ABSTRACT
Background
Calcific uraemic arteriolopathy (CUA; calciphylaxis) is a rare disease seen predominantly in patients receiving dialysis. Calciphylaxis is characterized by poorly healing or non-healing wounds, and is associated with mortality, substantial morbidity related to infection and typically severe pain. In an open-label Phase 2 clinical trial, SNF472, a selective inhibitor of vascular calcification, was well-tolerated and associated with improvement in wound healing, reduction of wound-related pain and improvement in wound-related quality of life (QoL). Those results informed the design of the CALCIPHYX trial, an ongoing, randomized, placebo-controlled, Phase 3 trial of SNF472 for treatment of calciphylaxis.
Methods
In CALCIPHYX, 66 patients receiving haemodialysis who have an ulcerated calciphylaxis lesion will be randomized 1:1 to double-blind SNF472 (7 mg/kg intravenously) or placebo three times weekly for 12 weeks (Part 1), then receive open-label SNF472 for 12 weeks (Part 2). All patients will receive stable background care, which may include pain medications and sodium thiosulphate, in accordance with the clinical practices of each site. A statistically significant difference between the SNF472 and placebo groups for improvement of either primary endpoint at Week 12 will demonstrate efficacy of SNF472: change in Bates-Jensen Wound Assessment Tool-CUA (a quantitative wound assessment tool for evaluating calciphylaxis lesions) or change in pain visual analogue scale score. Additional endpoints will address wound-related QoL, qualitative changes in wounds, wound size, analgesic use and safety.
Conclusions
This randomized, placebo-controlled Phase 3 clinical trial will examine the efficacy and safety of SNF472 in patients who have ulcerated calciphylaxis lesions. Patient recruitment is ongoing.
Keywords: calciphylaxis, controlled clinical trial, design, SNF472, rationale
INTRODUCTION
Calcific uraemic arteriolopathy (CUA or calciphylaxis) is a rare disease seen predominantly in patients with end-stage kidney disease (ESKD) [1, 2]. The annual incidence of calciphylaxis is ˂1% among patients on dialysis [3–5]. Calciphylaxis lesions, which most commonly appear in the truncal area and lower limbs, need to be differentiated from peripheral arterial disease that tends to be more distally distributed [6].
Calciphylaxis results from progressive calcification of skin arterioles, typically in areas with abundant adipose tissue such as the abdomen and thighs [1]. Subsequent subintimal fibrosis and thrombosis lead to arteriole occlusion and progressive necrosis, eventually resulting in skin ulceration [1]. These ulcerations are extremely painful, with pain often refractory to analgesics [7]. Ulcerated lesions are also susceptible to wound infection and sepsis, both of which can lead directly or indirectly to mortality [8–11].
Several studies have reported 1-year mortality rates in patients with calciphylaxis at 40–50% or higher [11–14]. A recent analysis of the UK Calciphylaxis Registry reported a 1-year mortality rate of 67% versus 10% in patients receiving dialysis without calciphylaxis [10]. Patients with calciphylaxis also have a high morbidity burden and poor quality of life (QoL) related to painful wounds, ambulatory difficulties and frequent hospitalizations [15, 16].
There are no specific treatment guidelines and no approved therapeutics or devices for the treatment of calciphylaxis. Current treatment strategies are based on anecdotal evidence and evidence from retrospective chart reviews and case series [1]. Three randomized clinical trials of sodium thiosulphate (STS) for calciphylaxis were previously attempted (NCT03150420, NCT02527213 and ISRCTN73380053) but all were terminated, and no results have been published from those studies.
We previously reported the results of a prospective open-label, single-arm Phase 2 clinical trial of 14 patients receiving dialysis who had calciphylaxis, all of whom received SNF472 by intravenous infusion during thrice-weekly haemodialysis for up to 12 weeks [17]. SNF472, the hexasodium salt of myo-inositol hexaphosphate (IP6), is a first-in-class inhibitor of vascular calcification. SNF472 has a novel mechanism of action: it physiochemically blocks formation and progression of vascular hydroxyapatite crystals, selectively inhibiting vascular calcification [18, 19]. SNF472 may also inhibit differentiation of vascular smooth muscle cells into osteoblast-like cells [19]. The Phase 2 clinical trial of SNF472 was the first prospective interventional clinical trial in patients with calciphylaxis to be published [17]. Patients treated with SNF472 experienced improvement in wound healing as measured with the 13-item Bates-Jensen Wound Assessment Tool (BWAT) [20], reduction in wound-related pain severity using a visual analogue scale (VAS) and improvement in wound-related QoL using the 17-item Wound-QoL questionnaire [21, 22]. SNF472 was well-tolerated, with no serious treatment-related adverse events.
These results informed the design and conduct of this larger Phase 3, randomized, placebo-controlled clinical trial to investigate the safety and efficacy of SNF472 treatment in patients with calciphylaxis. Herein we describe the design of this study and discuss elements of the study design implemented to overcome the challenges of conducting prospective, controlled clinical trials in this patient population.
MATERIALS AND METHODS
Study design
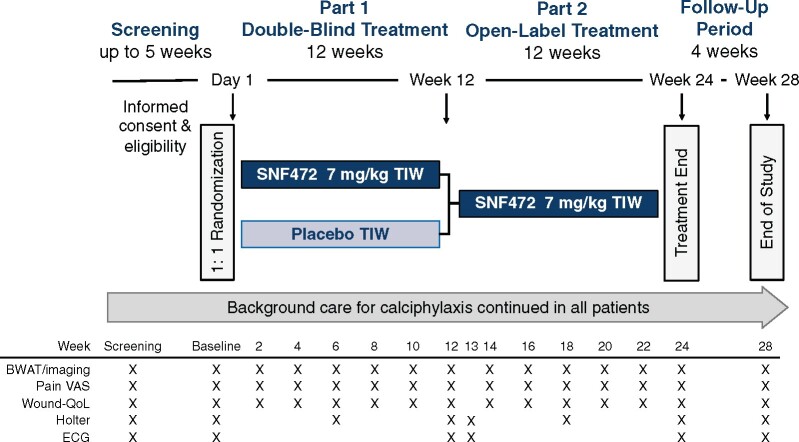
This Phase 3, global, multicentre clinical trial (NCT04195906; CALCIPHYX) will examine the efficacy and safety of SNF472 in adult patients receiving maintenance haemodialysis who have at least one ulcerated calciphylaxis lesion. The study will include a screening period of up to 5 weeks, a 12-week double-blind, randomized, placebo-controlled treatment period (Part 1) followed by a 12-week open-label treatment period (Part 2) in which all patients will receive SNF472, and a 4-week follow-up period (Figure 1). This study will be conducted in accordance with the declaration of Helsinki and the International Council for Harmonisation Guidelines on Good Clinical Practice. The first patient was enrolled in February 2020 and study completion is expected in approximately April 2022.
FIGURE 1:
Study design. After a screening period of up to 5 weeks, eligible patients will be randomized 1:1 to receive an infusion of SNF472 or placebo TIW, during haemodialysis. Double-blind study treatment will be administered for 12 weeks, followed by open-label SNF472 treatment for an additional 12 weeks, and a 4-week safety follow-up period. Background care, including pain medications and/or STS, will be stabilized during screening and no changes to the background care regimen will be made after randomization unless medically indicated in the opinion of the Investigator.
Patients meeting all eligibility criteria will be randomized in a 1:1 ratio to receive either SNF472 or matching placebo in a double-blind manner. Randomization will be stratified based on the provision of intravenous STS use (yes/no). Planned enrolment is 66 patients from ∼60 sites globally. The number of patients may be adjusted up to a maximum of 99 based on results of a sample size re-estimation, as discussed in Statistical analyses below. SNF472 solution (30 mg/mL) and matching placebo will be diluted in physiological saline and infused via the dialysis circuit over a period of ∼2.5–3 h thrice weekly (TIW) during regular dialysis sessions. Patients must be on stable background care, including pain medications and/or STS, during screening; no changes will be made after randomization unless medically indicated in the opinion of the Investigator. The Investigators should also consider whether these measures are appropriate for their patients during screening: replacement of warfarin with another anticoagulant, withdrawal of calcium-based phosphate binders (unless being used for hypocalcaemia), reduction in vitamin D administration and a haemodialysis regimen of 4 h TIW. Wound care will be at the Investigator’s discretion. Patients completing the study will return for a follow-up visit 4 weeks after the last dose of study medication.
Eligibility
Inclusion and exclusion criteria are provided in Table 1. Patients must be at least 18 years of age and receiving maintenance haemodialysis in a clinical setting for at least 2 weeks. The clinical diagnosis of calciphylaxis will be made by the site Investigator, based on the criteria of livedo reticularis, plaques or nodules, necrotic lesions covered by eschar or full thickness open wounds in a patient withESKD. Inclusion criteria require at least one calciphylaxis lesion with ulceration of the epithelial surface and a pain VAS score at least 50 of 100 at screening. Patients will be excluded from study participation if the primary lesion is due to causes other than calciphylaxis, life expectancy is ˂6 months, or kidney transplantation or parathyroidectomy is expected to occur within 6 months.
Table 1.
Study eligibility criteria
| Inclusion criteria |
|---|
| ≥18 years of age |
| Receiving maintenance haemodialysis in a clinical setting (i.e. excluding home haemodialysis) for at least 2 weeks prior to screening |
| Clinical diagnosis of calciphylaxis by the Investigator including ≥1 calciphylaxis lesion with ulceration of the epithelial surface. A central wound rating group will review wound images to confirm the primary lesion is due to calciphylaxis |
| Calciphylaxis wound-related pain shown by a pain VAS ≥50 of 100 |
| Primary lesion that can be clearly photographed for the purpose of protocol-specified wound healing assessments |
| Willing and able to understand and sign the informed consent form and willing to comply with all aspects of the protocol |
|
|
| Exclusion criteria |
|
|
| Patients whose primary lesion is due to causes other than calciphylaxis |
| History of treatment with bisphosphonates within 3 months of baseline (Week 1, Day 1) |
| Severely ill patients without a reasonable expectation of survival for at least 6 months based on the assessment of the Investigator |
| Patients with a scheduled parathyroidectomy during the study period |
| Expectation for kidney transplant within the next 6 months based on Investigator assessment or identification of a known living donor |
| Pregnant or trying to become pregnant, currently breastfeeding or of childbearing potential (including perimenopausal women who have had a menstrual period within 1 year) and not willing to either completely avoid sexual intercourse with a person of the opposite sex or use a highly effective method of birth control from screening through at least 30 days after last dose of study drug |
| Significant non-compliance with dialysis treatment evidenced by repeated missed dialysis sessions (including if due to hospitalizations where dialysis treatment is unavailable) or significant non-compliance with medication regimen, in the judgement of the Investigator |
| Any history of active malignancy within the last year (history of localized basal cell or squamous cell carcinoma that has been excised/appropriately treated or a fully excised malignant lesion with a low probability of recurrence will not be considered exclusionary) |
| Clinically significant illness other than calciphylaxis within 30 days prior to screening that, in the judgment of the Investigator, could interfere with interpretation of study results, impair compliance with study procedures or impact the safety of the patient (e.g. unstable angina, unstable heart failure, stroke, uncontrolled hypertension or other illness requiring hospitalization) |
| Participation in an investigational study and receipt of an investigational drug or investigational use of a licensed drug (with the exception of intravenous STS) within 30 days prior to screening. If participating in an investigational study of intravenous STS, all visits of that study must be completed prior to screening for this study. Note: off-label use of intravenous STS outside of an investigational study is not restricted |
| Past or current participation in another clinical study with SNF472 |
| History or presence of active alcoholism or drug abuse as determined by the Investigator within 6 months before screening or concurrent social conditions that, in the opinion of the Investigator, would potentially interfere with the patient’s study compliance |
| Mental impairment or history of or current significant psychiatric disease that, in the opinion of the Investigator, may impair ability to provide informed consent or impact compliance with study procedures |
| Any other condition or circumstance that, in the opinion of the Investigator, may make the patient unlikely to complete the study or comply with study procedures and requirements or may pose a risk to the patient's safety and well-being |
| Patients whose calciphylaxis lesions exhibit significant improvement, in the opinion of the Investigator, between the first and second screening visit |
Schedule of assessments
Study visits will occur twice during screening, TIW during each treatment period and once 4 weeks after the last dose of study medication. Key efficacy and safety assessments will occur at the times shown in Figure 1.
Wound healing will be assessed with the BWAT, which measures 13 items: size, depth, edges, undermining, necrotic tissue type, necrotic tissue amount, exudate type, exudate amount, skin colour surrounding wound, peripheral tissue oedema, peripheral tissue induration, granulation tissue and epithelialization (Table 2) [20]. Nine items will be rated from 1 to 5 and four items from 0 to 5, yielding a total score from 9 (best) to 65 (worst). Because the BWAT was originally developed to assess pressure ulcers, one of the primary endpoints of this study will use BWAT-CUA, an 8-item modification specifically developed by experts to evaluate eight prototypical features of calciphylaxis lesions (Table 2) [23, 24].
Table 2.
Components of the BWAT total score and BWAT-CUA
| BWAT item/scores | BWAT total | BWAT- CUA |
|---|---|---|
| Necrotic tissue type | Yes | Yes |
| 1 = None visible | – | – |
| 2 = White/grey non-viable tissue and/or non-adherent yellow slough | – | – |
| 3 = Loosely adherent yellow slough | – | – |
| 4 = Adherent, soft, black eschar | – | – |
| 5 = Firmly adherent, hard, black eschar | – | – |
| Necrotic tissue amount | Yes | Yes |
| 1 = None visible | – | – |
| 2 = <25% of wound bed covered | – | – |
| 3 = 25–50% of wound covered | – | – |
| 4 = >50% and <75% of wound covered | – | – |
| 5 = 75–100% of wound covered | – | – |
| Exudate type | Yes | Yes |
| 1 = Non | – | – |
| 2 = Bloody | – | – |
| 3 = Serosanguineous: thin, watery, pale red/pink | – | – |
| 4 = Serous: thin, watery, clear | – | – |
| 5 = Purulent: thin or thick, opaque, tan/yellow, with or without odour | – | – |
| Exudate amount | Yes | Yes |
| 1 = None, dry wound | – | – |
| 2 = Scant, wound moist but no observable exudate | – | – |
| 3 = Small | – | – |
| 4 = Moderate | – | – |
| 5 = Large | – | – |
| Skin colour surrounding wound | Yes | Yes |
| 1 = Pink or normal for ethnic group | – | – |
| 2 = Bright red and/or blanches to touch | – | – |
| 3 = White or grey pallor or hypopigmented | – | – |
| 4 = Dark red or purple and/or non-blanchable | – | – |
| 5 = Black or hyperpigmented | – | – |
| Peripheral tissue oedema | Yes | Yes |
| 1 = No swelling or oedema | – | – |
| 2 = Non-pitting oedema extends <4 cm around wound | – | – |
| 3 = Non-pitting oedema extends >4 cm around wound | – | – |
| 4 = Pitting oedema extends <4 cm around wound | – | – |
| 5 = Crepitus and/or pitting oedema extends >4 cm around wound | – | – |
| Peripheral tissue induration | Yes | Yes |
| 1 = None present | – | – |
| 2 = Induration <2 cm around wound | – | – |
| 3 = Induration 2–4 cm extending <50% around wound | – | – |
| 4 = Induration 2–4 cm extending >50% around wound | – | – |
| 5 = Induration >4 cm in any area around wound | – | – |
| Granulation tissue | Yes | Yes |
| 1 = Skin intact or partial thickness wound | – | – |
| 2 = Bright, beefy red; 75–100% of wound filled and/or tissue overgrowth | – | – |
| 3 = Bright, beefy red; <75% and >25% of wound filled | – | – |
| 4 = Pink, and/or dull, dusky red and/or fills ≤25% of wound | – | – |
| 5 = No granulation tissue present | – | – |
| Undermining | Yes | No |
| 0 = Healed, resolved wound | – | – |
| 1 = None | – | – |
| 2 = Undermining <2 cm in any area | – | – |
| 3 = Undermining 2–4 cm involving <50% wound margins | – | – |
| 4 = Undermining 2–4 cm involving >50% wound margins | – | – |
| 5 = Undermining >4 cm or tunnelling in any area | – | – |
| Size | Yes | No |
| 0 = Healed, resolved wound | – | – |
| 1 = Length × width <4 cm2 | – | – |
| 2 = Length × width 4 to <16 cm2 | – | – |
| 3 = Length × width 16.1 to <36 cm2 | – | – |
| 4 = Length × width 36.1 to <80 cm2 | – | – |
| 5 = Length × width >80 cm2 | – | – |
| Depth | Yes | No |
| 0 = Healed, resolved wound | – | – |
| 1 = Non-blanchable erythema on intact skin | – | – |
| 2 = Partial thickness skin loss involving epidermis and/or dermis | – | – |
| 3 = Full thickness skin loss involving damage or necrosis of subcutaneous tissue; may extend down to but not through underlying fascia; and/or mixed partial and full thickness and/or tissue layers obscured by granulation tissue | – | – |
| 4 = Obscured by necrosis | – | – |
| 5 = Full thickness skin loss with extensive destruction, tissue necrosis or damage to muscle, bone or supporting structures | – | – |
| Edges | Yes | No |
| 0 = Healed, resolved wound | – | – |
| 1 = Indistinct, diffuse, none clearly visible | – | – |
| 2 = Distinct, outline clearly visible, attached, even with wound base | – | – |
| 3 = Well-defined, not attached to wound base | – | – |
| 4 = Well-defined, not attached to base, rolled under, thickened | – | – |
| 5 = Well-defined, fibrotic, scarred or hyperkeratotic | – | – |
| Epithelialization | Yes | No |
| 1 = 100% wound covered, surface intact | – | – |
| 2 = 75% to <100% wound covered and/or epithelial tissue extends >0.5 cm into wound bed | – | – |
| 3 = 50% to <75% wound covered and/or epithelial tissue extends to <0.5 cm into wound bed | – | – |
| 4 = 25% to <50% wound covered | – | – |
| 5 = <25% wound covered | – | – |
The other primary endpoint, wound-related pain using the VAS, will be administered electronically: the patient will mark a position on a 10-cm horizontal line to indicate the worst wound-related pain experienced during the previous 24 h. The Wound-QoL questionnaire will be administered electronically and will consist of 17 questions on impairments during the previous 7 days [21, 22]. Patients will also complete a daily pain medication diary.
Adverse events will be recorded at each visit. Clinical laboratory assessments for safety (haematology, chemistry, ionized calcium, parathyroid hormone, high-sensitivity C-reactive protein) will be collected at baseline and every 6 weeks. Holter monitoring will be performed during dialysis during screening and at Weeks 1, 6, 12, 13 and 24. Electrocardiograms (ECGs) will be acquired during screening and at Weeks 1, 12, 13 and 24. An external Independent Data and Safety Monitoring Board will review unblinded safety data periodically.
Central wound rating group
A blinded central wound rating group will be responsible for confirming lesions are due to calciphylaxis and completing BWAT rating and qualitative review of lesion progress using the images collected by the sites. The study site will use a standardized tablet and Tissue Analytics Software (NetHealth, Pittsburgh PA, USA) to acquire images (both photos and videos) of calciphylaxis lesions. All images will be centrally reviewed for quality within 48 h of collection by an image quality reviewer (L.J.G.). Two wound experts (L.J.G. and T.E.S.) will evaluate all screening lesions for confirmation of patient inclusion.
Sites will receive training on BWAT and rating of the three items that rely on bedside evaluation: undermining, peripheral tissue oedema and peripheral tissue induration. The video will record the process of the site staff rating these three items. The central wound rating group will rate the remaining BWAT items based on the review of the wound photos and videos and will review the site’s ratings of undermining (erosion under the wound edges), peripheral tissue oedema and peripheral tissue induration, updating these ratings if necessary. The video will show removal of the wound dressing (if present) and the underside of the dressing to allow the central raters to score exudate type and amount. Size rating will be aided by automated measurements from imaging software for the wound size.
Ratings of images will occur after the patient completes Part 1 of the study. Central BWAT raters will not be aware of the sequence of visits or dates the images were acquired. Each central BWAT rater will successfully complete BWAT training and an examination (given by T.E.S.) before rating images from study patients. A second member of the central wound rating group (T.E.S. or a central BWAT rater) will perform a quality control review of each image.
In addition, two other reviewers will separately determine whether lesions have improved, worsened or remained the same between Week 1 (baseline) and Week 12, and between Weeks 1 and 24 based on qualitative review of the images. These reviewers will be blinded to study treatment and visit order, and they will not be involved in confirmation of calciphylaxis lesions or BWAT rating during the study. If needed, a third qualitative reviewer may be consulted to reach consensus.
Statistical analyses
The modified intention-to-treat (mITT) population, which will be used as the primary analysis set for efficacy, will consist of all enrolled patients who are randomized, receive at least one dose of study drug and have at least one post-randomization efficacy evaluation. The per-protocol population of patients in the mITT population who do not have major protocol violations, have evaluable primary efficacy data and with a pre-specified minimum study drug exposure will be used for supportive analyses of efficacy endpoints.
Study endpoints are listed in Table 3. This study will have two alternate primary efficacy endpoints, each considered of equal clinical relevance: absolute change from baseline to Week 12 in BWAT-CUA score for the primary lesion, and absolute change from baseline to Week 12 in pain VAS score. The study will be considered successful if there is a statistically significant improvement with SNF472 compared with placebo for at least one of these endpoints. To control Type I error inflation, a modified Hochberg procedure with two-sided alpha of 4% will be used for the alternate primaries. If both primary endpoints are met, then the alpha apportioned will be recycled and the secondary endpoints will be assessed hierarchically at the 5% alpha level, two-sided, as follows: Wound-QoL score, then BWAT total score for the primary lesion, then qualitative wound image evaluation for the primary lesion and then rate of change in opioid use. If only one primary endpoint is met, then these secondary endpoints will be assessed hierarchically at the 1% alpha level, two-sided. The comparisons of absolute change from baseline to Week 12 between treatment groups for each of the primary endpoints will be achieved using a mixed model for repeated measures analyses to estimate the difference in least squares means. Models will be stratified for STS use at baseline.
Table 3.
Study endpoints
| Alternate primary efficacy endpoints (Part 1) | Absolute change from baseline to Week 12 in BWAT-CUA score for the primary lesion |
| Absolute change from baseline to Week 12 in pain VAS score | |
| Secondary efficacy endpoints (Part 1) | Absolute change from baseline to Week 12 in Wound-QoL score |
| Absolute change from baseline to Week 12 in the BWAT total score for the primary lesion | |
| Qualitative wound image evaluation for the primary lesion (worsened, equal to or improved relative to baseline) at Week 12 | |
| Rate of change in opioid use as measured in MME from baseline to Week 12 | |
| Exploratory efficacy endpoints (Part 1) | Absolute change from baseline to Week 12 in wound size for the primary lesion |
| Absolute change from baseline to Week 12 in each BWAT item for the primary lesion | |
| Absolute change in BWAT-CUA, BWAT total, pain VAS and Wound-QoL score by visit | |
| Proportion of patients with new calciphylaxis lesions between baseline and Week 12 | |
| Absolute change from baseline to Week 12 in the BWAT-CUA score for the secondary and tertiary lesions | |
| Proportion of patients requiring an increase in pain medication related to their calciphylaxis lesion(s) between baseline and Week 12 | |
| Proportion of patients with a decrease in pain medication related to their calciphylaxis lesion(s) between baseline and Week 12 | |
| Absolute change from baseline to Week 12 in opioid use as measured in MME | |
| Exploratory efficacy endpoints (Part 2) | Absolute change from baseline to Week 24 versus Week 12 in the BWAT-CUA score for the primary lesion |
| Absolute change from baseline to Week 24 versus Week 12 in the pain VAS score | |
| Absolute change from baseline to Week 24 versus Week 12 in the Wound-QoL score | |
| Absolute change from baseline to Week 24 versus Week 12 in the BWAT total score for the primary lesion | |
| Proportion of patients within each category of the wound image evaluation at Week 24 versus Week 12 (worsened, equal to or improved relative to baseline) for the primary lesion | |
| Absolute change from baseline to Week 24 versus Week 12 in wound size for the primary lesion | |
| Absolute change from baseline to Week 24 versus Week 12 in each BWAT item for the primary lesion | |
| Absolute change from Week 24 to the follow-up visit in the BWAT-CUA score for primary lesion | |
| Absolute change from Week 24 to the follow-up visit in the pain VAS score | |
| Safety endpoints | Proportion of patients with adverse events, serious adverse events and deaths |
| Changes from baseline in laboratory parameters; QTc interval and other ECG parameters; Holter monitoring results; and vital signs | |
| Proportion of patients with a calciphylaxis wound-related infection, sepsis, hospitalization or any calciphylaxis wound-related complication |
BWAT (13 items), BWAT-CUA (8 items); MME, morphine milligram equivalents.
Sample size calculation
The calculation of sample size for this Phase 3 clinical trial was based upon the effect sizes and standard deviations observed for changes in BWAT-CUA and pain VAS from baseline to Week 12 in the Phase 2 study [17]. The effect sizes for BWAT-CUA and pain VAS were 6.3 and 24 U, respectively, with standard deviations of 6.5 and 31.4 U. Assuming similar results and based on 1 000 000 trial simulations, a sample size of 66 patients (33 per group) will provide an overall power of between 95.1% and 99.0% (corresponding to correlations between the BWAT-CUA and VAS test statistics of between 0.90 and 0) when the alternate primary endpoints are tested using a Hochberg closed test procedure with a 4% alpha level, two-sided. A sample size re-estimation will occur when primary endpoint data are available from approximately half of the initial sample (approximately 33 patients total). Conditional power will be calculated, and the sample size may be increased by up to 50% (maximum of 99 patients total) based on pre-specified rules [25]. To guard against Type I error inflation, the pre- and post-interim data will be combined using the approach of Cui et al. [26]. The sample size re-estimation will be completed by an external, independent statistical service provider. The sponsor, study sites and patients will continue to be blinded.
DISCUSSION
This Phase 3, double-blind, randomized, placebo-controlled, global, multicentre study will investigate whether SNF472 added to usual care for treatment of calciphylaxis in patients receiving maintenance haemodialysis improves wound healing, reduces wound-related pain and improves wound-related QoL. Informed by the Phase 2 open-label clinical trial, we designed and initiated this randomized, placebo-controlled Phase 3 clinical trial to further evaluate the safety and efficacy of SNF472 treatment for calciphylaxis. The study will be led by a Steering Committee composed of academic experts in nephrology and wound care, physician researchers and medical leaders in this field from major haemodialysis organizations who have extensive experience in treating patients with calciphylaxis.
There are currently no approved treatment options for calciphylaxis and a lack of other promising agents and clinical development programmes, while morbidity and mortality remain high. In addition, recruitment and execution of clinical trials are challenging in this patient population. Several clinical trials of STS were terminated early (NCT03150420, NCT02527213 and ISRCTN73380053). Except for the Phase 2 clinical trial with SNF472 [17], we are not aware of a prior rigorous assessment of calciphylaxis wound healing that has been published.
The sample size of 66 patients will provide power of >95% to demonstrate clinically meaningful improvement in wound healing and/or pain with SNF472 as compared with placebo. Given limited prior data, a sample size re-estimation is planned after approximately half of the initial sample provides evaluable data for the alternate primary endpoints. An independent data monitoring committee will review these data and will make a recommendation if the sample size should be increased based on pre-specified rules. Patients, study sites and the study sponsor will remain blinded until study completion. Increasing the sample size later, if needed, maximizes the likelihood that the study will achieve its enrolment goals while providing enough patients to achieve adequate statistical power for the primary endpoints.
Primary study endpoints for wound healing and wound-related pain will be used to demonstrate the efficacy of SNF472 if either of those endpoints shows a statistically significant benefit of SNF472 versus placebo. Use of both primary endpoints will provide a comprehensive and clinically relevant assessment of the efficacy of SNF472. Wound complications such as infection and sepsis are major contributors to mortality [8–11], and wound-related pain is of great importance to patients; improvement in either would be clinically meaningful. The 13-item BWAT is a validated tool to assess pressure ulcers and other chronic wounds [20], but BWAT was initially developed neither to assess changes in wounds over time, nor to assess calciphylaxis wounds. Clinician–researchers with expertise in calciphylaxis and wound healing collaborated to develop BWAT-CUA [23, 24], which will be used for the primary endpoint of calciphylaxis wound healing in this study. BWAT-CUA is a modified 8-item version of the tool focusing on prototypical and clinically relevant features of calciphylaxis at diagnosis and during healing. The other primary endpoint for wound-related pain will use the pain VAS, which has been validated extensively and is a commonly used assessment tool for pain in clinical practice and clinical trials [27].
Because calciphylaxis is a rare condition, numerous dialysis centres will participate in this study. Most study sites might enrol one or two patients each. To establish rigorous endpoint assessment, an expert central wound rating group will be responsible for wound evaluations to ensure consistency and accuracy of ratings across patients and across sites. Images (photos and videos) will be collected at each site with a standardized device and software. Site staff will rate three BWAT items (undermining, peripheral tissue oedema and peripheral tissue induration). A central wound rating group will rate the other 10 BWAT items and review the site’s ratings, revising if needed. Extensive training and standardized procedures will be used to ensure quality and consistency of both quantitative and qualitative wound ratings. Central BWAT raters will be blinded to both the study medication assignment and the sequence of images, each rater will be trained and verified by a calciphylaxis wound expert (T.E.S.) before rating images, and quality review of all ratings will be performed by another person in the central rating group.
The secondary efficacy endpoints will provide additional assessment of the effects of SNF472 on clinically meaningful outcomes of calciphylaxis. Wound-QoL is a validated 17-item questionnaire that assesses the effects of the wound on QoL and function [21, 22]. QoL is a key consideration in patients with calciphylaxis but is underreported in this population [15]. We previously used the Wound-QoL questionnaire to examine changes in wound-related QoL in the SNF472 Phase 2 clinical trial [17]. The results for this endpoint, combined with the primary endpoint for wound-related pain, will provide a comprehensive examination of patient-reported outcomes of calciphylaxis wounds, as well as the effect of SNF472 treatment on these outcomes. The central wound rating group will be responsible for the secondary efficacy endpoint of qualitative wound image evaluation for the primary lesion (worsened, equal to or improved relative to baseline). This endpoint will be included to further support results of quantitative wound healing assessments. Change in opioid use measured from baseline to Week 12 will also be a secondary endpoint. Opioid use will be based on daily pain medication diaries that each patient will maintain throughout the study. Sites will conduct weekly reviews of the diary, both to monitor changes and to ensure patient compliance with the diary. Opioids are commonly used to manage the severe pain associated with calciphylaxis, but patients receiving dialysis are at high risk of opioid overdose or toxicity due to opioid accumulation [28]. The potential to manage pain with less exposure to opioids could be particularly beneficial in these patients.
The study will also provide important data on the safety of SNF472 in patients with calciphylaxis. A recent randomized, placebo-controlled Phase 2 study of 274 patients with ESKD and cardiovascular calcification (CaLIPSO; Cal for calcium and IPSO for the item itself) reported that SNF472 treatment TIW for 52 weeks significantly reduced the progression of coronary artery calcium and aortic valve calcification compared with placebo, with similar adverse event profiles in the SNF472 and placebo groups [29].
No medication has been approved for the treatment of calciphylaxis; therefore, the study will be appropriately placebo controlled. To obtain additional efficacy and safety data, all patients completing 12 weeks of randomized double-blind treatment will be eligible to receive open-label SNF472 in Part 2 of the study, also 12 weeks in duration. All patients will receive standard background treatment as per treating physician, including pain medication and/or STS, throughout this study. Because STS is used to treat calciphylaxis in some regions, despite the lack of randomized clinical trials demonstrating efficacy [1, 10, 30], patients in this study may receive it and randomization will be stratified by baseline STS use. To minimize the influence of background treatment on study outcomes, doses of these background medications, including analgesics, will be stabilized at screening and modified only if clinically indicated during the study.
In summary, this rigorously designed study will provide evidence about the efficacy and safety of SNF472 in patients with ESKD and calciphylaxis. To achieve this goal, this study will use a double-blind placebo-control design, with standard background care in both groups, clinically meaningful primary and secondary endpoints, a sample size re-estimation during the study, a digital tool for imaging and a central wound rating group to standardize wound assessments, a quantitative assessment scale (BWAT-CUA) for wound healing that was specifically optimized for the evaluation of calciphylaxis lesions, and patient-reported outcomes for other clinically meaningful endpoints of wound-related pain and wound-related QoL. Patient recruitment is currently ongoing with additional study sites being added.
ACKNOWLEDGEMENTS
Sanifit Therapeutics thanks Dr Barbara Bates-Jensen for permission to use the BWAT in the SNF472 calciphylaxis studies. Jonathan Latham, a medical writer supported by funding from Sanifit Therapeutics, provided drafts and editorial assistance to the authors during preparation of this manuscript.
FUNDING
This study is supported by research funding from Sanifit Therapeutics.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the design of the study and the writing of the manuscript. All authors read and approved the final manuscript. The results presented in this paper have not been published previously in whole or part, except in abstract form.
CONFLICT OF INTEREST STATEMENT
S.S. is a consultant for Sanifit Therapeutics, and has received lecture fees from Vifor Pharma, Bayer and Napp Pharmaceuticals, received sponsorship to attend conference from AstraZeneca, attended advisory boards for Advicenne, Novartis and AstraZeneca, and received research funding from AstraZeneca and Ethicon. L.J.G. is a consultant for Sanifit Therapeutics. S.U.N. has received grant support from Hope Pharmaceuticals and Laboratoris Sanifit, and consulting fees from Epizon Pharma, Fresenius Renal Therapies, Ardelyx and Laboratoris Sanifit. T.E.S. has received research support from Sanifit Therapeutics. V.B. has nothing to declare. S.M.M. has received consulting fees from Amgen, Sanifit and Ardelyax, and grant support from Chugai Pharmaceuticals and Keryx. G.A. has nothing to declare. J.L.H. reports employment, options and stock in Fresenius. D.K.C. is an employee of Fresenius Medical Care. S.M., C.P. and A.G. are shareholders and employees of Sanifit Therapeutics. J.P. is a consultant for Sanifit Therapeutics and holds patents related to SNF472. K.J.C. is a paid independent consultant to Sanifit. G.M.C. is a consultant to Sanifit, Akebia, Ardelyx, AstraZeneca, Gilead, Reata and Vertex, holds stock or options in Ardelyx, CloudCath, Cricket, Durect, Outset and Physiowave, and has received research funding from Amgen and Janssen.
DATA AVAILABILITY STATEMENT
Patient-level data from this study are currently not available for external access.
Contributor Information
Smeeta Sinha, Renal Medicine, Salford Royal NHS Foundation Trust, Salford, UK; Faculty of Biology, Medicine, and Health, University of Manchester, Manchester, UK.
Lisa J Gould, South Shore Health Department of Surgery, South Shore Health Center for Wound Healing, Weymouth, MA, USA.
Sagar U Nigwekar, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Thomas E Serena, SerenaGroup Research Foundation, Cambridge, MA, USA.
Vincent Brandenburg, Cardiology and Nephrology, Rhein-Maas Hospital, Würselen, Germany.
Sharon M Moe, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
George Aronoff, Clinical Affairs, DaVita Kidney Care, Naples, FL, USA.
Dinesh K Chatoth, Fresenius Kidney Care, Waltham, MA, USA.
Jeffrey L Hymes, Global Head of Clinical Affairs, Fresenius Kidney Care, Waltham, MA, USA.
Stephan Miller, Department of Clinical Development, Sanifit Therapeutics, San Diego, CA, USA.
Claire Padgett, Department of Clinical Development, Sanifit Therapeutics, San Diego, CA, USA.
Kevin J Carroll, KJC Statistics Ltd, Cheshire, UK.
Joan Perelló, University Institute of Health Sciences Research (IUNICS- IDISBA), University of the Balearic Islands, Palma, Spain; Department of Clinical Development, Sanifit Therapeutics, Palma, Spain.
Alex Gold, Department of Clinical Development, Sanifit Therapeutics, San Diego, CA, USA; Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Glenn M Chertow, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
REFERENCES
- 1. Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med 2018; 378: 1704–1714 [DOI] [PubMed] [Google Scholar]
- 2. Brandenburg VM, Kramann R, Rothe H et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant 2017; 32: 126–132 [DOI] [PubMed] [Google Scholar]
- 3. Goel SK, Bellovich K, McCullough PA. Treatment of severe metastatic calcification and calciphylaxis in dialysis patients. Int J Nephrol 2011; 2011: 701603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Floege J, Kubo Y, Floege A et al. The effect of cinacalcet on calcific uremic arteriolopathy events in patients receiving hemodialysis: the EVOLVE Trial. Clin J Am Soc Nephrol 2015; 10: 800–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nigwekar SU, Solid CA, Ankers E et al. Quantifying a rare disease in administrative data: the example of calciphylaxis. J Gen Intern Med 2014; 29 (Suppl 3): S724–S731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bahrani E, Perkins IU, North JP. Diagnosing calciphylaxis: a review with emphasis on histopathology. Am J Dermatopathol 2020; 42: 471–480 [DOI] [PubMed] [Google Scholar]
- 7. Chinnadurai R, Sinha S, Lowney AC et al. Pain management in patients with end-stage renal disease and calciphylaxis- a survey of clinical practices among physicians. BMC Nephrol 2020; 21: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruderman I, Toussaint ND, Hawley CM et al. The Australian Calciphylaxis Registry: reporting clinical features and outcomes of patients with calciphylaxis. Nephrol Dial Transplant 2021; 36: 649–656 [DOI] [PubMed] [Google Scholar]
- 9. Gaisne R, Pere M, Menoyo V et al. Calciphylaxis epidemiology, risk factors, treatment and survival among French chronic kidney disease patients: a case-control study. BMC Nephrol 2020; 21: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chinnadurai R, Huckle A, Hegarty J et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis study. J Nephrol 2021; doi: 10.1007/s40620-020-00908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weenig RH, Sewell LD, Davis MD et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56: 569–579 [DOI] [PubMed] [Google Scholar]
- 12. McCarthy JT, El-Azhary RA, Patzelt MT et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc 2016; 91: 1384–1394 [DOI] [PubMed] [Google Scholar]
- 13. Nigwekar SU, Zhao S, Wenger J et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol 2016; 27: 3421–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabel CK, Nguyen ED, Chakrala T et al. Assessment of outcomes of calciphylaxis. J Am Acad Dermatol 2020; doi: 10.1016/j.jaad.2020.10.067 [DOI] [PubMed] [Google Scholar]
- 15. Riemer CA, El-Azhary RA, Wu KL et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol 2017; 177: 1510–1518 [DOI] [PubMed] [Google Scholar]
- 16. Olaniran KO, Percy SG, Zhao S et al. Palliative care use and patterns of end-of-life care in hospitalized patients with calciphylaxis. J Pain Symptom Manage 2019; 57: e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandenburg VM, Sinha S, Torregrosa JV et al. Improvement in wound healing, pain, and quality of life after 12 weeks of SNF472 treatment: a phase 2 open-label study of patients with calciphylaxis. J Nephrol 2019; 32: 811–821 [DOI] [PubMed] [Google Scholar]
- 18. Ferrer MD, Perez MM, Canaves MM et al. A novel pharmacodynamic assay to evaluate the effects of crystallization inhibitors on calcium phosphate crystallization in human plasma. Sci Rep 2017; 7: 6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perelló J, Ferrer MD, Del Mar Pérez M et al. Mechanism of action of SNF472, a novel calcification inhibitor to treat vascular calcification and calciphylaxis. Br J Pharmacol 2020; 177: 4400–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bates-Jensen BM, Vredevoe DL, Brecht ML. Validity and reliability of the pressure sore status tool. Decubitus 1992; 5: 20–28 [PubMed] [Google Scholar]
- 21. Augustin M, Baade K, Herberger K et al. Use of the WoundQoL instrument in routine practice: feasibility, validity and development of an implementation tool. Wound Med 2014; 5: 4–8 [Google Scholar]
- 22. Augustin M, Conde Montero E, Zander N et al. Validity and feasibility of the wound-QoL questionnaire on health-related quality of life in chronic wounds. Wound Repair Regen 2017; 25: 852–857 [DOI] [PubMed] [Google Scholar]
- 23. Sinha S, Gould L, Brandenburg V et al. Improvements in calcific uremic arteriolopathy wound healing during SNF472 treatment assessed with the BWAT-CUA [Abstract SA-PO689]. J Am Soc Nephrol 2018; 29: 914–915 [Google Scholar]
- 24. Gould LJ, Serena TE, Sinha S. Development of the BWAT-CUA scale to assess wounds in patients with calciphylaxis. Diagnostics 2021; 11: 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta CR, Pocock SJ. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med 2011; 30: 3267–3284 [DOI] [PubMed] [Google Scholar]
- 26. Cui L, Hung HM, Wang SJ. Modification of sample size in group sequential clinical trials. Biometrics 1999; 55: 853–857 [DOI] [PubMed] [Google Scholar]
- 27. Hjermstad MJ, Fayers PM, Haugen DF et al. ; European Palliative Care Research Collaborative (EPCRC). Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011; 41: 1073–1093 [DOI] [PubMed] [Google Scholar]
- 28. Seethapathy H, Brandenburg VM, Sinha S et al. Review: update on the management of calciphylaxis. QJM 2019; 112: 29–34 [DOI] [PubMed] [Google Scholar]
- 29. Raggi P, Bellasi A, Bushinsky D et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized Phase 2b study. Circulation 2020; 141: 728–739 [DOI] [PubMed] [Google Scholar]
- 30. Peng T, Zhuo L, Wang Y et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton) 2018; 23: 669–675 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient-level data from this study are currently not available for external access.