ABSTRACT
Background
Frailty is associated with poor outcomes for haemodialysis patients, but its prevalence is uncertain due to heterogeneous definitions. The aim of this study was to compare and contrast prevalence and features of commonly used frailty instruments in a British haemodialysis cohort.
Methods
The FITNESS (Frailty Intervention Trial iN End-Stage patientS on haemodialysis) study recruited adults aged ≥18 years after informed consent, with ≥3 months haemodialysis exposure and no hospital admission within 4 weeks unless for dialysis access. Study participants were clinically phenotyped with frailty instruments including the Frailty Index (FI), Frailty Phenotype (FP), Edmonton Frailty Scale (EFS) and Clinical Frailty Scale (CFS), alongside comprehensive baseline data collection of biochemical, clinical and social characteristics.
Results
Between 12 January 2018 and 18 April 2019, 485 haemodialysis patients were recruited. Baseline demographics were median age 63 years, male sex 58.6% and non-White ethnicity 42.1%. Prevalence of frailty was high; 41.9% of participants were frail by FP, 63.3% by FI, 50.2% by EFS and 53.8% by CFS. Female gender was associated with increased frailty, with no independent association observed with age or ethnicity. While correlation between frailty instruments was strong, intraclass correlation coefficient for frailty agreement was 0.628 (95% confidence interval 0.585–0.669) and only weak agreement between instrument pairs.
Conclusion
Frailty is highly prevalent among haemodialysis patients regardless of criteria used. However, our data suggest caution when interpreting heterogenous definitions of frailty for haemodialysis patients as they are not interchangeable. Consensus agreement on the optimal frailty definition for haemodialysis patients must balance ease of use with predictive ability for adverse outcomes before determining clinical application.
Keywords: age, epidemiology, ESRD, haemodialysis, physical activity, quality of life
INTRODUCTION
Frailty is a syndrome of accelerated ageing, characterized by multisystem dysregulation and vulnerability to stressor events such as infections or surgery [1]. Best studied in the general population, frailty is associated with poor outcomes including mortality, hospitalization, cognitive impairment and disability [2]. However, heterogeneous use of frailty tools is a major limitation for research investigation and clinical application.
The gold standard frailty definition is the comprehensive geriatric assessment (CGA) [3], but this has significant cost and logistic implications. Consequently, screening tools have been proposed, including the Fried Frailty Phenotype (FP) [4], Rockwood Frailty Index (FI) [5], Edmonton Frailty Scale (EFS) [6] and Clinical Frailty Scale (CFS) [7]. The FP distinguishes frailty from disability and comorbidity [8], and has been the predominant frailty measure used in research practice [9]. It is simple to administer but focuses upon measures of sarcopenia rather than a multidimensional approach [10–12]. Conversely, the FI does not distinguish frailty from disability or comorbidity, defining frailty as a composite of ‘deficits’ across multiple clinical, biochemical and social domains [13, 14]. This gives greater scope for interpretation but is more complex to administer; hence, the same group devised the EFS and CFS as more accessible deficit models.
Lack of diagnostic consensus is important as frailty is a major problem for kidney failure populations, with prevalence amplified compared with the general population independent of age. Frailty prevalence by FP criteria among community-dwelling elderly in the USA has been estimated between 6.9% and 16.3% [4, 15], compared with 31–42% of US haemodialysis recipients ≥18 years of age [16–18]. Outcome data, predominantly derived from US cohorts, demonstrate adverse morbidity and mortality outcomes in frail haemodialysis patients of all ages [16, 19, 20]. In summary, frailty in haemodialysis patients is common, clinically important and warrants further investigation to clarify clinical applications.
Therefore, we require better understanding of how frailty tools can be utilized for haemodialysis populations to improve delivery of care. Instruments define frailty through different mechanisms and there is a need to establish how these compare with each other in this high-risk population. As a first step, we must improve our understanding of prevalence stratified by different frailty instruments. In this report, we describe the prevalence, correlation and interaction between various frailty assessment measures at recruitment.
MATERIALS AND METHODS
Study design
A detailed description of the FITNESS (Frailty Intervention Trial iN End-Stage patientS on haemodialysis) study has been reported [21]. Briefly, this first stage is a cross-sectional assessment and long-term follow-up of study participants on maintenance haemodialysis with comprehensive frailty and bio-clinical phenotyping [22]. The study protocol was subject to favourable opinion by the South Birmingham Research Ethics Committee (Ref: 17/WM/0381) and institutional review board assessment of University Hospitals Birmingham NHS Foundation Trust (RRK6082).
Study setting
Patients were recruited from a single nephrology centre located in Birmingham, UK. The service provides haemodialysis to patients in a mixture of urban and rural settings, with a diverse range of ethnic and socio-economic groups. Eligible patients were identified by interrogation of hospital electronic patient records (EPRs) and from discussion with clinicians at each dialysis unit. Eligible patients were approached, given written and verbal information about the study and given sufficient opportunity to consider the information before giving consent for recruitment.
Eligibility criteria
Inclusion criteria included adults aged ≥18 years, receiving regular haemodialysis for at least 3-month duration and ability to give informed consent. The only exclusion criterion was inpatient care within 4 weeks of recruitment unless for vascular access purposes, to avoid confounding of baseline data with frailty secondary to recent hospitalization.
Baseline assessment
Baseline assessments took place at individual dialysis units before and during patients’ usual dialysis session. To negate potential effects of the long break from dialysis, we avoided the first haemodialysis session after the long weekend interval.
Prior to connection to dialysis, participants underwent a timed walk over 4 m, and grip strength dynamometer assessment. As the 4-m timed walk replaced the timed up-and-go test in the case of the EFS, we split the results into terciles and attributed zero points to those in the lowest tercile, one point to those in the middle tercile and two points to those in the top tercile. Once dialysis started, participants completed questionnaires on disability [activities of daily living (ADL)], demography and social history, and frailty-specific questionnaires. EPR was interrogated for comorbidities, drug history, dialysis vintage/adequacy, previous transplantation and biochemical data, providing sufficient data to calculate FP, FI and EFS assessments. Determination of socio-economic deprivation was based upon the Index of Multiple Deprivation, a multiple deprivation model calculated at the local area level, with 1 representing the most deprived and 5 the least deprived area, respectively [23].
Frailty definitions
Frailty was defined as described in our methodology paper [21] and detailed in Supplementary data, File S1. In summary, all patients were classified at baseline with the following frailty definitions; FP range 0–5 (frail ≥3, vulnerable 1–2 and robust 0), EFS range 0–17 (frail ≥8, vulnerable 6–7 and robust <6) and CFS range 1–9 (frail ≥5, vulnerable 4 and robust <4).
The FI was not originally designed to be split into categories, though many groups have subsequently done so to aid interpretation. For example, the primary care electronic FI (eFI) [24, 25] categorized scores of robust (≤0.12), mildly frail (>0.12 to ≤0.24), moderately frail (>0.24 to ≤0.36) and severely frail (>0.36). Due to the relatively low numbers of non-frail participants using this classification, and the lack of a ‘vulnerable’ category present in the other instruments, we reclassified the mildly frail group (>0.12 to ≤0.24) as vulnerable and scores ≥0.24 were classed as frail (≥0.24 mildly, ≥0.36 moderately and ≥0.48 severely frail).
Statistics
Statistical analysis was performed using STATA (StataCorp 2019, Stata Statistical Software: Release 16; StataCorp LLC, College Station, TX, USA) and R 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). Categorical data were presented as numbers and percentages, with continuous variables reported as medians and interquartile ranges (IQRs).
Differences between groups were compared using Chi-square or Fisher’s exact tests for categorical variables and Student’s t-tests or Mann–Whitney tests (parametric or non-parametric data, respectively) for all continuous variables. Correlations were analysed using Spearman’s test. Odds ratios (ORs) were calculated using logistic regression, with adjustment against clinically important variables including age, gender, ethnicity, Charlson Comorbidity Score and social deprivation. Agreement between frailty scores was analysed using Cohen’s Kappa or intraclass coefficient as appropriate. Strength of agreement was rated as >0.90 almost perfect agreement, 0.80–0.90 strong, 0.60–0.79 moderate, 0.40–0.59 weak, 0.21–0.39 minimal and ≤0.20 no agreement [26].
Missing data were assumed missing at random and handled via listwise deletion. A P-value <0.05 was considered significant in the statistical analysis.
RESULTS
Recruitment
In total, 500 participants gave informed consent to participate in the FITNESS study between 12 January 2018 and 18 April 2019. After initial approach, 15 patients withdrew from the study either prior to or during baseline recruitment, leaving 485 with data available for analysis. Figure 1 shows a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart outline study recruitment.
FIGURE 1:
PRISMA flowchart of study participation in the FITNESS study. The numbers for specific exclusion criteria do not add up to the total excluded number as some participants had more than one reason for ineligibility.
Baseline demographics and comorbidities
Table 1 highlights baseline demographics and comorbidities of our study participants. We identified significant health and socio-economic burden in our overall study cohort, but no difference between frailty instruments.
Table 1.
Baseline demographics of FITNESS study participants
| Parameter | Total cohort | Participants identified as frail by |
P-value | ||||
|---|---|---|---|---|---|---|---|
| FP | FI | EFS | CFS | ||||
| Participants identified as frail, n (%) of total cohort | – | 203 (41.9) | 307 (63.3) | 244 (50.3) | 261 (53.8) | – | |
| Age, median (IQR) | 63 (53–74) | 67 (56–77) | 63 (54–75) | 63 (53–74.5) | 65 (55–76) | 0.125 | |
| Ethnicity | White | 281 (57.9) | 121 (59.6) | 174 (56.7) | 132 (54.1) | 144 (55.2) | 0.909 |
| South Asian | 115 (23.7) | 51 (25.1) | 80 (26.1) | 61 (25.0) | 71 (27.2) | ||
| Black | 76 (15.7) | 27 (13.3) | 46 (15.0) | 47 (19.3) | 41 (15.7) | ||
| Other | 13 (2.7) | 4 (2.0) | 7 (2.3) | 4 (1.6) | 5 (1.9) | ||
| Male | 284 (58.6) | 106 (52.2) | 165 (53.8) | 127 (52.1) | 136 (52.1) | 0.974 | |
| BMI, median (IQR) | 26.8 (23.2–32.4) | 27.8 (22.7–33.6) | 27.9 (23.1–33.2) | 27.7 (23.1–33.6) | 27.9 (23.2–33.7) | 0.967 | |
| Cause of kidney failurea | Diabetes | 114 (23.5) | 64 (31.5) | 86 (28.0) | 71 (29.1) | 81 (31.0) | 0.800 |
| Ischaemic | 38 (7.8) | 18 (8.9) | 27 (8.8) | 20 (8.2) | 24 (9.2) | 0.983 | |
| Hypertension | 39 (8.4) | 14 (6.9) | 22 (7.2) | 21 (8.6) | 17 (6.5) | 0.823 | |
| IgA | 37 (7.6) | 12 (5.9) | 21 (6.8) | 19 (7.8) | 17 (6.5) | 0.882 | |
| PKD | 28 (5.8) | 7 (3.5) | 14 (4.6) | 11 (4.5) | 11 (4.2) | 0.933 | |
| FSGS | 24 (5.0) | 10 (4.9) | 13 (4.2) | 13 (5.3) | 10 (3.8) | 0.852 | |
| Reflux | 17 (3.5) | 11 (5.4) | 12 (3.9) | 10 (4.1) | 10 (3.8) | 0.826 | |
| Obstructive | 16 (3.3) | 7 (3.5) | 8 (2.6) | 7 (2.9) | 6 (2.3) | 0.896 | |
| AAV | 15 (3.1) | 5 (2.5) | 6 (2.0) | 5 (2.1) | 4 (1.5) | 0.741 | |
| Interstitial nephritis | 10 (2.1) | 4 (2.0) | 5 (1.6) | 3 (1.2) | 4 (1.5) | 0.946 | |
| Myeloma | 10 (2.1) | 2 (1.0) | 5 (1.6) | 3 (1.2) | 2 (0.8) | 0.835 | |
| Other | 86 (17.7) | 36 (17.7) | 60 (19.5) | 46 (18.9) | 47 (18.0) | 0.950 | |
| Unknown | 68 (14.0) | 23 (11.3) | 40 (13.0) | 28 (11.5) | 37 (14.2) | 0.749 | |
| Medical comorbidities | MI | 98 (20.2) | 53 (26.1) | 73 (23.8) | 61 (25.0) | 64 (24.5) | 0.946 |
| Heart failure | 52 (10.7) | 24 (11.8) | 35 (11.4) | 31 (12.7) | 33 (12.6) | 0.958 | |
| Stroke | 57 (11.8) | 36 (17.7) | 43 (14.0) | 37 (15.2) | 40 (15.3) | 0.725 | |
| PVD | 47 (9.7) | 27 (13.3) | 36 (11.7) | 26 (10.7) | 32 (12.3) | 0.854 | |
| Cancer | 56 (11.6) | 18 (8.9) | 34 (11.1) | 25 (10.3) | 26 (10.0) | 0.881 | |
| Smoking history | Current | 68 (14.1) | 28 (13.9) | 41 (13.4) | 36 (14.8) | 30 (11.5) | 0.971 |
| Previous | 132 (27.3) | 51 (25.3) | 78 (25.5) | 59 (24.3) | 68 (26.2) | ||
| Never | 284 (58.7) | 123 (60.9) | 187 (61.1) | 148 (60.9) | 162 (62.3) | ||
| Median Charlson Scoreb, median (IQR) | 4 (3–6) | 5 (4–7) | 5 (3–6) | 5 (3–6) | 5 (4–7) | 0.235 | |
| Dialysis details | Dialysis vintage, months, median (IQR) | 37 (17–76) | 49 (22–93) | 41 (18–82) | 35 (16–77) | 41 (20–81) | 0.219 |
| Line | 113 (23.3) | 54 (26.6) | 77 (25.1) | 67 (27.5) | 66 (25.3) | 0.916 | |
| Kt/V, median (IQR) | 1.6 (1.4–1.9) | 1.6 (1.4–1.9) | 1.6 (1.4–1.9) | 1.6 (1.4–1.9) | 1.6 (1.4–1.9) | 0.835 | |
| Transplant list status | Active | 58 (12.0) | 11 (5.4) | 27 (8.8) | 20 (8.2) | 22 (8.4) | 0.592 |
| Suspended | 15 (3.1) | 2 (1.0) | 9 (2.9) | 6 (2.5) | 6 (2.3) | ||
| Not listed | 412 (85) | 190 (93.6) | 271 (88.3) | 218 (89.4) | 233 (89.3) | ||
| % Employment status | Employed | 69 (14.3) | 5 (2.5) | 16 (5.2) | 13 (5.4) | 8 (3.1) | 0.337 |
| Unemployed | 148 (30.6) | 67 (33.2) | 114 (37.3) | 94 (38.7) | 90 (34.6) | ||
| Retired | 267 (55.2) | 130 (64.4) | 176 (57.5) | 136 (56.0) | 162 (62.3) | ||
| % Job rolec | Unskilled manual | 181 (39.3) | 93 (48.4) | 131 (45.8) | 101 (44.5) | 111 (45.7) | 0.999 |
| Skilled manual | 101 (21.9) | 37 (19.3) | 58 (20.3) | 49 (21.6) | 51 (21.0) | ||
| Clerical | 52 (11.3) | 24 (12.5) | 33 (11.5) | 27 (11.9) | 24 (9.9) | ||
| Managerial | 46 (10.0) | 14 (7.3) | 21 (7.3) | 18 (7.9) | 20 (8.2) | ||
| Professional | 81 (17.6) | 24 (12.5) | 43 (15.0) | 32 (14.1) | 37 (15.2) | ||
| % Education level | High school | 342 (70.7) | 153 (75.7) | 229 (74.8) | 185 (76.1) | 196 (75.4) | 0.884 |
| College/sixth form | 92 (19.0) | 39 (19.3) | 54 (17.7) | 41 (16.9) | 43 (16.5) | ||
| University | 50 (10.3) | 10 (5.0) | 23 (7.5) | 17 (7.0) | 21 (8.1) | ||
| Residence | Own home | 462 (95.9) | 188 (93.1) | 288 (94.4) | 224 (93.0) | 244 (94.2) | 0.993 |
| Warden-controlled flat | 12 (2.5) | 7 (3.5) | 10 (3.3) | 11 (4.6) | 9 (3.5) | ||
| Residential home | 5 (1.0) | 4 (2.0) | 4 (1.3) | 4 (1.7) | 3 (1.2) | ||
| Nursing home | 3 (0.6) | 3 (1.5) | 3 (1.0) | 2 (0.8) | 3 (1.2) | ||
| % With professional carersd | 36 (7.8) | 31 (16.1) | 35 (12.0) | 30 (13.0) | 36 (14.6) | 0.607 | |
Data are presented as n (%) unless otherwise indicated. aP-values for differences between those classified as Frail by each of the frailty scores studied. P-values obtained by Chi-square or Fischer’s exact as appropriate for categorical data, Kruskal–Wallis or Rank Sum test as appropriate for continuous data. Some participants had more than one cause of kidney failure listed.
CKD omitted from Charlson Score.
Current job or previous job if unemployed/retired.
For those living in own home.
A notable demographic feature of our study recruits was a significant proportion of individuals of non-White ethnicity (comprising 42.1% of total study cohort). In Supplementary data, Table S1, we highlight significant differences between ethnic groups in reasons for non-recruitment after invitation. For example, difficulty with language barrier was recorded particularly highly in South Asian and other ethnicity (not White, South Asian or Black) patients, while declined invitations and lack of mental capacity were proportionally higher in White and Black patients. However, our study participant cohort is broadly representative of our local haemodialysis population by ethnicity, gender and age as confirmed by comparison with registry data [27].
Prevalence of frailty by instrument
Figure 2 summarizes prevalence of frailty, vulnerability and robustness using the different instruments. The median FP was 2 (IQR 1–3), with 63 (13.0%) participants categorized as robust, 219 (45.2%) vulnerable and 203 (41.9%) as frail.
FIGURE 2:
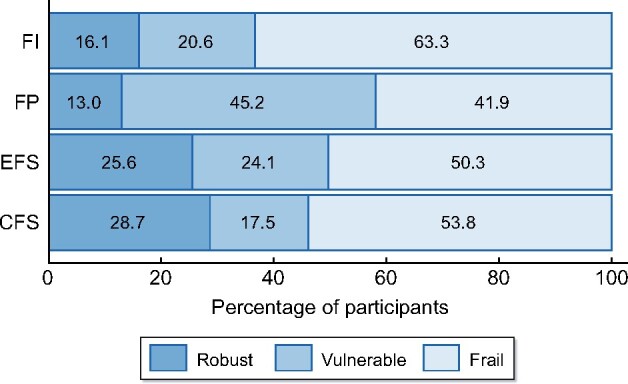
Percentage of participants classified as robust, vulnerable or frail by different frailty instruments: FI, FP, EFS and CFS.
The median FI was 0.305 (IQR 0.164–0.477). Using the eFI thresholds [24, 25] in our cohort, 78 (16.1%) of participants were robust, 100 (20.6%) were mildly frail, 116 (23.9%) moderately frail and 191 (39.4%) severely frail. Due to the relatively low numbers of non-frail participants using this classification, and the lack of a ‘vulnerable’ category present in the other instruments, we reclassified the mildly frail group (>0.12 to ≤0.24) as vulnerable. Using this FI classification, 16.1% of participants were robust, 20.6% vulnerable and 63.3% frail (comprising 23.9% mildly frail, 14.6% moderately frail and 24.7% severely frail). This new FI classification was used in all further analyses.
The median EFS was 8 (IQR 5–10), with 124 (25.6%) classed as robust, 117 (24.1%) vulnerable, 114 (23.5%) mildly frail, 93 (19.2%) moderately frail and 37 (7.6%) severely frail.
Finally, the median CFS was 5 (IQR 3–6), with 139 (28.66%) classed as robust, 85 (17.5%) vulnerable, 131 (27.0%) mildly frail, 103 (21.2%) moderately frail and 27 (5.6%) severely frail.
Association between frailty and key demographics
Frail participants by FP had a significantly higher median age in years than non-frail participants (67 versus 60 years, respectively, P < 0.001), as did frail participants adjudged by CFS (65 versus 60 years, respectively, P = 0.002). Those aged ≥65 years were more likely to be characterized as frail by CFS [OR 1.50, 95% confidence interval (CI) 1.05–2.16, P = 0.025] and FP (OR 1.82, 95% CI 1.27–2.63, P = 0.001) on univariable analysis. However, these associations were no longer significant when adjusted for clinically important variables such as Charlson Comorbidity Score, gender, ethnicity and social deprivation. There was no significant association between age ≥65 years and FI (OR 1.14, 95% CI 0.79–1.65, P = 0.49) or EFS (OR 0.95, 95% CI 0.66–1.35, P = 0.76) on either univariable or adjusted analyses. Frailty remained prevalent in younger patients, with 49.0% of participants <65 years being characterized as frail by CFS, 51% frail by EFS, 35% by FP and 61.9% by FI.
Female participants were more likely to be frail by all frailty instruments, with an OR of 1.57 (95% CI 1.09–2.26, P = 0.016) for FP frailty, 1.74 (95% CI 1.18–2.55, P = 0.005) for FI frailty, 1.72 (95% CI 1.20–2.48, P = 0.004) for EFS frailty and 1.79 (95% CI 1.24–2.59, P = 0.002) for CFS frailty. These associations were retained upon adjustment for Charlson Comorbidity Score, social deprivation, age ≥65 years and ethnicity; ORs were 1.76 for FP (95% CI 1.17–2.67, P = 0.007), 1.87 for FI (95% CI 1.22–2.85, P = 0.004), 1.72 for EFS (95% CI 1.16–2.58, P = 0.008) and 2.06 for CFS (95% CI 1.37–3.12, P = 0.001).
Table 2 shows there was no significant difference between ethnicities in frailty status regardless of the instrument used, despite variation in ranges of frailty prevalence estimates between ethnicities. Table 3 shows correlation between frailty scores and physical/physiological parameters.
Table 2.
Proportion of frailty within ethnicities (P-values derived by Chi-squared or Fisher’s exact as appropriate)
| Percentage of ethnicity classed as frail |
P-value | ||||
|---|---|---|---|---|---|
| White | South Asian | Black | Other | ||
| FP | 43.1 | 44.4 | 35.5 | 30.8 | 0.499 |
| FI | 61.9 | 69.6 | 60.5 | 53.9 | 0.388 |
| EFS | 47.0 | 53.0 | 61.8 | 30.8 | 0.054 |
| CFS | 51.3 | 61.7 | 54.0 | 38.5 | 0.180 |
Table 3.
Correlation between baseline frailty instruments and physical/psychological parameters
| FP | FI | EFS | CFS | |
|---|---|---|---|---|
| Charlson Comorbidity Score | 0.265 | 0.226 | 0.175 | 0.275 |
| Number of admissions previous 12 months | 0.099 | 0.103 | 0.344 | 0.047 |
| Number of drugs | 0.280 | 0.362 | 0.324 | 0.352 |
| Dialysis vintage (years) | 0.129 | 0.077 | −0.039 | 0.087 |
| Grip strength (strongest hand) | −0.576 | −0.470 | −0.398 | −0.434 |
| Grip strength (weakest hand) | −0.547 | −0.481 | −0.392 | −0.440 |
| MOCA | −0.295 | −0.346 | −0.364 | −0.310 |
| PHQ9 score | 0.419 | 0.537 | 0.477 | 0.361 |
| Health rate score | 0.421 | 0.513 | 0.587 | 0.387 |
| EQ5D | −0.434 | −0.494 | −0.411 | −0.392 |
MoCA, Montreal Cognitive Assessment; PHQ9, Patient Health Questionnaire-9; EQ5D, EuroQol EQ-5D-3L.
Correlation between frailty instruments
Table 4 shows correlations between frailty instruments. The FI showed strong correlation with both the CFS (Spearman’s ρ = 0.884, P < 0.001) and FP (Spearman’s ρ = 0.790, P < 0.001). The EFS showed strong correlation with the FI (ρ = 0.755, P < 0.001) and moderately strong correlation with the FP (ρ = 0.666, P < 0.001). The CFS was moderately correlated with FP (ρ = 0.665, P < 0.001) and the EFS (ρ = 0.646, P < 0.001).
Table 4.
Correlation of raw frailty scores by Spearman’s rho
| FP | FI | EFS | CFS | |
|---|---|---|---|---|
| FP | 0.790 | 0.666 | 0.665 | |
| FI | 0.790 | 0.755 | 0.884 | |
| EFS | 0.666 | 0.755 | 0.646 | |
| CFS | 0.665 | 0.884 | 0.646 |
All results significant at P < 0.001.
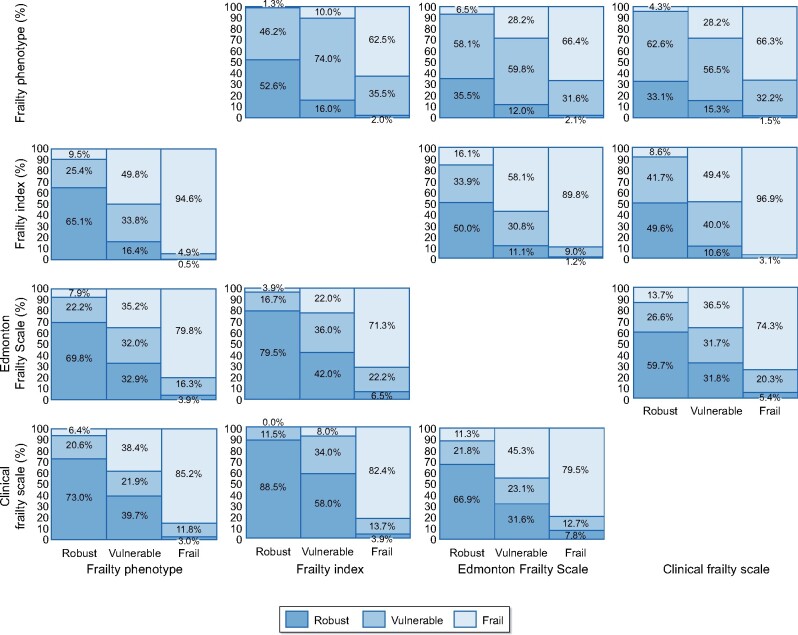
Agreement between instruments on severity of frailty
Figure 3 gives a visual representation of the agreement between scores of frailty severity. The intraclass correlation coefficient across the scores was 0.628 (95% CI 0.585–0.669). The FI showed weak agreement with FP (κ = 0.409, P < 0.001), EFS (κ = 0.414, P < 0.001) and CFS (κ = 0.539, P < 0.001). The FP showed minimal agreement with EFS (κ = 0.0.334, P < 0.001) and CFS (κ = 0.317, P < 0.001). The EFS and CFS showed minimal agreement (κ = 0.392, P < 0.001). Agreement between frailty instruments was weakest in the vulnerable category. Intraclass correlation coefficients by ethnicity were 0.622 (95% CI0.566–0.676) for White, 0.645 (95% CI 0.562–0.721) for South Asian, 0.602 (95% CI 0.495–0.702) for Black and 0.688 (95% CI 0.449–0.873) for other ethnicity participants.
FIGURE 3:
Agreement between frailty instruments.
DISCUSSION
FITNESS is the largest European, prospective cohort study with detailed baseline frailty phenotyping of haemodialysis patients linked to long-term outcomes. In our first analysis, we aim to compare and contrast multiple frailty instruments to determine agreement, correlation and utility for haemodialysis patients. Our main finding is a high prevalence of frailty among haemodialysis patients regardless of actual instrument used. Females are more likely to be classified as frail regardless of instrument used independent of other demographic characteristics. Finally, while we highlight strong correlation between raw frailty scores, agreement of frailty status between instruments is weak at best and suggests frailty tools are not interchangeable.
Our results indicate a higher burden of frailty than that reported in some studies. A recent Spanish study categorized 26.5% of 277 prevalent haemodialysis patients as frail by EFS [19], compared with 50.2% using the same measure in our cohort. A Dutch study found 48% of 125 incident haemodialysis recipients aged ≥65 years were frail by FP [28]. While this appears similar to our 42%, our cohort was not limited to older dialysis patients. Our prevalence appears more comparable to US cohorts, where between 31% and 42% of haemodialysis recipients are categorized as frail by FP regardless of age [16, 18]. We must exercise caution when comparing cohorts; there are likely to be many reasons why burden of frailty can differ between countries, including demographics, socio-cultural and/or environmental differences.
Frailty prevalence was significantly higher in females across all instruments studied in our haemodialysis cohort. Our data do not offer clues as to why females may be more frail than males, but this phenomenon is not limited to the haemodialysis population [4]. It is interesting to observe that in this multi-ethnic cohort, frailty instruments did not show any significant difference in prevalence between ethnic groups, which aligns with data from non-British dialysis cohorts. This is contrary to a study in the general population, where frailty prevalence (by eFI) varied between 14.0% in Black to 32.9% in Bangladeshi patients aged ≥65 years in the UK (overall prevalence of 18.1%) [29]. The underlying reasons for this discrepancy require further investigation, but may be related to the known reverse epidemiology and/or survival paradox observed for non-White patients on haemodialysis [30]. It is reassuring that there was no meaningful difference in frailty agreement between ethnicities; the original validation cohorts were North American with different ethnic breakdowns from the UK [4–7]. As expected, frailty greatly increased with age but remained a significant burden among younger haemodialysis patients.
We observed a large spread of prevalence across the different frailty instruments. Correlation between raw frailty scores was stronger than the agreement between these same instruments when divided into frailty categories. This suggests instruments are classifying large numbers of participants with frailty differently. This assertion is supported by the 21.5% difference in frailty prevalence between the highest and lowest estimations, the FI and FP, respectively. Our data demonstrated the weakest agreement between frailty instruments in the vulnerable category. This is of particular concern as this is the ‘decision zone’ where clinical conclusions may be drawn based upon whether a patient is frail or not-frail (i.e. vulnerable), which may lead to detrimental consequences for haemodialysis recipients. Discrepancy between estimated frailty prevalence and agreement between instruments demonstrates the difficulties in attempting to divide frailty into neat ‘silos’ and is in keeping with a syndrome that defies easy clinical—and indeed mechanistic—definition. The weak agreement between instruments on frailty classification suggests caution be applied when attempting to dichotomize individual patients into frailty groups.
Achieving consensus on the optimal frailty screening tool for haemodialysis patients requires a balance between ease of use versus utility. Frailty instruments range from quick (e.g. CFS) to more time-consuming (FI). Usability will be centre-specific and dependent upon infrastructure, multi-disciplinary support and EPR capabilities. A more important question is whether a frailty instrument that characterizes two-thirds of haemodialysis recipients as ‘frail’ provides added value to clinical decision-making. For example, if these instruments are used as screening tools for referral to support networks, then referring over half of dialysis recipients of all ages for further assessment would be impractical and counterproductive. Conversely, a frailty prevalence less than observed here would be more manageable but may risk underestimating ‘true’ frailty in our patients and depriving at-risk individuals from potential intervention. Van Loon et al. [28] demonstrated a frailty prevalence of 75% in incident elderly (aged ≥65 years) haemodialysis patients by the gold-standard CGA, with the FP displaying good positive predictive value but poor negative predictive value for CGA-defined frailty. We can speculate that the instruments returning higher rates of frailty within the FITNESS cohort, such as the FI, may represent a closer estimate of ‘true’ frailty prevalence in this cohort. However, the very strong correlation between raw FI and CFS scores may suggest that the CFS, properly applied using assessment of ADL disability, may be a suitable alternative. The CFS is simple and speedy to use, and therefore carries significant advantage in clinical practice over the more detailed FI. However, true prognostication value will be determined by linkage of each frailty instrument to long-term adverse outcomes, and that is planned as part of the FITNESS study.
To the best of our knowledge, this study is the first to directly compare commonly used frailty instruments in a large prevalent haemodialysis cohort. One of its major strengths is study recruitment representative of the local cohort, featuring a diverse mix of demographics, comorbidities and socio-economic backgrounds. This should provide reassurance for translation of these findings to clinical application in real-world cohorts. Limitations include the use of frailty assessment as a single baseline measure in a cross-sectional analysis rather than prospective serial measurements over time. This is important as the frailty syndrome appears to be a dynamic process [31]. Frailty characteristics may also not be translatable across different cohorts and should be interpreted with caution in non-British populations.
To conclude, our work identifies a high burden of frailty in a large prospective cohort of prevalent British haemodialysis patients regardless of frailty definition. At present, lack of consensus in frailty assessment for haemodialysis patients limits translatability between cohorts, prohibiting collaborations for clinical research. Our data, highlighting significant variability between different measures, reinforces the importance of achieving consensus on this issue. However, the optimal instrument requires a balance between ease of use, utility and predictive value for adverse outcomes. Prospective monitoring of the FITNESS cohort, with record linkage to clinical outcomes, will provide further insight into the clinical sequelae associated with different frailty instruments and aid such discussions to achieve consensus.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This work was supported by the Queen Elizabeth Hospital Charity (Fund Number 17-3-886).
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract form.
Supplementary Material
Contributor Information
Benjamin M Anderson, Department of Nephrology and Transplantation, Queen Elizabeth Hospital, Birmingham, UK; Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK.
Muhammad Qasim, Department of Nephrology and Transplantation, Queen Elizabeth Hospital, Birmingham, UK; Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK.
Gonzalo Correa, Department of Nephrology, Hospital del Salvador, Santiago, Chile.
Felicity Evison, Department of Health Informatics, Queen Elizabeth Hospital, Birmingham, UK.
Suzy Gallier, Department of Health Informatics, Queen Elizabeth Hospital, Birmingham, UK; PIONEER: HDR-UK hub in Acute Care, Birmingham, UK.
Charles J Ferro, Department of Nephrology and Transplantation, Queen Elizabeth Hospital, Birmingham, UK; Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK.
Thomas A Jackson, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK; Department of Healthcare for Older People, Queen Elizabeth Hospital, Birmingham, UK.
Adnan Sharif, Department of Nephrology and Transplantation, Queen Elizabeth Hospital, Birmingham, UK; Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK.
REFERENCES
- 1. Morley JE, Vellas B, van Kan GA et al. Frailty consensus: acall to action. J Am Med Dir Assoc 2013; 14: 392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sy J, Johansen KL. The impact of frailty on outcomes in dialysis. Curr Opin Nephrol Hypertens 2017; 26: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner G, Clegg A. Best practice guidelines for the management of frailty: A British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014; 43: 744–747 [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–727 [DOI] [PubMed] [Google Scholar]
- 6. Perna S, Francis MDA, Bologna C et al. Performance of Edmonton Frail Scale on frailty assessment: Its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr 2017; 17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fried LP, Ferrucci L, Darer J et al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004; 59: M255–M263 [DOI] [PubMed] [Google Scholar]
- 9. Bouillon K, Kivimaki M, Hamer M et al. Measures of frailty in population-based studies: An overview. BMC Geriatr 2013; 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT et al. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook 2010; 58: 76–86 [DOI] [PubMed] [Google Scholar]
- 11. De Vries NM, Staal JB, van Ravensberg CD et al. Outcome instruments to measure frailty: A systematic review. Ageing Res Rev 2011; 10: 104–114 [DOI] [PubMed] [Google Scholar]
- 12. Bergman H, Ferrucci L, Guralnik J et al. Frailty: An emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62: 731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellis G, Whitehead MA, Robinson D et al. Comprehensive geriatric assessment for older adults admitted to hospital: Meta-analysis of randomised controlled trials. BMJ 2011; 343: d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 2004; 52: 1929–1933 [DOI] [PubMed] [Google Scholar]
- 15. Woods NF, LaCroix AZ, Gray SL et al. Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 2005; 53: 1321–1330 [DOI] [PubMed] [Google Scholar]
- 16. McAdams-DeMarco MA, Law A, Salter ML et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013; 61: 896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sy J, McCulloch CE, Johansen KL. Depressive symptoms, frailty, and mortality among dialysis patients. Hemodial Int 2019; 23: 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johansen KL, Dalrymple LS, Glidden D et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 2016; 11: 626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia-Canton C, Rodenas A, Lopez-Aperador C et al. Frailty in hemodialysis and prediction of poor short-term outcome: Mortality, hospitalization and visits to hospital emergency services. Ren Fail 2019; 41: 567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johansen KL, Chertow GM, Jin C et al. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007; 18: 2960–2967 [DOI] [PubMed] [Google Scholar]
- 21. Anderson BM, Dutton M, Day E et al. Frailty Intervention Trial iN End-Stage patientS on haemodialysis (FITNESS): study protocol for a randomised controlled trial. Trials 2018; 19: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Relton C, Torgerson D, O’Cathain A et al. Rethinking pragmatic randomised controlled trials: Introducing the “cohort multiple randomised controlled trial” design. BMJ 2010; 340: c1066. [DOI] [PubMed] [Google Scholar]
- 23. Noble S, McLennan D, Noble M et al. The English Indices of Deprivation 2019. 2019. Available from https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (1 February 2021, date last accessed)
- 24. Boyd PJ, Nevard M, Ford JA et al. The electronic frailty index as an indicator of community healthcare service utilisation in the older population. Age Ageing 2019; 48: 273–277 [DOI] [PubMed] [Google Scholar]
- 25. Clegg A, Bates C, Young J et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012; 22: 276–282 [PMC free article] [PubMed] [Google Scholar]
- 27. UK Renal Registry. UK Renal Registry 22nd Annual Report. Data to 31 December 2018. Bristol, UK, 2020. Available from renal.org/audit-research/annual-report (1 February 2021, date last accessed)
- 28. van Loon IN, Goto NA, Boereboom FT et al. Frailty screening tools for elderly patients incident to dialysis. Clin J Am Soc Nephrol 2017; 12: 1480–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pradhananga S, Regmi K, Razzaq N et al. Ethnic differences in the prevalence of frailty in the United Kingdom assessed using the electronic Frailty Index. Aging Med (Milton) 2019; 2: 168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kucirka LM, Grams ME, Lessler J et al. Association of race and age with survival among patients undergoing dialysis. JAMA 2011; 306: 620–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang PO, Michel JP, Zekry D. Frailty syndrome: A transitional state in a dynamic process. Gerontology 2009; 55: 539–549 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.