ABSTRACT
Introduction
Acute tubulointerstitial nephritis (ATIN) is a common cause of acute kidney injury with various etiologies. It has been shown that autoimmune-related ATIN (AI-ATIN) has a higher recurrence rate and a greater likelihood of developing into chronic kidney disease compared with drug-induced ATIN, yet misdiagnosis at renal biopsy is not uncommon.
Methods
Patients who were clinicopathologically diagnosed as ATIN from January 2006 to December 2015 in Peking University First Hospital were enrolled. Clinical, pathological and follow-up data were collected. Serum samples on the day of renal biopsy were collected and tested for anti-C-reactive protein (CRP) antibodies. CRP and its linear peptides were used as coating antigens to detect antibodies. Statistical analysis was used to assess the diagnostic value of the antibodies.
Results
Altogether 146 patients were enrolled. The receiver operating characteristic–area under the curve of the anti-CRP antibody for the identification of late-onset AI-ATIN was 0.750 (95% confidence interval 0.641–0.860, P < 0.001) and the positivity was associated with ATIN relapse (adjusted hazard ratio = 4.321, 95% confidence interval 2.402–7.775, P < 0.001). Antibodies detected by CRP linear peptide 6 (PT6) were superior with regard to differentiating patients with AI-ATIN, while antibodies detected by peptide 17 (PT17) could predict ATIN relapse. Antibodies detected by these two peptides were positively correlated with the severity of tubular dysfunction and pathological injury.
Conclusions
Serum anti-CRP antibody could be used to differentiate late-onset AI-ATIN and predict relapse of ATIN at the time of renal biopsy. The CRP linear peptides PT6 and PT17 could be used as coating antigens to detect anti-CRP antibodies, which may provide more information for the clinical assessment of ATIN.
Keywords: acute kidney injury, acute tubulointerstitial nephritis, autoantibody, C-reactive protein, relapse
BACKGROUND
Acute tubulointerstitial nephritis (ATIN) is a clinicopathological syndrome characterized by tubular impairment and a reduction in the renal filtration rate as well as infiltration of inflammatory cells into the renal interstitium that is often accompanied by, but not dependent on, degeneration of the renal tubular epithelium [1, 2]. ATIN is found in 10–30% of kidney biopsies performed in the context of acute kidney injury (AKI), and there is an increasing trend [2–7]. The etiology of ATIN can be related to drugs, autoimmune disorders, malignancies, infections, metabolic disorders, toxins and undetermined causes. The most commonly recognized etiologies of ATIN are drug-induced ATIN (DATIN) and autoimmune-related ATIN (AI-ATIN). AI-ATIN primarily includes tubulointerstitial nephritis and uveitis (TINU) syndrome, primary Sjögren’s syndrome-induced ATIN (SS-ATIN), immunoglobulin G4 (IgG4)-related disease-induced ATIN (IgG4-ATIN) and ATIN caused by other autoimmune disorders [5, 8–13]. Unlike most DATINs, in which renal injury might be spontaneously restored after prompt withdrawal of the culprit drugs, AI-ATIN has a higher recurrence rate and a greater likelihood of developing into chronic kidney disease (CKD); therefore, long-term follow-up and enhanced immunosuppressive therapies are warranted [13–17]. However, patients with AI-ATIN might have kidney injury prior to other organ involvement, and autoimmune antibodies might not be present at the time of renal biopsy, which could lead to a misdiagnosis of DATIN, especially when patients have been taking various medications to treat non-specific symptoms. Early identification is crucial for close monitoring during follow-up to achieve a positive outcome of AI-ATIN [9, 13, 14, 18, 19].
Serum IgG antibodies against C-reactive protein (CRP) were found to be elevated in TINU patients, one of the most common causes for AI-ATIN; elevated levels were also identified in case reports of ATIN with other autoimmune causes [14, 20, 21]. However, whether anti-CRP antibodies could serve as a biomarker for AI-ATIN remains unknown. The aim of our study is to investigate the presence of anti-CRP antibodies in a prospective biopsy-proven ATIN cohort and explore its potential diagnostic value. In studies involving ATIN population performed thus far, anti-CRP antibody detection requires CRP derived from human fluid, which adds costs and restricts the application of the detection of anti-CRP antibodies for the assessment of ATIN. Therefore, we further assessed the value of synthesized CRP linear peptides as an alternative and comparable detecting antigen for anti-CRP antibody detection.
MATERIALS AND METHODS
Patients
Patients were enrolled from a prospective cohort who were clinically and pathologically diagnosed as ATIN from 1 January 2006 to 31 December 2015, at Peking University First Hospital. Patients with concurrent glomerular and vascular disease, ongoing immunosuppressive therapy before renal biopsy or insufficient serum specimen were excluded. The study adhered to the declaration of Helsinki and was approved by the Committee on Research Ethics of Peking University First Hospital. Informed consents were obtained from all participants.
Collection and evaluation of clinical, pathological and follow-up data
Clinical data were collected from the hospital medical records, including sex, age, present history, past medical history, medication history, serum creatinine (sCr), blood uric acid, urine total protein, glycosuria, urinary N-acetyl beta-d-glucosidase (NAG), urinary α1-microglobublin (A1M), blood white blood cell count, serum CRP, erythrocyte sedimentation rate, serum IgG, serum C3, serum C4 and autoimmune antibodies, including antinuclear antibodies, anti-extractable nuclear antigen antibodies, anti-neutrophil cytoplasmic antibodies, rheumatoid factor, Coombs’ test results, etc. The estimated glomerular filtration rate was calculated with the CKD Epidemiology Collaboration equation and is expressed in mL/min/1.73 m2 [22]. The disease course was defined by AKI, acute kidney disease (AKD) and CKD according to theKidney Disease: Improving Global Outcomes (KDIGO) criteria and the latest Acute Dialysis Quality Initiative consensus [23–25].
Standard processing of kidney biopsy specimens included light microscopy, immunofluorescence and electron microscopy. For light microscopy observation, all samples were stained with hematoxylin and eosin, periodic acid–Schiff, Masson’s trichrome and Jones methenamine silver. Semiquantitative scores for tubular simplification, interstitial edema, interstitial inflammatory infiltrates, tubulitis, tubular atrophy and interstitial fibrosis were calculated based on the Banff Working Classification criteria [23, 26, 27].
The etiology of ATIN was determined by at least three nephrologists, according to patient’s medication history, clinical characteristics and laboratory test results, as outlined in our previous study [13]. AI-ATIN patients included ATIN related to TINU syndrome, Sjögren’s syndrome, IgG4-related disease or other autoimmune disorders. DATIN was defined by definite histories of culprit drugs usage before kidney injury and no evidence of uveitis or systemic autoimmune diseases. ATIN caused by infections, metabolic disorders, toxins, malignancies or heredity were all grouped as having other causes.
Patients were followed up at least once per month for the first 6 months and then every 3 months after renal biopsy. Clinical and laboratory assessments were routinely performed. Ocular examinations and test for autoimmune antibodies were performed as needed. The etiology of ATIN was revaluated at every visit as mentioned above. Patients who were non-AI-ATIN at renal biopsy, yet presented with uveitis or other manifestations and laboratory abnormalities that met the definitions for TINU syndrome, Sjögren’s syndrome, IgG4-related disease or other autoimmune disorders during follow-up, were reclassified as AI-ATIN and termed as ‘late-onset AI-ATIN’. The change in etiological diagnosis was made before and independently of anti-CRP antibody assay.
ATIN relapse was defined by an acute increase (at least 2-fold) in the urinary levels of NAG concurrent with an increase (at least 50%) in the sCr level within 3 months, with the careful exclusion of systemic infections, medication, renal perfusion or post-renal obstruction as the cause for the increase in sCr.
Detection of anti-CRP antibodies by ELISA
Anti-CRP antibodies were detected as previously described with some modifications [21]. Briefly, human CRP (Sigma, St Louis, MO, USA) was diluted to 5.0 μg/mL with 0.05 mol/L bicarbonate buffer (pH 9.6) and coated onto polystyrene microtiter plates (Nalge NUNC, Rochester, NY, USA) at 4°C overnight. Bicarbonate buffer alone was used in antigen-free wells. Free binding sites were blocked with 1% protein-free blocking buffer (Sangon Biotech, Shanghai, China) in PBST for 2 h at room temperature. Serum samples were diluted to 1:100 with PBST. Alkaline phosphatase-conjugated goat anti-human IgG (Sigma) antibody at a dilution of 1:5000 in PBST was added to the plates for 30 min at 37°C. One milligram per milliliter p-nitrophenyl phosphate dissolved in 1 M diethanolamine (pH 9.8) with 0.5 mM MgCl2 was then added and allowed to react for 30 min. Spectrophotometric absorbance was read at 405 nm. The same duplicate positive control, negative control and blank control (PBST diluent without plasma) were added to every plate, reaching a fixed absorbance as quality controls between plates. Binding results are expressed as the percentage of the positive control sample, which was considered to be 100 arbitrary units (AUs). Coefficient of variations inter- and intra-assay for the negative control ˂6% was acceptable. The final coefficient of variation was 5.18% inter-assay and was 2.07% intra-assay for anti-CRP antibody.
Serum samples of 80 healthy donors with informed consent were used as normal controls. These healthy donors were from our Health Examination Center with normal urinalysis and blood tests and had been excluded for kidney diseases, acute infectious diseases, autoimmune diseases, chronic diseases, etc. The cutoff value for positivity was deemed as mean + 2 standard deviations (SD) of anti-CRP antibody in 80 healthy donors (20.02 AU).
Detection of serum IgG with CRP linear peptides
A panel of 22 peptides was designed covering the amino acid (AA) sequence of CRP (UniProtKB/Swiss-Prot—P02741), based on a series of 20-mer peptides overlapping by ∼10 AAs (Supplementary data, Table S1). Adjustments to peptide length were made to avoid splitting reported functional regions of CRP. Peptides were synthesized on an automatic peptide synthesizer using 9-fluorenyl-methyloxycarbonyl chemistry (Sangon Biotech). Except for using 10 μmol/L peptide as the coating antigen, the method of detecting serum IgG with the peptides was in accordance with the anti-CRP antibody described above. The same duplicate positive control, negative control and blank control (PBST diluent without plasma) were added to every plate, reaching a fixed absorbance as quality controls between plates. Binding results are expressed as the percentage of the positive control sample, which was considered to be 100 AUs. The coefficient of variation inter-assay was 3.61% for antibody detected by CRP linear peptide 6 (PT6-ab), 3.92% for antibody detected by CRP linear peptide 8 (PT8-ab), 2.95% for antibody detected by CRP linear peptide 12 (PT12-ab) and 4.05% for antibody detected by CRP linear peptide 17 (PT17-ab). The overall intra-assay coefficient of variation was ˂2.0%. Serum from 40 healthy donors was used as normal controls for this assay. The cutoff value for positivity was deemed as higher than mean + 2 SD of normal controls (n = 40), which was 24.28 AU for PT6-ab, 16.44 AU for PT8-ab, 15.36 AU for PT12-ab and 10.93 AU for PT17-ab.
Statistical analysis
The statistical analysis was performed using SPSS version 26.0 statistical software (IBM, Armonk, NY, USA). Quantitative data are expressed as the means ± SDs or medians with 25th and 75th percentiles and were compared by t-test, one-wayAnalysis of Variance(ANOVA), the Mann–Whitney test or the Kruskal–Wallis test as appropriate. Categorical data are expressed as frequencies (percentages), and were compared using the chi-squared test or Fisher’s exact test, as appropriate. Pearson and Spearman correlations were performed to analyze the relationships between the levels of antibodies and the clinical data, whenever appropriate. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC) was used to measure the diagnostic value of the antibody Using ATIN relapse as an endpoint, Kaplan–Meier curves were used to analyze the correlation of the antibody with ATIN relapse. Follow-up for ATIN relapse was censored at the last follow-up visit for each participant. Multivariable Cox regression model was performed to estimate hazard ratios (HRs) of the antibodies, adjusted for sex, age, sCr level and serum IgG level at the time of the renal biopsy. sCr and IgG levels were selected based on statistical significance in the univariate analysis. A two-sided P < 0.05 was defined as statistically significant.
RESULTS
In total, 146 patients with ATIN were enrolled (Figure 1), with an average age of 48.0 ± 12.5 years and a female predominance (70.5%; 103/146). The values of sCr reached 230 (143, 335) μmol/L at the time of renal biopsy and 262 (177, 441) μmol/L at the peak, with 89.7% (131/146) of the patients defined as having AKI or AKD at the time of diagnosis (Supplementary data, Table S2). Immunosuppressive therapy was prescribed to 91.1% (133/146) of the patients, which lasted for 7 (5–11) months.
FIGURE 1:
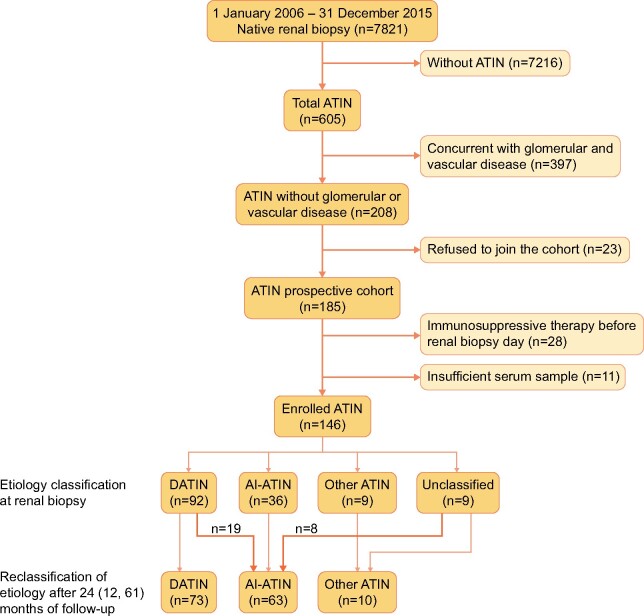
Flow diagram of ATIN patient enrollment and reclassification of etiology. Flow diagram of the study showing the inclusion and exclusion of patients with ATIN and the etiological spectrum changing during the follow-up course. Nineteen patients originally classified as DATIN at renal biopsy are reclassified as AI-ATIN after follow-up. Eight patients unclassified of etiology at renal biopsy are reclassified as AI-ATIN after follow-up.
Patients were followed for 24 (12–61) months. Twenty-seven patients were reclassified as AI-ATIN during follow-up due to late-onset uveitis or other autoimmune disorders as described above. After follow-up, 63 (43.2%) were identified as having AI-ATIN, including 28 (19.2%) with TINU syndrome, 15 (10.3%) with SS-ATIN, 4 (2.7%) with IgG4-ATIN and 16 (11.0%) with other types of AI-ATIN. Seventy-three (50.0%) had DATIN, and 10 had other causes. During follow-up, 61 (41.8%) patients relapsed, which mainly occurred in AI-ATIN, during the tapering or withdrawal of corticosteroids at 6 (4–10) months after the renal biopsy.
Antibody levels, positivity rate and early diagnostic value of anti-CRP antibodies in ATIN patients
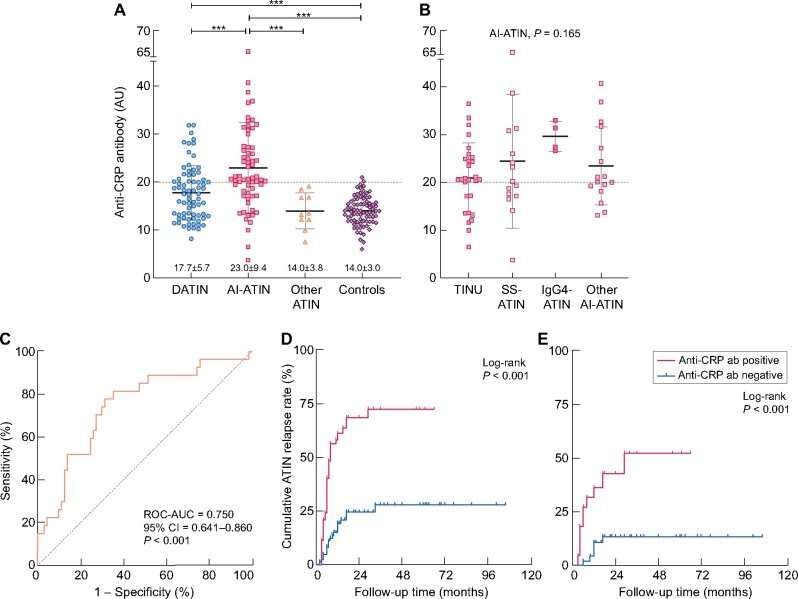
The levels of serum anti-CRP antibody were markedly higher in ATIN patients than in the normal controls (19.7 ± 8.0 AU versus 14.0 ± 3.0 AU, P < 0.001), and were higher in patients with AI-ATIN than in DATIN according to final etiologic diagnosis (23.0 ± 9.4 AU versus 17.7 ± 5.7 AU, P < 0.001, Figure 2A). There was no significant difference in the anti-CRP antibody levels among the various AI-ATIN subgroups (P = 0.165; Figure 2B). When using the mean + 2 SD of anti-CRP antibody in 80 healthy donors (20.02 AU) as the cutoff value for positivity, the positive rate for serum anti-CRP antibody was 42.5% (62/146) in all enrolled ATIN patients, with a higher positive rate in patients with AI-ATIN than in those with DATIN (63.5% versus 30.1%, P < 0.001). The ROC–AUC of the anti-CRP antibody for the identification of late-onset AI-ATIN in patients who were classified as having DATIN or unclassified ATIN at the time of renal biopsy was 0.750 [95% confidence interval (CI) 0.641–0.860, P < 0.001; Figure 2C]. Considering the final diagnostic categories, a positive assay of anti-CRP antibody could identify AI-ATIN with sensitivity of 63.5% and specificity of 73.5%. The positive predictive value was 64.5% and the negative predictive value was 72.6%.
FIGURE 2:
Levels of serum anti-CRP antibody and its diagnostic value for late-onset AI-ATIN and predictive value for ATIN relapse. (A) Levels of anti-CRP antibody in ATIN patients and healthy controls. Patients grouping is based on the final etiology reclassified during follow-up. ***P < 0.001. (B) Levels of anti-CRP antibody in subgroups of patients with AI-ATIN reclassified after follow-up. (C) The ROC–AUC of anti-CRP antibody for identifying late-onset AI-ATIN was 0.750 (0.641–0.860) in patients who were diagnosed as DATIN or ATIN for unknown reasons at renal biopsy. (D) Kaplan–Meier survival curves demonstrate a higher cumulative ATIN relapse rate in patients with positive anti-CRP antibody levels than in patients with negative anti-CRP antibody levels (P < 0.001). All ATIN patients were included in the analysis independently of etiologic diagnosis. (E) Kaplan–Meier survival curves demonstrate a higher cumulative ATIN relapse rate in DATIN patients with positive anti-CRP antibody levels than in patients with negative anti-CRP antibody levels (P < 0.001). DATIN patients with final etiologic diagnosis after follow-up were included.
Predictive ability of the anti-CRP antibody for ATIN relapse
Positivity of the anti-CRP antibody was associated with ATIN relapse (log-rank P < 0.001; Figure 2D). Cox regression analysis showed that positivity for the anti-CRP antibody was associated with ATIN relapse in all enrolled ATIN patients (unadjusted HR = 4.172, 95% CI 2.417–7.202, P < 0.001). After adjusting for age, sex, sCr level and serum IgG level at the time of renal biopsy, anti-CRP antibody remained an independent predictor of ATIN relapse (adjusted HR = 4.321, 95% CI 2.402–7.775, P < 0.001). Positivity of anti-CRP antibody was correlated with ATIN relapse in both patients with DATIN and those with AI-ATIN. The unadjusted HR for ATIN relapse was 4.746 (95% CI 1.722–13.078, P = 0.003; Figure 2E) in DATIN and 2.948 (95% CI 1.398–6.226, P = 0.005) in AI-ATIN, according to final etiologic diagnosis. Positivity for the anti-CRP antibody remained an independent predictor in patients initially diagnosed as DATIN (unadjusted HR = 5.963, 95% CI 2.656–13.387, P < 0.001; Supplementary data, Table S3).
Alternative detection assay for the anti-CRP antibody in patients with ATIN
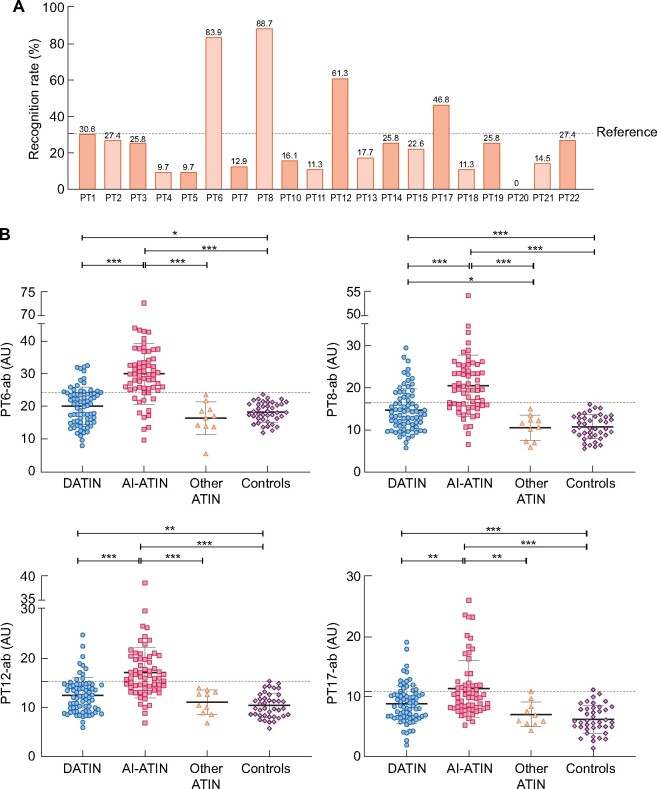
Among 20 synthesized linear CRP, 4 peptides were found to be specifically recognized when peptide 1 (PT1, signal peptide) was used as the nonspecific combining reference (Figure 3A): PT6 (AA 35–57), PT8 (AA 53–72), PT12 (AA 92–111) and PT17 (AA 143–162). We then detected antibodies with these four peptides in the rest of the patients (Figure 3B). The overall positivity rate for antibodies detected by PT6 (PT6-ab) was the highest in the population (45.2%, 66/146), followed by PT8 (PT8-ab, 43.2%, 63/146), PT12 (PT12-ab, 32.2%, 47/146) and PT17 (PT17-ab, 26.7%, 39/146).
FIGURE 3:
Recognition rate of 20 synthesized CRP linear peptides in anti-CRP-positive ATIN patients and PT6-ab, PT8-ab, PT12-ab and PT17-ab in ATIN patients with different etiology groups. (A) Recognition rate of 20 synthesized CRP linear peptides in ATIN patients with positive anti-CRP antibody. (B) Antibody levels were detected by PT6-ab, PT8-ab, PT12-ab and PT17-ab in ATIN patients. Patients grouping is based on the final etiology reclassified during follow-up. *P < 0.05; **P < 0.01; ***P < 0.001.
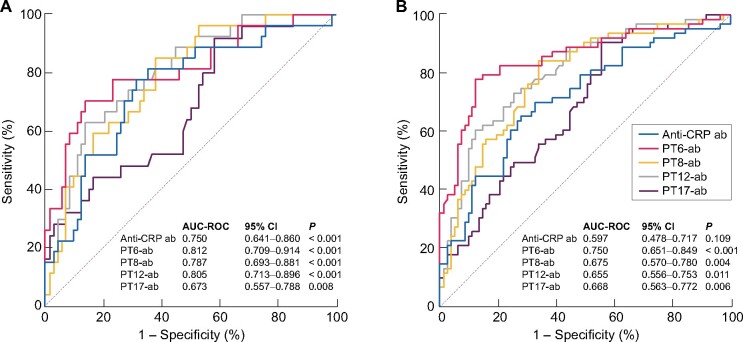
PT6-ab yielded positive results in 79.4% (50/63) of AI-ATIN patients and 21.9% (16/73) of DATIN patients (Figure 3B). It was superior to anti-CRP antibody detection and detection with the three other peptides for the early identification of late-onset AI-ATIN in patients who were diagnosed as DATIN or ATIN for unknown reasons at renal biopsy, with an AUC–ROC of 0.812 (95% CI 0.709–0.914, P < 0.001; Figure 4A). It also was superior with regard to differentiating patients with AI-ATIN at the time of renal biopsy from all other enrolled patients, with an AUC–ROC of 0.750 (95% CI 0.651–0.849, P < 0.001; Figure 4B).
FIGURE 4:
Diagnostic value of antibodies detected by CRP (anti-CRP ab), CRP linear PT6-ab, PT8-ab, PT12-ab and PT17-ab. (A) The ROCs of anti-CRP ab, PT6-ab, PT8-ab, PT12-ab and PT17-ab for identifying late-onset AI-ATIN in patients who were diagnosed as DATIN or ATIN for unknown reasons at renal biopsy. (B) The ROCs of anti-CRP ab, PT6-ab, PT8-ab, PT12-ab and PT17-ab for identifying patients with final etiologic diagnosis of AI-ATIN after reclassification during follow-up in all ATIN.
With regard to ATIN relapse, positivity for antibodies as detected by PT6, PT8, PT12 and PT17 could be potentially used as a predictor according to Cox regression analysis (Table 1). However, only positivity for PT17-ab had an HR comparable to that of anti-CRP antibody for ATIN relapse (unadjusted HR = 4.291, 95% CI 2.582–7.130, P < 0.001). After adjusting for age, sex, sCr level and serum IgG level, a positive result using PT17 remained an indicator of ATIN relapse (adjusted HR = 4.334, 95% CI 2.535–7.411, P < 0.001) comparable to anti-CRP antibody.
Table 1.
Unadjusted and adjusted HRs for the risk of ATIN relapse according to the positivity of antibodies
| Factors | Positivity of antibody alone |
Model 1a |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Anti-CRP antibody (+) | 4.172 | 2.417–7.202 | <0.001 | 4.321 | 2.402–7.775 | <0.001 |
| PT6-ab (+) | 2.356 | 1.406–3.948 | 0.001 | 2.165 | 1.245–3.764 | 0.006 |
| PT8-ab (+) | 2.368 | 1.417–3.957 | 0.001 | 2.188 | 1.253–3.821 | 0.006 |
| PT12-ab (+) | 2.276 | 1.376–3.767 | 0.001 | 2.109 | 1.299–3.709 | 0.010 |
| PT17-ab (+) | 4.291 | 2.582–7.130 | <0.001 | 4.334 | 2.535–7.411 | <0.001 |
Model 1, adjusting for age, gender, serum creatinine and serum IgG at renal biopsy. (+), positivity of the antibody, using mean + 2 SD of healthy controls as positive cut-off value. Cox analysis was performed in all ATIN.
Moreover, both PT6-ab and PT17-ab were found to have a positive correlation with tubular injury and dysfunction, while a similar correlation was not found for anti-CRP antibody (Table 2). PT6-ab was positively correlated with urinary NAG levels (r = 0.192, P = 0.026) and was negatively correlated with serum potassium values (r = −0.250, P = 0.002). PT17-ab was positively correlated with glycosuria (r = 0.259, P = 0.002), renal tubular acidosis (r = 0.199, P = 0.016), urinary A1M levels (r = 0.190, P = 0.034), the pathological semiquantitative scores for interstitial inflammatory infiltrates (r = 0.308, P < 0.001) and tubulitis (r = 0.177, P = 0.032). However, PT17-ab was negatively correlated with serum potassium (r = −0.267, P = 0.001) and serum uric acid (r = −0.180, P = 0.030). Therefore, in addition to determining etiology and predicting relapse, these two detection methods could also be used to assess the severity of ATIN.
Table 2.
Clinical and pathological correlations of anti-CRP antibody, PT6-ab and PT17-ab
| Anti-CRP antibodya |
PT6-aba |
PT17-aba |
|||||
|---|---|---|---|---|---|---|---|
| n | r | P-value | r | P-value | r | P-value | |
| Glycosuria | 146 | 0.059 | 0.477 | 0.108 | 0.195 | 0.259 | 0.002 |
| Renal tubular acidosis | 146 | 0.026 | 0.758 | 0.149 | 0.073 | 0.199 | 0.016 |
| Serum potassium | 146 | −0.119 | 0.153 | −0.250 | 0.002 | −0.267 | 0.001 |
| Urinary NAG | 134 | 0.117 | 0.177 | 0.192 | 0.026 | 0.109 | 0.210 |
| Urinary A1M | 127 | 0.132 | 0.138 | 0.169 | 0.057 | 0.189 | 0.034 |
| sCr at peak | 146 | 0.010 | 0.901 | −0.102 | 0.220 | −0.024 | 0.778 |
| sCr at renal biopsy | 146 | 0.030 | 0.716 | −0.083 | 0.319 | −0.001 | 0.993 |
| Serum uric acid | 146 | −0.046 | 0.584 | −0.152 | 0.067 | −0.180 | 0.030 |
| Serum CRP | 136 | 0.040 | 0.642 | 0.121 | 0.160 | 0.080 | 0.357 |
| Erythrocyte sedimentation rate | 136 | 0.245 | 0.004 | 0.270 | 0.001 | 0.227 | 0.008 |
| Serum IgG | 138 | 0.413 | <0.001 | 0.426 | <0.001 | 0.324 | <0.001 |
| Serum C3 | 134 | 0.054 | 0.534 | 0.198 | 0.022 | 0.038 | 0.662 |
| Serum C4 | 134 | −0.105 | 0.228 | −0.048 | 0.580 | 0.029 | 0.739 |
| Positivity of autoimmune antibodiesb | 146 | 0.213 | 0.010 | 0.249 | 0.002 | 0.166 | 0.045 |
| Loss of brush border, thinned epithelium (area) | 146 | 0.073 | 0.383 | 0.081 | 0.333 | 0.142 | 0.087 |
| Cellular debris, denuded basement membrane (area) | 146 | 0.090 | 0.282 | 0.087 | 0.297 | 0.072 | 0.389 |
| Tubular atrophy | 146 | −0.042 | 0.613 | 0.000 | 0.997 | −0.034 | 0.682 |
| Interstitium edema | 146 | −0.030 | 0.717 | −0.028 | 0.738 | 0.021 | 0.803 |
| Interstitial inflammatory infiltrates | 146 | 0.080 | 0.339 | 0.143 | 0.085 | 0.308 | <0.001 |
| Tubulitis | 146 | 0.078 | 0.352 | 0.098 | 0.240 | 0.177 | 0.032 |
| Interstitial fibrosis | 146 | −0.129 | 0.122 | −0.129 | 0.119 | −0.078 | 0.349 |
Antibody titers.
Positivity of autoimmune antibodies, including positivity of antinuclear antibodies, anti-extractable nuclear antigen antibodies, anti-neutrophil cytoplasmic antibodies, rheumatoid factor, Coombs’ test results, etc.
Bold indicates the correlations with statistic significance.
DISCUSSION
ATIN is a significant cause of AKI, and the incidence is increasing, especially in patients with multiple diseases and medications [2–7, 18, 28, 29]. As an inflammatory disease, unlike most cases of DATIN, patients with AI-ATIN showed higher chances of relapse. Although clinical evidence for immunosuppressive treatment therapy is not solid for DATIN up to now, AI-ATIN usually needed more enhanced and longer courses of immunosuppressive treatment [5, 8–11, 13, 15, 16, 30]. However, in our previous study, 46.2% of AI-ATIN patients were originally presented and treated as DATIN or for unclarified causes at renal biopsy [13]. Therefore, early identification of these cases of AI-ATIN would be very important. It might be helpful for clinicians making better management decisions, leading to better outcomes. However, studies on biomarkers for AI-ATIN have been very limited.
Previous studies found anti-CRP antibody was elevated in TINU syndrome patients and could be used as a predictive biomarker for late-onset uveitis in patients with ATIN. However, anti-CRP antibody was also found to be elevated in a fraction of patients with ATIN without uveitis based on case reports [14, 21, 31, 32]. In our biopsy-proven ATIN cohort, we proved that anti-CRP antibody was elevated in 42.5% patients with various etiologies at the time of the renal biopsy, and was valuable for the early diagnosis of late-onset AI-ATIN. It was also an indicator of a higher likelihood of ATIN relapse.
To date, studies on anti-CRP antibodies in patients with ATIN have used commercialized CRP derived from human fluid samples as the primary antigen, which increases the cost and may restrict the clinical application of the detection of anti-CRP antibodies for ATIN [14, 20, 21]. Using synthesized CRP peptides as antigens to detect the antibodies could substantially reduce the cost and may improve the specificity compared with using the entire protein as the antigen. Thus, we determined whether synthesized CRP linear peptides could be used as an alternative mean of assessing ATIN. Among 20 synthesized peptides, 4 peptides were found to be highly recognized by ATIN with elevated ant-CRP antibody level. These four peptides were close to each other either in sequence or in space of CRP protein (Supplementary data, Figure S1). Antibodies, when detected by PT6, were more specifically elevated in AI-ATIN, and were superior to anti-CRP antibody in AI-ATIN identification. Though PT6-ab was inferior to anti-CRP antibody detection for the prediction of ATIN relapse, PT17-ab could be a useful marker for identifying patients with ATIN who are at high risk for relapse. Moreover, PT6-ab and PT17-ab levels were associated with tubular dysfunction, and PT17-ab levels were associated with the pathological findings of interstitial inflammation and tubulitis. Antibodies detected with these two CRP peptides might provide more information to doctors for evaluation of the severity of ATIN.
In a recent study, a peptide consisting of AA 35–47 of CRP was used to detect antibodies in systemic lupus erythematosus patients. The antibody against that peptide inhibits the modulation of factor H by competitive combining CRP [33]. PT6 in our study included AA 35–47; thus, PT6-ab may have a similar mechanism of autoimmune activation in patients with ATIN as in patients with systemic lupus erythematosus. It is worth mentioning that tubular epithelial cells can express CRP when injured, and autoantibodies against CRP may exacerbate damage in situ [21, 34, 35]. The correlation of PT17-ab with interstitial inflammation and ATIN relapse may involve a process of the activation of autoimmune response. But whether these peptides are autoimmune epitopes in ATIN and how anti-CRP antibodies participate in the pathophysiological processes need further exploration.
This study was an observational study of anti-CRP antibodies in a biopsy-proven ATIN cohort of 146 patients from a single center. The positivity rate and diagnostic and predictive value of anti-CRP antibodies detected by all methods need to be validated in a prospective study and in a larger population. Due to the limited availability of serum samples, pathogenic study was not involved in this study. Further in-depth research is warranted.
In conclusion, we detect that the level of anti-CRP antibody was elevated in 42.5% of the ATIN patients diagnosed over a period of 10 years in our hospital. Anti-CRP antibody could be used to early differentiate late-onset AI-ATIN and predict relapse of ATIN. The CRP linear peptides PT6 and PT17 could be used as coating antigens to detect anti-CRP antibodies, which may be cost-effective for clinical usage and provide more information for the clinicians to assess and manage ATIN with different etiology.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This study was supported by grants from the National Natural Science Foundation of China (No. 91742205 and No. 81625004), the Beijing Young Scientist Program (BJJWZYJH01201910001006), the National Science and Technology Major Projects for ‘Major New Drugs Innovation and Development’ of China (No. 2017ZX09304028) and the Peking University Clinical Scientist Program by the Fundamental Research Funds for the Central Universities.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
Supplementary Material
Contributor Information
Jun-Wen Huang, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China.
Tao Su, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China.
Ying Tan, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China.
Jin-Wei Wang, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China.
Jia-Wei Tang, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China.
Su-Xia Wang, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Renal Pathology Room, Peking University First Hospital, Beijing, China; Laboratory of Electron Microscopy, Peking University First Hospital, Beijing, China.
Gang Liu, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Renal Pathology Room, Peking University First Hospital, Beijing, China.
Ming-Hui Zhao, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China.
Li Yang, Department of Medicine, Renal Division, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Renal Pathology Room, Peking University First Hospital, Beijing, China.
REFERENCES
- 1. Clarkson MR, Giblin L, O’Connell FP et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 2004; 19: 2778–2783 [DOI] [PubMed] [Google Scholar]
- 2. Raghavan R, Eknoyan G. Acute interstitial nephritis - a reappraisal and update. Clin Nephrol 2014; 82: 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goicoechea M, Rivera F, López-Gómez JM; Spanish Registry of Glomerulonephritis. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2013; 28: 112–115 [DOI] [PubMed] [Google Scholar]
- 4. Baker RJ, Pusey CD. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2004; 19: 8–11 [DOI] [PubMed] [Google Scholar]
- 5. Wilson GJ, Kark AL, Francis LP et al. The increasing rates of acute interstitial nephritis in Australia: a single centre case series. BMC Nephrol 2017; 18: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bomback AS, Markowitz GS. Increased prevalence of acute interstitial nephritis: more disease or simply more detection. Nephrol Dial Transplant 2013; 28: 16–18 [DOI] [PubMed] [Google Scholar]
- 7. Nussbaum EZ, Perazella MA. Diagnosing acute interstitial nephritis: considerations for clinicians. Clin Kidney J 2019; 12: 808–813 [Google Scholar]
- 8. Caravaca-Fontán F, Fernández-Juárez G, Praga M. Acute kidney injury in interstitial nephritis. Curr Opin Crit Care 2019; 25: 558–564 [DOI] [PubMed] [Google Scholar]
- 9. Muriithi AK, Leung N, Valeri AM et al. Biopsy-proven acute interstitial nephritis, 1993–2011: a case series. Am J Kidney Dis 2014; 64: 558–566 [DOI] [PubMed] [Google Scholar]
- 10. Praga M, González E. Acute interstitial nephritis. Kidney Int 2010; 77: 956–961 [DOI] [PubMed] [Google Scholar]
- 11. Navaratnarajah A, Sambasivan K, Cook TH et al. Predicting long-term renal and patient survival by clinicopathological features in elderly patients undergoing a renal biopsy in a UK cohort. Clin Kidney J 2019; 12: 512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raza MN, Hadid M, Keen CE et al. Acute tubulointerstitial nephritis, treatment with steroid and impact on renal outcomes. Nephrology (Carlton) 2012; 17: 748–753 [DOI] [PubMed] [Google Scholar]
- 13. Su T, Gu Y, Sun P et al. Etiology and renal outcomes of acute tubulointerstitial nephritis: a single-center prospective cohort study in China. Nephrol Dial Transplant 2018; 33: 1180–1188 [DOI] [PubMed] [Google Scholar]
- 14. Li C, Su T, Chu R et al. Tubulointerstitial nephritis with uveitis in Chinese adults. Clin J Am Soc Nephrol 2014; 9: 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moledina DG, Perazella MA. Drug-induced acute interstitial nephritis. Clin J Am Soc Nephrol 2017; 12: 2046–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moledina DG, Wilson FP, Kukova L et al. Urine interleukin-9 and tumor necrosis factor-α for prognosis of human acute interstitial nephritis. Nephrol Dial Transplant 2020; 10.1093/ndt/gfaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Storrar J, Woywodt A, Arunachalam C. AIN’t got no easy answers: recent advances and ongoing controversies around acute interstitial nephritis. Clin Kidney J 2019; 12: 803–807 [Google Scholar]
- 18. Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 2010; 6: 461–470 [DOI] [PubMed] [Google Scholar]
- 19. Moledina DG, Perazella MA. Treatment of drug-induced acute tubulointerstitial nephritis: the search for better evidence. Clin J Am Soc Nephrol 2018; 13: 1785–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jakuszko K, Krajewska M, Hałoń A et al. Pathogenic role of antibodies against monomeric C-reactive protein in tubulointerstitial nephritis and uveitis syndrome. Intern Med J 2014; 44: 809–812 [DOI] [PubMed] [Google Scholar]
- 21. Tan Y, Yu F, Qu Z et al. Modified C-reactive protein might be a target autoantigen of TINU syndrome. Clin J Am Soc Nephrol 2011; 6: 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 24. Chawla LS, Bellomo R, Bihorac A et al. ; Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 25. Kellum JA, Lameire N, Aspelin P et al. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury (AKI). Kidney Int Suppl 2011; 2: 20121 [Google Scholar]
- 26. Racusen LC, Solez K, Colvin RB et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723 [DOI] [PubMed] [Google Scholar]
- 27. Solez K, Colvin RB, Racusen LC et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8: 753–760 [DOI] [PubMed] [Google Scholar]
- 28. Cassol C, Satoskar A, Lozanski G et al. Anti-PD-1 immunotherapy may induce interstitial nephritis with increased tubular epithelial expression of PD-L1. Kidney Int Rep 2019; 4: 1152–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sury K, Perazella MA. The nephrotoxicity of new immunotherapies. Expert Rev Clin Pharmacol 2019; 12: 513–521 [DOI] [PubMed] [Google Scholar]
- 30. Muriithi AK, Leung N, Valeri AM et al. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int 2015; 87: 458–464 [DOI] [PubMed] [Google Scholar]
- 31. Pu L, Zhang P, Li G. IgG4-related acute interstitial nephritis and the potential role of mCRP autoantibodies: a case report. Ren Fail 2019; 41: 657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin ZS, Liu XL, Cui Z et al. Acute tubulointerstitial nephritis with germinal centers in antineutrophil cytoplasmic antibody-associated vasculitis: a case report and literature review. Medicine (Baltimore) 2019; 98: e18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li QY, Li HY, Fu G et al. Autoantibodies against C-reactive protein influence complement activation and clinical course in lupus nephritis. J Am Soc Nephrol 2017; 28: 3044–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jabs WJ, Lögering BA, Gerke P et al. The kidney as a second site of human C-reactive protein formation in vivo. Eur J Immunol 2003; 33: 152–161 [DOI] [PubMed] [Google Scholar]
- 35. Schwedler SB, Guderian F, Dämmrich J et al. Tubular staining of modified C-reactive protein in diabetic chronic kidney disease. Nephrol Dial Transplant 2003; 18: 2300–2307 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.