Abstract
Background
In addition to their proinflammatory effect, eosinophils have antiviral properties. Similarly, inhaled corticosteroids (ICS) were found to suppress coronavirus replication in vitro and were associated with improved outcomes in coronavirus disease 2019 (COVID-19). However, the interplay between the two and its effect on COVID-19 needs further evaluation.
Objective
To determine the associations among preexisting blood absolute eosinophil counts, ICS, and COVID-19–related outcomes.
Methods
We analyzed data from the Cleveland Clinic COVID-19 Research Registry (April 1, 2020 to March 31, 2021). Of the 82,096 individuals who tested positive, 46,397 had blood differential cell counts obtained before severe acute respiratory syndrome coronavirus 2 testing dates. Our end points included the need for hospitalization, admission to the intensive care unit (ICU), and in-hospital mortality. The effect of eosinophilia on outcomes was estimated after propensity weighting and adjustment.
Results
Of the 46,397 patients included in the final analyses, 19,506 had preexisting eosinophilia (>0.15 × 103 cells/μL), 5,011 received ICS, 9,096 (19.6%) were hospitalized, 2,129 required ICU admission (4.6%) and 1,402 died during index hospitalization (3.0%). Adjusted analysis associated eosinophilia with lower odds for hospitalization (odds ratio [OR] [95% confidence interval (CI)]: 0.86 [0.79-0.93]), ICU admission (OR [95% CI]: 0.79 [0.69-0.90]), and mortality (OR [95% CI]: 0.80 [0.68-0.95]) among ICS-treated patients but not untreated ones. The correlation between absolute eosinophil count and the estimated probability of hospitalization, ICU admission, and death was nonlinear (U-shaped) among patients not treated with ICS, and negative in treated patients.
Conclusions
The association between eosinophilia and improved COVID-19 outcomes depends on ICS. Future randomized controlled trials are needed to determine the role of ICS and its interaction with eosinophilia in COVID-19 therapy.
Key words: Severe acute respiratory syndrome coronavirus 2, COVID-19, Inhaled corticosteroids, Asthma, Chronic obstructive pulmonary disease, Eosinophilia
Abbreviations used: ACE2, Angiotensin converting enzyme 2; AEC, Absolute eosinophil count; BMI, Body mass index; CBC, Complete blood count; CCCRR, Cleveland Clinic COVID-19 Research Registry; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; ICS, Inhaled corticosteroids; ICU, Intensive care unit; RCT, Randomized controlled trial; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; T2, Type 2
What is already known about this topic? Low eosinophil counts during acute severe acute respiratory syndrome coronavirus 2 infection are associated with worse outcomes.
What does this article add to our knowledge? Baseline preexisting immune profile might affect COVID-19-related outcomes; the association between eosinophilia and disease outcomes varies by inhaled corticosteroid therapy.
How does this study impact current management guidelines? If confirmed by future randomized trials, eosinophilia can be used as a biomarker to guide therapy with inhaled corticosteroids in COVID-19.
Introduction
In March 2020, the coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic and has since caused more than 4.5 million deaths worldwide.1 Older age, male sex, Black race, tobacco use, and multiple comorbidities are associated with increased COVID-19 severity.2 Although the cause of sex- and age-based differences in COVID-19 severity remains unknown, it suggests that an individual’s baseline preexisting immune profile may determine the host immune response to SARS-CoV2 infection to influence outcomes.
Entry of SARS-CoV-2 into respiratory epithelial cells relies on two essential host proteins: angiotensin-converting enzyme 2 (ACE2) and transmembrane protease, serine 2.3 Expression of these critical host genes differs in patients with type 2 (T2) inflammation, which is characterized by the presence of eosinophilia and elevated FeNO.4 , 5 When considering SARS-CoV-2 infection, higher blood eosinophil counts were previously associated with better outcomes in patients with and without asthma.6, 7, 8 However, most of these epidemiologic studies relied on eosinophil counts obtained during the acute SARS-CoV-2 infection6 , 7 , 9 or were limited to small sample sizes.8
Severe COVID-19 is associated with a number of distinct immunologic signatures.10 SARS-CoV-2 infection reconfigures leukocyte phenotype in a severity-specific fashion, in which severe COVID-19 is associated with lymphopenia and T-cell exhaustion,11 , 12 neutrophil activation,13 , 14 and hematopoietic alterations resulting in immature and dysfunctional neutrophils.12 , 15 Severe disease is associated with depletion of CD16 monocytes, dendritic cells, and natural killer cells, and changes in the transcriptional phenotype of these cells as well.10 In contrast to patients with severe COVID-19, those with mild disease exhibit notably reduced proinflammatory plasma cytokines. These findings suggest that the immune response of those with mild disease efficiently eliminates viral infection, thus evading the hyperinflammatory state associated with severe disease.16 , 17
Although the effect of SARS-CoV-2 on the immune system has been well-studied, little is known about the effect of the baseline immune profile on COVID-19 outcomes or the interaction between high eosinophil counts and inhaled corticosteroids (ICS). Previous reports suggested that corticosteroid therapy, both inhaled and intranasal, might have a beneficial role in COVID-19 related to alterations in viral host interactions or anti-inflammatory effects.18, 19, 20, 21, 22, 23 A recent phase II open-label randomized controlled trial (RCT) of 146 individuals demonstrated that early administration of inhaled budesonide reduced the risk for progression to severe COVID-19 and overall COVID-19–related health care use.18 However, the effect of ICS on COVID-19–related outcomes in asthma patients is controversial and may be confounded by asthma severity and T2 inflammation.24, 25, 26, 27, 28 In this study, we evaluated preexisting complete blood cell counts with differential in a large, general population, prospectively collected COVID-19 registry to test the hypothesis that peripheral blood eosinophil counts influence risk for severe COVID-19 through interactions that are modulated by concurrent ICS therapy.
Methods
Subjects
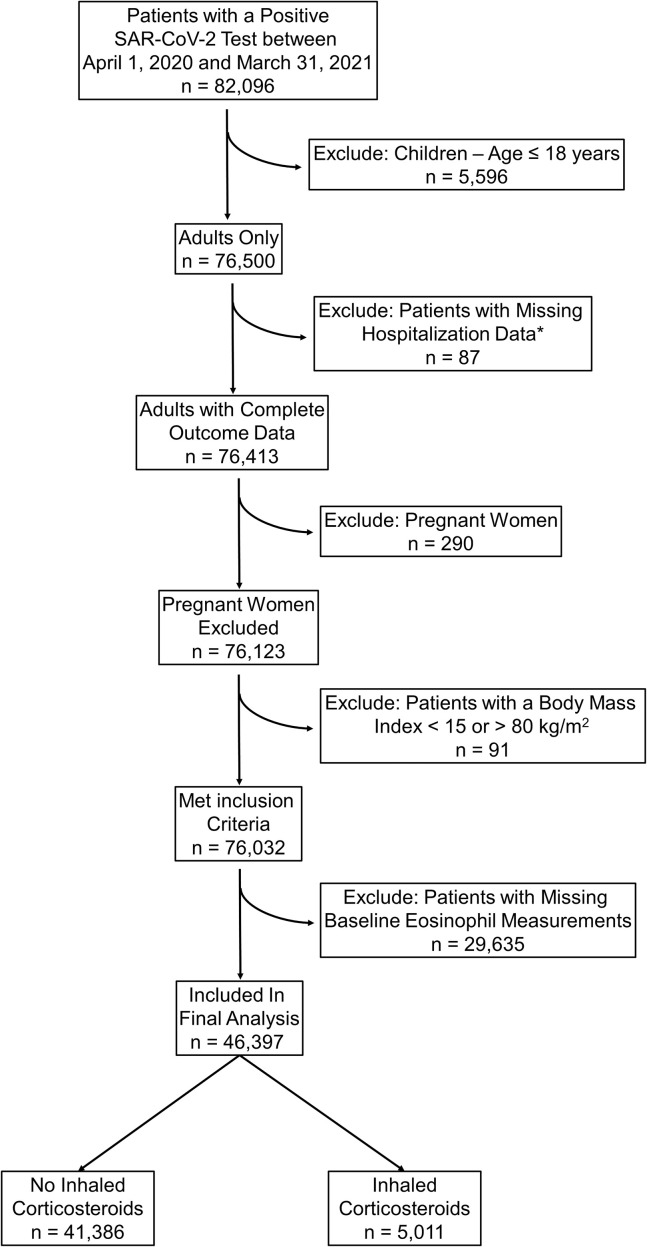
The Cleveland Clinic COVID-19 Research Registry (CCCRR) database23 , 29, 30, 31, 32, 33 was used to study 82,096 individuals who had a positive SARS-CoV-2 test between April 1, 2020 and March 31, 2021. We excluded 5,596 children aged 18 years and younger, 290 pregnant women, 87 patients with missing hospitalization data, and 91 patients with a body mass index (BMI) less than 15 or greater than 80 kg/m2 (see Appendix E1 and Figure E1 in this article’s Online Repository at www.jaci-inpractice.org).
Figure E1.
Flowchart of patients in final analysis. SAR-CoV-2, severe acute respiratory syndrome coronavirus 2.
Baseline complete blood count differential cell subtypes
Of the 76,032 individuals who met inclusion criteria, 46,397 had a complete blood count (CBC) with differential measured at least 2 weeks before the date of the SARS-CoV-2 test (defined as preexisting or baseline). Of those, 5,011 were prescribed ICS therapy and 41,386 did not have an ICS prescription in electronic health records.
Peri-testing differential cell subtypes
Of the 76,032 individuals who met inclusion, 12,944 had a CBC with differential measured within 48 hours of the SARS-CoV-2 test date. Of those, 9,650 had both a baseline (preexisting) and a peri-testing CBC with differential measurements available for analysis. Unless specified as peri-testing, all differential cell counts results in this article refer to baseline (preexisting) measurements.
Study outcomes and groups definition
The primary outcome was COVID-19–related hospitalizations. We also studied two secondary outcomes: the rate of COVID-19–related admissions to the intensive care unit (ICU) and mortality during index hospitalization. To study the effect of high eosinophil counts and ICS use on COVID-19–related outcomes, we performed two separate sensitivity analyses involving 3,066 patients with chronic obstructive pulmonary disease (COPD) or emphysema, and 6,739 asthmatic patients with available baseline absolute eosinophil count (AEC) measurements. Patients with asthma or COPD were identified using International Classification of Diseases, 10th Revision codes, and such diagnoses were verified by trained medical personnel using standardized protocols.32 These sensitivity analyses were done based on evidence that eosinophils are potentially protective against severe COVID-19.8 , 9 We chose a baseline AEC cutoff of 0.15 × 103 cells/μL to define eosinophilia because this threshold was previously found to drive treatment choices in asthma and COPD34, 35, 36, 37, 38 and was associated with better COVID-19 outcomes in asthma.8 We define eosinopenia (<0.1 × 103 cell/μL) based on previous reports associating this threshold with severe COVID-19.39 Analyses were stratified by ICS therapy to evaluate whether such therapy is associated with improved COVID-19 outcomes as previously reported.18 We did not consider new prescriptions for ICS ordered after the date of the SARS-CoV-2 test result.
The effect of COVID-19 on immune cell profile reconfiguration was assessed by evaluating the association between COVID-19 outcomes and peri-testing of CBC differential cell subtypes in 12,944 patients. We also examined the association between outcomes and changes from baseline in these cell subtypes in 9,650 patients.
Statistical methods
In this retrospective analysis of a prospectively collected COVID-19 registry, we present data as counts with percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. Two group comparisons of continuous nonnormally distributed variables were performed using Wilcoxon rank sum test and normally distributed continuous variables were compared using t test. Categorical variables were compared using χ2 test.
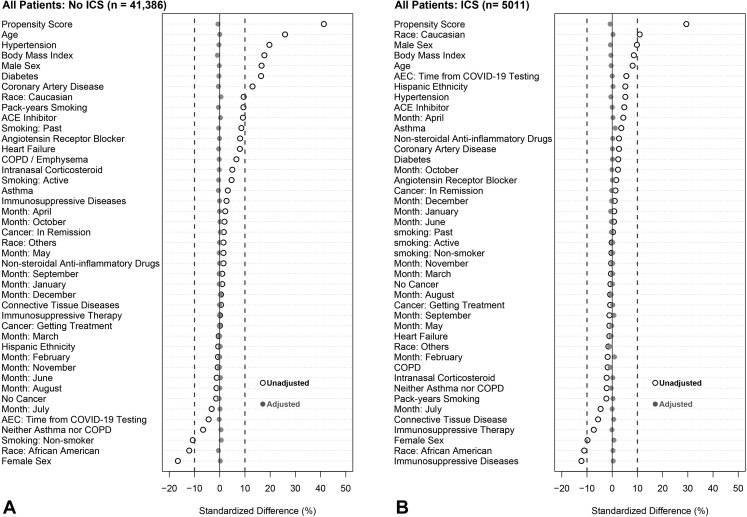
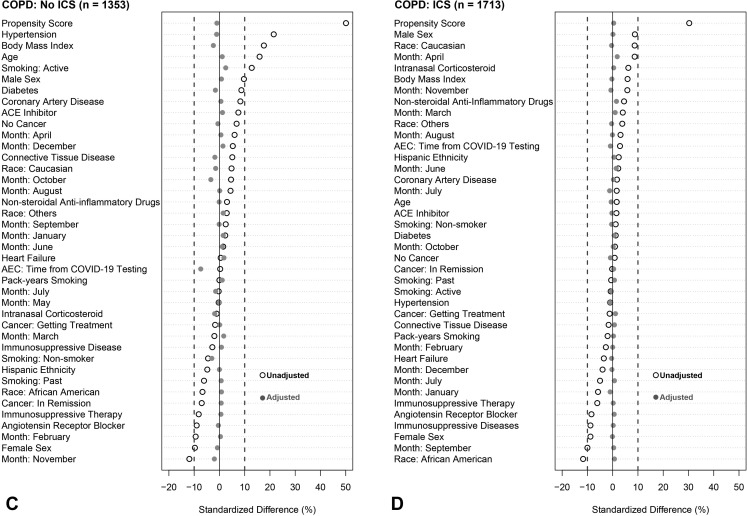
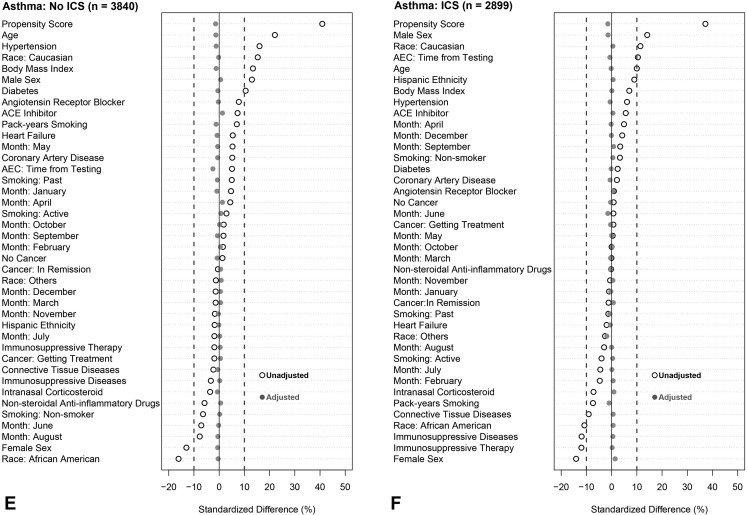
To account for observed covariate differences between patients with and without eosinophilia (ie, AEC > 0.15 × 103/μL), we used inverse weighting on the propensity score. The propensity score for each patient is the predicted probability of eosinophilia from a nonparsimonious logistic regression model using covariates known a priori to be associated with severe COVID-19.2 , 40 Such covariates include the month of testing, demographics, BMI (log transformed), smoking status, pack-years smoking history, comorbidities, time between the AEC test date and SARS-CoV-2 test date (log transformed), and relevant medication prescriptions including immunosuppressive therapy (see Figure E2 and Appendix E2 in this article’s Online Repository at www.jaci-inpractice.org). All covariates had variance inflation factor of less than 2, which suggests the absence of multicollinearity. Adjusted odds ratios (ORs) were then calculated to estimate the effect of eosinophilia on outcomes by weighting each patient with the inverse propensity score and controlling for the propensity as a covariate in the model. Similar propensity-weighted approaches were applied in all sensitivity analyses. To assess the nonlinearity of the association between blood eosinophil counts and the probability of poor COVID-19 outcomes (ie, hospitalization, ICU admission, or hospital mortality), we compared logistic regression models fitted with a restricted cubic spline function for the AEC (log10-transformed) with three knots with models assuming a linear association using the likelihood ratio test. Adjustment for the month of testing was included to avoid the chronological bias introduced by changes in SARS-CoV-2 testing policies, therapies and management protocols, and improvements in mortality.41 , 42
Figure E2.
Standardized mean differences before and after weighting on inverse propensity score and controlling for propensity score as a covariate in the model. Analysis was stratified into six groups: (A) all patients not receiving inhaled corticosteroids (ICS), (B) all patients receiving ICS, (C) patients with chronic obstructive pulmonary disease (COPD) not receiving ICS, (D) patients with COPD who were receiving ICS, (E) patients with asthma who were not receiving ICS, and (F) patients with asthma who were receiving ICS. An absolute standardized difference of 0% indicates no residual bias; values <10% indicate inconsequential bias. Open black circles show standardized mean differences for each covariate before weighting. Solid gray circles show differences after weighting. ACE, angiotensin-converting enzyme; AEC, absolute eosinophil count; COVID-19, coronavirus disease 2019.
All covariates included in the regression models were missing fewer than 3% of subjects. We carried out multiple imputation (five imputations) for missing variables using the Multivariate Imputation by Chained Equations package and pooled separate results using Rubin’s rules to obtain the final results.43 Multivariate Imputation by Chained Equations replaces each missing value with a plausible value drawn from a distribution specifically designed for each missing data point. We also repeated all analyses using the original complete non-imputed data (ie, excluding individuals with missing data). Based on the CCCRR registry sample size, a power analysis, completed subsequent to the initiation of this study, comparing two proportions between two groups of unequal sample size (eosinophil count <0.15 [×103/μL] vs >0.15 [×103/μL]), demonstrated that we had greater than 90% power to detect significant differences in hospitalization rates between patients with high versus low eosinophil counts at a significance level of α = 0.05 irrespective of the effect size (small, medium, or large). All P values were two-tailed, performed at a significance level of .05. All statistical analyses were conducted with R software (version 4.0.5, R Project for Statistical Computing, Vienna, Austria).
Results
Demographics and clinical characteristics
Of the 46,397 patients included in the final analysis, 9,096 were hospitalized (19.6%), 2,129 required ICU admission (4.6%), and 1,402 died during hospitalization (3.0%). In addition, 6,739 carried the diagnosis of asthma and 3,066 were diagnosed with COPD or emphysema. Baseline eosinophilia (>0.15 × 103 cells/μL) was present in 19,506, and ICS were used in 5,011 individuals.
Patient demographics and clinical characteristics varied considerably between individuals with and without eosinophilia. Median age of patients with eosinophilia was 56.3 years (IQR, 42.1-69.3 years) and 10,609 were female (54.4%). Median age of patients with low eosinophil levels was 50.6 years (IQR, 35.8-64.4 years) and 16,623 were female (61.8%) (Table I ). Clinical characteristics of patients with asthma and COPD are detailed in Tables E1 and E2 (in this article’s Online Repository at www.jaci-inpractice.org).
Table I.
Clinical characteristics and outcomes by eosinophilia (>0.15 × 103 /μL) for all patients in registry stratified by ICS
| Variable | All patients | P | No ICS | P | ICS | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Eosinophil count | <0.15 [×103/μL] | >0.15 [×103/μL] | <0.15 [×103/μL] | >0.15 [×103/μL] | <0.15 [×103/μL] | >0.15 [×103/μL] | |||
| n | 26,891 | 19,506 | 24,428 | 16,958 | 2,463 | 2,548 | |||
| Demographics | |||||||||
| Age, y | 50.6 [35.6-64.4] | 56.3 [42.1-69.3] | <.001 | 49.7 [34.8-63.6] | 55.5 [41.2-68.6] | <.001 | 59.3 [47.2-70.8] | 61.3 [48.9-72.5] | .002 |
| Female sex | 16,623 (61.8) | 10,609 (54.4) | 14,976 (61.3) | 9,024 (53.2) | <.001 | 1,647 (66.9) | 1,586 (62.2) | .002 | |
| Body mass index, kg/m2 | 29.1 [25.0-34.3] | 30.5 [26.3-35.9] | <.001 | 28.9 [24.9-34.0] | 30.3 [26.2-35.6] | <.001 | 30.8 [25.9-37.2] | 31.6 [26.9-37.8] | <.001 |
| Race∗ | <.001 | <.001 | <.001 | ||||||
| Black | 6,075 (22.6) | 3,549 (18.2) | 5,465 (22.4) | 3,030 (17.9) | 610 (24.8) | 520 (20.4) | |||
| White | 18,364 (68.3) | 14,117 (72.4) | 16,713 (68.4) | 12,285 (72.4) | 1,651 (67.0) | 1,832 (71.9) | |||
| Others | 2,452 (9.1) | 1,838 (9.4) | 2,250 (9.2) | 1,643 (9.7) | 202 (8.2) | 196 (7.7) | |||
| Hispanic ethnicity | 961 (3.6) | 697 (3.6) | .989 | 873 (3.6) | 622 (3.7) | .466 | 86 (3.5) | 92 (3.6) | .09 |
| Smoking history | <.001 | <.001 | .998 | ||||||
| Current | 1,902 (7.1) | 1,595 (8.2) | 1,705 (7.0) | 1,393 (8.3) | 197 (8.0) | 203 (8.0) | |||
| Past | 6,629 (24.7) | 5,543 (28.5) | 5,658 (23.2) | 4,536 (26.9) | 971 (39.5) | 1,007 (39.5) | |||
| Pack-years smoking | 11.0 [4.0-25.0] | 14.0 [5.0-30.0] | <.001 | 10.0 [3.5-22.0] | 12.0 [5.0-28.0] | <.001 | 20.0 [6.4-40.0] | 20.0 [7.5-40.0] | .564 |
| Eosinophil count (×103/μL)† | 0.08 [0.04-0.11] | 0.24 [0.20-0.33] | <.001 | 0.08 [0.05-0.11] | 0.23 [0.19-0.32] | <.001 | 0.08 [0.03-0.11] | 0.27 [0.20-0.38] | <.001 |
| Comorbidities | |||||||||
| Chronic obstructive pulmonary disease or emphysema | 1,524 (5.7) | 1,542 (7.9) | <.001 | 673 (2.8) | 680 (4.0) | <.001 | 851 (34.6) | 862 (33.8) | .611 |
| Asthma | 3,559 (13.3) | 3,180 (16.3) | <.001 | 2,145 (8.8) | 1,695 (10.0) | <.001 | 1,414 (57.5) | 1,485 (58.3) | .574 |
| Diabetes | 4,060 (15.1) | 4,178 (21.4) | <.001 | 3,383 (13.8) | 3,449 (20.3) | <.001 | 677 (27.5) | 730 (28.6) | .376 |
| Hypertension | 9,284 (34.5) | 8,582 (44.0) | <.001 | 7,916 (32.4) | 7,101 (41.9) | <.001 | 1,368 (55.5) | 1,482 (58.2) | .065 |
| Coronary artery disease | 2,083 (7.7) | 2,286 (11.7) | <.001 | 1,642 (6.7) | 1,803 (10.6) | <.001 | 441 (17.9) | 483 (19.0) | .356 |
| Heart failure | 1,699 (6.3) | 1,661 (8.5) | <.001 | 1,267 (5.2) | 1,225 (7.2) | <.001 | 432 (17.5) | 436 (17.1) | .717 |
| Cancer history | 3,051 (11.3) | 2,487 (12.8) | <.001 | 2,599 (10.6) | 2,034 (12.0) | <.001 | 452 (18.4) | 454 (17.8) | .65 |
| Connective tissue disease | 751 (2.8) | 571 (2.9) | .405 | 568 (2.3) | 415 (2.4) | .442 | 183 (7.4) | 156 (6.1) | .074 |
| Immunosuppressive disease | 2,417 (9.0) | 1,850 (9.5) | .07 | 1,939 (7.9) | 1,465 (8.6) | .011 | 478 (19.4) | 385 (15.1) | <.001 |
| Medications | |||||||||
| Nonsteroidal anti-inflammatory drugs | 4,098 (15.2) | 3,098 (15.9) | .06 | 3,684 (15.1) | 2,644 (15.6) | .16 | 414 (16.8) | 454 (17.8) | .365 |
| Angiotensin converting enzyme inhibitors | 2,436 (9.1) | 2,314 (11.9) | <.001 | 2,174 (8.9) | 2,003 (11.8) | <.001 | 262 (10.6) | 311 (12.2) | .089 |
| Angiotensin receptor blockers | 1,719 (6.4) | 1,685 (8.6) | <.001 | 1,416 (5.8) | 1,358 (8.0) | <.001 | 303 (12.3) | 327 (12.8) | .6 |
| Intranasal corticosteroids | 6,637 (24.7) | 5,339 (27.4) | <.001 | 5,437 (22.3) | 4,122 (24.3) | <.001 | 1,200 (48.7) | 1,218 (47.8) | .534 |
| Intensive care unit | 2,463 (9.2) | 2,547 (13.1) | <.001 | 0 | 0 | NA | 2,463 (100.0) | 2,548 (100.0) | NA |
| Immunosuppressive therapy‡ | 264 (1.0) | 190 (1.0) | .973 | 173 (0.7) | 123 (0.7) | .886 | 91 (3.7) | 67 (2.6) | .038 |
| Outcomes | |||||||||
| Hospitalization | 4,952 (18.4) | 4,144 (21.2) | <.001 | 4,099 (16.8) | 3,352 (19.8) | <.001 | 853 (34.6) | 792 (31.1) | .008 |
| Admission to intensive care unit | 1,153 (4.3) | 976 (5.0) | <.001 | 895 (3.7) | 769 (4.5) | <.001 | 258 (10.5) | 207 (8.1) | .005 |
| Hospital mortality | 734 (2.7) | 668 (3.4) | <.001 | 576 (2.4) | 535 (3.2) | <.001 | 158 (6.4) | 133 (5.2) | .08 |
ICS, inhaled corticosteroids; NA, not available.
Data are presented as n (%) for categorical variables and median [interquartile range] for continuous variables.
“Other” race category includes American Indians or Alaska Natives, Asian individuals, Native Hawaiian or Other Pacific Islanders, and individuals with multiple racial backgrounds.
Defined by a baseline blood absolute eosinophil count > 0.15 (x103 cells/ μL) obtained at least 2 weeks before severe acute respiratory syndrome coronavirus 2 test date.
Includes chronic systemic corticosteroid therapy.
Baseline eosinophilia is associated with improved COVID-19–related outcomes
In parallel with the higher rate of comorbidities and medication use, patients with eosinophilia had higher rates of hospitalization (21.2% vs 18.4%; P < .001), ICU admission (5% vs 4.3%; P < .001), and in-hospital mortality (3.4% vs 2.7%; P < .001) than those without preexisting eosinophilia in unadjusted comparisons (Table I). However, analyses adjusted using inverse probability weighting for demographics, BMI, smoking history, medications, comorbidities, the month of testing, and the time between the AEC test date and the SARS-CoV-2 test date (Figure E2) demonstrated that patients with eosinophilia (n = 19,506) were at a significantly lower odds for hospitalization (adjusted OR [95% confidence interval (CI)]: 0.96 [0.93-0.99]) and ICU admission (adjusted OR [95% CI]: 0.92 [0.87-0.98]) compared with patients without eosinophilia (n = 26,891). Preexisting eosinophilia was not associated with improved in-hospital mortality (adjusted OR [95% CI]: 0.94 [0.88-1.03], P = .19).
Association between COVID-19–related outcomes and baseline AEC is nonlinear and varies according to ICS use
Logistic regression fitted with a restricted cubic spline function for the AEC (log10-transformed) with three knots optimally described the nonlinear relationship between AEC and outcome measures (see Table E3 in this article’s Online Repository at www.jaci-inpractice.org). Using a likelihood ratio test, nonlinear models were found to be different from models assuming a linear association (P < .001).
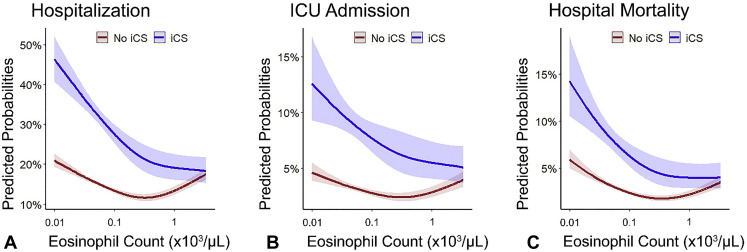
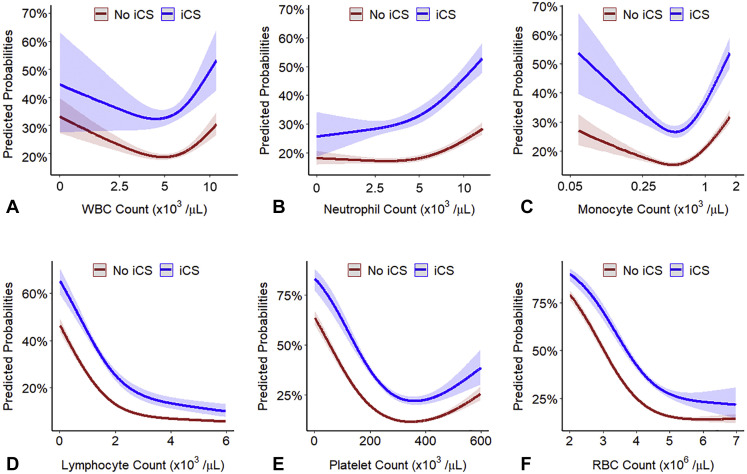
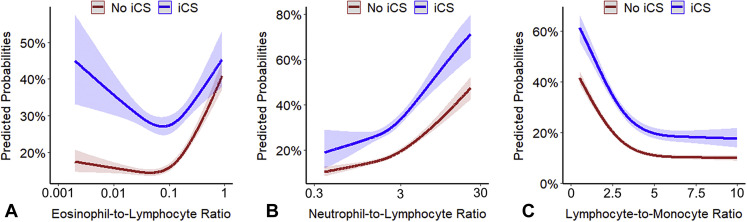
Interaction plots of the predicted probabilities of COVID-19–related hospitalization, ICU admission, and in-hospital mortality as a function of AEC, stratified by ICS use, demonstrate that the relationship between AECs and COVD-19–related outcomes is modified by ICS therapy (all interaction P < .001) (Figure 1 ). The predicted probability for COVID-19–related outcomes was higher among individuals with elevated AEC not treated with ICS, but not among ICS-treated patients. Interaction plots between other types of white blood cells and ICS did not show a significant interaction except for neutrophils (P for interaction = .002) (see Figure E3 in this article’s Online Repository at www.jaci-inpractice.org). A significant interaction also existed between ICS and the eosinophil-to-lymphocyte ratio (P for interaction < .001), but not with the neutrophil-to-lymphocyte ratio (P for interaction = .23) or the lymphocyte-to monocyte ratio (P for interaction = .58) (see Figure E4 in this article’s Online Repository at www.jaci-inpractice.org).
Figure 1.
Predicted probabilities of coronavirus disease 2019–related (A) hospitalization, (B) admission to the intensive care unit (ICU), and (C) hospital mortality as a function of baseline peripheral blood absolute eosinophil count (AEC), stratified by inhaled corticosteroids (ICS) use. These interaction plots show that the association between coronavirus disease 2019–related outcomes and AEC depends on the use of ICS (all P values for interaction < .001). The probabilities were calculated by fitting a logistic regression using a restricted cubic spline function for the AEC (log10-transformed). The 95% confidence intervals are indicated by the shaded area around the fitted line.
Figure E3.
Predicted probabilities of coronavirus disease 2019 (COVID-19)-related hospitalization as a function of (A) baseline white blood cell count (WBC), (B) absolute neutrophil count (ANC), (C) absolute monocyte count (AMC), (D) absolute lymphocyte count, (E) platelet count, and (F) red blood cell (RBC) count in peripheral blood stratified by inhaled corticosteroid (iCS) use. The probabilities were calculated by fitting a logistic regression using a restricted cubic spline function for each of the parameters. Log10 transformation was applied to nonnormally distributed variables (WBC, ANC, and AMC). The 95% confidence intervals are indicated by the shaded areas around the fitted lines.
Figure E4.
Predicted probabilities of coronavirus disease 2019 (COVID-19)-related hospitalization as a function of the (A) baseline eosinophil-to-lymphocyte ratio (ELR), (B) neutrophil-to-lymphocyte ratio (NLR), and (C) lymphocyte-to-monocyte ratio (LMR) in peripheral blood stratified by inhaled corticosteroid (iCS) use. The probabilities were calculated by fitting a logistic regression using a restricted cubic spline function for each of the parameters. Log10 transformation was applied to nonnormally distributed variables (ELR and NLR). The 95% confidence intervals are indicated by the shaded areas around the fitted lines.
Baseline eosinophilia is associated with improved COVID-19–related outcomes only in ICS-treated patients
Compared with patients without eosinophilia, propensity-weighted models showed that patients with eosinophilia had lower odds for hospitalization (adjusted OR [95% CI]: 0.86 [0.79-0.93]), ICU admission (adjusted OR [95% CI]: 0.79 [0.69-0.90]), and in-hospital mortality (adjusted OR [95% CI]: 0.80 [0.68-0.95]) among ICS-treated, but not untreated patients (Table II ). Stratified analysis showed similar protective effects in patients with COPD or emphysema or with asthma (Table II).
Table II.
Clinical outcome results of patients with preexisting eosinophilia∗ compared with patients without eosinophilia, stratified by ICS therapy and airway disease category
| Outcome | All patients |
Chronic obstructive pulmonary disease |
Asthma |
|||
|---|---|---|---|---|---|---|
| No ICS |
ICS |
No ICS |
ICS |
No ICS |
ICS |
|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| n | 41,320 | 5,009 | 1,352 | 1,714 | 3,840 | 2,899 |
| Unadjusted | ||||||
| Hospitalization | 1.22 (1.16-1.29) | 0.85 (0.76-0.96) | 1.01 (0.82-1.25) | 0.75 (0.62-0.90) | 1.02 (0.86-1.20) | 0.78 (0.66-0.91) |
| ICU admission | 1.25 (1.13-1.38) | 0.76 (0.62-0.92) | 0.95 (0.69-1.30) | 0.74 (0.57-0.95) | 1.01 (0.70-1.45) | 0.70 (0.53-0.93) |
| Hospital mortality | 1.35 (1.20-1.52) | 0.80 (0.63-1.02) | 0.86 (0.61-1.22) | 0.78 (0.58-1.04) | 1.07 (0.67-1.71) | 0.87 (0.61-1.25) |
| Adjusted for age, sex, ethnicity, race, and month of testing | ||||||
| Hospitalization | 0.99 (0.96-1.03) | 0.82 (0.75-0.89) | 0.96 (0.83-1.12) | 0.77 (0.67-0.88) | 0.87 (0.77-0.98) | 0.73 (0.64-0.81) |
| ICU admission | 1.00 (0.93-1.07) | 0.73 (0.63-0.83) | 0.92 (0.74-1.15) | 0.75 (0.63-0.90) | 0.85 (0.66-1.10) | 0.66 (0.54-0.80) |
| Hospital mortality | 0.95 (0.87-1.04) | 0.74 (0.62-0.87) | 0.78 (0.61-1.00) | 0.76 (0.62-0.94) | 0.78 (0.56-1.10) | 0.75 (0.58-0.97) |
| Adjusted for age, sex, race, ethnicity, smoking history, pack-years smoking, medications, comorbidities, time between absolute eosinophil count test date and severe acute respiratory syndrome coronavirus 2 test date, and month of testing† | ||||||
| Hospitalization | 0.98 (0.94-1.01) | 0.86 (0.79-0.93) | 0.99 (0.85-1.15) | 0.80 (0.70-0.91) | 0.89 (0.79-1.00) | 0.78 (0.69-0.87) |
| ICU admission | 0.97 (0.90-1.04) | 0.79 (0.69-0.90) | 0.86 (0.68-1.07) | 0.78 (0.65-0.93) | 0.84 (0.65-1.09) | 0.72 (0.59-0.87) |
| Hospital mortality | 0.99 (0.91-1.08) | 0.80 (0.68-0.95) | 0.83 (0.65-1.05) | 0.80 (0.70-0.91) | 0.86 (0.62-1.20) | 0.84 (0.66-1.09) |
CI, confidence interval; ICS, inhaled corticosteroids; ICU, intensive care unit; OR, odds ratio.
Baseline preexisting eosinophilia were defined by a blood absolute eosinophil count of greater than 0.15 × 103 cells/μL, obtained at least 2 weeks before the severe acute respiratory syndrome coronavirus 2 test date.
The effect of a high blood absolute eosinophil count greater than 0.15 × 103 cells/μL on hospital outcomes is estimated by weighting each patient with the inverse propensity score and controlling for the propensity score as a covariate in the model. Medications included nonsteroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, ICS, intranasal corticosteroids, and immunosuppressive therapy (including systemic corticosteroids). Comorbidities include asthma, chronic obstructive pulmonary disease or emphysema, diabetes, hypertension, coronary artery disease, heart failure and cancer (historical or current), immunosuppressive diseases, and connective tissue diseases.
Among patients with COPD who were treated with ICS, eosinophilia was associated with lower odds for hospitalization (adjusted OR [95% CI]: 0.80 [0.70-0.91]), ICU admission (adjusted OR [95% CI]: 0.78 [0.65-0.93]), and mortality (adjusted OR [95% CI]: 0.80 [0.70-0.91]). Similarly, patients with asthma and eosinophilia had lower odds for COVID-19–related hospitalization (adjusted OR [95% CI]: 0.78 [0.69-0.87]) and ICU admission (adjusted OR [95% CI]: 0.72 [0.59-0.87]). Eosinophilia was not associated with lower in-hospital mortality (adjusted OR [95% CI]: 0.84 [0.66-1.09]; P = .12) among asthmatic patients treated with ICS. Eosinophilia was not associated with better COVID-19–related outcomes among patients with asthma or patients with COPD not treated with ICS (Table II).
Results from analyses using the original non-imputed data (ie, complete cases) were consistent with findings from the main analyses using imputed data (see Table E4 in this article’s Online Repository at www.jaci-inpractice.org).
In contrast to eosinophilia, eosinopenia (<0.1 × 103 cells/μL) was associated with worse COVID-19 outcomes irrespective of ICS use (see Table E5 in this article’s Online Repository at www.jaci-inpractice.org).
Median [IQR] time between the AEC test date and the SARS-CoV-2 test date was 530 days (234-1,274 days). To minimize bias introduced by the time between the AEC and SARS-CoV-2 test dates, we included the time variable as a covariate in all of our models. Furthermore, we performed two additional stratified analyses for those who had AEC measurements obtained within 1 year (n = 15,084) and 2 years (n = 24,095) from the SARS-CoV-2 test date, and found similar results among these patients (see Table E6 in this article’s Online Repository at www.jaci-inpractice.org).
Fractional exhaled nitric oxide
Fractional exhaled nitric oxide measurements were available for 1,294 patients with asthma and correlated poorly with the AEC (r = 0.173; P < .001). After adjusting for AEC and the month of testing, FeNO measurements above 35 parts per billion were associated with lower hospitalization odds in 835 patients with asthma treated with ICS (adjusted OR [95% CI: 0.45 [0.28;-0.70]), but not in 468 asthmatic patients not receiving ICS therapy (adjusted OR [95% CI: 0.79 [0.32-1.77]). This beneficial association in the high-FeNO group treated with ICS was also significant after additional adjustments to demographics and comorbidities (see Table E7 in this article’s Online Repository at www.jaci-inpractice.org).
Increased peri-testing blood AECs are associated with improved COVID-19–related outcomes
Peri-testing AECs were obtained in 12,944 patients within 48 hours of having a positive SARS-CoV-2 test. Of those, 1,280 had a peri-testing AEC above 0.15 × 103 cells/μL (9.9%), 1,665 were receiving ICS (12.9%), 8,369 were hospitalized (64.7%), 2,023 were admitted to the ICU (25.6%), and 1,312 died during hospitalization (10.1%). Propensity-weighted analyses showed that eosinophilia was associated with lower odds for hospitalization (adjusted OR [95% CI]: 0.83 [0.78-0.87]), ICU admission (adjusted OR [95% CI]: 0.59 [0.54-0.63]), and in-hospital mortality (adjusted OR [95% CI]: 0.58 [0.0.53-0.64]).
COVID-19–related reduction of peri-testing AEC from baseline is associated with worse outcomes
Of the 9,650 patients with both baseline and peri-testing AEC measurements available, 1,536 were receiving ICS therapy, 6,436 were hospitalized, 1,551 were admitted to the ICU, and 1,035 died during hospitalization. Median drop from baseline in the AEC during SARS-CoV-2 infection was 0.09 × 103 cells/μL (IQR, 0.02-0.18).
After weighting on the inverse propensity score and adjustment, patients with more than a 0.09 × 103 cells/μL decrease in AEC from baseline had higher odds of being hospitalized (adjusted OR [95% CI]: 1.21 [1.14-1.28])), being admitted to the ICU (adjusted OR [95% CI]: 1.21 [1.12-1.30]), or dying during hospitalization (adjusted OR [95% CI]: 1.21 [1.12-1.30]) compared with those with a less significant drop in AEC.
Discussion
The main finding of our study was that the association between preexisting eosinophil counts and COVID-19–related outcomes depends on ICS therapy. Our results associating low blood eosinophil counts with poor COVID-19 outcomes support previous reports associating eosinophils with better outcomes in respiratory viral infections.44 In addition to their role in allergy, asthma, and parasitic infections, eosinophils have antiviral properties mediated by Toll-like receptor signaling pathways.44, 45, 46, 47, 48, 49 For example, ovalbumin sensitization was associated with an 80% reduction in the viral content of the lungs of guinea pigs infected with parainfluenza, which was reversed by anti-IL-5 antibodies.50
Our study demonstrated that higher eosinophil counts were protective against poor COVID-19 outcomes in patients treated with ICS, but not in those without a prescription. These findings support previous data suggesting a beneficial role for ICS.18 Although the mechanism remains largely unknown, recently published data suggest that corticosteroids may have direct and indirect effects on COVID-19 infection.19, 20, 21, 22 , 51 Dexamethasone binds directly to the ACE2 receptor and consequently inhibits the cellular entrance of SARS-CoV-2 after receptor binding to the spike protein.22 Corticosteroids might also have direct antiviral properties by targeting the viral replication–transcription complex.21 For example, ciclesonide and mometasone, two ICS used in human asthma, were found to suppress coronavirus replication in vitro.21 In addition to their direct effects, corticosteroids were presumed to modulate SARS-CoV-2 infection through genomic mechanisms. In fact, ICS use was associated with lower expression of ACE2 and transmembrane serine protease 2 in sputum cells in a subset of asthmatics enrolled in the National Institutes of Health– National Heart, Lung, and Blood Institute Severe Asthma Research Program19 , 51 and in airway epithelial cells obtained by bronchoscopy from individuals with COPD.20
Furthermore, ICS may indirectly modulate pulmonary inflammation by suppressing the immune response driven by T-helper cells.52 During SARS-CoV-2 infection, viral replication causes epithelial cells pyroptosis and the release of damage-associated molecular patterns, which results in proinflammatory cytokines and chemokines release by neighboring cells and alveolar macrophages. This causes monocyte, macrophage, and T-cell migration to the lungs. There, these cells are activated and promote further inflammation.53 In most patients, recruited virus-specific T cells eradicate the infection in the lung without causing significant damage. However, in patients with severe COVID-19, a defective immune response triggers the overproduction of proinflammatory cytokines (ie, cytokine storm) that mediates widespread lung damage, multiorgan failure, and sometimes death.53 Although ICS are beneficial in obstructive airways diseases by inhibiting T-cell migration and activation within the airways, it is not known whether this mechanism underlies the beneficial effect of ICS in COVID-19.
The contribution of eosinophils to lung pathology during COVID-19 is not well-understood. Eosinophil-associated lung damage was previously reported in respiratory syncytial virus and severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) vaccination studies.54 In contrast, autopsy specimens from patients with COVID-19 did not show significant eosinophil infiltration into the pulmonary tissue.55 Our data demonstrate a nonlinear (U-shaped) association between baseline AEC and COVID-19 outcomes in the absence of ICS therapy, which suggests that eosinophilia may contribute to disease severity. In contrast, eosinophilia is not associated with worse outcomes among ICS-treated patients. These findings suggest that ICS somehow modulate disease severity for those with high but not low eosinophil counts. Eosinophils are an important aspect of the innate immune system owing to their antiviral properties, which could explain why patients with low eosinophil counts have poor outcomes.45, 46, 47, 48 Furthermore, we recently demonstrated worse outcomes in COVID-19 patients with severe asthma.56 These findings suggest that the risk for severe COVID-19 is highest among individuals with eosinopenia who are treated with ICS. Further studies are needed to corroborate our findings and assess the effect of eosinophilia on the hyperinflammatory state seen in severe COVID-19.
Our data also revealed better outcomes in patients with elevated FeNO and support previous reports describing NO as a potent antiviral molecule.57 , 58 Once activated, Toll-like receptor pathways can promote inducible nitric oxide synthase, which mediates NO production.57 In vitro experiments showed that SARS-CoV-1 viral replication was inhibited by NO.58 However, it remains unknown whether the replication of SARS-CoV-2, a single-stranded RNA virus that shares most of the genome of SARS-CoV-1, is also inhibited by NO.58
As a biomarker of T2 inflammation, FeNO is commonly used to determine the likelihood of corticosteroid responsiveness, monitor airway inflammation, and uncover nonadherence to ICS.59 High FeNO was also associated with refractory airway T2 inflammation in a large subgroup of patients with asthma enrolled in the Severe Asthma Research Program.60 Although FeNO measurements above 35 parts per billion were associated with better outcomes among ICS users, it remains unclear whether this reflects refractory airway T2 inflammation or poor adherence to ICS therapy in this cross-sectional study. There is also likely a component of indication bias (confounding by indication) to this finding. In CCCRR, 835 patients receiving ICS (28.8%) had FeNO measurements available, compared with 459 not receiving ICS (12.0%).
Finally, we replicate previously published data associating eosinophil count, lymphocyte counts, the eosinophil-to-lymphocyte ratio, and neutrophil-to-lymphocyte ratio measurements obtained during acute viral infection with COVID-19 outcomes.6 , 8 , 10 , 12 , 53 To our knowledge, we are the first to associate baseline immune cell profiles with the response to SARS-CoV-2 infection progressing to hospitalization, ICU care, and death, and the impact of changes between baseline and peri-COVID-19 immune profiles on these severe outcomes. Although this will help us better identify patients at risk for severe COVID-19, further studies are needed to determine whether baseline differential cell types can be used as biomarkers to guide therapy. We are also the first to show an interaction between eosinophilia and ICS determining risk from severe COVID-19 outcomes. Accordingly, future RCTs studying the therapeutic benefit of ICS in COVID-19 should account for the presence (or absence) of eosinophilia.
Our study had several limitations. Like other registry-based observational studies, it could be subject to bias, particularly as it relates to the use of ICS in individuals with more severe forms of asthma and COPD, a subgroup more likely to elicit early diagnostic testing and care. Owing to its cross-sectional design, we cannot assess the influence of longitudinal changes in baseline (preexisting) blood eosinophil counts. Our analyses of the CCCRR are also limited by the lack of information regarding the dose or type of ICS used by patients, and adherence to therapy. This is an important limitation because it remains unclear whether the association between ICS and COVID-19 outcome is dose-dependent or medication (vs class) specific. Because we limited our analysis to patients cared for at the Cleveland Clinic, our analyses did not account for patients admitted to other hospitals and those who died after being discharged to long-term acute care facilities. Socioeconomic factors are strong determinants of COVID-19 outcomes, especially in underrepresented minority groups, and it is possible that ICS-treated patients also reflect a subgroup of asthma and COPD patients with improved health care access. Regardless, we believe our study has many strengths, including a large sample size, strict and predefined methods for data collection, and a thorough analysis of data that considers nonlinear relationships and adjusts for patient demographics, medications, comorbidities, the month of SARS-CoV-2 testing, and the time between the AEC and SARS-CoV-2 test dates.
Our study demonstrates that the baseline immune profile might determine the risk for COVID-19–related hospitalization, ICU admission, and in-hospital mortality. It also shows that the association between eosinophilia and COVID-19 outcomes depends on ICS therapy. Our study highlights the need to risk-stratify patients with COVID-19 better, identify new biomarkers, and plan future RCTs to study the effect of ICS.
Acknowledgments
We thank Mr Bradly Souder from the CCF Business Intelligence Department, Ms Laura Peterson, BA from Lerner Research Institute, and Mr Greg Strnad, MS from CCF Clinical Outcomes Research Center for their valuable input and help. J.G. Zein, R. Strauss, S.S. Chamat, and V.E. Ortega made substantial contributions to the conception or design of the work; J.G. Zein acquired, analyzed, and interpreted the data for the work; A. Milinovich extracted data from electronic health records; J.G. Zein, R. Strauss, A.H. Attaway, B. Hu, N. Jawhari, S.S. Chamat, and V.E. Ortega contributed to drafting the work or revising it critically for important intellectual content; J.G. Zein agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and J.G. Zein, R. Strauss, A.H. Attaway, B. Hu, A. Milinovich, N. Jawhari, S.S. Chamat, and V.E. Ortega gave the final approval of the version to be published.
Footnotes
This study was funded by the National Institutes of Health–National Heart, Lung, and Blood Institute (Grant K08 HL133381 [to J.G. Zein]).
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
Appendix E1: Description of the Registry
The Cleveland Clinic COVID-19 Research Registry
The Cleveland Clinic COVID-19 Research Registry was previously described by us and others.E1, E2, E3, E4, E5 The registry was started by the Cleveland Clinic on March 8, 2020 and includes all patients tested for coronavirus disease 2019 (COVID-19) within its health care system by trained medical personnel using standardized protocols.E4 Testing was performed by means of the Centers for Disease Control and Prevention’s reverse transcription polymerase chain reaction severe acute respiratory syndrome coronavirus 2 assay, which uses MagNA Pure (Roche, Branchbug, NJ, USA) extraction and ABI 7500 DX PCR (Thermo Fisher Scientific, Waltham, MA, USA) instruments.E4 The registry’s clinical characterization and data collection are consistent with clinical features previously published on COVID-19.E6, E7, E8, E9, E10 Uniform clinical templates were implemented in electronic health records (Epic, Epic Systems Corporation, Verona, WI, USA) across the Cleveland Clinic Health System to standardize patient care and facilitate data extraction.E1, E4, E5 Patients with a positive COVID-19 test were monitored by the COVID-19 home monitoring program, which consisted of telephone outreach by a registered nurse and self-monitoring through an app for patient entry of COVID-19 symptoms. Patients were asked for the presence of COVID-19–related symptoms, such as fever, cough, dyspnea, weakness, vomiting, diarrhea, or loss of appetite. Frequent comorbidities (eg, asthma, chronic obstructive pulmonary disease, diabetes mellitus, hypertension, coronary artery disease, heart failure, and immunosuppressive diseases), and certain medications (nonsteroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and immunosuppressive therapy) were documented in the database. The immunosuppressive diseases definition was adapted from the Agency of Healthcare Research and Quality definition of immunocompromised state diagnosis.E11 Outcomes related to hospitalizations and admission to the intensive care unit were extracted. Data from the electronic health records were verified manually by a trained research team using predefined processes published previously.E5
Institutional review board approval
This study and the Cleveland Clinic COVID-19 Research Registry were approved by the Cleveland Clinic Institutional Review Board (Institutional Review Board Nos. 20-283 and 20-391).
Appendix E2: Systemic Medications Used to Define Immunosuppressive Therapy
| Abatacept | Dexamethasone | Pediapred |
| Actemra | Dexone | Predicort |
| Adalimumab | Dexpak | Prednisolone |
| Afinitor | Enbrel | Prednisone |
| A-Hydrocort | Entocort EC | Prelone |
| A-Methapred | Entyvio | Prograf |
| Anakinra | Envarsus XR | Rapamune |
| Arava | Etanercept | Rayos |
| Aristocort | Everolimus | Remicade |
| Aristospan | Flo-Pred | Risankizumab-rzaa |
| AsmalPred | Florinef | Rituxan |
| Astagraf XL | Fludrocortisone | Rituximab |
| Azasan | Golimumab | Sandimmune |
| Azathioprine | Guselkumab | SangCya |
| Basiliximab | Humira | Secukinumab |
| Beclomethasone | Hydrocortisone | Skyrizi |
| Belimumab | Hydrocortone | Siliq |
| Benlysta | Imuran | Simponi |
| Betamethasone | Infliximab | Simulect |
| Brodalumab | Ixekizumab | Sirolimus |
| Bubbli-Pred | Kenaject | Stelara |
| Budesonide | Kenalog | Sterapred |
| Celestone | Kineret | Tacrolimus |
| CellCept | Leflunomide | Taltz |
| Certolizumab | Medrol | Tocilizumab |
| Cimzia | Meprolone | Tofacitinib |
| Cortef | Methotrexate | Tremfya |
| Cortisone | Methylpred | Triamcinolone |
| Cosentyx | Methylprednisolone | Triesence |
| CPC-Cort-D | Meticorten | Tysabri |
| Cyclosprine | Millipred | Ustekinumab |
| Daclizumab | Mycophenolate | Vedolizumab |
| Decadron | Myfortic | Veripred |
| Deflazacort | Natalizumab | Xeljanz |
| Deltasone | Neoral | Zema |
| Depo-Medrol | Orapred | Zinbryta |
| Dexacen | Orencia | Zortress |
Table E1.
Clinical characteristics and outcomes by eosinophilia (>0.15 ×103/μL) for patients with asthma in registry stratified by ICS use
| Variable | All patients with asthma | P | No ICS | P | ICS | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Eosinophil count | <0.15 [×103/μL] | >0.15 [×103/μL] | <0.15 [×103/μL] | >0.15 [×103/μL] | <0.15 [×103/μL] | >0.15 [×103/μL] | |||
| n | 3,559 | 3,180 | 2,145 | 1,695 | 1,414 | 1,485 | |||
| Demographics | |||||||||
| Age, y | 48.9 [34.1-62.6] | 52.9 [39.0-66.2] | <.001 | 42.8 [30.1-58.2] | 48.3 [34.2-62.3] | <.001 | 55.8 [44.4-67.2] | 58.0 [45.4-69.7] | .004 |
| Female sex | 2,610 (73.3) | 2,142 (67.4) | <.001 | 1,565 (73) | 1,138 (67.1) | <.001 | 1,045 (73.9) | 1,004 (67.6) | <.001 |
| Body mass index, kg/m2 | 31.2 [26.2-37.5] | 32.3 [27.3-38.4] | <.001 | 30.6 [25.8-36.9] | 32.1 [27.1-38.0] | <.001 | 31.9 [26.9-38.4] | 32.6 [27.4-39.0] | .036 |
| Race | <.001 | <.001 | .012 | ||||||
| Black | 1,081 (30.4) | 781 (24.6) | 676 (31.5) | 420 (24.8) | 405 (28.6) | 361 (24.3) | |||
| White | 2,089 (58.7) | 2,082 (65.5) | 1,208 (56.3) | 1,079 (63.7) | 881 (62.3) | 1,003 (67.5) | |||
| Others∗ | 389 (10.9) | 317 (10.0) | 261 (12.2) | 196 (11.6) | 128 (9.1) | 121 (8.1) | |||
| Hispanic ethnicity | 117 (3.3) | 92 (2.9) | .347 | 68 (3.2) | 59 (3.5) | .663 | 51 (3.6) | 33 (2.2) | .043 |
| Smoking history | .345 | .13 | .5 | ||||||
| Current | 320 (9.0) | 284 (8.9) | 208 (9.7) | 181 (10.7) | 112 (7.9) | 103 (6.9) | |||
| Past | 1,052 (29.6) | 992 (31.2) | 542 (25.3) | 465 (27.4) | 510 (36.1) | 527 (35.5) | |||
| Pack-year smoking | 10.0 [3.6-25.0] | 11.4 [4.0-27.0] | .379 | 7.5 [2.0-20.0] | 10.0 [4.0-22.5] | .004 | 15.0 [5.0-30.0] | 14.0 [4.7-30.0] | .153 |
| Eosinophil count (×103/μL)† | 0.08 [0.04-0.12] | 0.26 [0.20-0.36] | <.001 | 0.09 [0.05-0.12] | 0.24 [0.20-0.34] | <.001 | 0.08 [0.03-0.11] | 0.27 [0.20-0.38] | <.001 |
| Comorbidities | |||||||||
| Diabetes | 810 (22.8) | 835 (26.3) | .001 | 401 (18.7) | 390 (23.0) | .001 | 409 (28.9) | 445 (30.0) | .566 |
| Hypertension | 1,631 (45.8) | 1,676 (52.7) | <.001 | 815 (38.0) | 775 (45.7) | <.001 | 816 (57.7) | 901 (60.7) | .113 |
| Coronary artery disease | 420 (11.8) | 430 (13.5) | .037 | 180 (8.4) | 166 (9.8) | .147 | 240 (17.0) | 264 (17.8) | .601 |
| Heart failure | 384 (10.8) | 379 (11.9) | .155 | 150 (7.0) | 146 (8.6) | .071 | 234 (16.5) | 233 (15.7) | .563 |
| Cancer history | 457 (12.8) | 463 (14.6) | .044 | 212 (9.9) | 209 (12.3) | .018 | 245 (17.3) | 254 (17.1) | .913 |
| Connective tissue disease | 241 (6.8) | 186 (5.8) | .133 | 112 (5.2) | 85 (5.0) | .83 | 129 (9.1) | 101 (6.8) | .025 |
| Immunosuppressive disease | 510 (14.3) | 399 (12.5) | .035 | 231 (10.8) | 171 (10.1) | .528 | 279 (19.7) | 228 (15.4) | .002 |
| Medications | |||||||||
| Nonsteroidal anti-inflammatory drugs | 795 (22.3) | 659 (20.7) | .114 | 521 (24.3) | 373 (22.0) | .104 | 274 (19.4) | 286 (19.3) | .973 |
| Angiotensin converting enzyme inhibitors | 297 (8.3) | 334 (10.5) | .003 | 155 (7.2) | 158 (9.3) | .022 | 142 (10.0) | 176 (11.9) | .134 |
| Angiotensin receptor blockers | 317 (8.9) | 343 (10.8) | .011 | 134 (6.2) | 147 (8.7) | .005 | 183 (12.9) | 196 (13.2) | .881 |
| Intranasal corticosteroids | 1,528 (42.9) | 1,328 (41.8) | .343 | 746 (34.8) | 559 (33.0) | .257 | 782 (55.3) | 769 (51.8) | .063 |
| ICS | 1,414 (39.7) | 1,485 (46.7) | <.001 | 0 | 0 | NA | 1,414 (100.0) | 1,485 (100.0) | NA |
| Immunosuppressive therapy‡ | 102 (2.9) | 66 (2.1) | .046 | 38 (1.8) | 26 (1.5) | .657 | 64 (4.5) | 40 (2.7) | .011 |
| Outcomes | |||||||||
| Hospitalization | 850 (23.9) | 717 (22.5) | .205 | 385 (17.9) | 308 (18.2) | .892 | 465 (32.9) | 409 (27.5) | .002 |
| Admission to intensive care unit | 193 (5.4) | 149 (4.7) | .186 | 69 (3.2) | 55 (3.2) | 1 | 124 (8.8) | 94 (6.3) | .016 |
| Hospital mortality | 103 (2.9) | 92 (2.9) | 1 | 39 (1.8) | 33 (1.9) | .863 | 64 (4.5) | 59 (4.0) | .268 |
ICS, inhaled corticosteroids; NA, not available.
Data are presented as n (%) for categorical variables and median [interquartile range] for continuous variables.
“Other” race category includes American Indians or Alaska Natives, Asian individuals, Native Hawaiian or Other Pacific Islanders, and individuals with multiple racial backgrounds.
Defined by a baseline blood absolute eosinophil count of greater than 0.15 (×103 cells/ μL) obtained at least 2 weeks before severe acute respiratory syndrome coronavirus 2 test date.
Includes chronic systemic corticosteroid therapy.
Table E2.
Clinical characteristics and outcomes by eosinophilia (>0.15 ×103/μL) for patients with chronic obstructive pulmonary disease in registry stratified by ICS use
| Variable | All patients with COPD | P | No ICS | P | ICS | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Eosinophil count | <0.15 [×103/μL] | >0.15 [×103/μL] | <0.15 [×103/μL] | >0.15 [×103/μL] | <0.15 [×103/μL] | >0.15 [×103/μL] | |||
| n | 1,524 | 1,542 | 673 | 680 | 851 | 862 | |||
| Demographics | |||||||||
| Age, y | 68.8 [60.6-78.3] | 71.1 [61.7-78.9] | .013 | 69.2 [60.6-78.4] | 71.8 [62.1-79.6] | .007 | 68.7 [60.6-78.2] | 70.1 [61.2-77.7] | .342 |
| Female sex | 883 (57.9) | 827 (53.6) | .018 | 356 (52.9) | 330 (48.5) | .121 | 527 (61.9) | 497 (57.7) | .08 |
| Body mass index, kg/m2 | 29.2 [24.5-35.1] | 30.1 [25.5-36.0] | .001 | 28.8 [23.9-34.0] | 29.6 [25.4-35.6] | .001 | 29.9 [25.0-36.0] | 30.2 [25.6-36.4] | .184 |
| Race∗ | .046 | .541 | .067 | ||||||
| Black | 435 (28.5) | 381 (24.7) | 182 (27.0) | 167 (24.6) | 253 (29.7) | 214 (24.8) | |||
| White | 994 (65.2) | 1,051 (68.2) | 453 (67.3) | 470 (69.1) | 541 (63.6) | 581 (67.4) | |||
| Others | 95 (6.2) | 110 (7.1) | 38 (5.6) | 43 (6.3) | 57 (6.7) | 67 (7.8) | |||
| Hispanic ethnicity | 36 (2.4) | 38 (2.5) | .947 | 11 (1.6) | 16 (2.4) | .453 | 26 (3.1) | 23 (2.7) | .74 |
| Smoking history | .251 | .042 | .911 | ||||||
| Current | 206 (13.5) | 240 (15.6) | 97 (14.5) | 132 (19.6) | 109 (12.8) | 108 (12.5) | |||
| Past | 881 (57.9) | 861 (56.1) | 374 (55.7) | 353 (52.5) | 507 (59.6) | 508 (59.0) | |||
| Pack-years smoking | 30.0 [14.3-47.0] | 30.0 [14.0-48.5] | .72 | 27.0 [11.1-45.0] | 30.0 [15.0-45.0] | .166 | 30.0 [15.0-50.0] | 30.0 [12.5-50.0] | .505 |
| Eosinophil count (×103/μL)† | 0.08 [0.03-0.11] | 0.27 [0.20-0.38] | <.001 | 0.08 [0.03-0.11] | 0.25 [0.20-0.37] | <.001 | 0.08 [0.03-0.11] | 0.27 [0.21-0.40] | <.001 |
| Comorbidities | |||||||||
| Diabetes | 630 (41.3) | 673 (43.6) | .209 | 263 (39.1) | 296 (43.5) | .108 | 367 (43.1) | 377 (43.7) | .837 |
| Hypertension | 1,212 (79.5) | 1,273 (82.6) | .036 | 511 (75.9) | 569 (83.7) | <.001 | 701 (82.4) | 704 (81.7) | .752 |
| Coronary artery disease | 541 (35.5) | 579 (37.5) | .254 | 240 (35.7) | 269 (39.6) | .155 | 301 (35.4) | 310 (36.0) | .837 |
| Heart failure | 534 (35.0) | 525 (34.0) | .589 | 207 (30.8) | 211 (31.0) | .961 | 327 (38.4) | 314 (36.4) | .421 |
| Cancer history | 443 (29.1) | 416 (27.0) | .212 | 192 (28.5) | 175 (25.7) | .274 | 251 (29.5) | 241 (28.0) | .516 |
| Connective tissue disease | 117 (7.7) | 122 (7.9) | .861 | 38 (5.6) | 47 (6.9) | .397 | 79 (9.3) | 75 (8.7) | .736 |
| Immunosuppressive disease | 460 (30.2) | 424 (27.5) | .109 | 199 (29.6) | 194 (28.5) | .718 | 261 (30.7) | 230 (26.7) | .076 |
| Medications | |||||||||
| Nonsteroidal anti-inflammatory drugs | 296 (19.4) | 323 (20.9) | .314 | 138 (20.5) | 148 (21.8) | .617 | 158 (18.6) | 175 (20.3) | .397 |
| Angiotensin converting enzyme inhibitors | 241 (15.8) | 268 (17.4) | .264 | 108 (16.0) | 129 (19.0) | .179 | 133 (15.6) | 139 (16.1) | .83 |
| Angiotensin receptor blockers | 238 (15.6) | 196 (12.7) | .024 | 96 (14.3) | 78 (11.5) | .146 | 142 (16.7) | 118 (13.7) | .097 |
| Intranasal corticosteroids | 549 (36.0) | 581 (37.7) | .362 | 178 (26.4) | 177 (26.0) | .91 | 371 (43.6) | 404 (46.9) | .19 |
| ICS | 851 (55.8) | 862 (55.9) | 1 | 0 | 0 | NA | 851 (100.0) | 862 (100.0) | NA |
| Immunosuppressive therapy‡ | 65 (4.3) | 50 (3.2) | .163 | 19 (2.8) | 12 (1.8) | .263 | 46 (5.4) | 38 (4.4) | .399 |
| Outcomes | |||||||||
| Hospitalization | 823 (54.0) | 772 (50.1) | .032 | 328 (48.7) | 333 (49.0) | .975 | 495 (58.2) | 439 (50.9) | .003 |
| Admission to intensive care unit | 257 (16.9) | 219 (14.2) | .047 | 91 (13.5) | 88 (12.9) | .814 | 166 (19.5) | 131 (15.2) | .022 |
| Hospital mortality | 188 (12.3) | 159 (10.3) | .087 | 78 (11.6) | 70 (10.3) | .499 | 110 (12.9) | 89 (10.3) | .109 |
ICS, inhaled corticosteroids; NA, not available.
Data are presented as n (%) for categorical variables and median [interquartile range] for continuous variables.
“Other” race category includes American Indians or Alaska Natives, Asian individuals, Native Hawaiian or Other Pacific Islanders, and individuals with multiple racial backgrounds.
Defined by a baseline blood absolute eosinophil count > 0.15 (x103 cells/ μL) obtained at least 2 weeks before severe acute respiratory syndrome coronavirus 2 test date.
Includes chronic systemic corticosteroid therapy.
Table E3.
Multivariable logistic regression∗ fitted with restricted cubic spline function for absolute eosinophil count (log10-transformed) with three knots describing nonlinear relationship between eosinophil count and outcomes measures such as hospitalization, admission to intensive care unit, and in-hospital mortality
| Outcome | Estimate | SE | z Value | P |
|---|---|---|---|---|
| Hospitalization | ||||
| X | –0.646 | 0.066 | –9.841 | <2e-16 |
| X′ | 0.514 | 0.070 | 7.311 | 2.6e-13 |
| Intensive care unit admission | ||||
| X | –0.500 | 0.110 | –4.524 | 6.1e-06 |
| X′ | 0.383 | 0.118 | 3.255 | 0.001 |
| Died | ||||
| X | –0.784 | 0.130 | –6.035 | 1.6e-09 |
| X′ | 0.599 | 0.142 | 4.222 | 2.4e-5 |
Medications in the model include nonsteroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, inhaled corticosteroids, intranasal corticosteroids, and immunosuppressive therapy that includes systemic corticosteroids). Comorbidities in the model include diabetes, hypertension, coronary artery disease, heart failure and cancer (historical or current), immunosuppressive diseases, and connective tissue diseases.
Adjusted for demographics, body mass index, smoking, medications, comorbidities, and the month of testing.
Table E4.
Association between eosinophilia∗ and coronavirus disease 2019–related outcomes using complete cases (ie, excluding patients with missing data and without imputation)
| Outcome | All patients |
Chronic obstructive pulmonary disease |
Asthma |
|||
|---|---|---|---|---|---|---|
| No ICS |
ICS |
No ICS |
ICS |
No ICS |
ICS |
|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| n | 39,888 | 4,973 | 1,336 | 1,701 | 3,802 | 2,883 |
| Unadjusted | ||||||
| Hospitalization | 1.23 (1.17-1.29) | 0.85 (0.75-0.95) | 1.01 (0.82-1.25) | 0.75 (0.62-0.90) | 1.01 (0.86-1.19) | 0.77 (0.66-0.91) |
| ICU admission | 1.26 (1.14-1.39) | 0.75 (0.62-0.91) | 0.95 (0.69-1.30) | 0.74 (0.57-0.95) | 1.01 (0.70-1.45) | 0.70 (0.53-0.93) |
| Hospital mortality | 1.35 (1.20-1.52) | 0.80 (0.63-1.02) | 0.86 (0.61-1.22) | 0.78 (0.58-1.04) | 1.07 (0.67-1.71) | 0.87 (0.61-1.25) |
| Adjusted for age, sex, ethnicity, race, and month of testing. | ||||||
| Hospitalization | 0.97 (0.93-1.03) | 0.81 (0.73-0.91) | 0.96 (0.78-1.20) | 0.77 (0.63-0.93) | 0.84 (0.70-1.00) | 0.72 (0.62-0.85) |
| ICU admission | 0.97 (0.89-1.06) | 0.72 (0.60-0.88) | 0.93 (0.68-1.26) | 0.74 (0.58-0.96) | 0.84 (0.58-1.22) | 0.65 (0.49-0.85) |
| Hospital mortality | 0.92 (0.83-1.03) | 0.74 (0.59-0.93) | 0.80 (0.57-1.11) | 0.76 (0.58-1.01) | 0.78 (0.49-1.25) | 0.76 (0.53-1.09) |
| Adjusted for age, sex, race, ethnicity, smoking history, pack-years smoking, medications, comorbidities, time between absolute eosinophil count test date and severe acute respiratory syndrome coronavirus 2 test date, and month of testing† | ||||||
| Hospitalization | 0.96 (0.91-1.02) | 0.84 (0.74-0.94) | 0.98 (0.79-1.22) | 0.78 (0.64-0.95) | 0.84 (0.71-1.00) | 0.76 (0.65-0.89) |
| ICU admission | 0.96 (0.87-1.06) | 0.77 (0.64-0.94) | 0.89 (0.65-1.21) | 0.77 (0.60-0.99) | 0.86 (0.59-1.24) | 0.71 (0.54-0.94) |
| Hospital mortality | 0.98 (0.87-1.11) | 0.79 (0.63-1.00) | 0.85 (0.61-1.18) | 0.78 (0.59-1.04) | 0.81 (0.50-1.31) | 0.83 (0.57-1.20) |
CI, confidence interval; ICS, inhaled corticosteroids; ICU, intensive care unit; OR, odds ratio.
Baseline preexisting eosinophilia were defined by a blood absolute eosinophil count of greater than 0.15 × 103 cells/μL, obtained at least 2 weeks before the severe acute respiratory syndrome coronavirus 2 test date.
The effect of a high blood absolute eosinophil count greater than 0.15 × 103 cells/μL on hospital outcomes is estimated by weighting each patient with the inverse propensity score and controlling for the propensity score as a covariate in the model. Medications included nonsteroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, ICS, intranasal corticosteroids, and immunosuppressive therapy (including systemic corticosteroids). Comorbidities include asthma, chronic obstructive pulmonary disease or emphysema, diabetes, hypertension, coronary artery disease, heart failure and cancer (historical or current), immunosuppressive diseases, and connective tissue diseases.
Table E5.
Coronavirus disease 2019–related outcomes results of patients with different blood AEC categories compared with patients with eosinopenia defined by AEC less than 0.1 × 103 cell/μLE12
| All patients | n | Hospitalization |
Intensive care unit admission |
Hospital mortality |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| AEC (×103 cell/μL) | ||||
| <0.1∗ | 15,512 | 1 | 1 | 1 |
| 0.1-0.2 | 18,250 | 0.74 (0.70-0.79) | 0.83 (0.73-0.92) | 0.66 (0.58-0.77) |
| 0.2-0.3 | 6,933 | 0.79 (0.73-0.86) | 0.89 (0.77-1.02) | 0.77 (0.65-0.91) |
| >0.3 | 5,702 | 0.88 (0.81-0.96) | 0.91 (0.79-1.05) | 0.84 (0.71-0.99) |
| No inhaled corticosteroids | ||||
| <0.1∗ | 14,088 | 1 | 1 | 1 |
| 0.1-0.2 | 16,468 | 0.75 (0.71-0.81) | 0.84 (0.74-0.95) | 0.68 (0.58-0.79) |
| 0.2-0.3 | 6,082 | 0.82 (0.75-0.89) | 0.96 (0.82-1.12) | 0.80 (0.66-0.97) |
| >0.3 | 4,748 | 0.94 (0.86-1.03) | 0.95 (0.81-1.12) | 0.95 (0.79-1.16) |
| Inhaled corticosteroids | ||||
| <0.1∗ | 1,424 | 1 | 1 | 1 |
| 0.1-0.2 | 1,782 | 0.68 (0.58-0.81) | 0.74 (0.58-0.95) | 0.61 (0.44-0.83) |
| 0.2-0.3 | 851 | 0.65 (0.53-0.79) | 0.63 (0.46-0.86) | 0.67 (0.46-0.96) |
| >0.3 | 954 | 0.64 (0.53-0.79) | 0.75 (0.56-1.01) | 0.52 (35-76) |
AEC, absolute eosinophil count; CI, confidence interval; OR, odds ratio.
The analysis was adjusted for demographics, baseline AEC, month of testing, smoking status, pack-years smoking, medications, and comorbidities.
Patients with preexisting AEC measurements were stratified into four categories. Those with an AEC greater than 0.3, 0.2 to 03, and 0.1 to 0.2 were compared with patients with eosinopenia (defined by an AEC less than 0.1 × 103 cell/μL).
Table E6.
Hospitalization risk comparing patients with an AEC greater than 0.15 × 103/μL versus those with an AEC less than 0.15 × 103/μL
| Time between AEC test date and SARS-CoV-2 test date | n | All patients |
Chronic obstructive pulmonary disease |
Asthma |
|||
|---|---|---|---|---|---|---|---|
| No ICS | ICS | No ICS | ICS | No ICS | ICS | ||
| <2 y∗ | 24,095 | 0.97 (0.90-1.03) | 0.85 (0.75-0.97) | 1.05 (0.82-1.33) | 0.79 (0.64-0.98) | 0.89 (0.73-1.10) | 0.78 (0.65-0.93) |
| <1 y† | 15,058 | 0.93 (0.86-1.00) | 0.81 (0.70-0.95) | 1.17 (0.89-1.54) | 0.77 (0.61-0.98) | 0.93 (0.73-1.18) | 0.76 (0.62-0.94) |
AEC, absolute eosinophil count; ICS, inhaled corticosteroids; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The analysis was stratified by the time between the AEC test date and the severe acute respiratory syndrome coronavirus 2 test date inhaled corticosteroids therapy and airway disease category.
Effect of a high blood absolute eosinophil count greater than 0.15 × 103 cells/μL on hospital outcomes is estimated by weighting each patient with the inverse propensity score and controlling for the propensity score as a covariate in the model. Medications include nonsteroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, ICS, intranasal corticosteroids, and immunosuppressive therapy (that includes systemic corticosteroids). Comorbidities include asthma, chronic obstructive pulmonary disease/emphysema, diabetes, hypertension, coronary artery disease, heart failure and cancer (historical or current), immunosuppressive disease, and connective tissue disease.
Patients for whom AEC measurements were obtained within 2 years (median [interquartile range]: 291 [126-457] days) of the SARS-CoV-2 test date.
Patients for whom AEC measurements were obtained within 1 year (median [interquartile range]: 173 [55-275] days) of the SARS-CoV-2 test date.
Table E7.
Association between high fractional of exhaled nitric oxide measurements (FeNO >35 parts per billion) and hospitalization risk stratified by ICS use
| Adjustment | ICS |
No ICS |
|---|---|---|
| (odds ratio [95% confidence interval]) | (odds ratio [95% confidence interval]) | |
| n | 835 | 459 |
| Adjusted for baseline absolute eosinophil count and month of testing | 0.45 (0.28-0.70) | 0.79 (0.32-1.77) |
| Adjusted for demographics,∗ baseline absolute eosinophil count, and month of testing | 0.70 (0.53-0.92) | 0.86 (0.61-1.22) |
| Adjusted for demographics, baseline absolute eosinophil count,∗ month of testing, smoking status, pack-years smoking, medications, and comorbidities† | 0.72 (0.55-0.95) | 0.85 (0.60-1.20) |
ICS, inhaled corticosteroids.
Demographics include age, sex, ethnicity. and race.
The effect of a high FeNO (>35 parts per billion) on hospitalization risk is estimated by weighting each patient with the inverse propensity score and controlling for the propensity score as a covariate in the model. Medications include immunosuppressive therapy (that includes systemic corticosteroids), angiotensin converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and intranasal corticosteroids. Comorbidities include body mass index, smoking history (both current and remote), diabetes, hypertension, coronary artery disease, heart failure, cancer history, immunosuppressive disease, and connective tissue disease.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F., et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.05.004. 80-8.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camiolo M., Gauthier M., Kaminski N., Ray A., Wenzel S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.05.051. 315-24.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair A.P., Soliman A., Al Masalamani M.A., De Sanctis V., Nashwan A.J., Sasi S., et al. Clinical outcome of eosinophilia in patients with COVID-19: a controlled study. Acta Biomed. 2020;91 doi: 10.23750/abm.v91i4.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Borgne P., Abensur Vuillaume L., Alame K., Lefebvre F., Chabrier S., Berard L., et al. Do blood eosinophils predict in-hospital mortality or severity of disease in SARS-CoV-2 infection? A retrospective multicenter study. Microorganisms. 2021;9:334. doi: 10.3390/microorganisms9020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G., et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract. 2021;9 doi: 10.1016/j.jaip.2020.12.045. 1152-62.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho K.S., Howell D., Rogers L., Narasimhan B., Verma H., Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. 2021;127:42–48. doi: 10.1016/j.anai.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilk A.J., Lee M.J., Wei B., Parks B., Pi R., Martinez-Colon G.J., et al. Multi-omic profiling reveals widespread dysregulation of innate immunity and hematopoiesis in COVID-19. J Exp Med. 2021;218 doi: 10.1084/jem.20210582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martinez-Colon G.J., McKechnie J.L., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217 doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aschenbrenner A.C., Mouktaroudi M., Kramer B., Oestreich M., Antonakos N., Nuesch-Germano M., et al. Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients. Genome Med. 2021;13:7. doi: 10.1186/s13073-020-00823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulte-Schrepping J., Reusch N., Paclik D., Bassler K., Schlickeiser S., Zhang B., et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182 doi: 10.1016/j.cell.2020.08.001. 1419-40.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R., Scott M., Hagan T., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramakrishnan S., Nicolau D.V., Jr., Langford B., Mahdi M., Jeffers H., Mwasuku C., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.L., Montgomery M.T., et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finney L.J., Glanville N., Farne H., Aniscenko J., Fenwick P., Kemp S.V., et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021;147 doi: 10.1016/j.jaci.2020.09.034. 510-9.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95:763–772. doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Hu S., Wang J., Xue Z., Wang C., Wang N. Dexamethasone inhibits SARS-CoV-2 spike pseudotyped virus viropexis by binding to ACE2. Virology. 2021;554:83–88. doi: 10.1016/j.virol.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss R., Jawhari N., Attaway A.H., Hu B., Jehi L., Milinovich A., et al. Intranasal corticosteroids are associated with better outcomes in coronavirus disease 2019 (COVID-19) J Allergy Clin Immunol Pract. 2021;9 doi: 10.1016/j.jaip.2021.08.007. 3934-40.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.06.010. 307-14.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izquierdo J.L., Almonacid C., Gonzalez Y., Del Rio-Bermudez C., Ancochea J., Cardenas R., et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57:2003142. doi: 10.1183/13993003.03142-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halpin D.M.G., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh D., Halpin D.M.G. Inhaled corticosteroids and COVID-19-related mortality: confounding or clarifying? Lancet Respir Med. 2020;8:1065–1066. doi: 10.1016/S2213-2600(20)30447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultze A., Walker A.J., MacKenna B., Morton C.E., Bhaskaran K., Brown J.P., et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attaway A.A., Zein J., Hatipoglu U.S. SARS-CoV-2 infection in the COPD population is associated with increased healthcare utilization: an analysis of Cleveland clinic's COVID-19 registry. EClinicalMedicine. 2020;26:100515. doi: 10.1016/j.eclinm.2020.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe K.E., Zein J., Hatipoglu U., Attaway A. Association of smoking and cumulative pack-year exposure with COVID-19 outcomes in the Cleveland Clinic COVID-19 Registry. JAMA Intern Med. 2021;181:709–711. doi: 10.1001/jamainternmed.2020.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zein J., Whelan G., Erzurum S. Safety of influenza vaccine during COVID-19. J Clin Transl Sci. 2020;5:1–3. [Google Scholar]
- 32.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milinovich A., Kattan M.W. Extracting and utilizing electronic health data from Epic for research. Ann Transl Med. 2018;6:42. doi: 10.21037/atm.2018.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N., et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 35.Ortega H.G., Liu M.C., Pavord I.D., Brusselle G.G., FitzGerald J.M., Chetta A., et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 36.Katz L.E., Gleich G.J., Hartley B.F., Yancey S.W., Ortega H.G. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11:531–536. doi: 10.1513/AnnalsATS.201310-354OC. [DOI] [PubMed] [Google Scholar]
- 37.Bel E.H., Wenzel S.E., Thompson P.J., Prazma C.M., Keene O.N., Yancey S.W., et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 38.Ortega H.G., Yancey S.W., Mayer B., Gunsoy N.B., Keene O.N., Bleecker E.R., et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–556. doi: 10.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 39.Outh R., Boutin C., Gueudet P., Suzuki M., Saada M., Aumaitre H. Eosinopenia <100/muL as a marker of active COVID-19: an observational prospective study. J Microbiol Immunol Infect. 2021;54:61–68. doi: 10.1016/j.jmii.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwitz L.I., Jones S.A., Cerfolio R.J., Francois F., Greco J., Rudy B., et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16:90–92. doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
- 42.Dennis J.M., McGovern A.P., Vollmer S.J., Mateen B.A. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49:209–214. doi: 10.1097/CCM.0000000000004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Buuren S.G.-O.,K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 44.Rosenberg H.F., Dyer K.D., Domachowske J.B. Eosinophils and their interactions with respiratory virus pathogens. Immunol Res. 2009;43:128–137. doi: 10.1007/s12026-008-8058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drake M.G., Bivins-Smith E.R., Proskocil B.J., Nie Z., Scott G.D., Lee J.J., et al. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am J Respir Cell Mol Biol. 2016;55:387–394. doi: 10.1165/rcmb.2015-0405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 47.Wong C.K., Cheung P.F., Ip W.K., Lam C.W. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 48.Flores-Torres A.S., Salinas-Carmona M.C., Salinas E., Rosas-Taraco A.G. Eosinophils and respiratory viruses. Viral Immunol. 2019;32:198–207. doi: 10.1089/vim.2018.0150. [DOI] [PubMed] [Google Scholar]
- 49.Nagase H., Okugawa S., Ota Y., Yamaguchi M., Tomizawa H., Matsushima K., et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 50.Adamko D.J., Yost B.L., Gleich G.J., Fryer A.D., Jacoby D.B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kasela S., Ortega V.E., Martorella M., Garudadri S., Nguyen J., Ampleford E., et al. Genetic and non-genetic factors affecting the expression of COVID-19-relevant genes in the large airway epithelium. Genome Med. 2021;13:66. doi: 10.1186/s13073-021-00866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georas S.N. Inhaled glucocorticoids, lymphocytes, and dendritic cells in asthma and obstructive lung diseases. Proc Am Thorac Soc. 2004;1:215–221. doi: 10.1513/pats.200402-004MS. [DOI] [PubMed] [Google Scholar]
- 53.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borczuk A.C., Salvatore S.P., Seshan S.V., Patel S.S., Bussel J.B., Mostyka M., et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zein J.G., Mitri J., Bell J.M., Lopez D., Strauss R., Attaway A.H. The relationship of asthma severity to COVID-19 outcomes. J Allergy Clin Immunol Pract. 2022;10 doi: 10.1016/j.jaip.2021.10.041. 318-21.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdul-Cader M.S., Amarasinghe A., Abdul-Careem M.F. Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses. Arch Virol. 2016;161:2075–2086. doi: 10.1007/s00705-016-2904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dweik R.A., Boggs P.B., Erzurum S.C., Irvin C.G., Leigh M.W., Lundberg J.O., et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters M.C., Kerr S., Dunican E.M., Woodruff P.G., Fajt M.L., Levy B.D., et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol. 2019;143 doi: 10.1016/j.jaci.2017.12.1009. 104-13.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Attaway A.A., Zein J., Hatipoglu U.S. SARS-CoV-2 infection in the COPD population is associated with increased healthcare utilization: an analysis of Cleveland clinic's COVID-19 registry. EClinicalMedicine. 2020;26:100515. doi: 10.1016/j.eclinm.2020.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K.E., Zein J., Hatipoglu U., Attaway A. Association of smoking and cumulative pack-year exposure with COVID-19 outcomes in the Cleveland Clinic COVID-19 Registry. JAMA Intern Med. 2021;181:709–711. doi: 10.1001/jamainternmed.2020.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein J., Whelan G., Erzurum S. Safety of influenza vaccine during COVID-19. J Clin Transl Sci. 2020;5:1–3. [Google Scholar]
- Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinovich A., Kattan M.W. Extracting and utilizing electronic health data from Epic for research. Ann Transl Med. 2018;6:42. doi: 10.21037/atm.2018.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality. Appendix I: Immunocompromised state diagnosis and procedure codes. https://www.qualityindicators.ahrq.gov/Downloads/Modules/PSI/V60-ICD10/TechSpecs/PSI_Appendix_I.pdf Accessed August 7, 2021.
- Outh R., Boutin C., Gueudet P., Suzuki M., Saada M., Aumaitre H. Eosinopenia <100/muL as a marker of active COVID-19: an observational prospective study. J Microbiol Immunol Infect. 2021;54:61–68. doi: 10.1016/j.jmii.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]