Abstract
The nucleotides coding for the extracellular part of the G glycoprotein and the full SH protein of bovine respiratory syncytial virus (BRSV) were sequenced from viruses isolated from numerous outbreaks of BRSV infection. The isolates included viruses isolated from the same herd (closed dairy farms and veal calf production units) in different years and from all confirmed outbreaks in Denmark within a short period. The results showed that identical viruses were isolated within a herd during outbreaks and that viruses from recurrent infections varied by up to 11% in sequence even in closed herds. It is possible that a quasispecies variant swarm of BRSV persisted in some of the calves in each herd and that a new and different highly fit virus type (master and consensus sequence) became dominant and spread from a single animal in connection with each new outbreak. Based on the high level of diversity, however, the most likely explanation was that BRSV was (re)introduced into the herd prior to each new outbreak. These findings are highly relevant for the understanding of the transmission patterns of BRSV among calves and human respiratory syncytial virus among humans.
Bovine respiratory syncytial virus (BRSV) and human respiratory syncytial virus (HRSV) are classified in the Pneumovirinae subfamily of Paramyxoviridae (22). BRSV and HRSV are the major cause of respiratory disease in younger calves and children, respectively (6, 20). In temperate climates, most outbreaks of BRSV infection occur in the winter season, and even self-supplementary (closed) dairy herds experience recurrent infection (32). It is not known how Pneumoviridae viruses survive between outbreaks, and the mode(s) of transmission during the course of natural infection has not been definitively defined. It is generally believed that close contact is required (14), albeit transmission of BRSV by air may be possible (21).
BRSV and HRSV have been classified into several distinct genetic linkages based on sequence variability of the major glycoprotein (G protein). In humans, epidemiological studies have shown that multiple different HRSV strains can cocirculate in the same community during the same season and that the relative frequency of their isolation shifts from year to year (2, 16). Furthermore, very similar genotypes have been isolated in distant places in different years (13). The intensiveness of global travel, however, makes it difficult to assess the transmission patterns of HRSV on the basis of epidemiological data. Thus, in contrast to the situation in Europe and North America, very similar genotypes have been identified in two different years in a community in Cuba, which has very restricted travel both to and from and within the island (31).
The variability among BRSV isolates is considerably less than that among HRSV isolates, but separate linkages have been defined by mapping studies and sequencing of the G protein (12, 20). In contrast to HRSV, however, the epidemiological patterns of circulating BRSVs involved in recurrent outbreaks have to our knowledge not previously been characterized. The Danish cattle herds provide a very suitable population for assessment of the epidemiological patterns of BRSV, since Denmark has a relatively closed national population of cattle due to a very limited import of live animals. The aim of the present study was therefore to describe the epidemiological patterns of BRSVs isolated during recurrent outbreaks. This was achieved by DNA sequence analysis of the genes coding for the G protein and the SH protein of a large collection of BRSV isolates. BRSV specific RNA for direct nucleotide sequencing was obtained from lung tissue submitted to the Danish Veterinary Laboratory for diagnostic purposes between 1988 and 1999. Figure 1 details the numbering, geographic origin, date, and herd type of the samples. Total RNA was isolated directly from lung tissue, cDNAs were synthesized, and the nucleotides coding for the extracellular part of the G protein (nucleotides 210 to 660) were PCR amplified using AmpliTaq DNA polymerase and primers as previously described (20). Similarly, the full SH gene was amplified using primers situated in the M and G genes (the primer sequences and PCR conditions can be obtained from the authors on request). The nucleotide sequences of the PCR products were determined by cycle sequencing using an AmpliTaq FS dye terminator kit and analyzed on a 373A automatic sequencer (Applied Biosystems/Perkin-Elmer Cetus) as previously described (20). Consensus DNA sequences were generated using the ABI Prism Sequence Navigator program. DNA sequence alignment was performed using the Clustal W program (30). The program GeneDoc (23) was used to calculate the number of nucleotide differences and percent homology. The phylogenic analyses were performed by the use of the program Puzzle, version 4.0.2. (28), using the maximum likelihood method (11). The minimum length trees were found by branch and bound search.
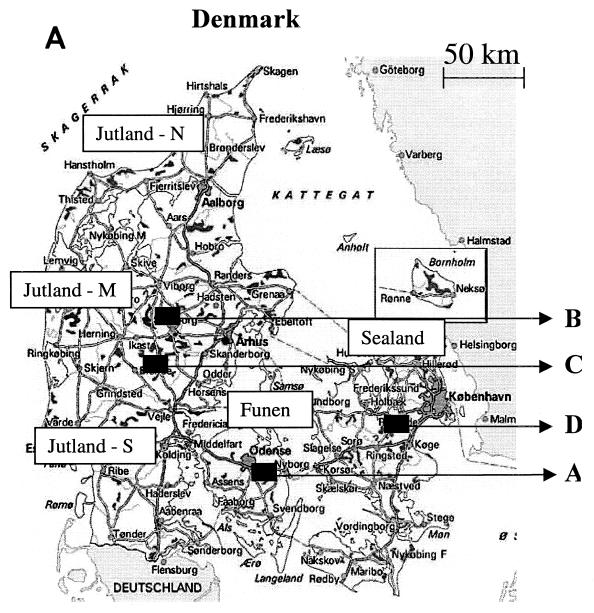
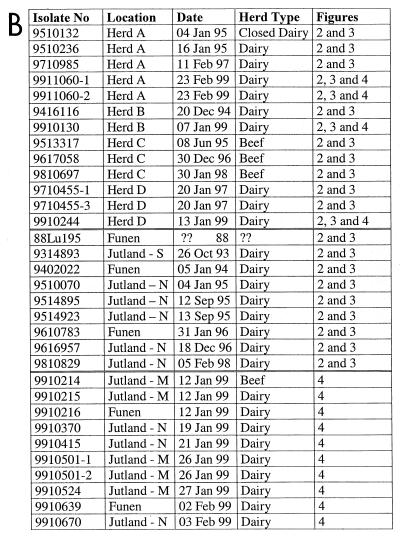
FIG. 1.
(A) Map of Denmark. The arrows indicate herds (A to D) from which BRSV was isolated during recurrent outbreaks. The different regions of Denmark are indicated as north (N), mid- (M), or south (S) Jutland; Funen; and Sealand. (B) All isolates included in the phylogenetic analysis. The first two digits of the isolate name indicate the year of isolation; the location indicates either herd designation or geographic location illustrated in panel A. Finally, the herd types and references to the phylogenetic tree(s) (figure number) in which the isolates are included are also listed.
To ensure that the experimental protocol generated sequence data reflecting the dominant consensus sequence, the level of sequence variability present in a single RNA preparation was assessed. Five separate cDNA preparations were generated from RNA extracted from each of two samples (herd A, 9510132 and 9911060-1). Three separate PCR runs (G protein) were then performed on each of these cDNA products followed by DNA sequencing of all 30 PCR products. In addition, a single PCR product from each of the same two isolates was cloned into the TA cloning vector (Invitrogen) and transfected into Escherichia coli according to the manufacturer's protocol. Five different clones, with inserts, from each of the two isolates were then sequenced. The DNA sequences generated for each of the two isolates were identical, apart from a single nucleotide difference in one of the clones (data not shown). These results revealed that the sequence obtained based on a single reaction represented the consensus sequence of the viral RNA pool (17). Furthermore, these results suggested a limited level of population diversity (quasispecies) of BRSV compared to other single-stranded-RNA viruses (9, 15, 25). It should be emphasized, however, that the present assessment was performed on viruses presumably isolated during the early phase of infection, at which time the mutant spectrum is limited (17). On the other hand, a prior bottleneck passage was avoided because the assessment was performed on virus RNA isolated directly from tissue without previous passage in cell culture.
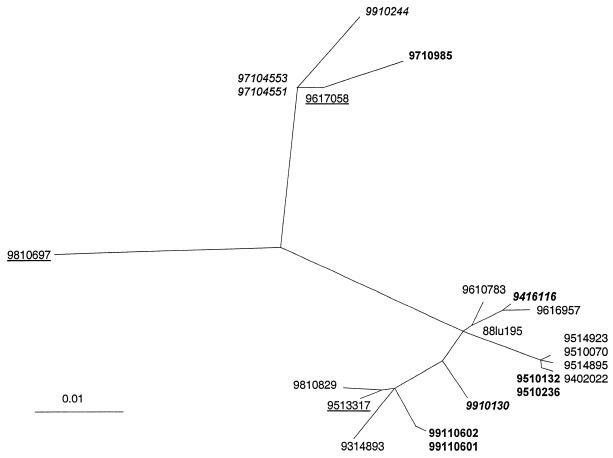
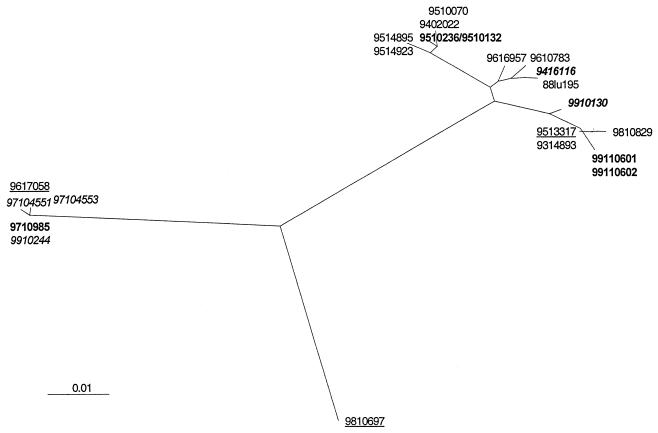
We have previously shown that there was no difference between phylogenetic trees based on the full G gene and those based on the extracellular part of the G protein (20). Nevertheless, it is possible that selection pressure on the G protein may influence the phylogenetic affiliation of the different isolates. Therefore, the full SH gene in addition to the G gene was analyzed on the same isolates. Almost identical trees were generated based on the G and SH genes, albeit some minor differences in the branching did exist (Fig. 2 and 3). Thus, the affiliations of the isolates with the major branches seemed to provide a valid reflection of the relatedness between the various isolates.
FIG. 2.
Phylogenetic tree based on the gene coding for the SH protein and describing the relationships among 22 BRSV isolates amplified from samples collected in Danish herds between 1988 and 1999. Branch lengths indicate the phylogenetic distances. The isolates from recurrent infections in herds A to D are indicated by boldface (herd A), boldface italics (herd B), underlining (herd C), and italics (herd D).
FIG. 3.
Phylogenetic tree based on the gene coding for the extracellular part of the G protein and describing the relationships among 22 BRSV isolates amplified from samples collected in Danish herds between 1988 and 1999. Branch lengths indicate the phylogenetic distances. The isolates from recurrent infections in herds A to D are indicated by boldface (herd A), boldface italics (herd B), underlining (herd C), and italics (herd D).
The variability of the full SH gene and the coding part of the SH gene was between 1 and 13% and 0 and 11%, respectively. The analyzed extracellular part of the G protein among the same isolates varied between 0 and 12%. These results confirm previous findings that the variability among the SH protein of global BRSV isolates is similar to the variability of the G protein (1). In contrast, the G protein of HRSV is far more variable than the SH protein (7, 18). The phylogenetic analysis also revealed that viruses isolated in Denmark during a period of 12 years formed a tree with several distinct branches and apparently without any relationship between the time of isolation and the clustering of isolates. Thus, these results confirmed the idea that several different distinct linkages of BRSV cocirculate in the field as previously reported for BRSV (20, 26) and HRSV (2, 24). In five herds, identical viruses were obtained up to 2 weeks apart (9510132/9510236, 9911060-1/-2, 9514895/9514923, 9710455-1/-3, and 9910502-1/-2 [Fig. 1]). This suggested that, on a herd basis, each BRSV outbreak was monotypic, i.e., initiated by a single virus type of apparently high fitness. This is in line with a recently published study on the epidemiological patterns of another single-stranded-RNA virus, bovine viral diarrhea virus (34). These results are also in accordance with previous findings that cell culture adaptation and passages in cells and/or calves did not induce extensive changes in the G gene of BRSV (20).
As mentioned, the mode(s) of transmission of BRSV from season to season within a herd has not been identified, but several findings support the view that BRSV survives either by herd persistence (continuous transfer of the virus between cows) or by persistence in individual cows (8, 32). HRSV and BRSV have been shown previously to persistently infect cells in vitro (4). HRSV has also been detected in the lungs of guinea pigs and mice 6 weeks and 100 days after experimental infection, respectively (27; P. J. Openshaw, personal communication). Furthermore, in an earlier study, acute BRSV infection was diagnosed in calves aged 2 to 6 months, which had been kept isolated since 10 days after birth (29). Despite these strong indications of persistence, attempts to reactivate Pneumovirus by treatment with immunosuppressive drugs have failed (19, 33). Alternatively, the virus may survive in reservoir hosts or in the external environment between seasons and then be transmitted iatrogenically (14) or by undefined carriers between herds in connection with outbreaks.
The presence of circulating distinguishable clusters of BRSV types in Denmark combined with the monotypic characteristics of viruses isolated during individual outbreaks presented the option of investigating whether an outbreak was due to persistence or to introduction from external sources. Therefore, the DNA sequence (G and SH genes) of the dominant virus type within a herd isolated in different years from each of four herds was included in the phylogenic analysis (herds A to D) (Fig. 1 to 3). In herd A, viruses from three different years (January 1995, February 1997, and February 1999) were situated on different branches. Similarly, viruses isolated from dairy herd B in 1994 and 1999 and from beef herd C in 1995, 1996, and 1998 clustered in distinctly different branches. In these herds, both the G and SH gene sequences varied by 3 to 11% among viruses from the same herd. In herd D, the G gene varied by only one nucleotide between viruses isolated in 1997 and 1999, while there were 10 nucleotide differences (3%) between the SH genes from viruses isolated in those years.
It is possible, though, that a quasispecies variant swarm of BRSV persisted in some of the calves in each herd and that a different highly fit virus type (master sequence) developed (15, 17). A sudden shift in master (and consensus) sequences is typically driven by positive selection pressure and often involves transmission of small virus populations or even a single virus particle (17). Thus, such genetic bottleneck transmissions of BRSV may take place when an immunologically naive calf first comes into contact with an older persistently infected animal. The older animal may previously have been infected by another type of BRSV, but the dominant virus type (master and consensus sequence) has changed, presumably due to selection pressure elicited by the immune system. In contrast, the massive virus transmissions characterizing acute (monotypic) outbreaks within a herd do not permit sudden changes in consensus sequence but instead should lead to selection of the most fit virus (10). This persistence-bottleneck theory combined with the high level of diversity (up to 11%) between viruses isolated from the same herd in different years would imply that the viable quasispecies population of BRSV is unusually heterogeneous. Accordingly, conventional phylogenetic trees cannot be used for assessment of epidemiological patterns of BRSV if this hypothesis is correct. A less controversial, and in our opinion more likely, interpretation is that the recurrent outbreaks were caused by different BRSV types introduced into herds A, B, C, and (probably) D prior to each new outbreak. This is interesting, since dairy herd A has been maintained as self-supplementary (closed) in the sense that no animals have been introduced from external sources since 1995. Also, herds B and D had had limited purchase of new cattle, whereas calves were introduced continuously into herd C (a beef herd). Thus, this explanation does not account for the source of the virus.
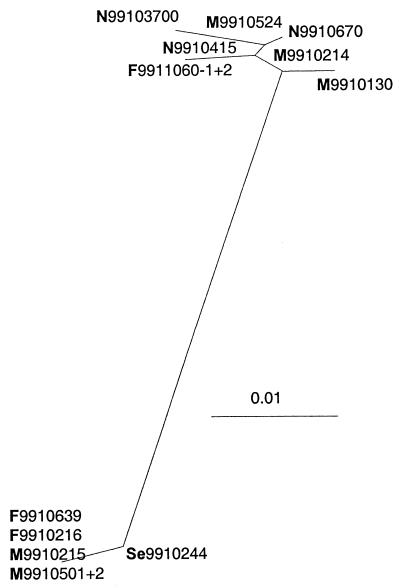
BRSV infections often occur as epidemic-like outbreaks one or (infrequently) two times during the winter (5). This may be explained by simultaneously (re)activation of the virus in individual persistently infected calves in several herds, possible triggered by factors such as sudden changes in outdoor temperature (3). Alternatively, these outbreaks may be caused by rapid horizontal spread of the virus from one or a few infected herds. Denmark experienced one of these major BRSV “epidemics” in January and February 1999. To characterize the epidemiological patterns of the virus types involved in this outbreak, the gene coding for the external part of the G protein was sequenced from viruses collected between 5 January and 22 February from 12 different herds from all parts of Denmark. As illustrated in Fig. 4, these isolates clustered in two distinct parts of a phylogenetic tree. The sequences within a cluster varied by 0 to 2% (nucleotide), whereas the variability of isolates between the two clusters varied by 9 to 11% (nucleotide). This indicated that the outbreak was initiated by at least two different circulating field types. There was no conclusive relationship—in time or geography—between isolates within a cluster. It was, however, interesting that isolates 9910215 and 9910639 were identical, since these two isolates were collected 3 weeks apart from herds situated less than 10 km apart. Likewise, the identical isolates 9510070 and 9514895/9514923 were collected half a year apart from two herds separated by less than 20 km (Fig. 2 and 3). This is in contrast to the pattern in herds A to D and may be coincidental but may also indicate either that the virus persisted in individual calves or that it was maintained in carriers or in the external environment.
FIG. 4.
Phylogenic tree based on the gene coding for the extracellular part of the G protein and describing the relationships among 12 BRSV isolates amplified from samples collected in Danish herds between 5 January and 22 February 1999 during a single epidemic of BRSV infection. Branch lengths indicate the phylogenetic distances. The geographic regions, referring to the map in Fig. 1, are indicated as follows: N, M, and S, northern, mid-, and south Jutland, respectively; F, Funen; and Se, Sealand.
In summary, to our knowledge the present study represents the first attempt to characterize BRSVs isolated during recurrent outbreaks by the use of DNA sequence data. The results showed that identical viruses were isolated within a herd during outbreaks and that viruses from recurrent infections varied by up to 11% even in closed herds. These findings are highly relevant for the understanding of the transmission of BRSV among calves and should also be considered when epidemiological data concerning HRSV transmission among humans are evaluated.
Nucleotide sequence accession number.
The GenBank accession numbers of the sequences reported in the present study are U92100, U92102, U92104 to U92107, AF248561 to AF248582, and AF248585 to AF248610.
Acknowledgments
The excellent technical assistance of Ivan Larsen, Flemming Jacobsen, Susanne Grell, and Jannie Pedersen is gratefully acknowledged.
The study was supported in part by grants from the Danish Ministry of Food, Agriculture and Fisheries and the Danish Research Centre for the Management of Animal Production and Health (CEPROS).
REFERENCES
- 1.Anderson K, King A M, Lerch R A, Wertz G W. Polylactosaminoglycan modification of the respiratory syncytial virus small hydrophobic (SH) protein: a conserved feature among human and bovine respiratory syncytial viruses. Virology. 1992;191:417–430. doi: 10.1016/0042-6822(92)90203-2. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L J, Hendry R M, Pierik L T, Tsou C, McIntosh K. Multicenter study of strains of respiratory syncytial virus. J Infect Dis. 1991;163:687–692. doi: 10.1093/infdis/163.4.687. [DOI] [PubMed] [Google Scholar]
- 3.Baker J C, Werdin R E, Ames T R, Markham R J F, Larson V L. Study on the etiologic role of bovine respiratory syncytial virus in pneumonia of dairy calves. J Am Vet Med Assoc. 1986;189:66–70. [PubMed] [Google Scholar]
- 4.Baldridge P, Senterfit L B. Persistent infection of cells in culture by respiratory syncytial virus. Proc Soc Exp Biol Med. 1976;151:684–688. doi: 10.3181/00379727-151-39286. [DOI] [PubMed] [Google Scholar]
- 5.Bryson D G, McFerran J B, Ball H J, Neill S D. Observations on outbreaks of respiratory disease in housed calves. (1) Epidemiological, clinical and microbiological findings. Vet Rec. 1978;103:485–489. doi: 10.1136/vr.103.22.485. [DOI] [PubMed] [Google Scholar]
- 6.Collins P L, McIntosh K, Chanock R M. Pneumovirinae. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 7.Collins P L, Olmsted R A, Johnson P R. The small hydrophobic protein of human respiratory syncytial virus: comparison between antigenic subgroups A and B. J Gen Virol. 1990;71:1571–1576. doi: 10.1099/0022-1317-71-7-1571. [DOI] [PubMed] [Google Scholar]
- 8.De Jong M C, Van der Poel W H, Kramps J A, Brand A, Van Oirschot J T. Quantitative investigation of population persistence and recurrent outbreaks of bovine respiratory syncytial virus on dairy farms. Am J Vet Res. 1996;57:628–633. [PubMed] [Google Scholar]
- 9.Domingo E, Escarmis C, Martinez M A, Martinez-Salas E, Mateu M G. Foot-and-mouth disease virus populations are quasispecies. Curr Top Microbiol Immunol. 1992;176:33–47. doi: 10.1007/978-3-642-77011-1_3. [DOI] [PubMed] [Google Scholar]
- 10.Domingo E, Holland J J. RNA virus mutations and fitness for survival. Annu Rev Microbiol. 1997;51:151–178. doi: 10.1146/annurev.micro.51.1.151. [DOI] [PubMed] [Google Scholar]
- 11.Fitch W M. Toward defining the course of evolution: minimal change for a specific tree topology. Syst Zool. 1971;20:406–416. [Google Scholar]
- 12.Furze J, Wertz G, Lerch R, Taylor G. Antigenic heterogeneity of the attachment protein of bovine respiratory syncytial virus. J Gen Virol. 1994;75:363–370. doi: 10.1099/0022-1317-75-2-363. [DOI] [PubMed] [Google Scholar]
- 13.Garcia O, Martin M, Dopazo J, Arbiza J, Frabasile S, Russi J, Hortal M, Perez Brena P, Martinez I, Garcia Barreno B. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J Virol. 1994;68:5448–5459. doi: 10.1128/jvi.68.9.5448-5459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall C B, Douglas R G J. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 15.Hedges J F, Balasuriya U B, Timoney P J, McCollum W H, MacLachlan N J. Genetic divergence with emergence of novel phenotypic variants of equine arteritis virus during persistent infection of stallions. J Virol. 1999;73:3672–3681. doi: 10.1128/jvi.73.5.3672-3681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendry R M, Pierik L T, McIntosh K. Prevalence of respiratory syncytial virus subgroups over six consecutive outbreaks: 1981–1987. J Infect Dis. 1989;160:185–190. doi: 10.1093/infdis/160.2.185. [DOI] [PubMed] [Google Scholar]
- 17.Holland J J, De La Torre J C, Steinhauer D A. RNA virus populations as quasispecies. Curr Top Microbiol Immunol. 1992;176:1–20. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- 18.Johnson P R, Jr, Olmsted R A, Prince G A, Murphy B R, Alling D W, Walsh E E, Collins P L. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987;61:3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khehra R S, Jones R C. Investigation into avian pneumovirus persistence in poults and chicks using cyclosporin A immunosuppression. Res Vet Sci. 1999;66:161–163. doi: 10.1053/rvsc.1998.0257. [DOI] [PubMed] [Google Scholar]
- 20.Larsen L E, Uttenthal A, Arctander P, Tjørnehøj K, Viuff B, Røntved C, Rønsholt L, Alexandersen S, Blixenkrone-Møller M. Serological and genetic characterisation of bovine respiratory syncytial virus (BRSV) indicates that Danish isolates belong to the intermediate subgroup: no evidence of a selective effect on the variability of G protein nucleotide sequence by prior cell culture adaption and passages. Vet Microbiol. 1998;62:265–279. doi: 10.1016/s0378-1135(98)00226-0. [DOI] [PubMed] [Google Scholar]
- 21.Mars M H, Bruschke C J, Van Oirschot J T. Airborne transmission of BHV1, BRSV, and BVDV among cattle is possible under experimental conditions. Vet Microbiol. 1999;66:197–207. doi: 10.1016/s0378-1135(99)00009-7. [DOI] [PubMed] [Google Scholar]
- 22.Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy. Sixth report of the International Committee on Taxonomy of Viruses. Arch Virol. 1995;1995(Suppl. 10):1–586. [Google Scholar]
- 23.Nicholas K B, Nicholas H B J. GeneDoc: analysis and visualization of genetic variation. EMBV News. 1997;4:14. [Google Scholar]
- 24.Peret T C, Hall C B, Schnabel K C, Golub J A, Anderson L J. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79:2221–2229. doi: 10.1099/0022-1317-79-9-2221. [DOI] [PubMed] [Google Scholar]
- 25.Radford A D, Turner P C, Bennett M, McArdle F, Dawson S, Glenn M A, Williams R A, Gaskell R M. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats. J Gen Virol. 1998;79:1–10. doi: 10.1099/0022-1317-79-1-1. [DOI] [PubMed] [Google Scholar]
- 26.Stine L C, Hoppe D K, Kelling C L. Sequence conservation in the attachment glycoprotein and antigenic diversity among bovine respiratory syncytial virus isolates. Vet Microbiol. 1997;54:201–221. doi: 10.1016/s0378-1135(96)01288-6. [DOI] [PubMed] [Google Scholar]
- 27.Streckert H J, Philippou S, Riedel F. Detection of respiratory syncytial virus (RSV) antigen in the lungs of guinea pigs 6 weeks after experimental infection and despite the production of neutralizing antibodies. Arch Virol. 1996;141:401–410. doi: 10.1007/BF01718305. [DOI] [PubMed] [Google Scholar]
- 28.Strimmer K, von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 2000;13:964–969. [Google Scholar]
- 29.Thomas L H, Stott E J, Jones P W, Jebbett N J, Collins A P. The possible role of respiratory syncytial virus and Pasteurella spp. in calf respiratory disease. Vet Rec. 1980;107:304–307. doi: 10.1136/vr.107.13.304. [DOI] [PubMed] [Google Scholar]
- 30.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valdes O, Martinez I, Valdivia A, Cancio R, Savon C, Goyenechea A, Melero J A. Unusual antigenic and genetic characteristics of human respiratory syncytial viruses isolated in Cuba. J Virol. 1998;72:7589–7592. doi: 10.1128/jvi.72.9.7589-7592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Poel W H, Kramps J A, Middel W G, Van Oirschot J T, Brand A. Dynamics of bovine respiratory syncytial virus infections: a longitudinal epidemiological study in dairy herds. Arch Virol. 1993;133:309–321. doi: 10.1007/BF01313771. [DOI] [PubMed] [Google Scholar]
- 33.Van der Poel W H, Langedijk J P, Kramps J A, Middel W G, Brand A, Van Oirschot J T. Serological indication for persistence of bovine respiratory syncytial virus in cattle and attempts to detect the virus. Arch Virol. 1997;142:1681–1696. doi: 10.1007/s007050050189. [DOI] [PubMed] [Google Scholar]
- 34.Vilcek S, Alenius S, Paton D J, Mittelholzer C, Belak S. Genetic clustering of bovine viral diarrhoea viruses in cattle farms: genetic identification and analysis of viruses directly from cattle sera. Vet J. 1999;158:33–38. doi: 10.1053/tvjl.1999.0363. [DOI] [PubMed] [Google Scholar]