Abstract
The Oxoid combination disk method for detecting extended-spectrum β-lactamases (ESBLs) depends on comparing the inhibition zones of cefpodoxime (10-μg) and cefpodoxime-plus-clavulanate (10- plus 1-μg) disks. The presence of clavulanate enlarged the zones for all of 180 ESBL-producing klebsiellae by ≥5 mm, whereas zones for cefpodoxime-susceptible isolates and cefpodoxime-resistant isolates with AmpC and K1 β-lactamases were enlarged by ≤1 mm. Good discrimination was achieved with either the NCCLS or the British disk method.
Extended-spectrum β-lactamases (ESBLs) are increasing sources of resistance to oxyimino-aminothiazolyl cephalosporins, especially in klebsiellae (1, 9). Most ESBLs are mutant TEM and SHV enzymes, but a few have different ancestries (4). Laboratory detection can be problematic, because some ESBLs do not confer obvious resistance to all their substrates in vitro and up to 35% of ESBL producers continue to be reported as susceptible to cefotaxime and ceftriaxone in Europe (1, 9). Accurate detection nevertheless is important because clinical failures arise even when the MICs of cephalosporins for ESBL producers are only 1 μg/ml (3, 13). Two detection strategies are in common use: (i) using ceftazidime or cefpodoxime as an indicator drug, and considering klebsiellae with reduced susceptibility to these drugs to be resistant to all oxyimino-aminothiazolyl cephalosporins (12) or (ii) screening for synergy between extended-spectrum cephalosporins and clavulanic acid. Synergy can be detected by double-disk tests (8), although the optimum separation of the disks is strain variable. Alternatively, commercial systems, such as Etests (5, 7) and Vitek (14), can be used. One obvious strategy is to compare the inhibition zones of cephalosporin disks with and without clavulanate added. This method has been used by several researchers (see, e.g., reference 10), and comparison of the zones given by cefotaxime (30-μg) and ceftazidime (30-μg) disks with or without clavulanate (10 μg) added is now advocated by the NCCLS (12). Such disks are available from several suppliers (Becton Dickinson, MAST, and Oxoid), and an evaluation was published recently (11). Cefpodoxime also seems an appropriate partner agent for clavulanate in such tests, since cefpodoxime itself is useful in ESBL screening tests. Accordingly, a cefpodoxime-based combination disk method has been developed (Oxoid, Basingstoke, Hampshire, United Kingdom) based on comparing the zones of cefpodoxime (10-μg) and cefpodoxime-plus-clavulanate (10- plus 1-μg) disks. We examined whether these disks reliably detected ESBL-positive klebsiellae and distinguished them from strains with AmpC enzymes and from Klebsiella oxytoca strains that hyperproduce chromosomal K1 (KOXY) β-lactamase.
Bacteria.
The bacteria tested comprised Klebsiella pneumoniae and K. oxytoca collected from intensive-care unit (ICU) patients in multicenter surveys covering Western and Southern Europe in 1994 and in 1997 to 1998 (1, 9). One hundred eighty ESBL producers were included, selected on the basis of ceftazidime/ceftazidime-plus-clavulanate MIC ratios of ≥16 by agar dilution. The ESBLs produced by these isolates were partially identified previously by isoelectric focusing and PCR–single-strand conformation polymorphism for SHV types (15, 16), or by isoelectric focusing and PCR-restriction fragment length polymorphism for TEM types (16). Genes for a few of these ESBLs were sequenced (16). Molecular studies invariably detected an ESBL gene when the presence of an ESBL enzyme had been inferred from synergy between ceftazidime and clavulanate (15, 16). Also included were 19 K. oxytoca isolates that hyperproduced their K1 chromosomal β-lactamase, as demonstrated by antibiogram and kinetic studies (6), and 5 klebsiellae deduced to have AmpC β-lactamases on the basis of synergy between ceftazidime and the monobactam Ro 48-1256 (9). Both of the latter groups of isolates gave ceftazidime/ceftazidime-plus-clavulanate MIC ratios of ≤4 by agar dilution (9). Control strains, which were randomly selected from the same survey collections (1, 9), comprised 25 isolates each of K. oxytoca and K. pneumoniae that lacked ESBLs or other potent β-lactamases. These organisms were highly susceptible (MICs, ≤2 μg/ml) to cefotaxime, ceftazidime, and cefpodoxime, and they gave ceftazidime/ceftazidime-plus-clavulanate MIC ratios of ≤4 by agar dilution (9).
Disk test methods.
The isolates were tested by the Oxoid combination disk method for ESBL Detection, which depends on comparing the inhibition zones given by cefpodoxime (CPD) (10-μg) and cefpodoxime-plus-clavulanate (CD01) (10- plus 1-μg) disks. Ceftazidime (CAZ) (30-μg) disks (Oxoid) were tested in parallel as a control (8). The disk tests were performed (i) with confluent growth on Mueller-Hinton agar (Oxoid), in accordance with the NCCLS recommendations for nonfastidious bacteria (12), and (ii) with semiconfluent growth on Iso-Sensitest agar (Oxoid), in accordance with the recommendations of the British Society for Antimicrobial Chemotherapy (BSAC Standardized Disc Sensitivity Testing Method, Newsletter of the BSAC, Summer 1999). Zone diameters were measured to the nearest millimeter. A difference of ≥5 mm between the zones of the CD01 (10- plus 1-μg) and CPD (10-μg) disks was taken to indicate ESBL production, as advocated by the manufacturer. The zones of the CAZ (30-μg) and CPD (10-μg) disks additionally were reviewed against the susceptibility criteria of the NCCLS and BSAC, and against NCCLS criteria for predicting ESBL production (12).
Inhibition zones for ESBL producers and susceptible controls.
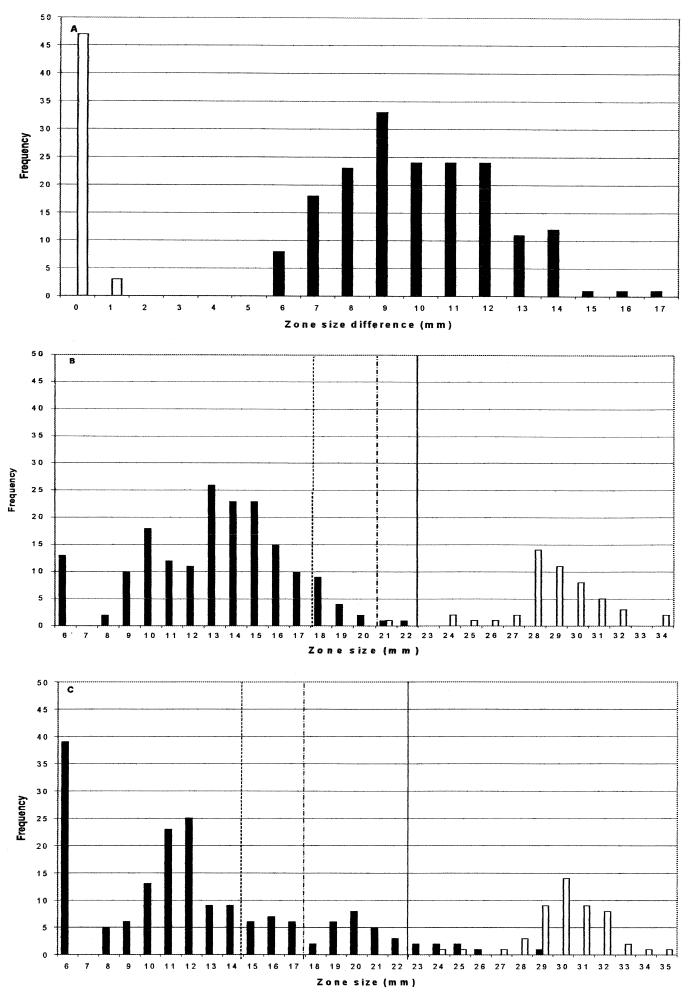
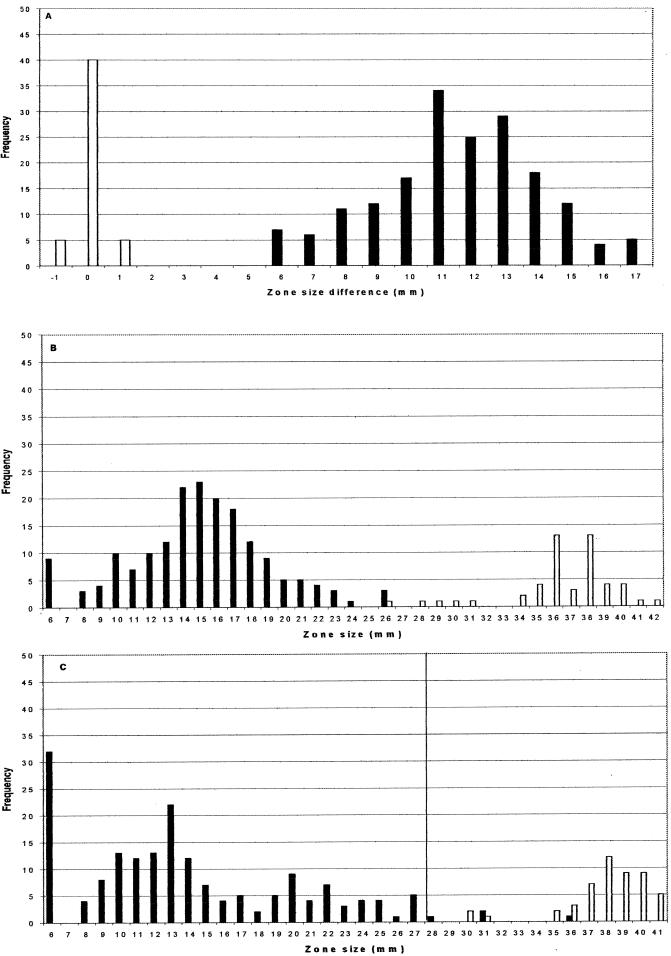
The distributions of inhibition zone differences (the CD01 [10- plus 1-μg] zone minus the CPD [10-μg] zone) for the ESBL producers and ESBL-negative controls by NCCLS methodology are shown in Fig. 1A; those obtained by BSAC methods are shown in Fig. 2A. Regardless of which method was used, all 180 ESBL producers gave zones at least 5 mm larger with the CD01 (10- plus 1-μg) disks than with the CPD (10-μg) disks. The differences in zone diameters between the clavulanate-containing and non-clavulanate-containing disks were larger by use of the BSAC methodology (mean difference, 11.6 mm; standard deviation, 7.8 mm) than by use of the NCCLS method (mean difference, 10.0 mm; standard deviation, 4.2 mm). None of the 50 ESBL-negative control isolates gave a difference in zone diameter of more than 1 mm between the two disk types by either method.
FIG. 1.
Zone distributions by NCCLS methodology. (A) Distribution of inhibition zone differences (CD01 zone minus CPD zone) for ESBL producers (solid bars) and non-ESBL producers (open bars). (B and C) Zone diameters of CPD (10-μg) disks and CAZ (30-μg) disks, respectively, for ESBL producers (solid bars) and non-ESBL producers (open bars). Zone diameters indicating resistance, intermediate resistance, and possible ESBL positivity according to the NCCLS are situated to the left of the dashed, dotted-and-dashed, and solid vertical lines, respectively.
FIG. 2.
Zone distributions by BSAC methodology. (A) Distribution of inhibition zone differences (CD01 zone minus CPD zone) for ESBL producers (solid bars) and non-ESBL producers (open bars). (B and C) Zone diameters of CPD (10-μg) disks and CAZ (30-μg) disks, respectively, for ESBL producers (solid bars) and non-ESBL producers (open bars). Zone diameters indicating resistance according to the BSAC are situated to the left of the solid vertical line.
The zones of CPD (10-μg) disks for ESBL producers and non-ESBL producers were better differentiated than those of CAZ (30-μg) disks, irrespective of whether NCCLS or BSAC methodology was used (compare Fig. 1B and C with Fig. 2B and C). This was so despite the fact that isolates had been categorized initially on the basis of synergy tests between ceftazidime and clavulanate. The NCCLS criterion (12) of predicting ESBL production from a zone of ≤22 mm for a CPD (10-μg) disk correctly identified all the producers (Fig. 1B), at the expense of mistakenly inferring production in 1 of 50 susceptible controls. The alternative criterion of inferring production from a zone of ≤22 mm for a CAZ (30-μg) disk identified 172 of 180 ESBL producers, without any false positives among the 50 controls (Fig. 1C). The NCCLS susceptibility criteria categorized 2 of 180 ESBL producers as cefpodoxime susceptible, and a further 15 as intermediate; NCCLS criteria also categorized 32 of 180 ESBL producers as ceftazidime susceptible and a further 19 as intermediate. The BSAC resistance breakpoint (≤27 mm) categorized 4 of 180 ESBL producers as susceptible to ceftazidime, with no nonproducers categorized as resistant. The Society makes no recommendation for CPD (10-μg) disks against Enterobacteriaceae, as 5-μg disks are routinely used for susceptibility testing with cefpodoxime in the United Kingdom.
Behavior of AmpC producers and of K. oxytoca strains hyperproducing K1 β-lactamase.
All five AmpC producers gave no zones with CPD (10-μg) disks by NCCLS methodology and zones no larger than 9 mm by the BSAC method. Zones for the CD01 (10- plus 1-μg) disks were within 1 mm of those for the CPD (10-μg) disks for these strains, regardless of whether NCCLS or BSAC methods were used. Zones with CAZ (30-μg) disks for the five AmpC producers ranged from 6 to 13 mm by the NCCLS method and from 6 to 19 mm by the BSAC method. Thus, irrespective of the method and the indicator cephalosporin, the AmpC producers were obviously resistant, and this resistance was not reversed by clavulanate, meaning that these strains were readily distinguishable from ESBL producers by the combination disk test. The behavior of the 19 K. oxytoca isolates that hyperproduced K1 β-lactamase was more problematic. Twelve of these K. oxytoca isolates gave zones with CPD (10-μg) disks that fulfilled the NCCLS criteria for predicting ESBL production (zone of ≤22 mm) and for intermediate resistance (≤20 mm); 11 gave zones signifying frank resistance (≤17 mm). Using BSAC methodology, the zones of the CPD (10-μg) disks for the 19 isolates ranged from 14 to 36 mm, with 11 of 19 isolates giving zones of ≤24 mm in diameter. The isolates that appeared least resistant to cefpodoxime were identical by both methods and corresponded to those previously found (6) to have the least degree of β-lactamase hyperproduction and to be least resistant to cefuroxime (data not shown). The zones with the CD01 (10- plus 1-μg) disks by both the NCCLS and BSAC methods were equal in diameter to those with the CPD (10-μg) disks, or up to 3 mm smaller, thus distinguishing resistant hyperproducers of this chromosomal β-lactamase from strains with ESBLs. All 19 hyperproducers appeared susceptible to CAZ (30-μg) disks in both BSAC and NCCLS tests, as anticipated for the phenotype (6).
In conclusion, comparison of the inhibition zones for the CPD (10-μg) and CD01 (10- plus 1-μg) disks distinguished ESBL-positive klebsiellae from susceptible strains without errors, regardless of whether the NCCLS or BSAC disk test methodology was used. M'Zali et al. (11) recently published the findings that use of ceftazidime (30-μg) and ceftazidime-plus-clavulanate (30- plus 10-μg) disks detected 86% of ESBL producers; that use of cefotaxime (30-μg) and cefotaxime-plus-clavulanate (30- plus 10-μg) disks detected 66% of producers; and that use of both disk pairs detected 93% of producers. Comparison of the two studies is tenuous, however, both because the test strains differed and because M'Zali et al. used the ratio of the zone diameters with and without clavulanate to infer ESBL production, rather than the difference in diameters.
In the present study, discrimination of ESBL producers and non-ESBL producers was also achieved, with a false-positive rate of 2% and no false negatives, by the simpler expedient of categorizing isolates as presumptive ESBL producers on the basis of zones of <22 mm with CPD (10-μg) disks in NCCLS tests. Application of the same criterion to CAZ (30-μg) disks led to a false-negative rate of 4.5%. Unlike these simpler strategies, the combination disk test additionally distinguished ESBL producers from strains with AmpC enzymes and from K. oxytoca isolates that overexpressed the K1 enzyme. This discrimination is valuable for surveillance and for patient care, where it guides the choice of further antibiotics to test (cephamycins and β-lactamase inhibitor combinations against ESBL producers, “fourth-generation” cephalosporins (cefpirone and cefepime) against AmpC producers, and ceftazidime against K1 hyperproducers). A limitation, irrespective of whether NCCLS or BSAC methods were followed, was that ca. 40% of K1 β-lactamase-hyperproducing K. oxytoca isolates appeared susceptible to the CPD (10-μg) disks. These strains would not have been confused with ESBL producers, but would have been categorized as cephalosporin susceptible unless tests were also performed with antibiotics that are better substrates for the K1 enzyme, notably aztreonam or cefuroxime.
Acknowledgments
We are grateful to Oxoid Ltd., UK for financial support.
We thank Meifang Yuan and Gioia Babini for provision of bacterial strains.
REFERENCES
- 1.Babini G S, Livermore D M. Antimicrobial resistance amongst Klebsiella spp. collected from intensive care units in Southern and Western Europe in 1997–1998. J Antimicrob Chemother. 2000;45:183–189. doi: 10.1093/jac/45.2.183. [DOI] [PubMed] [Google Scholar]
- 2.Babini G S, Yuan M, Livermore D M. Interactions of β-lactamases with sanfetrinem (GV 104326) compared to those with imipenem and with oral β-lactams. Antimicrob Agents Chemother. 1998;42:1168–1175. doi: 10.1128/aac.42.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brun-Buisson C, Legrand P, Philippon A, Montravers F, Ansquer M, Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987;ii:302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structures. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cormican M G, Marshall S A, Jones R N. Detection of extended-spectrum beta-lactamase (ESBL)-producing strains by the Etest ESBL screen. J Clin Microbiol. 1996;34:1880–1884. doi: 10.1128/jcm.34.8.1880-1884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheorghiu R, Yuan M, Hall L M C, Livermore D M. Bases of variation in resistance to β-lactams in Klebsiella oxytoca isolates hyperproducing K1 β-lactamase. J Antimicrob Chemother. 1997;40:533–541. doi: 10.1093/jac/40.4.533. [DOI] [PubMed] [Google Scholar]
- 7.Jan I S, Hsueh P R, Teng L J, Ho S W, Luh K T. Antimicrobial susceptibility testing for Klebsiella pneumoniae isolates resistant to extended-spectrum beta-lactam antibiotics. J Formos Med Assoc. 1998;97:661–666. [PubMed] [Google Scholar]
- 8.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 9.Livermore D M, Yuan M. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996;38:409–424. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin Infect Dis. 1997;24(Suppl. 1):S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 11.M'Zali F H, Chanawong A, Kerr K G, Birkenhead D, Hawkey P M. Detection of extended spectrum β-lactamases in members of the family Enterobacteriaceae: comparison of the MAST DD test, the double disk and the E-test ESBL. J Antimicrob Chemother. 2000;45:881–885. doi: 10.1093/jac/45.6.881. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests, 7th ed. Approved standard M2–A7 (M100–S10). Wayne, Pa: NCCLS; 2000. [Google Scholar]
- 13.Rice L B, Yaou J D C, Klimm K, Eliopoulos G M, Moellering R C. Efficacy of different β-lactams against an extended-spectrum β-lactamase-producing Klebsiella pneumoniae in the rat intra-abdominal sepsis model. Antimicrob Agents Chemother. 1991;35:1243–1244. doi: 10.1128/aac.35.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders, C. C., A. L. Barry, J. A. Washington, C. Shubert, E. S. Moland, M. M. Traczewski, C. Knapp, and R. Mulder. Detection of extended-spectrum beta-lactamase-producing members of the family Enterobacteriaceae with the Vitek ESBL test. J. Clin. Microbiol. 34:2997–3001. [DOI] [PMC free article] [PubMed]
- 15.Yuan M, Aucken H, Hall L M C, Pitt T L, Livermore D M. Epidemiological typing of klebsiellae with extended-spectrum β-lactamases from European intensive care units. J Antimicrob Chemother. 1998;41:527–539. doi: 10.1093/jac/41.5.527. [DOI] [PubMed] [Google Scholar]
- 16.Yuan M. Ph.D. thesis. London, United Kingdom: University of London; 1999. [Google Scholar]