, and the aqueous layer was washed again with 12 000 mL of TBME. The organic layers were combined, and the solvent was removed, in batches, with a 20 L rotatory and evaporator. Two thousand four hundred milliliters of isopropanol were added to the residue, then removed by a rotatory evaporator. The crude weight of the product (MDMA-free base) was 2524.0 g (94.57% peak area by HPLC).
The crude MDMA was then returned to the 50 L flask, along with 20 280 mL of 2-propanol. Stirring was initiated, and 2435 mL 5.4 M HCl in 2-propanol (13.1 mol) was added, dropwise, over 120 min. The mixture was then stirred for an additional 30 min, at room temperature. The white precipitate was captured via vacuum filtration, on a plate filter fitted with a filter cloth. The filter cake was washed twice with 2-propanol (2500 mL) and then dried under vacuum (100 mbar) for 18 h at 57.3 °C. After drying, 2280.4 g of crude MDMA·HCl remained (73.4% unadjusted yield, 99.26% peak area by HPLC).
Stage 4—Recrystallization of MDMA·HCl
To a 50 L reaction vessel was added 4107.3 g of crude MDMA·HCl and 41 000 mL of 2-propanol. The batch temperature was increased to 67.2 °C, while stirring, and the mixture was then stirred for 30 min at 67.2 °C until all of the solids dissolved. Stress tests had demonstrated stability for 72 h at 70–80 °C proving the thermal stability of MDMA·HCl.
The batch was then transferred through a 1.2 μm in-line filter capsule, using positive pressure, to a clean, 50 L reaction vessel, fitted with a jacket that had been preheated to 66.1 °C. In this new reaction vessel, the batch was cooled to 55.3 °C, over the course of 90 min. Then, 41.1 g of MDMA·HCl Form 1 seed crystal (0.18 mol, 0.008 equiv) was added, and the batch was stirred at the same temperature for 30 min. The batch was cooled to 15.2 °C at a rate of 3 °C/h and then stirred at this temperature for an additional 10 h.
The white suspension was removed from the mother liquor via vacuum filtration over a filter plate fitted with a filter cloth and then washed with 8220 mL of 2-propanol. The filter cake was transferred to a drying oven and dried under vacuum (140 mbar) for 19 h at 56.6 °C. The collected MDMA·HCl was a white solid weighing 3548.3 g (85.5% yield; 99.95% peak area, 99.64% w/w by HPLC). No single impurity exceeded 0.02% of the peak area by HPLC, and residual solvents (methanol, <6 ppm; 2-propanol, 490 ppm) were found to be within the target range.
Acknowledgments
The authors would like to thank MAPS staff and donors for fundraising and financial support. The authors would also like to thank Heather Clouting for her contribution and leadership in initiating the seminal CMC Department at MAPS PBC.
Author Contributions
L.H. and B.Y.-K. provided oversight on behalf of MAPS. K.P. and J.B.N. drafted the manuscript. All authors contributed to the critical review and final version of the manuscript. All authors have given approval to the final version of the manuscript.
This research was sponsored by the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501(c)(3) nonprofit organization. MAPS funded this study using private and foundation donations. MAPS Public Benefit Corporation (MAPS PBC), wholly owned by MAPS, was the trial organizer.
All information in this article is provided for academic and scientific purposes only.
The authors declare no competing financial interest.
References
- Stevens J.Storming Heaven; LSD and the American Dream: Paladin, 1987. [Google Scholar]
- Carhart-Harris R. L.; Goodwin G. M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. 10.1038/npp.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangini M. Treatment of alcoholism using psychedelic drugs: a review of the program of research.. J. Psychoact. Drugs 1998, 30, 381–418. 10.1080/02791072.1998.10399714. [DOI] [PubMed] [Google Scholar]
- Aday J. S.; Bloesch E. K.; Davoli C. C. 2019: A year of expansion in psychedelic research, industry, and deregulation. Drug Sci., Policy Law 2020, 6, 1–6. 10.1177/2050324520974484. [DOI] [Google Scholar]
- Nutt D. J.; King L. A.; Nichols D. E. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat. Rev. Neurosci. 2013, 14, 577–585. 10.1038/nrn3530. [DOI] [PubMed] [Google Scholar]
- Climko R. P.; Roehrich H.; Sweeney D. R.; Al-Razi J. Ecstacy: a review of MDMA and MDA. Int. J. Psychiatry Med. 1987, 16, 359–372. 10.2190/DCRP-U22M-AUMD-D84H. [DOI] [PubMed] [Google Scholar]
- Hutchinson C. MDMA-Assisted Psychotherapy for Posttraumatic Stress Disorder: Implications for Social Work Practice and Research. Clin. Soc. Work J. 2018, 25, 421–430. 10.1007/s10615-018-0676-3. [DOI] [Google Scholar]
- Mitchell J. M.; Bogenschutz M.; Lilienstein A.; Harrison C.; Kleiman S.; Parker-Guilbert K.; Ot’alora G. M.; Garas W.; Paleos C.; Gorman I.; Nicholas C.; Mithoefer M.; Carlin S.; Poulter B.; Mithoefer A.; Quevedo S.; Wells G.; Klaire S. S.; van der Kolk B.; Tzarfaty K.; Amiaz R.; Worthy R.; Shannon S.; Woolley J. D.; Marta C.; Gelfand Y.; Hapke E.; Amar S.; Wallach Y.; Brown R.; Hamilton S.; Wang J. B.; Coker A.; Matthews R.; de Boer A.; Yazar-Klosinski B.; Emerson A.; Doblin R. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021, 27, 1025–1033. 10.1038/s41591-021-01336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth A. L.; Grob C. S.; Struble C.; Feduccia A. A.; Walker N.; Jerome L.; Yazar-Klosinski B.; Emerson A. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology 2018, 235, 3137–3148. 10.1007/s00213-018-5010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa B.; Higbed L.; Nutt D. A Review of 3,4-methylenedioxy-methamphetamine (MDMA)-Assisted Psychotherapy. Front. Psych. 2019, 10, 138 10.3389/fpsyt.2019.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facts About the Current Good Manufacturing Practices (cGMPs). United States Food and Drug Administration website. Updated June 1, 2021. https://www.fda.gov/drugs/pharmaceutical-qualityresources/facts-about-current-good-manufacturing-practicescGMPs (Accessed July 15, 2021).
- Schniepp S. Applying GMPs in Stages of Development. Pharm. Technol. 2018, 42, 61–62. [Google Scholar]
- Merck E.assignee. Verfahren zur Darstellung von Alkyloxyarly-, Dialkyloxyaryl- und Alkylenedioxyarylaminopropanen bzw. deren am Stickstoff monoalkylierton Derivaten. German Patent No. DE274350C1914.
- Bernschneider R. S.; Oxler F.; Freudenmann R. W.. The origin of MDMA (‘Ecstasy’) – separating the facts from the myth. Die Pharmazie - Int. J. Pharm. Sci. 966–972.. [PubMed] [Google Scholar]
- Biniecki S.; Krajewski E. Preparation of dl- I-(3,4- mpethylenedioxyphenyl)-2-(methylamino)propane and dl-(3,4- dimethoxyphenyl)-2-(methylamino)propane. Acta Pol. Pharm. 1960, 17, 421–425. [Google Scholar]
- Shulgin A. T. The Background and Chemistry of MDMA. J. Psychoact. Drugs 1986, 18, 291–304. 10.1080/02791072.1986.10472361. [DOI] [PubMed] [Google Scholar]
- Schulze M. Synthesis of 2-Arylethylamines by the Curtius Rearrangement. Synth. Commun. 2010, 40, 1461–1476. 10.1080/00397910903097302. [DOI] [Google Scholar]
- Dunlap L. E.; Andrews A. M.; Olson D. E. Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine. ACS Chem. Neurosci. 2018, 9, 2408–2427. 10.1021/acschemneuro.8b00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather E.; Shimmon R.; McDonagh A. M. Organic impurity profiling of 3,4-methylenedioxymethamphetamine (MDMA) synthesised from catechol. Forensic Sci. Int. 2015, 248, 140–147. 10.1016/j.forsciint.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Renton R. J.; Cowie J. S.; Oon M. C. A study of the precursors, intermediates and reaction by-products in the synthesis of 3,4-methylenedioxymethylamphetamine and its application to forensic drug analysis. Forensic Sci. Int. 1993, 60, 189–202. 10.1016/0379-0738(93)90238-6. [DOI] [PubMed] [Google Scholar]
- Plummer C. M.; Breadon T. W.; Pearson J. R.; Jones O. A. H. The synthesis and characterisation of MDMA derived from a catalytic oxidation of material isolated from black pepper reveals potential route specific impurities. Sci. Justice 2016, 56, 223–230. 10.1016/j.scijus.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Chambers S. A.; DeSousa J. M.; Huseman E. D.; Townsend S. D. The DARK Side of Total Synthesis: Strategies and Tactics in Psychoactive Drug Production. ACS Chem. Neurosci. 2018, 9, 2307–2330. 10.1021/acschemneuro.7b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalasinsky K. S.; Hugel J.; Kish S. J. Use of MDA (the ″love drug″) and methamphetamine in Toronto by unsuspecting users of ecstasy (MDMA). J. Forensic Sci. 2004, 49, 1106–1112. 10.1520/JFS2003401. [DOI] [PubMed] [Google Scholar]
- Yazar-Klosinski B. B.; Mithoefer M. C. Potential Psychiatric Uses for MDMA. Clin. Pharmacol. Ther. 2017, 101, 194–196. 10.1002/cpt.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Q3C—Tables and List Guidance for Industry (Revision 3), pages 6–7. Published June 2017, Accessed July 6, 2021. https://www.fda.gov/media/71737/download.
- Song H. Y.; Yang J. Y.; Suh J. W.; Lee H. S. Acaricidal Activities of Apiol and Its Derivatives from Petroselinum sativum Seeds against Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Tyrophagus putrescentiae. J. Agric. Food Chem. 2011, 59, 7759–7764. 10.1021/jf201945y. [DOI] [PubMed] [Google Scholar]
- Singh P.; Berlinguet L. The synthesis of some derivatives of 3,4-methylenedioxybenzene. Can. J. Chem. 1964, 42, 1901–1905. 10.1139/v64-282. [DOI] [Google Scholar]
- Paterson D. L.; Barker D. Synthesis of the furo[2,3-b]chromene ring system of hyperaspindols A and B. Beilstein J. Org. Chem. 2015, 11, 265–270. 10.3762/bjoc.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. Mechanisms of Inhibitory and Regulatory Effects of Methylenedioxyphenyl Compounds on Cytochrome P450-Dependent Drug Oxidation. Curr. Drug Metab. 2000, 1, 67–84. 10.2174/1389200003339270. [DOI] [PubMed] [Google Scholar]
- Huot P.; Johnston T. H.; Lewis K. D.; Koprich J. B.; Reyes M. G.; Fox S. H.; Piggott M. J.; Brotchie J. M. Characterization of 3,4-Methylenedioxymethamphetamine (MDMA) Enantiomers In Vitro and in the MPTP-Lesioned Primate: R-MDMA Reduces Severity of Dyskinesia, Whereas S-MDMA Extends Duration of ON-Time. J. Neurosci. 2011, 31, 7190–7198. 10.1523/JNEUROSCI.1171-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psycho Chemist (pseudonym). Synthesis of Safrole. Erowidwebsite. https://erowid.org/archive/rhodium/chemistry/safrole.html (Accessed July 10, 2021).
- Alam M.; Wise C.; Baxter C. A.; Cleator E.; Walkinshaw A. Development of a Robust Procedure for the Copper-catalyzed Ring-Opening of Epoxides with Grignard Reagents. Org. Process Res. Dev. 2012, 16, 435–441. 10.1021/op200329x. [DOI] [Google Scholar]