Abstract
The consequences of the vaccination of neonatal calves with the widely used live-attenuated temperature-sensitive (ts) bovine herpesvirus type 1 (BHV-1) were investigated. The ts strain established acute and latent infections in all vaccinated calves either with or without passive immunity. Four of seven calves vaccinated under passive immunity became clearly BHV-1 seronegative by different serological tests, as did uninfected control calves after the disappearance of maternal antibodies, and they remained so for long periods. A cell-mediated immune response was detected by a BHV-1 gamma interferon assay, but this test failed to detect the seronegative latent carriers (SNLCs). While they are not detected, SNLCs represent a threat for BHV-1-free herds or countries. This study demonstrates that SNLCs can be easily obtained by inoculation with a live-attenuated BHV-1 under passive immunity and that latent carrier animals without any antibody do exist. Consequently, this situation could represent a good model to experimentally produce SNLCs.
Bovine herpesvirus type 1 (BHV-1), a member of the Alphaherpesviridae subfamily, is a pathogen of worldwide importance for cattle which is associated with several clinical manifestations and particularly with a respiratory syndrome called infectious bovine rhinotracheitis (23, 31, 42). Since the end of the 1970s, conventional vaccines and especially intranasal live-attenuated vaccines have efficiently contributed to control of the disease (10, 13, 22, 24, 31, 42). Currently, most artificial insemination centers have to be BHV-1 free, and BHV-1 eradication or control programs have been initiated in several European countries (1, 5, 37). One of the major problems in controlling this infection is the maintenance of the virus in a latent state after infection with both wild-type and live-attenuated BHV-1 strains (23, 34, 36).
Latently infected animals are usually identified by the detection of BHV-1-specific antibodies in their serum. However, the presence of maternal antibodies can interfere with an antibody response following either infection (2, 14) or vaccination (3, 18, 19). We recently demonstrated that a BHV-1 seronegative latent carrier (SNLC) can be experimentally obtained after infection of passively immunized calves with a virulent BHV-1 strain (16). From field observations, it has been postulated that SNLCs could also be produced when calves had been vaccinated with a live-attenuated temperature-sensitive (ts) BHV-1 in the presence of maternal antibodies (10). On the other hand, several authors have reported an absence of seroconversion after vaccination with attenuated BHV-1 strains and especially with the ts vaccine (7, 13, 18, 22, 30, 32, 37, 43), but in these cases the establishment of the latent state was never demonstrated. These observations suggest that the probability of producing SNLCs could be increased with an attenuated strain. The aim of this study, therefore, was to determine whether vaccination of passively immunized neonatal calves with the live-attenuated ts BHV-1 vaccine strain could generate SNLCs.
Nineteen calves originating from BHV-1-free dairy farms were used and were allocated to three groups. One group of five calves had received colostrum from their seronegative dams, and two groups of seven calves had received 2 to 3 liters of a single pool of colostrum (from a colostrum bank, Marloie, Belgium) containing anti-BHV-1 antibodies, within the first 12 h after birth. Throughout the study, precautions were taken to avoid the spread of virus between calves, as previously described (16). The five seronegative calves (group V, for vaccinated) and seven passively immunized calves (group CV, for vaccinated under colostral immunity) were inoculated intranasally (1 ml per nostril) with a total recommended dose of 105.4 PFU of the live-attenuated BHV-1 ts vaccine strain RLB 106 (Tracherhine; Pfizer Animal Health) (43). Because calves enter selection stations at the earliest when they are 1 week old, calves of groups V and CV were vaccinated at 4 days of age. Seven passively immunized calves were not vaccinated in order to follow the natural decrease of colostrally derived BHV-1 antibodies (group C, for colostrum).
Animals were monitored for 6.5 to 13 months. Blood samples were taken weekly from each animal for serological monitoring. Heparinized blood samples were also regularly taken to detect a cell-mediated immune response by an in vitro BHV-1-specific gamma interferon (IFN-γ) production assay, performed as described by Lemaire et al. (16). One calf of group CV (calf CV3) was removed from the study 14 weeks after inoculation (p.i.) for a medical reason unrelated to the study (umbilical hernia). At the end of the observation period, each animal was treated with dexamethasone (Fortecortine; Bayer) at 0.1 mg/kg intravenously on 3 consecutive days, in order to demonstrate BHV-1 latent infection. Group C control calves received a 5-consecutive-day treatment (24). After inoculation and experimental reactivation, nasal swabs were taken daily from each animal for 21 days. Between these two periods, nasal swabs were taken twice a week to detect any virus reexcretion. The presence of BHV-1 was detected and titrated by plaque assay on MDBK cells as previously described (15, 16). The experimental procedures were carried out in accordance with the Belgian law (AR 14/11/93) implementing the European Council directive number 86/609/ECC of 24 November 1986.
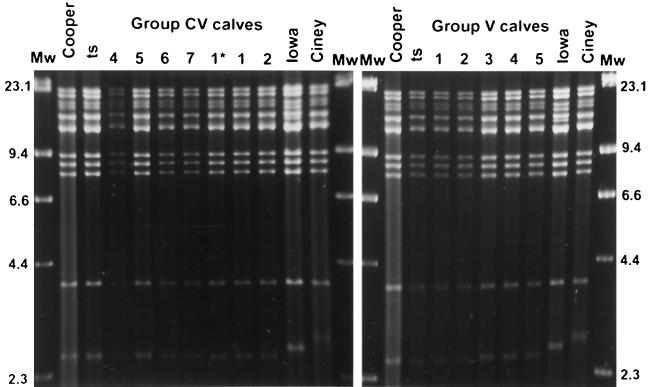
The presence or the absence of passively acquired specific antibodies had no effect on virus shedding after inoculation and on the establishment of latency. The ts strain was excreted at high titers and for a long period in both vaccinated groups, with or without a passive immunity and independently of the different levels of maternal antibodies. The mean (± the standard deviation) peak virus titers were similar in the CV and V groups and were 105.4±0.8 and 105.8±0.6 PFU/100 mg of nasal secretions on days 6 and 7 p.i., respectively. BHV-1 was recovered from nasal secretions for long periods, between 9 and 16 days for the V group and between 10 and 24 days for the CV group. In the latter group, the virus was isolated from the nasal swabs taken twice a week up to days 23, 25, and 36 p.i. in calves CV3, CV4, and CV5, respectively. A long period of virus shedding in the presence of passively acquired antibodies was also observed in other studies (15, 16, 31) and may be a result of the immune-evasive character of herpesviruses (17, 21). All inoculated calves were latently infected, and the live-attenuated ts vaccine strain was easily reactivated and reexcreted. Calf CV1 reexcreted the virus spontaneously on p.i. days 380, 384, and 385 (day of first dexamethasone injection), with a peak virus titer of 103.7 PFU/100 mg of nasal secretions on day 384. After dexamethasone treatment (PDT), BHV-1 was isolated in the nasal swabs from all inoculated calves, for 3 (calf CV4) to 7 (calf CV2) days in calves of group CV and for 6 days in all calves of group V. The peak titers were 103.6±1.8 and 105.7±0.7 PFU/100 mg of nasal secretions on PDT days 6 and 7 in groups CV and V, respectively. In contrast to the virus excretion after inoculation, an effect of the specific immune level was observed on virus reexcretion PDT, as previously described for virulent strains (23, 42). In group CV, only calf CV2, which presented at this time no detectable antibody and cellular responses (see below), had a reexcretion profile similar to that of calves of group V. All the reexcreted viruses were characterized as ts strain by relative temperature growth at 35 and 40°C (22, 43). The identity with the inoculated virus was confirmed by DNA restriction endonuclease analysis, as described by Lemaire et al. (15). HindIII restriction endonuclease patterns were similar in the two inoculated groups (CV and V) for the RLB 106 ts strain and for the BHV-1.1 reference strain Cooper but differed clearly from the field strain Ciney and the virulent strain Iowa (Fig. 1). No BHV-1 was isolated from group C throughout the study.
FIG. 1.
Restriction endonuclease HindIII DNA profiles of nasal viral isolates from the vaccinated calves (group CV and control group V) on the sixth and seventh days after their first dexamethasone injection (PDT) compared with DNA profiles of the BHV-1 ts vaccine strain, the BHV1.1 reference strain Cooper, the virulent strain Iowa, and the field strain Ciney. For calf CV1, the restriction endonuclease analysis was performed on DNA from viruses isolated before the dexamethasone treatment on p.i. day 380 (1*) and on the fourth day PDT (1).
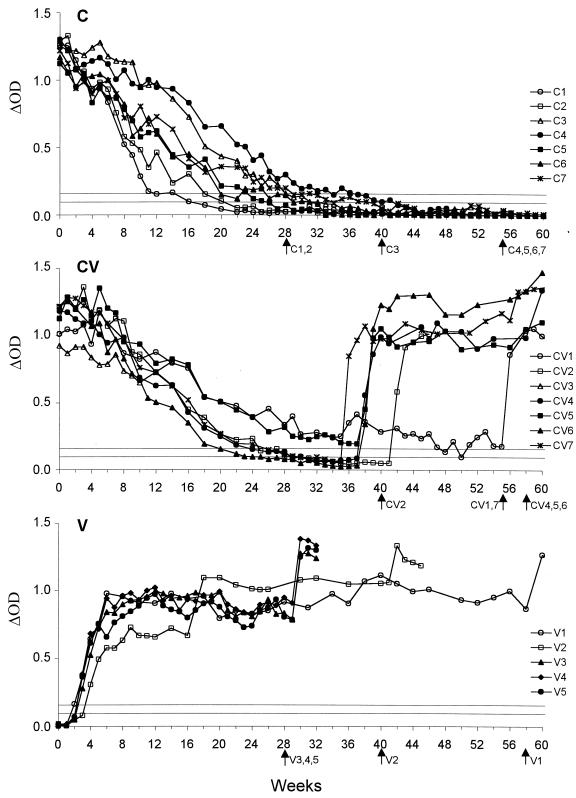
The presence of BHV-1 antibody throughout the study was determined by a classical indirect enzyme-linked immunosorbent assay (ELISA) (SERELISA IBR/IPV antibody Bi Indirect; Synbiotics), a blocking ELISA for the detection of antibodies against glycoprotein E (gE) of BHV-1 (BHV-1 gE antibody test HerdChek; Idexx) and a 24-h virus neutralization (VN) test performed as described by Lemaire et al. (16). The evolutions of BHV-1 antibody levels obtained with the indirect ELISA are shown in Fig. 2. Four calves inoculated under passive immunity (CV2, CV4, CV6, and CV7) became BHV-1 seronegative, like control calves (group C), after an average period of 7 months (Fig. 2 and Table 1). These calves remained seronegative for 3 to 11 weeks (Fig. 2). With the commercially available gE-blocking ELISA, all calves of groups C and CV became seronegative against BHV-1 gE at an average of 5 months p.i. (Table 1). Five calves inoculated under passive immunity (CV1, CV2, CV4, CV6, and CV7) remained clearly seronegative for 12 to 21 weeks. The sixth calf (CV5) was seronegative only at weeks 29, 30, and 33 p.i. (data not shown). With the VN test, an increase in antibody titer was observed between 4 and 6 weeks p.i. in the two calves of group CV (CV1 and CV3), which had the lowest BHV-1 VN titers (data not shown). Four calves (CV2, CV4, CV6, and CV7) reached undetectable BHV-1 VN titers at a mean of 6 months, also in the same manner as the uninfected calves (Table 1). Antibody titers in calf CV5 decreased continuously and reached low VN titers from 7 months p.i. (≤4 50% effective doses). The mean antibody half-life of calves CV2, CV4, CV5, CV6, and CV7 was similar to that observed in noninoculated control calves (Table 1). An increase (with seroconversion) was observed with all serological tests between 36 and 38 weeks p.i. in almost all calves, except in calf CV2, which remained seronegative until dexamethasone treatment (41 weeks p.i.). Calf CV1 showed a moderate increase and was again classified seronegative to gE on weeks 54 and 55 p.i.
FIG. 2.
Evolution of anti-BHV-1 antibody levels obtained by the commercially available indirect ELISA throughout the study, in each experimental group. Group C (top), seven passively immunized control calves; group CV (middle), after vaccination (week 0) with a live-attenuated ts vaccine strain of BHV-1 in seven passively immunized calves (calf CV3 was removed from the study at 14 weeks p.i.); group V (bottom), after vaccination (week 0) with a live-attenuated ts vaccine strain of BHV-1 in five seronegative control calves. The results are expressed as the difference in optical density between positive and control antigens (ΔOD). Horizontal lines indicate the cut-off values: the upper line represents the positive limit, and the lower line represents the negative limit; between the two lines the results are inconclusive. Arrows indicate the times corresponding to the dexamethasone treatment for the different animals.
TABLE 1.
Age when maternally immunized calves became seronegative to BHV-1a by three serological tests and BHV-1 neutralizing antibody half-lives
| Groupb | Mean age in weeks ± SD
|
Antibody half-life in days | ||
|---|---|---|---|---|
| Indirect ELISA | gE blocking ELISA | VN test | ||
| C | 30 ± 9 (n = 7) | 21 ± 6 (n = 7) | 24 ± 6 (n = 7) | 30 ± 6 (n = 7) |
| CV | 31 ± 2 (n = 4) | 22 ± 4 (n = 6) | 27 ± 5 (n = 4) | 32 ± 8 (n = 5)c |
Calves of group CV were inoculated with a live-attenuated vaccine strain at 4 days of age; therefore, the p.i. time for each animal can be considered as its age.
Group C, not vaccinated; group CV, vaccinated.
Antibody half-lives were calculated on the five calves which did not show any increase in antibody titers after inoculation.
All negative results were confirmed at least twice, and those obtained with the indirect ELISA in the four inoculated calves, CV2, CV4, CV6, and CV7, were also confirmed with a commercially available glycoprotein B (gB)-blocking ELISA (SERELISA IBR/IPV Ab Mono Blocking; Synbiotics) and by two reference serological tests (Veterinary and Agrochemical Research Center, Brussels): a blocking ELISA with BHV-1 polyclonal antibodies and a 24-h VN test (12, 26). Three to five serum samples taken between p.i. weeks 30 and 38 from these four inoculated calves were tested with the Danish test system (5) (Gezondheidsdienst voor Dieren, Deventer, The Netherlands). One calf (CV6, at weeks 34 and 36 p.i.) was found to be negative, and the other three presented inconclusive results. Some samples with inconclusive results were also tested with the gB-blocking reference test in use in The Netherlands (5) and were found to be negative.
The seronegative calves (group V) seroconverted between 2 and 4 weeks p.i. based on the indirect ELISA (Fig. 2) and between 3 and 5 weeks p.i. based on the gE-blocking ELISA. These antibody responses were considerably delayed compared to those observed after infection with virulent BHV-1 strains (38, 42). Such delayed antibody response in seronegative calves should be taken into account in BHV-1 control programs. After dexamethasone treatment, all uninfected control calves (group C) remained seronegative, whereas the inoculated calves (groups V and CV) showed a rise in their antibody level within 2 weeks (Fig. 2).
In contrast to the antibody response, the presence of passively acquired antibodies did not hinder the development of a cell-mediated immune response (Table 2). Except one nonimmunized calf (V4), which showed a very poor response, almost all inoculated calves (groups V and CV) showed a positive response in the IFN-γ assay starting 1 to 3 weeks p.i. until at least 5 weeks p.i. for two calves (V2 and CV6) and 10 weeks p.i. for the other ones. This interesting finding confirms that the IFN-γ assay is able to distinguish calves possessing only passively acquired antibodies from those latently infected with BHV-1 (16), even with an attenuated BHV-1 strain. As developed for bovine tuberculosis and brucellosis diagnosis (39, 41), the IFN-γ assay appears to be a good complementary test to the serological methods, at least in the acute phase of infection. However, the number of positive results decreased with time, and almost all inoculated calves showed negative stimulation index (SI) values at around 30 weeks p.i. (Table 2). In the absence of antigenic restimulation, the cell-mediated response seems to decrease to undetectable levels, as documented in a number of studies using the IFN-γ assay (15, 16) or lymphocyte proliferation assays (4, 28, 29). Therefore, the IFN-γ test was not able to detect the SNLCs, except for one calf (CV1), for which the test could detect only a seronegative response to gE. Significant increases in SI results were observed in calves inoculated under passive immunity between 35 and 38 weeks p.i. (except for calf CV2).
TABLE 2.
Results of specific IFN-γ production assay after inoculation with a live-attenuated BHV-1 vaccine strain in seronegative calves (group V) and in passively immunized calves (group CV)
| Calf | Stimulation indexa after BHV-1 inoculation at week:
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 10 | 14 | 18 | 22 | 26 | 27 | 28 | 30 | 34 | 35 | 37 | 38 | 40 | 41 | 44 | 48 | 52 | 55 | 58 | |
| Group V | |||||||||||||||||||||||||||
| V1 | 1.0 | 1.2 | 2.2 | 1.7 | 4.4 | 2.0 | 1.1 | NI | 1.1 | NI | 1.3 | 1.7 | 1.5 | — | — | 1.5 | 1.7 | — | — | 2.9 | — | 2.2 | 3.0 | 1.1 | 1.4 | 0.9 | 1.2b |
| V2 | 1.2 | 0.9 | 2.0 | NI | 1.4 | 2.0 | 1.4 | 2.1 | 0.8 | 0.8 | 3.1 | 2.1 | 1.8 | — | — | NI | 1.6 | — | 0.6 | — | 0.9b | ||||||
| V3 | 0.5 | 2.3 | 4.9 | 2.2 | 3.5 | 2.8 | 1.8 | 3.5 | 3.4 | 2.4 | 2.6 | 2.0 | 2.4 | — | 1.6b | ||||||||||||
| V4 | 1.0 | 1.3 | 1.3 | 1.9 | 1.3 | 1.4 | 1.0 | 2.0 | 1.6 | 1.6 | 1.9 | 1.2 | 1.2 | 1.1 | 1.4b | ||||||||||||
| V5 | 1.0 | 1.2 | 11.0 | 4.3 | 3.0 | 3.0 | 2.0 | 2.2 | 2.3 | 1.3 | 2.3 | 3.4 | 2.1 | 1.5 | 1.2b | ||||||||||||
| Group CV | |||||||||||||||||||||||||||
| CV1 | 1.0 | 1.3 | 1.3 | 8.7 | 2.0 | — | 3.1 | 5.0 | 7.8 | 4.6 | 5.4 | 2.8 | NI | — | — | 2.0 | 3.2 | 14.0 | — | — | 5.4 | 3.0 | NI | 6.1 | 5.7 | 31.0b | |
| CV2 | 1.0 | 1.5 | 2.8 | 4.5 | 4.7 | 4.4 | 3.7 | 2.1 | 2.7 | 1.2 | NI | NI | 1.0 | — | — | 1.2 | 1.2 | NI | — | — | 1.6b | ||||||
| CV3 | 1.0 | NI | 2.3 | 1.9 | 2.8 | — | 4.3 | 2.3 | 2.3 | 2.9c | |||||||||||||||||
| CV4 | 1.0 | 2.5 | 2.1 | 1.4 | 6.3 | 4.0 | 3.5 | 4.1 | 3.0 | 2.0 | 2.0 | 6.5 | 1.5 | — | — | 1.3 | 1.4 | 1.4 | 1.4 | — | — | 6.8 | 3.0 | — | 11.0 | 5.7 | 14.0b |
| CV5 | 1.0 | 1.4 | 2.5 | 2.2 | 2.3 | 1.7 | 3.6 | 2.0 | 3.7 | 2.2 | 2.0 | 1.6 | 1.1 | — | — | 1.4 | 1.3 | — | — | 29.0 | — | 5.3 | 5.4 | 4.1 | — | 5.5 | 3.3b |
| CV6 | 0.9 | 2.5 | 1.4 | 2.6 | 8.3 | 2.1 | 1.0 | 1.3 | 1.2 | NI | 1.3 | 1.3 | 1.5 | — | — | 1.2 | 0.8 | — | 1.3 | 16.0 | — | 23.0 | 12.0 | — | — | 2.8 | 3.4b |
| CV7 | 0.9 | NI | NI | 3.2 | 2.1 | NI | NI | 2.3 | NI | NI | 2.4 | 1.5 | 2.1 | — | — | 0.8 | 0.7 | 12.0 | — | 12.0 | 18.0 | 7.0 | 3.9 | 5.3 | 14.0 | 17.0b | |
Stimulation index: optical density (OD) of culture with BHV-1 antigen/OD of culture with cell control antigen. Positive results, greater than or equal to 2.0, are indicated in bold. This cut-off value was calculated from the results obtained in the seven noninoculated control calves (group C) throughout the study (mean plus three standard deviations). NI, not interpretable (results were considered not interpretable when the OD values in the control cultures were elevated (>0.4) (9); —, not tested.
End of the observation period (start of the dexamethasone treatment).
Calf CV3 was removed from the study 14 weeks p.i.
In this study, SNLCs were easily generated by vaccination of passively immunized neonatal calves with a conventional live-attenuated BHV-1 strain. After inoculation in the presence of a passive immunity (group CV), the antibody level of the five calves possessing the highest levels of BHV-1 antibodies decreased in the same manner as that of the control calves (group C). The mean antibody half-life was similar to that observed in group C, clearly indicating that the antibodies were of maternal origin. In total, four calves inoculated under passive immunity became clearly BHV-1 seronegative based on the indirect ELISA and the 24-h VN test, and six calves became seronegative based on the gE-blocking ELISA, at a mean 7, 6, and 5 months of age, respectively. As already mentioned, all these calves were latently infected. At approximately 8 to 9 months of age, almost all calves showed increases in both antibody titers and SI values. This increase of the specific immune response was most likely due to natural virus reactivation (20, 25, 33). This result may reflect a natural phenomenon. Indeed, recent studies of seroprevalence in infected herds revealed a window between 6 and 9 months of age when most of the animals tested were seronegative for BHV-1 (M. Dispas, personal communication). SNLC calves remained seronegative for long periods, up to 3 months with the indirect ELISA and up to 5 months with the gE-blocking ELISA, The negative results obtained with the highly sensitive indirect ELISA (6) were confirmed by the gB-blocking ELISA and by the serological reference tests used in Belgium were confirmed and for at least one calf by the Danish test system in another laboratory. In a comparative study, the Danish test system showed the highest sensitivity, the gE-blocking ELISA the lowest (5). However, the gE-blocking ELISA is an important diagnostic test, because it is the companion test of the new generation of vaccines deleted in the BHV-1 gE gene, which are used in BHV-1 eradication programs (5, 38). The results presented here indicate that in addition to animals that test false seronegative due to a lack of sensitivity of the serological test used (11, 35), truly seronegative animals without any antibodies do also exist. The epidemiological consequences of both types of SNLC are identical because such animals are not detected. There are currently no means to diagnose directly a latent infection other than dexamethasone treatment (24) or PCR examination of the trigeminal ganglion (27). Recently, PCR assays were successfully developed for the detection of BHV-1 DNA sequences in peripheral blood leukocytes in naturally infected cattle (8). However, positive results were not confirmed by demonstration of latency, and further investigations should be performed under experimental conditions. Another novel approach could be the detection of BHV-1 DNA in the tonsils (40).
In conclusion, the obtained results clearly demonstrate that SNLCs can be experimentally produced after vaccination of maternally immunized calves with a live-attenuated ts BHV-1, whatever the serological test used and despite a high sensitivity. Furthermore, it seems to be easier to produce SNLCs with an attenuated BHV-1 strain than with a virulent one (16). The existence of SNLCs is of primary economic importance in selection stations, artificial insemination centers, and BHV-1-free farms or regions, where a virus circulation among free animals can induce a disastrous seroconversion in a significant number of animals. Since this virus vaccine has been intensively and widely used, it will persist indefinitely in the cattle population (24). It is difficult, however, to evaluate the importance of this phenomenon in the field, because it is hard to detect such SNLCs. Vaccination with live-attenuated BHV-1 of calves possessing passively acquired specific antibodies could represent a good model to experimentally produce SNLCs for studies to improve the serological diagnostic tools or to develop new approaches in the detection of latency.
Acknowledgments
We thank L. Nols, J.-P. Georgin, A. Brichaud, C. Salvador, F. Seret, and M. Loncar for excellent technical assistance and M. Dispas (Brussels, Belgium) for helpful discussions. We also thank J. C. Bosch and J. J. de Wit (Deventer, The Netherlands) for the serological reference tests performed. Reagents for the in vitro stimulation in the IFN-γ assay and monoclonal antibodies for the IFN-γ ELISA were kindly provided by J. Godfroid (Brussels, Belgium) and by V. Weynants and J.-J. Letesson (Namur, Belgium), respectively. Pfizer Animal Health (Louvain-La-Neuve, Belgium) kindly provided the RLB 106 strain.
This study was supported by the Ministère des Classes Moyennes et de l'Agriculture, Administration Recherche et Développement.
REFERENCES
- 1.Ackermann M, Belak S, Bitsch V, Edwards S, Moussa A, Rockborn G, Thiry E. Round table on infectious bovine rhinotracheitis/infectious pustular vulvovaginitis virus infection diagnosis and control. Vet Microbiol. 1990;23:361–363. doi: 10.1016/0378-1135(90)90167-t. [DOI] [PubMed] [Google Scholar]
- 2.Bradshaw B J, Edwards S. Antibody isotype responses to experimental infection with bovine herpesvirus 1 in calves with colostrally derived antibody. Vet Microbiol. 1996;53:143–151. doi: 10.1016/s0378-1135(96)01242-4. [DOI] [PubMed] [Google Scholar]
- 3.Brar J S, Johnson D W, Muscoplat C C, Shope R E J, Meiske J C. Maternal immunity to infectious bovine rhinotracheitis and bovine viral diarrhea viruses: duration and effect on vaccination in young calves. Am J Vet Res. 1978;39:241–244. [PubMed] [Google Scholar]
- 4.Denis M, Kaashoek M J, van Oirschot J T, Pastoret P-P, Thiry E. Quantitative assessment of specific CD4+ T lymphocyte proliferative response in bovine herpesvirus 1 immune cattle. Vet Immunol Immunopathol. 1994;42:275–286. doi: 10.1016/0165-2427(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 5.de Wit J J, Hage J J, Brinkhof J, Westenbrink F. A comparative study of serological tests for use in the bovine herpesvirus 1 eradication programme in The Netherlands. Vet Microbiol. 1998;61:153–163. doi: 10.1016/s0378-1135(98)00166-7. [DOI] [PubMed] [Google Scholar]
- 6.Filleton R, Crevat D, Thibault J C. Proceedings of the 7th International Symposium of Veterinary Laboratory Diagnosticians. Buenos Aires, Argentina: World Association of Veterinary Laboratory Diagnosticians; 1994. Performances of a new diagnostic kit for the detection of antibodies against IBR/IPV in bovine serum; p. 170. [Google Scholar]
- 7.Frerichs G N, Woods S B, Lucas M H, Sands J J. Safety and efficacy of live and inactivated infectious bovine rhinotracheitis vaccines. Vet Rec. 1982;111:116–122. doi: 10.1136/vr.111.6.116. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs M, Hubert P, Detterer J, Rziha H-J. Detection of bovine herpesvirus type 1 in blood from naturally infected cattle by using a sensitive PCR that discriminates between wild-type virus and virus lacking glycoprotein E. J Clin Microbiol. 1999;37:2498–2507. doi: 10.1128/jcm.37.8.2498-2507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfroid J, Czaplicki G, Kerkhofs P, Weynants V, Wellemans G, Thiry E, Letesson J-J. Assessment of the cell-mediated immunity in cattle infection after bovine herpesvirus 4 infection, using an in vitro antigen-specific interferon-gamma assay. Vet Microbiol. 1996;53:133–141. doi: 10.1016/s0378-1135(96)01241-2. [DOI] [PubMed] [Google Scholar]
- 10.Hage J J, Glas R D, Westra H H, Maris-Veldhuis M A, van Oirschot J T, Rijsewijk F A. Reactivation of latent bovine herpesvirus 1 in cattle seronegative to glycoproteins gB and gE. Vet Microbiol. 1998;60:87–98. doi: 10.1016/s0378-1135(97)00152-1. [DOI] [PubMed] [Google Scholar]
- 11.Kramps J A, Magdalena J, Quak J, Weerdmeester K, Kaashoek M J, Maris-Veldhuis M A, Rijsewijk F A, Keil G, van Oirschot J T. A simple, specific, and highly sensitive blocking enzyme-linked immunosorbent assay for detection of antibodies to bovine herpesvirus 1. J Clin Microbiol. 1994;32:2175–2181. doi: 10.1128/jcm.32.9.2175-2181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramps J A, Perrin B, Edwards S, van Oirschot J T. A European inter-laboratory trial to evaluate the reliability of serological diagnosis of bovine herpesvirus 1 infections. Vet Microbiol. 1996;53:153–161. doi: 10.1016/s0378-1135(96)01243-6. [DOI] [PubMed] [Google Scholar]
- 13.Kucera C J, White R G, Beckenhauer W H. Evaluation of the safety and efficacy of an intranasal vaccine containing a temperature-sensitive strain of infectious bovine rhinotracheitis virus. Am J Vet Res. 1978;39:607–610. [PubMed] [Google Scholar]
- 14.Lemaire M, Meyer G, Ernst E, Vanherreweghe V, Limbourg B, Pastoret P-P, Thiry E. Latent bovine herpesvirus 1 infection in calves protected by colostral immunity. Vet Rec. 1995;137:70–71. doi: 10.1136/vr.137.3.70. [DOI] [PubMed] [Google Scholar]
- 15.Lemaire M, Schynts F, Meyer G, Thiry E. Antibody response to glycoprotein E after bovine herpesvirus type 1 infection in passively immunised, glycoprotein E-negative calves. Vet Rec. 1999;144:172–176. doi: 10.1136/vr.144.7.172. [DOI] [PubMed] [Google Scholar]
- 16.Lemaire M, Weynants V, Godfroid J, Schynts F, Meyer G, Letesson J-J, Thiry E. Effects of bovine herpesvirus type 1 infection in calves with maternal antibodies on immune response and virus latency. J Clin Microbiol. 2000;38:1885–1894. doi: 10.1128/jcm.38.5.1885-1894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemaire, M., E. Hanon, F. Schynts, G. Meyer, and E. Thiry. Specific passive immunity reduces the excretion of glycoprotein E-negative bovine herpesvirus type 1 vaccine strain in calves. Vaccine, in press. [DOI] [PubMed]
- 18.Lucas M H, Roberts D H, Sands J J, Westcott D G. The use of infectious bovine rhinotracheitis vaccine in a commercial veal unit: antibody response and spread of virus. Br Vet J. 1982;138:23–28. doi: 10.1016/s0007-1935(17)31185-5. [DOI] [PubMed] [Google Scholar]
- 19.Menanteau-Horta A M, Ames T R, Johnson D W, Meiske J C. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine virus diarrhea vaccines. Can J Comp Med. 1985;49:10–14. [PMC free article] [PubMed] [Google Scholar]
- 20.Mengeling W L. Anamnestic immune response of pigs to pseudorabies virus: latent virus reactivation versus direct oronasal and parenteral exposure to virus. J Vet Diagn Investig. 1991;3:133–136. doi: 10.1177/104063879100300205. [DOI] [PubMed] [Google Scholar]
- 21.Nagashunmugam T, Lubinski J, Wang L, Goldstein L T, Weeks B S, Sundaresan P, Kang E H, Dubin G, Friedman H M. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J Virol. 1998;72:5351–5359. doi: 10.1128/jvi.72.7.5351-5359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nettleton P F, Sharp J M. Infectious bovine rhinotracheitis virus excretion after vaccination. Vet Rec. 1980;107:379. doi: 10.1136/vr.107.16.379. [DOI] [PubMed] [Google Scholar]
- 23.Pastoret P-P, Thiry E, Brochier B, Derboven G, Vindevogel H. The role of latency in the epizootiology of infectious bovine rhinotracheitis. In: Wittmann G, Gaskell R M, Rziha H-J, editors. Latent herpesvirus infections in veterinary medicine. Boston, Mass: Martinus Nijhoff Publishers; 1984. pp. 221–227. [Google Scholar]
- 24.Pastoret P P, Thiry E. Diagnosis and prophylaxis of infectious bovine rhinotracheitis: the role of virus latency. Comp Immunol Microbiol Infect Dis. 1985;8:35–42. doi: 10.1016/0147-9571(85)90052-9. [DOI] [PubMed] [Google Scholar]
- 25.Perng G C, Slanina S M, Yukht A, Ghiasi H, Nesburn A B, Wechsler S L. Herpes simplex virus type 1 serum neutralizing antibody titers increase during latency in rabbits latently infected with latency-associated transcript (LAT)-positive but not LAT-negative viruses. J Virol. 1999;73:9669–9672. doi: 10.1128/jvi.73.11.9669-9672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrin B, Bitsch V, Cordioli P, Edwards S, Eloit M, Guerin B, Lenihan P, Perrin M, Ronsholt L, van Oirschot J T. A European comparative study of serological methods for the diagnosis of infectious bovine rhinotracheitis. Rev Sci Tech. 1993;12:969–984. doi: 10.20506/rst.12.3.724. [DOI] [PubMed] [Google Scholar]
- 27.Ros C, Belák S. Studies of genetic relationships between bovine, caprine, cervine, and rangiferine alphaherpesviruses and improved molecular methods for virus detection and identification. J Clin Microbiol. 1999;37:1247–1253. doi: 10.1128/jcm.37.5.1247-1253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rouse B T, Babiuk L A. Host defense mechanisms against infectious bovine rhinotracheitis virus: in vitro stimulation of sensitized lymphocytes by virus antigen. Infect Immun. 1974;10:681–687. doi: 10.1128/iai.10.4.681-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutten V P M G, Wentink G H, de Jong W A C, van Exsel A C A, Hensen E J. Determination of BHV-1 specific immune reactivity in naturally infected and vaccinated animals by lymphocyte proliferation assays. Vet Immunol Immunopathol. 1990;25:259–267. doi: 10.1016/0165-2427(90)90049-x. [DOI] [PubMed] [Google Scholar]
- 30.Schipper I A, Kelling C L. Evaluation of inactivated infectious bovine rhinotracheitis vaccines. Can J Comp Med. 1975;39:402–405. [PMC free article] [PubMed] [Google Scholar]
- 31.Straub O C. Infectious bovine rhinotracheitis. In: Dinter Z, Morein B, editors. Virus infections of ruminants. Amsterdam, The Netherlands: Elsevier Science Publishers; 1990. pp. 71–108. [Google Scholar]
- 32.Thiry E, Brochier B, Lansival B, Hanton G, Derboven G, Pastoret P P, Antoine H. Etude sur l'excrétion et la réexcrétion spontanée de deux souches vaccinales du virus de la rhinotrachéite infectieuse bovine (bovine herpesvirus 1) par des veaux sains maintenus en station de sélection. Ann Med Vet. 1983;127:625–634. [Google Scholar]
- 33.Thiry E, Brochier B, Hanton G, Derboven G, Pastoret P-P. Réactivation du virus de la rhinotrachéite infectieuse bovine (bovine herpesvirus 1, BHV-1) non accompagnée de réexcrétion de particules infectieuses, après injection de dexaméthasone, chez des bovins préalablement soumis au test d'hypersensibilité retardée au BHV-1. Ann Med Vet. 1983;127:377–381. [Google Scholar]
- 34.Thiry E, Brochier B, Saliki J, Pirak M, Pastoret P P. Excretion and reexcretion of thermosensitive and wild-type strains of infectious bovine rhinotracheitis virus after co-infection or two successive infections. Vet Microbiol. 1985;10:371–380. doi: 10.1016/0378-1135(85)90007-0. [DOI] [PubMed] [Google Scholar]
- 35.Thrusfield M. Serological epidemiology. In: Thrusfield M, editor. Veterinary epidemiology. London, United Kingdom: Butterworths; 1986. pp. 175–186. [Google Scholar]
- 36.Tikoo S K, Campos M, Babiuk L A. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv Virus Res. 1995;45:191–223. doi: 10.1016/s0065-3527(08)60061-5. [DOI] [PubMed] [Google Scholar]
- 37.van der Poel W H, Kramps J A, Quak J, Brand A, van Oirschot J T. Persistence of bovine herpesvirus-1-specific antibodies in cattle after intranasal vaccination with a live virus vaccine. Vet Rec. 1995;137:347–348. doi: 10.1136/vr.137.14.347. [DOI] [PubMed] [Google Scholar]
- 38.van Oirschot J T, Kaashoek M J, Maris-Veldhuis M A, Weerdmeester K, Rijsewijk F A. An enzyme-linked immunosorbent assay to detect antibodies against glycoprotein gE of bovine herpesvirus 1 allows differentiation between infected and vaccinated cattle. J Virol Methods. 1997;67:23–34. doi: 10.1016/s0166-0934(97)00073-6. [DOI] [PubMed] [Google Scholar]
- 39.Weynants V, Godfroid J, Limbourg B, Saegerman C, Letesson J-J. Specific bovine brucellosis diagnosis based on in vitro antigen-specific gamma interferon production. J Clin Microbiol. 1995;33:706–712. doi: 10.1128/jcm.33.3.706-712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler M T, Doster A, Jones C. Persistence and reactivation of bovine herpesvirus 1 in the tonsils of latently infected calves. J Virol. 2000;74:5337–5346. doi: 10.1128/jvi.74.11.5337-5346.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood P R, Corner L A, Plackett P. Development of a simple rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res Vet Sci. 1990;49:46–49. [PubMed] [Google Scholar]
- 42.Wyler R, Engels M, Schwyzer M. Infectious bovine rhinotracheitis/vulvovaginitis (BHV1) In: Wittmann G, editor. Herpesvirus diseases of cattle, horses and pigs. Boston, Mass: Kluwer Academic Publishers; 1989. pp. 1–72. [Google Scholar]
- 43.Zygraich N, Lobmann M, Vascoboinic E, Berge E, Huygelen C. In vivo and in vitro properties of a temperature sensitive mutant of infectious bovine Rhinotracheitis virus. Res Vet Sci. 1974;16:328–335. [PubMed] [Google Scholar]