ABSTRACT
Background
Although it is well known that low bone mineral density (BMD) is associated with an increased risk of cardiovascular disease (CVD) and mortality in the general population, the prognostic role of bone mineral density (BMD) has not been established in the chronic kidney disease (CKD) population. Therefore we aimed to evaluate the association between BMD and the risk of CVD and cardiovascular mortality in patients with predialysis CKD.
Methods
This prospective cohort study was conducted with 1957 patients with predialysis CKD Stages 1–5. BMD was measured using dual-energy X-ray absorptiometry and coronary arterial calcification (CAC) scores were evaluated using coronary computed tomography. The primary outcome was a major adverse cardiovascular event (MACE).
Results
When patients were classified based on total hip BMD T-score tertiles stratified by sex, the lowest BMD tertile was significantly associated with an increased risk of MACE {hazard ratio 2.16 [95% confidence interval (CI) 1.25–3.74]; P = 0.006}. This association was also shown with BMD at the femur neck but not with BMD at lumbar spine. In the subgroup of 977 patients with follow-up CACs at their fourth year, 97 (9.9%) showed accelerated CAC progression (>50/year), and BMD was inversely associated with accelerated CAC progression even after adjusting for the baseline CAC score [odds ratio 0.75 (95% CI 0.58–0.99); P = 0.039]. In addition, baseline CAC was associated with an increased risk of MACEs after adjusting for total hip T-score.
Conclusions
Low BMD was significantly associated with CAC progression and MACEs in patients with predialysis CKD.
Keywords: bone mineral density, cardiovascular disease, chronic kidney disease, coronary calcification, osteoporosis
GRAPHICAL ABSTRACT
Graphical Abstract.
INTRODUCTION
Low bone mineral density (BMD) is a common public health problem highly prevalent in postmenopausal women and the elderly and associated with fracture, disability and mortality [1–3]. For several decades, epidemiological studies have reported an inverse association between BMD and vascular calcification, cardiovascular disease (CVD) and mortality in the general population [4–8]. In addition, mounting evidence has ascertained the epidemiological and genetic linkage between low BMD and atherosclerotic CVD outcomes [9–12].
In patients with chronic kidney disease (CKD), BMD is lower than in the general population [13] and it tends to decrease as renal function declines [14]. The 2009 Kidney Disease: Improving Global Outcomes (KDIGO) guideline did not recommend the routine measurement of BMD, because BMD had not been considered to be a major determining factor of bone quality in most patients with CKD [15]. CKD mineral and bone disorder (CKD-MBD) is a complex bone disease caused by abnormal mineral metabolism and uremic milieu [16], and BMD can vary with the type of CKD-MBD [17]. Patients with CKD have a higher risk of fracture than the general population [18] and recent observational studies have reported that low BMD may be a risk factor for fracture in patients with predialysis and dialysis-dependent CKD [19–21]. Accordingly, the KDIGO guidelines for the management of CKD-MBD were updated in 2017 and suggest measuring BMD to assess fracture risk [22]. Nevertheless, the relationship between BMD and CVD is inconclusive in patients with CKD, despite the increasing association between CVD and low BMD in the general population.
CVD is the leading cause of death in patients with CKD [23]. In addition, coronary arterial calcification (CAC) is a common pathological change of atherosclerotic heart disease and an important predictor of cardiovascular mortality in patients with CKD [24, 25]. Previously, observational studies reported that low BMD is associated with CAC progression and an increased risk of cardiovascular events (CVEs) in patients with dialysis-dependent CKD [26, 27]. However, to the best of our knowledge, only cross-sectional studies have shown an inverse relationship between BMD and vascular calcification in patients with predialysis CKD [28, 29]. Therefore we aimed to evaluate the relationship between low BMD and cardiovascular outcomes in a large Korean predialysis CKD cohort. In addition, we also examined the association between low BMD and CAC progression in this cohort.
MATERIALS AND METHODS
Study design and population
This study was conducted with subjects participating in the KoreaN cohort study for Outcome in patients With CKD (KNOW-CKD). The detailed design, inclusion criteria and methods of the study were previously described (NCT01630486; http://www.clinicaltrials.gov) [30]. Between April 2011 and February 2016, adult patients ages 20–75 years with various causes of CKD were screened and a total of 2238 patients were included in the KNOW-CKD study. Because there might be artifacts in measuring CAC, we further excluded 135 patients with a history of coronary arterial disease with percutaneous coronary intervention [31]. In addition, 94 patients without a CAC measurement at baseline and 52 without a BMD measurement at baseline were also excluded. Thus a total of 1957 patients were included in this study. We first examined the relationship between BMD and cardiovascular outcomes in 1957 patients. Because patients were required to undergo CAC measurement in year 4 of follow-up according to the KNOW-CKD protocol, we further examined the relationship between BMD and CAC progression in a subgroup of 977 patients (Supplementary data, Figure S1). This study was performed in accordance with the Declaration of Helsinki and the study protocol was approved by the institutional review boards of the participating centers.
Data collection
Baseline sociodemographic data, anthropometric measurements, comorbidities and medication histories were extracted from the electronic data management system of the KNOW-CKD. Blood samples were obtained after at least 8 h of fasting and centrifuged within 1 h for serum separation. Blood and urine samples were sent to the central laboratory of the KNOW-CKD (Lab Genomics, Seongnam, Republic of Korea). Serum creatinine was measured using an isotope dilution mass spectrometry traceable method and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [32]. The serum intact parathyroid hormone (PTH) level was measured using an electrochemiluminescence immunoassay (Roche Diagnostics, Penzberg, Germany). The 1,25-dihydroxyvitamin D [1,25(OH)2D] level was measured using radioimmunoassay (Beckman Coulter, Fullerton, CA, USA).
Measurement of BMD and CAC
BMD was estimated using a dual-energy X-ray absorptiometry (DXA) system (Hologic, Marlbourough, MA, USA). We measured BMD for the total hip, femoral neck and lumbar spine (L1–L4) at baseline (Supplementary data, Figure S2) and those results were expressed as T-scores [standard deviation (SD) from the average BMD value in the healthy young population]. All patient CAC scores were calculated using electrocardiography-gated multidetector 3-mm sliced computed tomography (CT) and 40 consecutive images were obtained within 100 ms. CAC was defined as a calcific plaque with a density of ≥130 HU within a 1-mm2 area. A total CAC score was quantified using the Agatston scoring method [33]. CAC was measured at baseline and year 4 of the study and yearly changes in CAC were calculated as follows: (CAC score at year 4 − CAC score at baseline)/duration between the two measurements [34].
Primary outcome
The primary outcome of this study was the cardiovascular outcome defined as major adverse CVEs (MACEs). A MACE was defined as death from cardiovascular causes or any type of CVE, including acute myocardial infarction, unstable angina, receiving percutaneous coronary artery intervention or coronary bypass surgery, ischemic or hemorrhagic stroke, congestive heart failure and symptomatic arrhythmia, that required hospitalization during the follow-up period.
Definition of accelerated CAC progression
The association between BMD and CAC progression was evaluated in a subgroup of 977 patients with CAC measurement scores in year 4 of follow-up. We defined accelerated CAC progression as CAC progression >50/year, which was the 90th percentile of our cohort.
Statistical analyses
All patients were first classified into tertiles of total hip T-scores in each sex. Continuous variables are expressed as mean ± SD and compared using one-way analysis of variance. Linear-by-linear association was evaluated and presented using P for trend. The normality of distribution was determined using the Kolmogorov–Smirnov test. Nonparametric variables are expressed as medians and interquartile ranges and compared using the Jonckheere–Terpstra test for trend analysis among groups. Categorical variables are expressed as numbers and percentages and compared by the chi-square test. The relationship between the BMD tertiles and MACEs was further evaluated using the cause-specific hazard regression model. Because we have stopped checking the occurrence of mortality and CVE after initiation of dialysis, according to the KNOW-CKD protocol, incident end-stage renal disease (ESRD) before the occurrence of the primary outcome was considered as a competing risk. We also examined this analysis using the T-score as a continuous variable and BMD per se (g/cm2) instead of T-score tertiles. To evaluate the relationship between T-score and accelerated CAC progression, multivariable binary logistic regression analysis was conducted. All the above analyses were also conducted with BMD measured at the femur neck and lumbar spine. Statistical significance was defined as P < 0.05. All statistical analyses were conducted using SPSS version 23.0 (IBM, Armonk, NY, USA).
RESULTS
Baseline characteristics
Baseline characteristics of 1957 patients according to the total hip T-score tertiles in each sex are presented in Table 1. The mean age was 53.0 ± 12.2 years, which tended to be younger in the higher tertiles, and 59.8% were men. The history of cerebrovascular disease and congestive heart failure was comparable among tertiles. The baseline eGFR was 54.3 ± 31.2 mL/min/1.73 m2 and eGFR gradually increased in high T-score tertiles (P for trend <0.001). Serum calcium (Ca) levels tended to increase in high T-score tertiles, whereas serum phosphate (P), PTH and random urine protein:creatinine ratio (UPCR) tended to decrease (P for trend <0.001, all). The drug prescription of Ca-based P binders was more frequent in the lower-tertile groups (P for trend <0.001).
Table 1.
Baseline characteristics of patients according to BMD of total hip tertiles
| Variables | Total hip T-score tertiles |
Total | P for trend | ||
|---|---|---|---|---|---|
| Tertile 1 −3.6 to −0.1 (♂) −3.8 to −0.9 (♀) |
Tertile 2 0.0–0.9 (♂) −0.8–0.2 (♀) |
Tertile 3 1.0–4.7(♂) −0.3–3.7 (♀) |
|||
| Participants, n | 645 | 672 | 640 | 1957 | |
| Age (years), mean ± SD | 57.2 ± 11.5 | 52.7 ± 11.8 | 49.2 ± 11.9 | 53.0 ± 12.2 | <0.001 |
| Sex (male), n (%) | 382 (59.2) | 400 (59.5) | 388 (60.6) | 1170 (59.8) | 0.641 |
| Current smoker, n (%) | 93 (14.4) | 112 (16.7) | 107 (16.7) | 312 (16.0) | 0.268 |
| DM, n (%) | 232 (36.0) | 209 (31.1) | 171 (26.7) | 612 (31.3) | <0.001 |
| HTN, n (%) | 627 (97.2) | 634 (94.3) | 618 (96.6) | 1879 (96.0) | 0.551 |
| PVD, n (%) | 27 (4.2) | 10 (1.5) | 13 (2.0) | 50 (2.6) | 0.010 |
| Cerebrovascular disease, n (%) | 42 (6.5) | 38 (5.7) | 29 (4.5) | 109 (5.6) | 0.125 |
| Congestive heart failure, n (%) | 9 (1.4) | 6 (0.9) | 4 (0.6) | 19 (1.0) | 0.161 |
| Charlson comorbidity index, mean ± SD | 2.5 ± 1.5 | 2.2 ± 1.5 | 1.8 ± 1.6 | 2.2 ± 1.6 | <0.001 |
| BMI (kg/m2), mean ± SD | 23.7 ± 3.3 | 24.5 ± 3.2 | 25.4 ± 3.6 | 24.5 ± 3.4 | <0.001 |
| Waist:hip ratio, mean ± SD | 0.90 ± 0.07 | 0.89 ± 0.07 | 0.90 ± 0.07 | 0.90 ± 0.07 | 0.920 |
| SBP (mmHg), mean ± SD | 128.4 ± 17.3 | 128.0 ± 15.7 | 129.0 ± 16.6 | 128.4 ± 16.5 | 0.555 |
| DBP (mmHg), mean ± SD | 75.8 ± 11.6 | 77.5 ± 10.6 | 78.0 ± 11.3 | 77.1 ± 11.2 | <0.001 |
| CKD stage, n (%) | <0.001 | ||||
| Stage 1 | 64 (9.9) | 114 (17.0) | 159 (24.8) | 337 (17.2) | |
| Stage 2 | 92 (14.3) | 128 (19.0) | 162 (25.3) | 382 (19.5) | |
| Stage 3a | 89 (13.8) | 123 (18.3) | 110 (17.2) | 322 (16.5) | |
| Stage 3b | 149 (23.1) | 133 (19.8) | 121 (18.9) | 403 (20.6) | |
| Stage 4 | 183 (28.4) | 140 (20.8) | 74 (11.6) | 397 (20.3) | |
| Stage 5 | 68 (10.5) | 34 (5.1) | 14 (2.2) | 116 (5.9) | |
| Creatinine (mg/dL), mean ± SD | 2.1 ± 1.3 | 1.8 ± 1.0 | 1.5 ± 0.8 | 1.8 ± 1.1 | <0.001 |
| eGFR (mL/min/1.73 m2), mean ± SD | 44.1 ± 27.8 | 54.3 ± 30.8 | 64.9 ± 31.2 | 54.3 ± 31.2 | <0.001 |
| Ca (mg/dL), mean ± SD | 9.0 ± 0.6 | 9.2 ± 0.5 | 9.2 ± 0.5 | 9.1 ± 0.5 | <0.001 |
| P (mg/dL), mean ± SD | 3.8 ± 0.7 | 3.7 ± 0.7 | 3.6 ± 0.6 | 3.7 ± 0.7 | <0.001 |
| Albumin (g/dL), mean ± SD | 4.1 ± 0.5 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | <0.001 |
| Triglyceride (mg/dL), mean ± SD | 150.8 ± 92.6 | 156.1 ± 99.0 | 165.3 ± 108.3 | 157.5 ± 100.5 | 0.011 |
| HDL cholesterol (mg/dL), mean ± SD | 49.0 ± 16.6 | 49.7 ± 15.2 | 50.0 ± 14.8 | 49.6 ± 15.6 | 0.263 |
| CRP (mg/L)a | 0.6 (0.2–1.7) | 0.6 (0.2–1.6) | 0.6 (0.2–1.6) | 0.6 (0.2–1.7) | 0.285 |
| PTH (pg/mL)a | 64.1 (40.2–105.7) | 48.8 (33.2–79.5) | 44.0 (29.5–65.3) | 51.0 (33.0–82.8) | <0.001 |
| 1,25(OH)2D (ng/mL), mean ± SD | 28.2 ± 14.6 | 32.8 ± 18.6 | 33.3 ± 16.4 | 31.4 ± 16.8 | <0.001 |
| UPCR (g/g)a | 0.6 (0.2–1.6) | 0.4 (0.1–1.5) | 0.4 (0.1–1.2) | 0.5 (0.1–1.4) | <0.001 |
| ACEi, n (%) | 67 (10.4) | 78 (11.6) | 75 (11.7) | 220 (11.2) | 0.438 |
| ARB, n (%) | 512 (79.4) | 548 (81.5) | 511 (79.8) | 1571 (80.2) | 0.854 |
| Statin, n (%) | 342 (53.0) | 335 (49.9) | 305 (47.7) | 982 (50.2) | 0.055 |
| Ca-based P binder, n (%) | 75 (11.6) | 56 (8.3) | 39 (6.1) | 170 (8.7) | <0.001 |
| Baseline CAC scorea | 6.3 (0.0–140.2) | 0.0 (0.0–67.4) | 0.0 (0.0–24.2) | 0.5 (0.0–69.5) | <0.001 |
| BMD (g/cm2), mean ± SD | |||||
| Total hip | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.2 | <0.001 |
| Femur neck | 0.7 ± 0.2 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.2 | <0.001 |
| Lumbar spine | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.2 | <0.001 |
| T-scorea | |||||
| Total hip | −1.1 (−1.6 to −0.5) | 0.2 (−0.1–0.5) | 1.4 (1.0–2.0) | 0.2 (−0.6–1.0) | <0.001 |
| Femur neck | −1.5 (−2.1 to −1.0) | −0.3 (−0.7–0.1) | 0.9 (0.4–1.5) | −0.3 (−1.1–0.6) | <0.001 |
| Lumbar spine | −1.1 (−2.0 to −0.3) | −0.1 (−0.8–0.7) | 0.9 (0.1–1.7) | −0.1 (−1.0–0.9) | <0.001 |
Data are expressed as median and interquartile range and compared by Jonckheere–Terpstra test.
PVD, peripheral vascular disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; CRP, C-reactive protein; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Association between BMD and MACE
During the median follow-up of 4.2 years, a total of 115 MACEs occurred. When examining the association between BMD and MACEs using cause-specific hazard regression analyses, the risk of MACEs continuously increased in the lowest tertile compared with the highest tertile in all models (Table 2). In the final model (Model 3), after adjusting for confounding factors including baseline CAC score, patients in the lowest tertile had a hazard ratio (HR) of 2.16 [95% confidence interval (CI) 1.25–3.74; P = 0.006] compared with the highest tertile. Further analyses with T-score (as a continuous variable) and BMD per se (g/cm2) also showed a significant inverse relationship between BMD and MACEs. This significant relationship between BMD and MACEs was also present when BMD was measured at the femur neck, but was not significant with BMD at the lumbar spine (Supplementary data, Table S1). The relationship between BMD and MACEs was still consistently present in a subgroup of 977 patients with follow-up CAC measurement in year 4 of the study (Supplementary data, Table S2).
Table 2.
Relationship between total hip BMD and MACEs
| BMD | Unadjusted |
Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
T-score tertiles | ||||||||
| Tertile 1 | 2.42 (1.49–3.95) | <0.001 | 1.44 (1.07–1.93) | 0.016 | 2.03 (1.17–3.51) | 0.011 | 2.16 (1.25–3.74) | 0.006 |
| Tertile 2 | 1.53 (0.91–2.57) | 0.108 | 1.27 (0.94–1.72) | 0.117 | 1.26 (0.71–2.23) | 0.423 | 1.26 (0.71–2.22) | 0.428 |
| Tertile 3 | (Reference) | – | (Reference) | – | (Reference) | – | (Reference) | – |
| T-score as a continuous variable | 0.79 (0.68–0.91) | 0.002 | 0.80 (0.67–0.95) | 0.010 | 0.76 (0.63–0.91) | 0.003 | 0.76 (0.64–0.91) | 0.003 |
| BMD (per 0.1 g/cm2 increase) | 0.86 (0.76–0.96) | 0.007 | 0.85 (0.75–0.97) | 0.019 | 0.83 (0.72–0.95) | 0.008 | 0.83 (0.73–0.96) | 0.009 |
BMD, bone mineral density; MACE, major adverse cardiovascular events. Model 1: adjusted for age, sex, eGFR, smoking, waist:hip ratio, SBP, history of DM and cerebrovascular disease.
Model 2: Model 1 + serum Ca-P product, triglyceride, PTH (data were log transformed), UPCR (data were log transformed), use of statins and Ca-based P binder.
Model 3: Model 2 + baseline CAC score.
Association between BMD and baseline CAC
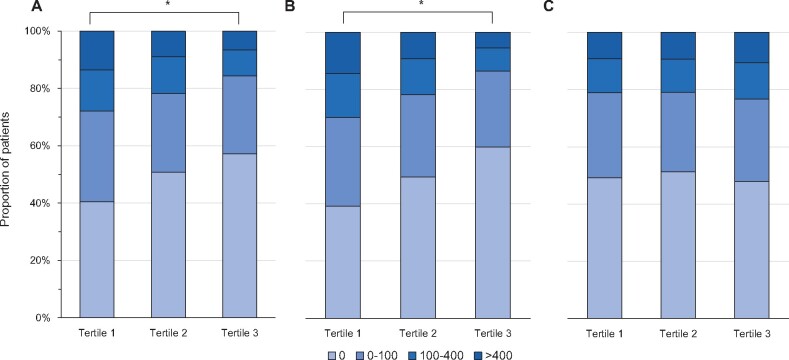
Among the participants, 989 (50.5%) patients had coronary Ca deposition (CAC >0) at baseline. There was a significant tendency for coronary Ca to present more often in the lower total hip T-score tertile groups (P for trend <0.001) (Figure 1A). Moreover, high CAC scores >100 were present in 180 (27.9%) patients in the lowest tertile at baseline and there was also a tendency that CAC scores >100 were more frequently seen in the low T-score tertile groups (P for trend <0.001). This inverse association was also seen in femur neck BMD (Figure 1B) but not in lumbar spine BMD (Figure 1C). Log-transformed CAC scores were also significantly decreased in high BMD tertiles in total hip and femur neck BMD (P for trend <0.001) but not in lumbar spine BMD (P for trend = 0.420) (Supplementary data, Figure S3). When we categorized patients into the presence of coronary Ca deposition at baseline, a significant association between BMD and MACEs was present in 989 patients with coronary Ca deposition at baseline, but not in those without (Supplementary data, Table S3).
FIGURE 1:
Box plot for baseline CAC score according to total hip T-score tertiles in 1957 patients with CKD: (A) total hip, (B) femur neck and (C) lumbar spine. *P < 0.001.
Association between BMD and CAC progression
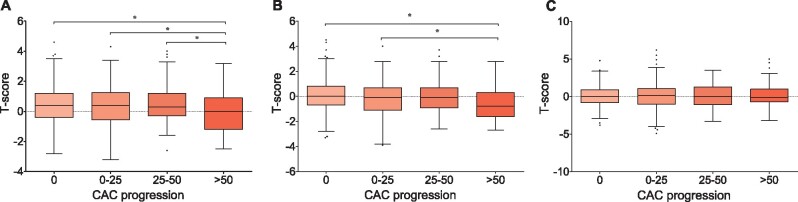
We further examined the relationship between BMD and CAC progression in 977 patients measured for follow-up CAC in year 4. The baseline characteristics of these 977 patients are presented in Supplementary data, Table S4. During the 4 years of follow-up, 435 (44.5%) patients had no CAC progression, 345 (35.3%) had CAC progression of <25/year, 64 (6.6%) had progression of 25–50/year and 97 (9.9%) had progression of >50/year. Importantly, patients with accelerated CAC progression (>50/year) had significantly lower levels of total hip and femur neck T-scores than nonprogressors (Figure 2A and B). However, T-scores from the lumbar spine were comparable among the CAC progression groups (P = 0.976; Figure 2C). In multistep logistic regression analyses, after stepwise adjustment of confounding factors including baseline CAC score, the total hip T-score was still inversely associated with accelerated CAC progression in Model 3 [odds ratio (OR) 0.75 (95% CI 0.58–0.99); P = 0.039] (Table 3).
FIGURE 2:
Box-and-whisker plot for baseline T-score according to CAC progression in 977 patients who had follow-up BMD at year 4. Boxes represent median and 25th–75th percentiles, whiskers represent upper and lower extreme values and dots represent outliers: (A) total hip, (B) femur neck and (C) lumbar spine. *P < 0.05.
Table 3.
Relationship between BMD and accelerated progression of CAC in subgroup of 977 patients who had follow-up CAC at year 4
| BMD | Unadjusted |
Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| T-score | ||||||||
| Total hip | 0.75 (0.63–0.89) | 0.001 | 0.70 (0.55–0.87) | 0.002 | 0.69 (0.54–0.88) | 0.003 | 0.75 (0.58–0.99) | 0.039 |
| Femur neck | 0.69 (0.58–0.82) | <0.001 | 0.75 (0.59–0.93) | 0.011 | 0.74 (0.58–0.95) | 0.016 | 0.83 (0.64–1.07) | 0.152 |
| Lumbar spine | 1.02 (0.89–1.18) | 0.759 | 0.93 (0.79–1.10) | 0.399 | 0.94 (0.79–1.11) | 0.446 | 0.97 (0.80–1.18) | 0.764 |
| BMD (per 0.1 g/cm2 increase) | ||||||||
| Total hip | 0.85 (0.75–0.96) | 0.012 | 0.82 (0.70–0.97) | 0.019 | 0.80 (0.67–0.96) | 0.016 | 0.85 (0.70–1.03) | 0.101 |
| Femur neck | 0.76 (0.66–0.86) | <0.001 | 0.80 (0.68–0.94) | 0.008 | 0.77 (0.65–0.92) | 0.003 | 0.84 (0.69–1.01) | 0.060 |
| Lumbar spine | 1.01 (0.91–1.12) | 0.881 | 0.96 (0.86–1.09) | 0.534 | 0.95 (0.84–1.08) | 0.451 | 0.97 (0.84–1.11) | 0.632 |
Model 1: adjusted for age, sex, eGFR, smoking, waist:hip ratio, SBP, history of DM and cerebrovascular disease.
Model 2: Model 1 + serum Ca-P product, triglyceride, PTH (data were log transformed), UPCR (data were log transformed), use of statin and calcium-based P binder.
Model 3: Model 2 + baseline CAC score.
Association between CAC and MACEs
As expected, there was a significant relationship between CAC and MACEs. In the cause-specific hazard regression model, baseline CAC scores >100 were associated with an increased risk of MACEs in all models (Table 4). In Model 3, adjusting for total hip T-score, patients with baseline CAC scores between 100 and 400 [HR 3.05 (95% CI 1.52–6.13); P = 0.002] and those with CAC scores >400 [HR 5.90 (95% CI 2.96–11.73); P < 0.001] had a significantly increased risk of MACEs than those without the presence of CAC.
Table 4.
Relationship between baseline CAC and MACEs
| Baseline CAC | Unadjusted |
Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| 0 | Reference | – | Reference | – | Reference | – | Reference | – |
| 0–100 | 2.19 (1.26–3.82) | 0.006 | 1.37 (0.74–2.52) | 0.315 | 1.47 (0.79–2.75) | 0.219 | 1.45 (0.78–2.71) | 0.237 |
| 100–400 | 4.98 (2.77–8.94) | <0.001 | 2.90 (1.48–5.66) | 0.002 | 3.13 (1.58–6.27) | 0.001 | 3.05 (1.52–6.13) | 0.002 |
| >400 | 11.69 (6.89–19.83) | <0.001 | 5.43 (2.80–10.55) | <0.001 | 6.09 (3.06–12.13) | <0.001 | 5.90 (2.96–11.73) | <0.001 |
Model 1: adjusted for age, sex, eGFR, smoking, waist:hip ratio, SBP, history of DM and cerebrovascular disease.
Model 2: Model 1 + serum Ca-P product, triglyceride, PTH (data were log transformed), UPCR (data were log transformed), use of statin and calcium-based P binder.
Model 3: Model 2 + baseline total hip T-score.
DISCUSSION
In this study we demonstrated that low BMD was associated with an increased risk of MACEs in patients with predialysis CKD. In addition, patients with low BMD had higher baseline CAC scores and faster CAC progression, both of which were significantly related to a high risk of MACEs. This relationship was shown when BMD was measured at the total hip and femur neck, but not when it was measured at the lumbar spine.
The pathophysiologic mechanisms that explain the relationship between low BMD and CVD are not yet fully elucidated. However, this relationship can be partially described as sharing common risk factors. Well-known cardiovascular risk factors, including old age, diabetes mellitus (DM), hypertension (HTN), smoking, low physical activity and chronic inflammation, are also well-described risk factors of low BMD and fracture [35]. In patients with CKD, not only are these factors more prevalent than in the general population, but nontraditional risk factors such as uremic milieu and disrupted mineral metabolism also exacerbate bone loss and CVD [36]. However, this study showed that low BMD was still independently associated with an increased risk of MACEs after adjusting for confounding factors including age, DM, HTN and CKD-MBD markers in patients with CKD.
Recent evidence suggests that several circulating factors from the bones affect abnormal vascular biology [37]. It is hypothesized that there is a functional interplay between the bones and vasculature, called the ‘bone–vascular axis’. Bone morphogenetic protein (BMP) is a potential candidate that induces the bone–vascular axis. A previous study reported that single nucleotide polymorphisms in the BMP7 gene were associated with inverse relationships between BMD and vascular calcification in the coronary, carotid and abdominal aortas in patients with DM [9]. Another experimental study also showed that BMP-2 accelerates the osteoblastic differentiation of vascular smooth muscle cells and induces vascular calcification in rats [38]. Meanwhile, another molecule acts as a protective factor for vascular calcification. Deficient matrix GLA protein as a negative regulator factor for vascular calcification in mice exhibiting inappropriate extraosseous calcification combined with osteopenia and fractures [39]. Osteoprotegerin, the soluble decoy receptor ofnuclear factor ϰB ligand, is an inhibitor of osteoclastogenesis and has been reported as a protective factor for vascular Ca deposition [40]. Therefore we surmised that several factors associated with bone loss might be associated with pathologic vascular calcification and increased cardiovascular outcomes in patients with CKD.
Furthermore, low BMD can be a partial sign of CKD-MBD, which encompasses the abnormalities of volume, mineralization and turnover in bone [16]. CKD-MBD can be developed from the early stage of CKD [41]. Low BMD can be observed both in high- and low-turnover diseases depending on the bone formation and resorption rate [36]. A high-turnover status is characterized by retarded osteoblastic differentiation and is manifested as a fibroblastic phenotype, which leads to bone marrow fibrosis and release of Ca and P into extracellular fluid [42]. These ions consequently promote the transformation of vascular smooth muscle cells into osteoblast-like cells and calcification of the vascular wall [43]. In addition, a low-turnover status shows a markedly reduced bone formation rate and low bone capacity as a buffer to handle the extra Ca load [43]. In addition, it is also reported that both high- and low-turnover bone diseases are associated with an increased risk of CVD in patients with CKD [44]. Of note, a previous study conducted by Barreto et al. [45] reported that a low trabecular bone volume confirmed by bone biopsy was associated with the development of CAC in dialysis-dependent patients. Moreover, the improvements of bone turnover from both high- and low-turnover bone diseases were related to lower CAC progression. Therefore, bone turnover management can be a useful therapeutic option for preventing CVD in patients with CKD. Further study is needed to clarify whether the improvement of bone turnover can increase BMD and reduce CVEs in the CKD population.
In this study, significant negative correlation between BMD and CAC was shown when DXA BMD was measured at the total hip and femur neck and not at the lumbar spine. A previous cross-sectional study also reported that femoral DXA BMD was inversely associated with aortic calcification but vertebral BMD was an unreliable measure in patients with CKD [29]. Because extra-osseous calcification and osteophytes are highly prevalent, these conditions can interfere with the precise measurement of vertebral BMD by DXA in patients with CKD. Therefore a quantitative CT scan, which is not affected by those conditions, may be a more accurate method for DXA BMD at the lumber spine in patients with CKD. A previous study demonstrated that vertebral BMD measured using a quantitative CT scan was significantly associated with CAC in patients with predialysis CKD and ESRD [28, 46]. Malluche et al. [26] also reported that total hip and femoral neck BMD had an inverse relationship with CAC in both measurements with DXA and quantitative CT in the dialysis population. However, lumbar BMD by quantitative CT was correlated with CAC, whereas lumbar BMD by DXA was not. Based on previous and present observations, predicting the risk of CVD using DXA BMD at the lumbar spine may be limited due to pre-existing extra-osseous soft tissue or vascular calcification.
There are some limitations for this study that should be discussed. First, although we suggested a significant relationship between BMD and CAC progression, the number of participants with measured CAC in year 4 was less than half. Thus there might be selection bias in this study. However, BMD was still associated with MACEs in this subgroup of 977 patients. Second, some factors affecting BMD, such as the concomitant use of steroids and bisphosphonates, were not considered in this study. Third, because this study was based on a nationwide multicenter cohort, measurements of BMD and CAC were conducted at each center and there might be intercenter variability. Finally, the incidence of MACEs in this study was lower than in other studies [47]. It is well known that there are ethnic differences in the risk of CVD among patients with CKD, and generally the Asian CKD population has a lower risk than the Western CKD population [48, 49].
In conclusion, low BMD measured at the total hip and femur neck was an independent predictor of the incidence of MACEs in patients with predialysis CKD. In addition, BMD was inversely associated with CAC and its progression during the 4-year follow-up period.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.
FUNDING
This work was supported by the research program funded by the Korea Disease Control and Prevention Agency (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101 and 2019E320102) and Soonchunhyang University Research Fund. Funding sources had no involvement in the study design; collection, analysis and interpretation of data; writing of the report; or the decision to submit the article for publication.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The dataset generated and analysed during this study is available at http://www.know-ckd.org/ckd/main/main.html.
Contributor Information
Hyoungnae Kim, Division of Nephrology, Soonchunhyang University Seoul Hospital, Seoul, Korea.
Joongyub Lee, Prevention and Management Center, Inha University Hospital, Incheon, Korea.
Kyu-Beck Lee, Department of Internal Medicine, Sungkyunkwan University School of Medicine, Kangbuk Samsung Hospital, Seoul, Korea.
Yeong-Hoon Kim, Department of Internal Medicine, Busan Paik Hospital, College of Medicine, Inje University, Busan, Korea.
Namki Hong, Department of Internal Medicine, Division of Endocrinology and Metabolism, Yonsei University College of Medicine, Seoul, Korea.
Jung Tak Park, Department of Internal Medicine, Yonsei University, Institute of Kidney Disease Research, College of Medicine, Seoul, Korea.
Seung Hyeok Han, Department of Internal Medicine, Yonsei University, Institute of Kidney Disease Research, College of Medicine, Seoul, Korea.
Shin-Wook Kang, Department of Internal Medicine, Yonsei University, Institute of Kidney Disease Research, College of Medicine, Seoul, Korea.
Kyu Hun Choi, Department of Internal Medicine, Yonsei University, Institute of Kidney Disease Research, College of Medicine, Seoul, Korea.
Kook-Hwan Oh, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
Tae-Hyun Yoo, Department of Internal Medicine, Yonsei University, Institute of Kidney Disease Research, College of Medicine, Seoul, Korea.
REFERENCES
- 1. Bliuc D, Nguyen ND, Milch VE et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009; 301: 513–521 [DOI] [PubMed] [Google Scholar]
- 2. Wright NC, Looker AC, Saag KG et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014; 29: 2520–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park EJ, Joo IW, Jang MJ et al. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2011. Yonsei Med J 2014; 55: 1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Browner WS, Seeley DG, Vogt TM et al. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet 1991; 338: 355–358 [DOI] [PubMed] [Google Scholar]
- 5. Sennerby U, Melhus H, Gedeborg R et al. Cardiovascular diseases and risk of hip fracture. JAMA 2009; 302: 1666–1673 [DOI] [PubMed] [Google Scholar]
- 6. Shen C, Deng J, Zhou R et al. Relation between bone mineral density, bone loss and the risk of cardiovascular disease in a Chinese cohort. Am J Cardiol 2012; 110: 1138–1142 [DOI] [PubMed] [Google Scholar]
- 7. Wiklund P, Nordstrom A, Jansson JH et al. Low bone mineral density is associated with increased risk for myocardial infarction in men and women. Osteoporos Int 2012; 23: 963–970 [DOI] [PubMed] [Google Scholar]
- 8. Choi SH, An JH, Lim S et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol (Oxf) 2009; 71: 644–651 [DOI] [PubMed] [Google Scholar]
- 9. Freedman BI, Bowden DW, Ziegler JT et al. Bone morphogenetic protein 7 (BMP7) gene polymorphisms are associated with inverse relationships between vascular calcification and BMD: the Diabetes Heart Study. J Bone Miner Res 2009; 24: 1719–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qu X, Huang X, Jin F et al. Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013; 166: 385–393 [DOI] [PubMed] [Google Scholar]
- 11. Ye C, Xu M, Wang S et al. Decreased bone mineral density is an independent predictor for the development of atherosclerosis: a systematic review and meta-analysis. PLoS One 2016; 11: e0154740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veronese N, Stubbs B, Crepaldi G et al. Relationship between low bone mineral density and fractures with incident cardiovascular disease: a systematic review and meta-analysis. J Bone Miner Res 2017; 32: 1126–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fidan N, Inci A, Coban M et al. Bone mineral density and biochemical markers of bone metabolism in predialysis patients with chronic kidney disease. J Investig Med 2016; 64: 861–866 [DOI] [PubMed] [Google Scholar]
- 14. Kim CS, Bae EH, Ma SK et al. Chronic kidney disease–mineral bone disorder in Korean patients: a report from the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). J Korean Med Sci 2017; 32: 240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kidney Disease: Improving Global Outcomes CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone Disorder (CKD-MBD). Kidney Int Suppl 2009; 7: 1–59 [DOI] [PubMed] [Google Scholar]
- 16. Moe S, Drueke T, Cunningham J et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69: 1945–1953 [DOI] [PubMed] [Google Scholar]
- 17. Hruska KA, Mathew S, Lund R. Osteoporosis and cardiovascular disease: lessons from chronic kidney disease. Clin Cases Miner Bone Metab 2008; 5: 35–39 [PMC free article] [PubMed] [Google Scholar]
- 18. Kwon YE, Choi HY, Kim S et al. Fracture risk in chronic kidney disease: a Korean population-based cohort study. Kidney Res Clin Pract 2019; 38: 220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yenchek RH, Ix JH, Shlipak MG et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 2012; 7: 1130–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iimori S, Mori Y, Akita W et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant 2012; 27: 345–351 [DOI] [PubMed] [Google Scholar]
- 21. West SL, Lok CE, Langsetmo L et al. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res 2015; 30: 913–919 [DOI] [PubMed] [Google Scholar]
- 22. Kidney Disease: Improving Global Outcomes CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 24. Matsuoka M, Iseki K, Tamashiro M et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol 2004; 8: 54–58 [DOI] [PubMed] [Google Scholar]
- 25. Xie Q, Ge X, Shang D et al. Coronary artery calcification score as a predictor of all-cause mortality and cardiovascular outcome in peritoneal dialysis patients. Perit Dial Int 2016; 36: 163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malluche HH, Blomquist G, Monier-Faugere MC et al. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol 2015; 26: 2534–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu TM, Lin CL, Shu KH et al. Increased risk of cardiovascular events in end-stage renal disease patients with osteoporosis: a nationwide population-based cohort study. Osteoporos Int 2015; 26: 785–793 [DOI] [PubMed] [Google Scholar]
- 28. Filgueira A, Carvalho AB, Tomiyama C et al. Is coronary artery calcification associated with vertebral bone density in nondialyzed chronic kidney disease patients? Clin J Am Soc Nephrol 2011; 6: 1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toussaint ND, Lau KK, Strauss BJ et al. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 2007; 23: 586–593 [DOI] [PubMed] [Google Scholar]
- 30. Oh KH, Park SK, Park HC et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): design and methods. BMC Nephrol 2014; 15: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalisz K, Buethe J, Saboo SS et al. Artifacts at cardiac CT: physics and solutions. Radiographics 2016; 36: 2064–2083 [DOI] [PubMed] [Google Scholar]
- 32. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agatston AS, Janowitz WR, Hildner FJ et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827–832 [DOI] [PubMed] [Google Scholar]
- 34. Budoff MJ, Young R, Lopez VA et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013; 61: 1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eastell R, Newman C, Crossman DC. Cardiovascular disease and bone. Arch Biochem Biophys 2010; 503: 78–83 [DOI] [PubMed] [Google Scholar]
- 36. Covic A, Vervloet M, Massy ZA et al. Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol 2018; 6: 319–331 [DOI] [PubMed] [Google Scholar]
- 37. Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol 2012; 8: 529–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakagawa Y, Ikeda K, Akakabe Y et al. Paracrine osteogenic signals via bone morphogenetic protein-2 accelerate the atherosclerotic intimal calcification in vivo. Arterioscler Thromb Vasc Biol 2010; 30: 1908–1915 [DOI] [PubMed] [Google Scholar]
- 39. Luo G, Ducy P, McKee MD et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997; 386: 78–81 [DOI] [PubMed] [Google Scholar]
- 40. Bennett BJ, Scatena M, Kirk EA et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/− mice. Arterioscler Thromb Vasc Biol 2006; 26: 2117–2124 [DOI] [PubMed] [Google Scholar]
- 41. Drueke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int 2016; 89: 289–302 [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez EA, Lund RJ, Martin KJ et al. Treatment of a murine model of high-turnover renal osteodystrophy by exogenous BMP-7. Kidney Int 2002; 61: 1322–1331 [DOI] [PubMed] [Google Scholar]
- 43. Schlieper G, Schurgers L, Brandenburg V et al. Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant 2016; 31: 31–39 [DOI] [PubMed] [Google Scholar]
- 44. Raggi P, Kleerekoper M. Contribution of bone and mineral abnormalities to cardiovascular disease in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3: 836–843 [DOI] [PubMed] [Google Scholar]
- 45. Barreto DV, Barreto FDC, de Carvalho AB et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis 2008; 52: 1139–1150 [DOI] [PubMed] [Google Scholar]
- 46. Chen Z, Qureshi AR, Ripsweden J et al. Vertebral bone density associates with coronary artery calcification and is an independent predictor of poor outcome in end-stage renal disease patients. Bone 2016; 92: 50–57 [DOI] [PubMed] [Google Scholar]
- 47. Chen J, Budoff MJ, Reilly MP et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2017; 2: 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka K, Watanabe T, Takeuchi A et al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int 2017; 91: 227–234 [DOI] [PubMed] [Google Scholar]
- 49. Kim H, Yoo TH, Choi KH et al. Baseline cardiovascular characteristics of adult patients with chronic kidney disease from the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). J Korean Med Sci 2017; 32: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated and analysed during this study is available at http://www.know-ckd.org/ckd/main/main.html.