Abstract
Purpose
Long-haul COVID-19 is a condition of unknown background occurring in COVID-19 survivors regardless of the severity of the SARS-CoV-2 infection itself. The aim of the study was to evaluate brain changes in patients suffering from variable symptoms of brain fog after COVID-19.
Material and methods
Eleven patients hospitalized due to symptoms of severe brain fog, i.e. insomnia, sudden impairment of cognitive function, headache, and depression, and 14 healthy volunteers underwent brain imaging including MR spectroscopy.
Results
Routine MR imaging revealed no specific macroscopic changes in keeping with brain fog. Considering that the clinical manifestation of brain fog is transient, the evaluation of the metabolic status of the brain remained the method of choice. The concentration of the major cerebral metabolites, i.e. NAA, Cho, and Cr, remained stable. However, changes in Glx and Lac concentration were observed in MR spectroscopy.
Conclusions
Following results along with clinical course of the brain for imply probable ischaemic background of symptoms.
Keywords: ischaemia, MRI, MRS, brain imaging, COVID-19, brain fog
Introduction
According to recent statistics, approximately 1/3 of COVID-19 survivors suffer from psychiatric and neurologic issues. One of them is so called “brain fog” – a clinical condition manifesting as transient problems with memory, multitasking, and sleeping disorders.
“Brain fog” is a cerebral manifestation of COVID-19. Its origin has not been fully understood yet; hence, multiparametric brain imaging may have an impact on future therapeutic management in this group of patients. Our study indicates transient cerebral hypoperfusion as a possible cause.
Recently several research studies have been conducted on the phenomena of brain fog assessing the cognitive symptoms and their severity among COVID-19 patients in a number of hospitals around the word in order to better understand the underlying causes of those symptoms and to look closely at the cognitive dysfunctions that are experienced by those patients [1-5]. However, still no clear definitions or explanations have been proposed when it comes to the term “brain fog”. There are many interpretations of the cause of this condition. However, there are several symptoms of cognitive impairment that can be referred to as “brain fog”, which have been observed in COVID-19 patients, such as decline in memory, difficulties concentrating, and trouble finding words [2,6,7]. It is necessary to point out that that those symptoms can persist for months and have a negative impact of patients when performing everyday activities [1,2,8]. Thus, there is a need for this subject to be studied in an extensive manner so that further knowledge can be obtained about the underlying mechanisms that cause the occurrence of “brain fog”.
Brain fog is one of the long-term side effects of COVID-19, which may appear regardless of the severity of the infection itself. Approximately 1/3 of survivors have symptoms of brain fog such as short-term memory decline, confusion, multitasking problems, or sleep disorders. The diagnosis remains clinical because no specific laboratory finding nor imaging feature has yet been found. The background of this condition remains unknown although there is a theory that the SARS-CoV2 virus penetrates the brain-blood barrier directly through the olfactory nerve causing cerebral inflammation. Another theory says that it is not the virus itself but the overreaction of the immune system which results in cerebral impairment. Another possible cause of brain injury is increased propensity to blood clotting due to infection. Based on published data regarding systemic and cerebral vasculitis in the course of COVID-19 [9] as well as increased incidence of stroke being under investigation [10] and other cerebral diseases based on vascular insufficiency in COVID-19 patients [11], we decided to investigate the macroscopic and biochemical status of the brain in patients suffering from brain fog in order to verify the occurrence of ischaemic lesions (i.e. lacunar infarctions) or substantial changes in concentration of cerebral metabolites.
Metabonomics – the analysis of the expression of metabolites in biosamples – is the research method which could allow an explanation the pathophysiological mechanisms of this condition and aid in the development of diagnostic tools [12]. MR spectroscopy is one of the techniques used for metabonomic mapping. It is noninvasive and feasible in patients with various neurological conditions.
Material and methods
Patient recruitment
We prospectively recruited patients with brain fog admitted to the neurology departments of our hospitals – CSK MSWiA in Warsaw and Medical Centre Hospital in Łańcut. The study was approved by the Institutional Research Ethics Committee, and written informed consent was obtained from the patient or assent from their relatives.
Inclusion criteria were defined as endured COVID-19 proven by positive test results (RT-PCR and/or antigen) followed by symptoms included in the spectrum of severe brain fog – defined as cognitive impairment described in detail in the following part of this chapter.
Patients who had metal inserts or were MR incompatible for other medical reasons, or who turned out to have a clinical condition mimicking brain fog, were excluded.
On admission, each patient underwent exclusion of COVID-19 as well as a blood test confirming recent COVID-19 infection, intoxication of alcohol and drugs (cannabis and cocaine), neurological urgencies such as stroke and intracranial bleeding, and chronic neurologic conditions such as migraine or neuroborreliosis (based on anamnesis).
Neuropsychological testing for cognitive impairment (referred to as brain fog)
Patients were screened by extensive neuropsychological tests for the presence of cognitive deficits using Addenbrooke’s cognitive examination III (ACE III), which is composed of tests of attention, orientation, memory, language, visual perceptual, and visuospatial skills; the Rey Complex Figure Test (RCFT), which evaluates different functions, such as visuospatial abilities, memory, attention, planning, working memory, and executive functions; the California Verbal Learning Test (CVLT), which assess the verbal learning and memory deficits; the Trail Making Test (TMT) Parts A & B, which measures attention, visual screening ability and processing speed; and subtests of Wechsler Adult Intelligence Scale (WAIS-R) – a similarities test to assess verbal reasoning, a coding test to measure visual-motor coordination, motor and mental speed, and a digit span test to examine attention, concentration, and mental control. Patients were also tested for any symptoms of anxiety and depression using the Hospital Anxiety and Depression Scale (HADS).
The neuropsychological testing was performed after detailed examination of the patient’s medical history, related to COVID-19, especially symptoms of brain fog and mental state. Screening took place in a quiet clinical setting to ensure no disturbance was present at the time, to obtain the most accurate results. Patients were given clear instructions and were informed of the right to withdraw from the tests at any time, in line with the code of ethics. All the patients were tested under the same conditions. None of the patients refused to take part in the neuropsychological tests, and all the patients showed the appropriate level of engagement and motivation at screening.
Patients with brain fog who suffered from long COVID-19 complained of wide-ranging fluctuating symptoms, such as general fatigue, slowness, and cognitive dysfunctions such as memory loss, speech deficit (lack of words, problems with fluency), and a decline in performance and learning abilities. Many of the patients also experienced confusion, difficulties concentrating, a sense that their thinking is slowed, depression, anxiety, and a reduction in their ability to carry out certain everyday activities or return to work many weeks after the initial COVID-19 diagnosis. According to the patients, the symptoms of cognitive impairment referred to as brain fog gradually appeared during the infection and continued to develop after the infection. Patients claimed that they had not suffered from the above-mentioned syndromes before the infection and that they first started to occur during the infection and persisted for a long time after. Some of those patients also stated that those symptoms had become less severe over time. Some patients also reported experiencing problems similar to psychosis (various problems with their senses, e.g. hearing non-existing sounds of instruments around them, noticing non-existing smells). However, most patients experienced mainly memory loss and problems with concentration.
The presence of cognitive impairment was confirmed during neuropsychological screening tests, which revealed dysfunctions in memory processing, poor concentration, executive functioning deficits, and episodic and visuospatial memory. In some of those patients an increased level of anxiety and depression was also seen. The general cognitive functioning of those patients was reduced.
Magnetic resonance imaging protocol
Patients underwent MRI within 3 days of admission. The images were acquired on a Philips 3T Ingenia scanner (Philips Healthcare, Best, The Netherlands) with self-shielding gradients (33 mT/m maximum) using a 32-chanel head coil. We obtained axial T2-weighted fast spin-echo and fluid attenuated inversion recovery imaging, axial diffusion-weighted imaging (DWI) with ADC maps, and axial T1-weighted point resolved spectroscopy (PRESS)-localized 1HMRS. Because we were focused on variability of concentration (ratios) of such compounds such as glutamate and glutamine, we performed short-time echo MRS. The PRESS-localized 1HMRS was performed with VOI 12 × 12 × 12 mm, echo time 35 ms, and repetition time 2000 ms. We used 3-pulse chemical shift selective water suppression and shimming, optimized on the slice of interest. Two additional saturation bands were placed around the PRESS box to minimize lipid contamination. The 1HMRS voxel grid was carefully centred on the slices in 3 perpendicular planes placed within the brain to avoid contamination of the spectra by a lipid signal from the bone marrow or subcutaneous tissue, but to include as much of the deep grey matter as possible. We coregistered 1HMRS and FLAIR data and visualized the single voxel MR spectroscopy box superimposed onto the corresponding anatomical images using in-house software. Spectroscopic processing was performed on a voxel-by-voxel basis after setting the residual water signal in each voxel to a chemical shift of 4.69 ppm. All spectroscopic data were modelled in the time domain by 7 Gaussian components (corresponding to choline, creatine, N-acetyl aspartate containing compounds, glutamine and glutamate, and the 2 components of lactate). We transformed the data to spectra for display and visual quality control. We discarded spectra with fitted line widths < 1 Hz or > 10 Hz, and metabolite peaks > 0.1 ppm offset from their expected values and from voxels containing > 20% cerebrospinal fluid. Each 1HMRS data set took ~7 minutes to acquire. Metabolites from both cerebral hemispheres were quantified using Cr as a reference; the Lac/Cr and Glx/Cr ratios were calculated.
Then, axial DWI with spin echo-planar imaging, fat-suppression, b values of 0 and 1000 s/mm2, TR 14528 ms, TE 93 ms, slice thickness 4 mm, number of excitations (NEX) 2, and matrix 192 × 165 were obtained. DWI assessment included both qualitative interpretation of DWI for lesion detection and possible quantitative measurement of ADC for lesion characterization. Eventually, the ADC values were not calculated on the ADC maps because none of the patients nor healthy volunteers manifested lesions with restricted diffusion.
A brief presentation of scanning parameters is shown in Table 1.
Table 1.
Scanning parameters
| Pulse sequence | TR/TE (msec/msec) | Slice thickness | Matrix | NEX |
|---|---|---|---|---|
| T2-weighted (FSE) | 3800/108 | 4 mm | 308 × 280 | 1 |
| FLAIR | 4800/125 | 3 mm | 192 × 165 | 2 |
| DWI | 4440/91 | 4 mm | 192 × 165 | 2 |
| 1HMRS-PRESS | 2000/31 |
Statistical analysis
We compared values between deep grey matter regions of both cerebral hemispheres between patients who were suffering from brain fog after confirmed COVID-19 and healthy volunteers, using the Kruskal-Wallis and Mann-Whitney U tests. Not all available data were used (e.g. a patient who suffered from SM was excluded). The abnormality of Glx and Lac concentration was compared with results acquired in the control group by brain subregion (Wilcoxon test). On this studies’ purpose the correlation with clinical data has not been made.
In order to quantify the concentration of metabolites the linear combination model (“LCModel”) method was used, because it includes automatic phase correction and baseline correction. We did not attempt to quantify absolute metabolite concentrations but decided to estimate ratios of Glx and Lac using Cr as a reference.
Patient recruitment
We initiated recruitment with 14 healthy controls and 14 non-brain-fog patients in both hospitals, but 1 patient with severe leukoaraiosis and 1 with multiple sclerosis were excluded, leaving 12 patients with brain fog. One patient was unable to tolerate MRI and produced no images, leaving 11 patients with performed imaging for analysis.
Results
The mean age was 47 years, range 21 to 88 years, including 13 males and 12 females. The groups did not differ in age or gender.
The mean Lac/Cr ratio for healthy controls was 0.153 (SD = 0.039, p < 0.05), Lac/Cr in the patient group was 0.106 (SD = 0.0349, p = 0.07). The mean Glx/Cr ratio for healthy controls was 0.530 (SD = 0.352, p < 0.05), Glx/Cr in the patient group was 9.489 (SD = 13.282, p < 0.05).
The results of the statistical analysis are presented in Figures 1 and 2.
Figure 1.
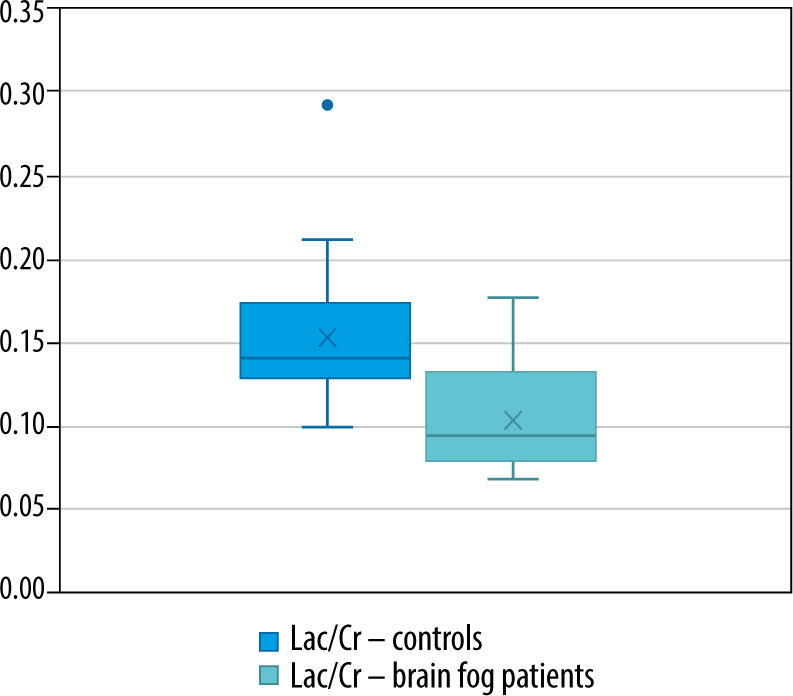
Box plots showing the distribution of the Lac/Cr in healthy controls and patients with brain fog symptoms
Figure 2.
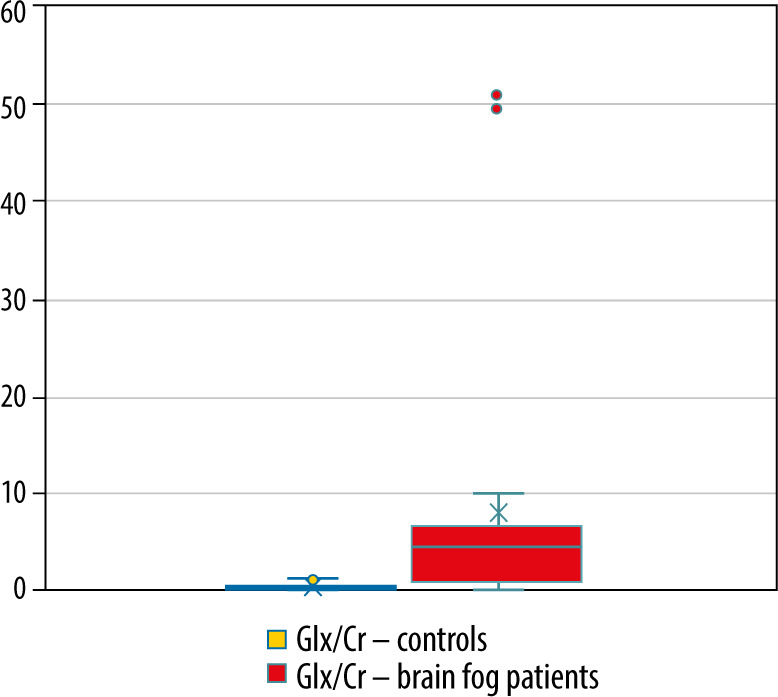
Box plots showing the distribution of the Glx/Cr in healthy controls and patients with brain fog symptoms
Discussion
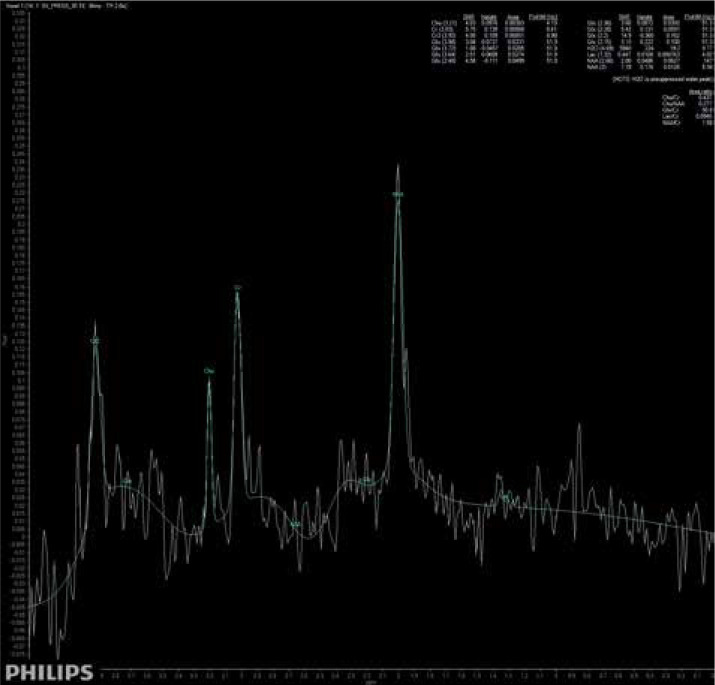
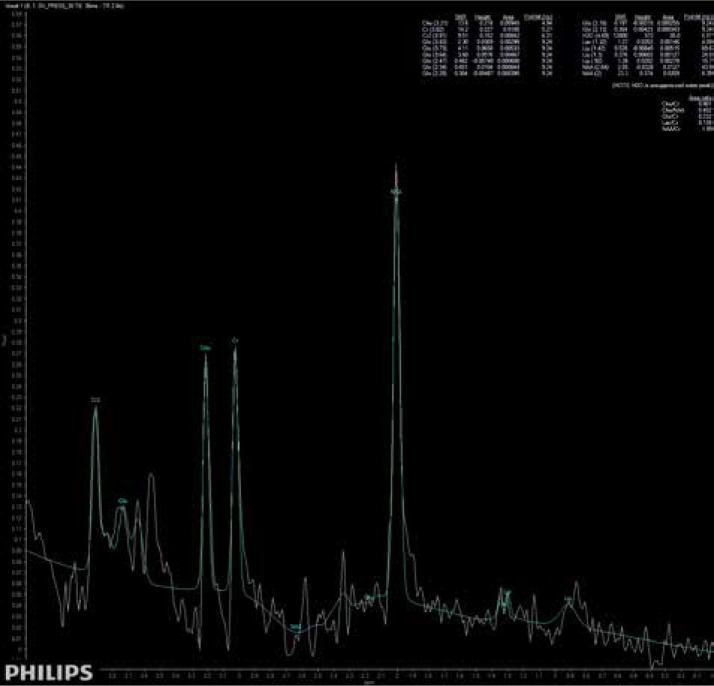
The main findings of the present study are that patients suffering from brain fog may exhibit subtle changes in brain metabolites, similar to those seen in patients suffering from insufficiency of brain blood circulation. Moreover, these patients do not present any other specific radiological symptoms of cerebral injury. A significantly higher concentration of Glx has been found in deep cerebral grey matter in patients suffering from brain fog, as shown in Figure 3, comparing to the metabolic status of a healthy volunteer, as shown in Figure 4. We extrapolate the knowledge of biochemical changes seen in stroke and recognize the metabolic features suggestive of cerebral hypoperfusion. As in stroke, the brain becomes low in energy substrates including glucose and oxygen, and resorts to anaerobic respiration. This leads to increased lactic acid, a byproduct of anaerobic glycolysis. Lactic acid has a negative impact on tissue metabolism and can potentially destroy cells [13,14]. In our case the lactic acid concentration was mildly reduced, which may be considered either as an effect of activation of mechanisms aiming on retrieval of intracellular homeostasis or as an error caused by the relatively small number of probes. The further cascade of cerebral injury is initiated when the energy production such as ATP fails, leading to failure of energy-dependent processes [13] and the release of excitatory neurotransmitters such as glutamate. It acts on receptors in neuronal cells and therefore produces an influx of calcium that activates enzymes that digest cellular proteins, lipids, and nuclear materials. Unlike in stroke, in brain fog the deterioration of cerebral function in limited. Macroscopic manifestation on neuronal disintegration of blood brain barrier was not seen in any of the cases.
Figure 3.
Short-time SV MRS spectrum acquired from the region of deep brain structures of a patient suffering from brain fog after COVID-19 infection; Cho – choline, Cr – creatine, Naa – N-acetyl-aspartate, Glx – glutamine/glutamate and GABA, Lac – lactates
Figure 4.
Short-time SV MRS spectrum acquired from the region of deep brain structures of a healthy control; Cho – choline, Cr – creatine, Naa – N-acetylaspartate, Glx – glutamine/glutamate and GABA, Lac – lactates
Lactates are not detectable under normal circumstances; however, in pathological conditions such as neoplasm, hypoxia, or ischaemia the Lac (lactates) are often detected.
Glutamate (Glu) overlaps with glutamine (Gln), and they are detected as a Glx peak. It can be elevated in MS [15], liver failure, and Raye’s syndrome [16]. In the current study, we analysed consecutive MR brain imaging scans in patients with brain fog proceeding from 2 major regional COVID-19 hospitals and found that these 11 patients had a higher concentration of Glx and mild reduction of Lac in the deep grey matter of both cerebral hemispheres compared to healthy controls not suffering from brain fog.
Brain fog remains a common long-term side effect of COVID-19 of unknown mechanism. A wide range of unspecific lingering symptoms and the impossibility to explain who will suffer from it and who will not point out the necessity of research into this matter. Taking into consideration our knowledge regarding cerebral manifestation of other viral infections, we anticipate a variety of possible scenarios. Brain fog is one of the long-covid symptoms occurring in all subpopulations of patients regardless of age, sex, severity of COVID-19, comorbidities, or vaccination status.
There may be several explanations as to why MR spectroscopy did not show an increase of Glx/Cr ratio in every patient. First of all, the brain fog may linger for a non-defined period. Therefore, some patients might have undergone MRS in the recovery phase when the level of Glx was normalizing. Secondly, we performed SV MRS only and not MV MRS acquiring the signal from the voxel positioned in the basal ganglia. This may be a drawback if vascular changes would not distribute evenly showing a predilection to specific cerebral regions. Thirdly, MR spectroscopy is a very demanding imaging method, and the lack of consistent results in other patients may be caused by various reasons such as motion artifacts, lack of proper suppression of water signal, or magnetic field heterogeneity.
Study limitations
Our study has several limitations. First, the only patients who were examined were suffering from severe brain fog, requiring hospital admission. Hence, we cannot confirm whether similar changes occur in patients with mild or moderate symptoms of brain fog; therefore, the findings need to be confirmed by a larger randomized clinical trial. The total number of examined brain fog patients was limited because the majority of them were not treated by a neurologist or a GP. Currently, there is no proper definition of “brain fog”, and no specific quantitative criteria can be taken into consideration in order to perform the statistical analysis of gathered data. We are aware that the presented results should be considered as preliminary, and we should compare them with the data from patients who suffered from COVID-19 and did not present further symptoms of brain fog. Then it would still require long-term follow-up to complete the mortality, stroke, neurological, and cognitive function assessment.
Cognitive screening tools to assess patients for cognitive symptoms after COVID-19 have some limitations because there are no tools that have been designed specifically for use in a COVID-19 population. Thus, cognitive screening tests developed for other patient populations must be implemented because there is an increasing need for COVID-19 patients to be tested for brain fog. It is also important to note that psychological and cognitive symptoms may occur simultaneously. It can be challenging to determine the aetiology of symptoms and whether a person is experiencing true cognitive impairment or impairment secondary to psychological distress related to the infection. Despite that, development of neuropsychological tools in the future, specifically designed for the purpose of diagnosing and assessing brain fog, may help many clinicians to determine the presence of brain fog in COVID-19 patients and propose adequate treatment and rehabilitation in order to improve the cognitive functioning of those patients [5,7].
None of the patients who were included in the study had a history of neurological or psychiatric disorders prior to COVID-19 infection. Having said that, we must admit that it is not possible to exclude that patients suffered from conditions that may result in changes in the Glx level within the brain not being properly diagnosed.
It should be also taken into account that the quantification of Glx and Lac was based on their relative amounts using Cr as a reference. It is possible that Cr concentration changes its concentration simultaneously and therefore the results are misleading.
Conclusions
A significantly higher concentration of Glx and mild reduction of Lac has been found in deep cerebral grey matter in patients suffering from brain fog, which needs to be further confirmed by future studies. Extrapolating knowledge from other inflammatory processes we would assume that the results or biochemical status of our patients’ brains returned to normal as the rehabilitation process finished and their symptoms disappeared.
This leads to several important conclusions:
brain fog is a result of transient inadequate brain blood administration,
possible insufficiency of cerebral microvasculature may be an explanation,
possible correlation with virus type and pathomechanism remains unclear,
possible occurrence of brain fog and increased risk of further impairment of cognitive function and long-term dementia needs to be investigated.
The presented study is the first to provide insights into the risk of transient brain ischaemia during brain fog. We propose to examine whether this poorly understood condition potentially deteriorates patients’ long-term neurological and cognitive functioning.
Conflicts of interest
The authors report no conflict of interest.
References
- 1.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hugon J, Msika E, Queneau M, et al. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol 2021; doi: 10.1007/s00415-021-10655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office of National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 April 2021. [Google Scholar]
- 4.Woo MS, Malsy J, Pöttgen J., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Communications 2020; 2: fcaa205. doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao J, Zhang J, Sun D, et al. Discriminating poststroke depression from stroke by nuclear magnetic resonance spectroscopy-based metabonomic analysis. Neuropsychiatr Dis Treat 2016; 12: 1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldrini M, Canoll PD, Klein RS. How COVID-19 affects the brain. JAMA Psychiatry 2021; doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callan C, Ladds E, Husain L, et al. “I can’t cope with multiple inputs”: qualitative study of the lived experience of ‘brain fog’ after Covid-19. BMJ Open 2021; doi: 10.1101/2021.08.07.21261740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frontera J, Lewis A, Melmed K, et al. Prevalence and predictors of prolonged cognitive and psychological symptoms following COVID-19 in the United States. Front Aging Neurosci 2021; 13: 690383. doi: 10.3389/fnagi.2021.690383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaschetto R, Cena T, Sainaghi PP, et al. Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J Clin Neurosci 2020; 79: 71-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke 2021; 16: 137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sklinda K, Dorobek M, Wasilewski P, et al. Radiological manifestation of neurological complications in the course of SARS-CoV-2 infection. Front Neurol 2021; 12: 711026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . In the wake of the pandemic: preparing for Long COVID 2021. Available at: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/publications-and-technical-guidance/2021/in-the-wake-of-the-pandemic-preparing-for-long-covid-2021. [PubMed]
- 13.Back T, Hemmen T, Schuler OG. Lesion evolution in cerebral ischemia. J Neurol 2004; 251: 388-397. [DOI] [PubMed] [Google Scholar]
- 14.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des 2008; 14: 3565-3573. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan R, Sailasuta N, Hurd R, et al. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 2005; 128 (Pt 5): 1016-1025. [DOI] [PubMed] [Google Scholar]
- 16.Kreis R, Pfenninger J, Herschkowitz N, Boesch C. In vivo proton magnetic resonance spectroscopy in a case of Reye’s syndrome. Intensive Care Med 1995; 21: 266-269. [DOI] [PubMed] [Google Scholar]