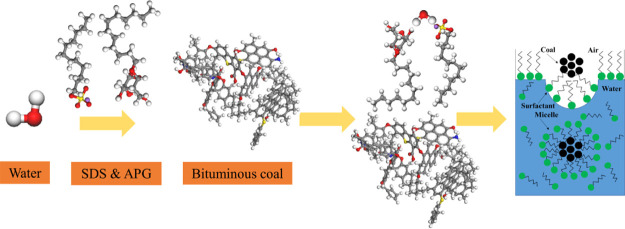
Abstract
To solve the problem of poor dust wettability during coal mine dust treatment, sodium dodecyl sulfate (SDS) and alkyl glycoside (APG1214) were selected for compounding. An efficient, environmentally friendly, economical wetting agent was prepared. First, through molecular dynamics simulation studies, it was determined that the tail group C of SDS and APG1214 was adsorbed on the surface of bituminous coal, and the head groups S and O were adsorbed on the surface of water. The simulation result is found to be consistent with the surfactant solution dust removal theory, which proves the confidence of simulation. Then, by comparing the interaction of water–SDS and APG1214–bituminous coal and water–bituminous coal systems and the number of hydrogen bonds, the wetting mechanism of the SDS and APG1214 solution on bituminous coal was revealed. Finally, the surface tension, contact angle, and wetting time of different SDS and APG1214 solutions were determined by experiments and they decreased with decreasing mass fraction of SDS at the same concentration. The surface tension of the SDS and APG1214 solution and the number of micelles affected the wettability of bituminous coal. The optimal concentration of the SDS and APG1214 solution was 0.7%, and the optimal ratio was SDS/APG1214 = 1:3.
Introduction
China is the largest coal producer and consumer. According to ⟨⟨BP Statistical Review of World Energy⟩⟩ in 2021, China’s total coal production and consumption in 2020 account for 50.7 and 54.3% of the world, respectively.1 This scenario would remain unaltered in the short term.2,3 However, the inhalation of the fine dust produced in the process of coal mining causes a number of respiratory diseases4−6 including chronic obstructive pulmonary disease, fibrosis associated with diffuse dust, artificial pneumoconiosis, and emphysema.7−10 In addition, dust hazards would become more severe with the continuous improvement in the level of coal mining mechanization.11
At present, the main method of dust removal in a coal mine of Lingshi County, Jinzhong, China, is spray dust suppression. The spray dust suppression is an inexpensive and convenient method.12−14 However, water is poor to the wettability of bituminous coal. At 20 °C, the surface tension of pure water is approximately 72.8 mN/m,15 whereas the critical surface tension for coal wetting is approximately 45 mN/m.16 Furthermore, the dust suppression efficiency is low. The addition of surfactants to water can reduce the surface tension of water and improve the wettability of coal.17,18 Therefore, the dust concentration and the instrument failure rate in the working environment of coal mine are lowered, the visibility is improved, and the health of the human body is maintained.
Scholars worldwide have conducted a substantial amount of research on the wettability of coal dust by different surfactants. Xu et al.19 experimentally measured the surface tension, wetting time, and infrared spectrum of coal dust and calculated the hydrophilic–lipophilic balance value to determine the wettability of coal dust by different anionic surfactants. Shi et al.15 studied the synergistic wetting performance of different nonionic and anionic surfactants on bituminous coal; measured the surface tension, contact angle, and settling time of bituminous coal; and formulated the optimal surfactant. Wang et al.20 analyzed the wetting process and mechanism of different surfactant solutions on various coal-dust surfaces by experimental measurements and theoretical calculations. The study provides a theoretical basis for the surfactant selection of different coal mines. The above studies were based on the experimental parameters for characterizing the wettability of coal dust. The wettability of different surfactants to the coal dust were determined, and the optimum surfactant was preferred for different coal dusts. However, the mechanism of coal dust wetting is not clear. In this paper, molecular dynamics simulation was used to study the wetting mechanism of sodium dodecyl sulfate (SDS) and alkyl glycoside (APG1214) solution on bituminous coal.
In this study, SDS and APG1214 were selected for preparing the SDS and APG1214 solution, considering the economy, environmental protection, and secondary dust.21−23 Materials Studio software was used to reveal the wetting mechanism of the SDS and APG1214 solution on bituminous coal, which can explain some phenomena and mechanisms on the atomic structure and microlevel, which cannot be observed in experiments.24−28 The distribution of tail group C, head group S, and O in the water—SDS and APG1214—bituminous coal system, the number of intermolecular hydrogen bonds, and the interaction energy of the water–SDS and APG1214–bituminous coal system and the water–bituminous coal system were obtained. Through experimental research, the surface tension, contact angle, and wetting time of SDS and APG1214 solutions at different concentrations and mass ratios were identified. Based on the above data, the optimal concentration and mass ratio of the SDS and APG1214 solution were determined.
Simulation and Experiment
Coal Sample
The coal sample is from in a coal mine of Lingshi County, Jinzhong, China, and its proximate analysis result is shown in Table 1. The coal sample belonged to the category of highly volatile 1/3 coking coal according to China’s coal classification,29 which is a kind of bituminous coal. The density and porosity of the coal are 1.27 g/cm and 4.87%, respectively.
Table 1. Proximate Analysis Result of the Coal Samplea.
| Mad (%) | Ad (%) | Vdaf (%) | FCd (%) |
|---|---|---|---|
| 0.72 | 16.04 | 31.96 | 57.13 |
Note: Mad = moisture (air-dry basis); Ad = ash (dry basis); Vdaf = volatile matter (air-dry basis); FCd = fixed carbon (dry basis).
Simulation Details
Materials Studio 2019 software was used to perform molecular dynamics simulation. Because the coal sample in the experiment belongs to bituminous coal, the paper selects bituminous coal model proposed by Wiser.30,31 Although the model is inconsistent with the model of bituminous coal in the experiment, it contains sulfur and nitrogen elements, and the oxygen-containing functional group related to bituminous coal wettability, such as phenolic hydroxyl groups, carboxyl groups, carbonyl groups, and so forth. Therefore, Wiser’s bituminous coal model is used to study the wetting mechanism of the SDS and APG1214 solution to experimental bituminous coal, and the simulation results are reliable. The structure is shown in Figure 1a. The molecular structures of SDS and APG1214 are shown in Figure 1b,c, respectively. Because the COMPASS II force field applies to organics (coal and the surfactant), small inorganic molecules (water), and polymers materials, all the calculations of this paper select the force field.
Figure 1.
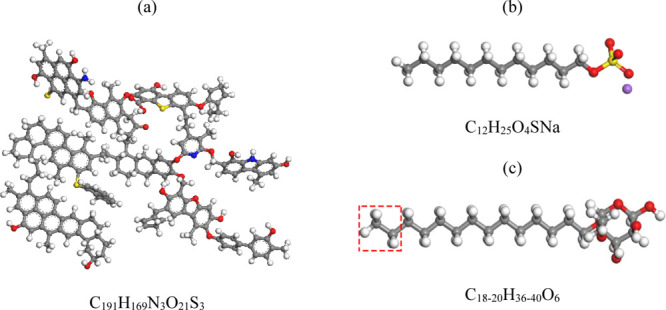
Molecular model of (a) bituminous coal, (b) SDS, and (c) APG1214. The red, gray, white, yellow, and purple balls represent O, C, H, S, and Na+, respectively.
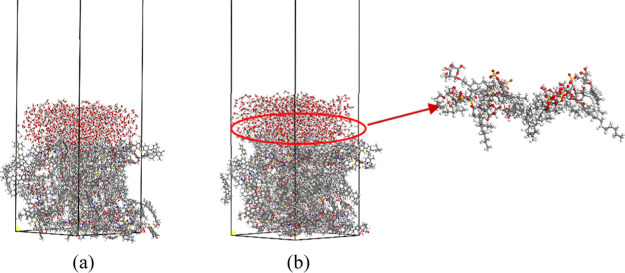
After obtaining the molecular structures of bituminous coal, SDS, and APG1214, the Forcite module was used for molecule structural optimization. The related parameters were set as follows: the task was geometry optimization, the method was smart, and the maximum convergence values of energy, force, displacement, and Max. iteration were 1.0 × 10–4 kcal/mol, 5 × 10–3 kcal/mol/Å, 5 × 10–5 Å, and 50 000, respectively. Second, the Amorphous Cell module was used to build the following crystal cells: a crystal cell with 20 bituminous coal molecules, a crystal cell with 1000 water molecules, and the wetting agent crystal cell consisting of 10 SDS molecules and 10 APG1214 molecules. The length and width of all crystal cells were 43 Å × 43 Å. Then, the above cells were optimized using geometry optimization and anneal approaches.32 Finally, the Build layers tool was used to set up the water–bituminous and water–SDS and APG1214–bituminous systems. The system size was 43 Å × 43 Å × 140 Å. In addition, a 75 Å vacuum layer was added above the systems to prevent any influence of the period boundary conditions, as shown in Figure 2 (a) and (b).
Figure 2.
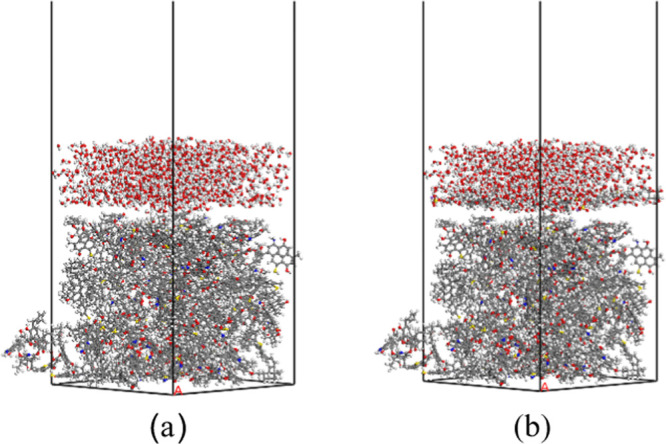
Water–bituminous coal system (a) and water–SDS and APG1214–bituminous coal system (b).
The geometry optimization of water–bituminous coal and water–SDS and APG1214–bituminous coal systems was performed. Then, the molecular dynamic simulation was carried out. The molecular dynamics simulation parameters were set identical to those for geometry optimization. The other parameters were set as follows: the ensemble was NVT, the temperature was 298 K, the time step was 1.0 fs, the total simulation time was 1000 ps (the energy and temperature around 1000 ps are stable, the systems have reached a balance), and the thermostat was Nosé–Hoover–Langevin.
Experiment
Experimental Materials
SDS used in the experiment (purity ≥ 90%) was provided by Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China. APG1214 (purity ≥ 50%) was produced by Yousuo Chemical Technology Co., Ltd., Shandong, China.
Experimental Process and Method
The SDS and APG1214 monomer solutions with mass fractions of 0.1, 0.3, 0.5, 0.7, and 0.9% were compounded. The compounding ratios of SDS and APG1214 were 4:0, 3:1, 2:2, 1:3, and 0:4. Tap water was used for spray dust removal in the field, so tap water was used to prepare the solution. A contact angle measuring instrument was used to measure the surface tension and contact angle, and each solution was measured three times to take the average value. The spreading coefficient was obtained by surface tension and contact angle.
The wetting effect of bituminous coal was characterized by measuring its wetting time. The operational process of the test system (as shown in Figure 3) was as follows: the surfactant solution (20 mL) was poured into a Petri dish and placed below a funnel with a certain height. Then, the balance was used to weigh 50 mg of bituminous coal samples. These were poured into Petri dishes through a funnel at a fixed height. The time required for the coal samples to be wetted completely was recorded. Each group of experiments was measured three times and averaged.
Figure 3.
Equilibrium structures of different systems: (a) water–bituminous coal system and (b) water–SDS and APG1214–bituminous coal system.
Results and Discussion
Molecular Dynamics Simulation Results
Surfactant Adsorption on the Surface of Bituminous Coal
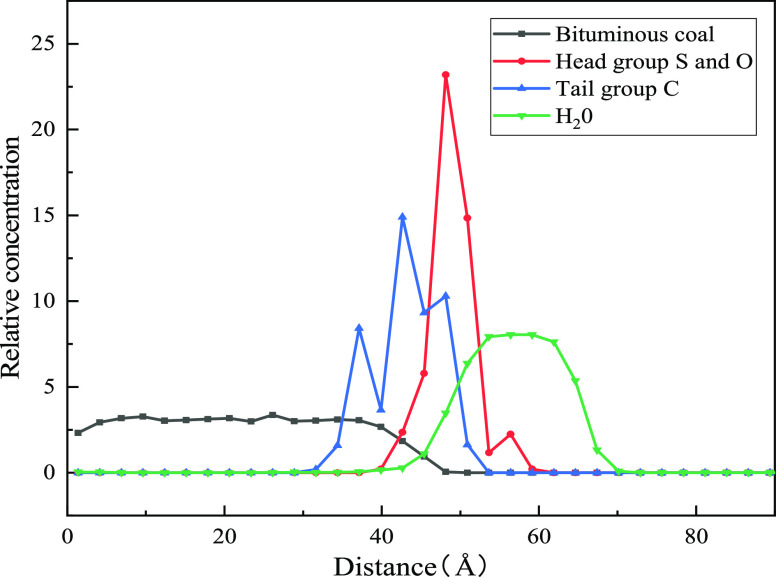
Figure 3 shows equilibrium structures of the water–bituminous coal and water–SDS and APG1214–bituminous coal systems. The hydrophilic group and hydrophobic group of SDS/APG 1214 surfactants adsorb on the surface of water and bituminous coal, respectively. Furthermore, the relative concentrations of water, bituminous coal, surfactant head group S and O, and tail group C in the direction of (0,0,1) to analyze the detailed adsorption behavior of two surfactants are presented in Figure 4. The S and O in the surfactant head group are close to the water surface, and the tail group C is close to the bituminous coal surface. The simulation result is consistent with surfactant solution dust removal theory. Therefore, the wetting mechanism of the surfactant to bituminous coal can be qualitatively analyzed by molecular dynamics simulation.
Figure 4.
Relative concentration distribution of water, bituminous coal, surfactant head group S and O, and tail group C in the z-direction.
Interaction energy
The molecular interaction energy can represent the strength of the intermolecular interaction. The value is negative, which indicates that the intermolecules are more prone to interactions. The higher the negative magnitude of the energy, the stronger is the interaction between molecules. When the value is positive, it is difficult to interact between the molecules.33Table 2 shows the interaction energy of different systems, in which the electrostatic interaction is dominant. The SDS and APG1214 surfactants are added in water, and the potential energy reduction between water molecules results in its cohesiveness decreased and activity increased. In addition, SDS and APG1214 are more easily adsorbed to the surface of the bituminous coal compared to water. Therefore, the addition of surfactants in water increased the wettability of bituminous coal.
Table 2. Interaction Energy of Different Systems (Epot is the Potential Energy, Enon is the Nonbonded Interaction Energy, EvdW is the van der Waals Energy, and Eelec is the Electrostatic Energy).
| systems | Epot (kcal/mol) | Enon (kcal/mol) | EvdW (kcal/mol) | Eelec (kcal/mol) |
|---|---|---|---|---|
| H2O (system a) | –7472.76 | –9048.18 | 2450 | –11498.18 |
| H2O (system b) | –6793.28 | –8341.19 | 2477.57 | –10818.19 |
| H2O–bituminous coal | –698.03 | –666.01 | –131.12 | –534.89 |
| SDS &APG1214–bituminous coal | –1389.91 | –1381.75 | –364.35 | –1017.4 |
| H2O–SDS &; APG1214–bituminous coal | –3819.68 | –3799.09 | –375.87 | –3423.22 |
Hydrogen Bonding
Water molecules contain a large number of hydrogen bonds. The number of hydrogen bonds changes when water molecules come into contact with surfactants and bituminous coal. The hydrogen bonding is a strong electrostatic interaction between atoms manifested at the macrolevel as a stronger interaction. The larger the number of hydrogen bonds between molecules, the stronger is the electrostatic interaction. To obtain the number of hydrogen bonds of among water, SDS and APG1214, and bituminous coal molecules, the hydrogen bonds in same molecules should be subtracted. The parameters of hydrogen bond calculations are as follows: the distance between the molecular hydrogen and receptor is less than 2.5 Å, and the angle among the donor, hydrogen, and the receptor is greater than 135°.24 After adding SDS and APG 1214 in water, the number of hydrogen bonds between water molecules was reduced by 37. This indicates that the cohesion between water molecules is reduced, and the activity is enhanced. The number of hydrogen bonds in water–bituminous coal and water–SDS and APG1214–bituminous coal systems is 58 and 127, respectively. The addition of SDS and APG1214 surfactants increases the intermolecular hydrogen bonding and enhances the intermolecular interaction. These, in turn, improve the wettability of bituminous coal.
Experimental Results
The wetting mechanism of bituminous coal by adding a surfactant comprising SDS and APG1214 in water was identified through molecular dynamics simulation. The optimal concentration and mass ratio of SDS and APG1214 solutions were determined by experimental means.
Surface Tension
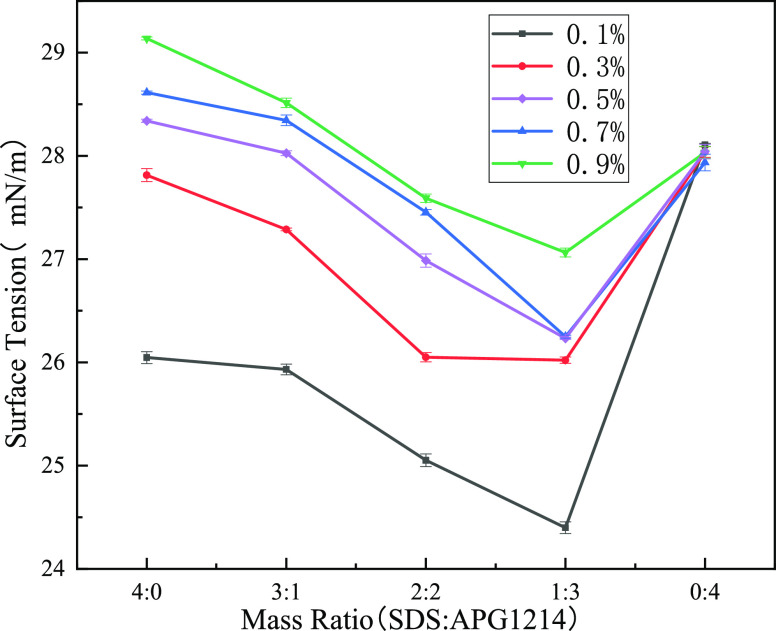
Figure 5 shows the surface tension of SDS and APG1214 solutions with different concentrations and ratios. The surface tension of the SDS and APG1214 solutions decreases with decreasing mass fraction of SDS. The surface tension is minimum when the mass ratio is 1:3. SDS is an anionic surfactant, and the overall charge is −1 after being dissociated in water. APG1214 is a nonionic surfactant that cannot be dissociated in water, and the whole is electrically neutral. Therefore, SDS molecules repel each other at the air–water interface,15,34,35 and the distance between the SDS molecules is larger than the APG1214 molecules. When SDS is compounded with APG1214, the gap of SDS is filled with APG1214. This results in an increase in the adsorption density of the surfactant on the water surface and a decrease in the surface tension.
Figure 5.
Surface tension of SDS and APG1214 solutions at different concentrations and mass ratios.
Contact Angle
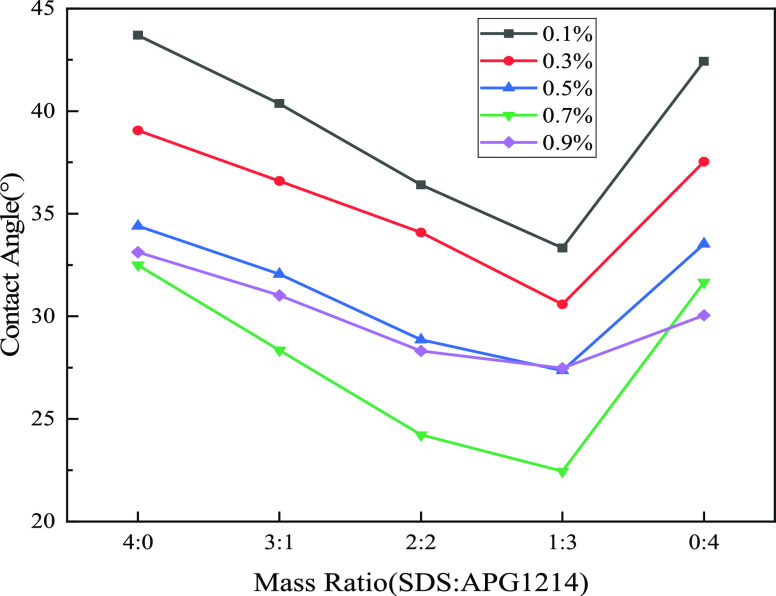
Figure 6 shows the contact angle of bituminous coal in the SDS and APG1214 solution at different concentrations and ratios. The smaller the contact angle, the better the wetting effect of bituminous coal. As shown in Figure 6, the contact angle of the bituminous coal increases with decreasing mass fraction of SDS in the SDS and APG1214 solutions. The minimum contact angle of the bituminous coal in the monomer surfactant is 30.65°. However, when the concentration of the SDS and APG1214 solution is 0.7%, the mass ratio is SDS/APG1214 = 1:3, and the contact angle decreases to 22.445°. For the single surfactant, the compound solutions can improve the hydrophobicity of the bituminous coal.
Figure 6.
Contact angle of bituminous coal at different concentrations and mass ratios.
Wetting Time
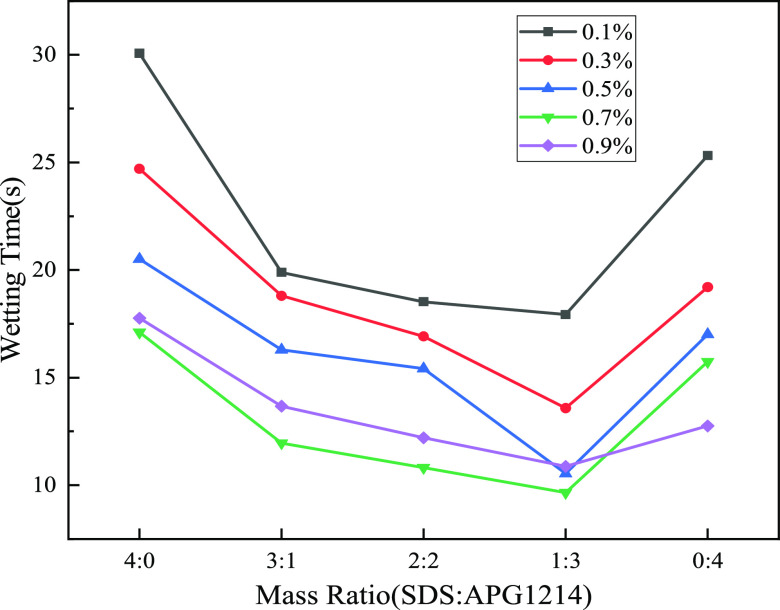
To visually analyze the wetting performance of different SDS and APG1214 solutions, the wetting time of bituminous coal was measured in addition to the above two parameters of surface tension and contact angle. Figure 7 shows the wetting time of bituminous coal in different SDS and APG1214 solutions. The optimal mass concentration is 0.7%, and the optimal ratio is SDS/APG1214 = 1:3, which is consistent with the measurement result of contact angle. The surface tension and the number of micelles determine the wettability of the composite solution. The lower the surface tension, the more easy it is that the droplet would deform and break and the better the wetting effect. With the increase in concentration, the surfactant self-polymerizes to form micelles (as shown in Figure 8). The solubilization of micelles causes insoluble bituminous coal particles to enter these, and the wetting improves (as shown in Figure 9).
Figure 7.
Wetting time of bituminous coal at different concentrations and mass ratios.
Figure 8.
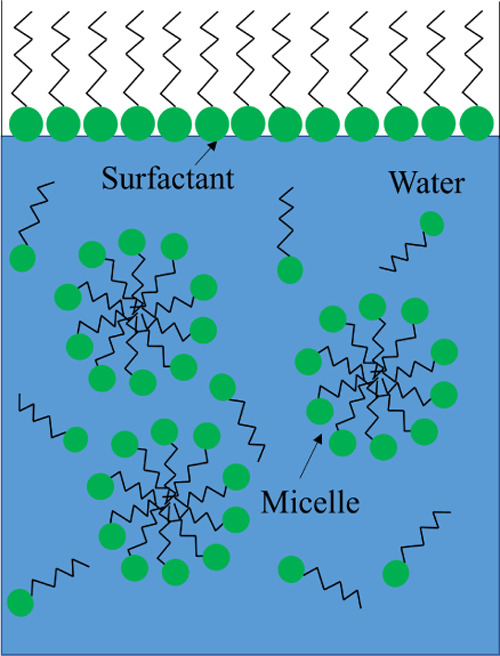
Surfactant forms micelles in solution.
Figure 9.
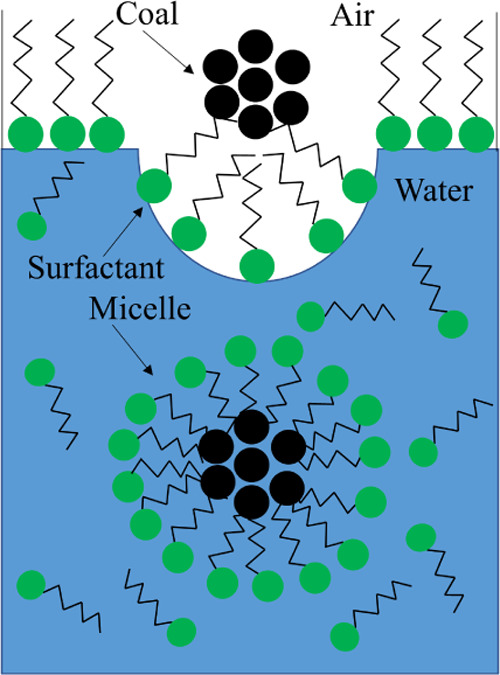
Dynamic soaking process of bituminous coal from the gas–liquid surface into surfactant micelles.
Conclusions
Through molecular dynamics simulation, the wetting mechanism of the SDS and APG1214 solution to bituminous coal was revealed: SDS and APG1214 are more easily adsorbed onto the surface of the bituminous coal compared to water; the cohesion between water molecules is reduced, and the activity is enhanced; the van der Waals force, electrostatic force, and the number of hydrogen bonds are increased in the water–SDS and APG1214–bituminous coal system.
The optimal concentration and mass ratio of the SDS and APG1214 solution for wetting bituminous coal were determined by surface, contact angle, and wetting time experiments. For various SDS and APG1214 solutions, the lower the surface tension and the SDS mass fraction, the more the number of micelles and the better the wettability of bituminous coal. The optimal concentration of the final SDS and APG1214 solution is 0.7%, and the optimal ratio is SDS/APG1214 = 1:3.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (grant number 52174195), Shanxi Province Foundation for Basic Research (grant number 20210302123122), Transformation of Scientific and Technological Achievements Programs of Higher Education Institutions in Shanxi (grant number 2020CG022).
Author Contributions
S.L. carried out the molecular genetic studies, participated in the sequence alignment, and drafted the manuscript. J.D. carried out the immunoassays. C.X. participated in the sequence alignment. L.S. participated in the design of the study and performed the statistical analysis. G.S. conceived the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
References
- Looney B.BP Statistical Review of World Energy 2021, 70th ed.; BP: London, U.K., 2021. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf.
- Cui K.; Shen F.; Han B.; Yuan J.; Suo X.; Qin T.; Liu H.; Chen J. Comparison of the Cumulative Incidence Rates of Coal Workers’ Pneumoconiosis between 1970 and 2013 among Four State-Owned Colliery Groups in China. Int. J. Environ. Res. Publ. Health 2015, 12, 7444–7456. 10.3390/ijerph120707444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney B. C.; Blackley D.; Hale J. M.; Halldin C.; Kurth L.; Syamlal G.; Laney A. S. Respirable coal mine dust in underground mines, United States, 1982-2017. Am. J. Ind. Med. 2019, 62, 478–485. 10.1002/ajim.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodny J.; Tutak M. Exposure to harmful dusts on fully powered longwall coal mines in Poland. Int. J. Environ. Res. Public Health 2018, 15, 1846. 10.3390/ijerph15091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J.; Wang L.; Au W.; Su M. Prevalence of coal workers’ pneumoconiosis in China: A systematic analysis of 2001-2011 studies. Int. J. Hyg. Environ. Health 2014, 217, 46–51. 10.1016/j.ijheh.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Hendryx M.; Islam M. S.; Dong G.-H.; Paul G. Air Pollution Emissions 2008-2018 from Australian Coal Mining: Implications for Public and Occupational Health. Int. J. Environ. Res. Public Health 2020, 17, 1570. 10.3390/ijerph17051570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go L. H. T.; Krefft S. D.; Cohen R. A.; Rose C. S. Lung disease and coal mining. Curr. Opin. Pulm. Med. 2016, 22, 170–178. 10.1097/mcp.0000000000000251. [DOI] [PubMed] [Google Scholar]
- Enterline P. E.; Lainhart W. S. The relationship between coal mining and chronic nonspecific respiratory disease. Am. J. Public Health 1967, 57, 484–495. 10.2105/ajph.57.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridbord K.; Costello J.; Gamble J.; Groce D.; Hutchison M.; Jones W.; Merchant J.; Ortmeyer C.; Reger R.; Wagner W. L. Occupational safety and health implications of increased coal utilization. Environ. Health Perspect. 1979, 33, 285–302. 10.1289/ehp.7933285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabila S. L.; Almberg K. S.; Friedman L.; Cohen R.; Ndlovu N.; Vorajee N.; Murray J. Occupational emphysema in South African miners at autopsy; 1975-2014. Int. Arch. Occup. Environ. Health 2018, 91, 981–990. 10.1007/s00420-018-1335-2. [DOI] [PubMed] [Google Scholar]
- Perret J. L.; Plush B.; Lachapelle P.; Hinks T. S. C.; Walter C.; Clarke P.; Irving L.; Brady P.; Dharmage S. C.; Stewart A. Coal mine dust lung disease in the modern era. Respirology 2017, 22, 662–670. 10.1111/resp.13034. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhou F.; Li S. Experimental study on the dust filtration performance with participation of water mist. Process Saf. Environ. Prot. 2017, 109, 357–364. 10.1016/j.psep.2017.04.006. [DOI] [Google Scholar]
- Zhou W.; Nie W.; Liu C.; Liu Q.; Hetang W.; Wei C.; Yan J.; Yin S.; Xiu Z.; Xu C. Modelling of ventilation and dust control effects during tunnel construction. Int. J. Mech. Sci. 2019, 160, 358–371. 10.1016/j.ijmecsci.2019.06.037. [DOI] [Google Scholar]
- Wang Q.; Wang D.; Wang H.; Shen Y.; Zhu X. Experimental investigations of a new surfactant adding device used for mine dust control. Powder Technol. 2018, 327, 303–309. 10.1016/j.powtec.2017.12.080. [DOI] [Google Scholar]
- Shi G.-Q.; Han C.; Wang Y.-m.; Wang H.-T. Experimental study on synergistic wetting of a coal dust with dust suppressant compounded with noncationic surfactants and its mechanism analysis. Powder Technol. 2019, 356, 1077–1086. 10.1016/j.powtec.2019.09.040. [DOI] [Google Scholar]
- Xu G.; Chen Y.; Eksteen J.; Xu J. Surfactant-aided coal dust suppression: a review of evaluation methods and influencing factors. Sci. Total Environ. 2018, 639, 1060–1076. 10.1016/j.scitotenv.2018.05.182. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Xu G.; Huang J.; Eksteen J.; Liu X.; Zhao Z. Characterization of coal particles wettability in surfactant solution by using four laboratory static tests. Colloids Surf., A 2019, 567, 304–312. 10.1016/j.colsurfa.2019.01.068. [DOI] [Google Scholar]
- Chang P.; Zhao Z.; Xu G.; Ghosh A.; Huang J.; Yang T. Evaluation of the coal dust suppression efficiency of different surfactants: A factorial experiment. Colloids Surf., A 2020, 595, 124686. 10.1016/j.colsurfa.2020.124686. [DOI] [Google Scholar]
- Xu C.; Wang D.; Wang H.; Ma L.; Zhu X.; Zhu Y.; Zhang Y.; Liu F. Experimental investigation of coal dust wetting ability of anionic surfactants with different structures. Process Saf. Environ. Prot. 2019, 121, 69–76. 10.1016/j.psep.2018.10.010. [DOI] [Google Scholar]
- Wang X.; Yuan S.; Jiang B. Experimental investigation of the wetting ability of surfactants to coals dust based on physical chemistry characteristics of the different coal samples. Adv. Powder Technol. 2019, 30, 1696–1708. 10.1016/j.apt.2019.05.021. [DOI] [Google Scholar]
- Zhou Y.; Huang H.; Ding Z.; Lu D. The Investigation of Preparing Sodium Lauryl Sulfate. Exp. Technol. Manag. 2006, 3, 41–43. [Google Scholar]
- Wang Q.; Wang D.; Wang H.; Xu C. Influence of alkyl polyglucoside and fatty alcohol ether sulfate on the foaming and wetting properties of sodium dodecyl benzene sulfonate for mine dust control. Powder Technol. 2019, 345, 91–98. 10.1016/j.powtec.2018.12.084. [DOI] [Google Scholar]
- Chai J. L.; Cui X. C.; Zhang X. Y.; Song M. M.; Wang J.; Lu J. J. Adsorption equilibrium and dynamic surface tension of alkyl polyglucosides and their mixed surfactant systems with CTAB and SDS in the surface of aqueous solutions. J. Mol. Liq. 2018, 264, 442–450. 10.1016/j.molliq.2018.05.055. [DOI] [Google Scholar]
- Guo J.; Zhang L.; Liu S.; Li B. Effects of hydrophilic groups of nonionic surfactants on the wettability of lignite surface: molecular dynamics simulation and experimental study. Fuel 2018, 231, 449–457. 10.1016/j.fuel.2018.05.106. [DOI] [Google Scholar]
- Li B.; Guo J.; Liu S.; Albijanic B.; Zhang L.; Sun X. Molecular insight into the mechanism of benzene ring in nonionic surfactants on low-rank coal floatability. J. Mol. Liq. 2020, 302, 112563. 10.1016/j.molliq.2020.112563. [DOI] [Google Scholar]
- Li B.; Liu S.; Fan M.; Zhang L. The effect of ethylene oxide groups in dodecyl ethoxyl ethers on low rank coal flotation: An experimental study and simulation. Powder Technol. 2019, 344, 684–692. 10.1016/j.powtec.2018.12.063. [DOI] [Google Scholar]
- Li B.; Liu S.; Guo J.; Zhang L.; Sun X. Increase in wettability difference between organic and mineral matter to promote low-rank coal flotation by using ultrasonic treatment. Appl. Surf. Sci. 2019, 481, 454–459. 10.1016/j.apsusc.2019.03.142. [DOI] [Google Scholar]
- Zhang L.; Sun X.; Li B.; Xie Z.; Guo J.; Liu S. Experimental and molecular dynamics simulation study on the enhancement of low rank coal flotation by mixed collector. Fuel 2020, 266, 117046. 10.1016/j.fuel.2020.117046. [DOI] [Google Scholar]
- General Administration of Quality Supervision . Inspection and Quarantine of the People’s Republic of China/China National Standardization Administration Committee. Chinese Classification of Coals. GB/T 5751-2009; China Standard Press: Peking, 2009.
- Wiser W. H.Conversion of bituminous coal to liquids and gases: chemistry and representative processes. In Magnetic resonance; Springer, 1984; Vol. 124, pp 325–350. ASIC, [Google Scholar]
- Zhang Z.; Wang C.; Yan K. Adsorption of collectors on model surface of Wiser bituminous coal: A molecular dynamics simulation study. Miner. Eng. 2015, 79, 31–39. 10.1016/j.mineng.2015.05.009. [DOI] [Google Scholar]
- Li B.; Guo J.; Liu S.; Albijanic B.; Zhang L.; Sun X. Molecular insight into the mechanism of benzene ring in nonionic surfactants on low-rank coal floatability. J. Mol. Liq. 2020, 302, 112563. 10.1016/j.molliq.2020.112563. [DOI] [Google Scholar]
- Zhang L.; Sun X.; Li B.; Xie Z.; Guo J.; Liu S. Experimental and molecular dynamics simulation study on the enhancement of low rank coal flotation by mixed collector. Fuel 2020, 266, 117046. 10.1016/j.fuel.2020.117046. [DOI] [Google Scholar]
- Wang X.; Yuan S.; Li X.; Jiang B. Synergistic effect of surfactant compounding on improving dust suppression in a coal mine in Erdos, China. Powder Technol. 2019, 344, 561–569. 10.1016/j.powtec.2018.12.061. [DOI] [Google Scholar]
- Wang C.; Cao X.-L.; Guo L.-L.; Xu Z.-C.; Zhang L.; Gong Q.-T.; Zhang L.; Zhao S. Effect of adsorption of catanionic surfactant mixtures on wettability of quartz surface. Colloids Surf., A 2016, 509, 564–573. 10.1016/j.colsurfa.2016.09.057. [DOI] [Google Scholar]